Purpose
Changes in the microarchitecture of subchondral bone (SB) and its mineral density (BMD) precede cartilage degeneration in osteoarthritis (OA); SB is also a potential target for OA therapies. An imaging modality enabling in-vivo quantitative assessment of bone health (structure and composition) simultaneously with evaluation of articular soft tissues could thus provide an early biomarker of OA. Spatial resolution better than 200 microns, consistent with the size of trabeculae, is required for accurate assessment of bone microarchitecture. Recently developed flat-panel detector (FPD) extremities cone-beam CT (CBCT, Fig. 1) provides an attractive platform for such capability owing to its high spatial resolution (already surpassing conventional CT [1]), simplified workflow, and capability for weight-bearing imaging.
Figure 1.
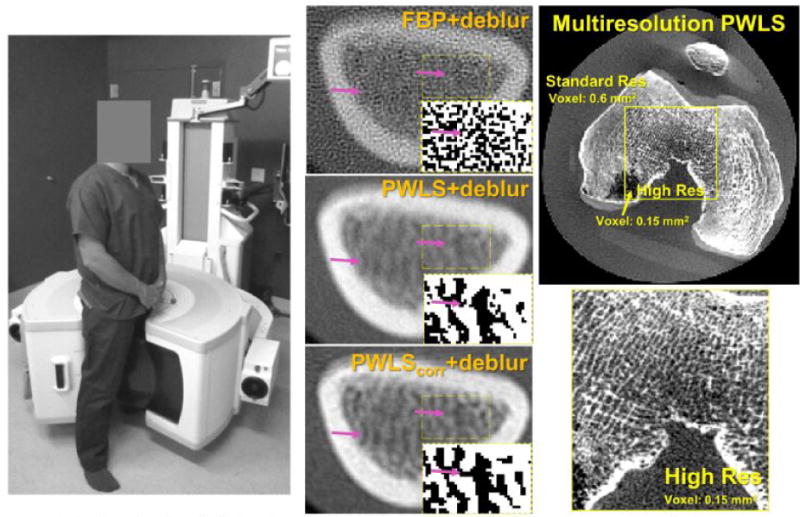
Left: extremities CBCT system. Center: FBP, PWLS with a noise model accounting for deblurring and PWLS with a noise model accounting for deblurring and correlations due to blur (PWLScorr) applied to deblurred projections of the distal radius. Insets show a thresholded map of the trabeculae inside the rectangular region-of-interest. Right: Multiresolution PWLS in a knee phantom with a zoom on the high resolution sub-volume.
We report the development of quantitative bone imaging capability on the dedicated extremities CBCT system, enabled by: (i) upgrade to a CMOS detector to improve baseline spatial resolution; (ii) a novel iterative model based reconstruction method developed to enhance resolution while mitigating noise amplification associated with conventional deblurring; and (iii) dual-energy (DE) imaging for determination of bone composition and BMD.
Methods
High resolution extremities CBCT: The prototype extremities CBCT scanner is shown in Fig. 1 (left). The system employs a FPD with 0.139 mm pixel pitch (PaxScan 2530, Varian) and a fixed anode x-ray source with 0.5 mm focal spot (XRS-125-10K-P, SourceRay) mounted on a sickle-shaped gantry allowing imaging of weight-bearing lower extremities in a natural stance and non-weight bearing upper and lower extremities. The field of view is ~20×20×20 cm, the scan time is ~20 sec, the patient dose is ~10 mGy and the spatial resolution is ~1.7 lp/mm (detail size of 300 microns) [1,2].
The extremities CBCT system will be upgraded to include a high resolution CMOS detector. Compared to FPDs, the current generation of CMOS sensors offers smaller pixels (75 – 100 micrometers) and lower electronic noise with comparable field-of-view (up to 30×30 cm). An analytical model of imaging performance based on cascaded systems analysis [2,3] was used in concert with experimentation to optimize detector pixel size, CsI:Tl scinitillator thickness (varied 150 – 600 microns), and focal spot size.
Resolution enhancement with Penalized Weighted Least Squares (PWLS) reconstruction: The PWLS algorithm acts on projection data deblurred with measured system blur and employs a Gaussian noise model with a covariance matrix accounting for the effect of deblurring and for noise correlations due to blur [4]. Accurate noise modeling in the reconstruction improves the resolution-noise tradeoff over conventional deblurred reconstructions.
The computational burden of reconstructing the entire volume on a fine voxel grid may be prohibitive, since PWLS requires complete support of the measured projections to be reconstructed. A multiresolution PWLS scheme was developed, where the forward model is factored into components that may differ not only in voxel size, but also in the resolution of the projection data. The sub-volumes can be reconstructed with different regularization strength. Specific bone sub-volumes were targeted for high resolution and reconstructed on a fine grid.
Dual-energy (DE) quantitative peripheral CBCT: DE imaging was performed using data acquired in rapid succession at low energy (LE, 60 kVp) and high energy (HE, 105 kVp). Three-material decomposition was applied to the LE and HE reconstructions to yield volume fractions of water, fat (marrow), and cortical bone (Fig. 2). Reconstructions involved rapid Monte Carlo based scatter correction as well as detector glare and beam hardening corrections. Evaluation of decomposition accuracy employed a water cylinder (~10 cm diameter) with inserts containing mixtures of K2HPO4 (emulating pure bone), water, and ethanol (emulating fat/marrow). Insert BMD100 contained a base mixture of 100 mg/ml of K2HOP4 (BMD=100 mg/ml). Insert BMD90 contained a mixture of 90% of the base BMD100 material and 10% marrow. Inserts BMD85 – BMD60 were defined analogously for decreasing volume concentrations of the base bone material. BMD0 represents pure marrow.
Figure 2.

Left: DE map of bone volume fraction superimposed on a single energy reconstruction of the DE phantom. Center: DE map of marrow volume fraction. Left: Measured (blue markers) and reference (black circles) BMD values for the six inserts of the DE phantom.
Results
Cascaded systems analysis of the presampling MTF indicates a limiting resolution (10% MTF) at ~3.5 lp/mm (150 micron detail size) for a CMOS-based extremities CBCT system with a 0.5 mm focal spot size and a 600 micron thick CsI:Tl, suggesting a ~2× improvement over the current CBCT prototype. Benefits of a thinner scintillator need to be weighed against the associated loss in DQE and are a subject of ongoing analysis.
Resolution enhancement with PWLS is illustrated in Fig. 1 (center). Resolution gains in deblurred FBP (top row) were offset by significant noise amplification. This amplification was mitigated by PWLS with a noise model accounting for deblurring (center). Additional inclusion of noise correlations in PWLScorr (bottom) reduced the regularization needed to achieve the same noise level (standard deviation of 0.0012 1/mm in both PWLS reconstructions) and thus improved resolution compared to PWLS (arrows indicate improved discrimination of trabeculae). Multiresolution PWLS is shown in Fig. 1 (right). The voxel size in the finely sampled inner region was 0.15 mm, compared to 0.6 mm in the outer region. No artifacts due to the variable voxel size are visible. Multiresolution PWLS enables reconstructions that simultaneously optimize image quality in the soft tissue regions (reduced noise at the cost of lower resolution) and in bone (high resolution).
Quantitative DE CBCT is illustrated in Fig. 2. Reference values were measured for each insert in air (without the water phantom). In each case, excellent accuracy of the BMD measurement (<5% error) was achieved. Changes in the concentration of marrow were detected down to 25% volume fraction (corresponding to increasing levels of bone marrow edema – i.e., displacement of marrow with fluid).
Conclusions
An optimized CBCT hardware configuration coupled with model-based reconstruction and DE imaging enhances the spatial resolution and quantitative accuracy of extremities CBCT to a level consistent with SB morphometry (100 μm detail size) and accurate assessment (<5% error) of subchondral BMD and detection of edema. Considering that CBCT already provides the capability to study the extremities under load and to volumetrically assess joint space width, the resulting quantitative imaging platform will extend capability to monitor a broad range of OA biomarkers in-vivo and offers important application in bone health assessment beyond OA – e.g., in osteoporosis or rheumatoid arthritis.
References
- 1.Carrino JA, Siewerdsen JH, Muhit AA, Zbijewski W, Stayman JW, Yorkston J, Packard N, Yang D, Senn R, Foos D, Thawait G. Dedicated Cone-Beam CT System for Extremity Imaging. Radiology. 2014;270(3):816–824. doi: 10.1148/radiol.13130225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zbijewski W, De Jean P, Prakash P, Ding Y, Stayman JW, Packard N, Senn R, Yang D, Yorkston J, Machado A, Carrino JA, Siewerdsen JH. A dedicated cone-beam CT system for musculoskeletal extremities imaging: design, optimization, and initial performance characterization. Medical Physics. 2011;38(8):4700–4713. doi: 10.1118/1.3611039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prakash P, Zbijewski W, Gang GJ, Ding Y, Stayman JW, Yorkston J, Carrino JA, Siewerdsen JH. Task-based modeling and optimization of a cone-beam CT scanner for musculoskeletal imaging. Medical Physics. 2011;38(10):5612–5629. doi: 10.1118/1.3633937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilley S, II, Stayman JW, Siewerdsen JH. Iterative CT Reconstruction using Models of Source and Detector Blur and Correlated Noise. Proceedings of International Conference on Image Formation in X-Ray Computed Tomography. 2014 [PMC free article] [PubMed] [Google Scholar]


