Abstract
Rationale
Stress experience during adolescence has been linked to the development of psychiatric disorders in adulthood, many of which are associated with impairments in prefrontal cortex function.
Objective
The current study was designed to determine the immediate and enduring effects of repeated social stress on a prefrontal cortex-dependent cognitive task.
Methods
Early adolescent (P28), mid-adolescent (P42), and adult (P70) rats were exposed to resident–intruder stress for 5 days and tested in an operant strategy-shifting task (OSST) during the following week or several weeks later during adulthood. Engagement of prefrontal cortical neurons during the task was assessed by expression of the immediate early gene, c-fos.
Results
Social stress during adolescence had no immediate effects on task performance, but impaired strategy-shifting in adulthood, whereas social stress that occurred during adulthood had no effect. The cognitive impairment produced by adolescent social stress was most pronounced in rats with a passive coping strategy. Notably, strategy-shifting performance was positively correlated with medial prefrontal cortical c-fos in adulthood but not in adolescence, suggesting that the task engages different brain regions in adolescents compared to adults.
Conclusions
Adolescent social stress produces a protracted impairment in prefrontal cortex-mediated cognition that is related to coping strategy. This impairment may be selectively expressed in adulthood because prefrontal cortical activity is integral to task performance at this age but not during adolescence.
Keywords: Social stress, Resident–intruder, Adolescence, Cognitive flexibility, Strategy-shifting, Coping, Prefrontal cortex
Introduction
Stress has been implicated in many psychiatric disorders including depression, schizophrenia, attentional deficit hyper-activity disorder, and obsessive–compulsive disorder (Findley et al. 2003; Kessler 1997; Marin et al. 2011; Nuechterlein et al. 1992; Wigal et al. 2012). These disorders are characterized by impairments in cognitive function, particularly, executive function that is regulated by the prefrontal cortex (PFC; Arnsten 2011; Clark et al. 2009; Jurado and Rosselli 2007). The PFC plays an integral role in cognitive flexibility, the ability to optimally adjust and maintain appropriate behavioral strategies in a changing environment (Coutlee and Huettel 2012; Kehagia et al. 2010). Stressors are thought to impair cognitive function as a result of structural and functional changes in the PFC (Arnsten 2009). For example, chronic restraint stress in rats decreased dendritic arborization, spine number, and size in the PFC, and this was associated with impaired cognitive flexibility (Liston et al. 2006; Radley et al. 2006, 2008). Similarly, chronic psychosocial stress in human subjects was associated with disrupted PFC functional connectivity and impaired cognitive flexibility (Liston et al. 2009).
Although stress during adulthood can influence cognitive function, its impact may be greater during specific windows of development when defense mechanisms and brain regions involved in cognition and emotion are still developing. The hypothalamic–pituitary–adrenal (HPA) axis response to stress is heightened during adolescence and does not habituate to chronic stress in the same manner as it does during adulthood (Gunnar et al. 2009; Romeo et al. 2006). Early life stress can also produce enduring effects, and this has been associated with the occurrence of psychiatric disorders in adulthood (Halligan et al. 2007; Lupien et al. 2009). Consistent with this, rats with adolescent stress experience display increased anxiety-related and depressive-like behaviors as well as impaired learning and memory in adulthood (Isgor et al. 2004; McCormick et al. 2008; Uys et al. 2006).
Social stressors are especially prevalent and detrimental to human mental health and well-being (Brown and Prudo 1981; Taylor et al. 2011). Social stress has been effectively modeled in rodents by the resident–intruder paradigm (Miczek 1979). This ethologically relevant stressor produces HPA axis dysfunctions and depressive-like and substance abuse-related behaviors (Buwalda et al. 2011; Covington and Miczek 2005; Rygula et al. 2008; Wood et al. 2010). Adolescent rats exposed to resident–intruder stress exhibit increased proactive defensive behaviors and increased noradrenergic tone. In contrast, adults exposed to the same stressor during adolescence or adulthood exhibit more passive defensive and social interaction behaviors and no increase in noradrenergic tone, underscoring how the stage of development during which social stress occurs and, when behavior is examined, are critical determinants of its impact (Bingham et al. 2011; Vidal et al. 2007).
To better understand the impact of social stress on cognitive function the current study evaluated the effects of social stress throughout development on performance in a medial PFC (mPFC)-dependent operant strategy-shifting task (OSST), adapted from and validated by Floresco et al. (2008). To determine whether stress effects on cognitive performance were related to effects on mPFC function, mPFC activity during task performance was also assessed by immunohistochemical quantification of the expression of the immediate early gene, c-fos.
Methods
Animals
Male Sprague–Dawley rats (Charles River, Wilmington, MA) served as social stress “intruder” rats or matched controls. Male Long–Evans retired breeders (550–850 g) served as residents (Charles River). Except where mentioned, rats were singly housed on a 12 h light/dark cycle with lights on at 7 AM. All experiments were carried out between 11 AM and 3 PM. Care and use of animals was approved by the Institutional Animal Care and Use Committee of the Children’s Hospital of Philadelphia.
Experimental design
Rats were exposed to stress or control manipulation 4 days after arrival. Stress or control manipulations occurred during early adolescence (PND 28–32, EA), mid-adolescence (PND 42–46, MA), or adulthood (PND 70–74, adult). These ages were selected to span the social and physical stages of early and mid-adolescence as designated previously (McCormick and Mathews 2010; Spear 2000; Sturman and Moghaddam 2011). Rats were exposed to 5 consecutive days of social stress or control manipulation. On the last day of social stress or control manipulation, EA, MA, and adult rats began food restriction to maintain 85 % free-feeding weight. OSST training began 3 days after the last experimental manipulation, and testing occurred after 3 days of training, 6 days after the final experimental manipulation. Additionally, a group of EA-stressed rats were tested as adults (EA–adults), and a group of MA-stressed rats were tested as adults (MA–adults), such that EA–adult and MA–adult animals were food-restricted, trained, and tested in the operant chamber at the same age as adult animals after a 6- or 4-week delay, respectively. A final group of adult-stressed rats (adult-delay) were food-restricted, trained, and tested in the operant chamber after a 5-week delay.
Social stress
The social stress and matched control methods were a modification of the resident–intruder model (Miczek 1979) and identical to that previously described except that rats were exposed for 5 consecutive days (Bingham et al. 2011). All animals were singly housed during social stress. However, EA–adult, MA–adult, and adult-delay rats were pair-housed with partners from their respective treatment group during the time period between the end of social stress and the beginning of food restriction and operant training/testing. EA, MA, and adult animals remained singly housed following social stress as they proceeded immediately to food restriction and operant training/testing. Defeat latency was recorded for each session and averaged across all five exposures to social stress for each intruder. Defeat latencies from sessions when animals were separated after five attacks without defeat were quantitatively treated as 900 s. The mean latencies for each rat were subjected to a cluster analysis to designate rats as short latency (SL) or long latency (LL).
Operant training and testing
Training and testing was carried out during the light portion of the 12-h light/dark cycle in two-lever operant chambers (Med-Associates, St. Albans, VT, USA), each within a sound-attenuating box. A stimulus light was positioned above each lever, and a house light was positioned top-center on the wall opposite the levers. Data was recorded and stored onto a PC computer via an interface module.
A 4-day operant training and testing protocol, adapted from Floresco et al. (2008), was initiated on the fourth day of food restriction. On Day 1, rats were shaped to lever press on a fixed-ratio 1 schedule on one lever (randomly chosen left/right) to a criterion of 50 presses within 30 min. On Day 2, rats were trained to the same criterion with a fixed-ratio 1 schedule on the opposite lever. On Day 3, rats were introduced to the trial structure of the task, under conditions with no discernable “rule.” On each trial, the house light and both stimulus lights were illuminated for 15-s during which rats could press one of the two levers for food reward. The correct lever was randomly selected to occur one, three, or five times in a row on a particular side, such that over many trials, it was equally likely to occur on either side. This encouraged rats to switch sides during training while not allowing them to use spatial or light cues to reliably predict the location of the correct lever. If the correct lever was pressed within 15 s of trial initiation, a single reward pellet was delivered, and all lights remained illuminated for 3 s followed by darkness for a 5-s timeout before initiation of the next trial. If the incorrect lever was pressed within 15 s of trial initiation, no reward was delivered, and all lights were immediately shut off for a 10-s timeout before initiation of the next trial. If neither lever was pressed within 15 s of trial initiation, all lights were shut off for a 5-s timeout before initiation of the next trial. Additionally, if either lever was pressed during a dark timeout period, the initiation of the following trial would be reset to occur 5 s after the time of this lever press. Trials continued until rats achieved 50 correct trials. Each animal’s side bias was determined to be toward the lever on the side that the animal pressed on the majority of trials during training. On Day 4, behavior was tested in a series of three consecutive discriminations: an initial side discrimination (SD), a side reversal discrimination (SR), and a shift to light discrimination (LD). Animals proceeded from one stage of the task to the next after achieving a criterion of eight consecutive correct choices, provided 30 trials had been attempted. This minimum of 30 trials stipulation was added to ensure that each animal experienced enough trials in each stage of the task for the transitions from one type of discrimination to the next to be cognitively meaningful. The trial structure and timing of light illuminations during each stage of the task were the same as they were during the previous day’s training session, with the exception that only one stimulus light was illuminated. For every pair of trials, on the first trial of the pair, the left or right stimulus light was randomly selected to be illuminated, and the opposite stimulus light was illuminated on the following trial, such that a typical 16 trial sequence might consist of the following pattern: LRRLLRLRRLRLLRRL. This was done to ensure that the light was never illuminated above the same lever on more than two consecutive trials. This pseudorandom selection pattern of light illuminations was applied in the same manner throughout each phase of the OSST. During the SD stage, the lever on the side opposite the animal’s side bias was designated to be the correct lever on every trial, regardless of the location of the stimulus light. During the SR stage, the correct lever on each trial was designated to be the lever opposite the correct lever during the initial side discrimination. During the LD stage, the correct lever was designated as the lever underneath the illuminated stimulus light on each trial. The task was ended after reaching criterion in the LD stage. Trials to criterion (TTC) and number of errors were recorded during each stage of the OSST for each rat. Omitted trials were not included in the TTC measure. Error types were characterized using logistic regression to determine whether treatments impacted perseveration on the previous rule or the acquisition and maintenance of the new rule. For the SR stage, every trial attempted by a particular animal was categorized as “correct” or “incorrect” and regressed by trial number. A logistic curve of best fit, representing the probability of a correct response with respect to trial number, was generated and the trial number after which the value of this curve became greater than or equal to chance performance value of 50 % was noted. Errors that occurred on or before this trial were characterized as perseverative errors, as they occurred while the animal was following the old rule with greater than chance probability. Errors that occurred after this trial were characterized as regressive errors, as these errors were made after the animal had disengaged from following the previous rule and was in the process of acquiring the new rule. For the LD stage, trials attempted were split into two categories: (1) trials when the stimulus light was illuminated above the previously correct lever during the SR, stage and (2) trials when the stimulus light was illuminated above the opposite lever. Errors from trials of the first category were classified as perseverative or regressive using the same method described above for the side reversal stage. Errors from trials of the second category were counted as random errors, as they were unrelated to the previously learned rule.
Immunohistochemistry
Thirty minutes after completing the OSST, half of the rats in each group were anesthetized with isoflurane and transcardially perfused with heparinized saline followed by 4 % paraformaldehyde for processing for immunohistochemical visualization of c-fos in the mPFC as previously described using a primary rabbit anti c-fos antibody (1:25,000) provided by Dr. Paul Sawchenko (The Salk Institute, La Jolla, CA; Snyder et al. 2012). The specific area in which c-fos was quantified included both prelimbic and infralimbic cortex and was identical to the region described by Snyder et al. (2012). Sections were microscopically visualized and digital images obtained by an individual blinded to the treatment group. Using Image J, immunoreactive profiles were sampled in the same area of prefrontal cortex of each section by creating a rectangular region of interest based on a representative section that included prelimbic and infralimbic cortex area. This same shape was superimposed on all other sections in the same region and c-fos profiles counted within this area. At least two sections per animal were used to count immunoreactive profiles and the number of profiles per section was averaged for each subject and the group mean determined from these values. Brains from the remaining rats that were not perfused were dissected and frozen after completion of the OSST to be analyzed for another study.
Statistical analysis
All data from animals that were stressed at the same age as they were tested in the OSST (EA, MA, adult) were analyzed independently from animals that were stressed at different ages but tested as adults (EA–adult, MA–adult, and adult). Effects of age on TTC were assessed across control rats by means of two-way analysis of variance (ANOVA, age of stress × stage) with repeated measures across stage. Effects of social stress and coping strategy on TTC were assessed by two-way ANOVAs (stress × stage) with repeated measures across stage performed within each experimental group. Effects of social stress and coping strategy on error type during the side reversal and shift to light stages were also assessed separately within each experimental group by performing two-way mixed ANOVAs (stress × error type) with error type as the within-subject factor. Where significant main effects or interactions were found, follow-up post hoc comparisons were performed using the Holm–Sidak method, unless otherwise noted.
Cluster analyses (JMP 9.0; SAS, Cary, NC) were applied separately to the defeat latencies of animals within each experimental group in order to categorize animals on the basis of their stress-coping strategy as short (SL) or long latency (LL) animals.
Results
Effects of social stress during development on cognitive performance
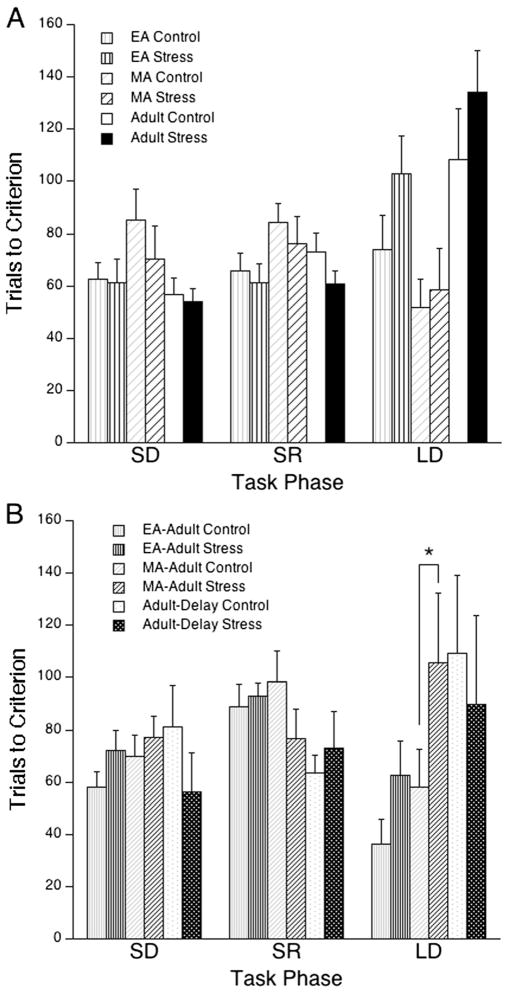
The mean time taken to complete the task was 87±2.3 min. A comparison between age groups and stress condition revealed that there was no difference in time taken to complete the task [F(9,135) = 1.14]. There was no effect of age, stress or age × stress interaction. EA (n=19 control, n=16 stress), MA (n=8 control, n=8 stress), and adult (n=20 control, n=28 stress) rats completed testing in the OSST. Some rats in each group did not finish the task including two EA control rats, four EA stressed rats, and one adult control rat. A two-way ANOVA (age × stage) with repeated measures across stage in control rats revealed a significant age × stage interaction [F(4,88) = 2.7, p<0.05] and post hoc comparisons showed that adult rats performed significantly worse than both EA and MA rats in the strategy-shifting phase of the task (p<0.05). Two-way ANOVAs (stress × stage) with repeated measures across stage in each age group revealed no significant effects of stress on task performance in rats tested 1 week following stress exposure.
Although social stress had no effect on cognitive performance that was assessed during the same developmental stage as the stress exposure, it had enduring effects in rats that were stressed as adolescents and assessed in adulthood (Fig. 1b). Some of these rats also did not finish the task including two EA–adult stressed rats, two MA–adult stressed rats, and one adult-delay stressed rat. A comparison of rats that were exposed to stress or control conditions in early adolescence (n= 11 control, n=11 stress), mid-adolescence (n=13 control, n= 12 stress), or adulthood (n=20 control, n=28 stress) and tested as adults revealed that early handling improved strategy-shifting performance [age of stress × stage interaction F(4,56) = 3.2, p<0.05]. Post hoc analysis showed that adult-delay rats performed significantly worse than either EA–adult rats (p<0.005) or MA–adult rats (p<0.05). A significant stress × stage interaction was found in MA–adult rats with social stress selectively impairing strategy-shifting performance [F(2,46) = 3.3, p<0.05; p<0.05 post hoc]. In EA–adult rats social stress generally impaired OSST performance, although this effect could not be attributed to a particular task phase [between-subject stress effect F(1,20) = 5.9, p<0.05].
Fig. 1.
Social stress selectively impaired strategy-shifting in MA–adult rats. The bars indicate the mean number of trials necessary to reach criterion for side discrimination (SD), side reversal discrimination (SR), and shift to light discrimination (LD) components of the task. a Rats tested 1 week after stress exposure. b Adolescent rats tested during adulthood and adult rats tested after a 5-week delay. Vertical lines represent SEM. *p<0.05
Effects of social stress on strategy-shifting error type
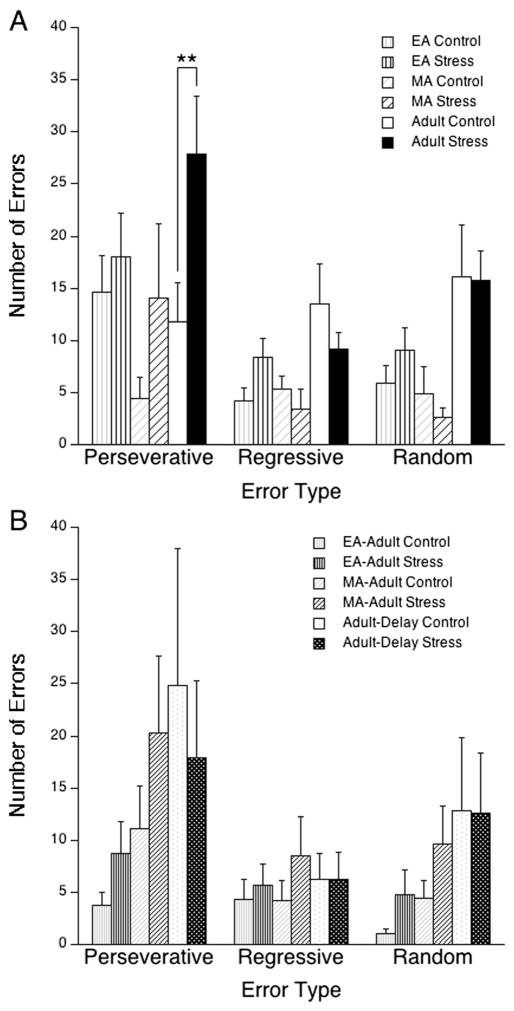
To better understand how adolescent social stress-affected cognitive performance, the effect of social stress on error type was analyzed. A two-way mixed ANOVA (group × error type) within control rats revealed a significant effect of error type [F(2,144) = 3.6, p<0.05], but no age × error type interaction [F(10,144) = 1.4, ns] during the strategy-shifting phase of the task and post hoc comparisons revealed that rats made significantly more perseverative than regressive errors (p<0.05). This suggests that across all groups tested, the performance of rats during the strategy-shifting phase of the OSST was significantly influenced by the previously learned strategy. Social stress was found, by two-way mixed ANOVA (stress × error type) performed within each age, to selectively increase perseverative errors in adult rats during strategy-shifting [F(2,92) = 4.6, p<0.05; p<0.01 post hoc; Fig. 2]. No effects of stress were observed on the type of errors committed during the SR phase of the task. Finally, the percentage of trials omitted during task performance was also assessed (Table 1) by two-way ANOVA (group × stress) which revealed a significant effect of group [F(4,142) = 2.4, p<0.05] and post hoc comparisons showed that EA rats omitted significantly more trials than adult rats (p<0.01) or MA–adult rats (p<0.05).
Fig. 2.
Social stress selectively increased strategy-shifting perseverative errors in adult rats. The bars indicate the mean number of perseverative, regressive, and random errors committed during the shift to light discrimination (LD) component of the task for a rats tested 1 week after stress exposure or b adolescent rats tested during adulthood and adult rats tested after a 5-week delay. Vertical lines represent SEM. *p<0.05
Table 1.
Percentage of trials omitted ± SEM during OSST performance in each experimental group
| Experimental group | Control | Stress |
|---|---|---|
| EA** | 11.5±2.2 % | 17.6±3.4 % |
| MA | 12.6±3.5 % | 9.3±1.7 % |
| Adult | 8.1±1.4 % | 8.8±1.0 % |
| EA–adult | 12.4±2.9 % | 9.4±1.9 % |
| MA–adult# | 9.3±1.4 % | 9.7±1.5 % |
p<0.05 (compared to EA);
p<0.01 (compared to adult)
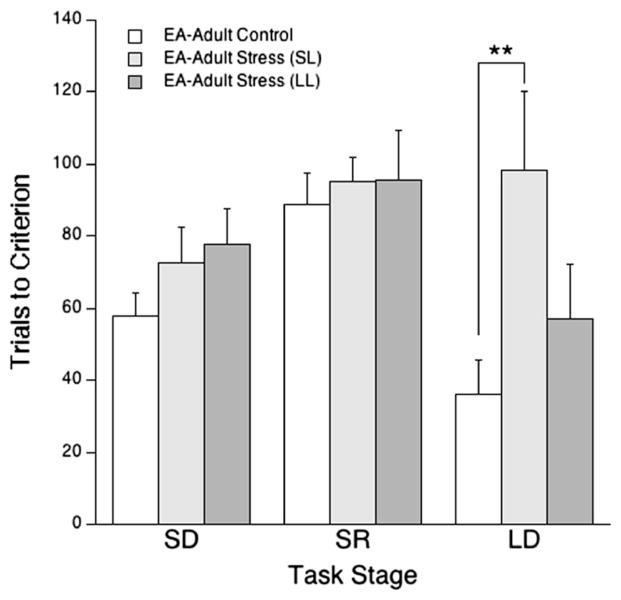
Effect of stress-coping strategy on cognitive performance
Table 2 shows the mean latency to defeat of each subpopulation for each experimental group. There was no difference in the proportion of SL/LL rats across experimental groups [χ2(4,79) = 2.4, p=0.7]. There was an effect of coping strategy on OSST performance in EA–adult rats such that those rats with a propensity to defeat (SL) exhibited impaired performance, particularly, during the strategy-shifting component of the task. There was a significant between-subject effect of latency group in EA–adult rats [F(2,22) = 4.4, p<0.05], and post hoc comparisons indicated that SL rats were specifically impaired by social stress with respect to control rats (p<0.05). Although statistical significance was not reached for a latency group × stage interaction [F(4,44) = 2.2, p<0.1], Tukey’s HSD post hoc test supported the interpretation that social stress impaired performance during strategy-shifting selectively in SL EA–adult rats with respect to control EA–adult rats (p<0.01; Fig. 3). No significant effects of coping strategy on strategy-shifting performance were found in any other experimental groups.
Table 2.
Mean latency (s) ± SEM to defeat for SL and LL rats in each experimental group
| Experimental group | SL | LL |
|---|---|---|
| EA | 278.0±56.5 (n=6) | 697.6±102.1 (n=13) |
| MA | 156.3±12.0 (n=3) | 541.8±179.6 (n=5) |
| Adult | 260.3±26.7 (n=9) | 527±22.0 (n=22) |
| EA–adult | 324.6±28.2 (n=7) | 621.7±51.3 (n=7) |
| MA–adult | 168.8±32.3 (n=4) | 518.8±62.0 (n=10) |
Fig. 3.
The short latency (SL) coping strategy was associated with impaired strategy-shifting in EA–adult rats. The bars indicate the mean number of trials necessary to reach criterion for side discrimination (SD), side reversal discrimination (SR), and shift to light discrimination (LD) components of the task. Vertical lines represent SEM. **p<0.01
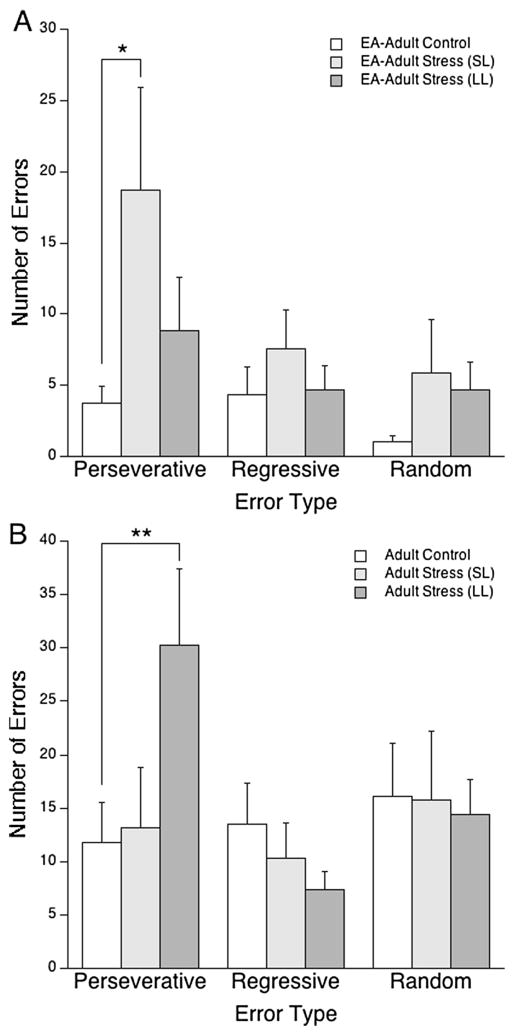
Effect of stress-coping strategy on error type
Although there was no latency group × error type interaction [F(4,44) = 1.4, ns] in EA–adult rats, Tukey’s HSD post hoc comparisons supported the interpretation that SL rats committed more perseverative errors than control rats in this group (p<0.05; Fig. 4a). Interestingly, a significant interaction between latency group and error type was found for adults during the strategy shift stage [F(4,86) = 3.0, p<0.05); Fig. 4b]. Social stress selectively increased perseverative errors in LL adult rats (p<0.01) as compared to controls.
Fig. 4.
The short latency (SL) and long latency (LL) coping strategies were associated with increased strategy-shifting perseverative errors in EA–adult and adult rats, respectively. The bars indicate the mean number of perseverative, regressive, and random errors committed during the shift to light discrimination (LD) component of the task for EA–adult (a) and adult (b) rats. Vertical lines represent SEM. *p<0.05; **p<0.01
Effects of social stress and age on task-associated activation of the medial prefrontal cortex
Table 3 summarizes the mean number of c-fos profiles in the mPFC in each group. There was no effect of stress on the number of c-fos profiles in the mPFC [F(1,38) = 0.1, ns] and no stress × group interaction [F(4,38) = 0.8, ns]. However, a significant main effect of group [F(4,38) = 6.0, p<0.001] was found, and post hoc comparisons indicated that MA animals had significantly less mPFC c-fos expression than other groups that were handled or stressed during adolescence (p<0.01) but not compared to the adults.
Table 3.
Effect of social stress on mPFC c-fos profile counts throughout development
| Experimental group | Control | Stress |
|---|---|---|
| EA** | 448.9±28.3 (n=4) | 476.5±55.3 (n=4) |
| MA | 214.7±45.4 (n=3) | 223.6±14.7 (n=4) |
| Adult | 344±47.5 (n=4) | 423.2±49.0 (n=8) |
| EA–adult*** | 464.7±70.0 (n=5) | 488.0±61.5 (n=8) |
| MA–adult*** | 602.4±95.6 (n=5) | 488.7±121.5 (n=3) |
p<0.01,
p<0.005 (compared to MA)
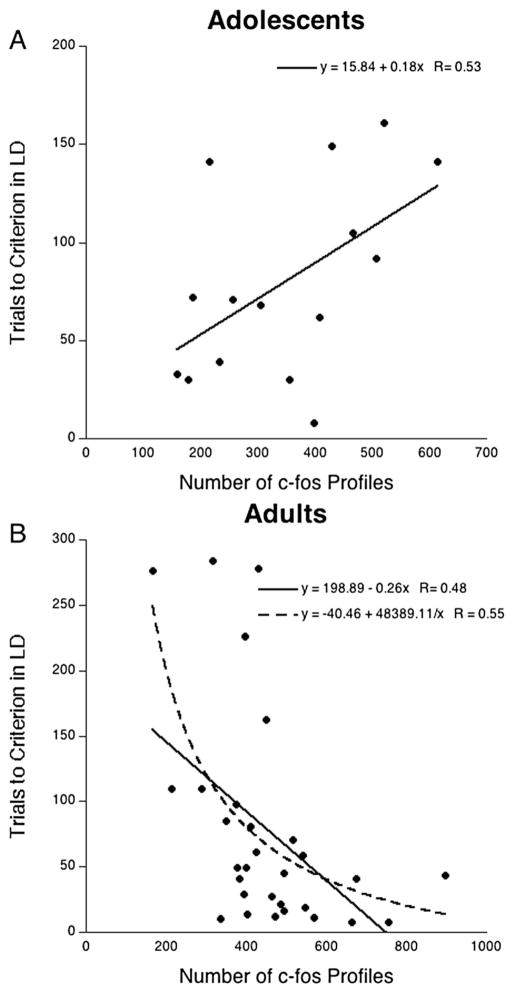
The relationship between c-fos profiles in the mPFC and trials to criterion during the strategy-shifting component of the task was then determined for rats tested in adolescence and for rats tested in adulthood. For rats tested during adolescence mPFC c-fos expression was negatively correlated with performance [positive between c-fos and trials to criterion; r(13) = 0.53, p<0.05], suggesting that mPFC activation may impair rather than facilitate performance during adolescence (Fig. 5a). In contrast, a significant positive correlation between mPFC c-fos expression and performance (negative between c-fos and trials to criterion) was found for rats tested in adulthood [r(28) = 0.48, p<0.01; Fig. 5b]. A reciprocal transformation of the number of mPFC c-fos profiles revealed an even stronger relationship with performance [r(28) = 0.55, p<0.005].
Fig. 5.
Expression of c-fos in the medial prefrontal cortex (mPFC) was differentially correlated with strategy-shifting performance depending upon the age of testing. a Each point in the scatterplot represents the number of c-fos profiles in the mPFC and trials to criterion during the shift to light discrimination (LD) for an individual rat that was tested during adolescence regardless of stress experience. The solid line represents the equation describing the linear relationship. b Each point in the scatterplot represents the number of c-fos profiles in the mPFC and trials to criterion during the LD for an individual rat that was tested during adulthood regardless of stress experience. The solid line represents the equation describing the linear relationship. The dotted line represents the equation describing the relationship based on a reciprocal transformation of the number of c-fos profiles
Discussion
The current study examined the short- and long-term impact of social stress experience and coping strategy throughout development on cognitive function. Notably, developmental and stress-related effects on task performance were generally isolated to the mPFC-dependent strategy-shifting phase of the OSST. The most prominent finding was that adolescent social stress produced a protracted impairment of performance in the strategy-shifting phase that did not manifest until adulthood. Taken with evidence from c-fos experiments that the mPFC is not engaged in task performance during adolescence, the findings suggest that adolescent social stress has enduring consequences on PFC development that are expressed as cognitive impairments in adulthood, a time when this brain region is integral to task performance. Importantly, a passive coping strategy was associated with vulnerability to these cognitive consequences of adolescent social stress.
Relationship to other studies
Previous studies demonstrated that stress experienced during adulthood can impair both prefrontal and hippocampal-dependent cognitive performance (Conrad et al. 1996; Liston et al. 2006). These cognitive impairments and the neuroplastic mechanisms underlying them were relatively transient, lasting only a few weeks (Conrad et al. 1999; Goldwater et al. 2009; Liston et al. 2009; Luine et al. 1994; Radley et al. 2005). Studies investigating the cognitive impact of stress throughout development suggest that it is typically less pronounced immediately after the stress, but is often expressed as behavioral or cognitive dysfunction during adulthood, consistent with the present results using social stress (Lupien et al. 2009; McCormick and Mathews 2010). For example, chronic variable stress in prepubertal animals impaired a hippocampal memory task and increased anxiogenic and depressive-like behaviors in adulthood (Isgor et al. 2004; Tsoory et al. 2007). Additionally, cognitive deficits associated with stress-related psychiatric disorders (i.e., anorexia nervosa) in adulthood are conspicuously absent during adolescence (Lang et al. 2014). To date, no studies have investigated the short- or long-term effects of adolescent social stress on prefrontal cortex-dependent cognitive tasks. Because the mPFC is stress-sensitive and continues to develop along with cognitive flexibility throughout adolescence, stress exposure during adolescence may have more pronounced effects compared to exposure during adulthood (Arnsten 2011; Arnsten and Shansky 2004; Cain et al. 2011; Kolb et al. 2012). This study was also unique in using social stress, a relevant stressor for humans, particularly, during adolescence (Buwalda et al. 2011).
Immediate effects of social stress
The most significant immediate effect of social stress on cognitive performance was an increase in the number of perseverative errors committed during the strategy-shifting in adult rats. Chronic stress in adult rats has been shown to induce atrophy of mPFC neurons and hypertrophy of neurons in the dorsolateral striatum (DLS), resulting in a bias toward habitual behavior and away from goal-directed performance (Dias-Ferreira et al. 2009). Lesions of the mPFC increase perseveration during strategy-shifting, and DLS lesions have been associated with impaired rule acquisition (Featherstone and McDonald 2004; Jacquet et al. 2013). Stress-induced frontostriatal reorganization favoring the DLS over the mPFC could account for an increase in perseverative errors without deficits in task performance.
Notably, social stress had no short-term effects on adolescent cognitive performance. This was somewhat surprising, given that the stress response is generally sensitized during adolescence (Romeo et al. 2006). We previously demonstrated that exposure to repeated resident–intruder stress during early adolescence but not mid-adolescence increases proactive defensive behaviors when measured within days of the last stress and this is associated with greater noradrenergic tone (Bingham et al. 2011). However, studies using other stressors also demonstrated that stress during adolescence had minimal acute cognitive or behavioral consequences (Hodes and Shors 2005; Isgor et al. 2004; Toth et al. 2008). As discussed below this may be attributed to task involvement of stress-insensitive brain regions at this age.
Interestingly, adolescent rats exhibited better strategy-shifting performance than adult rats. This is consistent with other evidence for greater cognitive flexibility during adolescence than adulthood, although it is at odds with other studies (Newman and McGaughy 2011; Simon et al. 2013).
Protracted effects of adolescent social stress
Although social stress experience during adolescence did not alter OSST performance when tested during the same developmental period, it resulted in cognitive impairments in adulthood. Mid-adolescence was particularly sensitive to protracted effects of social stress on the strategy-shifting mPFC-mediated component of the task. This is relevant because this is a dynamic period of mPFC development during which intense synaptic pruning occurs (Gourley et al. 2012; Rakic et al. 1994). This is also a time of changes in white matter development and connectivity between PFC and striatum, a circuit that supports executive control (Asato et al. 2010). Although these ongoing developmental changes heighten the vulnerability of mPFC to stress and might be expected to affect performance during adolescence (Selemon 2013), the c-fos results suggest that the mPFC is not engaged during the OSST task in adolescents. That the impairment is seen during only in adulthood is consistent with the role of the mPFC in the task at this time. The findings also indicate that the effects of adolescent social stress must be enduring rather than transient to be expressed in adulthood. In contrast, the perseverative impairment observed in adult rats tested immediately after social stress was not observed after a 5-week delay, suggesting that the cognitive impact of social stress experience during adulthood is transient. Interestingly, early adolescent social stress produced a more general impairment in OSST performance during adulthood that was less selective to a particular task phase. This may be attributed to the greater number of task-relevant brain regions that are developing at this time.
An unanticipated finding was that rats exposed to the control handling experience during adolescence displayed improved strategy-shifting performance in adulthood compared to rats that experienced control or stress manipulations as adults. Previous studies have shown that early life handling has enduring effects to decrease anxiogenic behaviors and improve cognition in adulthood (Caldji et al. 2000; Meaney et al. 1988). The current findings suggest that handling in adolescence is beneficial for cognitive flexibility but that social stress at this time removes that benefit. Alternatively, improved performance of these rats during the light discrimination phase could reflect increased salience of the light cue rather than strategy-shifting and an ability of social stress history to interfere with that. Nonetheless, the positive correlation between performance in that component of the task and PFC c-fos profiles in adolescent rats tested as adults is consistent with performance being related to strategy-shifting.
Relationship between coping strategy and cognitive consequences of social stress
Exposure of rats to repeated resident–intruder stress reveals two subpopulations that are distinguished by their latency to assume a subordinate defeat posture during the resident–intruder encounter (Wood et al. 2010). Rats in the LL group exhibit more upright postures in response to aggressive encounters by the resident, suggesting that this is a more proactive coping strategy and that the SL is more passive. Social stress has different behavioral and physiological consequences in rats that exhibit these distinct coping strategies (Berube et al. 2013; Coppens et al. 2011; Wood et al. 2010, 2012, 2013). In the present study, the propensity to assume the subordinate defeat posture during early adolescence was associated with impaired strategy-shifting and more perseverative errors during adulthood. This suggests that engaging the circuits that subserve this defensive behavior in early adolescence impairs the development of neural substrates underlying strategy-shifting in adulthood. Alternatively, engaging circuits underlying proactive coping in early adolescence may protect against stress. It is also possible that coping style is not a causative determinant of the impact of stress but rather a parallel trait. The association of a specific coping style with the consequences of social stress on cognitive function did not extend to mid-adolescence suggesting that resistance to defeat is protective only in early adolescent rats. Similarly, for adults exposed to social stress, the increase in perseverative errors observed during the strategy-shifting phase of the OSST was driven primarily by LL rats, suggesting that resisting defeat at this point in development does not confer protection from the cognitive effects of social stress.
Dependence of performance during the strategy-shifting phase on mPFC activation throughout development
We previously demonstrated that the number of c-fos profiles in the mPFC was negatively correlated with the trials to reach criterion (i.e., positively correlated to performance) in an attentional set shifting task (Snyder et al. 2012). In the present study, a similar correlation was demonstrated only for rats that were tested in adulthood. Unexpectedly, a negative correlation was found in animals that were tested during adolescence, suggesting that increased mPFC activity was associated with impaired strategy-shifting in these animals. Adolescent animals may be using alternative faster-developing brain regions associated with goal-directed behavior such as the basal ganglia that solve the task (Da Cunha et al. 2012).
Clinical implications
Adverse experiences during adolescence have been strongly linked to the development of psychiatric disorders in adulthood, many of which are associated with deficits in prefrontal cortex function (Arnsten 2011; Clark et al. 2009; Patchev et al. 2013). The current study provides evidence that prefrontal cortex-mediated cognition in adulthood can be impacted by social stress experience during adolescence. Elucidating neurobiological substrates underlying this link will reveal novel pharmacological targets for reversing stress-induced cognitive impairments. The dependency of this effect on coping strategy in rats that were stressed during early adolescence suggests there may be potential therapeutic benefits to teaching children coping strategies. Interestingly, when rats experienced stress as adults, the coping strategy that was protective during early adolescence was associated with increased cognitive rigidity. Thus, adaptive stress-coping strategies for young children may be quite different than those that are adaptive during adulthood. While social stress experience may be unavoidable, future research into the associations between stress-coping strategies throughout development and cognitive outcomes in human subjects may reveal pharmacological and other therapeutic strategies to effectively cope with social stress and limit its negative consequences.
Acknowledgments
This work was supported by the National Institute of Health grants MH093981, MH040008, and T32 MH14654 (KS). The authors would also like to acknowledge the assistance of Julia Valenziano.
Contributor Information
Kevin P. Snyder, The University of Pennsylvania, Philadelphia, PA 19104, USA
Mark Barry, The University of Pennsylvania, Philadelphia, PA 19104, USA.
Rita J. Valentino, Email: rjv@mail.med.upenn.edu, The University of Pennsylvania, Philadelphia, PA 19104, USA. The Children’s Hospital of Philadelphia, 402D Abramson Pediatric Research Center, Philadelphia, PA 19104, USA
References
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29:215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Shansky RM. Adolescence: vulnerable period for stress-induced prefrontal cortical function? Introduction to part IV. Ann N Y Acad Sci. 2004;1021:143–147. doi: 10.1196/annals.1308.017. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube P, Laforest S, Bhatnagar S, Drolet G. Enkephalin and dynorphin mRNA expression are associated with resilience or vulnerability to chronic social defeat stress. Physiol Behav. 2013;122:237–245. doi: 10.1016/j.physbeh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Bingham B, McFadden K, Zhang X, Bhatnagar S, Beck S, Valentino R. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology. 2011;36:896–909. doi: 10.1038/npp.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Prudo R. Psychiatric disorder in a rural and an urban population: 1. Aetiology of depression. Psychol Med. 1981;11:581–599. doi: 10.1017/s0033291700052880. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Geerdink M, Vidal J, Koolhaas JM. Social behavior and social stress in adolescence: a focus on animal models. Neurosci Biobehav Rev. 2011;35:1713–1721. doi: 10.1016/j.neubiorev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Cain RE, Wasserman MC, Waterhouse BD, McGaughy JA. Atomoxetine facilitates attentional set shifting in adolescent rats. Dev Cogn Neurosci. 2011;1:552–559. doi: 10.1016/j.dcn.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Coppens CM, Siripornmongcolchai T, Wibrand K, Alme MN, Buwalda B, de Boer SF, Koolhaas JM, Bramham CR. Social defeat during adolescence and adulthood differentially induce BDNF-regulated immediate early genes. Front Behav Neurosci. 2011;5:72. doi: 10.3389/fnbeh.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlee CG, Huettel SA. The functional neuroanatomy of decision making: prefrontal control of thought and action. Brain Res. 2012;1428:3–12. doi: 10.1016/j.brainres.2011.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berlin) 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Gomez-A A, Blaha CD. The role of the basal ganglia in motivated behavior. Rev Neurosci. 2012;23:747–767. doi: 10.1515/revneuro-2012-0063. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a simple discrimination task. Behav Brain Res. 2004;150:15–23. doi: 10.1016/S0166-4328(03)00218-3. [DOI] [PubMed] [Google Scholar]
- Findley DB, Leckman JF, Katsovich L, Lin H, Zhang H, Grantz H, Otka J, Lombroso PJ, King RA. Development of the Yale children’s global stress index (YCGSI) and its application in children and adolescents ith Tourette’s syndrome and obsessive–compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2003;42:450–457. doi: 10.1097/01.CHI.0000046816.95464.EF. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012;32:2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biol Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Horm Behav. 2005;48:163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal–juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jacquet M, Lecourtier L, Cassel R, Loureiro M, Cosquer B, Escoffier G, Migliorati M, Cassel JC, Roman FS, Marchetti E. Dorsolateral striatum and dorsal hippocampus: a serial contribution to acquisition of cue-reward associations in rats. Behav Brain Res. 2013;239:94–103. doi: 10.1016/j.bbr.2012.10.061. [DOI] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang K, Stahl D, Espie J, Treasure J, Tchanturia K. Set shifting in children and adolescents with anorexia nervosa: an exploratory systematic review and meta-analysis. Int J Eat Disord. 2014;47(4):394–399. doi: 10.1002/eat.22235. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Marin MF, Lord C, Andrews J, Juster RP, Sindi S, Arsenault-Lapierre G, Fiocco AJ, Lupien SJ. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem. 2011;96:583–595. doi: 10.1016/j.nlm.2011.02.016. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Adolescent development, hypothalamic–pituitary–adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of D-amphetamine and cocaine. Psychopharmacology (Berlin) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev Psychobiol. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J. Developmental processes in schizophrenic disorders: longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- Patchev AV, Rodrigues AJ, Sousa N, Spengler D, Almeida OF. The future is now: early life events preset adult behaviour. Acta Physiol (Oxf) 2013 doi: 10.1111/apha.12140. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic–pituitary–adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Havemann-Reinecke U, Rüther E, Hiemke C, Zernig G, Fuchs E, Flügge G. Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav Pharmacol. 2008;19:183–196. doi: 10.1097/FBP.0b013e3282fe8871. [DOI] [PubMed] [Google Scholar]
- Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behav Neurosci. 2013;127:23–32. doi: 10.1037/a0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev. 2011;35:1704–1712. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PJ, Gooding P, Wood AM, Tarrier N. The role of defeat and entrapment in depression, anxiety, and suicide. Psychol Bull. 2011;137:391–420. doi: 10.1037/a0022935. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 2007;17:245–256. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Uys JD, Marais L, Faure J, Prevoo D, Swart P, Mohammed AH, Stein DJ, Daniels WM. Developmental trauma is associated with behavioral hyperarousal, altered HPA axis activity, and decreased hippocampal neurotrophin expression in the adult rat. Ann N Y Acad Sci. 2006;1071:542–546. doi: 10.1196/annals.1364.060. [DOI] [PubMed] [Google Scholar]
- Vidal J, Bie J, Granneman RA, Wallinga AE, Koolhaas JM, Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 2007;92:824–830. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Truong C, Stehli A. The novel use of objective laboratory school tasks to measure stress responses in children with ADHD. Postgrad Med. 2012;124:49–57. doi: 10.3810/pgm.2012.09.2593. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berlin) 2012;222:325–336. doi: 10.1007/s00213-012-2648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Zhang XY, Reyes BA, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087–1094. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]