Abstract
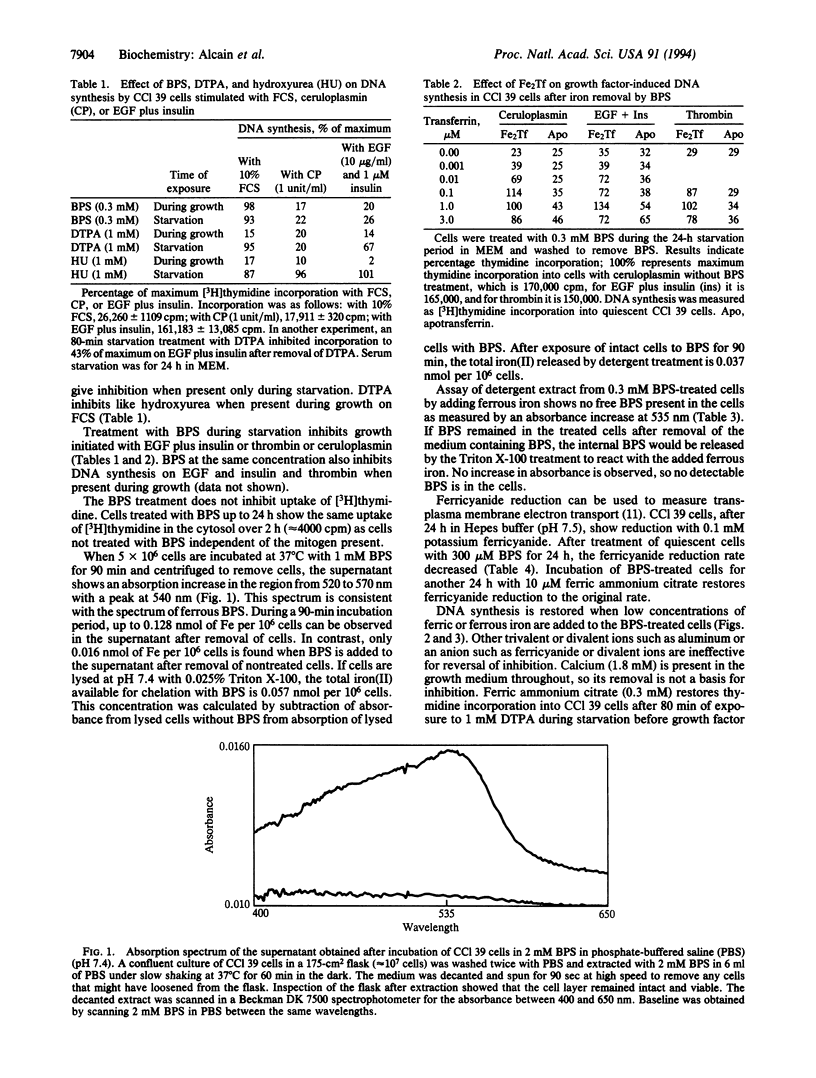
Treatment of Chinese hamster lung fibroblasts (CCl 39 cells) with the impermeable iron(II) chelator bathophenanthroline disulfonate (BPS) inhibits DNA synthesis when cell growth is initiated with growth factors including epidermal growth factor plus insulin, thrombin, or ceruloplasmin, but not with 10% fetal calf serum. The BPS treatment inhibits transplasma membrane electron transport. The treatment leads to release of iron from the cells as determined by BPS iron(II) complex formation over 90 min. Growth factor stimulation of DNA synthesis and electron transport are restored by addition of di- or trivalent iron to the cells in the form of ferric ammonium citrate, ferrous ammonium sulfate, or diferric transferrin. The effect with BPS differs from the inhibition of growth by hydroxyurea, which acts on the ribonucleotide reductase, or diethylenetriaminepentaacetic acid, which is another impermeable chelating agent, in that these agents inhibit growth in 10% fetal calf serum. The BPS effect is consistent with removal of iron from a site on the cell surface that controls DNA synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcaín F. J., Villalba J. M., Löw H., Crane F. L., Navas P. Ceruloplasmin stimulates NADH oxidation of pig liver plasma membrane. Biochem Biophys Res Commun. 1992 Jul 31;186(2):951–955. doi: 10.1016/0006-291x(92)90838-c. [DOI] [PubMed] [Google Scholar]
- Basset P., Quesneau Y., Zwiller J. Iron-induced L1210 cell growth: evidence of a transferrin-independent iron transport. Cancer Res. 1986 Apr;46(4 Pt 1):1644–1647. [PubMed] [Google Scholar]
- Bergeron R. J., Cavanaugh P. F., Jr, Kline S. J., Hughes R. G., Jr, Elliott G. T., Porter C. W. Antineoplastic and antiherpetic activity of spermidine catecholamide iron chelators. Biochem Biophys Res Commun. 1984 Jun 29;121(3):848–854. doi: 10.1016/0006-291x(84)90755-1. [DOI] [PubMed] [Google Scholar]
- Bollinger J. M., Jr, Edmondson D. E., Huynh B. H., Filley J., Norton J. R., Stubbe J. Mechanism of assembly of the tyrosyl radical-dinuclear iron cluster cofactor of ribonucleotide reductase. Science. 1991 Jul 19;253(5017):292–298. doi: 10.1126/science.1650033. [DOI] [PubMed] [Google Scholar]
- Bottomley S. S., Wolfe L. C., Bridges K. R. Iron metabolism in K562 erythroleukemic cells. J Biol Chem. 1985 Jun 10;260(11):6811–6815. [PubMed] [Google Scholar]
- Grebing C., Crane F. L., Löw H., Hall K. A transmembranous NADH-dehydrogenase in human erythrocyte membranes. J Bioenerg Biomembr. 1984 Dec;16(5-6):517–533. doi: 10.1007/BF00743243. [DOI] [PubMed] [Google Scholar]
- Löw H., Crane F. L., Grebing C., Isaksson M., Lindgren A., Sun I. L. Modification of transplasma membrane oxidoreduction by SV40 transformation of 3T3 cells. J Bioenerg Biomembr. 1991 Dec;23(6):903–917. doi: 10.1007/BF00786008. [DOI] [PubMed] [Google Scholar]
- Morgan E. H. Membrane transport of non-transferrin-bound iron by reticulocytes. Biochim Biophys Acta. 1988 Sep 1;943(3):428–439. doi: 10.1016/0005-2736(88)90374-4. [DOI] [PubMed] [Google Scholar]
- Nunez M. T., Cole E. S., Glass J. The reticulocyte plasma membrane pathway of iron uptake as determined by the mechanism of alpha, alpha'-dipyridyl inhibition. J Biol Chem. 1983 Jan 25;258(2):1146–1151. [PubMed] [Google Scholar]
- Ponka P., Grady R. W., Wilczynska A., Schulman H. M. The effect of various chelating agents on the mobilization of iron from reticulocytes in the presence and absence of pyridoxal isonicotinoyl hydrazone. Biochim Biophys Acta. 1984 Dec 20;802(3):477–489. doi: 10.1016/0304-4165(84)90367-2. [DOI] [PubMed] [Google Scholar]
- Reichard P., Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science. 1983 Aug 5;221(4610):514–519. doi: 10.1126/science.6306767. [DOI] [PubMed] [Google Scholar]
- Sun I. L., Sun E. E., Crane F. L. Stimulation of serum-free cell proliferation by coenzyme Q. Biochem Biophys Res Commun. 1992 Nov 30;189(1):8–13. doi: 10.1016/0006-291x(92)91517-t. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Thorstensen K., Aisen P. Release of iron from diferric transferrin in the presence of rat liver plasma membranes: no evidence of a plasma membrane diferric transferrin reductase. Biochim Biophys Acta. 1990 Apr 9;1052(1):29–35. doi: 10.1016/0167-4889(90)90053-g. [DOI] [PubMed] [Google Scholar]
- Thorstensen K. Hepatocytes and reticulocytes have different mechanisms for the uptake of iron from transferrin. J Biol Chem. 1988 Nov 15;263(32):16837–16841. [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. The role of transferrin in the mechanism of cellular iron uptake. Biochem J. 1990 Oct 1;271(1):1–9. doi: 10.1042/bj2710001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J. L., Paulson D. F., Webb K. S. 1,10-Phenanthroline reversibility inhibits proliferation of two human prostate carcinoma cell lines (PC-3 and DU145). Biochem Biophys Res Commun. 1984 Oct 30;124(2):538–543. doi: 10.1016/0006-291x(84)91587-0. [DOI] [PubMed] [Google Scholar]
- White G. P., Bailey-Wood R., Jacobs A. The effect of chelating agents on cellular iron metabolism. Clin Sci Mol Med. 1976 Mar;50(3):145–152. doi: 10.1042/cs0500145. [DOI] [PubMed] [Google Scholar]
- van der Ende A., du Maine A., Simmons C. F., Schwartz A. L., Strous G. J. Iron metabolism in BeWo chorion carcinoma cells. Transferrin-mediated uptake and release of iron. J Biol Chem. 1987 Jun 25;262(18):8910–8916. [PubMed] [Google Scholar]
- von der Heul C., Kroos M. J., de Jeu-Jaspars C. M., von Eijk H. G. The uptake of iron by reticulocytes. The influence of purification of the ghosts on iron-containing components in the ghost suspension. Biochim Biophys Acta. 1980 Oct 2;601(3):572–583. doi: 10.1016/0005-2736(80)90559-3. [DOI] [PubMed] [Google Scholar]



