Abstract
High-fructose feeding impairs copper status and leads to low copper availability, which is a novel mechanism in obesity-related fatty liver. Copper deficiency-associated hepatic iron overload likely plays an important role in fructose-induced liver injury. Excess iron in the liver is distributed throughout hepatocytes and Kupffer cells (KCs). The aim of this study was to examine the role of KCs in the pathogenesis of nonalcoholic fatty liver disease induced by a marginal-copper high-fructose diet (CuMF). Male weanling Sprague-Dawley rats were fed either a copper-adequate or a marginally copper-deficient diet for 4 wk. Deionized water or deionized water containing 30% fructose (wt/vol) was also given ad libitum. KCs were depleted by intravenous administration of gadolinium chloride (GdCl3) before and/or in the middle of the experimental period. Hepatic triglyceride accumulation was completely eliminated with KC depletion in CuMF consumption rats, which was associated with the normalization of elevated plasma monocyte chemoattractant protein-1 (MCP-1) and increased hepatic sterol regulatory element binding protein-1 expression. However, hepatic copper and iron content were not significantly affected by KC depletion. In addition, KC depletion reduced body weight and epididymal fat weight as well as adipocyte size. Plasma endotoxin and gut permeability were markedly increased in CuMF rats. Moreover, MCP-1 was robustly increased in the culture medium when isolated KCs from CuMF rats were treated with LPS. Our data suggest that KCs play a critical role in the development of hepatic steatosis induced by marginal-copper high-fructose diet.
Keywords: fructose, copper, iron, Kupffer cell, adipose tissue
nonalcoholic fatty liver disease (NAFLD) is now the most common liver disease in the United States. As of 2011, the prevalence of NAFLD and nonalcoholic steatohepatitis (NASH) was reported to be 46 and 12.2%, respectively, in middle-aged adults in the United States (47). The rapid rise in NAFLD suggests a possible role for environmental factors in the pathogenesis of this disease.
Inadequate copper intake (23, 26, 27) and increased fructose consumption represent two important nutritional problems in the United States (45). Recent studies suggest that high fructose intake may be an important risk factor for the development of NAFLD (3, 33). Decreased copper availability has also been observed in NAFLD patients, and a copper-deficient diet induces fatty liver in rodents (1, 2). Moreover, dietary fructose-copper interactions worsen copper status and are associated with the metabolic syndrome (13–15). Our recent research clearly showed that not only high dietary fructose but also modest fructose consumption worsened copper status in marginally copper-deficient rats and caused liver injury and fat accumulation (39, 40). One potential underlying mechanism for the hepatic abnormalities caused by low-copper/high-fructose diets is hepatic iron overload (7, 17, 39, 40). The release of iron from macrophages and hepatocytes is a copper-dependent process that involves ceruloplasmin, a copper-dependent ferroxidase (9). In fact, hepatic iron accumulation is observed in up to 34.5% of NAFLD patients (31). The term “insulin resistance-associated hepatic iron overload” (IRHIO) syndrome was coined a decade ago to describe the association between insulin resistance, NASH, and iron overload (29).
Maintenance of systemic iron homeostasis involves the balance between iron absorption, storage, and recycling. Most of the iron in the body is in the hemoglobin of erythrocytes. Senescent erythrocytes are phagocytosed by macrophages and recycled at a rate of 20–25 mg iron per day (20). Hepcidin is the master regulator of iron homeostasis, and it is secreted mainly by hepatocytes in response to iron stores, inflammation, and hypoxia (34). It regulates cellular iron release by acting on the iron exporter ferroportin-1 (12, 32). In addition, efficient iron release from hepatocytes/macrophages and enterocytes also requires ceruloplasmin and hephaestin, respectively, which are multicopper oxidases that oxidize Fe(II) to Fe(III), the form of iron that binds to Apo-transferrin (9). Therefore, copper deficiency may lead to iron accumulation in the liver (22). Excess iron in the liver secondary to copper deficiency is distributed throughout hepatocytes and Kupffer cells (KCs). Human research suggests that the cellular pattern of hepatic iron deposition is correlated with the histological severity in NAFLD patients (31).
KCs are the resident macrophages of liver, and they play critical roles in host defense and iron recycling. However, the exact role of KCs in the pathogenesis of NAFLD is unclear. Our previous studies clearly documented increased hepatic iron deposition in the marginal-copper high-fructose diet (CuMF) rat model (39, 40). However, the iron deposition pattern and the role of iron deposition in different cell types have not been defined in an animal model of NAFLD. The present study was conducted to examine the role of KCs in the development of NAFLD and the role of cell type-specific iron deposition in disease progression.
MATERIALS AND METHODS
Animal experiments.
Male weanling Sprague-Dawley rats (35–45 g) from the Harlan Laboratories (Indianapolis, IN) were fed (ad libitum) a purified AIN-76 diet with a defined copper content. The copper-adequate rats received 6 mg/kg copper, and the marginal-copper rats received 1.6 mg/kg of copper for 4 wk to achieve marginal copper deficiency. The animals were housed in stainless steel cages in a temperature- and humidity-controlled room with a 12:12-h light-dark cycle. Animals had free access to either deionized water or deionized water containing 30% fructose (wt/vol). Fructose-enriched drinking water was changed twice a week. KCs were depleted by tail vein injection of GdCl3 (10 mg/kg body wt) twice a week before (Pre-GdCl3) or after 2 wk (Post-GdCl3) of CuMF diet (35). In the Pre-GdCl3 group, animals received GdCl3 or saline solution injection three times prior to the beginning of experimental diet (CuMF) at 72, 48, and 24 h, then followed by twice-weekly injections until the end of the experiment (total 4 wk). In the Post-GdCl3 group, animals received GdCl3 injection twice weekly starting from 2-wk exposure to CuMF diet until the end of the experiment (total 2 wk). After fasting overnight, all the animals were killed under anesthesia with pentobarbital (50 mg/kg ip injection). Blood was collected from the inferior vena cava, and citrated plasma was stored at −80°C for further analysis. Portions of liver tissue were fixed with 10% formalin for subsequent sectioning, while others were snap-frozen with liquid nitrogen. All studies were approved by the University of Louisville Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care.
Hepatocyte and KC isolation and culture.
Primary hepatocytes were isolated from experimental rats according to a modified two-step collagenase-perfusion method (38). KCs were isolated by Percoll gradient centrifugation as described previously (38). The purity of KCs was 80%. KCs were suspended with RPMI 1640 containing 5 U/ml penicillin and 50 μg/ml streptomycin, supplemented with 10% FBS at 37°C in a humidified O2-CO2 (19:1) atmosphere, and seeded into 24-well plate at a density of 1 × 106/well.
Assessment of copper and iron status.
Plasma ceruloplasmin was measured on the basis of its oxidase activity (37). Plasma copper and liver copper were measured by Varian SpectrAA 880/GTA-100 graphite furnace atomic absorption spectrometer (AAS) (Worcester Polytechnic Institute, Worcester, MA). Liver iron concentration was measured by iCE3000 series flame AAS (Thermo Fisher Scientific, Waltham, MA).
Hepatic triglyceride assay.
Liver tissues were homogenized in ice-cold phosphate-buffered saline. Hepatic total lipids were extracted with chloroform/methanol (2:1) according to the method described by Bligh and Dyer (6). The lipid in chloroform was dried and redissolved in 2% Triton X-100 in water. Hepatic triglyceride content was determined by commercially available kits (Infinity, Thermo Electron, Melbourne, Australia).
Liver enzyme and plasma biochemical assays.
Liver enzyme and the plasma biochemical assays were performed with commercially available kits: aspartate aminotransferase (AST), glucose, cholesterol, triglyceride (Infinity, Thermo Electron, Melbourne, Australia), nonesterified fatty acids (NEFA) (Wako Chemicals, Richmond, VA), insulin (Lino Research, St. Charles, MO), hepcidin (Uscn Life Science, Houston, TX), and monocyte chemoattractant protein-1 (MCP-1) (Invitrogen, Camarillo, CA).
Determination of gut permeability and plasma endotoxin.
For ex vivo detection of intestinal permeability, a freshly isolated 10-cm section of ileum was rinsed with modified Krebs-Henseleit bicarbonate buffer (KHBB, pH 7.4). Then 100 μl FITC-dextran (molecular weight 4,000, FD-4, 40 mg/ml) was injected into the lumen before the gut was ligated to form a sac, as described previously (48). The gut sac was then placed in KHBB and incubated at 37°C for 20 min. The FD-4 that penetrated from the lumen into the incubation buffer was measured spectrofluorometrically with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. The FD-4 permeability was expressed as micrograms per centimeter per minute. Plasma samples were diluted twofold in pyrogen-free water and heated at 75°C for 10 min and cooled down to room temperature (48). Endotoxin was measured by using the limulus amoebocyte lysate kit (Lonza, Walkersville, MD) according to the manufacturer's instructions.
Histology and immunohistochemistry.
Formalin-fixed, paraffin-embedded liver and adipose tissue sections were cut at 5-μm thickness by a routine procedure. Sections were stained with hematoxylin and eosin (H&E). Adipocyte size was measured by use of Image J software (http://rsb.info.nih.gov/ij/). For immunohistochemical analysis, sections were incubated with anti-CD68 antibody (AbD Serotec, Raleigh, NC) overnight at 4°C. Staining was visualized with the horseradish peroxidase-conjugated DAKO staining system (DAKO InVision, Carpenteria, CA).
Isolation of RNA and real-time RT-PCR.
Total RNA was extracted from liver and adipose tissues by using TRIzol (Invitrogen, Carlsbad, CA). Primers were designed by use of Primer-BLAST except acetyl-CoA carboxylase-β (Acacb, PPR49745F) (SABiosciences, Valencia, CA) (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome) (Table 1). Real-time PCR was performed with an ABI Prism 7500 sequence detection system and SYBR Green master mix (Life Technologies, Grand Island, NY). The relative gene expression was analyzed by the 2−ΔΔCt method.
Table 1.
Primers used for real-time PCR analysis
| Gene | Sequence(5′-3′) | Refseq No. |
|---|---|---|
| Emr1 | Forward CATCCAGCAGATGGGAATTG | NM_001007557.1 |
| Reverse CTCCCAAGGGTGTTGGTACA | ||
| CD68 | Forward ACCTTTGGATTCAAACAGGAC | NM_001031638.1 |
| Reverse GCTGAGAATGTCCACTGTGCT | ||
| CD163 | Forward GGCATGCAATGGAAATGAGT | NM_001107887.1 |
| Reverse TCAGATCCGCTCCGTCTAAG | ||
| TNF-α | Forward ACTGAACTTCGGGGTGATCG | NM_012675.3 |
| Reverse TTTGCTACGACGTGGGCTAC | ||
| Il1b | Forward AGCAGCTTTCGACAGTGAGG | NM_031512.2 |
| Reverse TCATCTGGACAGCCCAAGTC | ||
| Ccl2 | Forward GTCTCAGCCAGATGCAGTT | NM_031530.1 |
| Reverse CTCTTGAGCTTGGTGACAAAT | ||
| Tlr4 | Forward CTGAGCTTCAACCCCCTGA | NM_019178.1 |
| Reverse TGCCATGCCTTGTCTTCAAT | ||
| Il6 | Forward AAGCCAGAGTCATTCAGAGC | NM_012589.2 |
| Reverse AGCCACTCCTTCTGTGACTC | ||
| Nfkb | Forward GCCTGTAACGGTGTTCCTG | NM_001008349.1 |
| Reverse TGTACTTCCTCCTTGTCTTCCA | ||
| Scd1 | Forward CCAAGAGATCTCCAGTTCCTACA | NM_139192.2 |
| Reverse GGACGGATGTCTTCTTCCAG | ||
| Fasn | Forward AGAAGCCCAGGAACAACTCA | NM_017332.1 |
| Reverse ACACAGGGACCGAGTAATGC | ||
| Srebf1 | Forward GAGCTACCCTTCGGTGAGG | NM_001276707.1 |
| Reverse GCTGAAGCATGTCTTCGATGT | ||
| Cpt1a | Forward AGACCGTGAGGAACTCAAACCCAT | NM_031559.2 |
| Reverse CACAACAATGTGCCTGCTGTCCTT | ||
| Acadl | Forward AGACCGTGAGGAACTCAAACCCAT | NM_012819.1 |
| Reverse CACAACAATGTGCCTGCTGTCCTT | ||
| Acadvl | Forward CTACAAGGCTGCATGGACAA | NM_012891.2 |
| Reverse ACTCAGACCACTGCCAATCC | ||
| Acadm | Forward CAATGGGGGCTTTTGATAGA | NM_016986.2 |
| Reverse CTCCTTGGTGCTCCACTAGC | ||
| Hmgcs2 | Forward CCGTCTAAGGAGGCCAATCC | NM_173094.2 |
| Reverse GATCCTATGGGGTCGCTGTG | ||
| Cpy4a1 | Forward AGAGGTGTTTGACCCTTCCAGGTT | NM_175837.1 |
| Reverse TTGTTTCCCAATGCAGTTCCTCGC | ||
| Mttp | Forward CCACCGAAGTGTTTCTCGAT | NM_001107727.1 |
| Reverse AGCCGTTATCGTGACTTGGA | ||
| Apob | Forward TCCTGCTTCTGTTCTTGGACACCA | NM_019287.2 |
| Reverse ACGTACTTCCGGAGGTGCTTGAAT | ||
| Actb | Forward GCGCAAGTACTCTGTGTGGA | NM_031144.3 |
| Reverse ACATCTGCTGGAAGGTGGAC |
Western blot.
Equal amounts of protein extracted from liver homogenate were loaded and resolved on 4–15% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membrane was blocked and probed with primary antibody for sterol regulatory element binding protein-1 (SREBP-1; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C and incubated with the corresponding horseradish peroxidase-conjugated secondary antibody. Protein signals were visualized by using the enhanced chemiluminescence system (Amersham Biosciences, Little Chalfont, UK). Band intensities were quantified by use of Image J software (http://rsb.info.nih.gov/ij/).
Statistical analysis.
All data were expressed as means ± SD and analyzed by one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple-comparison test and Student's t-test. The interactions between copper and fructose were examined by two-way ANOVA. Differences at P ≤ 0.05 were considered to be statistically significant.
RESULTS
Dietary marginal copper deficiency and high-fructose feeding induces liver injury and fat accumulation.
As expected, rats fed on the CuMF diet developed obvious liver injury and fat accumulation at 4 wk, as shown by elevated plasma AST (Fig. 1A), increased hepatic triglyceride level (Fig. 1B), and histology (H&E staining) (Fig. 1C). These findings are consistent with our previous data and further confirmed our previous results (39).
Fig. 1.
Effect of marginal copper-deficient diet+30% fructose drinking (CuMF) feeding on liver injury and fat accumulation. A: plasma aspartate aminotransferase (AST). B: hepatic triglyceride. C: representative photomicrographs of the hematoxylin and eosin (H&E) staining of liver section (×200). Data represent means ± SD (n = 5–8). * vs. adequate copper diet (CuA); # vs. marginal copper-deficient diet (CuM); $ vs. adequate copper diet+30% fructose drinking (CuAF) (P ≤ 0.05). †, interaction between copper and fructose is significant (P ≤ 0.05, 2-way ANOVA).
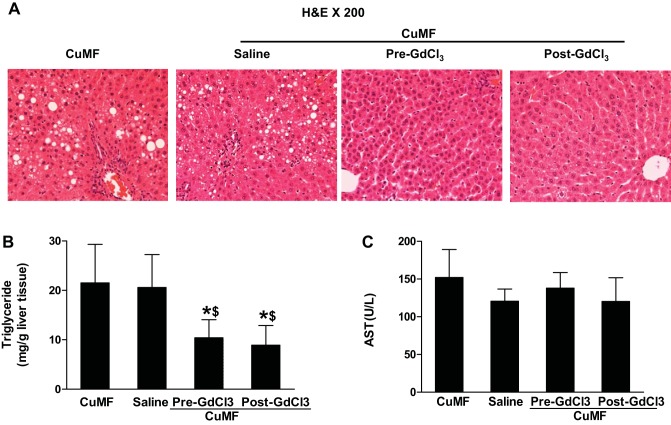
KC depletion completely eliminates hepatic fat accumulation, but not liver injury, in CuMF rats.
CuMF-induced fat accumulation was characterized mainly by macrovesicular steatosis and was located around the portal areas as shown by H&E. The increased hepatic fat accumulation in CuMF rats was not apparent with KC depletion (Fig. 2A). Consistent with histological findings, the hepatic triglyceride level was significantly increased in CuMF rats compared with controls. KC depletion, either before or in the middle of the CuMF protocol, completely prevented or resolved fat accumulation in the liver (Fig. 2B). Liver injury was assessed by plasma levels of liver enzymes [alanine aminotransferase (ALT) and AST]. Although ALT level was not significantly changed in CuMF rats compared with controls (data not shown), AST level was significantly increased. Moreover, this increase was not attenuated by KC depletion (Fig. 2C).
Fig. 2.
Effect of Kupffer cell (KC) depletion on the CuMF-induced hepatic fat accumulation and liver injury. A: representative photomicrographs of the H&E staining of liver section (×200). B: hepatic triglyceride. C: plasma AST. Data represent means ± SD (n = 6–8). * vs. CuMF; $ vs. CuMF+saline (P ≤ 0.05, 1-way ANOVA). GdCl3, gadolinium chloride. Pre-GdCl3, GdCl3 injection before CuMF; Post-GdCl3, GdCl3 injection after 2 wk of CuMF.
Hepatic expression of KC marker and proinflammatory genes with KC depletion.
The expression of hepatic KC marker genes Emr1 (F4/80), CD68, and CD163 was significantly decreased (by ∼50, 55, and 35%, respectively) with GdCl3 injection prior to fructose feeding (Pre-GdCl3). A similar effect was also observed at the later time point (Post-GdCl3), suggesting the successful depletion of KCs both before and after/during fructose feeding (Fig. 3A). It was further confirmed by markedly decreased hepatic CD68 expression with KC depletion, as shown by immunohistochemical staining (Fig. 3B). TNF-α and Il-1β mRNA expression were significantly downregulated with KC depletion in both Pre-GdCl3 and Post-GdCl3 groups. Toll-like receptor 4 (TLR4) mRNA was markedly downregulated in Post-GdCl3 group (Fig. 3C).
Fig. 3.
Effect of KC depletion on KC markers and proinflammatory gene expression in liver. A: liver Emr1 (F4/80), CD68, and CD163 mRNA expression. B: representative photomicrographs of immunohistochemical staining for rat liver CD68 (×100). C: proinflammatory gene expression. Data represent means ± SD (n = 6–8). * vs. CuMF+saline; $ vs. Pre-GdCl3 (P ≤ 0.05, 1-way ANOVA).
Effects of KC depletion on body weight, liver weight, epididymal fat weight, and plasma metabolic indexes.
After 4 wk exposure to dietary CuMF, rats in Pre-GdCl3 group showed significantly lower body weight and body weight gain compared with controls. Although body weight and weight gain were not significantly reduced in rats from the Post-GdCl3 group compared with controls, these variables were significantly higher in the Post-GdCl3 animals than in the Pre-GdCl3 group. Liver weight was significantly decreased with KC depletion in rats from Pre-GdCl3 group compared with control. However, liver-to-body weight ratio did not differ significantly. KC depletion also led to a significant decrease of epididymal fat weight as well as epididymal fat weight-to-body weight ratio compared with controls. Although the difference between groups in plasma indexes did not reach the statistical significance, there is a trend toward decreased plasma triglyceride, glucose, and homeostasis model assessment of insulin resistance (HOMA-IR) in the Pre-GdCl3 group compared with controls (Table 2).
Table 2.
Effects of KCs depletion on body weight, liver weight, epididymal fat weight, and plasma indexes
| CuMF |
||||
|---|---|---|---|---|
| Variable | Cu MF | Saline | PreGdCl3 | PostGdCl3 |
| BW, g | 242.3 ± 15.8 | 236.5 ± 22.6 | 198.7 ± 9.0*† | 225.3 ± 15.2‡ |
| BW gain, g | 190.0 ± 14.5 | 185.6 ± 21.1 | 149.5 ± 7.7*† | 175.4 ± 13.5‡ |
| Liver weight, g | 10.24 ± 1.31 | 9.58 ± 1.16 | 8.14 ± 0.71* | 9.36 ± 1.06 |
| Liver/BW ratio, % | 4.22 ± 0.35 | 4.06 ± 0.35 | 4.09 ± 0.26 | 4.15 ± 0.30 |
| Epididymal fat weight, g | 3.00 ± 0.51 | 2.53 ± 0.31 | 1.80 ± 0.31*† | 2.06 ± 0.57* |
| EFW/BW ratio, % | 1.23 ± 0.14 | 1.07 ± 0.10 | 0.91 ± 0.16* | 0.91 ± 0.21* |
| Plasma indexes | ||||
| Triglyceride, mg/dl | 43.2 ± 9.3 | 43.5 ± 15.1 | 32.7 ± 7.3 | 38.2 ± 13.4 |
| Cholesterol, mg/dl | 43.7 ± 7.4 | 41.6 ± 10.6 | 44.8 ± 11.9 | 59.9 ± 15.5 |
| NEFA, μM | 288.0 ± 63.3 | 341.7 ± 94.9 | 387.0 ± 86.6 | 294.2 ± 61.1 |
| Glucose, mg/dl | 110.0 ± 10.9 | 104.0 ± 24.4 | 87.7 ± 20.0 | 106.4 ± 11.2 |
| Insulin, ng/ml | 0.52 ± 0.13 | 0.48 ± 0.05 | 0.55 ± 0.17 | 0.51 ± 0.07 |
| HOMA-IR | 3.22 ± 0.51 | 2.98 ± 0.40 | 2.75 ± 0.49 | 3.27 ± 0.43 |
Data are expressed as means ± SD (n = 6–8).
vs. CuMF;
vs. CuMF+Saline;
vs. CuMF+PreGdCl3 (P < 0.05). BW, body weight; CuMF, marginal copper-deficient diet +30% fructose drinking; GdCl3, gadolinium chloride; PreGdCl3, GdCl3 start before the experimental diet; PostGdCl3, GdCl3 start from 2 wk of the experimental diet; EPW, epididymal fat weight; NEFA, nonesterified fatty acid; HOMA-IR, homeostasis model assessment of insulin resistance.
KC depletion reduces adipocyte size with altered adipose tissue proinflammatory profile in epididymal fat. As expected, intravenous injection of GdCl3 successfully depleted hepatic KCs, whereas adipose tissue macrophages were not significantly affected, as shown by the macrophage marker gene expression (Fig. 4A). Mean epididymal adipocyte size was significantly smaller in the two groups with KC depletion, with the more profound effect seen in the Pre-GdCl3 group (Fig. 4B). Although some of the proinflammatory cytokine gene expression was downregulated (IL-6) or had a trend toward being decreased (CCL2/MCP-1); however, it seems that the adipose tissue inflammation was not markedly improved with KC depletion, at least in the limited time period, from the profile of proinflammatory gene expression (Fig. 4C).
Fig. 4.
Effect of KC depletion on macrophage markers, adipocyte size, and proinflammatory gene expression in white adipose tissue (WAT). A: Emr1 (F4/80), CD68, and CD163 mRNA expression. B: representative photomicrographs of the H&E staining of epididymal fat section (×100) and measurement of adipocyte size. Adipocyte size (μm2) was measured and average cell size of >100 cells for each group was calculated. C: proinflammatory gene expression. Data represent means ± SD (n = 6–8). * vs. CuMF+saline; $ vs. Pre-GdCl3 (P < 0.05, 1-way ANOVA).
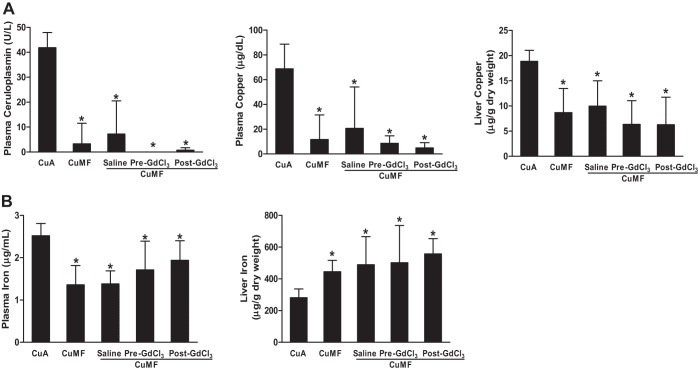
Effects of KC depletion on copper and iron status.
As expected, the plasma ceruloplasmin activity, as well as the plasma and liver copper levels, were significantly decreased in the CuMF rats compared with controls. KC depletion with GdCl3 slightly decreased the plasma ceruloplasmin activity, as well as the plasma and liver copper levels. However, the differences did not reach the statistical significance (Fig. 5A). Moreover, liver iron was significantly increased and plasma iron was significantly decreased in the CuMF rats, and they were not modified by KC depletion (Fig. 5B).
Fig. 5.
Effect of KC depletion on copper and iron status. A: plasma ceruloplasmin and copper and liver copper. B: plasma iron and liver iron. Data represent means ± SD (n = 6–8). * vs. CuA (P ≤ 0.05, 1-way ANOVA).
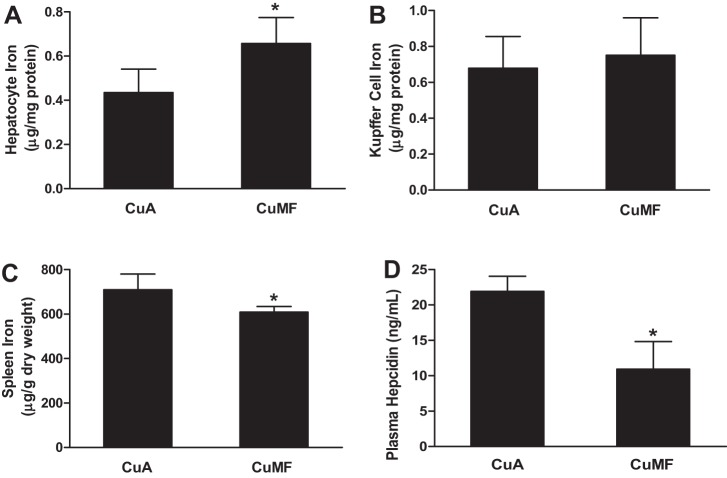
Increased hepatocyte iron and decreased spleen iron is associated with plasma hepcidin deficiency in CuMF rats.
KC depletion did not affect iron content in the whole liver tissue. To further determine the iron level in each cell type, we isolated hepatocytes and KCs from CuMF-treated rats. Hepatocyte iron was markedly increased (Fig. 6A), and spleen iron was significantly decreased in CuMF rats (Fig. 6C), suggesting iron redistribution from spleen to hepatocytes after exposure to CuMF diet. However, we did not detect a significant change in iron levels in KCs from CuMF rats compared with control, at least at this time point (Fig. 6B). Plasma hepcidin, an antimicrobial small peptide that regulates iron homeostasis, was significantly decreased in CuMF rats (Fig. 6D).
Fig. 6.
Iron content in hepatocytes, KCs, spleen, and plasma hepcidin. A: hepatocyte iron. B: KC iron. C: spleen iron. D: plasma hepcidin. Data represent means ± SD (n = 6–8). *P ≤ 0.05, Student's t-test.
Effect of KC depletion on hepatic expression of genes involved in lipid metabolism and SREBP-1 protein.
To further explore the mechanism(s) by which KC depletion prevented hepatic fat accumulation, hepatic expression of genes involved in fatty acid synthesis, fatty acid oxidation and very low-density lipoprotein (VLDL) secretion were determined by real-time RT-PCR. Among the multiple genes related to fatty acid oxidation, most genes regulate mitochondrial β-oxidation; only AcadVL and Cyp4a1 genes regulate peroxisomal and microsomal oxidation, respectively. Although most genes related to fatty acid oxidation were suppressed in CuMF rats as well as in CuMF rats with KC depletion, some (Hmgcs2 and Cyp4a1) were further downregulated with KC depletion (Fig. 7A). Srebp1 gene expression was not significantly changed in CuMF rats with or without KC depletion. Although fasn gene expression was not changed with diet, it was significantly upregulated by KC depletion in CuMF rats. Scd1 expression was inhibited in CuMF rats, and it was further downregulated by KC depletion (Fig. 7B). The expression of genes involved in VLDL secretion was also suppressed in CuMF rats as well as in CuMF rats with KC depletion. Moreover, ApoB expression was further downregulated with KC depletion (Fig. 7C). SREBP-1 is a critical transcriptional factor in hepatic lipogenesis. As shown in Fig. 7D, CuMF feeding led to an obvious increase of mature SREBP-1 compared with controls and this increase was blocked with KC depletion.
Fig. 7.
Effect of KC depletion on hepatic gene expression of lipid metabolism and sterol regulatory element binding protein 1 (SREBP-1) protein expression. A: fatty acid oxidation. B: fatty acid synthesis. C: VLDL secretion. Data represent means ± SD (n = 6–8). * vs. CuA; # vs. CuMF; $ vs. CuMF+saline (P ≤ 0.05). D: hepatic SREBP-1 expression was determined by Western blots. Optical density of the band was quantified by ImageJ software. The ratio to β-actin was calculated by assigning the value from adequate-copper diet controls as 1. Data represent means ± SD (n = 3). * vs. CuA; # vs. CuMF; $ vs. CuMF+saline (P ≤ 0.05, 1-way ANOVA).
Increased gut permeability in CuMF rats.
Gut permeability was evaluated by plasma endotoxin level and ex vivo measurement of ileum permeability to FD-4. As shown in Fig. 8A, plasma endotoxin was significantly increased in CuMF rats. Consistent with plasma endotoxin level, FD-4 permeability of ileum was also markedly increased in CuMF rats compared with control (Fig. 8B).
Fig. 8.

Plasma endotoxin and gut permeability. A: plasma endotoxin. Endotoxin was measured by the limulus amoebocyte lysate (LAL) kit. B: gut permeability. The penetration of intraluminal FD-4 to the incubation buffer was determined after incubation of ileum sac for 20 min. Data represent means ± SD (n = 5–7). *P ≤ 0.05, Student's t-test.
KC depletion prevents elevated plasma MCP-1 in CuMF rats.
Plasma MCP-1 level was significantly increased in CuMF rats. However, this increase was blocked by KC depletion, suggesting that KCs may directly or indirectly contribute to the increased plasma MCP-1 in CuMF rats (Fig. 9A).
Fig. 9.

Plasma MCP-1 and KC MCP-1 production in response to LPS and iron chelation. A: plasma MCP-1. Data represent means ± SD (n = 5–7). * vs. CuA; # vs. CuMF (P ≤ 0.05, 1-way ANOVA). B: MCP-1 in KC culture medium. KCs were seeded into a 24-well plate (as described in materials and methods) and pretreated with deferiprone (L1) or deferoxamine (DFO) (iron chelators) for 20 h, then treated with LPS 300 ng/ml for 24 h. (This experiment was repeated 3 times). MCP-1 in the culture medium and plasma was determined by ELISA. * vs. Control; # vs. LPS (P ≤ 0.05, 1-way ANOVA).
KC MCP-1 production in response to LPS and iron chelation.
To further determine KC function in response to LPS and the role of iron in this response, freshly isolated KCs from CuMF rats were treated with LPS in the presence/absence of an iron chelator. MCP-1 levels were predictably increased in response to LPS. Interestingly, this increase was significantly attenuated by pretreatment with an intracellular lysosomal iron chelator, suggesting that the MCP-1 signaling pathway was mediated, at least partially, by intracellular iron (Fig. 9B).
DISCUSSION
KCs are the resident macrophages in the liver, and they are a critical component of the innate immune response. In addition, KCs play a role in iron recycling. However, the role of KCs in the pathogenesis of NAFLD remains poorly understood. In the present study, we found that KC depletion completely blocked hepatic fat accumulation in CuMF rats but did not block the liver injury, suggesting that KCs play a pivotal role in hepatic lipid metabolism. Particularly, we found that KC depletion reduced body weight and epididymal fat weight as well as adipocyte size, predominantly in the Pre-GdCl3 group. Moreover, elevated plasma MCP-1 was inhibited with KC depletion in CuMF rats, suggesting that KCs may directly or indirectly contribute to the elevated levels of circulating MCP-1.
It is well documented that hepatic iron overload is a common feature associated with copper deficiency (17, 22). Excess iron in the liver was diffusely distributed throughout hepatocytes and KCs (22). To further dissect the differential role of iron deposition in KCs and hepatocytes in CuMF-induced liver injury and fat accumulation, we depleted KCs before or in the middle of feeding rats a CuMF diet. Interestingly, we found that although fat accumulation was completely prevented with KC depletion at both time points, hepatic copper and iron were not significantly affected by KC depletion. Next, we isolated KCs and hepatocytes and measured iron content. We did not detect significantly increased iron in the KCs of CuMF rats, at least at the time of euthanasia, compared with controls, probably owing to the limited time period of the experiment. Although the KC iron level was not increased in rats fed with CuMF diet for 4 wk, the plasma endotoxin level was significantly increased. In fact, previous studies have shown that high-fructose feeding may lead to bacterial overgrowth and increased gut permeability, with subsequent endotoxemia, which may contribute to KC activation and MCP-1 secretion (5, 41). To further demonstrate this, isolated KCs from CuMF rats were exposed to exogenous LPS in the presence or absence of an iron chelator. We found that MCP-1 was dramatically increased in the culture medium in response to LPS, and this increase was significantly attenuated by pretreatment with an iron chelator, suggesting that KCs may be one of the sources of elevated circulating MCP-1 and that LPS-induced MCP-1 secretion is, at least partially, iron dependent.
Although KC iron content was not significantly affected, hepatocyte iron was significantly increased, and spleen iron was markedly decreased in CuMF rats compared with controls. Moreover, plasma hepcidin, the master regulator of iron homeostasis, was significantly decreased in CuMF rats, suggesting that iron was redistributed under the condition of hepcidin deficiency (19, 21). Sex differences in the metabolic effects of fructose and/or copper deficiency have long been noted in the animal studies (16, 18) as well as in humans (4, 10), with male sex being sensitive to the deleterious effects of fructose and/or copper deficiency and female sex being protective. Hepatic iron overload is a likely mechanism underlying fructose/copper deficiency-induced metabolic syndrome. It is also well documented that the expression of hepcidin is suppressed by testosterone (28). Taken together, sex-related discrepancy in the metabolic effects of fructose might attribute to the dysregulated metabolism of copper and/or iron. Further studies on the potential molecular mechanisms are warranted.
A growing body of evidence has suggested that KCs may promote the development of diet-induced hepatic steatosis by suppression of hepatocyte fatty acid β-oxidation via secreting TNF-α and IL-1β (24, 42), or they may trigger NASH development via recruitment of monocytes/macrophages through MCP-1 (30, 44). In the present study, we found that KC depletion not only protects against the development of NAFLD but also reduces total body weight as well as white adipose tissue fat mass and weight. Moreover, KC depletion was associated with the normalization of plasma MCP-1 and hepatic SREBP-1 expression, both of which play important roles in obesity-related hepatic steatosis and insulin resistance. The role of MCP-1 in the onset of metabolic disorders is attributed to macrophage recruitment in either adipose tissue (25) or liver (30, 44). In agreement with this, our data showed that hepatic TNF-α and IL-1β, as well as TLR4, mRNA were significantly downregulated by KC depletion, which is consistent with previous findings (24, 42). In adipose tissue, IL-6 mRNA, but not TNF-α mRNA, was markedly downregulated. IL-6 is mainly produced from adipocytes, and adipose tissue macrophage is the major source of TNF-α (36, 43, 46). Both of these factors support the concept that the alteration of adipose tissue proinflammatory profile is likely due to the reduced adipocyte size. Inhibition of hepatic SREBP-1 expression by KC depletion suggests that hepatic lipogenesis may be regulated by KCs. However, how KC regulate SREBP-1 expression remains to be determined.
The fact that KC depletion leads to hepatic steatosis but not liver injury supports the concept that metabolic endotoxemia is the initiator of hepatic steatosis and obesity (“first hit”) (8, 11). However, low-copper status and hepatic iron overload were not improved by KC depletion, suggesting they might be the “second hit” leading to the liver injury and NASH progression (11).
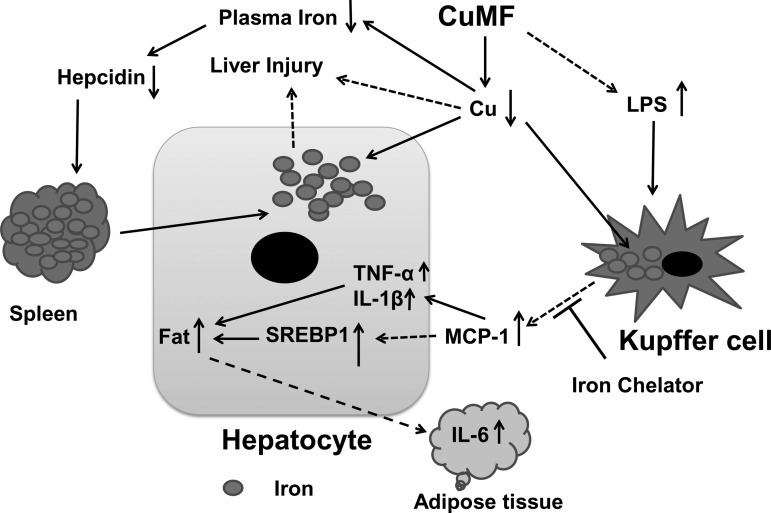
In summary, interaction between dietary fructose and marginal copper deficiency further impaired copper status and led to iron redistribution with increased deposition in hepatocytes and decreased levels in the spleen under the condition of hepcidin deficiency (21). KC depletion prevented hepatic steatosis with reduced white adipose tissue fat mass, suggesting a critical role of KCs in the onset of hepatic steatosis and adiposity induced by marginal-copper and high-fructose diet (Fig. 10). However, copper and iron status were not improved by KC depletion, suggesting the possible link between low copper, iron overload, and liver injury. Our data provided novel insights into the better understanding of NAFLD.
Fig. 10.
Schematic diagram of the critical role of KC in marginal-copper high-fructose diet-induced hepatic steatosis. Dashed arrows denote mechanisms that have not been fully defined.
GRANTS
This study was supported in part by National Institute on Alcohol Abuse and Alcoholism Grants RO1AA015970, PO1AA017103, R37AA010762, RO1AA018869, RC2 AA019385, P30AA019360 (C. J. McClain), RO1AA014623, RO1AA016013, RO1AA018844 (Z. Zhou); the Veterans Administration (C. J. McClain); National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-055030 (D. A. Schuschke) and RO1DK071765 (C. J. McClain); and the University of Louisville Clinical and Translational Pilot Program (C. J. McClain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.S., D.A.S., and C.J.M. conception and design of research; M.S., Z.Z., W.Z., J.Z., and Y.W. performed experiments; M.S., Z.Z., X.Z., and Y.W. analyzed data; M.S., Z.Z., and C.J.M. interpreted results of experiments; M.S. and X.Z. prepared figures; M.S. drafted manuscript; M.S., D.A.S., and C.J.M. edited and revised manuscript; M.S., D.A.S., and C.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Tomas Ganz for helpful discussion and Xingguo Sun, Sharon Gordon, Tom Burke, and Dr. David Barker for technical support. We thank Marion McClain for careful reading of this manuscript.
REFERENCES
- 1.Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A, Solioz M, Tilg H, Patsch W, Weiss G, Stickel F, Datz C. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am J Gastroenterol 105: 1978–1985, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, Scharinger L, Stickel F, Mourlane F, Weiss G, Datz C. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology 135: 680–688, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 22: 811–816, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr 72: 1128–1134, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 48: 983–992, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 7.Bureau I, Lewis CG, Fields M. Effect of hepatic iron on hypercholesterolemia and hypertriacylglycerolemia in copper-deficient fructose-fed rats. Nutrition 14: 366–371, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Collins JF, Prohaska JR, Knutson MD. Metabolic crossroads of iron and copper. Nutr Rev 68: 133–147, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couchepin C, Le KA, Bortolotti M, da Encarnacao JA, Oboni JB, Tran C, Schneiter P, Tappy L. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes Care 31: 1254–1256, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 114: 842–845, 1998. [DOI] [PubMed] [Google Scholar]
- 12.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol 9: 72–81, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Fields M, Ferretti RJ, Smith JC Jr, Reiser S. Effect of dietary carbohydrates and copper status on blood pressure of rats. Life Sci 34: 763–769, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Fields M, Ferretti RJ, Smith JC Jr, Reiser S. Impairment of glucose tolerance in copper-deficient rats: dependency on the type of dietary carbohydrate. J Nutr 114: 393–397, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Fields M, Ferretti RJ, Smith JC Jr, Reiser S. The interaction of type of dietary carbohydrates with copper deficiency. Am J Clin Nutr 39: 289–295, 1984. [DOI] [PubMed] [Google Scholar]
- 16.Fields M, Lewis C, Scholfield DJ, Powell AS, Rose AJ, Reiser S, Smith JC. Female rats are protected against the fructose induced mortality of copper deficiency. Proc Soc Exp Biol Med 183: 145–149, 1986. [DOI] [PubMed] [Google Scholar]
- 17.Fields M, Lewis CG. Hepatic iron overload may contribute to hypertriglyceridemia and hypercholesterolemia in copper-deficient rats. Metabolism 46: 377–381, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol 283: H2478–H2484, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Ganz T. Systemic iron homeostasis. Physiol Rev 93: 1721–1741, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med 62: 347–360, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med 2: a011668, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA 96: 10812–10817, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holden JM, Wolf WR, Mertz W. Zinc and copper in self-selected diets. J Am Diet Assoc 75: 23–28, 1979. [PubMed] [Google Scholar]
- 24.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 59: 347–357, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klevay LM. Is the Western diet adequate in copper? J Trace Elem Med Biol 25: 204–212, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Klevay LM. Searching for people low in copper. Metabolism 61: 1201–1204, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Latour C, Kautz L, Besson-Fournier C, Island ML, Canonne-Hergaux F, Loreal O, Ganz T, Coppin H, Roth MP. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology 59: 683–694, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, Le Gall JY, Brissot P, David V, Deugnier Y. Insulin resistance-associated hepatic iron overload. Gastroenterology 117: 1155–1163, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol 302: G1310–G1321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A, Kowdley KV; Nonalcoholic Steatohepatitis Clinical Research Network. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology 53: 448–457, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 48: 993–999, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, Burchardt ER, Rippe RA, Thurman RG. Attenuation of CCl4-induced hepatic fibrosis by GdCl3 treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol 281: G200–G207, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322: 1539–1543, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schosinsky KH, Lehmann HP, Beeler MF. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem 20: 1556–1563, 1974. [PubMed] [Google Scholar]
- 38.Smedsrod B, Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol 38: 213–230, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Song M, Schuschke DA, Zhou Z, Chen T, Pierce WM Jr, Wang R, Johnson WT, McClain CJ. High fructose feeding induces copper deficiency in Sprague-Dawley rats: a novel mechanism for obesity related fatty liver. J Hepatol 56: 433–440, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song M, Schuschke DA, Zhou Z, Chen T, Shi X, Zhang J, Zhang X, Pierce WM Jr, Johnson WT, Vos MB, McClain CJ. Modest fructose beverage intake causes liver injury and fat accumulation in marginal copper deficient rats. Obesity (Silver Spring, MD) 21: 1669–1675, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 50: 1094–1104, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Muller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology 51: 511–522, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772–783, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem 287: 40161–40172, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med 10: 160, 2008. [PMC free article] [PubMed] [Google Scholar]
- 46.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140: 124–131, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol 298: G625–G633, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]