Abstract
The hypothesis that cerebrovascular autoregulation was not impaired during head-up tilt (HUT) that followed brief exposures to varying degrees of prior head-down tilt (HDT) was tested in 10 healthy young men and women. Cerebral mean flow velocity (MFV) and cardiovascular responses were measured in transitions to a 60-s period of 75° HUT that followed supine rest (control) or 15 s HDT at −10°, −25°, and −55°. During HDT, heart rate (HR) was reduced for −25° and −55°, and cardiac output was lower at −55° HDT. MFV increased during −10° HDT, but not in the other conditions even though blood pressure at the middle cerebral artery (BPMCA) increased. On the transition to HUT, HR increased only for −55° condition, but stroke volume and cardiac output transiently increased for −25° and −55°. Total peripheral resistance index decreased in proportion to the magnitude of HDT and recovered over the first 20 s of HUT. MFV was significantly less in all HDT conditions compared with the control in the first 5-s period of HUT, but it recovered quickly. An autoregulation correction index derived from MFV recovery relative to BPMCA decline revealed a delay in the first 5 s for prior HDT compared with control but then a rapid increase to briefly exceed control after −55° HDT. This study showed that cerebrovascular autoregulation is modified by but not impaired by brief HDT prior to HUT and that cerebral MFV recovered quickly and more rapidly than arterial blood pressure to protect against cerebral hypoperfusion and potential syncope.
Keywords: push-pull effect, transcranial Doppler ultrasound, cerebrovascular autoregulation
the “push-pull effect” was first shown experimentally by Banks et al. (3) on the basis of rapid transitions on an acceleration platform from gravity oriented toward the head (−Gz) to gravity oriented toward the feet (+Gz). The −Gz to +Gz transition caused a greater reduction in mean arterial blood pressure (MAP) than was observed if normal 1 G gravitational stress was the baseline prior to +Gz acceleration, and the effect on MAP was increased as the duration of prior −Gz was increased (2, 3). Investigating the push-pull effect was of practical interest as a potential explanation for increased risk for loss of consciousness in jet fighter pilots during maneuvers (19, 20).
Responses of MAP during tilt table experiments with transitions from head-down (HDT) to head-up tilt (HUT) (10, 22, 23) were similar to those observed by Banks and colleagues (3) with an acceleration platform. The mechanism underlying the exaggerated reduction in MAP with the push-pull effect was speculated to be neural in origin, related in part to a delayed or inappropriate vascular response following large and rapid increases in carotid sinus pressure followed by rapid decreases (7). However, a potential role of cardiopulmonary baroreceptor response to the increased pressure with a transient movement of blood into the thorax (29) might be involved, while experiments with autonomic ganglionic blockade in dogs (30) and centrally acting α2-receptor blockade in humans (23) revealed the importance of myogenic responses in the lower limb vasculature. That is, the transient reduction in arterial pressure during HDT induced excessive vasorelaxation in the lower limbs that recovered slowly on return to HUT (23, 30).
Loss of consciousness with acceleration or with HUT is a direct consequence of a reduction in cerebral blood flow (28). While it is evident that a reduction in blood pressure at the level of the middle cerebral artery (BPMCA) with HUT would initially be associated with a decline in cerebral blood flow, cerebrovascular autoregulation counters the decrease in BPMCA with rapid reductions of cerebrovascular resistance (CVR), so the cerebral blood flow is quickly restored toward the baseline value (1, 12, 17, 24). During the push-pull maneuver, one study identified an increase in cerebrovascular resistance during brief exposure to −1 Gz HDT prior to +1 Gz HUT that contributed to the decline in cerebral blood flow. In contrast, a study with an 8-m-radius human centrifuge using transitions from +1 Gz or −2 Gz for 10 s to +2 Gz found that prior −2 Gz exaggerated the reduction in cerebral blood flow velocity and tissue oxygenation, but the adaptation of an index of cerebrovascular resistance remained intact and was not different between conditions (26). Thus, unlike the peripheral cardiovascular responses resulting from the push-pull effect (22, 23, 30), the cerebrovascular responses have not been clarified.
The current study was designed to investigate the primary hypothesis that cerebrovascular autoregulation would remain intact during HDT and respond rapidly and appropriately in a transition to HUT, enabling a quick recovery of cerebral blood flow. To test the impact of varying levels of “push” effect, three different angles of HDT were imposed prior to HUT. The dynamic cerebrovascular adaptation was assessed by an autoregulation correction index derived from MFV recovery relative to BPMCA decline.
MATERIALS AND METHODS
Participant description.
Ten healthy adult men and women, all nonsmokers (32.3 ± 6.3 yr, height 167.4 ± 7.2 cm, weight 62.2 ± 10.3 kg), volunteered for this study and provided written, informed consent after receiving a complete written and verbal description of the experimental procedures and potential risks, in accordance with the policies of the Office of Research Ethics of the University of Waterloo, which approved this study. All participants were instructed to refrain from consuming alcohol, caffeinated beverages, and engaging in vigorous exercise for 24 h prior to testing, and a light meal was preferred 2 h before testing.
Experimental design.
All tests were conducted on a manually controlled tilt table, which allowed transition of the body from −55° head down tilt (HDT) to 75° head up tilt (HUT) within 2 s. Shoulder blocks and a bicycle saddle were fixed and adjusted well to prevent the body from moving during rapid transition from HDT to HUT. Another function of the saddle was to ensure relaxation of the legs avoiding muscle contraction during HUT. Two straps around the chest and legs were used to firmly and comfortably secure the participants to the table.
After instrumentation and before actual testing, familiarization was provided between −55° head-down tilt for 15 s with a rapid transition to 75° head-up tilt (65°/s). This minimized psychological responses to the posture change.
After being in the supine position for 5 min, 2 min of baseline data was collected. Then the participants remained horizontal or were moved to one of three randomized HDT angles: −10°, −25°, and −55° (corresponding with vector components of −0.17, −0.42, −0.82 Gz) for 15 s prior to being moved rapidly to HUT (75°) for 1 min. To ensure the participant returned to a stable baseline before the next tilt, 5 min of supine rest was arranged between each tilt task.
Data acquisition.
Continuous estimates of arterial blood pressure (systolic, SBP; diastolic, DBP) were determined using finger-cuff plethysmography internally calibrated by a return to flow arm cuff (Finometer PRO, Finapres Medical Systems, Amsterdam, The Netherlands), and height-corrected to the heart. Arterial pressures were corrected to a standard arm cuff measurement by subtracting the difference between a 2-min period of resting beat-by-beat data and duplicate arm cuff values obtained on the same arm prior to initiating the finger pressure collections. Heart rate was measured from the R-R interval of the electrocardiogram (Colin Pilot, Colin Medical Instruments, San Antonio, TX). An estimate of cardiac output (QMF) was obtained from the finger arterial pulse wave with the Modelflow algorithm that incorporates age, sex, height, and weight as the factors to estimate stroke volume (SV) (14).
Blood pressure at the middle cerebral artery (BPMCA) was calculated as BPMCA = MAP − (distance in cm from heart to MCA × 0.735 mmHg/cmH2O). A water-filled catheter was positioned at the cerebral Doppler probe and connected to a pressure transducer placed at the height corrector of Finometer to establish the hydrostatic difference (in cm) between MCA and heart level.
Transcranial Doppler ultrasound (TCD) with a 2-MHz pulsed Doppler probe (DWL, Germany) was placed over the right temporal window to allow for the insonation of the M1 segment of the middle cerebral artery (MCA). The probe was held in place using an adjustable headband throughout the testing. Cerebrovascular resistance index (CVRi) was calculated as arterial pressure at the level of the MCA (BPMCA) divided by mean MCA mean flow velocity (MFV).
Breath-by-breath CO2 was sampled through a nasal cannula and analyzed by infrared spectroscopy (Colin Pilot, Colin Medical Instruments, San Antonio, TX). End-tidal CO2 (PetCO2) values were converted to millimeters Hg based on atmospheric temperature and pressure.
All signals were output at 1,000 Hz from a PowerLab (ADInstruments, Australia) and recorded onto a computer running LabChart 7.3.7 for future analysis. The signal from Finometer was shifted −1 s, and QMF was shifted one beat back to compensate for Finometer's internal digital signal processing delay. The signals were linearly interpolated at 1-s intervals.
Data analysis.
Baseline data were obtained from 30 s of steady conditions before tilting. During 15 s of HDT, the initial 7 s were averaged and referred to as HDT 1, and the final 8 s were called HDT 2. The first 20 s of HUT data were divided to four segments every 5 s, and later 40 s were divided to two segments every 20 s.
Dynamic regulation of cerebral blood flow was assessed by time to reach nadir and a cerebrovascular autoregulation correction index. The time to reach nadir (T1) was identified visually on linearly interpolated recording of TPR, CBFV, and CVRi with analysis completed independently by two researchers involved in this testing and averaged. The autoregulation correction index was calculated as (MFVmeasured − MFVpredicted)/(MFVbaseline − MFVpredicted), where MFVpredicted = MFVbaseline × (BPMCAmeasured/BPMCAbaseline).
Statistics.
All data were expressed as means ± SD. Statistical testing was established a priori as two separate phases: the first compared the HDT to the baseline and between conditions, the second phase compared the HUT period between conditions. For each phase, all variables were compared using a two-way repeated-measures ANOVA with main effects of condition of prior HDT (−10°, −25°, and −55°) and time. The time to nadir was assessed by one-way repeated-measures ANOVA. When significant effects were observed, the Holm-Sidak method was used for comparisons among the factors. The data were analyzed using SigmaPlot 12.5 (Systat Software). All the graphs were made by Origin 8.1 (OriginLab).
RESULTS
Central cardiovascular and PetCO responses.
During the HDT periods, there was a significant main effect of time (P = 0.001) and a significant condition × time (P = 0.002) interaction effect for each of HR, MAP, SV, and QMF (see Supplemental Table A1, available with the online version of this article). HR was significantly reduced relative to the supine baseline for the −25° and −55° conditions and was lower during −55° than −10° (Table 1, Supplemental Table A1). MAP had a significant main effect of time and was elevated in the first HDT period compared with both baseline and HDT2. Estimated QMF had significant main effects of condition and time, and an interaction of condition × time. QMF was reduced from baseline in the −25° condition only during the HDT1 period, while it was reduced at both HDT1 and HDT2 with −55°, and it was also lower than the other HDT angles. SV had significant condition, and condition × time interaction, and was reduced at −55° compared with baseline and relative to the other HDT angles. There were no effects of HDT on PetCO.
Table 1.
Cardiovascular and PetCO2 responses to head-up tilt after various head-down tilts
| Baseline | HDT1 | HDT2 | Duration at HUT, s |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 40 | 60 | ||||
| HR, beats/min | |||||||||
| Con | 66.8 ± 10.7 | 74.1 ± 12.1 | 74.6 ± 10.6 | 73.1 ± 9.5 | 74.3 ± 9.0 | 74.9 ± 9.2 | 77.0 ± 11.0 | ||
| −10° | 64.5 ± 12.3 | 63.3 ± 12.4 | 64.4 ± 14.2 | 73.8 ± 14.6 | 76.7 ± 16.0 | 74.7 ± 13.5 | 73.2 ± 12.5 | 74.5 ± 11.3 | 78.0 ± 10.1 |
| −25° | 65.2 ± 11.0 | 61.6 ± 10.4† | 61.6 ± 11.0† | 76.1 ± 13.1 | 78.5 ± 12.5 | 75.7 ± 13.6 | 71.9 ± 12.3 | 73.7 ± 13.3 | 77.6 ± 13.2 |
| −55° | 64.9 ± 10.8 | 58.5 ± 9.9†* | 59.7 ± 10.7†* | 81.2 ± 12.7*#& | 85.3 ± 13.0*#& | 80.9 ± 13.3& | 75.7 ± 11.6 | 76.5 ± 12.4 | 78.6 ± 12.6 |
| MAP, mmHg | |||||||||
| Con | 88.0 ± 11.0 | 86.4 ± 14.7 | 87.7 ± 15.0 | 90.8 ± 13.4 | 93.3 ± 12.9 | 91.9 ± 12.2 | 90.8 ± 11.8 | ||
| −10° | 86.8 ± 12.3 | 89.6 ± 10.7† | 86.0 ± 11.2 | 78.7 ± 13.7& | 81.3 ± 13.4 | 89.2 ± 13.4 | 91.0 ± 12.3 | 92.5 ± 10.6 | 91.8 ± 9.7 |
| −25° | 85.9 ± 12.2 | 88.5 ± 10.8 | 83.8 ± 12.0 | 72.6 ± 15.2&* | 78.9 ± 16.8& | 87.8 ± 13.7 | 92.4 ± 12.8 | 92.1 ± 11.4 | 90.4 ± 11.5 |
| −55° | 84.9 ± 12.5 | 88.1 ± 11.0† | 82.9 ± 13.0* | 62.5 ± 16.8&*# | 71.0 ± 20.4&*# | 84.6 ± 15.2& | 89.8 ± 13.9 | 91.1 ± 11.2 | 89.7 ± 10.9 |
| SV, ml | |||||||||
| Con | 80.4 ± 14.0 | 76.2 ± 16.7 | 68.5 ± 13.4 | 68.5 ± 14.7 | 65.1 ± 12.6 | 63.8 ± 11.7 | 61.5 ± 12.2 | ||
| −10° | 80.8 ± 14.0 | 80.1 ± 13.3 | 82.1 ± 14.4 | 85.5 ± 13.7& | 78.0 ± 15.0& | 72.4 ± 16.2 | 69.7 ± 13.0 | 64.2 ± 11.6 | 60.1 ± 11.1 |
| −25° | 79.5 ± 13.5 | 78.6 ± 13.4 | 81.3 ± 14.0 | 87.8 ± 15.2& | 82.1 ± 16.1& | 75.2 ± 15.4& | 72.0 ± 13.8 | 66.6 ± 12.7 | 62.5 ± 12.1 |
| −55° | 80.1 ± 13.4 | 75.4 ± 14.4* | 76.0 ± 13.6*# | 85.5 ± 15.0& | 83.6 ± 19.2& | 75.2 ± 18.6 | 70.6 ± 15.2 | 65.0 ± 14.1 | 60.7 ± 12.0 |
| QMF, l/min | |||||||||
| Con | 5.3 ± 1.2 | 5.4 ± 1.2 | 4.9 ± 1.0 | 4.9 ± 0.9 | 4.7 ± 0.7 | 4.7 ± 1.0 | 4.6 ± 1.0 | ||
| −10° | 5.1 ± 1.2 | 5.1 ± 1.3 | 5.2 ± 1.3 | 6.2 ± 1.3& | 5.8 ± 1.1& | 5.3 ± 1.2 | 5.0 ± 1.3 | 4.7 ± 1.2 | 4.6 ± 1.1 |
| −25° | 5.1 ± 1.2 | 4.8 ± 1.1† | 5.0 ± 1.1 | 6.5 ± 1.2& | 6.3 ± 1.3&#* | 5.5 ± 1.2& | 5.0 ± 1.0 | 4.8 ± 1.2 | 4.7 ± 1.2 |
| −55° | 5.1 ± 1.1 | 4.4 ± 1.0†*# | 4.5 ± 1.0†*# | 6.7 ± 1.3&* | 6.9 ± 1.1&* | 5.9 ± 1.3&* | 5.2 ± 1.3 | 4.8 ± 1.4 | 4.7 ± 1.3 |
| PetCO2, mmHg | |||||||||
| Con | 28.5 ± 3.0 | 26.9 ± 3.3 | 27.0 ± 3.5 | 27.7 ± 3.3 | 27.9 ± 3.1 | 28.0 ± 3.7 | 27.2 ± 3.9 | ||
| −10° | 29.3 ± 3.2 | 29.5 ± 3.0 | 29.4 ± 2.7 | 27.1 ± 3.0 | 27.2 ± 3.1 | 27.3 ± 2.7 | 27.4 ± 2.8 | 27.7 ± 3.0 | 28.0 ± 3.6 |
| −25° | 28.8 ± 3.1 | 28.9 ± 3.3 | 28.2 ± 3.2 | 27.6 ± 2.9 | 27.1 ± 3.3 | 27.2 ± 3.5 | 27.5 ± 3.5 | 27.6 ± 3.4 | 27.1 ± 3.4 |
| −55° | 28.9 ± 2.9 | 28.6 ± 3.6 | 28.6 ± 3.0 | 27.7 ± 3.0 | 27.5 ± 2.7 | 27.8 ± 3.6 | 28.3 ± 3.3 | 28.0 ± 3.1 | 27.5 ± 3.6 |
Data are expressed as means ± SD; n = 10.
HR, heart rate; MAP, mean arterial pressure; SV, stroke volume; QMF, cardiac output calculated by way of Modelflow; PetCO2, end-tidal Pco2; HDT1: HDT at 0–7 s; HDT2: HDT at 8–15 s.
HUT of 5: 0–5 s; 10: 6–10 s; 15:11–15 s; 20: 16–20 s; 40: 21–40 s; 60: 41–60 s. P < 0.05: significantly different †vs. baseline, &vs. control,
vs. −10° HDT condition, #vs. −25° at same period.
Cardiovascular responses were altered following rapid HUT with the magnitude of change dependent on the degree of preceding HDT. With the exception of PetCO, there were significant main effects during HUT of condition and of time as well as the interaction of condition × time for each of HR, MAP, SV, and QMF (see Supplemental Table A2, available with the online version of this article). HR was elevated in the first 15 s following −55° HDT compared with control and the other HDT angles (Table 1). MAP was markedly affected on HUT with significantly greater reductions noted with greater prior HDT angle at 5 s of HUT. At 10 s HUT, the −25° and −55° HDT conditions MAP remained reduced compared with control, and −55° was different from −25° and −10°, while at 15 s only, −55° remained lower than control. There were no differences in MAP after 20 s in HUT position. SV estimated by Modelflow was significantly elevated above control at 5 and 10 s for each of the prior HDT conditions and remained elevated for −25° and −55° at 15 s and for −25° at 20 s. QMF was elevated compared with control for all prior HDT conditions at 5 and 10 s and remained elevated for −25° and −55° at 15 s and for −55° at 20 s. QMF was also elevated in the −55° condition compared with −10° through the first 15 s and compared with −25° at 10 s. PetCO2 was not significantly different between conditions during HUT (Table 1).
Systemic total peripheral vascular resistance.
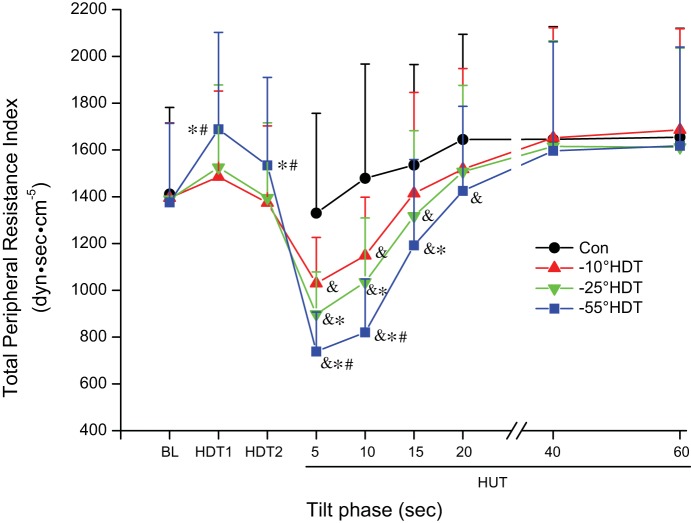
The index of total peripheral resistance (TPRi) measured during the prior HDT period had significant main effects of condition and time, as well as the interaction (see Supplemental Table A1). There was a greater elevation in −55° condition compared with −25° and −10° prior HDT at both the HDT1 and HDT2 time points (Fig. 1). With HUT, significant main effects of condition and time were observed along with the interaction effect (see Supplemental Table A2). The magnitude of decrease in TPRi was affected by prior HDT with significant reductions for all prior HDT compared with control in the first 10 s. Further, the decrease following −55° was greater compared with −10° and −25°, and the decrease following −25° was greater compared with −10° during the first 10 s of HUT. This decrease continued at 15 s in −25° and −55° HDT condition with the reduction following −55° HDT greater than that of −10°. At HUT for 20 s, only −55° was still different from control.
Fig. 1.
Index of systemic total peripheral resistance during supine baseline, 3 different degrees of head-down tilt (HDT), and rapid transitions to 75° head-up tilt (HUT). BL, supine baseline; Con, HUT without previous HDT; HDT1, HDT values between 0 and 7 s; HDT2, HDT at 8–15 s. 75° HUT at the times 5: 0–5 s; 10: 6–10 s; 15: 11–15 s; 20: 16–20 s; 40: 21–40 s; 60: 41–60 s. Significantly different &vs. Control, *vs. −10° HDT condition, #vs. −25° at same period, P < 0.05. Data are expressed as means ± SD.
Cerebrovascular responses.
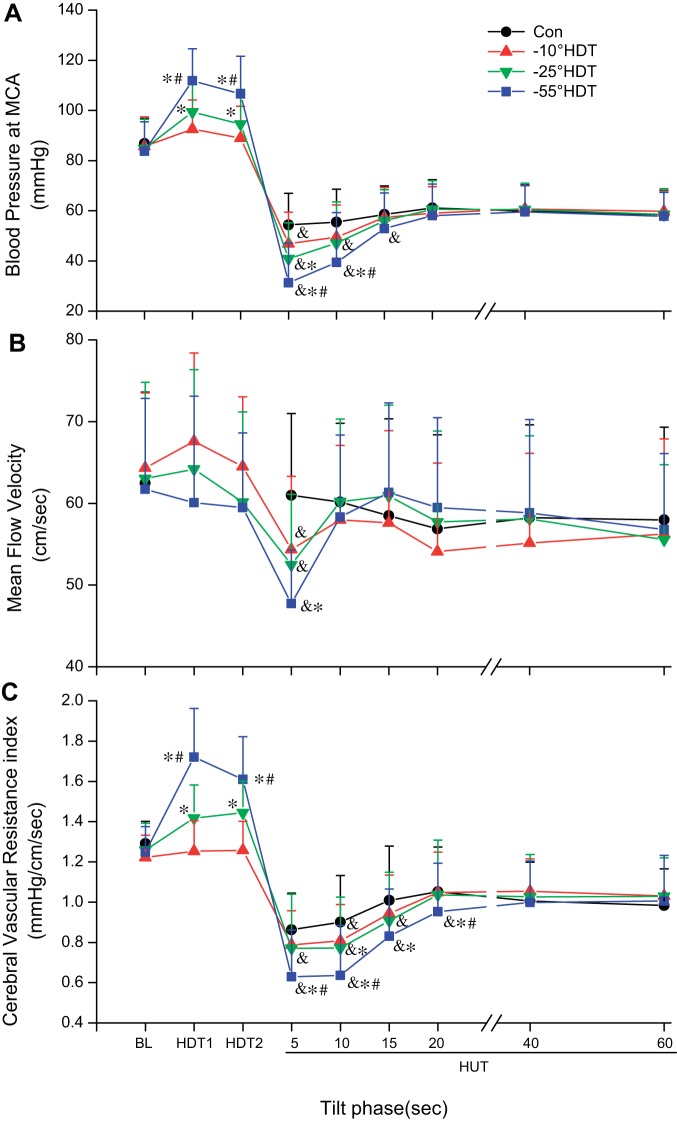
BPMCA was increased during HDT with significant main effects of condition and time as well as a significant interaction reflecting increases related to the greater HDT angle (Fig. 2A). Significant main and interaction effects were also observed during HUT, with BPMCA significantly lower than control in the first 15 s following −55° HDT, for 10 s after −25°, and for only 5 s following −10° HDT. The reduction in BPMCA for the −55° condition was greater than after −10° and −25° HDT in the first 10 s. A difference was also detected between −10° and −25° at 5 s. In all conditions, the BPMCA remained reduced during HUT with respect to the supine baseline.
Fig. 2.
Blood pressure at the level of the middle cerebral artery (MCA; A), mean cerebral blood flow velocity (B), and cerebrovascular resistance index (C) during supine baseline, 3 different degrees of head-down tilt (HDT), and rapid transitions to 75° head-up tilt (HUT). See Fig. 1 for abbreviations. Significantly different &vs. Control, *vs. −10° HDT condition, #vs. −25° at same period, P < 0.05.
Cerebral blood flow velocity.
There was a significant main effect of condition, but not of time nor an interaction effect for mean blood flow velocity in the MCA (MFV, see Supplemental Table A1), with −10° HDT being different from −25° and −55° (Fig. 2B). On moving to HUT, there was no significant difference between conditions, but time and the condition × time interaction were significant (see Supplemental Table A2). Only in the first 5 s of HUT was MFV reduced relative to control in all tilts that followed HDT, and a difference was detected between −10° and −55° (Fig. 2B). Overall, MFV was slightly reduced during the HUT compared with the supine baseline position.
Cerebrovascular resistance indexes.
Head-down tilt resulted in significant main effects of condition and time, as well as the interaction (see Supplemental Table A1) with increases of CVRi during −25° and −55°, with differences between the levels of HDT (Fig. 2C). On moving to HUT, there were significant main effects of condition and time as well as the interaction (see Supplemental Table A2). CVRi in control was significantly reduced, and the reduction in CVRi in the first 10 s was exaggerated after prior HDT with the greatest drop following −25° and −55° HDT. CVRi remained less than Control at 20 s in −55° HDT condition with differences between the prior tilt angles (Fig. 2C).
Peripheral and cerebrovascular response and recovery.
The time to reach the nadir response of TPRi on moving to HUT was significantly greater for each of the prior HDT angles compared with control (Table 2). There were no differences in the time to nadir for the MFV responses for control compared with any of the prior HDT angles. The time to nadir for CVRi was longer following prior −10° and −25° HDT, but not for prior −55° HDT.
Table 2.
Time to nadir after head-up tilt
| Time to Nadir, s |
|||
|---|---|---|---|
| TPRi | MFV | CVRi | |
| Control | 2.00 ± 1.2 | 3.90 ± 3.8 | 3.20 ± 2.8 |
| −10° | 4.70 ± 2.1§† | 2.00 ± 1.7 | 5.78 ± 2.6*‡ |
| −25° | 4.40 ± 1.3§‡ | 2.20 ± 1.5 | 6.10 ± 1.9§‡ |
| −55° | 3.90 ± 1.4§ | 2.50 ± 1.8 | 4.40 ± 1.4† |
Data are means ± SD, in seconds.
TPRi, index of total peripheral resistance calculated as mean arterial pressure/cardiac output (dyn·s·cm−5); MFV, mean cerebral blood flow velocity (cm/s); CVRi, cerebrovascular resistance index (mmHg/cm/s).
P < 0.05,
P < 0.01, significant difference vs. control condition.
P < 0.05,
P < 0.01, significant difference vs. MFV.
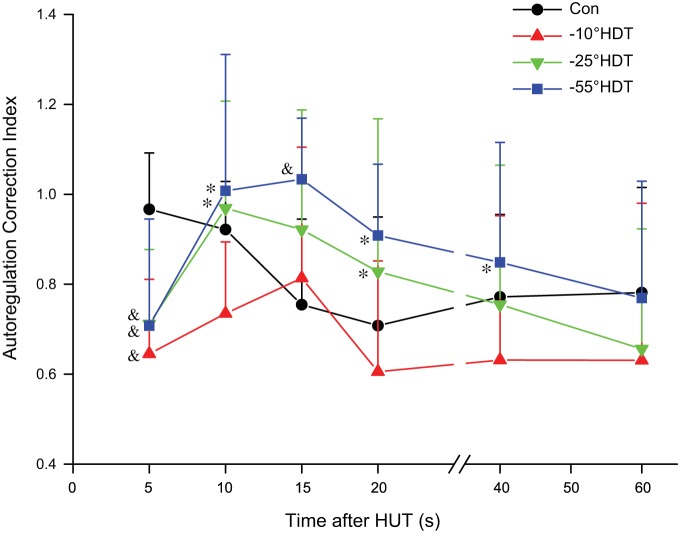
The calculated cerebrovascular autoregulation correction index (Fig. 3) displayed a complex pattern of adaptation assessed by two-way repeated-measures ANOVA revealing significant main effects of condition and a condition × time interaction. The within-condition time effects are presented in Supplemental Table A1. The differences between groups at each time point are summarized: at 5 s after HUT, each of the prior HDT angles (−10°, −25° and −55°) was significantly reduced compared with control; at 10 s after HUT, −55° and −25° were increased compared with −10°; at 15 s, −55° was greater than control; at 20 s, −55° and −25° were greater than −10°; and at 40 s, −55° was greater than −10°.
Fig. 3.
Autoregulation correction index referenced to supine baseline (see materials and methods) was calculated for control and 3 different degrees of prior head-down tilt (HDT) during 75° head-up tilt (HUT). See Fig. 1 for abbreviations. Significantly different &vs. Control, *vs. −10° HDT condition, P < 0.05.
DISCUSSION
Dynamic cerebrovascular regulation during a HUT maneuver was challenged in the current study by brief periods of preceding HDT at different angles. These tilt table maneuvers, which simulate the “push-pull effect,” have been known to challenge and delay regulation of arterial blood pressure (10, 22, 23). Indeed, in the current study the decrease in BPMCA on moving to HUT was greater and for a longer duration as the degree of prior HDT was increased. In the first 5 s, the recovery of MFV expressed by the autoregulation correction index lagged the control condition for all prior HDT conditions. However, by 10 s, autoregulation correction index did not differ from control for any of the prior HDT conditions, by 15 s, the index for the −55° HDT condition exceeded control, and at 20 s, both −55° and −25° HDT exceeded the −10° HDT condition. The consequence of the rapid recovery of autoregulation was that cerebral MFV was similar to the control condition before 10 s of HUT for all of the prior HDT angles. These results support our hypothesis that cerebrovascular autoregulation remained intact regardless of the prior HDT position. The current data contrast with a report of sustained, ∼20 s, increase in cerebrovascular resistance after HDT (31) and reveal that prior HDT as used in the current study does not negatively impact cerebrovascular regulation.
Systemic cardiovascular responses.
The push-pull paradigm causes a greater and more prolonged reduction in arterial blood pressure with the transition from HDT to HUT compared with the supine to HUT maneuver. The stimulation of the carotid baroreceptors in the HDT position could delay the appropriate vasoconstrictor response on moving to HUT (10). HDT also increases the pressure at the cardiopulmonary baroreceptors potentially contributing to suppression of sympathetic nerve activity during postural transition (29). However, alternative explanations for the vascular responses of the lower body on transitions from HDT to HUT have been suggested (23, 30). In the HDT position, distending pressures in the arteries and veins of the lower limbs are reduced. On the venous side, this means that with transition to HUT venous volume and pressures are initially low as the veins refill, allowing for transient increase in arterial-venous pressure gradient and blood flow. As well, arteries and arterioles in the lower parts of the body relax by myogenic mechanisms in response to the reduction in distending pressure as confirmed in carefully controlled studies in dogs (30) and humans (23). From these studies, Sheriff and colleagues concluded that the sympathetic nervous system is active in the vasculature of the legs but that it neither contributes to, nor guards against, the peripheral vascular response to the push-pull effect. The graded effect of prior HDT on the magnitude of decline in TPRi, determined from MAP/QMF, with HUT is consistent with a myogenic effect on resistance vessels in the leg.
The current study revealed additional information about the cardiac output response to the push-pull effect. Previously, Sheriff and colleagues (22, 23) measured, by Doppler ultrasound, increases in stroke volume and cardiac output on transition from −15° HDT to +30° HUT that were greater than from supine to HUT position. Similar observations were made with Modelflow estimates (10). In the current study, the Modelflow approach revealed in the transitions to 75° HUT that the magnitude of increase in cardiac output was greater as the magnitude of prior HDT increased. The mechanisms underlying the transient increase in cardiac output on HUT might relate to blood volume stored in the lungs during HDT and the reduction in right ventricular pressure associated with HUT, allowing greater left ventricular filling, as venous return is reduced on HUT (22, 25). A summation of the total volume of blood ejected by the heart in the first 15 s after HUT suggested a net increase of about 400 ml for the −55° HDT condition compared with control. It is also possible that the increase in stroke volume and cardiac output resulted from the reduction in afterload (MAP) even if cardiac filling pressure was reduced with HUT. The increase in heart rate contributed significantly to the increase in cardiac output only for the −55° HDT condition.
The reduction in MAP with the concomitant transient increase in cardiac output in the first 10-15 s after HUT established an interesting set of conditions for regulation of cerebral blood flow. Several studies have shown a strong correlation between MFV and cardiac output (27, 28), while others did not (4, 15, 16). In the current study, there was no relationship between cardiac output and MFV. In the first 5 s after HUT following prior HDT, cardiac output was elevated while MFV was reduced. MFV recovered while cardiac output was elevated and when cardiac output recovered to the control values. However, in the current study some caution should be applied to interpretation of estimates of cardiac output from the pulse contour analysis by the Modelflow algorithm as the method has bias when peripheral vascular resistance and arterial compliance are acutely modified (8).
Dynamic cerebral regulation.
This is the first study to investigate cerebrovascular regulation with rapid transitions to HUT from various HDT positions. We observed that even with the maximum drop in BPMCA of ∼75 mmHg within 2 s during the transition from −55° HDT to 75° HUT that CBFV recovered to control levels in less than 10 s. As the mean BPMCA after the −55° HDT was ∼31 mmHg in the initial 5 s of HUT, the ability to autoregulate might be quite remarkable, appearing to extend, in this group of healthy, young adults, below the range of about 50–170 mmHg normally indicated for static autoregulation (1).
There were differences in the cerebrovascular response to HUT that were dependent on the prior supine or HDT condition. In the control condition for the first 5 s after HUT from supine baseline, the measured MFV was only slightly less than baseline (62.8 vs. 61.6 cm/s) even though BPMCA was reduced by 32.4 mmHg. The calculated autoregulation correction index for control was close to 1.0 in this initial phase, but then it declined and stabilized near 0.8. In contrast for the prior HDT conditions, the autoregulation correction index was about 0.7 in the first 5 s, but rapidly increased so that by the second 5-s period, none of the prior HDT conditions differed from control. The greater autoregulatory response to HUT following the largest prior HDT angle (−55°) was necessary to restore MFV in the face of the greater and more prolonged reduction in BPMCA in this condition. Interestingly, at the 15-s point, the response following −55° was greater than control. Beyond this time, the autoregulatory responses tended to be more consistent across the prior supine or HDT conditions with all achieving approximately 70–80% correction. Reductions in MFV in upright posture compared with supine, as observed here, are well documented, but the mechanism is not clear, with possible contributions from reductions in cardiac output or arterial Pco2 (6, 13, 21), or simply an incomplete correction. The current study suggests that changes in magnitude and rate of application of perfusion pressure can influence at least the early autoregulatory responses.
Calculation of CVRi does not account for the perfusion pressure gradient across the cerebral circulation, as determined by not only arterial side pressure, but intracranial pressure (1), nor does it account for potential changes in cerebrovascular compliance. In the transition, the perfusion pressure gradient is not known and cannot be predicted. However, an alternative explanation to a rapid reduction in CVRi that could account for the relatively small reduction in CBFV with HUT, and its rapid recovery, derives from discussions of the “siphon effect” in the cerebral circulation. A Point-Counterpoint series on this topic provided a rationale for and against the mechanism for blood flow through the brain being described as a siphon (9, 11). Evidence against a siphon, and more in line with a vascular waterfall, could be obtained from the necessity to maintain arterial perfusion pressure in conditions of sustained HUT (28, 32), the requirements for elevated central arterial pressure in giraffes, or visual observation of collapsed internal jugular veins in HUT (9). However, consistent with a siphon effect, it is possible that in the transient period of the rapid tilt that a continuous column of blood from arterial to venous circulations was maintained (11), allowing blood to flow to the head against gravity under very low perfusion pressure.
The conditions of the early phase of the rapid HUT following HDT differ from the many studies that have investigated dynamic cerebrovascular regulation in postural transitions from supine to head-up, or sitting to standing (24, 32). A study describing a transition from −90° to +90° tilt reported that calculated Doppler resistance index was elevated during HDT and remained elevated for up to 20 s after HUT (31). The authors suggested that the increase in resistance index during HDT was protective, but that the sustained increase could contribute to cerebral hypoperfusion during the HUT. A subsequent preliminary investigation reported on the cerebrovascular response of four men at the midpoint of a 30-s period of twice force of gravity (+2 Gz) on an 8-m centrifuge when preceded by 10 s at −2 Gz (26). In contrast with the tilt table study (31) an increase in cerebrovascular resistance index was not observed with the transition from negative to positive acceleration; however, the early transient response was not reported in the centrifuge study. Thus the current study adds clarity to the understanding of the cerebrovascular response to the push-pull maneuver by showing that cerebrovascular regulation remains intact and is capable of responding rapidly to large changes in arterial perfusion pressure.
There are limitations with the measurement of cerebral blood flow velocity and the calculation of variables reflecting cerebrovascular function that must be acknowledged. First, the measurement is strictly of velocity without a measurement of MCA diameter. Recent studies have revealed changes in MCA diameter when arterial Pco2 varies across a modest range (5). Likewise, drug-induced changes in arterial pressure have been associated with changes in MCA diameter (18), although it was not clear from that study whether the pressure per se or the drugs caused the changes in diameter. Thus for the current study it is possible that myogenic alterations in cerebrovascular tone contributed to constriction of the MCA in HDT and dilation in HUT; however, there are no data to reveal such changes in the timeframe of this study. We calculated an autoregulation correction index directly from measured MFV and BPMCA. This determines the magnitude of adaptation assuming that the supine baseline is the reference value. A component of the adaptation is a dilation of cerebral resistance vessels, but changes in cerebrovascular compliance probably also contribute, especially during the transition phase.
Implications and conclusions.
The current study has shown that a brief 10–20% reduction in cerebral MFV occurred when a short exposure to HDT was followed by a rapid transition to 75° HUT. There was, however, a rapid recovery of MFV after the initial 5 s period for all previous HDT conditions, reflecting very efficient cerebrovascular autoregulation in support of our hypothesis and showing, contrary to a previous investigation (31), that any increase in CVRi during the HDT was not sustained on transition to HUT. The mechanism responsible for the rapid cerebrovascular autoregulation appeared to be primarily myogenic in origin. However, the rapid recovery of MFV needs to be placed in perspective. The current maneuvers established a maximum HDT stress of nearly −0.9 Gz and a maximum HUT stress less than +1 Gz. This contrasts with push-pull effects encountered with jet tactical flight maneuvers, in which the negative acceleration can exceed −2.0 Gz, and the positive acceleration can be as high as +7 Gz. Under these latter conditions, one would predict that CBFV would drop to much lower values potentially resulting in grayout or even blackout.
GRANTS
This work was supported through the Defense Medical Fund of China (CWS11J146), Military Science Foundation of China under Grant (13QNP126), and the Natural Sciences and Engineering Research Council (RGPIN-6473).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.Y., Y.G., and R.L.H. conception and design of research; C.Y., D.K.G., R.V., T.B., K.S.F., and R.L.H. performed experiments; C.Y., Y.G., D.K.G., R.V., T.B., K.S.F., and R.L.H. analyzed data; C.Y., Y.G., R.V., and R.L.H. interpreted results of experiments; C.Y., Y.G., and R.L.H. prepared figures; C.Y. and Y.G. drafted manuscript; C.Y., Y.G., D.K.G., R.V., T.B., K.S.F., and R.L.H. edited and revised manuscript; C.Y., Y.G., D.K.G., R.V., T.B., K.S.F., and R.L.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the volunteers for participation in the study. The authors are grateful to the anonymous reviewers for suggestions for the presentation of indicators of autoregulation.
REFERENCES
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Banks RD, Grissett JD, Saunders PL, Mateczun AJ. The effect of varying time at −Gz on subsequent +Gz physiological tolerance (push-pull effect). Aviat Space Environ Med 66: 723–727, 1995. [PubMed] [Google Scholar]
- 3.Banks RD, Grissett JD, Turnipseed GT, Saunders PL, Rupert AH. The “push-pull effect”. Aviat Space Environ Med 65: 699–704, 1994. [PubMed] [Google Scholar]
- 4.Brown CM, Dutsch M, Hecht MJ, Neundorfer B, Hilz MJ. Assessment of cerebrovascular and cardiovascular responses to lower body negative pressure as a test of cerebral autoregulation. J Neurol Sci 208: 71–78, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol 117: 1090–1096, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Deegan BM, Devine ER, Geraghty MC, Jones E, Olaighin G, Serrador JM. The relationship between cardiac output and dynamic cerebral autoregulation in humans. J Appl Physiol 109: 1424–1431, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doe CP, Self DA, Drinkhill MJ, McMahon N, Myers DS, Hainsworth R. Reflex vascular responses in the anesthetized dog to large rapid changes in carotid sinus pressure. Am J Physiol Heart Circ Physiol 275: H1169–H1177, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Dyson KS, Shoemaker JK, Arbeille P, Hughson RL. Modelflow estimates of cardiac output compared with Doppler ultrasound during acute changes in vascular resistance in women. Exp Physiol 95: 561–568, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Gisolf J, Gisolf A, van Lieshout JJ, Karemaker JM. The siphon controversy: an integration of concepts and the brain as baffle. Am J Physiol Regul Integr Comp Physiol 289: R627–R629, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Goodman LS, LeSage S. Impairment of cardiovascular and vasomotor responses during tilt table simulation of “push-pull” maneuvers. Aviat Space Environ Med 73: 971–979, 2002. [PubMed] [Google Scholar]
- 11.Hicks JW, Munis JR. The siphon controversy counterpoint: the brain need not be “baffling”. Am J Physiol Regul Integr Comp Physiol 289: R629–R632, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hughson RL, Edwards MR, O'Leary DD, Shoemaker JK. Critical analysis of cerebrovascular autoregulation during repeated head-up tilt. Stroke 32: 2403–2408, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Immink RV, Truijen J, Secher NH, Van Lieshout JJ. Transient influence of end-tidal carbon dioxide tension on the postural restraint in cerebral perfusion. J Appl Physiol 107: 816–823, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Langewouters GJ, Wesseling KH, Goedhard WJ. The static elastic properties of 45 human thoracic and 20 abdominal aortas in vitro and the parameters of a new model. J Biomech 17: 425–435, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Larsen FS, Strauss G, Knudsen GM, Herzog TM, Hansen BA, Secher NH. Cerebral perfusion, cardiac output, and arterial pressure in patients with fulminant hepatic failure. Crit Care Med 28: 996–1000, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 90: 298–306, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke 31: 1897–1903, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Zhu YS, Hill C, Armstrong K, Tarumi T, Hodics T, Hynan LS, Zhang R. Cerebral autoregulation of blood velocity and volumetric flow during steady-state changes in arterial pressure. Hypertension 62: 973–979, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaud VJ, Lyons TJ. The “push-pull effect” and G-induced loss of consciousness accidents in the U.S. Air Force. Aviat Space Environ Med 69: 1104–1106, 1998. [PubMed] [Google Scholar]
- 20.Michaud VJ, Lyons TJ, Hansen CM. Frequency of the “push-pull effect” in U.S. Air Force fighter operations. Aviat Space Environ Med 69: 1083–1086, 1998. [PubMed] [Google Scholar]
- 21.Serrador JM, Hughson RL, Kowalchuk JM, Bondar RL, Gelb AW. Cerebral blood flow during orthostasis: role of arterial CO2. Am J Physiol Regul Integr Comp Physiol 290: R1087–R1093, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Sheriff DD, Nadland IH, Toska K. Hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol 103: 452–458, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Sheriff DD, Nadland IH, Toska K. Role of sympathetic responses on the hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol 108: 523–532, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Sorond FA, Serrador JM, Jones RN, Shaffer ML, Lipsitz LA. The sit-to-stand technique for the measurement of dynamic cerebral autoregulation. Ultrasound Med Biol 35: 21–29, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toska K, Walloe L. Dynamic time course of hemodynamic responses after passive head-up tilt and tilt back to supine position. J Appl Physiol 92: 1671–1676, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Tran CC, Berthelot M, Etienne X, Dussault C, Jouanin JC, Van Beers P, Serra A, Guezennec CY. Cerebral oxygenation declines despite maintained orthostatic tolerance after brief exposure to gravitational stress. Neurosci Lett 380: 181–186, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Van Lieshout JJ, Pott F, Madsen PL, van Goudoever J, Secher NH. Muscle tensing during standing: effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke 32: 1546–1551, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, oxygenation. J Appl Physiol 94: 833–848, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 112: 157–165, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Wong BJ, Sheriff DD. Myogenic origin of the hypotension induced by rapid changes in posture in awake dogs following autonomic blockade. J Appl Physiol 105: 1837–1844, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang WX, Zhan CL, Geng XC, Lu X, Yan GD, Chu X. Cerebral blood flow velocity by transcranial Doppler during a vertical-rotating table simulation of the push-pull effect. Aviat Space Environ Med 71: 485–488, 2000. [PubMed] [Google Scholar]
- 32.Zuj KA, Arbeille P, Shoemaker JK, Hughson RL. Cerebral critical closing pressure and CO2 responses during the progression toward syncope. J Appl Physiol 114: 801–807, 2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.