Abstract
In recent years, it has become clear that the immune system contributes to the genesis of hypertension. Hypertensive stimuli, such as angiotensin II, DOCA-salt, and norepinephrine, cause T cells and monocytes/macrophages to accumulate in the kidney and vasculature. These cells release inflammatory cytokines, such as IL-6, interferon-γ, and IL-17, that promote renal and vascular dysfunction. These cytokines also promote angiotensinogen production in the proximal tubule and Na+ retention in the distal nephron and contribute to renal fibrosis and glomerular damage. For several years, we have observed accumulation of memory T cells in the kidney and vasculature. Given the propensity for memory cells to produce cytokines such as interferon-γ and IL-17, interventions to prevent the formation or renal accumulation of specific memory T cell subsets could prevent end-organ damage and blood pressure elevation in response to hypertensive stimuli.
Keywords: adaptive immunity, immunological memory, T cells, cytokines, CD70
hypertension affects more than one billion people worldwide and is an enormous healthcare burden in Western societies. It is a major risk factor for stroke, myocardial infarction, and heart failure (6, 8, 12). Perturbations of the central nervous system, vasculature, and kidney have all been implicated; however, the manner in which these interact remains poorly defined. During the past several years, research from our laboratory and others has shown that perturbations of the immune system play a major role in this disease (5). Hypertension is associated with the appearance of T cells in the kidney that release inflammatory cytokines, which promote Na+ retention and vascular dysfunction (16). Both ANG II and DOCA-salt challenge causes antigen-presenting cells, and in particular dendritic cells, to accumulate isoketal protein adducts. Recent studies have suggested that these oxidatively modified proteins serve as neoantigens and promote the release of cytokines, including IL-6, IL-1β, and IL-23 (7). These dendritic cells promote T cell proliferation and polarize T cells to an inflammatory phenotype. In keeping with this, mice lacking specific subsets of T cells are protected against the antidiuretic and antinatriuretic effects of ANG II (16).
The Concept of “Memory” in Hypertension
More than 50 years ago, Dickenson et al. (3) showed that normally subpressor doses of ANG II will ultimately elevate blood pressure when given for prolonged periods and termed this phenomenon “autopotentiation.” Recently, Xue et al. (17) showed in rats that the central actions of ANG II have “memory.” During an initial exposure to a low dose (10 ng·kg−1·min−1) of ANG II, Xue et al. (17) showed induction of mRNA of ANG II type 1 and type 2 receptors, mineralacorticoid receptor, and aldosterone synthase in the lamina terminalis, which then potentiated the hypertensive response, induced by a second high dose of ANG II (120 ng·kg−1·min−1) (17). This augmented hypertension during the second exposure could be blocked by central administration of an ANG II type 1 receptor antagonist during the first exposure.
We propose that in addition to this central nervous system memory response, there is also a component of immunological memory. We have consistently observed an increase of memory T cells in the blood, vasculature, and kidneys of hypertensive mice. The precise role of these, compared with newly activated CD4+ and CD8+ T cells, is poorly understood. In this minireview, a novel aspect of immunity in hypertension and its role in end-organ damage will be discussed.
Molecular Basis of the Adaptive Immune Memory Response
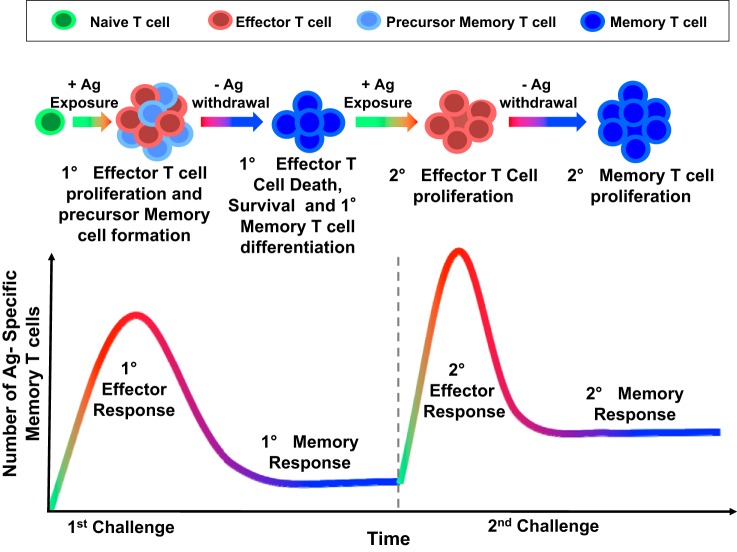
Immunological memory is a cardinal feature of adaptive immunity, which provides protection against an antigen that has been encountered previously and is the basis for vaccination against infection. The classical adaptive T cell immune response is characterized by an initial expansion of T cells upon antigen presentation (Fig. 1) (14). The majority of these effector T cells ultimately die; however, a few remaining cells become long-lived memory T cells. Some of these return to secondary lymphoid organs, such as lymph nodes and the spleen, and are referred to as central memory cells. Others remain in the periphery, particularly in the bone marrow, skin and mucosa, and are referred to as resident memory cells. Upon a second exposure to the antigen, both central memory and resident memory cells can be activated to become effector memory cells. This activation involves a rapid expansion of cell numbers, the production of cytokines, and a change in surface markers. In the mouse, naïve T cells are CD44lo/CD62Lhi/CCR7+. Central memory cells are CD44hi/CD62Lhi/CCR7+, and effector memory cells are CD44hi/CD62Llo/CCR7−. Resident memory cells are CD44hi/CD62L−/CD103+/CD69+ (11). Transcription factors, such as T-bet, eomesodermin, Blimp, Id3, and Bcl-6, all influence the effector and memory fate, as does regulation of the T cell metabolic state. This topic has recently been elegantly reviewed (2).
Fig. 1.
The dynamics of T cell immune responses. The classical adaptive T cell immune response is characterized by an initial expansion of naïve T cells upon antigen (Ag) presentation. The majority of activated effector T cells ultimately undergo apoptosis with a subset of antigen-specific memory T cells remaining. These memory cells are characterized by the presence of a T cell receptor that is capable of recognizing the original antigen and can respond rapidly and efficiently to a second antigenic stimulus.
There are several experimental interventions that have been used to disrupt the formation of memory T cells. As examples, mice lacking either IL-7 or IL-15 fail to maintain memory cells upon antigen rechallenge (13, 15). Likewise, the formation of CD8 and CD4 memory T cells also requires the interaction of CD27 on T cells with CD70 on activated antigen-presenting cells (10). This costimulatory interaction is similar to that of T cell CD28 with B-7 ligands, which is requried for naïve T cell activation. Mice lacking either CD27 or CD70 fail to develop memory T cells upon antigen rechallenge. Finally, CD8+ T cell memory is dependent on help from CD4+ T cells (1).
Insights From Memory Mouse Models
The concept of immunlogical memory might have important clinical implications in hypertension. Hypertensive stimuli are often intermittant and reoccuring. An example includes sleep apnea, which leads to repeated surges of sympathetic outflow. Repeated episodes of dietary indescretion might also lead to recurrant hypertensive challenges. Likewise, repeated episodes of emotional stress have been associated with hypertension (9). To study this in relevant animal models, it would be ideal to avoid repeated surgeries, for example, to implant osmotic minipumps. Mice genetically altered to develop hypertension in response to a dietary intervention that can be introduced, removed, and reintroduced would therefore be an excellent tool. We have engineered such a mouse, which expresses the renin-2 and angiotensinogen genes downstream of a promoter that can be induced by the additon of a hydrocarbon to the diet. Preliminary data indicate that this animal develops moderately severe hypertension upon dietary intervention. Another useful model is that similar to one used by Gonzalez-Villalobos et al. (4), which involves an initial challenge with the nitric oxide synthase inhibitor N-nitro-l-arginine methyl ester followed by a salt challenge. Another approach might involve adoptive transfer of memory T cells to naïve mice to determine if these worsen hypertension in response to generally subpressor stimuli. Another approach might involve adoptive transfer of dendritic cells from a hypertensive mouse to a naïve recipient. We have shown that this leads to marked hypertension in response to a generally subpressor dose of ANG II and that these cells preferentially activate memory cells from hypertensive mice (7). Finally, studies of mice lacking critical mediators such as CD70 and CD27 can prove useful.
Concluding Remarks
In summary, studies in mouse models of sustained hypertension will provide insights on the role and mechanism of action of immunological memory T cell formation. It is crucial to identify the tissue localization and longevity of antigen-specific memory T cells formed that could be major sources of potent inflammatory cytokines, such as IL-17A and interferon-γ. Accumulation of these cells over time in the kidney with repeated hypertensive challenges will promote renal damage. This is important because memory cells are very long lived and can sensitize individuals to repeated hypertensive stimuli, thus contributing to end-organ damage.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-HL-039006, P01-HL-058000, P01-HL-095070, P01-GM-015431, R01-HL-108701, and R01-HL-105294, VITA award HHSN268201400010C, and an American Heart Association Strategically Focused Research Network Award. H. A. Itani is the recipient of NIH Individual Postdoctoral Fellowship 1-F32-HL-124972-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.A.I. and D.G.H. conception and design of research; H.A.I. performed experiments; H.A.I. analyzed data; H.A.I. and D.G.H. interpreted results of experiments; H.A.I. and D.G.H. prepared figures; H.A.I. and D.G.H. drafted manuscript; H.A.I. and D.G.H. edited and revised manuscript; H.A.I. and D.G.H. approved final version of manuscript.
REFERENCES
- 1.Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol 4: 595–602, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol 15: 1104–1115, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickinson CJ, Lawrence JR. A slowly developing pressor response to small concentrations of angiotensin. Its bearing on the pathogenesis of chronic renal hypertension. Lancet 1: 1354–1356, 1963. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA. The absence of intrarenal ACE protects against hypertension. J Clin Invest 123: 2011–2023, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 57: 132–140, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH Jr, Messerli FH, Oparil S, Schork MA. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 354: 1685–1697, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension 41: 1178–1179, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation 110: 74–78, 2004. [DOI] [PubMed] [Google Scholar]
- 10.McKinstry KK, Strutt TM, Bautista B, Zhang W, Kuang Y, Cooper AM, Swain SL. Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2. Nat Commun 5: 5377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 31: 137–161, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases–where worlds meet. N Engl J Med 363: 1196–1198, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med 204: 951–961, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22: 745–763, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 1: 426–432, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64: 1108–1115, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior Ang II treatment. Hypertension 59: 459–466, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]