Abstract
In whole cell patch-clamp recordings, we characterized the L-type Ca2+ currents in bovine adrenal zona fasciculata (AZF) cells and explored their role, along with the role of T-type channels, in ACTH- and angiotensin II (ANG II)-stimulated cortisol secretion. Two distinct dihydropyridine-sensitive L-type currents were identified, both of which were activated at relatively hyperpolarized potentials. One activated with rapid kinetics and, in conjunction with Northern blotting and PCR, was determined to be Cav1.3. The other, expressed in approximately one-half of AZF cells, activated with extremely slow voltage-dependent kinetics and combined properties not previously reported for an L-type Ca2+ channel. The T-type Ca2+ channel antagonist 3,5-dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoro-piperidin-4-ylmethyl]-benzamide (TTA-P2) inhibited Cav3.2 current in these cells, as well as ACTH- and ANG II-stimulated cortisol secretion, at concentrations that did not affect L-type currents. In contrast, nifedipine specifically inhibited L-type currents and cortisol secretion, but less effectively than TTA-P2. Diphenylbutylpiperidine Ca2+ antagonists, including pimozide, penfluridol, and fluspirilene, and the dihydropyridine niguldipine blocked Cav3.2 and L-type currents and inhibited ACTH-stimulated cortisol secretion with similar potency. This study shows that bovine AZF cells express three Ca2+ channels, the voltage-dependent gating and kinetics of which could orchestrate complex mechanisms linking peptide hormone receptors to cortisol secretion through action potentials or sustained depolarization. The function of the novel, slowly activating L-type channel is of particular interest in this respect. Regardless, the well-correlated selective inhibition of T- and L-type currents and ACTH- and ANG II-stimulated cortisol secretion by TTA-P2 and nifedipine establish the critical importance of these channels in AZF cell physiology.
Keywords: adrenal fasciculata, cortisol, ACTH, ANG II, TTA-P2, L-type Ca2+ channel, Cav3.2, Cav1.3
in mammals, zona fasciculata cells of the adrenal cortex secrete glucocorticoids in a diurnal rhythm under the control of the pituitary peptide adrenocorticotropic hormone (ACTH) (57). Physical and psychological stress triggers additional bursts of corticosteroid secretion in response to activation of the hypothalamic-pituitary-adrenal axis (57). In some species, including bovine and human, angiotensin II (ANG II) also stimulates glucocorticoid production (10, 30, 43).
Glucocorticoids, including cortisol and corticosterone, regulate a variety of physiological processes, ranging from energy metabolism to long-term memory formation and emotional behavior (8, 10, 25, 32). Chronic excessive cortisol secretion, which occurs in prolonged stress or Cushing's disease, leads to systemic and central nervous system toxicity. In the central nervous system, cortisol-induced damage to the hippocampus and other brain areas causes memory impairments, as well as neuropsychiatric disorders (2, 27, 35).
Because cortisol functions pivotally in human health and disease, an understanding of the molecular mechanisms that regulate its production at the level of the adrenal zona fasciculata (AZF) cell is critical. In this regard, the biochemical and ionic signaling pathways by which ACTH and ANG II stimulate glucocorticoid synthesis are complex, involving multiple second messengers and the activation and synthesis of a number of steroidogenic proteins (54, 58). ACTH binds to a Gs protein-coupled melanocortin 2 receptor, leading to the synthesis of cAMP through activation of adenylate cyclase (45). ANG II interacts with a Gq protein-coupled angiotensin type 1 receptor, leading to the activation of phospholipase Cβ, the production of diacylglycerol and inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-diphosphate, and the release of Ca2+ from the endoplasmic reticulum (56).
In addition to the biochemical mechanisms that couple ACTH and ANG II receptor activation to corticosteroid synthesis, an important role for ion channels and depolarization-dependent Ca2+ entry has been established. Early intracellular recording from zona fasciculata tissue and isolated rabbit, cat, bovine, rat, and mouse AZF cells showed that these cells maintained membrane potentials (Vm) determined by the resting K+ permeability (36, 38, 39, 46, 51). Furthermore, ACTH depolarized and triggered action potential-like waveforms in mouse and rabbit AZF cells (36, 38, 39). Ca2+-dependent action potentials have also been recorded in cat, rat, and bovine AZF cells (1, 46, 51).
Studies combining patch-clamp recording and molecular cloning have identified Ca2+ and K+ channels expressed by bovine AZF cells. Specifically, bovine AZF cells express voltage-gated Cav3.2 and Kv1.4 channels and a novel leak-type K+ channel that set their resting Vm (41, 42, 44). The leak-type K+ channel, later identified as TREK-1 of the two-pore K+ channel family, was potently inhibited by ACTH and ANG II, producing membrane depolarization (14, 18, 41). Based on these findings, we proposed a model for cortisol secretion wherein ACTH and ANG II receptor activation was coupled to membrane depolarization and the activation of Cav3.2 channels through TREK-1 inhibition (14, 17, 33). The inhibition of ACTH- and ANG II-stimulated cortisol secretion by organic Ca2+ antagonists at concentrations that blocked Cav3.2 channels provided support for this model (17).
Although these studies significantly advanced our understanding of the role of electrical events, ion channels, and Ca2+ in corticosteroid secretion, major questions remained unanswered. First, although the inhibition of cortisol secretion by organic Ca2+ antagonists indicates a requirement for Ca2+ in ACTH- and ANG II-stimulated corticosteroidogenesis, these findings did not determine whether increasing intracellular Ca2+ concentration is sufficient to stimulate cortisol production. In this regard, the complex signaling pathways activated and the multiple second messengers generated in response to ACTH and ANG II receptor activation lead to rapid and delayed increases in cortisol synthesis, involving protein phosphorylation and the transcription of genes coding for steroidogenic proteins (26, 48, 54, 58). Because of the complexity of these responses, it is difficult to assign specific steroidogenic actions to Ca2+ in these cells.
The identity of the voltage-sensitive Ca2+ channels involved in regulating cortisol secretion has also been controversial. Although low voltage-activated T-type Ca2+ channels are the most prominent channels in nearly all bovine, human, and rat AZF cells, L-type Ca2+ channels are also expressed in at least some of these cells (15, 42, 60). Confusion regarding the role of L-type Ca2+ channels in the regulation of glucocorticoid secretion by ACTH and ANG II arises from several sources. First, in standard whole cell patch-clamp recordings, L-type Ca2+ currents often “run down” rapidly, coincident with the diffusion-controlled dilution of cytoplasmic components essential for L-type channel activity (28). The time-dependent decrease in L-type channel activity is therefore inversely related to cell size and slowed by patch pipettes with smaller tips (24). The rundown of L-type currents can also be delayed by strongly buffering intracellular Ca2+ to low levels, with Ba2+, rather than Ca2+, as the charge carrier, and by including 5–20 mM ATP in the pipette solutions (28).
A previous study of AZF Ca2+ channels focusing on the voltage-dependent gating and kinetic properties of T-type channels was done under conditions that did not promote the activity of L-type channels (42). Accordingly, in a large fraction of cells, only a T-type current later identified as Cav3.2 was detected. Later, in perforated patch-clamp recordings of bovine AZF cells, a small, noninactivating L-type current was detected in ∼40% of tested cells (22).
Previous studies aimed at determining the role of specific Ca2+ channel subtypes in ACTH- and ANG II-stimulated cortisol secretion have been compromised by the limited specificity of organic Ca2+ antagonists available at the time. In particular, dihydropyridines (DHPs) were introduced as extremely potent, selective L-type channel antagonists (53). However, in addition to inhibiting L-type Ca2+ channels, many DHPs are also relatively potent T-type Ca2+ channel blockers (21, 50). In bovine AZF cells, the potency of selected DHP Ca2+ channel antagonists as inhibitors of ACTH-stimulated cortisol secretion was well correlated with the inhibition of T-type Ca2+ current in these cells (17). In recent years, studies have identified DHP antagonists, including nifedipine, that selectively block L-type channels at concentrations that have little or no effect on T-type channels, particularly Cav3.2 (50).
The absence of specific antagonists has also limited our understanding of the role of T-type channels in cortisol secretion. However, organic antagonists reported to potently and selectively block T-type Ca2+ channels have recently become available (9, 11).
In the present study we have characterized the voltage-gated Ca2+ channel currents in bovine AZF cells under conditions that maximized the activity of L-type currents. It was discovered that, in addition to T-type Ca2+ currents, these cells also express two distinctive L-type currents, slowly and rapidly activating currents, both of which are activated at relatively hyperpolarized Vm. Specific antagonists of these T- and L-type currents were identified and then used to determine the contribution of these Ca2+ channel subtypes to ACTH- and ANG II-stimulated cortisol secretion.
MATERIALS AND METHODS
DMEM/F-12, antibiotics, and fetal bovine serum (FBS) were obtained from Invitrogen (Carlsbad, CA); phosphate-buffered saline, bovine plasma fibronectin, tocopherol, selenite, ascorbic acid, 8-bromoadenosine-3′,5′-cyclic monophosphate (8-BrcAMP), MgATP, NaGTP, ACTH-(1–24), ANG II, nifedipine, pimozide, fluspirilene, and (±)-BAY K8644 from Sigma (St. Louis, MO); S-(+)-niguldipine hydrochloride from Santa Cruz Biotechnology (Santa Cruz, CA); and penfluridol from Janssen Pharmaceutical (Beerse, Belgium). 3,5-Dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoro-piperidin-4-ylmethyl]-benzamide (TTA-P2; Alomone Labs, Jerusalem, Israel) was made up as a 10 mM stock solution in dimethyl sulfoxide; aliquots were kept at −20°C and diluted for use as indicated.
Isolation and culture of AZF cells.
Bovine adrenal glands from steers (2–3 yr of age) were purchased from a local slaughterhouse. Isolated AZF cells were obtained and prepared as previously described (16). After isolation, cells were resuspended in DMEM/F-12 (1:1) with 10% FBS (Invitrogen), 100 U/ml penicillin, 0.1 mg/ml streptomycin, and the antioxidants 1 μM tocopherol, 20 nM selenite, and 100 μM ascorbic acid (DMEM/F-12+) and plated for immediate use or resuspended in FBS-5% dimethyl sulfoxide, divided into 1-ml aliquots, and stored in liquid nitrogen for future use. To ensure cell attachment, dishes were treated with fibronectin (10 μg/ml) for 30 min at 37°C and then rinsed with PBS before cells were added. Cells were maintained at 37°C in a humidified atmosphere of 95% air-5% CO2.
Cortisol secretion and assay.
In experiments measuring cortisol secretion, bovine AZF cells were plated in fibronectin-coated 35-mm dishes at a density of 0.2–0.4 × 106 cells per dish in DMEM/F-12+. After a recovery period of 24 h, the medium was changed to defined medium, which consisted of DMEM/F-12 supplemented with 50 μg/ml BSA, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 1× insulin-transferrin-selenium-sodium pyruvate (ITS-A, Life Technologies) with or without additional agents for required times. Medium from experiments was assayed immediately after collection or frozen (−20°C) until all samples were collected. All experimental conditions were performed in triplicate. Cortisol secretion by AZF cells was measured using a cortisol enzyme immunoassay kit (catalog no. 11-CORHU-E01, Alpco Immunoassays, Salem, NH) according to the manufacturer's directions. If necessary, media samples were diluted using defined medium. Statistical analyses (see Figs. 3 and 9–13) were performed using the unpaired Student's t-test for comparison of two data sets or ANOVA with the Holm-Sidak method posttest for all pair-wise multiple comparisons.
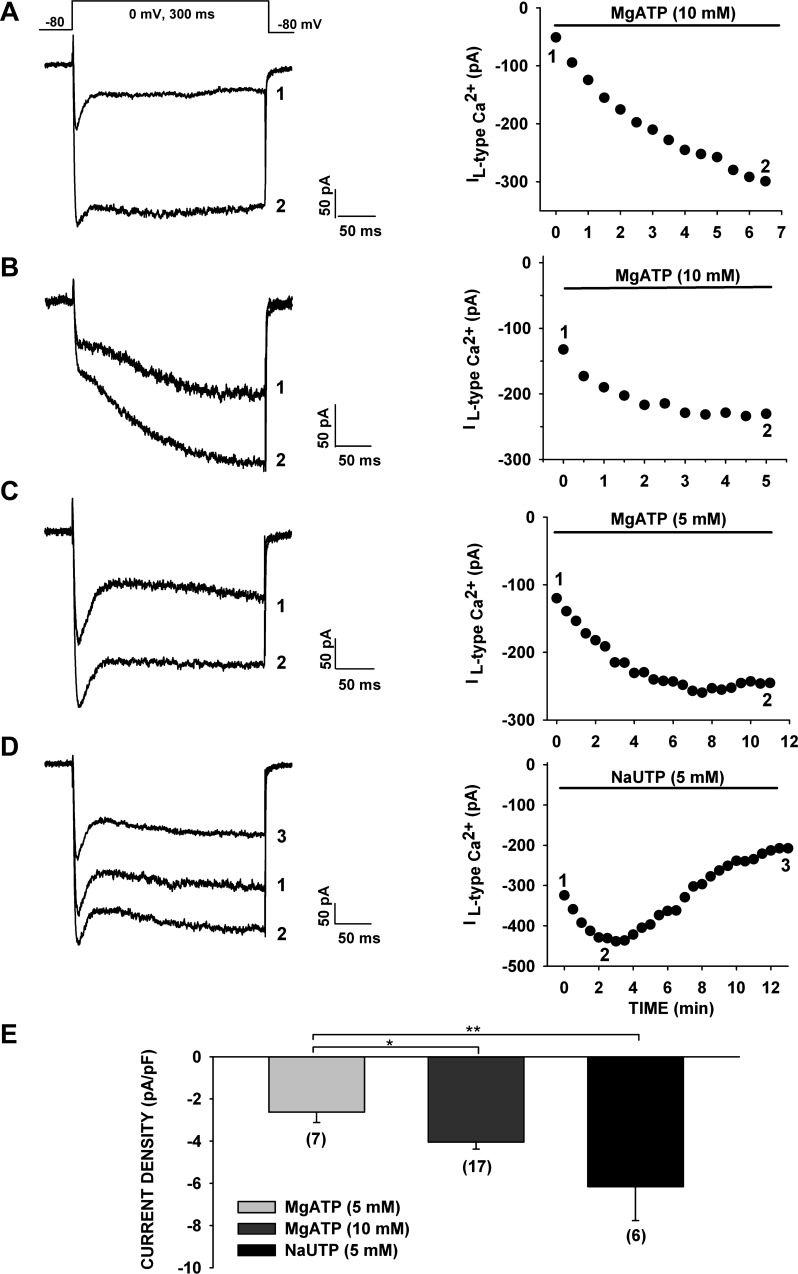
Fig. 3.
Effect of ATP and UTP on the time-dependent increase and rundown of noninactivating Ca2+ channel current. A–D: spontaneous time-dependent growth and decrease of rapidly and slowly activating L-type Ca2+ channel currents for pipette solutions containing 5 or 10 mM MgATP or 5 mM NaUTP. Currents were activated by voltage steps to 0 mV applied at 30-s intervals from a holding potential of −80 mV. Numbers (1, 2, and 3) on current traces (left) correspond to numbers on current amplitude plots (right). Noninactivating Ba2+ current amplitudes were measured by averaging 100 points (of 1,440 points/300 ms) near the end of a 300-ms test pulse. A: time-dependent amplitude of rapidly activating noninactivating current. B: time-dependent amplitude of slowly activating noninactivating current. C and D: currents recorded with pipettes containing 5 mM MgATP or 5 mM NaUTP. E: average maximum current density of noninactivating currents recorded with pipettes containing MgATP (5 or 10 mM) or NaUTP (5 mM). Values are means ± SE for number of determinations shown in parentheses. *P < 0.03, 5 mM MgATP vs. 10 mM MgATP. **P < 0.003, 5 mM NaUTP vs. 5 mM MgATP.
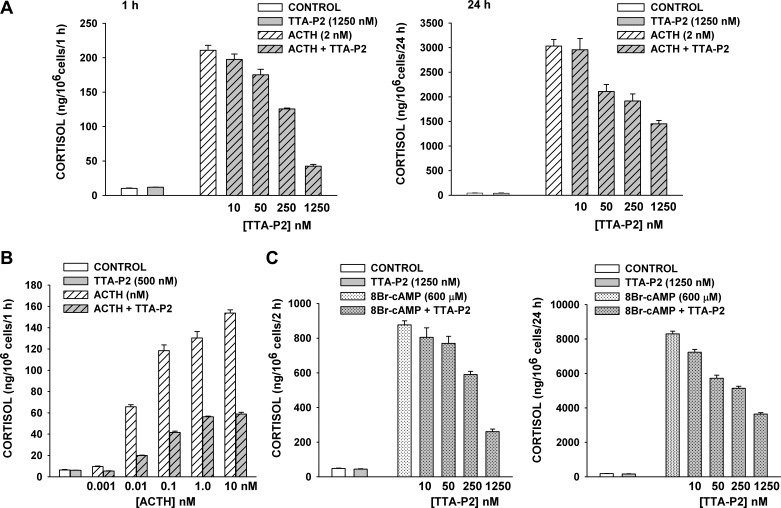
Fig. 9.
Effect of TTA-P2 on ACTH- and 8-BrcAMP-induced cortisol secretion from bovine AZF cells. At 24 h after bovine AZF cells were plated, medium was aspirated and replaced with defined medium without (control) or with ACTH, TTA-P2, or 8-BrcAMP. Samples containing TTA-P2 were pretreated with TTA-P2 alone for 30 min prior to addition of ACTH or 8-BrcAMP. Medium was collected at 1, 2, and 24 h, and cortisol concentration was determined by enzyme immunoassay. Values are means ± SE of duplicate determinations from triplicate plates. A: cortisol from media samples collected 1 h and 24 h after no treatment (control) or treatment with ACTH (2 nM), TTA-P2 (1,250 nM), or ACTH (2 nM) + TTA-P2 (10-1,250 nM). P < 0.001 for ≥50 nM TTA-P2 at 1 and 24 h. B: cortisol from media samples collected 1 h after no treatment (control) or treatment with TTA-P2 (500 nM) or ACTH (0.001–10 nM) + TTA-P2 (500 nM). P < 0.0003 for TTA-P2 inhibition at each ACTH concentration. C: cortisol in media samples collected 2 h and 24 h after no treatment (control) or treatment with 8-BrcAMP (600 μM), TTA-P2 (10-1,250 nM), or 8-BrcAMP (600 μM) + TTA-P2 (1,250 nM). At 2 h, P < 0.0001 for >50 nM TTA-P2; at 24 h, P < 0.003 for all TTA-P2 concentrations.
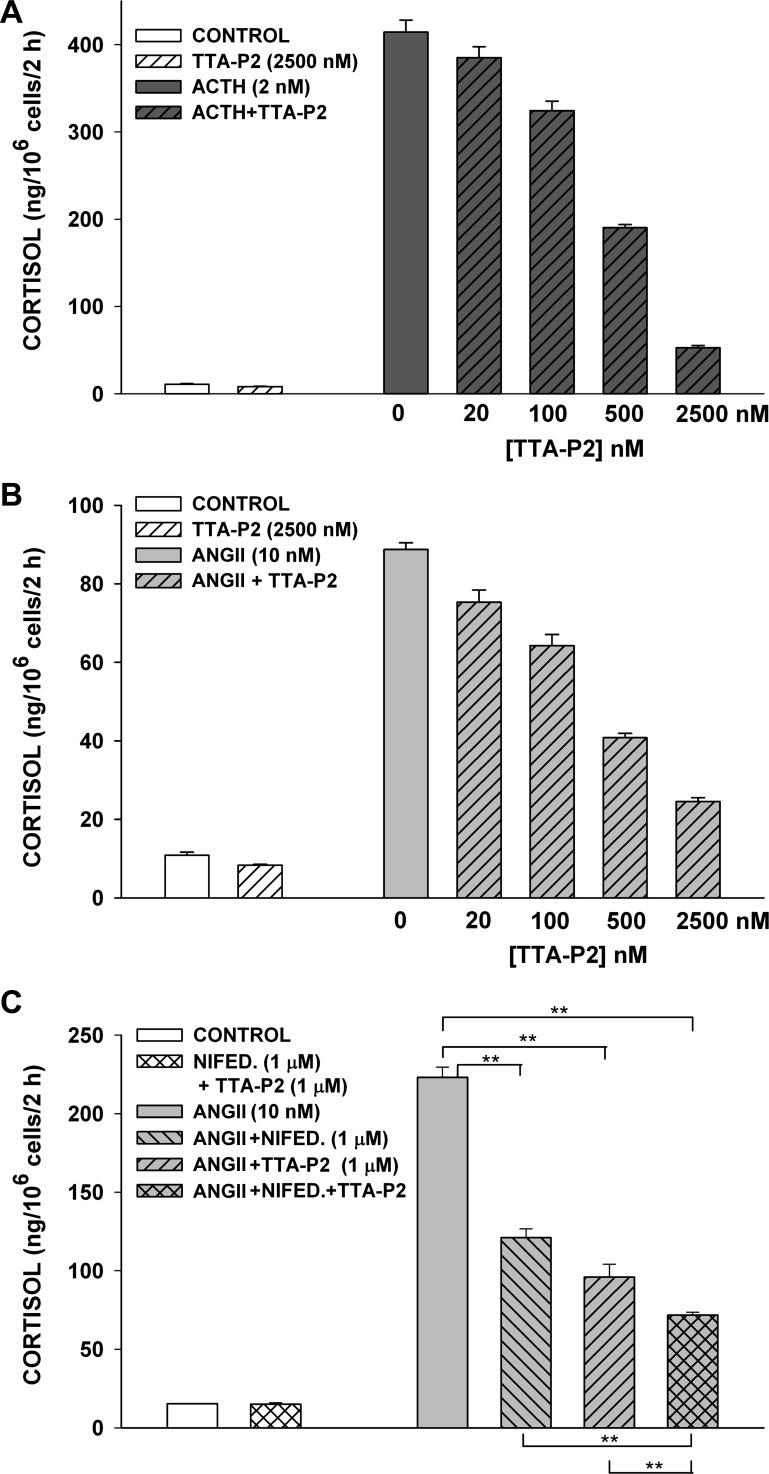
Fig. 13.
Inhibition of ACTH- and ANG II-induced cortisol secretion in bovine AZF cells by TTA-P2 and nifedipine. At 24 h after bovine AZF cells were plated, medium was aspirated and replaced with defined medium without (control) or with ACTH, ANG II, TTA-P2, or nifedipine. Samples containing TTA-P2 or nifedipine were pretreated with that agent alone for 30 min prior to addition of ACTH or ANG II. Medium was collected after 2 h, and cortisol concentration was determined by enzyme immunoassay. Values are means ± SE of duplicate determinations from triplicate plates. A: cortisol was measured from media samples collected 2 h after no treatment (control) or treatment with ACTH (2 nM) or ACTH (2 nM) + TTA-P2 (20-2,500 nM). P < 0.002 for >100 nM TTA-P2. B: cortisol was measured from media samples collected 2 h after no treatment (control) or treatment with ANG II (10 nM) or ANG II (10 nM) + TTA-P2 (20-2,500 nM). P < 0.009 for ≥20 nM TTA-P2. C: cortisol was measured from media samples collected 2 h after no treatment (control) or treatment with nifedipine (1 μM) + TTA-P2 (1 μM), ANG II (10 nM), or ANG II (10 nM) + nifedipine (1 μM) and/or TTA-P2 (1 μM). **P < 0.02.
Northern blot hybridization and measurement of mRNA.
Total RNA isolation and Northern blot procedures are described elsewhere (12). Briefly, 5–7 × 106 AZF cells were plated on 60-mm fibronectin-treated dishes in DMEM/F-12+. After 24 h, the medium was replaced with defined medium without (control) or with ACTH-(1–24) or 8-BrcAMP as required. At the end of the incubation period, total RNA was extracted using RNeasy columns (Qiagen, Valencia, CA), electrophoresed on a denaturing gel containing 10 μg of total RNA per lane, and transferred to a nylon transfer membrane (GeneScreen Plus, PerkinElmer Life Sciences, Waltham, MA). Cav1.3 probe was generated by RT-PCR using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI), specific primers for bovine Cav1.3, and total RNA isolated from bovine AZF cells as described above. Cav1.3 primers (AGTCCTCTTGGCTCTGTTCACCTG and AACCTTGTTGTCACTGTTGGCTATCTG) were obtained from the National Center for Biotechnology Information [accession no. NM_001193025.1; Bos taurus Ca2+ channel, voltage-dependent L-type α1D-subunit (CACNA1D) mRNA]. This ∼800-bp probe is specific for bovine CACNA1D mRNA and has low homology to other L-type Ca2+ channel mRNAs. Cav1.1-specific probe was generated from CACNA1S cDNA (catalog no. EMM1002-213248710, Thermo Fisher Scientific). Cav1.1 and Cav1.3 probes were labeled with [α-32P]dCTP by random primer labeling (Prime-It II, Stratagene, La Jolla, CA). Northern autoradiograms were imaged using a Typhoon 9200 variable-mode imager and quantitated using ImageQuant TL v2003.3 software (GE Healthcare Life Sciences, Piscataway, NJ).
Ca2+ channel current recording.
Patch-clamp recordings of voltage-gated Ca2+ currents from bovine AZF cells were made in the whole cell configuration as previously described with modifications to maximize L-type current expression and minimize rundown (42). The standard pipette solution consisted of 120 mM CsCl, 1 mM CaCl2, 2 mM MgCl2, 11 mM 1,2,-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 10 mM HEPES, 10 mM MgATP, and 0.2 mM GTP, with pH titrated to 7.2 with CsOH (11 mM BAPTA pipette). In some experiments, a second pipette solution containing 120 mM CsCl, 0.16 mM CaCl2, 2 mM MgCl2, 2 mM EGTA, 10 mM HEPES, 10 mM MgATP, and 0.2 mM GTP, with pH titrated to 7.2 with CsOH, was used (2 mM EGTA pipette). The external solution consisted of 117 mM tetraethylammonium chloride, 5 mM CsCl, 10 mM CaCl2 (Ca2+ ext) or 10 mM BaCl2 (Ba2+ ext), 2 mM MgCl2, 5 mM HEPES, and 5 mM glucose, with pH adjusted to 7.4 with tetraethylammonium hydroxide. Pipette solutions were filtered through 0.22-μm cellulose acetate filters.
Recording conditions and electronics.
At 2–8 hours after they were plated, AZF cells were used for patch-clamp experiments. To minimize Ca2+ channel rundown, whole cell recordings were made from larger AZF cells with use of higher-resistance patch electrodes. Typically, cells with diameters >15 μm and capacitances of 20–35 pF were selected. Coverslips upon which cells were plated (Bellco, Vineland, NJ) were transferred from 35-mm culture dishes to the recording chamber (1.5 ml volume), which was continuously perfused by gravity at a rate of 3–5 ml/min. For whole cell recordings, patch electrodes with resistances of 2.5–3.5 MΩ were fabricated from Corning 0010 glass (World Precision Instruments, Sarasota, FL). These electrodes routinely yielded access resistances of 4–8 MΩ and voltage-clamp time constants of <200 μs. Ca2+ currents were recorded at room temperature (22–25°C) according to the procedure of Hamill et al. (23) using a List EPC-7 patch-clamp amplifier.
Pulse generation and data acquisition were done using a personal computer and pCLAMP software with Digidata 1200 interface (Axon Instruments, Burlingame, CA). Currents were digitized at 2–10 kHz after they were filtered with an eight-pole Bessel filter (Frequency Devices, Haverhill, MA). Summed scaled hyperpolarizing steps of one-fourth pulse amplitude were used to subtract linear leak and capacity currents from current records. Data were analyzed using pCLAMP (Clampfit 9.2) and SigmaPlot (version 11.0) software.
RESULTS
Voltage-gated Ca2+ currents.
When whole cell Ca2+ currents were recorded from smaller AZF cells (<15 pF) in the presence of 10 mM Ca2+ with pipettes containing 1 mM MgATP, a low-voltage-activated, rapidly inactivating T-type Ca2+ current was typically the only measurable current observed (Fig. 1A). In cells where a small noninactivating Ca2+ current was initially present, it ran down within several minutes. In contrast, when recordings were made from larger cells (20–35 pF) with 10 mM Ba2+, in place of Ca2+, and pipettes contained 10 mM MgATP, larger noninactivating currents that did not run down were present in most cells (Fig. 1B). The unidentified noninactivating currents were activated at relatively negative voltages, resembling Cav1.3 or Cav1.4 L-type currents in other cells (29, 31, 37, 40, 59).
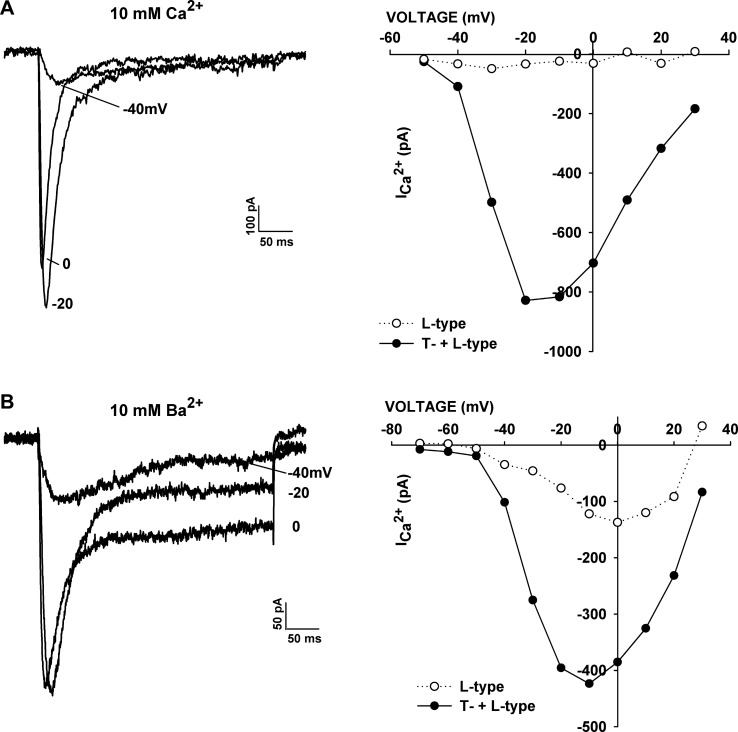
Fig. 1.
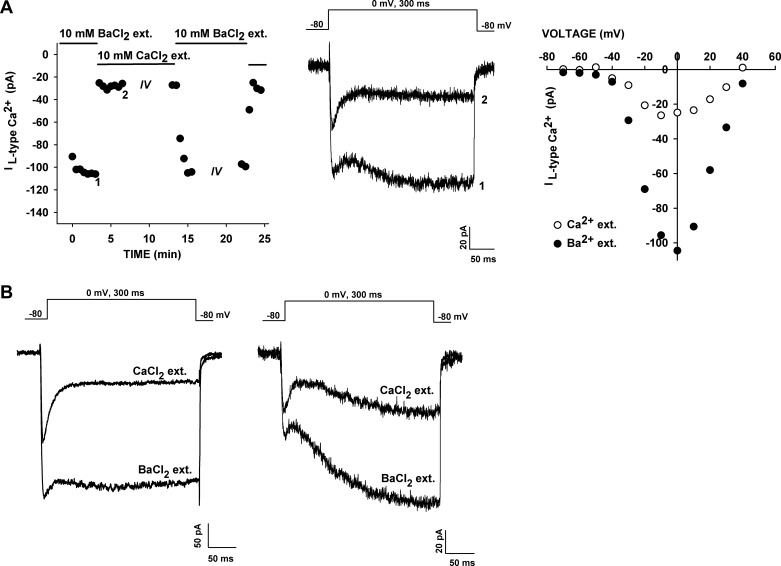
Current-voltage (I–V) relationships in Ca2+ and Ba2+ solutions. A and B: I–V relationships in whole cell patch-clamp recordings from bovine adrenal zona fasciculata (AZF) cells in external solutions containing 10 mM Ca2+ or 10 mM Ba2+. Currents were activated at 30-s intervals by 300-ms voltage steps applied to various test potentials from a holding potential of −80 mV. Current traces were recorded at the indicated test potentials. Amplitudes of peak (T-type + L-type) and noninactivating (L-type) currents (ICa2+) are plotted against test voltage. Noninactivating current amplitudes were measured by averaging 100 points (of 1,440 points/300 ms) near the end of a 300-ms test pulse.
Experiments in which recordings were made from the same cell using both Ca2+- and Ba2+-containing external solutions showed that the noninactivating current was markedly and specifically increased when Ba2+ was used as the charge carrier. In the experiment illustrated in Fig. 2A, substitution of Ca2+ with Ba2+ increased the amplitude of the maximum noninactivating current nearly fivefold, with little or no shift in the voltage-dependent activation. Overall, the Ba2+-to-Ca2+ current amplitude ratio for the noninactivating current was 4.66 ± 0.95 (n = 9). Furthermore, in Ca2+- and Ba2+-containing solutions, the noninactivating current was activated at relatively negative voltages (Fig. 2A, right).
Fig. 2.
Comparison of Ba2+ and Ca2+ conductances. Whole cell Ca2+ channel currents were recorded from AZF cells initially in 10 mM Ba2+ and then in external (ext) solution containing 10 mM Ca2+. A: Ca2+ channel currents were first recorded in 10 mM Ba2+ in response to voltage steps applied at 30-s intervals from a holding potential of −80 mV to a test potential of 0 mV. After substitution of 10 mM Ca2+ for 10 mM Ba2+ in the external solution, a I–V relationship was obtained by application of 300-ms voltage steps at 30-s intervals to various potentials from a holding potential of −80 mV. A second I–V relationship was obtained after return to 10 mM Ba2+ external solution. Left: noninactivating Ca2+ and Ba2+ current amplitudes plotted against time. Middle: Ba2+ (1) and Ca2+ (2) current traces at times indicated at left. Right: I–V relationships for noninactivating L-type Ca2+ and Ba2+ currents. B: Ba2+ and Ca2+ current traces for cells expressing rapidly (left) and slowly (right) activating noninactivating currents.
While recording Ca2+ channel currents from AZF cells, we noticed two distinct types of noninactivating current. One of these was a novel, very slowly activating current that was most easily observed when Ba2+ served as the charge carrier (Fig. 2B, right). In contrast, the activation kinetics of the more rapidly activating component were obscured by the large T-type current expressed in most cells (Fig. 2B, left).
The prominent expression of two types of voltage-gated, noninactivating Ca2+ currents, particularly the novel, very slowly activating current resembling the Cav1.1 L-type current expressed in skeletal muscle, was unexpected. A series of experiments were done in an effort to identify and characterize these currents and determine their role in the physiology of cortisol secretion.
Nucleotide-dependent increase in the noninactivating currents in whole cell recordings.
In many cells, various Ca2+ currents often run down within minutes of initiation of whole cell recordings. Rundown is accelerated when ATP is absent from the pipette and when Ca2+ is the charge carrier, especially in combination with weak intracellular Ca2+ buffering (19, 28). When Ca2+ channel currents were recorded in AZF cells using pipettes containing 10 mM ATP and 11 mM BAPTA, and with 10 mM Ba2+ substituted for Ca2+ in the external solution, the rapidly and slowly activating noninactivating currents increased significantly during the first minutes of recording (Fig. 3). The spontaneous time-dependent increase in the noninactivating currents was most easily observed in recordings from cells that expressed smaller T-type currents. In the experiment illustrated in Fig. 3A, the rapidly activating current increased spontaneously from its initial amplitude of ∼50 pA to >300 pA in 6.5 min. The experiment illustrated in Fig. 3B shows a recording from a cell that appeared to express only rapidly and slowly activating noninactivating currents. The combined current amplitude increased approximately twofold to a stable maximum during a 5-min recording.
Intracellular ATP has been shown to modulate the activity of voltage-activated Ca2+ channels by phosphorylation-dependent and -independent mechanisms (4, 61). We compared the effects of MgATP (5 and 10 mM) and UTP (5 mM) on the time-dependent expression of the noninactivating, presumed L-type Ca2+ currents in AZF cells. Raising the MgATP concentration from 5 to 10 mM increased the maximum noninactivating current density from −2.62 ± 0.50 (n = 7) to −4.05 ± 0.33 pA/pF (n = 17, P < 0.01; Fig. 3, A–C and E).
The noninactivating current reached a significantly larger maximum current density when pipettes contained 5 mM NaUTP than when they contained 5 or 10 mM MgATP (i.e., −6.16 ± 1.60 pA/pF, n = 6). However, the noninactivating current ran down much more rapidly when the pipettes contained NaUTP (Fig. 3, D and E).
Pharmacology of AZF cell Ca2+ channels: block of Ca2+ currents by TTA-P2 and nifedipine.
Previous studies aimed at characterizing the different Ca2+ channels of bovine AZF cells and their specific roles in the physiology of cortisol secretion have been compromised by the limited selectivity of available organic Ca2+ antagonists. TTA-P2 is an organic Ca2+ antagonist reported to selectively block T-type Ca2+ channels in rat sensory and thalamic neurons without affecting high-voltage-activated Ca2+ channels in these cells (9, 11). However, the potency and selectivity of TTA-P2 as an inhibitor of specific T- and L-type Ca2+ channel subtypes have not been established.
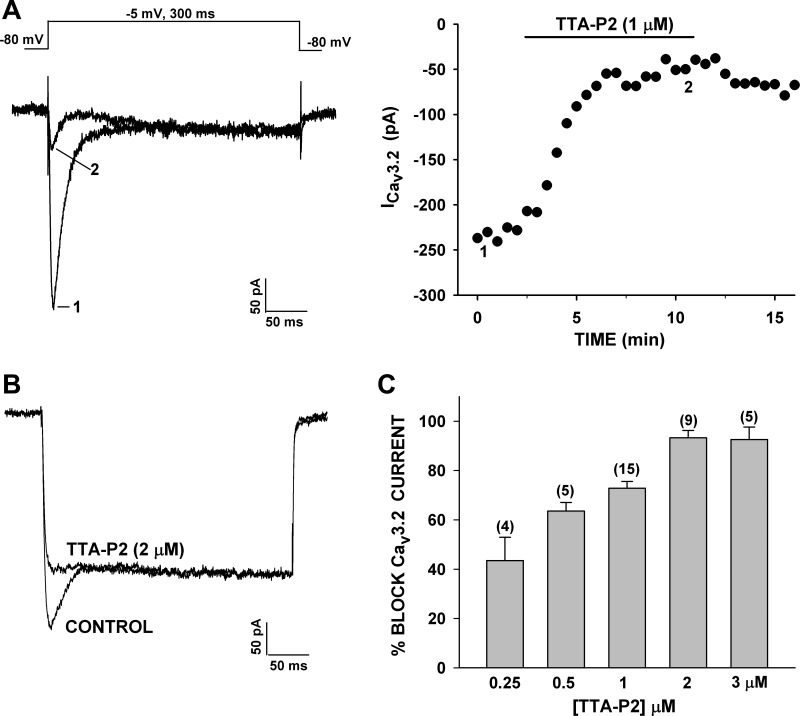
To measure the specific inhibition of Cav3.2 by TTA-P2, currents were recorded in 10 mM Ca2+ to minimize the L-type current. Cells with little or no noninactivating current were chosen for application of the antagonist. In these experiments, the difference between the amplitude of the peak currents before and after superfusion of TTA-P2 was used to calculate percent inhibition of Cav3.2 (Fig. 4A). In other experiments, Ca2+ currents were activated with short (10-ms) depolarizing steps, allowing Cav3.2 to be recorded in isolation as a slowly deactivating tail current (Fig. 5F). Use of these two methods to measure T-type current inhibition by TTA-P2 yielded similar results.
Fig. 4.
Specific block of T-type Ca2+ channel currents by 3,5-dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoro-piperidin-4-ylmethyl]-benzamide (TTA-P2). Concentration-dependent inhibition of Ca2+ channel currents by TTA-P2 was monitored in whole cell recordings. A: Ca2+ currents were recorded in 10 mM external Ca2+ in response to voltage steps to −5 mV from a holding potential of −80 mV before superfusion of the cell with 1 μM TTA-P2. Numbers (1 and 2) on traces (left) correspond to those on plot of peak current amplitude (right). B: Ca2+ currents were recorded in 10 mM Ba2+ in response to voltage steps to −5 mV, applied from a holding potential of −80 mV, before and after superfusion of the cell with 2 μM TTA-P2. Traces were recorded in control saline and after steady-state block by TTA-P2. C: concentration-dependent inhibition of CaV3.2 currents by TTA-P2. Ca2+ currents were recorded in the presence of 10 mM Ca2+ or 10 mM Ba2+. Bars show percent block of CaV3.2 current activated by short (10-ms) or long (300-ms) test pulses at 30-s intervals from −80 mV to test potentials of −5 or 0 mV. Values are means ± SE for number of determinations shown in parentheses.
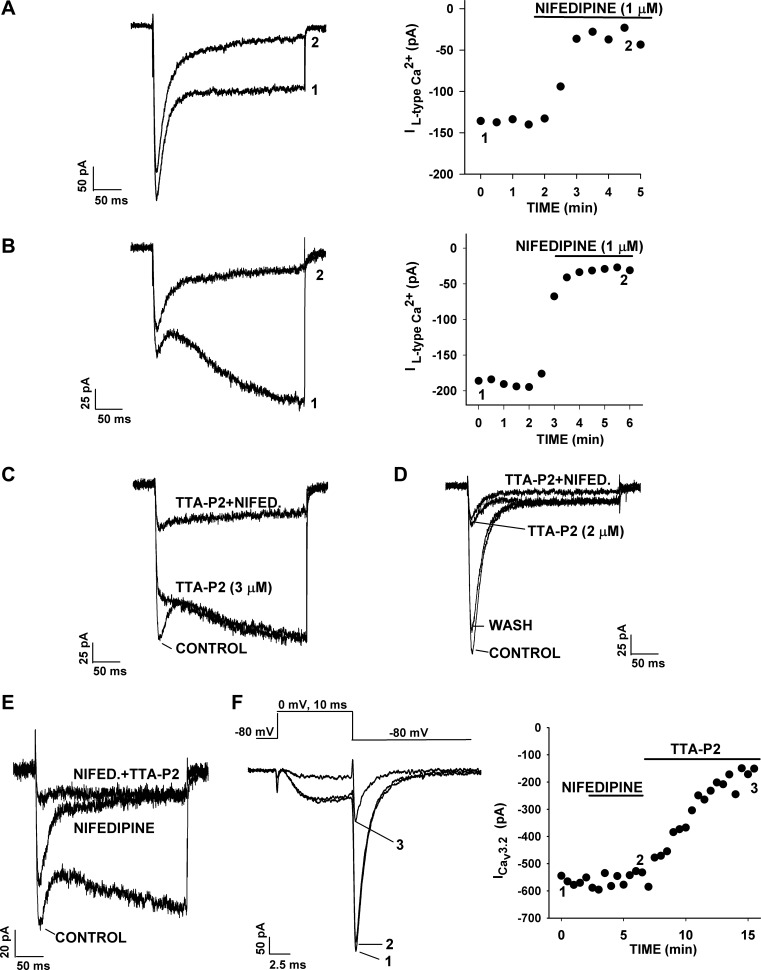
Fig. 5.
Specific inhibition of noninactivating and T-type currents by nifedipine and TTA-P2. Ca2+ channel currents were activated by 300-ms (A–E) or 10-ms (F) voltage steps from a holding potential of −80 mV at 30-s intervals. Cells were superfused with nifedipine or TTA-P2 alone or in combination. A and B: block of rapidly (A) or slowly (B) activating noninactivating L-type currents by nifedipine. Numbers (1 and 2) on traces (left) correspond to those on plot of current amplitudes (right). C and D: block of T-type and rapidly (D) or slowly (C) activating noninactivating Ca2+ currents by TTA-P2 (2 or 3 μM), followed by TTA-P2 + nifedipine (Nifed, 1 μM). E: block of L- and T-type currents by nifedipine (1 μM), followed by nifedipine + TTA-P2 (2 μM). F: block of deactivating T-type current by TTA-P2, but not nifedipine. Deactivating T-type current was recorded in response to 10-ms depolarizing steps. Cell was superfused with nifedipine (1 μM), followed by TTA-P2 (1 μM). Numbers (1, 2, and 3) on traces (left) correspond to those on plot of “tail” current amplitudes (right).
We found that TTA-P2 blocked Cav3.2 channels in bovine AZF cells, with an estimated IC50 of 350 nM. In contrast, at ≤3 μM, TTA-P2 did not affect the noninactivating Ca2+ currents (Fig. 4, A–C). Inhibition of Cav3.2 by TTA-P2 was slowly reversible. Thus, TTA-P2 was determined to be a potent and selective antagonist of Cav3.2 channels in bovine AZF cells.
DHP Ca2+ channel antagonists are among the most potent inhibitors of L-type Ca2+ channel subtypes. However, these DHPs also block T-type Ca2+ channels with variable potency (20, 50). In this regard, nifedipine is significantly less potent than other DHPs as an inhibitor of Cav3.2, blocking these channels with an IC50 of 21.3 μM (50). Nifedipine was used to determine whether the noninactivating currents of AZF cells were authentic L-type currents. Accordingly, nifedipine (1 μM) inhibited the rapidly and slowly activating components of the noninactivating Ca2+ channel currents, measured at the end of a 300-ms test pulse, by ∼90% but did not significantly reduce Cav3.2 (Fig. 5, A and B). In addition to demonstrating the selectivity of nifedipine, this result provides convincing evidence that both of the noninactivating currents in bovine AZF cells were due to DHP-sensitive L-type Ca2+ channels.
Other experiments in which TTA-P2 and nifedipine were applied individually and then in combination further demonstrate the selectivity of these agents and show that together they blocked T- and L-type currents in AZF cells reversibly and almost completely (Fig. 5, C–E). The experiment illustrated in Fig. 5F shows the separate effects of nifedipine and TTA-P2 on the slowly deactivating Cav3.2 “tail” current, activated in response to short (10-ms) depolarizing voltage steps. While nifedipine (1 μM) failed to reduce the deactivating tail current, TTA-P2 (1 μM) inhibited it by 80%.
Variability of expression and voltage-dependent activation of T- and L-type currents.
The recording of Ca2+ channel currents from AZF cells revealed significant variability in the fraction of cells expressing T- and L-type channels. While Cav3.2 was expressed in 97% (229 of 236) of cells, it was the sole measurable Ca2+ current in 18% (23 of 236) of these cells. By comparison, the slowly activating L-type current was recorded in 45.3% (107 of 236) of AZF cells. The rapidly activating L-type current was present in 82.2% (106 of 129) of cells where no slowly activating current was detectable. While the rapidly activating L-type current was also clearly expressed with the slowly activating current in many cells, the precise number of cells expressing both currents could not be determined with certainty (see below).
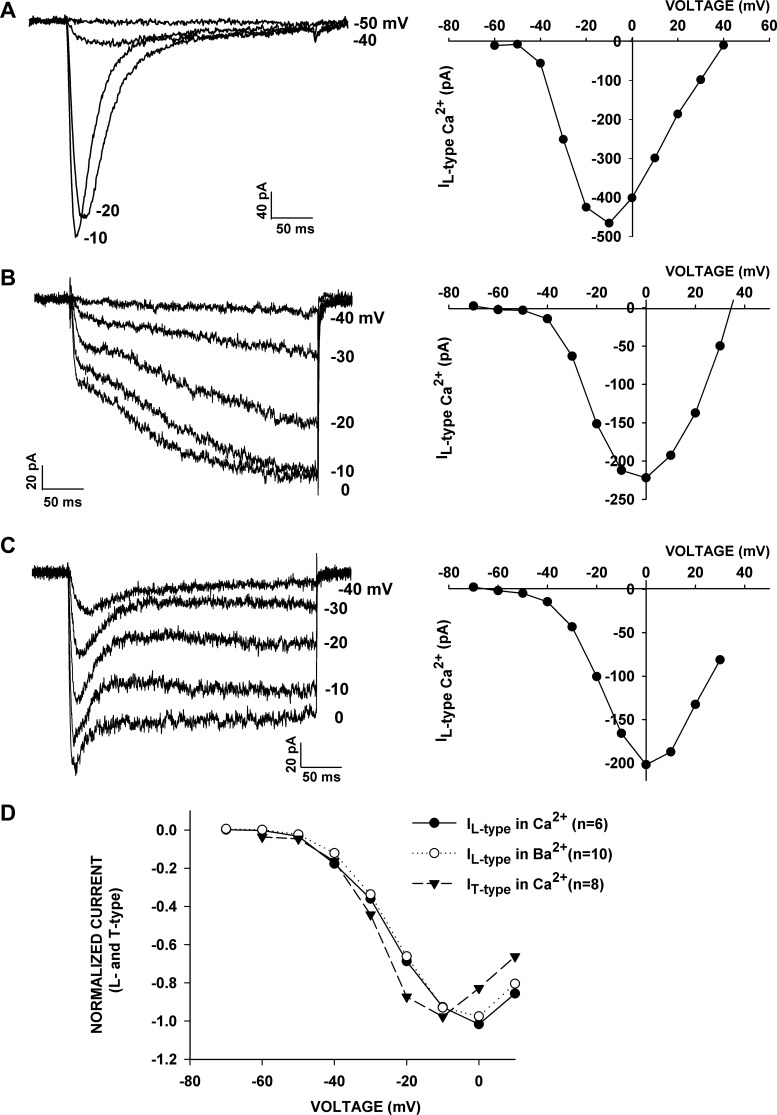
Surprisingly, the recording of current-voltage (I–V) relationships from cells that allowed the unambiguous measurement of T- and L-type currents indicate that each of the three channels is activated with similar voltage dependence. The experiments illustrated in Fig. 6 show I–V relationships for the Cav3.2 current and the slowly activating and rapidly activating L-type currents. For each cell, distinct inward inactivating or noninactivating currents were activated at potentials positive to −50 mV, while the maximum currents occurred at −10 mV for Cav3.2 and 0 mV for both L-type currents.
Fig. 6.
Similar voltage-dependent activation of T- and L-type currents. I–V relationships were obtained in whole cell recordings from AZF cells with external solutions containing 10 mM Ca2+ or 10 mM Ba2+. Currents were activated by 300-ms voltage steps applied at 30-s intervals to various test potentials from a holding potential of −80 mV. A: I–V relationship recorded in 10 mM Ca2+ for T-type current. B and C: I–V relationships recorded in 10 mM Ba2+ for slowly and rapidly activating L-type currents. Current amplitudes are averages of 100 (of 1,440) points near the end of a 300-ms test pulse. D: voltage dependence of T- and L-type currents in Ba2+ or Ca2+. Ca2+ and Ba2+ currents were activated as described above in 10 mM Ca2+ or 10 mM Ba2+. Normalized current amplitudes were averaged and plotted against test potentials for T-type currents in Ca2+, L-type currents in Ca2+, and L-type currents in Ba2+.
In Fig. 6D, the voltage-dependent activation of T-type currents recorded in 10 mM Ca2+ and L-type currents recorded in 10 mM Ca2+ or 10 mM Ba2+ are compared. The results show mean normalized currents for 6–10 cells plotted against test potential at −60 to +10 mV. These results show very little difference between voltage-dependent activation of L-type currents recorded in either Ca2+ [half-maximal potential (V½) = −25.6 mV] or Ba2+ (V½ = −25.0 mV). T-type Ca2+ currents recorded in 10 mM Ca2+ activated at slightly more negative potentials, with a V½ of −28.5 mV.
Voltage-dependent activation kinetics of L-type current.
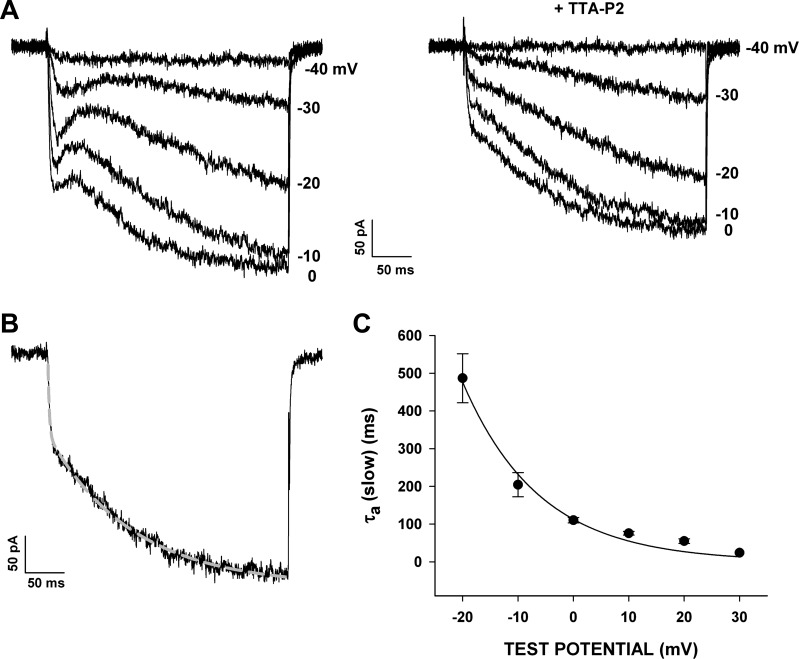
Even though the great majority of AZF cells express both T- and L-type currents that are activated at similar voltages, the potent and specific block of Cav3.2 by TTA-P2 allowed the slowly and rapidly activating L-type currents to be studied separately. The traces in Fig. 7A show currents recorded at various test potentials, before and after superfusion of the cell with TTA-P2 (2 μM).
Fig. 7.
Voltage-dependent activation kinetics of L-type currents. Voltage-dependent activation kinetics of rapidly and slowly activating L-type currents were determined using current traces from I–V relationships recorded in 10 mM Ba2+ after block of T-type current with TTA-P2 (2 μM). Individual traces were fit with an equation of the following form: I = IF(1 − e−t/τF) + IS(1 − e−t/τS), where IF and IS are fractions of L-type currents activated with fast and slow kinetics and τF and τS are the corresponding fast and slow time constants. A: AZF cell Ba2+ currents recorded at various test potentials before (left) and after (right) superfusion of the cells with TTA-P2 (2 μM). B: current trace and corresponding fit illustrating rapidly and slowly activating L-type currents in response to voltage steps to 0 mV. C: activation time constants (τS, means ± SE) obtained at various test potentials for 3 different cells plotted as a function of test voltage and fit with a single-exponential function.
The voltage-dependent activation kinetics of the rapidly and slowly activating L-type currents were characterized by fitting noninactivating currents with an equation of the following form: I = IF(1 − e−t/τF) + IS(1 − e−t/τS), where IF and IS are the fractions of L-type currents activated with fast and slow kinetics. As illustrated in Fig. 7B, L-type Ca2+ currents in AZF cells were activated with two markedly different time constants. Ca2+ channel currents were recorded in response to voltage steps to 0 mV in the presence of TTA-P2 (2 or 3 μM). In each of 17 cells expressing a slowly activating L-type current, a rapidly activating component of noninactivating current was also present. Overall, 61% of the L-type current in these cells activated with a fast time constant (τF) of 2.31 ± 0.2 ms, while 39% activated with a slow time constant (τS) of 120 ± 24.6 ms.
When measured at various test voltages, τS values varied from 486.6 ± 65.0 ms at −20 mV to 54.8 ± 6.1 ms (n = 4) at +30 mV. The function relating τS and Vm could be expressed as a single exponential with an e-fold change per 13.9 mV and a voltage-independent offset of 36 ms (Fig. 7C). Over this same range of test potentials, τF showed little voltage dependence and varied only from 1.88 ± 0.46 ms at −20 mV to 1.30 ± 0.27 ms (n = 4) at + 20 mV. These results demonstrate that bovine AZF cells express two L-type Ca2+ currents, the voltage-dependent activation kinetics of which can vary by >2 orders of magnitude. They also indicate that, in a great majority of cells expressing the slowly activating L-type current, a larger component of rapidly activating current is also present.
Identifying the L-type Ca2+ channels in AZF cells.
DHP-sensitive Ca2+ currents of bovine AZF cells displayed a distinctive combination of properties, including activation at negative voltages, spontaneous “run-up” in whole cell recordings, and extremely slow activation kinetics, which, taken together, do not match the profile of any of the four known L-type Ca2+ channels. Of particular interest, the extremely slowly activating L-type current in AZF cells resembles the Cav1.1 current, which is believed to be expressed exclusively in skeletal muscle.
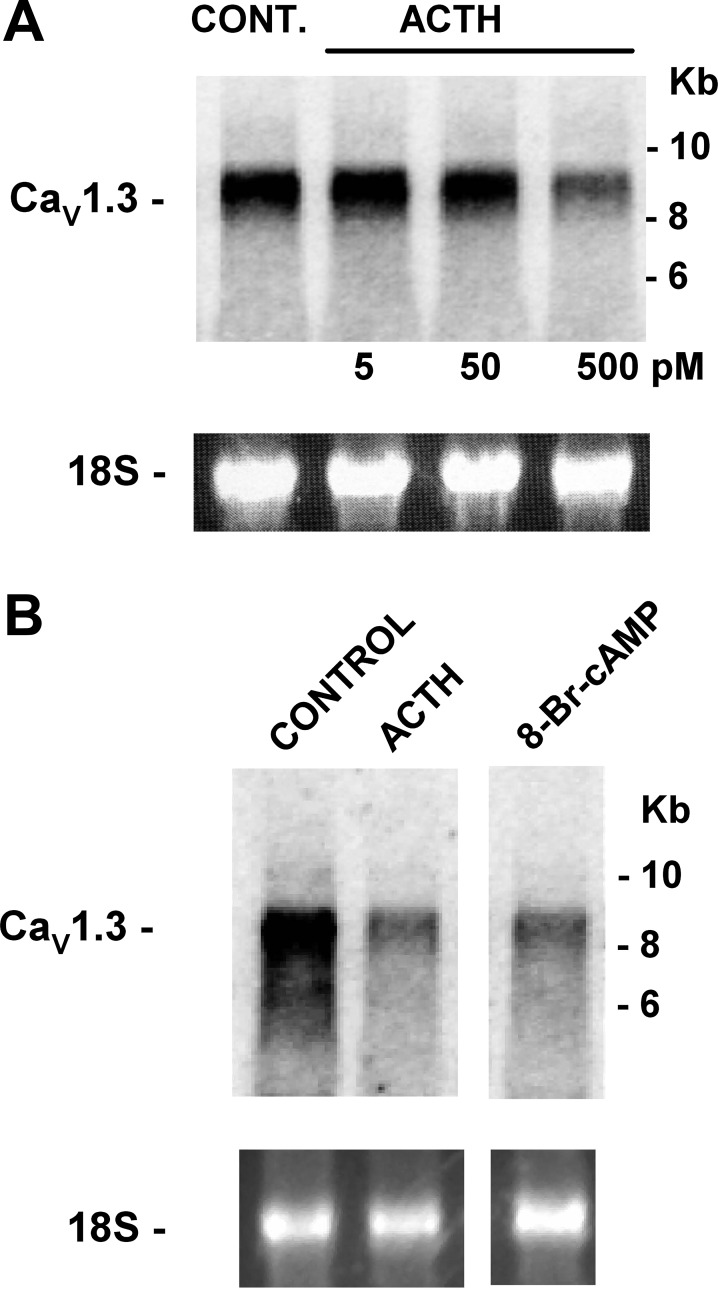
In nested-primer RT-PCR experiments using specific primers for bovine Cav1.1 [accession no. XM_002694242.2 (National Center for Biotechnology Information), B. taurus Ca2+ channel, voltage-dependent, L-type, α1S-subunit (CACNA1S)] and Northern blot experiments using a skeletal muscle Cav1.1 probe, we found that cDNA and mRNA for Cav1.1 channels were undetectable in bovine AZF cells, while robust expression of total RNA was observed for skeletal muscle (data not shown). In contrast, mRNA for the Cav1.3 channel was highly expressed. Interestingly, after 24 h of incubation, Cav1.3 mRNA was markedly downregulated by ACTH and 8-BrcAMP (Fig. 8).
Fig. 8.
CaV1.3 L-type ion channel gene expression in bovine adrenal cortex as shown by Northern autoradiograms with total RNA isolated from bovine AZF cells isolated and cultured for 24 h. Membranes were hybridized with specific probe for bovine CaV1.3 (CACNA1D) mRNA. Each lane contained 10 μg of total RNA. 18S rRNA bands from respective gels are shown as evidence of even loading. Northern autoradiograms were imaged after 24 h of exposure to an imaging screen. A: bovine AZF cells were incubated without [control (Cont)] or with ACTH (5–500 pM). Total RNA was isolated after 24 h. Northern blots were hybridized with specific probe for bovine Cav1.3 (CACNA1D) mRNA. B: bovine AZF cells were incubated without (control) or with ACTH (2 nM) or 8-bromo-cAMP (8-BrcAMP, 600 μM). Total RNA was isolated after 24 h. Northern blot was hybridized with specific probe for bovine CaV1.3 mRNA.
Regulation of cortisol secretion by T- and L-type Ca2+ channels.
Patch-clamp experiments established that bovine AZF cells express two distinct voltage-gated L-type Ca2+ channels and Cav3.2 T-type channels that are selectively blocked by nifedipine and TTA-P2, respectively. Since ACTH receptor activation is coupled to membrane depolarization and the activation of voltage-gated Ca2+ channels, experiments were done to determine the separate roles of T- and L-type channels in ACTH-stimulated cortisol production. ACTH stimulates cortisol synthesis through two temporally distinct cAMP-dependent mechanisms. The rapid phase occurs within minutes and is mediated by the mobilization and delivery of cholesterol to intracellular sites where steroidogenic enzymes reside (5, 54). The delayed increase in cortisol production is mediated through the enhanced transcription of genes coding for steroidogenic proteins, including steroid hydroxylases, which catalyze the stepwise synthesis of cortisol from cholesterol (26, 54).
TTA-P2 (10-1,250 nM) produced a concentration-dependent inhibition of the rapid and delayed components of ACTH-stimulated cortisol secretion. In the experiment illustrated in Fig. 9A, TTA-P2 inhibited ACTH-stimulated cortisol secretion at 1 and 24 h, with a potency similar to that observed for the inhibition of Cav3.2 current. At 1,250 nM TTA-P2, secretion was reduced by 79.9 ± 1.2% and 52.1 ± 2.2% at 1 and 24 h, respectively. Similar results were obtained in each of three experiments. These findings indicate that a large fraction of the rapid and delayed components of ACTH-stimulated cortisol secretion depends on Ca2+ entry into AZF cells through Cav3.2 channels.
Previously, we showed that ACTH inhibits TREK-1 and depolarizes bovine AZF cells, with IC50 values of 4.5 and 10.4 pM, respectively (41). In the present study, we found that TTA-P2 effectively inhibited cortisol secretion over a wide range of ACTH concentrations. In the experiment illustrated in Fig. 9B, TTA-P2 (500 nM) reduced secretion stimulated by ACTH (1 pM–10 nM) by 44.8–61.7%.
In AZF cells, ACTH stimulates the activation of adenylate cyclase and the synthesis of cAMP, which mediate biochemical and ionic events, leading to cortisol synthesis (54). In bovine and human AZF cells, inhibition of TREK-1 by cAMP leads to membrane depolarization and the activation of voltage-gated Ca2+ channels (17, 33, 41). Accordingly, we found that 8-BrcAMP stimulated large rapid and sustained increases in cortisol synthesis, which were inhibited by TTA-P2, with potency and effectiveness similar to that observed for the inhibition of ACTH-stimulated secretion. The experiment illustrated in Fig. 9C shows that TTA-P2 (10-1,250 nM) inhibited 8-BrcAMP-stimulated cortisol secretion at 2 and 24 h. At 2 and 24 h, 1,250 nM TTA-P2 reduced stimulated cortisol secretion by 70.3 ± 1.8% and 56.1 ± 1.0%, respectively.
Nifedipine inhibits ACTH-stimulated cortisol secretion.
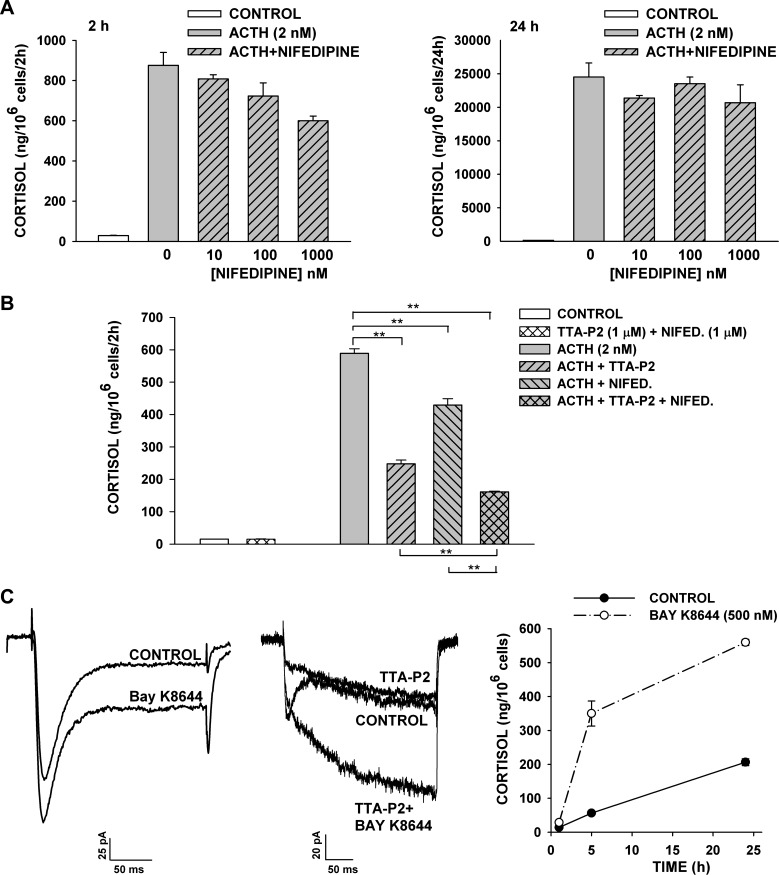
In patch-clamp experiments, we found that nifedipine potently inhibited rapidly and slowly inactivating L-type channels at concentrations that did not affect the Cav3.2 current. At 10 nM–1 μM, nifedipine inhibited ACTH-stimulated cortisol secretion, but less effectively than TTA-P2 (Fig. 10A). In this experiment, nifedipine (1 μM) inhibited ACTH-stimulated cortisol secretion at 2 and 24 h by 31.6 ± 2.7% and 15.6 ± 10.9%, respectively. In three experiments, nifedipine (2.5 μM) inhibited ACTH-stimulated cortisol secretion at 2 and 24 h by 24.0 ± 4.2% and 20.5 ± 5.0%, respectively.
Fig. 10.
Effect of nifedipine, TTA-P2, and BAY K8644 on cortisol secretion and L-type currents. At 24 h after bovine AZF cells were plated, medium was aspirated and replaced with defined medium without (control) or with ACTH, nifedipine, TTA-P2, or BAY K8644. Samples containing TTA-P2 or nifedipine were pretreated with agent alone for 30 min prior to addition of ACTH. Medium was collected at 2 and 24 h, and cortisol concentration was determined by enzyme immunoassay. Values are means ± SE of duplicate determinations from triplicate plates. A: cortisol from media samples collected 2 h and 24 h after no treatment (control) or treatment with ACTH (2 nM) or ACTH + nifedipine (1-1,000 nM). P < 0.007 for 1,000 nM nifedipine at 2 h. B: cortisol from media samples collected 2 h after no treatment (control) or treatment with TTA-P2 (1 μM) + nifedipine (1 μM), ACTH (2 nM), or ACTH (2 nM) + TTA-P2 (1 μM) and/or nifedipine (1 μM). **P < 0.001 for TTA-P2, nifedipine, and TTA-P2 + nifedipine inhibition of ACTH-stimulated secretion. C: BAY K8644 increases rapidly and slowly activating components of L-type current in AZF cells and stimulates cortisol secretion. Ca2+ currents were recorded in 10 mM Ba2+ in response to voltage steps to −5 mV, applied from a holding potential of −80 mV, before superfusion of the cell with 1 μM BAY K8644 (left) or 2 μM TTA-P2 followed by 2 μM TTA-P2 + 1 μM BAY K8644 (middle). Right: cortisol measured from media samples collected 0–25 h after no treatment (control) or treatment with BAY K8644 (500 nM).
In the same experiments, TTA-P2 was significantly more effective than nifedipine as an inhibitor of rapid and delayed ACTH-stimulated cortisol synthesis. In the experiment illustrated in Fig. 10B, TTA-P2 (1 μM) and nifedipine (1 μM), applied separately, inhibited ACTH-stimulated cortisol secretion at 2 h by 58.0 ± 2.0% and 27.2 ± 3.3%, respectively. When TTA-P2 and nifedipine were used in combination, inhibition increased to 72.6 ± 0.4%. Similar results were obtained in each of three separate experiments with TTA-P2 and nifedipine, used alone or in combination, each at 500 nM or 1 μM.
BAY K8644 enhances L-type currents and the rapid phase of cortisol secretion.
Experiments with nifedipine identified a role for L-type Ca2+ channels in ACTH-stimulated cortisol secretion. Accordingly, we found that the DHP Ca2+ channel agonist BAY K8644 increased both the rapidly and slowly activating components of noninactivating current in AZF cells and also stimulated cortisol secretion (Fig. 10C). However, in contrast to ionomycin, the BAY K8644-induced increase in secretion persisted for <5 h. These results also provide further proof that both noninactivating currents were DHP-sensitive L-type currents.
Effect of TTA-P2 and nifedipine on ionomycin-stimulated cortisol secretion.
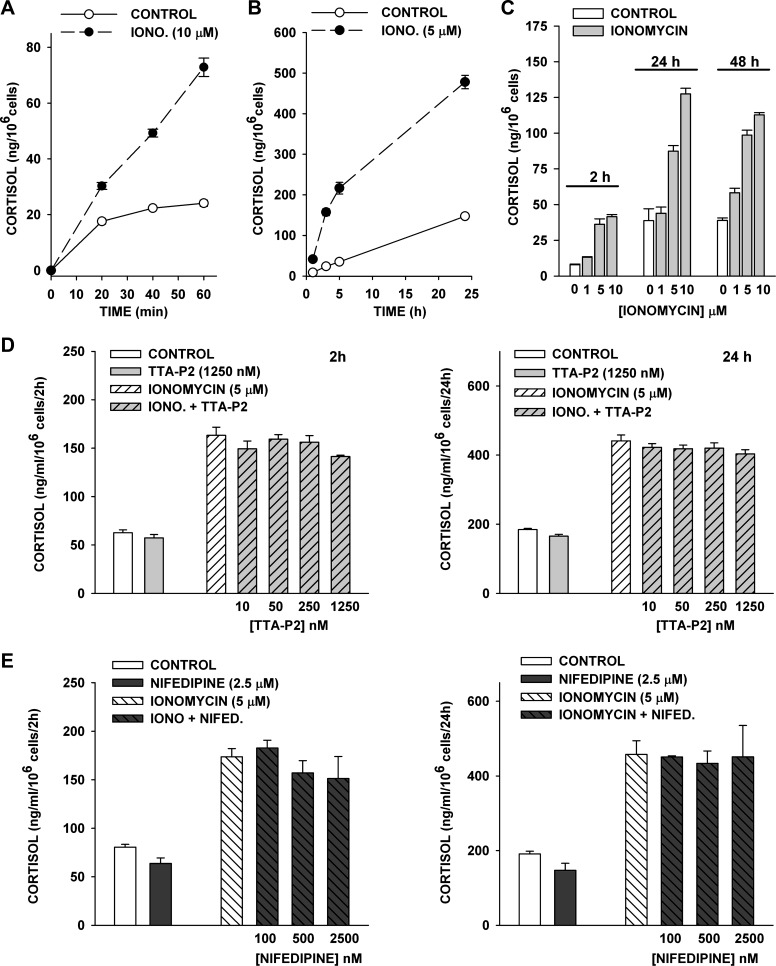
The inhibition of ACTH-stimulated cortisol secretion by TTA-P2 and nifedipine indicates that these agents likely function by blocking depolarization-dependent Ca2+ entry through T- and L-type Ca2+ channels. However, these results do not exclude the possibility that these agents act at other Ca2+-dependent sites in the pathway leading to cortisol synthesis. The Ca2+ ionophore ionomycin stimulates Ca2+ influx and release from intracellular stores independently of any direct effect on voltage-gated Ca2+ channels. We used ionomycin to determine whether TTA-P2 or nifedipine suppressed Ca2+-stimulated cortisol secretion independent of their inhibition of T- and L-type channels in AZF cells.
At 1–10 μM, ionomycin stimulated concentration-dependent increases in cortisol production. Increases in the rate of cortisol secretion were observed within 20 min and persisted for ≥24 h in defined medium (Fig. 11, A–C). These experiments indicate that increasing Ca2+ concentration in bovine AZF cells is sufficient to produce rapid and sustained increases in cortisol secretion.
Fig. 11.
Effect of TTA-P2 and nifedipine on ionomycin-stimulated cortisol secretion. At 24 h after bovine AZF cells were plated, medium was aspirated and replaced with medium containing ionomycin or ionomycin + TTA-P2 or nifedipine. Medium was collected at indicated times, and cortisol secretion was determined by enzyme immunoassay. Values are means ± SE of duplicate determinations from triplicate plates. A: cortisol was measured from media samples collected 20, 40, and 60 min after no treatment (control) or treatment with ionomycin (Iono, 10 μM). B: cortisol was measured from media samples collected 1, 3, 5, and 24 h after no treatment (control) or treatment with ionomycin (5 μM). C: cortisol was measured from media samples collected 2, 24, and 48 h after no treatment (control) or treatment with ionomycin (1, 5, or 10 μM). D: effect of TTA-P2. Cortisol was measured from media samples collected 2 h and 24 h after no treatment (control) or treatment with ionomycin (5 μM), TTA-P2 (1,250 nM), or ionomycin (5 μM) + TTA-P2 (1,250 nM). E: effect of nifedipine. Cortisol was measured from media samples collected 2 h and 24 h after no treatment (control) or treatment with ionomycin (5 μM), nifedipine (2.5 μM), or ionomycin (5 μM) + nifedipine (2.5 μM). P > 0.18 for data in D and E.
In contrast to their potent inhibition of ACTH-stimulated cortisol secretion, TTA-P2 and nifedipine were not effective inhibitors of ionomycin-stimulated secretion. At ≤250 nM, TTA-P2 failed to measurably reduce ionomycin-stimulated cortisol production at 2 or 24 h. However, in three experiments, TTA-P2 (1,250 nM) slightly, but not significantly, reduced ionomycin-stimulated cortisol secretion at 2 and 24 h by 14.5 + 0.5% (P > 0.07) and 8.6 + 1.9% (P > 0.1), respectively, while basal secretion was not affected (Fig. 11D).
Similarly, in three experiments, nifedipine at 100-2,500 nM failed to significantly inhibit ionomycin-stimulated cortisol secretion, while at the highest nifedipine concentration, basal secretion was inhibited at 2 and 24 h by 20.8 ± 6.9% and 22.9 ± 9.8%, respectively (Fig. 11E). These results indicate that TTA-P2 and nifedipine inhibit ACTH-stimulated cortisol secretion by blocking Ca2+ influx through specific T- and L-type channels.
Inhibition of Ca2+ channels and ACTH-stimulated cortisol secretion by other organic antagonists.
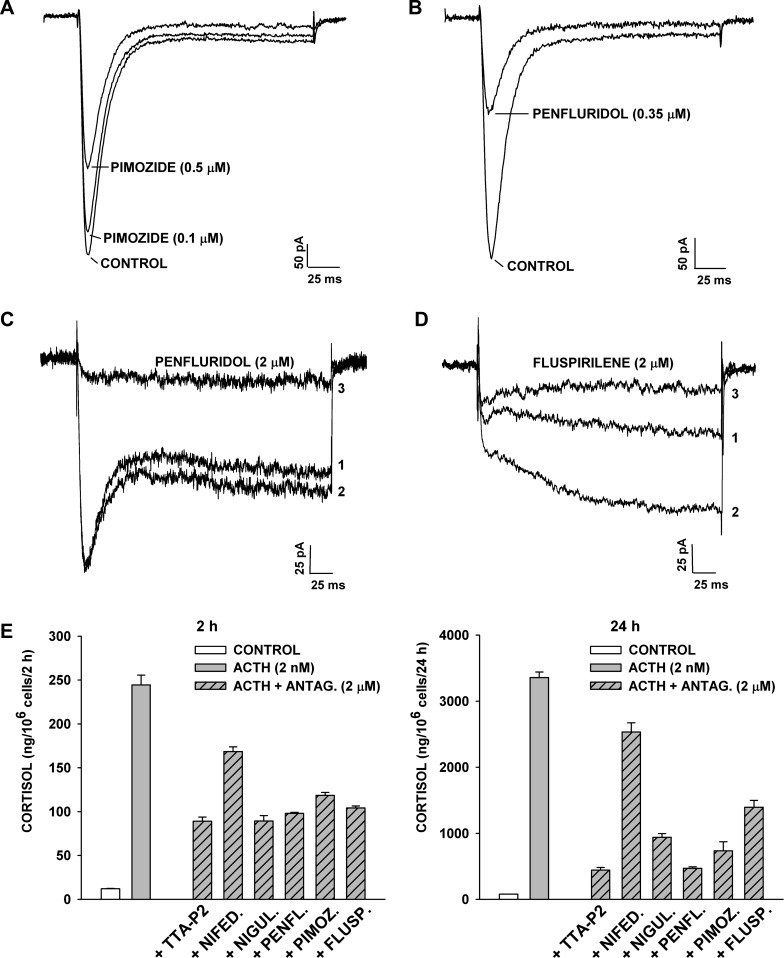
Diphenylbutylpiperidines (DPBPs), including pimozide and penfluridol, have been shown to inhibit T-type Ca2+ currents in bovine AZF cells, with IC50 values of 0.3 and 0.5 μM (17). In the present study we found that these two agents, as well as a third DPBP (fluspirilene), at 0.1–2 μM, also effectively inhibited the rapidly and slowly activating L-type channels of these cells. Of the three antagonists, penfluridol was the most potent, but all three agents inhibited T- and L-type currents by >85% at 2 μM (Fig. 12, A–D).
Fig. 12.
Inhibition of T- and L-type channels and cortisol secretion by diphenylbutylpiperidine antagonists. A–D: inhibition of Ca2+ currents. Whole cell Ca2+ channel currents were recorded from AZF cells in response to voltage steps applied at 30-s intervals from a holding potential of −80 mV. Cells were superfused with pimozide (A), penfluridol (B and C), and fluspirilene (D). External solution contained 10 mM Ca2+ (A and B) and 10 mM Ba2+ (C and D). Numbers on traces in C and D correspond to initial currents (1), maximum control currents (2), and current after block by penfluridol or fluspirilene (3). E: inhibition of ACTH-stimulated cortisol secretion. At 24 h after bovine AZF cells were plated, medium was aspirated and replaced with defined medium without (control) or with ACTH (2 nM), alone or in combination with TTA-P2, nifedipine, niguldipine (Nigul), penfluridol (Penfl), pimozide (Pimoz), or fluspirilene (Flusp), each at 2 μM. Antagonist (Antag)-treated cells were preexposed to that agent for 30 min prior to addition of ACTH. Medium was collected at 2 h and 24 h, and cortisol was measured. Values are means ± SE of duplicate determinations from triplicate plates. P < 0.002 for each antagonist at 2 and 24 h compared with ACTH-stimulated value.
We compared the three DPBPs with TTA-P2 as inhibitors of ACTH-stimulated cortisol secretion. The DPBPs resembled TTA-P2 with respect to their effectiveness as inhibitors of cortisol secretion at 2 and 24 h. They were significantly more effective than nifedipine in this respect (Fig. 12E).
Interestingly, we also discovered that the DHP Ca2+ antagonist niguldipine was far more effective than nifedipine as an inhibitor of cortisol secretion (Fig. 12E). Accordingly, in addition to inhibiting L-type Ca2+ currents, niguldipine is a potent inhibitor of Cav3.2 channels, with a reported IC50 of 0.9 μM (50).
Inhibition of ANG II-stimulated cortisol secretion by TTA-P2 and nifedipine.
Although the signaling pathway activated by ANG II is different from that activated by ACTH in bovine AZF cells, both peptides inhibit TREK-1 channels, leading to membrane depolarization and the activation of voltage-gated Ca2+ channels (14, 41). However, in contrast to ACTH, ANG II induces primarily a short-term increase in cortisol secretion. TTA-P2 inhibited ACTH- and ANG II-stimulated cortisol secretion with similar potency and effectiveness. In the experiment illustrated in Fig. 13, TTA-P2, at 20 nM–2.5 μM, inhibited ACTH- and ANG II-stimulated cortisol secretion similarly. At the maximal concentration, TTA-P2 inhibited ACTH- and ANG II-stimulated secretion by 87.3 ± 0.6% and 72.3 ± 1.1%, respectively (Fig. 13, A and B).
Nifedipine also inhibited ANG II-stimulated cortisol secretion. In the experiment illustrated in Fig. 13C, TTA-P2 (1 μM) and nifedipine (1 μM) inhibited ANG II-stimulated cortisol secretion by 57.0 ± 3.7% and 45.7 ± 2.5%, respectively. In combination, these two antagonists produced greater inhibition than either agent alone (67.8 ± 0.8%).
DISCUSSION
In this study it was discovered that, in addition to Cav3.2, bovine AZF cells express two L-type Ca2+ currents, both of which are activated at relatively hyperpolarized potentials. Patch-clamp and Northern blot experiments indicate that one of these L-type currents is Cav1.3. The second Ca2+ current displayed extremely slow voltage-dependent activation kinetics and combined properties not previously reported for any voltage-gated Ca2+ current. It was also discovered that TTA-P2 and nifedipine potently and specifically blocked the T- and L-type Ca2+ channels of AZF cells at concentrations identical to those that inhibited ACTH- and ANG II-stimulated cortisol secretion.
These results show that bovine AZF cells express a unique group of low-voltage-activated Ca2+ channels, including a novel slowly activating L-type channel, that function pivotally in the physiology of ACTH- and ANG II-stimulated cortisol secretion. They suggest a model wherein ACTH and ANG II receptor activation is coupled to Vm and cortisol secretion through the activation of multiple voltage-gated Ca2+ channels. In this model, activation of either peptide receptor leads to depolarization through inhibition of TREK-1 channels. Within the framework of this model, Ca2+ and cAMP function cooperatively as dual second messengers that couple ACTH receptor activation to rapid and delayed effects on cortisol synthesis.
It is not clear why bovine AZF cells require three separate voltage-gated Ca2+ channels, which are activated with similar voltage dependence but vary widely in their activation and inactivation kinetics. These Ca2+ channels, along with Kv1.4 and TREK-1 channels, may confer electrical properties on these cells that permit tight coupling of the ACTH concentration to Vm and cortisol secretion during the daily diurnal rhythm and under conditions of extreme stress. Ca2+ entry through sustained, graded depolarizations or Ca2+-dependent action potentials are likely possibilities. Regardless of the specific ionic mechanisms, the results of this study establish the importance of electrical events and T- and L-type Ca2+ channels in the physiology of cortisol secretion.
Properties and identity of L-type Ca2+ channels.
Whole cell recordings made under conditions designed to maximize and stabilize the L-type Ca2+ currents allowed us to identify novel properties of these currents not previously reported for L-type channels in these or other cells. The more than fourfold increase in L-type current amplitude observed upon substitution of Ba2+ for Ca2+ in the external solution was entirely due to conductance differences between the two ions, since Ca2+-dependent inactivation was eliminated by strongly buffering Ca2+ with 11 mM BAPTA. The absence of current inactivation during a 300-ms voltage step with Ca2+ as the charge carrier provides convincing evidence for this point.
The unexpected spontaneous run-up of fast and slowly activating L-type currents in whole cell recordings with pipettes containing 5–10 mM MgATP or 5 mM NaUTP indicates that the initial increase in current amplitude depends on nucleotide binding but is not specific for ATP. Furthermore, the increase does not require phosphorylation, since UTP is not a substrate for protein kinases. In contrast, the persistent increase requires ATP and, perhaps, ATP-dependent phosphorylation, since the L-type currents ran down within minutes in the presence of NaUTP. ATP has been reported to regulate cardiac L-type channels by phosphorylation-dependent and -independent mechanisms (4, 61). Whatever the mechanism, addition of ATP (5–20 mM) to the pipette has been reported to delay Ca2+ channel rundown (28).
Of the two L-type Ca2+ currents expressed by AZF cells, the rapidly activating current resembles the Cav1.3 current expressed in neurons, pacemaker cells of the heart, and some endocrine cells (7, 29, 31, 37). In each cell type, Cav1.3 channels are activated at relatively hyperpolarized potentials, display large Ba2+/Ca2+ conductance, and require micromolar concentrations of DHP antagonist for inhibition. Accordingly, in Northern blot experiments, Cav1.3 mRNA was readily detected in AZF cells. Furthermore, of the two L-type Ca2+ channels activated at relatively negative potentials, Cav1.3, but not Cav1.4, was detected in PCR experiments.
The identity of the novel, extremely slowly activating L-type channel is unknown. Although its activation kinetics strongly resemble those of the Cav1.1 skeletal muscle channel, no Cav1.1 mRNA was detected in Northern blot or PCR studies. Furthermore, the muscle Cav1.1 current is activated only at significantly more positive test potentials than the slowly activating current in AZF cells (31).
It is possible that the rapidly and slowly activating L-type currents in AZF cells could be variants of Cav1.3. L-type Ca2+ current activation kinetics are significantly slowed in the absence of an associated β-subunit (6, 49). In addition, skeletal muscle Cav1.1 currents in mouse myocytes have been reported to activate with both fast and slow activation time constants (47). The activation kinetics of the slowly activating L-type current in AZF cells were markedly voltage-dependent but reached a clear minimum at potentials positive to 0 mV, indicating that the gating of this channel involves voltage-dependent and -independent transitions along the pathway from the closed to the open state.
When recorded in 10 mM Ba2+ or Ca2+, the L-type Ca2+ currents in AZF cells were activated at potentials positive to −50 mV. At the more physiological Ca2+ concentration of 2 mM, these L-type currents would have been activated at even more hyperpolarized potentials, since divalent cations shift current-voltage curves to the right in a concentration-dependent manner (55). Under physiological conditions, these channels could be activated by small depolarizations near the resting potential, allowing for sustained, graded Ca2+ entry tightly coupled to Vm. In this regard, in addition to stimulating depolarization-dependent Ca2+ entry through bovine TREK-1 inhibition, ACTH could also enhance Ca2+ influx by shifting the voltage dependence of L-type channel activation in the hyperpolarizing direction.
A slowly activating L-type current has not been described in previous patch-clamp studies on bovine, rat, mouse, or human adrenocortical cells or cell lines. Failure to observe this current in some of these studies may have resulted from suboptimal recording conditions. When conditions were optimized in bovine AZF cells, the slowly and rapidly activating L-type currents spontaneously increase, rather than run down, in whole cell recordings. It is also possible that the slowly activating current is only expressed in AZF cells of some species. Importantly, using the same recording conditions, we recently identified a similar Ca2+ current in normal human AZF cells (unpublished observations).
Ca2+ channel pharmacology and cortisol secretion.
Previously, the lack of specific T-type channel antagonists hampered our ability to study L-type currents and their function in cortisol secretion. The identification of TTA-P2 as a potent and specific T-type channel blocker in AZF cells greatly facilitated our study of Ca2+ channels in these cells. Specifically, in the presence of TTA-P2, L-type Ca2+ currents and, especially, their activation kinetics could be viewed in isolation (Figs. 4 and 7). This was of critical importance, because the presence of the rapidly activating and inactivating T-type current obscured the early activating component of the slowly activating L-type current. The failure to selectively eliminate T-type channel currents in previous studies may have prevented the earlier discovery of the slowly activating L-type current in these or other AZF cells.
The discovery that TTA-P2 blocked Cav3.2 almost completely at concentrations that did not affect either L-type current in AZF cells allowed us to quantify the specific role of these T-type Ca2+ channels in the physiology of ACTH- and ANG II-stimulated cortisol secretion. The inhibition of up to 80% of ACTH- and 8-BrcAMP-stimulated cortisol secretion by TTA-P2 further validated the critical function of these channels in AZF cell physiology.
ANG II-stimulated cortisol secretion was inhibited by TTA-P2, with potency and effectiveness similar to that observed for ACTH. Although ANG II and ACTH function through different G protein-coupled receptors and signaling pathways, both inhibit TREK-1 and depolarize AZF cells similarly (41). Apparently, the activation of Cav3.2 channels is a pivotal mechanism shared by both peptides in stimulating cortisol production.
TTA-P2 was significantly more effective than nifedipine as an inhibitor of ACTH-stimulated cortisol secretion, indicating a more prominent role for T-type channels. This result is consistent with our observation that Cav3.2 is the most highly expressed Ca2+ current in AZF cells.
At low micromolar concentrations, nifedipine blocked both L-type currents in AZF cells with little or no effect on Cav3.2. In whole cell recordings, the extent of block increased markedly during the course of a 300-ms voltage step. This result is consistent with the previously reported voltage-dependent block of L-type Ca2+ channels by DHP antagonists, wherein their potency is markedly increased at depolarized potentials (3). The selectivity of nifedipine, in combination with its enhanced potency at depolarized potentials, makes it an excellent agent for determining the role of L-type channels in ACTH- and ANG II-stimulated cortisol secretion: both of these peptides depolarize bovine AZF cells by a maximum of at least 50 mV from their resting potential of −71 mV (41). Accordingly, at concentrations as low as 10 nM, nifedipine produced significant inhibition of ACTH-stimulated cortisol secretion. As expected, the nonselective DHP niguldipine, which potently blocks Cav3.2, as well as L-type, channels, was significantly more effective than nifedipine as an inhibitor of ACTH-stimulated secretion. The DPBPs also blocked Cav3.2 and rapidly and slowly activating L-type Ca2+ currents. Therefore, they resembled niguldipine and were more effective than nifedipine in inhibiting secretion.
Our observation that ACTH was ≥10 times more effective than ionomycin at stimulating cortisol secretion indicates that Ca2+ and cAMP in combination provide a far more powerful stimulus for secretion than Ca2+ alone. The >80% inhibition of ACTH-stimulated cortisol secretion by TTA-P2 and nifedipine in combination establishes a critical requirement for Ca2+ channels and cAMP in the steroidogenic response.
L-type Ca2+ channels and AZF cell electrical activity.
It is not known why AZF cells express L-type Ca2+ channels that activate with fast or extremely slow kinetics. However, the slowly activating channels would be open during a sustained depolarization but remain closed during single fast action potentials. Perhaps Ca2+ channel subtypes are differentially expressed in the AZF, creating subpopulations of cells with different electrical and secretory properties. Accordingly, the slowly activating L-type channel was detected in 45% of >200 cells.
Control of ion channel gene expression by ACTH.
This and previous studies demonstrate that ACTH and cAMP regulate the expression of genes coding for all the AZF cell Ca2+ and K+ channels. Interestingly, ACTH strongly induces Cav3.2 mRNA and current but suppresses Cav1.3 mRNA (34). Similarly, ACTH increases the expression of bovine TREK-1 but rapidly downregulates Kv1.4 mRNA and current (12, 13). These results indicate that, under conditions of prolonged stress, ACTH could remodel the electrical properties of AZF cells, leading to corresponding changes in cortisol secretion.
Conclusion.
Our results establish critical roles for T- and L-type channels in ACTH- and ANG II-stimulated cortisol secretion in bovine AZF cells. These Ca2+ channels couple peptide hormone receptor activation to membrane depolarization and cortisol secretion through bovine TREK-1 inhibition. Recently, we discovered that normal human AZF cells were indistinguishable from bovine AZF cells with respect to the ion channels expressed and the inhibition of TREK-1 by ACTH and ANG II (15). More recently, we found that human AZF cells express L-type Ca2+ channels similar to those of bovine cells and that TTA-P2 and nifedipine block ACTH-stimulated cortisol secretion in these cells (unpublished observations). Thus, bovine AZF cells appear to be identical to human cells with respect to the ionic mechanisms that mediate cortisol production.
It will be important to identify the L-type Ca2+ channels expressed in bovine and human AZF cells, particularly the novel, low-voltage-activated, slowly activating channel. In this regard, gain-of-function mutations in the CACNA1D Ca2+ channel of adrenal zona glomerulosa cells cause primary hyperaldosteronism in humans by shifting the voltage dependence of channel activation (52). It will be interesting to determine whether similar mutations in human AZF cells could be responsible for some cases of Cushing's syndrome.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant R56 DK-047875 (to J. J. Enyeart) and by the Department of Neuroscience, Wexner Medical Center, The Ohio State University.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.E. developed the concept and designed the research; J.J.E. and J.A.E. performed the experiments; J.J.E. and J.A.E. interpreted the results of the experiments; J.J.E. drafted the manuscript; J.J.E. and J.A.E. edited and revised the manuscript; J.J.E. and J.A.E. approved the final version of the manuscript; J.A.E. analyzed the data; J.A.E. prepared the figures.
REFERENCES
- 1.Barbara JG, Takeda K. Voltage-dependent currents and modulation of calcium channel expression in zona fasciculata cells from rat adrenal gland. J Physiol 488: 609–622, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin JP, Mombereau C, Faure P, Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science 339: 332–335, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Bean BP. Nitrendipine block of cardiac calcium channels: high affinity binding to the inactivated state. Proc Natl Acad Sci USA 81: 6388–6392, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belles B, Malecot CO, Hescheler J, Trautwein W. “Run-down” of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflügers Arch 411: 353–360, 1988. [DOI] [PubMed] [Google Scholar]
- 5.Bose H, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 417: 87–91, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a third calcium channel β-subunit. J Biol Chem 268: 3450–3455, 1993. [PubMed] [Google Scholar]
- 7.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3: a003947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat Neurosci 15: 1707–1714, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe W, Messinger RB, Leach E, Eckle VS, Obradovic A, Salajegheh R, Jevtovic-Todorovic V, Todorovic SM. TTA-P2 is a potent and selective blocker of T-type calcium channels in rat sensory neurons and a novel antinociceptive agent. Mol Pharmacol 80: 900–910, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clyne CD, Nicol MR, MacDonald S, Williams BC, Walker SW. Angiotensin II stimulates growth and steroidogenesis in zona fasciculata/reticularis cells from bovine adrenal cortex via the AT1 receptor subtype. Endocrinology 132: 2206–2212, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of ITwindow. J Neurosci 30: 99–109, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enyeart JA, Danthi SJ, Enyeart JJ. Corticotropin induces the expression of TREK-1 mRNA and K+ current in adrenocortical cells. Mol Pharmacol 64: 132–142, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Enyeart JA, Xu L, Enyeart JJ. A bovine adrenocortical KV1.4 K+ channel whose expression is potently inhibited by ACTH. J Biol Chem 275: 34640–34649, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Enyeart JJ, Danthi SJ, Liu H, Enyeart JA. Angiotensin II inhibits bTREK-1 K+ channels in adrenocortical cells by separate Ca2+- and ATP hydrolysis-dependent mechanisms. J Biol Chem 280: 30814–30828, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Enyeart JJ, Enyeart JA. Ca2+ and K+ channels of normal human adrenal zona fasciculata cells: properties and modulation by ACTH and AngII. J Gen Physiol 142: 137–155, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enyeart JJ, Gomora JC, Xu L, Enyeart JA. Adenosine triphosphate activates a noninactivating K+ current in adrenal cortical cells through nonhydrolytic binding. J Gen Physiol 110: 679–692, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enyeart JJ, Mlinar B, Enyeart JA. T-type Ca2+ channels are required for ACTH-stimulated cortisol synthesis by bovine adrenal zona fasciculata cells. Mol Endocrinol 7: 1031–1040, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Enyeart JJ, Xu L, Danthi S, Enyeart JA. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J Biol Chem 277: 49186–49199, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Fenwick EM, Marty A, Neher E. Sodium and calcium channels in adrenal chromaffin cells. J Physiol 331: 599–635, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furukawa T, Nukada T, Miura R, Ooga K, Honda M, Watanabe S, Koganesawa S, Isshiki T. Differential blocking action of dihydropyridine Ca2+ antagonists on a T-type Ca2+ channel (α1G) expressed in Xenopus oocytes. J Cardiovasc Pharmacol 45: 241–246, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa T, Nukada T, Namiki Y, Miyashita Y, Hatsuno K, Ueno Y, Yamakawa T, Isshiki T. Five different profiles of dihydropyridines in blocking T-type Ca2+ channel subtypes [Cav3.1 (α1G), Cav32 (α1H), and Cav33 (α1I)] expressed in Xenopus oocytes. Eur J Pharmacol 613: 100–107, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Guyot A, Dupre-Aucourturier S, Ojeda C, Rougier O, Bilbaut A. Two types of pharmacologically distinct Ca2+ currents with voltage-dependent similarities in zona fasciculata cells isolated from bovine adrenal gland. J Membr Biol 173: 149–163, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981. [DOI] [PubMed] [Google Scholar]
- 24.Horn R, Korn SJ. Prevention of rundown in electrophysiological recording. Methods Enzymol 207: 149–155, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda Y, Kumagai H, Skach A, Sato M, Yanagisawa M. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell 155: 1323–1336, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John ME, John MC, Boggaram V, Simpson ER, Waterman MR. Transcriptional regulation of steroid hydroxylase genes by corticotropin. Proc Natl Acad Sci USA 83: 4715–4719, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaouane N, Porte Y, Vallee M, Brayda-Bruno L, Mons N, Calandreau L, Marighetto A, Piazza PV, Desmedt A. Glucocorticoids can induce PTSD-like memory impairments in mice. Science 335: 1510–1513, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Kepplinger KJ, Romanin C. The run-down phenomenon of Ca2+ channels. In: Voltage-Gated Calcium Channels, edited by Zamponi GW. New York: Springer, 2005, p. 220–230. [Google Scholar]
- 29.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. α1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem 276: 22100–22106, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Lebrethon MC, Jaillard C, Defayes G, Begeot M, Saez JM. Human cultured adrenal fasciculata-reticularis cells are targets for angiotensin-II: effects on cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17α-hydroxylase, and 3β-hydroxysteroid-dehydrogenase messenger ribonucleic acid and proteins and on steroidogenic responsiveness to corticotropin and angiotensin-II. J Clin Endocrinol Metab 78: 1212–1219, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol 92: 2633–2641, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci 16: 698–705, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Enyeart JA, Enyeart JJ. ACTH inhibits bTREK-1 K+ channels through multiple cAMP-dependent signaling pathways. J Gen Physiol 132: 279–294, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Enyeart JA, Enyeart JJ. ACTH induces Cav3.2 current and mRNA by cAMP-dependent and cAMP-independent mechanisms. J Biol Chem 285: 20040–20050, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lupien SJ, deLeon M, deSanti S, Convit A, Tarshish C, Nair NP, Thakur M, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1: 69–73, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Lymangrover JR, Matthews EK, Saffran M. Membrane potential changes of mouse adrenal zona fasciculata cells in response to adrenocorticotropin and adenosine 3′,5′-monophosphate. Endocrinology 110: 462–468, 1982. [DOI] [PubMed] [Google Scholar]
- 37.Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci USA 100: 5543–5548, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews EK, Saffran M. Effect of ACTH on the electrical properties of adrenocortical cells. Nature 219: 1369–1370, 1968. [DOI] [PubMed] [Google Scholar]
- 39.Matthews EK, Saffran M. Ionic dependence of adrenal steroidogenesis and ACTH-induced changes in the membrane potential of adrenocortical cells. J Physiol 234: 64, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McRory JE, Hamid J, Doering CJ, Garcia E, Parker R, Hamming K, Chen L, Hildebrand M, Beedle AM, Feldcamp L, Zamponi GW, Snutch TP. The CACNA1F gene encodes an L-type calcium channel with unique biophysical properties and tissue distribution. J Neurosci 24: 1707–1718, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mlinar B, Biagi BA, Enyeart JJ. A novel K+ current inhibited by ACTH and angiotensin II in adrenal cortical cells. J Biol Chem 268: 8640–8644, 1993. [PubMed] [Google Scholar]
- 42.Mlinar B, Biagi BA, Enyeart JJ. Voltage-gated transient currents in bovine adrenal fasciculata cells. I. T-type Ca2+ current. J Gen Physiol 102: 217–237, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mlinar B, Biagi BA, Enyeart JJ. Losartan-sensitive AII receptors linked to depolarization-dependent cortisol secretion through a novel signaling pathway. J Biol Chem 270: 20942–20951, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Mlinar B, Enyeart JJ. Voltage-gated transient currents in bovine adrenal fasciculata cells. II. A-type K+ current. J Gen Physiol 102: 239–255, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science 257: 1248–1251, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Natke EJ, Kabela E. Electrical responses in cat adrenal cortex: possible relation to aldosterone secretion. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E158–E162, 1979. [DOI] [PubMed] [Google Scholar]
- 47.O'Connell KM, Dirksen RT. Prolonged depolarization promotes fast gating kinetics of L-type Ca2+ channels in mouse skeletal myotubes. J Physiol 529: 647–659, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25: 947–970, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain β-subunit of the L-type calcium channel. J Biol Chem 267: 1792–1797, 1992. [PubMed] [Google Scholar]
- 50.Perez-Reyes E, Van Deusen AL, Vitko I. Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs. J Pharmacol Exp Ther 328: 621–627, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinn SJ, Cornwall MC, Williams GH. Electrical properties of isolated rat adrenal glomerulosa and fasciculata cells. Endocrinology 120: 903–914, 1987. [DOI] [PubMed] [Google Scholar]
- 52.Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Akerstrom G, Bjorklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 45: 1050–1054, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scriabine A. Comparative pharmacology of 1,4-dihydropyridines and other Ca2+ channel ligands. J Cardiovasc Pharmacol 9 Suppl 1: S3–S7, 1987. [PubMed] [Google Scholar]
- 54.Simpson ER, Waterman MR. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol 50: 427–440, 1988. [DOI] [PubMed] [Google Scholar]
- 55.Smith PA, Aschroft FM, Fewtrell CM. Permeation and gating properties of the L-type calcium channel in mouse pancreatic beta cells. J Gen Physiol 101: 767–797, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev 84: 489–539, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Stewart PM, Krone NP. The adrenal cortex. In: Williams Textbook of Endocrinology, edited by Melmud S, Polonsky K, Kronenberg HM. Philadelphia, PA: Saunders Elsevier, 2011, p. 479–534. [Google Scholar]
- 58.Waterman MR. Biochemical diversity of cAMP-dependent transcription of steroid hydroxylase genes in the adrenal cortex. J Biol Chem 269: 27783–27786, 1994. [PubMed] [Google Scholar]
- 59.Xu W, Lipscombe D. Neuronal CaV1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 21: 5944–5951, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yanagibashi K, Kawamura M, Hall PF. Voltage-dependent Ca2+ channels are involved in regulation of steroid synthesis by bovine but not rat fasciculata cells. Endocrinology 127: 311–318, 1990. [DOI] [PubMed] [Google Scholar]
- 61.Yazawa K, Kameyama A, Yasui K, Li JM, Kameyama M. ATP regulates cardiac Ca2+ channel activity via a mechanism independent of protein phosphorylation. Pflügers Arch 433: 557–562, 1997. [DOI] [PubMed] [Google Scholar]