Abstract
Whole exome sequencing (WES) uses next generation sequencing technology to provide information on nearly all functional, protein-coding regions in an individual's genome. Due to the vast amount of information and incidental findings that can be generated from this technology, patient preferences must be investigated to help clinicians consent and return results to patients. Patients (n=19) who were previously clinically diagnosed with Lynch syndrome, but received uninformative negative Lynch syndrome genetic results through traditional molecular testing methods participated in semi-structured interviews after WES testing but before return of results to explore their views of WES and preferences for return of results. Analyses of interview results found that nearly all participants believed that the benefits of receiving all possible results generated from WES outweighed the undesirable effects. The majority of participants conveyed that relative to coping with a cancer diagnosis, information generated from WES would be manageable. Importantly, participants' experience with Lynch syndrome influenced their notions of genetic determinism, tolerance for uncertain results, and family communication plans. Participants would prefer to receive WES results in person from a genetic counselor or medical geneticist so that an expert could help explain the meaning and implications of the potentially large quantity and range of complicated results. These results underscore the need to study various populations with regard to the clinical use of WES in order to effectively and empathetically communicate the possible implications of this new technology and return results.
Keywords: Whole exome sequencing, Patient preferences, Return of results, Genetic counseling practice
Introduction
Whole exome sequencing (WES) is a powerful genetic test that uses next generation sequencing to provide information on nearly all functional, protein-coding regions, referred to as “exons,” in an individual's genome. Since most Mendelian disorders originate from mutations in exons, WES has the potential to uncover many more underlying genetic mutations than conventional genetic testing can encompass (Bamshad et al., 2011; Haimovich, 2011). With recent declines in price and increasing numbers of Clinical Laboratory Improvement Amendments (CLIA)-approved laboratories offering the testing clinically, WES has already begun to revolutionize genetic testing (Jamal et al., 2013). Thus far, WES has successfully identified underlying mutations for rare, monogenic syndromes (Choi et al., 2009; Ng et al., 2010), unveiled complex features of more common monogenic disorders (Grillo et al., 2013), and accelerated diagnostic discoveries (Worthey et al., 2011).
In the near future, WES is likely to become a routine clinical, and perhaps first-tier, test for patients who have suspected genetic diseases but have not yet been diagnosed with a particular condition. An illustrative example is Lynch syndrome, the most common cause of hereditary colorectal cancer. Individuals can be diagnosed with Lynch syndrome 1) based on family history criteria in addition to specific tumor findings, particularly high levels of microsatellite instability (MSI-H) and absence of one or more mismatch repair proteins based on immunohistochemistry (IHC) results, or 2) through current genetic testing for mutations or deletions in any of seven known mismatch repair genes (MLH1, MSH2, MSH6, MSH3, PMS1, PMS2, and EPCAM) (Ku et al. 2012; Lagerstedt-Robinson et al. 2007; Schneider, 2012). However, the sensitivity for conventional genetic testing for the genetic mutations causative of Lynch syndrome is only around 70 %, leaving many families without accurate ways to establish who is at risk (Lagerstedt-Robinson et al. 2007). Established effective cancer screening and prevention techniques (e.g. colonscopy, prophylactic hysterectomy and bilateral salpingo-oophorectomy, and esophagogastroduodenoscopy) for individuals with Lynch syndrome decrease morbidity and mortality rates while reducing costs (Burt et al. 2013).
In families without previously identified pathogenic mutations but where Lynch syndrome is suspected, the surveillance recommendation is for all family members to follow stringent screening guidelines (Lindor et al., 2006; Mvundura et al. 2010). Because Lynch syndrome screening guidelines are intensive, it is both time and cost-effective to correctly diagnose individuals so that appropriate screening is enacted. Equally as important is the avoidance of the risks and burdens of unnecessary cancer screening for relatives who do not have the familial mutations. If utilized earlier in the diagnostic odyssey, WES results may provide patients with diagnoses more rapidly than several sequential single gene tests or multi gene panels, thus ensuring better and more cost-effective patient care and treatment. Even when identifying conditions without available treatment, WES results may provide diagnostic information and answers for patients and their families.
Despite these transformative benefits, WES can also generate incidental findings that may be unrelated to the condition or phenotype under investigation. In an individual exome, it is estimated that WES will find several thousands of variants, which must be filtered to reflect only those variants of clinical significance (Bamshad et al., 2011; Singleton, 2011). Variants of clinical significance can include carrier status for recessive disorders, later-onset genetic conditions, and predispositions to cancer or common diseases such as diabetes, obesity, and coronary artery disease. In addition, a challenging number of variants of unknown significance or mutations in genes of unknown significance are expected from any sequenced exome.
These outcomes create a perplexing and uncharted scope of issues for both providers and patients, including questions regarding how and which results clinicians should report back to patients, who should be responsible for conducting pre- and post-test counseling sessions and patient follow-up, and who should have access to a patient's whole exome or genome sequencing results. Currently, there are few published guidelines that address patient consent and use of clinical WES, the return of WES results including incidental findings, and the ethical concerns raised by this new technology. The American College of Medical Genetics and Genomics (ACMG) issued a policy statement in 2012 (ACMG Board of Directors, 2012) regarding the clinical application of genomic sequencing that focuses on when to use whole exome or genome sequencing, and more recently in March 2013 issued recommendations as to when and which types of incidental findings to report (Green et al., 2013). Although broad recommendations are well intentioned, providers must nonetheless evaluate the utility of these guidelines with regard to special circumstances of each patient and use their clinical judgment in adhering to or deviating from these recommendations. Discordance among genetic specialists about whether specific incidental findings in clinical whole exome and genome sequencing should be reported to patients or not suggests that even professionals may not be fully prepared to deal with the implications of this new technology (Green et al., 2012).
At present, each laboratory performing WES sets its own policy for reporting incidental findings, and different labs have significantly different WES consent forms (Ambry Genetics, 2012; Baylor College of Medicine Medical Genetics Laboratories, 2013; Emory Genetics Laboratory, 2012). As clinical WES becomes more routine, laboratory consent forms and policies regarding the return of results may need to become more standardized to help clinicians facilitate more consistent pre- and post-test counseling sessions and help patients navigate through the massive amounts of information generated. In order to address the vast amount of potential results generated from WES with patients prior to testing, scalable categorical frameworks (e.g. categorizing genomic sequencing results and incidental findings into “bins” (Berg et al. 2011)) have been proposed to organize types of genomic sequencing results.
Research to assess patient perspectives on which types of results should be returned, how to best communicate results, and what to do with incidental findings is needed to develop effective practices for return of results. Previous research studies evaluating opinions about return of whole exome and genome sequencing results have focused on specific populations such as parents and families of pediatric patients, particular ethnic groups, and healthy members of the public (Tabor et al., 2012; Townsend et al., 2012; Yu et al. 2013). One meta-analysis reported that 90 % of participants, consisting of cancer patients, pregnant women, parents of children with suspected genetic disease, participants in various genetic research studies, and randomly selected residents of Sweden and the United States, wanted to receive all possible personal genetic results from the particular genetic testing research studies within which they were enrolled (Shalowitz & Miller, 2008). Another study appraising the opinions of clinical genetics professionals toward genome sequencing found that 96 % of subjects were interested in learning about only “clinically actionable” incidental findings and an equivalent percentage felt such information should be disclosed to adult patients (Lemke et al. 2012).
Despite the increase in genome sequencing studies, research has not been conducted with adult cancer patients who have previously received uninformative or negative genetic test results. The application of WES in cancer genomics is becoming more popular and feasible. Consequently, gaining insight into patient preferences related to WES is pertinent and pressing (Berg et al., 2011; Haimovich, 2011; Majewski et al. 2011). Additionally, questions remain as to how patients may interpret the value of genomic sequencing results after experiencing a difficult disease such as cancer.
To address such gaps and extend previous research on patient preferences regarding WES, this study explored the preferences of cancer patients who were clinically diagnosed with Lynch syndrome, but for whom traditional molecular tests were unable to detect a deleterious mutation. The specific questions addressed in this study include (1) Which results generated from WES does this patient population want to receive and why? (2) How and by whom do patients wish to receive their WES results and follow-up? (3) What concerns do these patients have about WES? (4) Do patients want direct access to their WES results? (5) Who, if anyone, would this patient population choose to share their results with and why?
Methods
Participants
Participants in this interview study were a subset of participants in a study of WES (Guiltinan et al. 2013). Participants in both studies were cancer patients recruited from the UCSF Cancer Risk Program and Gastrointestinal Cancer Prevention Program Registries. Individuals who met the following criteria were eligible for both studies: (1) high microsatellite instability (MSI) found in Lynch-associated tumors in the absence of one or more mismatch repair (MMR) proteins based on immunochemistry (IHC) results; (2) a family history suggestive of Lynch syndrome; (3) comprehensive germline molecular testing designated as “uninformative negative” of the known mismatch repair genes associated with Lynch syndrome; (4) previous cancer risk genetic counseling for Lynch syndrome; and (5) consent obtained for WES for research purposes. Sixty-eight individuals who met inclusion criteria were contacted by phone by either their previous genetic counselor or one of the primary investigators to discuss both studies and participation requirements. A brief description of WES and the types of results that could be generated were communicated to each potential participant during the initial phone call. Participants were informed that they would only receive cancer-related results generated from WES.
Those who consented to WES (n=32) were also invited to participate in a telephone interview. Consent to WES was initially conducted by phone; subsequently, written consent was obtained in person, by fax or email. A single consent form was used for both studies, using an “opt-out” option for the interview portion of the study. Telephone interviews were conducted after participants consented to WES, but prior to the receipt of results. No incentive for participation was provided to interview participants.
All procedures were approved by the California State University, Stanislaus Institutional Review Board and the University of California, San Francisco Committee on Human Research.
Procedures
The first author (KH), in consultation with an experienced cancer genetic counselor (AB) and a medical anthropologist (GJ), developed a semi-structured interview guide to collect both quantitative and qualitative data. Current literature on the practices and policies of clinical exome sequencing, as well as various commercial laboratory and academic medical center exome sequencing consent forms were consulted when developing the structure and content of the guide. The interview guide consisted of a brief description of WES, structured questions about participants' preferences regarding receiving WES results, and open-ended questions focused on access to and sharing of WES results. Participants also were given the opportunity to voice any concerns or questions about WES not addressed by specific questions. The interview guide was pilot tested on a sample population of four healthy adult individuals who do not have Lynch syndrome who were unfamiliar with WES. The interview guide was then modified to ensure clarity.
The first author conducted all telephone interviews over a 4-month period in 2012. Each topic in the interview guide was discussed with each participant; however, the semi-structured protocol allowed participants to discuss topics of particular interest and the interviewer to elicit specific individual perspectives, as is standard practice in qualitative health research (Berg, 2001).
Data Analysis
Interviews were professionally transcribed verbatim. For those questions with either binary or multiple answer choices, participant answers were quantified. The sample size varies across questions because of the semi-structured protocol. For open-ended questions, qualitative data analysis was conducted using standard methods. The first author initially read each transcript independently several times and developed a coding outline using a directed content analysis approach. Directed content analysis is a common approach for qualitative health research that allows the researcher to use existing theories or research to guide the identification of findings and concepts (Hsieh & Shannon, 2005). A second researcher read and coded each transcript independently. Analyses and coding outlines were compared and were found to be consistent with one another, with a greater than 85 % concordance rate between coders. As new themes emerged, the coding outline was modified and transcripts were reanalyzed accordingly. Segments of text were also grouped by themes and analyzed separately. The researchers independently analyzed the emergent categories before coming together to agree on the main themes.
Quantitative Results
Sample Demographics
Of the 32 participants enrolled in the larger research study performing WES, 19 individuals (59 %) consented to the interview portion of the research project. Individual semi-structured telephone interviews lasting from 25 to 50 min were audio recorded with each participant. As shown in Table 1, the majority of participants were White, female, and ranged in age between 31 and 70 years old. The mean age of the sample was 52.6 years. Seventeen participants (89 %) had at least a college degree or some college education.
Table 1. Demographic details of the cohort (n=19).
| Variable | n | Percent |
|---|---|---|
| Age, years | ||
| 18-30 | 1 | 5.3 |
| 31-50 | 9 | 47.3 |
| 51-70 | 8 | 42.1 |
| 71-90 | 1 | 5.3 |
| Gender | ||
| Female | 13 | 68.4 |
| Male | 6 | 31.6 |
| Ethnicity | ||
| White | 15 | 78.9 |
| Asian | 2 | 10.5 |
| African American | 1 | 5.3 |
| Hispanic | 1 | 5.3 |
| Highest level of education | ||
| Primary school | 1 | 5.3 |
| High school | 1 | 5.3 |
| Some college | 4 | 21.0 |
| College/University | 10 | 52.6 |
| Postgraduate | 3 | 15.8 |
| Occupation | ||
| Administrator/Professional | 4 | 21.0 |
| Tradesperson | 4 | 21.0 |
| Retired | 5 | 26.3 |
| Disabled | 4 | 21.0 |
| Homemaker | 1 | 5.3 |
| Student | 1 | 5.3 |
Prior Awareness of WES
To assess participants' baseline familiarity with WES, participants were asked whether they had heard of WES prior to consenting to this study. Eighteen participants (95 %) had not heard of WES; one participant (5 %) heard of genome sequencing through a news story.
Return of Results Preferences
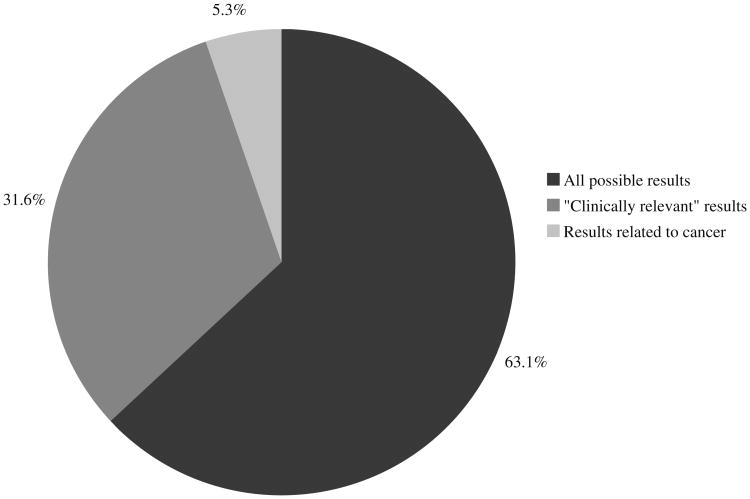
Although participants agreed to only receive cancer-related WES results during the consent process, participants were asked to indicate their hypothetical general preference for receiving WES results. As shown in Fig. 1, 12 of 19 participants (63 %) wished to receive all possible results generated from WES, while six of 19 (32 %) chose only results deemed “clinically relevant” to their medical care. Participant preferences regarding communication of WES results are depicted in Table 2. Of 19 participants, 12 (63 %) preferred to receive WES results from a genetic counselor and four (21 %) from a medical geneticist. Eleven participants (58 %) preferred results be given in person, acknowledging they would have a lot of questions regarding the results, including help understanding any medical terminology.
Fig. 1. Overall preference related to receiving WES results (n=19).
Table 2. Preferred communication of WES results (n=19).
| Variable | n | Percent |
|---|---|---|
| Informant | ||
| Genetic counselor | 12 | 63.1 |
| Medical geneticist | 4 | 21.0 |
| Other | 3 | 15.8 |
| Mode of initial results | ||
| In person | 11 | 57.9 |
| Letter with follow-up in person consultation | 3 | 15.8 |
| Letter with contact phone number | 3 | 15.8 |
| Over-the-telephone | 2 | 10.5 |
| Mode of follow-up or reclassification of results | ||
| In person | 4 | 21.0 |
| Letter with contact phone number | 7 | 36.8 |
| Email with contact phone number | 3 | 15.8 |
| Over the telephone | 2 | 10.5 |
| Any way possible | 3 | 15.8 |
A brief description of variants of unknown significance (VUS), which was written by three of the authors (JY, AB and KH) and pilot tested for clarity, was read to each participant. Participants were then asked to explain their preferences for receiving VUS results. Three of 19 participants (16%) did not wish to receive VUS results generated from WES; this was consistent with their preference to receive only “clinically relevant” results. All participants preferred to receive follow-up, such as VUS re-classifications or updated clinical implications, from a genetic professional; however, there was variation in participant preference for the mode of follow-up communication (see Table 2).
Regarding access to WES results, 13 of 19 participants (68 %) wanted direct access to their WES clinical reports (Table 3). Six participants (32 %) were comfortable with their health care provider solely having direct access to their results. Fourteen participants (74 %) expressed privacy or security concerns regarding personal genomic results on the World Wide Web, but ultimately desired password-protected access to results online in the future.
Table 3. Access to WES testing and results.
| Variable | n | Percent |
|---|---|---|
| Access to results (n=19) | ||
| Direct, self access | 13 | 68.4 |
| Health care provider access only | 6 | 31.6 |
| Online access to results (n=19) | ||
| In favor, but expressed privacy concerns | 14 | 73.7 |
| Not in favor | 5 | 26.3 |
| Direct-to-consumer WES (n=15) | ||
| In favor | 2 | 13.3 |
| Would consider based on cost | 3 | 20.0 |
| Not in favor | 10 | 66.7 |
Fifteen of 19 participants (79 %) were asked if they would consider WES through an outside company, referred to as “direct-to-consumer” genomic testing (see Table 3). Ten of 15 participants (67 %) felt strongly that they would not choose to undergo WES without their doctor's approval. Concerns arose due to the amount of results participants could glean from WES without help with interpretation of results. Worries about cost, insurance coverage, and the security of testing outside the clinical setting were also contributing factors against direct-to-consumer WES. Three of 15 (20 %) participants mentioned that cost would be their only reason against direct-to-consumer WES.
Sharing Test Results
Participants were asked whether they would share their personal WES results with family members or other health providers (Table 4). If affirmative, we explored patient preferences regarding modes of communication and reasoning for sharing results. Fourteen of 19 participants (74 %) wanted their WES results accessible to all their health providers, while three participants (16 %) wanted control over which providers could have access to their results. All 19 participants stated they would inform all relevant family members about the results that could impact their family members in some way. Sixteen of 19 participants (84 %) said they would disclose all WES information to family members, while three participants only wanted to share results that were medically actionable. Eleven of 19 participants (58 %) preferred telling their family members about WES results verbally in person. Three participants (16 %), all of whom were geographically separated from their relatives, favored providing family members with results over-the-telephone. Two participants (11 %) wanted their family members to accompany them to their results appointment; both had had previous, positive experiences bringing family members to their Lynch syndrome genetic test result counseling sessions.
Table 4. Preferences in sharing WES results (n=19).
| Variable | n | Percent |
|---|---|---|
| Health providers | ||
| All providers | 14 | 73.7 |
| Selected providers | 3 | 15.8 |
| No providers | 2 | 10.5 |
| Family members | ||
| All family members impacted by results | 19 | 100.0 |
| Selected family members | 0 | 0.0 |
| No family members | 0 | 0.0 |
| Types of results communicated to family members | ||
| All possible results | 16 | 84.2 |
| Medically actionable results | 3 | 15.8 |
| Mode of family communication | ||
| In person | 11 | 57.9 |
| Over the telephone | 3 | 15.8 |
| Letter | 3 | 15.8 |
| Accompany proband to results appointment | 2 | 10.5 |
Qualitative Themes
The key themes that emerged from our directed approach to content analysis were: 1) motivations for and concerns about receiving WES results; 2) influence of prior cancer experiences; 3) ownership of results; and 4) family communication.
1) Motivations for and Concerns about Receiving WES Results
Motivations for Receiving WES Results
Although not surprising given that all participants consented to WES prior to participating in the qualitative interview, participants tended to focus on the benefits of knowing information generated from WES. They often used the expression “knowledge is power” and made other similar statements. For example, one participant who was diagnosed with colon cancer at age 44, said, “The more I know, I mean, the better I can help make informed decisions along with my doctor” (Male, 46 years old).
All participants felt that WES results would motivate them to prevent various genetic conditions through adaptation of lifestyle, altering medical management, or seeking additional resources, particularly in relation to cancer predisposition or late-onset disease results.
Yeah, I would want to know because I would take up dancing or whatever you can do for Alzheimer's. I would study Alzheimer's and find out what the heck I could do, eat or exercise - I'd take up bridge if I had to! I would like to know if I'm gonna have breast cancer, for instance, because I would try to do whatever I could do to avoid it. (Female, 63 years old)
Some participants did not view WES as much different from other predictive medical tests that can guide effective medical management.
Right now, a doctor will test blood sugar levels to see if you're at risk for diabetes and you can take care of yourself. I think [WES] is just an extension of medical care and preventive medicine, to know that you have an increased risk and to get medical advice about what you can do to minimize your risk. (Female, 68 years old)
Participants believed that WES results that predispose or diagnose an individual with a condition even with no current available treatment might be valuable in preparing both financially and psychologically for the future.
I was the primary caretaker for my father and I'm familiar with health deterioration. I'm sure that if I found out that I was gonna go through a similar sequence, that it would be very disturbing to me, but I guess I feel like I would rather figure out how to cope with that and go through and decide what I wanted to do with my healthy years and, you know, looking into the future, be able to make arrangements so that I didn't bankrupt my family and I had some choice about what kind of care I was gonna get. I guess I would take that tradeoff. (Female, 68 years old)
Participants also considered the utility of receiving WES results in relation to planning other areas of their life, such as retirement and reproductive/family planning. Other participants were motivated to learn about their WES results because they were looking for an explanation for their clinical Lynch syndrome diagnoses.
The way I have lived, the way I've eaten does not warrant all of the things that have happened to me. It wasn't all my doing. So maybe if there was some explanation of why it's happening… I thought that one time genetic testing would be the end of it, so it's really sort of reassuring that somebody is continuing. (Female, 70 years old)
Concerns About Receiving WES Results
Participants did acknowledge some possible risks associated with learning WES results, recognizing that information about risk of late-onset disease and conditions without available treatment might be “emotional,” “difficult,” “depressing,” or “scary” to receive. For example, one participant stated, “That's a long time to know, to wait. It's something that could lead to misery…. There's no point in living in anticipation, but I wouldn't want to miss an opportunity if something came up that would help with it down the road” (Female, 45 years old). One 45-year-old male participant recognized that learning of WES results could “affect your day-to-day life,” and a 50-year-old male participant acknowledged, “I can't un-know that information.” One participant reflected on the potential for WES information to affect him deeply:
I guess I'm concerned that in some ways I might change the way that I'm living my life now unintentionally. Not even consciously, but having that knowledge may potentially change what I'm doing now that I don't actually want to change… especially if it was something that caused a later disease that I couldn't help. (Male, 45 years old)
Participants acknowledged their already intensive Lynch syndrome cancer screening practices; therefore, adding information about other health conditions might be overwhelming and compound their current medical management. Some had concerns about disease penetrance.
It would depend, you know, on the risk factor. If it was probable, yes, I'd want to know, even though there is no cure. But if it was a percentage, like a low percent, less than 5 % or something like that, no, I don't think I would. Not that it'd be stressful, but I think I'm so focused on maintaining a healthy lifestyle and, with colon cancer, and/or if I do have the Lynch syndrome, adding another thing to it might be a little much, especially if it was a rare disease that had no cure. (Male, 46 years old)
To help alleviate psychological burden and undue worry most participants (see Table 2) preferred to have a genetic counselor explain the meaning and implications of their results while providing psychosocial support.
I mean, it depends how [the results] are presented, I guess; but taken out of context I think they could potentially be a little bit alarming. So if it's just sort of presented as, ‘Hey, you've got this huge mutation in this gene,’ whoa, that seems to be bad, I don't know what that means. I'm not sure that that would necessarily be something I would like, versus somebody to sort of walk me through it and understand. (Male, 45 years old) When I had that original Lynch test, my oncologist gave me the results and like, they were negative, but I feel like if it had been positive, she didn't really know… it wasn't the most tender way. Whereas I felt like the genetic counselor was a little more gentle and a little more counseling aspect or something, and I think that's important with this subject because it's sensitive. (Female, 29 years old)
Participants contemplated the psychosocial implications of WES results, yet felt they would ultimately deal with the information positively. Some predicted that their first instinct upon learning WES results that were of uncertain clinical significance would be to do “research on PubMed,” research “clinical trials to participate in,” and “seek a second opinion” on the interpretation of their WES results.
I'd probably treat it like I do the colon cancer… do lots of research, find out if there's a relation to the colon cancer or to Lynch, and just continue to follow research on that or even push for research. (Male, 46 years old)
Other participants felt they would cope with psychologically difficult results through positive thinking, prayer, or reflection.
I don't think [the results] would really affect me ‘cause I have a positive attitude and, you know, I believe in miracles. So there's never a no-cure or a positive that life's over. So I wouldn't, I don't think, take it to heart really. (Female, 57 years old)
2) Influence of Prior Cancer Experiences
Participants' previous experiences with cancer and receiving uninformative negative Lynch syndrome genetic testing results seemed to affect their sense of self, their perceived ability to manage WES results, and their interpretation of genetic information.
Resilience and Self-Efficacy
Relative to dealing with cancer, many participants indicated that WES test results would be more manageable. One participant diagnosed with uterine cancer at age 28 said, “I've already had, I'd say one of the heaviest diseases you can get, so I'm not really scared at this point of anything else” (Female, 29 years old). Some participants articulated a high degree of self-efficacy (Bandura 1982), especially regarding the skills learned by managing a cancer diagnosis.
I've already changed my medical management and lifestyle with this new diagnosis and I've already, you know, dealt emotionally with cancer. Having all of this information gives me more, would give me more knowledge, more power in my own health care regime. (Female, 55 years old)
Interpreting Genetic Information
Influenced by their experience with Lynch syndrome, participants expressed nondeterministic understandings of genetics.
The majority felt that they could act upon WES results, for example by changing their medical management or adjusting their lifestyle choices.
I like information and anything that could potentially help me be more conscious of this potential reality, because it's nothing, it's all malleable, I know that. Like if you told me, oh yeah, you have a predisposition to BRCA, I really believe that a predisposition does not make it definite. (Female, 29 years old)
In regard to conditions without available treatment, one participant said he wants to know such information “even though quote-unquote, there's nothing you can do” (Male, 50 years old).
Due to their prior experience with genetic counseling and clinical Lynch syndrome diagnoses, participants seemed to have a good understanding of basic genetic concepts. Participants' level of genetic literacy appeared to help participants give meaning to VUS results. Most participants expressed tolerance for the uncertainty involved in receiving VUS results.
I'm sure a lot of people would obsess about the fact that they have a genetic defect of some kind when they don't know what the meaning of it is, but it could be that 10 years from now there will be a finding that would mean something and then they'll have the chance to know that. Other people may, you know, have the feeling that they don't want to live their lives with this information, that it's too scary, too debilitating. I respect that and I think people should be given a choice. I really clearly lean in the direction of wanting to know and thinking I deserve to know what the other people know about my body. (Female, 68 years old)
However, three participants did not wish to receive VUS results generated from WES, one of whom stated, “Unless you might have something that will impact your health in the future, I don't know if it's good or useful for just anybody to get to see their whole genetic makeup” (Female, 49 years old). The participants who preferred not to receive VUS results concomitantly preferred only to receive WES results that were “clinically actionable,” suggesting that they believed knowledge is only useful when it can be applied to take specific clinical action.
3) Ownership of Results
Access to Results
During the interviews, we informed participants of current clinical practices of returning WES results, in which a patient's ordering clinician receives all reported results and relays to the patient only the information that he or she decides is medically necessary. For instance, if a clinician adhered to the current ACMG recommendations, he or she would at least return the results listed on the ACMG's minimum list of incidental findings that must be reported despite patient preferences (Green et al., 2013). Most participants were not keen on this mode of returning results; but rather, preferred control over their WES results. Participant reasoning included having results for future reference, ease in sharing results with other or future health providers, providing family members access to relevant results, limiting practices such as providers withholding certain results, and a strong belief in patient empowerment.
I don't really see the argument of keeping it secret… it's like someone playing God with your life. I don't see why some scientists or researchers or medical people should have information about me that I don't have. I deserve to know what the other people know about my body. (Female, 68 years old)
The concern that personal genetic information would be known, but not disclosed, was common to most participants who said they believed they would be able to cope with any and all WES results provided.
Privacy and Genetic Discrimination Concerns
The majority of participants expressed privacy or security concerns with receiving personal genomic results via the World Wide Web, but desired future online access to their results so long as the results were protected. Those who did not want their results online also cited security concerns as the main rationale. In some cases, prior experiences with online health information influenced participants' views on obtaining genomic data via the Internet.
[My hospital] now has a system where you can look up test results and a lot of times it's very unclear what the test is and what the results are. I don't think that's a good way to get the information. I think it's really important initially to have a consultation with someone about it, especially if there are some significant findings and it's not all that clear. (Female, 68 years old)
4) Family Communication Plans
Participants were asked if, how and why they would share WES results with family members (see Table 4). When discussing family communication strategies, participants recalled their experiences communicating information about Lynch syndrome with their family members.
I'd tell them verbally, just talk to them. I'd sit them down and go over the results in the same way that I've done with this Lynch syndrome. (Male, 79 years old) [My family members] are really understanding of and involved with my Lynch syndrome and they ask a lot… it's best just to tell family the truth. Like with Lynch syndrome, and these ambiguous results, it was really difficult to tell them, but I know I would just have to find a way to tell them, to talk to them. They know everything and sometimes it's hard, but we have to find the words to tell them. I know they will understand, especially when it affects them. But it's easier to tell them everything from the beginning. (Female, 54 years old) In fact, with this diagnosis that I was recently presented with, I have shared everything that's been shared with me from the genetics department with my immediate family and also my extended family, and they are being very proactive in their own health care…. It has really stirred up a lot of good things, I feel, in my family. They're all wanting information. (Female 55 years old)
These participants had positive experiences sharing Lynch syndrome results, and therefore seem motivated to share any additional results generated from WES for the benefit of family members and because of the support they perceive they will receive from family.
Some participants were cautious about openly sharing all of their personal WES results with family, recognizing that “if it was going to do more harm than good by telling them certain results, there is a slight chance I would not tell them” (Male, 61 years old). Some individuals expressed hesitation to “dumping all of this information on family that either requires lots of interpretation to understand or is less medically certain” (Male, 45 years old). Another participant reflected on individual differences that contribute to how someone may react to the information.
I'm someone who can really handle information and not get too freaked out by it, but it's a fine delicate balance of like telling someone if they have something that's completely unrelated to, you know, what I've been going through. I think I'd ask them if they want to know and I would really think about it and probably get the opinion of a genetic counselor. (Female, 29 years old)
Disclosing Results to Children
Many participants were grateful to have the opportunity to share WES results with their offspring. One participant stated, “To be able to share that with my children and their family you know, I can't think of a better gift to give them for their own health” (Male, 59 years old). Participants felt that sharing information generated from WES, particularly carrier status results, would be valuable for future generations' reproductive plans and decision-making. Participants with school-aged children wished to wait to tell their children about the pertinent WES results that could impact their reproductive decisions.
It's not information I would share with my children until they became much older and could make their own decisions about having children. It would be something I'd want to know now so I could share that information with them when it was right. When they were of child-bearing years they could have that information. (Female, 45 years old)
One participant realized the psychological implications that could stem from sharing WES results with children, but focused on the support he could provide in doing so.
I think it would be important to discuss it with my sons and daughter, to let them know what they might, what they may be running into in the future. It wouldn't be easy, but I could just let them know that they're not alone in it, that somebody cares about it. (Male, 61 years old)
Discussion
This study supports several concepts established in the literature: most adult individuals who have been surveyed regarding genetic testing results want to receive all possible results, including incidental findings, generated from WES. Studies show that: individuals believe the benefits outweigh the risks of knowing such information (Bloss et al., 2010; Lemke et al., 2012; Murphy et al., 2008; Shalowitz & Miller, 2008); patients want direct access to their results but prefer posttest guidance and education from genetic specialists (Foster et al. 2009); and patients' reasons to undergo WES parallel their reasons for consenting to genetic susceptibility testing (i.e. for the benefit of their biological relatives, to inform prevention or management strategies for future illnesses, to reduce uncertainty about the cause of current condition, etc.) (Townsend et al., 2012; Uhlmann et al. 2009; Yu et al., 2013).
Analysis of interview data revealed several novel findings particular to our population of patients with clinical Lynch syndrome diagnoses but negative genetic test results, including a perception of a high degree of self-efficacy, the impact of prior experience dealing with uncertainty, and family communication strategies. Based on these novel findings, we suggest WES consent practices, pretest counseling strategies, and post-test return of results practices.
Interestingly, participants perceived that they had a great degree of control over their health, abilities to prevent disease, and even their genes. Their perceived self-efficacy suggests an internal locus of control, rather than an external locus of control in which outside factors control one's well-being (Lau, 1982). The confidence and sense of empowerment participants anticipated in their ability to cope positively with and use WES results for prevention is consistent with previous studies done on patients who consent to genetic testing for hereditary cancer syndromes (Vernon et al., 1999; Aktan-Collan et al., 2000; Hadley et al., 2003). Consistent with our study in which participants focused on prevention strategies, an internal locus of control has been found to facilitate positive health intentions and behaviors in cancer survivors (Park & Gaffey, 2007),
Yet participants may have overrated their ability to control and manipulate their genomic destinies. As Kruger and Dunning have found, people tend to overestimate their own skills, particularly when they possess less knowledge about the subject in question or when competence in a particular area would be self-serving (Dunning et al. 2003; Kruger & Dunning 1999). Particularly when discussing the hypothetical situation of receiving WES results related to genetic conditions without available treatment, participants tended to focus on preventing the onset of such conditions by changing lifestyle or through non-traditional healing methods. Participants may have been more prone to believe they could manipulate the onset of such genetic conditions because of their somewhat limited knowledge of genetics or because the ability to change their health-related behavior and health outcomes would be self-serving.
Unlike other genetic counseling specialties in which “nondirectiveness” is held as an ethos, genetic counseling about medically actionable results tends to emphasize appropriate treatment or preventative strategies at the best interest of the patient's health. For instance, Lynch syndrome genetic counseling strategies have a propensity to stress that knowledge of one's Lynch syndrome genetic status can be empowering through the implementation of effective cancer screening (Lindor et al., 2006; Uhlmann et al., 2009; Weil, 2000). Participants may have felt a sense of empowerment stemming from their existing Lynch syndrome screening practices, inflating their sense of efficacy and affecting their perceptions of how they could use WES results. That is, participants' prior understanding of the effectiveness of Lynch syndrome cancer screening and prevention may lead these individuals to overestimate their ability to control other aspects of their health.
Participants mentioned that, after learning of their cancer and clinical Lynch syndrome diagnoses, they altered their lifestyle in addition to increasing cancer-screening practices. Some felt that even if WES were to diagnose an untreatable or late-onset condition, holistic, lifestyle, and non-Western medicine treatments might aid in preventing or diminishing disease. Mastery of and control over one's genes was a prominent, yet concerning idea that arose during interviews with participants. Of course, individuals cannot control their genetic makeup or how it affects their health, but rather can only affect the external factors that may or may not modify the expression of genes. Therefore, clinicians returning WES results to patients must be careful in clearly communicating the natural history and implications certain genetic disorders are known to carry.
Contrary to current research suggesting individuals often associate genetic concepts with fate, participants in this study had less deterministic views about genetics (Gould & Heine, 2012; Parrott & Smith, 2013). Rather than addressing genes as the determinant of their health, many participants referred to their genetic susceptibility to disease. Participants focused on their ability to prevent or delay symptom onset through environmental or lifestyle changes. These findings suggest that having an experience of managing and coping with a difficult disease such as cancer, along with the knowledge gained in the process of Lynch syndrome genetic counseling, may increase one's perceived self-efficacy. In turn, more self-efficacious individuals may perceive genetic information as manageable and beneficial, therefore skewing their desire to receive all types of WES results. In the present study, participants' frame of reference and mental resiliency due to their cancer experience seemed to affect their preferences for receiving WES results.
Participants were familiar with traditional approaches to genetic testing and associated uncertainties due to their experience with Lynch syndrome genetic testing and counseling. Although parents of pediatric patients with prior uncertain or negative genetic results undergoing WES have been surveyed (Tabor et al., 2012), this is the first study to analyze adults with such experience. Our participants' prior genetic testing experiences may be related to their tolerance for ambiguity in WES results. Participants had prior experience making decisions regarding their own health management, communicating information to family members, and pursuing further testing based on ambiguous results. The high comfort level with ambiguity may relate to participants' positive, self-efficacious attitudes, or may be a learned coping response due to previous experience with uninformative negative Lynch syndrome results.
The ability to provide genetic information to relatives, offspring, and future generations was a motivating factor for consenting to WES. Although such motivations for seeking genetic testing are not uncommon, participants referred to previous Lynch syndrome-related family communication experiences. Participants acknowledged the courage it took to communicate their Lynch syndrome diagnoses with family, but that overall, family members reacted positively by proactively changing their healthcare management. Because knowledge about Lynch syndrome status offers individuals cancer screening and prevention opportunities, participants seemed most open to sharing information that their family could act upon.
Nevertheless, many realized the challenges that could stem from communicating the scope of WES results with their family. When it came to sharing susceptibility information about the more emotionally laden conditions, such as late-onset or untreatable diseases, participants were particularly protective of their children, younger siblings, and family members currently battling health problems. Participants felt strongly about telling family members that they have potentially impactful genetic information, but felt that each individual should ultimately decide what was to be shared. Such intrafamilial discussions may be difficult for patients to broach in a nondirective, unbiased manner after receiving results. Therefore, we recommend that WES pretest counseling sessions include discussions about family communication plans.
Practice Implications
Recently, the ACMG issued a report stating they “do not favor offering the patient a preference as to whether or not to receive the minimum list of incidental findings described in these recommendations” (Green et al., 2013). The minimum list of incidental findings includes high-penetrance, medically-actionable results and known and expected pathogenic mutations (i.e. not VUS results). Green et al. (2013) recognized that such recommendations might undermine a patient's autonomy but also asserted the “fiduciary duty to prevent harm by warning patients and their families about certain incidental findings and that this principle superseded concerns about autonomy.” Laboratories performing WES and following ACMG recommendations must report the “minimum list” of results, yet clinicians and patients may still come to agreements on what information will and will not be reported to patients.
Many participants in this study voiced concerns about their providers deciding which results they receive, arguing for autonomy in relation to their genes. The great majority showed a strong desire to receive all WES results included in the ACMG's “minimum list” and beyond; however, the fact that a few participants did not want to receive all results suggests some variability in individuals' thresholds for genomic information and perhaps the need to let individuals personally consent to which results to receive and which to not receive. Based on the opinions of patients in the current study, pretest genetic counseling for whole exome or genome sequencing should provide thorough education about the ACMG guidelines (i.e. that high-penetrance, medically-actionable results will be reported by the laboratory to the ordering clinician) so that patients are aware of what results they will be receiving. The data collected in this study supports providing patients the autonomy to choose which non-medically actionable WES results are reported from clinician to patient. Giving patients the choice to opt out of receiving any medically actionable results could result in ethical problems for providers, including withholding information that could help prevent serious disease and patients inadvertently learning of results from other providers that have access to the patient's medical records.
Townsend et al. (2012) found discordance between patients and clinicians regarding preferences for returning incidental findings from whole genome sequencing. Particularly, lay groups and patients believed strongly in autonomous decision-making, while clinicians emphasized the clinical relevance of results as the main criterion for disclosure. The majority of participants in this study were keen on individual choice and empowerment, as reinforced by their desire for direct access to WES clinical reports and transparency between clinicians and patients. Participants also acknowledged that others, including their family members, may not desire as much or the same types of genomic information because individuals may desire, interpret, and cope with genetic information differently. However, some participants notably preferred that experts filter and interpret results prior to patient disclosure in order to reduce undue worry. This discordance may stem from the quality of the relationship or level of trust patients have in their providers, or from patient recognition of the efficacy of their individual coping styles.
Very often, participant rationale for receiving WES information included drawing upon previous Lynch syndrome genetic counseling and cancer experiences. As clinical WES is applied as a first tier test, patients may have no experience to draw from when consenting to receiving results. Exploring a patient's coping styles and previous experience with ambiguity outside of genetic testing may be beneficial to encourage full exploration of their thoughts and feelings prior to initiating WES. Encouraging patients to draw upon coping strategies that have worked for them in prior similar situations is a widely accepted counseling strategy proven effective in other situations, and is especially applicable when discussing the range of possible uncertain results that could be generated from large-scale sequencing (Gaff & Bylund, 2010).
Some attributes of the medical field have fostered “genetic exceptionalism,” or treating genetic information differently from other medical information due to its unique implications (Evans et al. 2010). This is an important idea when educating patients on large-scale genome tests such as WES. Some participants in this study, however, made comparisons between WES results and other medical information, a view called “reverse genetic exceptionalism” (Evans et al., 2010). These results reveal that providers meeting with individuals prior to initiation of WES need to underscore the differences between genomic and other health information. Furthermore, while most participants in this study recognized the apparent magnitude difference in WES data compared to their previous experience testing for a handful of genes associated with Lynch syndrome, it is imperative that pretest counseling sessions divulge the possible implications that could result from receiving WES data in detail, ensuring adequate anticipatory guidance and self-reflection for patients.
No participants mentioned telling family members of their decision to undergo WES prior to testing. Sobel and Cowan (2000) investigated the impact of presymptomatic Huntington disease testing within a family systems theory frame, concluding that family involvement in the decision-making process should be strongly encouraged. Because WES can detect similarly impactful conditions, family involvement prior to consent may need to be explored in pretest counseling discussions. Discussing and considering family communication and the impact WES information could have on family members in advance could minimize family conflict after results are returned.
Study Limitations
This study had several limitations. From a demographic standpoint, participants represented a small sample size from the same general region, and were generally well educated, middle-aged, and white. All participants had previously participated in genetic counseling and Lynch syndrome genetic testing, and therefore may be more knowledgeable about genetics, consent, and test results than the general population. Participants may have been more likely to want WES results due to a wish to determine a genetic cause for their clinical Lynch syndrome diagnosis or to learn more about their condition. Also, participants generally had a positive outlook on receiving health information, which may be related to their prior experiences with cancer, high level of perceived self-efficacy and high level of health literacy. Given the small, homogeneous sample in this study, it is not possible to determine whether the views of this group are representative of all individuals diagnosed with uninformative negative Lynch syndrome genetic test results or representative of the general population. Thus generalizability of our findings is limited.
Recruitment of the interviewees may also have been biased in that only those interested in the topic and open about voicing their opinions were likely to participate. All of the interviewees were referred by the genetic counselor with whom they had previous contact, and these individuals may have been chosen by the genetic counselors because of their positive experience with genetic counseling, thus favorably inclining their views toward receiving genetic information.
Research Recommendations
Future studies on this subject should include a larger sample size and individuals from diverse ethnic backgrounds, varied education and socioeconomic levels, and a broader range of genetic knowledge and experience levels. Patients, providers, and policy makers should be consulted in determining recommendations for follow-up and updates from whole exome and genome sequencing. Based on the somewhat recent utilization of WES in clinical practice, it is not yet known whether learning of one's WES results will have positive, negative, or no effect on motivation to engage in lifestyle and medical management changes, family communication, or emotional burden. Patients undergoing clinical WES should be followed long-term to track health behavior changes and better understand the enduring psychological impact of receiving WES results. Such longitudinal studies will be imperative to deduce the most effective procedures for integrating whole exome and genome testing into clinical practices. Should whole exome and genome sequencing become first-tier genetic tests, studies should be conducted to examine the experiences and preferences of individuals without prior genetic testing experiences feel regarding genomic testing and the types of results it may provide.
Acknowledgments
This study was completed in partial fulfillment of the requirements for the first author's Master of Science degree from the California State University, Stanislaus (CSUS) Genetic Counseling Program. Thanks go to the program as well as the CSUS Biology Research Committee for funding. Thank you to Ambry Genetics for providing WES and bioinformatics analysis for the patients included in this study. Thank you to Illumina for donating the reagents required for WES. The investigators would like to thank the individuals who helped with the recruitment of subjects: Margo Thelander, Peggy Conrad, Kate Loranger, and Megan Myers. Special thanks to the 19 individuals who so openly and willingly shared their thoughts and opinions with us. Your comments are appreciated.
Footnotes
Disclosure of Conflict The authors have no conflicts of interest to disclose.
Contributor Information
Kelly Hitch, Email: kellyhitch89@gmail.com, California State University, Stanislaus, Turlock, CA, USA; 1125 6th Street, Apt. 2, Santa Monica, CA 90403, USA.
Galen Joseph, Univeristy of California, San Francisco, San Francisco, CA, USA.
Jenna Guiltinan, California State University, Stanislaus, Turlock, CA, USA; Ambry Genetics, Aliso Viejo, CA, USA.
Jessica Kianmahd, University of California, Los Angeles, Los Angeles, CA, USA.
Janey Youngblom, California State University, Stanislaus, Turlock, CA, USA.
Amie Blanco, Univeristy of California, San Francisco, San Francisco, CA, USA.
References
- Aktan-Collan K, Mecklin JP, Jarvinen H, Nystrom-Lahti M, Peltomaki P, Soderling I, et al. Predictive genetic testing for hereditary non-polyposis colorectal cancer: Uptake and long-term satisfaction. Internal Journal of Cancer. 2000;89(1):44–50. [PubMed] [Google Scholar]
- Ambry Genetics. Exome test requisition, preverification, & consent form – proband. 2012 Retrieved from http://www.ambrygen.com/sites/default/files/pdfs/forms/Exome_Req_Consent.pdf.
- ACMG Board of Directors. Points to consider in the clinical application of genomic sequencing. Genetics in Medicine. 2012;14(8):759–761. doi: 10.1038/gim.2012.74. [DOI] [PubMed] [Google Scholar]
- Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for mendelian disease gene discovery. Nature Reviews Genetics. 2011;12:745–754. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy mechanism in human agency. American Psychologist. 1982;37(2):122–147. doi: 10.1037/0003-066X.37.2.122. [DOI] [Google Scholar]
- Baylor College of Medicine Medical Genetics Laboratories. WESrequisiton. 2013 Retrieved from https://www.bcm.edu/geneticlabs/index.cfm?
- Berg BL. Qualitative research methods for the social sciences. 4th. Boston: Allyn and Bacon; 2001. [Google Scholar]
- Berg JS, Khoury MJ, Evans JP. Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge on bin at a time. Genetics in Medicine. 2011;13(6):499–505. doi: 10.1097/GIM.0b013e318220aaba. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Ornowski L, Silver E, Cargil M, Vanier V, Schork NJ, et al. Consumer perceptions of direct-to-consumer personalized genomic risk assessments. Genetics in Medicine. 2010;12(9):556–566. doi: 10.1097/GIM.0b013e3181eb51c6. [DOI] [PubMed] [Google Scholar]
- Burt RW, Cannon JA, David DS, Early DS, Ford JM, Giardiello FM, et al. National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology [Colorectal cancer screening] Version 2.2013. 2013 doi: 10.6004/jnccn.2010.0003. NCCN.org. [DOI] [PubMed]
- Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proceedings of the National Academy of Sciences USA. 2009;106:19096–19101. doi: 10.1073/pnas.0910672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning D, Johnson K, Ehrlinger J, Kruger J. Why people fail to recognize their own incompetence. Current Directions in Psychological Science. 2003;12(3):83–87. doi: 10.1111/1467-8721.01235. [DOI] [Google Scholar]
- Emory Genetics Laboratory. EmExome Requisition and Consent Form. 2012 Retrieved from http://genetics.emory.edu/egl/reqform/standard/exome.php.
- Evans JP, Burke W, Khoury M. The rules remain the same for genomic medicine: The case against “reverse genetic exceptionalism”. Genetics in Medicine. 2010;12(6) doi: 10.1097/GIM.0b013e3181deb308. [DOI] [PubMed] [Google Scholar]
- Foster MW, Mulvihill JJ, Sharp RR. Evaluating the utility of personal genomic information. Genetics in Medicine. 2009;11(8):570–574. doi: 10.1097/GIM.0b013e3181a2743e. [DOI] [PubMed] [Google Scholar]
- Gaff CL, Bylund CL. Family communication about genetics: Theory and practice. New York: Oxford University Press; 2010. [Google Scholar]
- Gould WA, Heine SJ. Implicit essentialism: geneticconcepts are implicitly associated with fate concepts. PLoS One. 2012;7(6):e38176. doi: 10.1371/journal.pone.0038176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RC, Berg JS, Berry GT, Biesecker LG, Dimmock DP, Evans JP, et al. Exploring concordance and discordance for return of incidental findings from clinical sequencing. Genetics in Medicine. 2012;14(4):405–410. doi: 10.1038/gim.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. American College of Medical Genetics and Genomics. 2013 doi: 10.1038/gim.2013.73. Retrieved from http://www.acmg.net/docs/ACMG_Releases_Highly-Anticipated_Recommendations_on_Incidental_Findings_in_Clinical_Exome_and_Genome_Sequencing.pdf. [DOI] [PMC free article] [PubMed]
- Grillo E, Lo Rizzo C, Bianciardi L, Bizzarri V, Baladassarri M, Spiga O, et al. Revealing the complexity of a monogenic disease: rett syndrome exome sequencing. PLoS One. 2013;8(2):e56599. doi: 10.1371/journal.pone.0056599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiltinan JS, Hitch K, Hechter E, Youngblom J, Blanco A. Application of whole exome sequencing to mine for novel genes associated with Lynch syndrome. Unpublished manuscript 2013 [Google Scholar]
- Hadley DW, Jenkins J, Dimond E, Nakahara K, Grogan L, Liewehr DJ, et al. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Archives of Internal Medicine. 2003;163(5):573–582. doi: 10.1001/archinte.163.5.573. [DOI] [PubMed] [Google Scholar]
- Haimovich AD. Methods, challenges, and promise of next-generation sequencing in cancer biology. Yale Journal of Biology andMedicine. 2011;84:439–446. [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Jamal SM, Yu JH, Chong JX, Dent KM, Conta JH, Tabor HK, et al. Practices and policies of clinical exome sequencing providers: Analysis and implications. American Journal of Medical Genetics. 2013;161(5):935–950. doi: 10.1002/ajmg.a.35942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J, Dunning D. Unskilled and unaware of it: How difficulties in recognizing one's own incompetence lead to inflated self-assessments. Journal of Personality and Social Psychology. 1999;77(6):1121–1134. doi: 10.1037//0022-3514.77.6.1121. [DOI] [PubMed] [Google Scholar]
- Ku CS, Cooper DN, Iacopetta B, Roukos DH. Integrating next-generation sequencing into the diagnostic testing of inherited cancer predisposition. Clinical Genetics. 2012;83(1):2–6. doi: 10.1111/cge.12028. [DOI] [PubMed] [Google Scholar]
- Lagerstedt-Robinson K, Liu T, Vandrovcova J, Halvarsson B, Clendenning M, Frebourg T, et al. Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics. Journal of the National Cancer Institute. 2007;99(4):291–299. doi: 10.1093/jnci/djk051. [DOI] [PubMed] [Google Scholar]
- Lau RR. Origins of health locus of control beliefs. Journal of Personality and Social Psychology. 1982;42(2):322–334. doi: 10.1037//0022-3514.42.2.322. [DOI] [PubMed] [Google Scholar]
- Lemke AA, Bick D, Dimmock D, Simpson P, Veith R. Perspectives of clinical genetics professionals toward genome sequencing and incidental findings: a survey study. Clinical Genetics. 2012:1–7. doi: 10.1111/cge.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. The Journal of the American Medical Association. 2006;296(12):1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- Majewski J, Schwartzentruber J, Lalonde E, Montpetit A, Jabado N. What can exome sequencing do for you? Journal of Medical Genetics. 2011;48:580–589. doi: 10.1136/jmedgenet-2011-100223. [DOI] [PubMed] [Google Scholar]
- Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, Hudson K. Public Expectations for Return of Results from Large-Cohort Genetic Research. The American Journal of Bio ethics. 2008;8(11):36–43. doi: 10.1080/15265160802513093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genetics in Medicine. 2010;12(2):93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, et al. Exome sequencing identifies the cause of amendelian disorder. Nature Genetics. 2010;42(1):30–35. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CL, Gaffey AE. Relationships between psychosocial factors and health behavior change in cancer survivors: An integrative review. Annals of Behavioral Medicine. 2007;34(2):115–134. doi: 10.1007/BF02872667. [DOI] [PubMed] [Google Scholar]
- Parrott R, Smith RA. Defining genes using “blueprint” versus “instruction” metaphors: Effects for genetic determinism, response efficacy, and perceived control. Journal of Health Communication. 2013 doi: 10.1080/10410236.2012.729181. [DOI] [PubMed] [Google Scholar]
- Schneider KA. Counseling about cancer: Strategies for genetic counseling. Hoboken: Wiley-Blackwell; 2012. [Google Scholar]
- Singleton AB. Exome sequencing: a transformative technology. Lancelet Neurology. 2011;10:942–946. doi: 10.1016/S1474-4422(11)70196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: Attitudes, practices, and future directions. Plos Medicine. 2008;5(5):714–720. doi: 10.1371/journal.pmed.0050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel SK, Cowan DB. Impact of genetic testing for Huntington's disease on the family system. American Journal of Medical Genetics. 2000;90(1):49–59. doi: 10.1002/(sici)1096-8628(20000103)90:1<49::aid-ajmg10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Tabor HK, Stock J, Brazg T, McMillin MJ, Dent KM, Yu JH, et al. Informed consent for whole genome sequencing: A qualitative analysis of participant expectations and perceptions of risks, benefits and harms. American Journal of Medical Genetics. 2012;158(Part A):1310–1319. doi: 10.1002/ajmg.a.35328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A, Adam S, Birch PH, Lohn Z, Rosseau F, Friedman JM. “I want to know what's in Pandora's box”: Comparing stakeholder perspectives on incidental findings in clinical whole genomic sequencing. American Journal of Medical Genetics. 2012;158(Part A):2519–2525. doi: 10.1002/ajmg.a.35554. [DOI] [PubMed] [Google Scholar]
- Uhlmann WR, Schuette JL, Yashar BM. A guide to genetic counseling. 2nd. Hoboken: Wiley-Blackwell; 2009. [Google Scholar]
- Vernon SW, Gritz ER, Peterson SK, Perz CA, Marani S, Amos CI, et al. Intention to learn results of genetic testing for hereditary colon cancer. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:353–360. [PubMed] [Google Scholar]
- Weil J. Psychosocial genetic counseling. New York: Oxford University Press; 2000. [Google Scholar]
- Worthey EA, Mayer AN, Syverson GD, Helbling D, Bonacci BB, Decker B, et al. Making a definitive diagnosis: Successful clinical application of WES in a child with intractable inflammatory bowel disease. Genetics in Medicine. 2011;13(3):255–262. doi: 10.1097/GIM.0b013e3182088158. [DOI] [PubMed] [Google Scholar]
- Yu JH, Crouch J, Jarnal SM, Tabor HK, Bamshad MJ. Attitudes of African Americans toward return of results from exome and whole genome sequencing. American Journal of Medical Genetics. 2013;9999(Part A):1–9. doi: 10.1002/ajmg.a.35914. [DOI] [PMC free article] [PubMed] [Google Scholar]