Abstract
The ubihydroquinone:cytochrome c oxidoreductase, or cytochrome bc1, is central to the production of ATP by oxidative phosphorylation and photophosphorylation in many organisms. Its three-dimensional structure depicts it as a homodimer with each monomer composed of the Fe-S protein, cytochrome b, and cytochrome c1 subunits. Recent genetic approaches successfully produced heterodimeric variants of this enzyme, providing insights into its mechanism of function. However, these experimental setups are inherently prone to genetic rearrangements as they carry repeated copies of cytochrome bc1 structural genes. Duplications present on a single replicon (one-plasmid system) or a double replicon (two-plasmid system) could yield heterogeneous populations via homologous recombination or other genetic events at different frequencies, especially under selective growth conditions. In this work, we assessed the origins and frequencies of genetic variations encountered in these systems and describe an improved variant of the two-plasmid system. We found that use of a recombination-deficient background (recA) minimizes spontaneous formation of co-integrant plasmids and renders the homologous recombination within the cytochrome b gene copies inconsequential. On the basis of the data, we conclude that both the newly improved RecA-deficient and the previously used RecA-proficient two-plasmid systems reliably produce native and mutant heterodimeric cytochrome bc1 variants. The two-plasmid system developed here might contribute to the study of “mitochondrial heteroplasmy”-like heterogeneous states in model bacteria (e.g., Rhodobacter species) suitable for bioenergetics studies. In the following paper (DOI 10.1021/bi400561e), we describe the use of the two-plasmid system to produce and characterize, in membranes and in purified states, an active heterodimeric cytochrome bc1 variant with unusual intermonomer electron transfer properties.
The ubihydroquinone:cytochrome c oxidoreductase (cytochrome bc1 or mitochondrial complex III) is a multisubunit membrane integral enzyme required for both photo synthetic (Ps) and respiratory (Res) electron transfer in many organisms. It oxidizes hydroquinones (QH2) to produce quinones (Q) and reduces metalloprotein electron carriers, which are often c-type cytochromes. This reaction is coupled with the translocation of protons across the membrane for ATP production. The three-dimensional structures of various cytochrome bc1 forms are symmetrical homodimers.1–4 In most bacteria, each monomer consists of three conserved subunits: cytochrome b, the Fe-S (also called Rieske) protein, and cytochrome c1. Cytochrome b has two b-type hemes (a low-potential bL heme and a high-potential bH heme) located on the p and n sides of the membrane. The Fe-S protein and cytochrome c1 carry a high-potential [2F–2S] cluster and a c-type heme, respectively.4
The modified Q cycle describes the mechanism of electron transfer and proton translocation within cytochrome bc1.5–7 Accordingly, two QH2 molecules are oxidized, one Q molecule is reduced, and the resulting electrons are conveyed to the terminal acceptors (e.g., cytochrome c oxidase or mitochondrial complex IV in respiration) via a complex series of reactions.8,9 As generally described, the Q cycle mechanism is confined to a single monomer of the dimeric cytochrome bc1 and does not consider any possible interaction between the monomers.10 A mechanism with pathways of electrons through a dimeric cytochrome bc1 was first formulated by De Vries et al.,11 and a dimeric organization of this enzyme was observed by electron microscopy.12 Similar ideas were rejuvenated after its dimeric architecture was established firmly via its three-dimensional structures.1–4 Various interesting features of the dimeric enzyme raised intriguing questions, including electron transfer possibilities between the monomers through the bL-bL hemes because of their proximity to each other, or conformational changes during the catalytic steps of a dimeric cytochrome bc1. These observations, together with experimental data13–18 and theoretical calculations,19 led to models that invoked allosteric interactions and electron transfer reactions between the monomers of cytochrome bc1.13–15
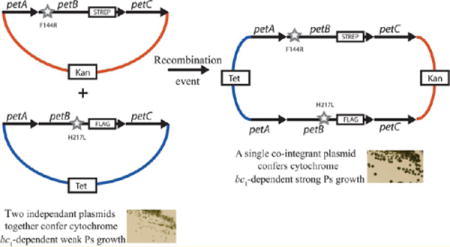
Recently, the intermonomer electron transfer issue was experimentally probed by various groups,20,21 including ours,22 using genetic approaches aimed at breaking the symmetry of the dimeric cytochrome bc1. Using Rhodobacter capsulatus, Osyczka’s group produced a cytochrome bc1 variant (b-bc1) in which two cytochromes b were genetically fused together with an amino acid linker. A single plasmid (termed the one-plasmid system) carrying this engineered petAB1-B2C operon yielded a heterodimeric cytochrome b-bc1, which was purified using an epitope tag added to the cyt b-b fusion subunit21 (Figure 1A). Independently, we developed a different genetic setup that duplicated the entire petABC operon (encoding the three catalytic subunits of cytochrome bc1) on two separate plasmids (termed the two-plasmid system), in which each petB gene is marked with a different epitope tag22 (Figure 1B). This system produced two-homodimeric (i.e., same tag and same mutation on both monomers) and one-heterodimeric (i.e., different tags and different mutations in each monomer) cytochrome bc1 variants in the same cell. Using judiciously chosen mutations inactivating the Qo site of one monomer and the Qi site of the other monomer, both one-plasmid and two-plasmid systems produced heterodimeric variants to expose the occurrence of the bL-bL heme intermolecular electron transfer21,22. Salient properties of these genetic systems were reviewed recently.23
Figure 1.
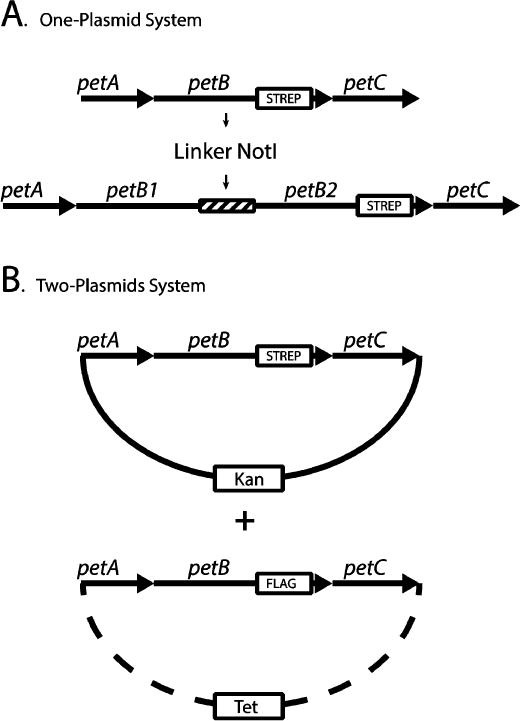
Genetic systems producing cytochrome bc1 heterodimers. (A) In the one-plasmid system, the petB gene was duplicated (petB1 and petB2) and linked together with the 12-amino acid NotI linker (ASIAGGRTASGP). The petB2 gene is C-terminally tagged with the Strep epitope (boxed). For the sake of simplicity, the remaining part of plasmid pMTS1 (KanR derivative of pRK415) carrying these constructs is not shown. (B) In the two-plasmid system, two petABC operons are carried by two plasmids (solid and dashed lines) marked with different antibiotic resistance cassettes, as indicated by boxes labeled Kan (kanamycin) and Tet (tetracycline). The petB genes of the Kan and Tet plasmids are C-terminally tagged with the Strep and Flag tags, respectively.
A more recent study by Hong et al.24 attempted to reproduce Osyczka’s work25 using Rhodobacter sphaeroides instead of R. capsulatus. These authors24 reported the occurrence of genetically rearranged forms of cytochrome b fusions that were thought to arise from homologous recombination. On the basis of these data, they questioned the validity of the one-plasmid system21 and the occurrence of intermonomer electron transfer in cytochrome bc1.21,22 Furthermore, they extended their skepticism to the two-plasmid system20,22 for which they had no experimental data. Unfortunately, in this work, the authors24 used inappropriate experimental conditions (i.e., Ps growth), which is strongly selective for better growing cells, invalidating their findings. The basis of the strong growth disadvantage seen with the one-plasmid system is not yet understood23 and may not necessarily originate from the intermonomer electron transfer in cytochrome bc1. Thus, no conclusion about the occurrence or rate of intermonomer electron transfer can be reached on the basis of the work by Hong et al.24
Following the course of our work, we report here a detailed assessment of the genetic rearrangements exhibited by R. capsulatus strains harboring the one- and two-plasmid systems. In each case, we monitored the growth phenotype that they confer, the frequency of the “revertants” that they form under selective (Ps) and nonselective (Res) growth conditions, and the molecular nature of the DNA rearrangements and sequence changes that they undergo. Furthermore, we isolated a R. capsulatus strain lacking the RecA-dependent homologous recombination pathway to assess its role in the observed genetic rearrangements. We found that a RecA− background minimized to different extents the frequency of DNA rearrangements observed with both the one- and two-plasmid systems. We conclude that the earlier RecA+ and the newly developed RecA− versions of the two-plasmid system reliably produce native and mutant forms of heterodimeric cytochrome bc1. In the following paper (DOI 10.1021/bi400561e), we describe the production, purification, and characterization of an active heterodimeric cytochrome bc1 with interesting electron transfer properties. Our findings are discussed with respect to the capabilities of the two-plasmid system to produce reliably heterodimeric multisubunit protein complexes and to generate heterogeneous populations of cytochrome bc1 in a bacterial model.
MATERIALS AND METHODS
Growth Conditions and Genetic Crosses
Escherichia coli strains were grown at 37 °C on LB medium supplemented with antibiotics [100 μg/mL ampicillin (Amp), 50 μg/mL kanamycin (Kan), and 12.5 μg/mL tetracycline (Tet)]. R. capsulatus wild-type and mutant strains were grown at 35 °C under respiratory (Res, aerobic dark) or photosynthetic (Ps, anaerobic light) conditions in liquid or solid enriched MPYE medium, supplemented with antibiotics as needed [10 μg/mL Kan, 2.5 μg/mL Tet, 10 μg/mL spectinomycin (Spe), and 1 μg/mL gentamicin (Gen)] as described previously.26 For Ps growth, completely filled culture vessels or solid medium-containing plates were placed in anaerobic jars with gas packs generating H2 and CO2 (Becton, Dickinson Inc.) and incubated in temperature-controlled Percival light incubators.
R. capsulatus strains harboring a single plasmid, or coharboring two plasmids, were obtained by conjugation between appropriate E. coli HB101 derivatives used as donors and R. capsulatus RecA+ strain MT-RBC1 with a complete chromosomal deletion of the petABC operon (a bc1− mutant)27 or its RecA− derivative BK-RBC1 as recipients. For the two-plasmid system, plasmids marked with different antibiotic resistance (KanR and TetR) genes were introduced into appropriate recipients by consecutive conjugative transfer. Strains coharboring two plasmids were maintained in the presence of one-half of the normal concentration of the antibiotics used (i.e., 5 μg/mL Kan and 1.25 μg/mL Tet).
Plasmids and Strains Used
All strains and plasmids used are listed in Table S1 of the Supporting Information. Molecular genetic techniques were performed using standard proce dures.28 All constructs were confirmed using appropriate restriction enzyme digestions and DNA sequencing. For the isolation of a RecA− derivative of MT-RBC1, the R. capsulatus ORF RCC01751 encoding the recA gene was first amplified via polymerase chain reaction (PCR) using the recAFor (5′-GTCGTCGCGGGTACCGAAGCGATA-3′ with the KpnI site – TCTAGA underlined) and recARev (5′-CGTCATCGGTGTTCTAGACGGTGACCA-3′ with the XbaI site underlined) primers. The PCR product thus obtained was digested with KpnI and XbaI restriction enzymes and cloned into the similarly digested plasmid pBluescript to yield plasmid pWX1 (Table S1 of the Supporting Information). The 600 bp SmaI-HindIII portion within recA carried by pWXI was deleted and replaced with a gentamycin (gen) resistance gene digested with the same restriction enzymes to yield pWX2. Plasmid pWX2 was digested with KpnI and XbaI, and the DNA fragment carrying the Δ(recA::gen) allele was cloned into a similarly digested pRK415, to yield plasmid pCWX3 (Table S1 of the Supporting Information). This plasmid was conjugated into gene transfer agent (GTA) overproducer strain Y262,29 and a chromosomal RecA null derivative of MT-RBC1 carrying the Δ(recA::gen) and Δ(petABC::spe) alleles, named BK-RBC1 (Table S1 of the Supporting Information), was obtained by GTA crosses.26 Strain BK-RBC1 subsequently received the one- or two-plasmid systems by conjugative transfer.
Construction of the One-Plasmid Genetic System
The one-plasmid system was generated as described in refs 21 and 25. The petAB1-B2C fusion plasmid (pBK6) contained a 12-amino acid (ASIAGGRTASGP) linker with a NotI site between petB1 and petB2 and a Strep tag at the C-terminus of petB2 (Figure 1A). First, the petA and petB1 genes were amplified via PCR using the pet-BamHI (5′-AAATATCTGTCGCTGGATCCGCTGCGCTATG-3′) and petL2 (5′-AACAGCCACTACGGCAATCCGGCGTCGATCGCCGGCGGCCGCACCG-3′) forward and reverse primers, with the NotI site underlined. Primer petL2 is located at the end of the petB gene where it overlapped Pro435 of cytochrome b and contained a NotI restriction site. The PCR product obtained was cloned into the pBluescript plasmid after digestion with the BamHI and NotI restriction enzymes, to yield plasmid pBK8 (Table S1 of the Supporting Information). Separately, a petB2 gene with a Strep (-S) tag at its C-terminus and the adjacent petC gene was amplified via PCR using petL1 (5′-GCCGGCGGCCGCACCGCATCGGGCCCGTCCGGAATTCCGCACGACCAT-3′, with the NotI site underlined) and pet-HindIII (5′-CGCCACACAGGAAGCTTTGATAGGCATCGA-3′) primers, respectively. The petL1 primer is located at the beginning of the petB2 gene where Ser2 of cytochrome b was linked to the second part of the NotI linker. The PCR product obtained was also cloned into the pBluescript plasmid after digestion with NotI and HindIII restriction enzymes, to yield plasmid pBK7 (Table S1 of the Supporting Information). Plasmids pBK7 and pBK8 were digested with the NotI and HindIII enzymes and the NotI and BamHI enzymes, respectively, to yield the petAB1-(NotI linker 1) and (NotI linker 2)-petB2C DNA fragments. These fragments were ligated into pMTSI (KanR derivative of plasmid pRK415) digested with the HindIII and BamHI enzymes to yield pBK6 (Table S1 of the Supporting Information). Plasmid pBK6 harbored the fused form of petB1-B2 (in the petAB1-Linker 1 NotI Linker 2-petB2C operon) with a NotI linker between petB1 and petB2 (Figure 1A).
Similarly, plasmid pBK32 (petAB1F144R-B2H217LC) containing the Qo site cyt b F144R mutation in petB130 and the Qi site cyt b H217L mutation in petB231 was constructed. Plasmid pBK8 (petA-petB1wt) was used as a template for PCR-mediated site-directed mutagenesis to generate the petB1F144R mutation using the F144For (5′-TGATGGGCACCGCCCGCATGGGCTACGTGC-3′) and F144Rev (5′-TGATGGGCACCGCCCGCATGGGCTACGTGC-3′) primers, yielding plasmid pBK22 (Table S1 of the Supporting Information). Plasmid pBK7 (petB2wt-petC) was used as a template for PCR-mediated site-directed mutagenesis to generate the petB2H217L mutation using the H217LFor2 (5′-ATCTGGGCCTTCCTCACCACCGGCAAC-3′) and H217LRev2 (5′-ATCTGGGCCTTCCTCACCACCGGCAAC-3′) primers, yielding plasmid pBK27 (Table S1 of the Supporting Information). As described above, plasmids pBK22 and pBK27 were digested with the NotI and HindIII enzymes and the NotI and BamHI enzymes, respectively. The petAB1F144R and petB2H217LC DNA fragments obtained were ligated into plasmid pMTS1 digested with HindIII and BamHI enzymes to yield pBK32 (Table S1 of the Supporting Information).
Construction of the Two-Plasmid Genetic System
The two-plasmid system was generated as described previously,22 except that the previously used pRK-pPET1 composite plasmid was replaced with the smaller pRK415 (TetR) derivative. Plasmid pMTS1-S or pMTS1-F carrying petABC with a Strep or a Flag tag at the carboxyl end of petB was digested with the HindIII and BamHI restriction enzymes, and the 3.5 kb DNA fragments containing the petABC operon were cloned into a similarly digested pRK415 (TetR), yielding plasmids pBK20 and pBK21, respectively. The previously described plasmids pPET1-F and pPET1-S22 were used as templates to introduce the cyt b H217L mutation into pPET1-F using primers H217LFor2 and H217LRev2 to yield pBK18 and the cyt b F144R mutation into pPET1-S using primers F144RFor and F144RRev to yield pBK19, respectively. Plasmid pBK19 was digested with EcoRI and HindIII enzymes to produce the petABF144RC fragment, which was used to replace its wild-type counterpart in pMTS1 (KanR) to yield plasmid pBK25-F. Similarly, plasmid pBK18 was digested with ApaLI and HindIII enzymes to produce the petABH217LC fragment, which was used to replace its wild-type counterpart in pBK20 to yield plasmid pBK28-S (Table S1 of the Supporting Information).
Biochemical Techniques
Cultures (1 L) of freshly controlled cells were grown by Res to prepare intracytoplasmic (chromatophore) membranes. Frozen washed cell pellets resuspended in MOPS-KCl buffer [50 mM MOPS (pH 7.0), 1 mM KCl, and 1 mM EDTA] were broken via two passages through a French press, as described in ref 32. Protein concentrations were determined using bicinchoninic acid with bovine serum albumin as a standard.33 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12.5%) was conducted as described in ref 34, and prior to being loaded, samples were solubilized in a loading buffer to obtain final concentrations of 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 0.1 M dithiothreitol, 25% glycerol, and 0.01% bromophenol blue by incubation at room temperature for 10 min. Immunoblot analyses were conducted as described in ref 35 using polyclonal antibodies against R. capsulatus cytochrome b and commercially available monoclonal anti-Flag (Sigma-Aldrich, Inc.) and anti-Strep II (Novagen, Inc.) antibodies.
Steady-state cytochrome bc1 activity was measured using decylbenzohydroquinone (DBH2) as an electron donor and horse heart cytochrome c as an electron acceptor at 25 °C. The reaction was initiated by enzyme addition and monitored at 550 nm for 1 min, and the portion of the initial rate that is famoxadone sensitive was taken as the enzyme activity. Optical spectra were recorded on a Cary 60 spectrophotometer (Agilent Technologies Inc.). Absorption difference spectra for the c- and b-type cytochromes were obtained using chromatophore membranes (0.3 mg of total protein/mL), oxidized with potassium ferricyanide, and reduced with sodium ascorbate or sodium dithionite, as appropriate.27
RESULTS
Isolation of a RecA− Mutant of R. capsulatus
During our previous work using the two-plasmid system, we observed that R. capsulatus strains that produce heterodimeric cytochrome bc1 variants yield large Ps+ colonies at a frequency of ~10−4 among a predominantly small Ps+/− population.22 We sought a RecA− mutant to evaluate the role of homologous recombination in the occurrence of the large Ps+ colonies. We thought that abolishing homologous recombination between the multiple copies of petABC might decrease the frequency of large Ps+ colonies produced by these strains. As a R. capsulatus RecA− mutant was unavailable, we scanned its genome and found ORF RCC01751 that is homologous to the recA gene encoding RecA in many organisms.36,37 We cloned and knocked out this chromosomal gene in a cytochrome bc1-null mutant (MT-RBC1) to isolate strain BK-RBC1 [Δ- (petABC::spe) Δ(recA::gen)] (Materials and Methods). E. coli RecA− strains exhibit slower growth than their wild-type counterparts and are hypersensitive to alkylating agents like methylmethanesulfonate (MMS) because of their DNA repair deficiency.38 As expected, BK-RBC1 was Ps− due to the absence of cytochrome bc1, grew slowly (~50% slower rate compared to that of its parent) under the Res growth conditions, and was sensitive to MMS unlike its parent MT-RBC1 [Δ(petABC::spe)]. The two isogenic MT-RBC1 (RecA+) and BK-RBC1 (RecA−) strains were used comparatively in all subsequent studies to assess the role of homologous recombination in the genetic rearrangements that occur in strains carrying the one- or two-plasmid systems.
One-Plasmid System
R. capsulatus strains pBK6/MT-RBC1 and pBK6/BK-RBC1 carrying plasmid pBK6 with the petAB1-B2C operon (two fused copies of wild-type petB) were taken to be “wild-type” analogues for the one-plasmid system (Figure 1A and Table S1 of the Supporting Information). They were compared with pMTS1/MT-RBC1 and pMTS1/BK-RBC1 (native petABC operon) with respect to their cytochrome bc1-dependent Ps growth ability, frequency of large Ps+ colonies that they yielded, and the molecular nature of the genetic rearrangements and amounts of heterodimeric enzymes that they produced, as described below.
Growth Properties of Strains Carrying the One-Plasmid System in RecA+ and RecA− Backgrounds
The Res growth phenotypes of pBK6/MT-RBC1 and pBK6/BK-RBC1 (petAB1-B2C) strains were similar to those of pMTS1/MT-RBC1 and pMTS1/BK-RBC1 (petABC). However, their Ps growth abilities were remarkably different (Figure 2A,B). The strains carrying pBK6 formed colonies under Ps growth conditions only upon prolonged incubation (~5 days for pBK6 vs ~2 days for pMTS1 derivatives, and longer incubation was needed for the RecA− derivatives). Thus, a fused petB1-petB2 construct was deficient for Ps growth, although it contained two wild-type petB alleles. The basis of this growth defect was not studied, but a possibility is that these strains may contain inadequate amounts of cytochrome bc1 to sustain Ps growth of R. capsulatus.39 In addition, pBK6-containing strains exhibited larger Ps+ colonies at frequencies of ~10−2 and ~10−3 in the RecA+ and RecA− backgrounds, respectively (Table S2A of the Supporting Information). Moreover, when plasmid pBK32 carrying the fused form of the petAB1F144R-B2H217LC operon with the Qo site cyt b F144R30 and Qi site cyt b H217L31 mutations [for detailed properties of these mutations, see the following paper (DOI 10.1021/bi400561e)] was tested, both pBK32/MT-RBC1 (RecA+) and pBK32/BK-RBC1 (RecA−) showed no Ps growth but produced few large Ps+ colonies (Figure 2C). Thus, neither pBK6-nor pBK32-containing strains were able to support adequate Ps growth, and the contribution of the RecA pathway upon abolishing the formation of large Ps+ colonies was approximately an order of magnitude (Table S2A of the Supporting Information).
Figure 2.
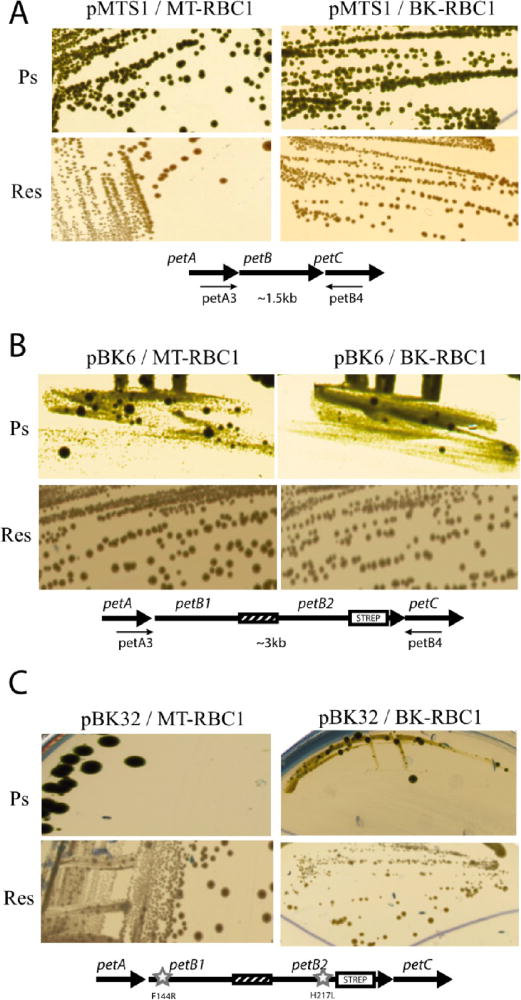
Growth phenotypes of strains carrying the one-plasmid system. Photosynthetic (Ps) and respiration (Res) growth on enriched medium of R. capsulatus strains MT-RBC1 (RecA+) and BK-RBC1 (RecA−) harboring plasmid (A) pMTS1 carrying a wild-type petABC operon, (B) pBK6 carrying petAB1-B2C, and (C) pBK32 carrying the mutated petAB1F144R-B2H217LC fusion operons. In each case, the appropriate operon constructs are shown below the colony pictures, and mutations cyt b F144R and cyt b H217L are indicated with stars. Primers petA3 and petB4 (see the text) were used for PCR amplification.
Genetic Rearrangements in Strains Carrying the One-Plasmid System in RecA+ and RecA− Backgrounds
In strains containing a plasmid-borne petAB1-B2C operon, genetic instability could originate from DNA events that occur either on the plasmid (e.g., pBK6 and pBK32) or on the host chromosome. Here, only the plasmid-borne events were examined. Plasmid DNA isolated from both large and small Ps+ colonies after subculture under Res conditions (i.e., one colony inoculated into 1 mL, and after overnight incubation, here transferred to 10 mL for another overnight incubation, and cells harvested for DNA extraction) was subjected to PCR amplification together with a control plasmid carrying the native petABC operon (pMTS1) using appropriate primers located on petA and petC, respectively (Figure 3A). The data showed that plasmids extracted from the large Ps+ colonies (lane 3) yielded a DNA fragment of a size (~1.5 kb) identical to that seen with a control plasmid (lane 2) carrying a single petB gene (Figure 1A). On the other hand, plasmids extracted from the small Ps+ colonies yielded a larger DNA fragment (~3.0 kb), indicating that they still carried the intact petB1-petB2 fusion (lane 4). Under Res growth conditions, colonies harboring the one-plasmid system showed no readily discernible phenotype on solid media (Figure 2B). However, a similar analysis using randomly chosen Res grown colonies indicated that they also exhibited heterogeneity, producing both the ~1.5 and ~3.0 kb DNA PCR fragments in variable amounts (Figure 3A, lanes 5–11). DNA sequencing of the plasmids yielding the 1.5 kb fragment confirmed that they carried only one copy of petB with its 3′ end still tagged with the DNA sequence corresponding to the Strep epitope used. Data similar to those described above were observed with the large Ps+ (Figure 2C) derivatives of pBK32/MT-RBC1 harboring the petA-B1F144RB2H217L-C operon (data not shown). DNA sequenc ing indicated that the plasmids extracted from these colonies contained only one copy of the wild-type petB gene without the cyt b F144R or cyt b H217L mutation but still contained the Strep tag. This finding implied that rare double-crossover events between the petB1F144R and petB2H217L copies occurred in these cells to yield a wild-type petB copy. Overall, the data indicated that complex intramolecular genetic events occur in strains carrying petB1-petB2 fusions under both selective (i.e., cytochrome bc1-dependent Ps) and nonselective (i.e., cyto chrome bc1-independent Res via an alternative quinol oxidase) growth conditions to yield heterogeneous populations of fused and nonfused forms of cytochrome b variants.
Figure 3.
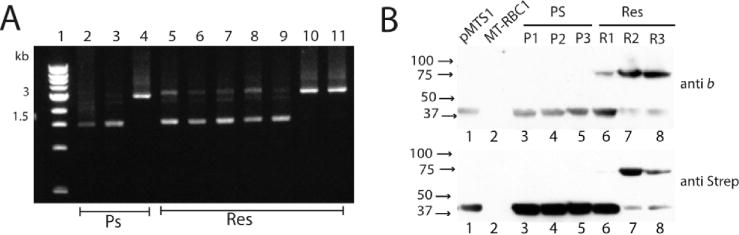
Genetic rearrangements in a strain carrying the one-plasmid system. (A) PCR-amplified DNA mixtures using the petA3 and petB4 primers were run on a 1% agarose gel, and each lane corresponds to plasmid DNA extracted from independent colonies of strain pBK6/MT-RBC1 grown under Ps and Res conditions (Materials and Methods): lane 1, DNA size markers; lane 2, control plasmid pMTS1 carrying the petABC operon; lane 3, plasmid extracted from a colony carrying the one-plasmid system and growing as a wild type under photosynthetic (Ps) conditions; lane 4, plasmid extracted from a colony carrying the one-plasmid system and growing poorly under Ps conditions; lanes 5–11, plasmids extracted from similar colonies growing under respiratory (Res) conditions. The 1.5 and 3 kb bands correspond to wild-type petB and the petB1-petB2 fusion, respectively. (B) SDS-PAGE and immunoblot analyses of chromatophore membranes (40 μg of total proteins) from cells harboring the one-plasmid system: lane 1, pMTS1/MT-RBC1; lane 2, MT-RBC1; lanes 3–8, Ps- and Res-grown colonies of pBK6/MT-RBC1 compared for their cytochrome b contents using anti-cytochrome b (anti b) and anti-Strep (anti Strep) antibodies.
Production of Heterodimeric Cytochrome b-bc1 with the One-Plasmid System
Cytochrome b-bc1 production by strain pBK6/MT-RBC1 carrying the petAB1-B2C operon grown under Ps or Res growth conditions was examined. Chromatophore membranes of appropriate strains were prepared and analyzed by SDS-PAGE and immunoblots using R. capsulatus cytochrome b polyclonal and Strep epitope monoclonal antibodies (Figure 3B). Compared with a strain lacking cytochrome bc1 (MT-RBC1, lane 2), membranes of pMTS1/MT-RBC1 carrying the native petABC operon produced an ~40 kDa band corresponding to a cytochrome b monomer detected by both antibodies (lane 1). A similar pattern was observed with membranes of all large Ps+ colonies of pBK6/MT-RBC1 (lanes 3–5). Importantly, membranes from randomly chosen Res-grown colonies of pBK6/MT-RBC1 produced variable amounts of the ~40 kDa band corresponding to cytochrome b and of a larger band corresponding to a relative molecular mass of 75–100 kDa under our experimental conditions (lanes 6–8). The molecular nature(s) of the higher-molecular mass band, possibly corresponding to the fused forms of cytochrome b dimers, was not pursued further. In a manner independent of the higher-molecular mass band, all strains harboring the petB1-B2 fusion also produced varying amounts of the ~40 kDa band corresponding to the size of the native cytochrome b monomer.
Two-Plasmid System
Strains carrying the two-plasmid system were also examined with respect to their cytochrome bc1-dependent Ps growth ability, the frequency of large (Ps+) and small (Ps+/−) colonies that they yielded, and the molecular nature of the genetic rearrangements and the amounts of heterodimeric enzymes that they produced (Figure 1B). The two-plasmid system used here was similar to that reported previously,22 except that two identical plasmids (i.e., with the same pRK replicon) were chosen instead of two different replicons (e.g., pRK and “pRK-composite” plasmids).
Growth Properties of Strains Carrying the Two-Plasmid System in a RecA+ or RecA− Background and Production of Heterodimeric Cytochrome bc1
As a wild-type control, KanR pMTS1 (petAB-SC) and TetR pBK21 (petAB-FC) were introduced into the RecA+ and RecA− backgrounds to yield the (pMTS1-S + pBK21-F/MT-RBC1) and (pMTS1-S + pBK21-F/BK-RBC1) strains, respectively (Table S1 of the Supporting Information). Comparison of the Ps and Res growth phenotypes on solid media of these strains with those carrying only one of the plasmids (pMTS1-S) showed no discernible difference in terms of colony size or population homogeneity under either growth condition (Figure 4A). The Ps growth conferred by this “wild-type” two-plasmid system was robust, unlike that conferred by the one-plasmid system carrying a fused “wild-type” petB1-petB2, which had very poor Ps growth (Figure 2B). In addition, both RecA+ and RecA− derivatives (pBK25-S + pBK28-F/MT-RBC1) and (pBK25-S + pBK28-F/BK-RBC1), respectively, harboring the two-plasmid system with the cyt b-S F144R and cyt b-F H217L mutations exhibited predominantly slightly smaller Ps+/− colonies together with rare large Ps+ colonies [following paper (DOI 10.1021/bi400561e)]. The frequency of the large Ps+ colonies was around 10−4 for both RecA+ and RecA− backgrounds (Table S2B of the Supporting Information). Furthermore, both Ps- and Res-grown liquid cultures of these strains formed approximately the same number of colonies in a manner independent of their sizes (n = 7 cultures, 1.5–1.9 × 108 and 2.2–2.3 × 108 colony forming units/mL under Ps and Res growth conditions, respectively). Thus, only the rare large Ps+ colonies probably underwent a genetic event(s) to exhibit a different (large vs small) growth phenotype (see below).
Figure 4.
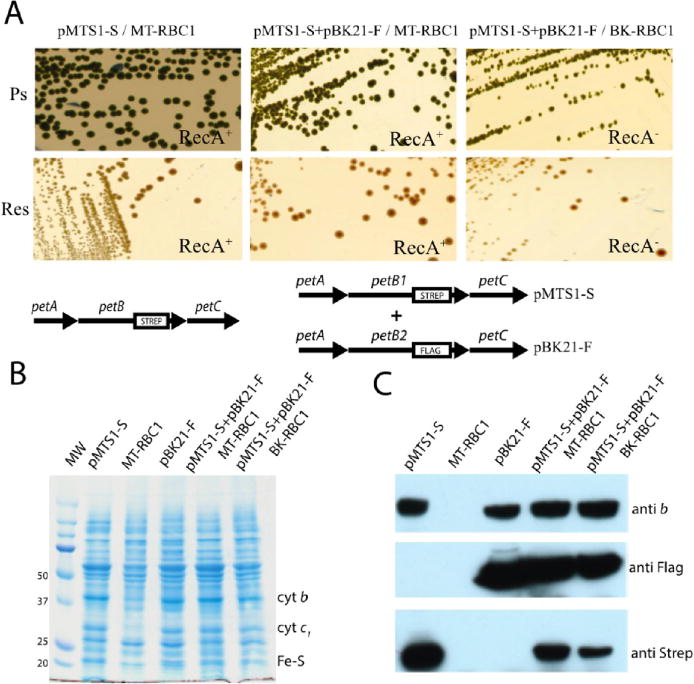
Growth and biochemical properties of strains carrying the two-plasmid system. (A) Photosynthetic (Ps) and respiration (Res) growth on enriched medium of wild-type R. capsulatus strain pMTS1/MT-RBC1 compared to that on MT-RBC1 (RecA+) and BK-RBC1 (RecA−) strains harboring the two-plasmid system (pMTS1-S + pBK21/MT-RBC1 and pMTS1-S + pBK21/BK-RBC1). In each case, the plasmids carrying the petABC operon are shown below the colony pictures. (B) SDS-PAGE and (C) immunoblot analyses of cytochrome bc1 subunits in a strain carrying the two-plasmid system. Chromatophore membranes (40 μg of total proteins per lane, from left to right) from pMTS1-S/MT-RBC1, MT-RBC1, pBK21-F/MT-RBC1, (pMTS1-S + pBK21)/MT-RBC1, and (pMTS1-S + pBK21)/BK-RBC1 were probed with polyclonal anti-cytochrome b and monoclonal anti-Flag and anti-Strep antibodies (see Materials and Methods).
Strains (pMTS1-S + pBK21-F/MT-RBC1) and (pMTS1-S + pBK21-F/BK-RBC1) carried two wild-type petABC operons in which only petB is tagged with Strep or Flag epitopes. Thus, cells would produce wild-type but tagged homodimeric (Strep-Strep and Flag-Flag) and heterodimeric (Strep-Flag and Flag-Strep) cytochrome bc1 variants. SDS-PAGE analyses (Figure 4B) indicated that, unlike a cytochrome bc1-lacking strain (MT-RBC1), the strains harboring only pMTS1-S or pBK21-F and those harboring the two-plasmid system in RecA+ (pMTSI-S + pBK21-F/MT-RBC1) or RecA− (pMTSI-S + pBK21-F/BK-RBC1) backgrounds produced all subunits of cytochrome bc1. Immunoblot analyses with antibodies against cytochrome b detected this subunit in all cases except MT-RBC1, whereas the anti-Strep and anti-Flag epitope-specific antibodies detected it only in pMTS1-S and pBK21-F, respectively, and in both RecA+ and RecA− strains with the two-plasmid system (Figure 4C).
Genetic Rearrangements Seen with Strains Carrying the Two-Plasmid System in a RecA+ or RecA− Back ground
The genetic rearrangements that occur in the two-plasmid system were examined by extracting plasmid DNA from appropriate cells grown under Res conditions. Extracted DNA was transformed into E. coli RecA− strain HB101, and KanR or TetR transformants were selected separately. The frequency of TetR clones found among the selected KanR clones and the frequency of KanR clones found among the selected TetR clones were scored after purification on the appropriate plates. The plasmids harbored by these clones were also analyzed by DNA sequencing (Table 1). When the DNA originated from the RecA+ strain (pMTS1-S + pBK21-F/MT-RBC1) grown under Res conditions, approximately one-half of the transformants were KanR TecS or KanS TecR (n = 6 independent experiments) and harbored the initial plasmids; 165 of 210 selected KanR transformants were also TetR, and 80 of 148 selected TetR transformants were also KanR (i.e., simultaneously KanR and TetR). The latter transformants remained KanR TetR despite extensive purification, suggesting that they were not cotransformants with two coresiding plasmids in the same cell (Table 1, entry a). DNA sequencing of the KanR TetS and KanS TetR plasmids indicated that the antibiotic resistance markers with respect to the epitope tags were frequently (3 of 8 and 0 of 3) exchanged between the plasmids to generate pMTS1-F and pBK21-S instead of pMTS1-S and pBK21-F (Table 1, entry a). Given that in most bacteria the RecA-dependent recombination pathway is involved in the formation of the co-integrant between identical plasmids and their resolution,40,41 the RecA− strain (pMTS1-S + pBK21-F/BK-RBC1) carrying the same plasmids was examined. Among the ~600 clones tested after KanR or TetR selection from three repeat cultures (n = 3), no KanR TetR colonies (0 of 224 and 0 of 341, respectively) were found (Table 1, entry b). DNA sequencing of the plasmids extracted from the KanR TetS and TetR KanS clones showed no antibiotic resistance marker or epitope tag exchange between the plasmids (0 of 3 in each case with n = 3). Thus, inactivation of the RecA pathway in a strain harboring the two-plasmid system drastically decreased the frequency of occurrence of KanR TetR co-integrant plasmid formation seen in the RecA+ background (Table 1, entry b).
Table 1.
Production of Native, Active, and Inactive Cytochrome bc1 Heterodimers Ps mutation
| two-plasmid system | Δ(petABC) | Ps growtha | plasmid selection in E. coli | tag exchange | mutation exchange |
|---|---|---|---|---|---|
| Wild-Type Heterodimers | |||||
| (a) pMTS1-S (KanR) + pBK21-F (TetR) | RecA+ | Ps+ | 165 of 210 KanR TetR | yes (3 of 8 KanR-Flag; 0 of 3 TetR-Strep) | NAc |
| 80 of 148 TetR KanR (n = 6)b | |||||
| (b) pMTS1-S (KanR) + pBK21-F (TetR) | RecA− | Ps+ | 0 of 224 KanR TetR | no (0 of 3 KanR-Flag; 0 of 3 TetR-Strep) | NAc |
| 0 of 341 TetR KanR (n = 3) | |||||
| Mutant Heterodimers | |||||
| (c) cyt b-S F144R + cyt b-F H217L | RecA+ | Ps+/− | 74 of 100 KanR TetR | yes (2 of 10 KanR-Flag; 3 of 13 TetR-Strep) | tag-linkedd |
| 36 of 50 TetR KanR (n = 3) | |||||
| small Ps colonies | RecA− | Ps+/− | 0 of 1734 KanR TetR | no (0 of 10 KanR-Flag; 0 of 10 TetR-Strep) | noe |
| (d) cyt b-S F144R + cyt b-F H217L | 0 of 1366 TetR KanR (n = 6) | ||||
| large Ps colonies | RecA− | Ps+ | 999 of 1000 KanR TetR | yes (9 of 10 KanR-Flag; 9 of 13 TetR Strep) | tag-linkedd |
| (e) cyt b-S F144R + cyt b-F H217L | 398 of 400 TetR KanR | ||||
| no Ps colonies | |||||
| (f) cyt b-S F144R + cyt b-F T288S | RecA+ | Ps− | NAc | NAc |
Ps+/− and Ps+, small and large colonies, respectively, formed under Ps growth conditions.
n is the number of cultures of R. capsulatus used to extract the plasmids; KanR and TetR indicate resistance to kanamycin and tetracycline, respectively, obtained after transformation of HB101 with plasmids from R. capsulatus. Clones were selected on Kan or Tet (underlined) and scored for the unselected marker (not underlined).
Not applicable.
Tag-linked, cytochrome b mutations and their associated tags remained together.
In the 10 KanR and 10 TetR plasmids sequenced, all mutations were associated with their tags. No tag or mutation exchange was seen among the 10 KanR and 10 TetR clones sequenced.
Similar analyses were also performed using a two-plasmid system carrying two cytochrome b mutations to assess the degree of segregation between the mutations on the different plasmids or between a given mutation and its associated epitope tag on the same plasmid. RecA+ and RecA− strains (pBK25-S + pBK28-F/MT-RBC1) and (pBK25-S + pBK28-F/BK-RBC1) (Table S1 of the Supporting Information) carrying a two-plasmid system with cyt b-S F144R30 and cyt b-F H217L mutations31 were examined. The data indicated that the formation of KanR TetR co-integrant plasmids and epitope tag exchange were seen again only in RecA+ [74 of 100 and 36 of 50 (n = 3)], not in RecA− [0 of 1734 and 0 of 1366 (n = 6)], backgrounds (Table 1, entries c and d). In the case of the RecA+ background, DNA sequencing of the plasmids extracted from KanR TetS [i.e., pBK25-S (cyt b-S F144R)] or KanS TetR [i.e., pBK28-F (cyt b-F H217L)] indicated that 2 of 10 of the KanR TetS clones contained the cyt b-F H217L mutation and 3 of 13 TetR KanS clones contained the cyt b-S F144R mutation (n = 3). Although the KanR and TetR markers were exchanged between the two plasmids, no segregation was seen between given mutations and their associated epitope on the same copy of petB. In other words, in all instances (~70 sequences), the cyt b F144R mutation remained linked to the Strep epitope tag and the cyt b H217L mutation to the Flag epitope tag, regardless of the exchange of antibiotic resistance markers between the plasmids (Table 1, entry c). With the RecA− background, indeed no KanR TetR colony and no segregation between the cytochrome b mutations and their associated epitope tags were seen among the ~3000 clones (n = 6) tested (Table 1, entry d). This finding suggested that in the RecA+ background, co-integrants between the two resident plasmids (pMTS1-S and pBK21-F or pBK25 and pBK28/MT-RBC1) of quasi-identical DNA sequences (except the cytochrome b mutations and their associated epitope tags) could be continuously formed and resolved, leading to frequent antibiotic marker exchanges between the plasmids.40,41 Importantly, because of the short distances that separate the cytochrome b mutations and their associated tags, the genetic rearrangements did not segregate them at a detectable rate.
Large Ps+ Clones Observed Using the Two-Plasmid System in a RecA− Background are Co-Integrants between the Two Plasmids
We inquired about the correlation between the occurrence of the large Ps+ colonies Although these colonies appear in both RecA+ and RecA− backgrounds, we analyzed them in the latter case in which the two-plasmid system is more robust (Table 1). Using strain pBK25-S + pBK28-F/BK-RBC1, we deliberately sought large Ps+ colonies, which appeared only after multiple subcultures, in agreement with their low frequency (approximately <10−4) (Table S2B of the Supporting Information). We purified these derivatives under Ps growth conditions and examined the genetic rearrangements that they underwent. Transformation of plasmid DNA extracted from these large Ps+ clones into an E. coli strain (HB101) almost exclusively yielded KanR TetR transformants independent of the initial KanR or TetR selection (only ~1 of 1000 clones was KanR TetS upon KanR selection, and 2 of 400 clones were TetR KanS upon TetR selection) (Table 1, entry e). Furthermore, DNA analyses revealed that the KanR TetR clones contained large co-integrant plasmids formed by the fusion of the two initial plasmids (Figure 5A, lane 5) but frequently not resolved back to the initial plasmids. Upon digestion with the appropriate restriction enzyme, religation, and transformation into E. coli, the large co-integrant plasmids regenerated the initial KanR TetS and TetR KanS plasmids. DNA sequence analyses of several (n = 23) such plasmids revealed that those carrying the cyt b-S F144R mutation remained associated with their Strep tag and those carrying the cyt b-F H217L mutation remained associated with their Flag tag. As observed with the RecA+ background, although exchange of antibiotic resistance markers (TetR and KanR) could be seen, in no instances did we detect any plasmid carrying a wild-type cytochrome b or an allele with both mutations (i.e., cyt b F144R and cyt b H217L carried by a single petB), or any segregation between a cytochrome b mutation and its associated epitope tag (Table 1, entry e). The data demonstrated that the large Ps+ colonies were not due to the loss of cytochrome b mutations by reversion or recombination and implied that they resulted from the formation of RecA-dependent co-integrants between the two resident plasmids. Importantly, the co-integrated plasmids also carried faithfully the initial cytochrome b mutations and their associated epitope tags and produced the expected heterodimeric cytochrome bc1 variants (Figure 6).
Figure 5.
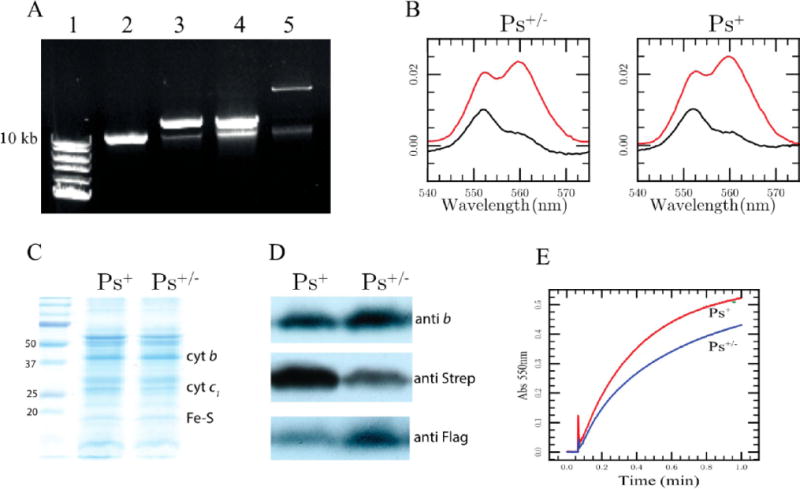
Large Ps+ colonies produced by a strain carrying the two-plasmid system contain large co-integrant plasmids. (A) Agarose gel showing the plasmid DNA extracted from RecA− cells carrying the pRK415 vector (lane 2), pMTSI-S (lane 3), pBK21-F (lane 4), and a co-integrant plasmid formed between two resident plasmids found in the large Ps+ colonies carrying both plasmids from strain pBK25-S + pBK28-F/BK-RBC1 (lane 5). In each case, the upper band corresponds to the pRK415 derivatives and the identity of the lower band is not defined. DNA size markers are shown in lane 1. (B) Optical redox difference spectra of total b-type (dithionite minus ferricyanide colored red) and c-type (ascorbate minus ferricyanide colored black) cytochrome contents of chromatophore membranes obtained from a large Ps+ and a small Ps+/− colony derivative of pBK25-S + pBK28-F/BK-RBC1. (C) SDS-PAGE and (D) immunoblot analyses of the chromatophore membranes obtained from the same large Ps+ and a small Ps+/− as described for panel B using cytochrome b polyclonal antibodies and commercially available monoclonal antibodies against the Strep and Flag epitope tags. (E) Steady-state DBH2:cytochrome c reduction activity (Materials and Methods) monitored at 550 nm exhibited by the same chromatophore membranes as in panel B, and the corresponding rates obtained using the same amounts of proteins (see the text).
Figure 6.
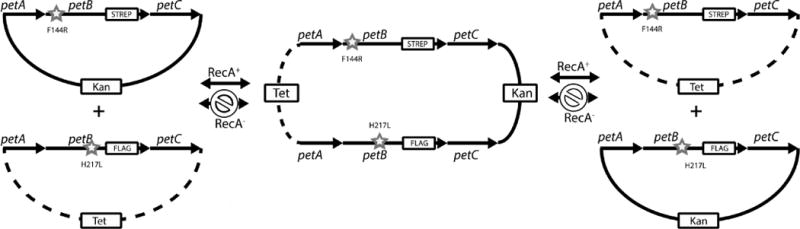
Formation of co-integrants between the resident plasmids in cells carrying the two-plasmid system. Continuous co-integrant plasmid formation and resolution via the homologous recombination (RecA) pathway between two plasmids carrying the same type of replicon coresiding in a cell are depicted. The frequency of occurrence of these single-recombination events is decreased drastically in the absence of the RecA protein (RecA− background). Segregations of the genetic markers carried by the co-integrant plasmids formed are distance-dependent. In a RecA+ background, exchange between the antibiotic resistance markers is detected upon the formation and resolution of the co-integrants, but no recombination or segregation between the cytochrome b mutations or the mutations and their associated epitope tags is seen, because of the short distances that separate these markers.
Better Ps+ growth of the large colonies suggested that they might contain increased amounts of active heterodimeric cytochrome bc1 variants. Optical redox difference spectra at 560 and 550 nm, visualizing the total amounts of cytochromes b and c, respectively, indicated that both the selected large Ps+ and the normal smaller Ps+/− clones had comparable amounts of total cytochrome bc1 (Figure 5B). Similar data were also obtained via SDS-PAGE analyses using chromatophore membranes of Ps+ and Ps+/− derivatives of pBK25-S + pBK28-F/BK-RBC1 (Figure 5C). However, immunoblot analyses using antibodies against cytochrome b and Strep and Flag epitopes revealed that these clones showed different levels of Strep and Flag epitope tag contents (Figure 5D). The Strep-tagged subpopulation (homodimers and heterodimers) was larger in the Ps+ clones than in the Ps+/− clones. Conversely, the amount of the Flag-tagged subpopulation (homodimers and heterodimers) was larger in the Ps+/− clones than in the Ps+ clones. Moreover, the chromatophore membranes of the large Ps+ colonies exhibited a cytochrome bc1 activity (0.33 and 0.24 μmol of cytochrome c reduced per minute per milligram of total membrane proteins, respectively, which correspond to 36 and 26%, respectively, of the wild-type activity) higher than that of the smaller Ps+/− colonies (Figure 5E). The data suggested that the formation of co-integrants between the two resident plasmids (Figure 6) increased the level of production of the Strep-tagged cytochrome b subunits in comparison to that of their Flag-tagged counterparts and consequently yielded an increased level of active heterodimeric cytochrome bc1 variants that supported better Ps+ growth.
DISCUSSION
In this work, we assessed the two genetic systems20–22 previously reported to successfully produce heterodimeric cytochrome bc1 variants. Because of the dimeric structure of the enzyme, these systems carry DNA duplications that are naturally prone to genetic rearrangements, including homolo gous and nonhomologous recombination.40,41 We aimed to elucidate the genetic events that could occur using these genetic systems and to evaluate their impact(s) on the production of heterodimeric cytochrome bc1 variants. A thorough assessment of the properties of these systems is of critical importance because the validity of the information gained about dimeric cytochrome bc1 directly depends on their experimental reliability and proper use. Their misuse could lead to erroneous conclusions, as reported in ref 24.
One-Plasmid System
Using the one-plasmid system, with a fusion of two “wild-type” or two mutant alleles of cytochrome b, we observed extremely slow or no Ps growth, respectively. Frequent genetic rearrangements were seen under both selective (Ps) and nonselective (Res) growth conditions. Both “wild-type” and mutant petB1-B2 fusions yielded better growing derivatives that carried a single copy of cytochrome b at a frequency of ~10−2 under our conditions. A RecA− background minimized the occurrence of these genetic rearrangements by approximately an order of magnitude (~10−3). Consequently, strains carrying the one-plasmid system seem to be under strong selective growth pressure and propagate as heterogeneous populations.25 In an attempt to reproduce the one-plasmid system in R. sphaeroides, the Crofts group reported that they obtained only wild-type-like derivatives while growing their strains under Ps conditions.24 This finding is not surprising, but rather expected, as the Ps growth conditions are strongly selective against even slightly Ps-defective mutants, including those carrying the one-plasmid system. Failure to produce a heterodimeric cytochrome bc1 under inappropriate experimental conditions using a fragile genetic system is expected. Therefore, the findings reported in ref 24 are inconclusive for the production of heterodimeric cytochrome b-bc1 variants or the occurrence and rate of intermonomer electron transfer in cytochrome bc1. Genetic systems using mutants should not be exposed to selective conditions. Otherwise, mutant populations rapidly “evolve” better-adapted variants that supersede the initial populations. Heterogeneous microbial populations are not suitable for many studies, especially for biophysical and biochemical characterizations.
The molecular basis underlying the strong selective pressure seen with strains carrying the one-plasmid system is unknown. Although they were not studied here beyond documenting that this system leads to marked population heterogeneity, a possible explanation is that the selection is not caused solely by inadequate cytochrome bc1 activity (e.g., slow intermonomer electron transfer) or exclusively by RecA-dependent recombination events, and its molecular basis may lie elsewhere. Indeed, even a wild-type petB1-petB2 fusion is defective for Ps growth. A Ps growth defect could only indicate that heterodimeric cytochrome b-bc1 variants are insufficiently functional (or not maintained in sufficient amounts) if this phenotype is directly correlated with the absence of an active cytochrome bc1. Nonetheless, meticulously controlled short culture periods seem to minimize the observed heterogeneity to allow purification of cytochrome b-bc1 variants.39 It has been reported that different cytochrome b fusions exhibit different “levels of engineering tolerance” because of the type of mutation(s) in cytochrome b or the size of the artificial linker sequence fusing the two copies of petB.25 Moreover, a variant of the one-plasmid system, which uses a R. sphaeroides cytochrome b fused with that of R. capsulatus (petBs-petBc instead of petB1-petB2), was recently described to provide enhanced stability and Ps-proficient growth.42
Two-Plasmid System
Our previously reported two-plasmid system based on two different replicons produced mutant heterodimeric cytochrome bc1 that supported slow Ps growth via the intermonomer electron transfer pathway.22 In an attempt to produce comparable amounts of homodimeric and heterodimeric cytochrome bc1 in a given cell, we tested a variant of this system in which the two plasmids used have the same type of replicon. However, biogenesis of cytochrome bc1 is complex and not well-known.43 The cytochrome b mutations and their associated epitope tags (e.g., Strep vs Flag) might interfere differently with the replication, expression, biogenesis, and degradation processes in vivo. Expecting that cells with a two-plasmid system would produce predetermined amounts of homodimers and heterodimers is unreasonable.24 In cells carrying two plasmids with the same type of replicons, the copy number of each plasmid might vary, although the total copy number of plasmids per cell is constant. Consequently, the amount of homo- and heterodimers produced can vary. In contrast, when a co-integrant plasmid is formed, the copies of the two plasmids are “equalized” as they become parts of the same molecule maintained at a given copy number, and the amounts of homo- and heterodimers are more similar, as seen here with the large Ps+ colonies.
The two-plasmid system yields a population of predom inantly small Ps+/− and fewer (at a frequency of ~10−4) large Ps+ colonies.22 RecA is important for frequent co-integrant formation and resolution; however, in the absence of RecA, co-integrants are still formed but apparently not resolved. More detailed studies are required to better define this process. In a population, the frequency of colonies with a different phenotype (e.g., small Ps+/− vs large Ps+) reflects how rapidly such colonies arise. This phenotype might originate from various molecular events, and this “reversion” should not be equated with a “wild-type” genotype. Hong et al.24 postulated that the small Ps+/− colonies might correspond to cells that have already reverted, rendering them partially Ps-proficient, and that the large Ps+ colonies acquired a second mutation that further improved their Ps growth ability. The observed frequency of this event already indicates that this cannot be the case. Moreover, cultures carrying the two-plasmid system produce the same number of colonies (independent of their size) on solid media under both Ps and Res growth conditions. With each colony being the result of successive divisions of a single cell, the data demonstrate that all cells are capable of growth under both selective (Ps) and nonselective (Res) growth conditions. The small Ps+/− colonies are formed at a frequency of ~1, demonstrating that they cannot be “revertants” even though their Ps phenotype (i.e., colony size) is different than a wild-type strain. Only the large Ps+ colonies that appear at a low frequency reflect cells that underwent genetic rearrangement(s) (i.e., revertants). Monitoring the frequency of these events is the key to ensuring the proper production of heterodimeric cytochrome bc1 variants using one- or two-plasmid systems.
Duplicated genetic materials usually undergo homologous recombination, which is a “distance-dependent” event. The frequency of homologous recombination segregating two genetic markers is lower when the markers are closer to each other. In the two-plasmid system carrying two Qo site defective cytochrome b mutations (cyt b-S F144R and cyt b-F T288S), no Ps+ colony was observed. Also, this strain did not produce any Ps+/− colonies as both Qo sites are defective (Table 1, entry f) [see also the following paper (DOI 10.1021/bi400561e)]. Similarly, the large number (~70) of plasmids thus far sequenced consistently showed the initial cytochrome b mutations linked to their associated epitope tags, in a manner independent of their phenotype. Thus, no recombination between two mutations in petB or a mutation and its associated epitope tag carried by petB is readily detected, whereas similar RecA-dependent exchanges between the antibiotic resistance markers (KanR and TetR) are often observed between the two plasmids (Table 1 and Figure 6). This finding is not unexpected given that the distance separating the cytochrome b mutations or a mutation and its associated epitope tag (~1 kb) is ~10 times shorter than that separating the KanR and TetR markers (~10 kb) on co-integrant plasmids. However, an increased recombination frequency might be seen between genetic markers that are physically far apart (e.g., a mutation located at the N-terminus of the Fe-S protein together with one at the C-terminus of cytochrome c1), although the use of a RecA− background might minimize such events (Figure 6).
A two-plasmid system that is very similar to that analyzed here was also used in Paracoccus denitrificans.20 That study suggested that one Qo site is sufficient for the activity of cytochrome bc1 and that the intermonomer electron transfer might occurs via the bL-bL hemes. This genetic setup has not been analyzed further, and the results obtained with R. capsulatus may not be directly translatable to the P. denitrificans system. The recombination pathways might differ between different organisms and their plasmids. The occurrence of co-integrant plasmids and the determination of the recombination frequency yielding a wild-type homodimeric cytochrome bc1 would be useful for further evaluation of this system. We note that the two-plasmid system developed here could be useful not only for the study of electronic communication between the monomers of a dimeric cytochrome bc1 but also for other homodimeric multisubunit enzymes. For example, it could be informative in the case of the dimeric cytochrome c nitrite reductase (ccNiR) or the dimeric photosynthetic reaction center type I (PSI) for which dimer interactions are not well-known. The three-dimensional structure of ccNiR is a homodimer in which the distance separating the hemes located at the edge of the dimer interface is short (~12 Å),44,45 whereas that of PSI is a pseudohomodimer containing similar but not identical (PsaA and PsaB) monomers.46,47
Finally, we note that the two-plasmid system, which can generate heterogeneous populations, has the potential to contribute to the study of the mitochondrial “heteroplasmy state” seen in mammalian cells. In the case of cytochrome bc1, analyses of heteroplasmic cytochrome b often require the generation of “cybrid” lines.48,49 Using the R. capsulatus two-plasmid system, generation of heterogeneous populations might provide an alternative approach, and comparative studies using bacteria and cybrids carrying human disease-causing mutations might be very informative.49,50
Supplementary Material
Acknowledgments
We thank Xiaomin Wu for isolation of the recA gene and construction of a RecA− mutant.
Funding
This work was supported by National Institutes of Health Grant GM 38237 and the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy, via Grant DE-FG02-91ER20052 to F.D.
ABBREVIATIONS
- Q
quinone
- SQ
semiquinone
- QH2
hydroquinone
- Res
respiration
- Ps
photosynthesis
- KanR
kanamycin resistance
- TetR
tetracycline resistance
Footnotes
Supporting Information
Plasmids and strains used in this work (Table S1) and photosynthetic (Ps) and respiratory (Res) colony forming abilities of appropriate strains that produce mutant cytochrome bc1 heterodimers (Table S2). This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The interest. authors declare no competing financial
References
- 1.Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA, Kim SH. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 3.Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 4.Berry EA, Huang LS, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F. X-ray structure of Rhodobacter capsulatus cytochrome bc1: Comparison with its mitochondrial and chloroplast counterparts. Photosynth Res. 2004:251–275. doi: 10.1023/B:PRES.0000036888.18223.0e. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976;62:327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- 6.Crofts AR, Meinhardt SW, Jones KR, Snozzi M. The role of the quinone pool in the cyclic electron-transfer chain of Rhodopseudomonas sphaeroides: A modified Q-cycle mechanism. Biochim Biophys Acta. 1983;723:202–218. doi: 10.1016/0005-2728(83)90120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990;265:11409–11412. [PubMed] [Google Scholar]
- 8.Darrouzet E, Valkova-Valchanova M, Moser CC, Dutton PL, Daldal F. Uncovering the [2Fe2S] domain movement in cytochrome bc1 and its implications for energy conversion. Proc Natl Acad Sci USA. 2000;97:4567–4572. doi: 10.1073/pnas.97.9.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darrouzet E, Daldal F. Movement of the iron-sulfur subunit beyond the ef loop of cytochrome b is required for multiple turnovers of the bc1 complex but not for single turnover Qo site catalysis. J Biol Chem. 2002;277:3471–3476. doi: 10.1074/jbc.M107974200. [DOI] [PubMed] [Google Scholar]
- 10.Crofts AR, Holland JT, Victoria D, Kolling DR, Dikanov SA, Gilbreth R, Lhee S, Kuras R, Kuras MG. The Q-cycle reviewed: How well does a monomeric mechanism of the bc1 complex account for the function of a dimeric complex? Biochim Biophys Acta. 2008;1777:1001–1019. doi: 10.1016/j.bbabio.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vries S, Albracht SP, Berden JA, Slater EC. The pathway of electrons through QH2:cytochrome c oxidoreductase studied by pre-steady-state kinetics. Biochim Biophys Acta. 1982;681:41–53. doi: 10.1016/0005-2728(82)90276-6. [DOI] [PubMed] [Google Scholar]
- 12.Weiss H, Leonard K, Neupert W. Puzzling subunits of mitochondrial cytochrome reductase. Trends Biochem Sci. 1990;15:178–180. doi: 10.1016/0968-0004(90)90155-5. [DOI] [PubMed] [Google Scholar]
- 13.Gopta OA, Feniouk BA, Junge W, Mulkidjanian AY. The cytochrome bc1 complex of Rhodobacter capsulatus: Ubiquinol oxidation in a dimeric Q-cycle? FEBS Lett. 1998;431:291–296. doi: 10.1016/s0014-5793(98)00768-6. [DOI] [PubMed] [Google Scholar]
- 14.Trumpower BL. A concerted, alternating sites mechanism of ubiquinol oxidation by the dimeric cytochrome bc1 complex. Biochim Biophys Acta. 2002;1555:166–173. doi: 10.1016/s0005-2728(02)00273-6. [DOI] [PubMed] [Google Scholar]
- 15.Cooley JW, Lee DW, Daldal F. Across membrane communication between the Qo and Qi active sites of cytochrome bc1. Biochemistry. 2009;48:1888–1899. doi: 10.1021/bi802216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Covian R, Gutierrez-Cirlos EB, Trumpower BL. Anti-cooperative oxidation of ubiquinol by the yeast cytochrome bc1 complex. J Biol Chem. 2004;279:15040–15049. doi: 10.1074/jbc.M400193200. [DOI] [PubMed] [Google Scholar]
- 17.Cooley JW, Ohnishi T, Daldal F. Binding dynamics at the quinone reduction Qi site influence the equilibrium interactions of the iron sulfur protein and hydroquinone oxidation Qo site of the cytochrome bc1 complex. Biochemistry. 2005;44:10520–10532. doi: 10.1021/bi050571+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covian R, Trumpower BL. Rapid electron transfer between monomers when the cytochrome bc1 complex dimer is reduced through center N. J Biol Chem. 2005;280:22732–22740. doi: 10.1074/jbc.M413592200. [DOI] [PubMed] [Google Scholar]
- 19.Shinkarev VP, Wraight CA. Intermonomer electron transfer in the bc1 complex dimer is controlled by the energized state and by impaired electron transfer between low and high potential hemes. FEBS Lett. 2007;581:1535–1541. doi: 10.1016/j.febslet.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellani M, Covian R, Kleinschroth T, Anderka O, Ludwig B, Trumpower BL. Direct demonstration of half-of-the-sites reactivity in the dimeric cytochrome bc1 complex: Enzyme with one inactive monomer is fully active but unable to activate the second ubiquinol oxidation site in response to ligand binding at the ubiquinone reduction site. J Biol Chem. 2010;285:502–510. doi: 10.1074/jbc.M109.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swierczek M, Cieluch E, Sarewicz M, Borek A, Moser CC, Dutton PL, Osyczka A. An electronic bus bar lies in the core of cytochrome bc1. Science. 2010;329:451–454. doi: 10.1126/science.1190899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanciano P, Lee DW, Yang H, Darrouzet E, Daldal F. Intermonomer electron transfer between the low-potential b hemes of cytochrome bc1. Biochemistry. 2011;50:1651–1663. doi: 10.1021/bi101736v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalfaoui-Hassani B, Lanciano P, Lee DW, Darrouzet E, Daldal F. Recent advances in cytochrome bc1: Inter monomer electronic communication? FEBS Lett. 2011;586:617–621. doi: 10.1016/j.febslet.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong S, Victoria D, Crofts AR. Inter-monomer electron transfer is too slow to compete with monomeric turnover in bc1 complex. Biochim Biophys Acta. 2012;1817:1053–1062. doi: 10.1016/j.bbabio.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czapla M, Borek A, Sarewicz M, Osyczka A. Fusing two cytochromes b of Rhodobacter capsulatus cytochrome bc1 using various linkers defines a set of protein templates for asymmetric mutagenesis. Protein Eng Des Sel. 2011;25:15–25. doi: 10.1093/protein/gzr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daldal F, Cheng S, Applebaum J, Davidson E, Prince RC. Cytochrome c2 is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci USA. 1986;83:2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atta-Asafo-Adjei E, Daldal F. Size of the amino acid side chain at position 158 of cytochrome b is critical for an active cytochrome bc1 complex and for photosynthetic growth of Rhodobacter capsulatus. Proc Natl Acad Sci USA. 1991;88:492–496. doi: 10.1073/pnas.88.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Russell DW. Molecular Cloning: A laboratory manual. 3. Cold Spring Harbor Laboratory Press; Plainview, NY: 2001. [Google Scholar]
- 29.Yen HC, Hu NT, Marrs BL. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J Mol Biol. 1979;131:157–168. doi: 10.1016/0022-2836(79)90071-8. [DOI] [PubMed] [Google Scholar]
- 30.Ding H, Daldal F, Dutton PL. Ion pair formation between basic residues at 144 of the cyt b polypeptide and the ubiquinones at the Qo site of the cyt bc1 complex. Biochemistry. 1995;34:15997–16003. doi: 10.1021/bi00049a013. [DOI] [PubMed] [Google Scholar]
- 31.Gray KA, Dutton PL, Daldal F. Requirement of histidine 217 for ubiquinone reductase activity (Qi site) in the cytochrome bc1 complex. Biochemistry. 1994;33:723–733. doi: 10.1021/bi00169a014. [DOI] [PubMed] [Google Scholar]
- 32.Gray KA, Davidson E, Daldal F. Mutagenesis of methionine-183 drastically affects the physicochemical properties of cytochrome c1 of the bc1 complex of Rhodobacter capsulatus. Biochemistry. 1992;31:11864–11873. doi: 10.1021/bi00162a027. [DOI] [PubMed] [Google Scholar]
- 33.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Davidson E, Ohnishi T, Tokito M, Daldal F. Rhodobacter capsulatus mutants lacking the Rieske FeS protein form a stable cytochrome bc1 subcomplex with an intact quinone reduction site. Biochemistry. 1992;31:3351–3358. doi: 10.1021/bi00128a007. [DOI] [PubMed] [Google Scholar]
- 36.Radding CM. Recombination activities of E. coli RecA protein. Cell. 1981;25:3–4. doi: 10.1016/0092-8674(81)90224-5. [DOI] [PubMed] [Google Scholar]
- 37.Cox MM. The bacterial RecA protein as a motor protein. Annu Rev Microbiol. 2003;57:551–577. doi: 10.1146/annurev.micro.57.030502.090953. [DOI] [PubMed] [Google Scholar]
- 38.Todd PA, Brouwer J, Glickman BW. Influence of DNA-repair deficiencies on MMS- and EMS-induced mutagenesis in Escherichia coli K-12. Mutat Res. 1981;82:239–250. doi: 10.1016/0027-5107(81)90153-6. [DOI] [PubMed] [Google Scholar]
- 39.Czapla M, Borek A, Sarewicz M, Osyczka A. Enzymatic Activities of Isolated Cytochrome bc1-like Complexes Containing Fused Cytochrome b Subunits with Asymmetrically Inactivated Segments of Electron Transfer Chains. Biochemistry. 2012;51:829–835. doi: 10.1021/bi2016316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedbrook JR, Ausubel FM. Recombination between bacterial plasmids leading to the formation of plasmid multimers. Cell. 1976;9:707–716. doi: 10.1016/0092-8674(76)90134-3. [DOI] [PubMed] [Google Scholar]
- 41.Potter H, Dressler D. On the mechanism of genetic recombination: The maturation of recombination intermedi ates. Proc Natl Acad Sci USA. 1977;74:4168–4172. doi: 10.1073/pnas.74.10.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czapla M, Cieluch E, Borek A, Sarewicz M, Osyczka A. Catalytically-relevant electron transfer between two hemes bL in the hybrid cytochrome bc1-like complex containing a fusion of Rhodobacter sphaeroides and capsulatus cytochromes b. Biochim Biophys Acta. 2013;1827:751–760. doi: 10.1016/j.bbabio.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith PM, Fox JL, Winge DR. Biogenesis of the cytochrome bc1 complex and role of assembly factors. Biochim Biophys Acta. 2012;1817:276–286. doi: 10.1016/j.bbabio.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Einsle O, Messerschmidt A, Stach P, Bourenkov GP, Bartunik HD, Huber R, Kroneck PM. Structure of cytochrome c nitrite reductase. Nature. 1999;400:476–480. doi: 10.1038/22802. [DOI] [PubMed] [Google Scholar]
- 45.Youngblut M, Judd ET, Srajer V, Sayyed B, Goelzer T, Elliott SJ, Schmidt M, Pacheco AA. Laue crystal structure of Shewanella oneidensis cytochrome c nitrite reductase from a high-yield expression system. JBIC, J Biol Inorg Chem. 2012;17:647–662. doi: 10.1007/s00775-012-0885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature. 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- 47.Jagannathan B, Golbeck JH. Breaking biological symmetry in membrane proteins: The asymmetrical orientation of PsaC on the pseudo-C2 symmetric Photosystem I core. Cell Mol Life Sci. 2009;66:1257–1270. doi: 10.1007/s00018-009-8673-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King MP, Attardi G. Human cells lacking mtDNA: Repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 49.Ghelli A, Tropeano CV, Calvaruso MA, Marchesini A, Iommarini L, Porcelli AM, Zanna C, De Nardo V, Martinuzzi A, Wibrand F, Vissing J, Kurelac I, Gasparre G, Selamoglu N, Daldal F, Rugolo M. The cytochrome b p. 278Y > C mutation causative of a multisystem disorder enhances superoxide production and alters supramolecular interactions of respiratory chain complexes. Hum Mol Genet. 2013;22:2141–51. doi: 10.1093/hmg/ddt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanciano P, Khalfaoui-Hassani B, Selamoglu N, Ghelli A, Rugolo M, Daldal F. Molecular mechanisms of superoxide production by complex III: A bacterial versus human mitochondrial comparative case study. Biochim Biophys Acta. 2013;1827:1332–1339. doi: 10.1016/j.bbabio.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang XW, Finlay DR, Guiney D, Helinski DR. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985;13:149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


