Abstract
Acylcarnitines, important lipid biomarkers reflective of acyl-CoA status, are metabolites that possess bioactive and inflammatory properties. This study examined the potential for long-chain acylcarnitines to activate cellular inflammatory, stress, and death pathways in a skeletal muscle model. Differentiated C2C12 myotubes treated with l-C14, C16, C18, and C18:1 carnitine displayed dose-dependent increases in IL-6 production with a concomitant rise in markers of cell permeability and death, which was not observed for shorter chain lengths. l-C16 carnitine, used as a representative long-chain acylcarnitine at initial extracellular concentrations ≥25 μM, increased IL-6 production 4.1-, 14.9-, and 31.4-fold over vehicle at 25, 50, and 100 μM. Additionally, l-C16 carnitine activated c-Jun NH2-terminal kinase, extracellular signal-regulated kinase, and p38 mitogen-activated protein kinase between 2.5- and 11-fold and induced cell injury and death within 6 h with modest activation of the apoptotic caspase-3 protein. l-C16 carnitine rapidly increased intracellular calcium, most clearly by 10 μM, implicating calcium as a potential mechanism for some activities of long-chain acylcarnitines. The intracellular calcium chelator BAPTA-AM blunted l-C16 carnitine-mediated IL-6 production by >65%. However, BAPTA-AM did not attenuate cell permeability and death responses, indicating that these outcomes are calcium independent. The 16-carbon zwitterionic compound amidosulfobetaine-16 qualitatively mimicked the l-C16 carnitine-associated cell stress outcomes, suggesting that the effects of high experimental concentrations of long-chain acylcarnitines are through membrane disruption. Herein, a model is proposed in which acylcarnitine cell membrane interactions take place along a spectrum of cellular concentrations encountered in physiological-to-pathophysiological conditions, thus regulating function of membrane-based systems and impacting cell biology.
Keywords: cell death, interleukin-6, skeletal muscle
plasma acylcarnitines have long been used as surrogate readouts reflecting tissue acyl-CoA pools (6). Thus, these indexes are used in newborn screening as diagnostic biomarkers of inherited disorders of metabolism involving enzymatic lesions in lipid and amino acid metabolism, in which there are increases or decreases in specific acyl-CoA tissue pools (7, 37). For example, long-chain fatty acid oxidation disorders (FAOD) are characterized by defects in mitochondrial oxidative enzymes, i.e., long-chain 3-hydroxyacyl-CoA dehydrogenase and carnitine palmitoyltransferase 2 (CPT2) deficiencies, and these lead to increased tissue, blood, and urine long-chain fatty acylcarnitines. Under certain FAOD conditions, plasma long-chain acylcarnitines can increase severalfold: l-C16 carnitine (C16 carnitine), for instance, has been reported to increase greater than five-fold, reaching levels between ∼5 and 44 μM in CPT2-deficient newborns (19, 23). Modest increases in tissue or blood long-chain acylcarnitines have also been noted following cardiac ischemia (17, 36, 38) and in type 2 diabetes/insulin resistance (1, 18, 20, 24, 26).
In addition to their use as biomarkers, there is recent evidence supporting the hypothesis that acylcarnitines have bioactivities. Our previous work has shown that long-chain fatty acylcarnitines activate proinflammatory signaling pathways in RAW 264.7 murine macrophages (1, 29) and in HCT-116 cells (29), and blunt the insulin signaling pathway in both murine C2C12 and human primary skeletal muscle cells (2). These activities were found to occur at initial extracellular concentrations of C14 carnitine and C16 carnitine as low as 5 μM. Thus, whether or not these metabolites contribute to, or exacerbate, disease phenotypes or normal physiological processes remains an open question. The mechanisms by which long-chain fatty acylcarnitines impinge upon inflammatory systems and insulin-associated cell signaling pathways in muscle have yet to be fully elucidated. Evidence to date suggests that, at least in macrophages, proinflammatory actions of acylcarnitines can occur in a MyD88-dependent manner (29), and MyD88 is considered an important adaptor protein that can serve as part of the membrane proximal signaling complexes, i.e., for many pattern recognition receptors (14). However, we found no evidence that specific Toll-like receptors or other pattern recognition receptors are driving the proinflammatory effects of C14- and C16-acylcarnitines (29).
In the course of studies examining the effect of long-chain fatty acylcarnitines on insulin resistance and inflammation, we noted changes in cell viability and function under conditions of high acylcarnitines (2, 29). In our previous work, it was observed that, at a higher concentration of C16 carnitine, release of interleukin-6 (IL-6) in myocytes (2) and adenylate kinase (AK, a cell permeability/death marker) in macrophages (29) was increased. Furthermore, in cardiac ischemia, heart muscle acylcarnitines accumulate (17, 36, 38) and long-chain acylcarnitines have been associated with increased cardiac cellular reactive oxygen species (ROS), apoptosis, and endoplasmic reticulum (ER) stress (32) and increases in intracellular calcium (39, 42). Considering that muscle is a site of robust acylcarnitine generation, we have begun to consider if long-chain acylcarnitines impact myocyte cell function. In the current cell culture experiments, it was hypothesized that the naturally occurring zwitterionic metabolites, long-chain acylcarnitines, can elicit cell stress and cell death responses in a model of skeletal muscle myotubes and that calcium-associated pathways play a role.
MATERIALS AND METHODS
Reagents.
Lipopolysaccharide (LPS) was purchased from List Biologicals (Campbell, CA). Lot-tested (lot no. K0109) premium-select fetal bovine serum (FBS) and horse serum were purchased from Atlanta Biologicals (Lawrenceville, GA) and Hyclone (Logan, UT), respectively. Dulbecco's Modified Eagle's Medium (DMEM), phenol red-free Hank's Balanced Salt Solution (HBSS), penicillin/streptomycin, sodium pyruvate, GlutaMax, and calcium green-1 AM, were all purchased from Life Technologies (Grand Island, NY). Ionomycin, BAPTA-AM, caspase-3 inhibitor II (Z-DEVD-FMK), and caspase inhibitor I (pan inhibitor, Z-VAD-FMK) were purchased from EMD Millipore (Billerica, MA), and cyclosporine A was purchased from Cell Signaling Technologies (Danvers, MA). Acyl-l-carnitines of varying chain lengths and l-carnitine were purchased from Advent Bio (Downers Grove, IL), amidosulfobetaine-16 (ASB-16) {3-[N,N-dimethyl(3-palmitoylaminopropyl)ammonio]-propanesulfonate} (catalog no. DG062, lot no. 092605) was purchased from G-Biosciences (St. Louis, MO), and Toxilight and IL-6 ELISA Assays were purchased from Lonza (Basel, Switzerland) and R&D Systems (Minneapolis, MN), respectively. 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide, disodium salt (XTT) sodium salt was purchased from Biotium (Hayward, CA) and phenazine methosulfate 98% (PMS) from Acros Organics (Geel, Belgium). Antibodies against phospho-p44/42 mitogen-activated protein kinase (MAPK) [extracellular signal-regulated kinase (ERK1/2)] (Thr202/Tyr204) (catalog no. 4370), total p44/42 MAPK (ERK) (catalog no. 9102), p-c-Jun NH2-terminal kinase (JNK) (catalog no. 4668), JNK (catalog no. 9252), p-p38 (catalog no. 4511), p38 (catalog no. 8690), lamin A/C (catalog no. 2032), and ER stress antibody sampler kit (catalog no. 9956) were all purchased from Cell Signaling Technology (Danvers, MA). The β-tubulin (Clone Tub 2.1, catalog no. ab11308) was purchased from Sigma-Aldrich (St. Louis, MO).
Cells and cell culture.
C2C12 (catalog no. CRL-1772 murine myoblast cell line) were purchased from ATCC (Manassas, VA). Cells were cultured in DMEM containing 10% FBS (premium select FBS, catalog no. S11595, lot no. K0109; Atlanta Biologicals, Lawrenceville, GA), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM GlutaMAX-I (Life Technologies, Grand Island, NY), and 100 μM l-carnitine (Advent Bio). Because penicillin and streptomycin were included in all treatment conditions, any potential effects on mitochondrial function or metabolism would not influence treatment-associated differences. C2C12 myoblasts were maintained at 37°C in a 5% CO2 atmosphere until >90% confluent at which time FBS was replaced with 10% horse serum (HyClone, Logan, UT) for 4–5 days to induce myotube differentiation.
Long-term acylcarnitine treatment.
Myotubes (4- to 5-day differentiated) were grown in 0.25% FBS starvation media for 2–4 h and then treated at various doses with different-chain-length acylcarnitines for 18 h, as indicated. Myotubes were pretreated for 1 h during starvation and overnight with sample treatment with BAPTA-AM, a calcium chelator, at the concentrations indicated. Conditioned media were harvested and frozen at −20°C and analyzed for IL-6. Media were further analyzed by Toxilight assay for presence of AK, a surrogate marker of cytotoxicity.
Short-term acylcarnitine treatment.
Fully differentiated myotubes were serum starved for 2–3 h in 0.25% FBS media and then treated with corresponding l-acylcarnitines at times and doses indicated. Plates were then placed on ice, media were removed and discarded, and cells were rinsed two times in ice-cold HBSS and lysed in 1× Cell Signaling Lysis Buffer + Pierce HALT phosphatase inhibitors (Rockford, IL). Sample supernatants were sonicated and subject to centrifugation at 12,000 g for 10 min at 4°C.
Immunoblotting.
Lysates were resolved via 4–12% Bis-Tris SDS-PAGE (Life Technologies) and transferred to polyvinyl difluoride membranes (Bio-Rad, Hercules, CA) using a Bio-Rad Trans-Blot Turbo. Membranes were blocked in a 1X PBS and 0.1% (vol/vol) Tween 20 (Fisher Scientific) (PBST) solution containing 2% wt/vol dry milk. Membranes were probed for 1 h at room temperature or overnight at 4°C with primary antibody in 1X PBST followed by incubation with horseradish peroxidase-conjugated secondary antibody (Southern Biotech, Birmingham, AL) at a 1:10,000 dilution in 1X PBST + 2% milk for 1 h at room temperature. Bands were visualized using Bio-Rad Clarity Western ECL reagent and imaged on a Bio-Rad ChemiDoc XRS system.
ER stress analysis.
Myotubes were grown in 96-well tissue culture plates and differentiated for 4 days. Cells were serum starved (0.25% FBS/DMEM) for 4 h and treated with C16 carnitine at 0, 5, 10, 25, 30, 40, 50, 75, and 100 μM and positive controls (staurosporine, thapsigargin, and tunicamycin) for 6 h in duplicate or triplicate. At 6 h, media were harvested, cells were rinsed two times with cold HBSS and lysed in 1X lysis buffer, and replicates were pooled. Media were analyzed for AK, and concentrations and lysate protein concentrations were measured and subjected to SDS-PAGE and Western blotting to determine levels of ER stress markers [cleaved caspase 3, inositol-requiring protein-1α (IRE-1α), binding immunoglobulin protein (BiP), and CCAAT/enhancer-binding protein homologous protein (CHOP)].
Live/dead assay.
C2C12 myoblasts were seeded into 96-well clear-bottom, black wall plates (BD Falcon) and differentiated as described above. The cells were serum starved in 0.25% FBS phenol red-free DMEM for 3–4 h before treatment for 6 h with various compounds in the same medium. Supernatants were removed, and 25 μl of HBSS with Ca2+/Mg2+ were added to each well. Twenty-five microliters of 2X live/dead dye (catalog no. R37601; Life Technologies) was added to each well 15 min before imaging. Imaging was done on a Nikon Eclipse Ti microscope with an automated platform and Zyla Andon camera, and the data acquired were analyzed using Nikon Elements HCT software. Three to four images were captured per well. The total GFP (green channel fluorescence) and RPE (red channel fluorescence) intensities were calculated by the Nikon Element HCT software. The ratio of GFP to RPE intensity was determined. A lower ratio indicates increased cell death.
XTT viability assay.
Myotubes were grown, starved, and treated as done above for live/dead assay. After 6 h treatment, media were removed, and fresh media with treatments were added, with 0.20 mg/ml XTT and 0.001 mM PMS activation reagent. Cells were returned to 37°C for 4 h. Absorbance measurements were read on a plate reader at 475 nm with a background correction of 690 nm.
Caspase assay.
C2C12 myotubes were pretreated with caspase inhibitors, caspase-3 inhibitor II (Z-DEVD-FMK) or caspase inhibitor I (Pan inhibitor, Z-VAD-FMK) (EMD Millipore), for 1 h after a serum starvation in 0.25% FBS for 2–3 h. C2C12 myotubes were cotreated with the respective caspase inhibitor and various compounds as indicated in the text for 6 or 20 h. Supernatants were collected and stored at −20°C. C2C12 were washed one time with cold HBSS and lysed in 25 μl of reporter lysis buffer per well (catalog no. E3971; Promega). The samples were stored at −20°C. For assay, lysate samples were thawed quickly at 50°C and mixed on an orbital shaker for 15 min at room temperature, after which 20 μl were transferred to white round-bottom 96-well plates, and 20 μl of the Caspase 3/7 Glo working reagent were added to each sample (catalog no. G8091; Promega). The plate was incubated (covered) at room temperature for 30 min on an orbital shaker. Luminescence was measured on a Synergy 2 plate reader.
Intracellular calcium readouts.
C2C12 myotubes in a black 96-well tissue culture plate were serum starved for 2–3 h in 0.25% FBS plus 10 μM calcium green-1 AM. Cells were rinsed two times in warm HBSS (phenol red free), and 100 μl phenol red-free 0.25% DMEM were added back. The plate was incubated at room temperature on a Synergy 2 plate reader, and 100 μl of a 2X treatment solution (containing acylcarnitines or other factors indicated in the text) were added to each well using the Synergy 2 injectors. Fluorescence readings were taken immediately following injection and continuing every 2 s for a 1-min duration. Baseline to maximum values was calculated by subtracting the initial fluorescent reading value from the highest value recorded.
Data analysis.
Each experiment was performed a minimum of three independent times, as indicated in results; typical experiments each included at least three replicates per treatment. Depending on the nature of the experiment, results were analyzed by one- or two-way ANOVA with Dunnett's (comparing against control) or Tukey's (comparing all treatments with one another) post hoc tests, and data are presented as means ± SE. Statistical analyses were performed using PrismGraph 6.0 (GraphPad Software, San Diego, CA).
RESULTS
Acylcarnitine chain-length effects on IL-6 production.
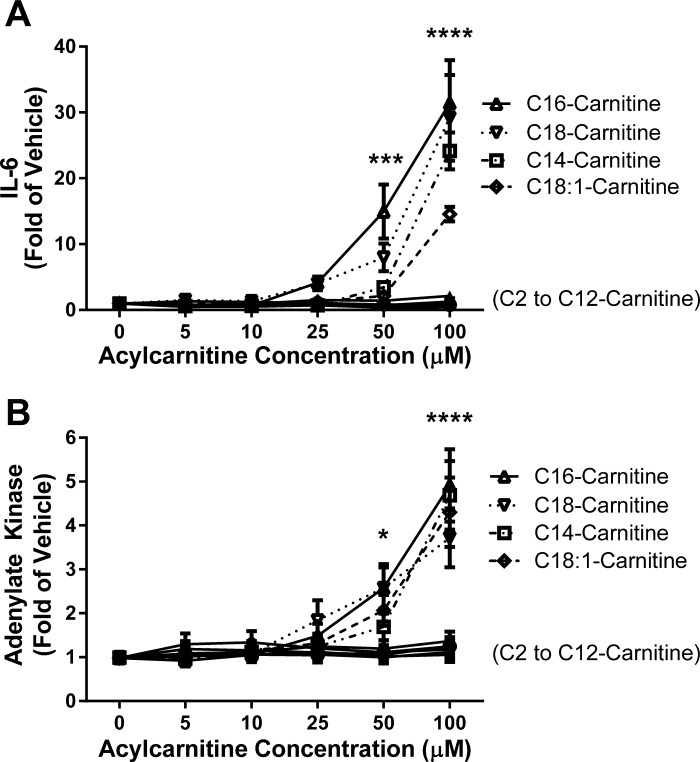
Because our previous studies showed that C14- and C16-acylcarnitine can elicit proinflammatory gene and cytokine expression in RAW 264.7 murine monocyte/macrophages and (at higher concentrations) in C2C12 murine myotube models (2, 29), we sought to fully characterize a panel of short-, medium-, and long-chain l-acylcarnitine treatments on media IL-6 cytokine and AK concentrations in the C2C12 myotube model. These markers were chosen as factors reflecting activation of inflammatory cascades (IL-6) or cytotoxicity typically tracking cell death (AK). l-Acylcarnitines of acyl chain lengths from C2- through C12- did not alter IL-6 cytokine production or AK release into the medium after 18 h of treatment (Fig. 1). Beginning with C14 chain-length acylcarnitine and continuing with the higher chain lengths tested (C16, C18, and C18:1 carnitines), l-acylcarnitine induced both IL-6 production and AK release in parallel. The minimum concentration tested that elicited an increase in IL-6 cytokine production was 25 μM C16- and C18- carnitine, with the caveat that other concentrations between 10 and 25 μM were not evaluated. These effects increased in a dose-dependent manner up to 100 μM, the highest concentration tested. AK tracked IL-6 production under all conditions except for C18:1 carnitine, where AK release increased at 50 μM without a corresponding increase in media IL-6.
Fig. 1.
l-Acylcarnitine chain length-dependent induction of the inflammatory cytokine interleukin-6 (IL-6) and secretion of adenylate kinase (AK) in a C2C12 murine myotube skeletal muscle cell model. Myotubes were serum starved for 3–4 h (0.25% FBS/DMEM) and then treated for 18 h with varying concentrations (0–100 μM) and chain lengths (C2 to C18:1) of l-acylcarnitines. Media concentrations of IL-6 (A) and AK (B) were measured by ELISA (R&D Systems Quantikine) and luminescence (Lonza Toxilight) assays, respectively; n = 6 or more over 3 experiments. Two-way ANOVA with Dunnett's test (post hoc) for dose response: *P < 0.05, ***P < 0.001, and ****P < 0.0001 vs. vehicle-treated control; mean ± SE. Data are expressed as fold of vehicle control. Mean vehicle IL-6 = 27.4 pg/ml.
Because C16 carnitine had the most robust effects on the C2C12 myotubes, all further experiments were performed using C16 carnitine as a representative long-chain acylcarnitine. Previously, the lots of FBS and C16 carnitine were subjected to endotoxin testing and found to be negative (29). These results indicate that the inflammatory IL-6 and AK responses are a direct result of C16 carnitine and not endotoxin contamination. Furthermore, in experiments not shown, LPS treatment of cells failed to elicit an AK response despite the expected robust IL-6 response.
Acylcarnitine activation of MAPK pathway.
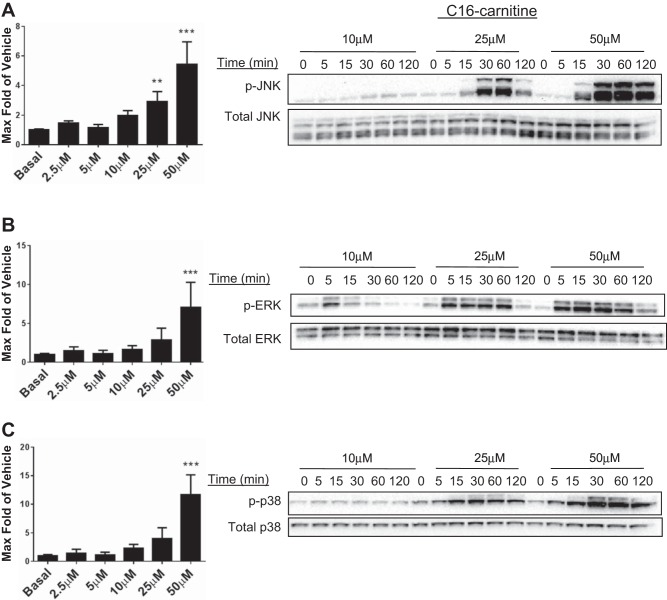
Our previous work demonstrated that C14 carnitine increases phosphorylation of the JNK and ERK MAPKs in RAW 264.7 murine macrophages. Furthermore, IL-6 cytokine production is known to be mediated in part by the MAPK cell stress signaling pathway in the C2C12 model (13). As shown in Fig. 2, C16 carnitine elicited an increase in phosphorylation of p38, JNK, and ERK in C2C12 myotubes. The pathway activation occurred in both time- and concentration-dependent manners that were consistent for all MAPK pathways; the effects appeared to be triggered by concentrations between 10 and 25 μM. This effect suggests that C16 carnitine can elicit global activation of MAPK pathways in the C2C12 model in a dose-dependent manner.
Fig. 2.
C16 carnitine increases phosphorylation of the c-Jun NH2-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 mitogen-activated protein kinase (p38-MAPK) in both a dose- and time-dependent manner. C2C12 myotubes were serum starved for 3–4 h (0.25% FBS/DMEM) before treatment with varying doses of C16 carnitine (0–50 μM) for the indicated times. Cell lysates were assessed via electrochemiluminescent ELISA (Meso Scale Discovery) and Western blot (representative blot from 4 separate experiments). Maximum phosphorylation of p-JNK (A), p-ERK (B), and p-p38 (C) over basal was quantitated by ELISA (p-JNK, p-ERK) and densitometry (p-p38). Maximum was calculated from n = 5/treatment over 4 experiments. One-way ANOVA with Dunnett's test: **P < 0.01 and ***P < 0.001 vs. basal; mean ± SE. Data are expressed as fold over basal.
C16 carnitine at higher concentrations elicits cell death within 6 h.
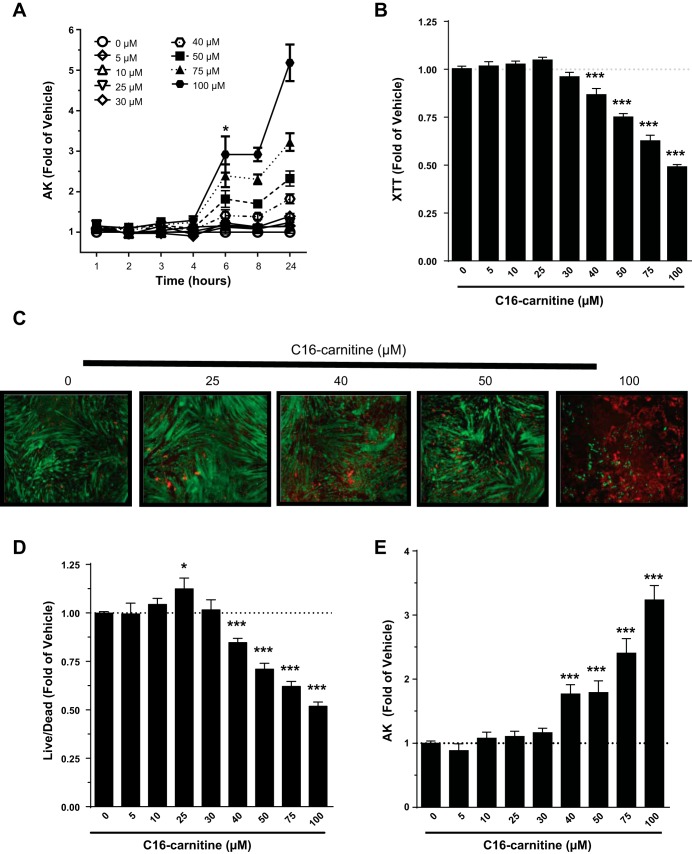
Because it was observed that C16 carnitine at concentrations greater than 25 μM cause significant increases in AK after 18 h (Fig. 1B), a time course of treatment was designed to determine when and at what concentration this effect occurs. By 6 h, media AK had increased at concentrations of 40 μM and higher (Fig. 3A), indicating that cells had permeabilized and begun to release intracellular contents into the media. As a secondary readout for cell damage, lactate dehydrogenase (LDH) was also assessed in the media. While variable, LDH increased in a dose-dependent manner with C16 carnitine treatment, similar to AK (data not shown).
Fig. 3.
Dose-dependent cell death occurs within 6 h of C16 carnitine treatment. C2C12 myotubes were serum starved for 4 h (0.25% FBS/DMEM) and then treated with varying concentrations of C16 carnitine (0–100 μM). Concentrations >40 μM resulted in increased AK release after 6 h (A). At 6 h, conversion of 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide, disodium salt (XTT) to its formazan product (B), a live/dead imaging assay (live: green, dead: red) (C and D), and release of AK (E) confirmed concentrations of C16 carnitine 40 μM and greater result in dose-dependent injury/death of C2C12 myotubes. C: representative images of at least 3 experiments. One-way ANOVA with Dunnett's test: *P < 0.05 and ***P < 0.001; mean ± SE. Data are expressed as fold over vehicle.
When cells are stressed and begin to die, mitochondrial function is compromised (21, 22). To further characterize the C2C12 response to C16 carnitine on cell stress and viability, XTT was used to assess mitochondrial redox potential at 6 h of treatment (Fig. 3B). The ability of mitochondria to convert XTT to formazan was significantly reduced starting at 40 μM C16 carnitine after a 6-h treatment, suggesting mitochondrial dysfunction. The results for AK, LDH, and XTT support significant cell damage and are suggestive of cell death. A live/dead imaging assay (Fig. 3, C and D) coupled to AK measurement (Fig. 3E) confirmed that higher concentrations of C16 carnitine cause C2C12 myotube cell death.
Long-chain acylcarnitines do not activate ER stress pathways but weakly activate caspases.
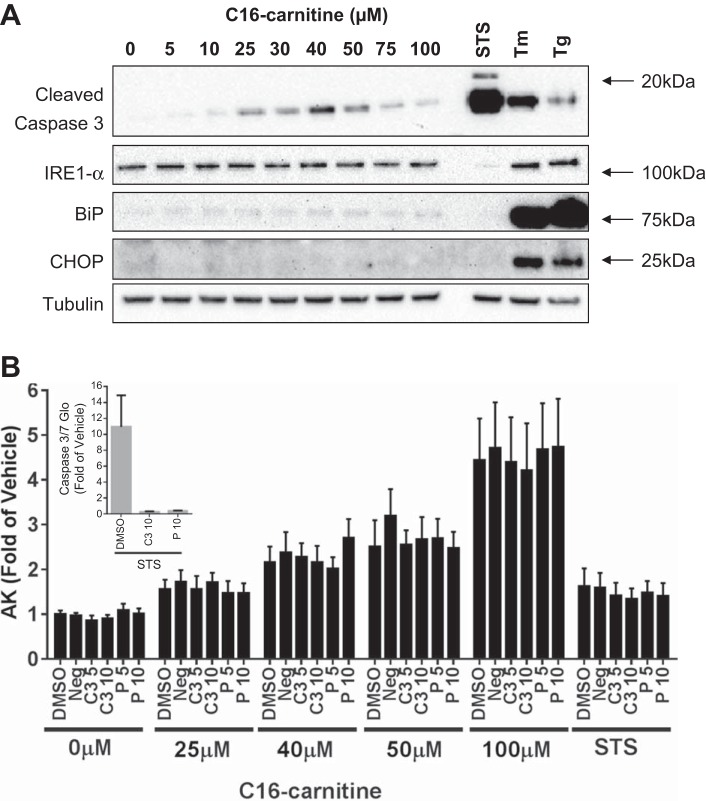
After observing that C16 carnitine can elicit myotube cell death, the mechanism by which death occurs was examined. Cellular death can occur through a number of well-described pathways, including activation of ER stress/unfolded protein response pathways that can occur through a disruption in lipid homeostasis (4). After 6 h of acylcarnitine treatment, none of the ER stress markers, IRE-1α, BiP, nor CHOP, were increased, in contrast to positive control treatments (Fig. 4A). Interestingly, C16 carnitine modestly increased the apoptotic marker cleaved caspase-3, beginning at 10–25 μM.
Fig. 4.
C16 carnitine weakly activates cleavage of caspase-3 but not endoplasmic reticulum (ER) stress and cannot be rescued from death by caspase inhibitors in C2C12 myotubes. C16 carnitine weakly elicits the cleavage of caspase-3 but does not appear to induce markers of ER stress [inositol-requiring protein-1α (IRE-1α), binding immunoglobulin protein (BiP), and CCAAT/enhancer-binding protein homologous protein (CHOP)] in myotubes. Positive controls: staurosporine (STS), Tunicamycin (Tm), and thapsigargin (Tg) (A). Inhibition of caspases does not affect release of AK (i.e., cell death) (B). Caspase inhibitors effectively inhibit staurosporine (STS)-induced caspase 3/7 activity (B, inset). Three independent experiments done in duplicate (n = 6/bar); mean ± SE. Data are expressed as fold over vehicle.
Staurosporine is a well-known activator of caspase-associated cell death and as expected triggered a large upregulation of cleaved caspase-3 in the cells (showing ∼10-fold increase vs. control; Fig. 4B, inset). Addition of either a caspase-3/7 or pan-caspase inhibitor was capable of preventing the cell from eliciting a staurosporine-mediated increase in caspase-3/7 (Fig. 4B, inset). However, neither of the caspase inhibitors was capable of protecting the cells from AK release following acylcarnitine treatment (Fig. 4B). Together, these results indicate that acylcarnitines are capable of activating cell stress pathways but that the observed cell death and membrane permeability to AK was not likely mediated through classical caspase-dependent apoptosis.
Acylcarnitine treatment rapidly increases intracellular calcium and promotes IL-6 production.
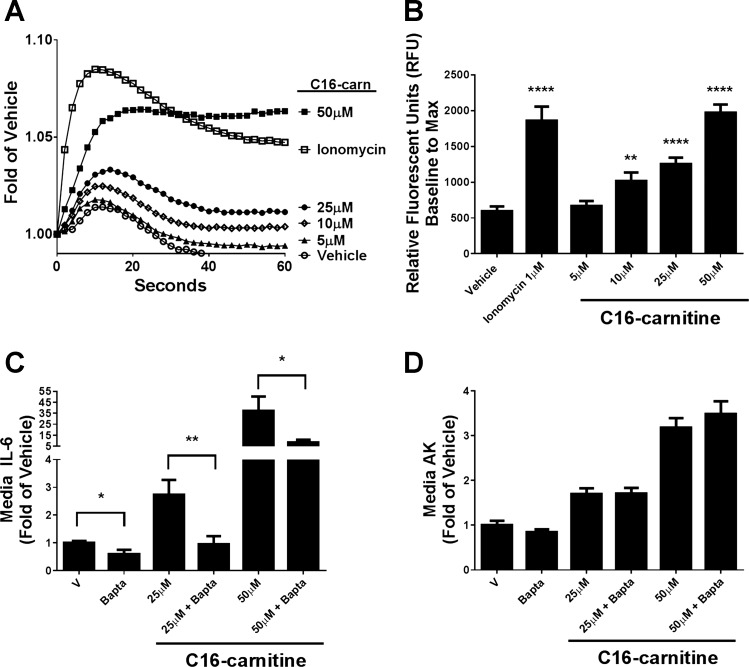
Increased intracellular calcium is required for caspase-3 activation (34) and has been associated with increases in acylcarnitines during cardiac ischemia (39). Additionally, increases in cytosolic calcium are well established to induce IL-6 transcription (3), which can be blunted by a calcineurin inhibitor (12). Thus, we assessed whether addition of exogenous C16 carnitine could elicit an increase in intracellular calcium in C2C12 myotubes. C16 carnitine rapidly elicited a dose-dependent increase in intracellular calcium when measured by a fluorescent calcium indicator using a microplate reader (Fig. 5, A and B). Whereas the rate of the calcium increase was slower than that of the positive control ionophore, ionomycin (Fig. 5A), the average maximum fluorescence from baseline was comparable between 50 μM C16 carnitine and 1 μM ionomycin (Fig. 5B). Increased calcium above vehicle control was detectable by 10 μM C16 carnitine.
Fig. 5.
Intracellular calcium is rapidly increased with C16 carnitine treatment, and its chelation blunts C16 carnitine-mediated IL-6 production but does not protect from cell death. Differentiated C2C12 myotubes were serum starved in 0.25% FBS/DMEM for 4 h and preloaded with calcium green-1 AM (10 μM) (A and B) or BAPTA-AM (7.5 μM) (C and D) for the last hour of starvation. Cells were treated with C16 carnitine (0, 5, 10, or 25 μM) or positive control ionomycin (1 μM) for 1 min, and maximum fluorescence over basal levels (B) was determined by plate reader as described in materials and methods. Fluorescence trace is representative of 5 experiments (A). Myotubes were treated for 18 h with C16 carnitine (0, 25, and 50 μM) with and without BAPTA-AM (7.5 μM) (C and D). Media IL-6 and AK were analyzed. One-way ANOVA with Tukey's test: *P < 0.05, **P < 0.01 and ****P < 0.0001 vs. basal; mean ± SE. Data are expressed as fold over vehicle.
We next investigated whether C16 carnitine-mediated increases in intracellular calcium were responsible for the increases in media IL-6. The intracellular calcium chelator BAPTA-AM was used to decrease free intracellular calcium. BAPTA-AM completely blunted the stimulation of IL-6 by 25 μM C16 carnitine and caused an ∼78% drop at 50 μM (Fig. 5C). Notably, intracellular calcium chelation had no effect on Toll-like receptor-mediated IL-6 production from LPS treatment (data not shown). Whereas BAPTA-AM strongly blunted the production of IL-6 with acylcarnitine treatment, it did not reduce the AK marker of cell death (Fig. 5D). The data indicate that changes in intracellular calcium are important for some, but not all, of the effects of C16 carnitine in myotubes.
Mechanism of acylcarnitine-mediated cell death.
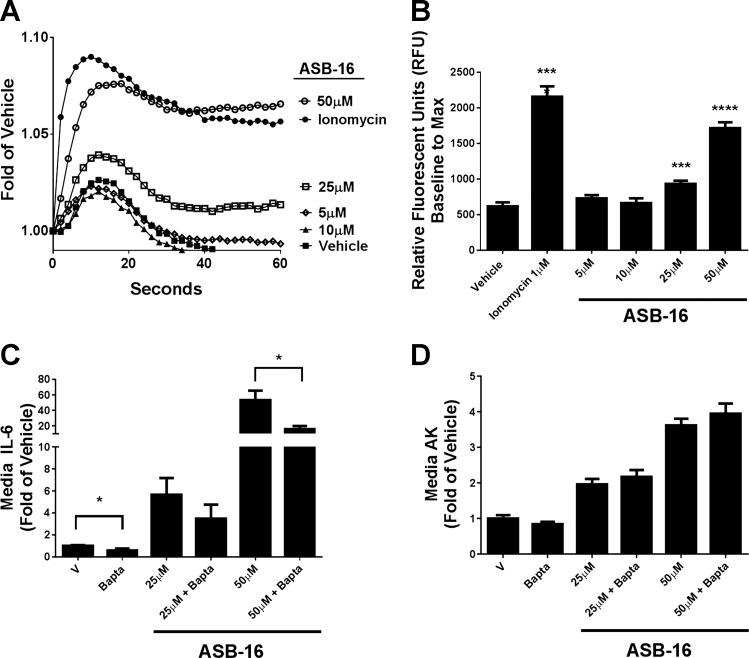
Observations from the current experiment and our previous studies of inflammation (29) and insulin sensitivity (2) in response to acylcarnitines have illustrated that types and magnitude of bioactivities differ dramatically when considering lower (i.e., 5–10 μM) and higher (i.e., >25 μM) concentrations of these metabolites. The latter consistently has led to cell permeability in both RAW cells and C2C12 myotubes, whereas this effect is not observed at lower long-chain acylcarnitine concentrations, despite proinflammatory or insulin-resistance phenotypes at the lower levels of the metabolites. It is possible that acylcarnitines elicit some or all of their effects through interaction with cellular membranes by virtue of their zwitterionic character, perhaps even at the lower concentrations. At very high concentrations, membrane integrity may be compromised, and cell stress responses ensue. To begin to evaluate this concept, we employed the 16-carbon zwitterionic compound ASB-16 that displays similar chemical characteristics with C16 carnitine (Fig. 6). In dose-response studies under identical conditions to those used for C16 carnitine, ASB-16 elicited comparable patterns for increasing intracellular calcium (Fig. 6A). Additionally, IL-6 and AK release were evident at similar concentrations to those seen for acylcarnitine (Fig. 6B). Although the studies were not designed to statistically compare ASB-16 and l-C16 carnitine, overall the molecules displayed qualitatively similar activities on the parameters tested. These results support the idea that at least some of the effects of acylcarnitine involve cell components sensitive to zwitterion interaction with cellular membranes.
Fig. 6.
Amidosulfobetaine-16 (ASB-16) elicits similar effects on IL-6 cytokine production and AK secretion in C2C12 myotubes as C16 carnitine. C2C12 cells were starved and preloaded as done previously and then treated with ASB-16 (0, 5, 10, or 25 μM) or ionomycin (1 μM). As seen with C16 carnitine, ASB-16 increased intracellular calcium fluorescence in a dose-dependent manner (A and B). ASB-16 also increased secretion of both IL-6 (C) and AK (D) in a dose-dependent manner. BAPTA-AM was also able to reduce IL-6 secretion but, as seen with C16 carnitine, could not prevent the myotubes from increased permeabilization to AK. *P ≤ 0.05, ***P ≤ 0.001, and ****P ≤ 0.0001.
DISCUSSION
A growing body of research related to inefficient or impaired long-chain fatty acid oxidation has implicated some lipid intermediates as being associated with insulin resistance, inflammation, and cell stress responses (1, 20). Recent studies by our laboratory have focused on the bioactive properties of acylcarnitines in eliciting an inflammatory response in murine macrophages (29) and in blunting insulin signaling in murine and human myotubes (2). These effects were qualitatively equivalent with either C14- or C16-acylcarnitines, suggesting a similar mechanism. One particularly striking finding was that higher concentrations of long-chain acylcarnitines (i.e., C14- or C16-acylcarnitines) elicited apparent cell death in macrophages; however, the pathophysiological relevance of these observations, if any, remains to be determined. Because skeletal muscle is a major source of whole body acylcarnitine production, we sought to identify the inflammatory and cell stress effects of long-chain acylcarnitines on murine C2C12 myotubes and explored the mechanism by which these effects might occur.
Herein, we provide evidence that C16 carnitine, a representative long-chain acylcarnitine elevated under certain disease conditions such as FAOD (23), cardiac ischemia (17, 36, 38), or more modestly in insulin resistance/type 2 diabetes (1, 18, 20, 24, 26), elicits the activation of cell death and stress pathways in a concentration-dependent manner in murine myotubes. C16 carnitine rapidly activated the JNK/ERK/p38 MAPK stress pathways (in concert with AK release), increased intracellular calcium, and elicited markers of cell death within 6 h. C16 carnitine also modestly activated the proapoptotic caspase-3 catalytic protein but did not increase markers of ER stress. These results are in line with a study by Mutomba and colleagues who reported activation of recombinant caspase-3 enzyme activity by palmitoylcarnitine (25). In the latter study, the idea that long-chain acylcarnitines can activate caspases was also supported by the observation that staurosporine-mediated apoptosis was blunted in cells lacking CPT1 (25). While speculative, results from the current study and the literature are consistent with the hypothesis that, under certain disease conditions or events, such as FAOD or severe lipotoxicity, increases in long-chain acylcarnitines could elicit muscle cell inflammation and stress.
Lipotoxicity is a well-described phenomenon that occurs when lipids and lipid intermediates accumulate abnormally throughout the body, as seen in insulin resistance and more severely in poorly controlled type 2 diabetes (30, 35). There are a host of cellular consequences to lipotoxic conditions, including increases in cellular ROS, ER stress, inflammation, and, in its most severe manifestation, cell death (5, 30). Increases in cardiac tissue acylcarnitines have been noted during ischemia-reperfusion and associated with a range of cellular complications, including derangements in ionic flux that control cardiac electrophysiology (9, 32, 40–42) and stress and death pathway activation (32). Although the skeletal muscle metabolic effects of lipotoxicity associated with type 2 diabetes or insulin resistance are well documented, the specific metabolites that trigger cell stress are less conclusive. There have been reports of increased caspase-3/apoptosis (33), ROS, and mitochondrial damage (31) in type 2 diabetes mellitus and insulin resistance. This current study, as well as a previous study (29), raise the possibility that, under certain conditions of inefficient β-oxidation, an abnormal increase in long-chain acylcarnitines contributes to lipid-associated cell stress.
The mechanisms-of-action of long-chain acylcarnitines on cell inflammation (29), insulin sensitivity (2), and cell stress responses has been elusive. Based on the aggregate of results, it is speculated that these effects involve associations with cell membranes (28) and fall along a spectrum from modest (i.e., impacting insulin signaling or inflammation) to severe (i.e., triggering cell death and loss of membrane integrity). At higher concentrations of long-chain acylcarnitines, disruption of membrane integrity may be associated with a host of negative consequences, including arrhythmias, myopathies, and necrosis, since loss of proper membrane function overrides the cells' ability to maintain homeostasis (10, 11). Long-chain acylcarnitines at high concentrations were also shown to trigger red blood cell lysis (8). Consistent with our perspective, it was observed that the zwitterionic compound ASB-16, often used to solubilize membranes and membrane proteins (16), displayed effects qualitatively similar to high-concentration long-chain acylcarnitines with respect to IL-6 release, cell death, and permeabilization in C2C12 myotubes. C16 carnitine and ASB-16 are structurally similar compounds. Furthermore, the C16 carnitine effects on cell death were seen at concentrations within the same order of magnitude as its 75- to 100-μM estimated critical micelle concentration (CMC) (27). As depicted in a working model (Fig. 7), it is proposed that the long-chain acylcarnitine interaction with cell membranes also manifests at lower, nonpermeabilizing concentrations that impact cell signaling outcomes. In other words, long-chain acylcarnitines at concentrations that are normally seen in vivo may hypothetically interact with cell membranes to impact cellular function and receptor signaling. In contrast, at pathophysiologically high concentrations, the membrane effects could also lead to cellular damage.
Fig. 7.
Proposed hypothesis and model for acylcarnitine action on cells and cell membranes. At modest concentrations, zwitterionic long-chain acylcarnitines interact with cellular membranes, which influences membrane-associated systems (i.e., certain receptors and receptor complexes) (left). This could be a natural physiological phenomenon that serves to link metabolism to cellular function. At least under certain conditions, the acylcarnitine-membrane associations can increase intracellular calcium, activate inflammatory pathways, or decrease insulin-stimulated phosphorylation of protein kinase B (Akt) and glucose uptake. At higher concentrations of long-chain acylcarnitines that elicit cell membrane disruption, cell permeabilization ensues (right). Under this condition, excessive tissue long-chain acylcarnitine accumulation, singly or in concert with other stressors, may contribute to pathology outcomes [i.e., episodic myopathies in fatty acid oxidation disorders (FAOD) or cardiomyocyte death following cardiac ischemia]. IR, insulin receptor; MyD88, myeloid differentiation primary response gene 88; CK, creatine kinase; P, phosphorylated; COX-2, cyclooxygenase-2.
While this concept provides a provocative perspective on acylcarnitine biology, a major limitation is that it remains to be determined if effects seen using the initial extracellular concentrations in cell culture studies mimic acylcarnitine actions at concentrations circa or within cells in vivo. It is notable that, in studies by Mutomba et al. (25), the lowest concentration of palmitoylcarnitine tested, 1 μM, activated recombinant purified caspase-3, supporting our assertion that long-chain acylcarnitine accumulation could trigger some cell stress mechanisms well below the CMC. We have also observed inflammatory phenotypes in immune cells treated with as low as 5 μM long-chain acylcarnitines (29). The working model of membrane associations would apply most clearly to long-chain acylcarnitines and would be less likely to explain bioactivities, if any, of shorter-chain acylcarnitines. Indeed, we observed a sharp increase of cell stress outcomes with increasing acyl chain length, consistent with the relative affinities for acylcarnitines for membranes, as indicated by a decrease in CMCs. For instance, Haeyaert et al. (15) reported CMCs of C12 carnitine (700 μM), C14 carnitine (100 μM), and C16 carnitine (40 μM). The absolute CMCs are highly dependent on the system being tested and the pH, yet, despite variability of these values in the literature, the chain-length associations would still hold. As a final consideration, it is likely that the free acylcarnitine concentrations in situ, and hence their cellular activities, are modified through binding to other lipids and proteins. Thus, future studies to examine the validity of the model components could include, for instance, determination, albeit challenging, of the in situ cellular concentrations of acylcarnitines (i.e., immune and myocyte cells), monitoring cellular effects of manipulation of intracellular generation of acylcarnitines (i.e., genetic modifications such as CPT2 knockdown, specific enzyme inhibitors), protein binding, and experiments that determine if acylcarnitine association with membrane preparations corresponds with receptor- or membrane protein-based outcomes.
In summary, we have shown that long-chain acylcarnitines have the potential to rapidly increase intracellular calcium and to activate skeletal muscle cell stress pathways, which under certain conditions elicit cell death. Our studies employed acylcarnitine administration externally to cells, which were in line with the high concentrations of long-chain acylcarnitines within plasma and muscle under conditions of FAOD (23, 43). However, it is acknowledged that the amounts used herein may differ from the concentrations found at the sarcolemma or within muscle cells. Thus, it remains speculative as to the potential role of long-chain acylcarnitine accumulation in the episodic myopathy typical of certain FAOD conditions. Whether acylcarnitines trigger cellular responses through specific receptors, membrane-associated proteins, nonspecific amphipathic interactions, or a combination of these remains to be fully elucidated.
GRANTS
This work was supported by the American Diabetes Association (1-12-BS-02), the National Institutes of Health (NIH) (R01-DK-078328 and R01-DK-078328-02S1), and United States Department of Agriculture-Agricultural Research Service intramural Project 5306-51530-019-00. This project was also supported by a T32 predoctoral training award (to C. S. McCoin), funded by the National Center for Advancing Translational Sciences, NIH, through Grant no. UL1-TR-000002 and linked award TL1-TR-000133.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The USDA is an equal opportunity provider and employer.
AUTHOR CONTRIBUTIONS
Author contributions: C.S.M., T.A.K., K.D.O.-M., P.J.O., and S.H.A. conception and design of research; C.S.M., T.A.K., and K.D.O.-M. performed experiments; C.S.M., T.A.K., and K.D.O.-M. analyzed data; C.S.M., T.A.K., K.D.O.-M., P.J.O., and S.H.A. interpreted results of experiments; C.S.M., T.A.K., K.D.O.-M., and S.H.A. prepared figures; C.S.M. and S.H.A. drafted manuscript; C.S.M., T.A.K., K.D.O.-M., and S.H.A. edited and revised manuscript; C.S.M., T.A.K., K.D.O.-M., P.J.O., and S.H.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Michael Paddy of the University of California Davis MCB LM Imaging Facility for technical support and training.
REFERENCES
- 1.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139: 1073–1081, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguer C, McCoin CS, Knotts TA, Thrush AB, Ono-Moore K, McPherson R, Dent R, Hwang DH, Adams SH, Harper ME. Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J 29: 336–345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen DL, Uyenishi JJ, Cleary AS, Mehan RS, Lindsay SF, Reed JM. Calcineurin activates interleukin-6 transcription in mouse skeletal muscle in vivo and in C2C12 myotubes in vitro. Am J Physiol Regul Integr Comp Physiol 298: R198–R210, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basseri S, Austin RC. Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem Res Int 2012: 841362, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biden TJ, Boslem E, Chu KY, Sue N. Lipotoxic endoplasmic reticulum stress, β cell failure, and type 2 diabetes mellitus. Trends Endocrinol Metab 25: 389–398, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Brass EP, Hoppel CL. Relationship between acid-soluble carnitine and coenzyme A pools in vivo. Biochem J 190: 495–504, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter KH, Wiley V. Application of tandem mass spectrometry to biochemical genetics and newborn screening. Clin Chim Acta 322: 1–10, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Cho KS, Proulx P. Lysis of erythrocytes by long-chain acyl esters of carnitine. Biochim Biophys Acta 193: 30–35, 1969. [DOI] [PubMed] [Google Scholar]
- 9.Corr PB, Creer MH, Yamada KA, Saffitz JE, Sobel BE. Prophylaxis of early ventricular fibrillation by inhibition of acylcarnitine accumulation. J Clin Invest 83: 927–936, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corr PB, Gross RW, Sobel BE. Arrhythmogenic amphiphilic lipids and the myocardial cell membrane. J Mol Cell Cardiol 14: 619–626, 1982. [DOI] [PubMed] [Google Scholar]
- 11.Drenckhahn D, Lüllmann-Rauch R. Experimental myopathy induced by amphiphilic cationic compounds including several psychotropic drugs. Neuroscience 4: 549–562, 1979. [DOI] [PubMed] [Google Scholar]
- 12.Farmawati A, Kitajima Y, Nedachi T, Sato M, Kanzaki M, Nagatomi R. Characterization of contraction-induced IL-6 up-regulation using contractile C2C12 myotubes. Endocr J 60: 137–147, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: role of the Jun NH2-terminal kinase. Am J Physiol Regul Integr Comp Physiol 285: R1153–R1164, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol 14: 546–558, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Haeyaert P, Verdonck A, Van Cauwelaert FH. Influence of acylcarnitines of different chain length on pure and mixed phospholipid vesicles and on sarcoplasmic reticulum vesicles. Chem Phys Lipids 45: 49–63, 1987. [DOI] [PubMed] [Google Scholar]
- 16.Henningsen R, Gale BL, Straub KM, DeNagel DC. Application of zwitterionic detergents to the solubilization of integral membrane proteins for two-dimensional gel electrophoresis and mass spectrometry. Proteomics 2: 1479–1488, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hull FE, Radloff JF, Sweeley CC. Fatty acid oxidation by ischemic myocardium. Recent Advan Stud Cardiac Struct Metab 8: 153–165, 1975. [PubMed] [Google Scholar]
- 18.Inokuchi T, Imamura K, Nomura K, Nomoto K, Isogai S. Changes in carnitine metabolism with ketone body production in obese glucose-intolerant patients. Diabetes Res Clin Pract 30: 1–7, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Isackson PJ, Bennett MJ, Lichter-Konecki U, Willis M, Nyhan WL, Sutton VR, Tein I, Vladutiu GD. CPT2 gene mutations resulting in lethal neonatal or severe infantile carnitine palmitoyltransferase II deficiency. Mol Genet Metab 94: 422–427, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JRB, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366: 177–196, 1998. [DOI] [PubMed] [Google Scholar]
- 23.McHugh D, Cameron CA, Abdenur JE, Abdulrahman M, Adair O, Al Nuaimi SA, Ahlman H, Allen JJ, Antonozzi I, Archer S, Au S, Auray-Blais C, Baker M, Bamforth F, Beckmann K, Pino GB, Berberich SL, Binard R, Boemer F, Bonham J, Breen NN, Bryant SC, Caggana M, Caldwell SG, Camilot M, Campbell C, Carducci C, Bryant SC, Caggana M, Caldwell SG, Camilot M, Campbell C, Carducci C, Cariappa R, Carlisle C, Caruso U, Cassanello M, Castilla AM, Ramos DE, Chakraborty P, Chandrasekar R, Ramos AC, Cheillan D, Chien YH, Childs TA, Chrastina P, Sica YC, de Juan JA, Colandre ME, Espinoza VC, Corso G, Currier R, Cyr D, Czuczy N, D'Apolito O, Davis T, de Sain-Van der Velden MG, Delgado Pecellin C, Di Gangi IM, Di Stefano CM, Dotsikas Y, Downing M, Downs SM, Dy B, Dymerski M, Rueda I, Elvers B, Eaton R, Eckerd BM, El Mougy F, Eroh S, Espada M, Evans C, Fawbush S, Fijolek KF, Fisher L, Franzson L, Frazier DM, Garcia LR, Bermejo MS, Gavrilov D, Gerace R, Giordano G, Irazabal YG, Greed LC, Grier R, Grycki E, Gu X, Gulamali-Majid F, Hagar AF, Han L, Hannon WH, Haslip C, Hassan FA, He M, Hietala A, Himstedt L, Hoffman GL, Hoffman W, Hoggatt P, Hopkins PV, Hougaard DM, Hughes K, Hunt PR, Hwu WL, Hynes J, Ibarra-Gonzalez I, Ingham CA, Ivanova M, Jacox WB, John C, Johnson JP, Jonsson JJ, Karg E, Kasper D, Klopper B, Katakouzinos D, Khneisser I, Knoll D, Kobayashi H, Koneski R, Kozich V, Kouapei R, Kohlmueller D, Kremensky I, la Marca G, Lavochkin M, Lee SY, Lehotay DC, Lemes A, Lepage J, Lesko B, Lewis B, Lim C, Linard S, Lindner M, Lloyd-Puryear MA, Lorey F, Loukas YL, Luedtke J, Maffitt N, Magee JF, Manning A, Manos S, Marie S, Hadachi SM, Marquardt G, Martin SJ, Matern D, Mayfield Gibson SK, Mayne P, McCallister TD, McCann M, McClure J, McGill JJ, McKeever CD, McNeilly B, Morrissey MA, Moutsatsou P, Mulcahy EA, Nikoloudis D, Norgaard-Pedersen B, Oglesbee D, Oltarzewski M, Ombrone D, Ojodu J, Papakonstantinou V, Reoyo SP, Park HD, Pasquali M, Pasquini E, Patel P, Pass KA, Peterson C, Pettersen RD, Pitt JJ, Poh S, Pollak A, Porter C, Poston PA, Price RW, Queijo C, Quesada J, Randell E, Ranieri E, Raymond K, Reddic JE, Reuben A, Ricciardi C, Rinaldo P, Rivera JD, Roberts A, Rocha H, Roche G, Greenberg CR, Mellado JM, Juan-Fita MJ, Ruiz C, Ruoppolo M, Rutledge SL, Ryu E, Saban C, Sahai I, Garcia-Blanco MI, Santiago-Borrero P, Schenone A, Schoos R, Schweitzer B, Scott P, Seashore MR, Seeterlin MA, Sesser DE, Sevier DW, Shone SM, Sinclair G, Skrinska VA, Stanley EL, Strovel ET, Jones AL, Sunny S, Takats Z, Tanyalcin T, Teofoli F, Thompson JR, Tomashitis K, Domingos MT, Torres J, Torres R, Tortorelli S, Turi S, Turner K, Tzanakos N, Valiente AG, Vallance H, Vela-Amieva M, Vilarinho L, von Dobeln U, Vincent MF, Vorster BC, Watson MS, Webster D, Weiss S, Wilcken B, Wiley V, Williams SK, Willis SA, Woontner M, Wright K, Yahyaoui R, Yamaguchi S, Yssel M, Zakowicz WM. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project. Genet Med 13: 230–254, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 18: 1695–1700, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutomba MC, Yuan H, Konyavko M, Adachi S, Yokoyama CB, Esser V, McGarry JD, Babior BM, Gottlieb RA. Regulation of the activity of caspases by L-carnitine and palmitoylcarnitine. FEBS Lett 478: 19–25, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 284: 22840–22852, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Requero MA, Goni FM, Alonso A. The membrane-perturbing properties of palmitoyl-coenzyme A and palmitoylcarnitine. A comparative study. Biochemistry 34: 10400–10405, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Requero MA, Gonzalez M, Goni FM, Alonso A, Fidelio G. Differential penetration of fatty acyl-coenzyme A and fatty acylcarnitines into phospholipid monolayers. FEBS Lett 357: 75–78, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Rutkowsky JM, Knotts TA, Ono-Moore KD, McCoin CS, Huang S, Schneider D, Singh S, Adams SH, Hwang DH. Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab 306: E1378–E1387, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol 14: 281–287, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 53: 1412–1417, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S, Shulman GI, Abel ED, Goldberg IJ. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest 120: 3443–3454, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamilarasan KP, Temmel H, Das SK, Al Zoughbi W, Schauer S, Vesely PW, Hoefler G. Skeletal muscle damage and impaired regeneration due to LPL-mediated lipotoxicity (Abstract). Cell Death Dis 3: e354, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tantral L, Malathi K, Kohyama S, Silane M, Berenstein A, Jayaraman T. Intracellular calcium release is required for caspase-3 and -9 activation. Cell Biochem Funct 22: 35–40, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Unger RH. Lipotoxic diseases. Ann Rev Med 53: 319–336, 2002. [DOI] [PubMed] [Google Scholar]
- 36.van der Vusse GJ, Prinzen FW, van Bilsen M, Engels W, Reneman RS. Accumulation of lipids and lipid-intermediates in the heart during ischaemia. Basic Res Cardiol 82, Suppl 1: 157–167, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Vianey-Liaud C, Divry P, Gregersen N, Mathieu M. The inborn errors of mitochondrial fatty acid oxidation. J Inherit Metab Dis 10: 159–198, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Whitmer JT, Idell-Wenger JA, Rovetto MJ, Neely JR. Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem 253: 4305–4309, 1978. [PubMed] [Google Scholar]
- 39.Wu J, Corr PB. Influence of long-chain acylcarnitines on voltage-dependent calcium current in adult ventricular myocytes. Am J Physiol Heart Circ Physiol 263: H410–H417, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Wu J, Corr PB. Palmitoyl carnitine modifies sodium currents and induces transient inward current in ventricular myocytes. Am J Physiol Heart Circ Physiol 266: H1034–H1046, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Wu J, McHowat J, Saffitz JE, Yamada KA, Corr PB. Inhibition of gap junctional conductance by long-chain acylcarnitines and their preferential accumulation in junctional sarcolemma during hypoxia. Circ Res 72: 879–889, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Yamada KA, Kanter EM, Newatia A. Long-chain acylcarnitine induces Ca2+ efflux from the sarcoplasmic reticulum. J Cardiovasc Pharmacol 36: 14–21, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Yasuno T, Osafune K, Sakurai H, Asaka I, Tanaka A, Yamaguchi S, Yamada K, Hitomi H, Arai S, Kurose Y, Higaki Y, Sudo M, Ando S, Nakashima H, Saito T, Kaneoka H. Functional analysis of iPSC-derived myocytes from a patient with carnitine palmitoyltransferase II deficiency. Biochem Biophys Res Commun 448: 175–181, 2014. [DOI] [PubMed] [Google Scholar]