Abstract
Background
Müllerian adenosarcoma is a rare mixed epithelial-mesenchymal tumor. An extragenital site of origin and sarcomatous overgrowth are associated with aggressive clinical behavior.
Case
We present a rare case of extragenital adenosarcoma with sarcomatous overgrowth and coexistent endometrioisis. She was treated with initial cytoreductive surgery and chemotherapy. She underwent a second surgery for management of a high-grade bowel obstruction, due to pathologically confirmed recurrent intraperitoneal adenosarcoma. A complete clinical response was achieved with liposomal doxorubicin, and the patient remains disease-free eighteen months after completion of chemotherapy.
Conclusion
Liposomal doxorubicin appears to be an active agent for the treatment of adenosarcoma with sarcomatous overgrowth. In addition, we conclude from our review of all reported cases of extragenital adenosarcoma that concurrent endometriosis may represent a favorable prognostic factor.
INTRODUCTION
Müllerian adenosarcoma is a mixed epithelial-mesenchymal tumor typically arising from the uterus [1]. The diagnostic criteria for adenosarcoma include proliferation of endometrioid glands and stroma, stromal condensation around glands or intraglandular polypoid projections, cytologic atypia of the stroma, and stromal mitoses. Although the adenosarcomas generally behave in a clinically indolent manner, adenosarcoma with sarcomatous overgrowth is a highly aggressive tumor. These tumors have a propensity to recur and metastasize, even in patients presenting with early-stage disease [2]. Beyond the uterus, adenosarcoma may originate from reproductive organs such as the vagina or ovary. Rarely, adenosarcoma may arise outside of the genital tract, and these tumors are referred to as extragenital adenosarcoma. [3].
We present an unusual case of extragenital adenosarcoma with sarcomatous overgrowth and concurrent endometriosis. The patient had metastatic disease at presentation with optimal cytoreduction of the tumor at the initial surgery. She developed progressive and platinum-resistant disease with clinical bowel obstruction necessitating a second tumor debulking procedure. She had a complete clinical response to six cycles of liposomal doxorubicin.
CASE
A 41-year-old African-American woman presented to the Emergency Room with right lower quadrant pain and nausea. She had no significant medical or family history. She had regular menses and used no medications or hormones. Physical examination revealed a palpable right-sided pelvic mass. Computed tomography (CT) of the chest, abdomen, and pelvis demonstrated a 10 × 7 cm right adnexal solid/cystic mass with internal septations and mural nodularity, small mesenteric lymph nodes and a small amount free fluid in the abdomen and pelvis. A pelvic ultrasonographic examination revealed a complex right ovarian cystic mass, measuring 16 × 14 × 13 cm with internal echoes and irregular borders. The uterus and left ovary appeared normal. All laboratory tests, including serum CA125, CA 19-9, and CEA were within normal limits.
Exploratory laparotomy revealed a 10 cm right-sided hemorrhagic cystic pelvic mass adherent to the mesentery of the terminal ileum, the right colon and the pelvic sidewall. Frozen section of the resected mass demonstrated endometriosis with stromal atypia. The uterus had a myomatous appearance. Both ovaries, fallopian tubes, and upper abdomen were grossly normal.
The resected pelvic mass consisted of tan-yellow hemorrhagic tissue. The permanent sections revealed areas of endometriosis that were juxtaposed with areas of sarcomatous stromal overgrowth with marked stromal atypia (Figure 1). Some of the endometriotic glands were cuffed by malignant stroma, a characteristic finding in adenosarcoma. The tumor cells were positive for desmin and focally positive for CD10 (Figure 2). Peritoneal cytology was negative for malignant cells.
Figure 1.
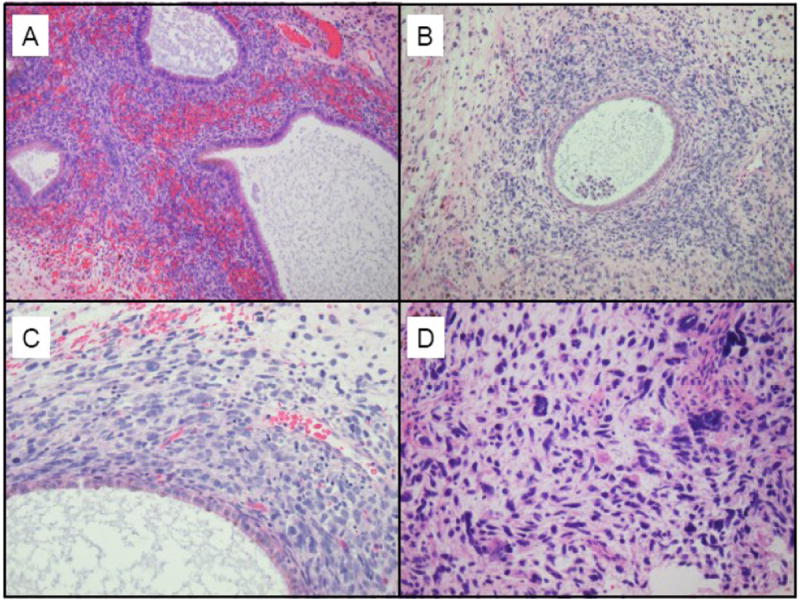
A: Benign endometriosis
B: Endometriotic glands are cuffed by malignant stroma, a characteristic finding of adenosarcoma
C: Higher power view of the malignant stroma, demonstrating nuclear pleomorphism, atypia, and hyperchromasia. In addition, the mitoses are in excess of 2 per 10 high power fields.
D: In the areas of sarcomatous overgrowth, the malignant cells contain enlarged, hyperchromatic nuclei with marked nuclear pleomorphism and numerous atypical mitotic figures
Figure 2.
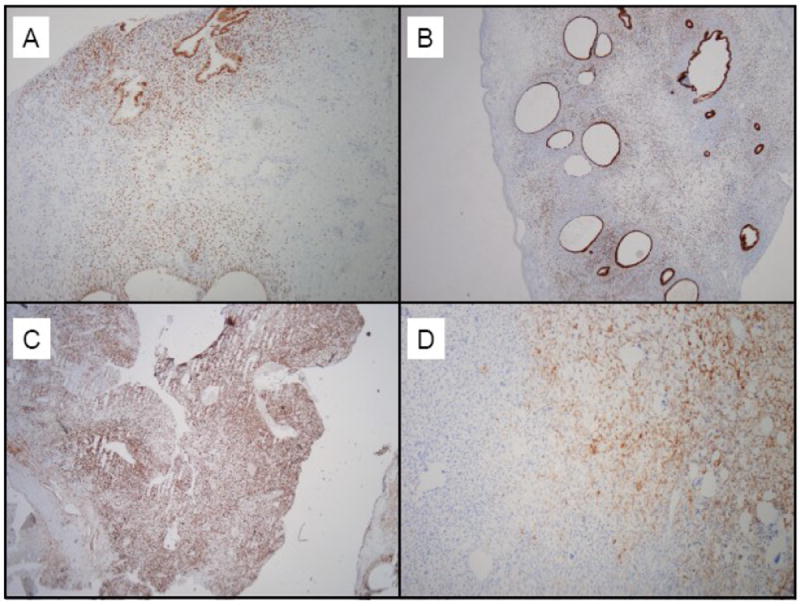
A: Estrogen receptor expression is seen in the endometriosis component
B: The glandular component demonstrates strong progesterone receptor expression
C: Tumor cells are diffusely immunopositive for desmin
D: Focal immunopositivity is seen for CD 10
The patient underwent a complete surgical staging including total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, resection of cul-de-sac and sigmoid colon nodules, and pelvic and para-aortic lymph node dissection. There was no gross residual disease at the completion of the surgery. Pathologic findings demonstrated high-grade sarcoma involving the cul-de-sac and sigmoid colon serosal nodules. The uterus contained secretory endometrium and adenomyosis without stromal atypia. The cervix, ovaries, fallopian tubes, omentum, pelvic and para-aortic lymph nodes were negative for tumor. The patient’s postoperative course was unremarkable.
The patient began adjuvant treatment with ifosfamide and cisplatin chemotherapy less than four weeks after surgery. A baseline Positron Emission Tomography (PET)/CT Scan demonstrated multiple FDG-avid intra-abdominal foci consistent with peritoneal metastases. After two cycles of chemotherapy, she was hospitalized for a high-grade small bowel obstruction. She underwent an exploratory laparotomy with lysis of adhesions and resection of an ileal mesenteric mass that was obstructing the small bowel at this point. Pathology demonstrated a high-grade pure sarcoma with increased cytological atypia and necrosis (Figure 3).
Figure 3.
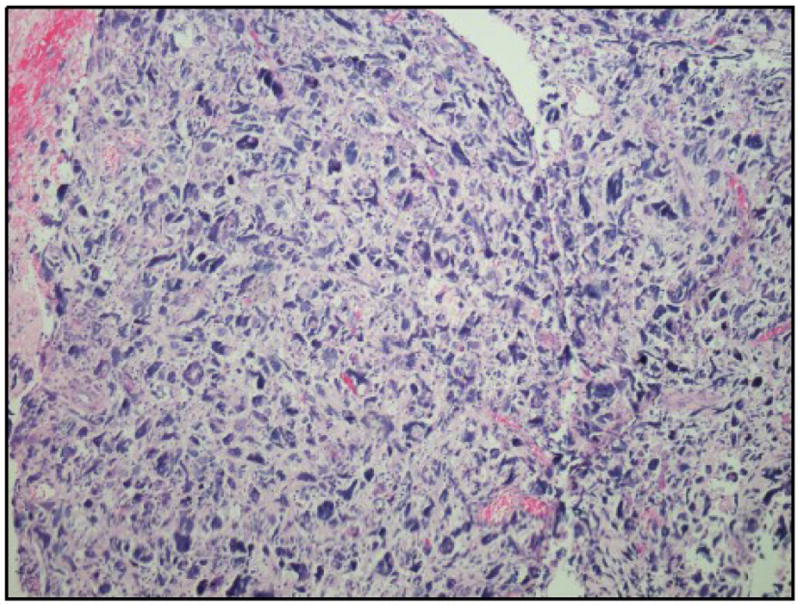
The ileal mesenteric mass consists of a high-grade pure sarcoma with increased cytological atypia compared with the previously resected tumor. Background necrosis is also present.
With confirmation of recurrent adenosarcoma, the patient’s chemotherapy regimen was changed to liposomal doxorubicin. She completed six cycles of chemotherapy without problems. A follow-up CT confirmed a complete response to treatment with resolution of the ascites and with no evidence of masses or lymphadenopathy. She remains clinically disease-free at eighteen months after completing treatment, confirmed on repeat PET/CT Scan.
COMMENT
In 1974, Clement and Scully first described müllerian adenosarcoma as an uncommon mixed tumor of the uterus [1]. Subsequently, the distinct clinical behavior of adenosarcoma based on the site of origin of the tumor has been demonstrated. In patients with uterine adenosarcoma, surgery alone is often curative. In these patients, recurrent tumor occurs in approximately 25% of cases, usually in the vagina or pelvis, and approximately 10% of patients die of disease. In contrast, extragenital adenosarcoma recurs in over 50% of patients, and is associated with a mortality rate of approximately 35% [4-6].
We reviewed the previously reported cases of adenosarcoma with respect to clinical and pathologic features associated with recurrence and death. The largest case series of 100 patients with uterine adenosarcoma was reported by Clement and Scully in 1990 [7]. Of the 88 cases with follow up data, only one quarter had disease recurrence, most commonly at the vagina, and only 10 patients died of disease. In this series, myometrial invasion was identified as a risk factor for the disease recurrence. Of note, only five cases had focal areas of high-grade, pure sarcoma, but as these foci constituted less than 10% the tumor, none were considered to have sarcomatous overgrowth. Ten cases of uterine adenosarcomas with sarcomatous overgrowth were reported separately by Clement [8]. These cases exhibited a high-grade sarcomatous component constituting at least 25% of the tumor. In contrast to the good prognosis observed in the preceding cohort without sarcomatous overgrowth, this variant of adenosarcoma demonstrated clinically aggressive behavior, with 6 of the 10 patients dying of disease. Also notable was the frequency of hematogenous metastases, observed in 40% of patients, compared to 2% in patients with typical adenosarcoma.
In a subsequent study of 31 patients with uterine adenosarcoma, published by the Gynecologic Oncology Group in 1992, extrauterine involvement and myometrial invasion were associated with higher rates of recurrence, while sarcomatous overgrowth alone was not a statistically significant poor prognostic factor [9]. However, two additional studies confirm Clement’s observation of a distinctly aggressive behavior associated with sarcomatous overgrowth. Verschraegen reported the outcome of 41 women diagnosed with adenosarcoma at the MD Anderson Cancer Center between 1982 and 1986 [10]. Of the 41 cases, 29% were extrauterine in origin. Thirty-eight percent of patients developed recurrent disease, of whom only one patient was alive without disease, and three were alive with recurrent disease at the time of the analysis. The major predictor of poor prognosis was found to be sarcomatous overgrowth. In 2001, Krivak reported the outcome of eleven patients with uterine adenosarcoma with sarcomatous overgrowth. The median survival for this group was only 13 months, comparable to the median survival of patients with uterine carcinosarcoma [2].
A large review by Stern of endometriosis-associated neoplasms revealed that adenosarcoma was the second most common malignancy (after clear cell carcinoma) to arise in the setting of extra-ovarian endometriosis [11]. In this review of 1000 patients with pathologically documented endometriosis, 3 cases of adenosarcoma were found to be associated with extra-ovarian endometriosis. In addition, in a study of 17 cases of gastrointestinal endometriosis by Yantiss, 4 cases of adenosarcoma were seen [12]. Of these four cases of adenosarcoma reported by Yantiss, follow-up was available for only 2 patients; both were alive and disease-free at 2 years and 3 years. Two prior reports of adenosarcoma arising in hepatic endometrioma were also noted [13, 14]. Follow-up was available for one of the two patients; she remained disease-free at 2 years. Several reports of vaginal adenosarcoma associated with endometriosis have also been reported, most recently reviewed by Toyoshima [15].
Prior studies of extragenital adenosarcoma have not evaluated the prognostic impact of associated endometriosis. We performed an Ovid Medline search, using the MeSH search term “adenosarcoma,” limited to the English language literature, human subjects, and female gender. To limit the scope to extragenital cases, we excluded those cases originating in the uterus, cervix, adnexa, and vagina. In addition to the 3 aforementioned adenosarcoma cases associated with gastrointestinal/hepatic endometriosis, we identified 18 cases of extragenital adenosarcoma for which treatment and follow-up information was reported [4-6, 16, 17]. We identified five previous cases associated with endometriosis. The sites of origin were the cul-de-sac in 2 patients, bladder in 1 patient, inguinal ligament in 1 patient, and multifocal (pelvic/peri-splenic) in 1 patient. In 2 of these 5 patients, sarcomatous overgrowth was described. In the first case, reported by Dincer, a patient with a long history of recurrent endometriomas developed multiple pelvic masses and a large peri-splenic mass [17]. The pelvic masses contained multiple foci of endometriosis and low-grade sarcoma, while the perisplenic mass contained a high-grade pure sarcoma accounting for 50% of its mass. This patient died of disease at 13 months. In the subsequent case, reported by Murugasu, the areas of sarcomatous overgrowth occupied only 15% of the tumor stroma, and would not be classified as sarcomatous overgrowth by the classical criteria proposed by Clement [4, 8]. This patient, along with 3 additional patients with endometriosis-associated adenosarcoma, remained disease-free and alive at the time of their report, with duration of follow-up of 1 year (2 patients), 2 years, and 3 years. Thus, four of five of these patients with endometriosis-associated extragenital adenosarcoma survived and were disease-free. In contrast, of the thirteen reported extragenital adenosarcoma patients without concurrent endometriosis, six patients died with disease (at 10 weeks, 11 weeks, 9 months, 10 months, 12 months, and 10 years), two patients died of other causes (at 2 months, and status post recurrence at 9 years), three patients are alive with disease (at 18, 22, and 45 months), and only two patients were disease-free at the time of their report (at 1 year, and 5 years).
These observations suggest that associated endometriosis may confer a better prognosis in patients with extragenital adenosarcoma. Including the 3 patients with gastrointestinal/hepatic endometriosis, along with the 5 case reports described above, the recurrence-free survival appears to be 88% (7 of 8) in patients with endometriosisassociated disease compared with approximately 17% (2 of 12 evaluable patients) in non-endometriosis associated extragenital sarcoma patients. However, we cannot exclude the possibility that the case reports found in the literature may be subject to reporting bias and therefore may not be representative of all cases of extragenital adenosarcoma and associated endometriosis. In addition, due to the large span of time during which these cases were identified, there are significant differences in the management of these patients, which may have influenced their clinical outcome.
To our knowledge, this is only the third reported case of extragenital adenosarcoma with sarcomatous overgrowth and concurrent endometriosis. The extragenital site of origin and extensive sarcomatous overgrowth portended an ominous prognosis for this patient. In addition, in our patient there was disease progression during adjuvant chemotherapy with ifosfamide and cisplatin. This may indicate resistance of the tumor to standard chemotherapeutic agents used in the treatment of mixed müllerian tumors including uterine carcinosarcomas. Other potentially active regimens include gemcitabine with docetaxel combination treatment, which is active in leiomyosarcomas, but appears to be significantly less active in other sarcomas [18, 19]. Therapeutic agents that inhibit growth factor signaling pathways, mammalian target of rapamycin, and angiogenesis pathways are also under evaluation for the potential treatment of sarcomas.
In the present case, we observed a complete clinical response to liposomal doxorubicin chemotherapy. This agent is tolerated well, with a convenient dosing schedule. Based on the excellent response and minimal toxicity observed in this case, we believe that liposomal doxorubicin may be considered for first-line chemotherapy in patients with residual or recurrent extragenital adenosarcoma. Although we did not specifically evaluate the use of this agent in an adjuvant setting, it is reasonable to consider adjuvant therapy in all patients with extragenital adenosarcoma with sarcomatous overgrowth, due to the high risk of recurrence and death, and by extrapolation, liposomal doxorubicin may be useful in this setting as well. Although additional clinical reports are lacking to confirm these recommendations, a previous case report noted a “marked response” to liposomal doxorubicin in the setting of recurrent uterine adenosarcoma [20]. Although extragenital adenosarcoma is associated with worse prognosis compared with uterine adenosarcoma, the shared histopathological features suggest that similar agents may be efficacious for adenosarcoma arising from different sites.
From our review of the literature, we also conclude that concurrent endometriosis may represent a favorable prognostic factor in patients with extragenital adenosarcoma. Endometriosis proliferates in an estrogen-dependent manner, and tumors arising from endometriotic lesions may retain hormone dependency. Indeed, immunohistochemical analysis demonstrates that many adenosarcomas express estrogen and progesterone receptors, although adenosarcomas with sarcomatous overgrowth are less likely to express hormone receptors [21, 22]. We hypothesize that endometriosis-associated adenosarcomas may be more likely to have a hormone-dependent phenotype that confers a better prognosis compared with non-endometriosis associated adenosarcoma. Molecular analysis of adenosarcomas and their associated endometriotic lesions may provide further insight into the pathogenesis of this uncommon disease.
Acknowledgments
Dr. Huang is an NCI-NICHD Scholar of the Reproductive Scientist Development Program, supported by the National Institutes of Health grant #5K12HD00849.
Footnotes
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clement PB, Scully RE. Mullerian adenosarcoma of the uterus. A clinicopathologic analysis of ten cases of a distinctive type of mullerian mixed tumor. Cancer. 1974;34:1138–49. doi: 10.1002/1097-0142(197410)34:4<1138::aid-cncr2820340425>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Krivak TC, Seidman JD, McBroom JW, MacKoul PJ, Aye LM, Rose GS. Uterine adenosarcoma with sarcomatous overgrowth versus uterine carcinosarcoma: comparison of treatment and survival. Gynecol Oncol. 2001;83:89–94. doi: 10.1006/gyno.2001.6334. [DOI] [PubMed] [Google Scholar]
- 3.Clement PB, Scully RE. Extrauterine mesodermal (mullerian) adenosarcoma: a clinicopathologic analysis of five cases. Am J Clin Pathol. 1978;69:276–83. doi: 10.1093/ajcp/69.1.276. [DOI] [PubMed] [Google Scholar]
- 4.Murugasu A, Miller J, Proietto A, Millar E. Extragenital mullerian adenosarcoma with sarcomatous overgrowth arising in an endometriotic cyst in the pouch of Douglas. Int J Gynecol Cancer. 2003;13:371–5. doi: 10.1046/j.1525-1438.2003.13187.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang HY, Changchien CC, Chen HH, Lin H, Huang CC. Extrauterine mullerian adenosarcoma associated with endometriosis and rectal villotubular adenoma: report of a case and review of the literature. Int J Gynecol Cancer. 2005;15:361–5. doi: 10.1111/j.1525-1438.2005.15230.x. [DOI] [PubMed] [Google Scholar]
- 6.Milam MR, Atkinson JB, Currie JL. Adenosarcoma arising in inguinal endometriosis. Obstet Gynecol. 2006;108:753–5. doi: 10.1097/01.AOG.0000192550.19403.0f. [DOI] [PubMed] [Google Scholar]
- 7.Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol. 1990;21:363–81. doi: 10.1016/0046-8177(90)90198-e. [DOI] [PubMed] [Google Scholar]
- 8.Clement PB. Mullerian adenosarcomas of the uterus with sarcomatous overgrowth. A clinicopathological analysis of 10 cases. Am J Surg Pathol. 1989;13:28–38. doi: 10.1097/00000478-198901000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kaku T, Silverberg SG, Major FJ, Miller A, Fetter B, Brady MF. Adenosarcoma of the uterus: a Gynecologic Oncology Group clinicopathologic study of 31 cases. Int J Gynecol Pathol. 1992;11:75–88. [PubMed] [Google Scholar]
- 10.Verschraegen CF, Vasuratna A, Edwards C, Freedman R, Kudelka AP, Tornos C, Kavanagh JJ. Clinicopathologic analysis of mullerian adenosarcoma: the M.D. Anderson Cancer Center experience. Oncol Rep. 1998;5:939–44. doi: 10.3892/or.5.4.939. [DOI] [PubMed] [Google Scholar]
- 11.Stern RC, Dash R, Bentley RC, Snyder MJ, Haney AF, Robboy SJ. Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol. 2001;20:133–9. doi: 10.1097/00004347-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Yantiss RK, Clement PB, Young RH. Neoplastic and pre-neoplastic changes in gastrointestinal endometriosis: a study of 17 cases. Am J Surg Pathol. 2000;24:513–24. doi: 10.1097/00000478-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 13.N’Senda P, Wendum D, Balladur P, Dahan H, Tubiana JM, Arrive L. Adenosarcoma arising in hepatic endometriosis. Eur Radiol. 2000;10:1287–9. doi: 10.1007/s003300000322. [DOI] [PubMed] [Google Scholar]
- 14.Jelovsek JE, Winans C, Brainard J, Falcone T. Endometriosis of the liver containing mullerian adenosarcoma: case report. Am J Obstet Gynecol. 2004;191:1725–7. doi: 10.1016/j.ajog.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Toyoshima M, Akahira J, Moriya T, Hayakawa S, Yaegashi N. Primary vaginal adenosarcoma with sarcomatous overgrowth. Gynecol Oncol. 2004;95:759–61. doi: 10.1016/j.ygyno.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Vara AR, Ruzics EP, Moussabeck O, Martin DC. Endometrioid adenosarcoma of the bladder arising from endometriosis. J Urol. 1990;143:813–5. doi: 10.1016/s0022-5347(17)40105-4. [DOI] [PubMed] [Google Scholar]
- 17.Dincer AD, Timmins P, Pietrocola D, Fisher H, Ambros RA. Primary peritoneal mullerian adenosarcoma with sarcomatous overgrowth associated with endometriosis: a case report. Int J Gynecol Pathol. 2002;21:65–8. doi: 10.1097/00004347-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329–34. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensley ML, Ishill N, Soslow R, Larkin J, Abu-Rustum N, Sabbatini P, Konner J, Tew W, Spriggs D, Aghajanian CA. Adjuvant gemcitabine plus docetaxel for completely resected stages I-IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecol Oncol. 2009;112:563–7. doi: 10.1016/j.ygyno.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 20.del Carmen MG, Lovett D, Goodman A. A case of Mullerian adenosarcoma of the uterus treated with liposomal doxorubicin. Gynecol Oncol. 2003;88:456–8. doi: 10.1016/s0090-8258(02)00093-8. [DOI] [PubMed] [Google Scholar]
- 21.Soslow RA, Ali A, Oliva E. Mullerian adenosarcomas: an immunophenotypic analysis of 35 cases. Am J Surg Pathol. 2008;32:1013–21. doi: 10.1097/PAS.0b013e318161d1be. [DOI] [PubMed] [Google Scholar]
- 22.Gallardo A, Prat J. Mullerian adenosarcoma: a clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am J Surg Pathol. 2009;33:278–88. doi: 10.1097/PAS.0b013e318181a80d. [DOI] [PubMed] [Google Scholar]


