Abstract
The ATP-sensitive K+ (KATP) channel is part of a class of inward rectifier K+ channels that can link local O2 availability to vasomotor tone across exercise-induced metabolic transients. The present investigation tested the hypothesis that if KATP channels are crucial to exercise hyperemia, then inhibition via glibenclamide (GLI) would lower hindlimb skeletal muscle blood flow (BF) and vascular conductance during treadmill exercise. In 27 adult male Sprague-Dawley rats, mean arterial pressure, blood lactate concentration, and hindlimb muscle BF (radiolabeled microspheres) were determined at rest (n = 6) and during exercise (n = 6–8, 20, 40, and 60 m/min, 5% incline, i.e., ∼60–100% maximal O2 uptake) under control and GLI conditions (5 mg/kg intra-arterial). At rest and during exercise, mean arterial pressure was higher (rest: 17 ± 3%, 20 m/min: 5 ± 1%, 40 m/min: 5 ± 2%, and 60 m/min: 5 ± 1%, P < 0.05) with GLI. Hindlimb muscle BF (20 m/min: 16 ± 7%, 40 m/min: 30 ± 9%, and 60 m/min: 20 ± 8%) and vascular conductance (20 m/min: 20 ± 7%, 40 m/min: 33 ± 8%, and 60 m/min: 24 ± 8%) were lower with GLI during exercise at 20, 40, and 60 m/min, respectively (P < 0.05 for all) but not at rest. Within locomotory muscles, there was a greater fractional reduction present in muscles comprised predominantly of type I and type IIa fibers at all exercise speeds (P < 0.05). Additionally, blood lactate concentration was 106 ± 29% and 44 ± 15% higher during exercise with GLI at 20 and 40 m/min, respectively (P < 0.05). That KATP channel inhibition reduces hindlimb muscle BF during exercise in rats supports the obligatory contribution of KATP channels in large muscle mass exercise-induced hyperemia.
Keywords: blood flow, conductance, glibenclamide
the appropriate redistribution of cardiac output at exercise onset is achieved by vascular smooth muscle cell (SMC) relaxation in arterioles supplying active skeletal muscle and SMC contraction in arterioles supplying quiescent tissue. Within the active skeletal muscle vascular bed, local metabolic byproducts of muscle contraction (e.g., ATP, H+, K+, adenosine, etc.) as well as endothelium-derived factors (e.g., nitric oxide and PGI2) are important for matching O2 supply with O2 demand during exercise (14, 21, 43) via modulation of vascular tone. The SMC resting membrane potential supports this balance by setting vascular tone as well as determining vasomotor sensitivity to depolarizing stimuli (i.e., sympathetic nerve activity). Modulation of membrane potential may therefore represent an important mechanism by which sympathetic vasoconstriction is attenuated in active skeletal muscle (24a, 47).
It is well recognized that inward rectifier K+ (Kir) channels influence membrane potential and are capable of hyperpolarizing the SMC membrane, resulting in an inhibitory effect on excitability and thus relaxation (33). Specifically, the vascular ATP-sensitive K+ (KATP) channel is activated, in part, by the accumulation of subsarcolemmal ADP and may therefore contribute to the integration of local O2 availability with vasomotor control across the greater than two orders of magnitude increase in muscle metabolism seen with exercise. Importantly, KATP channel activation has been shown to result in SMC hyperpolarization, relaxation of vascular smooth muscle, and attenuated α-adrenergic vasoconstriction (27, 31, 39, 45). In healthy human forearm muscle, the activation of KATP channels increases vasodilation in response to ischemia, indicating the potential for KATP channels to contribute substantially to skeletal muscle O2 delivery during exercise (7). Despite early studies demonstrating that blockade of KATP channels significantly attenuates reactive and functional hyperemia in humans and animals (1, 2, 6), more recent work has failed to confirm these results (16, 17, 42). As vasomotor control mechanisms may be fiber type dependent (e.g., Refs. 5, 10, and 22), it is quite possible that, within muscles with a specific fiber type composition, KATP channel activation may represent an obligatory mechanism supporting exercise-induced skeletal muscle hyperemia in vivo. Additionally, given that KATP channels are sensitive to metabolic status and that there exists considerable heterogeneity of metabolic characteristics among and within skeletal muscles, it is plausible that KATP channel-mediated vasodilation is dependent on skeletal muscle fiber type distribution, a concept that cannot be addressed in humans with current technology. The differences in fiber type profile between species, muscles, or states of dysfunction promote remarkably different muscle O2 tensions, which may determine ADP accumulation and the open probability of KATP channels in the skeletal muscle vasculature.
The purpose of the present investigation was to test the hypothesis that inhibition of KATP channels via glibenclamide (GLI) would reduce hindlimb skeletal muscle blood flow (BF) and vascular conductance (VC) and increase arterial blood lactate concentration during submaximal treadmill exercise in healthy rats. Furthermore, given the purported coupling of cellular metabolism with vasomotor tone by KATP channels, it was anticipated that the reductions in VC with GLI would associate directly with the percentage of type I and type IIa fibers across muscles.
MATERIALS AND METHODS
Ethical approval.
All procedures were approved by the Institutional Animal Care and Use Committee of Kansas State University under guidelines established by the National Institutes of Health and conducted according to animal use guidelines mandated by The Journal of Physiology (15). Twenty-seven adult male Sprague-Dawley rats (∼4 mo old, body mass: 366 ± 7 g) were maintained in accredited animal facilities (Association for the Assessment and Accreditation of Laboratory Animal Care) at Kansas State University on a 12:12-h light-dark cycle with food and water provided ad libitum. Rats were separated into either a rest group (n = 6) or three exercise groups (n = 6–8) and used for within-animal comparisons under control and KATP channel inhibition (GLI) conditions. Rats were acclimatized to running during a familiarization period composed of five to seven sessions on a custom-built motor-driven treadmill set at an incline of 5%. Each session consisted of running at progressive speeds from ∼20 to ∼60 m/min over a total duration of no more than 5 min.
The pharmacological sulphonylurea derivative GLI (494 g/mol, 5-chloro-N-{4-[N-(cyclohexylcarbamoyl)sulfamoyl]phenethyl}-2-methoxybenzamide, Sigma-Aldrich, St. Louis, MO) was used to achieve inhibition of vascular KATP channels. Briefly, 50 mg GLI was dissolved in 4 ml NaOH (0.1 M) under continuous sonication to produce a 12.5 mg/ml stock solution. A 5 mg/kg dose was drawn from the stock solution and diluted to ∼1 ml with heparinized saline. Thomas et al. (47) previously demonstrated that 20 mg/kg iv GLI reversed the effect of the same dose of diazoxide. However, it has been previously reported that GLI is a selective KATP channel blocker at concentrations below 5 μmol/l (3, 41). The current dose of 5 mg/kg for rats of a mean body mass of 366 g equates to a blood concentration of ∼140 μmol/l. Given that 98–99% of GLI is bound to plasma protein, the effective blood concentration of GLI is ∼2–3 μmol/l (19), which is in the range for GLI to be selective for KATP channels without possible inhibition of Ca+ channel current (41). This dosing strategy, based on SMC investigations, has been shown to elicit a degree of KATP channel blockade for swine in vivo (60–70%) (16).
Surgical instrumentation.
On the day of the final protocol, rats were anesthetized initially with a 5% isoflurane-O2 mixture and maintained on a 3% isoflurane-O2 mixture for the duration of the surgical instrumentation. Cannulation of both carotid and caudal arteries was performed with PE-10 connected to PE-50 (Intra-Medic polyethylene tubing, BD, Franklin Lakes, NJ). The catheters were then tunneled subcutaneously to the dorsal aspect of the cervical region, where they were exteriorized through a puncture wound in the skin. After the closure of incisions, the rat was removed from anesthesia and given a minimum recovery period of 2 h.
Experimental protocol.
After the recovery period, the exercise BF protocol was performed with the treadmill set at an incline of 5%. The rat was placed on the treadmill, and the carotid catheter was attached to a pressure transducer (P23ID, Gould Statham Instruments, Hato Rey, Puerto Rico) for measurements of mean arterial pressure (MAP) and heart rate (HR) while the caudal catheter was connected to a 1-ml syringe attached to a Harvard pump (model 907, Harvard, Holliston, MA). Exercise (i.e., ∼60–100% maximal O2 uptake) was initiated at a speed of 20 m/min (n = 8), 40 m/min (n = 6), or 60 m/min (n = 7) and remained steady for ∼3 min, at which time premicrosphere HR and pressures were recorded. At ∼3.5 min of total exercise time, blood withdrawal was initiated from the caudal catheter at a rate of 0.25 ml/min. The carotid catheter was then disconnected from the pressure transducer, and ∼0.5–0.6 106, 15-μm-diameter microspheres (57Co or 85Sr in random order, Perkin Elmer Life and Analytical Sciences, Waltham, MA) were rapidly infused into the aortic arch of the running animal for the determination of tissue BF. Upon reconnection of the carotid catheter to the pressure transducer, a second pressure reading was immediately recorded postmicrosphere infusion. An arterial blood sample (0.2 ml) was then drawn from the carotid artery catheter for the determination of blood gases, hematocrit, pH, lactate concentration, and glucose concentration. Exercise was terminated, and the rat was continuously monitored during a minimum 30-min rest period before the second bout began.
A postrecovery pressure was recorded to establish resting pressure and HR values. The KATP channel inhibitor GLI (5 mg/kg) was infused via the caudal artery catheter. Pressure was monitored continuously until GLI elicited a persistent rise in MAP, at which time the second exercise bout was initiated. The second bout and administration of microspheres were performed identically to the protocol described above. As previously demonstrated, subsequent control bouts of exercise for this protocol demonstrate high reproducibility of hemodynamic variables (MAP, HR, BF, and VC) (43). Upon exercise termination, the rat was euthanized with an overdose of pentobarbital (>50 mg/kg body mass) via the carotid artery catheter. For another group of rats (n = 6), administration of microspheres, blood sampling, and pressure recordings were performed at rest under control and GLI conditions as described above.
Determination of BF and VC.
Correct placement of the carotid catheter in the aortic arch was verified by anatomic dissection. Hindlimb muscles and muscle portions as well as the lungs, kidneys, and representative organs of the splanchnic region were removed, weighed, and placed in counting vials for the determination of radioactivity.
Radioactivity was measured for each tissue as well as the reference sample using a γ-scintillation counter (model 5230, Packard Auto Gamma Spectrometer, Downers Grove, IL). Taking into account the cross-talk fraction between isotopes enabled radioactivity to be determined for separate microsphere injections (57Co or 85Sr). Based on this radioactivity, BF to each tissue was determined for the individual conditions, control or GLI, by comparison with the reference sample of known flow rate and measured radioactivity (23, 30). Tissue BFs were expressed as milliliters per minute per 100 g of tissue, and results were also normalized to MAP and expressed as VC (in ml·min−1·100 g−1 tissue·mmHg−1). Adequate mixing of the microspheres for each BF determination was verified by a <15% difference in BF between the right and left kidneys or right and left hindlimbs.
Statistical analysis.
All variables were compared between and within groups using mixed two-way ANOVAs and Student-Newman-Keuls post hoc tests where appropriate. Muscle fiber type composition was based on the percentage of type I, type IIa, type IIb, and type IId/x fibers in the individual muscles and muscle parts of the rat hindlimb, as previously reported by Delp and Duan (13). Pearson product correlations were used to test the relationship between changes in VC and muscle fiber type. Significance was set at P < 0.05, and values are expressed as means ± SE.
RESULTS
There was no significant difference in body mass between groups (rest: 365 ± 5 g, 20 m/min: 367 ± 13 g, 40 m/min: 407 ± 26 g, and 60 m/min: 375 ± 15 g, P > 0.05).
Effects of GLI on HR, MAP, arterial blood gases, pH, hematocrit, lactate concentration, and glucose concentration.
At rest and during exercise at all speeds, MAP was higher with GLI compared with control treatment (Fig. 1). HR was lower with GLI only at rest and during exercise at 20 m/min. Administration of the vehicle did not change MAP (prevehicle: 127 ± 4 mmHg and postvehicle: 125 ± 4 mmHg, P > 0.05) or HR (prevehicle: 400 ± 18 beats/min and postvehicle: 436 ± 19 beats/min, P > 0.05) in a subset of rats (n = 6). Arterial O2 tension was higher with GLI at rest (control: 96.8 ± 2.2 mmHg and GLI: 107.7 ± 2.6 mmHg, P < 0.05) but not during exercise at any speed (P > 0.05 for all). Hematocrit was higher with GLI during exercise at 20 m/min only (control: 31 ± 1% and GLI: 33 ± 1%, P < 0.05), whereas pH was lower with GLI during exercise at 40 m/min only (control: 7.39 ± 0.02 and GLI: 7.35 ± 0.03, P < 0.05). During exercise at both 40 m/min (control: 102 ± 6 mg/dl and GLI: 89 ± 8 mg/dl) and 60 m/min (control: 87 ± 4 mg/dl and GLI: 75 ± 3 mg/dl), blood glucose concentration was lower with GLI (P < 0.05 for both). Blood lactate concentration was higher with GLI during exercise at 20 m/min (control: 2.0 ± 0.3 mmol/l and GLI: 4.1 ± 0.9 mmol/l, P < 0.05) and 40 m/min (control: 5.9 ± 0.5 mmol/l and GLI: 8.7 ± 1.4 mmol/l, P < 0.05) but not at rest or 60 m/min (P > 0.05 for both).
Fig. 1.

Glibenclamide (GLI) increased mean arterial pressure (MAP; B) at rest and during submaximal exercise at 20, 40, and 60 m/min, whereas heart rate (HR; A) was decreased at rest and 20 m/min. *P < 0.05 vs. control (CON).
Effects of GLI on skeletal muscle BF and VC.
At rest, there were no differences between groups in total hindlimb skeletal muscle BF or VC (P = 0.40; Fig. 2) or in BF and VC in any of the individual muscles or muscle portions (Table 1). During exercise at all speeds, both total hindlimb skeletal muscle BF and VC were lower with GLI compared with control treatment (Fig. 2). Specifically, GLI resulted in a lower BF in 13, 20, and 14 of the 28 individual hindlimb muscles or muscle portions during exercise at 20, 40, and 60 m/min, respectively (Table 2). VC was lower with GLI in 15, 21, and 18 of the 28 individual hindlimb muscles or muscle portions during exercise at 20, 40, and 60 m/min, respectively (Table 3). Furthermore, there was a significant correlation between the percentage of type I and type IIa fibers and the percent decrease in VC for the 28 muscles or muscle portions of the hindlimb during exercise at 20 m/min (r = −0.53), 40 m/min (r = −0.69), and 60 m/min (r = −0.50; Fig. 3).
Fig. 2.
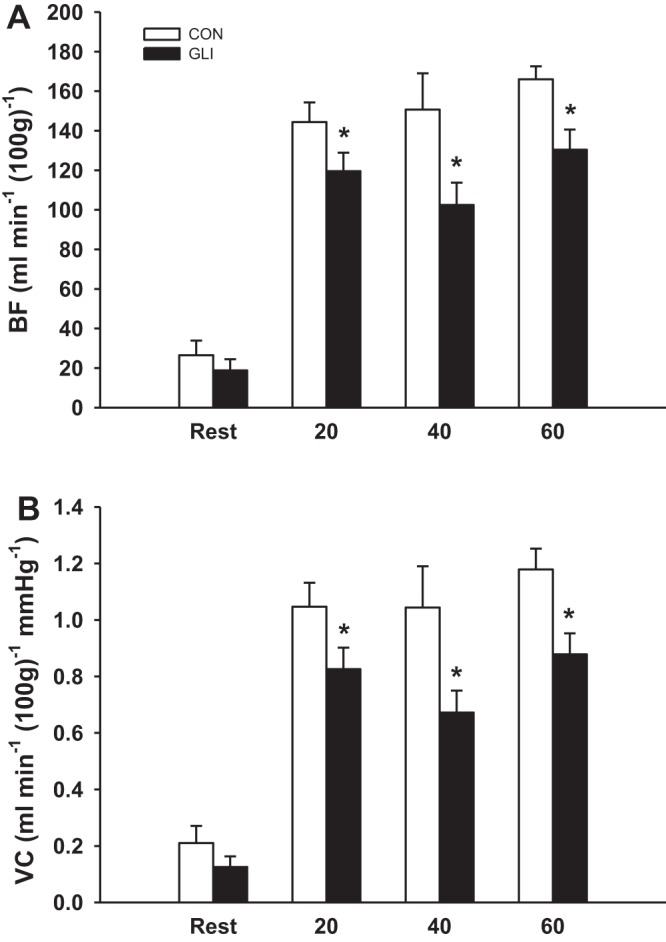
GLI decreased total hindlimb skeletal muscle blood flow (BF; A) and vascular conductance (VC; B) during submaximal exercise at 20, 40, and 60 m/min but not at rest compared with control. *P < 0.05 vs. control.
Table 1.
Effects of GLI on individual hindlimb skeletal muscle BFs and VCs for the rest group
| BF, ml·min−1·100 g−1 |
VC, ml·min−1·100 g−1·mmHg−1 |
||||
|---|---|---|---|---|---|
| Percentage of Type I + IIa Fibers According to Delp and Duan (13) | Control | GLI | Control | GLI | |
| Ankle extensors | |||||
| Soleus | 91 | 115 ± 20 | 91 ± 28 | 0.88 ± 0.15 | 0.62 ± 0.21 |
| Plantaris | 20 | 18 ± 4 | 16 ± 4 | 0.4 ± 0.03 | 0.11 ± 0.03 |
| Gastrocnemius, red | 86 | 57 ± 18 | 46 ± 22 | 0.44 ± 0.15 | 0.30 ± 0.14 |
| Gastrocnemius, white | 0 | 11 ± 3 | 16 ± 5 | 0.09 ± 0.03 | 0.10 ± 0.03 |
| Gastrocnemius, mixed | 9 | 20 ± 5 | 20 ± 9 | 0.16 ± 0.04 | 0.13 ± 0.06 |
| Tibialis posterior | 27 | 26 ± 10 | 11 ± 4 | 0.21 ± 0.08 | 0.07 ± 0.02 |
| Flexor digitorum longus | 32 | 57 ± 31 | 65 ± 48 | 0.41 ± 0.21 | 0.38 ± 0.26 |
| Flexor halicus longus | 29 | 16 ± 4 | 9 ± 2 | 0.12 ± 0.03 | 0.06 ± 0.01 |
| Ankle flexors | |||||
| Tibialis anterior, red | 37 | 59 ± 17 | 33 ± 12 | 0.45 ± 0.13 | 0.20 ± 0.06 |
| Tibialis anterior, white | 20 | 24 ± 8 | 17 ± 4 | 0.19 ± 0.06 | 0.11 ± 0.03 |
| Extensor digitorum longus | 24 | 22 ± 5 | 20 ± 5 | 0.16 ± 0.04 | 0.13 ± 0.03 |
| Peroneals | 33 | 27 ± 7 | 18 ± 7 | 0.22 ± 0.06 | 0.12 ± 0.04 |
| Knee extensors | |||||
| Vastus intermedius | 96 | 134 ± 34 | 101 ± 44 | 1.04 ± 0.28 | 0.70 ± 0.30 |
| Vastus medialis | 18 | 38 ± 14 | 30 ± 12 | 0.30 ± 0.12 | 0.20 ± 0.08 |
| Vastus lateralis, red | 65 | 88 ± 37 | 87 ± 45 | 0.71 ± 0.31 | 0.59 ± 0.29 |
| Vastus lateralis, white | 0 | 10 ± 2 | 14 ± 4 | 0.08 ± 0.01 | 0.10 ± 0.03 |
| Vastus lateralis, mixed | 11 | 24 ± 8 | 24 ± 8 | 0.19 ± 0.07 | 0.16 ± 0.05 |
| Rectus femoris, red | 34 | 38 ± 11 | 28 ± 13 | 0.29 ± 0.08 | 0.18 ± 0.09 |
| Rectus femoris, white | 0 | 21 ± 4 | 16 ± 5 | 0.17 ± 0.04 | 0.11 ± 0.03 |
| Knee flexors | |||||
| Biceps femoris anterior | 0 | 12 ± 3 | 12 ± 2 | 0.10 ± 0.03 | 0.08 ± 0.02 |
| Biceps femoris posterior | 8 | 20 ± 7 | 16 ± 5 | 0.16 ± 0.05 | 0.10 ± 0.03 |
| Semitendinosus | 17 | 23 ± 7 | 15 ± 4 | 0.19 ± 0.06 | 0.10 ± 0.02 |
| Semimembranosus, red | 28 | 25 ± 12 | 17 ± 4 | 0.20 ± 0.09 | 0.12 ± 0.03 |
| Semimembranosus, white | 0 | 13 ± 2 | 10 ± 1 | 0.10 ± 0.02 | 0.07 ± 0.01 |
| Thigh adductors | |||||
| Adductor longus | 95 | 97 ± 14 | 74 ± 20 | 0.76 ± 0.11 | 0.50 ± 0.15 |
| Adductor magnus and brevis | 11 | 31 ± 14 | 22 ± 7 | 0.25 ± 0.11 | 0.15 ± 0.05 |
| Gracilis | 23 | 23 ± 9 | 14 ± 3 | 0.18 ± 0.07 | 0.09 ± 0.02 |
| Pectineus | 31 | 29 ± 10 | 20 ± 6 | 0.23 ± 0.08 | 0.13 ± 0.04 |
Data are means ± SE; n = 6 at rest and 8 during exercise.
GLI, glibenclamide; BF, blood flow; VC, vascular conductance.
P < 0.05 vs. control.
Table 2.
Effects of GLI on individual hindlimb skeletal muscle BFs for exercise groups
| 20 m/min |
40 m/min |
60 m/min |
|||||
|---|---|---|---|---|---|---|---|
| Percentage of Type I + IIa Fibers According to Delp and Duan (13) | Control | GLI | Control | GLI | Control | GLI | |
| Ankle extensors | |||||||
| Soleus | 94 | 382 ± 19 | 277 ± 21* | 293 ± 63 | 152 ± 25*† | 312 ± 15 | 213 ± 28* |
| Plantaris | 20 | 253 ± 25 | 209 ± 21 | 254 ± 35 | 193 ± 32* | 258 ± 15 | 223 ± 10 |
| Gastrocnemius, red | 86 | 419 ± 40 | 337 ± 33* | 395 ± 70 | 238 ± 52* | 432 ± 11 | 351 ± 41 |
| Gastrocnemius, white | 0 | 53 ± 13 | 69 ± 9 | 82 ± 15 | 81 ± 11 | 93 ± 13 | 85 ± 11 |
| Gastrocnemius, mixed | 9 | 161 ± 9 | 152 ± 16 | 182 ± 28 | 137 ± 21* | 201 ± 9 | 167 ± 13* |
| Tibialis posterior | 27 | 192 ± 23 | 161 ± 16 | 229 ± 37 | 162 ± 20* | 285 ± 22 | 222 ± 21* |
| Flexor digitorum longus | 32 | 118 ± 17 | 105 ± 20 | 116 ± 8 | 86 ± 16 | 122 ± 11 | 124 ± 15 |
| Flexor halicus longus | 29 | 107 ± 14 | 87 ± 12* | 117 ± 21 | 84 ± 15* | 107 ± 12 | 96 ± 10 |
| Ankle flexors | |||||||
| Tibialis anterior, red | 37 | 383 ± 24 | 337 ± 39 | 420 ± 63 | 270 ± 40* | 405 ± 23 | 334 ± 40 |
| Tibialis anterior, white | 20 | 136 ± 12 | 139 ± 18 | 157 ± 24 | 93 ± 17* | 127 ± 14 | 117 ± 15 |
| Extensor digitorum longus | 24 | 70 ± 5 | 72 ± 5 | 128 ± 27† | 97 ± 15 | 94 ± 9 | 97 ± 13 |
| Peroneals | 33 | 169 ± 13 | 144 ± 19 | 172 ± 22 | 112 ± 9* | 149 ± 17 | 122 ± 10 |
| Knee extensors | |||||||
| Vastus intermedius | 96 | 419 ± 25 | 299 ± 18* | 407 ± 69 | 262 ± 40* | 456 ± 16 | 321 ± 31* |
| Vastus medialis | 18 | 249 ± 15 | 186 ± 11* | 245 ± 34 | 169 ± 27* | 277 ± 15 | 221 ± 15* |
| Vastus lateralis, red | 65 | 464 ± 27 | 306 ± 42* | 325 ± 48† | 196 ± 44* | 396 ± 26 | 291 ± 36* |
| Vastus lateralis, white | 0 | 56 ± 20† | 57 ± 14 | 68 ± 12 | 68 ± 14 | 99 ± 7 | 82 ± 11 |
| Vastus lateralis, mixed | 11 | 200 ± 12† | 139 ± 16* | 186 ± 21 | 123 ± 25* | 236 ± 20 | 180 ± 15* |
| Rectus femoris, red | 34 | 324 ± 29 | 233 ± 25* | 280 ± 33 | 196 ± 23* | 333 ± 21 | 263 ± 26* |
| Rectus femoris, white | 0 | 146 ± 15 | 113 ± 10* | 141 ± 15 | 113 ± 18 | 187 ± 12‡ | 146 ± 13* |
| Knee flexors | |||||||
| Biceps femoris anterior | 0 | 34 ± 4 | 41 ± 6 | 72 ± 16† | 62 ± 10 | 74 ± 5 | 63 ± 7 |
| Biceps femoris posterior | 8 | 106 ± 11 | 93 ± 10 | 119 ± 15 | 78 ± 17* | 138 ± 11 | 103 ± 15* |
| Semitendinosus | 17 | 77 ± 7 | 77 ± 15 | 73 ± 9 | 45 ± 4* | 50 ± 9 | 50 ± 8 |
| Semimembranosus, red | 28 | 137 ± 19 | 123 ± 14 | 173 ± 16 | 110 ± 17* | 215 ± 14 | 170 ± 20*‡ |
| Semimembranosus, white | 0 | 40 ± 6 | 47 ± 7 | 59 ± 10 | 41 ± 8* | 91 ± 15‡ | 76 ± 15*‡ |
| Thigh adductors | |||||||
| Adductor longus | 95 | 396 ± 18 | 251 ± 32* | 254 ± 50† | 111 ± 12*† | 273 ± 31 | 164 ± 22* |
| Adductor magnus and brevis | 11 | 142 ± 10 | 99 ± 6* | 118 ± 6 | 86 ± 9* | 147 ± 15 | 118 ± 13* |
| Gracilis | 23 | 81 ± 13 | 58 ± 7* | 69 ± 11 | 57 ± 12 | 66 ± 18 | 55 ± 11 |
| Pectineus | 31 | 104 ± 19 | 58 ± 8* | 112 ± 19 | 83 ± 25 | 77 ± 14 | 55 ± 5 |
Data (in ml·min−1·100 g−1) are means ± SE; n = 6 at rest and 8 during exercise.
P < 0.05 vs. control;
P < 0.05 vs. 20 m/min;
P < 0.05 vs. 40 m/min.
Table 3.
Effects of GLI on individual hindlimb skeletal muscle VCs for exercise groups
| 20 m/min |
40 m/min |
60 m/min |
|||||
|---|---|---|---|---|---|---|---|
| Percentage of Type I + IIa Fibers According to Delp and Duan (13) | Control | GLI | Control | GLI | Control | GLI | |
| Ankle extensors | |||||||
| Soleus | 91 | 2.76 ± 0.20 | 1.92 ± 0.20* | 2.04 ± 0.48 | 0.99 ± 0.15*† | 2.21 ± 0.11 | 1.44 ± 0.19* |
| Plantaris | 20 | 1.83 ± 0.21 | 1.45 ± 0.18* | 1.75 ± 0.26 | 1.26 ± 0.21* | 1.84 ± 0.12 | 1.50 ± 0.07 |
| Gastrocnemius, red | 86 | 3.02 ± 0.32 | 2.33 ± 0.27* | 2.75 ± 0.56 | 1.56 ± 0.34* | 3.07 ± 0.14 | 2.38 ± 0.30* |
| Gastrocnemius, white | 0 | 0.37 ± 0.08 | 0.47 ± 0.07 | 0.56 ± 0.11 | 0.53 ± 0.07 | 0.67 ± 0.11 | 0.58 ± 0.08 |
| Gastrocnemius, mixed | 9 | 1.15 ± 0.06 | 1.04 ± 0.13 | 1.26 ± 0.22 | 0.90 ± 0.14* | 1.44 ± 0.10 | 1.14 ± 0.10* |
| Tibialis posterior | 27 | 1.36 ± 0.14 | 1.10 ± 0.10 | 1.57 ± 0.25 | 1.06 ± 0.13* | 2.01 ± 0.14‡ | 1.50 ± 0.14* |
| Flexor digitorum longus | 32 | 0.84 ± 0.12 | 0.71 ± 0.13 | 0.80 ± 0.07 | 0.56 ± 0.10 | 0.88 ± 0.10 | 0.83 ± 0.09 |
| Flexor halicus longus | 29 | 0.75 ± 0.09 | 0.59 ± 0.09* | 0.80 ± 0.14 | 0.54 ± 0.09* | 0.77 ± 0.10 | 0.65 ± 0.07 |
| Ankle flexors | |||||||
| Tibialis anterior, red | 37 | 2.76 ± 0.20 | 2.32 ± 0.28 | 2.92 ± 0.50 | 1.77 ± 0.26* | 2.88 ± 0.17 | 2.24 ± 0.27* |
| Tibialis anterior, white | 20 | 0.98 ± 0.09 | 0.96 ± 0.12 | 1.10 ± 0.19 | 0.61 ± 0.11* | 0.90 ± 0.10 | 0.79 ± 0.10 |
| Extensor digitorum longus | 24 | 0.50 ± 0.04 | 0.49 ± 0.03 | 0.87 ± 0.17† | 0.63 ± 0.09* | 0.67 ± 0.08 | 0.65 ± 0.08 |
| Peroneals | 33 | 1.22 ± 0.10 | 0.99 ± 0.13* | 1.19 ± 0.17 | 0.73 ± 0.06* | 1.06 ± 0.12 | 0.82 ± 0.07* |
| Knee extensors | |||||||
| Vastus intermedius | 96 | 3.00 ± 0.18 | 2.06 ± 0.16* | 2.79 ± 0.48 | 1.69 ± 0.24* | 3.24 ± 0.15 | 2.15 ± 0.20* |
| Vastus medialis | 18 | 1.79 ± 0.13 | 1.29 ± 0.11* | 1.68 ± 0.24 | 1.10 ± 0.17* | 1.97 ± 0.14 | 1.49 ± 0.10* |
| Vastus lateralis, red | 65 | 3.34 ± 0.24 | 2.12 ± 0.34* | 2.23 ± 0.34† | 1.28 ± 0.28* | 2.81 ± 0.19 | 1.95 ± 0.23* |
| Vastus lateralis, white | 0 | 0.40 ± 0.15 | 0.40 ± 0.10 | 0.46 ± 0.07 | 0.44 ± 0.09 | 0.70 ± 0.06 | 0.56 ± 0.07 |
| Vastus lateralis, mixed | 11 | 1.44 ± 0.11 | 0.96 ± 0.13* | 1.29 ± 0.17 | 0.81 ± 0.16* | 1.67 ± 0.14 | 1.21 ± 0.09* |
| Rectus femoris, red | 34 | 2.35 ± 0.26 | 1.62 ± 0.21* | 1.92 ± 0.23 | 1.28 ± 0.15* | 2.36 ± 0.16 | 1.77 ± 0.17* |
| Rectus femoris, white | 0 | 1.05 ± 0.13 | 0.78 ± 0.08* | 0.97 ± 0.11 | 0.74 ± 0.12 | 1.32 ± 0.07‡ | 0.98 ± 0.08* |
| Knee flexors | |||||||
| Biceps femoris anterior | 0 | 0.24 ± 0.03 | 0.28 ± 0.04 | 0.49 ± 0.11† | 0.40 ± 0.06 | 0.53 ± 0.04 | 0.43 ± 0.05* |
| Biceps femoris posterior | 8 | 0.76 ± 0.08 | 0.64 ± 0.07 | 0.83 ± 0.12 | 0.51 ± 0.11* | 0.98 ± 0.09 | 0.70 ± 0.11* |
| Semitendinosus | 17 | 0.55 ± 0.05 | 0.52 ± 0.10 | 0.50 ± 0.06 | 0.30 ± 0.03*† | 0.36 ± 0.07 | 0.34 ± 0.06 |
| Semimembranosus, red | 28 | 0.98 ± 0.14 | 0.84 ± 0.09 | 1.20 ± 0.13 | 0.73 ± 0.12* | 1.53 ± 0.11 | 1.15 ± 0.14*‡ |
| Semimembranosus, white | 0 | 0.28 ± 0.04 | 0.32 ± 0.04 | 0.40 ± 0.07 | 0.28 ± 0.06* | 0.65 ± 0.12‡ | 0.51 ± 0.10* |
| Thigh adductors | |||||||
| Adductor longus | 95 | 2.85 ± 0.16 | 1.74 ± 0.25* | 1.77 ± 0.39† | 0.72 ± 0.07*† | 1.94 ± 0.22 | 1.11 ± 0.15* |
| Adductor magnus and brevis | 11 | 1.02 ± 0.08 | 0.68 ± 0.05* | 0.81 ± 0.06 | 0.56 ± 0.16* | 1.06 ± 0.13 | 0.80 ± 0.09* |
| Gracilis | 23 | 0.58 ± 0.09 | 0.39 ± 0.04* | 0.47 ± 0.07 | 0.37 ± 0.08 | 0.47 ± 0.13 | 0.37 ± 0.08 |
| Pectineus | 31 | 0.75 ± 0.14 | 0.40 ± 0.05* | 0.76 ± 0.12 | 0.54 ± 0.10 | 0.56 ± 0.12 | 0.37 ± 0.03 |
Data (in ml·min−1·100 g−1·mmHg−1) are means ± SE; n = 6 at rest and 8 during exercise.
P < 0.05 vs. control;
P < 0.05 vs. 20 m/min;
P < 0.05 vs. 40 m/min.
Fig. 3.
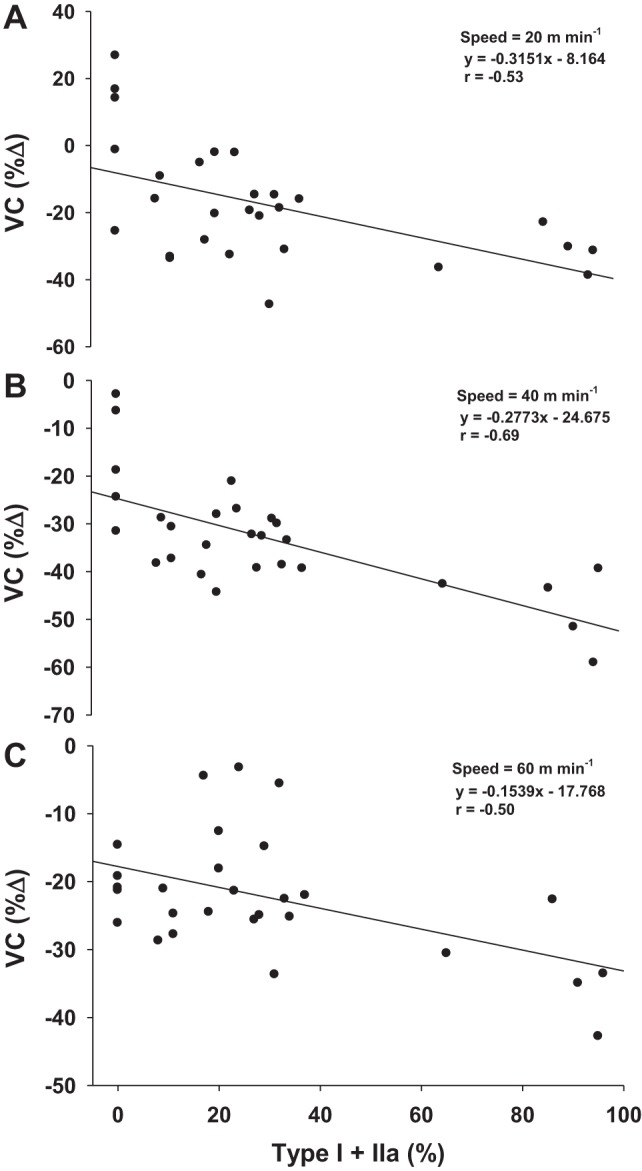
Percent decreases in exercising hindlimb VC with GLI were correlated with the percentage of type I and type IIa fibers of the muscles or muscle portions for speeds of 20 m/min (A), 40 m/min (B), and 60 m/min (C). P < 0.05 vs. control.
Effects of GLI on renal and splanchnic BF and VC at rest and during exercise.
Renal BF and VC were reduced by GLI at rest but not during exercise at any speed (Table 4). Among the representative organs of the splanchnic region, BF was reduced at rest to the spleen, adrenals, and large intestine with GLI. VC was reduced at rest to the stomach, adrenals, spleen, pancreas, small intestine, and large intestine with GLI. BF during exercise at 20 m/min was reduced with GLI to the pancreas and small intestine, and VC was reduced to the stomach, pancreas, and small intestine (Table 4). There were no differences in BF or VC between control and GLI treatment for any organs at 40 or 60 m/min. However, at 40 and 60 m/min, control renal, adrenal, pancreas, and small intestine BFs were lower compared with 20 m/min. Similarly, renal, stomach, pancreas, and large intestine VCs were lower at both 40 and 60 m/min compared with 20 m/min.
Table 4.
Effects of GLI on BF and VC in the kidneys and organs of the splanchnic region for rest and exercise groups
| Rest |
20 m/min |
40 m/min |
60 m/min |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | GLI | Control | GLI | Control | GLI | Control | GLI | |
| BF, ml·min−1·100 g−1 | ||||||||
| Kidney | 488 ± 50 | 380 ± 27* | 455 ± 42 | 417 ± 47 | 237 ± 107†‡ | 164 ± 49†‡ | 217 ± 25†‡ | 190 ± 26†‡ |
| Stomach | 100 ± 21 | 48 ± 8 | 101 ± 15 | 73 ± 27 | 86 ± 52 | 97 ± 72 | 35 ± 2 | 64 ± 43 |
| Adrenals | 718 ± 144 | 405 ± 45* | 448 ± 55‡ | 419 ± 69 | 253 ± 53†‡ | 262 ± 63 | 217 ± 24†‡ | 242 ± 21 |
| Spleen | 397 ± 82 | 307 ± 88* | 113 ± 28‡ | 115 ± 31‡ | 27 ± 14‡ | 21 ± 6‡ | 25 ± 4‡ | 16 ± 3‡ |
| Pancreas | 142 ± 26 | 108 ± 15 | 198 ± 31 | 92 ± 22* | 80 ± 27† | 40 ± 9† | 65 ± 7†‡ | 38 ± 7 |
| Small intestine | 304 ± 41 | 257 ± 37 | 304 ± 40 | 229 ± 40* | 155 ± 41†‡ | 107 ± 24†‡ | 141 ± 8†‡ | 88 ± 12†‡ |
| Large intestine | 218 ± 48 | 98 ± 14* | 153 ± 30 | 116 ± 28 | 107 ± 35‡ | 72 ± 23 | 83 ± 9‡ | 55 ± 13 |
| Liver§ | 31 ± 6 | 37 ± 9 | 20 ± 7 | 20 ± 6 | 21 ± 5 | 19 ± 5 | 26 ± 1 | 21 ± 3 |
| VC, ml·min−1·100 g−1·mmHg−1 | ||||||||
| Kidney | 3.82 ± 0.50 | 2.53 ± 0.24* | 3.30 ± 0.36 | 2.88 ± 0.36 | 1.66 ± 0.75†‡ | 1.08 ± 0.32†‡ | 1.53 ± 0.16†‡ | 1.28 ± 0.18†‡ |
| Stomach | 0.76 ± 0.15 | 0.32 ± 0.04* | 0.64 ± 0.08 | 0.33 ± 0.05* | 0.22 ± 0.06†‡ | 0.15 ± 0.03 | 0.24 ± 0.01†‡ | 0.14 ± 0.02 |
| Adrenals | 5.73 ± 1.32 | 2.69 ± 0.33* | 3.17 ± 0.35‡ | 2.92 ± 0.55 | 1.75 ± 0.40‡ | 1.70 ± 0.40 | 1.52 ± 0.14‡ | 1.61 ± 0.10 |
| Spleen | 3.08 ± 0.66 | 1.99 ± 0.54* | 0.83 ± 0.23‡ | 0.82 ± 0.24‡ | 0.19 ± 0.10‡ | 0.14 ± 0.04‡ | 0.18 ± 0.03‡ | 0.11 ± 0.02‡ |
| Pancreas | 1.13 ± 0.22 | 0.72 ± 0.12* | 1.46 ± 0.26 | 0.65 ± 0.16* | 0.56 ± 0.20†‡ | 0.26 ± 0.06 | 0.46 ± 0.05†‡ | 0.25 ± 0.05 |
| Small intestine | 2.35 ± 0.31 | 1.71 ± 0.27* | 2.23 ± 0.35 | 1.62 ± 0.31* | 1.08 ± 0.31‡ | 0.71 ± 0.17 | 1.00 ± 0.05‡ | 0.60 ± 0.09 |
| Large intestine | 1.72 ± 0.38 | 0.64 ± 0.07* | 1.11 ± 0.25‡ | 0.90 ± 0.21 | 0.79 ± 0.23†‡ | 0.50 ± 0.14†‡ | 0.58 ± 0.06†‡ | 0.41 ± 0.08†‡ |
| Liver§ | 0.24 ± 0.05 | 0.24 ± 0.06 | 0.14 ± 0.04 | 0.14 ± 0.04 | 0.14 ± 0.04 | 0.12 ± 0.03 | 0.18 ± 0.01 | 0.14 ± 0.02 |
Data are means ± SE; n = 6 at rest and 8 during exercise.
P < 0.05 vs. control;
P < 0.05 vs. rest;
P < 0.05 vs. 20 m/min.
Arterial, not portal, BF and VC.
DISCUSSION
The primary original findings of the present investigation were that inhibition of KATP channels in the rat via GLI during exercise increased MAP and decreased HR, reduced hindlimb skeletal muscle BF and VC, and increased blood lactate concentration. Furthermore, the decrease in VC with GLI reflected a fiber type-selective effect such that VC was reduced primarily to muscles composed of type I and type IIa fibers. The strength of the fiber type relationship persisted at higher speeds, where muscle recruitment increasingly represents the complete spectrum of fiber types. These data support that KATP channels contribute significantly to exercise-induced skeletal muscle hyperemia during treadmill exercise in rats.
The finding that GLI increased MAP at rest with a consequent decrease in HR is not surprising as this is consistent with previous reports at rest in conscious rats (18, 28), swine (16), and humans (17). Specifically, the higher MAP and lower HR at rest with KATP channel inhibition were coincident with large reductions in renal and splanchnic VC and suggest the occurrence of KATP channel inhibition. This is in agreement with studies demonstrating a significant contribution of KATP channels to basal vasomotor tone in the systemic circulation of conscious rats (18, 28, 36), hamsters (24, 40), and dogs (11, 48).
The similar hindlimb skeletal muscle BF and VC at rest with GLI is most likely due to the inhibition of KATP channels at physiological ATP concentrations or a compensatory vasodilation by redundant pathways (e.g., nitric oxide, PGI2, or baroreflex) to maintain appropriate basal vasomotor tone (21). Similar observations have been made across species (16, 17), although there is some evidence for modest skeletal muscle vascular KATP channel activity at rest (34).
Previously, KATP channel blockade in rats and hamsters has been shown to reduce the skeletal muscle hyperemic response to electrically induced contractions (40, 47). In this regard, our findings indicate that, in rats, KATP channel inhibition also reduces the in vivo skeletal muscle hyperemic response to treadmill running. This corroborates the notion that KATP channels represent an important mechanistic link between metabolism and vasomotor tone during exercise. The 20% decrease in hindlimb skeletal muscle VC during exercise at 20 m/min with GLI suggests that the rat locomotor muscle vascular bed contains a substantial pool of KATP channels that are activated even at moderate exercise intensities. Importantly, the 16% decrease in exercising hindlimb skeletal muscle BF occurred simultaneous with a doubling of arterial blood lactate concentration (2.0 to 4.1 mmol/l) with GLI at 20 m/min and a 44% increase at 40 m/min, an effect not seen at rest. Thus, not only is vasodilation attenuated in the hindlimb vasculature, but this decrement represents an impaired vasomotor control failing to meet the BF demands of active skeletal muscle. These intriguing findings support the hypothesis that KATP channels are obligatory for exercise-induced hyperemia with respect to exercise tolerance and work capacity, at least within the rat. Furthermore, the magnitude of the KATP channel role may actually be underestimated in the present study. We acknowledge that incomplete KATP channel blockade may be present given that the precise degree of inhibition was not established. Consequently, the data reported here may reflect only a portion of the KATP channel contribution to exercise-induced hyperemia. Regardless, the results clearly demonstrate the significant effects of GLI on skeletal muscle BF, VC, and arterial blood lactate concentration. The substantial increase of arterial blood lactate concentration at 20 and 40 m/min is not surprising given that GLI-induced decreases in BF were correlated with the percentage of type I and IIa fibers such that the predominantly oxidative muscles and muscle portions experienced the most consistent decrease in BF during exercise. The muscle fiber type composition explains ∼28%, 48%, and 25% of the VC decrease with GLI at 20, 40, and 60 m/min, respectively. The preferential recruitment of type I and type IIa fibers during the low-speed exercise protocol (∼55–65% maximal O2 uptake) is likely to account for a substantial portion of the fiber type-selective effect at 20 m/min. However, the relationship persisted even at 40 and 60 m/min, when a greater proportion of type IIb/dx fibers were recruited. Nitric oxide, which has a proportionally greater role in the control of oxidative fiber type vascular beds (22), has been shown to operate through the activation of KATP channels (29, 44). Thus, inhibition of KATP channels in the vasculature supplying type I and IIa fibers would result in a greater decrement of VC during exercise, as it may block a portion of this nitric oxide-mediated vasodilation. Investigating the sensitivity of the fiber type relationship to work rates above and below fundamental physiological tipping points, such as critical speed/power, lactate threshold, etc., may prove insightful.
With respect to the interpretation of the effects of GLI on altered BF and VC as purely local muscle effects, the possible impacts of KATP channel inhibition on sympathetic nerve discharge must be considered. The loss of KATP channel-mediated hyperpolarization could conceivably result in increased sympathetic nerve discharge for a given stimulus, which would account, in part, for the decrements in BF and VC demonstrated in the present study. However, we consider this unlikely because it has been demonstrated, in male Sprague-Dawley rats, that direct injection of GLI into the rostral ventrolateral medulla has no effect on blood pressure, HR, or renal sympathetic nerve activity (20). These findings support that GLI did not directly impact sympathetic nerve discharge in the present study and that the reductions in BF and VC are the result, specifically, of muscle vascular KATP channel inhibition.
It is important to note that the BF and VC results found in the present study run contrary to data from exercising swine (16). This may be the result of cross-species differences in vascular KATP channel expression and/or function. Indeed, while the octameric structure of vascular KATP channels is presumed to be Kir6.1 pore-forming units with associated sulfonylurea receptor (SUR)2B subunits, considerable heterogeneity of functional expression has been demonstrated through readily occurring heteromultimerization of the pore-forming units (Kir6.x) as well as SUR subunits (SURx), which confer the channel's ATP sensitivity. Accordingly, there is substantial variation in unitary conductance, which may impact the relative importance of the KATP channel in vasomotor control across species, tissue beds, and metabolic rates (46). This is particularly true for vascular KATP channels, as the range of unitary conductance appears to be much greater (∼20 to >200 pS) than for pancreatic β cells (∼50–90 pS) and some regions of the heart and brain (∼40–80 pS) (9). Further exploration of the discrepancies in channel molecular structure across tissues may lead to a better understanding of the functional consequences resulting from global KATP channel blockade.
The role of KATP channels in the control of human skeletal muscle BF has been elucidated primarily by the use of KATP channel agonists, namely, nicorandil and diazoxide, as well as the KATP channel inhibitor GLI. Seminal results include KATP channel agonists eliciting forearm vasodilation (6) and KATP channel blockade reducing the BF response to both reactive and functional hyperemia in some (1, 2, 7) but not all (16, 42) studies. The reason for the differential conclusions drawn from use of KATP channel agonists versus blockers is unclear. In healthy humans, KATP channels may not be obligatory for achieving adequate skeletal muscle BF during exercise given the great redundancy of vasodilatory mediators (21). The potential for parallel pathways such as nitric oxide- and PGI2-mediated vasodilation to adequately meet BF requirements is present at relatively low workloads. This notion is consistent with KATP blockade studies in humans involving the forearm musculature, which represents a small, nonlocomotory portion of the total body muscle mass. Furthermore, the putative variability of KATP channel expression among skeletal muscle vascular beds with vastly different metabolic characteristics may confound interpretation of the available human data. It appears that the vascular KATP channel subtype uniquely requires nucleotide diphosphates (i.e., ADP) for activation and thus is designated as a nucleotide-dependent K+ channel (4). As such, the channel would only demonstrate obligatory function where sufficiently low local muscle O2 tension results in pronounced vascular subsarcolemmal ADP accumulation. Under these conditions, even a small impact of KATP channel activation on SMC membrane potential could drive large increases in vasodilation. This is due to the remarkably steep relationship between membrane potential and Ca2+ influx in smooth muscle; changes of only a couple of millivolts can reduce intracellular Ca2+ concentration ∼30% (32). For this reason, we are in agreement with Schrage et al. (42), who recognized that the determination of KATP channel function across more diverse muscle vascular beds and a greater range of exercise intensities/paradigms is necessary to resolve the circumstances under which these channels impact O2 delivery. The in vivo measurements evaluating 28 muscles and muscle portions documented here demonstrate the novel information to be gained from this approach.
The results described herein suggest that the role of KATP channels may be potentiated under conditions of low local muscle O2 tension (i.e., when muscle fibers are recruited), which can increase vascular glycolytic flux and drive greater intracellular ADP accumulation. This environment is exacerbated in muscle fibers exposed to high metabolic demand as well as conditions characterized by skeletal muscle hypoxia, such as heart failure (38), type II diabetes (12, 35), and aging (37). Therefore, the potential for KATP channels to contribute significantly to exercise-induced skeletal muscle hyperemia has important implications for characterizing the etiology of blood-muscle O2 transport decrements in these conditions.
Experimental considerations.
A major strength of the current experimental design is that each animal acts as its own control for the effects of GLI, thus increasing statistical power and reducing animal use. However, the limitation of only two available isotopes precludes BF controls for the vehicle being performed in the same animal. To minimize animal use per Institutional Animal Care and Use Committee mandates, the effect of the vehicle on MAP and HR was assessed in a subset of animals (n = 6) with no change evident after administration of the vehicle. Furthermore, a similar NaOH/saline vehicle was shown to have no impact on the vascular hemodynamic response to contractions in Sprague-Dawley rats (47).
Since GLI is not selective for vascular smooth muscle KATP channels, it might be argued that GLI-induced KATP channel inhibition in the coronary vasculature or cardiac myocytes could account for the reduced BF during exercise demonstrated here. Measurements of coronary BF and VC were not available in the present investigation. However, decrements in cardiac output would not be expected to support a decrease in BF for select muscles or muscle portions, as demonstrated here. Likewise, the increase in MAP with GLI would not be consistent with decrements in cardiac output. These observations suggest that GLI does not impact coronary BF such that decrements in cardiac function (and reduced cardiac output) underlie the decrease in hindlimb skeletal muscle BF during submaximal treadmill exercise found in the present study. Additionally, given that inhibition of endothelial KATP channels can be achieved via GLI, the effects of KATP channel inhibition on vascular control may be mediated via altered endothelial function rather than a direct action on SMCs (26). However, the conclusion that KATP channels contribute to skeletal muscle vascular control during large muscle mass exercise in rats is independent of the ability to partition endothelial influence from direct SMC effects.
The existence of mitochondrial membrane KATP channels requires the consideration that altered muscle O2 consumption or extraction impacts skeletal muscle BF. The limited evidence available for whole body exercising O2 uptake indicates no change with GLI (25). This might be expected given that mitochondrial KATP channel blockade does not appear to alter the O2 cost of exercise per se. Furthermore, if an increase in O2 consumption occurred during treadmill exercise with GLI, the regulation of O2 supply-demand previously described would dictate an increase in hindlimb skeletal muscle BF with GLI rather than the decrease found in the present study. While O2 extraction for a given O2 uptake can increase with KATP channel blockade in cardiac muscle during submaximal exercise (16), this effect, as dictated by the Fick equation, serves to offset the GLI-induced reductions in BF. Crucially, the use of mitochondria-selective KATP channel blockers verifies that the GLI-induced reduction of myocardial O2 uptake in the canine during exercise is the result of reduced myocardial BF rather than a primary mitochondrial respiration effect (8). Thus, changes in O2 extraction or consumption appear to be driven by impaired vascular control rather than a direct result of mitochondrial KATP channel inhibition.
Conclusions.
Our principal novel findings show that inhibition of KATP channels via GLI (5 mg/kg) attenuates hindlimb skeletal muscle BF and VC and elevates blood lactate concentration during submaximal treadmill exercise. The effects of KATP channel inhibition were significant across the range of exercise speeds used and demonstrated a fiber type selectivity within muscles composed of highly oxidative fibers. These results provide compelling evidence that KATP channel-induced hyperpolarization constitutes an important mechanism of vasomotor control in exercising skeletal muscle, supporting that KATP channels represent an obligatory pathway for skeletal muscle vascular control during large muscle mass exercise in healthy rats.
GRANTS
This work was funded by Kansas State University SMILE, American Heart Association Midwest Affiliate Grant 10GRNT4350011, and National Heart, Lung, and Blood Institute Grant HL-108328 (to D. C. Poole).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.T.H., S.W.C., S.K.F., G.E.S., D.C.P., and T.I.M. conception and design of research; C.T.H., S.W.C., S.K.F., G.E.S., and T.I.M. performed experiments; C.T.H. analyzed data; C.T.H., S.W.C., S.K.F., G.E.S., D.C.P., and T.I.M. interpreted results of experiments; C.T.H. prepared figures; C.T.H. drafted manuscript; C.T.H., S.W.C., S.K.F., G.E.S., D.C.P., and T.I.M. edited and revised manuscript; C.T.H., S.W.C., S.K.F., G.E.S., D.C.P., and T.I.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank K. Sue Hageman for expert technical assistance and Dr. Daniel M. Hirai for scientific advice.
REFERENCES
- 1.Bank AJ, Sih R, Mullen K, Osayamwen M, Lee PC. Vascular ATP-dependent potassium channels, nitric oxide, and human forearm reactive hyperaemia. Cardiovasc Drugs Ther 14: 23–29, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Banitt PF, Smits P, Williams SB, Ganz P, Creager MA. Activation of ATP-sensitive potassium channels contributes to reactive hyperaemia in humans. Am J Physiol Heart Circ Physiol 271: H1594–H1598, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Beech DJ, Zhang H, Nakao K, Bolton TB. Single channel and whole-cell K-currents evoked by levcromakalim in smooth muscle cells from the rabbit portal vein. Br J Pharmacol 110: 583–590, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotidediphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol 110: 573–582, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behnke BJ, Armstrong RB, Delp MD. Adrenergic control of vascular resistance varies in muscles composed of different fiber types: influence of the vascular endothelium. Am J Physiol Regul Integr Comp Physiol 301: R783–R790, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bijlstra PJ, Lutterman JA, Russel FG, Thien T, Smits P. Interaction of sulphonylurea derivatives with vascular ATP-sensitive potassium channels in humans. Diabetologia 39: 1083–1090, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Bijlstra PJ, den Arend JA, Lutterman JA, Russel FG, Thien T, Smits P. Blockade of vascular ATP-sensitive potassium channels reduces the vasodilator response to ischaemia in humans. Diabetologia 39: 1562–1568, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Traverse JH, Zhang J, Bache RJ. Selective blockade of mitochondrial KATP channels does not impair myocardial oxygen consumption. Am J Physiol Heart Circ Physiol 281: H738–H744, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cole WC, Clément-Chomienne O. ATP-sensitive K+ channels of vascular smooth muscle cells. J Cardiovasc Electrophysiol 14: 94–103, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Copp SW, Hirai DM, Hageman KS, Poole DC, Musch TI. Nitric oxide synthase inhibition during treadmill exercise reveals fiber-type specific vascular control in the rat hindlimb. Am J Physiol Regul Integr Comp Physiol 298: R478–R485, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comtois A, Sinderby C, Comtois N, Grassino A, Renaud JM. An ATP-sensitive potassium channel blocker decreases diaphragmatic circulation in anesthetized dogs. J Appl Physiol 77: 127–134, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Cunha MR, Silva ME, Machado HA, Fukui RT, Correia MR, Santos RF, Wajchenberg BL, Rocha DM, Rondon MU, Negrão CE, Ursich MJ. Cardiovascular, metabolic and hormonal responses to the progressive exercise performed to exhaustion in patients with type 2 diabetes treated with metformin or glyburide. Diabetes Obes Metab 10: 238–245, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–70, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol 587: 713–719, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncker DJ, Oei HH, Hu F, Stubenitsky R, Verdouw PD. Role of KATP+ channels in regulation of systemic, pulmonary, and coronary vasomotor tone in exercising swine. Am J Physiol Heart Circ Physiol 280: H22–H33, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Farouque HM, Meredith IT. Effects of inhibition of ATP-sensitive potassium channels on metabolic vasodilation in the human forearm. Clin Sci 104: 39–46, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Gardiner SM, Kemp PA, March JE, Fallgren B, Bennet T. Effects of glibenclamide on the regional haemodynamic actions of α-trinositol and its influence on responses to vasodilators in conscious rats. Br J Pharmacol 117, 507–515, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George S, McBurney A, Cole A. Possible protein binding displacement interaction between glibenclamide and metolazone. Eur J Clin Pharmacol 38: 93–95, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Guo Q, Jin S, Wang XL, Wang R, Xiao L, He RR, Wu YM. Hydrogen sulfide in the rostral ventrolateral medulla inhibits sympathetic vasomotor tone through ATP-sensitive K+ channels. J Pharmacol Exp Ther 338: 458–465, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Hellsten Y, Nyberg M, Jensen LG, Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. J Physiol 590: 6297–6305, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol 77, 1288–1293, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Ishise S, Pegram BL, Yamamoto J, Kitamura Y, Frohlich ED. Reference sample microsphere method: cardiac output and blood flows in conscious rat. Am J Physiol Heart Circ Physiol 239: H443–H449, 1980. [DOI] [PubMed] [Google Scholar]
- 24.Jackson WF. Arteriolar tone is determined by activity of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol 265: H1797–H1803, 1993. [DOI] [PubMed] [Google Scholar]
- 24a.Keller DM, Ogoh S, Greene S, Olivencia-Yurvati A, Raven PB. Inhibition of KATP channel activity augments baroreflex-mediated vasoconstriction in exercising human skeletal muscle. J Physiol 561: 273–282, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen JJ, Dela F, Madsbad S, Vibe-Petersen J, Galbo H. Interaction of sulfonylureas and exercise on glucose homeostasis in type 2 diabetic patients. Diabetes Care Oct 22: 1647–1654, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Malester B, Tong X, Ghiu I, Kontogeorgis A, Gutstein DE, Xu J, Hendricks-Munoz KD, Coetzee WA. Transgenic expression of a dominant negative KATP channel subunit in the mouse endothelium: effects on coronary flow and endothelin-1 secretion. FASEB J 21: 2162–2172, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Mori HM, Chujo M, Tanaka E, Yamakawa A, Shinozaki Y, Mohamed MU, Nakazawa H. Modulation of adrenergic coronary vasoconstriction via ATP-sensitive potassium channel. Am J Physiol Heart Circ Physiol 268: H1077–H1085, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Moreau R, Komeichi H, Kirstetter P, Yang S, Aupetit-Faisant B, Cailmail S, Lebrec D. Effects of glibenclamide on systemic and splanchnic haemodynamics in conscious rats. Br J Pharmacol 112: 649–653, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol 486: 47–58, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Nakai T, Ichihara K. Effects of diazoxide on norepinephrine-induced vasocontraction and ischemic myocardium in rats. Biol Pharmacol Bull 17: 1341–1344, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol 259: C3–C18, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen JJ, Kristensen M, Hellsten Y, Bangsbo J, Juel C. Localization and function of ATP-sensitive potassium channels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 284: R558–R563, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of type II diabetes on muscle microvascular oxygen pressures. Respir Physiol Neurobiol 156: 187–195, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Parekh N, Zou AP. Role of prostaglandins in renal medullary circulation: response to different vasoconstrictors. Am J Physiol Renal Fluid Electrolyte Physiol 271: F653–F658, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Poole DC, Ferreira LF. Oxygen exchange in muscle of young and old rats: muscle-vascular-pulmonary coupling. Exp Physiol 92, 341–346, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302: H1050–H1063, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quast U, Guillon JM, Cavero I. Cellular pharmacology of potassium channel openers in vascular smooth muscle. Cardiovasc Res 28: 805–810, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Saito Y, McKay M, Eraslan A, Hester RL. Functional hyperaemia in striated muscle is reduced following blockade of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol 270: H1649–H1654, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Sadraei H, Beech DJ. Ionic currents and inhibitory effects of glibenclamide in seminal vesicle smooth muscle cells. Br J Pharmacol 115: 1447–1454, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrage WG, Dietz NM, Joyner MJ. Effects of combined inhibition of ATP-sensitive potassium channels, nitric oxide, and prostaglandins on hyperaemia during moderate exercise. J Appl Physiol 100: 1506–1512, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Symons JD, Stebbins CL, Musch TI. Interactions between angiotensin II and nitric oxide during exercise in normal and heart failure rats. J Appl Physiol 87: 574–581, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Tare M, Parkington HC, Coleman HA, Neild TO, Dusting GJ. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature 346: 69–71, 1990. [DOI] [PubMed] [Google Scholar]
- 45.Tateishi J, Faber JE. ATP-sensitive K+ channels mediate α2D-adrenergic receptor contraction of arteriolar smooth muscle and reversal of contraction in hypoxia. Circ Res 76: 53–63, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol 572: 617–624, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest 99: 2602–2609, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanelli G, Hussain SN. Effects of potassium channel blockers on basal vascular tone and reactive hyperaemia of canine diaphragm. Am J Physiol Heart Circ Physiol 266: H43–H51, 1994. [DOI] [PubMed] [Google Scholar]


