Abstract
Background
Limited studies exist on the outcome of thrombolytic therapy of acute ischemic stroke patients outside of clinical trials.
Aim
To assess the possible risk factors associated with in-hospital death and symptomatic intracerebral hemorrhage among patients who received intravenous tissue plasminogen activator.
Methods
A total of 7193 patients with a clinical diagnosis of acute ischemic stroke and a documented National Institutes of Health Stroke Scale score were treated with intravenous tissue plasminogen activator within 4·5 hours of time last known to be well. Generalized estimating equations modeling was used to assess the associations of in-hospital death and symptomatic intracerebral hemorrhage with clinical characteristics.
Results
Among 7193 patients treated with intravenous tissue plasminogen activator, 516 (7·2%) died during hospitalization. Factors associated with in-hospital death were older age, male gender, National Institutes of Health Stroke Scale score, history of myocardial infarction or coronary artery disease, and history of nonvalvular atrial fibrillation. Increasing age, higher National Institutes of Health Stroke Scale score, and history of dyslipidemia were associated with symptomatic intracerebral hemorrhage. There was no difference in the rates of in-hospital death or symptomatic intracerebral hemorrhage among patients treated with intravenous tissue plasminogen activator within three-hours of time last known to be well and those treated between three and 4·5 hours after this time.
Conclusions
In this study of acute ischemic stroke patients, older age, male gender, National Institutes of Health Stroke Scale score, history of myocardial infarction or coronary artery disease, and history of atrial fibrillation were associated with increased in-hospital death among patients receiving intravenous tissue plasminogen activator. Among patients treated with intravenous tissue plasminogen activator, in-hospital mortality and symptomatic intracerebral hemorrhage rates were similar between those treated within three-hours of time last known to be well and those treated between three and 4·5 hours after this time.
Keywords: acute stroke, stroke outcomes, symptomatic intracerebral hemorrhage, thrombolysis
Introduction
Each year, an estimated 795 000 people experience a new or recurrent stroke, and approximately 87% of all strokes are ischemic strokes. Intravenous tissue plasminogen activator (IV tPA) therapy is the only treatment approved by the Food and Drug Administration (FDA) for acute ischemic stroke (AIS), yet the overall use of IV tPA is low, ranging from 3·4% to 5·2% of all ischemic strokes in the United States (1,2). IV tPA has been shown to improve outcomes after acute ischemic stroke (3). Although several clinical trials have examined the complications of IV tPA, studies using large-scale non-clinical trial data are limited.
Determining which factors are associated with poor outcomes in a non-clinical trial setting is important for understanding the safety of IV tPA in ‘real-world’ experience. Clinical trials involving IV tPA had few very elderly (over age 80) patients. In fact, the tPA trial conducted by the National Institute of Neurological Disorders and Stroke (NINDS) included only 25 patients (of 312) that received IV tPA who were over the age of 80 (4). A recent review paper looked at 407 studies on the thrombolytic use of IV tPA and found IV thrombolysis was an effective treatment for stroke when delivered under appropriate clinical conditions up to 4·5 h from symptom onset (5). While IV tPA is only approved by the FDA for use within three-hours of onset of stroke symptoms, many professional organizations now advocate the use of IV tPA beyond three-hours (6), with additional cautions for patients with a history of both diabetes and prior stroke, patients over age 80, and those with a National Institutes of Health Stroke Scale (NIHSS) score over 25.
Aims
The aim of the present study was to assess possible risk factors associated with in-hospital death and symptomatic intracerebral hemorrhage (sICH) among patients who received IV tPA in the Centers for Disease Control and Prevention’s (CDC’s) Paul Coverdell National Acute Stroke Registry (PCNASR) between January 2008 and September 2012.
Methods
Data sources and study sample
Since 2001, the CDC has funded the development and implementation of acute stroke quality-of-care registries that are state-centric and hospital based. In 2004, the PCNASR was implemented in four state health departments (Georgia, Illinois, Massachusetts, and North Carolina), and in 2007, funding was extended to six state health departments (Georgia, Massachusetts, Michigan, Minnesota, North Carolina, and Ohio). The mission of the PCNASR is to measure, track, and improve the quality of care and access to care for stroke patients from onset of stroke symptoms through rehabilitation and recovery; decrease rates of premature death and disability from acute stroke; eliminate disparities in care; support the development of stroke systems of care that emphasize quality of care; improve access to rehabilitation and opportunities for recovery after stroke; and increase the workforce capacity and scientific knowledge for stroke surveillance within stroke systems of care. The details of the PCNASR program design have been previously published (7,8).
This analysis included all cases aged 18 and older collected in the PCNASR from January 2008 to September 2012 with a clinically assigned diagnosis of AIS and a recorded NIHSS score that had been treated with IV tPA within 4·5 h after time last known to be well (LKW time). Because we used the clinical diagnosis for case identification, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were not used in this analysis. sICH was defined by a CT scan showing intracranial hemorrhage within 36 h of IV thrombolysis and medical record documentation indicating clinical deterioration due to hemorrhage. Life-threatening hemorrhage was defined by bleeding within 36 hours of IV thrombolysis requiring more than three units of transfused blood within seven-days or earlier after receipt of IV thrombolysis or discharge, together with medical record documentation that bleeding was the reason for transfusion.
From 2008 to 2012 there were 152 097 patients with a clinical diagnosis of AIS in the PCNASR. Among them, 8227 (5·4%) received IV tPA within 4·5 h after LKW time, excluding those who received IV tPA at outside hospitals and were subsequently transferred to stroke centers. Of these, 1030 (12·5%) patients had missing NIHSS scores and four patients had missing gender information; these patients were excluded from the analysis. The final study sample was 7193 patients from 209 participating hospitals.
Statistical methods
The differences between categorical variables were examined using two-tailed Fisher’s exact or χ2-test, and the differences between continuous variables were examined using the Wilcoxon–Mann–Whitney rank test or the Kruskal–Wallis test. To account for the clustering of patients within hospitals, generalized estimating equations (GEE) modeling was used to assess the association between the outcomes (in-hospital death, sICH) and the prognostic factors. The adjusted model included the following covariates: age (<55 years, 55–64 years, 65–74 years, 75–79 years, 80+ years), gender, race-ethnicity (white vs. other), time of receipt of IV tPA (≤3 h vs. 3–4·5 h from LKW time), NIHSS score, hypertension status, smoking status, and medical history [dyslipidemia, myocardial infarction (MI) or coronary artery disease (CAD), heart failure (HF), diabetes, nonvalvular atrial fibrillation (AF), and prior stroke]. We used an upper limit of time from symptom onset to IV tPA initiation of 4·5 h. Patients with a history of hypertension or taking antihypertensive medication at time of the stroke were considered as having hypertension. Adjusted odds ratios (ORs) were obtained along with 95% confidence intervals (CIs). A P value of <0·05 was considered statistically significant. All analyses were done using SAS software (version 9·2; SAS Institute, Cary, NC, USA).
Results
Among 7193 patients who received IV tPA within 4·5 hours after LKW time, the median age was 71 years (range 18–103), and 30·5% of patients were 80 years or older (Table 1). Eighty-two percent of patients received IV tPA within three-hours after LKW time, 9% received IV tPA between 3 and 3·5 h after LKW time, and 9% received IV tPA between 3·5 and 4·5 h after LKW time (data not shown). The median NIHSS score was 11 (range 0–42). The majority of patients had hypertension, and the other comorbidities are presented in Table 1. We found that 5·6% of patients had a history of both diabetes and prior stroke (data not shown). The proportion of smokers was 21·4%, and the median length of stay was four-days (range 0–381). Fifty-two percent of patients were admitted to hospitals with more than 500 beds, 90·3% of them were treated in a hospital with a stroke unit, and 74·3% of them were treated in a teaching hospital (Table 1).
Table 1.
Characteristics of AIS patients who received IV tPA according to whether they died in the hospital
| Variable | Overall (n = 7193) | Died (n = 516) | Survived (n = 6677) | P value* |
|---|---|---|---|---|
| Age (years), median (range) | 71 (18–103) | 81 (28–100) | 70 (18–103) | <0·0001 |
| Age group, n (%) | ||||
| <55 years | 1362 (18·9) | 36 (7·0) | 1326 (19·9) | <0·0001 |
| 55–64 years | 1348 (18·7) | 56 (10·9) | 1292 (19·4) | |
| 65–74 years | 1465 (20·4) | 86 (16·7) | 1379 (20·7) | |
| 75–79 years | 821 (11·4) | 63 (12·2) | 758 (11·4) | |
| 80+ years | 2197 (30·5) | 275 (53·3) | 1922 (28·8) | |
| Male, n (%) | 3563 (49·5) | 240 (46·5) | 3323 (49·8) | 0·16 |
| Race-ethnicity, n (%) | ||||
| White | 5273 (73·3) | 403 (78·1) | 4870 (72·9) | 0·03 |
| Black | 1462 (20·3) | 83 (16·1) | 1379 (20·7) | |
| Other | 458 (6·4) | 30 (5·8) | 428 (6·4) | |
| Time from LKW to IV tPA ≤ 3 h, n (%) | 5904 (82·1) | 432 (83·7) | 5472 (82·0) | 0·34 |
| NIHSS score, median (range) | 11 (0–42) | 19 (0–41) | 10 (0–42) | <0·0001 |
| Medical history, n (%) | ||||
| Hypertension | 5757 (80·0) | 460 (89·1) | 5297 (79·3) | 0·0001 |
| Dyslipidemia | 3020 (42·0) | 217 (42·1) | 2803 (42·0) | 1·00 |
| MI or CAD | 1880 (26·1) | 186 (36·0) | 1694 (25·4) | <0·0001 |
| Heart failure | 751 (10·4) | 90 (17·4) | 661 (9·9) | <0·0001 |
| Diabetes | 1793 (24·9) | 142 (27·5) | 1651 (24·7) | 0·17 |
| Atrial fibrillation | 1489 (20·7) | 187 (36·2) | 1302 (19·5) | <0·0001 |
| Prior stroke | 1251 (17·4) | 98 (19·0) | 1153 (17·3) | 0·33 |
| Smoker, n (%) | 1537 (21·4) | 63 (12·2) | 1474 (22·1) | <0·0001 |
| Length of stay (days), median (range) | 4 (0–381) | 4 (0–91) | 4 (0–381) | 0·36 |
| Hospital bed size, n (%)† | ||||
| ≤300 | 1321 (20·1) | 96 (20·3) | 1225 (20·1) | 0·97 |
| 301–500 | 1813 (27·6) | 133 (28·1) | 1680 (27·6) | |
| >500 | 3429 (52·2) | 245 (51·7) | 3184 (52·3) | |
| Stroke unit, n (%)† | 5927 (90·3) | 422 (89·0) | 5505 (90·4) | 0·97 |
| Teaching hospital, n (%)† | 4879 (74·3) | 363 (76·6) | 4516 (74·2) | 0·25 |
Unadjusted.
Information missing for 34 hospitals.
AIS, acute ischemic stroke; IV tPA, intravenous tissue plasminogen activator; LKW, time last known to be well; NIHSS, National Institutes of Health Stroke Scale; MI, myocardial infarction; CAD, coronary artery disease.
In-hospital death
Among 7193 AIS patients treated with IV tPA, 516 (7·2%) died during the hospital admission. Unadjusted analyses showed no variation in the proportion of in-hospital deaths in relation to hospital bed size, presence of stroke unit in the hospital, or status as a teaching hospital (Table 1). There was no difference in the proportion of in-hospital deaths between those receiving thrombolysis within three-hours of LKW time and those receiving thrombolysis between 3 and 4·5 h after LKW time (P = 0·34).
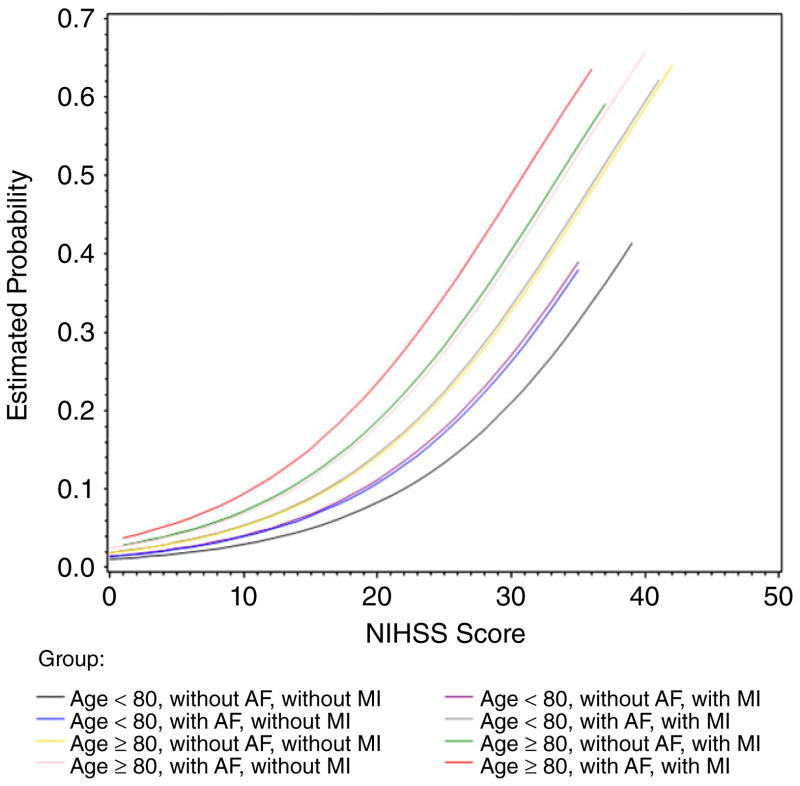
Adjusted analyses showed there were five factors associated with the risk of in-hospital death. Increased age was associated with an increased risk of in-hospital death. The adjusted odds ratio (OR) versus patients <55 years old was 1·73 (95% CI 1·15–2·61, P = 0·008) for patients age 65–74, 2·01 (95% CI 1·29–3·13, P = 0·002) for those age 75–79, and 2·92 (95% CI 1·99–4·29, P < 0·001) for those age 80 years and older. Males had a higher risk of in-hospital death than females (adjusted OR of 1·24; 95% CI 1·02–1·52, P = 0·03). The NIHSS score was significantly associated with the risk of in-hospital death, with an adjusted OR of 1·11 (95% CI 1·10–1·13, P < 0·001) for each unit increase. Patients with a history of MI or CAD or a history of AF had a higher risk of in-hospital death (adjusted ORs 1·28 and 1·25 respectively) than those without these comorbidities (Table 3). Further, by looking at whether patients were or were not age 80 years or older, at whether they had a history of MI or CAD or AF, and at their NIHSS score, we found that patients age 80 years and older with a history of AF and a history of MI or CAD had the highest risk of in-hospital death after thrombolysis compared with others (Fig. 1).
Table 3.
Adjusted odds ratios of predictors of in-hospital death and sICH
| Variables | In-hospital death
|
sICH
|
||||
|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | P value | Adjusted OR | 95% CI | P value | |
| Age group | ||||||
| <55 years | Reference | Reference | ||||
| 55–64 years | 1·42 | 0·92–2·18 | 0·11 | 1·19 | 0·60–2·33 | 0·62 |
| 65–74 years | 1·73 | 1·15–2·61 | 0·008 | 2·30 | 1·29–4·08 | 0·005 |
| 75–79 years | 2·01 | 1·29–3·13 | 0·002 | 3·01 | 1·62–5·60 | 0·001 |
| 80+ years | 2·92 | 1·99–4·29 | <0·001 | 3·09 | 1·73–5·51 | 0·0001 |
| Male | 1·24 | 1·02–1·52 | 0·03 | 1·20 | 0·95–1·53 | 0·13 |
| Race-ethnicity | ||||||
| Whites | Reference | Reference | ||||
| Others* | 1·03 | 0·81–1·30 | 0·80 | 0·77 | 0·59–1·01 | 0·06 |
| Time from LKW to IV tPA >3 h | 0·89 | 0·69–1·14 | 0·35 | 0·81 | 0·58–1·14 | 0·23 |
| NIHSS score | 1·11 | 1·10–1·13 | <0·001 | 1·06 | 1·05–1·08 | <0·0001 |
| Medical history | ||||||
| Hypertension | 0·81 | 0·63–1·05 | 0·11 | 1·07 | 0·75–1·52 | 0·70 |
| Dyslipidemia | 0·86 | 0·70–1·05 | 0·14 | 1·34 | 1·05–1·70 | 0·02 |
| MI or CAD | 1·28 | 1·04–1·58 | 0·02 | 0·91 | 0·70–1·18 | 0·46 |
| Heart failure | 1·17 | 0·90–1·51 | 0·25 | 0·90 | 0·64–1·27 | 0·56 |
| Diabetes | 1·14 | 0·92–1·42 | 0·25 | 1·29 | 0·999–1·66 | 0·051 |
| Atrial fibrillation | 1·25 | 1·01–1·57 | 0·046 | 1·19 | 0·91–1·56 | 0·20 |
| Prior stroke | 0·93 | 0·73–1·20 | 0·58 | 0·98 | 0·73–1·32 | 0·90 |
| Smoker | 0·92 | 0·68–1·23 | 0·57 | 0·73 | 0·51–1·06 | 0·10 |
Due to the small number of patients who were not white, we collapsed these patients into one group.
sICH, symptomatic intracerebral hemorrhage; OR, odds ratio; CI, confidence interval; LKW, time last known to be well; IV tPA, intravenous tissue plasminogen activator; NIHSS, National Institutes of Health Stroke Scale; MI, myocardial infarction; CAD, coronary artery disease.
Fig. 1.
The predicted probability of in-hospital death among patients receiving intravenous tissue plasminogen activator. NIHSS, National Institutes of Health Stroke Scale; AF, atrial fibrillation; MI, myocardial infarction.
Hemorrhagic complications of IV thrombolytic therapy
Overall, sICH was observed in 4·5% of patients receiving IV tPA. Compared with patients without sICH, patients with sICH were older (median ages 78 vs. 70), had higher NIHSS scores (median scores 16 vs. 10), and were more likely to have hypertension (86·4% vs. 79·7%), a history of dyslipidemia (50·8% vs. 41·6%), a history of diabetes (31·3% vs. 24·6%), or a history of AF (30·3% vs. 20·2%) (Table 2). Patients who had a history of both diabetes and prior stroke had a higher incidence of sICH (7·1%) than those who did not (5·5%, data not shown). However, among patients who had sICH, only 11·8% of patients were smokers, as compared with 21·8% among those without sICH. Hemorrhagic complication rates did not differ between patients who received tPA within ≤3 h of LKW time and those who received IV tPA within 3–4·5 h (Table 2). Adjusted analysis showed that increasing age, higher NIHSS score (adjusted OR 1·06; 95% CI 1·05–1·08, P < 0·0001), and history of dyslipidemia (adjusted OR 1·34; 95% CI 1·05–1·70, P = 0·02) were associated with sICH (Table 3). Patients with a history of both diabetes and prior stroke were not more likely to develop sICH (data not shown). Patients with sICH had a higher rate of in-hospital death (26·9%) than those who did not develop sICH (6·2%) (Table 2).
Table 2.
Characteristics of AIS patients who received IV tPA according to whether they developed hemorrhagic complications
| Variable | sICH
|
Life-threatening hemorrhage
|
||||
|---|---|---|---|---|---|---|
| Yes | No | P value* | Yes | No | P value* | |
| Overall | 323 (4·5) | 6870 (95·5) | 58 (0·8) | 7135 (99·2) | ||
| Age (years), median (range) | 78 (22–99) | 70 (18–103) | <0·0001 | 78 (23–96) | 70 (18–103) | 0·003 |
| Age group, n (%) | ||||||
| <55 years | 26 (8·0) | 1336 (19·4) | <0·0001 | 7 (12·1) | 1355 (19·0) | 0·005 |
| 55–64 years | 33 (10·2) | 1315 (19·1) | 3 (5·2) | 1345 (18·9) | ||
| 65–74 years | 74 (22·9) | 1391 (20·2) | 12 (20·7) | 1453 (20·4) | ||
| 75–79 years | 50 (15·5) | 771 (11·2) | 13 (22·4) | 808 (11·3) | ||
| 80+ years | 140 (43·3) | 2057 (29·9) | 23 (39·7) | 2174 (30·5) | ||
| Male, n (%) | 155 (48·0) | 3408 (49·6) | 0·61 | 33 (56·9) | 3530 (49·5) | 0·29 |
| Race-ethnicity, n (%) | ||||||
| White | 235 (72·8) | 5038 (73·3) | 0·85 | 46 (79·3) | 5227 (73·3) | 0·37 |
| Others | 88 (27·2) | 1832 (26·7) | 12 (20·7) | 1908 (26·7) | ||
| Time from LKW to IV tPA ≤ 3 h, n (%) | 258 (79·9) | 5646 (82·2) | 0·30 | 45 (77·6) | 5859 (82·1) | 0·39 |
| NIHSS score, median (range) | 16 (1–34) | 10 (0–42) | <0·0001 | 17 (2–26) | 10 (0–42) | <0·0001 |
| Medical history, n (%) | ||||||
| Hypertension | 279 (86·4) | 5478 (79·7) | 0·003 | 50 (86·2) | 5707 (80·0) | 0·32 |
| Dyslipidemia | 164 (50·8) | 2856 (41·6) | 0·001 | 22 (37·9) | 2998 (42·0) | 0·59 |
| MI or CAD | 95 (29·4) | 1785 (26·0) | 0·18 | 20 (34·5) | 1860 (26·1) | 0·18 |
| Heart failure | 42 (13·0) | 709 (10·3) | 0·14 | 8 (13·8) | 743 (10·4) | NA |
| Diabetes | 101 (31·3) | 1692 (24·6) | 0·008 | 14 (24·1) | 1779 (24·9) | 1·00 |
| Atrial fibrillation | 98 (30·3) | 1391 (20·2) | 0·0001 | 18 (31·0) | 1471 (20·6) | 0·07 |
| Prior stroke | 63 (19·5) | 1188 (17·3) | 0·29 | 13 (22·4) | 1238 (17·4) | 0·30 |
| Smoker, n (%) | 38 (11·8) | 1499 (21·8) | <0·0001 | 8 (13·8) | 1529 (21·4) | NA |
| Discharge disposition, n (%) | ||||||
| Home | 33 (10·2) | 2832 (41·2) | <0·0001 | 1 (1·7) | 2864 (40·1) | NA |
| Hospice | 37 (11·5) | 223 (3·2) | <0·0001 | 10 (17·2) | 250 (3·5) | <0·0001 |
| Acute care facility | 21 (6·5) | 189 (2·8) | 0·001 | 3 (5·2) | 207 (2·9) | NA |
| Other health care facility | 139 (43·0) | 2993 (43·6) | 0·86 | 8 (13·8) | 3124 (43·8) | NA |
| In-hospital death, n (%) | 87 (26·9) | 429 (6·2) | <0·0001 | 34 (58·6) | 482 (6·8) | <0·0001 |
Unadjusted.
AIS, acute ischemic stroke; sICH, symptomatic intracerebral hemorrhage; IV tPA, intravenous tissue plasminogen activator; LKW, time last known to be well; NIHSS, National Institutes of Health Stroke Scale; MI, myocardial infarction; CAD, coronary artery disease; NA, not applicable because of the small sample size.
Similarly to sICH, life-threatening hemorrhage was more common among older patients (median age of 78 vs. 70 for those without it), those with higher NIHSS scores (median score of 17 vs. 10 for those without it) and was associated with a greater rate of in-hospital death (58·6% vs. 6·8% for those without it, P < 0·0001; Table 2). Only 0·8% of patients developed life-threatening hemorrhage, and among the 323 patients who had sICH and the 58 patients who had life-threatening hemorrhage, 14 had both complications.
Overall, 38·7% of patients were discharged to home, 4·7% to hospice, 2·9% to an acute care facility, and 43·5% to other health care facilities (skilled nursing units, long-term care facilities, and rehabilitation hospitals) (data not shown). Patients with sICH were less likely to be discharged home and more likely to be discharged to hospice and acute care facilities (Table 2).
Discussion
We found that, consistent with results from other studies (9,10), in-hospital death among ischemic stroke patients who received IV tPA was associated with increasing age and stroke severity. Regression analyses showed increasing age, higher NIHSS score, and history of dyslipidemia were independent predictors of sICH. These predictors are also used in the iScore. The iScore is a scoring tool used to predict the risk of complications from IV tPA, developed by Stroke Outcomes Research Canada (http://www.sorcan.ca/iscore/index.html). However, although smoking is used by the iScore, we did not find smoking to be positively associated with sICH or in-hospital death after thrombolysis (11).
Even though increasing age was an independent risk factor for in-hospital death and sICH, several studies have suggested that age alone should not be a barrier to the treatment (9,10,12,13). These studies showed that age, particularly for those age 80 years and older, was not associated with poor outcome as defined by modified Rankin Scale (mRS) score at discharge (9,10,12–15). The IST-3 study, which was a randomized controlled trial, found thrombolysis given within six-hours of stroke improved functional outcome as defined by the Oxford Handicap Scale (OHS), and the benefit did not seem to be diminished in patients 80 years and older (16). We were unable to confirm this result because of the lack of information on mRS or OHS scores. However, in our study, 31% of patients who received IV tPA were over 80 years old; this was much higher than in previous studies (8% in the NINDS trial, 19% in the VISTA trial, and 10% in the SITS trial, respectively) (4,12,13). Furthermore, we did find that older age, whether analyzed as a continuous variable, a stratified variable, or a dichotomous variable with a cut-point of 80 years, was associated with a greater risk of in-hospital death and the development of sICH. A recent study found that when the sum of age and the NIHSS score (Sum) was greater than or equal to 100, patients receiving IV tPA developed sICH at more than double the rate of those whose Sum was less than 100 (17). Similarly, that study found that when Sum was greater than or equal to 100, the mortality rate at three-months was four times higher than when Sum was less than 100. However, that study only had 36 patients whose Sum was greater than or equal to 100. In our study, the rates of in-hospital death and sICH were 18·8% and 7·8%, respectively, for patients whose Sum was greater than or equal to 100 (n = 1241), compared to 4·8% and 3·8%, respectively, for patients whose Sum was less than 100 (n = 5952). Patients whose Sum was greater than or equal to 100 had significantly higher rates of in-hospital death and sICH than those with Sum less than 100 (P < 0·001 for both, data not shown).
NIHSS score was a potential predictor for both in-hospital death and sICH for patients receiving IV tPA. A recent study by Fonarow et al. indicated the NIHSS score was a substantial prognostic factor for short-term mortality, even in the absence of other clinical information (18). Several non-US studies also confirmed these findings (19,20). However, the completeness of recorded NIHSS scores was a challenge in all studies. In our study, 13% of patients who received IV tPA had missing NIHSS scores. In the PCNASR, we have seen a rapid improvement in the documentation of NIHSS score among acute ischemic stroke patients across the years (from 42% in 2008 to 71% in 2012; P-value for trend was < 0·001, data not shown), but the overall rate of documentation of NIHSS score remains sub-optimal. We found that a history of MI or CAD and a history of AF were also independent predictors of in-hospital death among patients who received IV tPA within 4·5 hours of LKW time, and being over 80 with either a history of AF or MI or one of CAD sharply increased the risk of in-hospital death after thrombolysis. Few studies looked at the association of a history of MI or CAD and mortality, but the rates of AF were comparable with and the association between AF and mortality was consistent with those reported in other studies (12–15). A German stroke study did not find AF to be an independent predictor of in-hospital mortality (unadjusted and adjusted analysis gave P-values of 0·19 and 0·76, respectively), reporting that 29·4% of patients who survived had AF, while 34·3% of patients who died had AF (9). However, in our study, only 19·5% of patients who survived had AF, which was much lower than in the German study. This difference might be due to differences in age distribution between the two registries. The patients in the German study were much younger, with only 23·9% aged ≥75 years, compared with 41·9% in the PCNASR.
Overall, sICH was observed in 4·5% of AIS patients in the PCNASR treated with IV tPA. This was much lower than the rates reported by several clinical trials but was comparable with results reported in stroke registries (21). One study showed the use of IV tPA for stroke in the oldest old (age 85+) did not increase the risk of sICH (22). In that study, the oldest old represented only 7%, a much smaller percentage than found in our study (18%, data not shown). Because of the small sample size in that study in this age group, there may not have been enough power for them to detect a significant difference.
Thrombolysis received in the extended time window (between 3 and 4·5 h from LKW time) was not associated with either in-hospital death or complications of thrombolytic therapies; however, this result differs from the CASES registry (23) and several published studies (6,16,24). The IST-3 study reported the greatest benefits in patients treated within three-hours, but there was not enough power to examine decay of benefit with time (16). The CASES registry showed there was a trend toward higher rates of death and sICH in patients treated within 3–4·5 h as compared with those treated in 0–3 h (22). In the CASES study, 88% of patients received tPA within three-hours, and the median NIHSS was 13 in the 3–4·5-h group and 15 in the 0–3 h group. In our study, 82% received tPA within three-hours, but the stroke severity was less for patients treated after both time intervals (median NIHSS 11 for the 0–3-h group and 9 for the 3–4·5-h group, respectively). This may in part account for the lack of association between the tPA time window and in-hospital death or sICH in our study. Several meta-analyses on trial data from all ECASS trials, the NINDS trial, and ATLANTIS suggested that thrombolysis within 3 to 4·5 h of LKW time improved functional outcomes but increased sICH rates, though without significantly increasing mortality (6,24). However, these studies had far fewer patients than our study. Our study could not control for selection bias in the decision to treat with IV tPA, which may explain differences between our study and clinical trials, but our study represents real-world experience with IV tPA.
Our study has several limitations. The PCNASR is a quality improvement program focusing on in-hospital care, and therefore the results may not be generalizable. The hospitals that participated in the PCNASR, as well as the program requirements for participation, changed over time. However, we controlled for hospital characteristics through the GEE models to minimize the bias. We did not have information on glucose level at the time of admission, computed tomography scan results, or whether antihypertensive medications, which have been shown in some studies to be related to the development of sICH (25), were required in the emergency department prior to administration of IV tPA. In addition, the definition of sICH in our study might be different as compared with other studies. Based on the analysis published by Gumbinger (26), our definition, which is similar to that used for the NINDS trial, may tend to overestimate the occurrence of sICH because it may include more minor cases of sICH. It is conceivable that our predictors for the development of sICH might change if laboratory and imaging results were included. Finally, regarding the concern that registry data are affected by case selection bias, CDC requires that funded states track the extent of case abstraction of participating hospitals within the state registries; however, we do not conduct a formal national audit of the data using a sole-source abstractor. While the potential for case selection bias exists, the majority of hospitals are making every effort to abstract all stroke cases. As noted above, we are unable to account for selection bias in the decision to treat with IV tPA, but because of that, we can provide information on real-world experience in the use of IV tPA. Limitations aside, the strength of this study is that we had a large cohort of patients treated in a variety of academic and nonacademic hospitals. Most existing studies had small numbers of patients, leading to inconclusive results.
In conclusion, given our findings that age, gender, NIHSS score, history of MI or CAD, and history of AF were associated with in-hospital death among AIS patients who received IV tPA, we conclude that whether a patient is age 80 or older and whether he or she has a history of AF and/or CAD are important factors to consider, along with the NIHSS score, when considering the use of IV thrombolysis. Finally, we confirmed significant associations between the NIHSS score and both mortality and sICH. While the overall documentation of stroke severity is high and has improved significantly in recent years, additional work needs to be done to document stroke severity in all patients receiving IV tPA.
Footnotes
Conflict of interest: The authors declare no potential conflict of interest.
This paper was part of an oral presentation at the 2013 International Stroke Conference; Honolulu, Hawaii, Feb. 6–8.
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–55. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke. Chest. 2012;141:e601S–36S. doi: 10.1378/chest.11-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longstreth WT, Jr, Katz R, Trischwell DL, Cushman M, Psaty BM. Intravenous tissue plasminogen activator and stroke in the elderly. Am J Emerg Med. 2010;28:359–63. doi: 10.1016/j.ajem.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeMers G, Meurer WJ, Shih R, Rosenbaum S, Vilke GM. Tissue plasminogen activator and stroke: review of the literature for the clinician. J Emerg Med. 2012;43:1149–54. doi: 10.1016/j.jemermed.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter CR, Keim SM, Milne WK, Meurer WJ, Barsan WG Best Evidence in Emergency Medicine Investigator Group. . Thrombolytic therapy for acute ischemic stroke beyond three hours. J Emerg Med. 2011;40:82–92. doi: 10.1016/j.jemermed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George MG, Tong X, McGruder H, et al. Paul Coverdell National Acute Stroke Registry Surveillance – four states, 2005–2007. Morb Mortal Wkly Rep. 2009;58:1–23. [PubMed] [Google Scholar]
- 8.George MG, Tong X, Yoon PW. Use of a registry to improve acute stroke care – seven states, 2005–2009. Morb Mortal Wkly Rep. 2011;60:206–10. [PubMed] [Google Scholar]
- 9.Heuschmann PU, Kolominsky-Rabas PL, Roether J, et al. Predictors of in-hospital mortality in patients with acute ischemic stroke treated with thrombolytic therapy. JAMA. 2004;292:1831–38. doi: 10.1001/jama.292.15.1831. [DOI] [PubMed] [Google Scholar]
- 10.Henriksen EH, Ljøstad U, Tveiten A, Næss H, Thomassen L, Mygland A. TPA for ischemic stroke in patients ≥80 years. Acta Neurol Scand. 2013;127:309–15. doi: 10.1111/ane.12008. [DOI] [PubMed] [Google Scholar]
- 11.Saposnik G, Kapral MK, Liu Y, et al. A risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011;123:739–49. doi: 10.1161/CIRCULATIONAHA.110.983353. [DOI] [PubMed] [Google Scholar]
- 12.Mishra NK, Ahmed N, Andersen G, et al. Thrombolysis in very elderly people: controlled comparison of SITS International Stroke Thrombolysis Registry and Virtual International Stroke Trials Archive. BMJ. 2010;341:c6046. doi: 10.1136/bmj.c6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra NK, Diener HC, Lyden PD, Bluhmki E, Lees KR. Influence of age on outcome from thrombolysis in acute stroke: a controlled comparison in patients from the Virtual International Stroke Trials Archives (VISTA) Stroke. 2010;41:2840–48. doi: 10.1161/STROKEAHA.110.586206. [DOI] [PubMed] [Google Scholar]
- 14.Mishra NK, Ahmed N, Davalos A, et al. Thrombolysis outcomes in acute ischemic stroke patients with prior stroke and diabetes mellitus. Neurology. 2011;77:1866–72. doi: 10.1212/WNL.0b013e318238ee42. [DOI] [PubMed] [Google Scholar]
- 15.Demchuk AM, Tanne D, Hill MD, et al. Predictors of good outcome after intravenous tPA for acute ischemic stroke. Neurology. 2001;57:474–80. doi: 10.1212/wnl.57.3.474. [DOI] [PubMed] [Google Scholar]
- 16.IST-3 Collaborative Group. . The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischemic stroke (the Third International Stroke Trial [IST-3]): a randomized controlled trial. Lancet. 2012;397:2352–63. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC. Stroke prognostication using age and NIH Stroke Scale: SPAN-100. Neurology. 2013;80:1–8. doi: 10.1212/WNL.0b013e31827b1ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonarow GC, Saver JL, Smith EE, et al. Relationship of National Institutes of Health Stroke Scale to 30-day mortality in Medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc. 2012;1:42–50. doi: 10.1161/JAHA.111.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Liu G, Zhang G, et al. A risk score based on Get With the Guidelines – stroke program data works in patients with acute ischemic stroke in China. Stroke. 2012;43:3108–09. doi: 10.1161/STROKEAHA.112.669085. [DOI] [PubMed] [Google Scholar]
- 20.Ayis SA, Coker B, Rudd AG, Dennis MS, Wolfe CD. Predicting independent survival after stroke: a European study for the development and validation of standardized stroke scales and prediction models of outcome. J Neuro Neurosurg Psychiatry. 2013;84:288–96. doi: 10.1136/jnnp-2012-303657. [DOI] [PubMed] [Google Scholar]
- 21.Seet RC, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis. 2012;34:106–14. doi: 10.1159/000339675. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Caldentey J, Leciñana MA, Simal P, et al. Intravenous thrombolytic treatment in the oldest old. Stroke Research and Treatment. 2012:article no.: 923676. doi: 10.1155/2012/923676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shobha N, Buchan AM, Hill MD. Thrombolysis at 3–4. 5 hours after acute ischemic stroke onset – evidence from the Canadian Alteplase for Stroke Effectiveness Study (CASES) registry. Cerebrovasc Dis. 2011;31:223–28. doi: 10.1159/000321893. [DOI] [PubMed] [Google Scholar]
- 24.Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4. 5 hours after acute ischemic stroke. Stroke. 2009;40:2438–41. doi: 10.1161/STROKEAHA.109.552547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lansberg MG, Thijs VN, Bammer R, et al. Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007;38:2275–78. doi: 10.1161/STROKEAHA.106.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gumbinger C, Gruschka P, Böttinger M, et al. Improved prediction of poor outcome after thrombolysis using conservative definitions of symptomatic hemorrhage. Stroke. 2012;43:240–2. doi: 10.1161/STROKEAHA.111.623033. [DOI] [PubMed] [Google Scholar]