Abstract
We tested the hypothesis that aortic perivascular adipose tissue (PVAT) from young low-density lipoprotein receptor-deficient (LDLr−/−) mice promotes aortic stiffness and remodeling, which would be mediated by greater PVAT-derived IL-6 secretion. Arterial stiffness was assessed by aortic pulse wave velocity and with ex vivo intrinsic mechanical properties testing in young (4–6 mo old) wild-type (WT) and LDLr−/− chow-fed mice. Compared with WT mice, LDLr−/− mice had increased aortic pulse wave velocity (407 ± 18 vs. 353 ± 13 cm/s) and intrinsic mechanical stiffness (5,308 ± 623 vs. 3,355 ± 330 kPa) that was associated with greater aortic protein expression of collagen type I and advanced glycation end products (all P < 0.05 vs. WT mice). Aortic segments from LDLr−/− compared with WT mice cultured in the presence of PVAT had greater intrinsic mechanical stiffness (6,092 ± 480 vs. 3,710 ± 316 kPa), and this was reversed in LDLr−/− mouse arteries cultured without PVAT (3,473 ± 577 kPa, both P < 0.05). Collagen type I and advanced glycation end products were increased in LDLr−/− mouse arteries cultured with PVAT (P < 0.05 vs. WT mouse arteries), which was attenuated when arteries were cultured in the absence of PVAT (P < 0.05). PVAT from LDLr−/− mice secreted larger amounts of IL-6 (3.4 ± 0.1 vs. 2.3 ± 0.7 ng/ml, P < 0.05), and IL-6 neutralizing antibody decreased intrinsic mechanical stiffness in LDLr−/− aortic segments cultured with PVAT (P < 0.05). Collectively, these data provide evidence for a role of PVAT-derived IL-6 in the pathogenesis of aortic stiffness and remodeling in chow-fed LDLr−/− mice.
Keywords: periaortic fat, aorta, cholesterol, inflammation, triglycerides
deaths due to cardiovascular diseases (CVD) are the leading cause of mortality in the United States and in other modern societies worldwide (21). Recent meta-analysis data have indicated that the increased risk for CVD and related events are, in part, attributable to stiffening of the aorta as assessed by aortic pulse wave velocity (aPWV) (3). Importantly, nearly 50% of Americans have total cholesterol values above the desirable level (21), which, in turn, promotes aortic stiffening (13, 23, 42). Thus, gaining insights for the mechanisms by which traditional risk factors, such as hypercholesterolemia, promote aortic stiffening is of clinical importance.
Aortic stiffness is, in part, due to changes in the expression of extracellular matrix proteins within the arterial wall and increased cross-linking of these proteins (15, 44). Collagen type I is a key load-bearing collagen isoform with increased expression in arteries with greater aortic stiffness (18). In contrast, elastin, a protein that provides elasticity to arteries, has an attenuated expression that also contributes to stiffening of the aorta (26, 38). Importantly, cross-linking of extracellular proteins by advanced glycation end products (AGEs) has been shown to promote stiffening of large elastic arteries (18, 33). However, it is not fully known how the expression of these aortic proteins change in response to hypercholesterolemia or the factors that may influence these pathological alterations.
A greater amount of aortic perivascular adipose tissue (PVAT) is associated with aortic stiffening and incident CVD events (4–6). A recent investigation in an age-related model of aortic stiffening has provided direct evidence for PVAT to influence arterial stiffness both in vivo and ex vivo, which was associated with alterations in aortic collagen type I expression and increased PVAT-derived inflammatory cytokine secretion (16). PVAT is an important source of adipokine production and secretion that has been shown to influence arterial function (24, 34). Notably, the proinflammatory cytokine IL-6 is related to arterial stiffness in adults and is secreted from PVAT in greater concentrations in older mice (16, 31). IL-6 is one proinflammatory cytokine shown to be secreted in higher concentrations from PVAT compared with other fat depots, thus highlighting the potential role for this PVAT-derived cytokine to promote arterial stiffening (10, 16). Importantly, under conditions of increased plasma cholesterol, PVAT has elevated IL-6 protein expression (12). It is currently unknown, however, whether increased cholesterol results in PVAT-induced aortic stiffening or arterial extracellular matrix remodeling or if PVAT secretes greater concentrations of IL-6 secretion to promote arterial stiffness.
We tested the hypothesis that chow-fed low-density lipoprotein receptor-deficient (LDLr−/−) mice with increased plasma cholesterol concentrations would have greater aortic stiffness as assessed by noninvasive aPWV and ex vivo mechanical properties testing, which would be related to increased collagen type I, greater AGE abundance, and reduced elastin expression in the aorta. We also hypothesized PVAT would mediate the arterial stiffening and alterations in aortic protein expressions observed in LDLr−/− mice. In addition, PVAT from LDLr−/− mice was hypothesized to secrete greater amounts of IL-6 that would contribute to arterial stiffness.
MATERIALS AND METHODS
Animals
Male wild-type (WT) and LDLr−/− mice on a C57BL/6 background at 4–6 mo of age were purchased from The Jackson Laboratory. All mice had ad libitum access to water and normal rodent chow and were housed in an animal facility on a 12:12-h light-dark cycle on the University of Kentucky campus. All animals were acclimated for at least 2 wk before experiments. The Animal Care and Use Committee of the University of Kentucky reviewed and approved all protocols in this study.
aPWV
Large artery stiffness was assessed by aPWV as previously described (16, 19, 33). Mice were anesthetized (2% isoflurane) and placed supine on a heating board with legs secured to ECG electrodes. Doppler probes (Indus Instruments) were used to noninvasively assess aortic velocities at the aortic arch and abdominal aorta. aPWV was calculated as the distance between the arch and abdominal probes divided by the difference in thoracic and abdominal preejection times. Data are presented as centimeters/second. Mice were euthanized after the aPWV measures by exsanguination via cardiac puncture while anesthetized with isoflurane.
Body Composition
All mice underwent an echoMRI to determine body composition (8). Briefly, conscious animals were placed in a plastic tube, which was inserted into the echoMRI scanner to assess fat mass and lean mass. Each scan was ∼2 min in duration per mouse. Data are presented as percent fat and lean mass.
Arterial Blood Pressure
Arterial blood pressure was assessed using the noninvasive CODA tail-cuff system (Kent Scientific) (14, 16). Briefly, mice were placed in restrainers and allowed to acclimate on a heated platform for 15–20 min. Blood pressure was recorded for 20 cycles on 3 consecutive days. Data for the 3 days were averaged.
Plasma Total Cholesterol, Triglycerides, and Glucose
Blood obtained from the cardiac puncture was collected with a syringe containing heparin after a 10-h fast, which was spun at 14,000 rpm for 10 min. Plasma was isolated and stored at −80°C. Total cholesterol (Wako Diagnostics), triglycerides (Cayman Chemical), and glucose (Abcam) were assessed according to the manufacturers' protocol.
Intrinsic Mechanical Testing
Ex vivo intrinsic mechanical properties of aortas from WT and LDLr−/− mice were assessed as previously described (16–18, 22). Aortic segments of the descending thoracic aorta were cleaned of the surrounding adipose tissue and placed onto pins in a chamber containing Ca2+- and Mg2+-free PBS. The myograph (DMT) was preheated to 37°C before experimentation. Force was recorded every 3 min as the aortic rings were stretched ∼10% until the arterial segment broke. The elastic modulus was calculated from the stress-strain curves as previously described (16, 18, 22). In brief, one-dimensional stress (t) was calculated as follows: t = λL/2HD, where L is the one-dimensioal load applied, H is wall thickness, and D is the length of vessel; strain (λ) was calculated as follows: λ = Δd/di, where Δd is the change in diameter and di = initial diameter. Wall thickness and diameter were assessed in histological sections, and aortic length was measured with calipers under a dissecting microscope. The elastic modulus, an index of mechanical stiffness, was determined as the greatest r2 value from the stress-strain curve.
Adipose Histology
Adipose from the perivascular descending thoracic aorta (PVAT) and subcutaneous and epididymal fat depots were collected and fixed in 4% paraformaldehyde. The adipose was embedded in paraffin, sectioned (5 μm), deparaffinized, and stained with hematoxylin and eosin (30). Using ImageJ software [National Institutes of Health (NIH)], adipocyte size and diameter for all adipose depots was measured in at least 100 cells from 2 sections for each sample. The data for each sample were averaged, which was used to determine the mean for each group. The data are presented as diameter (in μm) and area (in μm2).
Aortic Tissue Culture Experiments
Aortic PVAT culture experiments.
Additional segments of the descending thoracic aorta from WT and LDLr−/− mice were cultured in DMEM containing antibiotics for 72 h at 37°C and 5% CO2 either with or without the adjacent PVAT. Tissue culture media was changed daily. At the end of the 72-h culture, PVAT was removed from all aortic segments and underwent intrinsic mechanical properties testing (16).
PVAT conditioned media experiments.
Descending thoracic aorta PVAT from WT and LDLr−/− mice was cultured in DMEM containing antibiotics for 24 h at 37°C and 5% CO2 at a concentration of 20 mg fat/100 μl culture media. Conditioned media (10% of total DMEM volume) was used to treat aortas without PVAT from naïve WT mice for 72 h at 37°C and 5% CO2. Media was changed daily. Aortic segments were mechanically tested after the 72-h treatment period.
IL-6 culture experiments.
Descending thoracic aortas from LDLr−/− mice were cultured with or without neutralizing IL-6 antibody (25 μg/ml, R&D Systems) in DMEM containing antibiotics either in the presence or absence of the adjacent PVAT for 72 h at 37°C and 5% CO2. Aortas were mechanically tested after 72 h, and the adjacent PVAT was removed from all arteries before testing.
Additional descending thoracic aortas without PVAT from WT mice were treated in the presence or absence of recombinant mouse IL-6 (3 ng/ml, R&D Systems) in DMEM (with antibiotics) for 72 h with daily media changes. After 72 h of culture at 37°C and 5% CO2, the structural integrity of aortic segments was assessed with mechanical testing.
Western Blot Analysis
Aortic protein expression was assessed in samples by Western blot analysis as previously described (18, 19). Briefly, PVAT and the surrounding connective tissue were removed from the aorta, which was frozen in liquid nitrogen and stored at −80°C. Using RIPA lysis buffer containing protease and phosphatase inhibitors (Roche) and a phosphatase inhibitor cocktail (Sigma), the samples were homogenized. Equal amounts of protein (15 μg/lane) were loaded in a polyacrylamide gradient (4–12%) gel, separated by electrophoresis, and transferred to a polyvinylidene difluoride membrane (Bio-Rad). Antibodies specific for collagen type I (1:1,000, Abcam), α-elastin (1:100, Abcam), AGEs (1:1,000, Abcam), and α-smooth muscle actin (1:1,000, Abcam) were applied to membranes overnight at 4°C. Densitometry analysis of each band was performed with ImageJ software (NIH). Densities for collagen type I, AGEs, and α-elastin were normalized to each respective internal α-smooth muscle actin control. The normalized values are expressed relative to the WT control group and are presented as protein expression [in arbitrary units (AU)].
Immunohistochemistry
Immunohistochemistry was performed by standard procedures as previously described (16, 18, 19). Aortic segments were frozen in optimal cutting temperature compound (Tissue-Tek) by placing the samples in liquid nitrogen-cooled isopentane. Aortas were sectioned (8 μm) and fixed with acetone. Slides were stained in two batches on different days using the Dako EnVision+ System-HRP-DAB kit (DAKO) per the manufacturer's instructions. Primary antibodies for collagen type I (1:400), AGEs (1:50), and α-elastin (1:250) were incubated at 4°C for 30 min, a labeled polymer secondary antibody was applied for 30 min at room temperature, and diaminobenzidine was used for 2 min to visualize the staining. Increasing concentrations of ethanol (50–100%) were used to dehydrate the slides, which were subsequently cleared in xylene and coverslipped. A Nikon 80i microscope was used to acquire digital images with a ×40 objective, and densitometry analysis was performed on the medial and adventitial layers with ImageJ software (NIH). The density analysis was performed on the entire medial layer or adventitial layer using two to four tissue cross-sections for each sample. A preoptimized red-green-blue color model was used to determine the mean density for each of the arterial layers. Medial layer measurements were taken from the internal elastic lamina to the external elastic lamina, and the adventitial layer was measured from the external elastic lamina to the outermost part of the artery. Adipose tissue was removed before freezing and was not analyzed. To account for potential day-to-day variations in staining, data were normalized to the WT + PVAT group for each staining batch. The normalized data for each staining batch were combined and analyzed. The data are presented as relative density (in AU).
IL-6 ELISA
PVAT was cultured in DMEM containing antibiotics at a concentration of 20 mg fat/100 μl media for 24 h at 37°C and 5% CO2. Conditioned media was assessed for IL-6 with an ELISA as recommended by the manufacturer (R&D Systems).
Statistics
All data are presented as means ± SE and were analyzed with GraphPad Prism (GraphPad Software). An unpaired, two-tailed t-test was used to analyze the animal characteristics, aPWV, mechanical testing, and Western blot data. The cultured PVAT experiments with arterial mechanical testing and immunohistochemistry as well as the IL-6 inhibition experiments were analyzed with two-way ANOVA with appropriate post hoc analyses. An unpaired, one-tailed t-test was used to analyze the IL-6 ELISA and recombinant IL-6 mechanical testing data. Significance was set at P < 0.05.
RESULTS
Animal Characteristics
Body weight, heart weight, percent fat mass, percent lean tissue, systolic blood pressure, and plasma glucose were not significantly different between WT and LDLr−/− groups (Table 1). Plasma total cholesterol and triglycerides were greater in LDLr−/− compared with WT mice (both P < 0.05; Table 1). Compared with WT mice, aortic PVAT from LDLr−/− mice demonstrated an increase in adipocyte diameter and area (both P < 0.05; Table 2). No differences in adipocyte diameter or area were observed in either subcutaneous or epididymal fat depots between WT and LDLr−/− groups (Table 2).
Table 1.
Animal characteristics
| WT Mice | LDLr−/− Mice | |
|---|---|---|
| Body mass, g | 29 ± 1 | 28 ± 1 |
| Heart mass, mg | 161 ± 4 | 151 ± 14 |
| Fat mass, % | 17 ± 1 | 17 ± 1 |
| Lean mass, % | 74 ± 1 | 74 ± 1 |
| Systolic blood pressure, mmHg | 145 ± 5 | 144 ± 5 |
| Glucose, nmol/μl | 5.1 ± 0.6 | 4.2 ± 0.3 |
| Total cholesterol, mg/dl | 89 ± 3 | 206 ± 7* |
| Triglycerides, mg/dl | 94 ± 4 | 135 ± 9* |
Values are means ± SE. WT, wild-type; LDLr−/−, low-density lipoprotein receptor knockout.
P < 0.05 vs. WT mice.
Table 2.
Adipocyte diameter and area
| WT Mice | LDLr−/− Mice | |
|---|---|---|
| Aortic perivascular adipose tissue | ||
| Diameter, μm | 20 ± 1 | 28 ± 2* |
| Area, μm2 | 267 ± 42 | 429 ± 45* |
| Subcutaneous | ||
| Diameter, μm | 41 ± 3 | 50 ± 6 |
| Area, μm2 | 1,117 ± 135 | 1,729 ± 355 |
| Epididymal | ||
| Diameter, μm | 60 ± 3 | 61 ± 2 |
| Area, μm2 | 2,178 ± 137 | 2,282 ± 147 |
Values are means ± SE.
P < 0.05 vs. WT mice.
Large Elastic Artery Stiffness and Aortic Collagen Type I, AGEs, and α-Elastin
LDLr−/− compared with WT mice had greater large elastic artery stiffness, as assessed by aPWV (P < 0.05; Fig. 1A) and ex vivo intrinsic mechanical stiffness testing (P < 0.05; Fig. 1B).
Fig. 1.
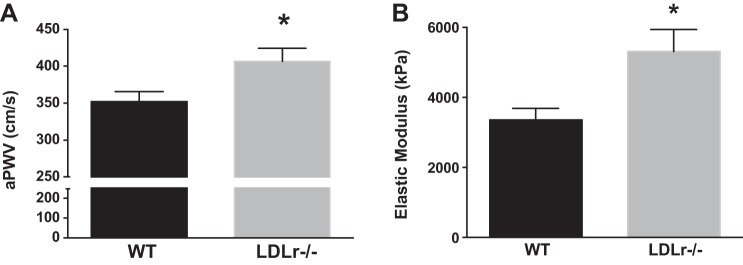
Large elastic artery stiffness. A and B: aortic pulse wave velocity (aPWV; A) and ex vivo intrinsic mechanical properties (B) in wild-type (WT) and low-density lipoprotein receptor knockout (LDLr−/−) mice. Values are means ± SE; n = 6–10 mice/group. *P < 0.05 vs. WT mice.
The extracellular matrix protein collagen type I (Fig. 2A) and AGEs (Fig. 2B) were greater in whole aortic lysates from LDLr−/− compared with WT mice (both P < 0.05). α-Elastin protein content was not significantly altered in whole aortic lysates between WT and LDLr−/− mice (P > 0.05; Fig. 2C).
Fig. 2.
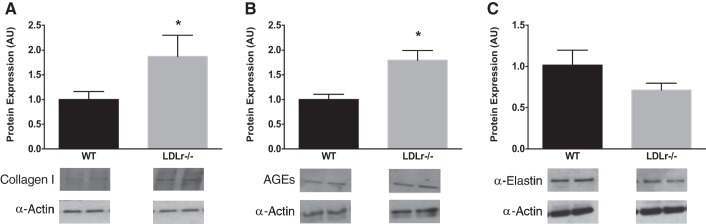
Aortic extracellular matrix proteins. A–C: collagen type I (A), advanced glycation end products (AGEs; B), and α-elastin (C) protein expressions in aortas from WT and LDLr−/− mice. Values are means ± SE; n = 4 mice/group. *P < 0.05 vs. WT mice.
Influence of PVAT on Arterial Stiffness and Aortic Collagen Type I, AGEs, and α-Elastin
Intrinsic mechanical stiffness was assessed in aortic segments from WT and LDLr−/− mice cultured in the presence (+) or absence (−) of aortic PVAT for 72 h. Intrinsic stiffness was increased in aortic segments from LDLr−/− + PVAT compared with WT + PVAT, which was reversed in LDLr−/− − PVAT cultured tissues (P < 0.05; Fig. 3A). To further examine the influence of aortic PVAT on artery stiffness, PVAT from the thoracic aorta of WT and LDLr−/− mice was incubated in media, which was used to treat aortas from naïve WT mice. Compared with WT mice, PVAT conditioned media from LDLr−/− mice increased aortic intrinsic mechanical stiffness (P < 0.05; Fig. 3B). Collectively, these experiments indicate that PVAT-derived factors promote arterial stiffness in LDLr−/− mice.
Fig. 3.
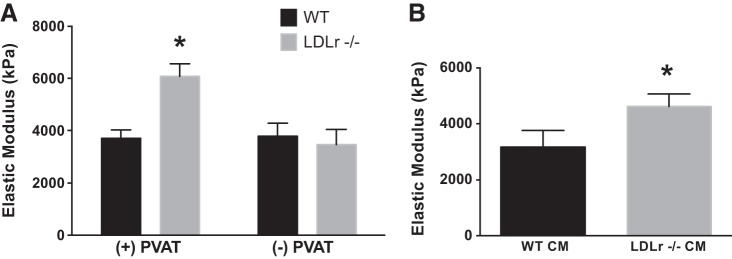
Effects of aortic perivascular adipose tissue (PVAT) and PVAT conditioned media (CM) on large elastic artery stiffness. A: ex vivo intrinsic mechanical stiffness in aortic segments cultured for 72 h in the presence (+) or absence (−) of PVAT from WT and LDLr−/− mice. B: intrinsic stiffness in aortic segments from WT mice treated with PVAT CM from WT or LDLr−/− mice for 72 h. Values are means ± SE; n = 5–7 mice/group. *P < 0.05 vs. WT + PVAT, WT − PVAT and LDLr−/− − PVAT; or vs. WT CM.
To determine the influence of PVAT on proteins related to arterial stiffness, immunohistochemistry was performed on aortic segments cultured from WT and LDLr−/− mice in either the presence or absence of PVAT for 72 h. Collagen type I was greater in the aortic medial (Fig. 4, A and C) and adventitial (Fig. 4, B and C) layers of LDLr−/− mice + PVAT compared with WT mice + PVAT (P < 0.05). Removal of PVAT from aortic segments from LDLr−/− mice normalized collagen type I expression in both medial and adventitial layers (P < 0.05).
Fig. 4.
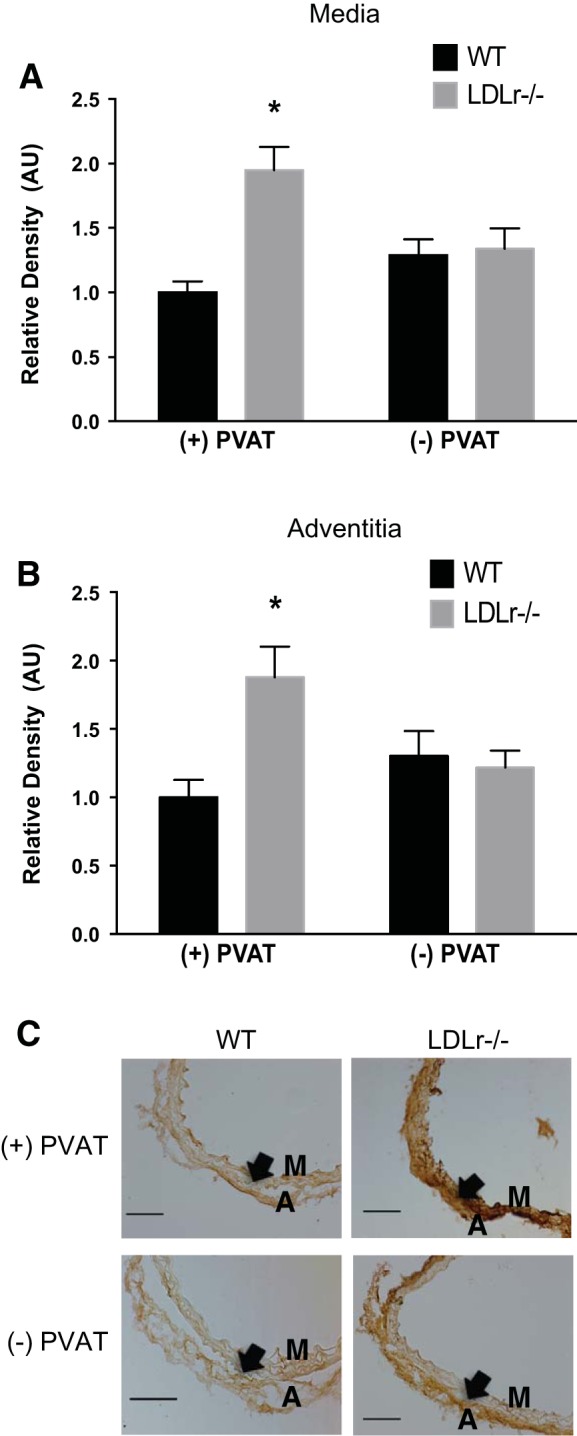
Effects of aortic PVAT on aortic collagen type I. A and B: medial (A) and adventitial (B) collagen type I in histological cross-sections (C) of aortic segments cultured for 72 h in the presence or absence of PVAT from WT and LDLr−/− mice. Values are means ± SE; n = 5–8 mice/group. *P < 0.05 vs. WT + PVAT, WT − PVAT, and LDLr−/− mice − PVAT. Arrows denote the medial-adventitial border. M, medial layer; A, adventitial layer. Scale bar = 100 μm.
Greater AGE abundance was observed in the medial (Fig. 5, A and C) and adventitial (Fig. 5, B and C) layers of the aorta cultured from LDLr−/− mice + PVAT compared with WT mice + PVAT (P < 0.05). Incubation of arteries from LDLr−/− mice without PVAT attenuated AGEs in the adventitia layer (P < 0.05) but had no effect on medial AGEs.
Fig. 5.
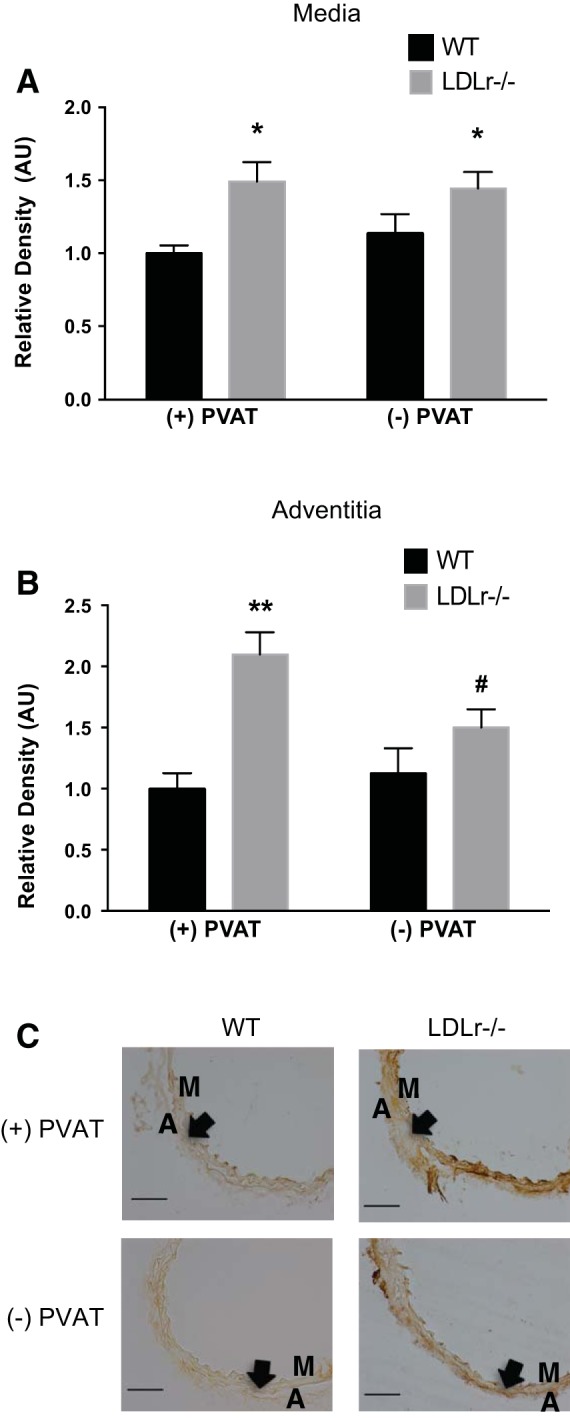
Effects of aortic PVAT on aortic AGEs. A and B: medial (A) and adventitial (B) AGEs in histological cross-sections (C) of aortic segments cultured for 72 h in the presence or absence of PVAT from WT and LDLr−/− mice. Values are means ± SE; n = 6–8 mice/group. *P < 0.05 for main effect of effect of strain in the medial layer; **P < 0.05 vs. WT + PVAT, WT − PVAT, and LDLr−/− − PVAT; #P < 0.05 vs. WT mice with PVAT. Arrows denote the medial-adventitial border. Scale bar = 100 μm.
α-Elastin expression was reduced in the medial layer (Fig. 6, A and C) of the aorta from LDLr−/− mice cultured + PVAT compared with WT mice cultured + PVAT (P < 0.05). When aortic segments from LDLr−/− mice were cultured in the absence of PVAT, α-elastin increased in the medial layer (P < 0.05; Fig. 6, A and C). There was a main effect for increased adventitial elastin expression in aortic segments cultured − PVAT (P < 0.05; Fig. 6, B and C).
Fig. 6.
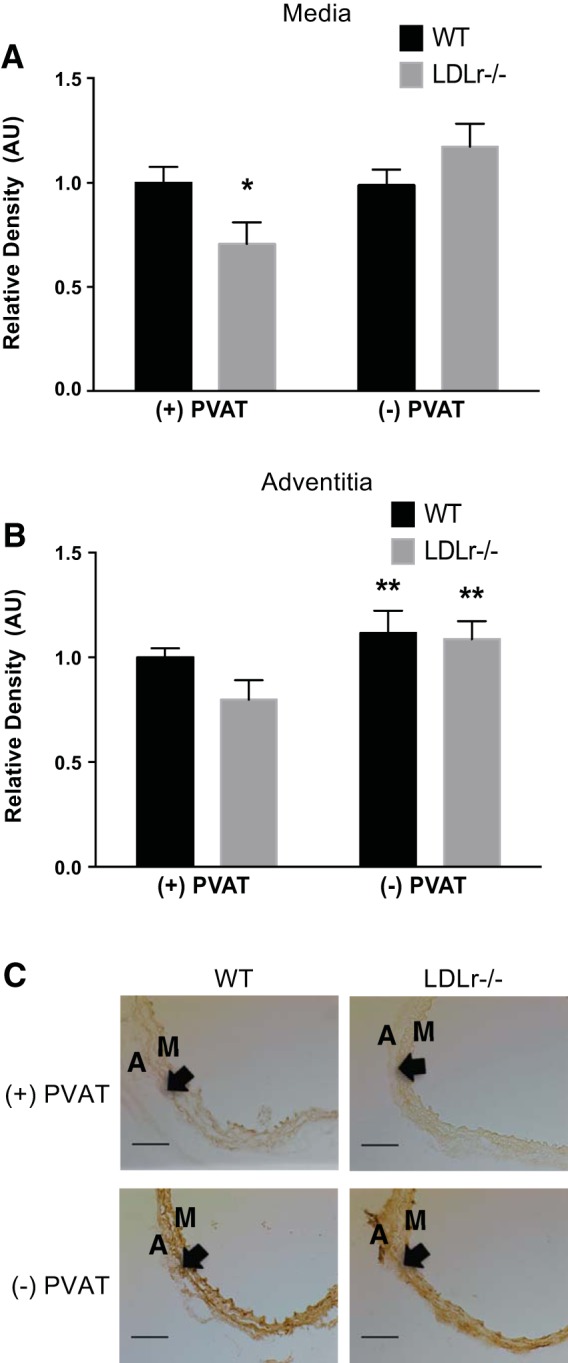
Effects of aortic PVAT on aortic α-elastin. A and B: medial (A) and adventitial (B) α-elastin in histological cross-sections (C) of aortic segments cultured for 72 h in the presence or absence of PVAT from WT and LDLr−/− mice. Values are means ± SE; n = 6–10 mice/group. *P < 0.05 vs. WT + PVAT, WT − PVAT, and LDLr−/− − PVAT; **P < 0.05 main of effect of PVAT. Arrows denote the medial-adventitial border. Scale bar = 100 μm.
PVAT IL-6 Secretion and Influence of IL-6 on Mechanical Stiffness
Unpublished cytokine array data from our laboratory indicate that IL-6 secretion is greater from PVAT of LDLr−/− compared with WT mice. To verify these potential changes, an ELISA was performed, which demonstrates greater PVAT IL-6 secretion from LDLr−/− versus WT mice (P < 0.05; Fig. 7A). To determine if the IL-6 secreted from PVAT promotes mechanical stiffness, a neutralizing antibody for IL-6 was cultured with aortas from LDLr−/− mice in the presence or absence of PVAT. Inhibition of IL-6 reduced intrinsic mechanical stiffness in LDLr−/− aortic segments + PVAT (P < 0.05; Fig. 7B) but did not further decrease arterial stiffness in aortic segments cultured − PVAT (Fig. 7B). Given IL-6 secretion was greater from PVAT of LDLr−/− mice and IL-6 inhibition attenuates mechanical stiffness, we determined whether the effects of IL-6 are sufficient to promote mechanical stiffness. Aortic segments from WT mice treated with IL-6, at a dose (3 ng/ml) similar to the concentration secreted from LDLr−/− PVAT, had greater intrinsic stiffness compared with control segments not treated with IL-6 (P < 0.05; Fig. 7C).
Fig. 7.
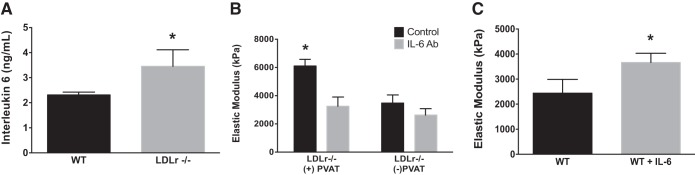
Aortic PVAT-derived IL-6 secretion and influence on ex vivo intrinsic mechanical stiffness. A: IL-6 concentration in media cultured with PVAT from WT and LDLr−/− mice for 24 h. B: intrinsic mechanical stiffness of aortic segments from LDLr−/− mice cultured in the presence or absence of PVAT with or without IL-6-neutralizing antibody (IL-6 Ab; 25 μg/ml for 72 h). C: ex vivo instrinsic stiffness of aortas from WT mice culture in the absence (WT) or presence of IL-6 (WT + IL-6; 3 ng/ml) for 72 h. Values are means ± SE; n = 4–8 mice/group. *P < 0.05 vs. all.
DISCUSSION
Identifying mechanisms that promote aortic stiffening in conditions with increased cholesterol concentrations is clinically relevant to develop novel therapeutic interventions. The present study in LDLr−/− mice with elevated plasma cholesterol and triglyceride concentrations demonstrate 1) increased aortic stiffness, as assessed by aPWV and intrinsic mechanical properties testing, which was associated with greater collagen type I deposition and AGE abundance in the aorta; 2) that PVAT directly contributes to aortic mechanical stiffening and aortic extracellular matrix remodeling; and 3) that PVAT secretes greater concentrations of IL-6, which, in turn, promotes arterial stiffness of the aorta. Thus, these findings provide initial causal insights for PVAT-derived IL-6 to increase arterial stiffness and possibly increase CVD risk in conditions with elevated cholesterol.
Arterial Stiffness
Aortic stiffness assessed by aPWV is an independent predictor of adverse cardiovascular events (3, 37). Our present findings demonstrate that LDLr−/− mice, which have increased circulating cholesterol and triglyceride concentrations compared with WT mice, demonstrate arterial stiffness, which, in turn, may increase cardiovascular risk. As such, adults with hypercholesterolemia have greater aortic stiffening compared with individuals with optimal cholesterol levels (27, 29, 39) and are at a greater risk of an adverse cardiovascular event (21). Notably, despite the increased aortic stiffness in LDLr−/− mice, these animals did not have elevated blood pressure. This absence of hypertension with increased total cholesterol has also been observed in adults (27, 29) and animals (41). These findings support the notion that cholesterol and triglyercerides, independent of changes in blood pressure, contribute to aortic stiffness. Thus, the development of novel therapeutic targets to attenuate arterial stiffening may, in turn, reduce cardiovascular risk.
Stiffening of the aorta is associated with quantitative changes in arterial extracellular matrix protein content (15, 44). In the present study using LDLr−/− mice, we demonstrate a similar change in arterial stiffening and extracellular matrix protein expression as observed in other animal models with increased cholesterol levels (1, 9, 40). Specifically, we observed increased collagen type I expression, which is an important collagen isoform contributing to load bearing and arterial stiffening (15). Additionally, AGE abundance was greater in aortas from LDLr−/− mice, which may promote arterial stiffness through cross-linking of extracellular proteins or signaling via the receptor of AGEs to increase inflammation and/or oxidative stress. Both inflammation and oxidative stress have been implicated in the development of arterial stiffening, and AGE signaling may contribute to these processes (7, 16, 17, 31). Finally, elastin content was not significantly changed in whole aortic samples from LDLr−/− mice. Collectively, our data suggest increases in collagen deposition and AGE abundance in whole artery lysates promote arterial stiffening in LDLr−/− mice.
Aortic PVAT
Our data provide the initial evidence for PVAT to promote arterial stiffness in LDLr−/− mice and are consistent with previous work demonstrating that PVAT contributes to age-related arterial stiffening (16). We demonstrate an effect of PVAT from LDLr−/− mice to contribute to arterial stiffness, where removal of this fat reduces mechanical stiffness. Importantly, our data show an effect of PVAT conditioned media from LDLr−/− mice to increase intrinsic mechanical stiffness of aortic segments from WT mice, indicating that secreted factors from PVAT promote the arterial stiffening. Thus, PVAT may be a novel target tissue to reverse and/or prevent arterial stiffness in clinical conditions of hypercholesterolemia and hypertriglyceridemia.
Clinical investigations in humans have shown greater amounts of PVAT are associated with aortic stiffness and increased CVD risk (4–6). Importantly, after adjusting for body mass index, adults at high risk for CVD events have a greater abundance of PVAT (4), suggesting that adipose surrounding the aorta may influence aortic stiffness independent of general adiposity status. The findings in our study support this notion as arterial stiffness was increased in LDLr−/− mice, which have a similar whole body lean and fat mass as WT mice. Interestingly, adipocyte diameter and area were increased in PVAT from LDLr−/− mice, but not in subcutaneous or epididymal fat depots. Our data collectively indicate that PVAT compared with other fat depots is selectively modified and directly contributes to arterial stiffening in LDLr−/− mice independent of total body fat mass.
Aortic stiffness was associated with structural remodeling in LDLr−/− mice, and we provide causal evidence for PVAT to influence arterial collagen, AGE, and elastin protein content. These findings are in agreement with our previous report (16) demonstrating an effect of PVAT to promote arterial collagen type I expression. The present study, however, extends previous findings observed with aging to the LDLr−/− mouse model and demonstrates an effect of PVAT to influence collagen type I, AGE, and elastin content in the aorta. More specifically, our data show that PVAT from LDLr−/− animals promote collagen type I expression in the medial and adventitial layers, whereas PVAT only contributes to AGE abundance in the adventitia of aortic segments from LDLr−/− mice. PVAT decreased elastin in the medial but not adventitial layers in aortas cultured from LDLr−/− mice. However, removal of PVAT increased elastin in both the medial and adventitial layers of the artery, indicating that PVAT from LDLr−/− mice modulates aortic elastin expression. Interestingly, our immunohistochemistry data indicate selective reduction in medial elastin that does not result in whole aorta changes in elastin content, as observed with our Western blots. These data collectively suggest that PVAT influences arterial protein expression in a cell-specific manner.
The finding for increased IL-6 secretion from PVAT is consistent with previous observations for greater inflammatory cytokine secretion and expression, including IL-6, from PVAT of old and high-fat diet-fed mice (2, 11, 16). Importantly, these findings were extended to provide direct evidence for PVAT-derived IL-6 to increase aortic stiffening of arteries from LDLr−/− mice. We also demonstrated an effect of IL-6, at a concentration similar to what was secreted by PVAT, to promote arterial stiffening of arteries from WT mice. IL-6 has been shown to increase collagen production in both the heart and arteries (25, 43), which suggests that IL-6 contributes to the enhanced aortic collagen expression observed in LDLr−/− mice. Although inflammation, including IL-6, has been implicated in the formation of AGEs (32), there is no direct evidence for IL-6 to increase AGE expression, and this requires further investigation. Thus, these findings demonstrate that PVAT-derived IL-6 may be an important signaling molecule to promote arterial stiffness via increases in aortic collagen type I and AGE expression in individuals with elevated cholesterol and triglyceride concentrations.
We hypothesized that increased plasma cholesterol concentrations would result in PVAT-related IL-6 secretion and arterial stiffening in LDLr−/− mice. The LDLr−/− mice in this study, however, also had increased plasma triglyceride concentrations. A previous investigation (12) has shown that a high-fat diet administered to rabbits increases circulating cholesterol and triglycerides and also induces IL-6 gene and protein expression as well as macrophage accumulation in PVAT. These data suggest that macrophage recruitment to PVAT may be a mechanism by which IL-6 secretion is increased in LDLr−/− mice, but this requires further investigation. In addition, apolipoprotein E knockout mice, which also demonstrate increased plasma cholesterol concentrations, have greater TNF-α gene expression in PVAT (35), suggesting that hypercholesterolemia induces TNF-α, which, in turn, promotes IL-6 production (36) and possibly secretion from PVAT. However, the mechanisms by which plasma cholesterol and triglycerides promote PVAT-derived IL-6 secretion are largely unknown.
Our experiments were focused on PVAT influencing the extracellular matrix and aortic intrinsic mechanical properties in LDLr−/− mice. It should be noted that plasma cholesterol and triglycerides influence the endothelium and vascular smooth muscle cells, which also contribute to arterial stiffness. As such, a recent investigation (20) has demonstrated an effect of vascular smooth muscle contributing to nearly 50% of age-related arterial stiffness. Although our findings provide initial evidence for PVAT in the development of mechanical stiffness in LDLr−/− mice, it is important to recognize that PVAT may influence the endothelium and vascular smooth muscle cells to promote arterial stiffening unders conditions of hypercholesterolemia and hypertriglyceridemia.
We used an ex vivo approach to determine the effects of PVAT on mechanical stiffness and extracellular matrix protein expression. A potential limitation of this approach is that once the aorta is removed from the body and placed in a culture dish, the artery begins to remodel independent of any additional external stimulus. This is true for all ex vivo experiments assessing artery function. We believe, however, our data from this ex vivo approach do provide important insights for the influence of PVAT in the pathogenesis of arterial stiffening in LDLr−/− mice. Importantly, previous findings have shown our ex vivo approach to assess PVAT-mediated arterial stiffness, as used in this study, to be supported by in vivo fat transplant approaches (16). Future investigations will need to utilize in vivo approaches to further establish PVAT as a mechanism contributing to arterial stiffness in the context of hypercholesterolemia and hypertriglyceridemia.
Conclusions
The results from this investigation provide evidence for LDLr−/− mice with increased circulating cholesterol and triglyceride concentration to have greater aortic stiffening and structural remodeling. Additionally, the present findings provide direct evidence for PVAT from LDLr−/− mice to promote intrinsic mechanical stiffening and aortic protein remodeling, which is associated with greater IL-6 secretion that, in turn, induces arterial stiffness. In summary, PVAT contributes to arterial stiffness in mice with increased circulating cholesterol and triglyceride concentrations and is a potentially novel therapeutic target that warrants further investigation.
GRANTS
Research reported in this publication was supported, in part, by National Institute of General Medical Sciences Institutional Development Award 8-P20-GM-103527-05 and by a University of Kentucky Summer Faculty Research Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.D., J.S.E., and B.S.F. conception and design of research; B.D., A.O., and B.S.F. performed experiments; B.D., A.O., and B.S.F. analyzed data; B.D., A.O., J.S.E., and B.S.F. interpreted results of experiments; B.D., A.O., J.S.E., and B.S.F. prepared figures; B.D., A.O., J.S.E., and B.S.F. drafted manuscript; B.D., A.O., J.S.E., and B.S.F. edited and revised manuscript; B.D., A.O., J.S.E., and B.S.F. approved final version of manuscript.
REFERENCES
- 1.Agianniotis A, Stergiopulos N. Wall properties of the apolipoprotein E-deficient mouse aorta. Atherosclerosis 223: 314–320, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci 68: 780–792, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkley TE, Leng X, Chughtai HL, Nicklas BJ, Kritchevsky SB, Ding J, Kitzman DW, Hundley WG. Periaortic fat and cardiovascular risk: a comparison of high-risk older adults and age-matched healthy controls. Int J Obes (Lond) 38: 1397–1402, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton KA, Pedley A, Massaro JM, Corsini EM, Murabito JM, Hoffmann U, Fox CS. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the Framingham Heart Study. J Am Heart Assoc 1: e004200, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britton KA, Wang N, Palmisano J, Corsini E, Schlett CL, Hoffmann U, Larson MG, Vasan RS, Vita JA, Mitchell GF, Benjamin EJ, Hamburg NM, Fox CS. Thoracic periaortic and visceral adipose tissue and their cross-sectional associations with measures of vascular function. Obesity (Silver Spring) 21: 1496–1503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci USA 109: 15888–15893, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter LG, Lewis KN, Wilkerson DC, Tobia CM, Ngo Tenlep SY, Shridas P, Garcia-Cazarin ML, Wolff G, Andrade FH, Charnigo RJ, Esser KA, Egan JM, de Cabo R, Pearson KJ. Perinatal exercise improves glucose homeostasis in adult offspring. Am J Physiol Endocrinol Metab 303: E1061–E1068, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S, Bedja D, Mishra S, Amuzie C, Avolio A, Kass DA, Berkowitz D, Renehan M. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E−/− mice and rabbits fed a high-fat and -cholesterol diet. Circulation 129: 2403–2413, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes influence of high-fat feeding. Circ Res 104: 541–549, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JY, Tsai PJ, Tai HC, Tsai RL, Chang YT, Wang MC, Chiou YW, Yeh ML, Tang MJ, Lam CF, Shiesh SC, Li YH, Tsai WC, Chou CH, Lin LJ, Wu HL, Tsai YS. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol 33: 839–846, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Wang X, Mai J, Zhao X, Liang Y, Gu M, Chen Z, Nie R, Wang J. C-reactive protein promotes vascular endothelial dysfunction partly via activating adipose tissue inflammation in hyperlipidemic rabbits. Int J Cardiol 168: 2397–2403, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Cheng HM, Ye ZX, Chiou KR, Lin SJ, Charng MJ. Vascular stiffness in familial hypercholesterolaemia is associated with C-reactive protein and cholesterol burden. Eur J Clin Invest 37: 197–206, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Daugherty A, Rateri D, Hong L, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp 15: 1291, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diez J. Arterial stiffness and extracellular matrix. Adv Cardiol 44: 76–95, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide-signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 13: 576–578, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: Role of normalization of advanced glycation end-products. Exp Gerontol 47: 588–594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Curcumin amerliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48: 269–276, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao YZ, Saphirstein RJ, Yamin R, Suki B, Morgan KG. Aging impairs smooth muscle-mediated regulation of aortic stiffness: a defect in shock absorption function? Am J Physiol Heart Circ Physiol 307: H1252–H1261, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129: e28–e292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphrey JD (editor). Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. New York: Springer, 2002. [Google Scholar]
- 23.Kanaki AI, Sarafidis PA, Georgianos PI, Kanavos K, Tziolas IM, Zebekakis PE, Lasaridis AN. Effects of low-dose atorvastatin on arterial stiffness and central aortic pressure augmentation in patients with hypertension and hypercholesterolemia. Am J Hypertens 26: 608–616, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Maenhaut N, Van de Voorde J. Regulation of vascular tone by adipocytes. BMC Med 9: 25, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mir SA, Chatterjee A, Mitra A, Pathak K, Mahata SK, Sarkar S. Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. J Biol Chem 287: 2666–2677, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nosaka H, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol 28: 204–212, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Pirro M, Schillaci g Savarese G, Gemelli F, Mannarino M, Raffaele Siepi D, Bagaglia F, Mannarino E. Attenuation of inflammation with short-term dietary intervention is associated with a reduction of arterial stiffness in subjects with hypercholesterolaemia. Eur J Cardiovasc Prevent Rehab 11: 497–502, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Pirro M, Schillaci G, Savarese G, Gemelli F, Vaudo G, Siepi D, Bagaglia F, Mannarino E. Low-grade systemic inflammation impairs arterial stiffness in newly diagnosed hypercholesterolaemia. Eur J Clin Invest 34: 335–341, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 29: 1458–1464, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, Yin X, Rong J, Vita JA, Newton-Cheh C, Levy D, Keaney JF Jr, Vasan RS, Mitchell GF, Benjamin EJ. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension 51: 1651–1657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation–a mini-review. Gerontology 58: 227–237, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, Nyhan SM, Lussman C, Gupta G, White AR, Daher JP, Shoukas AA, Levine BD, Berkowitz DE. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol 47: 565–572, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tano JY, Schleifenbaum J, Gollasch M. Perivascular adipose tissue, potassium channels, and vascular dysfunction. Arterioscler Thromb Vasc Biol 34: 1827–1830, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Tian Z, Miyata K, Tazume H, Sakaguchi H, Kadomatsu T, Horio E, Takahashi O, Komohara Y, Araki K, Hirata Y, Tabata M, Takanashi S, Takeya M, Hao H, Shimabukuro M, Sata M, Kawasuji M, Oike Y. Perivascular adipose tissue-secreted angiopoietin-like protein 2 (Angptl2) accelerates neointimal hyperplasia after endovascular injury. J Mol Cell Cardiol 57: 1–12, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Vanden Berghe W, Vermeulen L, De Wilde G, De Bosscher K, Boone E, Haegeman G. Signal transduction by tumor necrosis factor and gene regulation of the inflammatory cytokine interleukin-6. Biochem Pharmacol 60: 1185–1195, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol 289: H1209–H1217, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Ye P, Luo L, Xiao W, Qi L, Bian S, Wu H, Sheng L, Xiao T, Xu R. Association of serum lipids with arterial stiffness in a population-based study in Beijing. Eur J Clin Invest 41: 929–936, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Wang YX, Halks-Miller M, Vergona R, Sullivan ME, Fitch R, Mallari C, Martin-McNulty B, da Cunha V, Freay A, Rubanyi GM, Kauser K. Increased aortic stiffness assessed by pulse wave velocity in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol 278: H428–H434, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation 103: 448–454, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson IB, Prasad K, Hall IR, Thomas A, MacCallum H, Webb DJ, Frenneaux MP, Cockcroft JR. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol 39: 1005–1011, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Yu H, Clarke MC, Figg N, Littlewood TD, Bennett MR. Smooth muscle cell apoptosis promotes vessel remodeling and repair via activation of cell migration, proliferation, and collagen synthesis. Arterioscler Thromb Vasc Biol 31: 2402–2409, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: 932–943, 2005. [DOI] [PubMed] [Google Scholar]


