Abstract
Impaired verbal memory is common in MDMA (Ecstasy) polydrug users. The contributions of Ecstasy or polydrug exposure to reduced verbal memory are unclear, as is the neural basis for this cognitive deficit. Ecstasy users have reduced gray matter in brain regions mediating verbal memory (BA 18, 21 and 45). N-acetylaspartate (NAA) as a neuronal marker and myoinositol (mI) as a glial marker are inconsistently affected in Ecstasy users. We used 3 Tesla MRS in 17 recreational drug users to test the hypothesis that Ecstasy polydrug use would be associated with altered NAA or mI in BA 18, 21 and 45. No effects were seen for mI. Metabolite ratios for NAA (mean ± SD) were: BA 18--NAA/Cr (2.030 ± 0.188); BA 21--NAA/Cr (1.861 ± 0.325); BA 45--NAA/Cr (1.925 ± 0.329). Lifetime cannabis use was significantly associated with BA 45 NAA/Cr (r = −0.687, p = 0.014) but not with NAA in BA 18 or 21. In contrast, there were no statistically significant associations for lifetime use of Ecstasy, alcohol, or cocaine with NAA. These findings suggest that cannabis use may contribute to altered neuronal integrity in Ecstasy polydrug users in a brain region associated with verbal memory processing.
Keywords: drug abuse, magnetic resonance spectroscopy, neuroimaging, neurotoxicity
1.0 Introduction
MDMA (3, 4-methylenedioxymethamphetamine) is a widely-used club drug that produces potentially permanent central nervous system (CNS) serotonin neurotoxicity (Green et al. 2003). MDMA, as an illicit drug, is sold under the street name of Ecstasy. Estimates from health and law enforcement agencies indicate that Ecstasy remains highly popular, especially in North America, Western Europe and Oceania (United Nations, 2007). After a brief decline, use among young adults in the United States is increasing since the year 2005 and estimates of lifetime use of Ecstasy topped 12 million people in the most recent U.S. surveys (Substance Abuse and Mental Health Administration, 2008; Johnston et al., 2007).
Recreational Ecstasy use is a considerable public health concern. Animal studies of MDMA administration and associational studies in human recreational users suggest that MDMA produces long-lasting alterations in brain serotonin function. Animal studies in multiple species (depending on dose quantity and frequency) have indicated that MDMA administration in the laboratory setting leads to long-lasting reductions in multiple molecular markers of brain serotonin (reviewed in Baumann et al., 2007; Green et al., 2003). Given serotonin’s diffuse innervation pattern (covering the entire brain) and serotonin’s role in both cell-cell signaling and cytosekeletal integrity, MDMA-induced brain effects, especially those influencing serotonin function, may produce a range of primary and secondary brain structural, functional, and neurochemical changes (Cowan et al., In Press; Green et al., 2003). One caveat to the study of MDMA effects in recreational drug users is that illicitly distributed Ecstasy preparations may vary in their concentration of MDMA and other ingredients (Cole et al., 2002; Parrott, 2004; Tanner-Smith, 2006). And, because Ecstasy users worldwide are generally polydrug users (e.g. Scholey et al. 2004; Degenhardt et al. 2004; Gouzoulis-Mayfrank and Daumann 2006), it is often difficult to determine if a single drug or a combination of drugs may contribute to brain differences. However, and consistent with predictions from animal studies of MDMA effects, human neuroimaging studies have generally demonstrated evidence for altered brain serotonin markers in Ecstasy users (Buchert et al., 2004; McCann et al., 1998, 2005, 2008; Obrocki et al., 1999; Reneman et al., 2002; Semple et al., 1999). MDMA’s potential toxicity is underscored by consistent reports of impaired cognitive function in human Ecstasy users, with verbal memory most commonly affected (Halpern et al., 2004; Kalechstein et al., 2007; reviewed in Zakzanis et al., 2007; Laws et al., 2007). While most reports of psychological assessments of verbal memory in Ecstasy users are retrospective, at least one prospective study suggests that Ecstasy per se use may cause verbal memory impairments, among other affected domains (Schilt et al., 2007). The clinical significance of impaired verbal memory in Ecstasy users is not apparent. Effect sizes from meta-analysis studies suggest that Ecstasy use is associated with small to large effects on verbal memory function ((Zakzanis et al. 2007;Laws and Kokkalis 2007;Kalechstein et al. 2007). Verbal intelligence quotient (IQ), however, seems to be largely preserved in Ecstasy users (e.g. (Bhattachary and Powell 2001;Halpern et al. 2004). Brain regions mediating aspects of verbal memory and semantic memory (verbal memory for word meaning) include, among other regions, Brodmann Areas (BA) 18, 21, and 45 (Lee et al., 2002). These brain regions have reduced brain gray matter concentration in Ecstasy polydrug users when measured using voxel-based morphometry (VBM), a largely automated technique that analyzes structural brain images for brain gray matter distribution differences (Cowan et al., 2003). Ecstasy, cocaine, alcohol, and cannabis may contribute to these findings of altered regional bran gray matter concentration (Cowan et al., 2003). Therefore, indirect evidence from cognitive studies and functional and structural neuroimaging suggest that BA 18, 21 and 45 would be reasonable target brain regions for additional investigations in human Ecstasy polydrug users.
One method for the non-invasive investigation of potential neurotoxic effects of recreational drug exposure is magnetic resonance spectroscopy (MRS). This method has proven sensitive to detecting metabolite alterations across several classes of drugs, including cocaine, nicotine, and alcohol (Magalhaes, 2005). Prior reports using magnetic resonance spectroscopy (MRS) in human Ecstasy polydrug users have measured primarily N-acetylaspartate (NAA) and myoinositol (mI), putative neuronal and glial markers, respectively )(Chang et al., 1999a; reviewed in Cowan, 2007; Cowan et al., 2007; Daumann et al., 2004; Obergriesser et al., 2001; Reneman et al., 2001, 2002;). Potentially due to cohort, polydrug exposure, or methodology differences, these studies have not consistently demonstrated specific associations between Ecstasy use and metabolite levels. A recent prospective study found no effects of low dose Ecstasy use on metabolite levels (de Win et al., 2007). The above-mentioned MRS studies of potential toxic effects in humans have been largely conducted at an MRI scanner field strength of 1.5 Tesla (except for Cowan et al., 2007, which was conducted at a field strength of 4 Tesla). However, there are important theoretical advantages to employing higher field strength scanners for MRS, including the potential for increased sensitivity and specificity of metabolite isolation (Hetherington et al., 1997).
Given the demonstrated sensitivity of MRS to assay drug effects, the prior reports in Ecstasy polydrug users of altered NAA and mI, as well as altered verbal memory and corresponding evidence that brain regions mediating aspects of verbal memory function have reduced gray matter in Ecstasy users, we chose to further investigate Ecstasy and polydrug related effects on BA 18, 21 and 45. We predicted that NAA would be inversely correlated with drug exposure (i.e. neural toxicity leading to reduced NAA) and that mI would be positively correlated with drug exposure (i.e. neural toxicity leading to glial reactivity with secondary increase in mI). To test this hypothesis, we employed a targeted region of interest approach using MRS at high field strength (3 Tesla) combined with a cross-sectional correlational study design to examine NAA and mI concentrations in BA 18, 21, and 45 in human recreational drug users.
2.0 Methods
2.1 Participants
Seventeen recreational drug users (age 21.6 ± 2.7 years) were recruited via advertisements in local media and by word of mouth. Recruitment materials invited individuals 18–35 years of age that had used Ecstasy or other drugs to enroll in a brain research study. We enrolled polydrug users (enriched for Ecstasy use by the specific advertisements) to conduct within-group exposure/outcome assays of drug exposure. Ecstasy users worldwide show a clear pattern of heavier and broader polydrug exposure than that of recruited non-Ecstasy user control groups, limiting the utility of comparison to a specific control group e.g., (Scholey et al. 2004;Degenhardt et al. 2004;Gouzoulis-Mayfrank and Daumann 2006). This approach is especially relevant to costly neuroimaging studies when very large, or multiple comparison groups would be needed and to studies based on animal research demonstrating a dose-toxicity relationship (reviewed in Green et al., 2003). Because our study focused primarily on the question of long-lasting toxicity, participants were not required to have recently used Ecstasy or other drugs. Participants were compensated for participation but were told that no compensation would be provided if urine or alcohol screens were positive. Participants comprising the current report are part of a larger ongoing study examining brain structure, function and neurochemistry in Ecstasy users. The overall study is an observational, cross-sectional study with no behavioral or clinical interventions. Each participant took part in various components of the larger study. The cohort reported here does not overlap with an earlier MRS study by our group (Cowan et al., 2007).
2.2 Screening
Participants were screened by phone for provisional entry criteria and then completed an in-depth screening that included Structured Clinical Interview for DSM-IV and detailed substance abuse questionnaires using a time-line follow back method. The questionnaire also contained queries for drug use history for alcohol, cannabis, stimulants, hallucinogens, opiates, sedatives, dissociative anesthetics, anabolic steroids, and inhalants. The questionnaires contain items to indicate when a specific drug was last used, number of total lifetime episodes, episodes in the last month, whether the subject intended to use the drug in the future, and approximate amount of the drug used (Cowan et al., 2006, 2007). Participants were blind to all inclusion/exclusion criteria other than the requirement for Ecstasy or other drug use. All participants self-reported abstinence from recreational drug and alcohol use for a minimum of 4 days prior to the study day (excluding nicotine and caffeine). Study exclusions were prior head injury, pregnancy (QuPid One Step Pregnancy Test; Stanbio Laboratory, Inc. San Antonio, TX), positive urine drug screen (Triage Drugs of Abuse Panel, Biosite Diagnostics, San Diego, CA), positive alcohol breath screen (Alco Sensor III, Intoximeters, St. Louis, MO) at enrollment or on the scan day, history of current or past substance or alcohol dependence, history of current or past SCID-diagnosed DSM-IV Axis 1 psychiatric disorder (except substance-induced mood disorder or substance abuse), use of non-illicit psychoactive medications (whether prescription or over-the-counter) within six weeks prior to the study, endocrine abnormalities, history of loss of consciousness for over 30 minutes, as well as contraindications to MR scanning (claustrophobia, implanted medical devices, pacemaker, aneurysm clips, non-removable metallic piercings, other possible metal in the body including shrapnel and sheet metal filings).
2.3 Ethics approval
The study protocol was approved by the Vanderbilt University Institutional Review Board (IRB) and conformed to the World Medical Organization Declaration of Helsinki (http://www.wma.net/e/policy/b3.htm). Participants were provided with written informed consent approved by the Hospital's IRB.
2.4 Confidentiality
To protect participant confidentially, a Certificate of Confidentiality was obtained from the National Institute on Drug Abuse (NIDA) and participants were informed of the Certificate protections in the informed consent document.
2.5 Acquisition of Proton Spectra
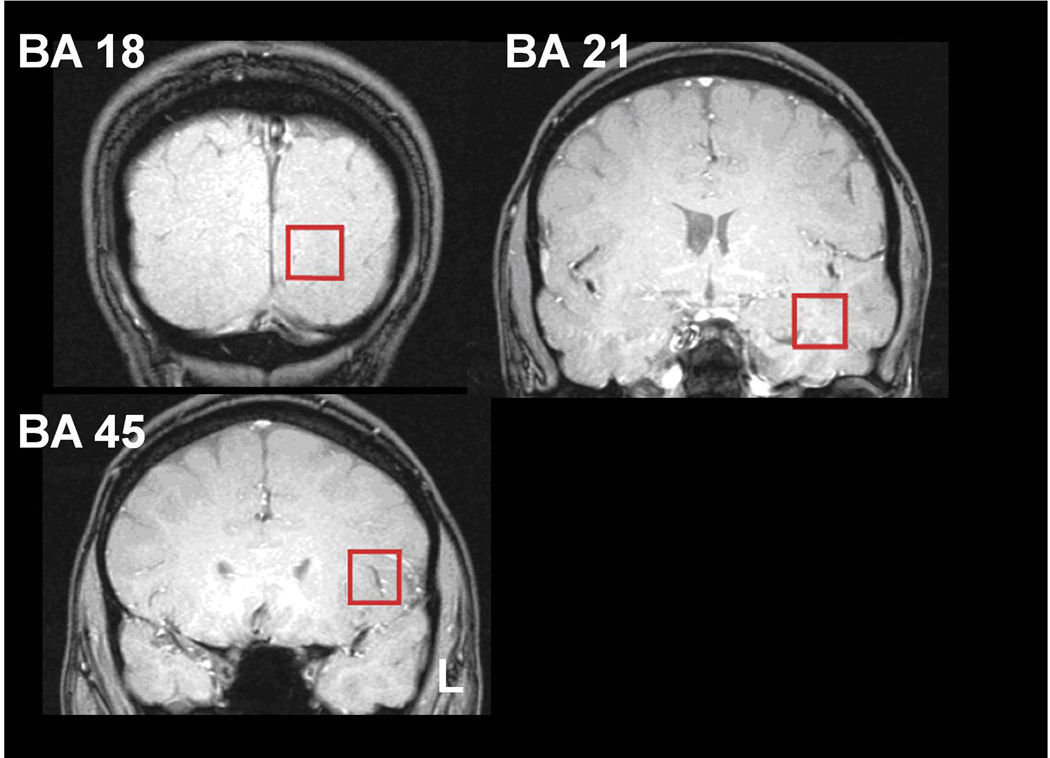
High resolution T1- weighted 3D anatomical images were used for centering of point-resolved spectroscopic sequence (PRESS) voxels in left BA 18, 21 and 45 (Figure 1) according to regions affected in the earlier VBM study (Cowan et al., 2003) and according to the Talairach and Tournoux atlas (Talairach and Tournoux, 1988). Voxel sizes were approximately 3.4 cm3 (approximately 1.5×1.5×1.5 cm in each plane). The anterior and posterior commissure points were used as references for voxel placement. PRESS measurements were made at 3 Tesla (T) on two scanners (GE Medical Systems, Milwaukee WI) or Philips Intera Achieva (Philips Healthcare, The Netherlands). Two echo times were used for each voxel, resulting in acquisition of two spectra each from areas 18 and 45. Due to technical limitations on short TE spectra in temporal lobe, only long-echo spectra for NAA were analyzed for BA 21. Long-echo (TE = 144 ms, both scanners) spectra were collected for observation of NAA and short-echo (TE = 30 ms for GE and TE = 32 ms for Philips) spectra were collected to measure mI. Water suppression was achieved using a chemical shift selective (CHESS) sequence. Repetition time (TR) was 2 seconds, with each spectrum consisting of 128 scans. Each area was shimmed separately using first order shims only. Typical unsuppressed water line widths were 8–10 Hz. Spectra with line widths greater than 15 Hz were rejected for GE data and metabolite measurements having Camer-Rao lower bounds <20% were reported for Philips data. Due to these rejection criteria, the sample size for analyzable spectra varied by brain region and metabolite (Table 3).
Figure 1. Representative voxel placement.
Coronal localizer scans indicating Brodman’s Areas (BA) regions of voxel placement (red square) for spectral acquisition. L = left side of brain.
Table 3.
Metabolite-Drug Use Correlations
| BA 18 NAA/Cr | BA 21 NAA/Cr | BA 45 NAA/Cr | |
|---|---|---|---|
| Lifetime Episodes | r (p) N* | r (p) N* | r (p) N* |
| Ecstasy | 0.407 (0.317) 8 | −0.100 (873) 5 | −0.406 (0.244) 10 |
| Cannabis | −0.171 (0.615) 11 | 0.690 (0.058) 8 | −0.687 (0.014) 12 |
| Alcohol | −0.116 (0.721) 12 | 0.567 (0.112) 9 | −0.423 (0.150) 13 |
| Cocaine | −0.036 (0.939) 7 | 0.800 (0.200) 4 | 0.180 (0.670) 8 |
data are presented as Spearman's rho (r), two tailed significance (p), number of subjects included in the correlation analysis (N).
2.6 Processing of Proton Spectra
Spectra were fit with a Marquart-Levenberg algorithm using either vendor-supplied software, SAGE (GE) or LC model (Philips). Ratios of NAA/Cr and mI/Cr were calculated from the spectral fits and are reported for each of the two anatomical regions. To control for the effects of the two scanners and slightly different acquisition methods, data were standardized to the overall group mean metabolite/Cr ratio so that NAA/Cr and mI/Cr correlation analyses are reported as standardized values.
2.7 Statistical Analysis
The drugs used most frequently by this cohort were Ecstasy, alcohol, cannabis, and cocaine. There were insufficient numbers of participants demonstrating past-year use of these drugs to permit reasonable correlation analyses. Therefore lifetime episodes (defined as each discrete 24-hour period) as well as estimated quantity of corresponding drug use (defined as the average amount of a drug used per episode multiplied by the number of episodes) were assessed for associations with the outcome variables (NAA/Cr and mI/Cr ratios). As expected, drug exposure was not normally distributed. Thus, associations between drug exposure and metabolite outcome measurements were quantified using Spearman correlation coefficients. Spearman correlations provide a robust measure of bivariate association when sample sizes are small and for distributions with extreme outliers (Pagano M and Gauvreau K 2000). A maximum two-tailed alpha of 0.05 was used for assessing statistical significance for all associations.
3.0 Results
Lifetime drug use (as episodes and quantity of drug consumed) and duration of abstinence are summarized for Ecstasy, alcohol, cannabis, and cocaine--the drugs most frequently used by the study sample (Table 1). Of the total cohort, only one subject met lifetime criteria for cannabis dependence and no other subjects met lifetime dependence criteria for any drug or alcohol. Two subjects met lifetime alcohol abuse criteria alone; one met lifetime abuse criteria for cannabis, stimulants, and hallucinogens; one met lifetime abuse criteria for alcohol and cocaine alone and current abuse criteria for cannabis. An example spectra from a single subject is illustrated in Figure 1. Mean (non-normalized) metabolite ratios are shown in Table 2. Overall, there were no statistically significant associations between lifetime use episodes of Ecstasy, alcohol, or cocaine for either NAA/Cr or mI/Cr ratios in BA 18, 21 or 45. Further, there were no consistent patterns observed for the direction of the associations between NAA/Cr or mI/Cr and Ecstasy use. The relationships between the lifetime episodes of drug exposure (analysis performed only in participants exposed to a particular drug) and metabolite ratios for NAA/Cr are depicted in Table 3. Lifetime episodes of cannabis use demonstrated a statistically significant inverse association with BA 45 NAA/Cr (r = −0.687, p = 0.014) but not with BA 45 mI/CR nor for either metabolite ratio in BA 18 or 21. Twelve subjects both used cannabis and had analyzable data for BA 45 NAA/Cr. With regard to the degree of cannabis use and the relationship to Ecstasy use in this group, 8 were also Ecstasy users. The Ecstasy and cannabis using group had the heaviest level of cannabis exposure with mean episodes of use of 595.5 (±873.6) episodes versus 120 (±80.4) episodes for the 4 subjects reporting cannabis, but not MDMA use. When the correlation analysis was repeated in the subset of 8 cannabis users also reporting Ecstasy use, there remained an association between cannabis use and BA 45 NAA/Cr (r = −0.595), but this finding was no longer statistically significant with the smaller sample size (p = 0.120). Associations of lifetime quantities of drug use with metabolite ratios were also assessed (not shown). There were no statistically significant correlations for Ecstasy, alcohol, and cocaine with NAA/Cr or mI/CR metabolite ratios in BA 18, 21 or 45. However, for cannabis, and in parallel with the association seen for lifetime episodes of cannabis use, the lifetime number of joints smoked showed a similar trend-level correlation with BA 45 NAA/Cr (r = −0.503, p = 0.095).
Table 1.
Lifetime drug use and duration of drug abstinence
| Lifetime Use Variables | Abstinence (days) | |||||
|---|---|---|---|---|---|---|
| Drug | N | Episodes | Quantity | Units | mean (s.d.) | minimum |
| Ecstasy | 12 | 71.75 (116.22) | 8330.00 (17216.42) | mg | 460.67 (303.84) | 21 |
| Alcohol | 16 | 372.91 (458.04) | 2117.05 (3607.24) | units | 14.36 (7.111) | 4 |
| Cannabis | 15 | 526.97 (773.21) | 993.31 (2101.82) | joints | 122.27 (172.76) | 4 |
| Cocaine | 10 | 8.10 (6.19) | 7.53 (13.84) | gm | 404.10 (362.99) | 48 |
Table 2.
Mean metabolite ratios by region
| Brodmann Area | |||
|---|---|---|---|
| Metabolite | 18 | 21 | 45 |
| NAA/Cr | 2.030 (0.188) | 1.861 (0.325) | 1.925 (0.329) |
| mI/Cr | 1.041 (0.539) | - | 0.709 (0.288) |
metabolite ratios presented as mean (S.D.).
4.0 Discussion
The intent of the present report was to test the hypotheses that NAA and mI in brain regions important in verbal memory (left BA 18, 21 and 45; Lee et al., 2002) and previously demonstrated to have reduced gray matter concentration in Ecstasy polydrug users (Cowan et al., 2003) would show evidence consistent with Ecstasy or polydrug-induced neurotoxicity. We found an inverse association between the degree of cannabis use and BA 45 NAA/Cr. This association was not observed for other brain regions or for mI. In contrast to the observed cannabis effects, there was no statistically significant association between the degree of lifetime Ecstasy, alcohol or cocaine use and NAA or mI in the tested regions. These results suggest that, at the levels of Ecstasy use reported by our cohort and in the brain regions studied, Ecstasy use, per se, may not be associated with neurotoxic effects in human recreational users as measured by NAA and mI.
Cannabis use has been associated with reduced NAA/Cr in dorsolateral prefrontal cortex (Hermann et al., 2007) and basal ganglia (Chang et al., 2006) and with hippocampal and amygdala volume loss (Yücel et al., 2008), but a similar association has not been previously reported in BA 45 to our knowledge. Since we did not find an association of cannabis exposure with NAA in BA 18 or 21, the current findings would suggest that cannabis does not have a generalized effect on cortical NAA. Notably, cannabis use showed a non-significant, trend-level positive correlation with NAA/Cr ratios in BA 21. Additional data examining other brain regions and structure function correlations in those regions seem warranted. With regard to polydrug effects in the VBM study (Cowan et al., 2003), both cannabis and cocaine use seemed to account for a portion of the observed effects on brain gray matter concentration in BA 45. However, it remains unclear whether this finding was due to the degree of inter-correlation of Ecstasy, cocaine and cannabis use or an actual specific effect of cannabis and cocaine on brain gray matter concentration.
Of note, we performed multiple statistical tests for the association of metabolites but did not adjust our statistical threshold for those comparisons. If we applied a Bonferroni correction adjusting for 20 tests across regions, metabolites, and drug use categories, then only associations at a significance threshold of p ≤ 0.0025 would survive. Given the prior reports of cannabis effects suggesting biological plausibility, the magnitude of the observed correlation for the cannabis effects (r = −0.687) and the uncorrected p value of 0.014, we believe it reasonable to conclude that this may be a real effect, and not the result of Type I experimental error.
Predictions derived from laboratory studies of MDMA administration are equivocal with regard to the consequences of MDMA exposure on brain metabolites (Cowan et al., 2008). NAA and mI have been previously studied in Ecstasy polydrug users with inconsistent findings (Chang et al., 1999; reviewed in Cowan, 2007; Daumann et al., 2004; Reneman et al., 2001, 2002; Cowan et al., 2007). Chang and colleagues (1999a) found increased occipital cortical mI but normal NAA in Ecstasy users while Reneman et al., (2002) employing similar methods and similar voxel placements reported no change in mI but reduced NAA in prefrontal regions. An additional MRS study targeting the same regions as Chang and Reneman found no effects of Ecstasy use on metabolites in a large cohort of Ecstasy users (de Win et al. 2008). A prospective study of incident Ecstasy use (employing voxel placements similar to those of Chang et al., (1999a), and Reneman et al., (2002), found no effects of low dose Ecstasy use on multiple metabolite measures, including NAA and mI (de Win et al., 2007). Daumann and colleages (2004) reported no significant differences between MDMA users and controls in hippocampal, mid-frontal, or mid-occipital brain regions. Obergreisser et al., (2001) found no significant differences in hippocampal NAA/Cr. Ecstasy use was not associated with altered NAA or mI in mid-line occipital cortex of moderate Ecstasy users (Cowan et al., 2007). In our earlier (Cowan et al., 2007) and current report the average Ecstasy use (Table 1) is modest in comparison to studies finding significant metabolite effects.
Both cocaine and alcohol have been variably associated with altered NAA in some brain regions in cocaine or alcohol-dependent individuals (Chang et al., 1999b; O’neill et al., 2001). However, the current study excluded subjects with a history of cocaine or alcohol dependence and use levels for both alcohol and cocaine were low.
Limitations of the study include the relatively small sample size, the inability to verify purity of Ecstasy or other polydrugs, and the retrospective associational study design. Drug purity concerns and other uncontrolled factors necessarily limit all studies of human substance use in a naturalistic environment (Green, 2004). The sample composition and size limitations of the current study do not permit us to exclude Type 2 experimental error for negative findings or to generalize beyond the current cohort. However, the Spearman test is robust to small sample sizes and significant effects were detected with regard to cannabis exposure. Finding an acceptable control group to reasonably match Ecstasy polydrug users is very difficult because Ecstasy users tend to use more of every class of drug than do their non-MDMA using peers (de Almeida and Silva, 2003; Gross et al., 2002; Pedersen and Skrondal, 1999; Schifano et al., 1998; Scholey et al., 2004; Wish et al., 2006) and within-group associational designs therefore have an important role in furthering our understanding of specific drug exposure with specific outcomes.
Given the vast numbers of Ecstasy polydrug users and evidence for the Ecstasy’s sustained worldwide popularity, much additional research examining the relative contributions of Ecstasy, cannabis, and other drugs to potential CNS toxicity is needed. Future studies examining correlated structure, function, and behavioral outcomes may be best suited to answer some questions. Linking behavioral effects to brain toxicity may have particular relevance if MDMA or polydrug toxicity is affected by genetic, behavioral, or other factors that lead to highly variable individual effects of the drug. Many limitations inherent in retrospective associational studies of Ecstasy users can potentially be overcome by prospective studies in groups at high-risk for future Ecstasy use (such as in the Netherlands XTC study; De Win et al., 2005). Due to cost limitations inherent in neuroimaging studies, however, such studies are not easily conducted in populations having low prevalence rates of Ecstasy use.
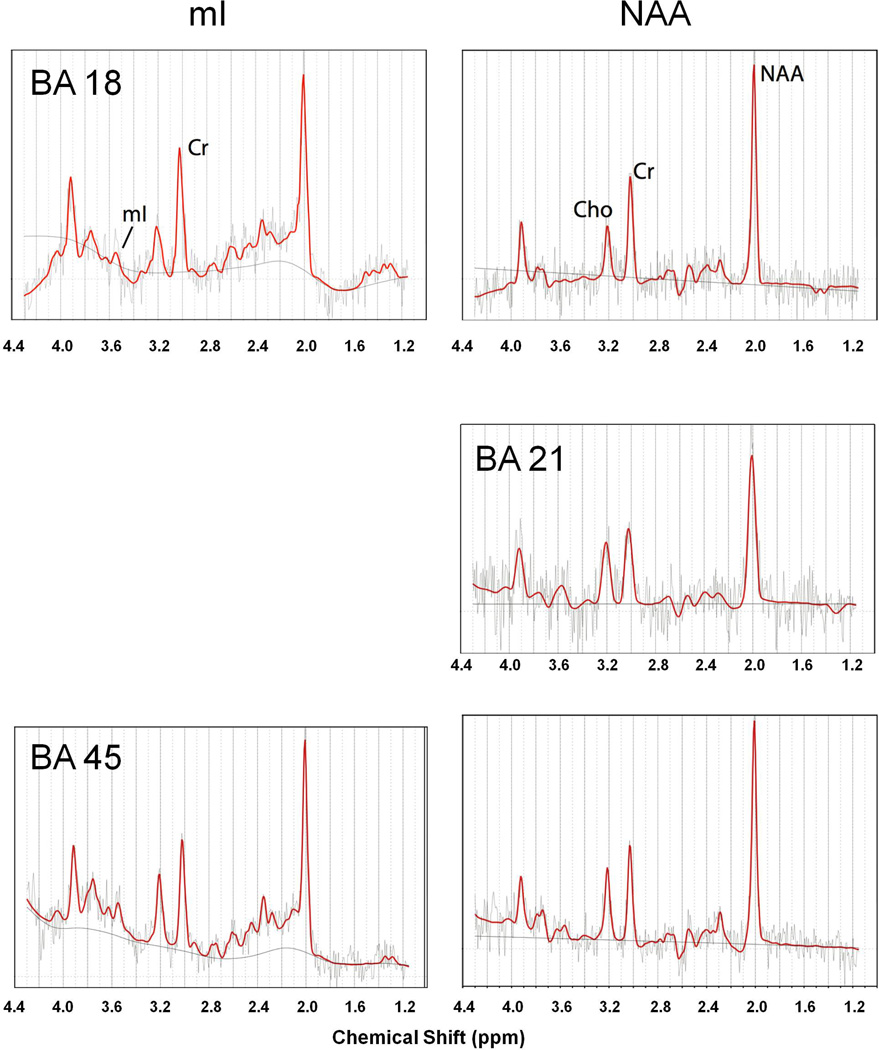
Figure 2. Representative fitted spectra.
Short (mI, left column) and long (NAA, right column) echo spectra from Brodmann areas (BA) 18 (top row), 21(middle row) and 45(bottom row) taken from a single subject. Spectra were fit using LCmodel (red lines). Horizontal axis indicates chemical shift in parts per million (ppm). Fitted baseline spline is indicated as solid line. Due to technical restrictions, no short echo (mI) spectra are provide for BA 21.
Acknowledgments
The authors would like to acknowledge the technical assistance of Amy Bauernfeind, Linda Todd, J. Gavin Lillevig, and Kimberly Morton. Deanne Roberts contributed to early versions of this manuscript. We would like to thank the following funding agencies: NIDA: DA015137, DA020149 and DA00366; NCRR: Vanderbilt CTSA UL1 RR024975; and VUIIS: Vanderbilt University Institute of Imaging Science.
Contributor Information
James M Joers, Email: jim.joers@vanderbilt.edu.
Mary S Dietrich, Email: Mary.dietrich@vanderbilt.edu.
References
- Bhattachary S, Powell JH. Recreational use of 3,4-methylenedioxymethamphetamine (MDMA) or 'ecstasy': evidence for cognitive impairment. Psychol. Med. 2001;31:647–658. doi: 10.1017/s0033291701003828. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Wilke F, Petersen K, Nebeling B, Obrocki J, Schulze O, Schmidt U, Clausen M. A voxel-based PET investigation of the long-term effects of "Ecstasy" consumption on brain serotonin transporters. Am J Psychiatry. 2004;161:1181–1189. doi: 10.1176/appi.ajp.161.7.1181. [DOI] [PubMed] [Google Scholar]
- Capela JP, Ruscher K, Lautenschlager M, Freyer D, Dirnagl U, Gaio AR, Bastos ML, Meisel A, Carvalho F. Ecstasy-induced cell death in cortical neuronal cultures is serotonin 2A-receptor-dependent and potentiated under hyperthermia. Neuroscience. 2006;139:1069–1081. doi: 10.1016/j.neuroscience.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Capela JP, Fernandes E, Remião F, Bastos ML, Meisel A, Carvalho F. Ecstasy induces apoptosis via 5-HT(2A)-receptor stimulation in cortical neurons. Neurotoxicology. 2007;28:868–875. doi: 10.1016/j.neuro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Grob CS, Poland RE. Cerebral (1)H MRS alterations in recreational 3, 4-methylenedioxymethamphetamine (MDMA, "ecstasy") users. J Magn Reson Imaging. 1999;10:521–526. doi: 10.1002/(sici)1522-2586(199910)10:4<521::aid-jmri4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR, Wagstaff GF. What is a dose of ecstasy? J Psychopharmacol. 2002;16:189–190. doi: 10.1177/026988110201600211. [DOI] [PubMed] [Google Scholar]
- Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology (Berl) 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Bolo NR, Dietrich M, Haga E, Lukas SE, Renshaw PF. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007;155:179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Lyoo IK, Sung SM, Ahn KH, Kim MJ, Hwang J, Haga E, Vimal RL, Lukas SE, Renshaw PF. Reduced cortical gray matter density in human MDMA (Ecstasy) users: a voxel-based morphometry study. Drug Alchohol Depend. 2003;72:225–235. doi: 10.1016/j.drugalcdep.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Haga E, deB Frederick B, Dietrich MS, Vimal RL, Lukas SE, Renshaw PF. MDMA use is associated with increased spatial BOLD fMRI visual cortex activation in human MDMA users. Pharmacol Biochem Behav. 2006;84:219–228. doi: 10.1016/j.pbb.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Roberts DM, Joers JM. Proceedings of the International Drug Abuse Research Society. Annals of the New York Academy of Sciences; Neuroimaging in human MDMA (Ecstasy) users: A cortical model. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumann J, Fischermann T, Pilatus U, Thron A, Moeller-Hartmann W, Gouzoulis-Mayfrank E. Proton magnetic resonance spectroscopy in ecstasy (MDMA) users. Neuroscience Letters. 2004;362:113–116. doi: 10.1016/j.neulet.2004.03.004. [DOI] [PubMed] [Google Scholar]
- de Almeida SP, Silva MT. Ecstasy (MDMA): effects and patterns of use reported by users in São Paulo. Rev Bras Psiquiatr. 2003;25:11–17. doi: 10.1590/s1516-44462003000100004. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Barker B, Topp L. Patterns of ecstasy use in Australia: findings from a national household survey. Addiction. 2004;99:187–195. doi: 10.1111/j.1360-0443.2003.00622.x. [DOI] [PubMed] [Google Scholar]
- de Win MM, Jager G, Booij J, Reneman L, Schilt T, Lavini C, Olabarriaga SD, Ramsey NF, Heeten GJ, van den Brink W. Neurotoxic effects of ecstasy on the thalamus. Br J Psychiatry. 2008;193:289–296. doi: 10.1192/bjp.bp.106.035089. [DOI] [PubMed] [Google Scholar]
- De Win MM, Jager G, Vervaeke HK, Schilt T, Reneman L, Booij J, Verhulst FC, Den Heeten GJ, Ramsey NF, Korf DJ, Van den Brink W. The Netherlands XTC Toxicity (NeXT) study: objectives and methods of a study investigating causality, course, and clinical relevance. Int J Methods Psychiatr Res. 2005;14:167–185. doi: 10.1002/mpr.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Win MM, Reneman L, Jager G, Vlieger EJ, Olabarriaga SD, Lavini C, Bisschops I, Majoie CB, Booij J, den Heeten GJ, van den Brink W. A prospective cohort study on sustained effects of low-dose ecstasy use on the brain in new ecstasy users. Neuropsychopharmacology. 2007;32:458–470. doi: 10.1038/sj.npp.1301225. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, 2nd, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151:533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. The confounding problem of polydrug use in recreational ecstasy/MDMA users: a brief overview. J. Psychopharmacol. 2006;20:188–193. doi: 10.1177/0269881106059939. [DOI] [PubMed] [Google Scholar]
- Green AR. MDMA: fact and fallacy, and the need to increase knowledge in both the scientific and popular press. Psychopharmacology (Berl) 2004;173:231–233. doi: 10.1007/s00213-004-1820-z. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Gross SR, Barrett SP, Shestowsky JS, Pihl RO. Ecstasy and drug consumption patterns: a Canadian rave population study. Can J Psychiatry. 2002;47:546–551. doi: 10.1177/070674370204700606. [DOI] [PubMed] [Google Scholar]
- Halpern JH, Pope HG, Jr, Sherwood AR, Barry S, Hudson JI, Yurgelun-Todd D. Residual neuropsychological effects of illicit 3,4-methylenedioxymethamphetamine (MDMA) in individuals with minimal exposure to other drugs. Drug Alcohol Depend. 2004;75:135–147. doi: 10.1016/j.drugalcdep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, Mann K. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007;61:1281–1289. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Pan JW, Chu WJ, Mason GF, Newcomer BR. Biological and clinical MRS at ultra-high field. NMR Biomed. 1997;10:360–371. doi: 10.1002/(sici)1099-1492(199712)10:8<360::aid-nbm477>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, 2nd, Mahoney JJ, 3rd, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology (Berl) 2007;189:531–537. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- Laws KR, Kokkalis J. Ecstasy (MDMA) and memory function: a meta-analytic update. Hum Psychopharmacol. 2007;22:381–388. doi: 10.1002/hup.857. [DOI] [PubMed] [Google Scholar]
- Lee AC, Robbins TW, Graham KS, Owen AM. "Pray or Prey?" dissociation of semantic memory retrieval from episodic memory processes using positron emission tomography and a novel homophone task. Neuroimage. 2002;3:724–735. doi: 10.1006/nimg.2002.1101. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC. Functional magnetic resonance and spectroscopy in drug and substance abuse. Top Magn Reson Imaging. 2005;16:247–251. doi: 10.1097/01.rmr.0000194048.43739.d4. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future Survey, NATIONAL RESULTS ON ADOLESCENT DRUG USE Overview of Key Findings. The University of Michigan Institute for Social Research. 2007 ( http://monitoringthefuture.org) [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA ("Ecstasy") on brain serotonin neurons in human beings. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Quantitative PET Studies of the Serotonin Transporter in MDMA Users and Controls Using [(11)C]McN5652 and [(11)C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/−)3,4-methylenedioxymethamphetamine ("ecstasy") users: relationship to cognitive performance. Psychopharmacology (Berl) 2008 Jul 27; doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obergriesser T, Ende G, Braus DF, Henn FA. Hippocampal 1H-MRSI in ecstasy users. Eur Arch Psychiatry Clin Neurosci. 2001;251:114–116. doi: 10.1007/s004060170044. [DOI] [PubMed] [Google Scholar]
- Obrocki J, Buchert R, Väterlein O, Thomasius R, Beyer W, Schiemann T. Ecstasy--long-term effects on the human central nervous system revealed by positron emission tomography. Br J Psychiatry. 1999;175:186–188. doi: 10.1192/bjp.175.2.186. [DOI] [PubMed] [Google Scholar]
- Pagano M, Gauvreau K. Principles of biostatistics. Pacific Grove, CA: Duxbury; 2000. [Google Scholar]
- Parrott AC. Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity. Psychopharmacology (Berl) 2004;173:234–241. doi: 10.1007/s00213-003-1712-7. [DOI] [PubMed] [Google Scholar]
- Pedersen W, Skrondal A. Ecstasy and new patterns of drug use: a normal population study. Addiction. 1999;94:1695–1706. doi: 10.1046/j.1360-0443.1999.941116957.x. [DOI] [PubMed] [Google Scholar]
- Reneman L, Endert E, de Bruin K, Lavalaye J, Feenstra MG, de Wolff FA, Booij J. The acute and chronic effects of MDMA ("ecstasy") on cortical 5-HT2A receptors in rat and human brain. Neuropsychopharmacology. 2002;26:387–396. doi: 10.1016/S0893-133X(01)00366-9. [DOI] [PubMed] [Google Scholar]
- Reneman L, Majoie CB, Schmand B, van den Brink W, den Heeten GJ. Prefrontal N-acetylaspartate is strongly associated with memory performance in (abstinent) ecstasy users: preliminary report. Biol Psychiatry. 2001;50:550–554. doi: 10.1016/s0006-3223(01)01177-5. [DOI] [PubMed] [Google Scholar]
- Schifano F, Di Furia L, Forza G, Minicuci N, Bricolo R. MDMA ('ecstasy') consumption in the context of polydrug abuse: a report on 150 patients. Drug Alcohol Depend. 1998;52:85–90. doi: 10.1016/s0376-8716(98)00051-9. [DOI] [PubMed] [Google Scholar]
- Schilt T, de Win MM, Koeter M, Jager G, Korf DJ, van den Brink W, Schmand B. Cognition in novice ecstasy users with minimal exposure to other drugs: a prospective cohort study. Arch Gen Psychiatry. 2007;64:728–736. doi: 10.1001/archpsyc.64.6.728. [DOI] [PubMed] [Google Scholar]
- Scholey AB, Parrott AC, Buchanan T, Heffernan TM, Ling J, Rodgers J. Increased intensity of Ecstasy and polydrug usage in the more experienced recreational Ecstasy/MDMA users: a WWW study. Addict Behav. 2004;29:743–752. doi: 10.1016/j.addbeh.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Semple DM, Ebmeier KP, Glabus MF, O'Carroll RE, Johnstone EC. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA ('ecstasy') users. Br J Psychiatry. 1999;175:63–69. doi: 10.1192/bjp.175.1.63. [DOI] [PubMed] [Google Scholar]
- Simantov R, Tauber M. The abused drug MDMA (Ecstasy) induces programmed death of human serotonergic cells. FASEB J. 1997;11:141–146. doi: 10.1096/fasebj.11.2.9039956. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) The National Survey on Drug Use and Health (NSDUH) 2008 Office of Applied Studies, http://oas.samhsa.gov. [PubMed]
- Tanner-Smith EE. Pharmacological content of tablets sold as "ecstasy": results from an online testing service. Drug Alcohol Depend. 2006;83:247–254. doi: 10.1016/j.drugalcdep.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human BrainThieme, Stuttgart, Germany. 1988. [Google Scholar]
- United Nations, Office on Drugs and Crime, World Drug Report. 2008 ( http://www.unodc.org/unodc/en/data-and-analysis/WDR-2008.html)
- Wang X, Baumann MH, Xu H, Morales M, Rothman RB. (+/−)-3,4-Methylenedioxymethamphetamine administration to rats does not decrease levels of the serotonin transporter protein or alter its distribution between endosomes and the plasma membrane. J Pharmacol Exp Ther. 2005;314:1002–1012. doi: 10.1124/jpet.105.088476. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Rothman RB. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse. 2004;53:240–248. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]
- Wish ED, Fitzelle DB, O'Grady KE, Hsu MH, Arria AM. Evidence for significant polydrug use among ecstasy-using college students. J Am Coll Health. 2006;55:99-04. doi: 10.3200/JACH.55.2.99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Campbell Z, Jovanovski D. The neuropsychology of ecstasy (MDMA) use: a quantitative review. Hum Psychopharmacol. 2007;22:427–435. doi: 10.1002/hup.873. [DOI] [PubMed] [Google Scholar]