Abstract
Smoking is a leading cause of mortality and morbidity worldwide. Smoking initiation often occurs during adolescence. This paper reviews and synthesizes adolescent development and nicotine dependence literatures to provide an account of adolescent smoking from onset to compulsive use. We extend neurobiological models of adolescent risk-taking, that focus on the interplay between incentive processing and cognitive control brain systems, through incorporating psychosocial and contextual factors specific to smoking, to suggest that adolescents are more vulnerable than adults to cigarette use generally, but that individual differences exist placing some adolescents at increased risk for smoking. Upon smoking, adolescents are more likely to continue smoking due to the increased positive effects induced by nicotine during this period. Continued use during adolescence, may be best understood as reflecting drug-related changes to neural systems underlying incentive processing and cognitive control, resulting in decision-making that is biased towards continued smoking. Persistent changes following nicotine exposure that may underlie continued dependence are described. We highlight ways that interventions may benefit from a consideration of cognitive-neuroscience findings.
Keywords: adolescence, incentive processing, cognitive control, nicotine dependence
1. Introduction
Smoking remains a leading cause of morbidity and mortality worldwide (American Cancer Society, 2010). The life expectancy for smokers is at least one decade shorter than for those who have never smoked (Jha et al., 2013) and for every smoker that dies from a smoking-related disease, another twenty Americans continue to live with a smoking-related disease (USDHHS, 2010). Quitting smoking is notoriously difficult, with unaided quit attempts resulting in relapse 90-95% of the time within one year of the quit attempt (Bancej et al., 2007; Hughes et al., 2004; Van Zundert et al., 2012). The personal and economic impact of smoking on public health and the immense challenge of quitting forcefully argue for increased understanding of the biological and environmental factors contributing to smoking initiation and early use.
A number of studies indicate that smoking initiation is most likely to occur during adolescence (Chen & Kandel, 1995; Lantz, 2003). Indeed, the majority of adults who smoke daily start smoking by the age of 18 (USDHHS, 2012). Despite recent declines, adolescent smoking rates remain generally high with 18% of 8th graders and 40% of 12th graders reporting having tried cigarettes at some time, and with 6.1% and 18.7% of 8th graders and 12th graders, respectively, reporting being daily smokers (Johnston et al., 2012). These statistics are particularly troubling given that early use of cigarettes during adolescence has been associated with heightened risk for later dependence (Kendler et al., 2013; Klein et al., 2013). Remarkably, even relatively low rates of cigarette consumption during adolescence (e.g., two to four cigarettes per week) increases the risk of becoming nicotine dependent in early adulthood (Riggs et al., 2007).
Characterizing processes underlying the uptake of smoking as well as the continued use of cigarettes through the adolescent period is integral to reducing the life-long health burden of smoking. Critically, adolescent smoking is a complex, multi-determined behavior, a fuller understanding of which necessitates an integrated biopsychosocial perspective. That is, to effectively identify individuals who may be at risk for experimentation and progressive use of cigarettes requires cross-disciplinary study at multiple levels of analysis, including (but not limited to) behavioral genetics, neuroscience, and epidemiology.
One key component to this perspective is knowledge of normative adolescent brain development and how experience or environmental perturbations, like nicotine exposure through cigarettes, may affect brain structure and function. In this paper, we review and synthesize literature on adolescent brain development, risk-taking and decision-making, and theories of nicotine dependence (from preclinical and human models) as a means to provide a more comprehensive neurobiological account of adolescent smoking from the initial cigarette to compulsive use. We also discuss persistent changes to incentive processing and cognitive control that encourages smoking beyond adolescence and impacts the ability to quit smoking during adulthood. To begin the task of integration across levels of analysis, we incorporate relevant findings beyond the neurobiological literature. Throughout, we highlight ways in which interventions aimed at smoking cessation may benefit from consideration of developmental neurobiological findings.
2. Model overview
First, we begin with an overview of our model. The model we present in this paper builds upon extraordinary progress in recent years in understanding adolescent brain development. Leading theories of adolescent brain development emphasize a unique sensitivity to motivational cues during this developmental period due to an adolescent-specific configuration of fronto-striatal circuitry (e.g., Luciana & Collins, 2012; Somerville & Casey, 2010). Due to the unique configuration of these systems (reviewed in section 3.4), behaviors towards immediately available, salient rewards are enhanced, while the ability to direct behavior towards long-term goals that may be inconsistent with the pursuit of immediately available rewards may be impaired. These neurobiological models have been used to explain the increase in risk-taking occurring during adolescence (e.g., Casey et al., 2008; Steinberg, 2008) and continue to be useful models for understanding adolescent risk behaviors (see Strang et al., 2013 for a discussion).
The model we present incorporates findings from the literature on adolescent smoking in an effort to extend this general model of adolescent risk-taking towards the formation of a model more specific to cigarette use during adolescence. Our model encompasses smoking initiation, the initial smoking experience, progression to regular smoking, and, finally, attempts at smoking cessation. First (section 3), we focus on the interplay between incentive processing and cognitive control processes in the brain, and suggest that the unique configuration and continued maturation of these systems during the adolescent period renders youth more vulnerable than adults to risky behavior like experimenting with cigarettes. Adolescents experience heightened approach motivation, relative to other age groups, towards novel stimuli such as cigarettes. Positive smoking expectancies – or beliefs about the effects of smoking – are suggested to enhance approach motivation towards cigarettes. While this heightened approach motivation is difficult to regulate due to the continued development of cognitive control abilities, adolescents at this stage of the addiction cycle have the capacity to exert control over their impulses, especially when they are motivated to do so. However, the motivation to do so may be lacking due to psychosocial risk-factors, some of which may be specific to the individual, such as peer influences on smoking, that impact decisions to smoke by rendering the prospect of smoking appealing. Further, we begin the task of extending contemporary neurobiological models by considering processes that take place once initial impulses to approach immediately available rewards have been suppressed and adolescents are successful in engaging deliberative decision-making processes (section 3.5). Contextual factors such as the availability of cigarettes, are also highlighted as a necessary component to consider in order to move contemporary neurobiological models from a general to a smoking-specific model of adolescent risk-taking (section 3.6).
Upon smoking, adolescents are more likely than other ages to continue smoking due to the increased positive effects induced by nicotine during this developmental period (reviewed in section 5). With continued use, adolescent smoking may be best understood as dependence, rather than risk-taking. We suggest that changes to mechanisms involving incentive processing and cognitive control regions may underlie this dependence (reviewed in section 6). Specifically, with continued cigarette use, adolescents experience heightened impulses to consume cigarettes in the context of drug-induced impairments in cognitive control. At this point, while they may now be motivated not to smoke cigarettes, the strong impulses to smoke and the experience of cognitive deficits renders decisions to remain abstinent from smoking difficult to execute.
Finally, our model considers persistent effects of smoking beyond acute withdrawal that may result exclusively from adolescent nicotine exposure (section 8). These persistent changes result in a protracted abstinence syndrome characterized by negative affect, cognitive-deficits, and increased reactivity towards nicotine and other drugs, even after long periods of abstinence, that may undermine smoking cessation and that highlights the need for preventive interventions to discourage smoking uptake during this vulnerable developmental period.
3. An overview of adolescent brain development and steps towards a smoking-specific model of adolescent smoking initiation
3.1. Adolescence as a time of increased risk-taking
Adolescence is widely recognized as a time of increased risk-taking compared to other age groups (Arnett, 1992; Spear, 2000). We broadly define risk-taking as engaging in behaviors high in subjective desirability but which also expose the individual to possible loss or harm (see Geier et al., 2010). The negative consequences of risk-taking, including vehicular accidents and substance abuse, are the major sources of death and disability during adolescence (Eaton et al., 2012).
Traditionally, the increased risk-taking in adolescence was viewed as the outcome of limited cognitive abilities (for review, see Boyer, 2006). Increases in risk-taking during adolescence, from this perspective, result from an inability to appreciate the risks involved in actions or the inability to effectively judge the probability of a risky outcome. Views from this approach include the adolescent vulnerability hypothesis (e.g., Arnett, 1992) that argues adolescents exhibit a cognitive deficiency, such that the adolescent regards him or herself as unique. This view of oneself as unique becomes a conviction that one will not die, that one is invulnerable to the consequences of behavior (Elkind, 1967). Research has failed to repeatedly support this purported cognitive limitation, however, with adolescents showing similar risk perceptions as adults and, in some instances, an increased perceived vulnerability to aversive outcomes (Fischhoff et al., 2000; Millstein & Halpern-Felsher, 2002). When asked to list the possible consequences of engaging in potentially risky behaviors, adolescents and adults exhibit similar response patterns (Beyth-Marom et al., 1993), illustrating that differences in the perceived consequences of risky behaviors do not underlie the increase in risky behaviors during adolescence. In fact, children as young as 5 years old have demonstrated a functional understanding of probability and expected value (Schlottman, 2001) and during a gambling task designed to assess abilities in probability estimation and reward evaluation, children, adolescents, and adults performed at a similar level (van Leijenhorst et al., 2008).
Such findings have lead researchers to view models focused on limitations in deliberative decision making to be inadequate in accounting for most adolescent risk-taking (for discussion see Reyna & Farley, 2006). Indeed, noting the capabilities of adolescents to encode mathematical probabilities about risks and rewards, fuzzy trace theory (Reyna & Brainerd, 2011) offers a compelling account of developmental differences in cognition that suggests that adolescents tend to be more rational in their decision-making, i.e. they analyze risky options, than adults who tend to incorporate gists, focusing on intuition rather than analytical details, into their decision-making process (Reyna et al., 2011). The theory contends that relying on deliberative decision-making is not necessarily a mark of mature decision-making and can actually result in risky behavior (Mills et al., 2008). The theory is noteworthy as it overcomes the often simplistic portrayal of interactions between emotional and analytic processes, noting that emotional aspects of cognition are not necessarily impulsive or primitive and that quick decisions relying on impulses are not always in conflict with decision outcomes that result from more deliberative processes. It is beyond the scope of this review to provide a complete overview of this model (interested readers are directed to Rivers et al., 2008 for a discussion of adolescent risk-taking from a fuzzy trace perspective) however its strength lies in its ability to reconcile the robust analytic decision-making abilities of adolescence and the real-world increase in risk-taking during adolescence.
3.2. Adolescent risk-taking in context
Noting the conflicting accounts of the inverted U-shaped pattern of risk-taking, peaking during adolescence, observed in real-world settings versus adolescent non-specific risk-taking observed in the laboratory (e.g. Weller et al., 2011) other theorists focus on the laboratory context in which risk-taking is often assessed and suggest that risk-taking in such contexts is not reflective of ‘real-world’ decision-making in that laboratory experiments usually involve hypothetical decisions in conditions of low emotional arousal (for discussion see Steinberg, 2004). Adolescent decision-making in risky contexts in the real world usually occurs under conditions of emotional arousal and, notably, in the presence of peers. Using two versions of the Columbia Card Task (CCT), Figner et al. (2009) investigated adolescent risk-taking under “hot” conditions - conditions in which affective processes were triggered - and “cold” conditions - conditions designed to avoid the triggering of affective processes (more deliberative decision-making). Adolescents exhibited increased risk-taking relative to adults in the “hot” but not the “cold” CCT. The increased risk taking was associated with less use of risk-relevant information, including probability, gain amount, and loss amount. In the cold condition, adolescents used information to the same extent as adults. Adult risk-taking was less variable across conditions suggesting a more balanced use of affective and deliberative processing. Similar developmental differences in performance across “hot” and “cold” decision-making tasks have been observed in other, more recent studies (e.g., van Duijvenvoorde et al., 2010; Johnson et al., 2012).
Another characteristic of real-world adolescent risk-taking is its social nature (for review see Albert et al., 2013). Indeed, the social nature of the initial smoking experience, often taking place in the presence of peers or siblings, has been widely noted (e.g., Friedman et al., 1985; Delorme et al., 2003). The social nature of adolescent risk-taking has been observed in laboratory tasks with stronger peer effects on risk-taking observed among adolescents than adults (Gardner & Steinberg, 2005). Chein et al. (2010) manipulated the social context during an incentivized simulated driving game in which participants made the decision to stop or to go at an intersection. Running through the intersection risked a collision with another vehicle but also held the potential of reaching the end of the track quicker and maximizing a monetary reward. In one condition, participants underwent the task alone while in another condition they were observed by peers. Adolescents, but not adults, exhibited greater risk taking (they drove through more yellow lights and crashed more often) when observed by their peers. Current interpretation of this phenomenon is that the presence of peers primes a reward-sensitive motivational state in adolescents more so than in adults (Albert et al., 2013). Indeed, social cues are motivationally relevant, with some inducing approach behavior (e.g., peer acceptance), and some inducing avoidance behavior (e.g., peer rejection) (see De Lorme et al., 2013 for discussion).
3.3. Interactions between affect and cognitive control
The studies cited in section 3.2 highlight the importance of understanding the functional interplay between affect and cognitive control to account for variability in adolescent decision-making across contexts. A key aspect of our model is the consideration of the joint contributions of an incentive-motivational system and a cognitive control system. The incentive-motivation system is supported by a well-studied circuitry originating in the ventral tegmental area, extending through the ventral striatum, and projecting to the medial and ventral regions of the prefrontal cortex (PFC), and the anterior cingulate cortex (O’Doherty, 2004; Schultz et al., 2000; Wise, 2002). The system supports reward valuation and the prediction of rewards and punishment (O’Doherty, 2004) and propels organisms to engage in incentive-motivated behaviors (Wahlstrom et al., 2010). Our model’s main focus is on the role of this system during adolescence in generating approach behaviors through appetitive motivation. Appetitive motivation refers to the state resulting from exposure to external stimuli with rewarding properties (Aarts et al., 2011). This state, resulting from mesolimbic dopamine (DA) projections to the ventral striatum, has been termed ‘wanting’ and generates approach behavior to stimuli signaling potential reward (Berridge, 2004).
In considering the role of cognitive control systems in the decision-making process, a useful distinction has been made between fast and slow (Metcalfe & Mischel, 1999), or impulsive and reflective (Bechara, 2005), systems. The impulsive system, supported by the aforementioned brain circuitry, is specialized for fast responses to stimuli while the reflective system is involved in more deliberative processing of stimuli. It would seem evolutionarily advantageous for an organism to determine rapidly, when confronted by a stimulus, whether approach or withdrawal is useful (Cacioppo et al., 1999), yet adaptive decision-making often requires the inhibition of a prepotent response based on the faster system to allow the engagement of reasoning abilities (Knoch & Fehr, 2007). The cognitive control system contributes to decision-making by inhibiting prepotent responses from the faster system and by providing the means to engage in deliberative reasoning. The neural circuitry underlying cognitive control differs depending on the aspect of cognitive control being examined and is generally widely distributed, although PFC has received the most attention (Luna et al., 2010). Studies on the interplay between these systems in adults are in line with this model. During a decision-making task involving choices involving monetary reward options varying by delay to delivery, McClure et al. (2004) observed greater fronto-parietal activity when subjects chose longer term options relative to times in which subjects chose more immediately available rewards. Areas of the limbic system associated with the midbrain DA system were preferentially activated during choices involving immediate rewards. Kuhnen and Knutson (2005) observed increased ventral striatum activity before risky choices relative to less risky choices. Low-frequency repetitive transcranial magnetic stimulation to the right dorsolateral PFC, a technique that temporarily disrupts function, resulted in riskier decision-making during a gambling task (Knoch et al., 2006).
3.4. Development of incentive processing and cognitive control during adolescence
The brain areas comprising these incentive-motivational and cognitive control systems undergo significant development during adolescence. Large-scale longitudinal neuroimaging studies have observed prepubertal increases in gray matter densities, followed by postpubertal loss (Giedd et al., 1999). The decreases in gray matter through adolescence is thought to partially result from the loss of underused synapses via synaptic pruning (Gogtay et al., 2004), a process which is thought to enhance information processing, capacity, and speed (see Luna et al., 2004). The developmental trajectory of gray matter reductions differs by region with brain areas associated with basic functions, such as motor and sensory brain areas, maturing first and areas involved in more complex functions, such as executive functions, maturing later (Gogtay et al., 2004). Considering the correlations between gray matter structure and cognitive functioning (e.g., Frangou et al., 2004; Van Petten et al., 2004), immaturities in these areas would be expected to result in limited incentive processing and cognitive control abilities.
Changes in white matter volume also occur through adolescence with linear increases occurring throughout childhood and adolescence (Giedd, 2008). Myelination underlies this increase in white matter volume and is a process that aids the functional integration of widely distributed circuitry (see Luna et al., 2010 for discussion). As efficient incentive processing requires the integration of many signals throughout the brain (Watanabe & Sakagami, 2007; Grace et al., 2007), the adolescent brain, still undergoing the process of myelination, may not be as efficient or rapid in accessing, and integrating, incentive signals. The continuing myelination during adolescence may also render top-down cognitive control mechanisms inefficient (Liston et al., 2006), potentially resulting in increased vulnerability to impulsive behaviors. Indeed, studies suggests that white matter structure is associated with the development of a host of cognitive functions during adolescence (e.g., Nagy et al., 2004).
Significant attention has been directed to developmental changes to the DA system because of its role in reward processing (for reviews see Ernst et al., 2009; Wahlstrom et al., 2010). DA tissue concentrations in both cortical and subcortical regions are at a relative high during adolescence (Goldman-Rakic & Brown, 1982; Irwin et al., 1994). Coupling these observations with findings suggesting an inverted u-shaped influence of DA activity on PFC functioning (for discussion, see Arnsten, 2009) with both deficient and excessive levels of DA impairing behavioral performance, Wahlstrom et al. (2010) hypothesize that DA levels in the PFC, under certain circumstances, exceed optimal levels, allowing activity in subcortical regions to dominate while PFC regions are “overdosed”. Furthermore, greater levels of DA activity in the nucleus accumbens may shift information flow in the nucleus accumbens toward greater limbic and less PFC input (Goto & Grace, 2008). Under such conditions, PFC input of incentive signals and top-down modulation of approach behavior towards more appropriate, goal-directed behavior may be compromised.
In line with these neurobiological findings, behavioral findings suggest that cognitive control abilities continue to develop through the adolescent period (e.g., Luna et al., 2004; Munoz et al., 1998; for review see Best et al., 2009). With age, performance on a wide-range of tasks indexing various components of cognitive control - including working memory and inhibitory control - becomes more accurate and consistent. Imaging data have also been used to argue for continued development of brain processes underlying cognitive control (e.g., Stevens et al., 2007; Velanova et al., 2008; for review see Luna et al., 2010). While adolescents can demonstrate adult-like behavior on cognitive control tasks, their functional circuitry during these tasks resembles that of adults performing a more difficult task (e.g., Scherf et al., 2006). There is also evidence for continued maturation through adolescence of the ability to engage regions necessary for the sustained maintenance of cognitive control sets which may account for the observation that adolescents are less consistent than adults during blocks of trials (Dosenbach et al., 2007; Velanova et al., 2009).
Although cognitive control abilities continue to develop throughout the adolescent period, especially in terms of the consistent execution of cognitive control (Velanova et al., 2009), adolescents are remarkably efficient and often demonstrate near-adult levels of performance on cognitive control tasks. Furthermore, researchers highlight the incongruity of the linear development of cognitive control from childhood through adolescence into adulthood, and the curvilinear development of risk-taking behavior - low in childhood, peaking in adolescence, and decreasing in adulthood (Casey & Caudle, 2013). As such, we argue that the role of immaturities in cognitive control for risky behavior provides an incomplete understanding of this phenomenon; limited cognitive control must be considered in the context of the heightened incentive motivation observed during this period (Luciana & Collins, 2012).
Studies on sensation- and novelty-seeking can inform research into incentive motivation. Sensation-seeking is the motivation to experience reinforcing stimuli and the willingness to risk aversive consequences in order to attain the desired experience (Zuckerman, 1994) and has been related to DA activity (for review see Roberti, 2004). Both sensation- and novelty-seeking may be viewed as a product of motivation to experience potential reward. From this perspective, risk taking might be viewed as a correlate of these behaviors, not the primary motivating influence, as the potential benefits of novelty- and sensation-seeking may also be accompanied by potential negative consequences. Increased novelty-seeking and exploratory behavior has been observed in adolescent rats and mice relative to adult animals (Douglas et al., 2003; Philpot & Wecker, 2008). In humans, adolescents also demonstrate increased approach behavior and decreased avoidance behavior relative to other age groups. Cauffman et al. (2010) demonstrated a curvilinear development pattern in approach behavior towards reward during a gambling task involving participants with ages ranging from 10 to 30. Reward-seeking peaked beginning in adolescence and declined in young adulthood. Adolescents also exhibited less harm avoidance tendencies suggesting that adolescents are more attentive to positive than negative outcomes. A peak in sensation-seeking, measured by items such as “I like new and exciting experiences, even if I have to break the rules”, has also been observed during adolescence (Romer et al., 2010; Steinberg et al., 2008).
In considering the development of incentive processing, it is also important to consider consummatory processing alongside reward-seeking behavior. The motivation to achieve an outcome is influenced by consummatory processing, or the way in which one responds to outcomes. There is behavioral evidence for increased hedonic responding to reward consumption during adolescence. Urosevic et al. (2012) provide longitudinal evidence for self-reported increases in positive affective responses to rewards from early to late adolescence, with evidence for a decline in the early 20s. During a monetary reward task, adolescents self-reported positive outcomes - notification of receipt of a monetary reward - as being more pleasurable than adults (Ernst et al., 2005). In a series of experiments, Wilmouth and Spear (2009) demonstrated increased hedonic reactions to sucrose in adolescent rats compared to adult rats. Note that the role of DA in generating hedonic responses has been disputed with its functioning thought to be more involved in generating incentive motivation rather than pleasure (see Berridge, 2004 for discussion). Instead, opioid activity in the shell of the nucleus accumbens is thought to mediate hedonic pleasure (Berridge, 2003; Kelley et al., 2002).
Imaging studies have been exceedingly useful in examining the development of incentive processing during the adolescent period. In investigating the adolescent response to incentives, it is important to consider the temporally distinct signals associated with incentive processing (Schultz et al., 2000; O’Doherty, 2004). Signals occurring prior to incentive delivery involve the processing of information associated with reward value, valence and anticipation, while signals occurring after incentive delivery include information associated with valence, magnitude, and prediction error (see Geier & Luna, 2009 for an extensive discussion). Adolescents show exaggerated ventral striatum responses during reward anticipation and receipt relative to adults (Ernst et al., 2005; Galvan et al., 2006; van Leijenhorst et al., 2010) and hypo-responsive striatal activity during the assessment of incentive value for upcoming trials (Geier et al., 2010). This pattern of activation suggests that adolescents may have limitations in reward assessment and a heightened reactivity in anticipation of reward, rendering them vulnerable to behavior directed by incentives when the value of the incentive has not been appropriately assessed. Note that this pattern of activity has not always been observed, with some studies reporting evidence for adolescent hypo-responsiveness during reward anticipation (e.g., Bjork et al., 2004). The field has converged on a hypothesis involving hyper-responsiveness during reward anticipation with the caveat that the nature of the task or context is important to consider (see Galvan, 2010 for an excellent discussion).
While the field has made great strides in examining the development of the circuitry underling incentive processing and cognitive control separately, the interplay between these systems is prominent in theories of decision-making. Few studies have examined the interplay between incentive processing and cognitive control systems, instead focusing on one or the other. A notable exception examined participants’ abilities to exert control over their actions when confronted with appetitive cues (Somerville et al., 2011). Relative to children and adults, adolescents exhibited a reduced capacity to suppress approach behavior to appetitive cues during a Go/No-Go task. Such reduced capacity was context-dependent and was not observed in response to neutral cues. Imaging results demonstrated that adolescents engaged the ventral striatum significantly more than children and adults. Furthermore, adolescents exhibited a marginally greater ventral striatum response to neutral facial expression, potentially suggesting reduced specificity of ventral striatal engagement. These results are in line with others and suggest an upregulation of motivated behavior in adolescents. On correctly performed No-Go trials to happy faces, adolescents exhibited a significant ventral-dorsal striatal coupling which may suggest that adolescents who activated the ventral striatum more strongly required greater dorsal striatal engagement to correctly suppress approach to the appetitive cues. Similar results were observed by Hare et al. (2008) and Tottenham et al. (2011).
Another way in which incentive processing and cognitive control brain systems interact is through processes in which cognitive control is enhanced by incentives. Incentives have been shown to improve performance on a wide variety of cognitive tasks in non-human primates (Takikawa et al., 2002) as well as human adults, including the Stroop task (Veling & Aarts, 2010), the Continuous Performance Test (Locke & Braver, 2008), indices of visual selective attention (Libera & Chelazzi, 2006), during a verbal delay recall task (Gilbert & Fiez, 2004), and oculomotor tasks (Duka & Lupp, 1997; Harsay et al., 2010). Increased performance on incentivized trials has been associated with increased activity in areas involved in cognitive control, including the dorsolateral PFC (Gilbert & Fiez, 2004) and the parietal and PFC (Locke & Braver, 2008).
The performance-enhancing effects of incentives have also been observed in adolescents during cognitive control tasks (Hardin et al., 2007) with adolescents reaching adult-like levels of performance under incentivized conditions (Jazbec et al., 2006). Enhanced activity in task-related brain regions was observed in adolescents during rewarded anti-saccade trials (Padmanabhan et al., 2011; Geier et al., 2010), further evidence of the ability of incentives to modulate cognitive control.
While such findings of enhanced cognitive control in the context of salient incentives may at first seem inconsistent with literature reporting immaturities in cognitive control functions in the context of approach motivation, there are a number of differences in the paradigms employed in these studies. In the antisaccade task, recruitment of inhibitory control is required to attain the available reward. Thus, in this case, the behavior being supported by motivation is directed towards immediate reward receipt, not the efficient integration of incentive signals involved in determining the appropriateness of the impulse to approach the reward, or goal-directed behavior directed away from the available reward. Indeed, Padmanabhan et al. (2011) have highlighted the potentially negative effects of this incentive modulation, suggesting that adolescent behavior geared towards immediate rewards may be enhanced before an adequate assessment of the incentive has occurred. However, this process may have the potential to redirect adolescent behavior towards more appropriate actions and so be relevant to interventions (discussed in section 4). Studies investigating whether cognitive control processes in the context of salient rewards, steering behavior towards a more appropriate behavioral outcome, can be enhanced by monetary, or other suitable, reward will be crucial in determining the potential for incentives to modify real-world, risk-taking behavior.
3.5. Decision-making beyond response inhibition
So far we have discussed the difficulties adolescents may have in inhibiting a prepotent response in affect-laden contexts. Once this response is inhibited, however, an adolescent may continue to seek the reward, deciding that the reward is worth the risk. Furthermore, in order to inhibit a prepotent response, adolescents must be motivated to do so. Thus interventions focusing on strengthening the capacity for impulse control are not likely to be a panacea for adolescent risk-taking. Adolescents have the capacity for effective decision-making, defined as being coherent in their decision-making (see Reyna & Farley, 2006 for discussion), at least to the extent that adults are. However, the information and subjective values - referred to here as the content of the decision-making process - may differ from those of adults (Scott et al., 1995). As a result, they may be less motivated to inhibit the impulse to smoke a cigarette in the first place and they may also be more likely to decide to smoke a cigarette even after undergoing deliberative decision-making.
One of the most intensively studied aspects of adolescent decision-making relevant to this discussion of motivation is the consideration of future consequences. Research indicates age differences in future orientation such that adolescents are more oriented to immediate rather than future consequences, relative to adults (Crone et al., 2003; Nurmi, 1991). In a large-scale study involving 935 individuals between the ages of 10 and 30 years, Steinberg et al. (2009) observed a weaker orientation to the future across multiple measures in young adolescents relative to individuals over 16 years of age. Peer influence may also influence the choices adolescents make. Peer conformity and perceived peer pressure towards misconduct peak during adolescence (Berndt, 1979; Clasen & Brown, 1985). Adolescents may bias their decision-making in order to fit in or to avoid rejection from their peer group (Brown et al., 1986). As discussed in section 3.4, the hedonic impact of stimuli may differ by age, which may result in differences in values ascribed to decision outcomes. Greater pleasure from stimuli, lesser concern for future outcomes, and greater susceptibility for peer influence places adolescents at increased risk for decision-making biased towards drug use, even when they are successful in inhibiting approach motivations in order to recruit more deductive reasoning.
Finally, future research must consider the effects of cues signaling the availability of potential reward and its accompanying affective state on decision-making after the initial impulse to approach has been inhibited. Pessoa’s (2009) model of motivation effects on cognitive control highlights the role of stimulus-driven effects on executive functioning. Although Pessoa focuses on negatively valenced emotional stimuli, the model may also be applied to reward-related stimuli. Drawing on resource models of executive functions (e.g., Norman & Bobrow, 1975), Pessoa suggests that the perception of emotion-laden items, in this case reward-related stimuli, may impair performance as resources are diverted from the executive functions involved in the decision-making process. This line of research, combined with findings demonstrating less information use among adolescents in affectively-charged situations (Gladwin et al., 2011), even when that information was readily available in the environment, highlights the need for research into adolescent information-use in “hot” contexts, even after the initial impulse to approach has been inhibited.
3.6. Individual differences in neurobiological, psychosocial, and contextual factors as risk-factors for smoking uptake
While there appears to be a normative increase in risk behaviors, including smoking, during the adolescent period, not all adolescents experiment with cigarettes. This suggests the need for a consideration of individual differences and a smoking-specific model of adolescent risk-taking that aims to address why some adolescents are more likely to turn to smoking than others.
In terms of individual differences in neurobiology, individuals with heightened activity in motivational neurocircuitry in response to cues signaling the potential availability of reward may be more likely to pursue rewards despite the potential risks while individuals exhibiting heightened activity during reward consumption may be prone to continuing the pursuit of the behavior (Bjork et al., 2011). In investigating individual differences in incentive processing, two proteins involved in terminating the action of intrasynaptic DA have received significant attention. Catechol-O-methylytansferase (COMT) catabolizes released DA and the DA transporter (DAT) recaptures extracellular DA into presynaptic terminals following release. The COMT gene contains a functional polymorphism that codes for the substitution of valine (val) by methionine (met) at codon 158. Due to the differential activity levels of the methionine and valine proteins at body temperature, with the valine protein acting optimally at body temperature while the methionine protein demonstrating lower activity at body temperature, individuals with two copies of the met allele have reduced COMT enzyme activity compared to individuals with two copies of the val allele (Chen et al., 2004). The DAT1 gene (SLC6A3) contains a variable number of tandem repeats (VNTR) in the 15th exon, occurring with greatest frequency in the 9- and 10-repeat forms (Heinz et al., 2000; Vandenbergh et al., 1992). The 10-repeat allele is related to increased expression of the gene (Mill et al., 2002; VanNess et al., 2005). Thus, individuals with two copies of the met allele and the 9-repeat allele of the DAT1 gene would be expected to have overall increased DA activity relative to those with the alternative genotypes.
There have been a slew of studies investigating the association between risk-taking behavior and polymorphisms associated with DA function (e.g., Amstadter et al., 2012; Mata et al., 2012). However, attempting to draw links between gene and behavior directly, without examining the effects of genetic variants on biological systems more proximate to genetic effects, such as neural activity, is difficult due to interactions between genes (e.g. Dreher et al., 2009) but also due to epigenetic processes and the generally small effects of individual genes. Imaging genetics studies which include a neural intermediate phenotype between genes and behavior are well placed to gain insight into the effects of genetic polymorphisms and risk-taking as polymorphism effects may be more readily observed at the neural level due to the subtle biological changes produced by genetic variants (see Buckholtz & Meyer-Lindenberg, 2008 and Hariri et al., 2006 for excellent discussions on the use of neural intermediate phenotypes in studies on genetic effects).
In one such imaging genetics study, Dreher et al. (2009) investigated the relationships between COMT and DAT1 polymorphisms and brain responses to the anticipation and consumption of uncertain reward. During reward anticipation, the number of met alleles was positively correlated with response of the ventral striatum and lateral PFC. In terms of DAT1, the 9-repeat carriers activated the ventral striatum more than 10-repeat carriers. During reward consumption, less activity in the orbitofrontal cortex was associated with the presence of val alleles. Increased lateral PFC activation was observed in 9-repeat carriers at the time of reward outcome. An interaction between COMT and DAT1 genes was observed in the ventral striatum and lateral PFC during reward anticipation and in the midbrain and lateral prefrontal and orbitofrontal cortices during reward delivery, with the highest activation exhibited by carriers of the DAT1 9-repeat allele and COMT met/met allele. A key strength of this study was its use of an intermediate phenotype and the combination of genetic and neural methods. Other imaging genetics studies have also observed individual differences in reward-related ventral striatum reactivity associated with DA related polymorphisms (e.g., Forbes et al., 2009).
Future imaging genetics studies using adolescent samples will be useful in examining individual differences in incentive motivation and cognitive control. For now, existing functional magnetic imaging studies provide an insight into the effects of individual differences in incentive processing and cognitive control on risk-taking. In a striking study, Casey et al. (2011) examined self-regulation during an emotional Go/No-Go task in adults assessed on delay of gratification in childhood and in their twenties and thirties. Participants were grouped into low and high delayers depending on their ability to delay gratification as children and young adults. No significant differences in cognitive control ability emerged between low and high delayers during a ‘cold’ Go/No-Go task. During an emotional, ‘hot’ version of the task, however, low delayers performed more poorly than high delayers when the task demanded response suppression to a happy face. This finding was specific to happy faces and was not found when suppressing responses to neutral or fearful faces. During an imaging component using the ‘hot’ version of the task, low delayers exhibited exaggerated recruitment of the ventral striatum during No-Go trials involving happy faces. These findings highlight relatively stable individual differences in the ability to self-regulate in ‘hot’ contexts. They also illustrate the ability for self-regulatory control to falter across the lifespan. Thus, what is unique to the adolescent period is not the faltering of self-regulation during ‘hot’ tasks but the increased demands placed on a still maturing cognitive control system due to a normative increase in incentive motivation (Luciana & Collins, 2012).
Other studies in adults have also demonstrated individual differences in reward sensitivity, indexed by mesolimbic DA activity, and its relevance to real-world decision-making, including consummatory behaviors following exposure to food cues (e.g., Lawrence et al., 2012). These individual differences are beginning to be explored in the adolescent literature. Bjork et al. (2011) demonstrated correlations between individual differences in reactivity of motivational neurocircuitry and psychosocial and behavioral problem symptoms in adolescents demonstrating not only the relevance of such processes to real-world risk-taking (see Newcomb & McGee, 1991 for longitudinal data exploring the role between sensation seeking and deviant behavior, including the use of licit and illicit drugs) but also the need to consider individual differences in motivational circuitry activity during this period of normatively heightened activity. Demos et al. (2012), in a sample of first year female college students, examined individual differences in nucleus accumbens activity to appetitive cues. Individual differences in reward-related brain activity to food and sexual images predicted subsequent weight gain and reported sexual activity at 6 months follow-up. The relationship between reward responsivity and behavior was unique to the different sets of stimuli and behaviors, e.g., reactivity to food images predicted food-related behavior and not sex-related behavior and vice versa. Such findings illustrate individual differences in reward reactivity and its predictive value. In a study examining the neural correlates of risk-taking, there was a significant association between nucleus accumbens activity and the likelihood of engaging in risky behavior in the near future among adolescents and adults (Galvan et al., 2007). Individual differences in anticipated consequences were related to accumbens activity with individuals anticipating positive consequences of risky behavior exhibiting increased activation among adolescents and adults. Among children and adolescents only, individuals anticipating negative consequences of engaging in risky behavior activated the region less. These individual differences in anticipated consequences and their association with activity in motivational neurocircuitry may render some adolescents more vulnerable to engaging in risky behaviors than others.
These studies demonstrate the relevance of individual differences in incentive motivation to real-world decision-making. They also highlight the need to consider the nature of the stimuli and the beliefs adolescents hold about the stimuli. Researchers have written about the need to consider the ‘ingredients’ of a situation that result in heightened incentive motivation (e.g., Bjork et al., 2010; Gladwin et al., 2011). In the context of smoking, much more work needs to be undertaken to determine what sets of beliefs and contexts result in an individual adolescent experiencing the impulse to smoke.
To begin this task, our model draws on a rich literature on smoking expectancies that represents an area in which the neurobiological and psychosocial literatures may be fruitfully bridged to offer a more smoking-specific account of adolescent risk-taking and insight into individual differences in smoking behavior during this period. Beliefs about these short- and long-term consequences of smoking are predictive of future smoking behaviors. Beliefs about the stress-reducing and relaxing consequences of smoking, for example, significantly predict the smoking stages of adolescents, with experimental smokers holding more positive beliefs about smoking than non-smokers and regular smokers holding more positive beliefs than both experimental and non-smokers (Wang et al., 1996). While the majority of studies examining the link between smoking expectancies and smoking behaviors have employed cross-sectional designs, there are a number of longitudinal studies that have been integral in demonstrating that health beliefs, including the perceptions of risk, including risk of heart attack and getting lung cancer, as well as perceptions of benefits, such as looking grown-up and becoming popular, predict smoking initiation among adolescents (Krosnick et al., 2006; Rodriguez et al., 2007; Song et al., 2009).
We suggest that positive smoking expectancies facilitate approach motivation within adolescents encountering cigarettes and may be a necessary component to explain smoking initiation from the perspective of neurobiological models focusing on incentive processing and cognitive control. Examining mesolimbic reactivity to smoking cues in adolescents at high- and low-risk for cigarette use, indexed by beliefs and anticipated consequences of smoking, across various contexts (e.g., peers present and peers absent) will be an important step for future research and will also be a way to move towards a smoking-specific model of adolescent risk-taking. Furthermore, a consideration of how such smoking expectancies develop will be crucial for model advancement and for prevention efforts, as this will highlighting factors that encourage the accrual of positive smoking expectancies.
The development of positive smoking expectancies likely involves biological, psychosocial, and ecological factors, necessitating a greater focus on the contexts of adolescent development and efforts to embed the existing neurobiological models of adolescent risk-taking within a broader, developmental framework that considers multiple systems within and without persons (see Magnusson & Cairns, 1996). For instance, while it has not yet been explicitly linked with adolescent brain development, the acquired preparedness model (Smith & Anderson, 2001) predicts that impulsivity acts as a risk-factor for substance abuse by facilitating the formation of more positive pre-initiation expectancies. This model has been supported by a prospective study of smoking initiation among college students (Doran et al., 2013) with impulsivity-related personality traits influencing risk of smoking initiation through the mediating factor of smoking expectancies (see Combs et al., 2012 for further support for the model in a younger sample). It may be fruitful to extend existing neurobiological models of adolescent risk-taking by incorporating findings from the acquired preparedness model given the normative increase in impulsivity during adolescents due to the unique configuration of fronto-striatal circuitry. Drawing on this model, it may be expected that individuals acquire and hold more positive smoking expectancies during adolescence than other developmental periods. The tendency to acquire positive smoking expectancies is likely be heightened in adolescents with the greatest mesolimbic reactivity to incentives.
The acquisition of positive smoking expectancies is also likely be influenced by the contexts in which adolescents are developing. Health behaviors have been demonstrated to spread within social networks (Christakis & Fowler, 2007). Socialization, by substance using peers, that encourages tolerance of substance use behaviors is thought to be an important factor in the spread of adolescent smoking (see Simons-Morton & Farhat, 2010 for review) and has been studied at the classroom level (Ennett et al., 2006). Indeed, prevention programs demonstrating effects on smoking behaviors have incorporated school-as-community components to discourage smoking partly by encouraging the development of more conservative attitudes towards smoking (see Sussman et al., 1997).
Research suggests that the focus on school contexts in the literature is warranted (Alexander et al., 2001). Other contexts that will be important to consider to determine the factors associated with the development of positive smoking expectancies include the family (Gilman et al., 2009; Vuolo & Staff, 2013) and, indeed, the community context adolescents are embedded within as a whole, given that communities differ in the levels of risk factors related to substance abuse present (Hawkins et al., 2004)
The contextual factor most specific to smoking initiation is the availability of cigarettes themselves. Low adolescent smoking prevalence is associated with the existence of smoking bans within schools (Piontek et al., 2008) and communities with high cigarette costs (Lovato et al., 2010) and low tobacco outlet density (Lipperman-Kreda et al., 2012). In the context of the model presented thus far, availability would affect adolescents’ decisions to smoke by affecting smoking expectancies, with higher frequencies of observing others smoke leading to greater perceptions that smoking is socially acceptable (Alesci et al., 2003). Low cigarette availability would also reduce the opportunities for offers to smoke cigarettes to be presented to adolescents – a factor that predicts smoking onset among middle-school nonsmokers (Ary & Biglan, 1988) and a reason given by adolescents for beginning to smoke (Sarason et al., 1992) – and, thus, limit the amount of situations in which adolescents are at risk to make the decision to smoke due to their susceptibility to act on impulse in the situations most associated with smoking initiation (e.g., among peers).
We make a final note in this section to suggest that this extended model may have particularly valuable explanatory power for smoking initiation among a subset of adolescents – those undergoing puberty early. Early puberty onset is associated with a younger age of smoking initiation (Wilson et al., 1994). Pubertal hormones are thought to influence incentive processing (for review, see Crone & Dahl, 2012) and thus, those undergoing puberty may experience heightened incentive motivation in the context of cognitive control processes that are in a particularly early stage of development. Furthermore, early-maturing girls tend to gravitate toward deviant peers (Westling et al., 2009), thus leading to earlier and greater opportunities to be exposed to pro-smoking norms and cigarette offers. A more thorough review of the role of puberty is beyond the scope of this paper but interested readers are directed to an excellent discussion about the complexities of brain-behavior interactions during puberty (see Peper & Dahl, 2013).
4. Adolescent brain development and steps towards a smoking-specific model of adolescent smoking initiation: Summary and implications for smoking prevention
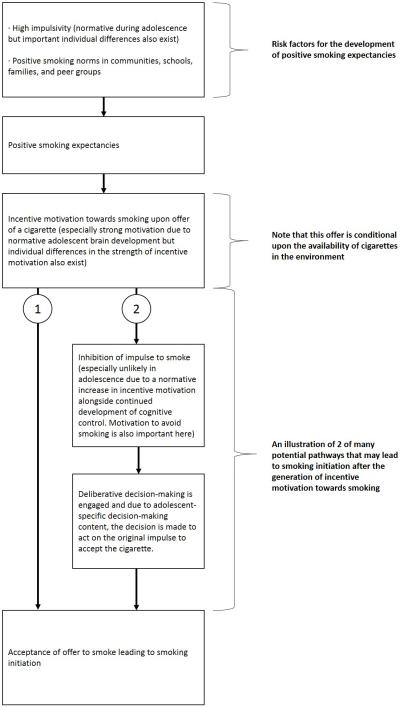
To summarize our model so far, due to normative developmental processes, including a heightened reactivity to novel and potentially rewarding stimuli, protracted maturation of cognitive control, as well as reduced future orientation, and heightened sensitivity to peers, adolescents are more likely than other developmental age groups to initiate cigarette smoking. Individual differences in neurobiology that exacerbate the imbalance between incentive processing and cognitive control processes in affect-laden contexts will render some adolescents more likely to take part in risky behaviors than others. Psychosocial factors specific to smoking, such as smoking expectancies, likely impact smoking-specific risk-taking behavior by facilitating dopaminergically-mediated impulses to smoke a cigarette when the anticipated consequences of smoking are positive. Such expectancies may also impact more deliberative decisions to smoke cigarettes once the initial impulse to smoke has been inhibited as positive smoking expectancies would likely increase the perceived value of smoking. Positive expectancies develop as a result of heightened sensitivity to rewards during this period but there are also particular contexts, including contexts in which cigarettes are readily available and in which positive social norms regarding smoking exist, that will encourage beliefs about smoking that render adolescents more likely to initiate smoking through their effects on smoking expectancies but also by creating situations in which adolescents may experience multiple offers to smoke (see fig. 1 for a schematic illustration of this model).
Figure 1. A schematic illustration of the processes leading to smoking initiation according to our model.
The normative increase in impulsivity during adolescence and contexts containing positive smoking norms act as risk factors for the development of positive smoking expectancies (section 3.6). Positive smoking expectancies facilitate the generation of incentive motivation towards smoking when adolescents encounter a cigarette. Encountering a cigarette is more likely in contexts in which there is a high availability of cigarettes. Furthermore, the incentive motivation experienced by adolescents is stronger than that experienced by other developmental groups due to normative brain development (section 3.4), although there are also important individual differences (section 3.6). At this point, there are many potential pathways that may lead an adolescent to act on the impulse to smoke the cigarette encountered. We present these paths to demonstrate that processes beyond the inhibition of impulsive responses are important to consider in models of adolescent risk-taking (see section 3.5). Two potential pathways are illustrated in this figure. In the first pathway (1), adolescents simply act on the initial impulse and smoke the cigarette. In the second pathway (2), the initial impulse is inhibited and deliberative decision-making is engaged. The inhibition of the initial impulse may be more difficult during adolescence relative to other age groups due to the continued maturation of cognitive control and the heightened incentive motivation occurring during this period. Furthermore, adolescents may lack the motivation to inhibit the initial impulse to smoke. Once deliberative decision-making is engaged, adolescent-specific content of the decision-making process (see section 3.5), leads the adolescent to act in line with their original impulse and to accept the cigarette.
In line with our model’s emphasis on psychosocial factors such as smoking expectancies and peer influence, interventions that have shown some effectiveness at reducing the prevalence of adolescent smoking have targeted smoking beliefs and information deficits, as well as the development of skills to resist social influences that encourage smoking (e.g., Botvin & Griffin, 2004; Flay, 2009; Sussman et al., 2002). However, there is little evidence for the long-term effectiveness of the majority of these programs (for review see Wiehe et al., 2005). Preventive interventions will benefit from the inclusion of findings from neurobiology in their designs. While interventions may make up an information deficit (Botvin et al., 1984; Rohrbach et al., 2010), it is unclear if this information will be drawn upon in the “hot” contexts in which smoking behavior often occurs. Future research examining the effects of interventions on incentive motivation when exposed to cigarette cues in the contexts of peers will be of great benefit. The rehearsal of learned techniques under “hot” conditions may render the skills learned during interventions more effective during the conditions under which cigarette initiation takes place. This may partially account for the increased effectiveness of interventions involving highly interactive intervention components (Black et al., 1998; Tobler & Stratton, 1997); however, this is a question for future research.
While the main focus of the adolescent neurobiological literature has focused on the relation between incentive processing and risk-taking, there is some evidence that the heightened reward sensitivity during this period can be an asset if motivation is directed towards positive behaviors. One area of positive behaviors that has received attention in recent years is that of prosocial behaviors. The mesolimbic reward system is engaged by charitable donations, in a manner similar to its engagement during the receipt of monetary reward (Harbaugh et al., 2007; Moll et al., 2006). In a study involving Latino and White youth, Latino participants, relative to White youth, showed more activity in the mesolimbic reward system when contributing money to their family than when gaining cash for themselves during a family assistance task (Telzer et al., 2010). These individual differences in reactivity to prosocial behaviors have been shown to relate to longitudinal declines in risk taking. Adolescents showing the greatest differences in ventral striatum activity to family contributions relative to personal reward receipt exhibited declines in risk taking over time (Telzer et al., 2013). While the mechanisms underlying such findings require further investigation, one hypothesis that warrants further investigation is the idea that youth who experience more reward as a result of prosocial behaviors experience less reward in the context of negative behaviors, including substance use and risk-taking. This line of research would benefit from integration with research examining the ability to divert sensation seeking tendencies away from detrimental activities, such as smoking, towards non-risky forms of sensation seeking (for review, see Roberti, 2004).
5. The initial smoking experience
In the previous sections of this paper we documented normative brain development and the role of this development in potentially rendering adolescents more vulnerable for smoking initiation. In this section, we review the literature of reward processes in adult and adolescent smokers, beginning with the initial smoking experience. Examining the initial smoking experience, including any developmental differences, is important in determining whether adolescents represent an at-risk population for compulsive cigarette use, as initial reinforcement consequences set the stage for subsequent cigarette use.
The initial smoking experience can include both positive and negative experiences. Positive qualities accompanying the experience include a sense of relaxation and a rush or ‘buzz’, while sensations such as coughing, nausea, and light-headedness are typically perceived as negative sensations (Friedman et al, 1985; Hirschman et al., 1984). Individuals experiencing an initial pleasant smoking experience are more likely to repeat drug use. Similarly, a lack of sensitivity to the aversive properties of smoking would encourage repeated use. Further, a decreased sensitivity to the use-limiting, aversive effects of drugs may allow individuals to tolerate higher levels of a drug and since oftentimes high levels of drug consumption are required for the establishment of addiction, such individuals would be at increased risk for developing addiction (Schramm-Sapyta et al., 2006).
In line with this model, an increasing body of research suggests that a pleasurable initial experience is a risk factor for continued smoking. In both longitudinal and retrospective studies, individuals who reported pleasurable initial experiences were more likely to continue smoking (Di Franza et al., 2007; Pomerleau et al., 1998). The experience of pleasure during the initial smoking experience, even when coupled with negative subjective effects, has been reported to be associated with rapid progression to regular smoking (Sartor et al., 2010) suggesting that pleasurable subjective reactions may play a larger role than aversive reactions in determining the progression to regular smoking. Di Franza et al. (2007) underline the importance of considering pleasurable initial experiences in future smoking behavior. Out of 45 factors studied – including a range of personality and environmental factors – relaxation associated with the initial experience was the strongest predictor of dependence and loss of autonomy over tobacco.
In preclinical work, early adolescent rats exhibit enhanced sensitivity to nicotine reward, compared to late adolescent and adult rats, in conditioned place preference (Brielmaier et al., 2007; Shram & Le, 2010; Torres et al., 2008) and oral self-administration procedures (Adriani et al., 2002). Animal studies have also indicated a reduced sensitivity to nicotine’s aversive effects in adolescents compared to adults (Shram et al., 2006; Wilmouth & Spear, 2004). Thus, adolescents seem to exhibit both increased sensitivity to the rewarding effects of nicotine and a reduced sensitivity to the aversive effects of nicotine. Research also suggests that the experience associated with nicotine may be modulated by the social context. Thiel et al. (2009) observed enhancement of conditioned place preference in adolescent male rats when nicotine and social interaction were paired with the testing chamber. Considering the social nature of adolescent risk-taking, adolescents may be more likely than adults to have a positive initial smoking experience, rendering them more at-risk for continued use.
The sensitivity model (Pomerleau, 1995) suggests that certain individuals are especially sensitive to nicotine. Buchmann et al. (2011) observed both developmental and individual differences in sensitivity to nicotine’s rewarding effects. Individuals who smoked their first cigarette at an earlier age had a more pleasant experience and this increased the probability of recurrent use. Furthermore, individuals who reported a pleasant initial smoking experience were more likely to smoke again and to be regular smokers in young adulthood.
Animal research suggests a genetic basis for the differences in initial sensitivity (Garg, 1969; Marks, Romm, et al., 1989) and that this genetic influence may function through neurotransmitter pathways (Marks, Stitzel, et al., 1989a Miner & Collins, 1989). Indeed, nicotine administration increases extracellular DA in the nucleus accumbens (Pontieri et al., 1996) and human studies provide preliminary evidence for a dopaminergically-mediated mechanism with genetic variants thought to influence DA receptor function observed to be associated with initial sensitivity to nicotine (Brody et al., 2006; Perkins et al., 2008).
6. From first cigarette to compulsive use
As well as being more likely to have a pleasurable initial smoking experience than other age groups, adolescent-onset smokers are also more susceptible to developing nicotine addiction than adult-onset smokers. Adolescents report symptoms of nicotine dependence within days to weeks of the onset of occasional smoking (DiFranza et al., 2000) and for many adolescents the time-course from smoking initiation to nicotine dependence is rapid (Dierker et al., 2012). There is also evidence of higher rates of daily cigarette consumption with early versus late smoking onset (Chen & Millar, 1998; Everett et al., 1999). Animal studies provide an insight into the rate of progression to nicotine dependence related to adolescent- or adult-onset of nicotine use that is difficult to establish in human populations. Studies examining the acquisition of nicotine self-administration in adult and adolescent rats demonstrate a faster rate of acquired nicotine self-administration among adolescent than adult rats (Chen et al., 2007), as well as higher rates of self-administration in adolescents than adults (Levin et al., 2003). Furthermore, receptor-binding assays following the completion of self-administration demonstrated differences in α4β2 nicotinic receptor binding across adolescent- and adult-onset conditions. Greater receptor binding was observed in the midbrain and striatum of adolescent-onset rats than adult-onset rats (Levin et al., 2007).
The motivational influences encouraging continued cigarette smoking are complex (Baker et al., 2004). Several accounts of drug addiction highlight drug-induced transformations to incentive processing and cognitive control systems (e.g., Bechara, 2005; Koob & Le Moal, 1997; Robinson & Berridge, 1993). Drawing on these theories, our model focuses on the interactions between the incentive processing and cognitive control systems, discussed in the normative development section (section 3), to examine aspects of adolescent nicotine dependence. In line with aspects of Bechara’s (2005) decision-making and impulse control theory of drug addiction, we argue that with continued nicotine use, alterations to the reward and cognitive control systems result in alterations to the decision-making process. With repeated use, cigarettes and their cues gain increased salience, resulting in activity in the mesolimbic system that drives approach behavior towards smoking behavior (Robinson & Berridge, 1993). Due to the continuing maturation of the PFC and its vulnerability to damage by nicotine (Goriounova & Mansvelder, 2012), the ability to exert top-down control over incentive motivation processes towards nicotine becomes compromised. Abstinent-related effects on incentive processing and cognitive control, which promote relapse, also contribute to continued drug use (see Eissenberg, 2004 for a discussion of negative reinforcement theories of cigarette use). Specific to adolescent-onset smoking, persistent effects of nicotine on these developing systems contribute to relapse to nicotine use after lengthy periods of abstinence.
Our model does not focus explicitly on withdrawal. For completeness, however, we briefly review findings relating to adolescent withdrawal. Relative to the adult experience of withdrawal, adolescents experience mild withdrawal symptoms (Smith et al., 2008a). Withdrawal symptoms also seem to be less relevant to promoting relapse during quit attempts in adolescent relative to adult smokers (Smith et al., 2008b). The diminished effects of withdrawal during adolescence relative to adulthood have also been observed in non-human studies, with adolescent rats and mice displaying fewer physical signs of withdrawal relative to adult animals (Kota et al., 2007; O’Dell et al., 2006). Such findings suggest other processes may be more important in motivating cigarette-use during this period of development (see O’Dell, 2009 for review) to which we now turn.
The incentive-salience theory of drug addiction proposes a primary role for drug-induced changes to the mesocorticolimbic system in the pathological motivation to consume drugs that defines addiction (Berridge & Robinson, 1998; Robinson & Berridge, 2008). The mesocorticolimbic system becomes hypersensitive towards drug-associated stimuli in a way that results in the attribution of incentive salience to these stimuli. Incentive salience results in the biasing of attentional processes towards drug-associated stimuli and, when perceived, drugs engender an incentive motivation - termed ‘wanting’ - for drugs.
In line with the incentive-salience theory, young adult and adult smokers demonstrate attentional and approach biases towards smoking cues (Bradley et al., 2004; Mogg et al., 2005; Munafo et al., 2003). Imaging studies are also providing evidence for abnormal incentive processing in adult smokers. Buhler et al. (2010) examined mesocorticolimbic activity to stimuli predicting monetary or cigarette rewards in nicotine-dependent and non-dependent smokers. Behaviorally, occasional, non-dependent smokers spent more effort to obtain money while dependent smokers demonstrated equivalent instrumental response rates for both money and cigarettes. Imaging data revealed higher reactivity of the mesocorticolimbic system to stimuli predicting monetary reward relative to cigarette reward in occasional smokers. In dependent smokers, anticipatory mesocorticolimbic brain activity did not differ for stimuli predicting monetary and cigarette reward suggesting either increased incentive salience of drug rewards or decreased incentive salience of nondrug rewards. While the literature on cue reactivity is much less extensive in adolescents than in adults, research is revealing similar responses to smoking stimuli in adolescent smokers (Lee et al., 2005; Rubinstein et al., 2011).
Not only do we see increased salience attributed to smoking cues but the reactivity to non-drug cues is also affected. Opponent-process theories of nicotine dependence emphasize drug-induced alterations in reward-threshold (Koob & LeMoal, 1997). During satiation, the drug-induced alterations in reward processing are masked due to nicotine’s continued ability to cause increases in DA transmission in areas of the brain associated with reward (Volkow et al., 2004). However, during abstinence, the drug-induced alterations in reward-processes may be ‘unmasked’. In line with this, Martin-Solch et al. (2001) examined reward processing in smokers and nonsmokers during a pattern-recognition task with response feedback using positron emission tomography. During feedback conditions in which feedback indicated monetary reward, DA regions including the striatum were activated in nonsmokers but not in smokers. These findings suggest that daily smokers react to non-drug rewards differently to non-smokers. Little research in this area has focused on these processes in adolescence. Peters et al. (2011) examined neural responses to reward anticipation in 14-year old adolescent smokers and a matched comparison group. During the anticipation stage of a modified monetary incentive delay task, adolescent smokers showed smaller neural responses in the ventral striatum and midbrain compared to matched comparison subjects. Similar hypo-responsivity was observed in a subset of smokers with mild smoking habits and, in the entire sample, fMRI activity in left striatal regions demonstrated negative correlations with smoking frequency. This pattern suggests a hypo-responsivity to non-drug reward anticipation preceding chronic nicotine use that is exacerbated by chronic drug use. An alternative interpretation is that individuals with the most robust hypo-active response are more likely to become chronic smokers. No group differences were observed during the outcome phase of the task.
The above studies did not take recency of smoking into account, giving an insight into the incentive processing of smokers in general but not into effects that may be related to abstinence and smoking satiety. There is evidence for an abstinence-induced anhedonic state in adult smokers across a range of paradigms. Epping-Jordan et al. (1998) observed decreases in brain reward function in adult rats during withdrawal from chronic nicotine administration. Less interference from appetitive words on a modified Stroop task was observed in smokers after experiencing overnight abstinence compared to their performance during a satiated state in which they smoked just before the task (Dawkins et al., 2006; Powell et al., 2011), providing evidence for decreased salience of non-drug rewards. A reduction in anhedonia, indexed by increased attentional bias to words with appetitive significance, was observed after nicotine administration in smokers after overnight abstinence (Powell et al., 2004). Abstinent smokers reported expectations to derive less enjoyment from a range of events and activities compared to when they are satiated and this reported anhedonia remained significant when subjectively rated withdrawal symptoms were controlled (Dawkins et al., 2006; Powell et al., 2002). Compared to satiated smokers, positively valenced film clips elicited lower ratings of happiness in abstinent smokers, suggesting that stimuli that are motivationally salient for the general population may elicit reduced positive affective responses during abstinence in regular smokers (Dawkins et al., 2007). A recent study has provided evidence for dissociable effects of smoking abstinence using a reward paradigm involving monetary reward, smoking reward in the form of earning cigarette puffs following the scan, and no-reward conditions (Sweitzer et al., in press). Abstinence, in this study, was associated with heightened activation in reward-related regions, including bilateral caudate head, ventral striatum, and medial PFC, during the anticipation of smoking rewards. Conversely, during the anticipation of monetary rewards, these regions demonstrated attenuated activation.
In line with these findings, research also suggests that simply perceiving an opportunity to access cigarettes in the near future significantly influences reward-related processing in adult smokers. Namely, the salience and incentive value of cues associated with nicotine reward appear to be enhanced when cigarettes are perceived to be available. For instance, adult smokers who anticipate a chance to consume cigarettes soon report strong cravings (Carter & Tiffany, 2001; Droungas et al., 1995; Juliano & Brandon, 1998; Sayette et al., 2003) and exhibit greater activation of the medial orbitofrontal cortex (McBride et al., 2006; Wilson et al., 2005) during exposure to smoking cues than those who expect a significant delay before being able to access cigarettes. Emerging evidence suggests that cigarette availability has the opposite effect on non-drug incentives and rewards, which appear to be devalued when encountered in the presence of an imminent opportunity to consume drugs. Namely, Wilson et al. (2008) found that adult smokers who were told they would be able to consume a cigarette during the study exhibited attenuated responses to monetary gains in the striatum, relative to those who anticipated having to wait several hours before having the opportunity to smoke – a pattern that is broadly consistent with the results obtained by Sweitzer et al. (in press). It is likely that the motivational shifts induced by perceived cigarette availability contribute to the maintenance of cigarette use and relapse in adult smokers (Wilson et al., 2014; in press). To our knowledge, the extent to which cigarette availability (perhaps concomitant with exposure to peers) affects reward-related processing in adolescent smokers has not been examined and remains an important target for future research.
The role of anhedonia in smoking relapse in adults after a quit attempt has been demonstrated, with anhedonia having the strongest influence on smoking cessation failure relative to other dimensions of depressive symptoms (Leventhal et al., 2008). Smokers experiencing high levels of anhedonia also report a greater number of past failed quit attempts (Leventhal et al., 2009).
There is some evidence that adolescents experience a similar anhedonic state following smoking abstinence with adolescent light smokers demonstrating attenuated responses to pleasurable food images, relative to non-smokers, in areas including the insula and inferior frontal region (Rubinstein et al., 2011). Further research in this area will be crucial to determine the nature of anhedonia experienced by adolescent smokers, especially in relation to the effects of abstinence and incentive types. While there is little research on the experience of anhedonia during abstinence in adolescents, research on the ability of nicotine to enhance the reinforcing properties of accompanying stimuli (Caggiula et al., 2009; Donny et al., 2003), an effect observed in adolescent rats (Weaver et al., 2012), provides suggestive evidence for a potential mechanism through which anhedonia may affect adolescent smoking behavior during abstinence. During periods of abstinence, stimuli that are motivationally salient for non-smokers may not be as pleasurable for smokers. This resulting state of anhedonia may act to promote a return to nicotine use through negative reinforcement. This mechanism requires more research both in its role in relapse and its presence in adolescents.
In addition to effects on incentive processing, there is also evidence for effects of nicotine on cognitive control. In adults, correlations between smoking status and performance on working memory, inhibitory control, and attention tasks have been observed, with smokers demonstrating poorer performance than non-smokers (Spilich et al., 1992; Spinella, 2002; Ernst, et al., 2001). Current smokers, relative to non-smokers, exhibit less neural activity in cortical areas involved in cognitive control during tasks indexing inhibitory control (Nestor et al., 2011). Furthermore, significantly smaller grey matter and lower grey matter density are observed in frontal brain regions in smokers, including regions involved in cognitive control, suggestive of nicotine-induced structural damage (Gallinat et al., 2006; Brody et al., 2004).
Smokers commonly report cognitive deficits, such as difficulty concentrating, following a quit attempt (Hughes, 2007; Ward et al., 2001). A range of abstinence-related cognitive deficits, including deficits in working memory, attention, and inhibitory control, have also been reported in laboratory studies (Kozink et al., 2010, Mendrek et al., 2006; Myers et al., 2008). Such cognitive deficits may contribute to maladaptive decision-making favoring drug-use over continued abstinence during a quit attempt as a diminished ability to inhibit the impulse to smoke acts, synergistically, with an increased drive to smoke (Bechara, 2005; Jentsch & Taylor, 1999). Further, re-exposure to nicotine following a period of abstinence has been shown to reverse these cognitive deficits (Davis et al., 2005; Myers et al., 2008) suggesting that, in line with negative-reinforcement theories of drug addiction (Eissenberg, 2004), relapse may also occur as an attempt to ameliorate abstinence-induced cognitive deficits.
Deficits in cognitive control have also been observed in adolescent smokers. Jacobsen et al. (2005) observed working memory impairments in adolescent smokers relative to non-smokers. During smoking cessation, adolescents exhibited further disruption of working memory and verbal memory. Jacobsen et al. (2007) examined auditory and visual attention in male and adolescent smokers and nonsmokers with and without prenatal exposure to maternal smoking. Exposure to tobacco smoke during prenatal or adolescent development was associated with deficits in auditory and visual attention in females. This effect was most pronounced in female smokers with prenatal exposure. Tobacco smoke exposure both prenatally and during adolescence among males was associated with marked deficits in auditory attention. Imaging results revealed greater activation of brain regions supporting auditory attention in adolescents in subjects with prenatal or adolescent tobacco exposure relative to those with no history of exposure, suggesting less efficient recruitment of these brain regions. Other neuroimaging data also demonstrate activity differences between smokers and non-smokers in brain regions involved in cognitive control (e.g., Musso et al., 2007). Further Imaging data will be useful to further examine these deficits.
The findings on incentive processing and deficits in cognitive control in smokers are also relevant considering the ability of incentives to enhance cognitive control. Contingency management approaches, for example, attempt to modify drug-related behavior by manipulating the relevant environmental contingencies associated with cigarette smoking (see Stanger & Budney, 2010 for a review of the use of contingency management interventions for adolescent substance use). The availability of a desired, non-drug reward may enhance the cognitive control required to resist the impulse to relapse into smoking. Contingency management approaches targeting adolescent smoking have shown some promise, demonstrating success in promoting abstinence (e.g., Reynolds et al., 2008; Krishnan-Sarin et al., 2006).
However, the experience of anhedonia during abstinence may undermine efforts to use non-drug incentives to promote continued abstinence. Indeed, youths with disorders affecting incentive processing, including bipolar disorder, anxiety, and depression, do not show the same incentive-modulated performance increases on cognitive control tasks evidenced in controls (Jazbec et al., 2005; Mueller et al., 2010). One of the few studies examining this effect in adolescents with substance use disorders (SUD) observed increased performance on an anti-saccade task during rewarded, relative to neutral, trials (Chung et al., 2011). This effect was not significant in the control group. This may suggest a hypersensitivity to reward in the SUD group. Alternatively, the control group may have been performing at effortful levels during neutral trials and there was less room for improvement in the rewarded condition (i.e., ceiling effects). At the neural level, SUD adolescents exhibited less activation, relative to controls, in brain regions known to support correct anti-saccade performance during the response preparation period during non-rewarded trials. Conversely, on rewarded trials SUD adolescents exhibited greater activation of these areas compared to controls. Thus, this study suggests that incentives can enhance response inhibition among SUD youth. However, this study focused on adolescents with poly-drug use and so the findings cannot necessarily be extended to nicotine use only. Furthermore, while adolescents were asked to abstain from alcohol and illicit substance use for at least 24 hours prior to imaging, abstinence was not biochemically verified. This area would greatly benefit from similar studies involving abstinent and satiated cigarette smokers. It would also benefit from the use of intensive, longitudinal, ecological momentary assessment studies to gain a greater insight into the trajectory of the abstinent-induced anhedonia and of incentive effects on cognitive control. Finally, a greater consideration of a range of potential non-drug incentives and their value to adolescent smokers is recommended.
7. From initial cigarette to compulsive use: model summary and recommendations for future research
Adolescents experience a more pleasurable initial smoking experience than adults, rendering them more likely to continue smoking beyond the first cigarette. Cigarette addicted adults experience impulses to approach cigarettes in the context of drug-induced impairments in cognitive control that may undermine smoking cessation (Bechara, 2005). This mechanism may be particularly strong during periods of smoking abstinence in which smokers demonstrate an anhedonic-like response to non-drug rewards while smoking-related cues gain increased incentive salience. While there is less extensive evidence for these mechanisms in adolescents, the above review suggests that similar mechanisms occur during this period. With adolescents, however, these mechanisms are occurring during a period of development in which normative brain maturation renders them especially vulnerable to self-regulatory failures due to robust approach motivation in the context of still-maturing cognitive control systems. Furthermore, by reviewing evidence for the neurotoxic effects of nicotine on adolescent brain development (section 8), we suggest another factor that renders adolescence particularly vulnerable to nicotine use.
Markedly absent from our model is the notion, common in models of adult addiction, that addiction to cigarettes and other drugs involves the transition from voluntary, goal-directed behavior to behavior that is automatic (Tiffany, 1990) or habitual (Everitt et al., 2001; Robbins & Everitt, 1999) in nature. In contrast to goal-directed actions, habits are rigid, stimulus bound, insensitive to outcome devaluation, and difficult to inhibit (Balleine & Dickinson, 1998; Everitt et al., 2001; Graybiel, 2008). Neurobiologically, a shift in the striatal mechanisms that exert primary influence over behavior (i.e., from ventral to dorsal striatal control over drug-seeking) is thought to play a critical role in the progression from goal-directed to habitual drug use (Everitt et al., 2001; Everitt & Robbins, 2013). As, the research on addiction as a form of habit-based learning (both human and animal) has, to our knowledge, focused on adults, it remains unclear whether the early symptoms of nicotine dependence exhibited by adolescent smokers reflect such striatal dynamics. There is, however, evidence that the striatum responds differently to reward-related information in general in adolescents compared to adults is emerging (e.g., Sturman & Moghaddam, 2012). Accordingly, research directly examining the potential role for habit-learning in the development of nicotine dependence among adolescents – and whether there are meaningful differences in habit-learning in the context of smoking among adolescents versus adults – would be valuable and inform the present model.
8. The persistent effects of adolescent smoking
One of the main characteristics of drug dependence is relapse to drug-taking behavior following periods of abstinence (Hyman & Malenka, 2001; O’Brien, 2003). Relapse rates among smokers attempting to quit are high. One study observed successful abstinent rates of only 3-5% in smokers not receiving treatment 6-12 months following smoking cessation (Hughes et al., 2004). Among smokers receiving treatment while attempting to quit, abstinence rates were also low (Alpert et al., 2013; Hajek et al., 2013). The majority of smoking cessation efforts are unsuccessful within the first week of abstinence (Hughes et al., 2004). However, a substantial number of smokers attempting to quit relapse after months and even years of abstinence (Herd et al., 2009; Segan et al., 2006; Wetter et al., 2004) suggesting persistent effects of smoking, beyond acute withdrawal, undermine smoking cessation efforts.
Relatively few human studies have examined the persistent effects of cigarette smoking, after smoking cessation, beyond acute withdrawal. This is most likely due to the high relapse rates in the first few days following smoking cessation (Hughes et al., 2004). However, researchers have argued for the consideration of the processes involved in late relapse in order to improve smoking cessation interventions (Piasecki et al., 2002). Studies involving both smokers and ex-smokers have examined the persistent effects of smoking during prolonged periods of abstinence. There is evidence for persistent changes in affect (Gilbert et al., 2002; Glassman et al., 2001), cognition (Deary et al., 2003; Munafo et al., 2005), responses to nicotine (Perkins, 2002), and neural activity (Nestor et al., 2011) that may undermine smoking cessation efforts.
These studies were not designed to dissociate the effects of smoking during adolescence from smoking during adulthood. Conducting such studies is difficult as the majority of smokers begin daily smoking during adolescence (Chen & Miller, 1998) and early smoking onset is associated with a decreased likelihood of quitting (Breslau & Peterson, 1996). Thus, recruiting young adult ex-smokers who began smoking during adolescence, and adult ex-smokers who began smoking in post-adolescence, remains a difficult task. It is also difficult to resolve issues of causality in the human studies on persistent effects that are available. For example, it is unclear whether the differences reported between smokers and ex-smokers that have aided ex-smokers in remaining abstinent are due to the amelioration of smoking-induced changes following cessation, or if the differences represent underlying dissimilarities that increased the likelihood of successful smoking abstinence. Longitudinal studies involving young adult ex-smokers who began smoking during adolescence and adult ex-smokers who began smoking post-adolescence would be an asset to this area.
Animal studies have been useful in examining the persistent effects of adolescent nicotine exposure. Strengths associated with this approach include the ability to control for confounding factors and to limit nicotine exposure to the developmental periods of interest. Using a variety of paradigms, research in this area has provided evidence for persistent effects on affect, cognition, drug-related behavior, and neurobiology following nicotine exposure. Furthermore, there is preliminary evidence that the observed effects are more pronounced following adolescent nicotine exposure than adult exposure. This suggests that adolescence represents a sensitive developmental period characterized by increased vulnerability to long-term changes induced by nicotine. This evidence is examined below.
Animal studies provide evidence for a protracted abstinence behavioral profile characterized by increased anxiety (Slawecki et al., 2005; Slawecki et al., 2003; Smith et al., 2006; Trauth et al., 2000), depressed mood (Iniguez et al., 2009), and anhedonia (Ribeiro-Carvalho et al., 2011). The effects of adolescent nicotine exposure persist beyond abstinence periods of 1 month for all three behavioral indices (Iniguez et al., 2009; Ribeiro-Carvalho et al., 2011; Smith et al., 2006). Studies including both adolescent-only and adult-only nicotine exposure groups provide preliminary evidence that the emergence of this profile is related to adolescent, but not adult-only, nicotine exposure (Iniguez et al., 2009; Smith et al., 2006; Ribeiro-Carvalho et al., 2011; Trauth et al., 2002).
Animal studies have also observed persistent effects of nicotine exposure during adolescence on future responses to drugs after extended abstinence including tolerance and sensitization to the effects of nicotine (Bracken et al., 2011; Brielmaier et al., 2007) and non-nicotine drugs such as cocaine (Hutchison & Riley, 2008; Kelley & Rowan, 2004) and amphetamine (Collins et al., 2004; Santos et al., 2009). Increased nicotine self-administration in adult rats exposed to nicotine during adolescence (Adriani et al., 2003; Levin et al., 2003) has also been observed. The increased vulnerability of adolescents to these effects is suggested by studies in which the persistent effects were more robust following exposure to nicotine during adolescence but not during adult-only exposure (Adriani et al., 2003; Bracken et al., 2011).
Few animal studies have explored the cognitive performance of animals following adolescent nicotine exposure. Observed long-term cognitive effects of adolescent nicotine exposure include deficits in serial pattern learning (Fountain et al., 2008), increased impulsivity and deficits in visuospatial attention (Counotte et al., 2009), and deficits in spatial- and recognition-memory (Mateos et al., 2011). The shortest nicotine abstinence period used in these studies was approximately 1 month, suggesting that the effects are persistent. Research in this area is relatively recent and the extent to which the reported deficits are age-dependent requires further examination. However, one study that included an adult comparison group observed the cognitive deficits due to nicotine exposure to be specific to adolescent exposure (Counotte et al., 2009) providing preliminary evidence for adolescence as a unique period of nicotine vulnerability.
The neuro-teratogenic status of nicotine has been well established (Levin & Slotkin, 1998; Slotkin, 1998). Recently, research has turned to the effects of nicotine on the adolescent brain and has found that nicotine can have neurotoxic effects on the developing adolescent brain. Persistent cellular damage has been observed in the midbrain, hippocampus, and cerebral cortex following adolescent nicotine exposure (Abreu-Villaca et al., 2003; Trauth, et al., 1999; Trauth et al., 2000) illustrating the sensitivity of the adolescent brain to nicotine neurotoxicity. Beyond indices of structural damage, studies have also observed persistent changes in the expression of nicotinic acetylcholine receptors (Trauth et al., 1999) and in acetylcholine synaptic functioning (Slotkin et al., 2007). Studies have also observed persistent effects in systems associated with cholinergic systems including enduring changes in noradrenergic and DA functioning (Counotte et al., 2009; Trauth et al., 2001) and serotonergic functioning (Slotkin et al., 2007; Xu et al., 2002).
The relationship between the persistent behavioral and neurobiological effects associated with adolescent nicotine exposure is still unclear. However, researchers have begun to examine the neurochemical alterations that may underlie the protracted abstinence behavioral profile observed in animals. Impaired serotonin synaptic function may underlie the enduring affective effects observed during protracted abstinence (Slotkin et al., 2007; Xu et al., 2002). Deficits in the expression of acetylcholine receptors are thought to be implicated in the increased sensitivity to nicotine in adulthood following adolescent nicotine exposure (Adriani et al., 2003) and reduced acetylcholine synaptic activity proceeding from adolescent nicotine exposure has been associated with the adult response to nicotine (Slotkin et al., 2008). Finally, long-lasting cognitive deficits resulting from adolescent nicotine exposure seem to be associated with enhanced release of DA in the medial PFC (Counotte et al., 2009).
In studies including both adolescent- and adult-exposure groups, persistent cellular damage observed after adolescent nicotine exposure was not observed after nicotine exposure in adults (Abreu-Villaca et al., 2003). The changes involving acetylcholine receptor expression after nicotine exposure have been found to be much less persistent following adult exposure compared to adolescent exposure (Adriani et al., 2003, Slotkin et al., 2008; Trauth et al., 1999). A line of research examining the combined effects of prenatal and adolescent nicotine exposure has suggested that double exposure may increase vulnerability to the persistent effects of nicotine (Slotkin et al., 2007).
9. Summary of the persistent effects of nicotine
Evidence suggests that adolescent nicotine exposure results in persistent changes in affect, cognition, drug-related behavior, and neurobiology. The persistent changes result in a protracted abstinence syndrome characterized by negative affect, cognitive-deficits, and increased reactivity towards nicotine and other drugs that may undermine smoking cessation. Many of these changes are not found in nicotine exposure limited to adulthood suggesting that nicotine exposure during adolescence may result in an increased probability of smoking continuation and relapse compared with adult nicotine exposure, even after long periods of abstinence. In terms of interventions, these findings of persistent effects highlight the need for preventive efforts and for interventions that delay the onset of smoking.
10. Conclusion
The unique configuration of the adolescent brain renders adolescents susceptible to impulsive decision-making in contexts in which cigarette smoking uptake is likely to occur (i.e., among peers). Psychosocial and contextual factors, including attitudes towards smoking, future orientation, and the availability of cigarettes, also contribute to the adolescent decision-making process, in a way that makes this phase of development a vulnerable period for the uptake of cigarette smoking. Upon smoking, adolescents may be more likely to continue smoking beyond the first cigarette due to the greater likelihood of a pleasant smoking experience during this developmental period. Acute and persistent effects of nicotine on the developing brain, some of which may be specific to adolescent-onset cigarette smoking, render it difficult for adolescents to quit smoking as they experience cognitive deficits and changes to incentive processing that bias decision-making in favor of continued cigarette use. While the unique configuration of the adolescent brain may act as a risk factor for the development of smoking behaviors, the adolescent-specific increase in incentive motivation may also provide opportunities for interventions to discourage smoking behavior.
References
- Aarts E, van Holstein M, Cools R. Striatal dopamine and the interface between motivation and cognition. Front. Psychol. 2011;2:1–11. doi: 10.3389/fpsyg.2011.00163. doi: 10.3389/fpsyg.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–49. doi: 10.1038/sj.npp.1300221. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet VD, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J. Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. doi: 0270-6474/03/234712-05$15.00/0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–24. doi: 10.1016/S0893-133X(02)00295-6. doi: 10.1016/S0893-13X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Albert D, Chein J, Steinberg L. The teenage brain: peer influences on adolescent decision making. Curr. Dir. Psychol. Sci. 2013;22:114–120. doi: 10.1177/0963721412471347. doi: 10.1177/0963721412471347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alesci NL, Forster JL, Blaine T. Smoking visibility, perceived acceptability, and frequency in various locations among youth and adults. Prev. Med. 2003;36:272–81. doi: 10.1016/s0091-7435(02)00029-4. doi: 10.1016/S0091-7435(02)00029-4. [DOI] [PubMed] [Google Scholar]
- Alexander C, Piazza M, Mekos D, Valente T. Peers, schools, and adolescent cigarette smoking. J. Adolesc. Health. 2001;29:22–30. doi: 10.1016/s1054-139x(01)00210-5. doi: 10.1016/S1054-139X(01)00210-5. [DOI] [PubMed] [Google Scholar]
- Alpert HR, Connolly GN, Biener L. A prospective cohort study challenging the effectiveness of population-based medical intervention for smoking cessation. Tob. Control. 2013;22:32–37. doi: 10.1136/tobaccocontrol-2011-050129. doi: 10.1136/tobaccocontrol-2011-050129. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . Cancer Facts and Figures 2010. American Cancer Society; Atlanta: 2010. [Google Scholar]
- Amstadter AB, MacPherson L, Wang F, Banducci AN, Reynolds EK, Potenza MN, Gelernter J, Lejuez CW. The relationship between risk-taking propensity and the COMT Val158Met polymorphism among early adolescents as a function of sex. J. Psychiatr. Res. 2012;46:940–945. doi: 10.1016/j.jpsychires.2012.04.010. doi: 10.1016/j.jpsychires.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev. Rev. 1992;12:339–373. doi: 10.1016/0273-2297(92)90013-R. [Google Scholar]
- Arnsten AF. Stress signaling pathways that impair prefrontal cortex structure and function. Nature Rev. Neurosci. 2009;1:410–422. doi: 10.1038/nrn2648. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary DV, Biglan A. Longitudinal changes in adolescent cigarette smoking behavior: onset and cessation. J. Behav. Med. 1988;11:361–382. doi: 10.1007/BF00844936. doi: 10.1007/BF00844936. [DOI] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu. Rev. Psychol. 2004;55:463–91. doi: 10.1146/annurev.psych.55.090902.142054. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4-5):407–419. doi: 10.1016/s0028-3908(98)00033-1. doi: 10.1016/S0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Bancej C, O’Loughlin J, Platt RW, Paradis G, Gervais A. Smoking cessation attempts among adolescent smokers: a systematic review of prevalence studies. Tob. Control. 2007;16:e8. doi: 10.1136/tc.2006.018853. doi: 10.1136/tc.2006.018853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision-making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Berndt TJ. Developmental changes in conformity to peers and parents. Dev. Psychol. 1979;15:608–616. doi: 10.1037/0012-1649.15.6.608. [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol. Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. doi: 10.1016/S0278-262(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH, Jones LL. Executive functions after age 5: changes and correlates. Dev. Rev. 2009;29:180–200. doi: 10.1016/j.dr.2009.05.002. doi: 10.1016/j.dr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyth-Marom R, Austin L, Fischhoff B, Palmgren C, Jacobs-Quadrel M. Perceived consequences of risky behaviors: adults and adolescents. Dev. Psychol. 1993;29:549–563. doi: 10.1037/0012-1649.29.3.549. [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Psychosocial problems and recruitment of incentive neurocircuitry by potential rewards: exploring individual differences in health adolescents. Dev. Cogn. Neurosci. 2011;1:570–577. doi: 10.1016/j.dcn.2011.07.005. doi: 10.1016/j.dcn.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: Comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennet SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DR, Tobler NS, Sciacca JP. Peer helping/involvement: an efficacious way to meet the challenge of reducing alcohol, tobacco, and other drug use among youth? J. Sch. Health. 1998;68:87–93. doi: 10.1111/j.1746-1561.1998.tb03488.x. doi: 10.111/j.1746-1561.1998.tb03488.x. [DOI] [PubMed] [Google Scholar]
- Botvin GJ, Griffin Life skills training: empirical findings and future directions. J. Primary Prevent. 2004;25:211–232. doi: 10.1023/B:JOPP.0000042391.58573.5b. [Google Scholar]
- Botvin GJ, Baker E, Renick NL, Filazzola AD, Botvin EM. A cognitive-behavioral approach to substance abuse prevention. Addict. Behav. 1984;9:137–147. doi: 10.1016/0306-4603(84)90051-0. doi: 10.1016/0306-4603(84)90051-0. [DOI] [PubMed] [Google Scholar]
- Boyer TW. The development of risk taking: a multi-perspective review. Dev. Rev. 2006;26:291–345. doi: 10.1016/j.dr.2006.05.002. [Google Scholar]
- Bracken AL, Chambers RA, Berg SA, Rodd SA, McBride WJ. Nicotine exposure during adolescence enhances behavioral sensitivity to nicotine during adulthood in Wistar rats. Pharmacol. Biochem. Behav. 2011;99:87–93. doi: 10.1016/j.pbb.2011.04.008. doi: 10.1016/j.pbb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behav. Pharmacol. 2004;15:29–36. doi: 10.1097/00008877-200402000-00004. doi: 10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am. J. Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. doi: 10.2105/AJPH.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol. Teratol. 2007;29:74–80. doi: 10.1016/j.ntt.2006.09.023. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, et al. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch. Gen. Psychiatry. 2006;63:808–816. doi: 10.1001/archpsyc.63.7.808. doi: 10.1001/archpsych.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol. Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. doi: 10.1016/S0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Brown BB, Clasen DR, Eicher SA. Perceptions of peer pressure, peer conformity dispositions, and self-reported behavior among adolescents. Dev. Psychol. 1986;22:521–530. doi: 10.1037/0012-1649.22.4.521. [Google Scholar]
- Buchmann AF, Blomeyer D, Jennen-Steinmetz C, Schmidt MH, Esser G, Banaschewski T, Laucht M. Early smoking onset may promise initial pleasurable sensations and later addiction. Addict. Biol. 2011;18:947–954. doi: 10.1111/j.1369-1600.2011.00377.x. doi: 10.111/j.1369-1600.2011.00377.x. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Buhler M, Vollstadt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Buchel C, Smolka MN. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol. Psychiatry. 2010;67:745–752. doi: 10.1016/j.biopsych.2009.10.029. doi: 10.1016/j.biopsych.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components form follows function. J. Pers. Soc. Psychol. 1999;76:839–855. doi: 10.1037/0022-3514.76.5.839. [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr. Sym. on Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp. Clin. Psychopharm. 2001;9:183–90. doi: 10.1037//1064-1297.9.2.183. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Caudle KC. The teenage brain: self control. Curr. Dir. Psychol. Sci. 2013;22:82–87. doi: 10.1177/0963721413480170. doi: 10.1177/0963721413480170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ. The teenage brain: an overview. Curr. Dir. Psychol. Sci. 2013;22:80–81. doi: 10.1177/0963721413480170. doi: 10.1177/0963721413486971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, et al. Behavioral and neural correlates of delay of gratification 40 years later. Proc. Natl. Acad. Sci. U.S.A. 2011;108:14998–15003. doi: 10.1073/pnas.1108561108. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, et al. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Dev. Psychol. 2010;46:193–207. doi: 10.1037/a0016128. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adoelscent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci. 2010;14:F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. doi: 10.111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004;75:807–21. doi: 10.1086/425589. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am. J. Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. doi: 10.2105/AJPH.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–48. [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–709. doi: 10.1038/sj.npp.1301135. doi:10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N. Engl. J. Med. 2007;357:370–9. doi: 10.1056/NEJMsa066082. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- Chung T, Geier C, Luna B, Pajtek S, Terwilliger R, Thatcher D, Clark DB. Enhancing response inhibition by incentive: comparison of adolescent with and without substance use disorder. Drug Alcohol Depen. 2011;115:43–50. doi: 10.1016/j.drugalcdep.2010.10.017. doi: 10.1016/j.drugalcdep.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen DR, Brown BB. The multidimensionality of peer pressure in adolescence. J. Youth Adolescence. 1985;14:451–468. doi: 10.1007/BF02139520. doi: 10.1007/BF02139520. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in preadolescent male but not female or adult male rats. Dev. Brain Res. 2004;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Combs JL, Spillane NS, Caudill L, Stark B, Smith GT. The acquired preparedness risk model applied to smoking in 5th grade children. Addict. Behav. 2012;37:331–334. doi: 10.1016/j.addbeh.2011.11.005. doi: 10.1016/j.addbeh.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer ANM, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Rev. Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crone EA, Vendel I, van der Molen MW. Decision-making in disinhibited adolescents and adults: insensitivity to future consequences or driven by immediate reward? Pers. Indiv. Differ. 2003;35:1625–1641. doi: 10.1016/S0191-8869(02)00386-0. [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J. Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addict. Behav. 2007;32:425–431. doi: 10.1016/j.addbeh.2006.05.010. doi: 10.1016/j.addbeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I – effects on incentive motivation. Psychopharmacology. 2006;189:355–367. doi: 10.1007/s00213-006-0588-8. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Pattie A, Taylor MD, Whiteman MC, Starr JM, Whalley LJ. Smoking and cognitive change from age 11 to age 80. J. Neurol. Neurosurg. Psychiatry. 2003;74:1006–1007. doi: 10.1136/jnnp.74.7.1006. doi: 10.1136/jnnp.74.7.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorme K, Bell MR, Sisk CL. The teenage brain: social reorientation and the adolescent brain - the role of gonadal hormones in the male Syrian hamster. Curr. Dir. Psychol. Sci. 2013;22:128–133. doi: 10.1177/0963721413479607. doi: 10.1177/0963721413479607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme DE, Kreshel PJ, Reid LN. Lighting up: young adults’ autobiographical accounts of their first smoking experiences. Youth Soc. 2003;34:468–496. doi: 10.1177/0044118X03034004004. [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L, Swendsen J, Rose J, He J, Merikangas K. Transitions to regular smoking and nicotine dependence in the adolescent national comorbidity survey (NCS-A) Ann. Beh. Med. 2012;43:394–401. doi: 10.1007/s12160-011-9330-9. doi: 10.1007/s12160-011-9330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Franza JR, Savageau JA, Fletcher K, Pbert L, O’Loughlin J, McNeill AD, et al. Susceptibility to nicotine dependence: the development and assessment of nicotine dependence in Youth 2 Study. Pediatrics. 2007;120:e974–e983. doi: 10.1542/peds.2007-0027. doi: 10.1542/peds.2007-0027. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, Coleman M. Initial symptoms of nicotine dependence in adolescents. Tob. Control. 2000;9:313–319. doi: 10.1136/tc.9.3.313. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Doran N, Khoddam R, Sanders PE, Schweizer CA, Trim RS, Myers MG. A prospective study of the acquired preparedness model: the effects of impulsivity and expectancies on smoking initiation in college students. Psychol. Addict. Behav. 2013;27:714–722. doi: 10.1037/a0028988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, et al. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol. Beh. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Dreher J-C, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc. Natl. Acad. Sci. U.S.A. 2009;106:617–622. doi: 10.1073/pnas.0805517106. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict. Behav. 1995;20:657–73. doi: 10.1016/0306-4603(95)00029-c. doi: 10.1016/0306-4603(95)00029-C. [DOI] [PubMed] [Google Scholar]
- Duka T, Lupp A. The effects of incentive on antisaccades: is a dopaminergic mechanism involved. Behav. Pharmacol. 1997;8:373–382. doi: 10.1097/00008877-199710000-00001. [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, et al. Youth risk behavior surveillance - United States, 2011. MMWR Surveill. Summ. 2012;61:1–162. [PubMed] [Google Scholar]
- Eissenberg T. Measuring the emergence of tobacco dependence: the contribution of negative reinforcement models. Addiction. 2004;99:5–29. doi: 10.1111/j.1360-0443.2004.00735.x. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Elkind D. Egocentrism in adolescence. Child Dev. 1967;38:1025–1034. doi: 10.2307/1127100. [PubMed] [Google Scholar]
- Ennett ST, Bauman KE, Hussong A, Faris R, Foshee VA, Cai L, DuRant RH. The peer context of adolescent substance use: findings from social network analysis. J. Res. Adolescence. 2006;16:159–186. doi: 10.1111/j.1532-7795.2006.00127.x. [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–9. doi: 10.1038/30001. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol. Biochem. Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in response to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Everett SA, Warren CW, Sharp D, Kann L, Husten CG, Crossett LS. Initiation of cigarette smoking and subsequent smoking behavior among US high school students. Prev. Med. 1999;29:327–333. doi: 10.1006/pmed.1999.0560. doi: 10.1006/pmed.1999.0560. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. doi: 10.1016/S0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J. Neurosci. 2007;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. J. Exp. Psychol. Learn. Mem. Cog. 2009;35:709–730. doi: 10.1037/a0014983. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Fischhoff B, Parker AM, de Brun WB, Downs J, Palmgren C, Dawes R, Manski CF. Teen expectations for significant life events. Public Opin. Quart. 2000;64:189–205. doi: 10.1086/317762. doi: 10.1086/317762. [DOI] [PubMed] [Google Scholar]
- Flay BR. School-based smoking prevention programs with the promise of long-term effects. Tob. Induc. Dis. 2009;5:1–18. doi: 10.1186/1617-9625-5-6. doi: 10.1186/1617-9625-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol. Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Kelley BM, Willey AR, Nolley E. Adolescent exposure to nicotine impairs adult serial pattern learning in rats. Exp. Brain Res. 2008;187:651–656. doi: 10.1007/s00221-008-1346-4. doi: 10.1007/s00221-008-1346-4. [DOI] [PubMed] [Google Scholar]
- Frangou S, Chitins X, Williams SC. Mapping IQ and gray matter density in healthy young people. NeuroImage. 2004;23:800–5. doi: 10.1016/j.neuroimage.2004.05.027. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Friedman LS, Lichtenstein E, Biglan A. Smoking onset among teens: an empirical analysis of initial situations. Addict. Behav. 1985;10:1–13. doi: 10.1016/0306-4603(85)90048-6. doi: 10.1016/0306-4603(85)90048-6. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Klaus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur. J. Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. doi: 10.111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Galvan A. The teenage brain: sensitivity to rewards. Curr. Dir. Psychol. Sci. 2013;22:88–93. doi: 10.1177/0963721413476512. doi: 10.1177/0963721413480859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front. in Hum. Neurosci. 2010;4:1–9. doi: 10.3389/neuro.09.006.2010. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev. Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risk decision making in adolescence and adulthood: an experimental study. Dev. Psychol. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Garg M. Variation in effects of nicotine in four strains of rats. Psychopharmacologia. 1969;14:432–438. doi: 10.1007/BF00403584. doi: 10.1007/BF00403584. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Luna B. The maturation of incentive processing and cognitive control. Pharmacol. Biochem. Behav. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. J. Adolescent Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neurosci. 1999;2:861–863. doi: 10.1038/13158. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilbert AM, Fiez JA. Integrating rewards and cognition in the frontal cortex. Cogn. Affect. Behav. Neurosci. 2004;4:540–552. doi: 10.3758/cabn.4.4.540. doi: 10.3758.CABN.4.4.540. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McCernon FJ, Rabinovich NE, Plath LC, Masson CL, Anderson AE, Sly KF. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. J. Consult. Clin. Psychol. 2002;70:142–52. doi: 10.1037//0022-006x.70.1.142. doi: 10.1037//0022-006X.70.1.142. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Rende R, Boergers J, Abrams DB, Buka SL, Niaura RS. Parental smoking and adolescent smoking initiation: an intergenerational perspective on tobacco control. Pediatrics. 2009;123:e274–e281. doi: 10.1542/peds.2008-2251. doi: 10.1542/peds.2008-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin TE, Figner B, Crone EA, Wiers RW. Addiction, adolescence, and the integration of control and motivation. Dev. Cogn. Neurosci. 2011;4:364–376. doi: 10.1016/j.dcn.2011.06.008. doi: 10.1016/j.dcn.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357:1929–32. doi: 10.1016/S0140-6736(00)05064-9. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LV, Toga AW, Rapport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. Doi:10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Res. 1982;256:339–349. doi: 10.1016/0165-3806(82)90146-8. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD. Nicotine exposure during adolescence alters the rules for prefrontal cortical synaptic plasticity during adulthood. Front. Synaptic Neurosci. 2012;4:1–9. doi: 10.3389/fnsyn.2012.00003. doi: 10.3389/fnsyn.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hajek P, Stead LF, West R, Jarvis M, Hartmann-Boyce J, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst. Rev. 2013;1:CD003999. doi: 10.1002/14651858.CD003999.pub4. doi: 10.1002/14651858.CD003999.pub4. [DOI] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. J. Child Psychol. Psychiatry. 2007;48:446–454. doi: 10.1111/j.1469-7610.2006.01722.x. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol. Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Harsay HA, Buitenweg JIV, Wijnen JG, Guerreiro MJS, Ridderinkhof K. Remedial effects of motivational incentive on declining cognitive control in healthy aging and Parkinson’s disease. Front. Aging Neurosci. 2010;2:1–12. doi: 10.3389/fnagi.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Van Horn ML, Arthur MW. Community variation in risk and protective factors and substance use outcomes. Prev. Sci. 2004;5:213–220. doi: 10.1023/b:prev.0000045355.53137.45. doi: 10.1023/B:PREV.0000045355.53. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. doi: 10.3389/fnagi.2010.00144. [DOI] [PubMed] [Google Scholar]
- Herd N, Borland R, Hyland A. Predictors of smoking relapse by duration of abstinence: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2009;104:2088–2099. doi: 10.1111/j.1360-0443.2009.02732.x. doi: 10.1111/j.1360-0443.2009.02732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman RS, Leventhal H, Glynn K. The development of smoking behavior: conceptualization and supportive cross-sectional survey data. J. Appl. Soc. Psychol. 1984;14:184–206. doi: 10.1111/j.1559-1816.1984.tb02231.x. [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob. Res. 2007;9:315–327. doi: 10.1080/14622200701188919. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hutchison MA, Riley AL. Adolescent exposure to nicotine alters the aversive effects of cocaine in adult rats. Neurotoxicol. Teratol. 2008;30:404–11. doi: 10.1016/j.ntt.2008.04.004. doi: 10.1016/j.ntt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nature Rev. Neurosci. 2001;2:695–703. doi: 10.1038/35094560. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Iniguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolanos-Guzman CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeil T, Chan P, Forno LS, et al. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3:251–265. [PubMed] [Google Scholar]
- Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32:2453–2464. doi: 10.1038/sj.npp.1301398. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Menci WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol. Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Exp. Brain Res. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol. Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. doi: 10.1007/PL00005483. [DOI] [PubMed] [Google Scholar]
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. New Engl. J. Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- Johnson SB, Dariotis JK, Wang C. Adolescent risk-taking under stressed and non-stressed conditions: conservative, calculating and impulsive types. J. Adolescent Health. 2012;51:S34–S40. doi: 10.1016/j.jadohealth.2012.04.021. doi: 10.1016/jadohealth.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Abuse, 1975-2011. Volume I: Secondary School Students. The University of Michigan; Ann Arbor, MI: 2012. [Google Scholar]
- Juliana LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Exp. Clin. Psychopharm. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. doi: 10.1037/1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Rowan JD. Long-term, low-level adolescent nicotine exposure produces dose-dependent changes in cocaine sensitivity and reward in adult mice. Int. J. Dev. Neurosci. 2004;22:339–48. doi: 10.1016/j.ijdevneu.2004.04.002. doi: 10.1016/j.ijdevneu.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol. Behav. 2002;76:365–77. doi: 10.1016/s0031-9384(02)00751-5. doi: 10.1016/S0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Damaj MI, Chen X. Early smoking onset and risk for subsequent nicotine dependence: a monozygotic co-twin control study. Am. J. Psychiatry. 2013;170:408–13. doi: 10.1176/appi.ajp.2012.12030321. doi: 10.1176/appi.ajp.2012.12030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H, Sterk CE, Elifson KW. Initial smoking experiences and current smoking behaviors and perceptions among current smokers. J. Addict. 2013;2013:1–9. doi: 10.1155/2013/491797. doi: 10.1155/2013/491797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Fehr E. Resisting the power of temptations: the right prefrontal cortex and self-control. Ann. N. Y. Acad. Sci. 2007;1104:123–24. doi: 10.1196/annals.1390.004. doi: 10.1196/annals.1390.004. [DOI] [PubMed] [Google Scholar]
- Knoch D, Gianotti LRR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J. Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;242:715–723. doi: 10.1126/science.278.5335.52. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J. Pharmacol. Exp. Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Kozink RV, Kollins SH, McClernon FJ. Smoking withdrawal modulates right inferior frontal cortex but not presupplementary motor area activation during inhibitory control. Neuropsychopharmacology. 2010;35:2600–2606. doi: 10.1038/npp.2010.154. doi: 10.1038/npp.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, McFetridge A, Cavallo DA. Contingency management for smoking cessation in adolescent smokers. Exp. Clin. Psychopharm. 2006;14:306–310. doi: 10.1037/1064-1297.14.3.306. doi: 10.1037/1064-1297.14.3.306. [DOI] [PubMed] [Google Scholar]
- Krosnick JA, Chang L, Sherman SJ, Chassin L, Presson C. The effects of beliefs about the health consequences of cigarette smoking on smoking onset. J. Commun. 2006;56:S18–S37. doi: 10.1111/j.1460-2466.2006.00281.x. [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lantz PM. Smoking on the rise among young adults: implications for research and policy. Tob. Control. 2003;12:i60–i70. doi: 10.1136/tc.12.suppl_1.i60. doi: 10.1136/tc.12.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence N, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage. 2012;63:415–422. doi: 10.1016/j.neuroimage.2012.06.070. 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (fMRI) study of cue-induced smoking craving in virtual environments. Appl. Psychophys. Biof. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine Tob. Res. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine Tob. Res. 2008;10:507–17. doi: 10.1080/14622200801901971. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol. Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Schwartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slotkin TA. Developmental neurotoxicity of nicotine. In: Slikker W, Chang LW, editors. Handbook of Developmental Neurotoxicology. Academic Press; San Diego, CA: 1998. pp. 578–615. [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cogn. Affect. Behav. Neurosci. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. doi: 10.3758/CABN.8.1.99. [DOI] [PubMed] [Google Scholar]
- Lovato CY, Zeisser C, Campbell HS, Watts AW, Halpin P, Thompson M, Eyles J, Adlaf E, Brown KS. Adolescent smoking: effect of school and community characteristics. Am. J. Prev. Med. 2010;39:507–14. doi: 10.1016/j.amepre.2010.08.019. doi: 10.1016/j.amepre.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Libera CD, Chelazzi L. Visual selective attention and the effects of monetary rewards. Psychol. Sci. 2006;17:222–227. doi: 10.1111/j.1467-9280.2006.01689.x. doi: 10.1111/j.1467-9280.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Lipperman-Kreda S, Grube JW, Friend KB. Local tobacco policy and tobacco outlet density: associations with youth smoking. J. Adol. Health. 2012;50:547–552. doi: 10.1016/j.jadohealth.2011.08.015. doi: 10.1016/j.jadohealth.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb. Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF. Incentive motivation, cognitive control, and the adolescent brain: is it time for a paradigm shift? Child Dev. Perspect. 2012;6:392–399. doi: 10.1111/j.1750-8606.2012.00252.x. doi: 10.1111/j.1750-8606.2012.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–13. doi: 10.1016/j.bandc.2009.08.005. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Magnusson D, Cairns RB. Developmental science: towards a unified framework. In: Cairns RB, Elder GH, Costello EJ, editors. Developmental Science. Cambridge University Press; Cambridge, UK: 1996. pp. 7–30. [Google Scholar]
- Marks MJ, Romm E, Campbell SM, Collins AC. Variation of nicotinic binding sites among inbred strains. Pharmacol. Biochem. Behav. 1989;33:679–89. doi: 10.1016/0091-3057(89)90407-3. doi: 10.1016/0091-3057(89)90407-3. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J. Pharmacol. Exp. Ther. 1989;235:619–28. [PubMed] [Google Scholar]
- Martin-Solch C, Magyar S, Kunig G, Missimer J, Schultz W, Leenders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers: a positron emission tomography study. Exp. Brain Res. 2001;139:278–286. doi: 10.1007/s002210100751. doi: 10.1007/s002210100751. [DOI] [PubMed] [Google Scholar]
- Mata R, Hau R, Papassotiropoulos A, Hertwig R. DAT1 polymorphism is associated with risk taking in the Balloon Analogue Risk Task (BART) PLoS ONE. 2012;7:e39135. doi: 10.1371/journal.pone.0039135. doi: 10.1371/journal.pone.0039135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli MP, Viveros M-P. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB1 cannabinoid receptors. J. Psychopharmacol. 2011;25:1676–1690. doi: 10.1177/0269881110370503. doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–38. doi: 10.1038/sj.npp.1301075. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: Comparison to non-smokers and effects of abstinence. Addict. Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol. Rev. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. doi: 10.1037/0033-295X.106.1.3. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3’ UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am. J. Med. Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. doi: 10.1002/ajmg.10948. [DOI] [PubMed] [Google Scholar]
- Mills B, Reyna VF, Estrada S. Explaining contradictory relations between risk perception and risk taking. Psychological Science. 2008;19:429–433. doi: 10.1111/j.1467-9280.2008.02104.x. doi: 10.1111/j.1467-9280.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- Millstein SG, Halpern-Felsher BL. Judgements about risk and perceived invulnerability in adolescents and young adults. J. Res. Adolesc. 2002;12:399–422. doi: 10.1111/1532-7795.00039. [Google Scholar]
- Miner LL, Collins AC. Strain comparison of nicotine-induced seizure sensitivity and nicotinic receptors. Pharmacol. Biochem. Behav. 1989;33:469–475. doi: 10.1016/0091-3057(89)90532-7. doi: 10.1016/0091-3057(89)90532-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Field M, Bradley BP. Attentional and approach bias for smoking cues in smokers: an investigation of competing theoretical views of addiction. Psychopharmacology. 2005;180:333–341. doi: 10.1007/s00213-005-2158-x. doi: 10.1007/s00213-005-2158-x. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Ng P, Temple V, Hardin MG, Pine DS, Leibenluft E, Ernst M. Perturbed reward processing in pediatric bipolar disorder: an antisaccade study. J. Psychopharmacol. 2010;24:1779–1784. doi: 10.1177/0269881109353462. doi: 10.1177/0269881109353462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC, Mackintosh B. Association of serotonin transporter genotype with selective processing of smoking-related stimuli in current smokers and ex-smokers. Nicotine Tob. Res. 2005;7:773–778. doi: 10.1080/14622200500259861. doi: 10.1080/14622200500259861. [DOI] [PubMed] [Google Scholar]
- Munafo M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified Stroop task. J. Psychopharmacol. 2003;17:310–6. doi: 10.1177/02698811030173013. doi: 10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp. Brain Res. 1998;121:391–400. doi: 10.1007/s002210050473. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Musso F, Bettermann F, Vucurevic G, Stoeter P, Konrad A, Winterer G. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology. 2007;191:159–169. doi: 10.1007/s00213-006-0499-8. doi: 10.1007/s00213-006-0499-8. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social reorientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. doi: 10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. NeuroImage. 2011;56:2258–2275. doi: 10.1016/j.neuroimage.2011.03.054. doi: 10.1016/j.neuroimage.2011.03.054. [DOI] [PubMed] [Google Scholar]
- Newcomb MD, McGee L. Influence of sensation seeking on general deviance and specific problem behaviors from adolescence to young adulthood. J. Pers. Soc. Psychol. 1991;61:614–628. doi: 10.1037//0022-3514.61.4.614. doi: 10.1037/0022-3514.61.4.614. [DOI] [PubMed] [Google Scholar]
- Norman DA, Bobrow DG. On data-limited and resource-limited processes. Cogn. Psychol. 1975;7:44–64. doi: 10.1016/0010-0285(75)90004-3. [Google Scholar]
- Nurmi J. How do adolescents see the future? A review of the development of future orientation and planning. Dev. Rev. 1991;11:1–59. doi: 10.1016/0273-2297(91)90002-6. [Google Scholar]
- O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am. J. Psychiatry. 2003;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- O’Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 56:263–278. doi: 10.1016/j.neuropharm.2008.07.039. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, et al. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology. 2006;186:612–9. doi: 10.1007/s00213-006-0383-6. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Geier CF, Ordaz SJ, Teslovich T, Luna B. Developmental changes in brain function underlying the influences of reward processing on inhibitory control. Dev. Cogn. Neurosci. 2011;1:517–529. doi: 10.1016/j.dcn.2011.06.004. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Dahl RE. The teenage brain: surging hormones - brain-behavior interactions during puberty. Curr. Dir. Psychol. Sci. 2013;22:134–139. doi: 10.1177/0963721412473755. doi: 10.1177/0963721412473755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington S, Jetton C, Karelitz JL, Wilson A, et al. Gene and gene by sex associations with initial sensitivity to nicotine in nonsmokers. Behav. Pharmacol. 2008;19:630–40. doi: 10.1097/FBP.0b013e32830c3621. doi: 10.1097/FBP.0b013e32830c3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nicotine Tob. Res. 2002;4:405–422. doi: 10.1080/1462220021000018425. doi: 10.1080/1462220021000018425. [DOI] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, et al. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am. J. Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L. Dependence of adolescent novelty-seeking behavior on response phenotype and effects of apparatus scaling. Behav. Neurosci. 2008;122:861–875. doi: 10.1037/0735-7044.122.4.861. doi: 10.1037/0735-7044.122.4.861. [DOI] [PubMed] [Google Scholar]
- Piontek D, Buehler A, Rudolph U, Metz K, Kroeger C, Gradl S, Floeter S, Donath C. Social contexts in adolescent smoking: does school policy matter? Health Edu. Res. 2008;23:1029–1038. doi: 10.1093/her/cym063. doi: 10.1093/her/cym063. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn. Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–9. doi: 10.1046/j.1360-0443.1998.93459515.x. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav. Genet. 1995;25:161–77. doi: 10.1007/BF02196925. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol. Psychiatry. 2002;51:151–163. doi: 10.1016/s0006-3223(01)01208-2. doi: 10.1016/S0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Powell J, Tait S, Lessiter J. Cigarette smoking and attention to signals of reward and threat in the Stroop paradigm. Addiction. 2011;97:1163–1170. doi: 10.1046/j.1360-0443.2002.00117.x. doi: 10.1046/j.1360-0443.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict. Behav. 2004:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Reyna VF, Brainerd CJ. Dual processes in decision making and developmental neuroscience: a fuzzy-trace model. Dev. Rev. 2011;31:180–206. doi: 10.1016/j.dr.2011.07.004. doi: 10.1016/j.dr.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna VF, Estrada SM, DeMarinis JA, Myers RM, Stanisz JM, Mills BA. Neurobiological and memory models of risky decision making in adolescents versus young adults. J. Exp. Psychol. Learn. Mem. Cog. 2011;37:1125–1142. doi: 10.1037/a0023943. doi: 10.1037/a0023943. [DOI] [PubMed] [Google Scholar]
- Reyna VF, Farley F. Risk and rationality in adolescent decision making: implications for theory, practice, and public policy. Psychol. Sci. Publ. Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Dallery J, Shroff P, Patak M, Leraas K. A web-based contingency management program with adolescent smokers. J. Appl. Behav. Anal. 2008;41:597–601. doi: 10.1901/jaba.2008.41-597. doi: 10.1901/jaba.2008.41-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Carvalho A, Lima CS, Nunes-Freitas AL, Filgueiras CC, Manhaes AC, Abreu-Villaca Y. Exposure to nicotine and ethanol in adolescent mice: effects on depressive-like behavior during exposure and withdrawal. Beh. Brain Res. 2011;221:282–289. doi: 10.1016/j.bbr.2011.03.014. doi: 10.1016/j.bbr.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Riggs NR, Chou C-P, Li C, Pentz MA. Adolescent to emerging adulthood smoking trajectories: when do smoking trajectories diverge, and do they predict early adulthood nicotine dependence? Nicotine Tob. Res. 2007;9:1147–1154. doi: 10.1080/14622200701648359. doi: 10.1080/14622200701648359. [DOI] [PubMed] [Google Scholar]
- Rivers SE, Reyna VF, Mills BA. Risk taking under the influence: a fuzzy-trace theory of emotion in adolescence. Dev. Rev. 2008;28:107–144. doi: 10.1016/j.dr.2007.11.002. doi: 10.1016/j.dr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Roberti JW. A review of behavioral and biological correlates of sensation seeking. J. Res. Pers. 2004;38:256–279. doi: 10.1016/S0092-6566(03)00067-9. [Google Scholar]
- Robinson TE, Berridge KE. The neural basis of drug craving: an incentive sensitization theory of addiction. Brain Res. Rev. 1993;18:129–140. doi: 10.1016/0165-0173(93)90013-p. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Phil. Trans. R. Soc. B. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez D, Romer D, Audrain-McGovern JA. Beliefs about the risks of smoking mediate the relationship between exposure to smoking and smoking. Psychosomatic Medicine. 2007;69:106–113. doi: 10.1097/PSY.0b013e31802e0f0e. doi: 10.1097/PSY.0b013e31802e0f0e. [DOI] [PubMed] [Google Scholar]
- Romer D, Duckworth AL, Sznitman S, Park S. Can adolescents learn self-control? Delay of gratification in the development of control over risk-taking. Prev. Sci. 2010;11:319–330. doi: 10.1007/s11121-010-0171-8. doi: 10.1007/s11121-010-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach LA, Sun P, Sussman S. One-year follow-up evaluation of the Project Towards No Drug Abuse (TND) dissemination trial. Prev. Med. 2010;51:313–319. doi: 10.1016/j.ypmed.2010.07.016. doi: 10.1016/j.ypmed.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Luks TL, Dryden WY, Rait MA, Simpson GV. Adolescent smokers show decreased brain responses to pleasurable food images compared with nonsmokers. Nicotine Tob. Res. 2011;13:751–755. doi: 10.1093/ntr/ntr046. doi: 10.1093/ntr/ntr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GC, Marin MT, Cruz FC, DeLucia R, Planeta CS. Amphetamine- and nicotine-induced cross-sensitization in adolescent rats persist until adulthood. Addict. Biol. 2009;14:1369–1600. doi: 10.1111/j.1369-1600.2009.00153.x. doi: 10.1111/j.1369-1600.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Mankowski ES, Peterson AV, Dinh KT. Adolescents’ reasons for smoking. J. Sch. Health. 1992;62:185–190. doi: 10.1111/j.1746-1561.1992.tb06039.x. doi: 10.1111/j.1746-1561.1992.tb06039.x. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lessov-Schlaggar CN, Scherrer JF, Buckholz KK, Madden PA, Pergadia ML, Grant JD, Jacob T, Xian H. Initial response to cigarettes predicts rate of progression to regular smoking: findings from an offspring-of-twins design. Addict. Behav. 2010;35:771–8. doi: 10.1016/j.addbeh.2010.03.004. doi: 10.1016/j.addbeh.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Hobel J. Effects of smoking opportunity on cue-elicited urge: a facial coding analysis. Exp. Clin. Psychopharm. 2003;11:218–27. doi: 10.1037/1064-1297.11.3.218. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schlottman A. Children’s probability intuitions: understanding the expected value of complex gambles. Child Dev. 2001;72:103–122. doi: 10.1111/1467-8624.00268. doi: 10.1111/1467-8624.00268. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol. Biochem. Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb. Cortex. 2000;10:272–283. doi: 10.1093/cercor/10.3.272. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Scott ES, Reppucci ND, Woolard JL. Evaluating adolescent decision making in legal contexts. Law Human Beh. 1995;19:221–244. doi: 10.1007/BF01501658. [Google Scholar]
- Segan CJ, Borland R, Greenwood KM. Can transtheoretical model measures predict relapse from the action stage of change among ex-smokers who quit after calling a quitline? Addict. Behav. 2006;31:414–428. doi: 10.1016/j.addbeh.2005.05.023. doi: 10.1016/j.addbeh.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Beh. Brain Res. 2010;206:240–4. doi: 10.1016/j.bbr.2009.09.018. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186:201–08. doi: 10.1007/s00213-006-0373-8. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Simons-Morton B, Farhat T. Recent findings on peer group influences on adolescent substance use. J. Prim. Prev. 2012;31:191–208. doi: 10.1007/s10935-010-0220-x. doi: 10.1007/s10935-010-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell AK, Khoury AE, Mathe AA, Ehlers CL. Increased CRF-like and NPY-like immunoreactivity in adult rats exposed to nicotine during adolescence: relation to anxiety-like and depressive-like behavior. Neuropeptides. 2005;39:369–377. doi: 10.1016/j.npep.2005.06.002. doi: 10.1016/j.npep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacol. Biochem. Behav. 2003;75:355–361. doi: 10.1016/s0091-3057(03)00093-5. doi: 10.1016/S0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Mackillop EA, Bodwell BE, Seidler FJ. Adolescent nicotine administration changes the responses to nicotine given subsequently in adulthood: adenylyl cyclase cell signaling in brain regions during nicotine administration and withdrawal, and lasting effects. Brain Res. Bull. 2008;76:522–30. doi: 10.1016/j.brainresbull.2008.03.001. doi: 10.1016/j.brainresbull.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Seidler FJ. Separate or sequential exposure to nicotine prenatally and in adulthood: persistent effects on acetylcholine systems in rat brain regions. Brain Res. Bull. 2007;74:91–103. doi: 10.1016/j.brainresbull.2007.05.007. doi: 10.1016/j.brainresbull.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J. Pharmacol. Exp. Ther. 1998;285:931–45. [PubMed] [Google Scholar]
- Smith AE, Cavallo DA, Dahl T, Wu R, George TP, Krishnan-Sarin S. Effect of acute tobacco abstinence in adolescent smokers compared with nonsmokers. J. Adolescent Health. 2008a;43:46–54. doi: 10.1016/j.jadohealth.2007.12.004. doi: 10.1016/j.jadohealth.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Cavallo DA, McFetridge A, Liss T, Krishnan-Sarin S. Preliminary examination of tobacco withdrawal in adolescent smokers during smoking cessation treatment. Nicotine Tob. Res. 2008b;10:1253–1259. doi: 10.1080/14622200802219357. doi: 10.1080/14622200802219357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Anderson KG. Adolescent risk for alcohol problems as acquired preparedness: A model and suggestions for intervention. In: Monti PM, Colby SM, O’Leary TA, editors. Adolescents, Alcohol, and Substance Abuse: Reaching Teens Through Brief Interventions. 2001. pp. 109–144. [Google Scholar]
- Smith LN, McDonald CF, Bergstrom HC, Brielmaier JM, Eppolito AK, Wheeler TL, Falco AM, Smith RF. Long-term changes in fear conditioning and anxiety-like behavior following nicotine exposure in adult versus adolescent rats. Pharmacol. Biochem. Behav. 2006;85:91–7. doi: 10.1016/j.pbb.2006.07.014. doi: 10.1016/j.pbb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AV, Morrell HER, Cornell JL, Ramos ME, Biehl M, Kropp RY, Halpern-Felsher BL. Perceptions of smoking-related risks and benefits as predictors of adolescent smoking initiation. Am. J. Public Health. 2009;99:487–92. doi: 10.2105/AJPH.2008.137679. doi: 10.2105/AJPH.2008.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spilich GJ, June L, Renner J. Cigarette smoking and cognitive performance. Br. J. Addict. 1992;87:1313–1326. doi: 10.1111/j.1360-0443.1992.tb02740.x. doi: 10.1111/j.1360-0443.1992.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Spinella M. Correlations between orbitofrontal dysfunction and tobacco smoking. Addict. Biol. 2002;7:381–384. doi: 10.1080/1355621021000005964. doi: 10.1080/1355621021000005964. [DOI] [PubMed] [Google Scholar]
- Stanger C, Budney AJ. Contingency management approaches for adolescent substance use disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2010;19:547–562. doi: 10.1016/j.chc.2010.03.007. doi: 10.1016/j.chc.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes and why? Ann. N. Y. Acad. Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev. Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav. Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang NM, Chein JM, Steinberg L. The value of the dual systems model of adolescent risk-taking. Front. Hum. Neurosci. 2013;223:1–4. doi: 10.3389/fnhum.2013.00223. doi: 10.3389/fnhum.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. Striatum processes reward differently in adolescents versus adults. Proc Natl Acad Sci U S A. 2012;109:1719–1724. doi: 10.1073/pnas.1114137109. doi: 10.1073/pnas.114137109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, Dent CW, Stacy AW. Project towards no drug abuse: a review of the findings and future directions. Am. J. Health Behav. 2002;26:354–65. doi: 10.5993/ajhb.26.5.4. doi: 10.5993/AJHB.26.5.4. [DOI] [PubMed] [Google Scholar]
- Sussman S, Craig S, Simon TR, Galaif ER. School-as-community activity selection at continuation high schools. Subst. Use Misuse. 1997;32:113–131. doi: 10.3109/10826089709027302. doi: 10.3109/10826089709027302. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Joel DJ, McGurrin P, Denlinger R, Forbes E, Donny EC. Dissociated effects of anticipating smoking versus monetary reward in the caudate as a function of smoking abstinence. Biol. Psychiatry. doi: 10.1016/j.biopsych.2013.11.013. in press. doi: 10.1016/j.biopsych.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Exp. Brain Res. 2002;142:284–291. doi: 10.1007/s00221-001-0928-1. doi: 10.1007/s00221-001-0928-1. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Dev. Cogn. Neurosci. 2013;3:45–52. doi: 10.1016/j.dcn.2012.08.004. doi: 10.1016/j.dcn.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Gaining while giving: an fMRI study of the rewards of family assistance among White and Latino youth. Soc. Neurosci. 2010;5:508–518. doi: 10.1080/17470911003687913. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interactions between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol. Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tobler NS, Stratton HH. Effectiveness of school-based drug prevention programs: a meta-analysis of the research. J. Primary Prevent. 1997;18:71–128. doi: 10.1023/A:1024630205999. [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol. Biochem. Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Casey BJ. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Front. Psychol. 2011;2:1–9. doi: 10.3389/fpsyg.2011.00039. doi: 10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. doi: 10.1016/S0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidlier FJ, Slotkin TA. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res. 2000;880:167–172. doi: 10.1016/s0006-8993(00)02823-7. doi: 10.1016/S0006-8993(00)02823-7. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. doi: 10.1016/S0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Urosevic S, Collins P, Muetzel R, Lim K, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev. Psychol. 2012;48:1488–1500. doi: 10.1037/a0027502. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Preventing Tobacco Use Among Youth and Young Adults: A report of the Surgeon General. Department of Health and Human Services; Atlanta, GA: 2012. [Google Scholar]
- van Duijvenvoorde ACK, Jansen BRJ, Visser I, Huizenga HM. Affective and cognitive decision-making in adolescents. Dev. Neuropsychol. 2010;35:539–554. doi: 10.1080/87565641.2010.494749. doi: 10.1080/87565641.2010.494749. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Zanolie K, van Meel CS, Westernberg PM, Rombouts SARB, Crone EA. What motivations the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb. Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Westenberg PM, Crone EA. A developmental study of risky decisions on the Cake Gambling Task: Age and gender analyses of probability estimation and reward evaluation. Dev. Neuropsychol. 2008;33:179–196. doi: 10.1080/87565640701884287. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Van Zundert RM, Ferguson SG, Shiffman S, Engels R. Dynamic effects of craving and negative affect on adolescent smoking relapse. Health Psychol. 2012;31:226–234. doi: 10.1037/a0025204. doi: 10.1037/a0025204. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT 1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. doi: 10.1016/S0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:1–11. doi: 10.1186/1471-2156-6-55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J. Neurosci. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veling H, Aarts H. Cueing task goals and earning money: relatively high monetary rewards reduce failures to act on goals in a Stroop task. Motiv. Emotion. 2010;34:184–190. doi: 10.1007/s11031-010-9160-2. doi: 10.1007/s11031-010-9160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Vuolo M, Staff J. Parent and child cigarette use: a longitudinal, multigenerational study. Pediatrics. 2013;132:1–10. doi: 10.1542/peds.2013-0067. doi: 10.1542/peds.2013-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72:146–159. doi: 10.1016/j.bandc.2009.10.013. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WQ, Fitzhugh EC, Eddy JM, Westerfield RC. Attitudes and beliefs of adolescent experimental smokers: a smoking prevention perspective. J. Alcohol Drug Educ. 1996;41:1–12. [Google Scholar]
- Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addict. Behav. 2001;26:311–327. doi: 10.1016/s0306-4603(00)00107-6. doi: 10.1016/S0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sakagami M. Integration of cognitive and motivational cortex information in the primate prefrontal cortex. Cereb. Cortex. 2007;17:101–109. doi: 10.1093/cercor/bhm067. doi: 10.1093/cercor/bhm067. [DOI] [PubMed] [Google Scholar]
- Weaver MT, Geier CF, Levin ME, Caggiula AR, Sved AF, Donny EC. Adolescent exposure to nicotine results in reinforcement enhancement but does not affect adult responding in rats. Drug Alcohol Depen. 2012;125:307–312. doi: 10.1016/j.drugalcdep.2012.03.006. doi: 10.1016/j.drugalcdep.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Denburg NL. Trajectory of risky decision making for potential gains and losses from ages 5 to 85. J. Behav. Decis. Making. 2011;24:331–344. doi: 10.1002/bdm.690. [Google Scholar]
- Westling E, Andrews JA, Hampson SE, Peterson M. Pubertal timing and substance use: the effects of gender, parental monitoring and deviant peers. J. Adolesc. Health. 2009;42:555–563. doi: 10.1016/j.jadohealth.2007.11.002. doi: 10.1016/j.jadohealth.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Welsch SK, Smith SS, Fouladi RT, Fiore MC, Baker TB. Prevalence and predictors of transitions in smoking behavior among college students. Health Psychol. 2004;23:168–77. doi: 10.1037/0278-6133.23.2.168. doi: 10.1037/0278-6133.23.2.168. [DOI] [PubMed] [Google Scholar]
- Wiehe SE, Garrison MM, Christakis DA, Ebel BE, Rivara FP. A systematic review of school-based smoking prevention trials with long-term follow-up. J. Adolescent Health. 2005;36:162–169. doi: 10.1016/j.jadohealth.2004.12.003. doi: 10.1016/j.adohealth.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacol. Biochem. Behav. 2009;92:566–573. doi: 10.1016/j.pbb.2009.02.009. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann. N. Y. Acad. Sci. 2004;1021:462–4. doi: 10.1196/annals.1308.065. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Killen JD, Hayward C, Robinson TN, Hammer LD, Taylor CB. Timing and rate of sexual maturation and the onset of cigarette and alcohol use among teenage girls. Arc. Pediatr. Adolesc. Med. 1994;148:789–95. doi: 10.1001/archpedi.1994.02170080019004. doi: 10.1001/archpedi.1994.02170080019004. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Delgado MR, McKee SA, Grigson PS, MacLean RR, Nichols TT, Henry SL. Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cognitive, Affective & Behavioral Neuroscience. doi: 10.3758/s13415-014-0285-8. in press. doi: 10.3758/s13415-014-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Smyth JM, MacLean RR. Integrating ecological momentary assessment and functional brain imaging methods: new avenues for studying and treating tobacco dependence. Nicotine Tob. Res. 2014;16:S102–110. doi: 10.1093/ntr/ntt129. doi: 10.1093/ntr/ntt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA, Delgado MR. Effects of smoking opportunity on responses to monetary gain and loss in the caudate nucleus. J. Abnorm. Psychol. 2008;117:428–434. doi: 10.1037/0021-843X.117.2.428. doi: 10.1037/0021-843X.117.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob. Res. 2005;7:637–645. doi: 10.1080/14622200500185520. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–40. doi: 10.1016/s0896-6273(02)00965-0. doi: 10.1016/S0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Cousins MM, Slikker W, Slotkin TA. Adolescent nicotine administration alters serotonin receptors and cell signaling mediated through adenylyl cyclase. Brain Res. 2002;951:280–292. doi: 10.1016/s0006-8993(02)03174-8. doi: 10.1016/S0006-8993(02)03174-8. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. Cambridge University Press; New York, NY: 1994. [Google Scholar]