Abstract
Clinical observations suggest that gut and dietary factors transiently worsen and, in some cases, appear to improve behavioral symptoms in a subset of persons with autism spectrum disorders (ASDs), but the reason for this is unclear. Emerging evidence suggests ASDs are a family of systemic disorders of altered immunity, metabolism, and gene expression. Pre- or perinatal infection, hospitalization, or early antibiotic exposure, which may alter gut microbiota, have been suggested as potential risk factors for ASD. Can a common environmental agent link these disparate findings? This review outlines basic science and clinical evidence that enteric short-chain fatty acids (SCFAs), present in diet and also produced by opportunistic gut bacteria following fermentation of dietary carbohydrates, may be environmental triggers in ASD. Of note, propionic acid, a major SCFA produced by ASD-associated gastrointestinal bacteria (clostridia, bacteroides, desulfovibrio) and also a common food preservative, can produce reversible behavioral, electrographic, neuroinflammatory, metabolic, and epigenetic changes closely resembling those found in ASD when administered to rodents. Major effects of these SCFAs may be through the alteration of mitochondrial function via the citric acid cycle and carnitine metabolism, or the epigenetic modulation of ASD-associated genes, which may be useful clinical biomarkers. It discusses the hypothesis that ASDs are produced by pre- or post-natal alterations in intestinal microbiota in sensitive sub-populations, which may have major implications in ASD cause, diagnosis, prevention, and treatment.
Keywords: autism spectrum disorder, short-chain fatty acids, food preservative, antibiotic, microbiome, carnitine, gastrointestinal, mitochondria, oxidative stress, glutathione, epigenetics, neuroinflammation, neurexin, gap junctions, lipids
Let food be thy medicine, and medicine be thy food Everything in excess is opposed to nature All disease begins in the gut
—Hippocrates
The Prophet said: Stomach is the home (source) of all illness
—Hadith (Islam)
When Jesus climbed out of the boat, a man possessed by an evil spirit came out from a cemetery to meet him. This man lived among the burial caves and could no longer be restrained, even with a chain. Whenever he was put into chains and shackles—as he often was—he snapped the chains from his wrists and smashed the shackles. No one was strong enough to subdue him. Day and night he wandered among the burial caves and in the hills, howling and cutting himself with sharp stones …
Then Jesus demanded, ‘What is your name?’ And he replied, ‘My name is Legion, because there are many of us inside this man’.
—Gospel of St. Mark, New Testament.
Autism spectrum disorders (ASDs) are a family of neurodevelopmental conditions of rapidly increasing incidence. The condition was originally prevalent in approximately 1 in 10,000 when first reported in the middle of the 20th century. At present, ASD occurs in one in 68 persons in the United States, and may be as many as one in 30 in Korea. More prevalent in males (4:1), ASD comprises behavioral symptoms, including communication and social impairments, sensory abnormalities, and restricted and repetitive behavior, often with self-injurious behavior (1). In many children and adults with ASD, comorbidities include restrictive eating, gastrointestinal symptoms, and seizure disorder (2, 3). Recent studies have suggested that ASD is not a primary brain disorder, but may be a ‘whole body’ disorder with broad systemic abnormalities in immune and metabolic function (4–8). Anecdotal reports also suggest these findings may be associated with possible regression after apparently normal development in a subset of children (9). This is particularly evident in some populations that have migrated from underdeveloped countries to more developed ones, such as Somali expatriates (10), which was also discussed in a recent Canadian documentary (‘The Autism Enigma’, Canadian Broadcasting Corporation, Cogent Benger Productions, 2011). How these disparate findings relate to ASD symptoms or pathogenesis is unclear.
It is becoming apparent that complex interactions between genetic, epigenetic, and environmental factors contribute to the development and expression of ASD. A wide number of genes involved in immune regulation, mitochondrial function, and neural circuit formation have been implicated (11). However, known genetic factors discovered thus far account for 10–20% of ASDs and concordance rates among monozygotic twins are less than 100%, suggesting an important role for environmental risk factors which act on the underlying genetic susceptibilities (12), possibly by altering expression of ASD-implicated genes or functional pathways (6, 13). We have proposed that many of these environmental factors may arise, directly or indirectly, from small molecule metabolites from microbial populations in the gut (6, 7, 14).
Enteric short-chain fatty acids – gut microbiota metabolites in health and disease
There is growing evidence that the diverse populations of microbes, which inhabit the human digestive tract, termed the gut microbiota (GM), play a major role in the modulation of diverse host metabolic and immune pathways in both health and disease (15, 16). This microbial ecosystem, which outnumbers host cells 10 to one and genetic material 100 to one. It behaves as a functional ‘organ’, playing a major role in gut–brain communication, immune function, metabolism, and even behavior (6, 7, 17–19). Enteric short-chain fatty acids (SCFAs) are a major class of signaling molecules produced from bacterial fermentation of dietary carbohydrates, odd-chain fatty acids, and some proteins (20, 21). The most abundant of these are acetic acid (AA), butyric acid (BA), and propionic acid (PPA) (20). These SCFAs directly affect the host digestive tract through phenotypic alteration of colonic epithelial cells and act as major energy substrates. They can act as tumor suppressor agents, in apoptotic cell death, and have recently been shown to be modulators of the enteric neuroendocrine system. SCFAs are also involved in gene regulation of anti-inflammatory processes both in vitro and in vivo (22–29).
Being weak organic acids miscible in both aqueous and lipid phase, the majority of SCFAs are absorbed both passively and via active transport by monocarboxylate transporters from the gut, which also transport ketones. Those not metabolized by colonocytes (principally BA) are transported via the portal circulation and metabolized in the liver before reaching the systemic circulation. Traditionally, hepatic clearance was thought to reduce systemic effects of SCFAs on systemic metabolic and regulatory pathways. However, the distal colon, where the majority of GM reside, bypasses the portal circulation enabling systemic access (6, 7, 20).
In addition to production through colonic bacterial fermentation, SCFAs may also be present in diet. Of note, PPA and its chemical derivatives have increasing use in agriculture and the food industry (20). PPA occurs naturally in many foods (i.e. Swiss cheese). It is a major animal silage and food preservative in wheat and dairy products, either as sodium or calcium salt (30, 31) or is produced by adding high fructose corn syrup substrate to propionibacteria cultures which are then inoculated into foods. Inulin propionate has recently been suggested as a weight loss agent (32, 33), and aspartame is known to increase PPA levels in rodent gut flora. Nitropropionic acid, a derivative of PPA produced by many plants and fungi, is a potential contaminant of processed rice and sugar cane, and also produced sometimes in ruminant fermentation. It is a potent mitochondrial toxin, capable of causing neurotoxicity, and its administration in rodents is an acceptable model for Huntington's chorea (34).
There is growing evidence that the systemic effects of SCFAs (especially PPA and BA) on host physiology are underappreciated. This may have been secondary to 1) their production across tissues of large surface area and relative inaccessibility (i.e. small and large intestine), 2) their rapid colonic uptake by monocarboxylate transporters, 3) their ability to intracellularly concentrate, particularly during acidotic states, and 4) their rapid metabolism, all of which make SCFA measurement difficult (6, 20).
SCFAs have a number of direct effects on gastrointestinal physiology. They are known to reduce gastric motility and increase the frequency of contractions, presumably via a reflex that involves direct contact of these SCFAs with the terminal ileum (35). In addition, PPA and BA increases contraction of colonic smooth muscle (36), dilates colonic arteries (37), activates mast cells (38), and increases the release of serotonin from gut enterochromaffin cells (39, 40). Specific free fatty acid G-protein-coupled receptors (GPCRs) have recently been located throughout the enteric nervous and immune (T reg) systems, and offer many opportunities for novel pharmacotherapeutic agents (29).
Most of the emerging literature supports the beneficial effects of SCFAs on weight control, lipid profiles, and colon health, which appear to be dose and tissue specific (see 6, 20 for reviews). In spite of the multiple beneficial effects of SCFA on host gastrointestinal activity, it is important to note that excessive quantities of PPA have been reported in acne (41), gingival inflammation (42), irritable bowel syndrome (43), and necrotizing enterocolitis (44). It is also elevated in the neurometabolic condition propionic acidemia (45). In this heterogeneous inborn error of fatty acid metabolism, which may be underreported (46), accumulation of PPA and possibly other SCFAs is associated with developmental delay, seizure and extrapyramidal findings, acidosis, hyperammonianemia, increased oxidative stress, and mitochondrial dysfunction, often accompanied by bouts of gastrointestinal symptoms (45). Furthermore, PPA and related SCFAs have broad effects on nervous system physiology, including activation of specific free fatty acid GPCR, neurotransmitter synthesis and release, intracellular pH/calcium gating, mitochondrial function, lipid metabolism, immune function, gap junction gating, and gene expression (6).
Potential links of SCFAs in autism
Recent evidence suggests potential, but unproven, links between dietary, metabolic, immune, infective, and gastrointestinal factors and ASDs. Although inheritable factors, mostly implicated in synaptic transmission, have been traditionally studied in ASDs (11), the fact that 1) known genetic factors thus far account for only 10–20% of cases, 2) there is less than 100% concordance in identical twins, and 3) there is a growing prevalence in the condition, collectively suggest an important role for environmental factors which act on the underlying genetic sensitivities (12). In particular, C-sections, hospitalization, early infections, and associated antibiotic exposure (47), which are risk factors for ASD, may alter the developing GM (16). Increased mean levels of PPA in stool of ASD children have been shown (48). Given that PPA is a key fermentation product of ASD-associated bacteria (clostridia, bacteroides, desulfovibrio) (49) and modulates many ASD-related biochemical processes, we have proposed that SCFAs represent a group of host GM metabolites that are plausibly linked to ASD and can induce widespread effects on gut, brain, immune and metabolic function, and behavior (6, 7).
Further to this, we (14, 50–55) and others (56–59) have shown that short-term central nervous system intracerebroventricular (ICV) and peripheral administration (intraperitoneal, subcutaneous or oral gavage) of PPA and, to a lesser extent, other SCFAs, at various developmental time periods in rodents, induce broad behavioral and brain effects remarkably consistent with findings in persons with ASD, and even predict potential biomarkers in patients.
ICV infusion of SCFAs in rats induces reversible behavioral and electrographic effects consistent with autism
Repeated (5–14 days) pulsed ICV infusions of buffered SCFAs (0.026, 0.052, 0.26 M, 4 µl, pH buffered to 7.5), approximating the levels found in propionic acidemia patients, elicit a number of reversible behavioral changes reminiscent of ASD. Within 2–30 min post-ICV infusion, PPA and, to a lesser extent, BA and AA-treated rats were found to show reversible repetitive dystonic behaviors, retropulsion, object preference, and behavioral perseveration. These behaviors are absent in rats receiving control compounds (isomolar l-propanol or phosphate-buffered saline vehicle). Behavioral scoring and electrographic recordings also provided evidence of seizure activity involving both cortical (neo/hippocampal) and subcortical (striatal spiking) abnormalities. With repeated SCFA treatment, some animals also showed evidence for ‘kindling’ of seizures (PPA,>BA>AC). These effects were not seen with the control compounds. Additional behavioral effects observed in PPA-, BA-, and AA-treated rats included hyperactivity in an automated open-field test, impairment in social behavior when tested in pairs in a large open-field, impairments in the reversal of spatial learning in the Morris Water Maze, object preference, and enhanced startle response magnitude to an acoustic stimulus. Of particular importance was the finding that rats treated with SCFAs showed very clear impairments in social interaction which were not a function of changes in locomotor activity (Fig. 1). Overall, PPA was the most effective in elicitation of ASD-like behaviors. Thus, enteric SCFA exposure in rodents mimics many behavioral findings in ASD.
Fig. 1.

Behavioral videos of propionic acid infusions in rats (click headings to view videos). Single intracerebroventricular (ICV) infusions (4 µl of 0.26 M solution over 4 min) of propionic acid (PPA), a metabolic end product of autism-associated enteric bacteria, produce bouts of reversible hyperactive and repetitive behavior (A) in adult rats, compared with phosphate-buffered saline (PBS) vehicle infused control rat (B). Rat pairs infused with PPA show markedly reduced social interaction and play behavior (C), compared with pairs of rats infused with PBS vehicle (D), which show typical social behavior. Ethovision behavioral tracking of control and PPA-treated rat pairs (E), showing further evidence of PPA-induced hyperactive, repetitive, and antisocial behavior. PPA-treated rat displays fixation on objects (F) and specific object preferences (i.e. block vs. sphere). PPA-infused rats also show turning, tics, dystonia, and retropulsion and electrographic evidence of complex partial seizures and basal ganglia spiking, consistent with findings in patients with autism spectrum disorders. With permission from MacFabe (6).
Potential mechanisms for these rapidly induced and reversible behaviors are complex, and include SCFA-mediated effects such as enhanced calcium-dependent glutamate, serotonin and dopamine release, inhibition of GABAergic receptors, activation of specific SCFA GPCR, increased glutamate receptor sensitivity, increased catecholamine synthesis, intracellular acidification, mitochondrial dysfunction, and closure of gap junctions (see 6, 7, 14, 50, 51, 60, 61 for reviews).
Regarding the latter, we have postulated that many of the effects of PPA may be due to its ability to reduce intracellular connectivity via the closure of gap junctions (6, 7, 14).
Gap junctions are intercellular channels which allow passage of ions and small molecules. They are composed of protein subunits known as connexins and are gated by a number of factors, including dopamine, calcium, and cytokines, all of which are influenced by PPA (14). Gap junctions play a major role in cellular differentiation and, in particular, peripheral nerve, cardiac, uterine, and gastrointestinal function. However, in the CNS, gap junction coupling is vital for the synchronization of neural electrical activity within discrete functional cell groups. Gap junction-mediated coupling is more extensive during early brain development and neuronal migration and is thought to play a major role in brain development. Astrocytes are extensively electrotonically connected by gap junctions, forming a physiological syncytium to uptake and spatially buffer calcium, glutamate, and potassium, to stabilize the extracellular CNS microenvironment (62).
Small molecules, many of which are apoptotic factors (calcium, sodium, lysophospholipids, inositol triphosphate), are capable of passing through these glial gap junctions (63, 64). Therefore, closed glial gap junctions may render neurons hyper excitable due to rising extracellular potassium and glutamate (64), while closed neuronal gap junctions would be neuroprotective (63). In turn, this decrease in gap junction coupling may lead to inhibited cortical pruning in development, consistent with the increased neuronal density found in ASD (14). Gap junction communication is involved in neurotransmission in the basal ganglia, prefrontal cortex, nucleus accumbens, and hippocampus: all areas that are implicated in seizure and movement disorders. Intrastriatal injections of gap junction blockers produce stereotypical movements, hyperlocomotion, and disruption of motor sequencing in rodents (65, 66). Furthermore, gap junction knockout mice show abnormal brain development, exaggerated responses to neurotoxic insults, seizure disorder, and abnormal behaviors (67).
Interestingly, gap junction blockers, such as volatile anesthetics, ethanol, oleamide, glycyrrhetinic acid, carbenoxylone, and SCFAs, also inhibit tight junctions in many cellular systems (68, 69), possibly contributing to altered barrier function in the placenta, brain, and GI tract in ASD (70). Given these findings, it seems plausible that PPA-induced alterations to gap junction and tight junction function may have widespread effects on behavior, neural development, and gut and placental function, and may play a role in ASD (6, 7, 14).
Brief systemic exposure of SCFAs at critical neurodevelopmental windows have sex specific enduring behavioral effects consistent with autism
Traditionally, most potential environmental factors implicated in ASD are thought to principally exert their effects at critical pre- and early post-natal neurodevelopmental windows, either alone, or synergistically with other factors (i.e. the ‘double-hit hypothesis’), possibly by epigenetic means (71). Further to this, we sought to examine the behavioral effects of early brief exposure to systemic PPA. This SCFA exposure occurred with or without the microbial cell wall product lipopolysaccharide (LPS), a known activator of innate immunity and another acceptable model of ASD (54, 55). Pregnant Long-Evans rats were subcutaneously injected once a day with PPA (500 mg/kg) on gestation days G12–16, LPS (50 µg/kg) on G15–16, or vehicle control on G12–16 or G15–16. Male and female offspring were injected with PPA (500 mg/kg) or vehicle twice a day, every second day from postnatal days (P) 10–18. Physical milestones and reflexes were monitored in early life with prenatal PPA and LPS. Developmental milestones including delays in eye opening, locomotor activity, and anxiety were assessed in adolescence (PND40–42) in the elevated plus maze (EPM) and open-field motor activity. Prenatal and postnatal treatments altered behavior in a sex-specific manner. Prenatal PPA decreased time spent in the center of the open-field in males and females while prenatal and postnatal PPA increased anxiety behavior on the EPM in female rats. Prenatal LPS did not significantly influence those behaviors. Evidence for the double-hit hypothesis was seen as females receiving a double hit of PPA (prenatal and postnatal) displayed increased repetitive behavior in the open-field.
In similar experiments, acoustic startle and pre-pulse inhibition were measured on PND 45, 47, 49, and 51. Prenatal and postnatal treatments altered startle behavior in a sex-specific manner. Prenatal LPS treatment produced hyper-sensitivity to acoustic startle in males, but not females and did not alter pre-pulse inhibition. Subtle alterations in startle responses that disappeared with repeated trials occurred with prenatal PPA and postnatal PPA treatment in both male and female offspring. Prenatal PPA treatment decreased pre-pulse inhibition in females, but not males. Finally, females receiving a double hit of PPA, prenatal and postnatal, showed sensitization to acoustic startle, providing evidence for the double-hit hypothesis. Furthermore, both male and female PPA-treated pups were impaired in a test of their nest seeking response, suggesting impairment in olfactory-mediated neonatal social recognition. As well, adolescent males, born to PPA-treated dams, approached a novel object more than control animals and showed increased levels of locomotor activity compared to prenatal PPA females. Prenatal LPS produced subtle impairments in social behavior in adult male and female rats. These findings raise the possibility that brief systemic prenatal exposure to elevated levels of GM products, such as PPA or LPS, can subtly influence neonatal, adolescent, and adult social behavior. Collectively, these findings show early exposure to SCFAs, with or without combined immune stimulation from other gut-derived compounds (LPS), are capable of inducing long term behavior effects consistent with ASD.
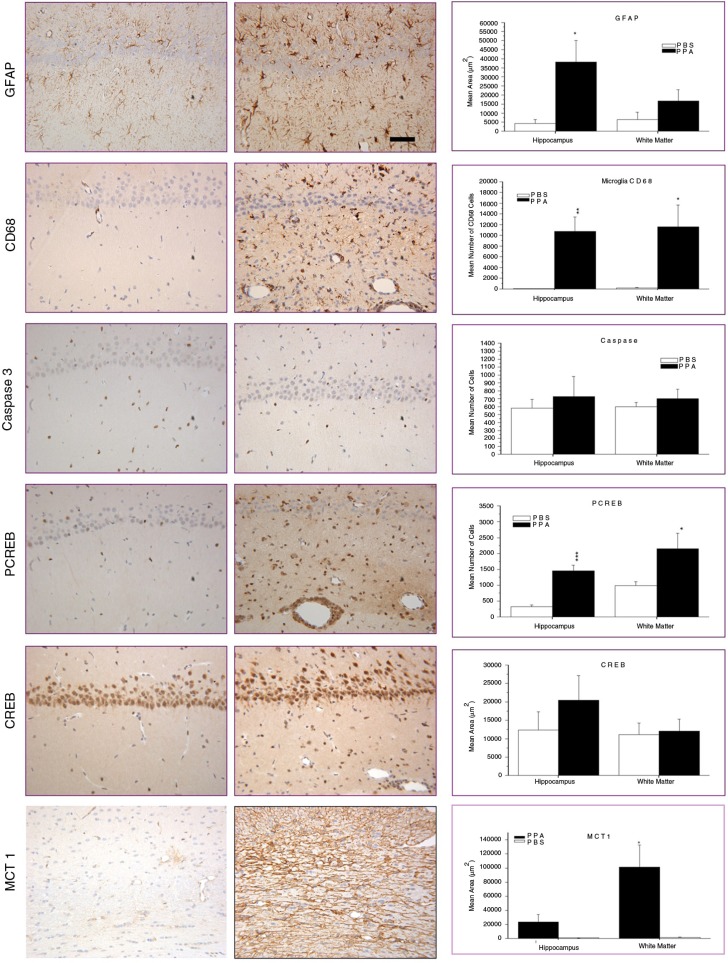
Central infusions of SCFAs produce neuroinflammatory and oxidative stress effects consistent with autism, and also activate neuroplastic memory (CREB) and fatty acid transport (monocarboxylate) systems. Twice-daily ICV infusions over 7–14 days of buffered PPA, BA, or AA (0.026, 0.052, 0.26 M, 4 µl, pH 7.5) produce broad brain changes reminiscent of ASD. Neuropathological analysis (hippocampus and external capsule white matter) (Fig. 2) revealed increases in reactive astrocytes (PPA, BA) and activated microglia (PPA), consistent with findings in ASD autopsy cases. In addition, further histochemical studies revealed increased monocarboxylate transporter/phosphorylated cyclic AMP respondent element binding protein (pCREB) immunoreactivity, a key factor in the epigenetic control of memory acquisition, in the absence of gross neuronal loss and apoptotic effects (caspase 3’), indicating broad effects in neuroplasticity and fatty acid metabolism (6, 7, 14, 50, 51, 60, 61).
Fig. 2.
Neuropathology (avidin–biotin complex immunohistochemistry) and semiquantitative image densitometry of coronal brain sections of dorsal hippocampus (CA2) and external capsule of adult rats with 14-day BID ICV infusions of propionic acid (PPA) or phosphate-buffered saline (PBS). PPA-induced significant reactive astrogliosis (anti-GFAP) and microglial activation (anti-CD68), without apoptotic neuronal cell loss (anti-cleaved caspase 3) in rat hippocampus, similar to finding in autopsy brain from patients with autism. Nuclear translocation of anti-CREB and an increase of anti phosphoCREB immunoreactivity are observed in neural, glial, and endovascular epithelium by PPA treatment, suggestive of gene induction. PPA increases monocarboxylate transporter 1 immunoreactivity, primarily in white matter external capsule, suggestive of alterations in brain short-chain fatty acid transport/metabolism. Black bars indicate PPA-treated animals; white bars indicate PBS (vehicle)-treated animals. Horizontal measurement bar = 100 µ. With permission from MacFabe (6).
Analyses of homogenates of brain regions produced evidence of increased oxidative stress and impaired glutathione (GSH) metabolism in discrete regions in PPA-treated animals. Biomarkers of protein and lipid peroxidation, total GSH as well as the activity of the antioxidant enzymes superoxide dismutase, catalase, GSH peroxidase, GSH reductase, and glutathione S-transferase (GST) were examined. Some brain regions of PPA-treated animals (neocortex, hippocampus, thalamus, striatum, cerebellum) showed increased lipid and protein oxidation accompanied by decreased total GSH in neocortex. Catalase activity was decreased in most brain regions of PPA-treated animals, suggestive of reduced antioxidant enzymatic activity against broad environmental zenobiotics implicated in ASD (metals, Tylenol administration). Collectively, these findings are consistent with those found in ASD patients (4–7, 14, 50, 60).
Lipid/mitochondrial/acylcarnitine profiles in PPA rodent model are consistent with findings in autism patients
We then wished to determine if there were any alterations in brain lipids associated with the ASD-like behavioral changes observed following intermittent ICV infusions of PPA, the related enteric metabolite BA or PBS vehicle. As in previous studies, both PPA and BA produced significant increases (p<0.001) in locomotor activity (total distance travelled and stereotypy). PPA and to a lesser extent BA infusions decreased the levels of total monounsaturates, total omega-6 fatty acids, total phosphatidylethanolamine plasmalogens, the ratio of omega-6:omega-3 and elevated the levels of total saturates in separated phospholipid species. In addition, total acylcarnitines, total long-chain (C12–24) acylcarnitines, total short-chain (C2–9) acylcarnitines, and the ratio of bound to free carnitine were increased following infusions with PPA and BA.
We applied electrospray ionization mass spectroscopy analysis to determine how brain and blood intact phospholipid species were altered during the induction of ASD-like behaviors in rats following ICV infusions with PPA. Animals were infused daily for 8 days, locomotor activity assessed, and animals were sacrificed during the induced behaviors. PPA infusions increased locomotor activity. Lipid analysis revealed treatment altered 21 brain and 30 blood phospholipid molecular species. Notable alterations were observed in the composition of brain sphingomyelin, diacyl mono and polyunsaturated phosphatidylcholine, phosphatidylinositol, phosphatidylserine, phosphatidylethanolamine, and plasmalogen phosphatidylcholine and phosphatidylethanolamine molecular species. These alterations suggest that SCFAs are able to cause broad changes in CNS lipid physiology, including membrane fluidity, cell signaling, redox capacity, and mitochondrial/carnitine function, consistent with findings in ASD patients (6, 7, 14, 50, 52, 60, 72).
Mitochondrial dysfunction found in the PPA rodent model predict novel biomarkers in a sub-set of autism patients
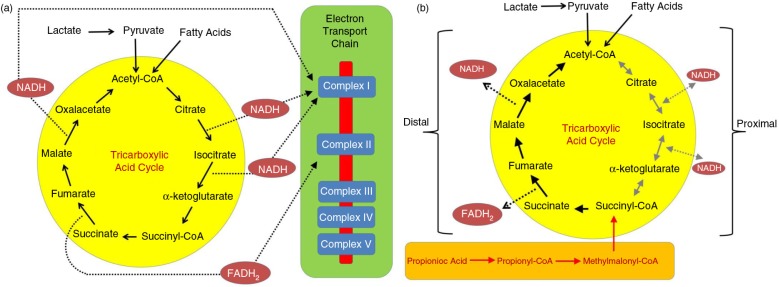
A comprehensive review has noted that ASD may occur with genetic and biochemical changes (lactate, pyruvate, carnitine alterations) consistent with mitochondrial disease (73). However, specific genetic mutations to explain mitochondrial disease are rare, suggesting that mitochondrial disease in some ASD patients may be environmentally acquired. As mentioned, our animal model similarly demonstrates many mitochondrial lipid changes associated with ASD, including a unique pattern of elevated short-chain and long-chain acyl-carnitines, suggesting broad alterations in fatty acid metabolism (6, 7, 52). To determine if these mitochondrial-related biomarkers are present in ASD patients, the laboratory results from a large cohort of children with ASD (n=213) who underwent screening for metabolic disorders, including mitochondrial and fatty acid oxidation disorders, in an autism clinic were reviewed (74). Acyl-carnitine panels were determined to be abnormal if three or more individual acyl-carnitine species were abnormal in the panel by repeated testing. Overall, 17% of individuals with ASD demonstrated consistently abnormal short- and long-chain acyl-carnitine panels consistent with the PPA rodent ASD model. Examination of electron transport chain function (muscle, fibroblast culture) and histological and electron microscopy examination of muscle suggest that PPA could be interfering with mitochondrial tricarboxylic acid metabolism (Fig. 3.). The function of the fatty acid oxidation pathway in fibroblast cultures and biomarkers for abnormalities in non-mitochondrial fatty acid metabolism were not consistently abnormal across the subgroup of ASD children, suggesting that the fatty acid metabolic abnormalities were secondary to tricarboxylic acid cycle abnormalities. GSH metabolism was abnormal in the ASD subset with acyl-carnitine panel abnormalities, similar to that found in the PPA rodent model (14, 60). These data suggest that there are similar pathological processes between a subset of ASD children and an animal model of ASD with acquired mitochondrial dysfunction. Future studies need to identify additional parallels between the PPA rodent model of ASD and this subset of ASD individuals with this unique pattern of acyl-carnitine and GSH abnormalities. Use of this animal model with ASD patients should lead to better insight into mechanisms behind environmentally induced ASD pathophysiology and should provide guidance for developing preventive and symptomatic treatments (6, 7, 14, 50, 52, 60, 72, 74, 75).
Fig. 3.
Effects of the enteric bacterial metabolite propionic acid (PPA) on the tricarboxylic acid cycle during (A) typical metabolism and (B) with high levels of PPA. PPA is metabolized to propionyl-CoA, which inhibits the proximal portion of the tricarboxylic acid cycle and enhances the distal portion of the tricarboxylic acid cycle. Effects of PPA on the citric acid cycle in the PPA rodent model of autism are consistent with those found in a subset of patients with ASD, along with further abnormalities in mitochondrial redox function, phospholipid, and acylcarnitine profiles. FADH2, flavin adenine dinucleotide; NADH, nicotinamide adenine dinucleotide. With permission from Ref. (74).
‘Common infections, chronic antibiotics, and clostridia colonization contribute to carnitine collapse, colitis, convulsions, and compulsions’ – impairment of carnitine metabolism from a variety of causes may be central to autism pathogenesis and regression
Of note, impairments in carnitine metabolism are a common feature in ASD and in our PPA rodent model (6, 52, 74, 76). Although the underlining cause of a relative carnitine deficiency reported in ASD remains unclear, we have noted that diverse neurodevelopmental conditions with gastrointestinal symptoms linked to ASD, such as Reye syndrome (77), valproate toxicity (78), propionic acidemia (79), and mitochondrial disorders (73), often collectively show disruptions in carnitine metabolism. Carnitine plays an underappreciated role in brain physiology and disease (80), particularly in brain astrocyte metabolism and GABAergic metabolism during early post-natal development (81). As carnitine is endogenously synthesized from lysine and methionine, persons with defects in methylation pathways, a common finding in ASD (82), would thus have impaired endogenous carnitine production, including those with an X-linked defect in the 6-N-trimethyllysine dioxygenase (TMLHE) enzyme responsible for the first step in carnitine biosynthesis, a risk factor for autism in males (83). These individuals would thus depend on dietary sources of carnitine, which is critical during periods of rapid development, and thus may be more sensitive to a number of conditions which impair gut carnitine uptake. Carnitine is transported across the gut-blood, blood–brain barriers and reabsorbed in the kidney via the Na+ dependent organic cation/carnitine transporter 2 (OCNT2) (84). Carnitine transport deficits have been implicated in colitis (85) and also lead to blood–brain barrier impairments, allowing non-neurotropic influenza A virus to enter the CNS, inducing a neonatal encephalopathy (86). Interestingly, long-term administration of common antibiotics (i.e. beta lactams) for routine pediatric infections, in addition to eliciting GM species favoring those which produce PPA, have also been shown to directly inhibit the OCNT2 transporter, including carnitine transport across gut-blood, and blood–brain barriers (84) and thus may elicit a relative systemic carnitine deficiency. Additionally, antibiotics given as forms of pivaloyl esters impair renal tubular carnitine reabsorption, potentially causing an increased urinary loss of carnitine. Such rapid impairments of carnitine metabolism could be significant during critical periods of early post-natal development of brain and gut, considering the reported high incidence of antecedent long-term early antibiotic use in some ASD patients (87–90), coupled with unique enteric PPA producing bacteria and gut carbohydrate malabsorption in regressive ASD (3, 87, 88). We have suggested this offers a potential explanation for autistic regression (6, 50, 74). It also explains the potential benefits of carnitine therapy and also of temporary behavioral improvements in some patients following vancomycin or metronidazole treatment, which transiently eradicates these bacteria and reduces PPA production (87, 88, 90, 91). Furthermore, removal of refined carbohydrates from the diet, which has been suggested as an empiric treatment to improve the behavioral fluctuations and gastrointestinal symptoms in ASD, may act by reducing substrate for these bacteria to produce PPA (14). Feeding of a high carbohydrate diet in rats is known to increase SCFA levels and produce anxiety and aggressive behavior (92). Interestingly, pre-eclamptic mothers, who have an increased risk of having offspring affected by ASD (93), have similar short and long acylcarnitine profiles (94) as those found in the ASD patients and the PPA rodent model. Although the overall relationships remain as yet unproven, we have proposed that the above observations link the decreased carnitine levels in some ASD patients with several genetic and environmental risk factors consistent with regression, gastrointestinal symptomatology, altered GM, lipid biomarkers, some empiric treatments (95), and with experimental findings obtained with the PPA model. Furthermore, oral carnitine and its derivative acetyl-l-carnitine have both neuroprotective (80, 96, 97) and coloprotective properties (98). We feel the use of these compounds as therapeutic agents in neurodevelopmental disorders, including ASD is warranted (14, 52, 73, 99). Furthermore, these observations would also support repeated carnitine/acylcarnitine screening of ‘patients at risk’. These would include infants with apparent developmental delay, seizure, and gastrointestinal dysfunction, particularly in the presence of maternal/infant hospital-acquired infection, early hospitalization (i.e. neonatal intensive care unit), or long-term antibiotic use (Table 1). However intriguing, it is important to note at this stage that it is unclear whether these complicated interactions are causative, compensatory, or confounders in ASD (100).
Table 1.
Potential causes and consequences of increased enteric short-chain fatty acid production and/or decreased breakdown and their relation to autism spectrum disorder.
| Causes | Consequences of SCFAs |
|---|---|
| Long term antibiotics for routine infections (maternal/infant) Treatment of maternal β hemolytic strep | Gut dysmotility/inflammation/carbohydrate malabsorbtion/altered gut permeability (tight junction impairment) |
| Hospitalisation (colonization of nosocomial bacteria) i.e. C-section, neonatal distress | Active uptake of SCFA to CNS (monocarboxylate transporters) |
| Prenatal drugs (valproate, ethanol) | pH dependent intracellular concentration of SCFAs |
| Opportunistic infection (Clostridium spp., Desulfovibrio spp.) | Neurotransmitter synthesis and release (catecholamines, enkephalins) CNS/sympathetic nervous system |
| Maternal/infant gut dysbiosis | Receptor activity (+NMDA, -GABA) SCFA G protein coupled receptors/Ca++ infiux |
| Organic acidemias (propionic/methymalonic, biotinidase/ holocarboxylase deficiency) | Gap junction closure, altered neurodevelopment, neuroinflammation |
| (B12/biotin deficiency) | Impaired mitochondrial function/increased oxidative stress |
| Genetic/acquired impaired carnitine synthesis/ absorption(TMLHE/OCTN2 genes, β- lactam antibiotics) | Reduced glutathione/increased sensitivity to xenobiotics (i.e. acetaminophen) |
| Mitochondrial disorder/dysfunction (inherited, acquired) | Decreased carnitine/altered lipid metabolism/membrane fiuidity |
| Colitis (impaired barrier/SCFA metabolism), i.e. celiac disease. Met-receptor tyrosine kinase mutation | Altered gene expression (CREB activation, histone deacetylase inhibition) |
| Increased refined carbohydrate consumption - substrate for bacterial fermentation | Antisocial/perseverative/anxiety-like behavior, seizure/movement disorder, Restrictive food interests/carbohydrate craving |
These findings, which are not mutually exclusive, may contribute to the pathophysiology, behavioral symptoms, and comorbidities of autism. With permission from MacFabe (6).
Epigenetic effects of SCFAs – potential links between genetic–environmental interactions in autism
One potential key mechanism where the metabolic products of an altered GM may contribute to ASD pathophysiology is via the alteration of gene expression associated with ASD mutations or ASD-implicated genetic pathways (6, 19, 101). Notably, SCFAs and their derivatives are known modulators of gene expression principally via their histone deacetylase inhibitor (HDACI) activity (102–108).
The rat pheochromocytoma (PC12) cell line is an extensively used in vitro cell system to examine molecular biological processes in neurobiology (109). Nankova and colleagues have used the PC12 line to examine the effects of SCFAs and their derivatives (i.e. valropic acid) on gene expression (110–112), particularly examining tyrosine hydroxylase (TH) gene, coding for a key enzyme in the synthesis of catecholamines, which is also implicated in ASD (113, 114). Moreover, CREB, a key factor in neurodevelopment, learning and memory (115), is a key determinant of catecholamine synthesis in PC12 cells, and shows increased CREB immunoreactivity in brains of PPA-treated rats (14). Furthermore, the anti-seizure/mood-stabilizing drug valproic acid, a known prenatal risk factor for ASD and produces an acceptable animal model for the condition, is structurally and pharmacologically similar to PPA, including HDACI properties (116–118) and produces similar effects as BA in PC12 cells (119).
We recently used rat PC12 cells as an in vitro system to extend our observations on the epigenetic effects of SCFAs to PPA (1–10 mM incubation over 48 h). Microarray technology was used to compare global changes in gene expression profiles following exposure to the structurally related SCFAs, PPA, and BA.
When PC12 cells were transiently transfected with plasmids having a luciferase reporter gene under the control of the TH promoter, PPA was found to induce reporter gene activity over a wide concentration range. CREB transcription factor was necessary for the transcriptional activation of the TH gene by PPA. At lower concentrations PPA also caused accumulation of TH mRNA and protein, indicative of increased cell capacity to produce catecholamines. PPA and BA induced broad alterations in gene expression including neurotransmitter systems, neuroplasticity and development, neuronal cell adhesion molecules (neurexin 1, neuroligin), inflammation, oxidative stress, lipid metabolism, mitochondrial function, and FMR1 (Fragile X) genes, all of which have been implicated in ASD (13). We are finding similar gene expression in preliminary studies in rats administered SCFA either centrally or in diet (unpublished observations). In conclusion, our data are consistent with a molecular mechanism through which SCFA metabolic products of the GM can epigenetically modulate cell function including genes related to ASD pathogenesis, further supporting their role as potential environmental epigenetic contributors to ASD.
Summary – can microbes control the mind? Future directions
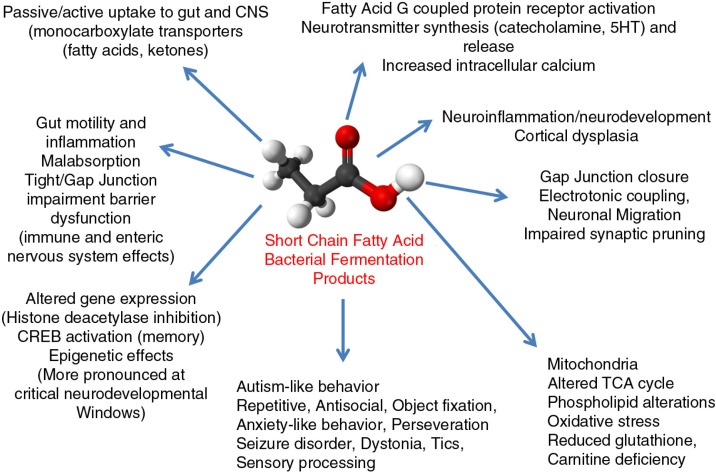
In summary, it can be seen that SCFA metabolic products of the GM have remarkable effects on host physiology including brain function and behavior (Fig. 4). Through our translational animal model, in vitro and clinical studies, it can be seen that SCFAs (PPA in particular) have unique properties, particularly involving neuroplasticity, memory acquisition, GPCR activation, gut physiology, tissue barrier permeability, oxidative stress, mitochondrial function, carnitine metabolism, and epigenetics, all of which have been implicated in ASD. Furthermore, there are many potential clinical scenarios for genetic–environmental interactions, which are consistent with enhanced exposure to or impaired metabolism of SCFAs in an individual at risk for ASD.
Fig. 4.
Broad physiological effects of enteric short-chain fatty acids on host physiology and brain function and behavior. These effects which are dose, tissue and temporally specific may be physiologically adaptive (immune/cellular energy regulation, food seeking, learning and memory, intra species social interaction), but may be pathological with increased production, decreased breakdown or increased early exposure of these bacterial metabolites during key periods of neurodevelopment.
It is important to note that, other than reports of increased PPA in stool samples (120), there are few studies that have systematically examined PPA and its related metabolites in ASD. The short half-life, rapid intracellular concentration of PPA by monocarboxylate transporters, metabolism via the TCA cycle, β-oxidation and incorporation into lipids, coupled with ‘difficult’ anatomical regions to clinically access (gut, brain) make SCFA measurement problematic, but possible. The evidence of many effects of PPA on diverse biological pathways being consistent with findings with ASD patients is intriguing but largely correlative. Novel translational models (animal models, artificial gut complex microbial colonies) (121) coupled with longitudinal human studies (100) are necessary to correlate these biomarkers to autistic regression or clinical improvement. These studies may also herald novel pharmacological treatments such as special diets, metabolic augmenters (carnitine), G-coupled receptor ligands, fatty acids, probiotics, or even GM repair (121, 122). However, cautious optimism is necessary for these future possible therapies, as we do not yet know long-term effects, or indeed which of these GM and metabolomics changes are contributory, causative, or simply a cofounder in ASD, or indeed any other condition (100).
However, given the acceptance and increased use of PPA in the food industry and agriculture, this does warrant further investigations and awareness of potential risks versus benefits of this SCFA. Of note, PPA's widespread use as a food preservative or weight loss agent, and its increased gut production following aspartame ingestion, may herald some caution, particularly during pregnancy and early postnatal development.
In a broader context, we have speculated that GM, through natural selection, have evolved to use their metabolites to adaptively modulate the physiology and ultimately behavior of the host, to promote survival (6, 7). This has been documented in behavioral neurobiology, with such examples as cordyceps fungus producing climbing behavior in ants, and Borna and rabies viruses eliciting salivary transmission and biting behavior in mammals (123, 124). In light of this, the observation of object fixation, restrictive eating of carbohydrates, diarrhea, and fecal smearing in patients with ASDs, which all could theoretically promote organism growth and spread, is intriguing. It is also worth noting that many of the effects of lower doses of SCFAs on gut physiology and immune function, at particular periods during the lifecycle are indeed beneficial to host and ultimately bacterial survival. Finally, it is important to note the ability of SCFAs to elicit anxiety-like, perseverative, repetitive, ritualistic, and antisocial behaviors that are common to many other neuropsychiatric conditions, such as obsessive compulsive, anxiety, attention deficit/hyperactive, mood, and eating disorders, posttraumatic stress disorder, irritable bowel syndrome, pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS), and schizophrenia, where infectious agents have been implicated as contributory (6, 7, 125–127). We have proposed that at least some of these neuropsychiatric conditions may in part represent potentially preventable or treatable metabolic disorders of impaired SCFA metabolism. By analogy, one can recall the tremendous strides which have been made in the management of diabetes, another apparently untreatable metabolic condition, which historically showed broad comorbid effects on multiple body systems including the CNS. Here, patients’ lives have been vastly improved subsequent to a better understanding of energy (glucose) metabolism, the exacerbating effect of infection, the role of diet, and the subsequent scientific developments of measurable metabolic biomarkers (glucometers), and pharmacological agents such as insulin and glyburide (7). With the collaborative efforts from experts from diverse scientific disciplines, it is the author's hope that similar strides can be made in ASD. In conclusion, it appears that enteric SCFAs play a major role in host physiology and provide further evidence that GM can modulate brain function and behavior in health and disease conditions, including ASD.
Acknowledgements
This manuscript was prepared as a summary of two lectures given at i) The Nobel Forum – ‘The Gut in Focus’ – Karolinska Institutet, Stockholm, Sweden (link: www.ki.se/mtc/kalender/gut-in-focus), and ii) at ‘1st International Symposium on the Microbiome in Health and Disease with a Special Focus on Autism’ Little Rock Arkansas, USA (link: www.microbiome-autism.com/). The author thanks all the investigators, collaborators, students, and technologists of the Kilee Patchell-Evans Autism Research Group, University of Western Ontario, Canada. Special thanks to Drs. Klaus-Peter Ossenkopp, Martin Kavaliers, Donald Peter Cain (behavior), and Fred Possmayer (biochemistry); graduate students/postdoctoral fellows Andrew Franklin, Jennifer Hoffman, Melissa Meeking, Jennifer Mepham, Stacey Holbrook; and Drs. Kelly Foley, Sandy Shultz (behavior), Karina Rodriguez-Capote (oxidative stress), and Raymond Thomas (lipid biochemistry). Expert technical assistance was provided by Roy Taylor (pathology), Francis Boon (behavior, surgery, EEG) and Lisa Tichenoff (Project Manager and generous assistance with manuscript preparation). The author is also indebted to collaborators Dr. Richard Frye (Arkansas Children's Hospital) and Bistra Nankova (New York Medical College), and especially Dr. Tore Midtvedt (Karolinska Institutet) for mentorship, support, and encouragement. Utmost thanks to David Patchell-Evans, for his tireless devotion to persons with autism, and his daughter, Kilee Patchell-Evans. Heartfelt thanks to countless parents and caregivers of persons with autism who have shared their stories. This research was supported by charitable contributions from GoodLife Children's Charities, Autism Canada, and Autism Research Institute to DFM. The author has not received any further funding or benefits from industry or elsewhere to conduct this study.
References
- 1.DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skokauskas N, Gallagher L. Psychosis, affective disorders and anxiety in autistic spectrum disorder: prevalence and nosological considerations. Psychopathology. 2010;43:8–16. doi: 10.1159/000255958. [DOI] [PubMed] [Google Scholar]
- 3.Williams BL, Hornig M, Buie T, Bauman ML, Cho PM, Wick I, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13:171–81. doi: 10.1016/j.pathophys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 5.James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23:2374–83. doi: 10.1096/fj.08-128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacFabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012;23:19260. doi: 10.3402/mehd.v23i0.19260. doi: http://dx.doi.org/10.3402/mehd.v23i0.19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacFabe D. Autism: metabolism, mitochondria, and the microbiome. Glob Adv Health Med. 2013;2:52–66. doi: 10.7453/gahmj.2013.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17:389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poling JS, Frye RE, Shoffner J, Zimmerman AW. Developmental regression and mitochondrial dysfunction in a child with autism. J Child Neurol. 2006;21:170–2. doi: 10.2310/7010.2006.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnevik-Olsson M, Gillberg C, Fernell E. Prevalence of autism in children born to Somali parents living in Sweden: a brief report. Dev Med Child Neurol. 2008;50:598–601. doi: 10.1111/j.1469-8749.2008.03036.x. [DOI] [PubMed] [Google Scholar]
- 11.Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–28. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nankova BB, Agarwal R, MacFabe DF, La Gamma EF. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells – possible relevance to autism spectrum disorders. PLoS One. 2014;9:e103740. doi: 10.1371/journal.pone.0103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149–69. doi: 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 16.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–70. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz HR, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour – epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 20.Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta. 2010;1801:1175–83. doi: 10.1016/j.bbalip.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Zoetendal EG, de Vos WM. Effect of diet on the intestinal microbiota and its activity. Curr Opin Gastroenterol. 2014;30:189–95. doi: 10.1097/MOG.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 22.Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res. 1994;54:3288–93. [PubMed] [Google Scholar]
- 23.Heerdt BG, Houston MA, Augenlicht LH. Short-chain fatty acid-initiated cell cycle arrest and apoptosis of colonic epithelial cells is linked to mitochondrial function. Cell Growth Differ. 1997;8:523–32. [PubMed] [Google Scholar]
- 24.Heerdt BG, Houston MA, Anthony GM, Augenlicht LH. Mitochondrial membrane potential (delta psi(mt)) in the coordination of p53-independent proliferation and apoptosis pathways in human colonic carcinoma cells. Cancer Res. 1998;58:2869–75. [PubMed] [Google Scholar]
- 25.Mariadason JM, Corner GA, Augenlicht LH. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000;60:4561–72. [PubMed] [Google Scholar]
- 26.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–28. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van IF. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–84. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 28.Schilderink R, Verseijden C, de Jonge WJ. Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Front Immunol. 2013;4:226. doi: 10.3389/fimmu.2013.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nohr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–64. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 30.Scotter MJ, Thorpe SA, Reynolds SL, Wilson LA, Strutt PR. Survey of baked goods for propionic acid and propionates. Food Addit Contam. 1996;13:133–9. doi: 10.1080/02652039609374391. [DOI] [PubMed] [Google Scholar]
- 31.Lind H, Jonsson H, Schnurer J. Antifungal effect of dairy propionibacteria – contribution of organic acids. Int J Food Microbiol. 2005;98:157–65. doi: 10.1016/j.ijfoodmicro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Palmnas MS, Cowan TE, Bomhof MR, Su J, Reimer RA, Vogel HJ, et al. Low-dose aspartame consumption differentially affects gut microbiota–host metabolic interactions in the diet-induced obese rat. PLoS One. 2014;9:e109841. doi: 10.1371/journal.pone.0109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2014:1–11. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francis K, Smitherman C, Nishino SF, Spain JC, Gadda G. The biochemistry of the metabolic poison propionate 3-nitronate and its conjugate acid, 3-nitropropionate. IUBMB Life. 2013;65:759–68. doi: 10.1002/iub.1195. [DOI] [PubMed] [Google Scholar]
- 35.Cuche G, Malbert CH. Short-chain fatty acids present in the ileum inhibit fasting gastrointestinal motility in conscious pigs. Neurogastroenterol Motil. 1999;11:219–25. doi: 10.1046/j.1365-2982.1999.00149.x. [DOI] [PubMed] [Google Scholar]
- 36.Mcmanus CM, Michel KE, Simon DM, Washabau RJ. Effect of short-chain fatty acids on contraction of smooth muscle in the canine colon. Am J Vet Res. 2002;63:295–300. doi: 10.2460/ajvr.2002.63.295. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut. 1990;31:1391–4. doi: 10.1136/gut.31.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karaki SI, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 39.Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585–94. doi: 10.1111/j.1365-2982.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 40.Mitsui R, Ono S, Karaki S, Kuwahara A. Propionate modulates spontaneous contractions via enteric nerves and prostaglandin release in the rat distal colon. Jpn J Physiol. 2005;55:331–8. doi: 10.2170/jjphysiol.RP000205. [DOI] [PubMed] [Google Scholar]
- 41.Zouboulis CC, Eady A, Philpott M, Goldsmith LA, Orfanos C, Cunliffe WC, et al. What is the pathogenesis of acne? Exp Dermatol. 2005;14:143–52. doi: 10.1111/j.0906-6705.2005.0285a.x. [DOI] [PubMed] [Google Scholar]
- 42.Niederman R, Zhang J, Kashket S. Short-chain carboxylic-acid-stimulated, PMN-mediated gingival inflammation. Crit Rev Oral Biol Med. 1997;8:269–90. doi: 10.1177/10454411970080030301. [DOI] [PubMed] [Google Scholar]
- 43.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–5. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Shoji H, Sato H, Nagata S, Ohtsuka Y, Shimizu T, et al. Effects of oral administration of bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J Pediatr Gastroenterol Nutr. 2007;44:252–7. doi: 10.1097/01.mpg.0000252184.89922.5f. [DOI] [PubMed] [Google Scholar]
- 45.Feliz B, Witt DR, Harris BT. Propionic acidemia: a neuropathology case report and review of prior cases. Arch Pathol Lab Med. 2003;127:e325–8. doi: 10.5858/2003-127-e325-PAANCR. [DOI] [PubMed] [Google Scholar]
- 46.Yorifuji T, Kawai M, Muroi J, Mamada M, Kurokawa K, Shigematsu Y, et al. Unexpectedly high prevalence of the mild form of propionic acidemia in Japan: presence of a common mutation and possible clinical implications. Hum Genet. 2002;111:161–5. doi: 10.1007/s00439-002-0761-z. [DOI] [PubMed] [Google Scholar]
- 47.Niehus R, Lord C. Early medical history of children with autism spectrum disorders. J Dev Behav Pediatr. 2006;27:S120–7. doi: 10.1097/00004703-200604002-00010. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57:2096–102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 49.Finegold SM. State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe. 2011;17:367–8. doi: 10.1016/j.anaerobe.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 50.MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res. 2010;217:47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Shultz SR, MacFabe DF, Martin S, Jackson J, Taylor R, Boon F, et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behav Brain Res. 2009;200:33–41. doi: 10.1016/j.bbr.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 52.Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF. Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem. 2010;113:515–29. doi: 10.1111/j.1471-4159.2010.06614.x. [DOI] [PubMed] [Google Scholar]
- 53.Ossenkopp KP, Foley KA, Gibson J, Fudge MA, Kavaliers M, Cain DP, et al. Systemic treatment with the enteric bacterial fermentation product, propionic acid, produces both conditioned taste avoidance and conditioned place avoidance in rats. Behav Brain Res. 2011;227:134–41. doi: 10.1016/j.bbr.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 54.Foley KA, Ossenkopp KP, Kavaliers M, MacFabe DF. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS One. 2014;9:e87072. doi: 10.1371/journal.pone.0087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foley KA, MacFabe DF, Vaz A, Ossenkopp KP, Kavaliers M. Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: implications for autism spectrum disorders. Int J Dev Neurosci. 2014;39:68–78. doi: 10.1016/j.ijdevneu.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Pettenuzzo LF, Schuck PF, Fontella F, Wannmacher CM, Wyse AT, Dutra-Filho CS, et al. Ascorbic acid prevents cognitive deficits caused by chronic administration of propionic acid to rats in the water maze. Pharmacol Biochem Behav. 2002;73:623–9. doi: 10.1016/s0091-3057(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 57.Brusque AM, Mello CF, Buchanan DN, Terracciano ST, Rocha MP, Vargas CR, et al. Effect of chemically induced propionic acidemia on neurobehavioral development of rats. Pharmacol Biochem Behav. 1999;64:529–34. doi: 10.1016/s0091-3057(99)00127-6. [DOI] [PubMed] [Google Scholar]
- 58.El-Ansary AK, Ben BA, Kotb M. Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation. 2012;9:74. doi: 10.1186/1742-2094-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Ghamdi M, Al-Ayadhi L, El-Ansary A. Selected biomarkers as predictive tools in testing efficacy of melatonin and coenzyme Q on propionic acid – induced neurotoxicity in rodent model of autism. BMC Neurosci. 2014;15:34. doi: 10.1186/1471-2202-15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacFabe DF, Rodriguez-Capote K, Hoffman JE, Franklin AE, Mohammad-Asef Y, Taylor A, et al. A novel rodent model of autism: intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am J Biochem Biotech. 2008;4:146–66. [Google Scholar]
- 61.Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, Taylor R, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54:901–11. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 62.Anders JJ. Lactic acid inhibition of gap junctional intercellular communication in in vitro astrocytes as measured by fluorescence recovery after laser photobleaching. GLIA. 1988;1:371–9. doi: 10.1002/glia.440010604. [DOI] [PubMed] [Google Scholar]
- 63.Frantseva MV, Kokarovtseva L, Naus CG, Carlen PL, MacFabe D, Perez Velazquez JL. Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. J Neurosci. 2002;22:644–53. doi: 10.1523/JNEUROSCI.22-03-00644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez-Velazquez JL, Frantseva MV, Naus CC. Gap junctions and neuronal injury: protectants or executioners? Neuroscientist. 2003;9:5–9. doi: 10.1177/1073858402239586. [DOI] [PubMed] [Google Scholar]
- 65.Moore H, Grace AA. A role for electrotonic coupling in the striatum in the expression of dopamine receptor-mediated stereotypies. Neuropsychopharmacology. 2002;27:980–92. doi: 10.1016/S0893-133X(02)00383-4. [DOI] [PubMed] [Google Scholar]
- 66.Juszczak GR, Swiergiel AH. Properties of gap junction blockers and their behavioural, cognitive and electrophysiological effects: animal and human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:181–98. doi: 10.1016/j.pnpbp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 67.Wiencken-Barger AE, Djukic B, Casper KB, McCarthy KD. A role for Connexin43 during neurodevelopment. GLIA. 2007;55:675–86. doi: 10.1002/glia.20484. [DOI] [PubMed] [Google Scholar]
- 68.Nagasawa K, Chiba H, Fujita H, Kojima T, Saito T, Endo T, et al. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J Cell Physiol. 2006;208:123–32. doi: 10.1002/jcp.20647. [DOI] [PubMed] [Google Scholar]
- 69.Miyoshi M, Usami M, Ohata A. Short-chain fatty acids and trichostatin A alter tight junction permeability in human umbilical vein endothelial cells. Nutrition. 2008;24:1189–98. doi: 10.1016/j.nut.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Li N, Neu J. Tight junctions, leaky intestines, and pediatric diseases. Acta Paediatr. 2005;94:386–93. doi: 10.1111/j.1651-2227.2005.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 71.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–94. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas RH, Meeking MM, Mepham JR, Tichenoff L, Possmayer F, Liu S, et al. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation. 2012;9:153. doi: 10.1186/1742-2094-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2011;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frye RE, Melnyk S, MacFabe DF. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry. 2013;3:e220. doi: 10.1038/tp.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frye RE, Rossignol DA. Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res. 2011;69:41R–7R. doi: 10.1203/PDR.0b013e318212f16b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Filipek PA, Juranek J, Nguyen MT, Cummings C, Gargus JJ. Relative carnitine deficiency in autism. J Autism Dev Disord. 2004;34:615–23. doi: 10.1007/s10803-004-5283-1. [DOI] [PubMed] [Google Scholar]
- 77.McArthur B, Sarnaik AP, Mitchell RA. Short-chain fatty acids and encephalopathy of Reye's syndrome. Neurology. 1984;34:831–4. doi: 10.1212/wnl.34.6.831. [DOI] [PubMed] [Google Scholar]
- 78.Coulter DL. Carnitine, valproate, and toxicity. J Child Neurol. 1991;6:7–14. doi: 10.1177/088307389100600102. [DOI] [PubMed] [Google Scholar]
- 79.Wajner M, Latini A, Wyse AT, Dutra-Filho CS. The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis. 2004;27:427–48. doi: 10.1023/B:BOLI.0000037353.13085.e2. [DOI] [PubMed] [Google Scholar]
- 80.Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog Lipid Res. 2010;49:61–75. doi: 10.1016/j.plipres.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Scafidi S, Fiskum G, Lindauer SL, Bamford P, Shi D, Hopkins I, et al. Metabolism of acetyl-L-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J Neurochem. 2010;114:820–31. doi: 10.1111/j.1471-4159.2010.06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.James SJ, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, et al. Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr. 2009;89:425–30. doi: 10.3945/ajcn.2008.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Celestino-Soper PB, Violante S, Crawford EL, Luo R, Lionel AC, Delaby E, et al. A common X-linked inborn error of carnitine biosynthesis may be a risk factor for nondysmorphic autism. Proc Natl Acad Sci USA. 2012;109:7974–81. doi: 10.1073/pnas.1120210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miecz D, Januszewicz E, Czeredys M, Hinton BT, Berezowski V, Cecchelli R, et al. Localization of organic cation/carnitine transporter (OCTN2) in cells forming the blood-brain barrier. J Neurochem. 2008;104:113–23. doi: 10.1111/j.1471-4159.2007.05024.x. [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto-Furusho JK, Mendivil-Rangel EJ, Villeda-Ramirez MA, Fonseca-Camarillo G, Barreto-Zuniga R. Gene expression of carnitine organic cation transporters 1 and 2 (OCTN) is downregulated in patients with ulcerative colitis. Inflamm Bowel Dis. 2011;17:2205–6. doi: 10.1002/ibd.21621. [DOI] [PubMed] [Google Scholar]
- 86.Yao D, Kuwajima M, Chen Y, Shiota M, Okumura Y, Yamada H, et al. Impaired long-chain fatty acid metabolism in mitochondria causes brain vascular invasion by a non-neurotropic epidemic influenza A virus in the newborn/suckling period: implications for influenza-associated encephalopathy. Mol Cell Biochem. 2007;299:85–92. doi: 10.1007/s11010-005-9046-x. [DOI] [PubMed] [Google Scholar]
- 87.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6–16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 88.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–53. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 89.Fallon J. Could one of the most widely prescribed antibiotics amoxicillin/clavulanate ‘augmentin’ be a risk factor for autism? Med Hypotheses. 2005;64:312–5. doi: 10.1016/j.mehy.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 90.Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429–35. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 91.Mellon AF, Deshpande SA, Mathers JC, Bartlett K. Effect of oral antibiotics on intestinal production of propionic acid. Arch Dis Child. 2000;82:169–72. doi: 10.1136/adc.82.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanstock TL, Clayton EH, Li KM, Mallet PE. Anxiety and aggression associated with the fermentation of carbohydrates in the hindgut of rats. Physiol Behav. 2004;82:357–68. doi: 10.1016/j.physbeh.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Mann JR, McDermott S, Bao H, Hardin J, Gregg A. Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord. 2010;40:548–54. doi: 10.1007/s10803-009-0903-4. [DOI] [PubMed] [Google Scholar]
- 94.Thiele IG, Niezen-Koning KE, van Gennip AH, Aarnoudse JG. Increased plasma carnitine concentrations in preeclampsia. Obstet Gynecol. 2004;103:876–80. doi: 10.1097/01.AOG.0000125699.60416.03. [DOI] [PubMed] [Google Scholar]
- 95.Frye RE, Delatorre R, Taylor H, Slattery J, Melnyk S, Chowdhury N, et al. Redox metabolism abnormalities in autistic children associated with mitochondrial disease. Transl Psychiatry. 2013;3:e273. doi: 10.1038/tp.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scafidi S, Racz J, Hazelton J, McKenna MC, Fiskum G. Neuroprotection by acetyl-L-carnitine after traumatic injury to the immature rat brain. Dev Neurosci. 2010;32:480–7. doi: 10.1159/000323178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel SP, Sullivan PG, Lyttle TS, Rabchevsky AG. Acetyl-L-carnitine ameliorates mitochondrial dysfunction following contusion spinal cord injury. J Neurochem. 2010;114:291–301. doi: 10.1111/j.1471-4159.2010.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fortin G, Yurchenko K, Collette C, Rubio M, Villani AC, Bitton A, et al. L-carnitine, a diet component and organic cation transporter OCTN ligand, displays immunosuppressive properties and abrogates intestinal inflammation. Clin Exp Immunol. 2009;156:161–71. doi: 10.1111/j.1365-2249.2009.03879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rossignol DA, Rossignol LW, Smith S, Schneider C, Logerquist S, Usman A, et al. Hyperbaric treatment for children with autism: a multicenter, randomized, double-blind, controlled trial. BMC Pediatr. 2009;9:21. doi: 10.1186/1471-2431-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.White RA, Bjornholt JV, Baird DD, Midtvedt T, Harris JR, Pagano M, et al. Novel developmental analyses identify longitudinal patterns of early gut microbiota that affect infant growth. PLoS Comput Biol. 2013;9:e1003042. doi: 10.1371/journal.pcbi.1003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Theije CG, Wu J, da Silva SL, Kamphuis PJ, Garssen J, Korte SM, et al. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur J Pharmacol. 2011;668:570–80. doi: 10.1016/j.ejphar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 102.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–6. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 103.Zhu WG, Otterson GA. The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr Med Chem Anticancer Agents. 2003;3:187–99. doi: 10.2174/1568011033482440. [DOI] [PubMed] [Google Scholar]
- 104.Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system: histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr Drug Targets CNS Neurol Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. [DOI] [PubMed] [Google Scholar]
- 105.Chen JS, Faller DV, Spanjaard RA. Short-chain fatty acid inhibitors of histone deacetylases: promising anticancer therapeutics? Curr Cancer Drug Targets. 2003;3:219–36. doi: 10.2174/1568009033481994. [DOI] [PubMed] [Google Scholar]
- 106.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–78. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–41. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 108.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2008;19:587–93. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 109.Shafer TJ, Atchison WD. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12:473–92. [PubMed] [Google Scholar]
- 110.DeCastro M, Nankova BB, Shah P, Patel P, Mally PV, Mishra R, et al. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res. 2005;142:28–38. doi: 10.1016/j.molbrainres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Shah P, Nankova BB, Parab S, La Gamma EF. Short chain fatty acids induce TH gene expression via ERK-dependent phosphorylation of CREB protein. Brain Res. 2006;1107:13–23. doi: 10.1016/j.brainres.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 112.Zia MT, Nankova BB, Krishnan K, Gueorguiev VD, Frenz CM, Sabban EL, et al. Role of Ca2+ in induction of neurotransmitter-related gene expression by butyrate. Neuroreport. 2004;15:1177–81. doi: 10.1097/00001756-200405190-00019. [DOI] [PubMed] [Google Scholar]
- 113.Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4:19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nakasato A, Nakatani Y, Seki Y, Tsujino N, Umino M, Arita H. Swim stress exaggerates the hyperactive mesocortical dopamine system in a rodent model of autism. Brain Res. 2008;1193:128–35. doi: 10.1016/j.brainres.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 115.Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Christensen J, Gronborg TK, Sorensen MJ, Schendel D, Parner ET, Pedersen LH, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol. 2000;22:319–24. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- 118.Cohen OS, Varlinskaya EI, Wilson CA, Glatt SJ, Mooney SM. Acute prenatal exposure to a moderate dose of valproic acid increases social behavior and alters gene expression in rats. Int J Dev Neurosci. 2013;31:740–50. doi: 10.1016/j.ijdevneu.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D'Souza A, Onem E, Patel P, La Gamma EF, Nankova BB. Valproic acid regulates catecholaminergic pathways by concentration-dependent threshold effects on TH mRNA synthesis and degradation. Brain Res. 2009;1247:1–10. doi: 10.1016/j.brainres.2008.09.088. [DOI] [PubMed] [Google Scholar]
- 120.Bresolin N, Freddo L, Vergani L, Angelini C. Carnitine, carnitine acyltransferases, and rat brain function. Exp Neurol. 1982;78:285–92. doi: 10.1016/0014-4886(82)90047-4. [DOI] [PubMed] [Google Scholar]
- 121.Allen-Vercoe E. Bringing the gut microbiota into focus through microbial culture: recent progress and future perspective. Curr Opin Microbiol. 2013;16:625–9. doi: 10.1016/j.mib.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scott MM, Deneris ES. Making and breaking serotonin neurons and autism. Int J Dev Neurosci. 2005;23:277–85. doi: 10.1016/j.ijdevneu.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 123.Kaushik M, Lamberton PH, Webster JP. The role of parasites and pathogens in influencing generalised anxiety and predation-related fear in the mammalian central nervous system. Horm Behav. 2012;62:191–201. doi: 10.1016/j.yhbeh.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 124.Kavaliers M, Choleris E, Agmo A, Pfaff DW. Olfactory-mediated parasite recognition and avoidance: linking genes to behavior. Horm Behav. 2004;46:272–83. doi: 10.1016/j.yhbeh.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 125.Sokol MS. Infection-triggered anorexia nervosa in children: clinical description of four cases. J Child Adolesc Psychopharmacol. 2000;10:133–45. doi: 10.1089/cap.2000.10.133. [DOI] [PubMed] [Google Scholar]
- 126.Suren P, Bakken IJ, Aase H, Chin R, Gunnes N, Lie KK, et al. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in norwegian children. Pediatrics. 2012;130:E152–8. doi: 10.1542/peds.2011-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kerbeshian J, Burd L. Is anorexia nervosa a neuropsychiatric developmental disorder? An illustrative case report. World J Biol Psychiatry. 2009;10:648–57. doi: 10.1080/15622970802043117. [DOI] [PubMed] [Google Scholar]