Abstract
Endothelial cell release of nitric oxide (NO) is a defining characteristic of nondiseased arteries, and abnormal endothelial NO release is both a marker of early atherosclerosis and a predictor of its progression and future events. Healthy coronaries respond to endothelial-dependent stressors with vasodilatation and increased coronary blood flow (CBF), but those with endothelial dysfunction respond with paradoxical vasoconstriction and reduced CBF. Recently, coronary MRI and isometric handgrip exercise (IHE) were reported to noninvasively quantify coronary endothelial function (CEF). However, it is not known whether the coronary response to IHE is actually mediated by NO and/or whether it is reproducible over weeks. To determine the contribution of NO, we studied the coronary response to IHE before and during infusion of NG-monomethyl-l-arginine (l-NMMA, 0.3 mg·kg−1·min−1), a NO-synthase inhibitor, in healthy volunteers. For reproducibility, we performed two MRI-IHE studies ∼8 wk apart in healthy subjects and patients with coronary artery disease (CAD). Changes from rest to IHE in coronary cross-sectional area (%CSA) and diastolic CBF (%CBF) were quantified. l-NMMA completely blocked normal coronary vasodilation during IHE [%CSA, 12.9 ± 2.5 (mean ± SE, placebo) vs. −0.3 ± 1.6% (l-NMMA); P < 0.001] and significantly blunted the increase in flow [%CBF, 47.7 ± 6.4 (placebo) vs. 10.6 ± 4.6% (l-NMMA); P < 0.001]. MRI-IHE measures obtained weeks apart strongly correlated for CSA (P < 0.0001) and CBF (P < 0.01). In conclusion, the normal human coronary vasoactive response to IHE is primarily mediated by NO. This noninvasive, reproducible MRI-IHE exam of NO-mediated CEF promises to be useful for studying CAD pathogenesis in low-risk populations and for evaluating translational strategies designed to alter CAD in patients.
Keywords: endothelial function, coronary artery disease, magnetic resonance imaging
endothelial cell release of nitric oxide (NO) is a defining characteristic of nondiseased vascular tissue; it inhibits platelet aggregation, attenuates inflammation, decreases cellular proliferation, and induces local vascular smooth muscle vasodilation (4). Most classic and novel cardiovascular risk factors converge to impair endothelial function, including dyslipidemia, insulin resistance, inflammation, tobacco abuse, and oxidative stress, as well as hemodynamic and genetic factors (4, 33). Critically, endothelial dysfunction is a marker for subclinical disease, an independent predictor of adverse cardiovascular events, and a potential target for medical interventions (21, 24, 28).
Traditionally, coronary endothelial function (CEF) is assessed by the direction and magnitude of changes in coronary arterial area and flow in response to endothelial-dependent vasomotor interventions (15). From the seminal description of paradoxical coronary vasoconstriction in response to a vasorelaxant substance in patients with coronary artery disease (CAD) (15), the assessment of endothelial-dependent coronary vasoreactivity during cardiac catheterization has been widely studied to characterize endothelial function. However, the invasive nature of coronary angiography with Doppler-flow measures used in the traditional assessment of CEF has limited clinical and research investigations, particularly those benefiting from repeated studies and/or studies in clinically stable or healthy subjects. Recently, the combination of new noninvasive 3.0-telsa (T) coronary MRI methods and isometric handgrip exercise (IHE), a purported endothelial-dependent stressor, has been reported as a means to noninvasively quantify CEF. In healthy subjects without CAD risk factors, IHE induced significant coronary dilatation and increased flow measured by increases in MRI-detected coronary cross-sectional area (CSA), peak diastolic coronary flow velocity (CFV), and diastolic coronary blood flow (CBF) (8, 9). In contrast, patients with CAD exhibited paradoxical vasoconstriction with reductions in CSA, CFV, and CBF (8). The noninvasive 3.0-T MRI-IHE approach detected coronary changes consistent with endothelial functional responses anticipated in healthy subjects and patients with CAD (8), but because IHE can in theory stimulate sympathetic, neural, and neurohumoral responses, the extent to which the MRI-detected coronary responses to IHE primarily reflect NO-mediated endothelial function was questioned (13). Although nuclear/positron emission tomography and MRI perfusion techniques can measure downstream myocardial perfusion in response to endothelial-independent and endothelial-dependent stressors (25, 34), if these MRI-IHE coronary responses are shown to be primarily NO mediated, they would be uniquely valuable for probing the local factors that might affect the pathophysiology of local plaque progression and rupture, since this approach quantifies local coronary segment vasodilatation. In addition, although MRI measures of CEF are unchanged during repeated IHE tests on the same day (10), it is not known whether the MRI-IHE test of CEF is reproducible over several days or weeks, as might be studied in an intervention trial. Such reproducibility of CEF measures over time is important because endothelial function is affected by a variety of external stimuli (36).
We therefore studied the normal coronary vascular response to IHE detected by MRI before and during the infusion of the endothelial NO synthase (eNOS) inhibitor (22) NG-monomethyl-l-arginine (l-NMMA) to test the hypothesis that the coronary response to IHE is largely mediated by NO and thus primarily reflects CEF. In addition, we assessed the reproducibility of noninvasive MRI measures of coronary endothelial vasoreactivity in both patients with CAD and healthy adults over a mean 8-wk period, an interval useful in planning future intervention trials.
METHODS
Patients
Subjects were outpatients with no contraindications to MRI. The Institutional Review Board at The Johns Hopkins School of Medicine approved the protocol. An Investigational New Drug application was approved by the Food and Drug Administration (No. 119574) for the administration of l-NMMA, and all participants provided written informed consent. Healthy subjects were those under the age of 50 yr without a history of CAD and traditional CAD risk factors and for those over age 50 with an Agatston coronary artery calcium score (1) of <10 by computed tomography. CAD subjects were outpatients with stable CAD (luminal stenosis of 30 to 70%) on coronary X-ray angiography or computed tomography angiography with no active angina or evidence of ischemia on their most recent stress imaging studies. The subjects were consecutively recruited de novo and were not reported in prior published studies.
Study Protocol
MRI was performed in the morning in the fasting state (>8 h) before administration of any prescribed vasoactive medications. Images were taken perpendicular to a proximal, well-visualized straight (∼20 mm) segment of a native coronary artery, as determined by double-oblique scout scanning (27), that had not undergone prior intervention or had a significant stenosis by invasive angiography. For the patients with CAD, the segment of the coronary artery selected for MRI measures of CSA, CFV, and CBF had no more than a 30% luminal stenosis.
For the l-NMMA study, each healthy volunteer (n = 10) underwent a first IHE period during which intravenous saline (placebo) was infused (Fig. 1). After postexercise recovery, each subject then received an intravenous infusion of l-NMMA at a dose of 0.3 mg·kg−1·min−1, as previously described (30). A new set of baseline coronary images was obtained after 5 min of l-NMMA infusion. A second IHE period was then initiated while l-NMMA infusion continued and coronary imaging was repeated at the same locations (Fig. 1). The entire l-NMMA infusion typically lasted 15–22 min. Heart rate and blood pressure were measured throughout using a noninvasive and MRI-compatible ECG and calf blood pressure monitor (Invivo, Orlando, FL). The rate pressure product (RPP) was calculated as systolic blood pressure × heart rate.
Fig. 1.
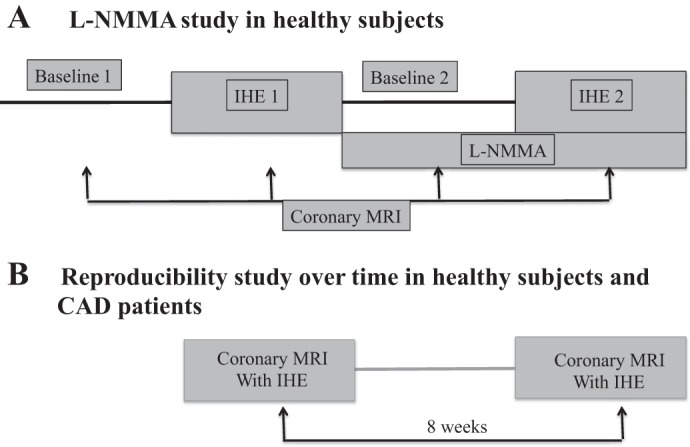
Protocol diagram illustrating MRI NG-monomethyl-l-arginine (l-NMMA) study (A) and the reproducibility study (B). Hemodynamic parameters, blood pressure, and heart rate were measured at the noted times. IHE, isometric handgrip exercise; CAD, coronary artery disease.
For the reproducibility study, 10 healthy subjects and 8 patients with CAD underwent two MRI-IHE exams at least 4 wk apart. There were no changes in clinical status or cardiovascular symptoms in patients with CAD between the two MRI exams. Both MRI-IHE exams were performed under similar fasting and experimental conditions. Special attention was given to repeat the study in the same coronary locations as in the first study, based on anatomic landmarks such as distance from coronary and branch vessel ostia.
MRI Acquisition Details
A commercial 3.0-T whole body MR scanner (Philips Healthcare, Best, The Netherlands) equipped with a 32-channel cardiac coil and multitransmit system was used. The imaging protocol and parameters have been detailed elsewhere (8). In each subject, a proximal or midsegment of a major coronary artery was selected for cross-sectional, anatomical (17), and flow velocity-encoded MRI (12), which were obtained using single breath-hold spiral cine sequences (31). Cine coronary imaging was performed at rest and repeated at the same location during IHE, performed using an MRI-compatible dynamometer (Stoelting, Wood Dale, IL) at 30% of maximum grip strength (32). In most cases, two coronary arteries per participant and/or different segments of the same vessel were imaged, and the results for each were quantified and reported. The total duration of MRI exams were ∼60 and 40 min for the l-NMMA and reproducibility protocols, respectively.
Image Analysis
Images were analyzed for stress-induced CSA changes with semiautomated software (Cine, General Electric, Milwaukee, WI) using the full-width, half-maximum algorithm, and contours were made around each vessel during a quiescent period in diastole. For flow measurements, velocity-encoded images were analyzed using commercially available software (QFLOW, Medis, The Netherlands). Peak diastolic CFV (mean velocity of coronary artery lumen pixels at peak diastolic flow) was used for the velocity measurements, and coronary artery peak diastolic blood flow (CBF) was calculated as coronary artery CSA × coronary artery peak diastolic velocity × 0.5 (5) and converted to milliliters per minute. Coronary segments with poor image quality (blurring due to artifact/patient motion) on either the baseline or follow-up exams were excluded. Data analysis for the l-NMMA study was performed by two independent investigators (A. G. Hays and M. Iantorno) blinded (and MRIs deidentified) to study group (placebo vs. l-NMMA) and stage of the protocol (rest vs. stress).
Statistical Analysis
Data are expressed as means ± SE unless otherwise specified. The χ2-test for normality was performed, and each data set was found to be normally distributed. Parametric testing (paired Student's t-test) was used to compare stress coronary artery CSA, CFV, and CBF measurements during IHE to the initial baseline measurements both with and without l-NMMA. The data from individual segments were treated as independent observations given the nature of the study (per segment analysis). Because l-NMMA results from different segments in the same individual may be correlated, in a separate analysis the results from different segments in a given individual were averaged and the data from each subject were treated as independent observations (per subject analysis). Linear regression analysis was performed to compare the first and second MRI-IHE exams in the reproducibility study, the mean differences were displayed with Bland-Altman plots, and intraclass correlations were calculated. Statistical analysis was performed with Medcalc software (version 14.8, Ostend, Belgium). Statistical significance was defined as a two-tailed P value ≤ 0.05.
RESULTS
Subject Characteristics
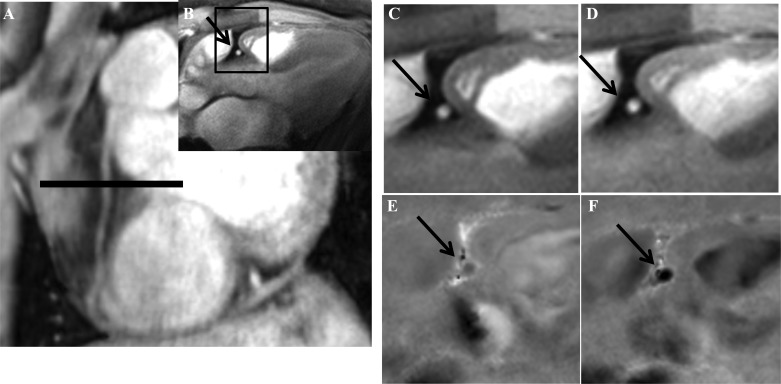
All 10 healthy subjects (mean age ± SD, 31 ± 9 yr; 5 women) completed the l-NMMA study. For the reproducibility study, the mean age for the healthy subjects was 37 ± 10 yr (4 women). Baseline characteristics of the CAD subjects in the reproducibility study are presented in Table 1. Of the 18 subjects enrolled in the reproducibility study, one healthy subject could not complete the second scan because of claustrophobia. For the l-NMMA study, 25 coronary segments [13 in the right coronary artery (RCA) and 12 in the left anterior-descending coronary artery (LAD)] were analyzed for CSA, CFV, and CBF in 10 subjects. For the reproducibility study, 17 participants completed both visits (7.7 ± 1.2 wk apart, mean ± SE; range, 4–23 wk). In these subjects a total of 29 segments were suitable for CSA analysis and 26 segments were of good quality for CFV and CBF analysis (3 segments were excluded because of artifacts or blurred borders in the flow scans). Of the segments analyzed for CSA in the 9 healthy individuals, 10 were in the RCA and 7 in the LAD whereas in the 8 patients with CAD, 6 were in the RCA and 6 in the LAD. An example of typical changes seen in CSA and CFV with IHE are shown in a healthy subject (Fig. 2). The CSA, CFV, and CBF responses to IHE were significantly attenuated in these patients with CAD compared with the responses in healthy subjects (Fig. 3), consistent with prior reports in other patients with CAD and healthy subjects (8, 9).
Table 1.
Demographics
| Characteristics | CAD Reproducibility Study (n = 8) |
|---|---|
| Age, yr (mean ± SD) | 60 ± 9 |
| Male sex, % | 8 (100) |
| Previous myocardial infarction | 4 (50) |
| Percutaneous intervention/stents | 3 (37) |
| Coronary artery bypass graft surgery | 0 |
| Dyslipidemia | 8 (100) |
| Hypertension | 5 (62) |
| Diabetes | 1 (13) |
| Angiotensin-converting enzyme inhibitor | 3 (37) |
| Statin | 8 (100) |
| β-Blocker | 3 (37) |
| Aspirin | 6 (75) |
| Coronary segments studied | |
| Right coronary artery | 6 |
| Left anterior-descending coronary artery | 6 |
| Total vessels studied | 12 |
CAD, coronary artery disease.
Fig. 2.
Anatomic and flow velocity coronary MR images at rest and during IHE. Scout scan obtained parallel to the right coronary artery (RCA) in a healthy subject is shown together with the location for cross-sectional imaging (A, black line) along with the cross-sectional image perpendicular to the RCA (B, black arrow) and the region selected for analysis (B, black square). Magnified cross-sectional area (CSA) of the RCA (shown by black arrow) was analyzed at rest (C) and during IHE (D). Magnified coronary flow velocity (CFV) of the RCA is shown at rest (E) and during IHE (F). The increase in area and darkness of the RCA with IHE (F) compared with that of baseline (E) represents the increase in velocity of coronary blood flowing down through the imaging plane.
Fig. 3.
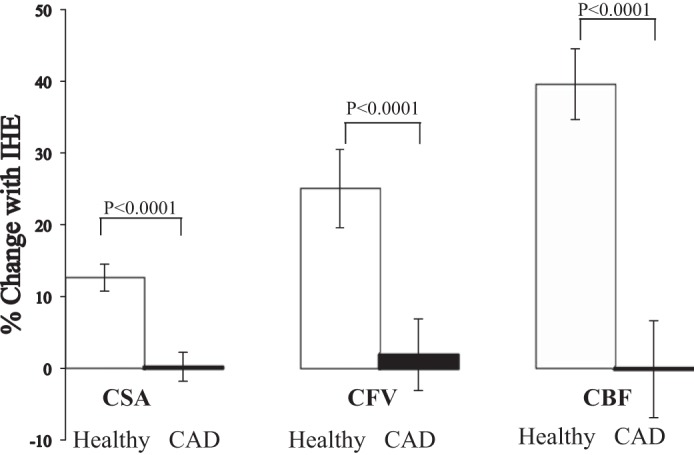
Coronary responses to IHE in healthy subjects and patients with CAD. Summary coronary artery vasoreactive changes to IHE stress (as %baseline values) for CSA, coronary blood velocity, and coronary blood flow (CBF) detected by MRI in healthy subjects (white bars) and patients with CAD (black bars).
Role of NO in Mediating the Coronary Response to IHE: the l-NMMA Study
Hemodynamic results.
Hemodynamic changes during each stage of the l-NMMA protocol (Fig. 1) are presented in Fig. 4. The baseline RPP increased with IHE and returned to baseline during the recovery period (Fig. 4). l-NMMA infusion increased mean arterial pressure (baseline, 80 ± 3 vs. l-NMMA, 96 ± 4 mmHg; P = 0.002) and decreased heart rate (baseline, 66 ± 3 vs. l-NMMA, 57 ± 3 beats/min; P = 0.002); however, mean RPP during l-NMMA infusion was not different from that before l-NMMA. The increase in RPP during IHE was similar in the absence and presence of l-NMMA (Fig. 4).
Fig. 4.
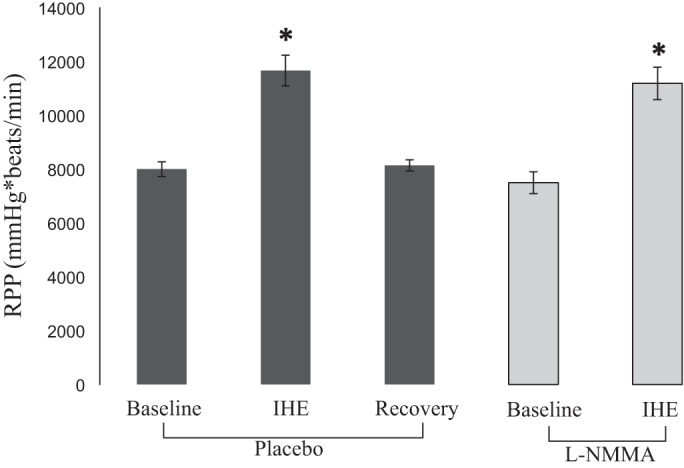
Hemodynamic parameters during l-NMMA infusion. Rate pressure product (RPP) is shown at baseline and during IHE before (placebo, dark gray bars) and during infusion of l-NMMA (light gray bars). *P < 0.05 compared with baseline RPP. Error bars indicate mean SE.
l-NMMA infusion blocks the normal coronary vasodilatory response to IHE.
Coronary arteries in healthy subjects significantly dilated in response to IHE (CSA baseline 1: 11.2 ± 0.6 vs. stress 1, 12.6 ± 0.7 mm2; P < 0.0001, Fig. 5), consistent with prior reports (8–10). Resting CSAs before the first IHE episode (during placebo infusion) and before the second IHE episode (during l-NMMA infusion) did not differ (CSA baseline 1, 11.2 ± 0.6 vs. baseline 2 with l-NMMA, 11.3 ± 0.7 mm2; P = 0.7). However, in contrast to the vasodilatory CSA response to IHE during placebo, there was no significant increase in CSA when IHE was repeated during l-NMMA infusion (baseline 2, 11.3 ± 0.7 vs. stress 2, 11.3 ± 0.7 mm2; P = 0.6). When comparing the IHE response between placebo and l-NMMA conditions, the CSA increase with IHE (as %baseline) was completely blocked by l-NMMA infusion (12.9 ± 2.5 vs. −0.3 ± 1.6%, placebo vs. l-NMMA; P < 0.001, Fig. 5).
Fig. 5.
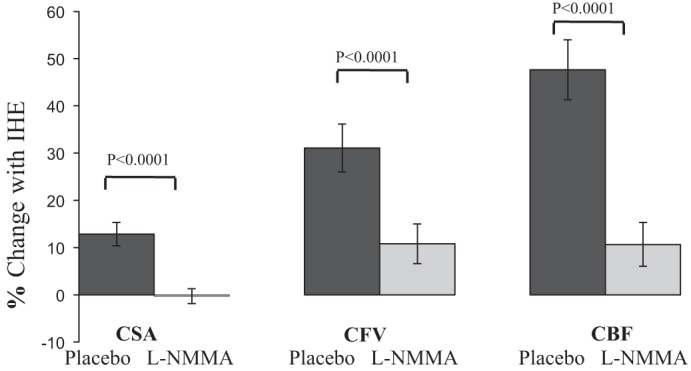
Intravenous infusion of l-NMMA blocks IHE-induced coronary vasodilation and the increase in CBF in healthy volunteers. Summary changes during IHE (%baseline values) for placebo (dark gray bars) and l-NMMA (light gray bars) conditions showing that l-NMMA completely blocks the coronary vasodilation and blunts the increase in diastolic coronary velocity and blood flow.
l-NMMA infusion blocks the normal increase in CFV and CBF with IHE.
Peak diastolic CFV significantly increased in response to IHE during placebo infusion, also consistent with prior reports (8–10), and was attenuated by l-NMMA as shown in Fig. 5. Likewise, peak diastolic CBF increased in response to IHE during placebo in healthy subjects (CBF, 39.2 ± 3.8 vs. 56.9 ± 5.1 ml/min, P < 0.0001), consistent with prior reports (8, 9). l-NMMA infusion did not change baseline CBF (39.2 ± 3.8 vs. 39.8 ± 3.7 ml/min; P = 0.6). Although CBF increased by ∼50% with IHE during placebo, l-NMMA infusion attenuated but did not completely block the CBF increase with IHE (47.7 ± 6.4 vs. 10.6 ± 4.6%, placebo vs. l-NMMA; P < 0.0001, Fig. 5). The findings that l-NMMA attenuated the normal IHE-induced increases in CFA, CFV, and CBF were consistently observed regardless of whether the data were analyzed on a per segment or per subject basis (data not shown).
Reproducibility over Weeks of the Coronary Response to IHE Detected by MRI
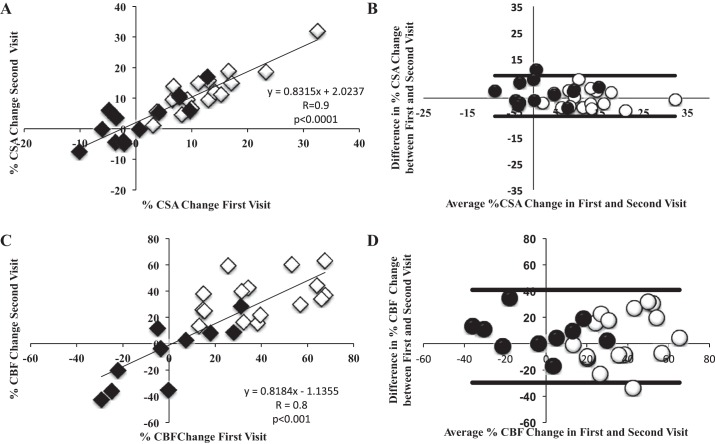
As expected, IHE induced significant hemodynamic changes in both healthy subjects (n = 9) and those with CAD (n = 8), and the change in RPP with stress was not significantly different between the first and second visits (11,805 ± 423 vs. 11,075 ± 496 mmHg·beats·min−1; P = 0.09). Among subjects (n = 17) that underwent repeat MRI after a mean ∼8-wk period, we observed no significant differences in IHE-induced changes in CSA, CFV, and CBF between the two studies (see Bland-Altman plots in Fig. 6). Importantly, there was a strong correlation between the %CSA change with IHE during the first and second visits weeks apart (R = 0.9; P < 0.0001) (Fig. 6). Similarly, there was a significant correlation between the %CFV and %CBF change at the first visit versus the second visit (R = 0.75; P < 0.01 and R = 0.8, P < 0.001, respectively; Fig. 6). The Bland-Altman analysis (Fig. 6) and intraclass correlation coefficients for %CSA, %CFV, and %CBF change with stress (intraclass correlation coefficients = 0.90, 0.75, and 0.80, respectively) indicated good confidence of agreement and little variability between the two measures.
Fig. 6.
Coronary artery vasoreactivity and blood flow response to IHE over a period of weeks both in healthy volunteers and patients with CAD. A: linear regression showing strong correlation between %CSA change during the first visit compared with second visit (A) and %peak diastolic CBF change between first and second visit (C). B and D: Bland-Altman plots comparing %CSA change and %CBF change between the 2 separate visits (n = 29) and indicate good confidence of agreement and little variability between the 2 measures. Lines represent 95% confidence of agreement. White circles/diamonds represent healthy volunteers, and black circles/diamonds represent patients with CAD.
DISCUSSION
Role of NO in the Coronary Vasomotor Response to IHE
We previously reported a novel approach to noninvasively quantify CEF that uses high-field (3.0-T) MRI combined with IHE and which detected increases in coronary CSA and peak diastolic CBF in healthy subjects but paradoxical vasoconstriction and decreases in blood flow in patients with CAD during IHE (8–10). These responses to IHE are consistent with classic coronary vasomotor responses to endothelial-dependent stressors; however, the extent to which the IHE response is in fact mediated by NO was not tested. Because the role of NO in the IHE response was not known and questioned (13), we administered l-NMMA, a direct eNOS inhibitor, and observed that it abolishes the normal coronary vasodilatory response and CBF increase that occurs with IHE, demonstrating that IHE is a predominately endothelial-dependent coronary stressor that can be used with MRI or potentially other noninvasive imaging modalities to probe NO-mediated CEF.
Our observation that the coronary vasoreactive response to IHE is NO mediated supports earlier vasomotor flow studies of the role of NO in the peripheral circulation. Sanders et al. (23) reported that atropine but not β-blockers blocked the forearm vascular resistance change in healthy volunteers during IHE, demonstrating a role for acetylcholine-mediated activation in handgrip exercise-mediated vasodilation. l-NMMA specifically inhibits the synthesis of NO in a concentration- and stereo-specific manner (22). Prior studies showed that l-NMMA blocks ∼70% of the brachial artery macrovascular vasodilatory response in healthy subjects, indicating that IHE results in endothelial-dependent, NO-dependent vasodilatation in peripheral arteries (35). Furthermore, the microvascular peripheral response is also blocked by l-NMMA infusion during IHE (29). Thus prior studies suggest that the peripheral vascular responses to IHE are mediated, at least in part, by NO and reflect endothelial function.
Despite studies suggesting a predominant role of NO mediating IHE-induced changes in peripheral vasoreactivity (23), there is a paucity of data regarding coronary vasoreactivity. The coronary arteries respond differently than do peripheral arteries to endothelial stressors (2, 3) and, in contrast to brachial arteries, are prone to atherosclerosis and plaque rupture. IHE has been used since the 1960s as a tool to study the cardiovascular response to stress, and the effects of IHE on the coronary vasculature were thought to be mediated by sympathetic vasoconstriction and vagal withdrawal (20). Brown et al. (2) using coronary angiography showed that significant vasoconstriction occurs during IHE in patients with CAD. However, when IHE was repeated after intracoronary nitroglycerin administration, vasodilation occurred, suggesting that decreased NO bioavailability contributes to an abnormal IHE response. In contrast to prior studies indicating an important role of NO in mediating the vascular response to IHE in brachial and coronary arteries, one study did not observe an effect of l-NMMA during IHE (19). That study differs from the current one in that handgrip exercise was greater (80 vs. 30% of maximal effort in the current study), and the additional stress may have triggered sympathetic and neurohumoral pathways not blocked by l-NMMA. Importantly, our population of healthy, young individuals without cardiovascular risk factors significantly differs from that in the prior study conducted in middle-aged patients with angina but whose cardiac angiograms did not demonstrate critical stenoses. Such patients often exhibit impaired NO production and endothelial dysfunction (28), and therefore, a NO synthase inhibitor is expected to have less of an effect in them than it does in healthy subjects with normal, robust coronary NO release, as studied here.
The significant attenuation of the normal coronary vasomotor response to handgrip with l-NMMA that we observed cannot be explained by changes in hemodynamics caused by l-NMMA itself or by a potential “memory effect” of the endothelium. We recently reported that two sequential IHE episodes (∼10 min apart) in the absence of l-NMMA elicit similar changes in CSA and CBF in healthy subjects [Fig. 7, reproduced from Hays et al. (10)], indicating that the attenuation of the vascular response during IHE by l-NMMA infusion during the second IHE (Fig. 5) is due to l-NMMA and not to a nonspecific “memory” effect of repeated IHE intervals. The significant attenuation of the normal increase in CSA and CBF in response to IHE by l-NMMA is in contrast to the modest attenuation of the increase in CBF by l-NMMA to low-dose adenosine but no attenuation with high-dose adenosine previously reported (30), suggesting that unlike adenosine, the predominant impact of IHE on coronary vasoreactivity is NO mediated. These findings have significant implications for CEF testing using IHE, which is not limited to only MRI, and may pertain to alternate imaging modalities, including positron emission tomography and computed tomography.
Fig. 7.
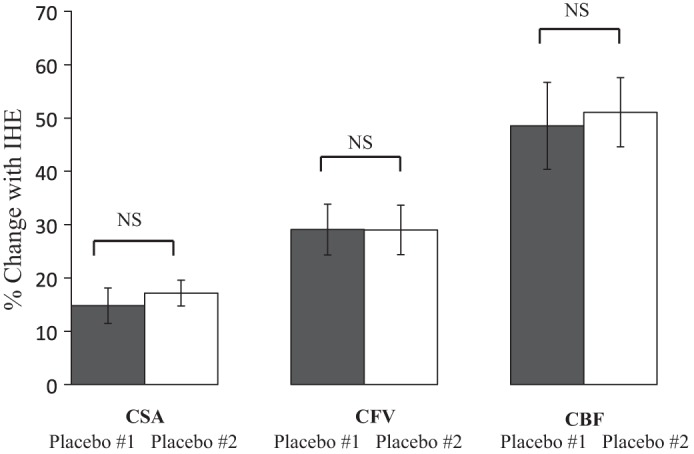
Sequential IHE episodes elicit similar responses in healthy subjects over time. Summary results for coronary changes during sequential IHE for placebo 1 (dark gray bars) and placebo 2 (white bars) showing that the coronary artery CSA, CFV, and CBF responses to repeated IHE are similar during sequential IHE episodes. Reproduced and modified with permission from Hays et al. (10).
Reproducibility of MRI Measures of CEF
We previously reported that MRI measures of CEF are unchanged during repeated IHE tests on the same day (10). However, the reproducibility over a longer time period was not previously investigated. We report here for the first time a reproducible response of CSA and CBF changes to IHE on different days and in this case over a mean 8-wk period. Endothelial function can change over the course of days and several weeks in response to a variety of stimuli including diet, exercise training, and therapeutic interventions (21), reflecting changes in expression of eNOS and altered NO bioavailability (7). The finding that the coronary MRI-IHE vasomotor responses for both CSA and CBF are reproducible over several weeks indicates that this noninvasive approach is potentially valuable for short- to medium-term intervention studies that target coronary endothelial dysfunction. Moreover, the MRI-IHE approach used in these studies is tolerated by both healthy subjects and patients with CAD and can be carried out in a reasonable time period (<45 min), with minimal risk since there is no intravenous contrast administration or radiation. The observation that the macrovascular CSA changes in response to IHE appear to be more reproducible and less variable over time than CBF responses (Fig. 6) suggests that the CSA end point may allow intervention trials with smaller sample sizes or detection of smaller group differences than the CBF end point. Furthermore, this MRI-IHE approach may facilitate the translation from the bench to the bedside of novel agents (6, 14) that may be safely and noninvasively tested in humans.
Limitations
The sample size of these studies is relatively modest but nonetheless statistically large enough to test the proposed hypotheses. l-NMMA profoundly blocked the normal coronary vasomotor response in only 25 segments in 10 subjects such that it was difficult to justify additional studies, especially with l-NMMA, a nonapproved agent. MRI measures of coronary area (16) and CFV (11, 18) were validated in prior studies, and our results are comparable with those reported using invasive techniques (2, 15, 24). Although systemic l-NMMA infusion caused a slight decrease in heart rate and increase in mean arterial pressure possibly mediated by neurohormonal mechanisms (26), the changes in RPP with IHE stress were not different during the placebo and l-NMMA IHE stress sessions. This suggests that the predominant effect of systemic l-NMMA to prevent IHE-induced coronary endothelial dilatation was due to its well-documented ability to inhibit NO rather than a modest sympathetic response that cannot be excluded.
Conclusions
In summary, we report here that the IHE response of the coronary arteries measured noninvasively with MRI is predominantly attenuated by l-NMMA, indicating that the normal coronary artery response to IHE is primarily NO mediated. In addition, MRI measures of NO-mediated CEF remain stable over an ∼8-wk period. This too is important for designing future studies designed to noninvasively assess the effects of therapeutic interventions on CEF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL120905 and HL125059, American Heart Association Grant 11SDG5200004, and the Clarence Doodeman Endowment of Johns Hopkins.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.G. and R.G.W. conception and design of research; A.G.H., M.I., S.S., A.S., and M. Schär performed experiments; A.G.H., M.I., and S.S. analyzed data; A.G.H., M.I., S.S., M. Schär, G.G., M. Stuber, and R.G.W. interpreted results of experiments; A.G.H. and M.I. prepared figures; A.G.H. and M.I. drafted manuscript; A.G.H., M.I., S.S., A.S., M. Schär, G.G., M. Stuber, and R.G.W. edited and revised manuscript; A.G.H., M.I., S.S., A.S., M. Schär, G.G., M. Stuber, and R.G.W. approved final version of manuscript.
REFERENCES
- 1.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Brown BG, Lee AB, Bolson EL, Dodge HT. Reflex constriction of significant coronary stenosis as a mechanism contributing to ischemic left ventricular dysfunction during isometric exercise. Circulation 70: 18–24, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 24: 1468–1474, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115: 1285–1295, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation 85: 1899–1911, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Gano LB, Donato AJ, Pasha HM, Hearon CM Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol 307: H1754–H1763, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol 56: 1657–1665, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Hays AG, Kelle S, Hirsch GA, Soleimanifard S, Yu J, Agarwal HK, Gerstenblith G, Schar M, Stuber M, Weiss RG. Regional coronary endothelial function is closely related to local early coronary atherosclerosis in patients with mild coronary artery disease: pilot study. Circ Cardiovasc Imaging 5: 341–348, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hays AG, Stuber M, Hirsch GA, Yu J, Schar M, Weiss RG, Gerstenblith G, Kelle S. Non-invasive detection of coronary endothelial response to sequential handgrip exercise in coronary artery disease patients and healthy adults. PLoS One 8: e58047, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hundley WG, Lange RA, Clarke GD, Meshack BM, Payne J, Landau C, McColl R, Sayad DE, Willett DL, Willard JE, Hillis LD, Peshock RM. Assessment of coronary arterial flow and flow reserve in humans with magnetic resonance imaging. Circulation 93: 1502–1508, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Keegan J, Gatehouse PD, Yang GZ, Firmin DN. Spiral phase velocity mapping of left and right coronary artery blood flow: correction for through-plane motion using selective fat-only excitation. J Magn Reson Imaging 20: 953–960, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Klocke FJ. Epicardial coronary artery vasomotion. J Am Coll Cardiol 56: 1666–1667, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Zhang H, Chen J, Dellsperger KC, Hill MA, Zhang C. Adiponectin abates diabetes-induced endothelial dysfunction by suppressing oxidative stress, adhesion molecules, and inflammation in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 303: H106–H115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 315: 1046–1051, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Manning WJ, Li W, Boyle NG, Edelman RR. Fat-suppressed breath-hold magnetic resonance coronary angiography. Circulation 87: 94–104, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Meyer CH, Hu BS, Nishimura DG, Macovski A. Fast spiral coronary artery imaging. Magn Reson Med 28: 202–213, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Nagel E, Bornstedt A, Hug J, Schnackenburg B, Wellnhofer E, Fleck E. Noninvasive determination of coronary blood flow velocity with magnetic resonance imaging: comparison of breath-hold and navigator techniques with intravascular ultrasound. Magn Reson Med 41: 544–549, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa Y, Kanki H, Ogawa S. Role of nitric oxide in coronary vasomotion during handgrip exercise. Am Heart J 134: 967–973, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Nutter DO, Schlant RC, Hurst JW. Isometric exercise and the cardiovascular system. Mod Concepts Cardiovasc Dis 41: 11–15, 1972. [PubMed] [Google Scholar]
- 21.Pugh CJ, Spring VS, Kemp GJ, Richardson P, Shojaee-Moradie F, Umpleby AM, Green DJ, Cable NT, Jones H, Cuthbertson DJ. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol 307: H1298–H1306, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Rees DD, Palmer RM, Hodson HF, Moncada S. A specific inhibitor of nitric oxide formation from l-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol 96: 418–424, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders JS, Mark AL, Ferguson DW. Evidence for cholinergically mediated vasodilation at the beginning of isometric exercise in humans. Circulation 79: 815–824, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101: 1899–1906, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Schindler TH, Facta AD, Prior JO, Cadenas J, Zhang XL, Li Y, Sayre J, Goldin J, Schelbert HR. Structural alterations of the coronary arterial wall are associated with myocardial flow heterogeneity in type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging 36: 219–229, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spieker LE, Corti R, Binggeli C, Luscher TF, Noll G. Baroreceptor dysfunction induced by nitric oxide synthase inhibition in humans. J Am Coll Cardiol 36: 213–218, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Stuber M, Botnar RM, Danias PG, Sodickson DK, Kissinger KV, Van Cauteren M, De Becker J, Manning WJ. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J Am Coll Cardiol 34: 524–531, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N, Ishibashi Y, Shimada T, Sakane T, Ohata S, Sugamori T, Ohta Y, Inoue S, Nakamura K, Shimizu H, Katoh H, Murakami Y. Impaired exercise-induced vasodilatation in chronic atrial fibrillation–role of endothelium-derived nitric oxide. Circ J 66: 583–588, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Tawakol A, Forgione MA, Stuehlinger M, Alpert NM, Cooke JP, Loscalzo J, Fischman AJ, Creager MA, Gewirtz H. Homocysteine impairs coronary microvascular dilator function in humans. J Am Coll Cardiol 40: 1051–1058, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Terashima M, Meyer CH, Keeffe BG, Putz EJ, de la Pena-Almaguer E, Yang PC, Hu BS, Nishimura DG, McConnell MV. Noninvasive assessment of coronary vasodilation using magnetic resonance angiography. J Am Coll Cardiol 45: 104–110, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Weiss RG, Bottomley PA, Hardy CJ, Gerstenblith G. Regional myocardial metabolism of high-energy phosphates during isometric exercise in patients with coronary artery disease. N Engl J Med 323: 1593–1600, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Wohrle J, Nusser T, Merkle N, Kestler HA, Grebe OC, Marx N, Hoher M, Kochs M, Hombach V. Myocardial perfusion reserve in cardiovascular magnetic resonance: Correlation to coronary microvascular dysfunction. J Cardiovasc Magn Reson 8: 781–787, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300: H1101–H1107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaron M, Roach V, Izkhakov E, Ish-Shalom M, Sack J, Sofer Y, Azzam I, Ray A, Stern N, and Tordjman KM. Effects of a typical acute oral calcium load on arterial properties and endothelial function in healthy subjects. Eur J of Clin Nut 68: 608–612, 2014. [DOI] [PubMed] [Google Scholar]