Abstract
Several studies have suggested a prominent (pro)inflammatory and harmful role of platelets in renal disease, and newer work has also demonstrated platelet release of proangiogenic factors. In the present study, we investigated the role of platelets in a mouse model of selective endothelial cell injury using either platelet depletion or the pharmacological P2Y12 receptor blocker clopidogrel as an interventional strategy. The concanavalin A/anti-concanavalin A model was induced in left kidneys of C57bl/6J wild-type mice after initial platelet depletion or platelet-inhibiting therapy using clopidogrel. FACS analysis of glycoprotein IIb/IIIa/P-selectin double-positive platelets and platelet-derived microparticles demonstrated relevant platelet activation after the induction of selective endothelial injury in mice. Enhanced platelet activation persisted for 5 days after disease induction and was accompanied by increased amounts of circulating platelet-derived microparticles as potential mediators of a prolonged procoagulant state. By immunohistochemistry, we detected significantly reduced glomerular injury in platelet-depleted mice compared with control mice. In parallel, we also saw reduced endothelial loss and a consequently reduced repair response as indicated by diminished proliferative activity. The P2Y12 receptor blocker clopidogrel demonstrated efficacy in limiting platelet activation and subsequent endothelial injury in this mouse model of renal microvascular injury. In conclusion, platelets are relevant mediators of renal injury induced by primary endothelial lesions early on, as demonstrated by platelet depletion as well as platelet inhibition via the P2Y12 receptor. While strategies to prevent platelet-endothelial interactions have shown protective effects, the contribution of platelets during renal regeneration remains unknown.
Keywords: renal microvascular endothelial injury, platelets, thrombotic microangiopathy, clopidogrel, platelet microparticles
the renal microvasculature represents a primary or secondary target in various types of glomerulonephritis. During the progression of kidney disease, loss of microvascular capillaries is accompanied by progressive renal scarring (7–9, 22, 41). To complement a variety of humoral factors, inflammatory cells frequently contribute to renal pathophysiology, finally leading to functional deterioration and the loss of intrinsic renal cells. Nonetheless, the actual contribution of platelets to renal disease remains a contentious issue, with some studies of experimental glomerulonephritis finding platelets being a source of inflammatory molecules and important proinflammatory effector cells, whereas some other clinical studies were not able to establish platelet inhibition as an effective therapy (3, 4, 26, 27).
After severe acute endothelial injury, platelets continuously interact with the endothelial surface and thereby play a central role in the development of subsequent thrombotic microangiopathy (TMA). In recent years, our pathophysiological knowledge regarding the development of TMAs has dramatically improved, and specific alterations of von Willebrand factor degradation or the complement system have been identified. In contrast, the influence of platelets has not been systematically investigated. To date, no therapeutic strategies are in existence to successfully modulate platelet or endothelial cell properties after initial endothelial injury as a potential protective approach.
We have previously established an inducible murine model of selective endothelial injury in the kidney (17) demonstrating subsequent features of TMA, including platelet activation and formation of microthrombi within the microvasculature. In the present study, we sought to define the role of platelets in this acute endothelial disease. We therefore applied two approaches to interfere with platelet-endothelial cell interactions: platelet depletion using anti-glycoprotein Ibα antibodies and pharmacological inhibition of the platelet P2Y12 receptor using the clinically established compound clopidogrel. We thereby prove the relevance of platelets for acute and ongoing endothelial injury and provide evidence that interference with platelet activation represents a protective interventional strategy.
MATERIALS AND METHODS
Animal model and experimental design.
All animal experiments were done according to American Physiological Society guidelines and were duly approved by local government authorities. Male C57bl/6J wild-type (WT) mice at the age of 9–12 wk (Charles River, Sulzfeld, Germany) were used for experiments. All animals were fed standard mice chow (Altromin 1324, Spezialfutterwerke) and received tap water ad libitum.
In all experiments, site-specific microvascular endothelial injury was induced as previously described (17). The anti-concanavalin A (anti-ConA) serum was generated in New Zealand White rabbits by Seqlab (Sequence Laboratories, Goettingen, Germany) and tested for antibody concentration to specifically induce the injury model in WT mice as previously described (17).
In the first experiment, we evaluated the time course of platelet activation in our disease model. Endothelial injury was induced in 10 C57Bl/6 mice, and 4 mice served as sham-operated (0.9% NaCl perfusion) controls. Mice were euthanized 1, 2, and 5 days after disease induction. Repetitive heparinized blood samples were collected by retroorbital bleedings and immediately processed for measurements of platelet activation and platelet-derived microparticles (PMPs) from blood samples by FACS.
In the second experiment, 10 WT mice received 100 μg anti-glycoprotein Ibα IgG (p0p3/p0p4) intraperitonealy the day before disease induction as previously described (41), whereas 5 additional WT mice received equal amounts of rat IgG (Sigma-Aldrich, Munich, Germany) and served as controls. These antibodies are known to induce rapid platelet depletion 24 h before disease induction (5, 36). Mice were euthanized 24 h after disease induction. On the day of euthanization, mice were anaesthetized using inhaled isoflurane, blood was collected via puncture of the inferior caval vein, and mice were then perfused via the heart with 0.9% (wt/vol) NaCl solution to remove blood components from both kidneys. Renal tissues were harvested and fixed either in methyl Carnoy's or zinc solution.
In the third experiment, 40 WT mice were subjected to clopidogrel treatment or solvent (placebo) via oral gavage once daily starting the day before disease induction up to days 1 or 3 (n = 8–12 mice/group). On the day of euthanization, we verified treatment efficacy by measuring tail bleeding time in each animal. Therefore, the time until the first break of the bloodstream was measured in prewarmed PBS solution at 37°C. Tissues were then harvested as described above and processed for further analysis.
FACS analysis.
To analyze platelets and PMPs, platelet-rich plasma of heparinized blood samples was attained after centrifugation at 1,500 g for 1.5 min at 21°C and incubated with phycoerythrin-conjugated anti-mouse CD41 (clone MWReg30, eBioscience, Frankfurt/Main, Germany) and allophycocyanin-conjugated anti-CD62 (clone Psel.KO2.3, eBioscience) at 21°C for 20 min. Samples were fixed by the addition of an equal volume of 1% paraformaldehyde in PBS and diluted 1:10 with PBS containing 0.1% BSA. FACS analysis was acquired using a FACSCanto II from BD, and data were analyzed using FlowJo data analysis software (FlowJo, Ahsland, OR).
Tissue processing and immunohistochemical staining.
Methyl carnoy's- or zinc-fixed tissues were embedded in paraffin and cut into 30 consecutive 3-μm sections spanning the distance of ∼150 μm and numbered from 1 to 30. To exclude artifacts due to the heterogeneity of the disease model, for all stainings, 3 tissue sections with a distance of at least 10 sectioning layers were used for light or immunofluorescence microscopy. Periodic acid-Schiff (PAS) and acid fuchsin orange G (AFOG) stainings of three 3-μm interval sections of each biopsy were performed to asses renal injury and fibrin deposition. For immunofluorescence microscopy, further sections were incubated with the following primary and secondary antibodies as indicated and also previously published (17, 19, 20, 22): glycoprotein Ibα, a rat monoclonal antibody against mouse glycoprotein Ib for specific staining of platelets (5); mouse endothelial cell antigen (MECA)-32, a rat monoclonal antibody against MECA-32 for specific detection of peritubular endothelial cells (16); CD31, a rat monoclonal antibody against mouse platelet-endothelial cell adhesion molecule-1 for specific detection of endothelial cells (46); PCNA, a murine monoclonal antibody against PCNA to detect actively proliferating cells (19A2, Merck Millipore) (23); and F4/80, a rat monoclonal antibody (Invitrogen, Life Technologies) to detect mouse macrophages/monocytes as inflammatory cell types. All antibodies were diluted in sterile PBS containing 1% (wt/vol) BSA. All tissue sections were incubated with primary antibodies overnight at 4°C in a wet chamber, and negative controls included omission of the referred antibodies. Afterward, fluorescent secondary antibodies (Invitrogen, Life Technologies), including Alexa fluor F488 and/or Alexa fluor 555 goat anti-rat IgG, were applied at room temperature in the dark. Cell nuclei were counterstained with 4′,6-diamino-2-phenylindole (DAPI; 1 μg/ml, Applichem).
Quantitative analysis of immunostaining and capillary rarefaction.
Computer-assisted image analysis (ImageJ software, National Institutes of Health) was used to quantify immunostained kidney sections after image acquisition with a digital microscope (BIOREVO BZ9000, Keyence, Neu-Isenburg, Germany) under ×200 or ×400 magnification.
Kidney injury and inflammation.
Glomerular platelet infiltration was assessed after staining for glycoprotein Ibα using a semiquantitative scoring system from 0 to 4, where 0 = absence of platelets, 1 = the presence of glomerular platelets in <10% of all glomeruli, 2 = the presence of platelets in up to 50% of glomeruli, 3 = >50% of glomeruli contained platelet thrombi often with peritubular capillary involvement, and 4 = severe glomerular and peritubular thrombosis up to 100% (22). Three distant sections of each kidney were evaluated, reflecting 15 cortical fields of vision at ×200 magnification. Glomerular injury was evaluated individually in at least 50 randomly selected glomeruli under ×400 magnification on PAS-stained tissue sections using a similar scoring system from 0 to 4, where, specifically, 0 = normal glomeruli without structural damage, 1 = glomerular matrix expansion and edema formation of <25% of the glomerulus, 2 = increased intraglomerular cell count and swelling up to 50%, 3 = obliteration or collapse of capillaries in up to 75% of the glomerular cross-section, and 4 = complete capillary loss and thrombosis. In AFOG-stained tissue sections (37), we quantified glomerular fibrin deposition (intense orange-red color) in at least 50 randomly selected glomeruli under ×400 magnification. Therefore, we used a scoring system from 0 to 4 analogous to the evaluation of platelet infiltration. All values are given as scores ± SD per glomerular cross-section. Furthermore, we counted all F4/80 and DAPI double-positive infiltrating monocytes and macrophages in 15 randomly selected cortical fields under ×400 magnification to assess the cell count per renal cortex excluding glomeruli.
Endothelial injury and cell proliferation.
After assessment of injury and inflammation, we evaluated the peritubular capillary rarefaction on digital images using a grid overlay (ImageJ software) consisting of 625-μm2-sized squares in at least 15 cortical images sparing glomeruli. This evaluation method has been previously published by our group using a special ocular with exactly the same grid size (17). Squares containing no MECA-32-positive capillary structures were counted. Capillary rarefaction is given as negative positive area ± SD per millimeter squared. These data directly reflect peritubular endothelial injury, where higher values indicate increased loss of capillaries (maximum = 100) and lower values indicate better capillary preservation. Glomerular capillary rarefaction was determined using a semiquantitative scoring system from 0 to 4 in at least 50 random glomeruli per murine kidney, where 0 = no capillary loss, 1 = <25%, 2 = up to 50%, 3 = up to 75%, and 4 = 100% absence of glomerular capillary structures. Values are given as mean scores ± SD per glomerular cross-section. In the next step, we assessed the proliferation rate during the time course of disease by counting all glomerular PCNA-positive cells in 50 randomly selected glomeruli and tubulointerstitial PCNA-positive cells in 15 cortical areas. In parallel, all MECA-32/PCNA double-positive proliferating endothelial cells were assessed. Data are given as mean scores ± SD per glomerular and (tubulo)interstitial cross-section.
TUNEL assay.
Necroptotic cells were detected by a TUNEL assay, as previously described, according to the manufacturer's instructions (30). The number of TUNEL-positive apoptotic cells was counted in 50 sequentially selected glomeruli and given as mean numbers either per glomerular cross-section or per millimeter squared after 20 peritubular areas of each section were counted.
Statistical analysis.
All data are expressed as means ± SD. We first tested for normal data distribution using a D'Agostino and Pearson omnibus normality test. FACS analysis data were analyzed using two-way ANOVA with a Bonferroni posttest. For all other data with normal distribution, an F-test was used to compare variances followed by an unpaired Student's t-test (PRISM software version 5.0, GraphPad). P values of <0.05 were considered statistically significant. Data failing the normality test were analyzed by a two-sided nonparametric Mann-Whitney U-test, and values of <0.05 were considered significant.
RESULTS
Rapid and long-lasting platelet activation after selective endothelial injury.
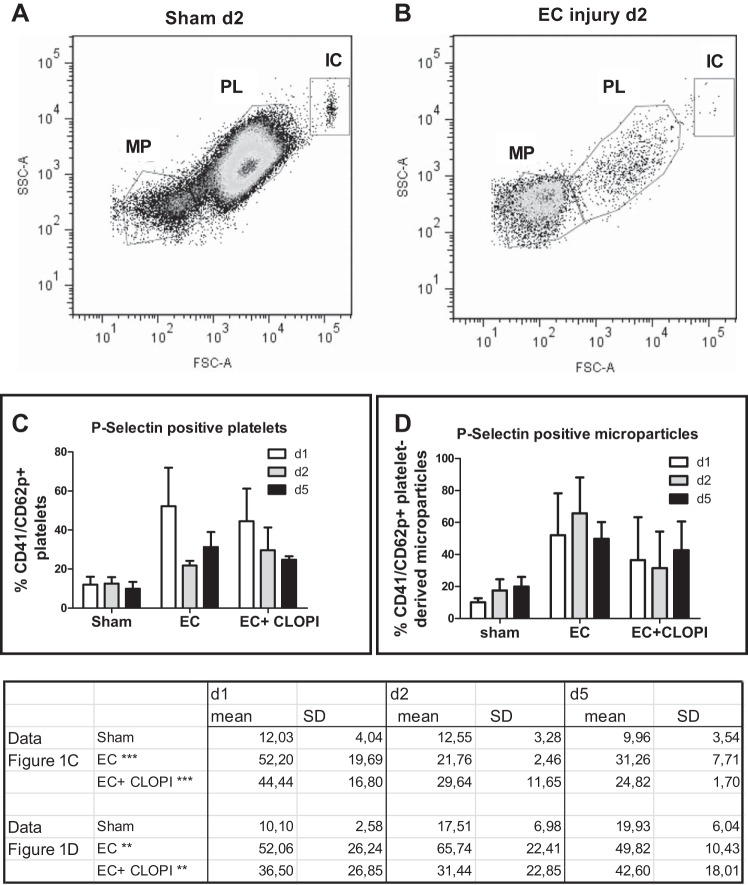
To prove the persistent activation of platelets in our disease model, we first investigated the extent of platelet activation and subsequent generation of PMPs in our murine renal microvascular injury model using FACS analysis of repetitively drawn blood samples. After incubation with antibodies directed against glycoprotein IIb/IIIa (CD41) and P-selectin (CD62), we were able to identify three distinct populations, including larger immune complexes containing platelet fragments, activated platelets, and microparticles (Fig. 1, A and B). Active disease led to a drastic decrease of platelet counts, as shown in Fig. 1B, which shows mice 2 days after the initial endothelial cell injury, versus Fig. 1A, where data from a sham control mouse are shown. Quantification demonstrated the significant increase of P-selectin-positive, activated platelets (Fig. 1C) and the generation of significant amounts of microparticles (Fig. 1D) compared with sham control mice. While these effects were clearly related to the onset of acute renal endothelial injury and subsequent TMA (by two-way ANOVA), no significant differences occurred due to the clopidogrel treatment. This significant degree of platelet activation and generation of PMPs were detected up to 5 days after disease induction.
Fig. 1.
Platelet activation and generation of platelet-derived microparticles (PMPs) after selective endothelial cell (EC) injury. A and B: in sham-operated mice (A) as well as in mice with selective EC injury (B), FACS analysis detected three distinct populations consisting of microparticles (MPs), platelets (PLs), and immune complexes (ICs). d, day. C: the numer of P-selectin positive, CD41-positive platelets was significantly higher in both groups undergoing EC injury compared with the sham control group, whereas no differences could be detected between the groups with and without clopidogrel (CLOPI) treatment (data table). D: in parallel, large amounts of microparticles were generated in mice after the induction of selective EC injury (data table), without differences between clopidogrel-treated and untreated mice. ***P < 0.001 and **P < 0.001 by two-way ANOVA.
We then decided to deplete platelets to prove the relevance of these cells for the initial microvascular injury in our disease model. Platelet depletion was induced 1 day before the initiation of endothelial injury, which led to a relevant hemorrhagic diathesis, making renal arterial perfusion in platelet-depleted mice very challenging. While all mice primarily survived the surgery, 50% of mice died 3–4 h after the end of anesthesia. Since we could not detect intra-abdominal bleedings, we believe that this was due to the intra-arterial volume load in the presence of a capillary leak leading to pulmonary edema.
Platelet depletion reduced renal injury and preserved microvascular capillaries.
On the day of euthanization, blood was drawn into heparin and immediately analyzed using an auto-analyzer (Beckman Instruments, Brea, CA) to verify the efficacy of platelet depletion. A significant reduction of the platelet count (normal values: ∼800–1,200 platelets/nl) was seen in platelet-depleted mice (64.5 ± 6.3 platelets/nl, P < 0.01 vs. control mice) as well as in nondepleted ConA/anti-ConA control mice (101 ± 10.2 platelets/nl).
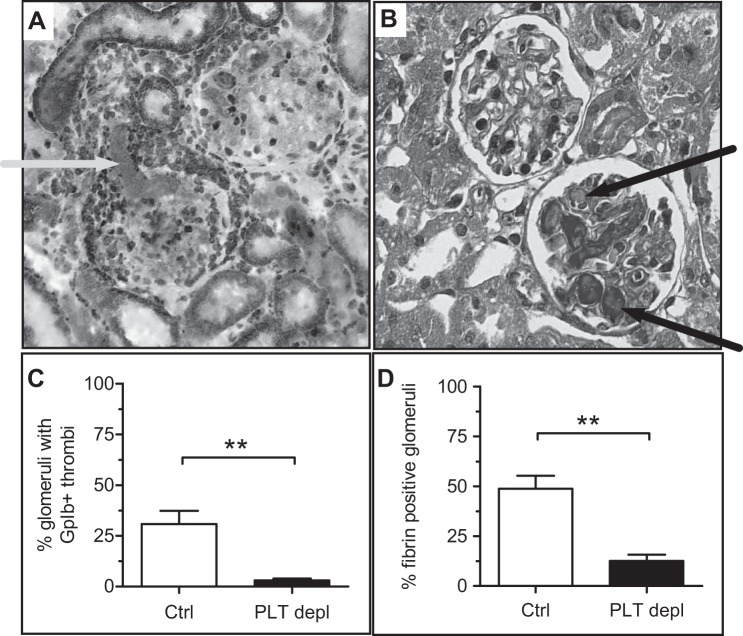
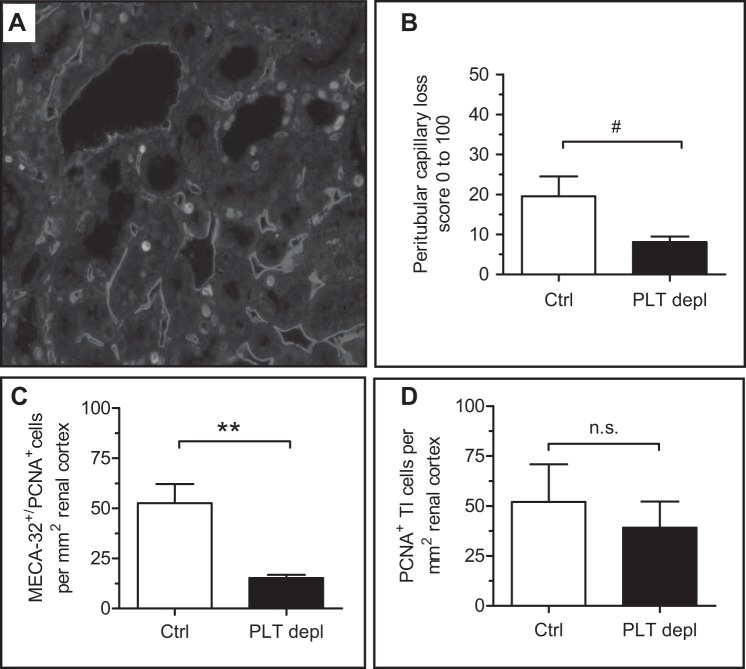
We then investigated the effect of our platelet depletion strategy on renal histology. First, we assessed platelet activation and thrombus formation after staining for glycoprotein Ibα (Fig. 2, A and B) and AFOG staining (Fig. 2, C and D), respectively. Data clearly showed the reduced deposition of platelets (Fig. 2B) and fibrin thrombi (Fig. 2D) in glomeruli of platelet-depleted mice compared with ConA/anti-ConA control mice (P < 0.01). Both findings also demonstrated that thrombocytopenia of control mice was due to the development of TMA and platelet consumption, whereas in platelet- depleted mice, it was predominantly a consequence of platelet depletion. To evaluate any possible protective effects on the microvascular endothelium, we performed fluorescence staining for MECA-32 (Fig. 3A), which showed a better preservation of peritubular capillaries after 24 h in platelet-depleted mice (P = 0.056, f < 0.05; Fig. 3B).
Fig. 2.
Reduced glomerular thrombus formation due to reduced injury after platelet depletion. A: staining for glycoprotein (GP)Ibα in a control (Ctrl) mouse 24 h after disease induction. B: platelet-derived thrombi and fibrin-positive glomeruli were assessed after acid fuchsin orange G (AFOG) staining. C and D: the deposition of platelets (C) and fibrin-rich thrombi (D) in glomeruli of platelet-depleted (PLT depl) animals compared with PBS-injected control animals was reduced. **P < 0.01.
Fig. 3.
Reduced capillary rarefaction and necessity of repair after platelet depletion. A: renal tissue sections were double stained with anti-mouse endothelial cell antigen (MECA)-32 and anti-PCNA antibodies to identify proliferating ECs. A control mouse 24 h after disease induction is shown. B: endothelial rarefaction was reduced in platelet-depleted mice. C: proliferating ECs were assessed as PCNA-positive/MECA-32-positive cells. D: proliferative response of tubulointerstitial cells was evaluated after PCNA staining. **P < 0.01; #f < 0.05. n.s., Not significant.
Since the primary endothelial cell injury needs to be compensated by a subsequent repair response, we then evaluated cell proliferation as a potential major intrinsic repair mechanism. In the first step, all proliferating tubulointerstitial (PCNA-positive) cells were counted, and, subsequently, all MECA-32/PCNA double-positive proliferating endothelial cells were assessed. In platelet-depleted mice, the proliferative response of endothelial cells was significantly reduced compared with ConA/anti-ConA control mice (P < 0.01; Fig. 3C), which was a consequence of reduced endothelial injury, as shown in Fig. 3B. In contrast to the endothelium, the overall proliferative response of tubulointerstitial cells was unchanged (Fig. 3D).
Pharmacological platelet inhibition prevents endothelial injury and subsequent TMA in the kidney.
Having provided evidence that platelets are direct mediators of endothelial injury in this disease model, we decided to use a clinically established compound, clopidogrel, to interfere with platelet activation via the P2Y12 receptor.
Treatment of mice by daily gavage of 75 mg/kg body wt clopidogrel was started 24 h before disease induction, which was continued on a daily basis thereafter. To verify treatment efficacy, tail bleeding times were measured in each animal on the day of euthanization. All clopidogrel-treated mice had a significantly prolonged tail bleeding time compared with control mice (day 1: P < 0.01 and day 3: P < 0.05; Table 1).
Table 1.
Platelet counts in control and platelet-depleted mice and tail bleeding times, F4/80-positive cell counts, and TUNEL-positive glomerular cells per cross-section in placebo- and clopidogrel-treated mice
|
Day 1 |
Day 3 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Control mice | Platelet-depleted mice | Placebo-treated mice | Clopidogrel-treated mice | Control mice | Platelet-depleted mice | Placebo-treated mice | Clopidogrel-treated mice |
| Platelet count, platelets/μl | 101,000 ± 10,212 | 64,500 ± 6,341 | N/A | N/A | ||||
| Tail bleeding time, s | 105.7 ± 99.2 | 246.4 ± 84.9† | 101.5 ± 48.5 | 213.2 ± 111.9* | ||||
| Count of F4/80+ cells, cells/mm2 renal cortex | 116 ± 62 | 22 ± 25‡ | 178 ± 69 | 124 ± 73 | ||||
| TUNEL-positive glomerular cells per cross-section, cells/mm2 | 0.0420 | 0.01333§ | 0.03667 | 0.03571 | ||||
Values are means ± SD. N/A, not applicable.
P < 0.05,
P < 0.01, and
P < 0.001 vs. placebo-treated mice;
P < 0.05 by F-test.
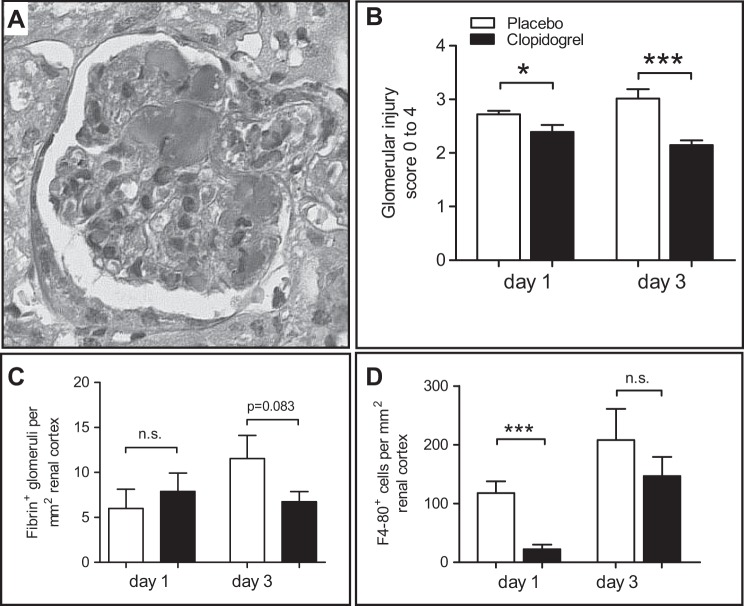
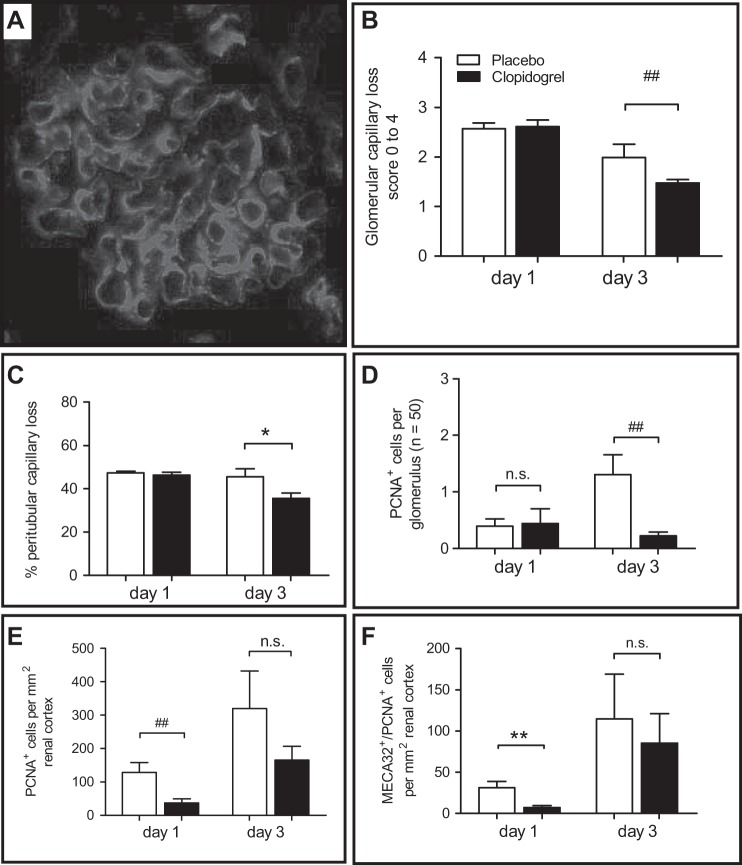
Evaluation of glomerular changes and thrombus formation were done on PAS-stained (Fig. 4A) and AFOG-stained tissue sections. On days 1 and 3, glomerular injury was significantly reduced in the clopidogrel-treated group (day 1: P < 0.05 and day 3: P < 0.001; Fig. 4B), whereas glomerular fibrin deposition was only reduced on day 3 (P < 0.05; Fig. 4C). To assess potential effects of inflammatory cells, we evaluated macrophage/monocyte infiltration of the renal cortex after staining for F4/80 as an indicator of the inflammatory response. In clopidogrel-treated mice, we detected a significantly lower number of F4/80-positive cells 24 h after disease induction (P < 0.001; Fig. 4D and Table 1), whereas no differences were detectable between both groups on day 3 (Table 1).
Fig. 4.
Clopidogrel treatment protects from glomerular injury and reduces the inflammatory response. A and B: for the evaluation of glomerular injury, we used Periodic acid-Schiff-stained tissue sections (A) and a semiquantitative scoring system from 0 to 4 representing the degree of injury (B). C: fibrin thrombus formation was evaluated after AFOG staining. D: monocytes/macrophages were evaluated after staining for F4/80. *P < 0.05 and ***P < 0.001; #P < 0.05 by Mann-Whitney U-test.
Since platelet depletion led to preservation of capillaries, we subsequently assessed the protective effects of clopidogrel treatment on glomerular and peritubular capillary injury on stainings of CD31 and MECA-32 (Fig. 5A). Analysis of differences showed a significant decrease of endothelial injury in glomeruli of clopidogrel-treated mice versus ConA/anti-ConA control mice (P = 0.059, f < 0.01; Fig. 5B) on day 3. In parallel, evaluation of peritubular capillaries on MECA-32-stained tissue sections also demonstrated a significantly decreased endothelial injury in the clopidogrel-treated group on day 3 (P < 0.01; Fig. 5C). Subsequent evaluation of cell proliferation as an indicator of the repair response showed decreased glomerular proliferation (P < 0.01; Fig. 5D) on day 3 and decreased tubulointerstitial cell proliferation on day 1 (P < 0.01; Fig. 5E) and day 3 (f < 0.05) as well as reduced peritubular endothelial cell proliferation on day 3 (P < 0.01; Fig. 5F). In parallel, we also detected a reduced number of necroptotic cells in glomeruli of clopidogrel-treated mice as judged by TUNEL staining on day 1 (f < 0.05; Table 1).
Fig. 5.
Clopidogrel treatment protects renal capillaries. A and B: staining of CD31 (A) was used to evaluate glomerular capillary rarefaction (B). C: tubulointerstitial capillary rarefaction was assessed after staining for MECA-32 as described in methods. D–F: glomerular (D), tubulointerstitial (E), and EC proliferation (MECA-32-positive/PCNA-positive cells) were evaluated after double staining for MECA-32 and PCNA (F). *P < 0.05 and **P < 0.01; ##P < 0.01 by by Mann-Whitney U-test.
DISCUSSION
Numerous experimental studies have shown a close correlation of microvascular endothelial injury and healing with the restitution of glomerular integrity, renal function, and progression of kidney disease (20, 22, 24, 29–32). While protective strategies for the microvascular endothelium are lacking, they might comprise an important approach to change the outcome of inflammatory kidney disease, especially in the presence of acute endothelial lesions. The latter may lead to the development of subsequent TMA, indicating that the interaction between endothelial cells and platelets can be very harmful and should be considered as a therapeutic target (17, 28, 34).
The present study was performed to 1) define the role of platelets in our ConA/anti-ConA murine model of selective primary endothelial injury (17) and 2) test whether the therapeutic interference with platelet-endothelial cell interactions through blockade or inhibition of platelet function represents a protective strategy in the kidney. By histology, an early and pronounced platelet influx with subsequent TMA was evident already (17).
To prove the pronounced systemic platelet activation, we evaluated the extent of platelet activation as reflected by the amount of P-selectin-positive platelets and PMPs. We found a clear-cut increase of platelet activation and generation of PMPs up to 5 days after the initial renal endothelial lesions, underscoring the fact that platelet activation is overlapping with the phase when endothelial repair is already ongoing. Although their role in kidney disease is widely unclear, PMPs have been characterized as catalytic procoagulant surfaces involved in thrombogenesis (13, 39). They have also been shown to contain a variety of inflammatory components, cytokines, and growth factors that are related to their involvement in inflammatory processes, immune responses, and angiogenesis (39, 40).
We then tested the relevance of platelets in this disease model using well-established anti-glycoprotein Ib antibodies to specifically induce >90% platelet depletion by Fc-independent mechanisms without systemic side effects. These antibodies have been previously used in long-term experiments with repetitive application, by our own group and others (5, 19, 36).
While a decrease of platelets was also evident in nondepleted mice, this latter decrease has to be put in the context of enhanced platelet activation and subsequent thrombus formation in these mice. Therefore, the low platelet count in depleted mice is related to the antibody application, whereas it is a consequence of consumption by active TMA in nondepleted mice.
To investigate the effect of reduced platelet-endothelial cell interactions on histology, we evaluated the degree of microvascular injury in the kidney. We found that platelet depletion significantly reduced renal injury in general and effectively preserved microvascular capillaries. Therefore, this is the first study directly demonstrating that platelets mediate loss of the endothelium in the kidney and that platelet depletion can protect from capillary rarefaction (28, 47). In recent years, many experimental and clinical studies have proven the efficacy of P2Y12 inhibition to prevent arterial thrombus formation and platelet activation (42). Hence, we subsequently chose this approach to investigate the effect of pharmacological platelet inhibition in our disease model. Since we wanted to know whether the inhibition of the P2Y12 receptor would also be able to sufficiently interfere with early platelet-endothelial cell interactions in our disease model, we started the gavage of clopidogrel the day before the initiation of disease.
In analogy to platelet depletion, P2Y12 inhibition prevented glomerular injury and fibrin deposition. During the time course of disease, glomerular as well as peritubular capillaries were protected by P2Y12 inhibition, which is consistent with our results of the platelet depletion approach. We showed that both the occurrence of necroptotic cells and (endothelial) cell proliferation, as a relevant intrinsic repair mechanism, were significantly reduced in the kidneys of mice that underwent platelet depletion or inhibition. Taken together, these data indicate the protective effect of platelet inhibition for the endothelium. In line with our findings, platelets are considered as being important effector cells of experimental inflammatory renal diseases. A variety of experimental studies in models of immune complex nephritis, anti-Thy1 nephritis, and Habu snake venom glomerulonephritis have demonstrated significant platelet activation, whereas few studies have proved this by direct interference with the platelet activation process or platelet depletion (9, 26, 27). Therefore, their exact relevance in distinct types of kidney disease is not clear. We have recently shown that platelets do not play a relevant role during passive crescentic glomerulonephritis in mice (18), a chronic inflammatory disease model with platelet activation. An important study by Johnson et al. (28) used the rat anti-ConA model of renal endothelial injury and showed that platelets play a mediating role in this endothelial disease, whereas they could not detect relevant, directly protective effects for the microvascular endothelium. Using another rat (anti-Thy1) glomerulonephritis model, Peters et al. (42) also showed protective effects using clopidogrel.
An experimental study (11) has also shown that platelets release a broad range of inflammatory mediators and cytokines inducing endothelial cell activation, leukocyte adhesion, and transmigration. In the present study, we found reduced numbers of F4/80-positive macrophages/monocytes in kidneys of clopidogrel-treated mice, indicating reduced inflammation early after injury induction. This finding indicates that the protective effects of clopidogrel treatment might also be mediated directly (but also indirectly) via its influence on local inflammation. Abele et al. (1, 2) have previously shown anti-inflammatory, protective effects of platelet inhibition due to clopidogrel treatment in an aortic allograft model. Recently, it has been proposed that the P2Y12 receptor might also play a role in amplifying the release of platelet's α-granules (12), since it directly interferes with P-selectin-driven platelet activation. The relevance of P-selectin-mediated platelet activation has been previously shown in the rat ConA model (47) and is reflected by the drastic increase of P-selectin-positive platelets and PMPs in our disease model. However, FACS analysis could not detect decreased systemic platelet activation by clopidogrel, which might relate to the limited group size of this approach. Moreover, we have previously shown that a large amount of circulating cytokines is released upon endothelial injury in this disease model (21), which is in accordance with studies in other rodent animal models showing the contribution of platelets to disease progression and inflammation and the anti-inflammatory as well as anti-fibrotic effects of clopidogrel (14, 35, 38, 42, 45).
In contrast, George et al. (15) demonstrated that platelets are also required for the maintenance of vascular integrity in inflammation and proposed that platelets locally deliver vasoactive mediators by release of their storage granules during transient interaction with the inflamed vessel wall. Subsequent experimental studies have described active sequestration of proangiogenic factors and molecules in platelet α-granules (25, 33), and in vitro platelets stimulate endothelial cells and promote the assembly of capillary-like structures (10, 43). Central repair mechanisms, such as cell proliferation, have been linked with platelet activation and secreted factors such as platelet-derived growth factor (6). Therefore, it is possible that the beneficial effect of platelet inhibition in our disease model could be harmful in situations when such factors are necessary to support proper regeneration.
In summary, in the present study, we demonstrated that depletion of platelets as well as blockade of the P2Y12 receptor reduced microvascular injury in a mouse model of selective endothelial injury and subsequent TMA in the kidney. This is a further proof of the general concept to interfere with platelet activation and platelet-endothelial cell interactions in distinct kidney diseases. Further studies will be necessary to investigate and differentiate the early and late effects of platelets on endothelial cell injury and repair.
GRANTS
This work was supported by Grant IZKF Erlangen TP B14 (to B. Hohenstein and C. P. M. Hugo), the Foundation Family Bouhon, and Deutsche Forschungsgemeinschaft HO 2522/6-1 (to B. Hohenstein). Partial support was also provided by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54602, DK-052783, and DK-45462 (to M. S. Goligorsky) and the Westchester Artificial Kidney Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.S., J.S., and K.M.L. performed experiments; C.S., K.M.L., and B.H. analyzed data; C.S., K.M.L., M.S.G., C.P.H., and B.H. interpreted results of experiments; C.S. prepared figures; C.S. drafted manuscript; C.S., K.M.L., M.S.G., B.N., C.P.H., and B.H. edited and revised manuscript; C.S., K.M.L., M.S.G., B.N., C.P.H., and B.H. approved final version of manuscript; M.S.G., B.N., C.P.H., and B.H. conception and design of research.
ACKNOWLEDGMENTS
The skilled technical assistance of S. Cabric, A. Stief, and S. Walther is gratefully acknowledged. In addition, the authors thank A. Vecchi (Istituto di Ricerche Farmacologiche, Milano, Italy) for providing PECAM-1 antibodies (Mec13.3).
REFERENCES
- 1.Abele S, Spriewald BM, Ramsperger-Gleixner M, Wollin M, Hiemann NE, Nieswandt B, Weyand M, Ensminger SM. Attenuation of transplant arteriosclerosis with clopidogrel is associated with a reduction of infiltrating dendritic cells and macrophages in murine aortic allografts. Transplantation 87: 207–216, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Abele S, Weyand M, Wollin M, Hiemann NE, Harig F, Fischlein T, Ensminger SM. Clopidogrel reduces the development of transplant arteriosclerosis. J Thorac Cardiovasc Surg 131: 1161–1166, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barnes JL. Platelets in glomerular disease. Nephron 77: 378–393, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Barnes JL, Venkatachalam MA. The role of platelets and polycationic mediators in glomerular vascular injury. Semin Nephrol 5: 57–68, 1985. [PubMed] [Google Scholar]
- 5.Bergmeier W, Rackebrandt K, Schroder W, Zirngibl H, Nieswandt B. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood 95: 886–893, 2000. [PubMed] [Google Scholar]
- 6.Boor P, Ostendorf T, Floege J. PDGF and the progression of renal disease. Nephrol Dial Transplant 29, Suppl 1: i45–i54, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Couser WG. Mediation of immune glomerular injury. J Am Soc Nephrol 1: 13–29, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Couser WG. Pathogenesis of glomerular damage in glomerulonephritis. Nephrol Dial Transplant 13, Suppl 1: 10–15, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Couser WG, Johnson RJ. Mechanisms of progressive renal disease in glomerulonephritis. Am J Kidney Dis 23: 193–198, 1994. [DOI] [PubMed] [Google Scholar]
- 10.D'Amore P, Shepro D. Stimulation of growth and calcium influx in cultured, bovine, aortic endothelial cells by platelets and vasoactive substances. J Cell Physiol 92: 177–183, 1977. [DOI] [PubMed] [Google Scholar]
- 11.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 357: 2482–2494, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Evans DJ, Jackman LE, Chamberlain J, Crosdale DJ, Judge HM, Jetha K, Norman KE, Francis SE, Storey RF. Platelet P2Y(12) receptor influences the vessel wall response to arterial injury and thrombosis. Circulation 119: 116–122, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest 115: 3355–3362, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia AE, Mada SR, Rico MC, Dela Cadena RA, Kunapuli SP. Clopidogrel, a P2Y12 receptor antagonist, potentiates the inflammatory response in a rat model of peptidoglycan polysaccharide-induced arthritis. PLoS One 6: e26035, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George JN. Platelets. Lancet 355: 1531–1539, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hallmann R, Mayer DN, Berg EL, Broermann R, Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn 202: 325–332, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Hohenstein B, Braun A, Amann KU, Johnson RJ, Hugo CP. A murine model of site-specific renal microvascular endothelial injury and thrombotic microangiopathy. Nephrol Dial Transplant 23: 1144–1156, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Hohenstein B, Daniel C, Johnson RJ, Amann KU, Hugo CP. Platelets are not critical effector cells for the time course of murine passive crescentic glomerulonephritis. Platelets 24: 267–274, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Hohenstein B, Daniel C, Johnson RJ, Amann KU, Hugo CP. Platelets are not critical effector cells for the time course of murine passive crescentic glomerulonephritis. Platelets 24: 267–274, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Hohenstein B, Kasperek L, Kobelt DJ, Daniel C, Gambaryan S, Renne T, Walter U, Amann KU, Hugo CP. Vasodilator-stimulated phosphoprotein-deficient mice demonstrate increased platelet activation but improved renal endothelial preservation and regeneration in passive nephrotoxic nephritis. J Am Soc Nephrol 16: 986–996, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Hohenstein B, Kuo MC, Addabbo F, Yasuda K, Ratliff B, Schwarzenberger C, Eckardt KU, Hugo CP, Goligorsky MS. Enhanced progenitor cell recruitment and endothelial repair after selective endothelial injury of the mouse kidney. Am J Physiol Renal Physiol 298: F1504–F1514, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hohenstein B, Renk S, Lang K, Daniel C, Freund M, Leon C, Amann KU, Gachet C, Hugo CP. P2Y1 gene deficiency protects from renal disease progression and capillary rarefaction during passive crescentic glomerulonephritis. J Am Soc Nephrol 18: 494–505, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Hugo C, Pichler R, Meek R, Gordon K, Kyriakides T, Floege J, Bornstein P, Couser WG, Johnson RJ. Thrombospondin 1 is expressed by proliferating mesangial cells and is up-regulated by PDGF and bFGF in vivo. Kidney Int 48: 1846–1856, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Iruela-Arispe L, Gordon K, Hugo C, Duijvestijn AM, Claffey KP, Reilly M, Couser WG, Alpers CE, Johnson RJ. Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol 147: 1715–1727, 1995. [PMC free article] [PubMed] [Google Scholar]
- 25.Italiano JE Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 111: 1227–1233, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RJ. The glomerular response to injury: progression or resolution? Kidney Int 45: 1769–1782, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RJ. Platelets in inflammatory glomerular injury. Semin Nephrol 11: 276–284, 1991. [PubMed] [Google Scholar]
- 28.Johnson RJ, Alpers CE, Pritzl P, Schulze M, Baker P, Pruchno C, Couser WG. Platelets mediate neutrophil-dependent immune complex nephritis in the rat. J Clin Invest 82: 1225–1235, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Johnson RJ. Vascular endothelial growth factor: a new player in the pathogenesis of renal fibrosis. Curr Opin Nephrol Hypertens 12: 43–49, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Kim YG, Suga SI, Kang DH, Jefferson JA, Mazzali M, Gordon KL, Matsui K, Breiteneder-Geleff S, Shankland SJ, Hughes J, Kerjaschki D, Schreiner GF, Johnson RJ. Vascular endothelial growth factor accelerates renal recovery in experimental thrombotic microangiopathy. Kidney Int 58: 2390–2399, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, Almog N, Kieran MW, Folkman J. Platelets actively sequester angiogenesis regulators. Blood 113: 2835–2842, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legendre CM, Licht C, Loirat C. Eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 369: 1379–1380, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Zhang Y, Ren H, Zhang Y, Zhu X. Effect of clopidogrel on the inflammatory progression of early atherosclerosis in rabbits model. Atherosclerosis 194: 348–356, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med 196: 887–896, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mihatsch MJ, Bremer J. Acid fuchsin orange G-stain (AFOG) for glomerular protein deposits. Clin Nephrol 9: 259, 1978. [PubMed] [Google Scholar]
- 38.Molero L, Lopez-Farre A, Mateos-Caceres PJ, Fernandez-Sanchez R, Luisa Maestro M, Silva J, Rodriguez E, Macaya C. Effect of clopidogrel on the expression of inflammatory markers in rabbit ischemic coronary artery. Br J Pharmacol 146: 419–424, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol 26: 2594–2604, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol 11: 156–164, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RJ. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int 54: 416–425, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Peters H, Eisenberg R, Daig U, Liefeldt L, Westenfeld R, Gaedeke J, Kramer S, Neumayer HH. Platelet inhibition limits TGF-β overexpression and matrix expansion after induction of anti-thy1 glomerulonephritis. Kidney Int 65: 2238–2248, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Pipili-Synetos E, Papadimitriou E, Maragoudakis ME. Evidence that platelets promote tube formation by endothelial cells on matrigel. Br J Pharmacol 125: 1252–1257, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stegner D, Nieswandt B. Platelet receptor signaling in thrombus formation. J Mol Med (Berl) 89: 109–121, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan BP, Wang R, Tawfik O, Luyendyk JP. Protective and damaging effects of platelets in acute cholestatic liver injury revealed by depletion and inhibition strategies. Toxicol Sci 115: 286–294, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A, et al. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol 63: 247–254, 1994. [PubMed] [Google Scholar]
- 47.Zachem CR, Alpers CE, Way W, Shankland SJ, Couser WG, Johnson RJ. A role for P-selectin in neutrophil and platelet infiltration in immune complex glomerulonephritis. J Am Soc Nephrol 8: 1838–1844, 1997. [DOI] [PubMed] [Google Scholar]