Abstract
Background
Colorectal cancer (CRC) is serious, yet a minority of US adults receive within-guideline screening exams.
Methods
A random selection of patients attending clinics in 3 different settings completed a survey on CRC-related barriers, knowledge, and beliefs.
Results
Participants with fewer barriers, better knowledge, and more positive beliefs toward screening were significantly more likely to be within screening guidelines. A physician’s screening recommendation was significantly related to screening in patients < 65 years, but was not significant for older patients.
Conclusions
Large-scale studies are needed. Results can be used to develop multifaceted, tailored education programs to improve CRC screening in primary care.
In 2006, it was estimated that 148,610 people would be diagnosed and 55,170 would die from colorectal cancer (CRC), making it the third leading type of cancer and the second leading cause of cancer death in the United States.1 These incidence rates are higher for certain segments of the population, namely, older and minority populations. The 1996–2000 CRC incidence rate for all ages was 55.1 per 100,000, with a rate of 18.6 per 100,000 for individuals under 65 years and 309.4 per 100,000 for 65 years and older.2 CRC incidence rates are higher among African Americans than Whites, and 5-year survival rates for those diagnosed with CRC are worse for African Americans.3,4
A number of studies have indicated that CRC screening can significantly reduce the morbidity and mortality rates attributable to this disease.5–9 Unfortunately, despite clear evidence supporting the effectiveness of CRC screening, adherence to screening recommendations has remained poor, and most health care providers do not follow guidelines strictly.10 To increase screening rates and subsequently reduce CRC morbidity and mortality, it is necessary to understand factors that influence screening behavior. Patients’ knowledge, beliefs, and barriers regarding CRC and CRC screening and physicians’ recommendations have been found to influence a decision to be screened.11–13 Further exploration of these factors could enhance our understanding of CRC screening behaviors and lead to the development and implementation of more effective cancer education interventions.
The objectives of this study were to identify (1) the percent of patients in diverse primary care clinics who receive CRC screening within American Cancer Society (ACS)-established14 guidelines, (2) primary care patients’ knowledge, beliefs, and barriers regarding CRC and CRC screening, and (3) the factors associated with completion of a screening test in a diverse patient population. Results could be used to design an education program to improve the use of CRC screening in primary care clinics.
MATERIALS AND METHODS
The Ohio State University (OSU) Primary Care Network is a collection of 15 Family Medicine and General Internal Medicine clinics dispersed throughout the central Ohio region that differ widely in size and patient demographics. From this group, we selected 3 clinics with significant variation in size, location, and patient demographics to participate in the study. One site was located in the inner city, 1 in the suburbs, and the third in an urban/university setting.
During the time frame of the study, the majority of patients across the 3 clinics were female (63%), and only the suburban site served a predominantly married population (65%). The clinics served a varied racial/ethnic patient population: Patients of the inner-city site were primarily African American (57%), the suburban practice site was primarily White (87%), and the University clinic site more closely mirrored US racial/ethnic demographics (58% White). Table 1 describes the patient demographics of participating clinics.
TABLE 1.
Characteristics of Patient Population (2002–2003) by Clinic Site
| Clinic Site | No. Patients Aged >50 y | Patient Age(y) | Patient Gender* | Marital Status† | Race |
|---|---|---|---|---|---|
| Inner City | 1,410 | 51–64 = 61% | M = 29% | M = 43% | African American = 57% |
| >64 = 39% | F = 71% | S = 39% | White = 41% | ||
| W = 18% | Other‡ = 2% | ||||
| Suburban | 1,733 | 51–64 = 73% | M = 40% | M = 65% | African American = 9% |
| >64 = 27% | F = 60% | S = 23% | White = 87% | ||
| W = 12% | Other = 4% | ||||
| Urban/University | 2,764 | 51–64 = 65% | M = 42% | M = 48% | African American = 36% |
| >64 = 35% | F = 58% | S = 40% | White = 58% | ||
| W = 12% | Other = 6% |
M indicates male; F, female.
M indicates married; S, single; W, widowed.
Other indicates interracial/multiracial, American Indian, Native American, Alaskan Native, and not specified race.
Physicians at the suburban site were attending physicians (n = 6), whereas both attending and resident physicians worked at the university (7 attendings, 18 residents) and inner-city (3 attendings, 2 residents) clinics. Patients of nurse practitioners (n = 1 suburban and inner city, n = 3 university) were also eligible to participate in the study. Eligible patients included individuals at least 51 years of age who sought health care services between September and December 2002. Although ACS guidelines suggest screening average-risk patients at age 50, we established an eligibility criteria of 51 years because patients would not be outside of screening guidelines until reaching that age. The study received approval from The OSU Institutional Review Board prior to implementation.
On 5 random days within a 6-week time period, 5 women and 5 men were randomly selected from medical appointment records at each clinic and invited to participate in the study by a research assistant. The research assistant approached 169 eligible patients to reach the recruitment goal of 150 patients (89% participation rate). A total of 74 women and 76 men completed the survey. The survey was completed in the waiting room prior to the medical visit. Participants received a $10 gift certificate from a local grocery store as a token of appreciation for their time.
Survey
The survey was developed in a previous research investigation. 15 Patients were asked if they had ever completed a CRC screening test, including fecal occult blood test (FOBT), flexible sigmoidoscopy (FS), and/or colonoscopy. If a patient completed a test, information was obtained regarding test dates and the reason for the test. The Appendix contains the scale and scoring system used to assess patients’ knowledge, beliefs, and barriers regarding CRC and CRC screening.
Analysis
Of the 150 participants who completed the questionnaire, data from 104 participants were used in the analyses. We excluded 5 patients because they were younger than 51 years, 15 had missing data and their age could not be determined, 21 were symptomatic and had undergone a CRC test for diagnostic purposes, and 5 patients were excluded due to completion of a test for unknown reasons. A participant was determined to be within screening guidelines if they self-reported having an FOBT in the past year, an FS in the last 5 years, or a colonoscopy in the past 10 years. To determine the not–within-guidelines category, we collapsed patients who reported having none of the 3 tests within guidelines (N = 38) with those who indicated a combination of “No” or “Don’t Know” response or who had missing responses across the 3 tests (N = 30). Of the 104 patients included in this analysis, 36 (35%) were within CRC screening guidelines and 68 (65%) patients were not within screening guidelines.
Summary statistics and frequencies were calculated for demographics (age, gender, race, education, marital status, working status, and insurance status) and physician recommendation for CRC screening. Chi-square tests (2-tailed) were used to test for relationships between each variable and CRC screening within guidelines. Fisher’s exact test was used when cell counts were small (≤5). Logistic regression15 compared screening outcome (yes/no) on each variable after adjusting for practice site, age (<65, ≥65 years), and race (White, non-White).
Composite scores were created for endoscopy barriers, endoscopy beliefs, FOBT barriers, FOBT beliefs, and CRC knowledge by summing over multiple survey items and converting to a 0 to 10 scale. t tests and logistic regression, adjusting for practice site, age, and race, were used to compare belief, barrier, and knowledge scores between individuals within or not within CRC screening guidelines.
We tested 3-way associations between age, physician recommendation, and CRC screening using logistic regression with CRC screening within guidelines as outcome and age, recommendation for a CRC test, and their interaction term as predictors. Odds ratios (Ors) and confidence intervals (CIs) were calculated for physician recommendation for CRC screening in the 2 age groups (<65, ≥65 years), and the 2 ORs were compared, testing for the interaction between physician recommendation and age. The same model was used, with race instead of age, to assess the 3-way associations between race (White, non-White), physician recommendation, and CRC screening receipt.
Predictive logistic regression modeling using purposeful forward selection16 was used to determine which set of factors were most associated with CRC screening after adjusting for practice site, age (<65, ≥65 years) and race (White, non-white), which were forced in the model as covariates. All demographic variables, physician recommendation, and barriers, beliefs, and knowledge scores were considered for each model along with their interactions. Data management and statistical analyses were performed with the SAS System for Windows version 9.1.17
RESULTS
Overall CRC screening rates by demographics and physician recommendation are listed in Table 2. Patients were diverse, with 34% over the age of 64, 46% reporting a non-White race, 16% reporting less than a high school education, and 47% not currently married. Only 41% of the participants reported they were currently employed full- or part-time.
TABLE 2.
Colorectal Cancer Screening Rates by Demographics and Physician Recommendation for a CRC test*
| Variable | CRC Screening Within Guidelines (N = 36) |
No CRC Screening Within Guidelines (N = 68) |
Total | Unadjusted Chi-Square Test P Value† |
Adjusted Logistic Regression P Value‡, † |
|
|---|---|---|---|---|---|---|
| Age (y) | 51–64 | 24 (34.8%) | 45 (65.2%) | 69 | ||
| ≥65 | 12 (34.3%) | 23 (65.7%) | 35 | — | — | |
| Sex | Female | 13 (25.5%) | 38 (74.5%) | 51 | ||
| Male | 23 (43.4%) | 30 (56.6%) | 53 | .055 | — | |
| Education | <High school | 5 (31.3%) | 11 (68.8%) | 16 | ||
| High school graduate/GED | 15 (27.3%) | 40 (72.7%) | 55 | |||
| College graduate | 5 (35.7%) | 9 (64.3%) | 14 | |||
| Some graduate school | 11 (64.7%) | 6 (35.3%) | 17 | .049§ | .103 | |
| Race‖ | Black/Other | 13 (27.7%) | 34 (72.3%) | 47 | ||
| White | 22 (40.0%) | 33 (60.0%) | 55 | — | — | |
| Marital status | Married | 23 (41.8%) | 32 (58.2%) | 55 | ||
| Single (not married) | 13 (27.1%) | 35 (72.9%) | 48 | — | .080 | |
| Working status | Part- or full-time | 13 (30.2%) | 30 (69.8%) | 43 | ||
| Retired | 16 (44.4%) | 20 (55.6%) | 36 | |||
| Volunteer, unemployed, disabled | 7 (28.0%) | 18 (72.0%) | 25 | — | — | |
| Insurance | None | 1 (33.3%) | 2 (66.7%) | 3 | ||
| Other insurance (82% reported either Medicaid or Medicare) | 10 (30.3%) | 23 (69.7%) | 33 | |||
| Private insurance | 25 (36.8%) | 43 (63.2%) | 68 | — | — | |
| MD recommend | No | 4 (10.3%) | 35 (89.7%) | 39 | ||
| Yes | 31 (55.4%) | 25 (44.6%) | 56 | <.001§ | <.001 | |
| Clinic | Inner city | 9 (25.7%) | 26 (74.3%) | 35 | ||
| Suburban | 12 (37.5%) | 20 (62.5%) | 32 | |||
| Urban/university | 15 (40.5%) | 22 (59.5%) | 37 | — | — |
Total N = 104. CRC indicates colorectal cancer; GED, general equivalency diploma.
P values less than or equal to .10 are reported.
P values are from a logistic regression model on CRC screening adjusting for practice site, age, and race.
P values are from Fisher’s Exact Test, used where any cell-count was less than 5.
Included African American (n = 41); interracial/multiracial (n = 3); American Indian, Native American, Alaskan Native (n = 1); Other (not specify; n = 1).
Only 35% (n = 36) completed a CRC test for screening purposes. The percent of participants who had a CRC screening within guidelines was 43.4% of men (95% CI, 30.1%–56.7%) and 25.5% of women (95% CI, 13.5%–37.4%). Although not statistically significant, higher screening rates were observed in married participants (41.8%) compared to those not married (27.1%; P = .080). Participants who completed some graduate school reported the highest rate of screening (64.7%) within guidelines as compared to other education levels. Patient report of a physician’s recommendation for a CRC screening test was significantly associated with CRC screening within guidelines (P < .001).
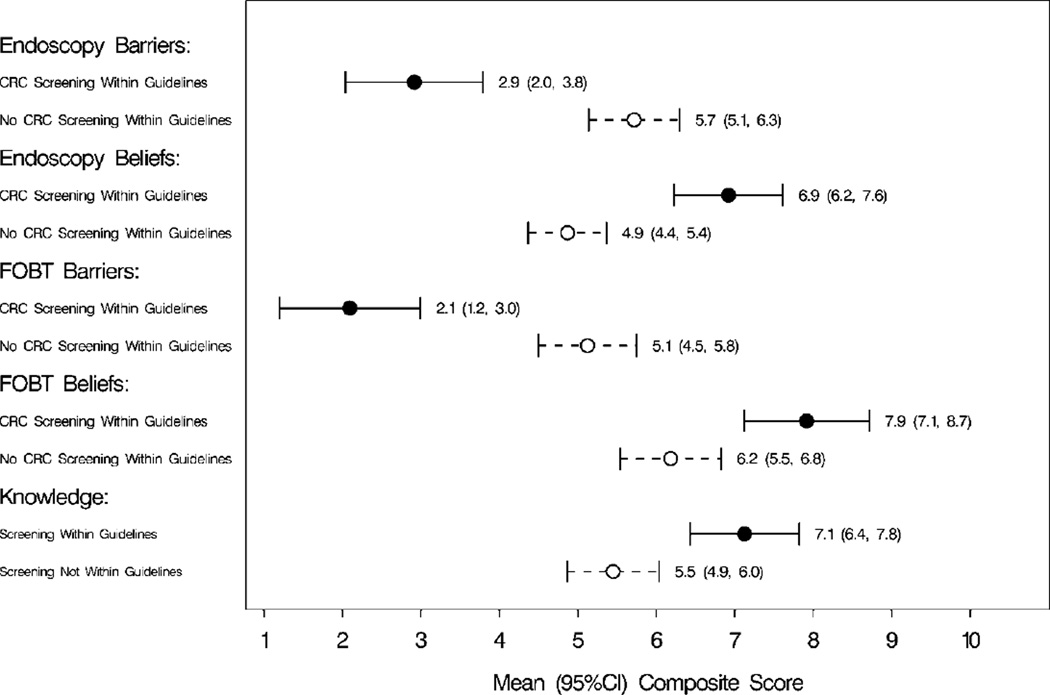
Table 3 lists means and confidence intervals for barriers, beliefs, and knowledge scores along with P values comparing subjects who did or did not complete a CRC screening test within guidelines. Results indicated that participants within guidelines for CRC screening had significantly fewer barriers for endoscopy (P < .001) and FOBT (P < .001), more positive beliefs about endoscopy (P < .001) and FOBT (P = .002), and better CRC knowledge (P = .001) after adjusting for primary care practice site and the age and race of the patient. Figure 1 is a graphic representation of the confidence intervals in Table 3.
TABLE 3.
Means and Confidence Intervals for Barriers, Beliefs, and Knowledge Scores by Completion of CRC Screening Within Guidelines*
| Variable | N | No CRC Screening Within Guidelines |
N | CRC Screening Within Guidelines |
Unadjusted t test P Values† |
Adjusted Logistic Regression P Value‡ |
|---|---|---|---|---|---|---|
| Endoscopy barriers | 48 | 5.72 (5.14, 6.29) | 32 | 2.92 (2.04, 3.79) | <.001 | <.001 |
| Endoscopy beliefs | 51 | 4.87 (4.37, 5.37) | 33 | 6.92 (6.22, 7.61) | <.001 | <.001 |
| FOBT barriers | 60 | 5.13 (4.50, 5.75) | 31 | 2.10 (1.20, 2.99) | <.001 | <.001 |
| FOBT beliefs | 55 | 6.18 (5.54, 6.82) | 27 | 7.92 (7.12, 8.71) | .001 | .002 |
| Knowledge | 60 | 5.45 (4.86, 6.04) | 36 | 7.13 (6.43, 7.82) | .001 | .001 |
CRC indicates colorectal cancer; FOBT, fecal occult blood test.
P values are from a 2-sided, 2-sample t test for comparison of means.
P values are from a logistic regression model on CRC screening adjusting for practice site, age, and race.
Figure 1.
Confidence Interval Plots: Barriers, Beliefs, and Knowledge Scores by Completion of CRC Screening Within Guidelines* *CRC indicates colorectal cancer; FOBT, fecal occult blood test; CI, confidence interval.
Table 4 describes the relationship between physician recommendation of a screening test and completion of CRC screening within guidelines by patient age and race. Participants less than 65 years old and who received a physician’s recommendation for CRC screening had 14.9 times the odds of completing CRC screening compared to a participant less than 65 years old who did not receive a physician’s recommendation for CRC screening. For participants 65 years and older who received a physician’s recommendation for CRC screening, the odds of completing screening was not significantly different compared to participants 65 years and older who did not receive a physician recommendation for CRC screening. Additionally, the difference in the ORs between age groups was not statistically significant.
TABLE 4.
Association Between Physician Recommendation of CRC Screening Test and Completion of CRC Screening Within Guidelines by Age and Race of the Patient*
| Variable | MD Recommended CRC Screening |
CRC Screening Within Guidelines |
Unadjusted OR (95% CI) and P Value |
Adjusted OR (95% CI) and P Value† |
|---|---|---|---|---|
| Difference in OR’s Between Age Groups‡ | P = .157 | P = .192 | ||
| Age < 65 | No | 2/28 (7.1%) | 20.4 (4.2–99.8) | 14.9 (3.7–90.2) |
| Yes | 22/36 (61.1%) | P < .001 | P < .001 | |
| Age ≥ 65 | No | 2/11 (18.2%) | 3.7 (0.6–21.6) | 3.6 (0.6–22.2) |
| Yes | 9/20 (45.0%) | P = .148 | P = .173 | |
| Difference in OR’s Between Race Groups‡ | P = .533 | P = .555 | ||
| White | No | 2/20 (10.0%) | 14.3 (2.8–72.7) | 14.1 (2.7–73.0) |
| Yes | 19/31 (61.3%) | P = .001 | P = .002 | |
| Non-White | No | 2/18 (11.1%) | 6.8 (1.2–36.1) | 6.8 (1.2–38.2) |
| Yes | 11/24 (45.8%) | P = .025 | P = .029 |
CRC indicates colorectal cancer; OR, odds ratio; CI, confidence interval. OR and P values from logistic regressions of age or race, doctor’s recommendation, and their interaction on CRC screening.
Estimates from models adjusting for practice site and race or age.
Significance of interaction between age or race and doctor’s recommendation in each logistic model.
The OR for the relationship between receiving a physician’s recommendation for CRC screening and being within CRC screening guidelines was slightly higher for White patients compared to non-White patients. However, the difference in the ORs between race groups was not statistically significant.
Predictive modeling determined that the best set of factors to use in predicting whether participants reported completing a CRC screening test within guidelines, after adjusting for practice site, age, and race, was a physician recommendation for a CRC screening test, endoscopy barriers score, and endoscopy beliefs score. A 1-unit increase in the endoscopy barriers score was associated with decreased odds of screening (OR = 0.65, 95% CI, 0.47–0.92, P = .014), and a 1-unit increase in the endoscopy beliefs score was associated with an increase in the odds of screening (OR = 1.67, 95% CI, 1.12–2.49, P = .013). Participants with a doctor’s recommendation showed odds of completing a CRC screening test of 11.24 (95% CI, 2.21–57.21, P = .004) times the odds of other participants after adjusting for endoscopy barriers score, endoscopy beliefs score, practice site, age, and race.
DISCUSSION
The US Preventive Services Task Force has ranked CRC screening as a high-priority clinical service.18 CRC screening can significantly reduce morbidity and mortality and is also cost effective.19 The cost effectiveness of screening has been found to range between $10,000 and $25,000 per life-year saved.20
One purpose of our study was to investigate CRC screening rates in a diverse primary care patient population to identify components of an educational program that could be implemented to improve CRC screening in primary care practices. The rate of CRC screening within guidelines was 35% in our study, which is considerably lower than rates found in other studies and those that reported from the Behavioral Risk Factor Surveillance Survey for the United States (48%).21,22 However, previous studies often have not discriminated between tests for screening and diagnostic purposes.23 Failure to investigate the reasons patients complete a CRC test can lead to inflated screening rates and an underestimation of the scope of the problem.
Patient beliefs, barriers, and knowledge regarding CRC and CRC screening were strongly related to screening behavior after adjusting for primary care practice size and the age and race of the patients. These findings highlight the value of developing sound educational interventions that address the key role of barriers, beliefs, and knowledge in patients’ screening behavior.
As with other studies, we found that physician recommendation was a strong determinant of colorectal cancer screening. 24 In addition to adjusting for the primary care practice site and the age and race of the patient, a multivariable model adjusting for patients’ beliefs and barriers about CRC screening determined that a physician recommendation for CRC screening continued to play a significant role in patient behavior.
Evidence for the association between age and responsiveness to physicians’ screening recommendations was a unique finding. The relationship between CRC screening recommendation and completing screening within guidelines was significant for patients under the age of 65 but not significant for older patients. Although this difference cannot be verified due to a small sample size, this finding may reflect the pessimistic perception of older adults toward cancer and its diagnosis as well as problems related to communication between physicians and elderly patients.25 A recent study indicated that increasing patient age was associated with a lower likelihood that screening was discussed during the medical visit.26 If clinicians view older patients as less responsive to their screening recommendations, they may not prioritize these discussions and may focus on other issues during a time-constrained office visit.
Results in this study also provided some evidence that a physician recommendation for CRC screening may occur more frequently or be more influential with White patients as compared to non-White patients. Previous studies have indicated that African American patients perceive their physician to be less participatory during the medical visit.27 Because a decision to complete CRC screening may be helped by a shared decision-making approach, our results may reflect this bias in provider-patient communication.28
One limitation of this study was the small sample size. With 104 subjects in the analysis, we had 80% power for estimating reasonable confidence intervals (± 9.2 percentage points) and for finding medium to large differences in proportions (0.3) or means (effect size = .6) between 2 groups. Three-way associations and modeling should be considered exploratory in which the results are preliminary evidence for further study.
Another limitation involves a reliance on self-report data. However, previous studies have indicated that self-report compares favorably to other methods of assessing screening rates.29 Missing data and “Don’t know” responses to questions on the self-administered questionnaires decreased the validity of our not-within-guidelines data. In addition, because data were collected from 1 geographical region, generalizability is limited.
These results point to the need for large-scale studies. Recommendations for future research include random selection of a large sample of patients who seek care at multiple clinical sites from diverse geographical regions. Use of interview-administered questionnaires and chart reviews to verify patient self-report would also enhance future studies.
Multifaceted educational programs designed to improve doctor-patient communication with older and minority populations, improve patients’ CRC knowledge and beliefs, and reduce barriers to receiving CRC screening may yield benefits beyond those of interventions focusing on 1 area. To achieve goals specified in Health People 2010,30 a concerted effort to develop and test innovative, yet practical interventions that systematically address the needs of patients, health care providers, and systems of care is needed. The results of this study point to potentially useful directions in this important effort.
Acknowledgments
Supported by Grants from the American Cancer Society, Ohio Division and The National Institutes of Health, P30 CA16058-30.
APPENDIX
Barriers, Beliefs, and Knowledge Scale*
| Yes | No | |||
|---|---|---|---|---|
| Barriers items† | ||||
| Someone encouraged me. | −1 | +1 | ||
| Someone discouraged me. | +1 | −1 | ||
| I have insurance. | −1 | +1 | ||
| Strongly Agree | Agree | Disagree | Strongly Disagree | |
| Screening takes too much time. | +2 | +1 | −1 | −2 |
| I don’t know where to get screened. | +2 | +1 | −1 | −2 |
| Screening costs too much. | +2 | +1 | −1 | −2 |
| Discomfort keeps me from getting screened. (FS)‡ | +2 | +1 | −1 | −2 |
| Beliefs items§ | ||||
| If you feel OK, a flex sig or FOBT won’t find anything. | −2 | −1 | +1 | +2 |
| The test is uncomfortable. (FS)‡ | −2 | −1 | +1 | +2 |
| Enemas are a bother. (FS)‡ | −2 | −1 | +1 | +2 |
| It’s safe. | +2 | +1 | −1 | −2 |
| It hurts. (FS)‡ | −2 | −1 | +1 | +2 |
| It’s useful. | +2 | +1 | −1 | −2 |
| It’s embarrassing. | −2 | −1 | +1 | +2 |
| It’s messy. (FOBT)‖ | −2 | −1 | +1 | +2 |
| It’s too hard. (FOBT)‖ | −2 | −1 | +1 | +2 |
| It’s disgusting. (FOBT)‖ | −2 | −1 | +1 | +2 |
| Afraid if they find something, part of my colon would be removed. (FOBT)‖ | −2 | −1 | +1 | +2 |
| Knew | Didn’t Know | |||
| Knowledge items¶ | ||||
| The name of the test as a test useful in detecting colon cancer. | +1 | −1 | ||
| What a Flex Sig or FOBT is. | +1 | −1 | ||
| How often to be screened. | +1 | −1 | ||
| Agree | Disagree | |||
| After a couple tests are ok, don’t need to be tested anymore. | −1 | +1 | ||
| Colon cancer runs in families. | +1 | −1 | ||
| Only people who eat a lot of high fat foods will get colon cancer. | −1 | +1 | ||
| Strongly Agree | Agree | Disagree | Strongly Disagree | |
| People have no control over whether colon cancer will be detected early. | −2 | −1 | +1 | +2 |
| Taking vitamins can help prevent colon cancer. | +2 | +1 | −1 | −2 |
| Regular exercise can help prevent colon cancer. | +2 | +1 | −1 | −2 |
| A person can tell if he/she has colon cancer without going to the doctor for tests. | −2 | −1 | +1 | +2 |
| Colon cancer can’t be cured, so there’s no reason to get screened. | −2 | −1 | +1 | +2 |
FS indicates flexible sigmoidoscopy; FOBT, fecal occult blood test. Barriers, Beliefs, and Knowledge scores were calculated by summing over scores for individual items in each scale as shown in the table. Raw summary scores were then converted to a 0 to 10 scale for use in descriptive statistics and analyses.
A higher score for Barriers indicates more barriers to getting an FS or FOBT.
Only asked on the FS section of the survey.
A higher score for Beliefs indicates positive beliefs about FS or FOBT screening.
Only asked on the FOBT section of the survey.
A higher score for Knowledge indicates more knowledge about tests and colorectal screening.
Footnotes
Presented at the North American Primary Care Research Group conference, Banff, Alberta, Canada, October 2003.
References
- 1.American Cancer Society: Cancer Facts and Figures, 2006. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 2.Surveillance, Epidemiology and End Results (SEER) Program. National Background Incidence Rates for 1999. Bethesda, MD: National Cancer Institute; 2002. [Google Scholar]
- 3.US Department of Commerce, Bureau of the Census. 2000 Census of Population, General Population Characteristics, Ohio. Washington, DC: US Bureau of the Census; 2000. [Google Scholar]
- 4.Ries LAG, Eisner MP, Kosary CL, et al., editors. SEER Cancer Statistics Review, 1975–2000. Bethesda, MD: National Cancer Institute; Available at: http://seer.cancer.gov/csr/1975_2000/. Accessed August 31, 2006. [Google Scholar]
- 5.Towler BP, Irwig L, Glaszious P, et al. Screening for colorectal cancer using the fecal occult blood test, hemoccult. Cochrane Database Syst Rev. 2000;2:CD001216. doi: 10.1002/14651858.CD001216. [DOI] [PubMed] [Google Scholar]
- 6.Mandel JS, Bond JH, Church TR, et al. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–437. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 7.Kronborg O, Fenger C, Olsne J, et al. Randomized study of screening for colorectal cancer with fecal-occult blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 8.Selby JV, Friedman GD, Quesenberry CP, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg A, Delco F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133:573–584. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 10.Chu KC, Tarone RE, Chow WH, et al. Temporal patterns in colorectal cancer incidence, survival, and mortality from 1950 through 1990. J Natl Cancer Inst. 1994;86:997–1006. doi: 10.1093/jnci/86.13.997. [DOI] [PubMed] [Google Scholar]
- 11.Greiner KA, Engleman KK, Hall MA, et al. Barriers to colorectal cancer screening in rural primary care. Prev Med. 2004;38:269–275. doi: 10.1016/j.ypmed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Shokar NK, Vernon SW, Weller SC. Cancer and colorectal cancer: knowledge, beliefs, and screening preferences of a diverse patient population. Fam Med. 2005;37:341–347. [PubMed] [Google Scholar]
- 13.Cornelius LJ, Smith PL, Simson GM. What factors hinder women of color from obtaining preventive health care? Am J Public Health. 2002;92:535–539. doi: 10.2105/ajph.92.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56:11–25. doi: 10.3322/canjclin.56.1.11. [DOI] [PubMed] [Google Scholar]
- 15.Paskett ED, D’Agostino R, Tatum C, et al. Colorectal cancer screening practices among low-income women. Clin J Women’s Health. 2000;1:3–9. [Google Scholar]
- 16.Hosmer D, Lemeshow S. Applied Logistic Regression. 2nd ed. New York: John Wiley & Sons Inc; 2000. [Google Scholar]
- 17.SAS System for Windows [computer program]. Version 9.1. SAS/ STAT SOFTWARE. Cary, NC: SAS Institute Inc; 2002. [Google Scholar]
- 18.Coffield AB, Maciosek MV, McGinnis JM, et al. Priorities among recommended clinical preventive services. Am J Prev Med. 2001;21:1–9. doi: 10.1016/s0749-3797(01)00308-7. [DOI] [PubMed] [Google Scholar]
- 19.Walsh JM, Terdiman JP. Colorectal cancer screening: Scientific review. JAMA. 2003;289:1288–1296. doi: 10.1001/jama.289.10.1288. [DOI] [PubMed] [Google Scholar]
- 20.Pignone M, Saha S, Hoerger T, et al. Cost effectiveness analyses of colorectal cancer screening: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian S, Klosterman M, Amonkar MM, et al. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38:536–550. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Behavioral Risk Factor Surveillance System (BRFSS) Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion; Available at: http://www.cdc.gov/brfss/. Accessed August 31, 2006. [Google Scholar]
- 23.Vernon SW, Meissner H, Klabunde C, et al. Measures for ascertaining use of colorectal cancer screening in behavioral, health services, and epidemiological research. Cancer Epidemiol Biomarkers Prev. 2004;13:898–905. [PubMed] [Google Scholar]
- 24.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 25.Greene MG, Adelman RD. Physician-older patient communication about cancer. Patient Educ Couns. 2003;50:55–60. doi: 10.1016/s0738-3991(03)00081-8. [DOI] [PubMed] [Google Scholar]
- 26.Patel P, Forjuoh SN, Avots-Avotins AA, et al. Identifying opportunities for improved colorectal cancer screening in primary care. Prev Med. 2004;39:239–246. doi: 10.1016/j.ypmed.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Cooper-Patrick L, Gall JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282:583–589. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]
- 28.Volk RJ, Cass AR, Spann SJ. A randomized controlled trial of shared decision making for prostate cancer screening. Arch Fam Med. 1999;8:333–340. doi: 10.1001/archfami.8.4.333. [DOI] [PubMed] [Google Scholar]
- 29.Hall HI, Van Den Eeden SK, Tolsma DD, et al. Testing for prostate and colorectal cancer: comparison of self-report and medical record audit. Prev Med. 2004;39:27–35. doi: 10.1016/j.ypmed.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services. Healthy People 2010: With understanding and Improving Health and Objectives for Improving Health. 2nd ed. Washington, DC: US Government Printing Office; 2000. [Google Scholar]