Abstract
Purpose
Musculoskeletal symptoms are the most common adverse effects of aromatase inhibitors (AIs) and can result in decreased quality of life and discontinuation of therapy. Omega-3 fatty acids (O3-FAs) can be effective in decreasing arthralgia resulting from rheumatologic conditions and reducing serum triglycerides.
Patients and Methods
Women with early-stage breast cancer receiving an AI who had a worst joint pain/stiffness score ≥ 5 of 10 using the Brief Pain Inventory–Short Form (BPI-SF) were randomly assigned to receive either O3-FAs 3.3 g or placebo (soybean/corn oil) daily for 24 weeks. Clinically significant change was defined as ≥ 2-point drop from baseline. Patients also completed quality-of-life (Functional Assessment of Cancer Therapy–Endocrine Symptoms) and additional pain/stiffness assessments at baseline and weeks 6, 12, and 24. Serial fasting blood was collected for lipid analysis.
Results
Among 262 patients registered, 249 were evaluable, with 122 women in the O3-FA arm and 127 in the placebo arm. Compared with baseline, the mean observed BPI-SF score decreased by 1.74 points at 12 weeks and 2.22 points at 24 weeks with O3-FAs and by 1.49 and 1.81 points, respectively, with placebo. In a linear regression adjusting for the baseline score, osteoarthritis, and taxane use, adjusted 12-week BPI-SF scores did not differ by arm (P = .58). Triglyceride levels decreased in patients receiving O3-FA treatment and remained the same for those receiving placebo (P = .01). No between-group differences were seen for HDL, LDL, or C-reactive protein.
Conclusion
We found a substantial (> 50%) and sustained improvement in AI arthralgia for both O3-FAs and placebo but found no meaningful difference between the groups.
INTRODUCTION
Despite the well-proven efficacy of aromatase inhibitors (AIs) for the treatment of hormone-sensitive breast cancer,1 some patients experience—and may even stop treatment early because of—undesirable adverse effects.2 Observational studies have shown that AI-related arthralgia is more prevalent than originally reported.3,4 In a cross-sectional survey of 200 consecutive postmenopausal women receiving adjuvant AI therapy for breast cancer, 94 (47%) reported AI-related joint pain, and 88 (44%) reported joint stiffness, with the bulk of the symptoms reported in the hands and knees.3 Risk factors include time since cessation of menstrual function, previous taxane chemotherapy, and prior hormone-replacement therapy.3,5,6 Of concern, only approximately 50% of women receive adjuvant hormonal therapy for the full duration at the optimal schedule.7
The use of omega-3 fatty acids (O3-FAs) for rheumatoid arthritis as well as other inflammatory conditions has been studied for more than 20 years. In a mouse model, fish oil, which is a rich source of O3-FAs—particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—decreased the incidence as well as severity of joint pain and inflammation.8 EPA and DHA inhibit the conversion of arachidonic acid to prostaglandin and leukotrienes, which results in reduced inflammation.9,10 Several randomized controlled trials have suggested that patients receiving O3-FAs or eating diets rich in O3-FAs have fewer symptoms of rheumatoid arthritis.11–15 A meta-analysis in 2007 found significant changes in patient-assessed pain, morning stiffness, number of painful and/or tender joints, and nonsteroidal anti-inflammatory drug consumption.16 O3-FAs have also been found to be equivalent to nonsteroidal anti-inflammatory drugs in patients with acute and chronic back pain.17 Finally, O3-FAs have been evaluated for the prevention of cardiovascular disease and have been shown to reduce serum triglyceride levels, which also may be increased in women receiving AIs.18–21
There are currently no proven treatments for AI-related arthralgia. We conducted a multicenter randomized double-blind placebo-controlled clinical trial to test the hypothesis that O3-FAs reduce pain and stiffness in women undergoing adjuvant AI therapy for early-stage breast cancer.
PATIENTS AND METHODS
The study was activated in February 2012 and closed to accrual in February 2013. Patients were informed of the investigational nature of the study and signed informed consent. The study was conducted after appropriate approval by individual institutional review boards at 52 sites, in compliance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Patient Characteristics
Postmenopausal women with a history of stage I to III hormone-sensitive breast cancer receiving adjuvant AI therapy for ≥ 90 days were screened. Those with a score of ≥ 5 of 10 on the Brief Pain Inventory–Short Form (BPI-SF) worst pain/stiffness measure (item 2) who reported that the symptoms started or worsened after initiation of AI therapy were eligible. Patients were required to have a Zubrod performance status of 0 to 2. Those who had received O3-FA supplements within 3 months before enrollment were excluded, as were those with a history of fracture or surgery involving the affected joint within the prior 6 months. Patients receiving oral narcotics or topical analgesics within the prior 14 days were excluded, and those receiving oral steroids or who received intra-articular steroid injections within the prior 28 days were excluded.
Study Design
A randomized double-blind multicenter trial comparing OA-FAs 3.3 g per day (six capsules; Ocean Nutrition, Dartmouth, Nova Scotia, Canada) versus matching placebo daily for 24 weeks was conducted. Each active capsule contained 560 mg of EPA plus DHA in a 40-to-20 ratio. Each placebo capsule contained a blend of soybean and corn oil. Both the active and placebo capsules were colored with carob and flavored with natural lemon-lime. Patient random assignment was stratified by prior history of arthritis (yes v no) and prior taxane use (yes v no). Fasting serum was collected at baseline and weeks 12 and 24, stored at −80°C, and tested centrally for total cholesterol, HDL, LDL, triglycerides, and C-reactive protein (CRP).
Patient-Reported Outcome Measures
Because there are no well-validated measures specifically for AI-induced arthralgia, we selected a general pain scale used in patients with cancer—the BPI-SF—to assess the primary end point.20 Numeric rating scales such as the BPI are among the most common, valid, and reliable measures used to assess cancer pain.22 The BPI-SF is a 14-item questionnaire that asks patients to rate pain over the prior week and the degree to which it interfered with activities on a 0-to-10 scale, where higher scores indicate more pain. We used the worst pain (item 2) as the primary end point measure. This form was modified to include stiffness.
We also used scales to assess joint pain, stiffness, and functional status in the knees and hands as secondary end points. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC; version 3.1) is a validated measure for assessing osteoarthritis of the knees and/or hips and consists of 24 questions related to three subscales: pain (0 to 500), stiffness (0 to 200), and physical function (0 to 1,700).23 The Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands (M-SACRAH) consists of three domains assessing pain, stiffness, and functional status in those with hand osteoarthritis and rheumatoid arthritis, using 100-mm visual analog scales.24 For both instruments, higher scores represent higher symptom burden. The Functional Assessment of Cancer Therapy–Endocrine Symptoms (FACT-ES) measures physical, social and family, emotional, and functional well-being and endocrine symptoms.25 The FACT scales have five response levels (“not at all” to “very much”), where higher scores reflect better well-being and fewer symptoms. This scale provided a measure of the broader impact of join pain and stiffness symptoms. Two single-item 7-point global rating of change scales for joint pain and stiffness were used to evaluate patients' perception of change in symptoms.26
Fasting Serum Analyses
Lipids and CRP were measured using the Cobas Integra 400 plus automated analyzer (Roche, Basel, Switzerland). Total cholesterol, HDL, and triglycerides were measured using individual enzymatic colorimetric methods. For cholesterol, interassay precision is 1.90%, intra-assay precision is 0.51%, and the quantification limit is 0.116 mg/dL. For HDL, interassay precision is 1.00%, intra-assay precision is 1.13%, and the quantification limit is 3 mg/dL. For triglycerides, interassay precision is 1.90%, intra-assay precision is 1.60%, and the quantification limit is 8.85 mg/dL. LDL is calculated from these measurements using the Friedewald equation. CRP (high sensitivity) was measured with an immunoturbimetric assay; interassay precision is 3.1%, intra-assay precision is 1.30%, and the quantification limit is 0.1 mg/L.
Statistical Considerations
The primary hypothesis of the study was that O3-FAs would decrease joint pain and/or stiffness associated with the use of AIs in patients with breast cancer compared with placebo at 12 weeks, as measured by the BPI worst pain and/or stiffness item. The study stipulated a two-sided α = 0.05, with an estimated 5% nonadherence (reducing nominal effect size) and 20% dropout rate (increasing total required sample size) at the primary end point evaluation time of 12 weeks after random assignment. For a 2-point difference and 3.5-point standard deviation at 12 weeks, with other parameters as specified, 222 eligible patients were required for 90% power under a two-arm normal design.
Secondary analyses examined BPI worst pain scores at weeks 6 and 24. Also, outcomes for the M-SACRAH, WOMAC, FACT-ES, and global rating of change scales were assessed at weeks 6, 12, and 24. These secondary end points were examined using multiple linear regression, adjusting for the baseline score and prespecified stratification factors. We also analyzed adverse events by intervention arm.
Cholesterol, LDL, HDL, triglycerides, and CRP were measured at baseline and weeks 12 and 24. Raw means at each time point and means of the difference from baseline to week 12 were calculated by treatment arm. Paired t tests were used to calculate unadjusted P values comparing week 12 with baseline measures within treatment arm, and two-sample t tests were used to calculate unadjusted P values comparing differences in serum measures, from baseline to week 12, between treatment arms. Counts of patients at each time point with elevated levels of each serum measure were calculated, and χ2 tests were used to calculate unadjusted P values comparing the proportions of patients with elevated levels between treatment arms.
RESULTS
Accrual, Eligibility, and Evaluability
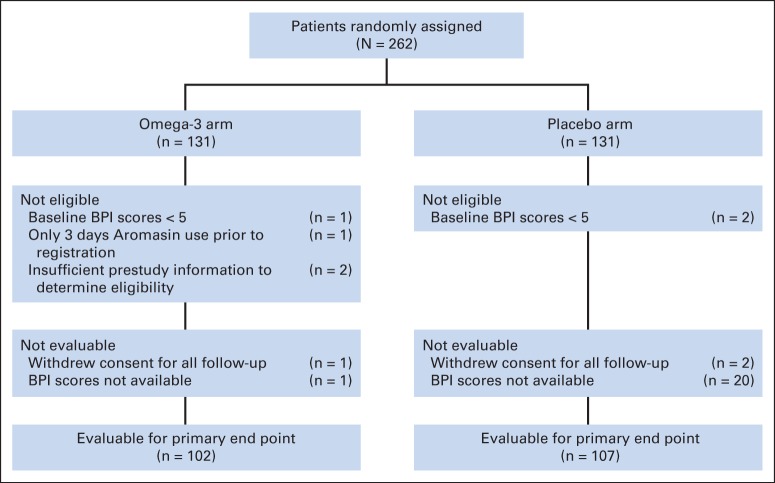
Accrual to this study was rapid, with a total of 262 patients registered from 52 sites between February 2012 and February 2013. Six patients were ineligible (one because of 3 days of exemestane use before registration, three because of low baseline BPI score, and two because of insufficient prestudy information; Fig 1). In addition, seven patients withdrew consent for all study follow-up and were considered not analyzable, leaving 249 evaluable patients (O3-FA arm, n = 122; placebo arm, n = 127). Nine of these patients were considered major deviations because they received only 0 to 1 week of intervention.
Fig 1.
CONSORT diagram. BPI, Brief Pain Inventory.
Patient Characteristics
Patient characteristics by intervention assignment are listed in Table 1. No notable imbalances by arm were observed by age, ethnicity, prior osteoarthritis, or prior taxane use, although fewer black women were randomly assigned to the O3-FA arm (4% v 12%).
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | Total (N = 249) |
O3-FA Arm (n = 122) |
Placebo Arm (n = 127) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Median | 59.2 | 59.5 | 59.1 | |||
| Hispanic ethnicity | ||||||
| Yes | 16 | 6 | 7 | 6 | 9 | 7 |
| No | 225 | 90 | 112 | 92 | 113 | 89 |
| Unknown | 8 | 3 | 3 | 2 | 5 | 4 |
| Race | ||||||
| White | 217 | 87 | 113 | 93 | 104 | 82 |
| Black* | 20 | 8 | 5 | 4 | 15 | 12 |
| Asian | 4 | 2 | 1 | 1 | 3 | 2 |
| Native American | 1 | < 1 | 0 | 0 | 1 | 1 |
| Multiracial | 2 | 1 | 1 | 1 | 1 | 1 |
| Unknown | 5 | 2 | 2 | 2 | 3 | 2 |
| Osteoarthritis | ||||||
| Yes | 57 | 23 | 28 | 23 | 29 | 23 |
| No | 192 | 77 | 94 | 77 | 98 | 77 |
| Prior taxane | ||||||
| Yes | 144 | 58 | 71 | 58 | 73 | 57 |
| No | 105 | 42 | 51 | 42 | 54 | 43 |
| Location of joint pain*† | ||||||
| Knee | 178 | 71 | 87 | 69 | 91 | 75 |
| Hand | 169 | 68 | 90 | 71 | 79 | 65 |
| Ankle | 91 | 37 | 34 | 27 | 57 | 47 |
| Wrist | 84 | 34 | 38 | 30 | 46 | 38 |
| Shoulder | 97 | 39 | 51 | 40 | 46 | 38 |
| Other | 130 | 52 | 65 | 51 | 65 | 53 |
| Self-reported comorbid conditions | ||||||
| Diabetes | 32 | 13 | 19 | 15 | 13 | 11 |
| Hypertension | 101 | 41 | 48 | 38 | 53 | 43 |
| High cholesterol | 77 | 31 | 45 | 35 | 32 | 26 |
| Heart disease | 13 | 5 | 9 | 7 | 4 | 3 |
| AI | ||||||
| Anastrozole | 146 | 59 | 73 | 57 | 73 | 60 |
| Exemestane | 29 | 12 | 14 | 11 | 15 | 12 |
| Letrozole | 74 | 30 | 40 | 31 | 34 | 28 |
| Years since starting AI therapy | ||||||
| Median | 1.2 | 1.1 | 1.3 | |||
| Years since last menstrual period | ||||||
| Median | 9.4 | 9.9 | 8.8 | |||
| Weight, kg | ||||||
| Median | 79.0 | 79.8 | 77.3 | |||
| Fish consumption | ||||||
| Mean total servings per week | ||||||
| Baseline | 1.6 | 1.7 | 1.5 | |||
| Week 6 | 1.7 | 1.9 | 1.4 | |||
| Week 12 | 2.1 | 2.0 | 2.2 | |||
| Week 24 | 1.7 | 2.0 | 1.5 | |||
Abbreviations: AI, aromatase inhibitor; O3-FA, omega-3 fatty acid.
Patient characteristics were well balanced by arm for all factors shown except location of joint pain and black race.
Percent columns sum to > 100% because patients could report > one location of joint pain.
Dropout and Nonadherence
The study design assumed a dropout rate of 20% and nonadherence rate of 5% (or total noncompliance rate of 25%) for the primary end point assessment time of 12 weeks after registration. BPI worst pain scores were not available for 20 (16%) of the 122 evaluable patients in the O3-FA arm (dropout, n = 18; nonadherence, n = 2); in the placebo arm, scores were not available for 20 (16%) of 127 eligible patients (dropout, n = 19; nonadherence, n = 1). Therefore, dropout and nonadherence were lower than anticipated and did not differ by arm.
Primary End Point: BPI Worst Pain/Stiffness at Week 12
In total, 84% (102 of 122) of patients in the O3-FA arm and 84% (107 of 127) in the placebo arm had week-12 BPI worst pain scores available for analysis. The mean observed BPI worst pain score was 1.74 points lower (reduced pain) at 12 weeks compared with baseline in the O3-FA arm and 1.49 points lower in the placebo arm (Table 2). In multivariable analysis, adjusted week-12 BPI worst pain scores were 0.17 points lower on average (implying slightly less pain) in the O3-FA arm versus the placebo arm (95% CI, −0.79 to 0.44; P = .58; Table 2). The effect of intervention on BPI worst pain did not differ according to stratification factors or baseline BPI score (data not shown). Results were similar at weeks 6 and 24 (Fig 2). A 2-point change from baseline was achieved in 61% of women receiving O3-FAs and 57% of women receiving placebo. Similar results were seen with regard to pain interference and the global rating of change scales (Table 2).
Table 2.
Mean BPI Worst Pain/Stiffness Score, Pain Interference, and Global Rating of Change
| Analysis | No. of Patients | Observed Results |
Adjusted Resultsa |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baselineb |
Follow-Up |
Within Group |
Between Group |
Follow-Up |
Differencec | 95% CI | Pd | |||||||
| Mean | 95% CI | Mean | 95% CI | Difference | Pe | Differencef | Pg | Mean | 95% CI | |||||
| BPI worst pain score | ||||||||||||||
| Week 6 | −0.33 | .32 | −0.27 | −0.88 to 0.34 | .38 | |||||||||
| O3-FAs | 108 | 7.09 | 6.80 to 7.39 | 5.41 | 4.94 to 5.87 | −1.69 | < .001 | 5.38 | 4.77 to 5.99 | |||||
| Placebo | 115 | 6.98 | 6.71 to 7.25 | 5.63 | 5.17 to 6.08 | −1.36 | < .001 | 5.65 | 5.04 to 6.26 | |||||
| Week 12 | −0.24 | .38 | −0.17 | −0.79 to 0.44 | .58 | |||||||||
| O3-FAs | 102 | 7.08 | 6.77 to 7.39 | 5.34 | 4.85 to 5.84 | −1.74 | < .001 | 5.30 | 4.68 to 5.91 | |||||
| Placebo | 107 | 6.92 | 6.65 to 7.20 | 5.43 | 4.97 to 5.89 | −1.50 | < .001 | 5.47 | 4.86 to 6.09 | |||||
| Week 24 | −0.43 | .52 | −0.36 | −1.08 to 0.38 | .34 | |||||||||
| O3-FAs | 94 | 7.06 | 6.75 to 7.38 | 4.83 | 4.28 to 5.38 | −2.23 | < .001 | 4.77 | 4.04 to 5.50 | |||||
| Placebo | 98 | 6.88 | 6.59 to 7.16 | 5.07 | 4.53 to 5.61 | −1.81 | < .001 | 5.13 | 4.40 to 5.86 | |||||
| BPI pain interference | ||||||||||||||
| Week 6 | −0.06 | .82 | −0.05 | −0.57 to 0.47 | .84 | |||||||||
| O3-FAs | 108 | 4.14 | 3.73 to 4.55 | 3.22 | 2.74 to 3.69 | −0.92 | < .001 | 3.22 | 2.84 to 3.59 | |||||
| Placebo | 115 | 4.13 | 3.72 to 4.54 | 3.27 | 2.82 to 3.72 | −0.86 | < .001 | 3.27 | 2.91 to 3.63 | |||||
| Week 12 | 0.10 | .74 | 0.11 | −0.44 to 0.66 | .70 | |||||||||
| O3-FAs | 102 | 4.17 | 3.74 to 4.60 | 3.14 | 2.64 to 3.64 | −1.03 | < .001 | 3.12 | 2.73 to 3.51 | |||||
| Placebo | 107 | 4.12 | 3.70 to 4.55 | 2.99 | 2.55 to 3.44 | −1.13 | < .001 | 3.01 | 2.63 to 3.40 | |||||
| Week 24 | −0.14 | .65 | −0.15 | −0.39 to 0.70 | .58 | |||||||||
| O3-FAs | 94 | 4.05 | 3.62 to 4.48 | 2.57 | 2.10 to 3.05 | −1.48 | < .001 | 2.58 | 2.19 to 3.00 | |||||
| Placebo | 98 | 4.09 | 3.64 to 4.54 | 2.75 | 2.28 to 3.21 | −1.34 | < .001 | 2.74 | 2.35 to 3.13 | |||||
| Global rating of change in joint pain from baselineh | ||||||||||||||
| Week 6 | 0.10 | .56 | 0.10 | −0.24 to 0.44 | .57 | |||||||||
| O3-FAs | 109 | 0.72 | 0.47 to 0.96 | — | < .001 | 0.72 | 0.47 to 0.96 | |||||||
| Placebo | 112 | 0.62 | 0.39 to 0.85 | — | < .001 | 0.62 | 0.38 to 0.86 | |||||||
| Week 12 | 0.27 | .16 | 0.26 | −0.10 to 0.63 | .16 | |||||||||
| O3-FAs | 98 | 0.76 | 0.47 to 1.04 | — | < .001 | 0.75 | 0.49 to 1.02 | |||||||
| Placebo | 104 | 0.49 | 0.25 to 0.73 | — | < .001 | 0.49 | 0.24 to 0.75 | |||||||
| Week 24 | 0.08 | .74 | 0.07 | −0.35 to 0.49 | .74 | |||||||||
| O3-FAs | 90 | 0.77 | 0.46 to 1.08 | — | < .001 | 0.77 | 0.46 to 1.07 | |||||||
| Placebo | 95 | 0.69 | 0.40 to 0.99 | — | < .001 | 0.70 | 0.40 to 0.99 | |||||||
| Global rating of change in joint stiffness from baselineh | ||||||||||||||
| Week 6 | 0.13 | .43 | 0.13 | −0.20 to 0.46 | .44 | |||||||||
| O3-FAs | 109 | 0.59 | 0.35 to 0.82 | — | < .001 | 0.59 | 0.35 to 0.82 | |||||||
| Placebo | 114 | 0.46 | 0.23 to 0.69 | — | < .001 | 0.46 | 0.23 to 0.69 | |||||||
| Week 12 | 0.27 | .12 | 0.28 | −0.07 to 0.62 | .12 | |||||||||
| O3-FAs | 98 | 0.68 | 0.41 to 0.95 | — | < .001 | 0.68 | 0.43 to 0.93 | |||||||
| Placebo | 103 | 0.41 | 0.18 to 0.63 | — | < .001 | 0.41 | 0.16 to 0.65 | |||||||
| Week 24 | 0.00 | 1.00 | −0.003 | −0.41 to 0.41 | .99 | |||||||||
| O3-FAs | 91 | 0.65 | 0.35 to 0.94 | — | < .001 | 0.65 | 0.35 to 0.94 | |||||||
| Placebo | 97 | 0.65 | 0.36 to 0.94 | — | < .001 | 0.65 | 0.36 to 0.94 | |||||||
Abbreviations: BPI, Brief Pain Inventory; O3-FA, omega-3 fatty acid.
From linear regression adjusting for baseline BPI score and stratification factors.
Among patients with week-6, -12, or -24 BPI worst pain score, respectively.
Difference derived from O3-FAs minus placebo.
Between-group P values.
P value for paired t tests for difference between baseline and week-12 measures within treatment arm.
Difference from change between follow-up and baseline score for O3-FAs minus change between baseline and follow-up for placebo (ie, difference in differences).
P value for unpaired t tests for difference in change measures between treatment arms.
Questionnaire evaluates changes in symptoms since last visit, so no baseline scores were collected.
Fig 2.
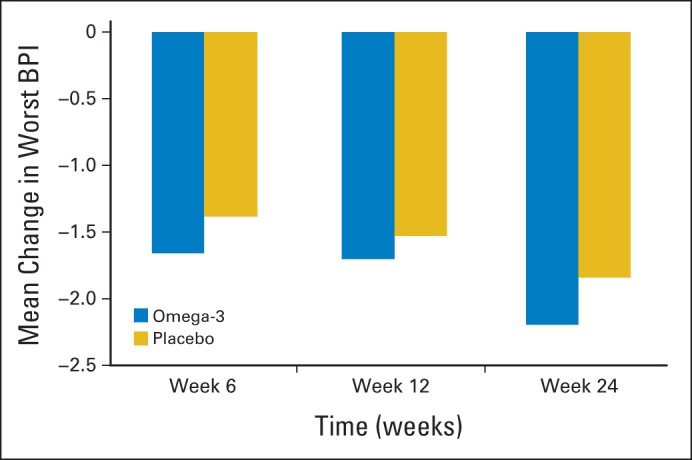
Adjusted change in mean Brief Pain Inventory (BPI) worst pain/stiffness score from baseline based on linear regression adjusting for taxane use, prior history of osteoarthritis, and baseline BPI score. Significant reduction in pain from baseline for both omega-3 fatty acid and placebo groups at 6, 12, and 24 weeks (P < .01). No significant between-group differences.
Secondary End Points
The mean observed change in the M-SACRAH, WOMAC, and FACT-ES measures reflected somewhat reduced symptoms in the O3-FA arm compared with the placebo arm at each of the assessment times using the M-SACRAH and WOMAC measures and somewhat increased symptoms in the O3-FA arm compared with the placebo arm at each assessment time using the FACT-ES measure (Appendix Table A1, online only). There was no evidence that any of these differences were statistically significant in multivariable regression analyses. There were no differences by arm in analgesic use over time.
Serum Analysis
Most of the serum measures remained at the same level over time or changed consistently between treatment arms (Table 3). However, triglycerides decreased in patients receiving O3-FA treatment (difference, −22.1 mg/dL) and stayed the same for those receiving placebo (difference, 0.1 mg/dL). The differences over time by arm were statistically significant (P = .01). At 12 weeks, HDL increased by 2.9 mg/dL in the O3-FA arm (P = .007); however, there was no statistically significant difference over time by arm (P = .25). At baseline, > 70% of women had LDL levels > 100 mg/dL, and approximately 50% had total cholesterol levels > 200 mg/dL (Table 4).
Table 3.
Between- and Within-Group Differences in Cholesterol and CRP in Women Receiving O3-FAs Versus Placebo
| Treatment | Baseline |
Week 12 |
Week 24 |
Difference (week 12 − baseline) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | P* | P† | |
| Cholesterol, mg/dL | ||||||||||||||
| All | 175 | 201.7 | 38.7 | 172 | 201.3 | 40.3 | 146 | 201.3 | 38.0 | 169 | −1.0 | 27.8 | .66 | |
| O3-FAs | 85 | 201.6 | 37.5 | 80 | 200.9 | 39.2 | 74 | 202.9 | 34.9 | 79 | −2.0 | 31.2 | .56 | |
| Placebo | 90 | 201.9 | 40.0 | 92 | 201.7 | 41.5 | 72 | 199.7 | 41.2 | 90 | −0.1 | 24.5 | .97 | |
| CRP, mg/L | ||||||||||||||
| All | 175 | 4.1 | 5.5 | 172 | 5.2 | 8.9 | 146 | 4.1 | 5.5 | 169 | 1.1 | 8.2 | .71 | |
| O3-FAs | 85 | 4.1 | 4.4 | 80 | 5.5 | 9.3 | 74 | 4.0 | 5.5 | 79 | 1.3 | 7.8 | .13 | |
| Placebo | 90 | 4.2 | 6.3 | 92 | 5.0 | 8.6 | 72 | 4.3 | 5.6 | 90 | 0.9 | 8.6 | .34 | |
| HDL, mg/dL | ||||||||||||||
| All | 175 | 55.5 | 16.9 | 172 | 57.4 | 17.8 | 146 | 57.3 | 18.7 | 169 | 1.9 | 8.9 | .25 | |
| O3-FAs | 85 | 56.2 | 16.6 | 80 | 59.1 | 18.5 | 74 | 59.6 | 19.3 | 79 | 2.7 | 8.8 | .007 | |
| Placebo | 90 | 54.8 | 17.2 | 92 | 55.8 | 17.1 | 72 | 55.1 | 18.0 | 90 | 1.1 | 9.0 | .23 | |
| LDL, mg/dL | ||||||||||||||
| All | 172 | 117.0 | 31.7 | 169 | 116.6 | 33.3 | 143 | 116.1 | 30.8 | 165 | −0.7 | 23.4 | .60 | |
| O3-FAs | 85 | 117.7 | 32.9 | 79 | 118.8 | 35.1 | 73 | 118.4 | 30.3 | 78 | 0.3 | 27.5 | .93 | |
| Placebo | 87 | 116.4 | 30.6 | 90 | 114.6 | 31.7 | 70 | 113.7 | 31.3 | 87 | −1.7 | 19.2 | .42 | |
| Triglycerides, mg/dL | ||||||||||||||
| All | 175 | 144.6 | 88.3 | 172 | 134.4 | 84.1 | 146 | 134.0 | 79.1 | 169 | −10.3 | 56.8 | .01 | |
| O3-FAs | 85 | 138.4 | 69.3 | 80 | 115.9 | 61.4 | 74 | 122.5 | 62.7 | 79 | −22.1 | 52.1 | < .001 | |
| Placebo | 90 | 150.5 | 103.2 | 92 | 150.5 | 97.2 | 72 | 145.7 | 92.1 | 90 | 0.0 | 59.0 | 1.00 | |
Abbreviations: CRP, C-reactive protein; O3-FA, omega-3 fatty acid; SD, standard deviation.
P value for paired t tests for difference between baseline and week-12 measures within treatment arm.
P value for unpaired t tests for difference in change measures between treatment arms.
Table 4.
Proportion of Patients With Abnormal* Lipid Profiles at Baseline and 12 and 24 Weeks by Treatment Arm
| Assay | Baseline |
Week 12 |
Week 24 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | P | No. | % | P | No. | % | P | |
| Cholesterol ≥ 200 mg/dL | .72 | .74 | .41 | ||||||
| O3-FAs | 43 | 50.6 | 42 | 52.5 | 41 | 55.4 | |||
| Placebo | 48 | 53.3 | 46 | 50.0 | 35 | 48.6 | |||
| CRP ≥ 5 mg/L | .46 | .35 | .37 | ||||||
| O3-FAs | 24 | 28.2 | 27 | 33.8 | 14 | 18.9 | |||
| Placebo | 21 | 23.3 | 25 | 27.2 | 18 | 25.0 | |||
| HDL ≤ 40 mg/dL | .33 | .48 | .32 | ||||||
| O3-FAs | 9 | 10.6 | 10 | 12.5 | 9 | 12.2 | |||
| Placebo | 14 | 15.6 | 15 | 16.3 | 13 | 18.1 | |||
| LDL ≥ 100 mg/dL | .92 | .49 | .86 | ||||||
| O3-FAs | 61 | 71.8 | 54 | 68.4 | 49 | 67.1 | |||
| Placebo | 63 | 72.4 | 57 | 63.3 | 46 | 65.7 | |||
| Triglycerides ≥ 150 mg/dL | .66 | .11 | .31 | ||||||
| O3-FAs | 32 | 37.6 | 19 | 23.8 | 20 | 27.0 | |||
| Placebo | 31 | 34.4 | 32 | 34.8 | 25 | 34.7 | |||
Abbreviations: CRP, C-reactive protein; O3-FA, omega-3 fatty acid.
As defined by National Institute of Health, National Heart Lung and Blood, guidelines.
Adverse Events
Among the 249 evaluable patients, 10 were not evaluable for adverse events because of either receiving no intervention7 or lack of adverse event reporting.3 Among the 239 patients for whom adverse events were assessed, there was one case each of grade 3 diarrhea, dyspepsia, and pain in the extremity in the O3-FA arm and one case each of grade 3 arthralgia, pain, peripheral motor neuropathy, and rash maculo-papular toxicity in the placebo arm (Appendix Table A2, online only).
Adverse events potentially related to AI joint pain/stiffness included arthralgia, range of motion decrease, and pain. There were 27 cases of grade ≥ 1 of these adverse events among patients receiving O3-FAs (23%) and 33 cases among those receiving placebo (27%; P = .68); there were 13 cases of grade ≥ 2 of these adverse events among those receiving O3-FAs (11%) and 25 cases among those receiving placebo (20%; P = .09). However, baseline symptoms were eligibility criteria, and the change in these adverse events from baseline is not known.
DISCUSSION
In this multicenter placebo-controlled trial, we found a substantial (approximately 50%) and sustained improvement in AI-associated arthralgia with both O3-FAs and placebo, but we found no meaningful difference between the groups. Surprisingly, the absolute reduction in both arms was similar to the effects seen in the intervention arms of other randomized studies.27–30 Women receiving O3-FAs had a significant lowering of triglyceride levels compared with those receiving placebo, which is a known benefit of O3-FAs. This observation reassures us that patients were compliant, and the supplement was active. In addition, we found an increase in serum HDL from baseline in the O3-FA group; however, the difference in HDL at 12 weeks was not different than with placebo.
The improvement in symptoms in both the treatment and placebo groups was unexpected. The magnitude of the expected placebo effect reported in the literature can vary from 6% to 59%31–33 and can be higher in symptom-management studies. We found an effect of > 50%. This larger-than-expected placebo effect may have been a result of the natural history of arthralgia, which can improve over time. It is also possible that the placebo contained ingredients (soy/corn oil) that were active in reducing arthralgia. It is also possible there was some contamination in the placebo arm, although the lower triglyceride levels in the O3-FA arm suggests that any contamination was limited.
With regard to the placebo effect, two randomized trials have been completed reporting no placebo effect. The first is a randomized sham-controlled blinded trial to assess the effect of a 6-week intervention of acupuncture in 38 women with AI-associated joint symptoms. The study reported that the true acupuncture group had a 50% decrease in pain compared with no change in the sham acupuncture group.27 Similarly, a randomized trial of an exercise intervention in 121 women with AI arthralgia reported that pain scores had decreased by 24% at 12 months among women randomly assigned to exercise versus no change among women randomly assigned to usual care.30 Similar to our study, both the acupuncture and exercise intervention trials used BPI score of 3 to 4 as an inclusion criterion, used the BPI as an outcome measure, and showed a decrease in mean pain score in the therapeutic arms similar to that in the O3-FA arm in our trial. The exercise trial was not blinded, and the acupuncture trial used sham acupuncture as the placebo.
Although several studies have been conducted to evaluate interventions to treat AI arthralgia, no clear treatment has emerged. The studies have been limited by small sample size, inconsistent definitions, and lack of objective outcome measures. A randomized trial evaluating vitamin D for the prevention of AI arthralgia reported that 61% of controls and 38% of those receiving vitamin D had an increase in pain after initiation of treatment.34 In addition, duloxetine,35 glucosamine chondroitin,36 testosterone,37 prednisolone,38 and switching therapies39 have all been evaluated in uncontrolled trials. Prospective double-blind studies are currently evaluating duloxetine and transdermal testosterone. Future studies may consider evaluating the effect of soy.
The mechanism of AI-associated arthralgia is unknown; however, preliminary evidence suggests there is an inflammatory component, which was a factor influencing the evaluation of O3-FAs. In a study conducted by Dizdar et al,40 women receiving AIs had increased tendon thickness and higher rates of effusions in hand joints/tendons on musculoskeletal sonography, compared with women who never received AIs.40–42 There may also be an autoimmune component to the syndrome; in mouse models where aromatase was knocked out, the mice manifested symptoms similar to Sjögren's syndrome.43 A better understanding of the mechanism of action may assist in the identification of interventions to control or prevent this common adverse effect that can result in decreased adherence and diminished quality of life.
Anastrozole results in significantly fewer thromboembolic and cerebrovascular events compared with tamoxifen and a similar incidence of ischemic cardiovascular events.2 However, in 2008, the US Food and Drug Administration issued a black-box warning for anastrozole as a result of data from the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial suggesting that women with pre-existing ischemic heart disease were at increased risk of ischemic cardiovascular events with anastrozole compared with tamoxifen (17% v 10%).2,44 In comparison with placebo after tamoxifen in the MA.17 adjuvant trial, patients receiving letrozole experienced significant increases from baseline in total cholesterol, LDL cholesterol, and lipoprotein A.21 In our study, we found a surprisingly high baseline rate of unfavorable lipid levels in those receiving an AI. Although it was encouraging that the group randomly assigned to O3-FAs had significant lowering of triglyceride levels and a suggestion of an increase in HDL levels, further research is necessary to determine optimal monitoring and treatment of hypercholesterolemia in this population of cancer survivors.
In summary, we found no evidence that O3-FAs improved joint pain or stiffness associated with AIs any more than placebo; however, we did find a large reduction in symptoms in both the treatment and placebo groups. Currently, there are no proven therapies for the treatment or prevention of AI-induced arthralgia; therefore, determination of optimal approaches to define, treat, and prevent this adverse effect is necessary. The high prevalence of unfavorable lipid profiles in this cohort suggests that more efforts are needed to manage long-term comorbidities in cancer survivors.
Supplementary Material
Appendix
Table A1.
Mean M-SACRAH, WOMAC, and FACT-ES Scores at 6, 12, and 24 Weeks
| Analysis | No. of Patients | Observed Results |
Adjusted Results* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline† |
Follow-Up |
Difference‡ | Follow-Up |
Difference‡ | 95% CI | P | |||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||||
| M-SACRAH | |||||||||||
| Week 6 | −3.5 | −1.77 | −6.16 to 2.63 | .43 | |||||||
| O3-FAs | 107 | 35.6 | 31.1 to 40.1 | 28.7 | 24.4 to 33.0 | 29.6 | 25.2 to 34.0 | ||||
| Placebo | 115 | 38.3 | 34.4 to 42.3 | 32.2 | 28.3 to 36.2 | 31.4 | 27.0 to 35.8 | ||||
| Week 12 | −0.6 | 0.72 | −4.17 to 5.60 | .77 | |||||||
| O3-FAs | 100 | 34.9 | 30.3 to 39.6 | 28.1 | 23.2 to 33.0 | 28.8 | 23.9 to 33.7 | ||||
| Placebo | 107 | 37.3 | 33.2 to 41.4 | 28.7 | 24.9 to 32.6 | 28.1 | 23.2 to 33.0 | ||||
| Week 24 | −2.3 | −0.99 | −6.32 to 4.34 | .72 | |||||||
| O3-FAs | 92 | 35.2 | 30.4 to 39.9 | 24.2 | 19.3 to 29.1 | 24.8 | 19.5 to 30.2 | ||||
| Placebo | 97 | 37.8 | 33.4 to 42.2 | 26.5 | 22.4 to 30.5 | 25.8 | 20.5 to 31.2 | ||||
| WOMAC | |||||||||||
| Week 6 | −0.4 | −1.19 | −5.74 to 3.37 | .61 | |||||||
| O3-FAs | 108 | 49.1 | 45.2 to 53.0 | 38.2 | 33.7 to 42.8 | 37.8 | 33.2 to 42.3 | ||||
| Placebo | 115 | 47.9 | 43.9 to 51.9 | 38.6 | 34.2 to 42.9 | 39.0 | 34.4 to 43.5 | ||||
| Week 12 | −0.7 | −2.13 | −7.30 to 3.04 | .42 | |||||||
| O3-FAs | 100 | 49.5 | 45.4 to 53.6 | 35.3 | 30.4 to 40.2 | 34.5 | 29.4 to 39.7 | ||||
| Placebo | 107 | 47.4 | 43.3 to 51.6 | 36.0 | 31.6 to 40.4 | 36.7 | 31.5 to 41.8 | ||||
| Week 24 | −1.3 | −2.41 | −8.13 to 3.31 | .41 | |||||||
| O3-FAs | 93 | 49.0 | 44.7 to 53.3 | 31.4 | 26.4 to 36.4 | 30.8 | 25.1 to 36.6 | ||||
| Placebo | 97 | 47.3 | 43.0 to 51.6 | 32.7 | 28.1 to 37.4 | 33.2 | 27.5 to 39.0 | ||||
| FACT-ES | |||||||||||
| Week 6 | −1.1 | 1.37 | −1.47 to 4.21 | .35 | |||||||
| O3-FAs | 108 | 87.4 | 84.2 to 90.5 | 92.5 | 89.2 to 95.8 | 93.8 | 91.0 to 96.7 | ||||
| Placebo | 115 | 90.6 | 87.6 to 93.7 | 93.6 | 90.7 to 96.6 | 92.4 | 89.6 to 95.3 | ||||
| Week 12 | −0.2 | 2.14 | −1.16 to 5.44 | .21 | |||||||
| O3-FAs | 100 | 87.8 | 84.5 to 91.1 | 93.8 | 90.4 to 97.2 | 95.0 | 91.7 to 98.3 | ||||
| Placebo | 107 | 90.9 | 87.8 to 94.0 | 94.0 | 90.7 to 97.2 | 92.8 | 89.5 to 96.1 | ||||
| Week 24 | −0.3 | 1.44 | −2.07 to 4.95 | .42 | |||||||
| O3-FAs | 93 | 88.4 | 84.9 to 91.8 | 96.9 | 93.3 to 100.4 | 97.8 | 94.3 to 101.3 | ||||
| Placebo | 97 | 90.8 | 87.4 to 94.3 | 97.2 | 93.8 to 100.6 | 96.3 | 92.8 to 99.8 | ||||
| Analgesic use§ | |||||||||||
| Week 6 | −0.13 | −0.09 | −0.81 to 0.63 | .80 | |||||||
| O3-FAs | 68 | 5.49 | 4.79 to 6.18 | 4.82 | 4.13 to 5.52 | 4.84 | 4.30 to 5.38 | ||||
| Placebo | 82 | 5.54 | 4.78 to 6.30 | 4.95 | 4.14 to 5.77 | 4.93 | 4.44 to 5.43 | ||||
| Week 12 | −0.42 | −0.53 | −1.39 to 0.33 | .23 | |||||||
| O3-FAs | 64 | 5.70 | 5.00 to 6.40 | 4.84 | 4.08 to 5.60 | 4.79 | 4.16 to 5.41 | ||||
| Placebo | 69 | 5.55 | 4.78 to 6.32 | 5.26 | 4.43 to 6.10 | 5.31 | 4.71 to 5.92 | ||||
| Week 24 | −0.004 | 0.10 | −0.83 to 1.03 | .84 | |||||||
| O3-FAs | 62 | 4.52 | 4.68 to 6.16 | 4.92 | 4.12 to 5.72 | 4.97 | 4.30 to 5.65 | ||||
| Placebo | 65 | 5.65 | 4.84 to 6.45 | 4.92 | 4.21 to 5.63 | 4.87 | 4.21 to 5.53 | ||||
Abbreviations: BPI, Brief Pain Inventory; FACT-ES, Functional Assessment of Cancer Therapy–Endocrine Symptoms; M-SACRAH, Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands; O3-FA, omega-3 fatty acid; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
From linear regression adjusting for baseline BPI score and stratification factors (osteoarthritis, prior taxane exposure).
Among patients with week-6, -12, or -24 worst pain scores, respectively.
Difference derived from O3-FAs minus placebo. (for M-SACRAH and WOMAC).
Frequency of analgesic use, where 1 = ≤ one pill per week, 2 = two to three pills per week, 3 = four to six pills per week, 4 = one pill/ per day, and 5 = ≥ one pill per day. Value is sum of frequencies of use of acetaminophen, ibuprofen, other nonsteroidal anti-inflammatory drugs, narcotics, other pain medication, and aspirin. At each time point, < 5% of participants took narcotics.
Table A2.
Adverse Events
| Adverse Event | O3-FA Arm (n = 115) |
Placebo (n = 124) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Abdominal pain | 114 | 1 | 0 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| ALP increased | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| Allergic reaction | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 0 | 1 | 0 | 0 | 0 |
| Arthralgia | 96 | 7 | 12 | 0 | 0 | 0 | 102 | 5 | 16 | 1 | 0 | 0 |
| Back pain | 114 | 0 | 1 | 0 | 0 | 0 | 122 | 2 | 0 | 0 | 0 | 0 |
| Bloating | 110 | 3 | 2 | 0 | 0 | 0 | 122 | 2 | 0 | 0 | 0 | 0 |
| Blood or lymph disorder—other | 114 | 1 | 0 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| Bone pain | 114 | 1 | 0 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| Breast pain | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| Chest pain—cardiac | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 0 | 1 | 0 | 0 | 0 |
| Constipation | 113 | 2 | 0 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| Cough | 114 | 1 | 0 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| Diarrhea | 100 | 10 | 4 | 1 | 0 | 0 | 118 | 5 | 1 | 0 | 0 | 0 |
| Dizziness | 114 | 1 | 0 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| Dry mouth | 115 | 0 | 0 | 0 | 0 | 0 | 122 | 2 | 0 | 0 | 0 | 0 |
| Dry skin | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 0 | 1 | 0 | 0 | 0 |
| Dysgeusia | 112 | 3 | 0 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| Dyspepsia | 95 | 11 | 8 | 1 | 0 | 0 | 112 | 7 | 5 | 0 | 0 | 0 |
| Dysphagia | 115 | 0 | 0 | 0 | 0 | 0 | 122 | 2 | 0 | 0 | 0 | 0 |
| Epistaxis | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| Esophagitis | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 0 | 1 | 0 | 0 | 0 |
| Fatigue | 113 | 1 | 1 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| Fever | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 0 | 1 | 0 | 0 | 0 |
| Flatulence | 113 | 1 | 1 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| Flu-like symptoms | 114 | 1 | 0 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| GERD | 114 | 1 | 0 | 0 | 0 | 0 | 123 | 0 | 1 | 0 | 0 | 0 |
| GI disorders—other (specify) | 114 | 1 | 0 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| Headache | 114 | 1 | 0 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| Hot flashes | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| Insomnia | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| Mucositis oral | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 0 | 1 | 0 | 0 | 0 |
| Nausea | 106 | 9 | 0 | 0 | 0 | 0 | 110 | 12 | 2 | 0 | 0 | 0 |
| Pain | 115 | 0 | 0 | 0 | 0 | 0 | 122 | 0 | 1 | 1 | 0 | 0 |
| Pain in extremity | 113 | 1 | 0 | 1 | 0 | 0 | 122 | 1 | 1 | 0 | 0 | 0 |
| Paresthesia | 114 | 1 | 0 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| Peripheral motor neuropathy | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 0 | 0 | 1 | 0 | 0 |
| Pruritus | 115 | 0 | 0 | 0 | 0 | 0 | 122 | 0 | 2 | 0 | 0 | 0 |
| Purpura | 114 | 1 | 0 | 0 | 0 | 0 | 124 | 0 | 0 | 0 | 0 | 0 |
| ROM decreased | 109 | 6 | 0 | 0 | 0 | 0 | 117 | 2 | 5 | 0 | 0 | 0 |
| Rash acneiform | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 0 | 1 | 0 | 0 | 0 |
| Rash maculopapular | 114 | 1 | 0 | 0 | 0 | 0 | 123 | 0 | 0 | 1 | 0 | 0 |
| Respiratory, thoracic, or mediastinal disorder | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 0 | 1 | 0 | 0 | 0 |
| Sore throat | 115 | 0 | 0 | 0 | 0 | 0 | 123 | 1 | 0 | 0 | 0 | 0 |
| Weight gain | 112 | 3 | 0 | 0 | 0 | 0 | 119 | 4 | 1 | 0 | 0 | 0 |
| Maximum-grade any adverse event | 57 | 30 | 25 | 3 | 0 | 0 | 74 | 19 | 27 | 4 | 0 | 0 |
NOTE. Investigators were instructed to document only those adverse events related to blinded protocol treatment (O3-FAs or placebo) and joint pain/stiffness resulting from aromatase inhibitor treatment.
Abbreviations: ALP, alkaline phosphatase; GERD, gastroesophageal reflux disease; O3-FA, omega-3 fatty acid; ROM, range of motion.
Footnotes
See accompanying editorial on page 1870; listen to the podcast by Dr Loprinzi at www.jco.org/podcasts
Supported by the Breast Cancer Research Foundation, by the National Cancer Institute (NCI) Division of Cancer Prevention, and by NCI Community Oncology Research Program Research Base Grant No. 1UG1CA189974-01.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01385137.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Dawn L. Hershman, Katherine D. Crew, Heather Greenlee, Lori M. Minasian, Carol M. Moinpour
Financial support: Dawn L. Hershman
Administrative support: Dawn L. Hershman
Collection and assembly of data: Dawn L. Hershman, Danielle Awad, Shaker R. Dakhil, Danika L. Lew
Data analysis and interpretation: Dawn L. Hershman, Joseph M. Unger, Katherine D. Crew, Julie Gralow, Heather Greenlee, Danika L. Lew, Cathee Till, James L. Wade III, Frank L. Meyskens, Carol M. Moinpour
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Multicenter Placebo-Controlled Trial of Omega-3 Fatty Acids for the Control of Aromatase Inhibitor–Induced Musculoskeletal Pain: SWOG S0927
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Dawn L. Hershman
No relationship to disclose
Joseph M. Unger
No relationship to disclose
Katherine D. Crew
No relationship to disclose
Danielle Awad
No relationship to disclose
Shaker R. Dakhil
No relationship to disclose
Julie Gralow
Consulting or Advisory Role: Roche/Genentech, Novartis
Research Funding: Roche/Genetech (Inst), Novartis (Inst), Amgen (Inst)
Heather Greenlee
Consulting or Advisory Role: EHE International
Danika L. Lew
No relationship to disclose
Lori M. Minasian
No relationship to disclose
Cathee Till
No relationship to disclose
James L. Wade III
Employment: Johnson & Johnson (I)
Stock or Other Ownership: Seattle Genetics, Celgene
Frank L. Meyskens
No relationship to disclose
Carol M. Moinpour
No relationship to disclose
REFERENCES
- 1.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 3.Crew KD, Awad D, Brafman L, et al. Prospective evaluation of joint symptoms in postmenopausal women initiating aromatase inhibitors for early stage breast cancer. Presented at the 32nd San Antonio Breast Cancer Symposium; December 10-13, 2009; San Antonio, TX. [Google Scholar]
- 4.Henry NL, Jacobson JA, Banerjee M, et al. A prospective study of aromatase inhibitor-associated musculoskeletal symptoms and abnormalities on serial high-resolution wrist ultrasonography. Cancer. 2010;116:4360–4367. doi: 10.1002/cncr.25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sestak I, Cuzick J, Sapunar F, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: A retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–872. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 7.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leslie CA, Gonnerman WA, Ullman MD, et al. Dietary fish oil modulates macrophage fatty acids and decreases arthritis susceptibility in mice. J Exp Med. 1985;162:1336–1349. doi: 10.1084/jem.162.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleland LG, French JK, Betts WH, et al. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. J Rheumatol. 1988;15:1471–1475. [PubMed] [Google Scholar]
- 10.Sundrarjun T, Komindr S, Archararit N, et al. Effects of n-3 fatty acids on serum interleukin-6, tumour necrosis factor-alpha and soluble tumour necrosis factor receptor p55 in active rheumatoid arthritis. J Int Med Res. 2004;32:443–454. doi: 10.1177/147323000403200501. [DOI] [PubMed] [Google Scholar]
- 11.Kremer JM, Bigauoette J, Michalek AV, et al. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet. 1985;1:184–187. doi: 10.1016/s0140-6736(85)92024-0. [DOI] [PubMed] [Google Scholar]
- 12.Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis: Clinical and immunologic effects. Arthritis Rheum. 1990;33:810–820. doi: 10.1002/art.1780330607. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A randomized, double blind trial. Eur J Clin Invest. 1992;22:687–691. doi: 10.1111/j.1365-2362.1992.tb01431.x. [DOI] [PubMed] [Google Scholar]
- 14.Sköldstam L, Börjesson O, Kjällman A, et al. Effect of six months of fish oil supplementation in stable rheumatoid arthritis: A double-blind, controlled study. Scand J Rheumatol. 1992;21:178–185. doi: 10.3109/03009749209099218. [DOI] [PubMed] [Google Scholar]
- 15.Nordström DC, Honkanen VE, Nasu Y, et al. Alpha-linolenic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled and randomized study—Flaxseed vs. safflower seed. Rheumatol Int. 1995;14:231–234. doi: 10.1007/BF00262088. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129:210–223. doi: 10.1016/j.pain.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Maroon JC, Bost JW. Omega-3 fatty acids (fish oil) as an anti-inflammatory: An alternative to nonsteroidal anti-inflammatory drugs for discogenic pain. Surg Neurol. 2006;65:326–331. doi: 10.1016/j.surneu.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Kastelein JJ, Maki KC, Susekov A, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: The EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8:94–106. doi: 10.1016/j.jacl.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Ascherio A, Rimm EB, Stampfer MJ, et al. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med. 1995;332:977–982. doi: 10.1056/NEJM199504133321501. [DOI] [PubMed] [Google Scholar]
- 20.Jula A, Marniemi J, Huupponen R, et al. Effects of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men: A randomized controlled trial. JAMA. 2002;287:598–605. doi: 10.1001/jama.287.5.598. [DOI] [PubMed] [Google Scholar]
- 21.Wasan KM, Goss PE, Pritchard PH, et al. Lipid concentrations in postmenopausal women on letrozole after 5 years of tamoxifen: An NCIC CTG MA.17 sub-study. Breast Cancer Res Treat. 2012;136:769–776. doi: 10.1007/s10549-012-2294-z. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MP, Turner JA, Romano JM, et al. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 23.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 24.Sautner J, Andel I, Rintelen B, et al. Development of the M-SACRAH, a modified, shortened version of SACRAH (Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands) Rheumatology (Oxford) 2004;43:1409–1413. doi: 10.1093/rheumatology/keh360. [DOI] [PubMed] [Google Scholar]
- 25.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy–Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 26.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: A review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17:163–170. doi: 10.1179/jmt.2009.17.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crew KD, Capodice J, Greenlee H, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early stage breast cancer. J Clin Oncol. 2010;28:1154–1160. doi: 10.1200/JCO.2009.23.4708. [DOI] [PubMed] [Google Scholar]
- 28.Engan T, Krane J, Johannessen DC, et al. Plasma changes in breast cancer patients during endocrine therapy: Lipid measurements and nuclear magnetic resonance (NMR) spectroscopy. Breast Cancer Res Treat. 1995;36:287–297. doi: 10.1007/BF00713400. [DOI] [PubMed] [Google Scholar]
- 29.Friedrichs K, Jänicke F. Aromatase inhibitors: New possibilities in treatment of breast carcinoma [in German] Praxis (Bern 1994) 1998;87:584–588. [PubMed] [Google Scholar]
- 30.Elisaf MS, Bairaktari ET, Nicolaides C, et al. Effect of letrozole on the lipid profile in postmenopausal women with breast cancer. Eur J Cancer. 2001;37:1510–1513. doi: 10.1016/s0959-8049(01)00155-1. [DOI] [PubMed] [Google Scholar]
- 31.Wampold BE, Minami T, Tierney SC, et al. The placebo is powerful: Estimating placebo effects in medicine and psychotherapy from randomized clinical trials. J Clin Psychol. 2005;61:835–854. doi: 10.1002/jclp.20129. [DOI] [PubMed] [Google Scholar]
- 32.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 33.Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;12:CD001395. doi: 10.1002/14651858.CD001395.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 35.Henry NL, Banerjee M, Wicha M, et al. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer. 2011;117:5469–5475. doi: 10.1002/cncr.26230. [DOI] [PubMed] [Google Scholar]
- 36.Nabholtz JM, Gligorov J. Cardiovascular safety profiles of aromatase inhibitors: A comparative review. Drug Saf. 2006;29:785–801. doi: 10.2165/00002018-200629090-00003. [DOI] [PubMed] [Google Scholar]
- 37.Lewis S. Do endocrine treatments for breast cancer have a negative impact on lipid profiles and cardiovascular risk in postmenopausal women? Am Heart J. 2007;153:182–188. doi: 10.1016/j.ahj.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 38.Kubo M, Onishi H, Kuroki S, et al. Short-term and low-dose prednisolone administration reduces aromatase inhibitor-induced arthralgia in patients with breast cancer. Anticancer Res. 2012;32:2331–2336. [PubMed] [Google Scholar]
- 39.Monnier A. Effects of adjuvant aromatase inhibitor therapy on lipid profiles. Expert Rev Anticancer Ther. 2006;6:1653–1662. doi: 10.1586/14737140.6.11.1653. [DOI] [PubMed] [Google Scholar]
- 40.Dizdar O, Ozçakar L, Malas FU, et al. Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitor–related arthralgia. J Clin Oncol. 2009;27:4955–4960. doi: 10.1200/JCO.2008.20.5435. [DOI] [PubMed] [Google Scholar]
- 41.Hozumi Y, Hakamata Y, Sasanuma H, et al. Effects of anastrozole on lipid metabolism compared with tamoxifen in rats. Breast Cancer Res Treat. 2002;76:131–136. doi: 10.1023/a:1020571617274. [DOI] [PubMed] [Google Scholar]
- 42.Sestak I, Sapunar F, Cuzick J. Aromatase inhibitor-induced carpal tunnel syndrome: Results from the ATAC trial. J Clin Oncol. 2009;27:4961–4965. doi: 10.1200/JCO.2009.22.0236. [DOI] [PubMed] [Google Scholar]
- 43.Laroche M, Borg S, Lassoued S, et al. Joint pain with aromatase inhibitors: Abnormal frequency of Sjogren's syndrome. J Rheumatol. 2007;34:2259–2263. [PubMed] [Google Scholar]
- 44.US Food and Drug Administration. Safety: Drug safety labeling changes. http://www.fda.gov/Safety/MedWatch/SafetyInformation/Safety-RelatedDrugLabelingChanges/ucm125179.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.