Abstract
Analysis of single molecules in living cells has provided quantitative insights into the kinetics of fundamental biological processes; however, the dynamics of messenger RNA (mRNA) translation have yet to be addressed. We have developed a fluorescence microscopy technique that reports on the first translation events of individual mRNA molecules. This allowed us to examine the spatiotemporal regulation of translation during normal growth and stress and during Drosophila oocyte development. We have shown that mRNAs are not translated in the nucleus but translate within minutes after export, that sequestration within P-bodies regulates translation, and that oskar mRNA is not translated until it reaches the posterior pole of the oocyte. This methodology provides a framework for studying initiation of protein synthesis on single mRNAs in living cells.
During translation, mRNAs are bound by the ribosome. Measurements of ribosome occupancy of mRNAs and protein abundance provide a genome-wide view of translation regulation (1, 2). Fluorescence microscopy complements these global approaches because it allows analysis of gene expression with single-molecule resolution in living cells and provides mechanistic insights obscured by ensemble measurements (3, 4). Imaging methods have been developed that allow newly synthesized proteins to be discerned from the preexisting population or enable actively translating ribosomes to be identified within the cell; however, these approaches are limited by low signal-to-noise ratio and lack the resolution to correlate these events with specific mRNA molecules (5). Here, we describe a single-molecule assay that allows untranslated mRNAs to be distinguished unequivocally from previously translated ones and provides a foundation for investigating the spatiotemporal regulation of translation in living cells.
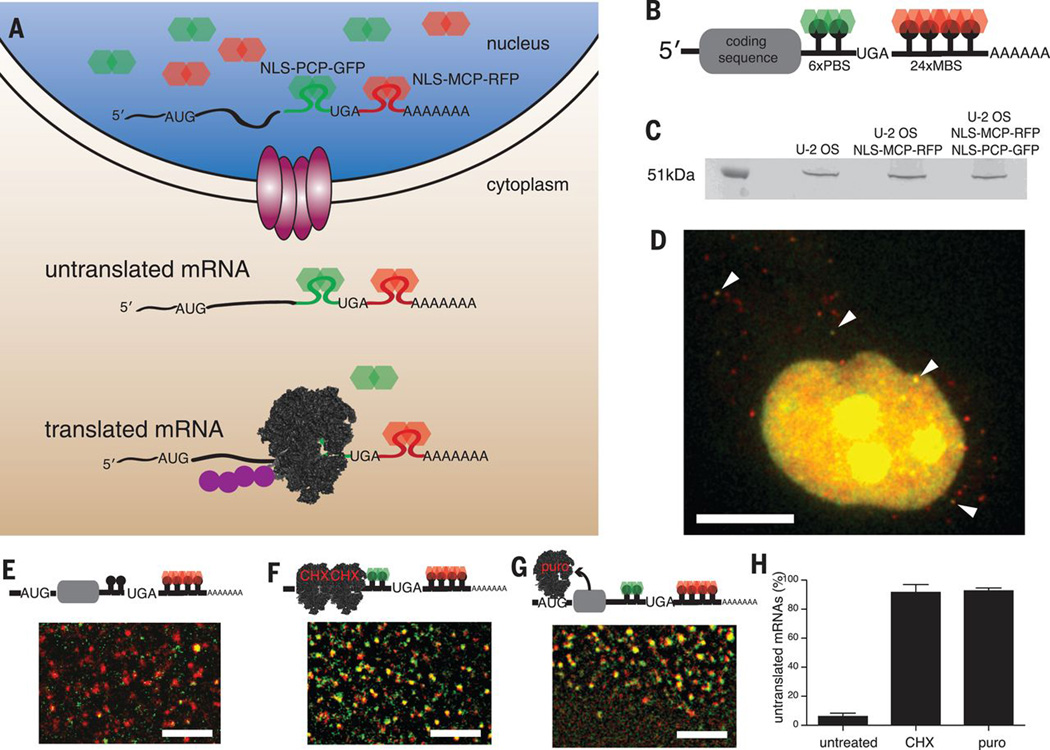
Because the ribosome or its associated factors must displace endogenous RNA-binding proteins during the first round of translation, we reasoned that it would be possible to construct an RNA biosensor whose fluorescent signal would depend on this process. The orthogonal bacteriophage PP7 and MS2 stem-loops were used to label a transcript within both the coding sequence (PP7) and the 3’ untranslated region (UTR) (MS2) with spectrally distinct fluorescent proteins (6). Simultaneous expression of the PP7 coat protein fused to a nuclear localization sequence (NLS) and green fluorescent protein (NLS-PCP-GFP) and the MS2 coat protein fused to an NLS and red fluorescent protein (NLS-MCP-RFP) resulted in nuclear transcripts labeled with both fluorescent proteins (Fig. 1A). Upon export of the reporter mRNA, the first round of translation displaces NLS-PCP-GFP from the transcript, as the ribosome traverses the coding region that contains the PP7 stem-loops. The NLS limits the concentration of free NLS-PCP-GFP in the cytoplasm, yielding translated mRNAs that are labeled with only NLS-MCP-RFP bound to the stem-loops in the 3’ UTR (Fig. 1A and B). We refer to this technique as translating RNA imaging by coat protein knock-off (TRICK).
Figure 1. Imaging translation of mRNAs in living cells.
(A) Schematic of TRICK assay. (B) Schematic of TRICK reporter transcript. 6xPP7 stem-loops (PBS) inserted in-frame with the C terminus of a protein-coding sequence and 24xMS2 stem-loops (MBS) in the 3’ UTR. (C) Expression of TRICK reporter mRNA in U-2 OS cells. The protein encoded by the TRICK reporter (51.4 kD) is translated in U-2 OS cells, and expression is not affected by NLS-MCP-RFP and NLS-PCP-GFP. (D) U-2 OS cell expressing TRICK reporter. Arrows indicate untranslated nuclear mRNA and three untranslated mRNAs detected in the cytoplasm. Scale bar, 10 µm. (E) Cytoplasmic region of untreated U-2 OS cells. (F) Addition of cycloheximide (100 µg ml−1) and (G) addition of puromycin (100 µg ml−1) during ponA induction of TRICK reporter mRNAs. Scale bar (E to G), 2 µm. (H) Percentage of untranslated TRICK mRNAs in U-2 OS cells. In untreated cells, 5.8 ± 1.4% of mRNAs colocalize with both NLS-PCP-GFP and NLS-MCP-RFP compared to 91.0 ± 3.0% for cycloheximide-treated and 92.6 ± 1.0% for puromycin-treated cells. n = 5 cells for each condition.
Efficient translation of a 6xPP7 stem-loop cassette required optimization of the distance between adjacent stem-loops, stem-loop folding, and codon usage so that they would not block or stall elongation of the ribosome, which might elicit decay of the transcript (7) (Fig. 1C). The polypeptide encoded by the PP7 stem-loops has a molecular mass of ~14 kD and is not homologous to any known protein. Binding of NLS-MCP-RFP to the 3’ UTR had no effect on translation, and binding of NLS-PCP-GFP to the PP7 stem-loop cassette in the coding region also did not result in reduced translation of the reporter mRNA (Fig. 1C and fig. S1). Similarly, binding of the fluorescent proteins to the reporter mRNA also did not alter the stability of the transcript (fig. S2).
The TRICK reporter mRNA was expressed in a U-2 OS human osteosarcoma cell line stably expressing NLS-PCP-GFP and NLS-MCP-RFP. Fluorescence-activated cell sorting isolated cells with small amounts of both fluorescent proteins, allowing detection of all reporter mRNAs (figs. S3 and S4). The cells were imaged on a fluorescence microscope equipped with two registered cameras, allowing simultaneous visualization of single mRNA molecules in both channels. In the nucleus, single mRNAs were fluorescently labeled with both red and green proteins and thus appeared yellow (Fig. 1D). In contrast, almost all of the mRNAs appeared as red particles in the cytoplasm, indicating that only NLS-MCP-RFP was bound (Fig. 1D and E). Quantification of the steady-state number of yellow mRNAs in the cytoplasm revealed that ~94% of TRICK reporter mRNAs had been translated at least once (Fig. 1E and H). To confirm that loss of NLS-PCP-GFP from cytoplasmic transcripts was translation-dependent, we induced transcription of the TRICK reporter by ponasterone A (ponA) in the presence of translational inhibitors (8). Adding either cycloheximide, which inhibits elongation, or puromycin, which causes premature termination, for 30 min before induction of TRICK reporter mRNA expression resulted in an increase in the number of untranslated mRNAs in the cytoplasm (Fig. 1F to H, and movies S1 to S3). Consistent with the imaging, polysome analysis indicated that NLS-PCP-GFP was absent from actively translating mRNAs, whereas NLS-MCP-RFP could be detected within polysomes (fig. S5). This demonstrated that translation of the PP7 stem-loops by the ribosome was required for displacement of the green signal from the mRNA.
Although translation is thought to occur exclusively in the cytoplasm, recent studies suggest that protein synthesis can occur in the nucleus (9, 10). Because the TRICK assay can distinguish between untranslated and translated mRNAs, we imaged TRICK reporter mRNAs in the nucleus 30 min after ponA induction. Single-particle tracking (SPT) of nuclear mRNAs determined that they undergo both corralled (D = 0.02 µm2 s−1) and random diffusion (D = 0.09 µm2 s−1), similar to the movements observed for other nuclear mRNAs (11, 12). We found 91.3 ± 0.9% of mRNAs labeled with both colors, which is not significantly different from the fraction of double-labeled mRNAs in the cytoplasm of cells treated with translational inhibitors (P = 0.75, unpaired t test) (fig. S6A and B, and movie S4). We cannot, however, exclude the possibility that the fusion protein rebound the PP7 stem-loops immediately after translation. If translation were occurring in the nucleus, addition of small amounts of cycloheximide would increase polysome formation, causing occlusion of the PP7 stem-loops and thereby preventing NLS-PCP-GFP from rebinding (13) (fig. S7A). Similar to experiments in the absence of cycloheximide, 90.7 ± 0.6% of nuclear mRNAs were labeled with both colors when cells were treated with 1 µg ml−1 cycloheximide (P = 0.44, unpaired t test) (fig. S7B and C, and movie S5). Although it is possible that nuclear translation could occur for specific mRNAs, this was not observed for the TRICK reporter. These findings are consistent with the previous observation that mRNAs containing premature stop codons are exported before undergoing decay in the cytoplasm (14).
The rapid diffusion of mRNAs in the cytoplasm and photobleaching prevented us from imaging a single mRNA from the time it entered the cytoplasm until it was translated (figs. S8 and S9). Untranslated mRNAs, however, could be detected after export from the nucleus and were observed throughout the cytoplasm (fig. S8). To verify these live-cell observations, we measured the spatial distribution of untranslated reporter mRNAs in fixed cells, using a combined immunofluorescence–fluorescence in situ hybridization (IF-FISH) approach. FISH probes targeted to the MS2 stem-loops allowed detection of all reporter mRNAs, whereas a GFP nanobody was used to identify the untranslated ones (fig. S10, A and B). In agreement with live-cell results, we observed a large percentage of cytoplasmic translated mRNAs (93.7%). As mRNAs diffuse away from the nucleus, their chances to collide with the 43S preinitiation complex and become translated increase over time. Indeed, we observed that the fraction of untranslated mRNAs decreased gradually as the distance from the nucleus increased (fig. S10C). Spatial profiles of untranslated mRNAs demonstrated that some mRNAs diffused micrometers away from the nucleus before undergoing translation, indicating that translation does not occur immediately upon export, but occurs minutes after the mRNA has entered the cytoplasm (the time before an mRNA translates should scale as L2/D, where L ~ 5 µm is the radial extent of the untranslated mRNA profile and D = 0.02 to 0.13 µm2 s−1 is the range of diffusion coefficients; fig. S9). Furthermore, we find no evidence for enrichment or depletion at specific cytosolic locations, suggesting that translation can occur homogeneously throughout the cytoplasm.
We next investigated how stress conditions affect translation. Upon a variety of cellular stresses, signaling pathways inhibit translation through phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), resulting in disassembly of polysomes and formation of cytoplasmic stress granules and processing bodies (P-bodies), cytoplasmic organelles whose role in RNA metabolism is not well understood (15, 16). The mRNAs and proteins that constitute these organelles are dynamic and rapidly exchange with the cytosol (17, 18). However, mRNAs containing 5’ terminal oligopyrimidine (TOP) motifs accumulate in stress granules upon amino acid starvation, suggesting that certain mRNA classes may be differentially regulated within these compartments (19). To characterize the spatiotemporal regulation of 5’ TOP mRNA translation during stress, a tetracycline-inducible HeLa cell line expressing a 5’ TOP TRICK reporter mRNA with green (NLS-PCP-GFP) and red (NLS-MCP-Halo; JF549) fluorescent proteins required for single-molecule RNA imaging were stressed with arsenite. 5’ TOP TRICK mRNAs were detected as single molecules distributed throughout the cytosol or located within stress granules and P-bodies. Only mRNAs sequestered within P-bodies formed large clusters. This association with P-bodies was specific for the 5’ TOP TRICK mRNAs because a reporter that lacked the 5’ TOP motif did not form multimeric assemblies within these cytoplasmic foci (Fig. 2A to C).
Figure 2. P-bodies are sites of translation regulation during stress in HeLa cells.
(A and B) IF-FISH of cells expressing Δ5’ TOP TRICK reporter mRNA [(A), gray] or 5’ TOP TRICK reporter mRNA [(B), gray] during arsenite stress (0.5 mM) contain stress granules (TIAR, green) and P-bodies (DDX6, red). Arrows: mRNA clusters in P-bodies. (C) Fraction of cytoplasmic Δ5’ TOP (n = 19 cells) and 5’ TOP (n =17 cells) mRNAs located within P-bodies after 60 min of arsenite (0.5 mM) stress (P = 0.0009, unpaired t test). (D and E) Live-cell image of 5’ TOP TRICK reporter mRNA during arsenite stress (D) and relief of stress (E). In stressed cells, mRNAs (red, green) in cytosol and P-bodies (cyan) are untranslated. In relieved cells, many mRNAs (red, green) in cytosol have been translated whereas mRNAs retained in P-bodies (cyan) remain untranslated. Arrow: clustered mRNAs. Scale bar (A, B, D, E), 10 µm. (F) Percentage of untranslated mRNAs (cytosol and P-bodies) during stress (n = 9 cells) and relief of stress (n = 10 cells). Upon relief of stress, 5’ TOP mRNAs in P-bodies are not translated (P = 0.31, unpaired t test); mRNAs in the cytosol have undergone translation (P < 0.0001, unpaired t test).
To address the translational regulation of cytosolic mRNAs and those clustered in P-bodies, we induced transcription of the 5’ TOP TRICK reporter mRNA for a short period before addition of arsenite. This resulted in an increase in the number of untranslated mRNAs in the cytoplasm to be detected compared to unstressed cells, consistent with an inhibition of eIF2.GTP.Met-tRNAiMet formation (Fig. 2D and F). The untranslated 5’ TOP TRICK reporter mRNAs in the cytoplasm were detected as either single mobile mRNAs or static clusters within P-bodies. Photobleaching of the clustered mRNAs indicated that they were stably associated with P-bodies (fig. S11). Upon removal of arsenite, 5’ TOP TRICK mRNAs in the cytosol underwent translation; however, the clustered transcripts retained in P-bodies remained untranslated, indicating that these cellular structures can provide a distinct level of regulation (Fig. 2E and F, and movies S6 to S7).
Messenger ribonucleoprotein (mRNP) granules form not only during cellular stress, but also as part of normal regulatory pathways. In Drosophilalocalized expression of Oskar protein at the posterior pole of the oocyte is essential for correct body patterning and germ cell formation (20). Precise spatiotemporal translational regulation is crucial during long-range transport of oskar mRNA (osk) from the nurse cells, where the mRNA is transcribed, to the posterior pole of the oocyte, where Oskar protein first appears during mid-oogenesis (stage 9) (21, 22). Additional mechanisms ensure degradation of ectopically expressed Oskar protein; hence, absence of the protein does not indicate lack of translation of its mRNA (23).
To monitor translation, we generated an osk-TRICK reporter mRNA by placing 12xPP7 stem-loops within the coding region of a construct that contained 6xMS2 stem-loops in the 3’ UTR (fig. S12) (24). Introducing 12xPP7 stem-loops into the open reading frame of osk mRNA did not inhibit translation of the reporter transcript, and the fusion protein was expressed at levels comparable to that of the wild-type protein (Fig. 3A). In early-stage oocytes of flies coexpressing osk-TRICK mRNA, NLS-MCP-RFP, and NLS-PCP-GFP, osk-TRICK mRNA was labeled by both NLS-PCP-GFP and NLS-MCP-RFP, indicating translational repression consistent with the absence of Oskar protein (Fig. 3B). In later stages, the NLS-PCP-GFP fluorescent signal was reduced at the posterior pole and Oskar protein was detected by immunofluorescence, consistent with translation of a portion of the transcripts (Fig. 3B and C). This methodology provides a framework for analyzing the cascade of regulatory mechanisms required for local translation during Drosophila development. It will also be informative in neurons where regulation of the first round of translation has been shown to be important for local protein synthesis in axons and dendrites (25, 26).
Figure 3. Stage specific translational activation of osk mRNA in Drosophila oocytes.
(A) 12xPP7 stem-loops in open reading frame of osk mRNA did not inhibit translation in Drosophila oocytes. Both long and short isoforms of wild-type Oskar protein, and Oskar-TRICK fusion protein were detected in ovary extracts. (B) osk-TRICK mRNA monitors stage-specific translational activation in oocytes. Immunodetection of Oskar protein (blue) in egg-chambers simultaneously expressing osk-TRICK mRNA, NLS-PCP-GFP, and NLS-MCP-RFP. Boxed areas: stage 7 oocyte (left) and pole plasm area of a stage 10 oocyte (right). (C) Reduction in NLS-PCP-GFP correlates with Oskar protein abundance and oocyte size. Quantification of fluorescent signals from NLS-PCP-GFP per NLS-MCP-RFP and Oskar protein at the pole plasm of individual oocytes. The size and color of dots indicate the measured area of the oocyte. Pearson correlation (r), linear regression (blue line), and 95% confidence area (gray). Scale bar, 50 µm.
This methodology pinpoints the precise time and place of the first translation event of single mRNA molecules. It reveals the translation control of mRNAs sequestered within cytoplasmic organelles or when and where the translation of a key cell fate determinant occurs in an organism undergoing development. The kinetics of translational regulation can now be coupled with single-molecule imaging of proteins to provide insights into mechanisms of regulation that were previously unapproachable by ensemble biochemical or genetic approaches (27). Observing regulation of mRNA translation in single living cells will lead to a better understanding of disease mechanisms.
Supplementary Material
Acknowledgments
This was work supported by the Novartis Research Foundation (J.A.C); NIH rants NS83085, EB013571, and GM57071 (R.H.S); Howard Hughes Medical Institute (R.H.S and T.L.); European Molecular Biology Laboratory (EMBL) (A.E.); and a postdoctoral fellowship from the EMBL Interdisciplinary Postdoc Program (EIPOD) under Marie Curie COFUND actions (F.W.). We thank C. Damgaard (University of Aarhus) for providing the rpL32-β-globin plasmid; S. Shenoy (Albert Einstein College of Medicine), L. Gelman and S. Bourke (Friedrich Miescher Institute), and EMBL Advanced Light Microscopy Facility for microscopy support; D. Ciepielewski (Nikon) for providing access to NIS tracking software; C. Eliscovich (Albert Einstein College of Medicine) for advice on IF-FISH; L. Lavis (Janelia Farm) for providing JF549 dye; M. Beal (Biosearch Technologies) for Stellaris FISH probes; A. Arnold (FMI) for assistance with polysome analysis; I. Gáspár (EMBL) for pHsp83 vector; and S. Chao, U. Meier, and J. Warner for helpful discussions.
References
- 1.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 3.Larson DR, Zenklusen D, Wu B, Chao JA, Singer RH. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science. 2011;332:475–478. doi: 10.1126/science.1202142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raj, van Oudenaarden A. Single-molecule approaches to stochastic gene expression. Annu. Rev. Biophys. 2009;38:255–270. doi: 10.1146/annurev.biophys.37.032807.125928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao JA, Yoon YJ, Singer RH. Imaging Translation in Single Cells Using Fluorescent Microscopy. Cold Spring Harb. Perspect. Biol. 2012;4:a012310. doi: 10.1101/cshperspect.a012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hocine S, Raymond P, Zenklusen D, Chao JA, Singer RH. Single-molecule analysis of gene expression using two-color RNA labeling in live yeast. Nat. Methods. 2013;10:119–121. doi: 10.1038/nmeth.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doma MK, Parker R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature. 2006;440:561–564. doi: 10.1038/nature04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.No D, Yao TP, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Jubran K, Wen J, Abdullahi A, Roy Chaudhury S, Li M, Ramanathan P, Matina A, De S, Piechocki K, Rugjee KN, Brogna S. Visualization of the joining of ribosomal subunits reveals the presence of 80S ribosomes in the nucleus. RNA. 2013;19:1669–1683. doi: 10.1261/rna.038356.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David, Dolan BP, Hickman HD, Knowlton JJ, Clavarino G, Pierre P, Bennink JR, Yewdell JW. Nuclear translation visualized by ribosome-bound nascent chain puromycylation. J. Cell Biol. 2012;197:45–57. doi: 10.1083/jcb.201112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shav-Tal Y, Darzacq X, Shenoy SM, Fusco D, Janicki SM, Spector DL, Singer RH. Dynamics of single mRNPs in nuclei of living cells. Science. 2004;304:1797–1800. doi: 10.1126/science.1099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mor, Suliman S, Ben-Yishay R, Yunger S, Brody Y, Shav-Tal Y. Dynamics of single mRNP nucleocytoplasmic transport and export through the nuclear pore in living cells. Nat. Cell Biol. 2010;12:543–552. doi: 10.1038/ncb2056. [DOI] [PubMed] [Google Scholar]
- 13.Stanners P. The effect of cycloheximide on polyribosomes from hamster cells. Biochem. Biophys. Res. Commun. 1966;24:758–764. doi: 10.1016/0006-291x(66)90390-1. [DOI] [PubMed] [Google Scholar]
- 14.Trcek T, Sato H, Singer RH, Maquat LE. Temporal and spatial characterization of nonsense-mediated mRNA decay. Genes Dev. 2013;27:541–551. doi: 10.1101/gad.209635.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchan JR, Parker R. Eukaryotic stress granules: The ins and outs of translation. Mol. Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kedersha N, Ivanov P, Anderson P. Stress granules and cell signaling: More than just a passing phase? Trends Biochem. Sci. 2013;38:494–506. doi: 10.1016/j.tibs.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mollet S, Cougot N, Wilczynska A, Dautry F, Kress M, Bertrand E, Weil D. Translationally repressed mRNA transiently cycles through stress granules during stress. Mol. Biol. Cell. 2008;19:4469–4479. doi: 10.1091/mbc.E08-05-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damgaard CK, Lykke-Andersen J. Translational coregulation of 5′ TOP mRNAs by TIA-1 and TIAR. Genes Dev. 2011;25:2057–2068. doi: 10.1101/gad.17355911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ephrussi, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358:387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- 21.Kim-Ha J, Kerr K, Macdonald PM. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995;81:403–412. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- 22.Markussen FH, Michon AM, Breitwieser W, Ephrussi A. Translational control of oskar generates short OSK, the isoform that induces pole plasma assembly. Development. 1995;121:3723–3732. doi: 10.1242/dev.121.11.3723. [DOI] [PubMed] [Google Scholar]
- 23.Morais-de-Sá E, Vega-Rioja A, Trovisco V, St Johnston D. Oskar is targeted for degradation by the sequential action of Par-1, GSK-3, and the SCF – Slimb ubiquitin ligase. Dev. Cell. 2013;26:303–314. doi: 10.1016/j.devcel.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin MD, Jiao X, Grima D, Newbury SF, Kiledjian M, Chou TB. Drosophila processing bodies in oogenesis. Dev. Biol. 2008;322:276–288. doi: 10.1016/j.ydbio.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 25.Colak D, Ji SJ, Porse BT, Jaffrey SR. Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell. 2013;153:1252–1265. doi: 10.1016/j.cell.2013.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130:179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Tanenbaum ME, Gilbert LA, Qi LS, Weissman JS, Vale RD. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 2014;159:635–646. doi: 10.1016/j.cell.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzpatrick JA, Yan Q, Sieber JJ, Dyba M, Schwarz U, Szent-Gyorgyi C, Woolford CA, Berget PB, Waggoner AS, Bruchez MP. STED nanoscopy in living cells using Fluorogen Activating Proteins. Bioconjug. Chem. 2009;20:1843–1847. doi: 10.1021/bc900249e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulon, Ferguson ML, de Turris V, Palangat M, Chow CC, Larson DR. Kinetic competition during the transcription cycle results in stochastic RNA processing. eLife. 2014;3:e03939. doi: 10.7554/eLife.03939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson DR, Fritzsch C, Sun L, Meng X, Lawrence DS, Singer RH. Direct observation of frequency modulated transcription in single cells using light activation. eLife. 2013;2:e00750. doi: 10.7554/eLife.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu B, Chao JA, Singer RH. Fluorescence fluctuation spectroscopy enables quantitative imaging of single mRNAs in living cells. Biophys. J. 2012;102:2936–2944. doi: 10.1016/j.bpj.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lionnet T, Czaplinski K, Darzacq X, Shav-Tal Y, Wells AL, Chao JA, Park HY, de Turris V, Lopez-Jones M, Singer RH. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nat. Methods. 2011;8:165–170. doi: 10.1038/nmeth.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm JB, English BP, Chen J, Slaughter JP, Zhang Z, Revyakin A, Patel R, Macklin JJ, Normanno D, Singer RH, Lionnet T, Lavis LD. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods. 2015;12:244–250. doi: 10.1038/nmeth.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weidenfeld, Gossen M, Löw R, Kentner D, Berger S, Görlich D, Bartsch D, Bujard H, Schönig K. Inducible expression of coding and inhibitory RNAs from retargetable genomic loci. Nucleic Acids Res. 2009;37:e50. doi: 10.1093/nar/gkp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol. Cell. 1998;2:437–445. doi: 10.1016/s1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- 37.Vanzo NF, Ephrussi A. Oskar anchoring restricts pole plasm formation to the posterior of the Drosophila oocyte. Development. 2002;129:3705–3714. doi: 10.1242/dev.129.15.3705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.