Abstract
Objectives
A phase II trial designed to evaluate the safety and efficacy of weekly topotecan and docetaxel in heavily treated patients with recurrent uterine or epithelial ovarian cancers.
Methods
Eligible patients with recurrent epithelial ovarian or uterine cancers were treated with weekly topotecan 3.5 mg/m2 and docetaxel 30 mg/m2 for 3 consecutive weeks. Cycles were repeated every 4 weeks for 6 cycles or until evidence of disease progression, unacceptable toxicity, or death. Response was assessed as per RECIST or Rustin’s criteria. Time to best response and overall survival were calculated using Kaplan–Meier statistical methods.
Results
Twenty-seven patients registered, of which 24 were evaluable for response. The majority of patients had received 2 prior chemotherapy regimens. Of the total 86 cycles of chemotherapy that were administered, there were three grade 4 (all neutropenia) and ten grade 3 toxicities. Six of the grade 3 non-hematologic toxicities were unrelated to treatment. There were 8 dose delays and 4 dose reductions. The overall response rate was 25% (95% CI: 7.7%–42.3%, 8% CR, 17% PR), and 38% of the patients had clinical benefit (95% CI: 18.1%–56.9%; CR+PR+13% SD). The median duration of response was 8.5 months (range 3–19 months). The median overall survival was 18.5 months (range 1.8–50.7 months).
Conclusion
The combination of weekly topotecan and docetaxel has clinical benefit and is well tolerated in this heavily treated patient population. Patients with platinum-resistant tumors had clinical benefit and should be considered for further study with this regimen.
Keywords: Ovarian cancer, Uterine cancer, Chemotherapy, Topotecan, Docetaxel, Clinical trial
Introduction
Patients with recurrent uterine and recurrent epithelial ovarian cancer (EOC) are often heavily treated with different chemotherapy combinations and they may not tolerate additional standard dose chemotherapeutic regimens. One of the goals of treatment at the time of recurrence is to try to stabilize disease without worsening the patient’s quality of life.
Recent data has demonstrated efficacy of topotecan and docetaxel in uterine and ovarian cancers. Phase I/II studies have demonstrated that topotecan has activity in advanced and recurrent uterine cancer with overall response rates ranging from 9 to 23% [1-5]. Reported side effects were mainly hematologic, specifically, neutropenia and thrombocytopenia. These were managed effectively with hematopoietic stimulating factors.
Taxanes have also been reported to be effective in the treatment of advanced and recurrent uterine cancer [6-13]. Endometrial cancer cell lines have demonstrated sensitivity to paclitaxel [8]. Response rates of 35%–37% have been reported in two separate phase II trials of paclitaxel in advanced endometrial cancers [6,7,10]. The SCOTROC trial demonstrated equivalence of docetaxel when substituted for paclitaxel in the treatment of primary ovarian cancer with significantly decreased peripheral neuropathy [14]. Phase II studies have demonstrated a 21–31% response rate of docetaxel in advanced or recurrent uterine cancer [9,11]. Gunthert et al. reported no patients with grade 3 or 4 toxicity while Katsumata, et al. reported significant grade 3 or 4 neutropenia as the major toxicity.
Topotecan is a commonly used agent in patients with recurrent ovarian cancer. Response rates of between 14% and 50% have been reported in the literature, including a large Gynecologic Oncology Group (GOG) study [15-17]. In addition, weekly dosing schedules suggest similar activity in ovarian cancer patients with less toxicity, especially myelosuppression [16-18].
Both topotecan and docetaxel are commonly used agents in recurrent ovarian cancer but not always in combination. Docetaxel has been shown to have response rates of 7–30% in platinum-refractory patients [19-22]. Docetaxel has also demonstrated clinical response in patients classified as paclitaxel-refractory, confirming an incomplete cross-resistance between these two agents [23-24].
Because of the broad-spectrum antitumor activity of topotecan and docetaxel that may not be cross-resistant, several phase I trials have been conducted with this combination regimen in various tumor types [25-31]. Docetaxel was administered on day 1 followed by daily intravenous topotecan on days 1–3 versus 1–5. Dose limiting toxicity for each regimen was myelosuppression, consisting of both neutropenia and/or thrombocytopenia requiring prophylactic growth factor support or dose reduction.
Weekly dosing of this combination regimen may preserve the synergy of this combination while decreasing toxicity. Both patients with recurrent uterine and ovarian cancers have response rates less than 30% with current second or third line chemotherapy regimens after front line therapy, and the clinical outcomes are similar [32,33]. The commonly utilized chemotherapy regimens after failing first line therapy are similar in recurrent uterine and ovarian cancer patients. Therefore, we initiated a single-institution Phase II trial of weekly docetaxel and topotecan in heavily treated patients with recurrent uterine and ovarian cancer to evaluate the safety and efficacy of this doublet regimen.
Materials and methods
This is an open-labeled phase II trial. The primary outcome is response to treatment. The secondary aim is to evaluate the safety and toxicity profile of the regimen in this patient population.
Patients
Eligible patients must have had recurrent uterine, epithelial ovarian, fallopian, or primary peritoneal cancer. Recurrent uterine cancer was defined as measurable disease that was documented by biopsy or surgery. Recurrent ovarian, fallopian, or primary peritoneal cancer was defined as measurable disease by imaging or by Rustin’s criteria [34]. In patients who had prior radiation therapy, the disease recurrence could have been inside or outside the radiation field. Patients were required to be able to have the capacity to consent to the trial, have an ECOG performance status of less than or equal to 2 and no more than grade 1 peripheral neuropathy. They received no chemotherapy or radiotherapy within 4 weeks prior to starting the trial.
Patients were excluded if they had impaired hematologic, renal, or hepatic functions including platelet count less than 100,000/mm3, absolute neutrophil counts (ANC) less than 1500/mm3, hemoglobin less than 8.0 g/dL, serum creatinine clearance less than or equal to 50 ml/minute, bilirubin less than or equal to the upper limit of normal, and AST, ALT, and alkaline phosphatase less than or equal to 1.5 times the upper limit of normal. Patients could not have chronic or active hepatitis or other uncontrolled medical conditions. Patients with known severe hypersensitivity to either study medication or the formulants were excluded.
Patients were removed from the study if they had disease progression, an unacceptable adverse event, or by an independent decision by the patient or treating gynecologic oncologist. All patients signed written, informed consent prior to initiating therapy. Institutional review board approval for the protocol and the consent were obtained prior to patient enrollment.
Treatment plan
Patients were clinically evaluated prior to each chemotherapy cycle. Serum chemistry, liver function tests, and complete blood count were performed every week. Tumor markers were drawn every 4 weeks. Radiographic assessment with CT scan of the abdomen and pelvis (and chest, when appropriate) was performed before initiation of therapy, after 3 cycles of chemotherapy, and at the end of the treatment to evaluate tumor measurements. Radiologic target lesions for therapeutic evaluation were identified and designated prior to initiating therapy.
Patients were treated with topotecan 3.5 mg/m2 followed by docetaxel 30 mg/m2 on day 1 and then weekly. Each cycle consisted of weekly therapy for 3 weeks (d1, 8, and 15) followed by a 1-week rest for a total of 6 cycles, progressive disease, dose-limiting toxicity, or death. Blood transfusions and G-CSF support were given as clinically indicated based on standard NCCN guidelines [35]. Toxicity was graded based on the standard National Cancer Institute Common Toxicity Criteria, version 2.0. Dose delays were permitted for up to 2 weeks for grade 3 or 4 hematologic or gastrointestinal toxicity. Chemotherapy doses were omitted or decreased based on hematologic parameters. Weekly doses were held for an ANC less than 999/mm3 or platelets less than 50,000/mm3. Subsequent doses were reduced by 20–30% based on the severity of the neutropenia or thrombocytopenia as deemed necessary by the primary treating physician. Elevations of AST, ALT, or alkaline phosphatase greater than 2.5 times the upper limits of normal resulted in dose omission or delay. Docetaxel was withheld or delayed for bilirubin levels greater than the upper limits of normal, grade 2 or greater stomatitis or peripheral neuropathy. Hypolacrimation was treated with artificial tears and docetaxel was withheld. For other grade 3 or 4 non-hematologic toxicities (excluding grade 3 or 4 nausea, vomiting, or alopecia), both treatment doses were held until resolution of toxicity to grade 2 or less with a subsequent 20% dose reduction.
Response was measured and confirmed using the standard RECIST and modified Rustin’s criteria where appropriate [34,36]. Response was categorized as a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) by established criteria. The proportion of patients who responded to treatment was computed along with corresponding 95% confidence interval.
Results
The patient characteristics are presented in Table 1 Twenty-nine patients were enrolled in the study from August, 2004 until February, 2007 and 27 were treated per protocol. One patient died before beginning treatment, and one withdrew consent. Of the 27 patients,15 had recurrent EOC, 3 had primary peritoneal cancer, and 9 had recurrent uterine cancer (6 UPSC, 3 endometrioid adenocarcinoma). All 27 patients had prior combination chemotherapy regimens: 9 had 1 prior regimen, 16 had 2 prior, 1 had 3 prior, and 1 had 4 prior regimens. Two patients had received prior radiation therapy in addition to prior chemotherapy.
Table 1.
Patient demographics and baseline disease characteristics, N=29.
| Median age (years) | 63 (47–79) |
| ECOG performance status | |
| 0 | 0 |
| 1 | 25 |
| 2 | 4 |
| Primary tumor site | |
| Ovary | 16 |
| Peritoneum | 3 |
| Uterine | 10 |
| Histology | |
| Serous | 22 |
| Endometrioid | 4 |
| Clear cell | 2 |
| Carcinosarcoma | 1 |
| Stage | |
| I–II | 7 |
| III | 17 |
| IV | 5 |
| Suboptimal-debulking | 3 |
| Platinum resistant | 11 |
| Prior # of chemotherapy regimens | |
| 0 | 2 |
| 1 | 9 |
| 2 | 16 |
| >=3 | 2 |
| Prior radiation therapy | 7 |
Abbreviation: ECOG = Eastern Cooperative Oncology Group.
A total of 86 cycles of chemotherapy were administered. There were three grade 4 toxicities and ten grade 3 toxicities (Table 2). Six patients were admitted for grade 3 non-hematologic toxicity while on protocol: three for abdominal pain (two due to disease progression, one from diverticulitis), two with lower back pain (both with bone metastases), and one patient with unilateral lower leg swelling and pain due to deep venous thrombosis and cellulitis. The three grade 4 toxicities were related to severe neutropenia. A total of 18 patients required additional supportive therapy due to myelosuppression or at the discretion of the investigator. Five patients received blood transfusions and 16 received erythropoietin-containing products for chemotherapy-related anemia. Two patients required G-CSF support for neutropenia. There were 8 dose delays and 4 dose reductions in total. Two dose reductions were due to grade 3 hepatic toxicity (elevated SGPT and LDH in one patient and elevated bilirubin in another patient). The other two dose reductions were due to grade 3 thrombocytopenia.
Table 2.
Dose reduction and delay-related toxicity (out of a total of 86 cycles administered).
| Grade | 3 | 4 |
|---|---|---|
| Gastrointestinal — bloating | 1 | 0 |
| Pain | 2 | 0 |
| Neuro-sensory back/leg pain | 2 | 0 |
| Leg swelling | 1 | 0 |
| LFT elevation | 2 | 0 |
| Thrombocytopenia | 2 | 0 |
| Neutropenia | 0 | 3 |
Abbreviation: LFT = liver function tests.
Three patients could not be assessed for response because they voluntarily withdrew from the study after receiving one dose of chemotherapy on study. Out of the 24 evaluable patients, 2 patients had a complete response, 4 had a partial response, 3 had stable disease, and 15 had progressive disease. The overall response rate was 25% (95% CI: 7.7%–42.3%, 8%CR, 17% PR) and 38% of the patients had clinical benefit (95% CI: 18.1%–56.9%; an additional 13% SD). The median time to best response was 2.5 months (range 1.1–6 months). The median duration of response was 8.5 months (range 3– 19 months). The median overall survival was 18.5 months (range 1.8–50.7 months, see Fig. 1 for Kaplan–Meier survival curve). There were no treatment related deaths. Two patients who had a PR and one with stable disease were originally platinum-resistant.
Fig. 1.
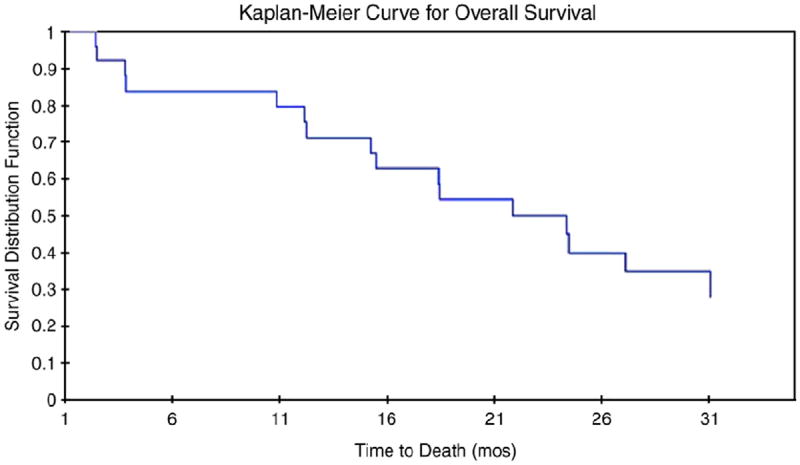
Kaplan–Meier curve for overall survival (N = 24). Median survival was 18.5 months (range 1.8–50.7 months) for patients treated with weekly topotecan and docetaxel. The median time to best response was 2.5 months (range 1.1–6 months).
Discussion
To our knowledge, this is the first trial to evaluate the weekly combination of docetaxel and topotecan in heavily treated patients with recurrent uterine, epithelial ovarian, and primary peritoneal cancers. Weekly topotecan (3.5 mg/m2) and docetaxel (30 mg/m2) was a well-tolerated regimen in this heavily chemotherapy treated patient population. Treatment was delivered on schedule in the majority of treatment cycles. The majority of the grade 3 toxicities were related to disease progression or other medical problems and non chemotherapy-related toxicities. All three of the grade four toxicities were related to neutropenia. Excluding four of the grade 3 or 4 toxicities related to disease progression, nine out of the 86 cycles (10%) resulted in clinically relevant toxicities. In addition, dose reductions or treatment delays were infrequent.
ICON 4 established the superiority of doublet versus monotherapy in appropriate subsets of patients with platinum-sensitive ovarian cancer [37]. Patients who received a paclitaxel and platinum regimen had an improved overall survival (median 5 months) and progression free survival (median 3 months) as compared to patients who received platinum monotherapy. Published response rates for weekly topotecan monotherapy in patients with recurrent ovarian cancer range from13.6 to 47.8% [16,17]. Up to 21% of patient with advanced or recurrent endometrial cancer responded with weekly docetaxel monotherapy [9]. Our study also included more heavily pretreated subjects than most previously-reported studies. In addition, the overall survival we observed with the combination of weekly topotecan and taxotere is higher (18.5 months) when compared to published data in prior phase II studies with similar patient populations [9,16].
Three patients with platinum-resistant ovarian cancer responded to treatment (2 PR, 1 SD). Albeit not statistically relevant, this suggests that the combination of docetaxel and topotecan may deserve further study in platinum-resistant patients, a group of patients where topotecan and docetaxel monotherapy has demonstrated a variable rate of activity [23,38-42].
Both preclinical and phase I studies build the basis for using topotecan and docetaxel combination. They both have a broad spectrum of activity. In vitro data has shown this combination may have synergistic effects on tumor death [43,44]. Docetaxel may have the greatest synergistic effects with topotecan when given at the time of highest topotecan-induced G2-phase cell arrest [45]. Both of these drugs are also metabolized by the CYP 3A4 system, possibly potentiating toxicity. However, such toxicities were not seen in this study.
There are several limitations of this study. We combined patients with recurrent uterine, ovarian, and primary peritoneal cancers in this study since the responses to therapy in this setting are similar. Also, while the underlying biology of these tumor types is likely different, women with these tumors have similar clinical courses, often with advanced upper abdominal, lymphatic, and distant metastases. In addition, the total number of patients recruited to this study was small, limiting multivariate or subset analyses.
The combination of weekly docetaxel and topotecan appears to have activity in recurrent uterine, ovarian, and primary peritoneal cancers. In addition, the safety profile of this regimen is acceptable in a heavily pretreated population where optimal treatment options are challenging secondary to toxicity. The results of our study suggest that further evaluation of this regimen in patients with platinum-resistant ovarian cancer and recurrent high risk uterine cancers is warranted.
Acknowledgments
Conflict of interest statement
This study was funded, in part, by Investigator-Initiated Grants (M.H.E.) from Sanofi-Aventis and GlaxoSmithKline Oncology for research-related costs.
Footnotes
Clinicaltrials.gov Unique Identifier #NCT00231855. A portion of this clinical trial data was previously presented at the 2007 Society of Gynecologic Oncologists Annual Meeting San Diego, CA
References
- 1.Wadler S, Levy D, Lincoln S, Soori G, Schink J, Goldberg G. Topotecan is an active agent in the first-line treatment of metastatic or recurrent endometrial carcinoma: Eastern Cooperative Oncology Group Study E3E93. J Clin Oncol. 2003;21:2110–4. doi: 10.1200/JCO.2003.12.093. [DOI] [PubMed] [Google Scholar]
- 2.Miller D, Blessing J, Lent S, Waggone S. A phase I/II trial of topotecan in patients with advanced, persistent, or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Proc Am Soc Clin Oncol. 2001;2468a doi: 10.1006/gyno.2002.6804. [DOI] [PubMed] [Google Scholar]
- 3.Finkler N, Holloway R. A phase I/II trial of weekly topotecan in the treatment of advanced recurrent metastatic endometrial cancer. Proc Am Soc Clin Oncol. 2002;2504a [Google Scholar]
- 4.Chambers J, Rutherford T, Schwartz P, Carcangiu M, Chambers S. A pilot study of topotecan for the treatment of serous endometrial cancer. Proc Am Soc Clin Oncol. 2001;872a doi: 10.1046/j.1525-1438.2003.13022.x. [DOI] [PubMed] [Google Scholar]
- 5.Traina TA, Sabbatini P, Aghajanian C, Dupont J. Weekly topotecan for recurrent endometrial cancer: a case series and review of literature. Gynecol Oncol. 2004;95:235–41. doi: 10.1016/j.ygyno.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Lissoni A, Zanetta G, Losa G, Gabriele A, Parma G, Mangioni C. Phase II study of paclitaxel as salvage treatment in advanced endometrial cancer. Ann Oncol. 1996;7:861–3. doi: 10.1093/oxfordjournals.annonc.a010768. [DOI] [PubMed] [Google Scholar]
- 7.Ball H, Blessing J, Lentz S, Mutch D. A phase II trial of paclitaxel in patients with advanced or recurrent adenocarcinoma of the endometrium: a Gynecologic Ocology Group study. Gynecol Oncol. 1996;62:278–81. doi: 10.1006/gyno.1996.0227. [DOI] [PubMed] [Google Scholar]
- 8.Rantanen V, Grenman S, Kulmala J, Grenman R. Endometrial cancer cell lines are sensitive to paclitaxel. Anticancer Res. 1996;16:475–9. [PubMed] [Google Scholar]
- 9.Gunthert AR, Ackerman S, Beckmann AW, Camara O, Kiesel L, et al. Phase II study of weekly docetaxel with recurrent or metastatic endometrial cancer: AGO-uterus 4. Gynecol Oncol. 2007;104:86–90. doi: 10.1016/j.ygyno.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Hirai Y, Hasumi K, Onose R, Kuramoto H, Kuzuya K, et al. Phase II trial of 3-hour infusion of paclitaxel in patients with adenocarcinoma of endometrium: Japanese Multicenter Study Group. Gynecol Oncol. 2004;94:471–6. doi: 10.1016/j.ygyno.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Katsumata N, Noda K, Nozawa S, Kitagawa R, Nishimura R, et al. Phase II trial of docetaxel in advanced or recurrent endometrial cancer: a Japanese Cooperative Study. British J Cancer. 2005;93:999–1004. doi: 10.1038/sj.bjc.6602817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scudder SA, Liu PY, Wilczynski SP, Smith HO, Jiang C, Hallum AV, 3rd, et al. Paclitaxel and carboplatin with amifostine in advanced, recurrent, or refractory endometrial adenocarcinoma: a phase II study of the Southwest Oncology Group. Gynecol Oncol. 2005;96:610–5. doi: 10.1016/j.ygyno.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Secord AA, Havrilesky LJ, Carney ME, Soper JT, Clarke-Pearson DL, Rodriguez GC, et al. Weekly low-dose paclitaxel and carboplatin in the treatment of advanced or recurrent cervical and endometrial cancer. Int J Clin Onc. 2007;12:31–6. doi: 10.1007/s10147-006-0619-9. [DOI] [PubMed] [Google Scholar]
- 14.Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, et al. Phase III randomized trial of docetaxel–carboplatin versus paclitaxel–carboplatin as first-line chemotherapy for ovarian carcinoma. JNCI. 2004;96:1682–91. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 15.McGuire WP, Blessing JA, Bookman MA, Lentz SS, Dunton CJ. Topotecan has substantial antitumor activity as first-line salvage therapy in platinum-sensitive epithelial ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2000;18:1062–7. doi: 10.1200/JCO.2000.18.5.1062. [DOI] [PubMed] [Google Scholar]
- 16.Levy T, Inbar M, Menczer J, Grisaru D, Glezerman M, et al. Phase II study of weekly topotecan in patients with recurrent or persistent epithelial ovarian cancer. Gynecol Oncol. 2004;95:686–90. doi: 10.1016/j.ygyno.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Spannuth WA, Leath IIICA, Huh WK, Barnes MN, III, Davidson SA, et al. A phase II trial of weekly topotecan for patients with secondary platinum-resistant recurrent epithelial ovarian carcinoma following the failure of second line therapy. Gynecol Oncol. 2007;104:591–5. doi: 10.1016/j.ygyno.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Morris RT. Weekly topotecan in the management of ovarian cancer. Gynecol Oncol. 2003;90:S34–8. doi: 10.1016/s0090-8258(03)00470-0. [DOI] [PubMed] [Google Scholar]
- 19.Kavanagh JJ, Kudelka AP, de Leon CG, Tresukosol D, Hord M, Finnegan MB, et al. Phase II study of docetaxel in patients with epithelial ovarian carcinoma refractory to platinum. Clin Cancer Res. 1996;2:837–42. [PubMed] [Google Scholar]
- 20.Francis P, Schneider J, Hann L, Balmaceda C, Barakat R, Phillips M, et al. Phase II trial of docetaxel in patients with platinum-refractory advanced ovarian cancer. J Clin Oncol. 1994;12:2301–8. doi: 10.1200/JCO.1994.12.11.2301. [DOI] [PubMed] [Google Scholar]
- 21.Katsumata N, Tsunematsu R, Tanaka K, Terashima Y, Ogita S, et al. Phase II trial of docetaxel in platinum pre-treated patients with advanced epithelial ovarian cancer: a Japanese Cooperative study. Annals Oncol. 2000;11:1531–6. doi: 10.1023/a:1008337103708. [DOI] [PubMed] [Google Scholar]
- 22.Birkenblit A, Seiden MV, Matulonis UA, Penson RT, Krasner CN, Roche M, et al. A phase II trial of weekly docetaxel in patients with platinum-resistant epithelial ovarian, primary peritoneal serous cancer, or fallopian tube cancer. Gynecol Oncol. 2004;95:624–31. doi: 10.1016/j.ygyno.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Rose PG, Blessing JA, Ball HG, Hoffman J, Warshal D, DeGeest K, et al. A phase II study of docetaxel in paclitaxel-resistant ovarian and peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:130–5. doi: 10.1016/s0090-8258(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 24.Tinker AV, Gebski V, Fitzharris B, Bucks M, Stuart-Harris R, et al. Phase II trial of weekly docetaxel for patients with relapsed ovarian cancer who have previously received paclitaxel. Gynecol Oncol. 2007;104:647–53. doi: 10.1016/j.ygyno.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Lu C, Shin D, Jung M. Phase I evaluation of docetaxel and topotecan for patients with advanced solid tumors. Proc Am Soc Clin Oncol. 2000;19:523a. [Google Scholar]
- 26.Aijaz A, Patel D, Puccio C, et al. A pilot trial of docetaxel, topotecan, and filgastim (G-CSF) in patients (PTS) with recurrent epithelial cancer of the ovary and peritoneum. Proc Am Soc Clin Oncol. 2000;19:407a. [Google Scholar]
- 27.Zamboni WC, Egorin MJ, Van Echo DA, Day RS, Meisenberg BR, et al. Pharmacokinetic and pharmacodynamic study of the combination of docetaxel and topotecan in patients with solid tumors. J Clin Oncol. 2000;18:3288–94. doi: 10.1200/JCO.2000.18.18.3288. [DOI] [PubMed] [Google Scholar]
- 28.Tkaczuk KH, Zamboni WC, Tait NS, Meisenberg BR, Austin Doyle L, et al. Phase I study of docetaxel and topotecan in patients with solid tumors. Cancer Chemother Pharmacol. 2000;46:228–442. doi: 10.1007/s002800000180. [DOI] [PubMed] [Google Scholar]
- 29.Tsao AS, Shin DM, Palmer JL, Lee JS, Glisson BS. Phase I evaluation of docetaxel and topotecan for patients with advanced solid tumors. Cancer. 2004;100:2240–5. doi: 10.1002/cncr.20238. [DOI] [PubMed] [Google Scholar]
- 30.Dubey S, Hutson P, Alberti D, Arzoomanian R, Binger K, et al. Phase I study of docetaxel and topotecan in patients with advanced malignancies. J Oncol Pharm Pract. 2005;11:131–8. doi: 10.1191/1078155205jp161oa. [DOI] [PubMed] [Google Scholar]
- 31.Posey JA, Wang H, Hamilton JE, Delgrosso A, Zhang R, et al. Phase-I dose escalation and sequencing study of docetaxel and continuous infusion topotecan in patients with advanced malignancies. Cancer Chemother Pharmacol. 2005;56:182–8. doi: 10.1007/s00280-004-0925-8. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Matulonis U. Rational use of cytotoxic chemotherapy for recurrent ovarian cancer. JNCCN. 2006;4:947–53. doi: 10.6004/jnccn.2006.0078. [DOI] [PubMed] [Google Scholar]
- 33.Fiorelli JL, Herzog TJ, Wright JD. Current treatment strategies for endometrial cancer. Expert Rev Anticancer Ther. 2008;8:1149–57. doi: 10.1586/14737140.8.7.1149. [DOI] [PubMed] [Google Scholar]
- 34.Rustin GJS, Quinn M, Thigpen T, et al. New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. 2004;96:487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 35.http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- 36.Therasse P, Arbuck SJ, Eisenhauer EA, Wanders J, Kaplan RS, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. JNCI. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 37.The ICON and AGO collaborators. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 38.Abushahin F, Singh DK, Lurain JR, Grendys EC, Rademaker AW, et al. Weekly topotecan for recurrent platinum resistant ovarian cancer. Gyencol Oncol. 2008;108:53–7. doi: 10.1016/j.ygyno.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 39.O’Malley DM, Azodi M, Makkencherry A, McAlpine J, Kelly M, et al. Weekly topotecan in heavily pretreated patients with recurrent epithelial ovarian cancer. Gynecol Oncol. 2005;98:242–8. doi: 10.1016/j.ygyno.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 40.Levy T, Inbar M, Menczer J, Grisaru D, Glezerman M, et al. Phase II study of weekly topotecan in patients with recurrent or persistent epithelial ovarian cancer. Gynecol Oncol. 2004;95:686–90. doi: 10.1016/j.ygyno.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Safra T, Menczer J, Bernstein R, Shpigel S, Inbar MJ, et al. Efficacy and toxicity of weekly topotecan in recurrent epithelial ovarian and primary peritoneal cancer. Gynecol Oncol. 2007;105:205–10. doi: 10.1016/j.ygyno.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, et al. Recurrent ovarian cancer: randomized phase vs topotecan. J Clin Oncol. 2001;19:3312–22. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 43.Chou TC, Motzer RJ, Tong Y, Bosl GJ. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. JNCI. 1994;86:1517–24. doi: 10.1093/jnci/86.20.1517. [DOI] [PubMed] [Google Scholar]
- 44.Jonsson E, Fridborg H, Nygren P, Larsson R. Synergistic interactions of combinations of topotecan with standard drugs in primary cultures of human tumor cells from patients. Eur J Clin Pharmacol. 1998;54:501–14. doi: 10.1007/s002280050505. [DOI] [PubMed] [Google Scholar]
- 45.Taron M, Plasencia C, Abad A, Martin C, Guillot M. Cytotoxic effects of topotecan combined with various active G2/M-phase anticancer drugs in human tumor-derived cell lines. Invest New Drugs. 2000;18:139–47. doi: 10.1023/a:1006325929424. [DOI] [PubMed] [Google Scholar]


