Abstract
Transcutaneous and epidural electrical spinal cord stimulation techniques are becoming more valuable as electrophysiological and clinical tools. Recently, we observed selective activation of proximal and distal motor pools during epidural spinal stimulation. In the present study, we hypothesized that the characteristics of recruitment curves obtained from leg muscles will reflect a relative preferential activation of proximal and distal motor pools based on their arrangement along the lumbosacral enlargement. The purpose was to describe the electrophysiological responses to transcutaneous stimulation in leg muscles innervated by motoneurons from different segmental levels. Stimulation delivered along the rostrocaudal axis of the lumbosacral enlargement in the supine position resulted in a selective topographical recruitment of proximal and distal leg muscles, as described by threshold intensity, slope of the recruitment curves, and plateau point intensity and magnitude. Relatively selective recruitment of proximal and distal motor pools can be titrated by optimizing the site and intensity level of stimulation to excite a given combination of motor pools. The slope of the recruitment of particular muscles allows characterization of the properties of afferents projecting to specific motoneuron pools, as well as to the type and size of the motoneurons. The location and intensity of transcutaneous spinal electrical stimulation are critical to target particular neural structures across different motor pools in investigation of specific neuromodulatory effects. Finally, the asymmetry in bilateral evoked potentials is inevitable and can be attributed to both anatomical and functional peculiarities of individual muscles or muscle groups.
Keywords: human, transcutaneous spinal cord stimulation, evoked potentials, electrophysiological assessment, neurorehabilitation
transcutaneous and epidural electrical stimulation techniques applied to the intact or injured spinal cord are emerging as valuable tools for electrophysiological and clinical assessment (10, 12, 13, 21, 39). Given the increasingly recognized clinical importance of spinal stimulation as a means to facilitate recovery of motor function after paralysis (21, 39), a better understanding of excitability of the sensory-motor pathways related to the spinal circuitry using transcutaneous and epidural stimulation is critical.
Spinally evoked potentials using transcutaneous spinal stimulation are most frequently studied with the cathode positioned over the T11–T12 intervertebral space (10, 12, 23, 28), which in the most cases provides simultaneous activation of knee and ankle flexors and extensor muscles. There are also several reports describing the responses to transcutaneous stimulation at different spinal levels along the rostrocaudal axis of the lumbosacral area (3, 36, 40, 58). Furthermore, Roy et al. (47) demonstrated the degree to which transcutaneous stimulation can preferentially activate sensory and motor roots, depending on the stimulation site; whereas, Krenn et al. (32) investigated selective activation of quadriceps and triceps surae muscles. These studies reported that the threshold of activation of the proximal muscles was lower than for the distal muscles when stimulating at the more rostral spinal levels, whereas the threshold for the distal muscles' recruitment occurs at lower intensities than for proximal muscles with stimulation at more caudal levels (32, 40, 47). However, no studies have investigated in details and characterized the topographical recruitment of multiple proximal and distal motor pools, depending on the location of the stimulation site.
Recently, we observed selective activation of proximal and distal motor pools in individuals with complete motor paralysis who were implanted with epidural electrodes during stimulation over the lumbosacral enlargement (48). It was demonstrated that the pathways mediating evoked responses include both monosynaptic and oligosynaptic components. Limiting stimulation within an electrode array to electrodes localized over the spinal segments L1–L2, L3–L4, and L5–S1 allowed for more selective recruitment of the motor pools, whereas a wide-field stimulation over L1–L2 and L5–S1 resulted in a more generalized pattern of activation among proximal and distal motor pools (Ref. 48: cf., Fig. 1).
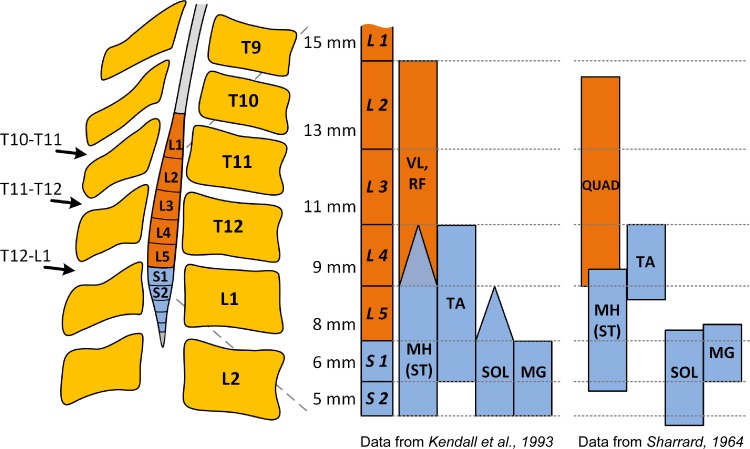
Fig. 1.
Reconstruction showing the approximate location of transcutaneous electrical spinal stimulation over the lumbosacral enlargement, and the location of the motor pools based on the segmental charts provided by Kendall et al. (27) and Sharrard (52). Figure created using data from Refs. 27 and 52. Triangle endings in Kendall's chart denote agreement of three to four sources out of six from the anatomical and clinical data, whereas square ending bars denote motor pools' localization agreed on five or six sources. The numbers shown left at each spinal segment are the average length in millimeters of the segment (52). VL, vastus lateralis; RF, rectus femoris; MH, medial hamstring; ST, semitendinosus; TA, tibialis anterior; SOL, soleus; MG, medial gastrocnemius; QUAD, quadriceps.
We suggested that the complex behavior and characteristics of the spinally evoked potentials using transcutaneous spinal stimulation can be similar to the potentials evoked using epidural stimulation. At the same time, the amount of selectivity at different motor pools' recruitment, as well as the contribution of specific neural structures during transcutaneous stimulation, remain unclear and can be different than during epidural stimulation. Appropriate utilization of these differences may be critical for the therapeutic and functional efficacy of these approaches.
In this study, we characterize the relative selectivity of recruitment of different motor pools innervating leg muscles in uninjured individuals using transcutaneous stimulation along the rostrocaudal axis of the lumbosacral enlargement, at sites similar to the previous study with epidurally implanted electrodes (48) (Fig. 1). We hypothesized that variation in multiple characteristics of the evoked potentials would reflect a relative preferential activation of proximal and distal leg muscles based on the proximal-distal position of the sensory motor pathways and motoneuron pools. The resulting spatiotemporal activation patterns should yield an electrophysiological map that reflects the responsiveness of multiple motor pools, which may, in turn, provide a neurophysiological marker for selecting the more effective stimulation sites for facilitating performance of a given motor task. Here, we describe the electrophysiological features of responses in leg muscles to transcutaneous electrical spinal stimulation at different segmental levels.
METHODS
Participants.
Experiments were conducted in 15 volunteers (7 men and 8 women; mean ± SD: age 29.9 ± 5.2 yr, height 168.3 ± 7.8 cm, body mass 68.8 ± 13.7 kg; 14 right-handed and 1 left-handed). None of the participants had any history of neurological or orthopedic disorders. Participants provided written, informed consent to the experimental procedures, which were approved by the University of Louisville (KY) institutional review board.
Transcutaneous electrical spinal stimulation.
A custom-built constant-current stimulator with a range of 0–100 mA was used to deliver transcutaneous spinal cord stimulation. The stimulation was administered using a conductive rubber electrode with a diameter of 18 mm placed on the skin between the spinous processes at the midline over the vertebral column as a cathode, and two 5 × 9 cm self-adhesive electrodes (Pro-Patch) located symmetrically on the skin over the iliac crests as anodes (10, 12, 20). The spaces between the spinous processes of the T10 and T11, T11 and T12, and T12 and L1 vertebrae were marked following palpation in a prone position. Once the cathode was affixed over the particular interspinous space, the participant was moved to a supine position and stayed relaxed during the experiment. In addition, we prompted the participants to keep their head position as stable as possible and avoid turning it on either side and ensured the symmetrical position of the limbs. The stimulation was delivered as single, 1-ms monophasic square-wave pulses every 6 s. Using 2-mA increments, the stimulation intensity was increased from 2 to 100 mA, or the maximum tolerable intensity. A minimum of three stimuli were delivered at each intensity. Recruitment curves were collected at three stimulation locations (Fig. 1).
EMG recording and data collection.
Surface electromyogram (EMG) signals were recorded bilaterally using bipolar surface electrodes (Motion Lab Systems, Baton Rouge, LA) placed longitudinally on the soleus (SOL), medial gastrocnemius (MG), tibialis anterior (TA), vastus lateralis (VL), rectus femoris (RF), and medial hamstrings (MH) muscles with fixed interelectrode distance of 17 mm. Indifferent or ground reference electrode was placed over the distal part of the left tibia bone and connected to the 16-channel portable unit, which carried the EMG signals to the desktop interface unit. The EMG signals were differentially amplified using MA300 EMG System (Motion Lab Systems, Baton Rouge, LA) with a band-pass filter of 10 Hz to 2 kHz (−3 dB). Finally, the EMG data were digitized at a sampling rate of 5,000 Hz.
Data analysis.
The digitized SOL, MG, TA, VL, RF, and MH EMG time series were full-wave rectified after subtraction of the mean background EMG. Magnitudes of the spinally evoked potentials were calculated by measuring the area under the curve, within a time window manually selected for each muscle. The onset of the time window was defined from the overlaid responses based on the earliest inclination from the baseline across all stimulation intensities. The duration of the time window for different muscles varied between 20 and 40 ms and was kept the same for a given muscle across different stimulation levels. Magnitudes were plotted as a function of stimulation intensity to determine the recruitment curve for each muscle at each location of spinal stimulation. As reported in the studies utilizing epidural spinal cord stimulation (18, 34, 48), two components of the evoked potentials can in some cases be distinguished in plantar flexors and dorsiflexors based on their latencies. Early (ER) and medium responses (MR), which may be attributed to the prevalent involvement of different pathways, were analyzed in such a case. To identify their latencies and magnitudes, the responses were examined at each stimulation intensity from the overlaid waveforms, displayed on a computer monitor upon higher magnification and resolution. The onset of the ER was determined based on the earliest inclination from the baseline in a given muscle at the magnitudes greater than the average plus 3 SDs of the baseline. A separation between two biphasic waveforms characterized by a change in the shape and magnitude of the components was determined as the onset of the MR. In occurrences when it was difficult to distinguish visually the ER and MR, the onset of MR was defined based on the duration of ER at different stimulation intensities within a given muscle and location of the same participant (48) (cf., Fig. 4A). For each component, the area under the full-wave-rectified curve was measured to represent their magnitudes within selected time windows, which were kept the same across different stimulation levels. The appearance of ER and MR was irregular across muscles in different individuals. That is the reason why the main findings are reported as the sum of area of ER and MR. In the pooled data, the evoked potential magnitude for each muscle was reported as a percentage of the maximum value for a given muscle across three stimulation locations; the stimulation intensity then was normalized to that corresponding to the MH threshold response that occurred during stimulation at the interspinous space of T11–T12. Normalization of the values to that of the MH response was chosen because of the interjacent location of the MH motor pools in the lumbosacral enlargement (Fig. 1). As such, we suggested that MH motor pools in all individuals would be always sufficiently activated, irrespective of both rostral or caudal placement of the stimulating electrode.
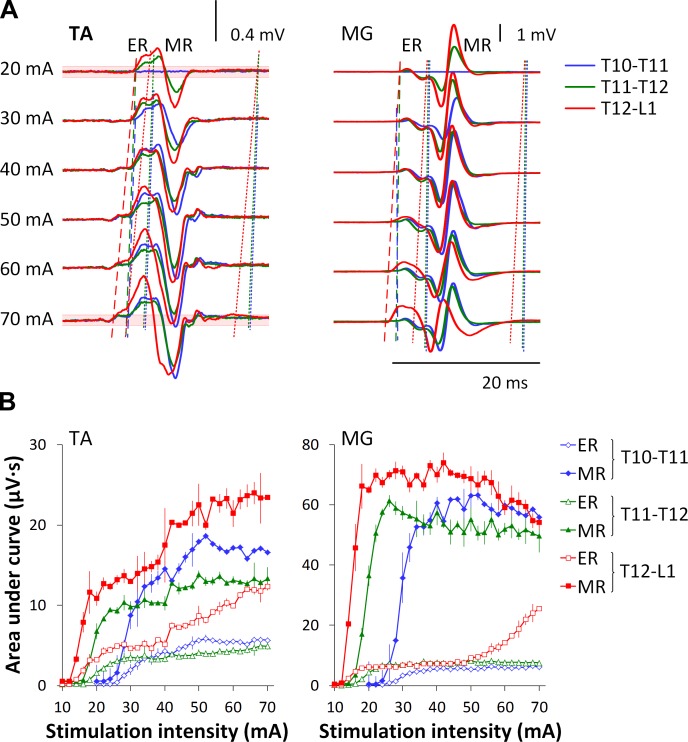
Fig. 4.
Evoked potentials in one participant during transcutaneous electrical spinal stimulation delivered at different intensity at three spinal levels. A: the average of three nonrectified responses is shown for each stimulation intensity. Dashed lines of different colors show the onset of the early responses (ER) determined from the ensemble-averaged waveforms and corresponding to each spinal level of stimulation. Note the changes of the onset with increment of the stimulation intensity. Dotted lines indicate the epochs of medium responses (MR). Note well-distinguished separation between ER and MR at lower stimulation intensities, and a masked, “smoothened” division between them at higher intensities. Red shadowed areas near the baseline on the top and bottom traces on the left indicate the average magnitude plus 3 SDs before the stimulus during stimulation at T12–L1. B: area of the ERs and MRs during stimulation delivered at each stimulation location.
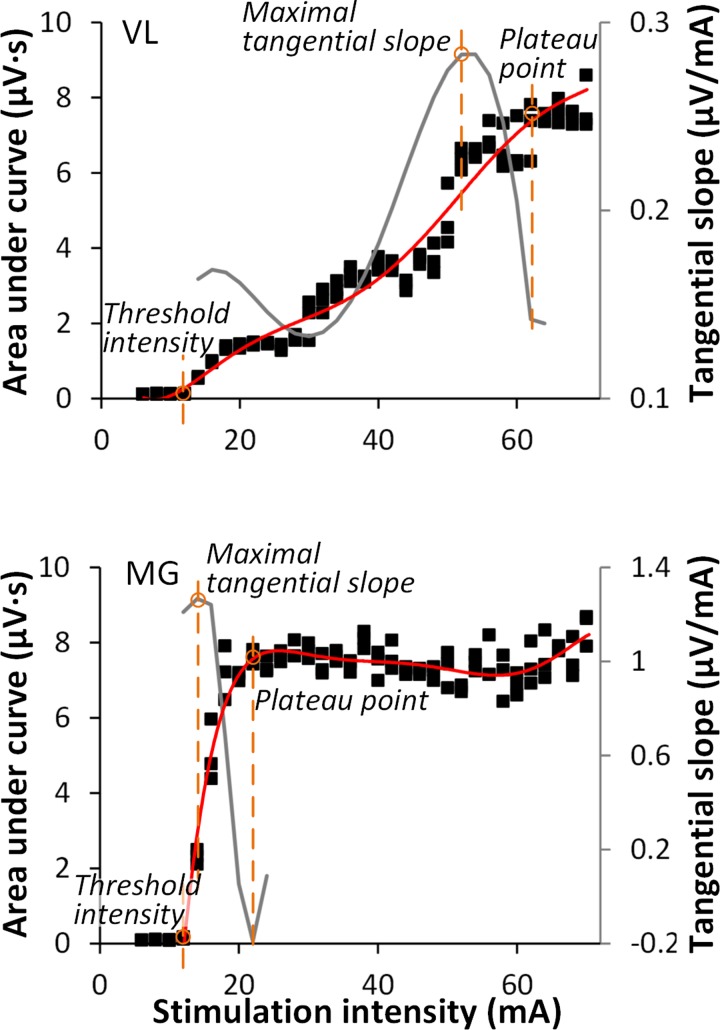
Four parameters were derived from the recruitment curves: 1) threshold intensity, 2) maximal tangential slope, 3) plateau point intensity, and 4) plateau point magnitude (Fig. 2). Threshold intensity was defined as the minimum stimulation intensity that produced the responses with the magnitudes greater than the average plus 3 SDs of the baseline in at least two of three trials. Threshold intensity was chosen as a measurement to provide the initial insight on the sensitivity of individual motor pools, as well as to reveal the sequence of recruitment of different pools in relation to each other. The maximal tangential slope was determined by fitting a sixth-order polynomial function to the ascending limb of the recruitment curve (29) and finding the greatest value of the first derivative. To improve fitting, the polynomial was initiated 6 mA below the threshold intensity. The maximal tangential slope of the recruitment curve can provide the estimate of the rate of Ia afferent recruitment (16, 30, 49) in different muscles in various locations of the stimulation. The plateau point intensity and magnitude were determined based on the minimum decrement of the second derivative of the polynomial, that is, where the slope of the recruitment curve is most rapidly decreasing. These parameters were chosen as the measurements attributed to the maximum or submaximum responses, because in some cases it was difficult to reach a “true plateau” in the magnitude of the responses due to either stimulator limitations or tolerance of participants (see, for instance, Fig. 2, VL). In addition, bilateral asymmetry was calculated for individual variables as a difference between left and right sides. The calculation was performed such that the dividend was always a larger value and the quotient was smaller; the negative values in results indicate that bilateral asymmetry was shifted to the left leg, whereas the positive values indicate the shift to the right leg. The pooled results are summarized in boxplots with the box as the 25–75th percentile, and the whiskers as the highest and lowest values.
Fig. 2.
Examples of recruitment curves (solid squares, left Y-axis) of VL and MG recorded in one participant. A sixth-order polynomial function (red line) was fitted to each curve; tangential slope was then computed as the first derivative (gray line, right Y-axis). Specific parameters were taken from the recruitment curve for experimental comparison: threshold intensity, maximal tangential slope, and plateau point.
Threshold intensity, maximal tangential slope, and plateau point intensity and magnitude were submitted to a 6 (muscles: VL, RF, MH, TA, SOL, and MG) by 3 (stimulation locations: T10–T11, T11–T12, and T12–L1) ANOVA. In this design, both the muscles and stimulation locations were within-participant factors. A post hoc t-tests with Bonferroni correction were made to decompose any significant effects (α = 0.05). The results for the pooled data are presented as mean values and SD.
RESULTS
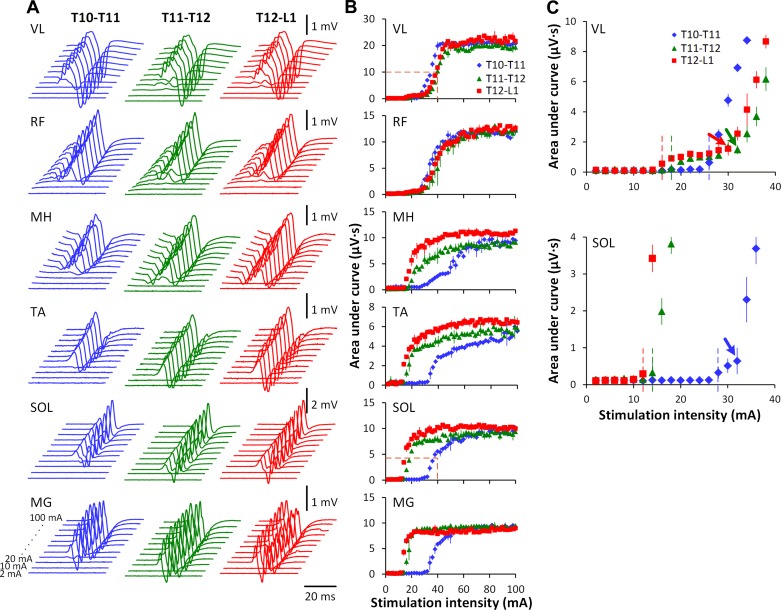
Stimulation over the lumbosacral enlargement resulted in single twitches in multiple leg muscles. Figure 3 demonstrates the evoked potentials (Fig. 3A) and corresponding recruitment curves (Fig. 3B) obtained at different stimulation intensities at three spinal locations in one participant. The order of activation in different muscles in relation to each other was dependent on the rostrocaudal location of stimulation. For instance, during stimulation at T10–T11 at lower stimulation intensities, the magnitude of response of VL and RF was higher than for MG, SOL, and MH, whereas, during stimulation at T12–L1 at the same intensities, this relationship was reversed. The threshold of activation of MG, SOL, TA, as well as MH, was also dependent on the location of stimulation, with thresholds increasing progressively with more rostral site of the stimulation. In proximal and distal muscles, recruitment curve characteristics varied based on stimulation location. Figure 3C shows the initial ascending portions of the recruitment curves of VL and SOL. Increasing intensities of stimulation at T10–T11 was characterized by a rapid increase in the magnitude of the evoked responses in the VL, whereas, at T11–T12 and T12–L1, the evoked potentials were very low, even at stimulation intensities ranging from 16 to 30 mA. The recruitment of SOL, on the contrary, was rapid during the stimulation at T11–T12 and T12–L1 and included a similar low-magnitude suprathreshold phase at T10–T11 as described for VL at T11–T12 and T12–L1.
Fig. 3.
Evoked potentials in one participant during transcutaneous electrical spinal stimulation delivered between the spinous processes of the T10 and T11, T11 and T12, and T12 and L1 vertebrae. A: the average of three nonrectified responses in right muscles at each stimulation intensity from 2 to 100 mA. Shown is the time window between 10 and 55 ms following the stimulus. B: recruitment curves of right muscles at each location of spinal stimulation. Orange dotted lines on VL and SOL indicate the initial rise of the recruitment curves. C: enlarged ascending fragments of the recruitment curves of VL and SOL designated in B. Dotted lines of different colors show the muscle activation threshold corresponding to each spinal level of stimulation. Arrows indicate the inflection points in the ascending fragments recruitment curves.
Figure 4 shows the onset and composition of evoked potentials in TA and MG in one participant during stimulation delivered at three locations. During stimulation at T10–T11 and T11–T12, the onset of the response did not change noticeably at different intensities, whereas, during stimulation at T12–L1, the onset changed considerably as a function of stimulation intensity (Fig. 4A). It is also noteworthy that the separation between ER and MR was more distinguishable at lower stimulation intensities and hardly detectable at higher intensities. In such cases, calculations were performed based on the time windows obtained at lower stimulation intensities within a given muscle and location. Figure 4B depicts recruitment curves of ER and MR during stimulation delivered at three locations. In TA, the increase of stimulation intensity was accompanied by a gradual increase of both ER and MR magnitude. In MG, increasing stimulation intensity from the medium to higher values was accompanied by an increase of the ER and considerable decrease of the MR during stimulation at T12–L1. The intensities required to reach threshold decreased considerably with a more caudal stimulation site (Fig. 4B).
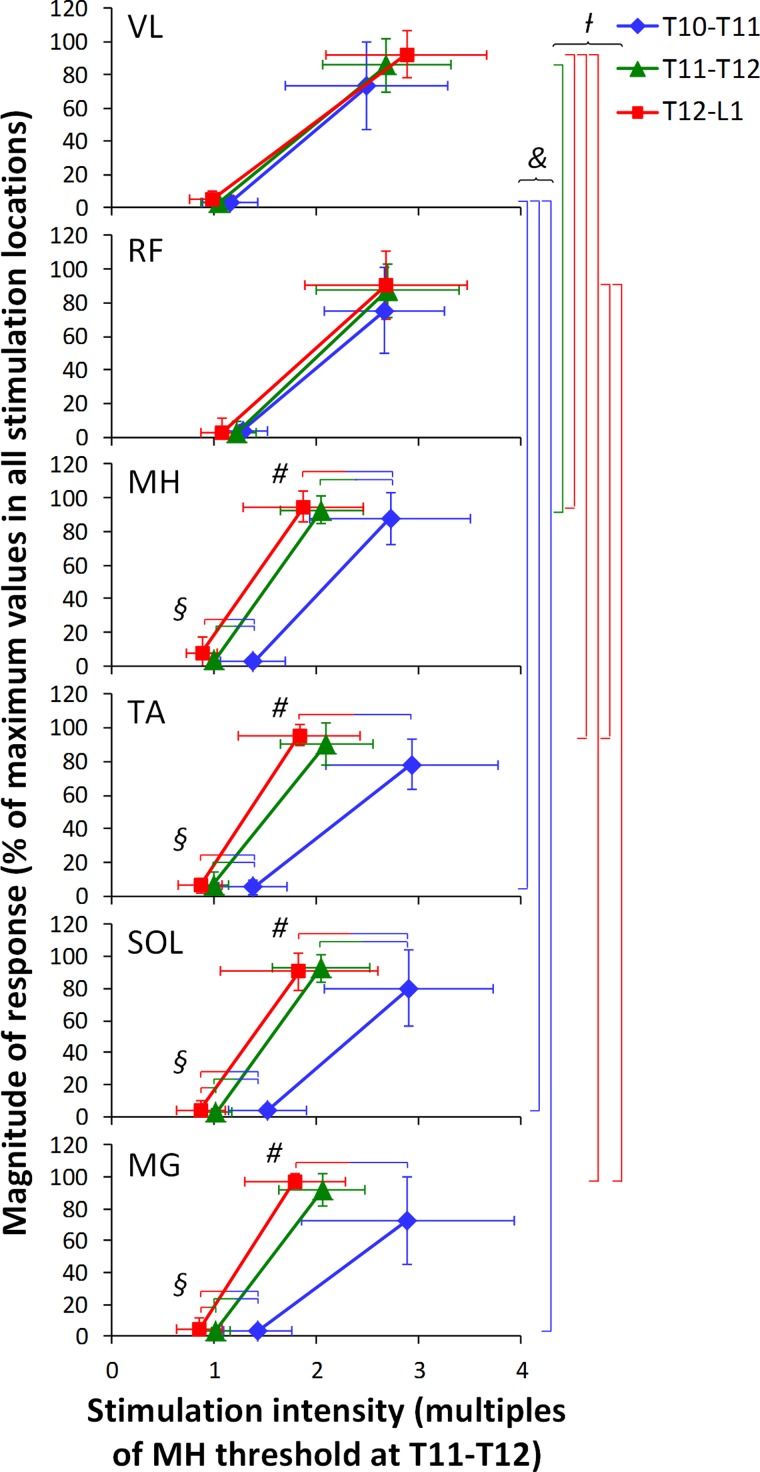
Figure 5 demonstrates the pooled data of threshold intensity, as well as plateau point intensity and magnitude derived from the recruitment curves. The analysis of the threshold intensity revealed main effects for the muscle (F2,14 = 3.7, P = 0.036), stimulation location (F2,14 = 25.1, P < 0.001), as well as the interaction between muscle and stimulation location (F4,14 = 8.4, P = 0.015). To account the number of comparisons, the α-value was corrected to 0.0006 (α = 0.05/81). The main effect for the stimulation location showed that the intensities required to evoke threshold responses were greater at T10–T11 compared with T11–T12 and T12–L1 (P < 0.05). For the interaction between muscle and stimulation location, the differences in the stimulation intensities required to produce threshold responses were revealed within MG, SOL, TA, MH (shown by bicolored horizontal lines), as well as in VL vs. TA, SOL, and MG within T10–T11 stimulation location (shown by blue vertical lines) (P < 0.05) (Fig. 5).
Fig. 5.
Pooled data of the thresholds and plateau points in right leg muscles at three stimulation locations. The magnitude of responses was normalized to the maximum area of each muscle across three stimulation locations. The stimulation intensity was normalized to the motor threshold of MH at T11–T12. Colored vertical bars on the right indicate statistically significant differences in the stimulation intensities required to produce threshold (&) or plateau point (/) responses between different muscles at each location (i.e., blue bars indicate the difference in VL vs. TA, SOL, and MG at T10–T11; green bar indicates the difference in VL vs. MH at T11–T12; and red bars indicate the difference in VL vs. MH, TA, and MG, as well as RF vs. TA and MG at T12–L1). Bicolored horizontal bars indicate significant differences in the stimulation intensities required to produce threshold (§) or plateau responses (#) in a single muscle between different stimulation locations (i.e., red-blue bars indicate the difference between T12–L1 and T10–T11; green-blue bars indicate the difference between T11–T12 and T10–T11; and red-green bars indicate the difference between T12–L1 and T11–T12) (P < 0.05).
The analysis of the plateau point intensity yielded main effects for the stimulation location (F2,14 = 19.9, P < 0.001) and the interaction between muscle and stimulation location (F4,14 = 39.8, P < 0.001). To account for the number of comparisons, the α-value was corrected to 0.0008 (α = 0.05/63). The stimulation intensities required to reach the plateau point were greater at T10–T11 compared with T11–T21 and T12–L1 (P < 0.05). The interaction between muscle and stimulation location differed within MG, SOL, TA, MH (shown by bicolored horizontal lines), as well as within T11–T12 and T12–L1 stimulation locations (shown by green and red vertical lines, respectively) (P < 0.05) (Fig. 5). Plateau point magnitude yielded main effect for the stimulation location (F2,14 = 4.9, P = 0.026). The α-value was corrected to 0.02 (α = 0.05/3). The average of the evoked potential magnitude was smaller during the stimulation at T10–T11, compared with T11–T12 and T12–L1 (P < 0.05).
Figure 6 presents the pooled data of the maximal tangential slope of the ascending portions of recruitment curves of right leg muscles at different locations of stimulation. The maximal tangential slope yielded main effects for the muscle (F2,14 = 8.6, P = 0.007), stimulation location (F2,14 = 9.6, P = 0.005), as well as the interaction between muscle and stimulation location (F4,14 = 18.2, P = 0.04). The α-value was corrected to 0.0006 (α = 0.05/81). The effect for the muscle showed lower slope of the recruitment curve of RF and TA, compared with MG, SOL, and MH (P < 0.05), and is depicted by black horizontal bars (Fig. 6). The slope of the recruitment curve was greater during the stimulation at locations T11–T12 and T12–L1 (0.35 ± 0.2 and 0.45 ± 0.2 μV/mA, respectively), compared with T10–T11 (0.58 ± 0.3 μV/mA) (P < 0.05). For the interaction between muscle and stimulation location, the differences within SOL and MG (shown by bicolored horizontal bars), as well as within the stimulation locations (shown by colored horizontal bars) were significant (P < 0.05) (Fig. 6).
Fig. 6.
Pooled data of the maximal tangential slope of the ascending portions of recruitment curves of right leg muscles at different locations of stimulation. *Black horizontal bars indicate statistically significant differences of the slope between the muscles: RF vs. MH, SOL, MG; MH vs. TA; and TA vs. SOL, MG. ≠|Blue, green, and red horizontal bars indicate statistically significant differences of the slope between different muscles at T10–T11 (i.e., TA vs. SOL, MH), T11–T12 (i.e., RF vs. SOL, MG, as well as TA vs. SOL, MG), and T12–L1 (i.e., RF vs. MG), respectively. Y|Blue-red bars indicate the differences of the slope between T10– T11 and T12–L1 in SOL and MG (P < 0.05).
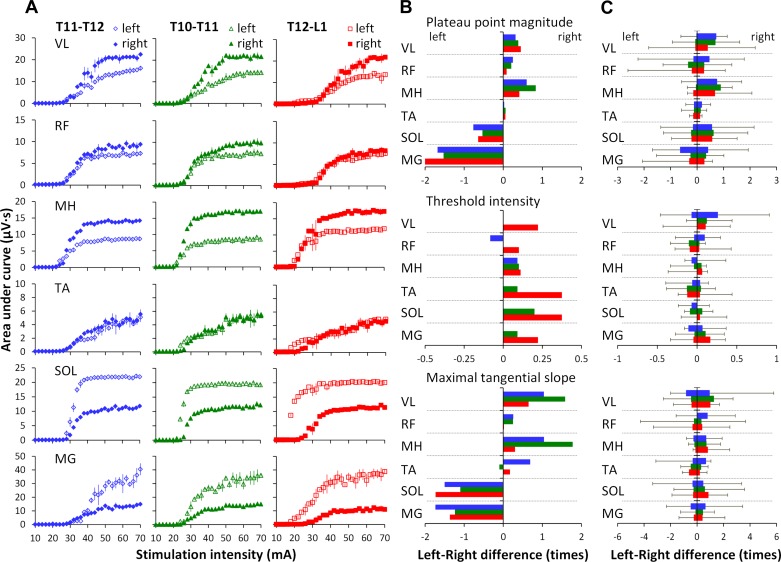
Figure 7, A and B, demonstrates the example of asymmetry between bilateral muscles in one individual during stimulation at different locations. It can be seen that the characteristics of the recruitment curves varied greatly in different muscles (Fig. 7A). Bilateral asymmetry was calculated for the plateau point magnitude, threshold intensity, and maximal tangential slope as a difference between left and right sides: the negative values indicate that bilateral asymmetry was shifted to the left, whereas the positive values indicate the shift to the right leg. Interestingly, with the graphical presentation according to the motor pools' topographical alignment in the lumbosacral enlargement, a pattern of reciprocity between proximal and distal muscles can be for the plateau point magnitude, as well as the maximal tangential slope (Fig. 7B). However, a consistent pattern of bilateral asymmetry was not revealed across the experimental group: the amount, direction, and proximal-distal muscles' relationship varied to a large extent among different individuals (Fig. 7C).
Fig. 7.
A: recruitment curves of left and right muscles at each location of spinal stimulation in one participant. Individual (B) and pooled data (C) illustrating the asymmetry level between left and right muscles in the plateau point magnitude (top), threshold intensity (middle), and maximal tangential slope (bottom).
DISCUSSION
The stimulation intensities required to evoke threshold responses, as well as to reach the magnitude plateau, were greater at more rostral locations of stimulation. Transcutaneous electrical stimulation of rostral and caudal areas of lumbosacral enlargement resulted in a selective topographical recruitment of proximal and distal leg muscles, based on their threshold intensity, maximal slope of the recruitment curves, and plateau point intensity and magnitude. The maximal slope of the recruitment curves varied among muscles. Finally, considerable levels of bilateral asymmetry in spinally evoked potentials were observed, varying among participants.
Spatiotemporal patterns of motor pool activation along the rostrocaudal axis of the spinal cord.
Using epidural stimulation of rostral and caudal areas of lumbar spinal cord, our laboratory has previously demonstrated a predominant activation of proximal and distal muscles' motoneuron pools (48). In the present study, we hypothesized that the optimal site for activation of a particular motor pool within the lumbosacral enlargement can be derived from changes in the relationship of multiple motor pools' recruitment characteristics across three stimulation locations. We found that transcutaneous spinal stimulation delivered even within a relatively narrow range between T10 and L1 vertebrae results in a different order of activation of the proximal and distal motor pools. Our data are generally consistent with previous reports (32, 47, 58), as well as with the anatomy and myotomal maps of the spinal cord and lumbosacral roots (2, 25, 27, 44, 51, 52, 55) (Fig. 1). We observed that stimulation over the areas that approximately correspond to L2–L4 or L4–S2 spinal segments differentially modulates the order of activation of the proximal and distal motor pools (Fig. 5).
We have also found more specific modulation of the relative threshold intensities and rate of recruitment of the MG, SOL, TA, and MH, compared with little changes in VL and RF at different spinal levels (Figs. 5 and 6). This difference may not be surprising in light of the anatomical arrangement of the VL and RF motor pools along the lumbosacral enlargement, and the average length of the corresponding L2 and L4 spinal segments (Fig. 1). At all three locations used, the stimulation always activated a considerable portion of the VL and RF nerve roots, as suggested by a minimal variation in the threshold and slope of the recruitment curve. Compared with VL and RF, more distal motor pools located in smaller and fewer spinal segments had greater threshold of activation during stimulation over the T10–T11 intervertebral level and reached their plateau point at relatively lower intensities during stimulation over the T12–L1 intervertebral level.
It is also noteworthy that, for all motor pools, the absolute intensities required to evoke threshold responses, as well as to reach the magnitude plateau, increased with more rostral stimulation delivery (Figs. 4B and 5). It is plausible that these results may be attributed to differences in the geometry of the thoraco-lumbar spine, as well as alignment of the corresponding dorsal roots, leading to changes in the relative position of the stimulating electrode, thereby influencing the flow of current during stimulation.
The analysis of the maximal slope of the recruitment curves of individual muscles, which, based on the H-reflex, has been suggested to indicate the rate of Ia afferent recruitment (16, 30, 49), revealed significant levels of both the interlocation and intermuscle variations. The interlocation variation of the rate of recruitment may also be explained by the difference in the anatomical arrangement of the motor pools, as described in the previous paragraph and, therefore, is dependent on the length of the corresponding spinal segments, as well as the distance between the stimulation location and projecting dorsal roots.
The intermuscle variation of the maximal slope of the recruitment curves can be attributed to the difference in diameter and number of low-threshold Ia afferents projecting to specific motoneuron pools, as well as to the type and size of the motoneurons (45). Our findings support these assertions. For instance, MG, SOL, and MH have larger diameter Ia afferents, compared with the VL and RF (37, 45). It is also known that motoneurons innervating slow-twitch units receive preferential distribution of Ia excitatory inputs (14). Furthermore, the number of connections between the motoneuron and the Ia afferent (synaptic density per unit area) varies inversely with the size of the motoneuron (54). The difference in the maximal slope of the recruitment curve between different muscles may also depend on the tonic level of presynaptic inhibition of Ia terminals (45), and in this context our data seem to concur with the occurrence and variations in the size of the maximal H-reflex demonstrated among different muscles at rest in previous studies (45, 50, 61). Whether this represents a functional separation in connections from Ia afferents to flexor and extensor muscles is presently unclear (61). It has been suggested that the motoneurons of individual muscles could have different central connections and recruitment patterns, depending on the intrinsic properties optimized for the performance of a specific functional task (22, 35). It is worth noting that the slowest slope gain occurred in the RF and TA, which are located anteriorly, and counteract posterior shifts of the center of mass, whereas the fastest slope gain occurred in posteriorly located muscles, such as SOL, MG, and MH, whose primary role during quiet standing is to resist the gravity-induced torque pulling the body forward due to the anterior location of the center of mass relative to the ankle joint axis. These anti-gravity muscles are requisite for continuous and accurate postural adjustments; their synergy and connection were demonstrated at both functional (23) and neurophysiological (45) levels. Finally, it is intriguing that the analysis has revealed considerable differences in the VL and RF recruitment characteristics compared with MH and distal muscles (Figs. 5 and 6). One explanation of this difference can be the activation of nerve fibers in the lumbar plexus through the anodes placed over the iliac crests. While activation of the lumbar plexus cannot be ruled out, modeling studies suggest that this is doubtful (33). It seems more likely that heteronomous inhibitory connections from the antagonistic MH might have a different influence on the recruitment characteristics of VL and RF.
Possible neural structures activated during transcutaneous spinal stimulation.
In the modeling study, it has been shown that transcutaneous stimulation can depolarize at least a subset of the same neural structures as recruited by implanted epidural electrodes (33). Data obtained from experiments with epidural stimulation (48) demonstrate that, with increasing intensity, the stimulus response relationship of the early and medium components in many muscles shares some characteristics with the H-reflex and M-wave interaction; that is, it is characterized by the initial increase of the MR, followed by a decrease in its magnitude at low-to-moderate stimulation intensities (similar to the H-reflex), and continuous increase of the ER magnitude, followed by a plateau at moderate-to-high stimulation intensities (similar to the M-wave) (Ref. 48; cf., Fig. 5). These findings let us suggest that epidural stimulation results in the recruitment of both sensory and motor fibers. Similar to those findings, our data indicate that transcutaneous spinal electrical stimulation can involve diverse elements along the spinal cord, based on the location and intensity (Figs. 3C and 4). An analysis of ER and MR recruitment curves in MG at different stimulation locations in one individual has revealed that, during stimulation at T12–L1, their relationship was reciprocal: the increment of ER was accompanied by a substantial decrement of MR (Fig. 4B). Although these findings have to be interpreted with caution, they suggest that lower stimulation intensities result in initial preferential recruitment of lower threshold afferent fibers accompanied to some extent with involvement of motor axons, whereas, with increasing intensity, more motor axons may become activated, leading to the decreased latency of the response and causing an occlusion effect of the afferent pathways. This notion concurs with prior reports obtained in experiments with transcutaneous (40) and epidural (48) spinal cord stimulation, as well as in modeling studies (33). Another plausible explanation of the reciprocal interplay between the ER and MR is the finding obtained in animal models (15) and human subjects (42) on the existence of inhibitory circuits from cutaneous receptors of the lumbosacral back contained within the spinal cord. In this light, it is possible that incremental increases in the stimulation intensity would result in a decrease in the magnitude of longer latency component of the evoked potentials. In experiments with H-reflex, Burke et al. (7) suggested that the composite excitatory postsynaptic potential of the H-reflex in the motoneurons of SOL had a sufficiently long rising phase to permit oligosynaptic inputs to reach the motoneuron. There is also considerable evidence from animal models (26) and in humans (41, 45) for the existence of oligosynaptic pathways from group Ia afferents onto homonymous and synergistic muscles. Based on the evidence from previous experiments with transcutaneous (12) and epidural spinal stimulation (48), there are also mounting reasons to suggest the contribution of oligosynaptic pathways to spinally evoked potentials. We suggest that, with increasing of the stimulation intensity, in addition to the Ia afferents, the involvement of smaller diameter afferents, including group Ib, larger diameter cutaneous afferents, group II muscle spindle afferents, and even intraspinal connections and spinal interneurons (34), as well as direct motor activation (47), may all contribute to the spinally evoked potentials with progressively higher stimulation intensities. Not all of these structures are susceptible to neuromodulatory effects, such as facilitation or inhibition during conditioning experiments (11), and might be influenced by inhibitory pathways (46) or heteronymous facilitation (8). Our data demonstrate the feasibility of the approach when responses can and should be evoked at a particular stage of recruitment across different muscles. Such approach may be of importance during neurophysiological or functional assessment, when activation of particular structures among different motor pools is advantageous.
Symmetry of evoked potentials in individual muscles.
We also investigated the symmetry of the bilateral evoked potentials in individual muscles. Bilateral anatomical asymmetries have been previously observed in numerous animal models (4, 5, 60). In systematic experiments with cats, it has been proposed that only the differences in the magnitude of reflex responses exceeding 15–20% can signify “true biological asymmetry” (24). We revealed that, even with careful bilateral symmetrical placement of both EMG and reference electrodes, as well as the stimulating electrode over the midline, the asymmetry between left and right that exceeds 100% is often inevitable. In the tested population, 14 participants were right-handed, one was left-handed, and there was no distinct pattern in occurrence of the asymmetry: its extent between the left and right responses varied considerably in proximal and distal muscles among different individuals. Contradictory results are found in the literature on the correlation between the asymmetries in the right and left SOL H-reflex recruitment curves and the dominant side of participants (9, 19, 43, 56). Recently, it has been suggested that lateral dominance does not seem to affect difference in the H-reflex characteristics, such as mean amplitude and level of variability, and it is likely that lateral dominance is less important in the leg than in the arm (38). We did not record the leg dominance in our experiments and suggest this as an additional measure in future experiments.
The existence of asymmetry in experiments with transcutaneous spinal cord stimulation has been noted previously (10, 40). These variations could arise from interindividual variability in the segmental innervation (44, 55), the anatomical asymmetries, including differences in the composition of the peripheral nerve fibers and the proportion of the type Ia, Ib, and II afferents (1, 6, 38), as well as from the asymmetry of motoneuron excitability (56). In addition, as it has been supported by recent studies examining SOL H-reflex operant conditioning (57, 59), the differences between the contralateral responses can be attributed to the functional peculiarities of individual muscles or muscle groups. By exploring the bilateral asymmetry using transcutaneous spinal cord stimulation, our data further contribute to the investigation of the phenomenon and indicate that spinally evoked potentials can serve in physiological, anatomic, and clinical studies, evaluating the degree of symmetry of the respective spinal reflex circuits. This is a highly relevant issue from the clinical perspective, given that almost all spinal injuries are functionally asymmetric. Further separate study of this phenomenon with test-retest reliability measurements, as well as in relation to the peripheral nerves' stimulation, is warranted.
Methodological considerations.
Our findings on the stimulus-response relationships at different configurations were similar in all participants. Although the previously published anatomical localization of segments of the human lumbosacral spinal cord has been confirmed by functional magnetic resonance imaging (31), the present findings have to be implemented, acknowledging potential interindividual variability of the anatomical relationship between spinal cord and vertebral column (53, 58), as well as in the segmental myotomal charts of muscle innervation (44, 55). As such, we suggest that the vertebral levels presented in our study will be used as approximate guidance, and the exact spatiotemporal maps of the proximal and distal leg muscles should be determined individually during probing over the lumbosacral area, based on the recruitment characteristics described. In addition, variations in threshold and magnitude of the evoked potentials among different individuals can result from variations in the underlying skin resistance, different amounts of subcutaneous fat, muscles, vertebral bone, and intervertebral ligaments.
Finally, different characteristics of spinal reflexes highly depend on the descending and segmental conditions, the level of muscle activity, and on the task performed (10, 12, 17, 45). Although the neuromodulatory effects of transcutaneous electrical spinal cord stimulation on the locomotor networks has been demonstrated (17, 20), there is a need to investigate to what extent the present data on the spinal segment-specific effects obtained in a supine resting position are relevant during different motor tasks, including gait rehabilitation or recovery of postural function.
Conclusion.
The present data show that transcutaneous electrical spinal cord stimulation can be used to differentially activate motor pools and projecting dorsal roots based on their anatomical arrangements along the rostrocaudal axis of the lumbosacral enlargement. We demonstrated that, during transcutaneous stimulation, preferential activation of spinal structures at specific segments is possible, even with the considerably wider configuration of the stimulating and reference electrodes and thus a less focused current flow compared with epidural stimulation. Selective recruitment of proximal and distal motor pools can be titrated at lower intensities. As during epidural spinal stimulation, with variation of the stimulation intensity and location, different neural structures may be activated. The maximal slope of the recruitment of particular muscles allows characterization of the properties of afferents projecting to specific motoneuron pools, as well as to the type and size of the motoneurons. The spatiotemporal patterns of spinal motor activation may have important clinical as well as electrophysiological implications, especially when there is a need to target particular motor pools. Finally, the asymmetry in bilateral evoked potentials is inevitable and can be attributed to both anatomical and functional peculiarities of individual muscles or muscle groups.
GRANTS
This work was supported by the Frazier Rehab Institute and Kentucky One Health, Kentucky Spinal Cord & Head Injury Research Trust Grant no. 11-7, National Institute of General Medical Sciences Grant 8 P30 GM-103507, Russian Foundation for Basic Research Grant 13-04-12030 ofi-m, and Russian Scientific Fund Project Nos. 14-15-00788 (development of the method for transcutaneous spinal cord stimulation) and 14-45-00024 (analysis of evoked potentials).
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.G.S. and Y.P.G. conception and design of research; D.G.S., D.A.A., C.J.D., K.M.G., V.L.S., and Y.P.G. performed experiments; D.G.S. and K.M.G. analyzed data; D.G.S., D.A.A., V.R.E., and Y.P.G. interpreted results of experiments; D.G.S. prepared figures; D.G.S. drafted manuscript; D.G.S., D.A.A., C.J.D., K.M.G., V.L.S., C.A.A., V.R.E., and Y.P.G. edited and revised manuscript; D.G.S., D.A.A., C.J.D., K.M.G., V.L.S., S.J.H., V.R.E., and Y.P.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the research volunteers for valuable contributions to this study.
REFERENCES
- 1.Aggelopoulos NC, Edgley SA. Segmental localisation of the relays mediating crossed inhibition of hindlimb motoneurones from group II afferents in the anaesthetized cat spinal cord. Neurosci Lett 185: 60–64, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Altman J, Bayer SA. Development of the Human Spinal Cord: An Interpretation Based on Experimental Studies in Animals. Oxford, UK: Oxford University Press, 2001, p. xi, 0542. [Google Scholar]
- 3.Andriianova E. [Peculiarities of multisegmental monosynaptic responses of leg muscles in subjects with lumbar nerve compression]. Fiziol Cheloveka 36: 80–88, 2010. [PubMed] [Google Scholar]
- 4.Avendano C, Lagares A. A stereological analysis of the numerical distribution of neurons in dorsal root ganglia C4-T2 in adult macaque monkeys. Somatosens Mot Res 13: 59–66, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Barker D, Chin NK. The number and distribution of muscle-spindles in certain muscles of the cat. J Anat 94: 473–486, 1960. [PMC free article] [PubMed] [Google Scholar]
- 6.Baxendale RH, Rosenberg JR. Crossed reflexes evoked by selective activation of muscle spindle primary endings in the decerebrate cat. Brain Res 115: 324–327, 1976. [DOI] [PubMed] [Google Scholar]
- 7.Burke D, Gandevia SC, McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol 339: 535–552, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol 52: 435–448, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Chandran AP, Maini BK, Marya RK. Long latency inhibition of H-reflex recovery by cutaneous tactile stimulation in man: a cutaneous transcortical reflex. Neuroscience 27: 1037–1048, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Courtine G, Harkema SJ, Dy CJ, Gerasimenko YP, Dyhre-Poulsen P. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J Physiol 582: 1125–1139, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81: 35–45, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Dy CJ, Gerasimenko YP, Edgerton VR, Dyhre-Poulsen P, Courtine G, Harkema SJ. Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J Neurophysiol 103: 2808–2820, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgerton VR, Harkema S. Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev Neurother 11: 1351–1353, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgerton VR, Smith JL, Simpson DR. Muscle fibre type populations of human leg muscles. Histochem J 7: 259–266, 1975. [DOI] [PubMed] [Google Scholar]
- 15.Frigon A, Thibaudier Y, Johnson MD, Heckman CJ, Hurteau MF. Cutaneous inputs from the back abolish locomotor-like activity and reduce spastic-like activity in the adult cat following complete spinal cord injury. Exp Neurol 235: 588–598, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funase K, Imanaka K, Nishihira Y. Excitability of the soleus motoneuron pool revealed by the developmental slope of the H-reflex as reflex gain. Electromyogr Clin Neurophysiol 34: 477–489, 1994. [PubMed] [Google Scholar]
- 17.Gerasimenko Y, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Roy RR, Lu DC, Edgerton VR. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J Neurophysiol 113: 834–842, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods 157: 253–263, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Goode DJ, Glenn S, Manning AA, Middleton JF. Lateral asymmetry of the Hoffmann reflex: relation to cortical laterality. J Neurol Neurosurg Psychiatry 43: 831–835, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorodnichev RM, Pivovarova EA, Pukhov A, Moiseev SA, Savokhin AA, Moshonkina TR, Shcherbakova NA, Kilimnik VA, Selionov VA, Kozlovskaia IB, Edgerton VR, Gerasimenko IP. [Transcutaneous electrical stimulation of the spinal cord: non-invasive tool for activation of locomotor circuitry in human]. Fiziol Cheloveka 38: 46–56, 2012. [PubMed] [Google Scholar]
- 21.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffer JA, Loeb GE, Sugano N, Marks WB, O'Donovan MJ, Pratt CA. Cat hindlimb motoneurons during locomotion. III. Functional segregation in Sartorius. J Neurophysiol 57: 554–562, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Hofstoetter US, Minassian K, Hofer C, Mayr W, Rattay F, Dimitrijevic MR. Modification of reflex responses to lumbar posterior root stimulation by motor tasks in healthy subjects. Artif Organs 32: 644–648, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Hultborn H, Malmsten J. Changes in segmental reflexes following chronic spinal cord hemisection in the cat. I. Increased monosynaptic and polysynaptic ventral root discharges. Acta Physiol Scand 119: 405–422, 1983. [DOI] [PubMed] [Google Scholar]
- 25.Ivanenko YP, Poppele RE, Lacquaniti F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J Neurophysiol 95: 602–618, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Jankowska E, McCrea D, Mackel R. Oligosynaptic excitation of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol 316: 411–425, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendall FP, McCreary EK, Provance PG. Muscles, Testing and Function: With Posture and Pain. Baltimore, MD: Williams & Wilkins, 1993, p. xv, 451. [Google Scholar]
- 28.Kitano K, Koceja DM. Spinal reflex in human lower leg muscles evoked by transcutaneous spinal cord stimulation. J Neurosci Methods 180: 111–115, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Klimstra M, Zehr EP. A sigmoid function is the best fit for the ascending limb of the Hoffmann reflex recruitment curve. Exp Brain Res 186: 93–105, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Komiyama T, Kawai K, Fumoto M. The excitability of a motoneuron pool assessed by the H-reflex method is correlated with the susceptibility of Ia terminals to repetitive discharges in humans. Brain Res 826: 317–320, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Kornelsen J, Stroman PW. fMRI of the lumbar spinal cord during a lower limb motor task. Magn Reson Med 52: 411–414, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Krenn M, Toth A, Danner SM, Hofstoetter US, Minassian K, Mayr W. Selectivity of transcutaneous stimulation of lumbar posterior roots at different spinal levels in humans. Biomed Tech (Berl) 58, Suppl 1, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Ladenbauer J, Minassian K, Hofstoetter US, Dimitrijevic MR, Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehabil Eng 18: 637–645, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol 96: 1699–1710, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Loeb GE. Motoneurone task groups: coping with kinematic heterogeneity. J Exp Biol 115: 137–146, 1985. [DOI] [PubMed] [Google Scholar]
- 36.Maertens de Noordhout A, Rothwell JC, Thompson PD, Day BL, Marsden CD. Percutaneous electrical stimulation of lumbosacral roots in man. J Neurol Neurosurg Psychiatry 51: 174–181, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Exp Brain Res 96: 534–544, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Mezzarane RA, Kohn AF. Bilateral soleus H-reflexes in humans elicited by simultaneous trains of stimuli: symmetry, variability, and covariance. J Neurophysiol 87: 2074–2083, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Minassian K, Hofstoetter U, Tansey K, Mayr W. Neuromodulation of lower limb motor control in restorative neurology. Clin Neurol Neurosurg 114: 489–497, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minassian K, Persy I, Rattay F, Dimitrijevic MR, Hofer C, Kern H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 35: 327–336, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 28: 144–160, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Nadeau S, Jacquemin G, Fournier C, Lamarre Y, Rossignol S. Spontaneous motor rhythms of the back and legs in a patient with a complete spinal cord transection. Neurorehabil Neural Repair 24: 377–383, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Nativ A, Frank J, Allard F. The effect of handedness on spinal and supra-spinal reflex excitability. Electroencephalogr Clin Neurophysiol 72: 157–164, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Phillips LH 2nd, Park TS. Electrophysiologic mapping of the segmental anatomy of the muscles of the lower extremity. Muscle Nerve 14: 1213–1218, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge, UK: Cambridge University Press, 2005, p. xxii, 642. [Google Scholar]
- 46.Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res 42: 337–350, 1981. [DOI] [PubMed] [Google Scholar]
- 47.Roy FD, Gibson G, Stein RB. Effect of percutaneous stimulation at different spinal levels on the activation of sensory and motor roots. Exp Brain Res 223: 281–289, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Sayenko DG, Angeli C, Harkema SJ, Edgerton VR, Gerasimenko YP. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J Neurophysiol 111: 1088–1099, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekiguchi H, Nakazawa K, Akai M. Recruitment gain of antagonistic motoneurons is higher during lengthening contraction than during shortening contraction in man. Neurosci Lett 342: 69–72, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Semmler JG, Turker KS. Compound group I excitatory input is differentially distributed to motoneurons of the human tibialis anterior. Neurosci Lett 178: 206–210, 1994. [DOI] [PubMed] [Google Scholar]
- 51.Sharrard WJ. The distribution of the permanent paralysis in the lower limb in poliomyelitis; a clinical and pathological study. J Bone Joint Surg Br 37-B: 540–558, 1955. [DOI] [PubMed] [Google Scholar]
- 52.Sharrard WJ. The segmental innervation of the lower limb muscles in man. Ann R Coll Surg Engl 35: 106–122, 1964. [PMC free article] [PubMed] [Google Scholar]
- 53.Soleiman J, Demaerel P, Rocher S, Maes F, Marchal G. Magnetic resonance imaging study of the level of termination of the conus medullaris and the thecal sac: influence of age and gender. Spine (Phila Pa 1976) 30: 1875–1880, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Stein R, Bertoldi R. The size principle: a synthesis of neurophysiological data. In: Motor Unit Types, Recruitment and Plasticity in Health and Disease. Progress in Clinical Neurophysiology, edited by Desmedt JE. Basel: Karger, 1981, vol. 9, p. 85–96. [Google Scholar]
- 55.Stewart JD. Electrophysiological mapping of the segmental anatomy of the muscles of the lower extremity. Muscle Nerve 15: 965–966, 1992. [PubMed] [Google Scholar]
- 56.Tan U. Lateral asymmetry of H-reflex recovery curves in cat: evidence for a spinal motor asymmetry. Int J Neurosci 24: 45–52, 1984. [DOI] [PubMed] [Google Scholar]
- 57.Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29: 5784–5792, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Troni W, Di Sapio A, Berra E, Duca S, Merola A, Sperli F, Bertolotto A. A methodological reappraisal of non invasive high voltage electrical stimulation of lumbosacral nerve roots. Clin Neurophysiol 122: 2071–2080, 2011. [DOI] [PubMed] [Google Scholar]
- 59.Wolpaw JR, Lee CL, Calaitges JG. Operant conditioning of primate triceps surae H-reflex produces reflex asymmetry. Exp Brain Res 75: 35–39, 1989. [DOI] [PubMed] [Google Scholar]
- 60.Ygge J, Aldskogius H, Grant G. Asymmetries and symmetries in the number of thoracic dorsal root ganglion cells. J Comp Neurol 202: 365–372, 1981. [DOI] [PubMed] [Google Scholar]
- 61.Zehr PE. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 86: 455–468, 2002. [DOI] [PubMed] [Google Scholar]