Abstract
Objective
To describe a surgical technique utilizing a regenerative approach and internal fixation for reconstruction of critical size bone defect non-union mandibular fractures.
Study design
Case series
Animals
Dogs (n = 6) that had internal fixation of defect non-union mandibular fracture.
Methods
In 5 of the 6 cases the repair was staged and extraction of teeth performed during the first procedure. After 21-98 days (mean 27 days) a pharyngotomy intubation and temporary maxillomandibular fixation were performed. Using an extraoral approach, a locking titanium miniplate plate was contoured and secured. A compression resistant matrix (CRM) infused with rhBMP-2, was implanted in the defect. The implant was then covered with a soft tissue envelope followed by routine closure.
Results
All dogs had healed with intact gingival covering over the mandibular fracture site defect and had immediate return to normal function and correct occlusion. Hard-tissue formation was observed clinically within 2 weeks and solid cortical bone formation within 3 months. Computed tomographic findings in one case at 3 months postoperatively demonstrated that the newly regenerated mandibular bone had 92% of the bone density and porosity compared to the contralateral side. Long-term follow-up revealed excellent outcome.
Conclusion
Mandibular reconstruction using internal fixation and CRM infused with rhBMP-2 is an excellent solution for the treatment of critical size defect non-union fractures in dogs.
Clinical Relevance
In dogs with a mandibular critical size defect non-union fractures, reconstruction using rhBMP-2 and a CRM should be considered as a viable surgical option.
Introduction
Individual mandibular fractures occasionally fail to heal and a non-union occurs. When this complication occurs the opposed ends of the fracture have failed to unite and to ossify.1 The amount of healing that occurs varies from fibrous connective tissue, cartilaginous bridge that does not mineralize, or absolute lack of bridging.1 According to the Weber-Cěch classification, a defect non-union occurs when a small or major section of the bone is lost during a trauma, due to sequestration or following surgery.1,2 The resulting gap between the remaining viable bone ends is too great to be bridged without surgical intervention.1,2 Common predisposing causes to non-union include comminution, infection, ischemia, hyperemia, excessive manipulation and hardware placement, periosteal stripping too early or excessive mobility and imperfect reduction.1 Radiologically, features such as absence of callus, evidence of a fracture gap, sclerosis of the fractures end, and displacement are typically present.
A common result of defect non-union fractures is malocclusion due to mandibular drift.3-6 Malocclusion may result in difficulty in eating and drinking, prehension and pain of the contralateral temporomandibular joint (TMJ).3-5,7 Therefore, the primary objective for repair of mandibular fractures, including non-union, is a quick return to normal function and restoration of normal occlusion.8 However, while mandibular reconstruction represents the ideal solution the aspects of this technique including choice of graft material to bridge the defect and matching anatomic geometry make this approach challenging.9,10 Autologous bone grafts and bone graft substitutes are examples of the techniques available to address the problem.6,9,11,12 However, these are still far from ideal due to donor site morbidity, especially in small dogs.9,13,14 Bone morphogenetic proteins (BMPs) are multifunctional growth factors within the transforming growth factor β (TGF-β) super family that were identified by Urist over 40 years ago based on their ability to initiate ectopic bone formation.15-18 Later, Reddi proposed that BMPs are responsible for the initiation of a cascade of developmental events, in which progenitor cells are induced to differentiate into bone cells thus resulting in new bone formation.19,20 This unique feature of BMP has allowed for their successful use as therapeutic agents for bone repair.18 Indeed, much work has been done with the clinical use of recombinant human BMPs (rhBMPs) in the field of spinal fusion, fracture healing, and engineering of dental tissues.21,22 Currently, rhBMP-2 and rhBMP-7 delivered via absorption onto collagen matrices are FDA approved for spinal fusion.21,23-25
Specifically, we report our experience gained from applying a collagen and calcium ceramic matrix impregnated with rhBMP-2 to effect bone regeneration in 6 dogs undergoing reconstruction for the treatment of mandibular defect non-union fractures.
Materials and Methods
Case recruitment and treatment planning
Cases with defect non-union fractures were evaluated for the degree of malocclusion and functionality. In all 6 cases a significant degree of malocclusion was present. In all cases the date of the initial fracture was unknown, as all animals were adopted from a shelter without further history. In 2 cases the duration of the non-union was known to be approximately 2 and 5 years, respectively. Full-mouth dental radiographs were obtained in all cases and computed tomography with tridimensional reconstruction was performed in 5 cases (Figures 1-2).
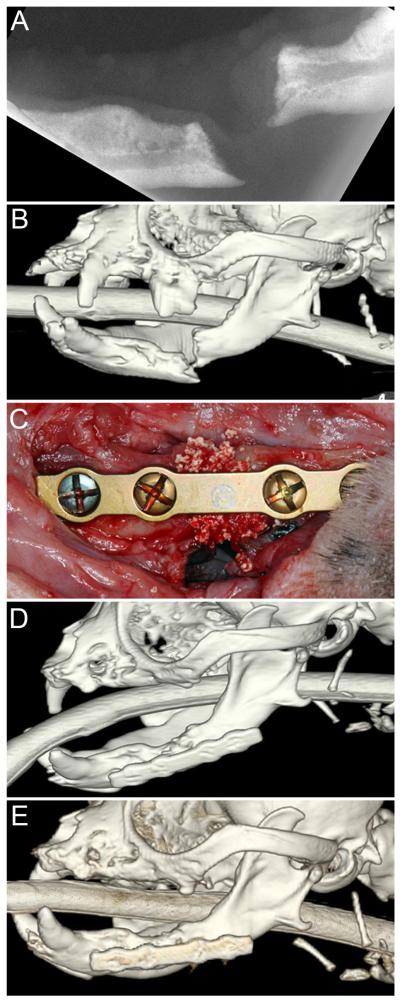
Figure 1.
Defect non-union mandibular fracture of 2-year duration in a 4.3 kg, 7.3-year-old dog: (A) dental radiograph of the affected mandible; (B) tridimensional CT reconstruction; (C) 2-mm locking plate fixation with rhBMP-2 in a CRM; (D) 3-month and (E) 14-month follow-up tridimensional CT reconstructions showing solid and stable new-bone formation.
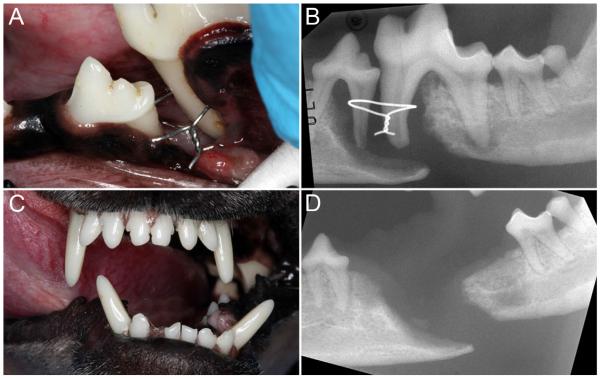
Figure 2.
Defect non-union mandibular fracture with teeth (and an orthopedic wire) in the fracture line, of unknown duration in a 6.0 kg, 3.0-year-old dog: (A) clinical image showing the defect, exposed roots and orthopedic wire; (B) dental radiograph of the affected mandible; (C) malocclusion visible at the 3-week re-check following dental extractions and debridement; (D) dental radiograph at the 3-week re-check showing the critically-sized defect.
Staging, extractions and debridement
In 5 cases the repair was staged. During the first procedure periodontal treatment was performed and in 4 cases the teeth (2-3, mean 2.5) in or adjacent to the non-union fracture site were extracted. The teeth involved included premolar tooth P2 (n=1), P3 (n=1), P4 (n=4), and molar tooth M1 (n=3), M2 (n=1). In one case there were no teeth related to the non-union but extensive periodontal treatment and multiple extractions elsewhere were performed. One dog was edentulous and the procedure was completed in one session. The non-union site was debrided in all cases, which included removal of an intraosseous wire in one case (Figure 2).
Scaffold and rhBMP-2 preparation
The CRM (collagen sponge with embedded granules of hydroxyapatite (HA) and tricalcium phosphate (TCP) (MasterGraft Matrix® Medtronic, Memphis, TN) and rhBMP-2 (Pfizer, Cambridge, MA) were used in this study. The volume of the defect was measured in 3 dimensions and a sufficient amount of collagen sponge (i.e., to provide a half to three-quarters of the mandibular height and a length 2 mm greater than defect span) was measured. Fifteen minutes prior to implantation the sponge was infiltrated with a 0.5 mg/mL solution of rhBMP-2 at a volume corresponding to 50% of the volume of the prepared scaffold. For example, for a scaffold that was 2 cm in length, 0.5 cm mandibular width and 1 cm mandibular height (2 × 0.5 × 1), the total defect volume is 1 cm3; thus, 0.5 mL of the rhBMP-2 solution was used.
Surgical technique
In all cases, ampicillin (20 mg/kg intravenously [IV]) was administered at the time of induction. The dogs were intubated via pharyngotomy as previously described.26 The mandibles and maxillas were brought into the desired closed-mouth occlusion and the mandibular and maxillary canines wired together using 28-gauge wire (Figure 3).
Figure 3.
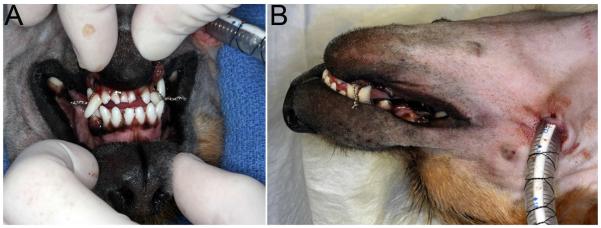
(A) Rostral and lateral images of the same patient depicted in Figure 2, showing the occlusion maintained with maxillomandibular fixation and pharyngotomy intubation. A small oral fenestration is visible, which was subsequently sutured intra-orally.
Using an extraoral approach, a single locking titanium 2-mm 4 or 6-hole miniplate (Synthes® Maxillofacial, Paoli, PA) was adapted to the desired anatomical contour of the mandible in a ventrolateral position while avoiding tooth root damage. No tooth roots were damaged by the screws. The fracture edges were debrided to remove sclerotic bone and attached soft tissues using a surgical hand-piece designed for major oral surgery (INTRAsurge 300, KaVo America Corp., Lake Zurich, IL) combined with an ostectomy bur (Lindemann bur, Hu-Friedy, Chicago, IL) or with rongeurs. The plate was then secured to the bone with 2-3 locking titanium screws in each segment of the fracture. The surgical area was copiously irrigated with sterile saline. Measurements of the defect were used to guide the preparation of the appropriate size scaffold, which was soaked in a solution of rhBMP-2 as described previously. The soaked scaffold was then implanted in the defect and the surrounding soft tissues were sutured around the plate and sponge to provide a soft tissue envelope. Care was taken to avoid fenestration into the oral cavity but when it occurred the oral mucosa was sutured. The subcutaneous tissues and skin were closed routinely. The dogs were kept on soft food for two weeks after surgery and were administered amoxicillin/clavulanic acid 20 mg/kg orally (Clavamox, Pfizer Animal Health, NY) twice daily for 1-2 weeks postoperatively. Postoperative pain management typically consisted of a combination of a non-steroidal anti-inflammatory drug and an opioid analgesic.
Diagnostic imaging
Radiographs of the mandible were obtained immediately postoperatively and at 2-4 weeks, 8 weeks, and 12 weeks after surgery. Radiographs were obtained at later time points if indicated. Transverse, 0.625-mm, collimated x-ray computed tomography (CT) images of the skull were acquired in one patient at 3 and 14 months after surgery using a LightSpeed 16 (GE Healthcare, Milwaukee, WI) CT scanner with kVp = 120 and auto-mA. All images were reconstructed using a bone filter. A CT calibration phantom containing 5 reference rods of known density (Mindworks Software, Inc.; San Francisco, CA) was included in the field of view during CT image acquisition. All radiographs and CT images were evaluated qualitatively by a board-certified veterinary radiologist (DDC) using DICOM image viewing software (OsiriX v. 5.0.2, Geneva, Switzerland). CT images were also evaluated quantitatively using data analysis software (MATLAB R2011a; Mathworks®, Natick, MA). For quantitative measurements, 4 transverse CT images were selected at regular intervals along the length of the mandibular repair. The porosity and radiographic mineral density were measured for the native mandible and mandibular repair tissue using freeform regions of interest that included the entire cross section of the mandible, but excluded the tooth roots or metal surgical implants. The 4 measurements were averaged to reduce error associated with measurement and image-to-image variability.
Results
All dogs had good physical condition and results of hematological, serum biochemical analysis and urinalysis were generally considered normal. In 6 small breed dogs, aged 2 – 11 years (mean 7.3 years), and weight 3.4 kg – 6 kg (mean 4.8 kg) a single 2-mm locking miniplate (Synthes® Maxillofacial, Paoli, PA) was used to stabilize defect non-union fractures of 5 - 18 mm (mean 9.2 mm). The follow-up period was between 6 – 21 months (mean 12 months).
Clinical evaluation
All dogs had appropriate occlusion immediately postoperatively and throughout the duration of the follow-up period. Besides restriction of heavy chewing (e.g., no raw hide chewing or rough games) for 3 months, all dogs returned to normal activity following surgery. At 2 weeks postoperatively, hard tissue spanning the entire defect site was palpable and covered by intact gingiva. There was no noticeable oozing from the intraoral and extraoral incision sites during the reported follow-up periods. At 4 weeks postoperatively, the defect felt completely solid and no abnormalities were noticed. During the rest of the follow-up period there was no recurrence of the fractures and functional occlusion.
Radiological evaluation
Immediate postoperative radiographs were available for all dogs and follow-up radiographs available for 5/6 dogs. Representative radiographs are presented in Figures 1-2 and 4. One dog exhibited a slight decrease in opacity of the implant material 2 weeks postoperatively. All dogs with recheck radiographs demonstrated increased opacity of the repair site with smoothly margined new bone formation bridging between the implant material and native mandible on radiographs obtained 4 weeks after surgery or later (Figure 4). The margins of the repair site became increasingly smooth and the transition between implant material and native mandible became progressively indistinct over time. For one dog with radiographs obtained 7 months after surgery, focal irregularity persisted at the dorsal margin of the mandibular repair site, but the implant material otherwise appeared remodeled and well integrated with native mandible.
Figure 4.
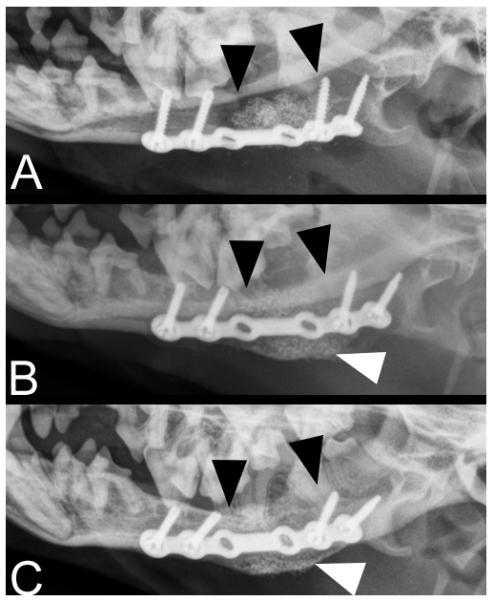
Radiographs of a defect non-union mandibular fracture, of unknown duration in a 5.2 kg, 2.0-year-old dog: (A) immediately postoperatively; (B) 4 weeks; and (C) 8 weeks after surgery. The black arrowheads indicate the approximate borders of the implanted rhBMP-2 CRM scaffold. Smoothly margined new bone formation is evident at the ventral aspect of the mandibular repair site (white arrowheads) and the gap between the implant material and native mandible is indistinct at 4 and 8 weeks after surgery.
On postoperative CT images obtained for one of the cases, there was radiologic evidence of new bone formation and integration of the implant material with the native mandible at 3 and 14 months after surgery (Figure 5). The porosity of the mandibular repair site was similar to native mandible at 3 and 14 months after surgery (26.8% and 27.4% porosity at 14 months for native and repair sites, respectively). The radiographic mineral density of the mandibular repair site measured 0.90 times that of the native mandible at 3 months and measured 0.96 times the density of the native mandible at 14 months.
Figure 5.
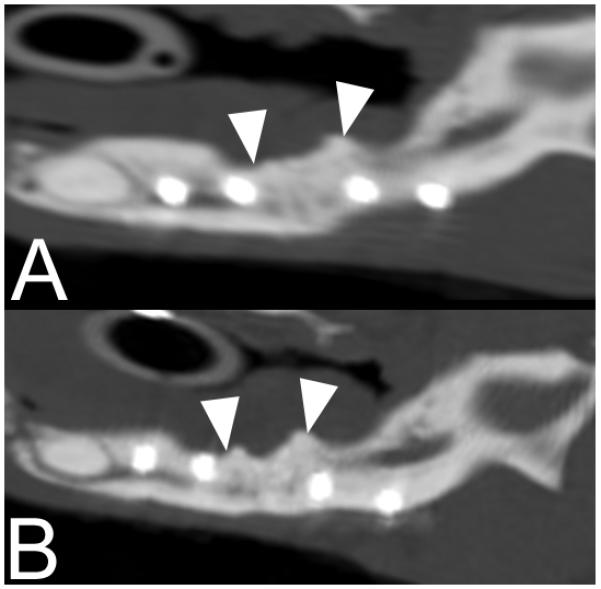
Sagittal reconstructed CT images of the left mandible of the patient depicted in Figure 1 at 3 months (A) and 14 months (B) after surgery. White arrowheads indicate the approximate borders of the original implant material. No gap is visible at the repair site and the implant material appears to be integrated with native mandible.
Discussion
To our knowledge, this is the first report of case series on the use of rhBMP-2 delivered via adsorption into a CRM for regenerating bone across chronic defect non-union fractures in dogs. Moreover, we report our surgical, clinical, and radiological experience with the use of rhBMP-2 in reconstruction of chronic defect non-union mandibular fractures. Importantly, this report emphasizes the benefits of utilizing rhBMP-2 delivered in CRM for their profound and predictable regenerative capacity in mandibular reconstruction in dogs.
Overall, using this combined surgical and regenerative strategy in small dogs was able to rapidly return chronically afflicted dogs to normal occlusion and function. This was because the surgical approach allowed proper restoration of normal anatomy and occlusion, and bone regeneration restored proper biomechanics. Palpable bone quickly formed using the CRM infused with the appropriate dosage of rhBMP-2. By 3 and 14 months this tissue radiographically approximated the density of and appeared integrated to native bone. Histologically, previous reports confirmed CRM infused with rhBMP-2 to result in well-mineralized trabecular bone reflective of healthy bone turn-over and remodeling.5,27,28 Importantly, this report agrees with several human case reports demonstrating that successful reconstruction of critical size mandibular defects could be achieved without the use of an autograft or other form of bone grafts.9,10,29 9,10,29 In experimental animal studies that used the same regenerative system (i.e., CRM and rhBMP-2), successful spinal fusion and mandibular reconstruction in non-human primates, dogs, and rabbits due to robust formation of bone approximating native tissue was observed.25,27,30 However, this report is unique in that we have successfully treated chronic mandibular defects that are pathophysiologically different from fresh defects.31
The therapeutic outcome following the use of rhBMP-2 critically depends on the delivery vehicle, quantity, concentration and time of application.32,33 The use of rhBMP-2 without a carrier is contraindicated and the selection of the matrix used for delivery must be carefully considered.34 In this study and others, the CRM proved to be appropriate for the delivery and release of rhBMP-2 at the defect site.5,9,25,29 With regards to the concentration, a study that evaluated the application of rhBMP-2 in a rat critical bone defect model has found that the degree of bone formation is dose dependent.25,28,30,35 However, increasing the dose of rhBMP-2 beyond a certain threshold concentration does not improve bone quality, and may promote lower quality bone and invoke a detrimental inflammatory response.28 In this study we used a uniform dose of 0.5 mg/ml with a 50% soak volume and bone approximating native geometry and density formed within the critical size defect and was well integrated to adjacent native tissue. However, in cases where a higher dosage of rhBMP-2 was applied in dogs there was initial excessive bone formation but this resolved within several months.7,27,36 Although we did not evaluate a series of concentrations, we conclude that the dose generally used in this study is clinically appropriate. Not only is the dose of rhBMP-2 critical to obtain bone formation, there must be appropriate cells and these cells must have the ability to respond to the cytokine. Thus, the success of rhBMP-2 application in our approach was due to the presence of appropriate stem cells in the local environment and their ability to differentiate into bone forming cells.9 Although, it is accepted that with increasing age the amount of stem cells available decrease,27,37 the osteogenic capabilities of rhBMP-2 are not negatively affected by increasing age.27 In agreement with this, we observed excellent clinical outcome suggesting that the presence and osteogenic ability of the resident stem cells in older age dogs is sufficient.
The procedure was staged to resolve infection related to periodontitis, remove teeth in the fracture line or affected by severe periodontitis, and remove previous failed implants and granulation tissue in the defect non-union. The rationale was to provide a more favorable environment for a subsequent regenerative procedure. Bacterial culture was not performed as the tissues appeared healthy at the time of the second surgery.
To avoid subsequent surgery we recommend that a single 2-mm titanium locking miniplate, placed in the mid-part of the mandibular body be used. This approach avoids iatrogenic damage to the teeth roots, is sufficient to buttress the defect, does not result in plate failure, and avoids plate exposure through the mucosa.
In conclusion, despite the regenerative capacity of skeletal tissue, the biological process sometimes fail and fractures may heal in non-union.31 Using rhBMP-2 with CRM has been demonstrated to be crucial in the initiation of bone healing cascade and formation of new bone.15,16,29,31,38 The combined surgical and regenerative methodology reported here achieved predictable, timely reconstruction of defect non-union fractures in small breed, older dogs. The use of rhBMP-2 should not be taken lightly as this is a very potent molecule that has wide-ranging functions and versatility and is dose dependent.28,39 Finally, incorporating regenerative technology into the surgical arena of managing defect non-union fractures is providing exciting possibilities that eliminate or minimize the morbidity associated with bone grafting and allow for a quick return to normal function.
Acknowledgment
The authors thank Pfizer® for the generous donation of rhBMP-2 and scaffold for companionate animal use. The authors also thank Mr. John Doval for the medical illustrations and to Dr. Tanya Garcia-Nolen for MATLAB code and guidance. Finally, the authors wish to thank Professor Hari Reddi for reviewing the manuscript.
The work was performed at the Department of Surgical and Radiological Sciences, School of Veterinary Medicine, and the Department of Biomedical Engineering, College of Engineering, University of California - Davis.
Financial support: Some of the materials were kindly donated by Pfizer and Medtronic for compassionate animal use.
Footnotes
The authors do not have conflict of interest with any of the materials or companies described in the manuscript.
REFERENCES
- 1.Sumner-Smith G. Non-union of fractures. In: Sumner-Smith G, Fackelman GE, editors. Bone in Clinical Orthopedics. ed 2 Stuttgart; 2002. pp. 349–358. [Google Scholar]
- 2.Marretta SM. Maxillofacial fracture complications. In: Verstraete FJ, Lommer MJ, editors. Oral and Maxillofacial Surgery in Dogs and Cats. ed 1 Saunders; Edinburgh: 2012. pp. 333–342. [Google Scholar]
- 3.Arzi B, Verstraete FJ. Mandibular rim excision in seven dogs. Vet Surg. 2010;39:226–231. doi: 10.1111/j.1532-950X.2009.00630.x. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Am Y, Verstraete FJ. Elastic training for the prevention of mandibular drift following mandibulectomy in dogs: 18 cases (2005-2008) Vet Surg. 2010;39:574–580. doi: 10.1111/j.1532-950X.2010.00703.x. [DOI] [PubMed] [Google Scholar]
- 5.Spector DI, Keating JH, Boudrieau RJ. Immediate mandibular reconstruction of a 5 cm defect using rhBMP-2 after partial mandibulectomy in a dog. Vet Surg. 2007;36:752–759. doi: 10.1111/j.1532-950X.2007.00332.x. [DOI] [PubMed] [Google Scholar]
- 6.Lantz GC. Mandibulectomy techniqes. In: Verstraete FJ, Lommer MJ, editors. Oral and Maxillofacial Surgery in Dogs and Cats. ed 1 Elsevier; Edinburgh: 2012. pp. 467–480. [Google Scholar]
- 7.Boudrieau RJ, Mitchell SL, Seeherman H. Mandibular reconstruction of a partial hemimandibulectomy in a dog with severe malocclusion. Vet Surg. 2004;33:119–130. doi: 10.1111/j.1532-950X.2004.04019.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith MM, Legendre LFJ. Maxillofacial fracture repair using noninvasive techniques. In: Verstraete FJ, Lommer MJ, editors. Oral and Maxillofacial Surgery in Dogs and Cats. ed 1 Saunders; Edinburgh: 2012. pp. 275–284. [Google Scholar]
- 9.Carter TG, Brar PS, Tolas A, et al. Off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) for reconstruction of mandibular bone defects in humans. J Oral Maxillofac Surg. 2008;66:1417–1425. doi: 10.1016/j.joms.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Moghadam HG, Urist MR, Sandor GK, et al. Successful mandibular reconstruction using a BMP bioimplant. J Craniofac Surg. 2001;12:119–127. doi: 10.1097/00001665-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Burchardt H, Enneking WF. Transplantation of bone. Surg Clin North Am. 1978;58:403–427. doi: 10.1016/s0039-6109(16)41492-1. [DOI] [PubMed] [Google Scholar]
- 12.Hollinger JO, Brekke J, Gruskin E, et al. Role of bone substitutes. Clin Orthop Relat Res. 1996:55–65. doi: 10.1097/00003086-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Heary RF, Schlenk RP, Sacchieri TA, et al. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery. 2002;50:510–516. doi: 10.1097/00006123-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Marx RE, Morales MJ. Morbidity from bone harvest in major jaw reconstruction: a randomized trial comparing the lateral anterior and posterior approaches to the ilium. J Oral Maxillofac Surg. 1988;46:196–203. doi: 10.1016/0278-2391(88)90083-3. [DOI] [PubMed] [Google Scholar]
- 15.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 16.Urist MR, Strates BS. The classic: Bone morphogenetic protein. Clin Orthop Relat Res. 2009;467:3051–3062. doi: 10.1007/s11999-009-1068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alaoui-Ismaili MH, Falb D. Design of second generation therapeutic recombinant bone morphogenetic proteins. Cytokine Growth Factor Rev. 2009;20:501–507. doi: 10.1016/j.cytogfr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009;20:475–480. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Reddi AH, Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972;69:1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddi AH. Cell biology and biochemistry of endochondral bone development. Coll Relat Res. 1981;1:209–226. doi: 10.1016/s0174-173x(81)80021-0. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 22.Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005;16:329–345. doi: 10.1016/j.cytogfr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 24.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 25.Herford AS, Lu M, Buxton AN, et al. Recombinant human bone morphogenetic protein 2 combined with an osteoconductive bulking agent for mandibular continuity defects in nonhuman primates. J Oral Maxillofac Surg. 2012;70:703–716. doi: 10.1016/j.joms.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 26.Lantz GC. Verstraete FJ, Lommer MJ, editors. Pharyngotomy and pharyngostomy. Oral and Maxillofacial Surgery in Dogs and Cats. (ed 1) 2012:543–546. [Google Scholar]
- 27.Boyne PJ, Salina S, Nakamura A, et al. Bone regeneration using rhBMP-2 induction in hemimandibulectomy type defects of elderly sub-human primates. Cell Tissue Bank. 2006;7:1–10. doi: 10.1007/s10561-005-2242-9. [DOI] [PubMed] [Google Scholar]
- 28.Zara JN, Siu RK, Zhang X, et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011;17:1389–1399. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herford AS, Boyne PJ. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2) J Oral Maxillofac Surg. 2008;66:616–624. doi: 10.1016/j.joms.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 30.He D, Genecov DG, Herbert M, et al. Effect of recombinant human bone morphogenetic protein-2 on bone regeneration in large defects of the growing canine skull after dura mater replacement with a dura mater substitute. J Neurosurg. 2010;112:319–328. doi: 10.3171/2009.1.JNS08976. [DOI] [PubMed] [Google Scholar]
- 31.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King GN, Cochran DL. Factors that modulate the effects of bone morphogenetic protein-induced periodontal regeneration: a critical review. J Periodontol. 2002;73:925–936. doi: 10.1902/jop.2002.73.8.925. [DOI] [PubMed] [Google Scholar]
- 33.Pang EK, Im SU, Kim CS, et al. Effect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect model. J Periodontol. 2004;75:1364–1370. doi: 10.1902/jop.2004.75.10.1364. [DOI] [PubMed] [Google Scholar]
- 34.Medtronic. 2.1 Physician labeling (3/12/2010) Infuse bone graft for certain oral maxillofacial and dental degenerative uses. 2010 Important medical information. http://www.medtronic.com/patients/lumbar-degenerative-disc-disease/important-safety-information/index.htm.
- 35.Kraiwattanapong C, Boden SD, Louis-Ugbo J, et al. Comparison of Healos/bone marrow to INFUSE(rhBMP-2/ACS) with a collagen-ceramic sponge bulking agent as graft substitutes for lumbar spine fusion. Spine (Phila Pa 1976 ) 2005;30:1001–1007. doi: 10.1097/01.brs.0000160997.91502.3b. [DOI] [PubMed] [Google Scholar]
- 36.Lewis JR, Boudrieau RJ, Reiter AM, et al. Mandibular reconstruction after gunshot trauma in a dog by use of recombinant human bone morphogenetic protein-2. J Am Vet Med Assoc. 2008;233:1598–1604. doi: 10.2460/javma.233.10.1598. [DOI] [PubMed] [Google Scholar]
- 37.Katagiri T, Yamaguchi A, Komaki M, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990;1:60–68. doi: 10.1097/00001665-199001000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Reddi AH, Reddi A. Bone morphogenetic proteins (BMPs): from morphogens to metabologens. Cytokine Growth Factor Rev. 2009;20:341–342. doi: 10.1016/j.cytogfr.2009.10.015. [DOI] [PubMed] [Google Scholar]