Abstract
Objectives
The Centers for Disease Control and Prevention recently released new surveillance definitions for ventilator-associated events, including the new entities of ventilator-associated conditions and infection-related ventilator-associated complications. Both ventilator-associated conditions and infection-related ventilator-associated complications are associated with prolonged mechanical ventilation and hospital death, but little is known about their risk factors and how best to prevent them. We sought to identify risk factors for ventilator-associated conditions and infection-related ventilator-associated complications.
Design
Retrospective case-control study.
Setting
Medical, surgical, cardiac, and neuroscience units of a tertiary care teaching hospital.
Patients
Hundred ten patients with ventilator-associated conditions matched to 110 controls without ventilator-associated conditions on the basis of age, sex, ICU type, comorbidities, and duration of mechanical ventilation prior to ventilator-associated conditions.
Interventions
None.
Measurements
We compared cases with controls with regard to demographics, comorbidities, ventilator bundle adherence rates, sedative exposures, routes of nutrition, blood products, fluid balance, and modes of ventilatory support. We repeated the analysis for the subset of patients with infection-related ventilator-associated complications and their controls.
Main Results
Case and control patients were well matched on baseline characteristics. On multivariable logistic regression, significant risk factors for ventilator-associated conditions were mandatory modes of ventilation (odds ratio, 3.4; 95% CI, 1.6–8.0) and positive fluid balances (odds ratio, 1.2 per L positive; 95% CI, 1.0–1.4). Possible risk factors for infection-related ventilator-associated complications were starting benzodiazepines prior to intubation (odds ratio, 5.0; 95% CI, 1.3–29), total opioid exposures (odds ratio, 3.3 per 100 μg fentanyl equivalent/ kg; 95% CI, 0.90–16), and paralytic medications (odds ratio, 2.3; 95% CI, 0.79–80). Traditional ventilator bundle elements, including semirecumbent positioning, oral care with chlorhexidine, venous thromboembolism prophylaxis, stress ulcer prophylaxis, daily spontaneous breathing trials, and sedative interruptions, were not associated with ventilator-associated conditions or infection-related ventilator-associated complications.
Conclusions
Mandatory modes of ventilation and positive fluid balance are risk factors for ventilator-associated conditions. Benzodiazepines, opioids, and paralytic medications are possible risk factors for infection-related ventilator-associated complications. Prospective studies are needed to determine if targeting these risk factors can lower ventilator-associated condition and infection-related ventilator-associated complication rates.
Keywords: hospital epidemiology, mechanical ventilation, patient safety, ventilator-associated events, ventilator-associated pneumonia
In January 2013, the Centers for Disease Control and Prevention (CDC) released new surveillance definitions for ventilator-associated events (VAE) to replace their longstanding surveillance definitions for ventilator-associated pneumonia (VAP). The VAE framework purposefully expands the scope of surveillance from pneumonia alone to include all complications of mechanical ventilation severe enough to trigger sustained increases in ventilatory support (1). VAEs are strongly associated with prolonged mechanical ventilation, extended intensive care and hospital lengths of stay, and higher hospital mortality rates (2–6). Very little is known, however, about VAEs’ specific risk factors and how best to prevent VAEs.
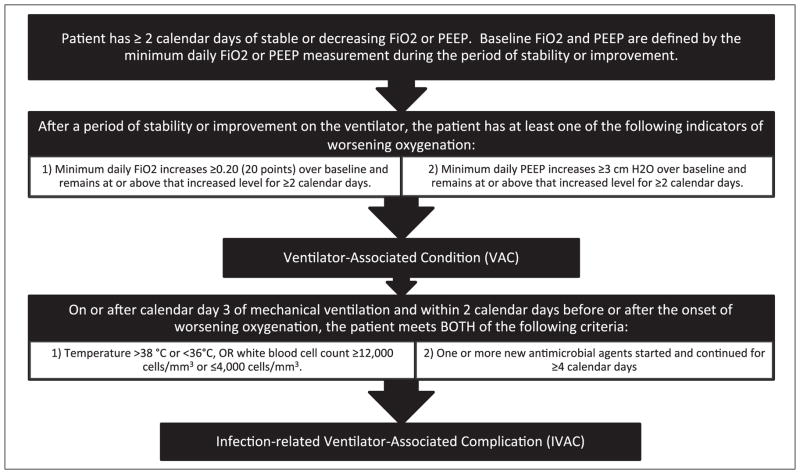
The VAE framework includes a hierarchy of definitions beginning with “ventilator-associated conditions” (VACs). VAC is defined as more than or equal to 2 days of increased ventilator settings after more than or equal to 2 days of stable or improving settings (Fig. 1). The second VAE target is “infection-related ventilator-associated complications” (IVACs), defined as the subset of VACs with concurrent inflammatory signs and more than or equal to 4 days of new antibiotics. The third VAE tier is possible or probable pneumonia. Patients with IVAC and concurrent purulent sputum or positive pulmonary cultures have possible pneumonia. Patients with IVAC and concurrent purulent sputum plus positive pulmonary cultures have probable pneumonia.
Figure 1.
The Centers for Disease Control and Prevention’s National Healthcare Safety Network ventilator-associated condition and infection-related ventilator associated complication criteria. PEEP = positive end-expiratory pressure.
VAE definitions allow for the possibility of automated surveillance using electronic clinical data but can be applied electronically or manually. Note that VAEs are surveillance concepts not clinical diagnoses. VAEs reflect rather than inform immediate patient management. This is because VAEs are only apparent in retrospect after ventilator increases have been sustained for at least 2 days, or for IVAC, once antibiotics have been continued for at least 4 days. VAE surveillance is intended to give a population-level estimate of complication rates rather than real-time diagnostic information to inform immediate patient management.
There is a rich literature on risk factors and strategies to prevent VAP, but their applicability to VAC and IVAC is unknown. Qualitative analyses suggest that most VACs are caused by pneumonia, atelectasis, acute pulmonary edema, acute respiratory distress syndrome, pulmonary embolism, aspiration, and abdominal distension (2, 5). Classic VAP prevention strategies may mitigate the 25–40% of VACs attributable to pneumonia but are unlikely to prevent the other conditions associated with VAC.
In addition, classic VAP prevention measures may not be the highest yield strategies to improve outcomes for mechanically ventilated patients. This is partly because VAP rates are now very low in most ICUs, partly because the attributable mortality of VAP is low, and partly due to ongoing uncertainty regarding the true impact of VAP prevention strategies (7–10). Multiple VAP prevention measures have been shown to decrease VAP rates, but very few shorten duration of mechanical ventilation or lower mortality rates (11–13). Given that VAC is more frequent than VAP and the strong association between VAC and adverse outcomes, prevention measures directed against VAC may prove to be higher yield strategies to improve population outcomes.
There is consequently a pressing need to define risk factors for VAC and IVAC and to test whether prevention strategies targeting risks specific to VAC and IVAC lead to lower rates and better outcomes for patients. We conducted a matched case-control study to identify potentially modifiable risk factors for VAC and IVAC.
METHODS
We retrospectively identified all VACs that occurred in Brigham and Women’s Hospital during calendar year 2011 using an electronic database of daily ventilator settings maintained by the hospital’s respiratory therapy department. We matched each VAC patient to a patient without VAC on the basis of age, sex, ICU type, Charlson score, and time to VAC onset. Because VAC events require at least two calendar days of increased ventilator settings, we required control patients to be ventilated for more than or equal to the paired case patient’s time from intubation to VAC onset plus 1 day. For each pair, a “match date” was assigned to the control patient to match the case patient’s time from intubation to VAC onset. The study was reviewed and approved by the Institutional Review Board of Brigham and Women’s Hospital.
We reviewed patients’ paper and electronic medical records to identify demographics, comorbidities, medications, laboratory values, blood products, nutrition support, daily fluid balances, ventilator settings, ventilator bundle adherence rates, and outcomes. We derived comorbidities from International Classification of Diseases, Ninth Edition, and Diagnosis-Related Group codes using the methods of Charlson et al (14) and Elixhauser et al (15). For processes of care, such as blood product support, modes of nutrition, daily fluid balances, ventilator settings, and ventilator bundle components, we evaluated care over both the 3-day and 7-day periods preceding the VAC onset date (for cases) or match date (for controls). We summarized performance for both time frames as the mean daily performance rate and as an all-or-nothing yes-no binary variable. For patients ventilated for less than 3 or 7 days prior to VAC, we only included their ventilator days until VAC onset.
We gathered data on daily ventilator bundle adherence using a database populated from standardized checklists prospectively completed by bedside nurses and respiratory therapists on a daily basis. Our hospital’s ventilator bundle includes elevation of the head of the bed to more than or equal to 30 degrees, daily oral care with chlorhexidine, mechanical or chemical thromboembolism prophylaxis, stress ulcer prophylaxis, daily sedative interruptions, and daily spontaneous breathing trials. Mean Rapid Shallow Breathing Index values were also abstracted whenever available. We recorded average daily minimum and maximum tidal volumes and mode of ventilation at the time of minimum and maximum tidal volume. We summarized patients’ mode of ventilation as both the proportion of days on a mandatory ventilator mode and an all-or-nothing variable for patients on a mandatory ventilator mode on all days surveyed. We defined all modes of mechanical ventilation other than pressure support as mandatory.
We calculated patients’ daily fluid balance as the difference between daily total inputs and outputs. For patients on continuous venovenous hemofiltration (CVVH), we recorded their net volume change per day as marked on their CVVH flow sheets. We classified patients’ mode of feeding as nil per os, total parenteral nutrition, or tube feeds for patients receiving enteral nutrition via oropharyngeal, nasopharyngeal, gastric, or jejunal tubes. We collected blood product administration in units by both product type and as the sum of all products received.
We gathered medication data from both electronic orders and medication administration records with additional chart review as needed to resolve discrepancies. Benzodiazepine and propofol doses were converted to midazolam equivalents, yielding total doses for both benzodiazepines alone and the sum of benzodiazepines and propofol combined. All opioids were converted to fentanyl equivalents. Our formulae for converting sedative and opioid exposures into midazolam and fentanyl equivalents are summarized in Table 1 (16, 17). We evaluated paralytic exposure three ways: exposure to any paralytic except succinylcholine from intubation to VAC or match date, exposure to any cisatracurium from intubation to VAC or match date, and cumulative cisatracurium exposure in mg/kg during the 3- and 7-day periods preceding VAC onset. Finally, we tabulated patients’ daily minimum Richmond Agitation Sedation Scale scores.
TABLE 1.
Sedative and Opioid Dose Conversion Formulae
| Opioids |
| Hydromorphone dose × 200/3 = Fentanyl equivalent (μg) |
| Morphine dose × 10 = Fentanyl equivalent (μg) |
| Oxycodone dose × 5 = Fentanyl equivalent (μg) |
| Total opioid = Fentanyl + hydromorphone + morphine + oxycodone (μg fentanyl equivalents) |
|
|
| Benzodiazepines |
| Diazepam × 0.106 = Midazolam equivalent (mg) |
| Lorazepam × 3.03 = Midazolam equivalent (mg) |
| Clonazepam × 6.06 = Midazolam equivalent (mg) |
| Alprazolam × 6.06 = Midazolam equivalent (mg) |
| Total benzodiazepine = Midazolam + diazepam + lorazepam + clonazepam + alprazolam (mg midazolam equivalents) |
|
|
| Sedatives |
| Propofol × 0.063 = Midazolam equivalent (mg) |
| Total sedative = Total benzodiazepine + propofol (mg midazolam) |
Statistical Analysis
We performed bivariate analyses examining the associations between each covariate and VAC and IVAC, respectively. We used conditional logistic regression to account for matching. We ranked covariates based on their p values from the likelihood ratio test and then developed separate multivariable conditional logistic regression models for VAC and IVAC. We initially included all variables with p values less than or equal to 0.1 as well variables of particular clinical interest and then sequentially removed the least significant variables and clinically overlapping variables (such as fluids in and net fluid balance) until the models converged. If removing a variable led to a substantial change in the estimated effect size for any of the remaining variables, we returned the variable to the model. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
There were 2,990 patients on mechanical ventilation in an ICU during the study period. There were 172 VACs and 70 IVACs (13.0 and 5.2 events per 1,000 ventilator days, respectively). We successfully matched 110 VAC cases to controls. Of the 110 VACs with successful matches, 38 met criteria for IVAC. Median time to both VAC and IVAC was 4 days (interquartile range, 3–7 d).
Baseline Characteristics
VAC and IVAC case and control patients’ baseline characteristics and outcomes are presented in Table 2. VAC cases and controls were well matched with regard to age, sex, race, weight, type of ICU, Charlson score, comorbidities, medications at the start of mechanical ventilation, and initial laboratory values. There were, however, some differences. Cases were less likely to have a history of congestive heart failure (10% vs 19%, p = 0.06) and alcohol abuse (1% vs 10%, p = 0.002). Cases were more likely to have sepsis at the time of intubation (40% vs 27%, p = 0.04) and to have been prescribed bronchodilators (71% vs 61%, p = 0.03). Cases had significantly lower mean aspartate aminotransferase levels at admission (233 vs 495, p = 0.03), but the rest of their liver function tests were similar to controls.
TABLE 2.
Characteristics of Patients With Ventilator-Associated Conditions, Infection-Related Ventilator-Associated Complications, and Their Matched Controls
| Variable | Ventilator-Associated Condition Cases | Controls | p | Infection-Related Ventilator-Associated Complication Cases | Controls | p |
|---|---|---|---|---|---|---|
| Mean age, yr (SD) | 60 (14) | 60 (14) | NS | 58 (15) | 59 (15) | NS |
|
| ||||||
| Male sex (%) | 69 (63) | 69 (63) | NS | 27 (71) | 27 (71) | NS |
|
| ||||||
| Race (%) | ||||||
| Asian | 3 (3.0) | 3 (2.0) | NS | 2 (5.7) | 1 (2.6) | NS |
| Black | 9 (8.9) | 11 (10.6) | NS | 4 (11.4) | 1 (2.6) | NS |
| Hispanic | 3 (3.0) | 5 (4.9) | NS | 1 (2.9) | 0 (0) | NS |
| White | 86 (85) | 85 (82) | NS | 28 (80) | 36 (95) | 0.05 |
|
| ||||||
| Weight, kg (SD) | 88 (27) | 87 (23) | NS | 87 (31) | 88 (25) | NS |
|
| ||||||
| Unit (%) | ||||||
| Cardiac surgery | 5 (4.6) | 5 (4.6) | NS | 1 (2.6) | 1 (2.6) | NS |
| Coronary care unit | 8 (7.3) | 8 (7.3) | NS | 2 (5.3) | 2 (5.3) | NS |
| Medical ICU | 38 (35) | 38 (35) | NS | 12 (32) | 12 (32) | NS |
| Neuroscience ICU | 11 (10) | 11 (10) | NS | 5 (13) | 5 (13) | NS |
| Surgical ICU | 35 (32) | 35 (32) | NS | 15 (39) | 15 (39) | NS |
| Thoracic surgery ICU | 13 (12) | 13 (12) | NS | 3 (7.9) | 3 (7.9) | NS |
|
| ||||||
| Charlson score, mean (SD) | 3.1 (2.5) | 3.1 (2.5) | NS | 2.3 (2.2) | 2.7 (2.5) | 0.04 |
|
| ||||||
| Comorbidities (%) | ||||||
| Congestive heart failure | 11 (10) | 21 (19) | 0.06 | 1 (2.6) | 7 (18) | 0.004 |
| Chronic lung disease | 12 (11) | 9 (8.2) | NS | 3 (7.9) | 1 (2.6) | NS |
| Diabetes mellitus | 6 (5.5) | 8 (7.3) | NS | 1 (2.6) | 2 (5.3) | NS |
| Renal failure | 7 (6.4) | 10 (9.1) | NS | 2 (5.3) | 2 (5.3) | NS |
| Liver failure | 6 (5.5) | 3 (2.7) | NS | 1 (2.6) | 1 (2.6) | NS |
| Metastatic cancer | 11 (10) | 10 (9) | NS | 3 (7.9) | 4 (11) | NS |
| Coagulopathy | 16 (15) | 19 (17) | NS | 5 (13) | 7 (18) | NS |
| Obesity | 5 (4.6) | 4 (3.6) | NS | 1 (2.6) | 2 (5.3) | NS |
| Alcohol abuse | 1 (0.9) | 11 (10) | 0.002 | 0 (0) | 5 (13) | 0.01 |
|
| ||||||
| Sepsis at intubation (%) | 44 (40) | 30 (27) | 0.04 | 15 (39) | 13 (34) | NS |
|
| ||||||
| Medications ordered between day of admission and day of intubation (%) | ||||||
| Antacids | 109 (99) | 109 (99) | NS | 38 (100) | 38 (100) | NS |
| Antibiotics | 103 (94) | 103 (94) | NS | 33 (87) | 37 (97) | 0.02 |
| Anticonvulsants | 22 (20) | 24 (22) | NS | 9 (24) | 10 (26) | NS |
| Benzodiazepines | 89 (81) | 89 (81) | NS | 34 (89) | 27 (71) | 0.05 |
| Bronchodilators | 78 (71) | 67 (61) | 0.03 | 23 (61) | 23 (61) | NS |
| Diuretics | 56 (51) | 57 (52) | NS | 15 (39) | 21 (55) | NS |
| Immunomodulators | 15 (14) | 13 (12) | NS | 7 (18) | 4 (11) | NS |
| Paralytics | 41 (37) | 35 (31) | NS | 16 (42) | 11 (29) | NS |
| Pressors | 88 (80) | 85 (77) | NS | 29 (76) | 30 (79) | NS |
| Propofol | 97 (88) | 98 (89) | NS | 35 (92) | 34 (89) | NS |
| Steroids | 45 (41) | 38 (35) | NS | 16 (42) | 13 (34) | NS |
|
| ||||||
| Laboratory results on intubation, mean (SD) | ||||||
| Creatinine, mg/dL | 1.6 (1.5) | 1.6 (1.3) | NS | 1.5 (1.2) | 1.3 (0.9) | NS |
| Troponin-T, ng/mL | 0.45 (1.0) | 0.72 (2.31) | NS | 0.55 (1.45) | 0.32 (0.79) | 0.05 |
| Alanine aminotransferase, U/L | 144 (381) | 364 (1,109) | NS | 135 (287) | 554 (1,335) | NS |
| Aspartate aminotransferase, U/L | 233 (710) | 495 (1,395) | 0.03 | 226 (459) | 832 (2,160) | NS |
| Albumin, g/dL | 2.6 (0.67) | 2.6 (0.71) | NS | 2.7 (0.7) | 2.6 (0.6) | NS |
| Maximum WBC, /mm3 | 15 (8.3) | 15 (7.7) | NS | 16 (7.8) | 14 (5.9) | NS |
| Hematocrit, % | 27 (6.4) | 27 (6.0) | NS | 27 (6.3) | 28 (6.0) | NS |
| Prothrombin time, s | 20 (7.5) | 19 (7.2) | NS | 22 (11) | 19 (6.9) | NS |
| Partial thromboplastin time, s | 54 (34) | 52 (33) | NS | 58 (36) | 47 (26) | NS |
|
| ||||||
| Median days to event (IQR) | 4 (3–7) | 4 (3–7) | NS | 4 (3–7) | 4 (3–7) | NS |
|
| ||||||
| Outcomes | ||||||
| Median ventilator days (IQR) | 11 (8–20) | 10 (7–17) | 0.02 | 15 (10–22) | 10 (7–19) | 0.02 |
| Median hospital days (IQR) | 24 (15–35) | 25 (18–37) | NS | 27 (18–39) | 23 (18–34) | NS |
| Mortality, % | 47 (43) | 25 (23) | < 0.001 | 17 (45) | 9 (24) | 0.08 |
NS = not significant, IQR = interquartile range.
Boldface text highlights comparisons where the p value for the difference between cases and controls ≤ 0.10.
On bivariate analysis, patients with IVAC were more likely to be nonwhite (20% vs 5%, p = 0.05), less likely to have congestive heart failure (3% vs 18%, p = 0.004), less likely to be on antibiotics at the start of mechanical ventilation (87% vs 97%, p = 0.02), and more likely to be on benzodiazepines at the start of ventilation (89% vs 71%, p = 0.05). IVAC cases also had higher mean troponin-T values at admission (0.55 vs 0.32, p = 0.05).
Both VAC and IVAC cases were ventilated for significantly more days than matched controls. VAC cases also had higher hospital mortality rates (43% vs 23%; odds ratio [OR], 3.00; 95% CI, 1.57–6.22). The hospital mortality trend was similar for IVAC but not statistically significant (45% vs 24%; OR, 2.14; 95% CI, 0.90–5.61). There were no differences in mean duration of hospitalization for VAC or IVAC compared with matched controls.
Care Characteristics
Potentially modifiable processes of care for VAC and IVAC cases versus controls are presented in Table 3 (an expanded version of Table 3 describing care characteristics over additional time frames is available in Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/A960). There were no significant differences between VAC cases and controls in ventilator bundle adherence rates, route of nutrition, type and volume of blood products, sedative choices, and sedative amounts. VAC cases, however, received significantly more fluids (mean 4.9 L vs 3.8 L per day, p = 0.003) and were more likely to have a net positive fluid balance (mean 2.4 L vs 1.5 L per day, p = 0.004) compared with controls. VAC patients were also significantly more likely to be on mandatory ventilator modes at the time of maximum daily tidal volume (36% vs 19%, p = 0.003).
TABLE 3.
Ventilator-Associated Condition and Infection-Related Ventilator-Associated Complication Bivariate Risk Factor Analysis
| Variable | VAC Cases | Controls | OR (95% CI) | p | Infection-Related Ventilator-Associated Complication Cases | Controls | OR (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
| Classic ventilator bundle components: mean % adherence over 3 d prior to VAC (SD) | ||||||||
| Head of bed elevation | 77 (28) | 74 (32) | 1.01 (1.00–1.02) | NS | 73 (34) | 75 (35) | 1.00 (0.98–1.01) | NS |
| Oral care with chlorhexidine | 77 (26) | 82 (30) | 1.01 (1.00–1.02) | NS | 75 (29) | 75 (33) | 1.00 (0.98–1.02) | NS |
| Deep venous thrombosis prophylaxis | 80 (27) | 74 (31) | 1.01 (1.00–1.02) | NS | 77 (32) | 77 (32) | 1.00 (0.98–1.02) | NS |
| Stress ulcer prophylaxis | 85 (24) | 79 (28) | 1.01 (1.00–1.02) | 0.08 | 83 (28) | 80 (29) | 1.01 (0.98–1.02) | NS |
| Sedative interruption | 47 (38) | 45 (35) | 1.00 (0.99–1.01) | NS | 44 (39) | 49 (37) | 1.00 (0.98–1.01) | NS |
| Spontaneous breathing trial | 22 (31) | 21 (30) | 1.00 (0.99–1.01) | NS | 21 (30) | 22 (31) | 1.00 (0.98–1.02) | NS |
|
| ||||||||
| Nutrition over 3 d prior to VAC (%) | ||||||||
| Nothing by mouth 100% of the time | 36 (33) | 36 (33) | 1.00 (0.49–2.06) | NS | 10 (26) | 11 (29) | 0.86 (0.28–2.58) | NS |
| Tube feeding—any exposure | 59 (54) | 68 (62) | 0.64 (0.34–1.19) | NS | 26 (68) | 26 (68) | 1.00 (0.34–2.92) | NS |
| Parenteral nutrition—any exposure | 15 (14) | 11 (10) | 1.50 (0.62–3.83) | NS | 2 (5.3) | 3 (7.9) | 0.67 (0.09–4.02) | NS |
|
| ||||||||
| Blood products: mean (units over 3 d prior to VAC) (SD) | ||||||||
| RBC | 0.63 (1.3) | 0.56 (1.6) | 1.04 (0.85–1.30) | NS | 0.7 (1.5) | 0.7 (2.3) | 1.01 (0.75–1.36) | NS |
| Fresh-frozen plasma | 0.44 (1.3) | 0.40 (1.4) | 1.03 (0.81–1.31) | NS | 0.5 (1.3) | 0.7 (2.1) | 0.92 (0.61–1.28) | NS |
| Platelets | 0.13 (0.4) | 0.15 (0.6) | 0.92 (0.50–1.64) | NS | 0.1 (0.3) | 0.3 (1.0) | 0.70 (0.20–1.47) | NS |
|
| ||||||||
| Sedative and paralytic medications: mean total dose over 7 d prior to VAC (SD) | ||||||||
| Total sedatives (propofol + benzodiazepines), mg midazolam equivalents/kg | 4.7 (5.5) | 5.9 (6.7) | 0.96 (0.91–1.01) | 0.10 | 6.1 (6.8) | 7.0 (7.4) | 0.98 (0.91–1.05) | NS |
| Total benzodiazepine, mg midazolam equivalents/kg | 1.0 (1.2) | 1.0 (1.5) | 1.00 (0.85–1.18) | NS | 0.9 (1.1) | 0.9 (1.2) | 1.01 (0.67–1.53) | NS |
| Propofol, mg midazolam equivalents/kg | 3.7 (5.2) | 4. (6.6) | 0.96 (0.91–1.01) | NS | 5.2 (6.8) | 6.1 (7.5) | 0.98 (0.91–1.05) | NS |
| Total opioid, 100 μg fentanyl equivalents/kg | 0.66 (0.95) | 0.62 (0.89) | 1.06 (0.77–1.50) | NS | 0.67 (0.64) | 0.49 (0.54) | 2.64 (0.90–9.6) | 0.08 |
| Dexmedetomidine, mg/kg | 0.3 (1.5) | 0.7 (4.6) | 0.95 (0.82–1.04) | NS | 0.1 (1.3) | 1.2 (7.1) | 0.95 (0.68–1.05) | NS |
| Cisatracurium, mg/kg | 0.3 (1.3) | 0.4 (1.4) | 0.98 (0.80–1.19) | NS | 0.6 (1.9) | 0.5 (1.9) | 1.02 (0.79–1.35) | NS |
|
| ||||||||
| Any cisatracurium within the 7 d prior to VAC (%) | 13 (12) | 11 (10) | 1.20 (0.52–2.84) | NS | 8 (21) | 4 (11) | 2.33 (0.65–10.83) | NS |
|
| ||||||||
| Richmond Agitation Sedation Scale: 7-day mean prior to VAC (SD) | –2.8 (1.4) | –2.6 (1.7) | 0.85 (0.64–1.08) | NS | –3.1 (1.2) | –2.9 (1.3) | 0.75 (0.44–1.19) | NS |
|
| ||||||||
| Fluid balance: 3-day mean/d prior to VAC (SD) | ||||||||
| Ins, L | 4.9 (3.7) | 3.8 (2.6) | 1.20 (1.05–1.41) | 0.003 | 4.7 (3.8) | 4.3 (3.1) | 1.07 (0.90–1.31) | NS |
| Outs, L | 2.5 (2.1) | 2.5 (1.4) | 1.01 (0.85–1.21) | NS | 2.4 (1.5) | 2.8 (1.5) | 0.66 (0.36–1.07) | 0.10 |
| Net balance, L | 2.4 (3.4) | 1.5 (2.3) | 1.20 (1.06–1.40) | 0.004 | 2.4 (3.8) | 1.6 (2.5) | 1.13 (0.95–1.43) | NS |
|
| ||||||||
| Fluid balance: 7-day mean/d prior to VAC (SD) | ||||||||
| Ins, L | 4.8 (3.5) | 3.8 (2.5) | 1.19 (1.05–1.38) | 0.004 | 4.7 (3.8) | 4.1 (3.0) | 1.08 (0.92–1.31) | NS |
| Outs, L | 2.4 (1.6) | 2.3 (1.3) | 1.07 (0.87–1.34) | NS | 2.4 (1.4) | 2.6 (14) | 0.79 (0.46–1.25) | NS |
| Net balance, L | 2.4 (3.3) | 1.6 (2.1) | 1.16 (1.03–1.35) | 0.015 | 2.3 (3.7) | 1.6 (2.3) | 1.10 (0.93–1.36) | NS |
|
| ||||||||
| Ventilator settings: 7-day mean prior to VAC onset (SD) | ||||||||
| Minimum tidal volume, mL/kg | 5.3 (1.6) | 5.1 (1.4) | 1.12 (0.91–1.39) | NS | 5.5 (1.3) | 5.1 (1.4) | 1.51 (0.95–2.59) | 0.08 |
| Maximum tidal volume, mL/kg | 7.7 (2.4) | 7.6 (2.4) | 1.01 (0.89–1.15) | NS | 8.0 (1.9) | 7.5 (2.3) | 1.14 (0.89–1.48) | NS |
| % of days on mandatory ventilatory modes at minimum TV | 62 (40) | 54 (38) | 1.01 (1.00–1.02) | 0.05 | 61 (37) | 54 (37) | 1.01 (0.99–1.02) | NS |
| % of days on mandatory ventilatory modes at maximum TV | 54 (41) | 42 (37) | 1.01 (1.00–1.02) | 0.007 | 52 (37) | 44 (37) | 1.01 (0.99–1.02) | NS |
| Mandatory ventilatory modes 100% of days at minimum TV (%) | 48 (44) | 35 (32) | 2.00 (1.05–4.02) | 0.04 | 13 (34) | 10 (26) | 1.50 (0.54–4.47) | NS |
| Mandatory ventilatory modes 100% of days at maximum TV (%) | 40 (36) | 21 (19) | 2.73 (1.41–5.69) | 0.003 | 10 (26) | 7 (18) | 1.60 (0.53–5.29) | NS |
VAC = ventilator-associated conditions, OR = odds ratio, NS = not significant, TV = tidal volume.
Boldface text highlights comparisons where the p value for the difference between cases and controls ≤ 0.10.
There were no differences between IVAC cases and controls in ventilator bundle adherence rates, route of nutrition, and type or volume of blood products administered. IVAC patients trended toward more total opioids (67 μg fentanyl equivalents/kg vs 49 μg/kg, p = 0.08). There was also trends toward less fluid output (mean 2.4 L vs 2.8 L per day, p = 0.10) and higher average minimum daily tidal volumes (5.5 mL/kg vs 5.1 mL/kg, p = 0.08).
Multivariable Models
On multivariable conditional logistic regression (Table 4), significant predictors of VAC were mandatory modes of mechanical ventilation (OR, 3.4; 95% CI, 1.6–8.0) and net daily fluid balance (OR, 1.2 per L; 95% CI, 1.0–1.4). Both history of congestive heart failure and liver disease were negative predictors. We were unable to include history of alcohol abuse in the multivariable model because there were too few events to allow the model to converge.
TABLE 4.
Multivariable Analysis of Risk Factors for Ventilator-Associated Conditions and Infection-Related Ventilator-Associated Complications
| Variable | OR | 95% CI |
|---|---|---|
| Ventilator-associated condition multivariable risk factor analysis | ||
| Mandatory ventilator modes 100% of days at maximum tidal volume | 3.4 | 1.6–8.0 |
| Net fluid balance (L) | 1.2 | 1.03–1.4 |
| Stress ulcer prophylaxis | 1.01 | 0.998–1.03 |
| Propofol started at intubation | 0.48 | 0.20–1.07 |
| Congestive heart failure | 0.40 | 0.15–0.95 |
| Liver disease | 0.12 | 0.01–0.85 |
|
| ||
| Infection-related ventilator-associated complication multivariable risk factor analysis | ||
| Benzodiazepines started between admission and intubation | 5.0 | 1.3–29 |
| Total opioid administered (per 100 μg fentanyl equivalents/kg) | 3.3 | 0.90–16 |
| Paralytic administered while intubated | 2.3 | 0.79–8.0 |
| Minimum tidal volume (mL/kg) | 1.5 | 0.91–2.9 |
| Net fluid balance (L) | 1.1 | 0.90–1.5 |
OR = odds ratio.
Boldface text highlights comparisons where the 95% confidence interval excludes 1.0.
In the multivariable model for IVAC (Table 4), starting benzodiazepines prior to intubation increased IVAC risk (OR, 5.0; 95% CI, 1.3–29). Additional positive risk factors for IVAC with near-significant ORs were total opioid exposures (OR, 3.3 per 100 μg fentanyl equivalent/kg; 95% CI, 0.90–16), paralytic exposures (OR, 2.3; 95% CI, 0.79–8.0), larger minimum tidal volumes (OR, 1.5 per mL/kg; 95% CI, 0.91–2.9), and positive daily fluid balances (OR, 1.1 per L positive; 95% CI, 0.90–1.5).
DISCUSSION
CDC’s new VAE definitions allow objective and reproducible surveillance for morbid complications of mechanical ventilation. This study is the first, however, to identify risk factors for VAC and IVAC that might inform prevention strategies. We identified two significant risk factors for VAC and three possible risk factors for IVAC. Mandatory ventilator modes and greater net fluid balance increased the likelihood of VAC. Initiating benzodiazepines prior to intubation, higher opioid exposures, and paralytics were possible risk factors for IVAC.
Fluid Status
The association between VAC and excess fluids is consistent with both observational and randomized controlled trial data. Higher central venous pressures are correlated with adverse outcomes in critically ill patients. Conservative fluid resuscitation leads to more ventilator-free days compared with liberal resuscitation strategies (18, 19). The association between VAC and excess fluids is also consistent with the analysis of Hayashi et al (5) who found that VAC patients received more furosemide than non-VAC patients and with the analysis of Klompas et al (2) who found that about a quarter of VACs were attributable to pulmonary edema. These observations may also explain why history of congestive heart failure was protective against VAC in the multivariable model. Clinicians may administer fluids more cautiously in patients with a history of heart failure. A post hoc analysis of our data confirmed that patients with a history of congestive heart failure were given one third less fluids than patients without congestive heart failure (mean 2.9 vs 4.6 L per day). Improving fluid management may prevent VACs and improve patients’ outcomes.
Mandatory Mode of Ventilation
Mandatory ventilator modes were another significant predictor for VAC in our study. Mandatory ventilation may increase the risk of VAC and related morbidity by increasing ventilator dyssynchrony, barotrauma, and ventilator-induced lung injury (VILI). VILI is already an established predictor of adverse outcomes in ventilated patients (20–22). VAC may be a surveillance marker for VILI.
The association between VAC and mandatory ventilator modes may be confounded by severity of pulmonary disease, neurological suppression, paralytic medications, or heavy sedation rather than an independent cause for VAC. Patients with these exposures can be very difficult to ventilate and may require frequent adjustments in ventilator settings that could inadvertently trigger VAC criteria. None of these alternative factors were significant on multivariable analysis, but prospective interventional studies are needed to elucidate these relationships more clearly. Of note, it is possible that some well-established care standards that have already been shown to improve patient outcomes work in part by minimizing patients’ exposures to mandatory ventilator modes, including spontaneous breathing trials and sedative weaning protocols (23–27).
Sedation, Analgesia, and Paralysis
The third category of potentially significant risk factors included paralytics, sedatives, and opioid analgesics. On multivariable analysis, benzodiazepines prior to intubation were significantly correlated with IVAC. Opioids and paralytics trended toward significant associations; the robust effect size and lower CIs close to 1.0 suggest that these may be true risk factors and the lack of statistical significance was due to small sample size.
Sedative and analgesic administration may increase VAE risk through multiple mechanisms. Higher levels of sedation increase the risk for delirium, agitation, re-intubation, and aspiration (28). In addition, greater doses of sedatives prolong duration of mechanical ventilation and hence time at risk for ventilator-associated complications. Higher levels of sedation may also increase the need to use mandatory modes of ventilation, which in turn we found to be an independent risk factor for VAC.
The negative association between history of liver disease and VAC may be related to sedative prescribing practices: clinicians may be more cautious about prescribing sedating agents to patients with impaired liver function. A post hoc analysis of our data confirmed that patients with a history of liver disease were prescribed approximately 75% less benzodiazepines and propofol compared with patients without liver disease in the 7 days prior to VAC onset. Sedative management strategies may also explain the negative bivariate association between alcohol abuse and VAC. Patients with a history of alcohol abuse tend to be more tolerant of benzodiazepines than other patients, and clinicians may be more cautious about prescribing opioids to this population. Post hoc analysis of our data confirmed that patients with a history of alcohol abuse were prescribed more benzodiazepines but fewer opioids and more dexmedetomidine compared with patients without alcohol abuse.
There is substantial evidence that daily sedative interruptions decrease duration of mechanical ventilation, length of stay, and perhaps mortality (27, 29–32). In our study, sedative interruptions protected against neither VAC nor IVAC. However, the daily rate of sedative interruption adherence was less than 40% for both VAC and non-VAC patients. Very low rates of sedative interruptions may have attenuated our capacity to detect benefits.
The possible association between IVAC and both sedatives and paralytics matches prior studies reporting a correlation between sedation and infections in the intensive care setting (29, 32). Nseir et al (33) have proposed several mechanisms by which sedatives and opioids may increase risk for infection in the ICU. These include prolonged exposure to risk factors for infection such as central venous and urinary catheters, microaspiration of gastric contents, intestinal dysmotility and associated microbiological imbalance, microcirculatory changes that might contribute to multisystem organ failure, and direct immunomodulatory effects. Minimizing sedatives and paralytics are therefore additional possible strategies to prevent VAC and IVAC.
Ventilator Bundles
We found no significant association between VAC and any component of the ventilator bundle, including semirecumbent positioning, oral care with chlorhexidine, mechanical or chemical thromboembolism prophylaxis, stress ulcer prophylaxis, sedative interruptions, and spontaneous breathing trials. We did not have sufficient possible VAP or probable VAP cases to evaluate whether bundle components decreased risk for these events. However, since each VAP case must also by definition have IVAC and VAC, we would have expected to see some protective signal toward VAC and IVAC.
The ventilator bundle has been the subject of considerable controversy due to the lack of clear evidence that it improves patient outcomes (11, 34, 35). A new bundle optimized to prevent VAC and other complications of mechanical ventilation may now be warranted. This study helps identify possible strategies to include in such a bundle such as conservative fluid management, minimizing sedative and paralytic exposures, and minimizing the use of mandatory ventilator modes. These interventions are all consistent with emerging best practices for the prevention and/or management of acute respiratory distress syndrome, pulmonary edema, delirium, and early liberation from mechanical ventilation (16, 19, 27).
Limitations
The findings of our study must be interpreted within the context of the study’s limitations. We gathered data retrospectively from a single center. We analyzed relatively few events, particularly IVACs, therefore limiting our power to identify potential risk factors. The physician reviewer was not blinded to patients’ case versus control status. Matching was largely successful in aligning demographics, overall severity of illness, and different ICU settings, but several nonmodifiable variables were not well matched, including congestive heart failure and alcohol abuse. We were unable to match all VAC patients to controls, potentially skewing our picture of the VAC population.
CONCLUSIONS
In conclusion, this study identifies potentially modifiable patterns of care associated with VAC and IVAC. Mandatory ventilator modes and positive fluid balance are significant risk factors for VAC. Benzodiazepines and possibly opioids and paralytics are risk factors for IVAC. These risk factors are potentially fruitful targets for intervention and prevention. Prospective studies are now warranted to test whether strategies targeting these risk factors can reduce VAE rates and improve patients’ outcomes.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Lewis is employed by University of California at San Francisco Infectious Disease Division. Dr. Li received reseach support from the Centers for Disease Control and Prevention (CDC). Dr. Klompas has received grant support from CDC and honoraria for lectures on ventilator-associated pneumonia surveillance from Premier Healthcare Alliance. He lectured for Infectious Disease Association of California and received support for travel from Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, and American Society of Microbiologists. His institution received grant support from the CDC. Mr. Murphy has disclosed that he does not have any potential conflicts of interest.
References
- 1.Magill SS, Klompas M, Balk R, et al. Developing a new, national approach to surveillance for ventilator-associated events. Crit Care Med. 2013;41:2467–2475. doi: 10.1097/CCM.0b013e3182a262db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klompas M, Khan Y, Kleinman K, et al. CDC Prevention Epicenters Program: Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One. 2011;6:e18062. doi: 10.1371/journal.pone.0018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klompas M, Kleinman K, Khan Y, et al. CDC Prevention Epicenters Program: Rapid and reproducible surveillance for ventilator-associated pneumonia. Clin Infect Dis. 2012;54:370–377. doi: 10.1093/cid/cir832. [DOI] [PubMed] [Google Scholar]
- 4.Prospero E, Illuminati D, Marigliano A, et al. Learning from Galileo: Ventilator-associated pneumonia surveillance. Am J Respir Crit Care Med. 2012;186:1308–1309. doi: 10.1164/ajrccm.186.12.1308. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi Y, Morisawa K, Klompas M, et al. Toward improved surveillance: The impact of ventilator-associated complications on length of stay and antibiotic use in patients in intensive care units. Clin Infect Dis. 2013;56:471–477. doi: 10.1093/cid/cis926. [DOI] [PubMed] [Google Scholar]
- 6.Muscedere J, Sinuff T, Heyland DK, et al. Canadian Critical Care Trials Group: The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest. 2013;144:1453–1460. doi: 10.1378/chest.13-0853. [DOI] [PubMed] [Google Scholar]
- 7.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297:1583–1593. doi: 10.1001/jama.297.14.1583. [DOI] [PubMed] [Google Scholar]
- 8.Klompas M, Platt R. Ventilator-associated pneumonia-the wrong quality measure for benchmarking. Ann Intern Med. 2007;147:803–805. doi: 10.7326/0003-4819-147-11-200712040-00013. [DOI] [PubMed] [Google Scholar]
- 9.Klompas M. Eight initiatives that misleadingly lower ventilator-associated pneumonia rates. Am J Infect Control. 2012;40:408–410. doi: 10.1016/j.ajic.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Tejerina E, Esteban A, Fernández-Segoviano P, et al. Accuracy of clinical definitions of ventilator-associated pneumonia: Comparison with autopsy findings. J Crit Care. 2010;25:62–68. doi: 10.1016/j.jcrc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Klompas M. The paradox of ventilator-associated pneumonia prevention measures. Crit Care. 2009;13:315. doi: 10.1186/cc8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouadma L, Wolff M, Lucet JC. Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis. 2012;25:395–404. doi: 10.1097/QCO.0b013e328355a835. [DOI] [PubMed] [Google Scholar]
- 13.Bonten MJ. Healthcare epidemiology: Ventilator-associated pneumonia: Preventing the inevitable. Clin Infect Dis. 2011;52:115–121. doi: 10.1093/cid/ciq075. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:278–280. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 17.Devlin JW, Roberts RJ. Pharmacology of commonly used analgesics and sedatives in the ICU: Benzodiazepines, propofol, and opioids. Anesthesiol Clin. 2011;29:567–585. doi: 10.1016/j.anclin.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg AL, Dechert RE, Park PK, et al. NIH NHLBI ARDS Network: Review of a large clinical series: Association of cumulative fluid balance on outcome in acute lung injury: A retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24:35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- 19.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 20.Gillette MA, Hess DR. Ventilator-induced lung injury and the evolution of lung-protective strategies in acute respiratory distress syndrome. Respir Care. 2001;46:130–148. [PubMed] [Google Scholar]
- 21.dos Santos CC, Slutsky AS. Protective ventilation of patients with acute respiratory distress syndrome. Crit Care. 2004;8:145–147. doi: 10.1186/cc2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slutsky AS, Ranieri VM. Mechanical ventilation: Lessons from the ARDSNet trial. Respir Res. 2000;1:73–77. doi: 10.1186/rr15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332:345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 25.Kollef MH, Shapiro SD, Silver P, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567–574. doi: 10.1097/00003246-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Marelich GP, Murin S, Battistella F, et al. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: Effect on weaning time and incidence of ventilator-associated pneumonia. Chest. 2000;118:459–467. doi: 10.1378/chest.118.2.459. [DOI] [PubMed] [Google Scholar]
- 27.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 28.Jackson DL, Proudfoot CW, Cann KF, et al. [Accessed April 25, 2013];Research a systematic review of the impact of sedation practice in the ICU on resource use, costs and patient safety [Internet] 2010 Available at: http://www.biomedcentral.com/content/pdf/cc8956.pdf.
- 29.Kollef MH, Levy NT, Ahrens TS, et al. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 30.Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 31.Schweickert WD, Gehlbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272–1276. doi: 10.1097/01.ccm.0000127263.54807.79. [DOI] [PubMed] [Google Scholar]
- 32.Arroliga A, Frutos-Vivar F, Hall J, et al. International Mechanical Ventilation Study Group: Use of sedatives and neuromuscular blockers in a cohort of patients receiving mechanical ventilation. Chest. 2005;128:496–506. doi: 10.1378/chest.128.2.496. [DOI] [PubMed] [Google Scholar]
- 33.Nseir S, Makris D, Mathieu D, et al. Intensive care unit-acquired infection as a side effect of sedation. Crit Care. 2010;14:R30. doi: 10.1186/cc8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan EY, Ruest A, Meade MO, et al. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: Systematic review and meta-analysis. BMJ. 2007;334:889. doi: 10.1136/bmj.39136.528160.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA. 1996;275:308–314. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.