Abstract
The mechanisms responsible for exercise-induced reductions in baseline heart rate (HR), known as training bradycardia, remain controversial. Therefore, changes in cardiac autonomic regulation and intrinsic sinoatrial nodal (SAN) rate were evaluated using dogs randomly assigned to either a 10- to 12-wk exercise training (Ex, n = 15) or an equivalent sedentary period (Sed, n = 10). Intrinsic HR was revealed by combined autonomic nervous system (ANS) blockade (propranolol + atropine, iv) before and after completion of the study. At the end of the study, SAN function was further evaluated by examining the SAN recovery time (SNRT) following rapid atrial pacing and the response to adenosine in anesthetized animals. As expected, both the response to submaximal exercise and baseline HR significantly (P < 0.01) decreased, and heart rate variability (HRV; e.g., high-frequency R-R interval variability) significantly (P < 0.01) increased in the Ex group but did not change in the Sed group. Atropine also induced significantly (P < 0.01) greater reductions in HRV in the Ex group compared with the Sed group; propranolol elicited similar HR and HRV changes in both groups. In contrast, neither intrinsic HR (Ex before, 141.2 ± 6.7; Ex after, 146.0 ± 8.0 vs. Sed before, 143.3 ± 11.1; Sed after, 141.0 ± 11.3 beats per minute), the response to adenosine, corrected SNRT, nor atrial fibrosis and atrial fibrillation inducibility differed in the Ex group vs. the Sed group. These data suggest that in a large-animal model, training bradycardia results from an enhanced cardiac parasympathetic regulation and not from changes in intrinsic properties of the SAN.
Keywords: exercise training, heart rate variability, sinoatrial node, bradycardia, cardiac autonomic regulation, parasympathetic nervous system, beta-adrenergic receptors
although training bradycardia is a well-established consequence of endurance exercise training (18, 28, 62), the mechanisms responsible for this reduction in baseline heart rate (HR) remain controversial (17). Exercise training-induced changes in HR could result from either changes in the autonomic regulation of cardiac pacemaker cells (i.e., increased parasympathetic neural regulation, reduced sympathetic neural regulation, or both), reduced spontaneous sinoatrial nodal (SAN) cell depolarization rates (i.e., changes in the intrinsic or inherent pacemaker rate, intrinsic HR), or some combination of the two mechanisms.
The effect of exercise training on cardiac autonomic regulation has been extensively studied in both humans and animals (8, 18, 28, 30, 62). Several studies provide strong evidence that endurance exercise training increases cardiac parasympathetic regulation and decreases sympathetic activation (8, 18, 28, 30, 62), and furthermore, that these changes in cardiac autonomic regulation are believed to be largely responsible for training-induced decreases in resting HR (18, 62). For example, most but not all studies have shown that exercise training consistently increases various indices of HR variability (HRV) (8, 12, 13, 18, 24, 52, 53), whereas intrinsic HR, as revealed by pharmacological blockade (i.e., combined beta-adrenergic and muscarinic receptor antagonists) of the autonomic nervous system (ANS), was similar in exercise-trained and sedentary humans or animals (23, 55, 62). Ordway and associates (48) further demonstrated that training bradycardia could not be induced in cardiac denervated dogs.
In contrast, D'Souza and co-workers (25), using a murine model, recently demonstrated that HR reductions associated with exercise training persisted after autonomic blockade in intact mice and in denervated SAN preparations, but were eliminated by the pharmacologic inhibition of an important pacemaker current (the so-called funny current, If). They further found that HCN4 protein expression and its corresponding ionic current (If) were reduced in exercise-trained mice compared with a sedentary group. They proposed that training bradycardia in athletes results from molecular remodeling of SAN function rather than as a consequence of changes in cardiac autonomic regulation (17). In contrast to longitudinal studies that largely support a predominant contribution cardiac autonomic component (42, 53, 55), cross-sectional studies comparing elite athletes with sedentary subjects also tend to support an important role for intrinsic HR changes (15, 38, 41, 57, 58) in training bradycardia.
Several methodological considerations make direct comparison between these studies difficult. For example, there are important cardiac electrophysiological and autonomic neural regulatory differences in species with high (rodents) and low (dogs, humans) resting heart rates (37, 46, 51, 54), whereas anatomical and functional studies suggest that the canine SAN is a more realistic model for the human SAN than that of small mammals (26).
Therefore, the present study investigated the effects of endurance exercise training on autonomic neural regulation of HR and SAN function in the dog, a species that shares similar cardiac autonomic neural and electrophysiological properties with humans (26, 37, 46, 51, 54). As such, the present study tested the hypothesis that training-induced bradycardia results from alterations in cardiac parasympathetic regulation rather than from changes in intrinsic pacemaker rate in a large, clinically relevant, animal model. Because it has recently been shown that intense exercise training promotes cardiac chamber fibrosis, thus increasing the risk for arrhythmias in the rat (5), the effect of more moderate endurance exercise training on cardiac chamber fibrosis was also investigated as an ancillary study.
METHODS
All animal procedures used in the present study were approved by the Ohio State University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (revised 1996) published by the National Academies Press (Washington, DC).
Submaximal exercise protocol.
Twenty-five heartworm-free, mixed-breed dogs (1-4 yr old, 13 male, 12 female) weighing 20.1 ± 0.5 kg (range, 15.5–26.6 kg) were used in this study. The animals were trained to run on a motor-driven treadmill and habituated to the laboratory environment for at least 1 wk before data collection began. HR and HRV [as an index of cardiac parasympathetic regulation (9)] response to submaximal exercise was evaluated before and again after the completion of the 10- to 12-wk exercise training or sedentary period (see below). Electrocardiographic (ECG) readings were obtained from electrodes placed on either side of the animal's chest and securely fastened with an elastic bandage. This exercise test consisted of six 3-min stages as follows: 4.8 kilometers per hour (kph), 0% grade; 6.4 kph, 0% grade; 6.4 kph, 4% grade; 6.4 kph, 8% grade; 6.4 kph, 12% grade; and 6.4 kph, 16% grade (7, 10, 11). Data were collected beginning 3 min before exercise onset, throughout exercise, and for the first 3 min following exercise cessation. The submaximal exercise test was repeated three times (once per day) both before and after the completion of the 10- to 12-wk exercise training or sedentary period (see below).
Intrinsic rate determination.
On a subsequent day, the animals were placed nonsedated and unrestrained on a laboratory table in a light- and sound-attenuated room. A catheter was placed percutaneously in a cephalic vein to administer drugs. As with the submaximal exercise test, transthoracic ECG readings were obtained and used to measure HR and HRV. Data collection began after a stabilization period of 10–15 min. Baseline data were collected for 5 min, and then either propranolol HCl (1.0 mg/kg) or atropine sulfate (50 μg/kg) was given as an intravenous bolus using a counterbalanced design; data were collected for 5 min or until steady-state values had been achieved. The second drug (propranolol or atropine) was then given and data were collected for 5 min or until steady-state values had been achieved. One week later the studies were repeated with the drugs given in reverse order. The dose of drugs given had been previously shown to produce a complete inhibition of beta-adrenergic or muscarinic receptors (7, 10, 11). These studies were repeated after the completion of the 10- to 12-wk exercise training or equivalent sedentary period (see below).
Exercise training protocol.
After collection of baseline data, animals were randomly assigned to one of two groups: exercise training (Ex, n = 15, 8 male, 7 female) or sedentary (Sed, n = 10, 5 male, 5 female). The exercise training protocol has been previously described (7, 12, 13). Briefly, dogs in the Ex group ran on a motor-driven treadmill for 10–12 wk, 5 days/wk at approximately 70–80% of estimated maximum HR. Exercise intensity and duration progressively increased as follows: week 1, 20 min at 4.8 kph/0% grade; week 2, 40 min at 5.6 kph/10% grade; week 3, 40 min at 6.4 kph/10% grade; week 4, 60 min at 6.4 kph/10% grade; week 5, 60 min at 6.4 kph/12% grade; week 6, 75 min at 6.4 kph/12% grade, week 7, 90 min at 6.4 kph/12% grade; weeks 8–12, 90 min at 6.4 kph/14% grade. Each exercise session included 5-min warm-up and 5-min cool-down periods (running at a low intensity, 0% grade and speed, 4.8 kph). The dogs in the Sed group were placed in a transport cage for equivalent time periods without receiving exercise.
Sinoatrial node function and atrial conduction.
SAN function was evaluated at least 2 days after completion of the final submaximal exercise and intrinsic rate studies (i.e., after the exercise training or sedentary periods). A catheter was placed percutaneously in a cephalic vein and the animals were anesthetized using sodium pentobarbital (30–40 mg/kg, iv). Endotracheal intubation was performed and the animals were placed on a positive-pressure respirator (55-0175; Harvard Apparatus, South Natick, MA). The heart was then exposed via a right thoracotomy. A set of bipolar electrodes was placed in the right atrium above the right coronary artery and used to record an atrial electrogram. A second set of electrodes was attached to the tip of the right atrial appendage and used to pace the atria. After a stabilization period of 15–20 min, SAN recovery time (SNRT) was determined. The atria were paced at 2× threshold (4–6 volts) for 1 min at 3.3, 5.0, 7.5, and 10 Hz (pulse duration 1 ms) using a solid-state square-wave stimulator (s44; Grass Instruments, Quincy, MA). Each stimulation frequency was repeated two to four times, and 1–2 min elapsed between each test frequency. SNRT, the time from the last paced atrial beat to the first poststimulation beat, was corrected for HR (cSNRT) using the technique described by Chadda et al. (19). The interval from the pacing stimulus artifact to the onset of the atrial depolarization recorded in the atrial electrogram was used as a marker of atrial conduction time. After completion of the pacing, high (25 mg) and low (5 mg) doses of adenosine were then injected (intravenous bolus). Five minutes elapsed between injections. The stimulation and adenosine studies were then repeated 5 min after autonomic blockade (combined atropine + propranolol); half of the animals received propranolol followed by atropine, whereas the remaining half received atropine followed by propranolol.
Immunoblot Analysis.
At the end of the SAN functional studies, the heart was immediately removed. The SAN was then identified, rapidly frozen in liquid nitrogen, and stored at −80° for future analysis. Protein analysis of tissue lysates was performed as previously described (20). Briefly, the tissue was thawed and placed into ice-cold homogenization buffer [in mmol/l: 50 Tris·HCl, 10 NaCl, 320 sucrose, 5 EDTA, 2.5 EGTA, supplemented with 1:1,000 protease inhibitor cocktail and 1:1,000 phenylmethanesulfonyl fluoride (Sigma, St Louis, MO)]. After quantification, tissue lysates were analyzed on Mini-PROTEAN tetra cell (BioRad, Hercules, CA) on a 4% to 15% precast TGX gel (BioRad). Gels were transferred to a nitrocellulose membrane using the Mini-PROTEAN tetra cell. Membranes were blocked for 1 h at room temperature using a 3% BSA solution or 5% milk solution and incubated with primary antibody overnight at 4°C. Densitometry analysis was carried out using ImageLab software (BioRad). For all experiments, protein values were normalized against an internal loading control [glyceraldehyde-3-phosphate dehydrogenase (GAPDH)].
Histological analysis.
At the end of the SAN functional studies, tissue samples were obtained from all four cardiac chambers. Cardiac tissues samples (∼2 × 2 cm) were fixed in 10% phosphate-buffered formalin, paraffin-embedded, and then sectioned perpendicular to the epicardium (Ex n = 5, Sed n = 6). The sections were stained for tissue fibrosis with Masson's trichrome staining. The slides were scanned, and the high-resolution (0.5 × 0.5 μm2) images were uploaded to ImageJ software for fibrotic analysis and quantification. The data are reported as a percentage of fibrotic area out of the total tissue area in the four cardiac chambers as previously described (29).
Data analysis.
All data are reported as means ± SE. The data were digitized (1 kHz) and recorded using an MP-100 data acquisition system (Biopac Systems, Goleta, CA). Data were averaged over 30-s intervals before, during (for each exercise level), and following termination of submaximal exercise. Data from the first submaximal exercise test was excluded and the results from the second and third trial were averaged to obtain one value for each animal. In a similar manner, ECG parameters for the autonomic intervention studies (i.e., intrinsic rate studies) were determined during the last 5 s before drug administration and over a 5-s interval when steady-state values had been obtained after administration of each autonomic agent (i.e., about 5 min after drug administration). The following ECG parameters were measured: R-R interval (from which HR was calculated), P wave duration, PR interval, QRS duration, QT interval and descending portion of the T wave, and Tpeak-Tend [Tp-e, an index of ventricular repolarization heterogeneity (47)]. Both QT interval and Tp-e were corrected for prevailing HR using Van de Water's correction factor [e.g., QTc = QT − 0.087(RR −1,000) (60)].
HRV data were obtained using a Delta-Biometrics (Urbana-Champaign, IL) vagal tone monitor triggering off the electrocardiogram R-R interval. This device employs the time-series signal processing techniques as developed by Porges et al. (50) to estimate the amplitude of respiratory sinus arrhythmia. Details of this analysis have been described previously (10, 11). Briefly, the ECG signal was digitized at 1 kHz, and sequential R-R intervals were timed to the nearest millisecond. The nonperiodic baseline fluctuations were removed using a moving third-order, 21-point polynomial function. This procedure prevented leakage of trends and harmonics of nonsinusoidal periodic activity (i.e., transient changes) into the respiratory frequency component. Once the these filtering procedures had been performed, the output of the moving polynomial was processed with a digital band-pass filter to extract the variance in the 0.24- to 1.04-Hz frequency band. The variance measure was then transformed to its natural logarithm to normalize the distribution of the variance estimates to limit the impact of large differences (i.e., outlying values.). The following variables were recorded for each 30-s period: mean R-R interval, total R-R interval variance (from which standard deviation was calculated, R-Rsd), R-R interval range (longest-to-shortest R-R interval, R-Rrange), and high-frequency component of the R-R variance (HF, 0.24 to 1.04 Hz).
The data were compared using ANOVA for repeated measures (NCSS statistical software, Kaysville, UT). For example, the effect of exercise training on baseline HR, HRV, intrinsic HR, and ECG parameters were analyzed using a two-factor mixed design ANOVA [group (two levels: Sed, Ex) × time (two levels: Pre, Post)] with repeated-measures on one factor [time] measures. In a similar fashion, the effects of exercise training on the HR and HRV responses to submaximal exercise were analyzed using a three-factor mixed design ANOVA [group (two levels: Ex, Sed) × time (two levels: Pre, Post) × exercise level (seven levels)] with repeated measures on two factors (exercise level, time). Homogeneity of covariance (sphericity assumption, equal correlates between the treatments) was tested using the Mauchley test and, if appropriate, adjusted using the Huynh-Feldt correction. If the F value exceeded a critical value (P < 0.05), post hoc comparisons of the data were then carried out using a Tukey-Kramer multiple comparison test. Cardiac chamber fibrosis and protein levels were compared using a t-test (sedentary vs. exercise-trained). Atrial arrhythmias (induced by high-frequency atrial pacing) were classified using the Lambeth Conventions (21). The incidence of atrial arrhythmias was evaluated using Fisher's exact test.
RESULTS
Effect of exercise training on HR and HRV responses to submaximal exercise.
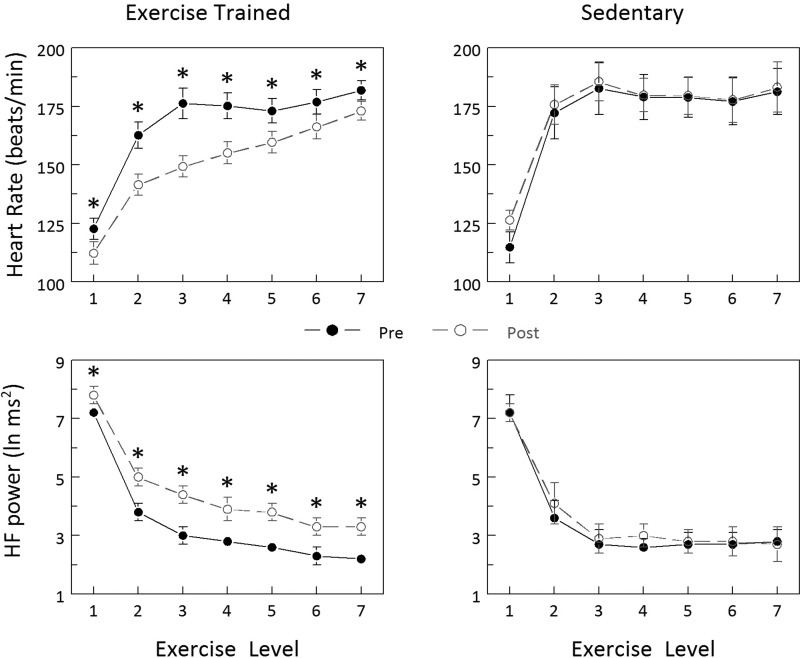
HR and HRV (the HF component of the R-R interval variability) responses to exercise training and sedentary periods are displayed in Fig. 1. Exercise training significantly reduced the HR response to submaximal exercise (Pre vs. Post P < 0.0005), whereas the response was not altered in the Sed group (Pre vs. Post P = 0.349). Correspondingly, submaximal exercise elicited significantly smaller changes in all three indices of HRV in the Ex group (Pre vs. Post: HF P < 0.0004; R-Rsd P < 0.00007, data not shown; R-Rrange P < 0.0004, data not shown), whereas the response was unchanged in the Sed group (Pre vs. Post: HF P = 0.338, R-Rsd P = 0.103, data not shown; R-Rrange P = 0.356, data not shown).
Fig. 1.
Effect of exercise training on heart rate (HR) and heart rate variability (HRV) responses to submaximal exercise. Exercise training decreased HR and increased HRV (HF power) at each exercise level, whereas these variables did not change in sedentary animals. Exercise levels: 1, 0 kph/0% grade; 2, 4.8 kph/0%; 3, 6.4 kph/0%; 4, 6.4 kph/4%; 5, 6.4 kph/8%; 6, 6.4 kph/12%; 7, 6.4 kph/16%. Pre and Post indicate, respectively, before and at the end of the 10- to 12-wk study period. HF power, high-frequency component of the R-R interval variability (0.24 to 1.04 Hz). *P < 0.01 Pre vs. Post values.
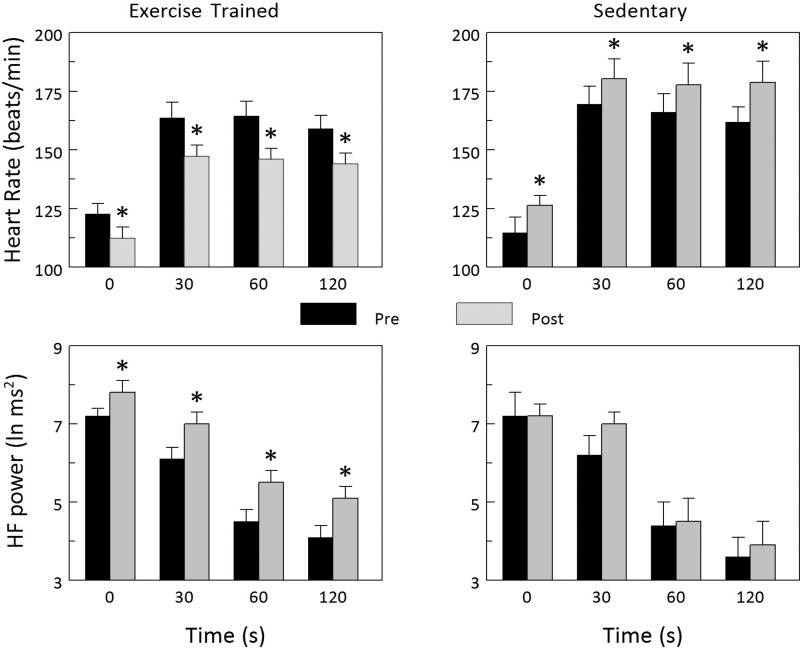
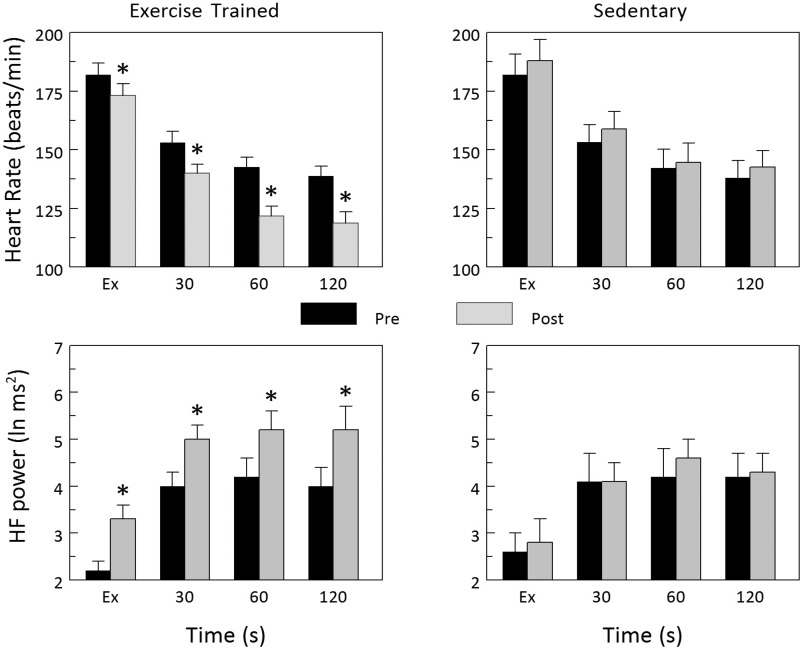
The HR and HRV responses to exercise onset (the first 2 min during exercise) and recovery (the first 2 min following exercise termination) before and after the exercise training or sedentary period are displayed in Figs. 2 and 3, respectively. Exercise training significantly reduced the HR response to the onset of submaximal exercise (Pre vs. Post P < 0.002), whereas the HR response was significantly increased in the Sed group (Pre vs. Post P < 0.002). Correspondingly, submaximal exercise elicited significantly smaller changes in all three indices of HRV in animals in the Ex group (Pre vs. Post: HF P < 0.001, R-Rsd P < 0.0001, data not shown; R-Rrange P < 0.01, data not shown), whereas the response was unchanged in the Sed group (Pre vs. Post: HF P = 0.318, R-Rsd P = 0.068, data not shown; R-Rrange P = 0.317, data not shown). In a similar manner, HR more rapidly returned to baseline levels (faster recovery) after completion of the exercise training (P < 0.00005), whereas HR recovery did not change in the Sed group (P = 0.140). Correspondingly, all three indices of HRV returned to baseline faster (a more rapid vagal reactivation) after completion of exercise training (Pre vs. Post: HF P < 0.003, R-Rsd P < 0.002, data not shown; R-Rrange P < 0.02, data not shown), whereas these variables did not change in the Sed animals (Pre vs. Post: HF P = 0.481, R-Rsd P = 0.692, data not shown; R-Rrange P = 0.642, data not shown). When considered together, these data confirm that a so-called trained state had been achieved by the end of the 10- to 12-wk exercise program.
Fig. 2.
Effect of exercise training on HR and HRV responses to the onset of submaximal exercise. The onset of exercise elicited significantly smaller increases in HR that were accompanied by significantly smaller reductions in the high-frequency component of the R-R interval variability (HF power) after completion of the 10- to 12-wk exercise training protocol. In contrast, exercise onset elicited significantly larger increases in HR at the end of the sedentary period. These data are consistent with either an enhanced parasympathetic neural regulation or reduced sympathetic neural response to exercise onset in exercise-trained animals, whereas the exaggerated response to exercise onset in the sedentary animals may reflect an enhanced cardiac parasympathetic withdrawal, or augmented sympathetic neural response to the exercise (perhaps due to deconditioning), or both. Data were averaged over each 30-s period beginning with the last 30 s before exercise onset (time = 0) and for the first 120 s during exercise. *P < 0.01 Pre vs. Post values.
Fig. 3.
Effect of exercise training on HR and HRV responses to the termination of submaximal exercise. Both HR and the high-frequency component of the R-R interval variability (HF power) returned toward baseline values much more rapidly after completion of the 10- to 12-wk exercise training protocol. In contrast, the recovery from submaximal exercise was not altered in the sedentary group. These data suggest that exercise training-mediated increases in HR recovery following the termination of exercise resulted from a more rapid reactivation of cardiac parasympathetic regulation. Data were averaged over each 30-s period beginning with the last 30 s of the submaximal exercise test (time = Ex) and for the first 120 s following the termination of exercise. *P < 0.01 pre vs. post values.
Effect of exercise training on baseline HR, HRV, and ECG parameters.
The effects of exercise training on baseline HR and HRV are displayed in Table 1. Exercise training elicited significant reductions in baseline HR (Pre vs. Post: HR P < 0.044) that were accompanied by significant increases in HRV (Pre vs. Post: HF P < 0.004, R-Rsd P < 0.0009, R-Rrange P < 0.025), whereas these variables were not altered in the Sed group (Pre vs. Post: HR P = 0.305, HF P = 0.281, R-Rsd P = 0.645, R-Rrange P = 0.06).
Table 1.
Effect of exercise training on baseline HRV and ECG variables
|
Ex |
Sed |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| HR, beats/min | 113.9 ± 4.3 | 104.3 ± 4.3* | 112.6 ± 7.5 | 116.6 ± 4.7 |
| HF, ln ms2 | 7.1 ± 0.2 | 8.2 ± 0.2* | 7.4 ± 0.5 | 7.0 ± 0.6 |
| R-Rsd, ms | 61.3 ± 4.0 | 100.5 ± 9.2* | 62.2 ± 7.0 | 63.6 ± 7.3 |
| R-Rrange, ms | 225.3 ± 13.4 | 276.2 ± 12.1* | 226.8 ± 21.7 | 194.6 ± 11.7 |
| R-R, ms | 524.7 ± 33.9 | 587.1 ± 22.3* | 556.8 ± 40.7 | 522.1 ± 21.5 |
| P wave, ms | 46.1 ± 1.6 | 45.8 ± 1.8 | 45.8 ± 1.1 | 49.3 ± 2.0 |
| PR, ms | 112.2 ± 3.8 | 116.3 ± 3.9 | 116.0 ± 3.7 | 120.8 ± 2.8 |
| QRS, ms | 72.4 ± 2.2 | 69.9 ± 2.2 | 75.2 ± 3.6 | 73.6 ± 3.7 |
| QTc, ms | 250.7 ± 4.3 | 253.3 ± 2.8 | 256.6 ± 3.8 | 256.0 ± 4.1 |
| cTp-e, ms | 87.8 ± 3.9 | 77.6 ± 2.4* | 77.6 ± 4.5 | 81.2 ± 3.7 |
HRV, heart rate variability; ECG, electrocardiographic; Ex, exercise-trained group; Sed, sedentary group; Pre and Post, before and after the 10- to 12-wk study period, respectively; HR, heart rate; HF, high-frequency component of the R-R variance (0.24 to 1.04 Hz); R-Rsd standard deviation from the total R-R interval variance; R-Rrange, longest-to-shortest R-R interval; QTc, QT interval corrected for heart rate (van de Water's correction factor; see Ref. 60; e.g., QTc = QT − 0.087(R-R − 1,000); cTp-e, Tpeak-Tend corrected for heart rate.
P < 0.05 pre vs. post.
Effects of exercise training on baseline ECG variables are also shown in Table 1. Exercise training increased the R-R interval (Pre vs. Post P < 0.045) and reduced the duration of the descending portion of the T wave (Tp-e corrected for HR Pre vs. Post P < 0.022); these data are consistent with a more homogeneous repolarization after completion of an exercise conditioning program. No other ECG differences were noted in the Ex group. In a similar manner, no ECG parameters were altered in the Sed group.
Effect of exercise training on autonomic neural blockade: intrinsic HR.
Changes in baseline HR and HRV induced by beta-adrenergic or muscarinic receptor blockade both before and after completion of the 10- to 12-wk exercise training or sedentary period are found in Table 2 and Table 3, respectively. Beta-adrenoceptor blockade (propranolol HCl) elicited a small reduction (Ex P < 0.00002, Sed P < 0.0002) in baseline HR but did not alter baseline HRV in either the Sed (HF P = 0.171, R-Rsd P = 0.345, R-Rrange P = 0.191) or the Ex group (HF P = 0.500, R-Rsd P = 0.313, R-Rrange P = 0.510) (Table 2). Furthermore, the response to propranolol was not altered by either exercise training or the sedentary period (Ex Pre vs. Post: HR P = 0.826, HF P = 0.858, R-Rsd P = 0.510, R-Rrange P = 0.958; Sed HR P = 0.054, HF P = 0.987, R-Rsd P = 0.497, R-Rrange P = 0.211). Muscarinic receptor blockade (atropine sulfate) provoked large increases (Ex P < 10−6, Sed P < 0.00008) in HR that were accompanied by corresponding reductions in HRV (Ex HF, R-Rsd, and R-Rrange all P < 10−6; Sed HF P < 10−6, R-Rsd P < 0.00002, R-Rrange P < 0.000002) (Table 3). In contrast to beta-adrenoceptor blockade, atropine elicited larger reductions in HRV at the end of the 10- to 12-wk exercise training program (HF P < 0.042, R-Rsd P < 0.0014, R-Rrange P < 0.013); the HRV response was not altered in the Sed group (HF P = 0.329, R-Rsd P = 0.574, except R-Rrange P < 0.025). These data suggest that the HRV increase induced by exercise training resulted from enhanced cardiac parasympathetic regulation rather than as a consequence of decreased cardiac sympathetic (beta-adrenergic) activation.
Table 2.
Effect of cardiac beta adrenergic receptor blockade on HR and HRV
|
Pre |
Post |
|||||
|---|---|---|---|---|---|---|
| Baseline | β-AR Block* | Δ† | Baseline | β-AR Block* | Δ† | |
| HR, beats/min | ||||||
| Ex | 114.0 ± 5.1 | 95.6 ± 7.7‡ | −18.4 ± 3.2 | 108.1 ± 4.0§ | 89.1 ± 2.9‡ | −19.1 ± 3.4 |
| Sed | 115.4 ± 6.7 | 98.6 ± 5.2‡ | −16.8 ± 3.8 | 122.4 ± 5.9 | 95.6 ± 2.8‡ | −26.8 ± 4.7 |
| HF, ln ms2 | ||||||
| Ex | 7.2 ± 0.2 | 7.1 ± 0.3 | −0.2 ± 0.3 | 7.8 ± 0.2§ | 7.7 ± 0.3 | −0.1 ± 0.3 |
| Sed | 7.1 ± 0.5 | 6.7 ± 0.7 | −0.4 ± 0.4 | 7.4 ± 0.4 | 7.0 ± 0.6 | −0.4 ± 0.4 |
| R-Rsd, ms | ||||||
| Ex | 61.3 ± 4.0 | 59.3 ± 7.2 | −0.5 ± 6.6 | 83.3 ± 3.8§ | 74.7 ± 9.0 | −8.6 ± 7.8 |
| Sed | 62.2 ± 9.9 | 61.7 ± 13.8 | −2.2 ± 6.1 | 63.6 ± 7.3 | 53.6 ± 7.3 | −9.9 ± 10.0 |
| R-Rrange, ms | ||||||
| Ex | 233.0 ± 11.5 | 226.2 ± 20.5 | −6.9 ± 20.9 | 246.5 ± 14.0 | 237.9 ± 13.1 | −8.7 ± 20.0 |
| Sed | 221.3 ± 26.5 | 174.4 ± 29.8 | −46.9 ± 16.5 | 220.9 ± 13.3 | 219.7 ± 33.2 | −1.2 ± 29.7 |
β-AR block, beta-adreneroceptor blockade (propranolol HCl, 1.0 mg/kg, iv).
Change from baseline.
P < 0.01 baseline vs. β-AR block.
P < 0.01 Pre vs. Post.
Table 3.
Effect of cardiac muscarinic receptor blockade on HR and HRV
|
Pre |
Post |
|||||
|---|---|---|---|---|---|---|
| Baseline | Vagal Block* | Δ† | Baseline | Vagal Block* | Δ† | |
| HR, beats/min | ||||||
| Ex | 113.4 ± 4.2 | 187.3 ± 6.2‡ | 73.9 ± 6.2 | 105.2 ± 3.9§ | 174.3 ± 7.3‡ | −69.1 ± 3.4 |
| Sed | 113.0 ± 10.3 | 193.6 ± 9.3‡ | 80.6 ± 10.4 | 123.9 ± 5.9 | 192.8 ± 11.8‡ | −69.4 ± 12.3 |
| HF, ln ms2 | ||||||
| Ex | 7.1 ± 0.2 | 0.1 ± 0.1 | −6.9 ± 0.3‡ | 8.2 ± 0.2§ | 0.5 ± 0.2‡ | −7.6 ± 0.3§ |
| Sed | 7.4 ± 0.5 | 0.4 ± 0.1 | −7.1 ± 0.5‡ | 7.0 ± 0.6 | 0.3 ± 0.2‡ | −6.7 ± 0.5 |
| R-Rsd, ms | ||||||
| Ex | 57.8 ± 3.4 | 7.8 ± 1.0‡ | −50.0 ± 3.7 | 100.5 ± 9.2§ | 9.9 ± 1.5 | −90.6 ± 9.7§ |
| Sed | 62.6 ± 11.2 | 6.7 ± 0.7‡ | −56.0 ± 11.3 | 67.1 ± 7.0 | 7.9 ± 1.0 | −59.2 ± 6.8 |
| R-Rrange, ms | ||||||
| Ex | 225.3 ± 13.3 | 30.7 ± 3.6‡ | −194.6 ± 13.7 | 276.2 ± 12.1§ | 37.7 ± 5.5 | −238.5 ± 13.4§ |
| Sed | 226.8 ± 21.7 | 24.2 ± 2.6‡ | −202.6 ± 22.3 | 194.6 ± 11.8 | 33.1 ± 5.2 | −161.5 ± 14.4§ |
Vagal block, muscarinic receptor blockade (atropine sulfate 50 μg/kg, iv).
Change from baseline.
P < 0.01 baseline vs. muscarinic block;
P < 0.01 Pre vs. Post.
Intrinsic pacemaker rate was revealed following combined cardiac autonomic blockade (propranolol + atropine). The effects of combined autonomic blockade on HR and HRV are displayed in Table 4. HR was significantly (Ex group P < 0.0004, Sed group P < 0.015) higher after autonomic blockade compared with predrug values (Table 4), demonstrating that dogs exhibit a large baseline cardiac parasympathetic tone. More importantly, autonomic blockade completely abolished the reductions in HR and increases in HRV that were induced by exercise training, such that there were no longer differences in the Sed and Ex groups. Furthermore, intrinsic HR was similar before and after completion of exercise training (P = 0.338) or the sedentary period (P = 0.423); that is, intrinsic HR was not altered by exercise training. Total ANS block also elicited larger reductions in HRV after exercise training (HF P < 0.028, R-Rsd P < 0.00004, R-Rrange P = 0.078) but not at the end of the sedentary period (HF P = 637, R-Rsd P = 0.733, R-Rrange P = 0.311), data that are again suggestive of an altered autonomic regulation in the exercise-trained group.
Table 4.
Effect of complete cardiac autonomic neural blockade on HR and HRV: intrinsic rate
|
Pre |
Post |
|||||
|---|---|---|---|---|---|---|
| Baseline | ANS Block* | Δ† | Baseline | ANS Block* | Δ† | |
| HR, beats/min | ||||||
| Ex | 113.7 ± 3.9 | 141.1 ± 5.9‡ | 27.4 ± 6.6 | 106.7 ± 3.8 | 141.5 ± 7.0‡ | 34.8 ± 8.2 |
| Sed | 114.2 ± 7.8 | 143.5 ± 10.7‡ | 29.3 ± 13.6 | 122.9 ± 4.9 | 142.7 ± 12.0‡ | 19.8 ± 13.8 |
| HF, ln ms2 | ||||||
| Ex | 7.2 ± 0.2 | 0.4 ± 0.1‡ | −6.8 ± 0.2 | 8.0 ± 0.2§ | 0.5 ± 0.2‡ | −7.5 ± 0.2§ |
| Sed | 7.3 ± 0.5 | 0.4 ± 0.2‡ | −6.8 ± 0.4 | 7.2 ± 0.4 | 0.4 ± 0.1‡ | −6.8 ± 0.4 |
| R-Rsd, ms | ||||||
| Ex | 59.6 ± 2.7 | 5.1 ± 0.7‡ | −54.5 ± 2.9 | 91.9 ± 5.6§ | 4.7 ± 0.5‡ | −87.2 ± 5.7§ |
| Sed | 67.4 ± 9.9 | 4.8 ± 0.6‡ | −62.6 ± 10.0 | 65.3 ± 4.4 | 5.1 ± 0.9‡ | −60.3 ± 4.9 |
| R-Rrange, ms | ||||||
| Ex | 229.2 ± 10.4 | 21.0 ± 2.9‡ | −208.2 ± 11.2 | 261.4 ± 10.2§ | 20.7 ± 2.2‡ | −240.7 ± 10.5§ |
| Sed | 224.0 ± 21.9 | 19.5 ± 2.4‡ | −204.6 ± 22.0 | 207.8 ± 11.2 | 22.0 ± 3.1‡ | −185.7 ± 13.4 |
Autonomic nervous system block, combined beta-adrenergic (propranolol HCl, 1.0 mg/kg, iv) and muscarinic (atropine sulfate, 50 μg/kg, iv) receptor blockade.
Change from baseline.
P < 0.01 baseline vs. ANS block.
P < 0.01 Pre vs. Post.
Effect of exercise training on SAN function.
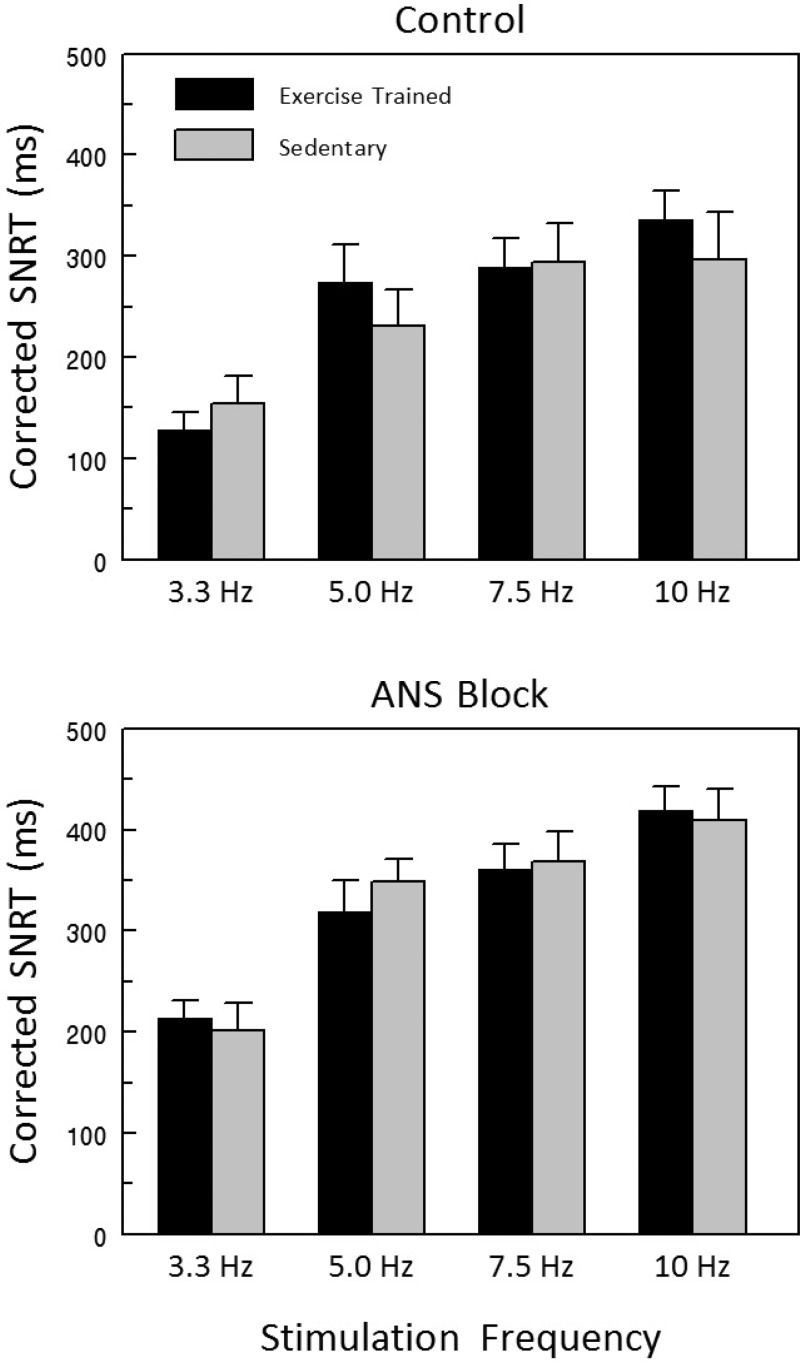
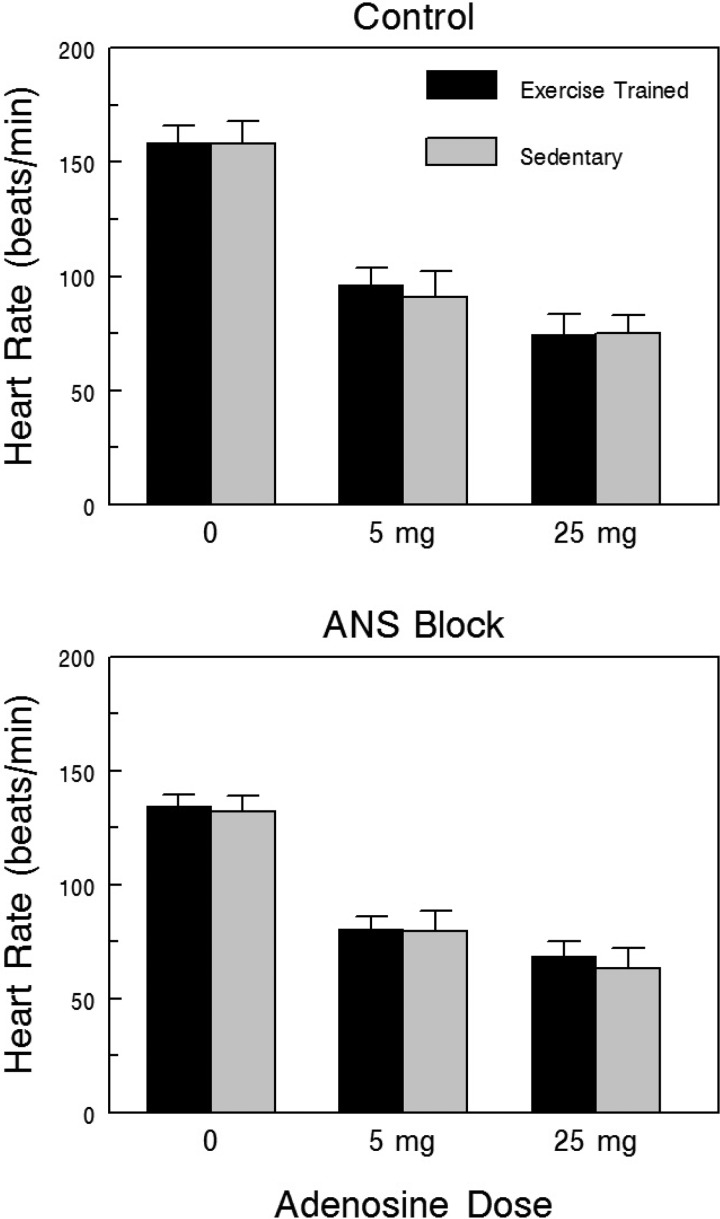
Effects of rapid atrial pacing on SAN recover time (SNRT, the time from the last atrial paced beat to the first after stimulation atrial electrogram peak) corrected for heart rate (cSNRT) are displayed in Fig. 4. The data were collected with and without ANS block to remove any confounding influence of changes in autonomic regulation on the properties of the SAN. After ANS block, cSNRT increased significantly and to a similar extent as stimulus frequency increased in both the Ex and Sed groups (frequency effect P < 10−6), such that there were no differences noted between the two groups (group effect P = 0.913, group × frequency interaction P = 0.237). Similar responses were also noted before ANS blockade (frequency effect P < 10−6, group effect P < 0.570, group × frequency effect P = 0.177). Atrial conduction time (the interval between the stimulus artifact and first atrial electrogram peak) was similar in the Ex and Sed groups both before (Ex 27.9 ± 3.0 vs. Sed 31.8 ± 4.3 ms) and after ANS blockade (Ex 30.8 ± 2.7 vs. Sed 35.2 ± 5.9 ms) (group P = 0.423, treatment P = 0.137, group × treatment interaction P = 0.924).
Fig. 4.
Effect of exercise training on sinoatrial node recovery time (SNRT) corrected for HR before and after complete autonomic neural blockade. Corrected SNRT significantly increased with increasing atrial stimulus frequency both before and after combined autonomic nervous system (ANS) blockade (propranolol HCl, 1.0 mg/kg + atropine sulfate 50 μg/kg). However, exercise training did not alter the SAN response to rapid atrial pacing; that is, there were no differences between the exercise-trained and sedentary groups. These data suggest that the intrinsic properties of the SAN pacemaker were not altered by exercise. SNRT was corrected for HR using the technique described by Chadda et al. (19).
Effects of adenosine, a potent negative chronotropic agent (32), on HR with and without ANS block, are shown in Fig. 5. Adenosine dose-dependently reduced HR to a similar extent in the Sed and Ex groups both before (dose effect P < 10−6, group effect P = 0.839, group × dose interaction P = 0.796) and after ANS blockade (dose effect P < 10−6, group effect P = 0.710, group × dose interaction P = 0.770). When considered together, these data further suggest that SAN responsiveness was not altered by exercise training.
Fig. 5.
Effect of exercise training on the HR response to adenosine before and after complete ANS blockade. Adenosine elicited significant reductions in HR with increasing doses of adenosine both before and after combined ANS blockade (propranolol HCl, 1.0 mg/kg + atropine sulfate 50 μg/kg). However, exercise training did not alter the HR response to adenosine; that is, there were no differences between the exercise-trained and sedentary groups. These data suggest that the sinoatrial function was not altered by exercise.
Effect of exercise training on protein expression.
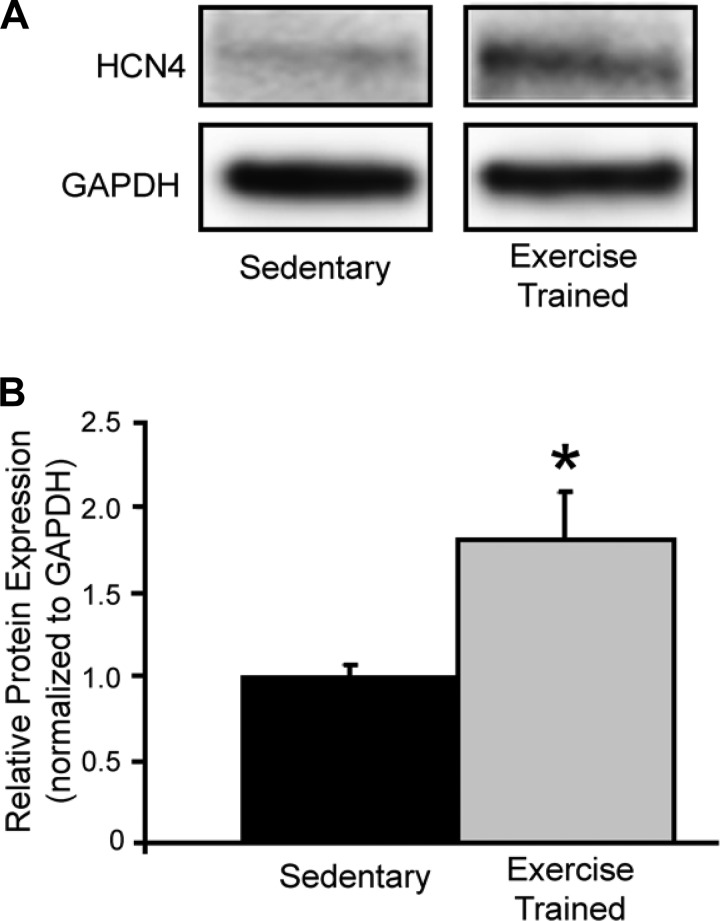
Representative examples and the composite data for HCN4 [responsible for the so-called funny current, If, a major cardiac pacemaker current (4)] are displayed in Fig. 6. SAN HCN4 protein levels (corrected for protein content with GAPDH) were significantly elevated (P < 0.05) in animals in the Ex group compared with those in the Sed group.
Fig. 6.
Effect of exercise training on sinoatrial HCN4 protein levels. A: representative examples of immunoblots for HCN4. B: composite data. HCN4 and was significantly elevated in the exercise-trained compared with the sedentary animals. *P < 0.05 exercise-trained vs. sedentary.
Effect of exercise training on cardiac chamber fibrosis.
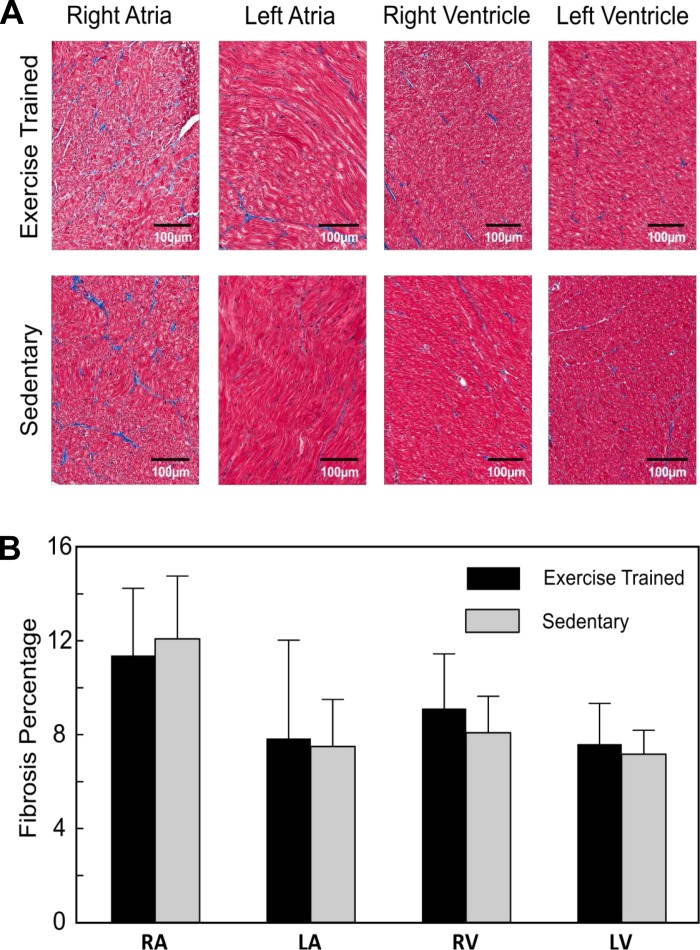
Representative examples of cardiac chamber tissue sections stained for fibrosis are shown in Fig. 7A, whereas the composite data for all four cardiac chambers are shown in Fig. 7B. Fibrosis levels did not differ between animals in the Sed and Ex groups in any cardiac chamber (right atrium P = 0.71, left atrium P = 0.88, right ventricle P = 0.46, left ventricle P = 0.67).
Fig. 7.
Effect of moderate-endurance exercise training on cardiac chamber fibrosis. Exercise training did not alter cardiac chamber fibrosis because the amount of fibrosis was similar in both the sedentary and exercise-trained animals. A: representative histological sections for each cardiac chamber from one exercise-trained and one sedentary animal. B: composite data for the exercise-trained (n = 5) and sedentary animals (n = 6). RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle.
Effect of training on induction of atrial fibrillation.
The highest atrial pacing rate (10 Hz, for 0.5 to 1 min) often induced atrial fibrillation (AF). There was no difference in the incidence of AF (P = 0.47) in either the Ex group (8 of 15, 57.1%) or the Sed group (5 of 9, 53.3%). The duration of AF was also similar in both groups (Ex 5.3 ± 1.7 vs. Sed 4.7 ± 1.1 s). Autonomic neural blockade reduced the incidence of AF to a similar extent in both groups (after ANS block: Ex group, 3 of 15, 20.0% vs. Sed group, 3 of 9, 33.3%). These data suggest that despite an enhanced cardiac parasympathetic regulation, exercise training did not alter the susceptibility to electrically induced AF (probably due to the absence of atrial structural remodeling; i.e., no change in atrial fibrosis).
DISCUSSION
The major findings of the present study are as follows: 1) in agreement with previous studies (12, 13), endurance exercise elicited significant reductions in both baseline HR and the HR response to submaximal exercise (including the responses to exercise onset and termination) and, furthermore, that these HR reductions were accompanied with corresponding increases in both total HRV and the high-frequency component of R-R interval variability [a noninvasive maker of cardiac parasympathetic regulation (9)]; 2) the muscarinic antagonist atropine elicited larger reductions in HRV in exercise-trained compared with sedentary animals, whereas beta-adrenoceptor blockade (propranolol) elicited similar HR and HRV changes both before after the completion of the 10- to 12-wk exercise training protocol; 3) exercise training did not alter intrinsic HR (revealed by complete ANS blockade) because it was similar in the Ex and Sed groups both before and after the completion of the study; 4) exercise training did not alter SAN function (measured by cSNRT, atrial conduction time, P wave duration, PR interval, or HR response to adenosine) because these variables were similar in the Ex and Sed groups both before and after completion of the study. These data suggest that exercise training did not induce an upregulation of either adenosine A1 receptors or adenosine-dependent potassium currents, Ikado; 5) in contrast to previous results (25), SAN HCN4 expression did not decrease, but it increased in animals in the Ex group compared with those in the Sed group; and finally, 6) moderate exercise training neither altered cardiac chamber fibrosis content nor increased the susceptibility to atrial fibrillation induced by high-frequency (10 Hz for 0.5–1 min) atrial pacing. When considered together, these data strongly suggest that exercise training-induced bradycardia results from an enhanced cardiac parasympathetic regulation rather than as a consequence of changes in intrinsic HR or SAN function.
Effect of endurance exercise training on cardiac autonomic regulation and intrinsic heart rate.
It is well established that both baseline HR and HR response to submaximal exercise are reduced by aerobic exercise training programs (18, 28, 62). Indeed, this training bradycardia is often used to demonstrate that an exercise program was effective (i.e., cardiovascular training had been achieved). As previously mentioned, two mechanisms that are not necessarily mutually exclusive have been proposed to explain this observation: namely, training-induced HR reductions result from either shifts in the autonomic balance toward dominant parasympathetic regulation, or from changes in the intrinsic properties of the cardiac pacemaker cells (decreased intrinsic diastolic depolarization rate).
Substantial evidence demonstrates that endurance exercise enhances cardiac parasympathetic regulation and reduces cardiac sympathetic regulation in both healthy individuals (18, 28, 62) and in patients with heart disease (35, 40). Indeed, endurance exercise training reversed the adverse autonomic remodeling induced by myocardial infarction in patients (40) and in a canine model of sudden cardiac death (12, 14, 34). However, the contribution of these changes in cardiac autonomic regulation to training bradycardia is more controversial. In agreement with the present study, several longitudinal training studies report that intrinsic HR as revealed by ANS blockade was similar before and after the completion of exercise training programs (42, 53, 55). Furthermore, exercise training also consistently elicited increases in both time and frequency domain indices of HRV, changes that were eliminated by autonomic blockade (12, 13, 18, 24, 56, 62). In the present study, exercise training elicited increases in both total HRV and the HF component of the R-R interval variability; increases that were eliminated by muscarinic blockade but not beta-adrenoceptor blockade. Indeed, atropine elicited much larger decreases in HRV following exercise training than were noted before training, whereas HRV was not altered over time in the Sed group. These data are consistent with an exercise training-induced enhancement of the parasympathetic regulation of baseline HR.
Perhaps the strongest evidence in support of the autonomic hypothesis is the observation that exercise training (confirmed by increases in skeletal muscle citrate synthase activity) failed to induce reductions in baseline HR in dogs with complete cardiac denervation (48). Similar findings have been reported in patients who have undergone cardiac transplant (6, 39). Training bradycardia also could not be induced in sympathectomized rats (46), whereas the lower basal HR noted in atria isolated from exercise-trained mice compared with atria from sedentary animals was found to result from enhanced vagal responsiveness that was mediated via the activation of nitric oxide synthase-1 pathways (22). Thus an intact cardiac autonomic innervation may be required for the induction/maintenance of training bradycardia.
In addition to changes in cardiac autonomic regulation, exercise training has also been shown to alter cardiac electrophysiological properties (1, 16, 36). For example, exercise training has been shown to increase ventricular potassium repolarization currents in vitro (16, 36) and to change T-wave morphology and duration in vivo (7), reversing the electrophysiological remodeling induced by myocardial infarction (7, 16) and heart failure (1). These changes in repolarizing currents could reduce regional differences in ventricular repolarization and may explain the reduction of the descending portion of the T-wave (Tp-e) that was noted in the present study. With regards to atrial electrophysiological properties, exercise training did not alter P wave duration, PR interval, atrial conduction time, or SAN function (as measured by cSNRT or the HR response to the potent negative chronotrophic agent adenosine) in the present study. Furthermore, HCN4 expression [the channel protein responsible for the important pacemaker current, If (4)] increased rather than decreased in the Ex group compared with the Sed group. An increase in HCN4 expression would tend to increase rather than decrease the spontaneous pacemaker rate because drugs that selectively inhibit If reduce basal HR [(4) and the HR response to exercise (27)]. Because HCN4 channels are significantly regulated by cAMP level (4), HCN4 upregulation in the SAN may represent a positive adaptation to exercise and may also explain faster HR recovery following exercise observed in dogs in the Ex vs. Sed groups in the present study and in previous studies (13).
When the autonomic blockade and electrophysiological results are considered together, these data suggest that in the dog, training bradycardia results from an augmentation of cardiac parasympathetic regulation rather than as a consequence of changes in the intrinsic properties of the SAN.
However, the findings of the present study contrast with some previous studies. Cross-sectional studies that compared elite or life-long athletes with age-matched sedentary subjects frequently demonstrated a lower HR after ANS blockade in the athletes compared with the sedentary group (15, 38, 41). Furthermore, Scott et al. (52) found that the amplitude of the respiratory sinus arrhythmia [a marker of respiratory induced changes in cardiac vagal control (9)] was similar in endurance athletes and age-matched nonathletes. In a similar manner, in rats, a 10-wk exercise program (treadmill running) induced a significant resting bradycardia that persisted in isolated perfused hearts (45), whereas atropine did not reverse the training-induced changes in R-R interval or SNRT in isolated rabbit hearts (59, 63). More recently and in contrast to previous murine studies (22, 23), D'Souza and coworkers (25) found that training-induced bradycardia persisted after pharmacologic autonomic blockade both in intact mice and in isolated SAN preparations. They further reported that both expression of the HCN4 channels and their corresponding pacemaker current (If) were reduced in atria of exercise-trained compared with sedentary mice; If block also abolished HR differences noted in both trained and sedentary mice. Thus they concluded that training-induced changes in the electrophysiological properties of the SAN may reset the intrinsic pacemaker to lower values both at rest and during exercise (17).
A number of factors may explain these seemingly disparate findings. First, the results obtained from cross-sectional studies that compared elite athletes or recreational athletes with a long history of exercise participation with age-matched sedentary groups may be influenced by genetic factors or a self-selection bias, a complication eliminated in longitudinal studies in which intrinsic rate is measured in the same individuals over time. Second, the mammalian heart contains parasympathetic ganglia with postganglionic neurons that innervate both atria (2). These neurons display ongoing activity and can modify cardiac activity even after acute decentralization in isolated heart preparations (2). Thus changes in parasympathetic regulation could still contribute to a lower basal HR in hearts isolated from exercise-trained animals. Indeed, an enhanced vagal responsiveness was demonstrated in atria isolated from exercise-trained mice compared with hearts from sedentary animals (22). Furthermore, exercise-training bradycardia could not be induced in animals with confirmed complete cardiac denervation in vivo (48). Third, species differences may also contribute to the disparity in the results noted in animals with high (rodents) and low (dogs, humans) resting heart rates. Cardiac electrophysiological and mechanical properties, as well as parasympathetic/sympathetic balance, vary widely across species (37, 44, 46, 51, 54). For example, cardiac parasympathetic regulation dominates in animals with low baseline heart rates (dogs, humans), whereas the sympathetic nervous system plays a more important role in HR regulation in animals with high resting HR (rodents, nonhuman primates) (51, 54). Some rodent species also lack important ionic currents (i.e., rats lack both IKr and IKs), which are responsible for repolarization in humans (37, 46). It is also important to stress that the canine SAN is both functionally and anatomically more similar to the human SAN than are the SANs of small mammals (26).
Two additional factors may have negatively affected the results obtained in the murine study. First, the mice were housed at 22°C (25), but thermoneutrality for the mouse is ∼30°C (43). The thermal stress associated with housing mice below their thermoneutral zone adversely alters cardiovascular regulation such that these animals are hypertensive, hypermetabolic, and sleep-deprived (43). Importantly, baseline HR of mice at 30°C is ∼375 beats/min and increases ∼25 beats/min for every 1°C below 30°C such that HR decreases following complete autonomic neural blockade (revealing the intrinsic HR) in mice housed at 22°C, but it increases in mice exposed to 30°C (43). These data are consistent with a shift from parasympathetic to sympathetic dominance in the cold-stressed animals (43), which may mask changes in parasympathetic regulation induced by exercise training. Second, swimming is not a natural behavior for mice and, as a consequence, forced swimming represents an aversive (i.e., drowning avoidance) rather than a physiological stimulus (e.g., voluntary wheel running) that once again would favor an excess sympathetic activation. Thus the translation of findings obtained in any animal model (particularly those with high resting HRs and small hearts) to the clinic must be made with appropriate caution.
Limitations of the study.
A few limitations with the present study could influence interpretation of the results. First, cardiac autonomic activity was indirectly assessed using noninvasive markers of HRV and pharmacologic blockade. The relationship between HRV and cardiac autonomic regulation, particularly with regards to cardiac sympathetic activity, is controversial and remains the subject of ongoing investigation (9). Because a quantitative assessment of cardiac autonomic regulation can be made only from direct nerve recordings, HRV data should always be interpreted with care. Second, both respiratory rate and tidal volume can alter HRV (33). Thus exercise training-induced changes in these respiratory variables could have contributed to the lower HRV values noted following exercise training. However, because ANS blockade completely eliminated the HR and HRV differences noted between animals in the Ex and Sed groups, a major contribution of exercise-induced changes in respiration (mechanical component) to training bradycardia seems unlikely. Finally, the SAN functional studies were performed in anesthetized, open-chest animals. Anesthesia can alter cardiac autonomic regulation (31), or exert direct depressant actions on cardiac pacemaker cells (or both), which could mask any exercise training-induced changes in the properties of these cells. Indeed, pentobarbital anesthesia increased baseline HR and decreased HRV in animals in both the Ex (HR, 158.9 ± 7.4 beats/min; HF 0.3 ± 0.3 ln ms2) and Sed (HR, 158.0 ± 10.1 beats/min; HF 0.2 ± 0.2 ln ms2) groups, and furthermore, this HR increase was attenuated by following ANS blockade (Ex, 134.5 ± 5.1; Sed, 132.2 ± 6.7 beats/min). However, because HR was similar in animals in both Sed and Ex groups following autonomic blockade, these data are more consistent with anesthesia/surgery-induced changes in cardiac autonomic activity (increased sympathetic and decreased parasympathetic regulation) rather than due to direct actions on the pacemaker cells.
Clinical implications.
Cardiac autonomic regulation is adversely altered in many pathological conditions including diabetes, hypertension, heart failure, and myocardial ischemia/infarction, thus increasing the risk for malignant cardiac arrhythmias (8, 9). Exercise training can restore a more normal cardiac autonomic balance by enhancing parasympathetic regulation and decreasing sympathetic responsiveness, thereby protecting against ventricular arrhythmias (12, 14, 34). However, enhanced atrial vagal tone may increase the risk for atrial fibrillation. Indeed, highly trained athletes, particularly older individuals, often exhibit a greater incidence of atrial arrhythmias and AF than is observed in the general population (61). Exercise-induced increases in cardiac fibrosis, as have been reported in rodents exposed to a long-duration, high-intensity exercise training regimen (approximately 85–90% maximum for 16 wk) (5) would further increase the risk for AF. In the present study, moderate-endurance exercise did not alter fibrosis levels in any cardiac chamber or the incidence of electrically induced atrial fibrillation. Thus moderate exercise, in contrast to long-duration, high-intensity exercise, may represent a safe and effective nonpharmacologic means of improving cardiac autonomic regulation even in high-risk patient populations.
Conclusions and future directions.
In summary, endurance exercise training elicited reductions in HR that were associated with increased cardiac parasympathetic (increased HRV and greater responses to muscarinic antagonist atropine) regulation without changes in the intrinsic properties of sinoatrial function (similar HR following complete autonomic blockade, SAN recovery time, and response to adenosine in sedentary and exercise-trained animals). Moderate exercise training also did not alter either cardiac chamber fibrosis content or increase the susceptibility to atrial fibrillation induced by high-frequency atrial pacing. When considered together, these data strongly suggest that exercise training-induced bradycardia results from an enhanced cardiac parasympathetic regulation rather than as a consequence of changes in intrinsic HR or SAN function. Furthermore, moderate exercise does not induce cardiac fibrosis or increase the risk for atrial fibrillation in this canine model.
Although the present study provides strong, albeit indirect, evidence that training bradycardia results from an enhanced parasympathetic regulation, the mechanisms that trigger this change in cardiac autonomic neural regulation were not investigated in the present study. Changes in either central or peripheral neural efferent activity as well as signal processing at the cellular level could all contribute to the enhanced parasympathetic response induced by exercise training. For example, exercise training could provoke either an increase in parasympathetic efferent activity (originating within the central nervous system or cardiac parasympathetic ganglia) or an upregulation of muscarinic receptors and/or downstream signaling pathway proteins (such that similar nerve activity elicits an increased response to acetylcholine release from nerve terminals). Indeed, exercise training can alter cardiac autonomic (both muscarinic and beta-adrenergic) receptor number/sensitivity (3, 14, 34) and improve central neural inhibitory pathways, thereby reducing sympathetic efferent activity in rats with heart failure (49). The contribution of changes in central neural/peripheral efferent nerve activity and the cellular responses to this efferent activity to training bradycardia in both healthy subjects and in patients with cardiovascular disease merit further investigation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.E.B. and V.V.F. conception and design of research; G.E.B., K.L.C., T.C., N.L., P.W., and V.V.F. performed experiments; G.E.B., P.J.M., and V.V.F. analyzed data; G.E.B., P.J.M., and V.V.F. interpreted results of experiments; G.E.B. prepared figures; G.E.B. drafted manuscript; G.E.B., P.J.M., and V.V.F. edited and revised manuscript; G.E.B., K.L.C., T.C., N.L., P.W., P.J.M., and V.V.F. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants HL-086700, HL-115580, HL-084583, HL-083422, and HL-114383.
REFERENCES
- 1.Ali A, Mehrs MR, Malik FS, Lavie CJ, Bass D, Milani RV. Effects of aerobic exercise training on indices of ventricular repolarization in patients with chronic heart failure. Chest 116: 83–87, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Armour JA. Clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93: 165–176, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Barbier J, Rannou-Bekono F, Marchais J, Berthon PM, Delamarche P, Carré F. Effect of exercise training on β1, β2, β3, adrenergic and M2 muscarinic receptors in the Rat heart. Med Sci Sports Exerc 36: 949–954, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Baruscotti M, Bucchi A, DiFrancesco. Physiology and pharmacology of cardiac pacemaker (“funny”) current. Pharmacol Ther 107: 59–79, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Benito B, Gay-Jordi G, Serrano-Mollar A, Gausch E, Shi Y, Tardif JC, Brugada Nattel SJ, Mont L. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 123, 13–22, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bernardi L, Radaelli A, Passino C, Falcone C, Auguadro C, Martinelli L, Rinaldi M, Vigano M, Finardi G. Effects of physical training on cardiovascular control after heart transplantation. Int J Cardiol 118: 356–362, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol Ther 111: 808–835, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Billman GE. Cardiac autonomic remodeling and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol 297: H1171–H1193, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Billman GE. Heart variability - a historical perspective. Front Physiol 2: 86, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billman GE, Dujardin JP. Dynamic changes in cardiac vagal tone as measured by time-series analysis. Am J Physiol Heart Circ Physiol 258: H896–H902, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Billman GE, Hoskins RS. Time-series analysis of heart rate variability during submaximal exercise. Evidence for reduced cardiac vagal tone in animals susceptible to ventricular fibrillation. Circulation 80: 146–157, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Billman GE, Kukielka M. Effects of endurance exercise training on heart rate variability and susceptibility to sudden cardiac death: protection is not due to enhanced cardiac vagal regulation. J Appl Physiol 100: 896–906, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Billman GE, Kukielka M. Effect of endurance exercise training on the heart rate onset and heart rate recovery responses to submaximal exercise in animals susceptible to ventricular fibrillation. J Appl Physiol 102: 231–240, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Billman GE, Kukielka M, Kelley R, Mustafa-Bayoumi M, Altschuld RA. Endurance exercise training attenuates cardiac β2-adrenoceptor responsiveness and prevents ventricular fibrillation in animals susceptible to sudden death. Am J Physiol Heart Circ Physiol 290: H2590–H2599, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Bonaduce D, Petretta M, Cavallaro V, Apicella C, Ianniciello A, Romano M, Breglio R, Marciano F. Intensive training and cardiac autonomic control in high level athletes. Med Sci Sports Exerc 30: 691–696, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Bonilla IM, Belevych A, Sridhar A, Nishijima Y, Ho TH, He Q, Kukielka M, Terentyev D, Terentyeva R, Györke S, Carnes CA, Billman GE. Endurance exercise training normalizes repolarization and calcium handling abnormalities preventing ventricular fibrillation in a canine model of sudden cardiac death. J Appl Physiol 113: 1772–1783, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyett MR, D'Souza A, Zhang H, Morris GM, Dobrzynski H, Monfredi O. Viewpoint: is resting bradycardia in athletes the result of remodeling of the sinoatrial node rather than high vagal tone? J Appl Physiol 114: 1351–1335, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Carter JB, Banister EW, Blaber AP. Effect of endurance exercise on autonomic control of heart rate. Sports Med 33: 33–46, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Chadda KD, Banka VS, Bodenheimer MM, Helfant RH. Corrected sinus node recovery time experimental physiologic and pathologic determinants. Circulation 51: 797–801, 1975. [DOI] [PubMed] [Google Scholar]
- 20.Curran J, Makara MA, Little SC, Musa H, Liu B, Wu X, Polina L, Alecusan J, Wright P, Li J, Billman GE, Boyden PA, Gyorke S, Band H, Hund TJ, Mohler PJ. EHD-3-dependent endosome pathway regulates cardiac membrane excitability and physiology. Circ Res 115: 68–78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA, Clements-Jewery H, Lambiase PD, Billman GE, Janse MJ, Pugsley MK, Ng GA, Camm AJ, Roden DM, Walker MJ. The Lambeth conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther 139: 213–248, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Danson EJ, Paterson DJ. Enhanced neuronal nitric oxide synthase expression is central to cardiac vagal phenotype in exercise-trained mice. J Physiol 546: 225–232, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Angelis K, Wichi RB, Jesus WR, Moreira ED, Morris M, Kreiger EM, Irigoygen MC. Exercise training changes autonomic cardiovascular balance in mice. J Appl Physiol 96: 2174–2178, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Dixon EM, Kamath MV, McCartney N, Fallen EL. Neural regulation of heart rate variability in endurance athletes and sedentary controls. Cardiovasc Res 26: 713–719, 1992. [DOI] [PubMed] [Google Scholar]
- 25.D'Souza AD, Bucchi A, Johnsen AB, Logantha SJ, Monfredi O, Yanni J, Prehar S, Hart G, Cartwright E, Wisloff U, Dorbryznski H, DiFrancesco D, Morris GM, Boyett MR. Exercise training reduces resting heart rate via downregulation of funny channel HCN4. Nat Commun 5: 3775, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedorov VV, Glukhov AV, Chang R. Conduction barriers and pathways of the sinoatrial pacemaker complex: their role in normal rhythm and atrial arrhythmias. Am J Physiol Heart Circ Physiol 302: H1773–H1783, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Frishman WH, Pepine CJ, Weiss RJ, Baiker WM. Addition of zatebradine, a direct sinus node inhibitor, provides no greater exercise tolerance benefit in patients with angina taking extended-release nifedipine: results of a multicenter, randomized double-blind, placebo-controlled, parallel-group study. The Zatebradine Study Group. J Am Coll Cardiol 26: 305–312, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Fu Q, Levine BD. Exercise and the autonomic nervous system. In: Handbook of Clinical Neurology Vol 117 (3rd series) Autonomic Nervous System, edited by Buijs RM, Swaab DF. Philadelphia: Elsevier, 2013, chapt. 13, pp 147–190. [DOI] [PubMed] [Google Scholar]
- 29.Glukhov AV, Hage L, Hansen BJ, Pedraza-Toscono A, Vargas-Pinto P, Weiss R, Carnes CA, Billman GE, Fedorov VV. Sinoatrial node reentry in a canine chronic left ventricular infarct model: role of intranodal fibrosis and heterogeneity of refractoriness. Circ Arrhythm Electrophyiol 6: 984–994, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregoire J, Tuck S, Yammoto Y, Hughson R. Heart rate variability at rest and exercise: influence of age, gender and physical training. Can J Appl Physiol 21: 455–470, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Halliwill JR, Billman GE. Effect of general anesthesia on cardiac vagal tone. Am J Physiol Heart Circ Physiol 262: H1719–H1724, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Headrick JP, Pearl JN, Reichelt ME, Haseler LJ. Adenosine and its receptors in the heart: regulation, retaliation, and adaptation. Biochim Biophys Acta 1808: 1413–1428, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol Heart Circ Physiol 241: H620–H629, 1981. [DOI] [PubMed] [Google Scholar]
- 34.Holycross BJ, Kukielka M, Nishijima Y, Altschuld RA, Carnes CA, Billman GE. Exercise training normalizes β-adrenoceptor expression in dogs susceptible to ventricular fibrillation. Am J Physiol Heart Circ Physiol 293: H2702–H2709, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Iellamo F, Legramante JM, Massro M, Raimondo G, Galante A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease. Circulation 102: 2588–2592, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Jew KN, Olsson C, Mokelke EA, Palmer BM, Moore RL. Endurance training alters outward K+ current characteristics in rat cardiomyocytes. J Appl Physiol 90: 1327–1333, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Kaese S, Frommeyer G, Verheule S, van Loon G, Gehrmann J, Breithardt G, Eckhardt L. The ECG in cardiovascular-relevant animal models of electrophysiology. Herzschrittmacherther Elektrophysiol 24: 84–91, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Katona PG, McLean M, Dighton DH, Guz A. Sympathetic and parasympathetic cardiac control in athletes and non-athletes at rest. J Appl Physiol 52: 1652–1657, 1982. [DOI] [PubMed] [Google Scholar]
- 39.Kavanagh T, Mertens DJ, Shephard RJ, Beyene J, Kennedy J, Campbell R, Sawyer P, Yacoub M. Long-term cardiorespiratory results of exercise training following cardiac transplantation. Am J Cardiol 91: 190–194, 2003. [DOI] [PubMed] [Google Scholar]
- 40.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 106: 945–949, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Lewis SF, Nylander E, Gad P, Areskog N. Non-autonomic component of in bradycardia of endurance trained men at rest and during exercise. Acta Physiol Scand 109: 297–305, 1980. [DOI] [PubMed] [Google Scholar]
- 42.Lewis S, Thompson P, Areskog NH, Marconyak M, Vodak P, deBusk R, Haskell W. Endurance training and heart rate control studied by combined parasympathetic and beta-adrenergic blockades. Int J Sports Med 1: 42–49, 1980. [Google Scholar]
- 43.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. Translating animal model research: does it matter that our rodents are cold? Physiology 29: 413–420, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Milani-Nejad D, Janssen PM. Small and large animal models of cardiac contraction: research advantages and disadvantages. Pharmacol Ther 141: 235–249, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nylander E, Sigvardsson K, Kilbom A. Training bradycardia and intrinsic heart rate in rats. Eur J Appl Physiol 48: 189–199, 1982. [DOI] [PubMed] [Google Scholar]
- 46.O'Hara T, Rudy Y. Quantitative comparison of cardiac ventricular myocyte electrophysiology and response to drugs in human and nonhuman species. Am J Physiol Heart Circ Physiol 302: H1023–H1030, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Opthof T, Coronel R, Wilms-Schopman FJ, Plotnikov AN, Shlapakova IN, Danilo P Jr, Rosen MR, Janse MJ. Dispersion of repolarization in canine ventricle and electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm 4: 341–348, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Ordway GA, Charles JB, Randall DC, Billman GE, Wekstein DR. Heart rate adaptation to exercise training in cardiac denervated dogs. J Appl Physiol 52: 1586–1590, 1982. [DOI] [PubMed] [Google Scholar]
- 49.Patel KP, Zheng H. Central neural control of sympathetic activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol 302: H527–H537, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porges SW, Bohrer RE, Cheung MN, Drasgow F, McCabe PM, Keren G. New time-series statistics for detecting rhythmic co-occurrence in the frequency domain: the weighted coherence and its application to psychophysiological research. Psychol Bull 88: 580–587, 1980. [PubMed] [Google Scholar]
- 51.Scher AM, Ohm WW, Bumgarner K, Boynton R, Young AC. Sympathetic and parasympathetic control of heart rate in the dog, baboon, and man. Fed Proc 31: 1219–1225, 1972. [PubMed] [Google Scholar]
- 52.Scott AS, Eberhard A, Ofir D, Benchetrit Dinh TP, Calabrese P, Lesiuk V, Perrault H. Enhanced cardiac vagal efferent activity does not explain training-bradycardia. Auton Neurosci 112: 60–68, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Seals DR, Chase PB. Influences of physical training on HR variability and baroreflex circulatory control. J Appl Physiol 66: 1886–1895, 1989. [DOI] [PubMed] [Google Scholar]
- 54.Shen YT, Vatner DE, Gagnon HE, Vatner SF. Species differences in regulation of α-adrenergic receptor function. Am J Physiol Regul Integr Comp Physiol 257: R1110–R1116, 1989. [DOI] [PubMed] [Google Scholar]
- 55.Shi X, Stevens GH, Foresman BH, Stern SA, Raven PB. Autonomic nervous control of the heart: endurance exercise training. Med Sci Sports Exerc 27: 1406–1413, 1995. [PubMed] [Google Scholar]
- 56.Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. The power spectral analysis of heart variability athletes during dynamic exercise–part I. Clin Cardiol 18: 583–586, 1995. [DOI] [PubMed] [Google Scholar]
- 57.Smith ML, Hudson DL, Graitzer HM, Raven PB. Exercise training bradycardia the role of the autonomic balance. Med Sci Sports Exerc 21: 40–44, 1989. [DOI] [PubMed] [Google Scholar]
- 58.Stein R, Moraes RS, Cavalcanti AV, Ferlin EL, Zimmerman LI, Ribeiro JP. Intrinsic sinus and atrioventricular node electrophysiologic adaptations in endurance athletes. J Am Coll Cardiol 329: 1033–1038, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Such L, Rodriguez A, Alberola A, Lopez L, Ruiz R, Artal L, Pons I, Pons ML, Garcia C, Chorro FJ. Intrinsic changes on automatism, conduction, and refractoriness by exercise in isolated rabbit hearts. J Appl Physiol 92: 225–229, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Van de Water A, Verheyen J, Xhonnex R, Reneman RS. An improved method to correct QT interval of the electrocardiogram for changes in heart rate. J Pharmacol Methods 22: 207–217, 1989. [DOI] [PubMed] [Google Scholar]
- 61.Wilhelm M, Roten L, Tanner H, Wilhelm I, Schmid JP, Saner H. Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am J Cardiol 108: 580–585, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Zanesco A, Anutnes E. Effects of exercise on the cardiovascular system: pharmacological approaches. Pharmacol Ther 114: 307–317, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Zarzoso M, Such-Miquel L, Parra G, Brines-Ferrando L, Such L, Charro FJ, Guerrero J, Guill A, O'Conner JE, Alberola A. The training-induced changes in on automatism, conduction and myocardial refractoriness are not mediated by parasympathetic postganglionic neuron activity. Eur J Appl Physiol 112: 2185–2193, 2012. [DOI] [PubMed] [Google Scholar]