Abstract
Background
Subthalamic nucleus (STN) deep brain stimulation (DBS) represents a well-established treatment for patients with advanced Parkinson's disease (PD) insufficiently controlled with medical therapies. This study presents the long-term outcomes of patients with PD treated with STN-DBS using an MRI-guided/MRI-verified approach without microelectrode recording.
Methods
A cohort of 41 patients who underwent STN-DBS were followed for a minimum period of 5 years, with a subgroup of 12 patients being followed for 8–11 years. Motor status was evaluated using part III of the Unified Parkinson's Disease Rating Scale (UPDRS-III), in on- and off-medication/on-stimulation conditions. Preoperative and postoperative assessments further included activities of daily living (UPDRS-II), motor complications (UPDRS-IV), neuropsychological and speech assessments, as well as evaluation of quality of life. Active contacts localisation was calculated and compared with clinical outcomes.
Results
STN-DBS significantly improved the off-medication UPDRS-III scores, compared with baseline. However, UPDRS scores increased over time after DBS. Dyskinesias, motor fluctuations and demands in dopaminergic medication remained significantly reduced in the long term. Conversely, UPDRS-III on-medication scores deteriorated at 5 and 8 years, mostly driven by axial and bradykinesia subscores. Quality of life, as well as depression and anxiety scores, did not significantly change at long-term follow-up compared with baseline. In our series, severe cognitive decline was observed in 17.1% and 16.7% of the patients at 5 and 8 years respectively.
Conclusions
Our data confirm that STN-DBS, using an MRI-guided/MRI-verified technique, remains an effective treatment for motor ‘off’ symptoms of PD in the long term with low morbidity.
Keywords: PARKINSON'S DISEASE, NEUROSURGERY
Introduction
High-frequency deep brain stimulation (DBS) of the subthalamic nucleus (STN) is a well-established surgical treatment for symptoms of Parkinson's disease (PD) insufficiently controlled with medical therapies. In 2005, STN DBS was granted the label of efficacious symptomatic treatment at the Movement Disorder Society (MDS) evidence-based medicine review update of treatments for PD.1 The European Federation of Neurological Societies (EFNS) and MDS—European Section recent collaboration to produce evidence-based recommendations for the treatment of PD accredited DBS with level A evidence for the management of disabling tremor, severe motor complications, unpredictable on–off periods, dyskinesias (including biphasic dyskinesias), off-periods and early-morning dystonia.2 STN DBS has been used for the treatment of patients with PD since the mid-1990s.3 4 Short-term and medium-term outcomes are well documented by several studies, showing substantial improvements in motor symptoms and quality of life, and reduction in dopaminergic medication requirements.5–8 Far fewer studies have reported on the effects of microelectrode-guided STN DBS after prolonged follow-up periods.9–11 According to a recent review including STN DBS studies with long-term follow-up data, substantial benefits for some of the motor symptoms, namely, tremor, rigidity, as well as motor complications, were maintained after 5 years.12 However, the same studies noted that akinesia, axial signs and cognition often deteriorated in the long term, most likely in keeping with the natural progression of the disease and the involvement of non-dopaminergic systems.
The major risks associated with the surgical procedure include intracranial bleeding and infection of the implanted material. Microelectrode recording (MER) identifies the neuronal firing patterns of the STN and currently represents the most commonly employed technique to assist and validate target localisation.6 Our group has previously published 12-month safety and efficacy data on a large series of patients with PD (n=79) treated with STN DBS using a standardised surgical technique based on individual MRI-guided and MRI-verified targeting without MER. This approach reduces the number of brain penetrations required and the associated risk of morbidity.13 14 Here, we report the long-term outcome in a cohort of individuals who underwent STN DBS at our institution (n=41) and were followed for a minimum period of 5 years, with a subgroup of patients (n=12) being followed for 8–11 years. To our knowledge, this is the first study presenting long-term outcomes of patients treated with high-frequency STN DBS using MRI-guided and MRI-verified approach without MER.
Methods
Study design
Clinical assessments were done in a non-blinded fashion. All data were prospectively collected and retrospectively analysed.
Patients
Forty-five consecutive patients with PD were implanted with bilateral STN DBS electrodes between November 2002 and September 2006 at the National Hospital for Neurology and Neurosurgery. Forty-one patients were followed for at least 5 years and were thus included in the present study. For a subset of 12 of these patients, follow-up data beyond 7 years were available. The remaining four of the initial cohort could not complete 5-year follow-up visits due to death (n=3) or being bed bound following stroke 3 years after surgery (n=1), in all cases unrelated to DBS surgery, and were therefore not included in the present study.
Each patient underwent a multidisciplinary evaluation to decide on the suitability of STN DBS surgery. All patients had a diagnosis of idiopathic PD according to the UK Brain Bank criteria, for at least 5 years, age younger than 70 years, and suffered from disabling motor complications despite optimal medical treatment. A formal levodopa challenge confirmed dopaminergic drug responsiveness. Detailed neuropsychological and neuropsychiatric assessments excluded patients with significant cognitive impairment and/or ongoing psychiatric comorbidities. A structural MRI was obtained to exclude surgical contraindications. Written informed consent was obtained from each patient before surgery. Table 1 summarises the characteristics of patients at baseline.
Table 1.
Characteristics of patients at baseline
| 5-year follow-up (n=41) |
8-year follow-up* (n=12) |
|
|---|---|---|
| Gender distribution | 27 men, 14 women | 8 men, 4 women |
| Age at onset (mean±SD, years) | 43.3±9.8 | 40.4±9.8 |
| Age at surgery (mean±SD, years) | 56.2±8.4 | 52.8±10.1 |
| Disease duration (mean±SD years) | 12.9±5.8 | 12.3±4 |
| L-dopa equivalent dose (LED) | 1471±515 | 1475±525 |
*Baseline features between the total cohort and the patients followed beyond 8 years did not differ significantly.
Surgical procedure and stimulation programming
Details of our neurosurgical procedure, using an MRI-guided and MRI-verified technique, have been previously described.13 15 Briefly, implantation of bilateral quadripolar DBS electrodes (3389 Medtronic, Minneapolis, MN) was performed under local anaesthesia, in the off-medication condition (see below) to allow clinical evaluations during electrode placement. Monopolar stimulation through the contacts of the DBS electrode was sequentially performed to assess for additional therapeutic effects and/or the presence of side effects. One patient did not tolerate the off-medication condition, and surgery was performed under general anaesthesia. The STN was visualised on stereotactic T2-weighted MRI sequences and directly targeted using manual calculation and/or planning software (FrameLink, Medtronic, Minneapolis, Minnesota, USA), without MER. An immediate postoperative stereotactic MRI was performed to verify accurate electrode placement. The pulse generator(s) (Soletra or Kinetra, Medtronic, Minneapolis, Minnesota, USA) was implanted in the subclavicular area and connected subcutaneously to the electrodes under general anaesthesia either immediately or a few days after electrode implantation.
Stimulation was initiated within the first postoperative week, and optimal settings were selected through a screening process. Stimulation and medication were further titrated according to clinical response over subsequent visits.
Clinical assessments
All 41 patients were assessed preoperatively and postoperatively at 1 year (mean±SD 1.1±0.3 years; range 0.8–2 years) and at 5 years (mean±SD 5.2±0.5; range 4.5–6 years). Twelve patients were further assessed at 8 years (mean±SD 8.1±1.6; range 7.2–11 years). Each visit included motor, neuropsychological and speech assessments, as well as evaluation of quality of life, which were carried out the same day or within two consecutive days for each patient.
Motor status was evaluated using part III of the Unified Parkinson's Disease Rating Scale (UPDRS-III). Prior to surgery, patients were assessed in the practically defined ‘off state’ after overnight withdrawal of antiparkinsonian drugs, and the ‘on state’, following a levodopa challenge using a suprathreshold dose of oral levodopa. After bilateral STN DBS, motor assessments were sequentially performed at the following conditions, in open fashion: off medication/on stimulation (with stimulation switched on after 12 h medication withdrawal); on medication/on stimulation (1 h after the administration of a suprathreshold dose of levodopa while stimulation was reintroduced). Subscores for individual cardinal features of PD were derived by summation of the relevant items from the UPDRS-III as follows: tremor (items 20–21), rigidity (item 22), bradykinesia (items 23–26, 31) and axial (18, 19, 27–30).
Evaluations also included the UPDRS parts II (activities of daily living (ADL)) and IV (motor complications). UPDRS-IV was divided further into two subscores; items 32–34 to assess dyskinesias and items 35–39 to reflect motor fluctuations (on–off phenomena).
Quality of life was assessed with the Parkinson's Disease Questionnaire 39 (PDQ-39). The 39 items encompass the following domains: mobility, ADL, emotional health, stigma, social support, cognition, communication and bodily discomfort. A summary score from 0 to 100 was calculated for the PDQ-39. This summary index (SI) is the arithmetic mean of the scores for the individual domains.16
Global cognitive functions were tested with the Mattis Dementia Rating Scale (DRS-2). Further analyses were done on the scale components (attention, initiation/perseveration, construction, conceptualisation and memory). The Age-Corrected Mayo’s Older Americans Normative Studies (MOANS) Scaled Score (AMSS) was computed from raw total and subscores.17 The test was performed at best clinical condition, under regular medication at baseline, and under current medication and on-stimulation at postoperative follow-ups.
The presence of dementia was clinically defined according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).
The Beck Depression Inventory (BDI) and the Beck Anxiety Inventory (BAI) were used to evaluate mood.
Speech was assessed by a speech therapist using the sentence task of the Assessment of Intelligibility for Dysarthric Speech (AIDS); this is the percentage of words correctly understood by a native English speaker blinded to patient status.18
Each patient's medication dose was recorded at each time point, and levodopa equivalent dose (LED) was derived using a standard formula.19
Stimulation settings were documented and the average voltage, pulse width and frequency were calculated for all active contacts for each patient.
Adverse events were systematically documented.
Active contact localisation
Preoperative stereotactic T2-weighted scans were used to visualise the MRI-defined STN in both the axial and the coronal planes. The STN was subdivided into five segments: superior (A), anterior-medial (B), central (C), posterolateral (D) and inferior (E). The stereotactic coordinates of each active contact were calculated from postoperative stereotactic MR images and transposed to the preoperative scan. A functional neurosurgeon, blinded to the clinical outcome, then determined which STN segment the active contact was most closely related to, as previously described.18 The position of each active contact was further classified according to the established functional subsections of the STN.20 21 Consequently, contacts associated with A or D segments were considered as stimulating the sensorimotor area of the STN, segment C the associative and in B or E the limbic area of the STN.
Statistical analysis
Clinical outcomes for continuous values are presented as mean±SD. All numeric values were checked for normality using One-Sample Kolmogorov–Smirnov analysis. Continuous data comparing baseline and postoperative scores were analysed by means of the Student t test, if normally distributed. Alternatively, the Wilcoxon signed-rank test was used for non-parametric data. Differences between independent groups were tested with one-way analysis of variance or the Kruskal–Wallis test, according to data distribution.
Correlations of clinical parameters with motor response to DBS and changes in global cognitive function at 5 and 8 years postoperatively were assessed by Spearman correlation coefficients. Linear regression analysis, as guided by univariate analysis outcome, was used to explore the impact of baseline factors to the abovementioned changes.
The motor response to DBS was calculated as follows: (baseline off-medication score minus postoperative off-medication on-stimulation score)/baseline off-medication score)×100 (positive scores denote improvement).
Change in global cognitive function was calculated as follows: (baseline DSR-2 AMSS minus postoperative DRS-2 AMSS)/baseline DSR-2 AMSS×100 (positive scores denote deterioration).
To allow clinically meaningful comparisons between clinical outcomes controlled by continuous bilateral stimulation, a combined contact score, taking into account both left and right electrodes, was adopted for each patient as follows: (1) both active contacts assigned to the sensorimotor area or one contact assigned to the sensorimotor area and one to the associative; (2) both active contacts assigned to the associative or one to the sensorimotor area and one to the limbic area and (3) both in the limbic area or one in the associative and one in the limbic area of the STN.
All reported p values are two-sided at a significance level of 0.05. All data were analysed using the SPSS statistical package (SPSS, Release V.21.0 Chicago, Illinois, USA).
Results
Motor outcomes
Off medication
STN DBS significantly improved the UPDRS-III score with respect to baseline at all follow-up points. However, an increase in UPDRS scores was observed over time on DBS (table 2, see online supplementary figure). Analysis of UPDRS-III subitems showed that STN DBS provided significant and persistent relief from tremor (72.6% reduction at 1 year, 73.9% at 5 years and 77.2% at 8 years) and rigidity (53.9% reduction at 1 year, 45.2% at 5 years and 50% at 8 years). Significant, but less remarkable, improvements were seen in bradykinesia (45.8% reduction at 1 year, 16.5% at 5 years and 23.2% at 8 years) and axial symptoms by 53.6% at 1 year, 41.2% at 5 years and 21.6% at 8 years. Speech intelligibility significantly deteriorated at 5 (−43.7%) and 8 years (−21.4%).
Table 2.
Effects of STN DBS during the off-medication condition
| Mean±SD scores* (possible range) |
Baseline (n=41) |
Baseline of 8-year follow-up (n=12) |
1 year post-op | 5 years post-op | 8 years post-op | p Value (1 year vs baseline) | p Value (5 years vs baseline) | p Value (8 years vs baseline) |
|---|---|---|---|---|---|---|---|---|
| Total UPDRS-III (0–108) |
50.3±15.8 | 57±15.2 | 22.9±12.8 | 33.7±8.7 | 36.2±16.9 | <0.001 | <0.001 | <0.001 |
| Tremor (0–28) | 7.3±4.1 | 7.9±3.6 | 2±2.6 | 1.9±1.6 | 1.8±1.8 | <0.001 | <0.001 | <0.001 |
| Rigidity (0–20) | 11.5±4.4 | 13±4.1 | 5.3±3.6 | 6.3±2.9 | 6.5±5 | <0.001 | <0.001 | <0.001 |
| Bradykinesia (0–36) | 21.8±7.1 | 25±6.5 | 11.8±7.1 | 18.3±5 | 19.2±8.4 | <0.001 | 0.03 | 0.019 |
| Axial (0–24) | 9.7±4.6 | 11.1±5.4 | 4.5±3.5 | 7.7±3.1 | 8.7±4.7 | <0.001 | 0.027 | 0.03 |
| UPDRS-II (ADL) (0–52) | 23.3±7.4 | 29.4±6.8 | 15.6±8.4 | 20.7±7 | 21±7.5 | <0.001 | 0.194 | 0.043 |
| On–off fluctuation (0–7) | 4.7±1.4 | 5.6±1.2 | 2.3±1.8 | 2.5±1.8 | 2.8±1.8 | <0.001 | <0.001 | 0.006 |
| Speech intelligibility (0–100) | 91.9±11.8 | 80.8±18.7 | 70.2±7.2 | 51.7±4.6 | 63.5±2.1 | >0.05 | <0.001 | <0.001 |
*For all outcome measures, higher scores indicate worse condition but for speech intelligibility, where higher scores are indicative of better performance.
ADL, activities of daily living; DBS, deep brain stimulation; STN, subthalamic nucleus; UPDRS, Unified Parkinson's Disease Rating Scale.
On medication
UPDRS-III scores during the on-medication–on-stimulation condition did not change significantly at 1 year postoperatively in comparison to baseline. However, deterioration was observed after 5 and 8 years; this was significant at 5 years, but in the smaller sample did not reach significance at 8 years (table 3).
Table 3.
Effects of STN DBS during the on-medication condition
| Mean±SD scores* (possible range) |
Baseline (n=41) |
Baseline of 8- year follow-up (n=12) |
1 year post-op | 5 years post-op | 8 years post-op | p Value (1 year vs baseline) | p Value (5 years vs baseline) | p Value (8 years vs baseline) |
|---|---|---|---|---|---|---|---|---|
| Total UPDRS-III (0–108) | 14±8.7 | 16.5±9.8 | 14±10 | 23.9±10.1 | 28.7±16.1 | 0.805 | <0.001 | 0.063 |
| Tremor (0–28) | 1.4±2.3 | 1.5±1.7 | 0.8±1.3 | 0.91±1.1 | 1.5±1.1 | 0.184 | 0.37 | 0.664 |
| Rigidity (0–20) | 4.2±3.1 | 4.5±3.4 | 3±3.1 | 3.4±2.7 | 5.8±5.1 | 0.038 | 0.388 | 0.600 |
| Bradykinesia (0–36) | 6.3±4.2 | 7.7±5 | 7.4±5.2 | 12.7±5.6 | 14.7±7 | 0.335 | <0.001 | 0.025 |
| Axial (0–24) | 2.5±2.1 | 3±2.1 | 2.9±2.4 | 6.1±3.5 | 6.6±3.8 | 0.475 | <0.001 | 0.006 |
| UPDRS-II (ADL) (0–52) | 6.2±5 | 8.18±4.3 | 7.5±4.8 | 13.2±7.5 | 15.2±8.1 | 0.251 | <0.001 | 0.011 |
| Dyskinesias (0–13) | 3.6±2.5 | 4.4±2.5 | 1.8±2.2 | 1.39±1.8 | 2.1±2.1 | 0.007 | 0.002 | 0.053 |
| Speech intelligibility (0–100) | 89±3.6 | 80.8±18.7 | 67.5±6.8 | 45.8±11 | 40.8±19.1 | <0.05 | <0.001 | <0.001 |
*For all outcome measures, higher scores indicate worse condition but for speech intelligibility, where higher scores are indicative of better performance.
ADL, activities of daily living; DBS, deep brain stimulation; STN, subthalamic nucleus; UPDRS, Unified Parkinson's Disease Rating Scale.
Rigidity subscore was significantly improved at 1 year (28.6%). Tremor and rigidity were improved at 5 years, although this was not significant. On the other hand, both bradykinesia and axial subscores deteriorated significantly at 5 and 8 years. Speech intelligibility declined significantly at 5 (−48.5%) and 8 years (−39.6%).
ADL
ADL in the off-medication state (UPDRS-II) improved with DBS at 1, 5 and 8 years. However, ADL scores in the on-medication state did not improve after STN stimulation (tables 2 and 3).
PDQ-39
PDQ-39 SI (score) improved by 26.1% at 1 year (p=0.008), but had almost returned to baseline 5 and 8 years post-DBS (table 4). Improvement in four of the eight PDQ-39 subdomains persisted up to 8 years following surgery: mobility, ADL, stigma and bodily discomfort. However, the support subdomain remained generally unchanged over time. Comparison analyses showed an improvement in bodily discomfort subdomain; but only at 5-year follow-up there was a statistically significant difference (p=0.013). Conversely, emotional well-being and cognition subdomains tended to decline at 5 and 8 years compared with baseline, although this was not significant. Communication was the only subdomain that deteriorated significantly at 5 (p=0.002) and 8 years (p=0.002) in comparison to preoperative mean scores.
Table 4.
STN-DBS effects on PDQ-39 summary index (SI) and different subdomains
| Mean scores±SD (possible range) |
Baseline (n=41) |
Baseline of 8-year follow-up | 1 years post-op | 5 years post-op | 8 years post-op | p Value (5 years vs baseline) | p Value (8 years vs baseline) |
|---|---|---|---|---|---|---|---|
| PDQ-39 SI (0–100)* | 38.2±17.2 | 45.6±19.2 | 28.9±14.2 | 37.5±18.5 | 44.9±15.1 | 0.886 | 0.932 |
| Mobility | 50.9±24.9 | 55.9±29.8 | 35.8±27.6 | 42.4±29.0 | 51.8±28.9 | 0.414 | 0.713 |
| Activities daily living | 39.6±18.8 | 49.6±20.4 | 23.0±14.7 | 36.3±22.7 | 39.0±17.0 | 0.759 | 0.131 |
| Emotional | 21.8±14.9 | 23.1±15.6 | 18.0±18.2 | 22.3±20.2 | 23.9±16.0 | 0.961 | 0.866 |
| Stigma | 24.1±21.8 | 31.8±23.5 | 17.9±16.9 | 16.3±16.6 | 22.2±19.9 | 0.162 | 0.364 |
| Support | 12.7±13.7 | 15.9±12.0 | 8.0±11.0 | 14.5±19.7 | 14.4±21.8 | 0.711 | 0.837 |
| Cognition | 21.9±14.8 | 23.3±17.0 | 20.4±13.4 | 28.0±15.8 | 24.4±12.3 | 0.709 | 0.870 |
| Communication | 27.7±24.2 | 33.3±28.6 | 31.1±21.7 | 40.8±22.6 | 61.4±22.8 | 0.002 | 0.002 |
| Bodily discomfort | 46.2±26.2 | 54.6±27.7 | 28.3±20.8 | 33.0±21.9 | 47.7±21.8 | 0.013 | 0.520 |
*Higher scores indicate worse condition.
DBS, deep brain stimulation; STN, subthalamic nucleus.
Drug-related motor complications
Significant improvements in drug-related motor complications were sustained over time as measured by the subitems of UPDRS-IV. Dyskinesias ameliorated by 50.0% at 1 year, 61.4% at 5 years and 52.3% at 8 years (table 2). On–off motor fluctuations reduced by 51.1% at 1 year, 46.8% at 5 years and 50.0% at 8 years after surgery (table 2).
Neuropsychological evaluations
Results of neuropsychological evaluations are shown in table 5. DRS-2 AMSS scores were stable at 1 year postoperatively, but tended to decline at 5 and 8 years. Nevertheless, p values at 8 years did not reach the chosen level of significance for either total scores or the separate cognitive subdomains. Attention and conceptualisation subscores did not significantly change at 5 years, while initiation/perseveration, construction and memory got significantly worse (table 5).
Table 5.
Neuropsychological evaluations
| Mean scores±SD (possible range) |
Baseline (n=41) |
Baseline of 8-year follow-up | 1 year post-op | 5 years post-op | 8 years post-op | p Value (5 years vs baseline) | p Value (8 years vs baseline) |
|---|---|---|---|---|---|---|---|
| Total DRS-2 raw (0–144)* | 141.3±.2.2 | 141.6±2.7 | 140.8±5.3 | 134.4±13.4 | 137.8±5.5 | 0.01 | 0.087 |
| Total DRS-2 AMSS | 12.4±1.9 | 12.9±2.2 | 12.7±3 | 9.9±3.7 | 11.2±3.4 | 0.001 | 0.404 |
| Attention | 11.8±1.3 | 12.1±1.0 | 11.9±1.6 | 11.8±2 | 11.2±2.0 | 0.937 | 0.477 |
| Initiation/perseveration | 10.3±1.4 | 9.7±2.3 | 10.2±2.6 | 8.2±3.2 | 8.9±3.0 | 0.001 | 0.374 |
| Construction | 10±0 | 10.0±0.0 | 9.6±1.4 | 9±2.3 | 9.6±1.1 | 0.03 | 0.374 |
| Conceptualisation | 11.1±1.4 | 12.0±0.0 | 11.6±1.1 | 10.2±2.5 | 11.6±0.9 | 0.180 | 0.374 |
| Memory | 11.9±1.8 | 11.6±1.9 | 11.8±2.7 | 9.1±3.6 | 10.4±2.5 | 0.002 | 0.690 |
| BDI (0–63)† | 10±5.4 | 14.0±7.1 | 8.3±4.2 | 11±6.3 | 11.1±3.2 | 0.084 | 0.531 |
| BAI (0–63)† | 14.5±8.6 | 20.0±9.2 | 10.3±7.3 | 12.4±8.4 | 16.1±7.0 | 0.458 | 0.313 |
*Higher scores indicate better cognitive performance.
†Higher scores indicate more severe symptoms of depression and anxiety.
BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory.
Dementia as defined by DSM-IV criteria was present in one patient (2.4%) at 1-year post-DBS, seven patients at 5 years (17.1%) and two patients (16.7%) among the ones followed by 8 years. There were no significant changes in BDI and BAI at 5 and 8 years.
Medical treatment, stimulation parameters and device replacements
The mean preoperative LED decreased by 35.4% at 1 year (from 1471.1±515.2 to 949.6±572.7, p<0.001), 45.1% (from 1471.1±515.2 to 668.5±359.3, p<0.001) at 5 years and 48.6% (from 1475±525 to 698.8±541.8, p=0.009) at 8 years. One patient was completely drug-free 1 year after surgery, and remained drug-free after 5 and 8 years.
There were no significant changes in the stimulation amplitude (voltage) from 1 year (3.1±0.7 V) to 5 (3.2±0.7 V) or 8 years (3.11±0.9). Pulse width did not vary significantly over time (1 year 60.4±2.4 µs; 5 years 63.8±12.5 µs and 8 years 61.5±5 µs). The mean stimulation frequency increased slightly but not to a significant degree (1 year 139.5±18.9 Hz; 5 years 142.7±24.1 Hz and 8 years 149.5±14.1 Hz). Bilateral monopolar stimulation was applied in 35 patients (85.4%). Four patients received unilateral double monopolar stimulation (9.7%) and two received bilateral double monopolar stimulation in the 5-year follow-up (4.9%), and one (8.3%) had unilateral double monopolar stimulation in the 8-year follow-up group, and the remaining patients were on bilateral monopolar stimulation.
No implantable pulse generator (IPG) replacement occurred among the patients followed at 5 years. Out of the 12 patients followed in the 8-year group (range 7.2–11 years), two had two IPG replacements and seven had one, under general anaesthesia without complications. The remaining three had no IPG replacements. Bilateral new Soletra IPGs were replaced in one patient and Kinetra IPG in the others, after a mean period of 5.9±1 years.
Active contacts position
At 5 years, 44.1%, 35.3% and 20.6% of active contacts were most closely associated with the motor, associative and limbic regions of the STN, respectively. In the subgroup of patients with 8-year follow–up, 62.5% of active contacts were associated with the motor and 37.5% the associative regions of the STN.
Correlations of clinical outcomes
The magnitude of the motor response to DBS correlated positively with younger age at disease onset (p=0.02). Younger age at surgery demonstrated a non-significant trend towards better motor response to DBS (p=0.07). Worse UPDRS-III off scores at baseline (both total and subscores) and more severe on–off fluctuations also correlated with significantly better postoperative motor responses. Younger age at surgery and better preoperative levodopa responsiveness, as measured by levodopa challenge, showed a positive trend towards a better motor response to DBS (p=0.07 and 0.064). After multivariate regression analysis, only higher tremor subscores in the off condition were found to be an independent factor of better clinical outcome (p=0.001).
Deterioration in DRS-2 scores was associated with older age at baseline, worse motor state in the on condition (UPDRS-III on), worse quality of life at baseline and higher medication requirements as measured by LED. None of the abovementioned factors reached a level of statistical significance when checked in the multivariate analysis.
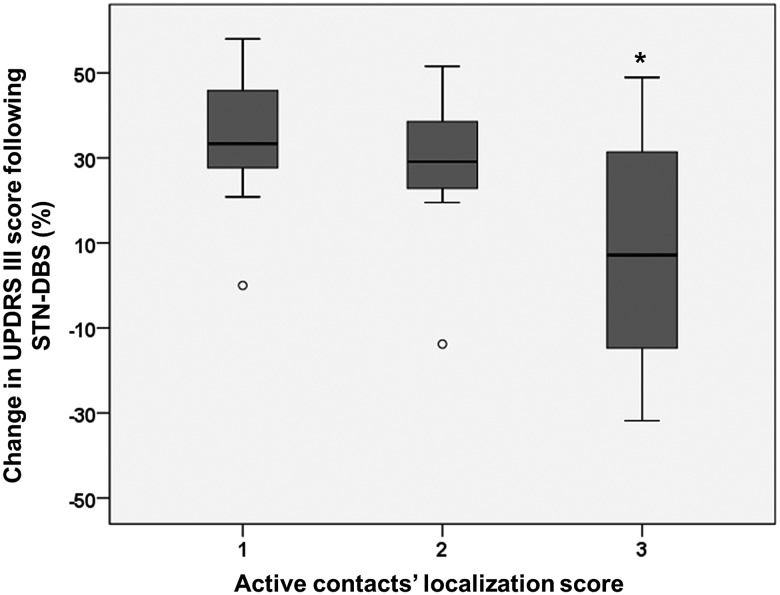
A better motor outcome was documented when contacts’ location calculated score was 1 (= both active contacts assigned to the sensorimotor area of the STN or one contact assigned to the sensorimotor area and one to the associative) in comparison to 2 (= both active contacts assigned to the associative area of the STN or one to the sensorimotor area and one to the limbic area) and 3 (= both in the limbic area or one in the associative and one in the limbic area of the STN). Significantly worse motor outcomes at 5 years were correlated with contact calculated score 3 in comparison to 1 (p=0.046) (figure 1).
Figure 1.
Percentage of changes in the Unified Parkinson's disease Rating Scale (UPDRS-III) following subthalamic nucleus (STN) deep brain stimulation (DBS) at 5 years in relation to active contact location calculated score (1=both active contacts assigned to the sensorimotor area of the STN or one contact assigned to the sensorimotor area and one to the associative; 2=both active contacts assigned to the associative area of the STN or one to the sensorimotor area and one to the limbic area; 3=both in the limbic area or one in the associative and one in the limbic area of the STN).
Adverse effects
All patients underwent stereotactic MRI immediately after lead implantation. There were no haemorrhages (either symptomatic or asymptomatic). In this group of patients, there were no hardware infections in the primary procedure or at impulse generator replacement. There were no DBS malfunctions, lead fractures or lead migrations. Two patients had their electrodes repositioned due to insufficient response from the first operation.
Five patients had a transient delirium/confusion episode during the first days after surgery. One patient suffered seizures during the 48 h after surgery but did not develop epilepsy. There was no severe depression, suicide or attempted suicide. There were four cases of impulse control disorder likely drug related, occurring at least 1 year after surgery, and one case of hypomania that required hospital admission.
Discussion
We report a long-term follow-up evaluation of the effect of high-frequency STN DBS in patients with advanced PD, who were operated on using a MRI-guided and MRI-verified approach without MER. Beneficial antiparkinsonian effects of DBS on rigidity and tremor in the off-medication state persisted after a follow-up period of 5 and 8 years. Improvements on dyskinesias and motor fluctuations also persisted in the long term.
Motor scores
In our series, the motor scores in the ‘off-medication’ state assessed by UPDRS-III were significantly reduced by stimulation, leading to an amelioration of the frequency and severity of ‘off’ periods that remained significantly improved in the long term. Comparison of the UPDRS-III scores in the ‘off’ medication state obtained at baseline with the one at 5 and 8 years in the ‘off’ medication and on stimulation condition revealed sustained improvement, as has been previously reported.10 22 23 On the other hand, UPDRS-III on medication significantly deteriorated at 5 and 8 years, mostly driven by axial symptoms and bradykinesia subscores.9 23 This outcome was paralleled by deterioration in the ability to perform daily living activities measured by UPDRS-II ‘on medication’. As previously reported, the deterioration in axial symptoms, for example, speech and postural instability, is characteristic of the natural history of PD, likely due to non-dopaminergic deficits.9
Reduction of dopaminergic therapy responsiveness, a well-recognised feature of advanced PD, combined with extension of the PD pathological process beyond the nigro-striatal dopaminergic system, could be partially responsible for this decline in the long-term response to DBS during the on-medication condition.24 25 Whether the longer-term deterioration of gait and balance merely reflects disease progression or is partially caused by chronic high-frequency STN DBS merits further investigation.
Our series demonstrated a positive correlation between younger age at PD onset and better long-term motor outcome after STN DBS. However, shorter disease duration or younger age at surgery did not seem to favourably impact the long-term motor response to DBS, although a trend was noted for younger age. It has been shown that younger age at onset is generally associated with a slower disease progression, likely in keeping with higher prevalence of some genetic forms of PD.26
As previously reported, delayed deterioration in speech can occur following STN DBS.9 18 Indeed, a decline in speech intelligibility was observed in this cohort, both during off-medication and on-medication conditions. Interestingly, this deterioration was more pronounced during the on-medication condition in comparison to the off-medication condition.
Cognitive scores
The proportion of patients with severe cognitive decline in our series was 17.1% at 5 years and was remarkably similar (16.7%) at 8 years. These rates are relatively lower in comparison to what has been previously reported (43% in a cohort of patients followed up >7 years after bilateral STN DBS).22 However, the cohort of patients described by these authors had higher age at surgery (61.5±5.7 vs 52.8±10.1 in our 8-year group) and longer disease duration at STN DBS implantation (22.8±2.3 vs 12.3±4 in our group). Nevertheless, a multicentre study that followed 35 STN DBS patients (mean disease duration at surgery 13.5 years, and mean age at surgery 59.6) for 5–6 years reported cognitive decline in 22.8%, a figure that is closer to that found in our series.23
Quality of life
A number of studies have reported on health-related quality of life after STN DBS showing favourable outcomes over short-term follow-up.27–30 To our knowledge, this series is the first to report long-term follow-up of quality of life in patients with PD treated with STN DBS.
In keeping with previously published data, health-related quality of life measured by PDQ-39 was significantly improved after STN-DBS in 1 year in our series. Total PDQ-39 scores have returned to baseline levels, although remained slightly better, at 5- and 8-year follow-up. Subdomains such as mobility, ADL, stigma, support and bodily discomfort were the ones showing a more sustained improvement over time. Conversely, emotional well-being, cognition and communication subdomains declined at 5 and 8 years.
The development of non-levodopa-responsive features could be, at least partially, responsible for the decline in quality of life from 1 year to 5- and 8-year follow-up in our series.
Anxiety and depression
It has been suggested that STN DBS may have an antidepressant effect in PD over time.31 Although higher levels of transient depressive disorders have been reported after the intervention,32 especially in the short term, it is mostly reported that depression and anxiety tend to improve in the first years after STN DBS.33 Nonetheless, depression and anxiety scores tend to reach baseline levels in studies reporting longer follow-up results. Accordingly, in our cohort, there was no significant change in depression or anxiety at long-term follow-up relative to baseline.
MRI-guided and MRI-verified DBS
The low morbidity of the MRI-guided and MRI-verified approach to surgery employed in this series demonstrates a high standard of safety while maintaining therapeutic efficacy.34 We are not aware of any other report on the long-term follow-up of patients with PD who had surgery with MRI-guided and MRI-verified STN DBS without use of MER. The optimal target point within the visualised STN is still disputed.35 Initially, we targeted the centre of the visualised STN as described by Bejjani et al.36 Ongoing audit of lead location and associated clinical results eventually resulted in the intended target point being located 2 or 3 mm posterolateral to that described by Bejjani. Thus, we currently target the most superior, posterior and lateral portion of the nucleus, thought to be the sensorimotor STN.20 21 These areas are equivalent to those labelled 1 and 2. Our data suggest that there is a significant correlation between lead location associated with these regions of the STN and superior improvement in motor outcomes. Other authors have reported that lead location in relation to the STN on postoperative imaging can predict clinical outcome.37 38 Our findings extend this concept to long-term clinical outcome.
The risk of symptomatic haemorrhage inevitably rises with increasing numbers of micro-electrode or macro-electrode brain penetrations. MRI-guided and MRI-verified surgery has been associated with a significantly reduced risk of haemorrhagic complications in functional neurosurgery.34 Accordingly, none of the patients in this series had visible haemorrhage on their postoperative imaging, confirming that a meticulous approach to MRI-guided and MRI-verified DBS is safe and accurate, with clinical outcomes comparable to other techniques, yet with a reduced risk associated with surgery.
Despite the lack of double-blind assessments and the relatively small sample size, our results suggest that motor improvements following STN DBS can be sustained beyond 8 years.
Supplementary Material
Acknowledgments
We would like to thank Professor Lisa Cipolotti, Dr Priyanka Pradhan and the neuropsychology department, as well as Dr Leonora Wilkinson and Dr Donna Page, for contribution to the cognitive data; and Dr Steve Tisch, Dr Paul Silberstein, Dr Raul Martinez-Fernandez, Dr Etienne Holl, Dr Erika Petersen and Ellie Borrell for contribution to clinical and imaging data. Finally, we wish to thank the patients and their families, without whose support none of this research would have been possible.
Footnotes
Contributors: IA-O and ZK made equal contributions in analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published. ET, JC, HA, IM-T and MJ were responsible for data collection and final approval of the version to be published. TF and MH were responsible for data collection, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published. LZ and PL were responsible for conception and design, data collection, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
Funding: This work was undertaken at UCL/UCLH and was funded in part by the Department of Health NIHR Biomedical Research Centres funding scheme. The Unit of Functional Neurosurgery, UCL Institute of Neurology, Queen Square, London, is supported by the Parkinson's Appeal and the Sainsbury's Monument Trust.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Goetz CG, Poewe W, Rascol O, et al. Evidence-based medical review update: pharmacological and surgical treatments of Parkinson's disease: 2001 to 2004. Mov Disord 2005;20:523–39. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira JJ, Katzenschlager R, Bloem BR, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson's disease. Eur J Neurol 2013;20:5–15. [DOI] [PubMed] [Google Scholar]
- 3.Limousin P, Pollak P, Benazzouz A, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 1995;345:91–5. [DOI] [PubMed] [Google Scholar]
- 4.Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Eng J Med 1998;339:1105–11. [DOI] [PubMed] [Google Scholar]
- 5.Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord 2006;21(Suppl 1):S290–304. [DOI] [PubMed] [Google Scholar]
- 6.Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet 2010;9:581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 9.Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 2003;349:1925–34. [DOI] [PubMed] [Google Scholar]
- 10.Fasano A, Romito LM, Daniele A, et al. Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain 2010;133:2664–76. [DOI] [PubMed] [Google Scholar]
- 11.Castrioto A, Lozano AM, Poon Y-Y, et al. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch neurol 2011;68:1550–6. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Oroz MC, Moro E, Krack P. Long-term outcomes of surgical therapies for Parkinson's disease. Mov Disord 2012;27:1718–28. [DOI] [PubMed] [Google Scholar]
- 13.Foltynie T, Zrinzo L, Martinez-Torres I, et al. MRI-guided STN DBS in Parkinson's disease without microelectrode recording: efficacy and safety. JNNP 2011;82:358–63. [DOI] [PubMed] [Google Scholar]
- 14.Zrinzo L, Foltynie T, Limousin P, et al. Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg 2012;116:84–94. [DOI] [PubMed] [Google Scholar]
- 15.Holl EM, Petersen EA, Foltynie T, et al. Improving targeting in image-guided frame-based deep brain stimulation. Neurosurgery 2010;67:437–47. [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson C, Fitzpatrick R, Peto V, et al. The Parkinson's Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing 1997;26:353–7. [DOI] [PubMed] [Google Scholar]
- 17.Lucas JA, Ivnik RJ, Smith GE, et al. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol 1998;20:536–47. [DOI] [PubMed] [Google Scholar]
- 18.Tripoliti E, Zrinzo L, Martinez-Torres I, et al. Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology 2011;76:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams-Gray CH, Foltynie T, Brayne CEG, et al. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 2007;130:1787–98. [DOI] [PubMed] [Google Scholar]
- 20.Yelnik J, Bardinet E, Dormont D, et al. A three-dimensional, histological and deformable atlas of the human basal ganglia. I. Atlas construction based on immunohistochemical and MRI data. NeuroImage 2007;34:618–38. [DOI] [PubMed] [Google Scholar]
- 21.Lambert C, Zrinzo L, Nagy Z, et al. Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted imaging. NeuroImage 2012;60:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merola A, Zibetti M, Angrisano S, et al. Parkinson's disease progression at 30 years: a study of subthalamic deep brain-stimulated patients. Brain 2011;134:2074–84. [DOI] [PubMed] [Google Scholar]
- 23.Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study of subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord 2010;25:578–86. [DOI] [PubMed] [Google Scholar]
- 24.Müller J, Wenning GK, Jellinger K, et al. Progression of Hoehn and Yahr stages in Parkinsonian disorders: a clinicopathologic study. Neurology 2000;55:888–91. [DOI] [PubMed] [Google Scholar]
- 25.Lang AE, Obeso JA. Challenges in Parkinson's disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol 2004;3:309–16. [DOI] [PubMed] [Google Scholar]
- 26.Schrag A, Ben-Shlomo Y, Brown R, et al. Young-onset Parkinson's disease revisited--clinical features, natural history, and mortality. Mov Disord 1998;13:885–94. [DOI] [PubMed] [Google Scholar]
- 27.LaGrange E, Krack P, Moro E, et al. Bilateral subthalamic nucleus stimulation improves health-related quality of life in PD. Neurology 2002;59:1976–8. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Martin P, Valldeoriola F, Tolosa E, et al. Bilateral subthalamic nucleus stimulation and quality of life in advanced Parkinson's disease. Mov Disord 2002;17:372–7. [DOI] [PubMed] [Google Scholar]
- 29.Siderowf A, Jaggi JL, Xie SX, et al. Long-term effects of bilateral subthalamic nucleus stimulation on health-related quality of life in advanced Parkinson's disease. Mov Disord 2006;21:746–53. [DOI] [PubMed] [Google Scholar]
- 30.Witt K, Daniels C, Reiff J, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: a randomised, multicentre study. Lancet Neurol 2008;7:605–14. [DOI] [PubMed] [Google Scholar]
- 31.Takeshita S, Kurisu K, Trop L, et al. Effect of subthalamic stimulation on mood state in Parkinson's disease: evaluation of previous facts and problems. Neurosurg Rev 2005;28:179–86; discussion 187. [DOI] [PubMed] [Google Scholar]
- 32.Houeto JL, Mesnage V, Mallet L, et al. Behavioural disorders, Parkinson's disease and subthalamic stimulation. JNNP 2002;72:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castelli L, Perozzo P, Zibetti M, et al. Chronic deep brain stimulation of the subthalamic nucleus for Parkinson's disease: effects on cognition, mood, anxiety and personality traits. Eur Neurol 2006;55:136–44. [DOI] [PubMed] [Google Scholar]
- 34.Zrinzo L, Foltynie T, Limousin P, et al. Image-verified deep brain stimulation reduces risk and cost with no apparent impact on efficacy. Mov Disord 2012;27:1585–6; author reply 1586–7. [DOI] [PubMed] [Google Scholar]
- 35.Coenen VA, Prescher A, Schmidt T, et al. What is dorso-lateral in the subthalamic Nucleus (STN)?--a topographic and anatomical consideration on the ambiguous description of today's primary target for deep brain stimulation (DBS) surgery. Acta Neurochir (Wien) 2008;150:1163–5; discussion 1165. [DOI] [PubMed] [Google Scholar]
- 36.Bejjani BP, Dormont D, Pidoux B, et al. Bilateral subthalamic stimulation for Parkinson's disease by using three-dimensional stereotactic magnetic resonance imaging and electrophysiological guidance. J Neurosurg 2000;92:615–25. [DOI] [PubMed] [Google Scholar]
- 37.Paek SH, Yun JY, Song SW, et al. The clinical impact of precise electrode positioning in STN DBS on three-year outcomes. J Neurol Sci 2013;327:25–31. [DOI] [PubMed] [Google Scholar]
- 38.Wodarg F, Herzog J, Reese R, et al. Stimulation site within the MRI-defined STN predicts postoperative motor outcome. Mov Disord 2012;27:874–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.