Summary
Posterior reversible encephalopathy syndrome (PRES) is a clinical/radiological syndrome characterized by symptoms that can include seizure, headache, impaired vision and hypertension, and can be confirmed by magnetic resonance imaging. Numerous reports have emerged that describe PRES in cancer patients. The list of medications linked to PRES can include traditional cytotoxic chemotherapeutics (e.g., cisplatin, cyclophosphamide, and high-dose corticosteroids), newer agents that target the vascular endothelial growth factor pathway (e.g., bevacizumab, sunitinib, and pazopanib), and supportive care mediations (e.g., granulocyte colony stimulating factors and erythropoietin). We report, for the first time, a case of PRES that is secondary to treatment with enzalutamide, a potent androgen receptor antagonist used in the treatment of metastatic castration-resistant prostate cancer. Enzalutamide is approved for the treatment of both docetaxel-pretreated and chemotherapy-naïve metastatic castration-resistant prostate cancer. Enzalutamide has been previously linked to the increased risk of seizures. Clinicians should be aware that, in rare cases, patients treated with enzalutamide could potentially be at risk for PRES. If symptoms suggestive of PRES arise in patients receiving enzalutamide, the drug should be discontinued immediately and the diagnostic process should be initiated.
Keywords: enzalutamide, castration resistant prostate cancer, adverse event, posterior reversible encephalopathy syndrome
Introduction
Posterior reversible encephalopathy syndrome (PRES, also known as reversible posterior leukoencephalopathy syndrome) is a rare but serious clinical/radiological syndrome. Patients may present with clinical symptoms that include confusion, altered consciousness, seizures, headache, impaired vision, and acute hypertension.[1,2] However, because many of the clinical signs and symptoms of PRES are nonspecific, diagnosis is confirmed by neuroimaging findings. Magnetic resonance imaging (MRI) typically demonstrates bilateral hyperintensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images, which is indicative of subcortical edema in parietal and occipital lobes.[2,3] Fortunately, PRES is reversible in the majority of patients who are diagnosed and appropriately treated, with clinical signs and symptoms dissipating and MRI findings normalizing within days to weeks.[1-4]
Enzalutamide is an oral, next-generation anti-androgen that inhibits binding of androgen ligands to the androgen receptor (AR) and prevents both AR translocation into the nucleus and DNA binding and co-activator recruitment at the transcription complex.[5] After the phase III AFFIRM trial demonstrated a positive efficacy and safety profile for enzalutamide, it was approved by the U.S. FDA in August 2012 for the treatment of docetaxel-pretreated metastatic castration-resistant prostate cancer (mCRPC).[6] The AFFIRM study revealed that rates of fatigue, diarrhea, hot flashes, musculoskeletal pain, headache, and seizure were higher in the enzalutamide-treated patients. In September 2014, the FDA expanded the approval for enzalutamide to include treatment of chemotherapy-naïve mCRPC based on results from the phase III PREVAIL trial.[7] In the PREVAIL study, rates of fatigue, arthralgia, back pain, hot flushes, and hypertension were higher in the enzalutamide-treated patients. Here we report, for the first time, a case of enzalutamide-induced PRES.
Case Report
A 76-year-old man was started on androgen deprivation with leuprolide acetate for diffuse bone metastases two years prior to this admission. His past medical history was significant for chronic renal insufficiency (baseline creatinine of 2.0 mg/dl) and a history of multiple urinary tract infections, but no history of hypertension, headache, seizure, or stroke. After 17 months on androgen deprivation therapy, the serum prostate-specific antigen (PSA) level increased from 1.6 ng/ml to 8.7 mg/ml. Due to the development of mCRPC, the patient was initially treated with sipuleucil-T, and then with enzalutamide (160 mg once daily). After four months of treatment with enzalutamide, the PSA level decreased from 14.9 ng/ml to 0.7 ng/ml.
After four months of enzalutamide treatment, the patient presented to the emergency department with complaints of altered mental status that worsened during preceding two-week period. He presented with confusion and experienced word-finding difficulty. The patient’s medication regimen included enzalutamide, calcium, vitamin D, and hydrocodone-acetaminophen (taken as needed). Physical examination revealed elevated blood pressure of 188/97 mm Hg. The patient exhibited moderate to severe cognitive linguistic deficits, including impaired memory skills, reasoning skills, auditory comprehension, executive functioning, and written expression. A brain MRI revealed significantly abnormal T2/FLAIR signals, which is indicative of diffuse vasogenic edema (more severe in the left hemisphere) involving the occipital, temporal, parietal, and frontal lobes. There was also involvement of the corpus callosum, and a 7-mm left-to-right midline shift was observed. Multiple foci of microhemorrhage were observed throughout the brain; however, there was no evidence of acute infarct or extra-axial fluid collection. No discreet masses, indicative of new brain metastases, were detected (Figure 1A).
Figure 1.
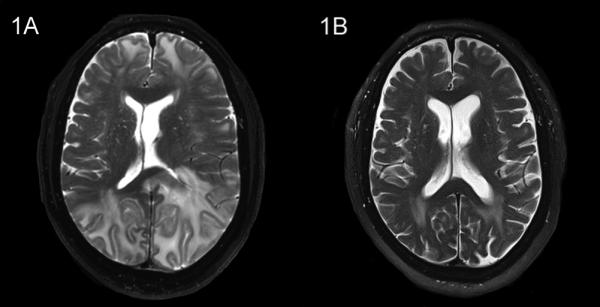
Brain MRI of the patient with PRES. (1A) The MRI at initial presentation revealed hyperintense signals on T2 sequences bilaterally in the occipital, parietal, temporal, and frontal lobes, which are indicative of diffuse vasogenic edema. Involvement of the corpus callosum, resulting in a 7-mm left-to-right midline shift, was also noted. (1B) After five weeks, a repeat MRI showed nearly complete resolution of abnormalities.
The patient was admitted to the hospital where enzalutamide was discontinued. He was treated with dexamethasone. He was also treated with an antihypertensive regimen of hydralazine 25 mg three times daily and carvedilol, which was subsequently switched to amlodipine 10 mg daily, and hydralazine treatment was continued. His blood pressure returned to normal levels within three days. His neurological examination results and mentation improved steadily throughout his one-week hospitalization period, and he became fully oriented and conversational. He was discharged from the hospital on a regimen than included amlodipine, hydralazine and dexamethasone 2 mg twice daily. The dexamethasone was subsequently discontinued after one week. A follow-up brain MRI, performed five weeks after discharge, revealed significant improvement of abnormal T2/FLAIR signals bilaterally in the frontal, temporal, and occipital lobes, with only mildly abnormal signal still detected in the posterior lobe (Figure 1B). The MRI also revealed no midline shift or mass lesions and no evidence of intracranial hemorrhage, acute infarct, or extra-axial fluid collection. Four months after this episode of PRES, the patient was reported to be doing well, without recurrence of any neurologic symptoms. The patient had remained off enzalutamide and had not required any additional treatment for mCRPC other than continuing androgen deprivation therapy.
Discussion
The development of PRES has been correlated previously with hypertensive conditions ranging from severe acute hypertension to pre-eclampsia/eclampsia and hypertensive emergencies. Moreover, it has been associated with acute porphyria, Guillain-Barré syndrome, thrombotic thrombocytopenic purpura, solid organ transplant, and cancer.[2,3] In addition, PRES has been associated with the use of cyclosporine, erythropoietin, intravenous immunoglobulin, high-dose dexamethasone, and traditional cytotoxic chemotherapeutics (e.g., carboplatin, cisplatin, cyclophosphamide, cytarabine, doxorubicin, and gemcitabine).[2,3,8-10] Recently, several case reports have emerged linking PRES to targeted anti-tumor agents that inhibit vascular endothelial growth factor and its receptors.[11]
The hypothesized etiologies of medication-induced PRES are poorly understood. One proposed model of pathogenesis centers on cerebral vascular autoregulation failure and endothelial dysfunction, which can often be secondary to medication-induced injury. Acute and severe cases of hypertension, resulting from injury to the vascular endothelium, may exceed cerebral vascular autoregulation. Sudden elevations in blood pressure, which exceed cerebral vascular autoregulation, cause pathological vasodilation of arterioles and result in blood-brain barrier dysfunction. Ultimately, blood-brain barrier dysfunction can lead to extravasation of fluids into the brain parenchyma.[1,4,12,13] A lack of adrenergic innervation in the posterior cerebral circulation appears to render that area vulnerable to vasogenic edema.[12]
The mechanisms underlying the development of PRES as a result of exposure to enzalutamide are unknown. We propose that enzalutamide-induced inhibition of gamma-aminobutyric acid A (GABAA)-gated chloride channels may explain the onset of PRES. One of the rare, yet serious, adverse events observed in the AFFIRM study was seizure that occurred in 5 (0.6%) out of 800 patients in the enzalutamide arm (while none of 399 patients in the placebo arm experienced seizure).[6] Animal studies suggest that lowered seizure threshold is a consequence of the ability of enzalutamide to cross the blood-brain barrier where it results in off-target inhibition of GABAA-gated chloride channels.[14] The AFFIRM study also revealed that the incidence of hypertension was twice as high in patients treated with enzalutamide than in those treated with placebo (6.6% versus 3.3%, respectively).[6] The GABAA inhibition in areas of the brain responsible for maintenance of sympathetic vasomotor tone (e.g., the paraventricular nucleus of the hypothalamus) has been shown to increase arterial blood pressure.[15] Another proposed alternate cause of PRES is related to cerebral vasospasm that leads to cerebral ischemia.[16] In preclinical models, the inhibition of GABAA receptors has been associated with increased regional cerebral blood flow and blood-brain barrier disruption in cerebral ischemia.[17] These data support the hypothesis that off-target inhibition of GABAA receptors by enzalutamide, especially in areas of the central nervous system (CNS) that are responsible for sympathetic tone, may lead to hypertension that exceeds cerebral vascular autoregulation and ultimately results in vasogenic edema. Furthermore, the blood-brain barrier disruption that occurs during this process may result in increased CNS concentrations of enzalutamide and, therefore, may accelerate the development of PRES. It is also possible that enzalutamide may induce PRES through an “off‐target” effect on molecules other than GABAA receptors in the CNS.
To our knowledge, this is the first reported case of enzalutamide-induced PRES. The patient’s clinical signs and symptoms and the MRI findings led to the diagnosis of PRES and the subsequent discontinuation of enzalutamide with a favorable clinical outcome. Prompt recognition and treatment of PRES are imperative because its clinical sequelae are not always reversible and can become both life-threatening and permanent.[18] Clinicians should be alert to the possibility of PRES, in addition to seizure, as an adverse event of enzalutamide that involves the CNS.
Acknowledgments
The authors would like to thank Dr. Amy Dorman for her expertise in reviewing and editing this case report. D.J.C. was supported by T32GM086330 from the National Institute of General Medicine Sciences (NIGMS).
Y.E.W. received research funding from Astellas and Janssen.
Footnotes
Conflict of Interest
D.J.C. stated no conflicts of interest.
References
- 1.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205–210. doi: 10.1001/archneurol.2007.46. [DOI] [PubMed] [Google Scholar]
- 3.Marinella MA, Markert RJ. Reversible posterior leucoencephalopathy syndrome associated with anticancer drugs. Intern Med J. 2009;39(12):826–834. doi: 10.1111/j.1445-5994.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- 4.Tajima Y, Isonishi K, Kashiwaba T, Tashiro K. Two similar cases of encephalopathy, possibly a reversible posterior leukoencephalopathy syndrome: serial findings of magnetic resonance imaging, SPECT and angiography. Intern Med. 1999;38(1):54–58. doi: 10.2169/internalmedicine.38.54. [DOI] [PubMed] [Google Scholar]
- 5.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–11977. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–4338. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okeda R, Kawamoto T, Tanaka E, Shimizu H. An autopsy case of drug-induced diffuse cerebral axonopathic leukoencephalopathy: the pathogenesis in relation to reversible posterior leukoencephalopathy syndrome. Neuropathology. 2007;27(4):364–370. doi: 10.1111/j.1440-1789.2007.00771.x. [DOI] [PubMed] [Google Scholar]
- 9.Delanty N, Vaughan C, Frucht S, Stubgen P. Erythropoietin-associated hypertensive posterior leukoencephalopathy. Neurology. 1997;49(3):686–689. doi: 10.1212/wnl.49.3.686. [DOI] [PubMed] [Google Scholar]
- 10.Irvin W, MacDonald G, Smith JK, Kim WY. Dexamethasone-induced posterior reversible encephalopathy syndrome. J Clin Oncol. 2007;25(17):2484–2486. doi: 10.1200/JCO.2007.10.9991. [DOI] [PubMed] [Google Scholar]
- 11.Chelis L, Souftas V, Amarantidis K, Xenidis N, Chamalidou E, Dimopoulos P, Michailidis P, Christakidis E, Prassopoulos P, Kakolyris S. 2012. Reversible posterior leukoencephalopathy syndrome induced by pazopanib. [DOI] [PMC free article] [PubMed]
- 12.Vaughn C, Zhang L, Schiff D. Reversible posterior leukoencephalopathy syndrome in cancer. Curr Oncol Rep. 2008;10(1):86–91. doi: 10.1007/s11912-008-0013-z. [DOI] [PubMed] [Google Scholar]
- 13.Strandgaard S, Paulson OB. Cerebral autoregulation. Stroke. 1984;15(3):413–416. doi: 10.1161/01.str.15.3.413. [DOI] [PubMed] [Google Scholar]
- 14.Foster WR, Car BD, Shi H, Levesque PC, Obermeier MT, Gan J, Arezzo JC, Powlin SS, Dinchuk JE, Balog A, Salvati ME, Attar RM, Gottardis MM. Drug safety is a barrier to the discovery and development of new androgen receptor antagonists. Prostate. 2011;71(5):480–488. doi: 10.1002/pros.21263. [DOI] [PubMed] [Google Scholar]
- 15.Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007;320(2):615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344–1346. [PMC free article] [PubMed] [Google Scholar]
- 17.Chi OZ, Hunter C, Liu X, Chi Y, Weiss HR. Effects of GABA(A) receptor blockade on regional cerebral blood flow and blood-brain barrier disruption in focal cerebral ischemia. J Neurol Sci. 2011;301(1-2):66–70. doi: 10.1016/j.jns.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Stott VL, Hurrell MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern Med J. 2005;35(2):83–90. doi: 10.1111/j.1445-5994.2004.00750.x. [DOI] [PubMed] [Google Scholar]


