Abstract
Background
Prenatal exposure to alcohol has a variety of morphologic and neurobehavioral consequences, yet more than 10% of women continue to drink during pregnancy, placing their offspring at risk for fetal alcohol spectrum disorders (FASD). Identification of at-risk pregnancies has been difficult, in part, because the presence and severity of FASD are influenced by factors beyond the pattern of alcohol consumption. Establishing maternal characteristics, such as maternal age, that increase the risk of FASD is critical for targeted pregnancy intervention.
Methods
We examined the moderating effect of maternal age on measures of attention in 462 children from a longitudinal cohort born to women with known alcohol consumption levels (absolute ounces of alcohol per day at conception) who were recruited during pregnancy. Analyses examined the impact of binge drinking, as average ounces of absolute alcohol per drinking day. Smoking and use of cocaine, marijuana, and opiates were also assessed. At 7 years of age, the children completed the Continuous Performance Test, and their teachers completed the Achenbach Teacher Report Form.
Results
After controlling for covariates, stepwise multiple regression analyses revealed a negative relation between levels of prenatal binge drinking and several measures of attention. The interaction between alcohol consumption and maternal age was also significant, indicating that the impact of maternal binge drinking during pregnancy on attention was greater among children born to older drinking mothers.
Conclusion
These findings are consistent with previous findings that children born to older alcohol-using women have more deleterious effects of prenatal alcohol exposure on other neurobehavioral outcomes.
Keywords: Attention, Prenatal Alcohol, Prenatal Exposure, Maternal Age
THE FETAL ALCOHOL spectrum disorders (FASD; Bertrand et al., 2004; Hoyme et al., 2005; Sokol et al., 2003), including fetal alcohol syndrome (FAS), include specific neural and craniofacial malformations, growth restriction, and neurobehavioral and cognitive deficits (Hoyme et al., 2005; Kodituwakku, 2007; Nash et al., 2006; Sokol et al., 2003; Spadoni et al., 2007). The estimated incidence of FAS ranges from 0.3 to 2.0 per 1,000 live births in the general population (CDC, 2002a, 2002b; May and Gossage, 2001), with a higher incidence among certain groups depending upon socio-demographic, behavioral, clinical, and other risk factors (Abel, 1995; CDC, 2002a, 2002b; May et al., 2007, 2008). The combined incidence of all FASD is higher and estimated at about 10 per 1,000 live births (Manning and Hoyme, 2007; O’Leary, 2004; Sampson et al., 1997).
While awareness of the risk of alcohol use during pregnancy has increased substantially since 1989 when warning labels began to appear on alcohol beverage containers, more than half of women of child-bearing age continue to consume alcoholic beverages, and over 11% report continued drinking during pregnancy (CDC, 2009; Stratton et al., 1996). The rates of certain patterns of consumption that put fetuses at greater risk for FASD, especially self-reported binge drinking—defined as 5 or more drinks per occasion (CDC, 2002a, 2002b)—have remained essentially unchanged at around 2% since 1991 (CDC, 2009). However, levels of drinking also vary with maternal age. Older pregnant women (>34 years old) are 37% more likely to report drinking during pregnancy than younger women (CDC, 2009). However, self-reported rates of binge drinking were not significantly different between younger pregnant women (18 to 24 years old), at 2.5%, and older pregnant women (>34 years old), at 1.8%(CDC, 2009).
In this paper, we are examining attention problems because they are among the more frequently reported outcomes associated with FASD (Brown et al., 1991; Carmichael Olson et al., 1998; Coles et al., 1997; Fryer et al., 2007; Kodituwakku, 2007). Deficits have been identified in sustained attention (Coles et al., 2002; Lee et al., 2004), as well as executive function, memory, IQ, fine motor skills, and other neurodevelopmental domains (Coles et al., 1997; Kodituwakku, 2007; Kodituwakku et al., 1995; Mattson et al., 1998). Coles and colleagues (1997) reported other alcohol-related attention deficits in visual / spatial skills, encoding of information, and flexibility in problem solving. Yet there are inconsistencies in the occurrence of attention problems and hyperactivity in prenatal alcohol-exposed children. Nanson and Hiscock (1990) reported increased hyperactivity and slower reaction times, while Roebuck and colleagues (1999) and Coles and colleagues (1997) did not.Carmichael Olson and colleagues (1992) found deficits in focused attention but not as severe as those found in children diagnosed with attention deficit hyperactivity disorder (ADHD). These variations in reported attention deficits may be related to multiple factors including which prenatal alcohol exposure variable was assessed, differential difficulty of the task assessing attention (Kodituwakku, 2007), sample size, or additional moderating variables, such as maternal age. In addition to maternal age and how risk drinking is assessed, several other factors are influential in the variable expression of attention effects in FASD and ADHD, such as how attention is measured and cohort characteristics (e.g., age, demographics, etc.). These are considered further in the Discussion.
Alcohol-related neurobehavioral deficits are certainly also influenced by differences in vulnerability and individual susceptibility, even at comparable levels and rates of maternal drinking. The variable expression of prenatal alcohol-related effects may be because of differential genetic susceptibility, exposure periods, or drinking patterns (Abel, 1995; American College of Obstetrics & Gynecology, 2006; Chiodo et al., 2009; Ernhart et al., 1987; Henderson et al., 2007; Jacobson and Jacobson, 1994, 1999; Maier and West, 2001; Martínez-Frías et al., 2004; NIAAA, 2005a; Olney, 2004; Sokol et al., 1986; Stratton et al., 1996; West et al., 1994), as well as nutritional or other risk factors. One factor that may influence the impact of prenatal alcohol on neurobehavioral outcome is the pattern of alcohol consumption. Detection of patterns of maternal drinking that place fetuses at risk for FASD is critical to diagnosis, treatment, and prevention but is challenging and often insufficient during pregnancy. We recently showed that outcomes depend on which measure of prenatal alcohol exposure is used and that a metric that accounts for several different measures of risk-level drinking during pregnancy is a better predictor of neurobehavioral effects of prenatal alcohol, including focus and divided attention, than the individual alcohol consumption measures alone (Chiodo et al., 2010).
Older age appears to be another maternal characteristic that is related to differential susceptibility to the effects of prenatal alcohol exposure. Jacobson and colleagues (1998, 2004) and Burden and colleagues (2005) showed greater adverse performance in attention and working memory tasks for infants and children born to older drinking mothers (≥30 years old) compared to younger mothers (Burden et al., 2005). Prenatal alcohol exposure-related deficits in working memory were greater among children born to women ≥30 years of age. Despite a small sample, children born to older mothers performed significantly-worse in 4 of 7 measures of attention, memory and general cognitive ability. For example, there were increased errors of omission in a Digit Cancellation task, working memory deficits in the digit span test, and lower scores on the Wechsler Intelligence Scale for Children–Revised (WISC-R) Arithmetic subscale, and others, while only one outcome—executive function measured by the Tower of London task—was significantly impaired for children whose mothers were <30 years of age.
In this study, we examined the impact of maternal age at the time of the first prenatal clinic visit on measures of attention in early school-age children. We hypothesized that the deficits in attention outcomes related to prenatal alcohol exposure would be greater among children born to older mothers, that is, ≥30 years of age. Understanding the influence of maternal age on the relation between prenatal alcohol and neurobehavioral outcome might assist in the development of focused primary care interventions for older drinking mothers.
METHOD
Sample
The sample consisted of children born to prospectively identified inner city African American women participating in a longitudinal pregnancy study which recruited women receiving prenatal care at a university antenatal clinic. Study inclusion criteria were singleton birth between September 1989 and August 1991 and continued residence in the Detroit area. Exclusion criteria included multiple-gestation, children born to women known to be HIV positive, or born with congenital malformations. Offspring from repeat pregnancies to the same participating mother were also excluded. As African American women constituted more than 90% of the antenatal clinic population, participation was limited to this group. After an exhaustive search, the 656 eligible children located in the Detroit area at 7 years of age comprised the potential study sample; 94% of these families agreed to participate and 85% completed laboratory testing (N = 556; 49.1% female). At the 7-year follow-up, 6 children were deceased, and 4 children were recognized to have nonalcohol-related congenital malformations and excluded. Within the Detroit area, families were not geographically stable. Most of the families (86.1%) had moved at least once since the child was born, and there was an average of 3.1 home address changes.
The final sample consisted of 462 of the 556 children for whom teacher data were also available. Analyses were performed comparing those 462 children included in these analyses and the 94 who were not included on several demographic and child variables [e.g., maternal IQ, socioeconomic status (SES), child gender and age] as well as prenatal alcohol exposure and attentional outcomes. Among the 15 variables examined, the 2 groups differed on child IQ, total Home Observation for Measurement of the Environment (HOME) score, and child age. Children not included in the analyses had higher IQ scores (t = 5.5, p < 0.001), higher total HOME scores (t = 2.5, p = 0.012), and were slightly younger (t = 3.4, p = 0.001). There were no differences between the groups on either the predictor or outcome variables.
Prenatal Alcohol Exposure
As detailed in Nordstrom-Bailey and colleagues (2004) and Hannigan and colleagues (2010), pregnant women were queried extensively by trained researchers at each prenatal visit to estimate pattern, quantity and frequency of current and peri-conceptional alcohol consumption using a structured interview developed specifically to assess alcohol use during pregnancy (Sokol et al., 1985). A 2-week recall by beverage type was obtained with questions linked to specific drinking habits and particular times of the day and days of week and included queries about binge drinking. From these data, several alcohol exposure variables were calculated as ounces of absolute alcohol per day (AAD) or per drinking day (AADD) at around conception, at the first antenatal visit, and averaged across pregnancy. The current analyses focused on ounces of absolute alcohol per drinking day across pregnancy (AADDXP). In addition, at the first prenatal visit, the 25- item Michigan Alcoholism Screening Test was administered (MAST; Selzer, 1971). At each visit, the adverse effects of alcohol consumption during pregnancy on the fetus were explained and women advised to stop or at least reduce their alcohol intake.
Other Prenatal Drug Use
At each prenatal visit, the use of cocaine, heroin, marijuana, and nonmedical opiates was also ascertained by maternal self-report and women were classified as users or nonusers. Prenatal tobacco exposure was quantified as the typical number of cigarettes the mothers reported they smoked each day.
Procedures
At 7 years of age, following the date of the child’s expected entry into first grade and after informed parental consent, the child and primary caregiver (usually the biologic mother) were tested in our laboratory. Female research assistants, blind to the child’s prenatal exposure status, interviewed each child and mother independently. Permission was given to obtain teacher assessments of child behavior. A third research assistant, also unaware of the child’s exposure status, collected data from the child’s teacher. These procedures and the many measures used in these analyses are detailed in Nordstrom- Bailey and colleagues (2004).
Child Outcomes
The computer-administered version of the Continuous Performance Test (CPT), developed to evaluate sustained attention (Conners, 1995), was used to generate distinct measures of inattention and impulsivity. In this 15-minutes task, a series of letters are presented sequentially to the child who presses a keyboard spacebar for every letter presented with the exception of “X.” The child is instructed not to press the spacebar when the letter “X” is presented. Commission errors (pressing the bar when an “X” appeared) were considered a measure of impulsivity, and omission errors (failing to press the bar when a letter other than “X” appeared) were considered a measure of inattention. Table 1 presents the CPT outcomes. In addition, mean hit reaction time to correct responses (processing speed), d′ (“d prime”), and β score were obtained. Slower reaction time with an increased error rate also indicates inattention. The d′ statistic is an assessment of attention measured as how consistently the child is correct in discriminating a “target” from a “nontarget” in this task. D-Prime (d′) T-scores at or above 60 indicate poor ability to discriminate and attend to the target stimulus (Conners, 1995). Individuals with high response rates (yielding low β scores; T-scores ≤40) are more impulsive and greater risk-takers, whereas those with low response rates (high β scores; T-scores ≥60) are considered more cautious (Conners, 1995). Finally, for each child the number of T-scores for the various indicators (i.e., T-scores for errors of omission, errors of commission, d′, β score, hit reaction time, response variability, and standard error block change) that were ≥2 SDs above the mean was summed to obtain an overall attention score (“ADHD Score”—an approximation of clinical severity). Thus, a child could have a score of 0, indicating none of their scores were indicative of an attention deficit, or a score of 7, suggesting that all attention problem indicators were elevated. Although this is not a standard CPT variable, it is an approximation of an ADHD profile. In general, one or more T-scores ≥60 is an indication that the child had difficulty on this task and attention problems are probable. Increased numbers of elevated T-scores are indicative of greater attention deficiency (Conners, 1995).
Table 1.
Conners Continuous Performance Test (CPT) Variables Assessed
| Variable | Domain assessed | Measurement |
|---|---|---|
| Mean hit reaction time | Processing speed | Time to correct response |
| Errors of omission | Inattention | Number of missed target responses |
| Errors of commission | Impulsivity | Number of nontarget responses |
| d′ (d-prime) | Attention | Consistent discrimination of target / nontarget responses |
| β score | Impulsivity | Rate of responding |
| ADHD score | Overall attention | Number of T-scoresa ≥2 SD above the mean |
T-scores: errors of omission, errors of commission, d′, β score, hit reaction time, response variability, and standard error block change.
The Conners version of the CPT was used in this study because it allows speed of presentation variability, which is often lacking in other CPT paradigms (Conners, 1995). Thus, the Conners CPT is able to assess deficiencies in both the slow and fast presentations of the stimuli, which is very important for assessment of hyperactivity. In addition, the Conners CPT employs a larger number of target responses to avoid a “floor effect” (Conners, 1995).
The widely utilized Teacher Report Form (TRF) was used to assess teacher-reported child behavior problems in the classroom (Achenbach, 1991). In addition to a total behavioral problem score, 8 syndrome scales were examined: aggressive behavior, delinquent behavior, anxious / depressed, withdrawn, somatic complaints, attention problems, social problems, and thought problems. Aggressive and delinquent behavior syndrome scales were summed for a total externalizing behavior score, while anxious / depressed, somatic complaints, and withdrawn comprised the internalizing behavior problem scale. Among the TRF scales, the attention problem syndrome scale was the only outcome of interest in this study.
Control Variables
At the 7-year follow-up visit, a structured interview with the caregiver assessed postnatal family drug, alcohol, and cigarette use, demographic information, including education, SES (Hollingshead, 1975), quality of the home environment (a laboratory-adapted version of the HOME; Caldwell and Bradley, 1984), as well as self-reported caregiver psychopathology (Derogatis et al., 1973) and an assessment of maternal verbal IQ (Wechsler, 1981). The children’s whole-blood lead levels were also assessed.
Data Analyses
Checks were performed for missing and out-of-range data, and for deviations from normality. To examine the impact of maternal age on the relations between prenatal alcohol exposure and measures of child attention, a series of multiple regression analyses were performed, controlling for confounding variables listed above. Because a control variable cannot be a confounder unless it is related to both exposure and outcome, association with either exposure or outcome can be used as the criterion for statistical adjustment (Schlesselman, 1982). In this study, control variables were selected for inclusion based on their relation to the outcome, which has the additional advantage of increasing precision by also including covariates unrelated to exposure and maternal age (Kleinbaum et al., 1988). Pearson’s correlation coefficients (“r”) were used to examine the relation of each control variable to each outcome. All control variables that were even modestly related to each outcome (p < 0.10) were adjusted statistically by regressing the attention outcome and the control variables related to that outcome. All covariates (related to an attention outcome p < 0.10) were entered together in the first step along with maternal age and the alcohol exposure measure (AADDXP). The maternal age × alcohol interaction term was entered in the second step. In the regression analyses, continuous measures of both levels of alcohol drunk per drinking day (AADDXP) and maternal age variables were used. The relations of alcohol consumption measures, maternal age, and attention were considered significant at alpha <0.05, after controlling for the potential confounders. In the tables presenting the regression analyses, the bivariate correlations are shown as Pearson’s “r.” The relation of attention and the interaction term is shown as the “β” statistic, the standardized regression coefficient (Table 3).
Table 3.
Relation Between Prenatal Alcohol Exposure and Measures of Attention
| Attention measures | Pregnancy drinking per drinking day
|
Across pregnancy average
|
|||||
|---|---|---|---|---|---|---|---|
| Maternal age
|
oz Alc / drinking day
|
Interaction term
|
oz Alc / day
|
||||
| r | β | r | β | r | β | β | |
| CPT | |||||||
| Errors of omissiona | 0.03 | −0.06 | 0.13** | −0.37 | 0.15*** | 0.52* | −0.16 |
| Errors of commissionb | −0.06 | −0.12 | 0.03 | −0.03 | 0.03 | 0.08 | 0.16 |
| ADHD T-score (# >2 SD’s above mean)c | 0.10† | 0.02 | 0.02 | −0.36† | 0.06 | 0.38† | 0.17 |
| d′ (d prime)d | 0.04 | 0.13* | −0.12** | 0.27 | −0.13** | −0.42† | −0.03 |
| β scoree | −0.01 | −0.18** | 0.10† | −0.63** | 0.12** | 0.78*** | 0.48 |
| Mean hit reaction timef | 0.16*** | 0.15* | 0.01 | −0.13 | 0.03 | 0.10 | −0.06 |
| TRF | |||||||
| Attention problemsg | 0.09 | 0.05 | 0.03 | −0.20 | 0.06 | 0.21 | −0.14 |
CPT, Continuous Performance Test; HOME, Home Observation for Measurement of the Environment; SES, socioeconomic status; TRF,
Teacher Report Form.
p < 0.10.
p < 0.05.
p < 0.01.
p < 0.001.
Covariates assessed by outcome:
Prenatal cocaine, 7-year blood lead levels, caregiver education, HOME score, Peabody Picture Vocabulary Test (PPVT).
Prenatal cocaine, 7-year blood lead levels, child gender, caregiver marital status, prenatal marijuana, 7-year caregiver marijuana.
Prenatal cocaine, 7-year blood lead levels, caregiver education, prenatal nicotine, age of child.
Prenatal cocaine, 7-year blood lead levels, HOME score, child gender, PPVT.
Prenatal cocaine, 7-year blood lead levels, caregiver education, SES, PPVT.
7-year blood lead levels, prenatal nicotine, child gender.
Prenatal cocaine, 7-year blood lead levels, caregiver education, prenatal nicotine, HOME score, SES, age of child.
RESULTS
Data from the TRF were not available for 56 (10.1%) of the 556 children assessed on the CPT in the laboratory at 7 years of age. Data from children with Performance IQs <65 (28 children; an additional 7.6%) were removed from the analyses to minimize attention problems that might be secondary to profound general cognitive delays or dysfunction. The final sample included 462 children. Within this sample, just over half of the caregivers had the equivalent of a high school diploma for both the younger and older women (57.7 and 57.5% high school graduate or General Education Degree (GED) respectively). In addition, 75% of the women were unmarried and the majority of caregivers were the biologic mothers (81.6%). Most families (58%) were in the lowest SES group based on income and employment (Hollingshead, 1975). Mean child whole-blood lead level was 5.0 μg / dl; however, 25 children had values >10 μg/ dl and 2 had values >15 μg/ dl (see Chiodo et al., 2007). Table 2 presents these maternal, family and child characteristics for both maternal age groups, <30 years of age vs. ≥30 years of age at the time of the first prenatal assessment. Older mothers were significantly more likely to use alcohol, cocaine, and nicotine during the prenatal period. There were no differences between older and younger mothers in marijuana usage or in any family or child characteristic. Average child total TRF T-scores as reported by the teacher were within the normal range. However, 20.1% of all children had clinical or borderline behavior problem scores. In addition, 11.5% of the sample was identified by the teacher as having clinical levels of attention behavior problems (TRF attention problems T-score ≥70), while an additional 7.1% had borderline levels of attention problems (T-scores ≥67 and ≤69). There was no significant relation between maternal drinking and the TRF attention problems syndrome scale (Table 3).
Table 2.
Sample Characteristics by Maternal Age Status
| Maternal age <30 | Maternal age ≥30 | t or χ2 | |
|---|---|---|---|
| Maternal characteristics | N = 334 | N = 128 | |
| Maternal age at the first prenatal visit (years) | 22.4 ± 4.2 | 33.7 ± 3.0 | −27.7*** |
| Alcohol during pregnancy (oz AADXP) | 0.11 ± 0.3 | 0.2 ± 0.6 | −2.9** |
| Alcohol per drinking occasion during pregnancy (oz AADDXP) | 1.1 ± 1.4 | 1.4 ± 1.2 | −1.9† |
| Cigarettes during pregnancy (# / day) | 6.5 ± 8.3 | 12.8 ± 11.0 | −6.6*** |
| Cocaine during pregnancy (% used) | 33% | 52% | 16.0*** |
| Marijuana during pregnancy (% used) | 35.6 | 35.9 | 0.0 |
| Caregiver characteristics (7-year assessment) | |||
| Primary caregiver (% biological mother) | 82.9% | 85.2% | 0.3 |
| Marital status (% married) | 26.5% | 26.6% | 0.0 |
| SESa | 29.7 ± 10.0 | 28.7 ± 10.0 | 1.0 |
| Home observation for measurement of the environment | 31.8 ± 5.9 | 32.8 ± 5.9 | −1.6 |
| Educationb | 4.6 ± 1.4 | 4.7 ± 1.4 | −0.5 |
| Current caregiver alcohol use (oz AAD) | 0.30 ± 0.6 | 0.31 ± 0.6 | 0.0 |
| Current caregiver cocaine use (yes / no) | 0.3% | 4.0% | 9.9* |
| Current caregiver marijuana use (yes / no) | 14.8% | 12.7% | 3.8 |
| Child characteristics (7-year assessment) | |||
| Age (years) | 6.9 ± 0.2 | 6.9 ± 0.3 | 1.5 |
| Gender (% male) | 49.4% | 51.6% | 0.1 |
| TRF total behavior problems T-score | 55.8 ± 11.4 | 57.0 ± 12.1 | 1.1 |
| TRF attention problems syndrome T-score | 57.9 ± 9.5 | 59.6 ± 9.6 | 3.5† |
| CPT | |||
| Total hits | 273.7 ± 35.9 | 269.2 ± 33.6 | 1.3 |
| Mean hit reaction time | 555.1 ± 100.8 | 579.0 ± 103.6 | 4.6* |
| Errors of commission | 21.3 ± 3.4 | 20.4 ± 6.2 | 1.4 |
| Errors of omission | 50.3 ± 35.9 | 54.8 ± 33.6 | 1.3 |
| ADHD score (# t’s 2SD+) | 1.2 ± 1.5 | 1.5 ± 1.6 | 3.2† |
| Mean blood lead (Pb) levels | 4.8 ± 2.8 | 5.3 ± 3.5 | 2.2 |
AAD, absolute alcohol per day; AADXP, absolute alcohol per day across pregnancy; AADDXP, absolute alcohol per drinking day across pregnancy; SES, socioeconomic status; TRF, Teacher Report Form; CPT, continuous performance test.
p < 0.10.
p < 0.05.
p < 0.01.
p < 0.001.
Based on Hollingshead, 1975.
1 = <7th grade; 2 = junior high; 3 = some high school; 4 = GED; 5 = high school grad; 6 = some college; 7 = college grad.
Regression analyses controlling for covariates identified a significant maternal age by across-pregnancy alcohol consumption levels per drinking day (AADDXP) interaction on the number of errors of omission (i.e., not responding to a “target”) and the β score (Table 3). In addition, there was a marginally significant effect (0.05 < p < 0.10) for d′ and the summary ADHD score, providing further support for the presence of a maternal age × prenatal alcohol exposure interaction on attention.
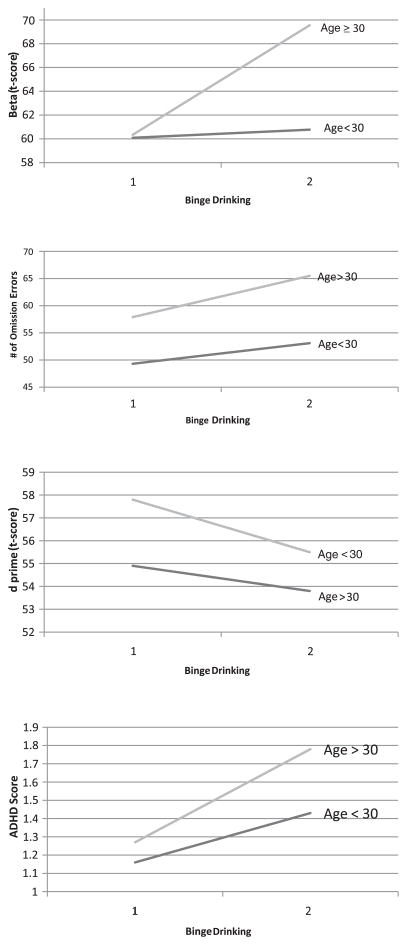
Further depiction of this relation is provided in Fig. 1 which provides a closer examination of each attention measure. For each figure, alcohol consumption level per drinking day (AADDXP) was dichotomized at 5 drinks per occasion. Those who drank <5 drinks per drinking day were coded as a 1, while those who drank ≥5 drinks per drinking day were coded as a 2 (see Fig. 1). Children born to older mothers (i.e., ≥30 years old) who binge drank have the highest β score, suggesting they performed very slowly and cautiously. In addition, these children have the highest numbers of errors of omission. On the other hand, there was no significant interaction between maternal age and AADDXP for errors of commission. Thus, these alcohol-exposed children were not behaving impulsively in this task, pressing the keys cautiously (high β score) but still making more mistakes. In addition, children of older women who binge drank had the lowest d′ scores, indicating they had more difficulty sustaining attention across the task. The presence of an interaction between maternal age and AADDXP on the summary ADHD score suggests that children of older mothers performed more poorly on these attention measures in comparison to children born to mothers <30 years of age. Children of older women (≥30 years old) have poorer attention scores overall than children born to younger mothers when exposed prenatally to higher levels of alcohol.
Fig. 1.
Attention outcomes across age and binge drinking groups (1 = AADDXP <2.5; 2 = AADDXP ≥2.5). AADDXP, absolute alcohol per drinking day across pregnancy.
Although previous examination of the CPT and IQ have not been significant (Hoerig, 1999; Pepin and Loranger, 1996), these 2 domains are significantly related in this sample (r’s range = −0.04 to −0.20). Owing to the significant relation between the CPT and IQ, regression analyses were redone including Wechsler Preschool and Primary Scale of Intelligence (WPPSI) Full IQ, also assessed at the age 7- year assessment, as a covariate. In both analyses, the maternal age × alcohol interaction term remained significant, suggesting that these alcohol-related attention deficits are specific to attentional abilities and were unrelated to the impact of prenatal alcohol exposure on general intelligence.
In addition to a significant maternal age × alcohol exposure interaction, there was a significant main effect of maternal age for 2 of the attention variables (d′ and β; β = 0.13, p = 0.05 and β = −0.18, p = 0.007, respectively). These results indicate that although children of older women performed more poorly than children of younger women, regardless of drinking status (the main effect), the children of older drinking women also performed more poorly than children of younger drinking women on both attention measures (the interaction).
DISCUSSION
Children born to drinking mothers 30 years of age or older but not children born to younger drinking mothers (<30 years old), showed statistically significant deficits in attention measures on the CPT at age 7 years. Regression analyses identified a significant interaction between maternal age and a measure of levels of alcohol consumed per drinking day across pregnancy (AADDXP) on several indicators of attention, but not impulsivity. This occurred over and above significant decreases in CPT performance in children of older women, regardless of drinking during pregnancy.
This finding is consistent with research identifying differential susceptibility to prenatal alcohol exposure on other cognitive outcomes in young children because of older maternal age. Jacobson and colleagues (1998) identified this interaction for behavioral maturation in infants assessed by the Bayley Scales of Development and Elicited Play. In infants born to women drinking alcohol in binge-like patterns of 5 or more drinks per occasion, there was no increased incidence of functionally significant deficits in infants born to younger women (<30 years old). However, infants born to older women (≥30 years of age) drinking at these levels were 2- to 5-times more likely to be functionally impaired on the Bayley Scales and Elicited Play (Jacobson et al., 1998). These same investigators later showed that maternal age also moderated the cognitive effect of prenatal alcohol exposure on IQ at 7.5 years of age (Jacobson et al., 2004). Among children born to older mothers (i.e., ≥30 years of age), there were statistically significant decreases in Full Scale IQ related to the amount of alcohol consumed per day during pregnancy, as well as a decrease in the Freedom of Distractibility IQ scale (FD). To ensure that this reduction in attention abilities was not actually because of general IQ, Jacobson and colleagues (1994) examined an additional composite Freedom from Distractibility- Verbal Comprehension score (FD-VC). Analyses revealed a significant alcohol-related effect for children born to older women only, further supporting the idea that specific attention effects related to alcohol exposure are moderated by maternal age. Burden and colleagues (2005) showed that working memory was also differentially susceptible to prenatal alcohol exposure as a function of maternal age. Our current results are consistent with this and demonstrate that children of older women appear to be more vulnerable to the effects of prenatal alcohol on attention as well.
A brief overview of previous studies of attention deficits after prenatal alcohol exposure suggests that several factors besides maternal age may also influence the likelihood of demonstrating prenatal alcohol-related attention problems. These include, for example, diagnosis (esp., ADHD) of comparison groups (e.g., Mick et al., 2002; Nanson and Hiscock, 1990), presence of dysmorphia in the alcohol-exposed children (Coles et al., 1997), prenatal cigarette exposure, parental ADHD, “social adversity” (Mick et al., 2002), the age of the children, and the specific outcome measure (Boyd et al., 1991; Coles et al., 1997, 2002; Mattson et al., 2006; Mick et al., 2002; Nanson and Hiscock, 1990). Others have discussed in more detail how important a careful definition of the components of attention is to understanding the apparently variable responses to prenatal alcohol exposure (e.g., Burden et al., 2005; Coles et al., 2002; Lee et al., 2004). Finally, child IQ may not influence attention deficits in FASD. This seems to be the case as attention problems in children with FAS/FAE and low IQ (i.e., 78) or with ADHD and normal IQ (i.e., 104 to 110) were similar (Nanson and Hiscock, 1990). Also Jacobson and colleagues (2004) demonstrated an independence of attention and IQ effects following prenatal alcohol exposure. On the other hand, specific deficits in cognitive or executive functioning have been related to the patterns of attention problems in children exposed to “heavy” prenatal alcohol (e.g., Vaurio et al., 2008).
The reason for increased susceptibility of children of older mothers is not clear but various reasonable hypotheses have been considered. Older mothers are likely to have been drinking longer than younger mothers and so are more likely to have greater tolerance to alcohol and more alcohol-related health problems, including liver dysfunction, all of which can lead to higher levels of alcohol in their fetuses. Chronic drinking in older mothers is also more likely than for younger mothers to be related to primary and secondary under-nutrition and so may lead to a greater reduction in the availability, absorption and activity of nutrients critical for fetal development (Fisher, 1988; Lieber, 1988). In addition, the ratio of body fat to water increases with advancing maternal age, thereby exposing both mother and fetus to relatively higher peak blood alcohol concentrations per unit of alcohol consumed over a longer period (Vestal et al., 1977). However, the relation between increased maternal body fat and fetal alcohol levels needs further investigation. Finally, others have suggested that parity, a variable that increases significantly in relation to maternal age may also play a role in the incidence of FAS (Abel, 1998; Abel and Hannigan, 1995).
Prenatal alcohol is known to cause a variety of adverse effects on the developing fetus and at this time there is no clearly defined minimum amount of alcohol known to be harmful to embryos or fetuses, nor any defined “safe” level of drinking during pregnancy that may be promulgated as a reasonable public health message (Abate et al., 2008; ACOG, 2006; Henderson et al., 2007; Roebuck et al., 1999; Sampson et al., 2000; Sokol et al., 2003). What is becoming clearer, however, is that the pattern of alcohol consumption— higher amount, faster rate, and/ or greater frequency of drinking—has important and proximate teratogenic consequences (Abel and Hannigan, 1995; Elliott and Bower, 2004; Olney, 2004; West et al., 1994). Jacobson and colleagues (1994, 1998) showed virtually no developmental effects on birth weight, the Bayley Mental and Psychomotor indices, and cognitive processing speed, with drinking <7 drinks per week on average, but substantial deficits were found in children exposed to more than this. Several researchers have reported that binge drinking and chronic alcoholism both are associated with neurobehavioral effects and decreases in IQ in the children born to these mothers (Ikonomidou et al., 2000; Sampson et al., 1989; Streissguth et al., 1989). Yet not all fetuses exhibit effects of prenatal alcohol exposure even at comparable rates of maternal drinking. The cause of these differential susceptibilities has not been elucidated but has been attributed to influences ranging from genetic predisposition, nutritional inadequacy and variation in the vulnerability of different brain regions (Maier and West, 2001).
As previously recommended, women ought to be warned that with increasing maternal age, fetuses may be more severely affected by alcohol exposure, even when the mother’s alcohol intake during pregnancy has not increased from previous pregnancy, and even if prior pregnancies and older children may appear to have been unaffected. Health care professionals need to be aware that increased maternal age among their pregnant patients (i.e., >30 years) increases the susceptibility of the fetus to effects of alcohol. In addition, physicians need to be able to appropriately tailor their interventions to patients during standard clinical visits about the relative risks of maternal drinking to fetuses when the mothers are older. Additional studies to evaluate how maternal age moderates prenatal alcohol-induced deficits in attention and other behavior or cognitive outcomes are needed to more fully understand the risks and consequences of maternal alcohol use during pregnancy.
One study limitation needs to be addressed, potentially poor generalizability. The current cohort included only low SES urban African American women and their children. We should also note that although significant, the effect size is small (Cohen’s F2 = 0.03 and 0.02 for β and errors of commission respectively). For these attention measures, the alcohol × maternal age interaction term accounts for 3% (β) and 2% of the variance (errors of commission). In addition, the cohort in which the initial maternal age effects were obtained (Jacobson et al., 1994) had similar homogenous demographics. Thus, these findings need to be reassessed in more heterogeneous populations. We also note the lack of significance in teacher-reported attention findings (i.e., the TRF). This lack of concordance is not too surprising as other researchers have found nonsignificant relations between the CPT and the TRF in children with ADHD (DuPaul et al., 1992; Halperin et al., 1991; Lovejoy and Rasmussen, 1990), suggesting that they are in part assessing different constructs.
In conclusion, this study demonstrates poorer attention performance in 7-year-old children exposed to alcohol prenatally born to older but not to younger drinking mothers. These findings may justify targeting older drinking mothers for particular attention in primary care settings because their fetuses are at greater risk than those of younger drinking mothers for alcohol-related deficits in attention.
References
- Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: evidence against “safe” amounts of prenatal exposure. Exp BiolMed. 2008;233:139–154. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol. 1995;17:437–443. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Abel EL. Fetal Alcohol Abuse Syndrome. Plenum Press; New York, NY: 1998. [Google Scholar]
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1995;17:445–462. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Achenbach TM. Manual for Teacher’s Report Form and 1991 Profile. University of VT, Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- American College of Obstetrics & Gynecology . [Accessed January 28, 2008];Drinking and reproductive health: a fetal alcohol spectrum disorders prevention tool kit. 2006 Available at: http://www.acog.org/departments/healthIssues/FASDToolKit.pdf.
- Bertrand J, Floyd RL, Weber MK, O’Connor M, Riley EP, Johnson KA, Cohen DE the National Task Force on FAS/FAE. Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. Centers for Disease Control and Prevention; Atlanta, GA: 2004. [Google Scholar]
- Boyd TA, Ernhart CB, Greene TH, Sokol RJ, Martier S. Prenatal alcohol exposure and sustained attention in the preschool years. Neurotoxicol Teratol. 1991;13:49–55. doi: 10.1016/0892-0362(91)90027-t. [DOI] [PubMed] [Google Scholar]
- Brown RT, Coles CD, Smith IE, Platzman KA, Silverstein J, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age: II. Attention and behavior. Neurotoxicol Teratol. 1991;13:369–376. doi: 10.1016/0892-0362(91)90085-b. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29:443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Administration manual, revised edition: home observation for measurement of the environment. University of Arkansas at Little Rock; Little Rock, AK: 1984. [Google Scholar]
- Carmichael Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Carmichael Olson H, Sampson PD, Barr H, Streissguth AP, Bookstein FL. Prenatal exposure to alcohol and school problems in late childhood: a longitudinal prospective study. Dev Psychopathol. 1992;4:341–359. [Google Scholar]
- CDC. Fetal Alcohol Syndrome – Alaska, Arizona, Colorado and New York, 1995–1997. MMWR. 2002a;51:433–435. [PubMed] [Google Scholar]
- CDC . Alcohol use among women of childbearing age—United States, 1991—1999. MMWR. 2002b;51:273–276. [PubMed] [Google Scholar]
- CDC. Alcohol use among pregnant and nonpregnant women of childbearing age—United States, 1991–2005. MMWR. 2009;58:529–532. [PubMed] [Google Scholar]
- Chiodo LM, Covington C, Sokol RJ, Hannigan JH, Jannise J, Ager J, Greenwald M, Delaney-Black V. Blood lead levels and specific attention effects in young children. Neurotoxicol Teratol. 2007;29:538–546. doi: 10.1016/j.ntt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Janisse J, Delaney-Black V, Sokol RJ, Hannigan JH. A metric of maternal prenatal risk drinking predicts neurobehavioral outcomes in preschool children. Alcohol Clin Exp Res. 2009;33:634–644. doi: 10.1111/j.1530-0277.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Sokol RJ, Delaney-Black V, Janisse J, Hannigan JH. Validity of the T-ACE in pregnancy in predicting child outcome and risk drinking. Alcohol. 2010;44(4) doi: 10.1016/j.alcohol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res. 2002;26:263–271. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek R, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- Conners CK. Conners’ Continuous Performance Test. Multi-Health Systems; Toronto: 1995. [Google Scholar]
- Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale: preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- DuPaul GJ, Anastopoulos AD, Shelton TL, Guevremont DC, Metevia L. Multi-method assessment of attention deficit hyperactivity disorder: the diagnostic utility of clinic-based tests. J Clin Child Psychol. 1992;21:394–402. [Google Scholar]
- Elliott EJ, Bower C. FAS in Australia: fact or fiction? J Paediatr Child Health. 2004;40:8–10. doi: 10.1111/j.1440-1754.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- Ernhart CB, Sokol RJ, Martier S, Moron P, Nadler D, Ager J, Wolf A. Alcohol teratogenicity in the human: a detailed assessment of specificity, critical period, and threshold. Am J Obstet Gynecol. 1987;156:33–39. doi: 10.1016/0002-9378(87)90199-2. [DOI] [PubMed] [Google Scholar]
- Fisher SE. Selective fetal malnutrition: the fetal alcohol syndrome. J Am Coll Nutrition. 1988;7:101–106. doi: 10.1080/07315724.1988.10720225. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119:e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager J, Greenwald MK, Delaney-Black V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2010;44(4) doi: 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J, Kesmodel U, Gray R. Systematic review of the fetal effects of prenatal binge-drinking. J Epidemiol Community Health. 2007;61:1069– 1073. doi: 10.1136/jech.2006.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerig DC. Evaluating the relationship among memory, attention, and intelligence with elementary-age children who have learning disabilities. Diss Abstr Int Sec A Hum Soc Sci. 1999;59(8-A):2924. [Google Scholar]
- Hollingshead AB. Unpublished paper. Yale University, Department of Social Work; New Haven, CT: 1975. Four factor index of social status. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal alcohol exposure and neurodevelopmental development: where is the threshold? Alcohol Health Res World. 1994;18:30–36. [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Drinking moderately and pregnancy. Effects on child development. Alcohol Res Health. 1999;23:25–30. [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ. Effects of fetal alcohol exposure on infant reaction time. Alcohol Clin Exp Res. 1994;18:1125–1132. doi: 10.1111/j.1530-0277.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Ager JW., Jr Relation of maternal age and pattern of pregnancy drinking to functionally significant cognitive deficit in infancy. Alcohol Clin Exp Res. 1998;22:345–351. doi: 10.1111/j.1530-0277.1998.tb03659.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE. Applied Regression Analysis and Other Multivariable Methods. 2. PWS-Kent; Boston, MA: 1988. [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev. 2007;31:192– 201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Wathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1995;19:1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Lee KT, Mattson SN, Riley EP. Classifying children with heavy prenatal alcohol exposure using measures of attention. J Int Neuropsychol Soc. 2004;10:271–277. doi: 10.1017/S1355617704102142. [DOI] [PubMed] [Google Scholar]
- Lieber CS. The influence of alcohol on nutritional status. Nutrition Rev. 1988;46:241–254. doi: 10.1111/j.1753-4887.1988.tb05443.x. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- Manning MA, Hoyme EH. Fetal alcohol spectrum disorders: a practical clinical approach to diagnosis. Neurosci Biobehav Rev. 2007;31:230–238. doi: 10.1016/j.neubiorev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Martínez-Frías ML, Bermejo E, Rodríguez-Pinilla E, Frías JL. Risk for congenital anomalies associated with different sporadic and daily doses of alcohol consumption during pregnancy: a case–control study. Birth Defects Res A Clin Mol Teratol. 2004;70:194–200. doi: 10.1002/bdra.20017. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20:361– 369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: a summary. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NCO, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Hendricks LS, Snell C, Tabachnick BG, Sttellavato C, Buckley DG, Brooke L, Viljoen DL. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res. 2008;32:738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case–control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 2002;41:378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Nanson JL, Hiscock M. Attention deficits in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1990;14:656–661. doi: 10.1111/j.1530-0277.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Nash K, Rovet J, Greenbaum R, Fantus E, Nulman I, Koren G. Identifying the behavioural phenotype in fetal alcohol spectrum disorder: Sensitivity, specificity and screening potential. Arch Womens Ment Health. 2006;9:181–186. doi: 10.1007/s00737-006-0130-3. [DOI] [PubMed] [Google Scholar]
- NIAAA . Surgeon general updates warning about alcohol use during pregnancy. NIAAA Newsletter. 2005a Spring;6:2. [Google Scholar]
- Nordstrom-Bailey B, Delaney-Black V, Covington CY, Ager J, Janisse J, Hannigan JH, Sokol RJ. Prenatal exposure to binge drinking and cognitive and behavioral outcomes at age 7 years. Am J Obstet Gynecol. 2004;191:1037–1043. doi: 10.1016/j.ajog.2004.05.048. [DOI] [PubMed] [Google Scholar]
- O’Leary CM. Foetal alcohol syndrome: diagnosis, epidemiology and developmental outcomes. J Paediatr Child Health. 2004;40:2–7. doi: 10.1111/j.1440-1754.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- Olney JW. Fetal alcohol syndrome at the cellular level. Addict Biol. 2004;9:137–149. doi: 10.1080/13556210410001717006. discussion 151. [DOI] [PubMed] [Google Scholar]
- Pepin M, Loranger M. Computer Administered Aptitude Battery Software Program. Multi-Health Systems; Toronto: 1996. [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res. 1999;23:1070–1076. [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Barr HM, Bookstein FL. Neurobehavioral effects of prenatal alcohol: Part II. Partial least squares analysis. Neurotoxicol Teratol. 1989;11:477–491. doi: 10.1016/0892-0362(89)90025-1. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Barr HM. On categorizations in analyses of alcohol teratogenesis. Environ Health Perspect. 2000;108 (Suppl 3):421–428. doi: 10.1289/ehp.00108s3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Schlesselman JJ. Case-Control Studies, Design, Conduct and Analysis. Oxford University Press; New York, NY: 1982. [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: The quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Ager J, Martier S, Debanne S, Ernhart C, Kuzma J, Miller SI. Significant determinants of susceptibility to alcohol teratogenicity. Ann N Y Acad Sci. 1986;477:87–102. doi: 10.1111/j.1749-6632.1986.tb40323.x. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorders. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Sokol R, Martier S, Ernhart C. Identification of alcohol abuse in the prenatal clinic. In: Chang NC, Chao HM, editors. NIAAA Research Monograph 17: Early Identification of Alcohol Abuse. DHHS Publication, U.S. Department of Health and Human Resources; Washington, DC: 1985. pp. 85–128. [Google Scholar]
- Spadoni AD, McGee CL, Fryer SL, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Neurosci Biobehav Rev. 2007;31:239–245. doi: 10.1016/j.neubiorev.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PD, Barr HM. Neurobehavioral effects of prenatal alcohol: Part III. PLS analyses of neuropsychologic tests. Neurotoxicol Teratol. 1989;11:493–507. doi: 10.1016/0892-0362(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure of attention-deficit / hyperactivity disorder. J Int Neuropsychol Soc. 2008;14:119–129. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, Mezey E. Aging and ethanol metabolism. Clin Pharmacol Ther. 1977;21:343–354. doi: 10.1002/cpt1977213343. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale— Revised. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- West JR, Chen WJ, Pantazis NJ. Fetal alcohol syndrome: the vulnerability of the developing brain and possible mechanisms of damage. Metab Brain Dis. 1994;9:291–322. doi: 10.1007/BF02098878. [DOI] [PubMed] [Google Scholar]