Abstract
Past experiments and current paradigms of cholesterol homeostasis suggest that cholesterol 7α-hydroxylase plays a crucial role in sterol metabolism by controlling the conversion of cholesterol into bile acids. Consistent with this conclusion, we show in the accompanying paper that mice deficient in cholesterol 7α-hydroxylase (Cyp7−/− mice) exhibit a complex phenotype consisting of abnormal lipid excretion, skin pathologies, and behavioral irregularities (Ishibashi, S., Schwarz, M., Frykman, P. K., Herz, J., and Russell, D. W. (1996) J. Biol. Chem. 261, 18017–18023). Aspects of lipid metabolism in the Cyp7−/− mice are characterized here to deduce the physiological basis of this phenotype. Serum lipid, cholesterol, and lipoprotein contents are indistinguishable between wild-type and Cyp7−/− mice. Vitamin D3 and E levels are low to undetectable in knockout animals. Stool fat content is significantly elevated in newborn Cyp7−/− mice and gradually declines to wild-type levels at 28 days of age. Several species of 7α-hydroxylated bile acids are detected in the bile and stool of adult Cyp7−/− animals. A hepatic oxysterol 7α-hydroxylase enzyme activity that may account for the 7α-hydroxylated bile acids is induced between days 21 and 30 in both wild-type and deficient mice. An anomalous oily coat in the Cyp7−/− animals is due to the presence of excess monoglyceride esters in the fur. These data show that 7α-hydroxylase and the pathway of bile acid synthesis initiated by this enzyme are essential for proper absorption of dietary lipids and fat-soluble vitamins in newborn mice, but not for the maintenance of serum cholesterol and lipid levels. In older animals, an alternate pathway of bile acid synthesis involving an inducible oxysterol 7α-hydroxylase plays a crucial role in lipid and bile acid metabolism.
Two pathways of bile acid biosynthesis have been described in the mammalian liver. One pathway is initiated in the endoplasmic reticulum of the hepatocyte by the enzyme cholesterol 7α-hydroxylase (referred to hereafter as 7α-hydroxylase; cholesterol 7α-monooxygenase (EC 1.14.13.17)), which converts cholesterol (cholest-5-en-3β-ol) into 7α-hydroxycholesterol (cholest-5-ene-3β,7α-diol). Subsequent enzymatic steps lead to the formation of the primary bile acids cholic acid and chenodeoxycholic acid (reviewed in Ref. 1). A second pathway is initiated in the mitochondria by the enzyme sterol 27-hydroxylase, which converts cholesterol into 27-hydroxycholesterol (cholest-5-ene-3β,27-diol) (2). This intermediate is acted on by an oxysterol 7α-hydroxylase to form 7α,27-dihydroxycholesterol (cholest-5-ene-3β,7α,27-triol) (3–7), which is subsequently converted into primary bile acids.
The pathway initiated by 7α-hydroxylase is thought to be the major route by which bile acids are synthesized in the liver. This assumption arises in part because the 7α-hydroxylase pathway was discovered first (8) and because the level of 7α-hydroxylase enzyme activity is tightly controlled by feedback regulation (1). The importance of this pathway is underscored by the finding that expression of an exogenous 7α-hydroxylase gene in hamsters via infection with a recombinant adenovirus leads to a marked increase in bile acid formation (9).
To gain further insight into the role of 7α-hydroxylase in bile acid metabolism, a line of mice deficient in this enzyme was created by gene targeting methods (10). Young 7α-hydroxylase-deficient mice (Cyp7−/−) exhibit a complex phenotype consisting of an increased rate of postnatal death, fat malabsorption, wasting, skin abnormalities, and vision problems (10). The absence of bile acids in newborn animals combined with fat-soluble vitamin deficiency is considered the most likely explanation for this phenotype. A peculiar feature of murine 7α-hydroxylase deficiency is that the phenotype is only present in newborn mice: once a deficient animal reaches the age of ~3 weeks, symptoms wane to the point that adult animals are indistinguishable from wild-type mice (10).
We now explain this phenotype by showing that the mitochondrial bile acid pathway is not present at birth, but appears at day 21 in both wild-type and Cyp7−/− mice, thereby obviating the requirement for 7α-hydroxylase. Induction correlates with the appearance of oxysterol 7α-hydroxylase activity in the liver and results in the formation of bile with an altered composition of bile acids relative to animals in which both pathways are functioning. By studying the levels of vitamins D3 and E, we have formulated the hypothesis that the bile acid products of the mitochondrial pathway are not as efficient as those of the endoplasmic reticulum pathway in mediating vitamin E absorption. Thus, the requirement for two pathways may reflect a need to synthesize bile acids of diverse chemical structures in order to ensure maximum solubilization of different dietary fats and vitamins.
EXPERIMENTAL PROCEDURES
Animal Methods
Bile was drawn from the gallbladders of mice euthanized with sodium pentobarbital. Samples were stored frozen at −20 °C until analyzed. Blood for lipoprotein, cholesterol, and triglyceride analysis was drawn by cardiac puncture or exsanguination via the ascending carotid artery and clotted at room temperature for 30 min. The sample was centrifuged at 16,000 × g for 50 min, and the serum was decanted from the pelleted blood cells. The levels of cholesterol, triglyceride, and lipoprotein were determined as described previously (11).
Vitamin Analysis
Vitamin E levels were measured in epididymal or ovarian fat samples using high pressure liquid chromatography methods as described previously (12). Vitamin D metabolites were assayed as described previously (13, 14), except that bovine mammary gland vitamin D receptor was used in place of calf thymus gland vitamin D receptor. In brief, serum samples were supplemented with trace quantities of radioactive 25-hydroxyvitamin D3 to quantify the yield of this metabolite during the ensuing purification. Following extraction of vitamin D metabolites from serum with acetonitrile and back-extraction with phosphate buffer, the vitamin D metabolites were purified and separated via chromatography on C18 and silica Sep-Pak cartridges (Waters). After removing an aliquot of the purified fractions for determination of yield, assay of 25-hydroxyvitamin D3 was performed via competitive protein binding using a 1:5000 dilution of human serum (vitamin D-binding protein) as the binding agent. The sensitivity of this assay is 0.2 ng or a serum value of 1 ng/ml assuming 80% yield and a single determination. The intra- and interassay coefficients of variation are 5 and 8%, respectively. Because this purification scheme does not separate the D2 (ergocalciferol) and D3 (cholecalciferol) forms of the metabolites, the concentration measured in serum represents total vitamin D.
Bile Acid Analysis
The extraction, separation, derivatization, and analyses of bile acids in bile using gas chromatography-mass spectrometry and liquid secondary ionization mass spectrometry were carried out as described previously (15).
Enzyme Assay
Oxysterol 7α-hydroxylase activity was determined in 0.5-ml incubation mixtures containing 0.06 nmol of 25-[26,27-3H2]hydroxycholesterol (77 Ci/mmol), 250μg of microsomal protein, 0.75 μmol of NADPH, 50 mM Tris acetate, pH 7.4, 1 mM EDTA, 2 mM dithiothreitol, and 0.03% (v/v) Triton X-100. After incubation for 15 min at 37 °C, the reactions were terminated by the addition of 6 ml of methylene chloride. The organic phase was evaporated to dryness under nitrogen; the lipid pellet was dissolved in 40 μl of acetone and analyzed by thin-layer chromatography on Silica Gel LK5D 150-Å plates (Whatman) in a solvent system containing toluene/ethyl acetate (2:3).1
Chemical Synthesis
A sample of authentic cholest-5-ene-3β,7α,25-triol was synthesized using a modification of a method described previously (16, 17). Briefly, cholest-5-ene-3β,25-diol was photochemically converted into 5α-hydroperoxycholest-6-ene-3β,25-diol in the presence of oxygen and hematoporphyrin. The product was purified by silica gel chromatography with a 46% yield and incubated in chloroform to allow rearrangement to the 7α-hydroperoxide derivative. This material was reduced with sodium borohydride to yield the desired product, which was purified by preparative thin-layer chromatography in a toluene/ethyl acetate (2:3) solvent system. The final yield of cholest-5-ene-3β,7α,25-triol was 3–5%.
Lipid Analysis
Scissors were used to barber fur from the abdomens or backs of mice. A 10-mg aliquot of fur was extracted with 1.5 ml of chloroform/methanol (2:1) for 2 h at 4 °C with continuous agitation. The resulting solvent was transferred to a fresh polypropylene tube and evaporated to dryness under a nitrogen stream. The pellet was dissolved in 50 μl of chloroform/methanol (2:1) and analyzed by thin-layer chromatography on Silica Gel LK5D 150-Å plates in a solvent system containing toluene/ethyl acetate (7:3).
For stool lipid analysis, 100-mg aliquots of droppings collected from animals of the indicated ages were mixed with a small amount of [carboxyl-14C]triolein (112 mCi/mmol) and dried for 1 h in a vacuum oven at 70 °C. The solid matter was extracted with 2 ml of chloroform/methanol (2:1) for 30 min at 60 °C, passed through a Whatman No. 1 filter, and brought to a final volume of 4 ml with chloroform/methanol (2:1). The material was back-extracted with 1 ml of H2O, and the organic phase was evaporated to dryness. The pellet was resuspended in 2 ml of chloroform/methanol (2:1) and transferred to preweighed vials. The solvent was evaporated, and the vial was taken to a constant weight by drying in a vacuum oven at 70 °C. The difference in weight between the starting empty vial and the vial containing the dried lipid was the fecal lipid amount, which was expressed as a percentage of the weight of the starting fecal sample. The percent recovery of radiolabeled triolein (85–92%) was determined by subjecting the vial to scintillation counting.
Chemical Analysis of Lipids
Gas chromatography-mass spectrometry of mouse fur lipids was performed before and after saponification as described previously (18). Native lipids were converted into trimethylsilyl ethers prior to analysis (19). Briefly, samples were treated with pyridine/hexamethyldisilazane/chlorotrimethylsilane (3:2:1) at 60 °C for 30 min. The solvent was evaporated under a stream of nitrogen, and the residue was dissolved in hexane for gas chromatography-mass spectrophotometry analyses. Saponified samples were converted into methyl esters by treatment with diazomethane (19), further derivatized with trimethylsilane as described above, and then subjected to chemical analysis.
Diets
The normal diet was mouse/rat diet 7001 (Harlan Teklad, Madison, WI) and contained ≥4% (w/w) fat, ≥24% (w/w) protein, and ≤5% (w/w) fiber. Where indicated, this diet was supplemented with 1% (w/w) cholic acid (Sigma). Vitamin supplements (CritterVites, Mardel Labs, Glendale Heights, IL) containing both water-soluble vitamins (thiamine, 180 mg/kg; riboflavin, 300 mg/kg; pantothenic acid, 600 mg/kg; niacin, 1500 mg/kg; vitamin B12, 1500 μg/kg; vitamin B6, 150 mg/kg; folic acid, 100 mg/kg; and ascorbic acid, 9000 mg/kg) and fat-soluble vitamins (vitamin A, 300,000 IU/kg; vitamin D3, 50,000 IU/kg; vitamin E, 750 IU/kg; and menadione, 250 mg/kg) were added to water bottles at the concentration (1 g/liter) recommended by the manufacturer. Vitamin supplements were replaced on a daily basis.
RESULTS
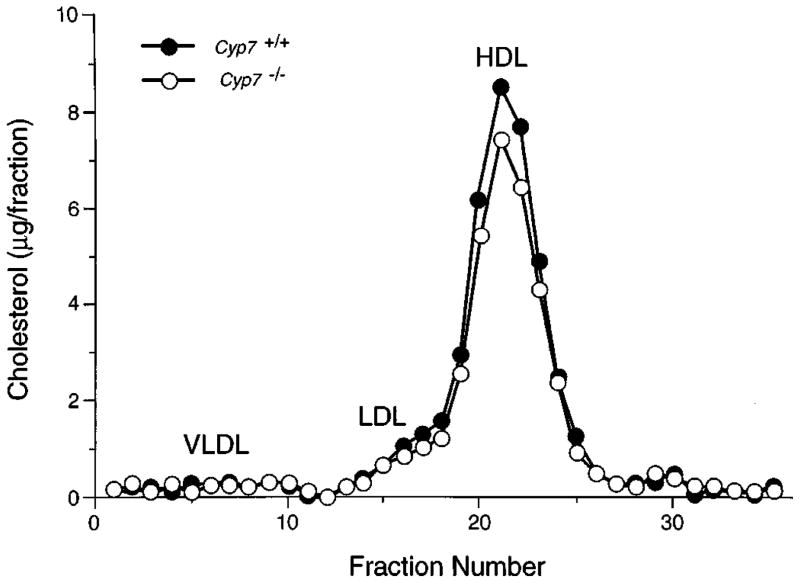
To determine the effects of 7α-hydroxylase deficiency on serum lipid levels, blood was sampled from animals of different genotypes and ages, and the serum lipids were analyzed. The data of Table I show that although there is wide interanimal variation, serum triglyceride and cholesterol contents are similar in wild-type and deficient mice, regardless of the age of the animals. In agreement with these measurements, the profiles of lipoprotein particles in the serum were similar in individual animals of different Cyp7 genotypes (Fig. 1). In experiments not shown, the concentrations of cholesterol in several tissues (liver, spleen, kidney, lung, and heart) were not significantly different between 15-day-old wild-type and mutant mice.
Table I.
Serum cholesterol and triglyceride levels in wild-type and Cyp7−/− mice
| Age | Cyp7 genotype | Cholesterola | Triglyceridea |
|---|---|---|---|
|
| |||
| days | mg/dl | mg/dl | |
| 6 | +/+ | 102.2 ± 16.5 | 177.6 ± 104.7 |
| −/− | 85.6 ± 22.3 | 140.0 ± 42.2 | |
| 15 | +/+ | 170.9 ± 30.7 | 93.0 ± 42.8 |
| −/− | 157.5 ± 44.3 | 151.7 ± 63.3 | |
| 23 | +/+ | 88.4 ± 10.0 | 54.2 ± 10.7 |
| −/− | 99.7 ± 19.6 | 82.3 ± 28.0 | |
| 60–90 | +/+ | 91.5 ± 7.9 | 64.4 ± 7.9 |
| −/− | 91.7 ± 5.6 | 39.7 ± 7.7 | |
| 150–180 | +/+ | 107.1 ± 15.7 | 76.3 ± 19.1 |
| −/− | 122.5 ± 20.0 | 82.5 ± 27.2 | |
Values represent means ± S.D. derived from measurements made in the sera of 4–10 animals of the indicated age and genotype.
Fig. 1. Lipoprotein analysis in wild-type and 7α-hydroxylase-deficient mice.
Plasma was obtained from one 8-month-old female mouse of the indicated Cyp7 genotype and fractionated on a Superose 6 column. The cholesterol content was determined and plotted as a function of column fraction. The positions at which known lipoproteins eluted from the column are indicated. VLDL, very high density lipoprotein; LDL, low density lipoprotein; HDL, high density lipoprotein.
Many of the phenotypic characteristics of 7α-hydroxylase-deficient mice, including early death, skin abnormalities, and eye and vision problems, are reminiscent of fat-soluble vitamin deficiencies. This hypothesis is supported by the finding that these symptoms can be alleviated by supplementing the water supply of nursing mothers with a vitamin mixture (10). To determine if a fat-soluble vitamin deficiency could be directly demonstrated, the levels of vitamins D3 and E were measured in the serum and fat, respectively, of mutant and wild-type mice. The data of Table II show that Cyp7−/− mice contain low levels of vitamins D3 and E. These deficiencies are detected in mice of different ages and in nursing mothers. Vitamin supplementation alone partially restored levels of vitamins D3 and E, whereas dietary supplementation with vitamins and a bile acid (cholic acid) more fully restored vitamin levels (Table II).
Table II.
Serum vitamin D3 and tissue vitamin E levels in wild-type and Cyp7−/− mice
| Agea,b | Cyp7 genotype | Serum vitamin D3c
|
Tissue vitamin Ed
|
||||
|---|---|---|---|---|---|---|---|
| Chow | Chow + vitamins | Chow + vitamins, cholic acid | Chow | Chow + vitamins | Chow + vitamins, cholic acid | ||
|
| |||||||
| days | ng/ml | ng/mg triglyceride | |||||
| 6 | +/+ | 20.0 | —e | — | — | — | — |
| −/− | 2.0 | 5.0 | 12.0 | — | — | — | |
| 16 | +/+ | 18.0 | — | — | 11.6 | — | — |
| −/− | 3.7 | 4.0 | 9.0 | 0.3 | <0.1 | 12.0 | |
| 23 | +/+ | 8.0 | — | — | 24.4 | — | — |
| −/− | 1.0 | 4.0 | 9.0 | <0.1 | <0.1 | 53.3 | |
| 34–38 | +/+ | 17.7 ± 1.1 | — | — | 10.4 ± 2.0 | — | — |
| −/− | 15.7 ± 2.4 | — | — | 5.6 ± 3.6 | — | — | |
| 120–180 | +/+ | 17.5 ± 2.5 | — | — | 90.7 ± 25.4 | — | — |
| −/− | 20.0 ± 3.3 | 11.0 ± 4.4 | 28.5 ± 2.5 | 4.4 ± 4.4 | 11.6 ± 2.5 | 146.1 ± 91.8 | |
Serum or fat tissue was pooled from 3–12 male and female mice to derive the values shown for the 6-, 16-, and 23-day time points.
Values for 34–38 and 120–180-day mice represent means ± S.E. derived from three to five animals.
25-Hydroxyvitamin D3 levels were measured in serum as described under “Experimental Procedures.”
Vitamin E levels were measured in epididymal or ovarian fat pads as described under “Experimental Procedures.”
—, not done.
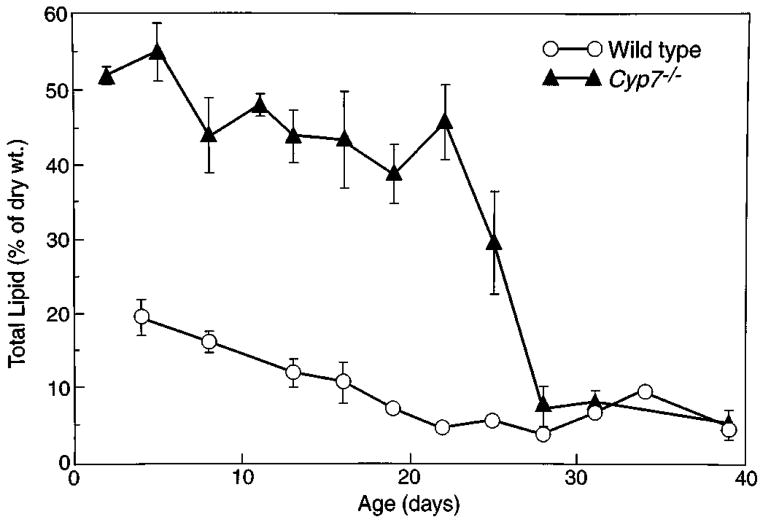
A second characteristic feature of newborn 7α-hydroxylase-deficient mice is their excretion of clay-colored stools, which are reminiscent of fat malabsorption (steatorrhea). To confirm this diagnosis, the stool fat content was monitored as a function of age in Cyp7−/− mice. The data of Fig. 2 show that newborn mice have enormously elevated levels of fat in their stools and that this elevation persists through approximately postnatal day 22, at which time the stool fat content begins to decrease and eventually (by day 28) approximates that of wild-type animals. Weaning took place on day 30 in these experiments; thus, the decline in fat content occurred while the animals were maintained on a high fat diet (mother’s milk). The reduction in stool fat content on or about postnatal day 22 coincides with the time at which the survival rate of mutant mice is dramatically increased (10). Those few animals that reach this age thereafter experience a normal life span. These findings are suggestive of a major change in bile acid metabolism occurring around postnatal day 22 in the Cyp7−/− mice.
Fig. 2. Stool fat content as function of age in wild-type and 7α-hydroxylase-deficient mice.
Total lipid content in stool was measured as described under “Experimental Procedures” and plotted as a function of animal age. Values obtained from wild-type (○) and Cyp7−/− mice (▲) are shown. All animals were weaned on day 30 of the experiment.
The chemical composition of bile from adult wild-type and Cyp7−/− animals (~3 months of age) was examined next. Animals were maintained on normal unsupplemented chow for ≥4 weeks; the major bile duct was cannulated; and bile was collected over a period of 30–60 min from two mice of each genotype. The withdrawn samples were analyzed by gas chromatography-mass spectrometry to identify individual bile acids. The results of these analyses are shown in Table III. The concentration of bile acids in the bile of wild-type and mutant mice was similar and ranged from 9.9 to 14.6 mM. Bile from wild-type animals contained at least 11 separable and measurable bile acid derivatives, of which cholic acid and β-muricholic acid were the predominant species, accounting together for ~75% of the total bile acids. In the Cyp7−/− mice, the concentration of cholic acid, but not β-muricholic acid, was decreased by more than half relative to that found in the wild-type animals (Table III). In addition, several bile acids were increased in concentration relative to levels in wild-type animals. For example, hyodeoxycholic acid (3α,6α-dihydroxy-5β-cholanoic acid) was not detected in wild-type bile, but this compound represented 14–25% of the total bile acid in the Cyp7−/− animals. Similarly, chenodeoxycholic acid and α-muricholic acid were detected only in the mutant mice (Table III).
Table III.
Analysis of bile acids in bile of individual wild-type and Cyp7−/− adult mice
| Retention timea | Bile acidb |
Cyp7 genotype
|
|||
|---|---|---|---|---|---|
| +/+ | +/+ | −/− | −/− | ||
|
| |||||
| MU | μmol/liter | ||||
| 31.81 | 3,12-Dihydroxy bile acid | 312 | 425 | 107 | 189 |
| 32.00 | 3α,7α,12α-Trihydroxy-5α-cholanoic acid (allo-cholic acid) | 482 | 651 | 174 | 236 |
| 32.13 | 3α,7α-Dihydroxy-5β-cholanoic acid (chenodeoxycholic acid) | NDc | ND | 431 | 350 |
| 32.18 | 3α,6β,7α-Trihydroxy-5β-cholanoic acid (α-muricholic acid) | ND | ND | 766 | 672 |
| 32.23 | 3α,7α,12α-Trihydroxy-5β-cholanoic acid (cholic acid) | 6710 | 7609 | 1918 | 2434 |
| 32.29 | 3α,6α-Dihydroxy-5β-cholanoic acid (hyodeoxycholic acid) | ND | ND | 2486 | 1713 |
| 32.51 | 3α,7β-Dihydroxy-5β-cholanoic acid (ursodeoxycholic acid) | 150 | 304 | 168 | 162 |
| 32.84 | 3α,7α,12α-Trihydroxy-5β-homocholanoic acid (homocholic acid) | 155 | 178 | ND | ND |
| 33.07 | 12-Oxo-3α-hydroxy-5β-cholanoic acid | 157 | 217 | 899 | 746 |
| 33.21 | 3α,6β,7β-Trihydroxy-5β-cholanoic acid (β-muricholic acid) | 1778 | 3131 | 1717 | 3060 |
| 33.52 | 3α,7β,12α-Trihydroxy-5α-cholanoic acid | 132 | 237 | 171 | 146 |
| 33.72 | Oxodihydroxy bile acid | 276 | 380 | 163 | 139 |
| 33.80 | 7-Oxo-3α,12α-Dihydroxy-5β-cholanoic acid | 260 | 290 | ND | 340 |
| 34.29 | 3α,6α,7β-Trihydroxy-5β-cholanoic acid (ω-muricholic acid) | 555 | 1203 | 902 | 1714 |
| Total bile acids | 10,967 | 14,625 | 9902 | 11,901 | |
Bile acids are listed based on retention times as methyl ester-trimethylsilyl ethers relative to a homologous series of n-alkanes, referred to as the methylene unit (MU) value.
Chemical structures were established by electron ionization-gas chromatography-mass spectrometry.
ND, not detected.
The data of Table IV summarize the results obtained after chemical analyses of bile acids in stool samples of adult normal and Cyp7−/− animals (~4 months of age). Feces were collected for a period of several days from five individual animals of each genotype, weighed, and then extracted and analyzed for bile acids. The average weight of stool collected from the wild-type mice was 1.32 ± 0.12 g/day (mean ± S.E.) versus 1.35 ± 0.05 g/day for the Cyp7−/− mice. The concentration of total bile acids in the droppings of wild-type mice was 1095.8 ± 88.9 μg/g of feces, whereas the droppings from Cyp7−/− mice contained 369.8 ± 18.2 μg/g of feces (Table IV). The diminished bile acid concentration in the stool samples of the mutant animals reflected a uniform decline in almost all of the individual bile acid species detected in the analyses (Table IV). For example, deoxycholic acid, which is the most abundant bile acid in mouse stool, was reduced from 316.2 ± 28.6 μg/g in the wild-type animals to 47.4 ± 12.6 μg/g in the Cyp7−/− animals (Table IV).
Table IV.
Analysis of fecal bile acids in individual wild-type and Cyp7−/− adult mice
| Retention timea | Bile acidb |
Cyp7 genotype
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| +/+ | +/+ | +/+ | +/+ | +/+ | −/− | −/− | −/− | −/− | −/− | ||
|
| |||||||||||
| MU | μg/gfeces | ||||||||||
| 31.18 | 3α-Hydroxy-5β-cholanoic acid (lithocholic acid) | 12 | 23 | 26 | 22 | 31 | 8 | 6 | 6 | ND | 4 |
| 31.61 | Unknown bile acid | NDc | 12 | 23 | 18 | 18 | ND | ND | 4 | ND | ND |
| 31.76 | 3,12-Dihydroxy bile acid | 21 | 27 | 26 | 34 | 32 | 3 | 6 | 5 | ND | 4 |
| 31.88 | 3α-12α-Dihydroxy-5β-cholanoic acid (deoxycholic acid) | 242 | 276 | 319 | 411 | 333 | 41 | 73 | 78 | 10 | 35 |
| 32.00 | 3α,7α,12α-Trihydroxy-5α-cholanoic acid (allo-cholic acid) | 18 | 11 | 11 | ND | 9 | ND | ND | 6 | 8 | 8 |
| 32.15 | 3α,7α-Dihydroxy-5β-cholanoic acid (chenodeoxycholic acid) | ND | 8 | 11 | ND | 10 | 8 | 6 | 6 | 7 | 4 |
| 32.20 | 3α,6β,7α-Trihydroxy-5β-cholanoic acid (α-muricholic acid) | 45 | 36 | 56 | 57 | 69 | ND | ND | ND | ND | ND |
| 32.26 | 3α,7α,12α-Trihydroxy-5β-cholanoic acid (cholic acid) | 127 | 137 | 158 | 181 | 132 | 43 | 62 | 70 | 188 | 117 |
| 32.29 | 3α,6α-Dihydroxy-5β-cholanoic acid (hyodeoxycholic acid) | 60 | 106 | 129 | 112 | 114 | 69 | 64 | 71 | 59 | 51 |
| 32.45 | Unknown bile acid | 13 | 20 | 24 | 20 | 20 | 10 | 7 | 8 | 7 | 8 |
| 32.51 | 3α,7β-Dihydroxy-5β-cholanoic acid (ursodeoxycholic acid) | ND | ND | 11 | ND | ND | 6 | ND | 14 | ND | ND |
| 32.60 | 3β,12α-Dihydroxy-5α-cholanoic acid | 12 | 16 | 17 | 21 | 20 | ND | ND | ND | ND | ND |
| 32.84 | 3α,7α,12α-Trihydroxy-5β-homocholanoic acid (homocholic acid) | 8 | ND | 14 | 15 | 10 | ND | 4 | ND | ND | ND |
| 33.06 | 12-Oxo-3α-hydroxy-5β-cholanoic acid | 65 | 44 | 85 | 75 | 61 | 26 | 35 | 30 | 23 | 26 |
| 33.22 | 3α,6β,7β-Trihydroxy-5β-cholanoic acid (β-muricholic acid) | 76 | 66 | 77 | 141 | 81 | 45 | 54 | 53 | 71 | 75 |
| 33.79 | 7-Oxo-3α,12α-dihydroxy-5β-cholanoic acid | 11 | ND | 9 | 16 | 6 | ND | 5 | 6 | 6 | 5 |
| 34.30 | 3α,6α,7β-Trihydroxy-5β-cholanoic acid (ω-muricholic acid) | 117 | 140 | 157 | 194 | 136 | 39 | 34 | 29 | 29 | 35 |
| 34.55 | 3α,6α,7β-Trihydroxy-5α-cholanoic acid (allo-ω-muricholic acid) | 27 | 33 | 41 | 43 | 34 | 6 | 7 | 4 | 6 | 6 |
| Total bile acids | 854 | 955 | 1194 | 1360 | 1116 | 304 | 363 | 390 | 414 | 378 | |
Bile acids are listed based on their retention times as methyl ester-trimethylsilyl ethers relative to a homologous series of n-alkanes, referred to as the methylene unit (MU) value.
Chemical structures were determined by electron ionization-gas chromatography-mass spectrometry.
ND, not detected.
Several conclusions were reached from the data summarized in Tables III and IV. First, Cyp7−/− animals that survive to adulthood when maintained on unsupplemented chow have normal concentrations of total bile acids in their bile (Table III), but reduced concentrations of bile acids in their stool (Table IV). Second, the composition of bile in terms of the individual bile acid species is different in the bile and stool of wild-type and Cyp7−/− animals. Third, adult animals deficient in 7α-hydroxylase nevertheless synthesize and secrete 7α-hydroxylated bile acids into their bile and excrete these compounds in their stool.
Bile acids with a 7α-hydroxyl group could arise in the mutant mice as a consequence of 7α-hydroxylase activity present in the intestinal flora or from the activity of another endogenous sterol 7α-hydroxylase enzyme. The data of Fig. 3 (lanes 1 and 3) demonstrate the presence of an enzyme activity in the livers of both wild-type and Cyp7−/− mice that is capable of 7α-hydroxylating cholest-5-ene-3β,25-diol (25-hydroxycholesterol) to form cholest-5-ene-3β,7α,25-triol. This oxysterol 7α-hydroxylase enzyme required NADPH as a cofactor (lanes 2 and 4). The specific activity of the enzyme was 6 pmol of product formed per min/mg of liver microsomal protein in adult wild-type mice and 5 pmol/min/mg of protein in Cyp7−/− mice.
Fig. 3. Oxysterol 7α-hydroxylase activity in wild-type and 7α-hydroxylase-deficient mice.
Liver microsomes were prepared from mice of the indicated Cyp7 genotype and incubated with [3H]cholest-5-ene-3β,25-diol (25-hydroxycholesterol) in the presence or absence of NADPH. After 15 min at 37 °C, sterols were extracted and analyzed by thin-layer chromatography. An autoradiogram of the experiment is shown.
The experiment shown in Fig. 4 was performed to confirm that the product of this enzyme activity was cholest-5-ene-3β,7α,25-triol. An authentic standard representing the predicted product was chemically synthesized as described under “Experimental Procedures.” This standard was chromatographed on a thin-layer plate either separately (lane 1) or together (lanes 2 and 3) with the radiolabeled products derived by incubating liver extracts with [3H]cholest-5-ene-3β,25-diol. A comparison of the position of the radiolabeled product on the chromatogram (determined by autoradiography) to that of the standard (determined by phosphomolybdic acid staining) revealed that the two sterols comigrated in this solvent system. Similar results were obtained when product analysis was carried out in two other solvent systems (chloroform/methanol/H2O/ethyl acetate (50:20:4:15) and toluene/ethyl acetate (2:3)) on silica gel plates and when the sample was analyzed by chromatography on C18 plates using an acetonitrile/tetrahydrofuran (6:4) solvent system. Finally, analysis of the product by mass spectrometry confirmed the structure of the molecule as cholest-5-ene-3β,7α,25-triol (data not shown).
Fig. 4. Authentication of oxysterol 7α-hydroxylase product.
Cholest-5-ene-3β,7α,25-triol, an anticipated product of the oxysterol 7α-hydroxylase enzyme activity in mouse liver, was chemically synthesized as described under “Experimental Procedures.” In lane 1, an aliquot of this compound was subjected to thin-layer chromatography in a solvent system containing toluene/ethyl acetate (2:3), and the lane was stained with phosphomolybdic acid (PMA). In lane 2, an aliquot of the authentic standard was mixed with the sterol products arising from incubation of mouse liver microsomes with [3H]cholest-5-ene-3β,25-diol (25-hydroxycholesterol), and the mixture was subjected to thin-layer chromatography and staining. In addition to steroids, dithiothreitol and Triton X-100, two components of the enzyme assay, were also stained by phosphomolybdic acid. In lane 3, an autoradiogram of lane 2 is presented. Comparison of the results obtained by autoradiography (lane 3) with those obtained by staining (lanes 1 and 2) reveals comigration of the authentic standard with the radiolabeled product of the enzyme.
We next determined the ability of different sterols to inhibit the oxysterol 7α-hydroxylase enzyme activity detected in the mutant mice. As shown by the data of Fig. 5, the product of the enzyme, cholest-5-ene-3β,7α,25-triol, specifically inhibited the reaction with an apparent IC50 of 9.0 μM. The product of the cholesterol 7α-hydroxylase enzyme, cholest-5-ene-3β,7α-diol (7α-hydroxycholesterol), did not inhibit the oxysterol 7α-hydroxylase activity, nor did an irrelevant triol compound, cholest-5-ene-3β,17α,20-triol (Fig. 5A). These data were quantitated by phosphoimage analysis and plotted as a function of oxysterol 7α-hydroxylase activity versus inhibitor concentration (Fig. 5B). Interpolation predicts an IC50 of 9 μM for the product of the enzyme.
Fig. 5. Product inhibition of oxysterol 7α-hydroxylase activity.
Liver microsomes prepared from wild-type mice were incubated with 0.12 μM [3H]cholest-5-ene-3β,25-diol (25-hydroxycholesterol), 1.5 mM NADPH, and the indicated concentrations of hydroxysterols. After a 15-min incubation, the formation of [3H]cholest-5-ene-3β,7α,25-triol was determined by thin-layer chromatography. A, an autoradiogram of the resulting thin-layer chromatogram is shown together with the positions to which substrate and product migrated. B, the results shown in A were quantitated by phosphoimaging and plotted as a standard inhibition curve. Among the three sterols tested, only the product of the oxysterol 7α-hydroxylase enzyme, cholest-5-ene-3β,7α,25-triol, was inhibitory.
To determine whether survival of the Cyp7−/− deficient animals correlates with the appearance of the oxysterol 7α-hydroxylase enzyme activity, the ontogeny of expression in the liver was determined. The data of Fig. 6A illustrate a representative experiment showing that the formation of [3H]cholest-5-ene-3β,7α,25-triol is low to absent in the livers of wild-type or mutant mice throughout postnatal days 1–25, but is substantially increased after this time period. A more detailed time course study reveals that the enzyme is gradually induced between postnatal days 20 and 50 (Fig. 6B). In the experiments shown in Fig. 6B, animals of both genotypes were weaned on day 24, suggesting that the induction of oxysterol 7α-hydroxylase activity might correlate with the shift from a high fat diet (mother’s milk) to a low fat diet (normal chow). However, in experiments not shown, we found that enzyme induction was independent of weaning in both wild-type and Cyp−/− mice.
Fig. 6. Ontogeny of oxysterol 7α-hydroxylase expression in mice.
Liver microsomes were prepared from wild-type (+/+) or 7α-hydroxylase-deficient mice (−/−) of the indicated ages and assayed for oxysterol 7α-hydroxylase activity. A, shown are autoradiograms of thin-layer chromatography plates. Oxysterol 7α-hydroxylase activity was first detected above background in 3 (day 22) to 4 (day 30)-week-old animals and remained detectable in the livers of older animals. B, data from a more extensive survey of animals of different ages and Cyp7 genotypes were quantitated by phosphoimaging and plotted as oxysterol 7α-hydroxylase enzyme activity versus age in days. Animals were weaned on day 24 in this experiment.
A clearly visible and striking phenotype associated with a deficiency of 7α-hydroxylase in mice is the development of an oily coat in newborn animals and nursing females (10). To determine if the unusual coat appearance was due to excess secretion of water or lipids, their concentrations in fur were examined. As determined by the Fischer method, the water content of wild-type mouse fur was 10.6 ± 0.1%, and that of nursing homozygous mothers with an oily coat was 9.9 ± 0.2% (data not shown). Qualitative thin-layer chromatography analysis of solvent-extractable lipids suggested that animals of both genotypes had near equal amounts of squalene, cholesterol, and cholesterol esters in their fur (Fig. 7). However, two lipids (designated X and Y in Fig. 7) with mobilities intermediate between cholesterol and squalene were present in excess in Cyp7−/− animals with greasy coats. A third region of the chromatogram (designated Z in Fig. 7) also appeared to contain more lipid in the homozygous animals, although the difference in the levels of this spot was not as great or as reproducible between wild-type and Cyp7−/− animals as that found for spots X and Y. Fur shaved from the abdomens of homozygous animals had a greater excess of spots X and Y relative to that obtained from the backs of the mice (Fig. 7).
Fig. 7. Qualitative analysis of fur lipids in wild-type and mutant mice.

The fur of nursing females of the indicated Cyp7 genotype was cut from the abdomen or back. Total lipids were extracted with chloroform/methanol (2:1), concentrated by evaporation, and then analyzed by thin-layer chromatography in a solvent system containing toluene/ethyl acetate (7:3). After development, the chromatogram was stained with phosphomolybdic acid and photographed. Squalene and cholesterol standards were chromatographed in adjacent lanes of the plate. The spot in the fur samples that comigrated with the squalene standard contained a mixture of squalene and cholesterol esters. At least three lipids (designated X, Y, and Z) were present at higher levels in the abdomen and back fur sheared from the Cyp7−/− animals.
To determine the chemical structures of spots X and Y, these compounds were purified by thin-layer chromatography and subjected to mass spectrometry analyses as described under “Experimental Procedures.” An initial study revealed that saponification of spots X and Y yielded a similar pattern of lipids as determined by thin-layer chromatography on C18 plates, indicating that the two spots contained structurally related esters. Chemical analyses indicated that spots X and Y each contained a mixture of monoglyceride esters of palmitate (16: 0), stearate (18:0), and oleate (18:1). The position and stereochemistry of the double bond in the oleate moiety were not determined. Fatty acids were esterified to carbon 1 or 3 of glycerol, but not carbon 2. Analysis of saponified samples demonstrated the presence of the predicted free fatty acids. We postulate that esterification at position 1 versus 3 of glycerol may underlie the differences in the mobilities between spots X and Y. Finally, an excess of these same monoglyceride esters was detected in the stool of the knockout animals (data not shown).
DISCUSSION
The data of this paper support two surprising conclusions. First, 7α-hydroxylase is not required in the mouse to maintain serum cholesterol and triglyceride levels in the normal range. Second, an alternate pathway of bile acid synthesis involving an oxysterol 7α-hydroxylase is induced between the third and fourth weeks of life in this species.
An alteration in steady-state plasma cholesterol and triglyceride levels was not observed in the Cyp7−/− mice (Fig. 1). Plasma lipid levels were normal in newborn animals that lacked detectable levels of oxysterol 7α-hydroxylase (i.e. animals less than 21 days old) and in older animals that contained this enzyme activity. At least two explanations may account for these findings. First, the response of the homeostatic regulatory mechanisms may be sufficient to maintain serum lipid and cholesterol levels even when key catabolic pathways are knocked out (7α-hydroxylase) or not yet induced (oxysterol 7α-hydroxylase). Second, alternate mechanisms that do not involve conversion of cholesterol into bile acids may exist that allow for cholesterol disposal and metabolism. Evidence to support both of these hypotheses is presented here.
In newborn Cyp7−/− animals, the synthesis of bile acids is presumed to be low to nonexistent. A direct test of this idea has not yet been possible due to technical difficulties in working with the tiny amounts of bile present in very young animals. Nevertheless, in the absence of bile acids, the solubilization of exogenous sterols and lipids should decrease, resulting in lower serum levels of these compounds. Thus, the normal levels of cholesterol and triglycerides in the young Cyp7−/− mice may reflect the absence of dietary lipids in the circulation and a subsequent compensation by the cholesterol supply pathways.
With respect to homeostasis in adult animals, although the total concentration of bile acids at ~12–16 weeks of age in the bile of older Cyp7−/− mice is similar to that in wild-type mice (Table III and Ref. 22), the types of bile acids are different, and their excretion in stool is reduced by 80% (Table IV). The altered composition of the bile acid pool may decrease the solubilization of dietary cholesterol and lipids, thus reducing the input of the exogenous (dietary) pathway to the steady state. Alternatively, the bile acid pool size may be compromised, as reflected by the reduced excretion of bile acids in stool, which in turn may decrease solubilization of dietary lipids. Since the concentration of bile acids in bile appears normal, another factor that regulates bile acid pool size such as the total volume of bile or the recycling efficiency of the entero-hepatic circulation may be altered in the knockout mice. Finally, the modified composition may also lead to a more efficient excretion of cholesterol into bile, thereby maintaining cholesterol homeostasis. Future feeding studies carried out with individual bile acids together with cholesterol balance studies may shed light on these possible explanations.
With respect to alternate cholesterol and lipid disposal mechanisms, Cyp7−/− mice develop oily coats due to hypersecretion of lipids and sterols. This phenotype appears in newborn mice (10) at a time when bile acid biosynthesis is inferred to be very low based on the genetic absence of 7α-hydroxylase and on the absence of induction of the oxysterol 7α-hydroxylase enzyme (Fig. 6). In addition, the phenotype is reversed by dietary supplementation with bile acid (10). We postulate that in the absence of sufficient bile acids, the Cyp7−/− animals secrete lipids that then appear in their fur (Fig. 7). The secreted compounds are monoglyceride esters, which are not normally found in mouse fur (20, 21, 23). In the knockout animals, they may arise by direct excretion from the skin to the fur. Alternatively, their presence in the stool may lead to an indirect transfer to and accumulation in fur. Additional studies are currently being carried out to determine the biosynthetic origins of these lipids and how they accumulate in fur.
The spectrum of phenotypes associated with 7α-hydroxylase deficiency largely disappears in animals that survive to post-natal day 21 (10). This reversal can now be explained by the induction of an alternate bile acid biosynthetic pathway beginning around the third week of life (Fig. 6). The alternate pathway most likely corresponds to the acidic or mitochondrial pathway described by Axelson and Sjövall (2). The first step in this pathway takes place in the mitochondria and is the conversion of cholesterol into cholest-5-ene-3β,27-diol (27-hydroxy-cholesterol) by the sterol 27-hydroxylase enzyme (3). This oxysterol intermediate is then 7α-hydroxylated by the oxysterol 7α-hydroxylase to produce cholest-5-ene-3β,7α,27-triol, which is thereafter converted into a spectrum of C24 bile acids (2). An oxysterol 7α-hydroxylase enzyme activity has been previously characterized in pig liver (4), rat liver (5), rat ovary (6), and human fibroblasts (7). In the mouse, this enzyme activity is present in the liver (Fig. 3) and at lower levels in the kidney, brain, and ovary (data not shown). The murine enzyme is active against cholest-5-ene-3β,25-diol (25-hydroxycholesterol), as are the pig and rat enzymes (4, 5), and is specifically inhibited by the product (Fig. 5).
The mouse oxysterol 7α-hydroxylase activity is initially detected in the liver at 3 weeks of age, gradually increases until ~8 weeks of age, and thereafter remains constant (Fig. 6). The induction of oxysterol activity is correlated with survival of the Cyp7−/− animals (10), with a decrease in stool fat content (Fig. 2), with an increase in tissue stores of vitamins D3 and E (Table II), and, in adult animals, with the occurrence of many species of 7α-hydroxylated bile acids in gallbladder bile (Table III). These findings strongly suggest that the oxysterol 7α-hydroxylase plays an active role in the biosynthesis of bile acids, which in turn participate in the metabolism of fat-soluble vitamins and lipids. The chemical reactions of the oxysterol 7α-hydroxylase pathway remain to be worked out; however, analyses of the bile acids present in the bile and feces of Cyp7−/− mice (Tables III and IV) suggest that the end products of this biosynthetic pathway may be different from those of the 7α-hydroxylase-initiated pathway. For example, hyodeoxycholic acid is a predominant component of bile in the mutant animals, suggesting that this bile acid is a major product of the oxysterol 7α-hydroxylase pathway. In the pig, hyodeoxycholic acid is a secondary bile acid derived from hyocholic acid (3α,6α,7α-tri-hydroxy-5β-cholanoic acid) by bacterial action in the intestine (24), and it is conceivable that the mouse hyodeoxcholic acid shares a similar origin. However, we were unable to detect hyocholic acid in the bile or stool of the Cyp7−/− animals (Tables III and IV), which suggests that hyodeoxycholic acid may be a primary bile acid in the mouse.
The stimulus for the induction of the oxysterol 7α-hydroxylase pathway has not yet been identified. Induction on day 22 does not appear to depend on weaning per se since a normal ontogeny of oxysterol 7α-hydroxylase activity was detected when pups were left with their mothers for ≥30 days. During this period, the pups derive most of their sustenance from milk since they are too small to reach the chow pellets on the cage top. Thus, a switch from a high fat diet (milk) to a low fat diet (chow) most likely does not provide a direct induction signal. In the rat, a marked increase in liver (25) and intestinal (26) bile acid transporters occurs at approximately the same time as induction of the oxysterol 7α-hydroxylase in the mouse, which raises the interesting possibility that a coordinate change in bile acid metabolism occurs at ~3 weeks of life. By using young Cyp7−/− mice and an oxysterol 7α-hydroxylase assay, it should prove possible to develop a bioassay with which to identify the putative induction signal for these changes.
The alternate bile acid pathway may play at least two physiological roles in the mouse. In one role, the oxysterol 7α-hydroxylase pathway acts as a backup for the classical 7α-hydroxylase pathway. In a second role, the oxysterol 7α-hydroxylase-initiated pathway may serve to increase the diversity of bile acid species in bile and thus to alter the solubility characteristics of this digestive fluid. The importance of bile composition with respect to bile acid species is suggested by the vitamin E measurements reported in Table II. In the adult Cyp7−/− animals (120–180 days old) with a fully functioning oxysterol 7α-hydroxylase bile acid synthesis pathway (Fig. 6), the capacity to solubilize and store vitamin E in fat is much reduced compared with wild-type animals, even when the mutant mice are fed an excess of the vitamin. This capacity is completely restored when cholic acid is added together with the vitamin supplements (Table II). Since Cyp7−/− mice have a lower level of cholic acid in bile (Table III), the results suggest that this bile acid is important for vitamin E uptake and, by extension, that the absorption of other nutrients can be affected by bile composition. The characterization of the oxysterol 7α-hydroxylase enzyme and pathway in wild-type and Cyp7−/− mice should provide additional insight into their physiological roles.
Acknowledgments
We thank Daphne Davis, Kristi Cala, Kevin Anderson, and Nancy O’Connell for excellent technical assistance; Steve Turley for help with lipid and sterol analyses; and Mike Brown and Joe Goldstein for critical review of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grant HL20948 and by grants from the W. M. Keck Foundation, the Henning and Johan Throne-Holst Foundation for Nutrition Research (to E. G. L.), and the Lucille P. Markey Charitable Trust (to J. H.).
The compositions of all solvent systems are indicated as volumetric ratios.
References
- 1.Russell DW, Setchell KDR. Biochemistry. 1992;31:4737–4749. doi: 10.1021/bi00135a001. [DOI] [PubMed] [Google Scholar]
- 2.Axelson M, Sjövall J. J Steroid Biochem. 1990;36:631–640. doi: 10.1016/0022-4731(90)90182-r. [DOI] [PubMed] [Google Scholar]
- 3.Björkhem I. J Lipid Res. 1992;33:455–471. [PubMed] [Google Scholar]
- 4.Toll A, Wikvall K, Sudjana-Sugiaman E, Kondo K, Björkhem I. Eur J Biochem. 1994;224:309–316. doi: 10.1111/j.1432-1033.1994.00309.x. [DOI] [PubMed] [Google Scholar]
- 5.Axelson M, Shoda J, Sjövall J, Toll A, Wikvall K. J Biol Chem. 1992;267:1701–1704. [PubMed] [Google Scholar]
- 6.Payne DW, Shackleton C, Toms H, Ben-Shlomo I, Kol S, deMoura M, Strauss JF, Adashi EY. J Biol Chem. 1995;270:18888–18896. doi: 10.1074/jbc.270.32.18888. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Larsson O, Sjövall J. Biochim Biophys Acta. 1995;1256:353–359. doi: 10.1016/0005-2760(95)00045-e. [DOI] [PubMed] [Google Scholar]
- 8.Danielsson H, Einarsson K, Johansson G. Eur J Biochem. 1967;2:44–49. doi: 10.1111/j.1432-1033.1967.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 9.Spady DK, Cuthbert JA, Willard MN, Meidell RS. J Clin Invest. 1995;96:700–709. doi: 10.1172/JCI118113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishibashi S, Schwarz M, Frykman PK, Herz J, Russell DW. J Biol Chem. 1996;261:18017–18023. doi: 10.1074/jbc.271.30.18017. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traber MG, Kayden HJ. Am J Clin Nutr. 1987;46:488–495. doi: 10.1093/ajcn/46.3.488. [DOI] [PubMed] [Google Scholar]
- 13.Popoff SN, Osier LK, Zerwekh JE, Marks SC. Bone (Elmsford) 1994;15:515–522. doi: 10.1016/8756-3282(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 14.Sakhaee K, Baker S, Zerwekh JE, Poindexter J, Garcia-Hernandez PA, Pak CYC. J Urol (Paris) 1994;152:324–327. doi: 10.1016/s0022-5347(17)32730-1. [DOI] [PubMed] [Google Scholar]
- 15.Setchell KDR, Yamashita H, Rodrigues CMP, O’Connell NC, Kren BT, Steer CJ. Biochemistry. 1995;34:4169–4178. doi: 10.1021/bi00013a004. [DOI] [PubMed] [Google Scholar]
- 16.Schenck GO, Golinick K, Neumüller OA. Ann Chem (Justus Liebigs) 1957;603:46–59. [Google Scholar]
- 17.Schenck GO, Neumüller OA, Eisfeld W. Angew Chem Int Ed Engl. 1958;19:595. [Google Scholar]
- 18.Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Anal Biochem. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 19.Lund E, Boberg KM, Byström S, Ölund J, Carlström K, Björkhem I. J Biol Chem. 1991;266:4929–4937. [PubMed] [Google Scholar]
- 20.Nicolaides N, Fu HC, Ansari MNA. Lipids. 1970;5:299–307. doi: 10.1007/BF02531461. [DOI] [PubMed] [Google Scholar]
- 21.Grigor MR. In: Lipid Metabolism in Mammals. Snyder F, editor. Plenum Press; New York: 1977. pp. 209–235. [Google Scholar]
- 22.Uchida K, Takase H, Nomura Y, Takeda K, Takeuchi N, Ishikawa Y. J Lipid Res. 1984;25:236–245. [PubMed] [Google Scholar]
- 23.Stewart ME, Downing DT. Adv Lipid Res. 1991;24:263–301. doi: 10.1016/b978-0-12-024924-4.50013-4. [DOI] [PubMed] [Google Scholar]
- 24.Bergström S, Danielsson H, Göransson A. Acta Chem Scand. 1959;13:776–784. [Google Scholar]
- 25.Suchy FJ, Balistreri WF. Pediatr Res. 1982;16:282–285. doi: 10.1203/00006450-198204000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. J Clin Invest. 1995;95:745–754. doi: 10.1172/JCI117722. [DOI] [PMC free article] [PubMed] [Google Scholar]