Abstract
Episodic memory is a complex construct at both the phenotypic and genetic level. Ample evidence supports age-related cognitive stability and change being accounted for by general and domain-specific factors. We hypothesized that general and specific factors would underlie change even within this single cognitive domain. We examined six measures from three episodic memory tests in a narrow age cohort at middle and late middle age. The factor structure was invariant across occasions. At both timepoints two of three test-specific factors (story recall, design recall) had significant genetic influences independent of the general memory factor. Phenotypic stability was moderate to high, and primarily accounted for by genetic influences, except for one test-specific factor (list learning). Mean change over time was nonsignificant for one test-level factor; one declined; one improved. The results highlight the phenotypic and genetic complexity of memory and memory change, and shed light on an understudied period of life.
Keywords: heritability, genetic correlation, free recall, factorial invariance, genetic complexity
The general (g) factor is a robust phenomenon that typically accounts for approximately 40% of the variance among cognitive abilities (Deary, Penke, & Johnson, 2010). Here we use the terms g or general factor simply to refer to a higher-order factor that represents the common variance among some set of cognitive measures regardless of the theoretical model of cognition. Tucker-Drob, Briley, Starr and Deary (2014) summarized 14 longitudinal studies, including their own, that addressed the question of whether individual differences in cognitive aging result from general or domain-specific changes. A common factor accounted for an average of 51% of the variance in cognitive change over time in these studies of middle-aged and mostly older adults. One-half or more of the variance in cognitive abilities or in cognitive changes must, therefore, be accounted for by domain-specific abilities and domain-specific change.
Despite overwhelming evidence for the influence of genes on cognition, little is known about the extent to which genetic or environmental influences account for individual differences in general or domain-specific cognitive changes. Indeed, 13 of the 14 studies enumerated by Tucker-Drob. Briley, et al. (2014) were phenotypic studies that cannot address this issue. The remaining study by Tucker-Drob, Reynolds, Finkel, and Pederson (2014) showed general and domain-specific genetic influences underlying age-related patterns of cognitive change in adults over age 65 years. Test-specific influences were not examined.
Just as g is an index derived from multiple domains, domain scores are composites of individual test scores within a particular domain. At this “macro” level of analysis, once each domain composite is created, it is treated as a unitary construct and within-domain heterogeneity or complexity tends to be ignored. This approach is also not particularly amenable to illuminating complexity at the genetic level because domain composites are created without knowledge of the genetic architecture of the tests.
In our view, understanding of the complex neural and genetic underpinnings of cognitive aging would be enhanced by assessing more specific cognitive processes. We therefore, applied a more micro-level approach by examining cognitive change within just a single cognitive domain. The domain we selected was episodic memory—remembering experiences and events with information about spatial and temporal contexts (Squire, Knowlton, & Musen, 1993; Tulving, 1972)—and we examined only free recall within this domain.
Of the 14 studies of general and specific cognitive abilities enumerated by Tucker-Drob, Briley, et al. (2014), 13 included memory measures. Sometimes tests were grouped even though they may not have fit well together based on their neural correlates (e.g., working and episodic memory), or may have involved different subtypes of episodic memory (e.g., story recall and list learning). Many studies included immediate recall only, combined immediate and delayed or verbal and visual-spatial recall a priori, and/or included only verbal memory. As already noted, these composites were also created without knowledge of the genetic architecture of the tests. However, episodic memory is a complex construct at both the phenotypic and genetic level (Bearden et al., 2012; Kremen et al., 2014; Papassotiropoulos & de Quervain, 2011). Here we used multivariate genetic approaches to understand more fully the complexity of age-related changes in episodic memory. Instead of grouping the measures in advance, we used twin modeling to determine the genetic and environmental architecture (Kremen et al., 2011).
In a previous cross-sectional analysis, we (Kremen et al., 2014) applied multivariate twin analysis to six measures from three widely used episodic memory tests: California Verbal Learning Test-II (CVLT-2; Delis, Kramer, Kaplan, & Ober, 2000); Wechsler Memory Scale-III (WMS-III; Wechsler, 1997) Logical Memory (LM); and WMS-III Visual Reproductions (VR). Because factor structures are not necessarily the same at the phenotypic and genetic levels, our approach was to directly determine the best-fitting genetic model (Kremen et al., 2014; Panizzon et al., 2014). Memory measures were adjusted for general cognitive ability (GCA) in order to determine variance associated specifically with episodic memory.
In twins aged 51 to 60 years, the best-fitting model was a multi-strata common pathway model that had a higher-order general episodic memory factor with a heritability of .60, and three test-level factors with heritabilities of .34 for CVLT-II, .48 for LM, and .43 for VR. Significant genetic influences that were independent of the general episodic memory factor accounted for 28% of the variance in the LM factor and 30% of the variance in the VR factor. A nonsignificant 6% of the variance in the CVLT-II factor was accounted for by specific genetic influences. Immediate and delayed recall comprised each test-level factor, indicating that those measures could be combined. Models with separate immediate and delayed recall factors, or verbal and visual-spatial factors had poor fits to the data. Although the general episodic memory factor is a reasonable summary measure, important variance in episodic memory remained beyond that accounted for by the summary measure. We speculated that these independent episodic memory factors might not all change in the same way with age.
In the present study, we conducted a mean 5.73-year follow-up of free recall in episodic memory. We hypothesized that general and specific factors would account for change even within this one aspect of this one cognitive domain. Using genetically-informed longitudinal analyses, we sought to determine if the genetic architecture of episodic memory changed from middle to late middle age. We also examined different patterns of change over time for different memory tests, and whether the variance of that change was significant. We included immediate and delayed recall conditions for separate verbal and visual-spatial tests. Finally, we note that all but the phenotypic Tucker-Drob, Briley, et al. study (2014) had wide age ranges. Our relatively age-homogeneous sample makes it much more likely that the results reflect true within-person differences in change over time, as opposed to cohort effects (Hofer & Sliwinski, 2001).
Method
Participants
A total of 1237 individual twins participated in wave 1 of the Vietnam Era Twin Study of Aging (VETSA; Kremen, Franz, & Lyons, 2013; Kremen et al., 2006): 349 monozygotic [MZ] pairs; 265 dizygotic [DZ] pairs; and 9 unpaired. At wave 2, 5.73 years (SD=0.69) later, there were 1014 individuals with data at both time points: 269 MZ pairs; 203 DZ pairs; and 70 unpaired. In order to increase our baseline sample, data were also collected from 54 new participants at wave 2: 15 MZ pairs, 9 DZ pairs, and 3 pairs whose zygosity could not be determined. Although these 54 attrition replacement participants were tested at wave 2, they underwent the wave 1 protocol, they were from the same twin registry as the other participants, and their age at testing (M=56.22 years, SD=0.69) was within the same age range as the other participants were at wave 1. For those who had data at both time points, the mean age was 55.81 years (SD=2.48) at wave 1 and 61.55 years (SD=2.43) at wave 2. Mean educational attainment was 13.84 years (SD=2.11).
VETSA participants form a national sample of American men who are similar to their age peers with respect to health and lifestyle characteristics based on U.S census and Center for Disease Control and Prevention data (Kremen et al., 2013; Kremen et al., 2006; Schoeneborn & Heyman, 2009). They are all part of the Vietnam Era Twin Registry, which includes twins (both members of a pair) who served in some branch of military service at sometime between 1965 and 1975. Thus, participation in wave 1 took place approximately 35 years later. Note that these are Vietnam era, not Vietnam veterans; that is, military service does not mean service in Vietnam. Indeed, 78 percent of the sample reported no combat experience. They are community-dwelling adults; although they are veterans, they do not constitute a VA patient sample. The study was approved by institutional review boards at the participating institutions and carried out in accordance with the Declaration of Helsinki.
Procedures
Participants were randomly selected from an earlier study of psychological health. They were not selected on the basis of any health or demographic characteristics. The only inclusion criteria were that they be between the ages of 51 and 59 at the time of recruitment, and both members of a pairs had to be willing to participate in wave 1. There was no requirement that both members of a pair participate in subsequent waves. The goal of the VETSA is to carry out a longitudinal behavior genetic study of cognitive and brain aging beginning in midlife. The design included a large sample with narrow age range so that all participants were of similar age at wave 1, and there could be a focus on differences in within-individual change over time with minimal impact of age differences among the participants.
Identical protocols were administered at two study sites, the University of California, San Diego and Boston University. Each individual had the choice of coming to either site, although brothers most often went to the same site on the same day. A small number of participants were tested in their hometowns because they could not or did not wish to travel. The complete assessment protocol has been described elsewhere (Kremen et al., 2013; Kremen et al., 2006).
For 92% of the sample, zygosity was determined on the basis of 25 microsatellite markers. For the remaining participants, the DNA-based zygosity determination failed or, in rare instances, a participant chose not to provide any biospecimen. In those cases, zygosity was based on the standard method of using questionnaire and blood group data (Eisen, Neuman, Goldberg, & Rice, 1989). The questionnaire and blood group information was available on all participants, and had 95% agreement with the DNA-based zygosity determination. Given this level of accuracy, we considered it preferable to include twins with questionnaire-based zygosity rather than dropping 8% of the sample without DNA-based zygosity. In cases of discrepancies, the DNA-based results always took precedence.
Tests and Measures
Episodic memory
We included both verbal and visual-spatial episodic memory measures. The California Verbal Learning Test-Second Edition (CVLT-2; Delis et al., 2000) includes a list of 16 words that is repeated on five trials. A second list is then presented once, and is followed by different free recall, cued recall, and recognition conditions for the first list. Measures included in the present analyses were the number of correct words on the short and long delay free recall conditions. The short delay conditions come immediately after the recall trial for the second list. The long delay conditions begin 20–25 minutes after the short delay conditions. The Wechsler Memory Scale-III (WMS-III; Wechsler, 1997) Logical Memory (LM) subtest includes the combined score for recall of two stories. Immediately after the each story is read, participants recall as much of it as they can. After an interval of 25–35 minutes, participants are again asked to recall as much as possible from one story and then the other. We used the immediate and delayed recall scores in the present analysis. The standard administration calls for reading the second story twice, but we read the second story only one time (as is done in WMS-IV) because we were not interested in the effects of repetition on information retrieval in this test. The WMS-III Visual Reproductions (VR) subtest consists of five designs that are presented for 10 seconds each and then drawn from memory. After a 25–35 minute interval, participants are asked to again draw each design from memory. Scores used in the present analysis were the immediate and delayed recall totals for the five designs. The identical memory tests and scores were used in both waves of the study.
Here we restrict the term “measure” to refer specifically to the each of the observed variables (i.e., the six individual episodic memory measures). We restrict the term “test” to refer to the three episodic memory tests (CVLT-II, LM, and VR) from which the memory measures were derived. In later sections we refer to “test-level factors” and “test-specific factors” to indicate the factors that emerged, each of which included individual measures from the CVLT-II, LM, and VR tests, respectively. The distinction between test-level and test-specific factors is explained in the paragraph describing the longitudinal factor model.
General cognitive ability (GCA)
It is well known that GCA is a highly heritable cognitive phenotype with which almost all specific cognitive abilities are positively correlated (Panizzon et al., 2014). To address the issue of confounding of genetic influences on episodic memory by genetic influences on GCA, we adjusted all memory measures for GCA prior to the twin analyses (Kremen et al., 2014). The adjusted (residual) scores were then used in the twin analyses. The measure of current GCA was the Armed Forces Qualification Test (AFQT; Bayroff & Anderson, 1963), administered on the same day as the memory tests. The AFQT is a 100-item test that assesses four cognitive domains: verbal ability (vocabulary); spatial processing (mental folding and unfolding of boxes; arithmetic ability; and reasoning about tools and mechanical relations. With a test-retest correlation of .75 over a 35-year period in VETSA participants, the AFQT has high reliability (Lyons et al., 2009). It also has very good validity based on its correlation of approximately .85 with IQ and other general intellectual ability measures (Lyons et al., 2009). AFQT scores are percentiles, but the raw percentiles were converted into their normal deviates in the statistical analyses. The mean AFQT percentile was 64 (interquartile range: 50–81) in VETSA wave 1 and 63 (interquartile range: 49–81) in wave 2. These scores are comparable to a mean IQ of 102–105. Wave 1 memory scores were adjusted for wave 1 AFQT scores, and wave 2 memory scores were adjusted for wave 2 AFQT scores. This procedure ensured that episodic memory scores were adjusted for concurrent GCA, and that change in memory performance was not the result of change in GCA.
Statistical Analysis
To elucidate the genetic and environmental architecture of episodic memory stability and change we examined the results from three distinct multivariate twin models. The first model, the ACE Cholesky, perfectly recaptures the observed genetic and environmental relationships within and across measures. The variance of each measure is decomposed into additive genetic influences (A), shared or common environmental influences (C) that make twins similar to one another, and individual-specific or unique environmental influences (E) that make twins different from one another, including measurement error (Eaves, Last, Young, & Martin, 1978; Neale & Cardon, 1992). It should be noted that if substantial variation due to non-additive genetic effects such as dominance or epistasis exists, the contribution of shared environmental factors may be underestimated. An extended twin family study would be able to resolve such additional sources of variation (Keller, Medland, & Duncan, 2010).
In addition, the ACE Cholesky further decomposes the covariance between measures into genetic and environmental influences, i.e., components of the covariance. This process allows for determination of the degree to which the phenotypic correlation between any two measures is driven by shared genetic and/or environmental influences, as well as the estimation of genetic and environmental correlations (i.e., the degree of shared genetic and environmental variance, respectively). For the present analyses the ACE Cholesky was used to determine the degree to which each episodic memory measure was determined by genetic and environmental influences, the cross-time phenotypic correlations for each measure (phenotypic stability), and the degree to which the cross-time phenotypic correlations were genetically and environmentally driven. Because the ACE Cholesky is a fully saturated model of genetic and environmental variance and covariance, it was also used as the comparison model to determine the overall fit of our two other multivariate models.
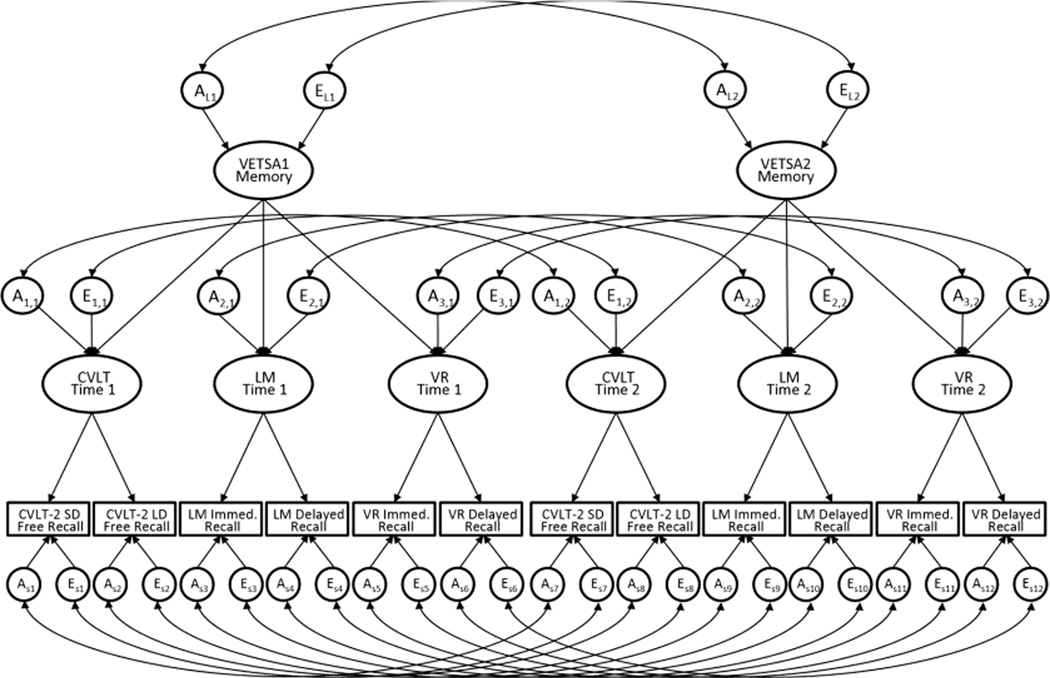
The second model, the Longitudinal Factor Model, extends our previously described higher-order common pathways model of episodic memory to two measurement timepoints (Kremen et al., 2014), and provides the opportunity to test the invariance of the factor structure across time as well as the stability of the latent phenotypes. As depicted in Figure 1, the identical factor structure is specified to the data from VETSA1 and VETSA2, and the genetic and environmental determinants of all latent factors, and the observed variables are allowed to correlate across time. Invariance of the factor structure was evaluated by systematically constraining elements of the model (factor loadings, variable-specific residual variances, test-specific factor residual variances, and intercepts) to be equal across time and then determining whether these constraints resulted in a significant deterioration in fit from the unconstrained model (Steiger, Shapiro, & Browne, 1985; Widaman, Ferrer, & Conger, 2010). In addition to evaluating the invariance of the factor structure, the Longitudinal Factor Model was used to determine the degree to which the higher-order, test-level, and test-specific factors at each timepoint are influenced by genetic and environmental determinants, the cross-time phenotypic correlations for each latent factor, and the degree to which the cross-time phenotypic correlations are genetically and environmentally driven. The higher-order factors, VETSA 1 Memory and VETSA 2 Memory, are the general memory factors representing the common variance among the tests at each timepoint. We distinguish between test-level and test-specific factors as follows: variance in test-level factors is contributed by both the test-specific factor and the higher-order general episodic memory factor; variance in the test-specific factors excludes the contribution of the general episodic memory factor.
Figure 1. Genetic architecture of episodic memory change.
The model is based on the factor structure found in the Time 1 assessment (Kremen et al., 2014). A = Additive genetic variance; E = Unique environmental variance; CVLT = California Verbal Learning Test – II; Time 1, 2 = VETSA 1, 2; LM = Logical Memory; VR = Visual Reproductions; SD = Short delay; LD = Long delay. AL2 = A for latent factor at Time 2; A3,1 = A for test-specific factor 3 at Time 1; Es5 = E specific for measure 5, which is VR Immed. Recall at Time 1; other abbreviations follow the same pattern. Variables for all A and E were fixed at 1 for model identification; common environmental variance (C) components are not shown for simplicity of presentation. Wherever there is an A or E component, there would also be a C component with an analogous set of curved arrows. Circles and ellipses represent latent influences and latent variables, respectively. Rectangles represent measured variables. Straight arrows represent loadings on factor or variables. Curved arrows represent correlations.
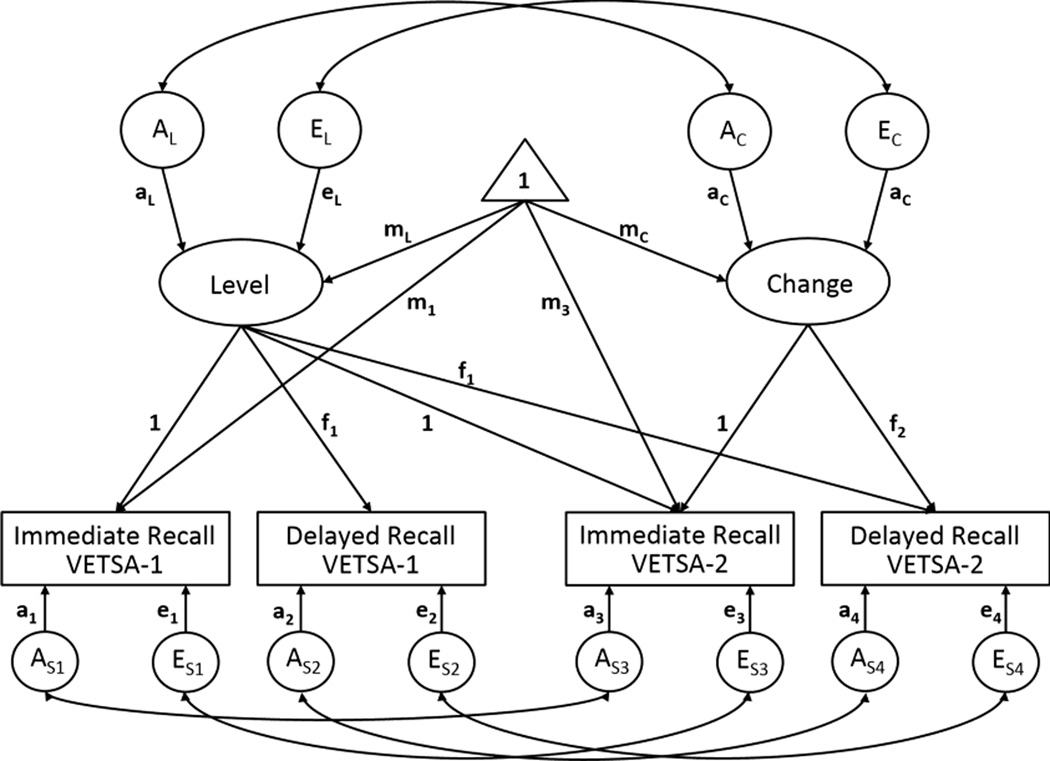
Finally, the Multivariate Latent Change Model allows for the estimation of average change across time for the test-level factors as well as individual differences in change (i.e., change variance). As described by McArdle and Nesselroade (1994), the latent change model utilizes multiple indicators of a latent variable at two timepoints to estimate latent level (intercept) and change factors. An example of a latent change model as applied to data from one memory test, and extended to include the genetic and environmental determinants of the latent factors and observed variables is presented in Figure 2. In the present analyses we utilized the immediate and delayed recall conditions of each memory test as the multiple indicators of performance on a single test, the latent factor. The higher-order factors in Figure 3B are labeled as General Level and General Change. General memory level represents the common variance among the levels for each test factor at VETSA 1. General memory change represents the common variance among the measures of change for each test factor. Thus, referring to change in the general memory factor refers to the common variance in change among the test factors. To identify the model, the following constraints were imposed: loadings from each level factor were equated across time; variable-specific genetic and common environmental influences were equated across time; and genetic and common environmental correlations among the variable-specific variance components were constrained to 1.0.
Figure 2. Latent change model for one memory test.
Rectangles represent measured variables. Circles represent latent genetic and environmental influences. Ellipses represent latent variables/phenotypes. The triangle denotes the estimation of means. Single headed arrows indicate regression coefficients; double-headed arrows indicate covariance. Common environmental influences are not presented in order to simplify the presentation. In order for the model to be identified the following constraints were made: residual/variable specific genetic influences are equal across time; residual/variable specific common environmental influences are equal across time; covariance among the residual genetic and common environmental factors are equal to 1 (rg = 1, rc = 1); and factor loadings are equal across time.
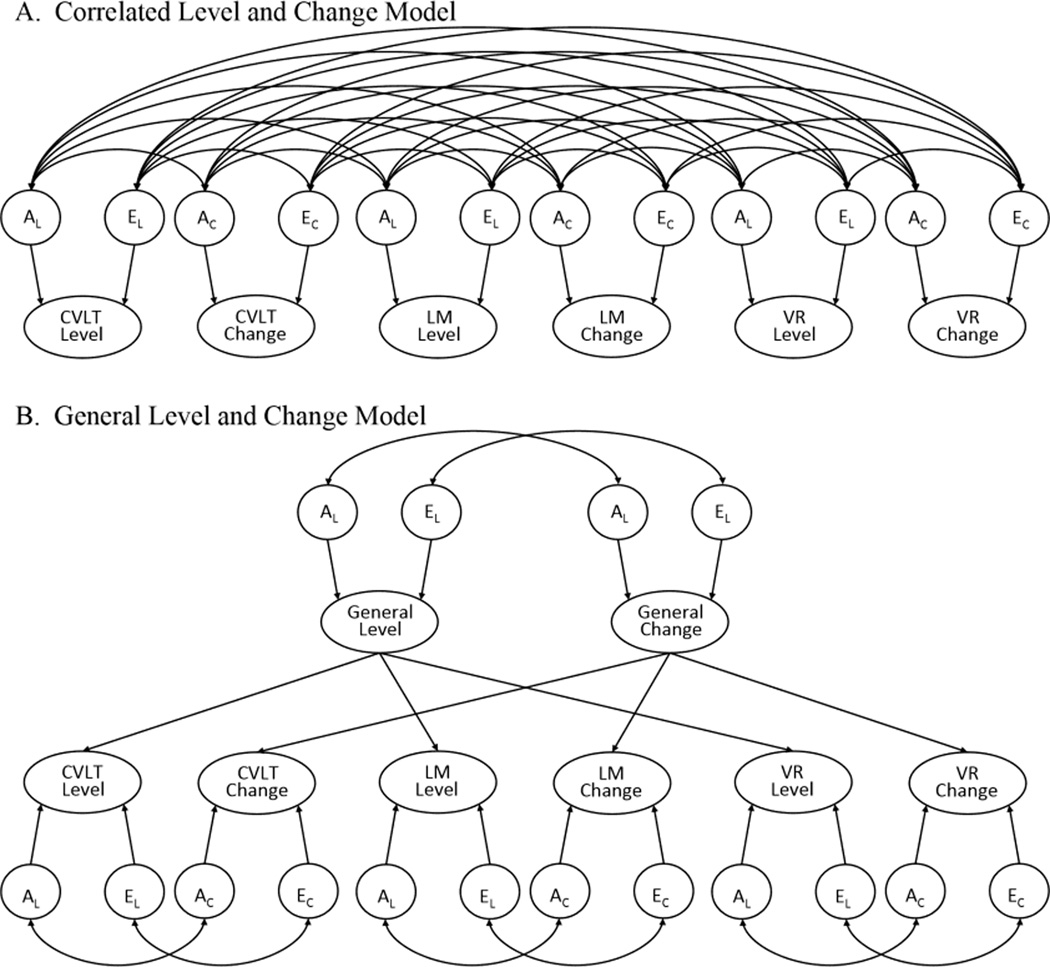
Figure 3. Multivariate latent change models of episodic memory.
CVLT = California Verbal Learning Test – II, LM = Logical Memory, VR = Visual Reproductions. Common environmental influences, measured variables, and means structure have been omitted in order to simplify the figure.
In addition to the means and variances of the level and change factors, the Multivariate Latent Change Model allows estimation of the degree to which each level and change factor is influenced by genetic and environmental determinants, the phenotypic correlation between level and change, and the degree to which the level-change correlation is genetically versus environmentally driven. When applied to the data from multiple memory tests (see Figure 3), one can estimate the degree to which level and change factors correlate across all tests (Figure 3A) as well as whether global processes influence level and change (Figure 3B).
Analyses were conducted using the statistical modeling software OpenMx (Boker et al., 2011). To simplify the specification of start values and parameter boundaries, all variables were standardized according to VETSA1 means and standard deviations, which permits estimation of changes in mean and variance of level while keeping all variables on the same metric. Model testing was conducted in a full-information maximum likelihood framework, which allows for the efficient use of all data—including attrition replacement participants—when multiple waves or measures are analyzed (Lange et al., 1976; Little & Rubin, 2002). Thus, all participants were included in the analyses even if they had data from only one timepoint. Doing so allows for more precise estimates of wave 1 means, variances, and heritabilities without affecting the covariance between waves 1 and 2. Evaluation of model fit was performed using the likelihood-ratio chi-square test (LRT), which is calculated as the difference in the −2 log-likelihood (−2LL) of a model relative to that of a comparison model. The LRT for the full Longitudinal Factor Model and the LRT for the full Multivariate Latent Change Model were calculated based on comparison with the ACE Cholesky. LRT for submodels within the different model structures were based on comparison with the respective full model. In addition to the LRT, we utilized the Bayesian Information Criterion (BIC) as a parsimony-based indicator of model fit (Akaike, 1987; Williams & Holahan, 1994). The BIC indexes both goodness-of-fit and parsimony, with more negative values indicating a better balance between them, and has been found to outperform the commonly used Akaike Information Criterion (AIC) with regard to model selection in the context of complex multivariate models (Markon & Krueger, 2004).
Results
Observed Memory Variables Across Time
Table 1 presents phenotypic correlations among the twelve individual observed memory measures at both VETSA 1 and 2, as derived from the full ACE Cholesky. Two features are noteworthy. First, although all are free recall episodic memory measures, the correlations among measures from different tests at a given timepoint are smaller than one might expect for measures purported to assess variants of the same construct. Cross-test correlations ranged from .16 to .37, while within-test correlations were substantially higher, ranging from .57 to .84. Second, the cross-time correlations for specific memory measures, frequently referred to as stability coefficients (Hertzog & Nesselroade, 2003; Hertzog & Schaie, 1986), indicate moderate to high stability over time for each of the individual measures, with slightly lower stability for the VR measures. Estimates of the genetic and environmental correlations for these variables followed the same general pattern (see Supplementary Table 1). Cross-time genetic correlations for the individual memory measures ranged from .91 to .98, and cross-time unique environmental correlations ranged from .22 to .35. Cross-time common environmental correlations were not readily interpretable because the variances were quite low at each timepoint.
Table 1.
Phenotypic Correlations Among Observed Memory Variables
| VETSA 1 (Mean age 55 years) |
VETSA 2 (Mean age 61 years) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| VETSA 1 | ||||||||||||
| 1. CVLT-II Short Delay FR | 1.0 | |||||||||||
| 2. CVLT-II Long Delay FR | .80 | 1.0 | ||||||||||
| 3. Logical Memory IR | .28 | .33 | 1.0 | |||||||||
| 4. Logical Memory DR | .31 | .36 | .84 | 1.0 | ||||||||
| 5. Visual Reproductions IR | .16 | .19 | .17 | .17 | 1.0 | |||||||
| 6. Visual Reproductions DR | .24 | .28 | .19 | .23 | .57 | 1.0 | ||||||
| VETSA 2 | ||||||||||||
| 7. CVLT-II Short Delay FR | .56 | .57 | .23 | .28 | .13 | .21 | 1.0 | |||||
| 8. CVLT-II Long Delay FR | .55 | .58 | .28 | .33 | .11 | .21 | .81 | 1.0 | ||||
| 9. Logical Memory IR | .22 | .27 | .58 | .57 | .12 | .15 | .30 | .34 | 1.0 | |||
| 10. Logical Memory DR | .24 | .30 | .56 | .61 | .11 | .16 | .33 | .37 | .84 | 1.0 | ||
| 11. Visual Reproductions IR | .13 | .18 | .10 | .09 | .46 | .38 | .17 | .17 | .17 | .15 | 1.0 | |
| 12. Visual Reproductions DR | .23 | .28 | .16 | .19 | .39 | .48 | .30 | .28 | .20 | .21 | .57 | 1.0 |
Note. Correlations are derived from the full ACE Cholesky model. Values in bold font represent the phenotypic stability coefficients of the individual observed memory measures. All correlations are significantly greater than zero based on 95% confidence intervals. CVLT-II = California Verbal Learning Test – II; FR = Free Recall; IR = Immediate Recall; DR = Delayed Recall.
Standardized variance components for the observed memory measures, also derived from the full ACE Cholesky, as well as the relative contributions of genetic and environmental influences to the phenotypic stability of each measure are presented in Table 2. At VETSA 1 heritability estimates ranged from .24 for VR immediate recall to .46 for LM delayed recall. Common environmental influences ranged from .04 to .15 and were significantly different from zero for the CVLT-II short and long delay free recalls and the VR immediate recall. At VETSA 2 the same general pattern of heritability estimates was observed; VR immediate recall had the lowest heritability (a2 = .21), whereas LM delayed recall had the highest heritability (a2 = .50). For the majority of memory measures, common environmental influences tended to be lower at VETSA 2, with the exception of the estimate for VR immediate recall (c2 = .12) which remained significantly greater than zero. Unique environmental influences ranged from .49 for VETSA 1 LM delayed recall to .67 for VETSA 2 VR immediate and delayed recall. The three rightmost columns in Table 2 show the genetic and environmental contributions to the phenotypic stability. For example, the phenotypic stability (rp) for CVLT-II long delay free recall was .58. The genetic and common environmental contributions to phenotypic stability were respectively 60%, (rpA*rp = .35/.58) and 9%, (rpC*rp = .05/.58). For each memory measure, genetic influences contributed the most to the phenotypic stability, ranging from 44% for VR immediate recall to 77% for LM delayed recall, with a median of 57%.
Table 2.
Standardized Variance Components and Contributions to the Phenotypic Stability of the Observed Memory Variables
| VETSA 1 (Mean age 55 years) |
VETSA 2 (Mean age 61 years) |
Contributions to phenotypic stability |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observed Variables | a2 | c2 | e2 | a2 | c2 | e2 | rp | rpA | rpC | rpE |
| CVLT-II Short Delay FR | .26 (.12, .40) |
.12 (.01, .25) |
.62 (.55, .70) |
.38 (.21, .49) |
.04 (.00, .19) |
.58 (.50, .66) |
.56 (.51, .60) |
.30 (.16, .40) |
.05 (−.02, .18) |
.21 (.16, .27) |
| CVLT-II Long Delay FR | .30 (.15, .45) |
.15 (.03, .29) |
.55 (.48, .62) |
.46 (.29, .55) |
.03 (.00, .17) |
.51 (.44, .60) |
.58 (.54, .62) |
.35 (.21, .45) |
.05 (−.03, .17) |
.18 (.13, .24) |
| Logical Memory IR | .40 (.25, .50) |
.04 (.00, .17) |
.56 (.48, .63) |
.43 (.24, .55) |
.05 (.00, .21) |
.52 (.45, .61) |
.58 (.54, .62) |
.39 (.24, .48) |
.03 (−.02, .16) |
.15 (.10, .21) |
| Logical Memory DR | .46 (.31, .56) |
.05 (.00, .17) |
.49 (.43, .57) |
.50 (.36, .59) |
.02 (.00, .14) |
.48 (.41, .56) |
.61 (.56, .64) |
.47 (.35, .54) |
.01 (−.03, .11) |
.13 (.09, .19) |
| Visual Reproductions IR | .24 (.08, .38) |
.12 (.01, .26) |
.64 (.56, .72) |
.21 (.05, .37) |
.12 (.01, .26) |
.67 (.58, .76) |
.46 (.41, .51) |
.20 (.06, .34) |
.12 (.00, .24) |
.14 (.08, .21) |
| Visual Reproductions DR | .31 (.14, .42) |
.05 (.00, .18) |
.64 (.57, .73) |
.22 (.08, .37) |
.01 (.00, .25) |
.67 (.59, .75) |
.48 (.43, .53) |
.25 (.11, .36) |
.06 (−.01, .18) |
.17 (.11, .23) |
Note. Estimates are based on the full ACE Cholesky model. 95% confidence intervals are presented in parentheses under each estimate. a2 = Additive genetic variance; c2 = Common/shared environmental variance; e2 = Unique environmental variance; rp = Phenotypic correlation; rpA = Genetic contribution to the phenotypic correlation; rpC = Common/shared environment contribution to the phenotypic correlation; rpE = Unique environment contribution to the phenotypic correlation; CVLT-II = California Verbal Learning Test – II; FR = Free Recall; IR = Immediate Recall; DR = Delayed Recall.
Longitudinal Factorial Invariance of Episodic Memory
In order to determine whether our previously identified factor structure for episodic memory was invariant over time, we fit a series of higher-order common pathway models. Results of this model fitting sequence are summarized in Table 3. We began by fitting an unconstrained longitudinal factor model (see Figure 1) in which the same higher-order common pathway model was applied to the VETSA 1 and VETSA 2 data. The model allowed for cross-time covariance between the variance components of the general factor, the test-level factors, and the residual variance components of the observed variables while imposing no constraints on any of the estimated parameters. Fit indices for the ACE Cholesky were: −2LL=30426.39, df=13435, BIC=-57296.35. The unconstrained longitudinal factor model resulted in a good fit relative to the ACE Cholesky (LRT = 101.04, Δdf = 134, BIC=-58,070.26, p = .98), establishing configural invariance (Widaman et al., 2010) of the memory data across time. Relative to this full model we tested whether increasingly rigorous constraints could be imposed upon the model without resulting in a significant change in fit. As shown in Table 3, factor loadings, variable-specific variance components, test-specific variance components, and intercepts could all be equated across time without a significant loss of model fit. Thus, the model met criteria for strict factorial invariance (Steiger et al., 1985; Widaman et al., 2010).
Table 3.
Model Fitting Results for Tests of Longitudinal Factorial Invariance
| Model | −2LL | df | BIC | LRT | Δdf | p |
|---|---|---|---|---|---|---|
| ACE Cholesky | 30426.39 | 13435 | −57296.35 | -- | -- | -- |
| Unconstrained longitudinal factor model | 30527.43 | 13569 | −58070.26 | 101.04 | 134 | .98 |
| Constrained factor loadings | 30530.62 | 13574 | −58099.71 | 3.19 | 5 | .67 |
| Constrained variable specific residual variance components | 30539.90 | 13592 | −58207.96 | 12.47 | 23 | .96 |
| Constrained test-specific factor residual variance components | 30542.25 | 13601 | −58264.38 | 14.82 | 32 | >.99 |
| Constrained intercepts | 30554.73 | 13604 | −58271.49 | 27.30 | 35 | .82 |
Note. Each model includes the constraints applied to the preceding models. The fit of the unconstrained longitudinal factor model is tested relative to the ACE Cholesky. The fits of all subsequent models are tested relative to the unconstrained longitudinal factor model. −2LL = negative 2 log-likelihood; BIC = Bayesian information criterion; LRT = likelihood ratio test; Δdf = change in degrees of freedom; p = significance of LRT.
Standardized variance components for the latent factors derived from the unconstrained longitudinal factor model, the cross-time phenotypic correlations between those factors, as well as the relative contributions of genetic and environmental influences to those factors are presented in Table 4. Heritability estimates for the general episodic memory factor were .53 at VETSA 1 and .72 at VETSA 2. For test-level factors, which include the genetic and environmental influences attributable to the higher-order factors (middle rows), heritability estimates ranged from .37 for VETSA 1 CVLT-II to .55 for VETSA 2 LM. Although we observed slight increases in the genetic influences for general and test-level factors from VETSA 1 to VETSA 2, as well as slight decreases in common environmental influences, these differences did not represent statistically significant changes.
Table 4.
Standardized Variance Components and Relative Contributions to the Phenotypic Stability of the Latent Memory Variables
| VETSA 1 (Mean age 55 years) |
VETSA 2 (mean age 61 years) |
Contributions to phenotypic stability |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Latent Variables | a2 | c2 | e2 | a2 | c2 | e2 | rp | rpA | rpC | rpE |
| General Memory | .53 (.21, .80) |
.17 (.00, .45) |
.30 (.19, .42) |
.72 (.46, .84) |
.01 (.00, .22) |
.27 (.16, .41) |
.83 (.77, .88) |
.60 (.34, .75) |
.03 (−.05, .26) |
.20 (.10, .29) |
| Test-Level Factors (Including the contribution of the general memory factor) | ||||||||||
| CVLT-II | .37 (.17, .55) |
.11 (.00, .29) |
.52 (.44, .60) |
.51 (.35, .60) |
.01 (.00, .14) |
.48 (.40, .57) |
.70 (.66, .74) |
.43 (.26, .53) |
.02 (−.04, .17) |
.25 (.18, .32) |
| Logical Memory | .47 (.34, .58) |
.05 (.00, .16) |
.48 (.41, .55) |
.55 (.43, .63) |
.00 (.00, .10) |
.45 (.37, .53) |
.67 (.63, .71) |
.50 (.39, .57) |
.01 (−.02, .11) |
.16 (.10, .22) |
| Visual Reproductions | .46 (.30, .57) |
.03 (.00, .16) |
.51 (.41, .61) |
.47 (.27, .58) |
.01 (.00, .19) |
.52 (.42, .63) |
.68 (.61, .73) |
.46 (.29, .54) |
.01 (−.02, .14) |
.21 (.13, .30) |
| Test-Specific Factors (Excluding the contribution of the general memory factor)* | rp* | rpA* | rpC* | rpE* | ||||||
| CVLT-II | .09 (.00, 21) |
.02 (.00, .15) |
.36 (.27, .45) |
.11 (.00, .23) |
.00 (.00, .13) |
.32 (.23, .42) |
.25 (.13, .34) |
.10 (−.01, .20) |
.01 (−.02, .12) |
.14 (.07, .21) |
| Logical Memory | .31 (.20, .39) |
.00 (.00, .09) |
.39 (.31, .46) |
.34 (.22, .43) |
.00 (.00, .10) |
.37 (.29, .45) |
.42 (.35, .49) |
.32 (.22, .39) |
.00 (−.01, .08) |
.10 (.05, .16) |
| Visual Reproductions | .34 (.20, .45) |
.00 (.00, .11) |
.45 (.34, .56) |
.30 (.10, .41) |
.00 (.00, .19) |
.46 (.34, .57) |
.49 (.41, .56) |
.32 (.16, .40) |
.00 (−.02, .13) |
.17 (.09, .26) |
Note. Estimates are based on the unconstrained longitudinal factor model. 95% confidence intervals are presented in parentheses under each estimate. a2 = Additive genetic variance; c2 = Common/shared environmental variance; e2 = Unique environmental variance; rp = Phenotypic correlation; rpA = Genetic contribution to the phenotypic correlation; rpC = Common environment contribution to the phenotypic correlation; rpE = Unique environment contribution to the phenotypic correlation; CVLT-II = California Verbal Learning Test – II.
These estimates were calculated indirectly, but they are equivalent to phenotypic correlations and contributions to the phenotypic correlations. For example, rp* = .25 is calculated by taking the phenotypic covariance for the VETSA 1 and 2 CVLT-2 factors excluding the general factor (.18), and dividing that by the phenotypic covariance for the CVLT-2 factors including the general factor (.52). .18/.52 = .35, indicating that 35% of the covariance between the CVLT-2 factors is accounted for the influence of CVLT-2 factors specifically and the remaining 65% is accounted for by the influence of the general memory factors. Translating the covariances into correlations (standardized covariances), we calculated 35% of the rp of .70 for the CVLT-2 factors including the general memory factor, which is an rp. of .25 for the factors after excluding the general memory factor. The actual covariance estimates for the last four columns of this table are shown in Supplemental Table 2.
The general memory factors were strongly correlated across time (rp = .83), with the majority of the covariance (rpA/rp=72%) attributable to common genetic influences. Cross-time phenotypic correlations between the test-level factors were slightly smaller than that of the general memory factor (ranging from .67 to .70), but were larger than those observed for the individual memory measures (shown in Table 2). As with the general memory factor, the majority of the cross-time covariance was accounted for by genetic influences (rpA/rp=61–75%).
The lower rows of Table 4 present variance components and cross-time phenotypic correlations of the test-specific factors, after excluding the contributions of the VETSA 1 and VETSA 2 general memory factors. Considerable residual variance remained. For example, 47% (i.e., .09+.02+.36) of the variance in the VETSA 1 CVLT-II factor was accounted for by influences specific to that factor and independent of the general factor. The same was true for 43% of the variance in the VETSA 2 CVLT-II factor and 71–76% of the variance in the LM and VR factors. Cross-time stability coefficients for each of the test-specific factors were reduced but were nevertheless still significant after accounting for general memory, as indicated in the changes from rp to rp*. Most of the total phenotypic cross-time stability for LM and VR was due to influences that are independent of general memory (rp*/rp=63–72%). Values in the lower rows of Table 4 are denoted by asterisks because they were calculated indirectly from the covariances (see note in Table 4 and Supplementary Table 2). For the LM and VR factors, genetic influences specific to those tests continued to have a significant impact on the cross-time phenotypic correlations, accounting for 76% and 65% (i.e., (rpA*/rp*) of the remaining covariance; the analogous genetic influences on CVLT-II were nonsignificant. Thus, stability in memory performance is by no means strictly the product of the general memory factor, but is also due to test-specific genetic and environmental influences.
Latent Change in Episodic Memory
As noted earlier, the longitudinal factor model allows one to make inferences about changes in latent phenotypes over time, but it does not directly assess change. We therefore fit a multivariate latent change model in order to evaluate the degree of change in episodic memory performance between VETSA 1 and VETSA 2, as well as determine whether change was the result of a global or test specific process. Results of that model are shown in Tables 5 and 6.
Table 5.
Means, Variances, and Phenotypic Correlations Among the Latent Initial Level (Intercept) and Change Factors
| Mean | Variance | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|---|
| CVLT-II | |||||||
| 1. Level (intercept) | 0.00 (−0.06, 0.06) |
0.72 (0.63, 0.81) |
-- | ||||
| 2. Change | 0.05 (−0.01, 0.12) |
0.46 (0.37, 0.57) |
−.35 (−.42, −.28) |
-- | |||
| Logical Memory | |||||||
| 3. Level (intercept) | 0.00 (−0.06, 0.06) |
0.74 (0.66, 0.83) |
.40 (.34, .45) |
−.08 (−.14, −.02) |
-- | ||
| 4. Change | −0.10 (−0.15, −0.05) |
0.49 (0.40, 0.58) |
−.07 (−.13, −.02) |
.24 (.16, .31) |
−.39 (−.45, −.33) |
-- | |
| Visual Reproductions | |||||||
| 5. Level (intercept) | 0.00 (−0.05, 0.05) |
0.45 (0.36, 0.54) |
.35 (.29, .40) |
−.07 (−.12, −.02) |
.26 (.19, .32) |
−.05 (−.09, −.01) |
-- |
| 6. Change | 0.10 (0.05, 0.18) |
0.29 (0.18, 0.43) |
−.06 (−.11, −.02) |
.18 (.10, .27) |
−.04 (−.08, −.01) |
.13 (.06, .21) |
−.40 (−.50, −.32) |
Note. CVLT-II = California Verbal Learning Test – II. All values are derived from the higher-order multifactorial latent change model. 95% confidence intervals are shown in parentheses.
Table 6.
Standardized Variance Components and Contributions to the Initial Level (Intercept)-Change Correlation From the Higher-Order Latent Change Model
| Level |
Change |
Contributions to level-change correlation |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Latent Variables | a2 | c2 | e2 | a2 | c2 | e2 | rp | rpA | rpC | rpE |
| General Memory | .50 (.16, .81) |
.20 (.00, .49) |
.30 (.18, .44) |
.15 (.00, .82) |
.32 (.00, .68) |
.53 (.14, .87) |
−.24 (−.38, −.07) |
.16 (−.28, .36) |
−.24 (−.48, .08) |
−.16 (−.36, .00) |
| Test-Level Factors (Including the contribution of the general memory factors) | ||||||||||
| CVLT-II | .37 (.15, .55) |
.11 (.00, .31) |
.52 (.43, .60) |
.05 (.00, .29) |
.12 (.00, .26) |
.83 (.68, .95) |
−.35 (−.42, −.28) |
.09 (−.11, .19) |
−.11 (−.23, .04) |
−.33 (−.44, −.23) |
| Logical Memory | .46 (.31, .58) |
.06 (.00, .17) |
.48 (.41, .57) |
.03 (.00, .16) |
.05 (.00, .13) |
.92 (.83, .98) |
−.39 (−.45, −.33) |
.05 (−.07, .13) |
−.05 (−.11, .02) |
−.39 (−.47, −.31) |
| Visual Reproductions | .45 (.27, .59) |
.05 (.00, .18) |
.50 (.39, .62) |
.02 (.00, .21) |
.04 (.00, .20) |
.94 (.76, .99) |
−.40 (−.50, −.32) |
−.01 (−.16, .11) |
−.03 (−.13, .05) |
−.36 (−.51, −.21) |
| Test-Specific Factors (Excluding the contribution of the general memory factors)* | rp* | rpA* | rpC* | rpE* | ||||||
| CVLT-II | .10 (.00, .22) |
.01 (.00, .16) |
.35 (.26, .45) |
.00 (.00, .16) |
.01 (.00, .16) |
.65 (.39, .82) |
−.25 (−.33, −.16) |
.02 (−.09, .09) |
.00 (−.11, .04) |
−.27 (−.38, −.16) |
| Logical Memory | .31 (.18, .40) |
.00 (.00, .09) |
.40 (.32, .48) |
.00 (.00, .07) |
.00 (.00, .06) |
.83 (.69, .93) |
−.33 (−.42, −.26) |
.02 (−.05, .08) |
.00 (−.05, .03) |
−.35 (−.45, −.28) |
| Visual Reproductions | .34 (.17, .46) |
.00 (.00, .13) |
.43 (.32, .55) |
.00 (.00, .17) |
.01 (.00, .15) |
.89 (.70, .97) |
−.37 (−.48, −.24) |
−.03 (−.19, .05) |
.00 (−.08, .05) |
−.34 (−.48, −.19) |
Note: Estimates are based on the higher-order latent change model. 95% confidence intervals are presented in parentheses under each estimate. a2 = Additive genetic variance; c2 = Common/shared environmental variance; e2 = Unique environmental variance; rp = Phenotypic correlation; rpA = Genetic contribution to the phenotypic correlation; rpC = Common environment contribution to the phenotypic correlation; rpE = Unique environment contribution to the phenotypic correlation; CVLT-II = California Verbal Learning Test – II.
See Table 4 for an explanation of how these values are calculated from covariance estimated. The actual covariance estimates for the last four columns of this table are shown in Supplemental Table 3.
Table 5 presents the means, variances, and phenotypic correlations among the latent level (intercept) and change factors. There was no significant group mean change in the CVLT-II factor, but there was a significant mean decline in the LM factor. A significant mean improvement in VR suggested a practice effect for that test. Initial level (intercept) and change were correlated for each test-level factor such that higher VETSA 1 scores were associated with larger declines. Overall phenotypic change was only mildly correlated across test-level factors. Although mean change was in the opposite direction for some test factors, change was positively correlated among all three memory factors. That is, the mean of one distribution was shifted upward or downward, but the overall relationship between change in each of them was positive. The other noteworthy finding in Table 5 is that there was significant variance in the change over time for each of the three test-level factors.
The values under Level in Table 6 are the same as those under VETSA 1 in Table 4, except for slight differences due to differences in model specification. The Change columns in Table 6 show that there was essentially no evidence for the heritability of change in any of the factors, and the vast majority of change in all cases was accounted for by unique environmental influences. The E term in twin models includes error, but we know that there is systematic change in all cases because the change variance as indicated in Table 5 was significant. The bottom three rows of Table 6 show that relatively little of the variance in change was accounted for by the general memory factor. For CVLT-II, for example, the total change variance was .66 (i.e., .00+.01+.65). Hence, the general factor accounted for 34% of the variance in change on the CVLT-II test-level factor. The general factor accounted for only 17% and 10% of the variance in change in the LM and VR test-level factors, respectively.
The genetic and environmental contributions to the phenotypic correlations (rp) between level (intercept) and change are presented in Table 6 (see also Supplementary Table 3). The correlations between level and change were mostly accounted for by unique environmental influences. In addition, most of the covariance between level and change for the test-specific factors was accounted for by influences that were independent of the general memory factor (rp*/rp=71–93%).
Because it is possible that age or duration of follow-up might moderate change in episodic memory, we also fit a full-moderation model (a latent change model that included age at VETSA1 and testing interval on the latent level and slope factors) for each of the three test factors. We then tested whether the moderation parameters could be fixed at zero without a significant change in model fit. Consistent with our cross-sectional data (Kremen et al., 2014) indicating that performance level was slightly poorer with increasing age, there was a significant moderation effect of age at VETSA 1 for CVLT (p<.009) and VR (p<.005). More relevant to the present study, there were no moderation effects of age or interval on slope (.10<ps<.67). Thus, in this age range, change in episodic memory did not differ as a function of age or follow-up duration.
Discussion
The factor structure of episodic memory recall was invariant across 5.73 years from middle to late middle age. At both timepoints there was a heritable general episodic memory factor and three test-level memory factors, each comprising immediate and delayed recall scores. Cross-time stability was primarily due to genetic influences. There was also reliable cross-time change variance, largely accounted for by unique environmental influences. Substantial stability and change effects were independent of the general memory factor.
Episodic Memory Stability
Phenotypic stability coefficients for our test-level factors were moderate to high (.67–.70, Table 4). These correlations are somewhat lower than other story recall and word list recall factors (both rs=.88) from the 6-year follow-up of Hertzog et al. (2003). Differences might be because their participants underwent mid-interval testing, read verbal test materials rather than only hearing them, and had to provide gist rather than specific recall for stories. Most of the phenotypic stability of the episodic memory factors as well as the observed memory measures from middle to late middle age was attributable to genetic influences.
Although the general memory factor was the most reliable over time, it still left considerable unaccounted-for variance in episodic memory stability. Unpacking the test-level factors, we found test-specific influences that were independent of the general memory factor accounted for a majority of the stability of the LM and VR factors and nearly 40% of the stability of the CVLT-II factor (Table 4). Thus, the stabilities of the general memory factor and three commonly used memory tests involve different, non-overlapping sets of genetic influences. There was no evidence for new genetic influences in any episodic memory factors or measures at VETSA 2. It is, of course, possible that the factor structure might change or that new genetic influences may come into play in later years, but that was not the case during this interval encompassing the transition from middle to late middle age.
With only two timepoints, it is not possible to detect nonlinear (quadratic) change. On the other hand, it seems unlikely that there would be nonlinear change over this interval of less than six years. There could be significant genetic influences on memory change over a longer interval, and there is some evidence for genetic influences on episodic memory change in older adults that are nonlinear (McArdle & Plassman, 2009; Reynolds et al., 2005). However, we do not know how typical these patterns are, given that we are aware of only these two twin studies that have examined nonlinear trajectories and genetic influences on memory change.
As we noted previously (Kremen et al., 2014), a brief review of cross-sectional genetic association studies of memory by Papassotiropoulos and de Quervain (2011) indicated that in some studies the same gene may be associated with multiple memory tests whereas in other studies a gene may be associated with one memory test but not another. For example, the brain-derived neurotrophic factor (BDNF) genes was associated with LM but not CVLT performance (Egan et al., 2003), whereas the kidney and brain expressed protein (KIBRA) gene was associated with performance on multiple episodic memory tests (Papassotiropoulos et al., 2006). Unfortunately, given the problems of replication in genetic association studies, it is nearly impossible to know whether a gene being associated with one test and not another represents test-specific genetic influences on each test or a failure to replicate. Moreover, although episodic memory phenotypes are most likely highly polygenic, candidate gene studies can examine only a very limited number of genes. Combining different tests across multiple studies for large-scale genome-wide association studies may be the answer, but that goal will be problematic if different genes influence different memory tests. The twin method cannot specify which genes or how many genes are involved, but it complements genetic association studies because it does account for the total aggregate effect of all genes.
The neural substrates of memory stability over time may also differ for different test-specific factors. All of the tests in the present study are hippocampal-dependent memory tests, but episodic memory involves a network of multiple brain regions (Schedlbauer, Copara, Watrous, & Ekstrom, 2014; Walhovd et al., 2005). Different network components may differentially affect performance on the different episodic memory factors or measures. For example, there is evidence that prefrontal cortical regions are relatively more important for temporal order memory compared with relatively greater importance of parietal cortical regions for memory of spatial layout (Schedlbauer et al., 2014). Memory for temporal order is likely to be useful for LM story recall, and memory for spatial memory layout would, of course, be useful for VR design recall. Consistent with the idea of different genetic influences on the LM and VR factors, our own work (Chen et al., 2013; Chen et al., 2012), has shown that there are different genetic influences on the area and thickness of subregions of prefrontal and parietal cortices.
Episodic Memory Change
Consistent with the possibility that we raised in our age 55 examination (Kremen et al., 2014), the direction of mean level change in episodic memory over this time period differed for the different episodic memory factors. Although mean level changes were small over this 5.73-year period beginning in midlife, there was significant variability of change for all indices even when there was no significant mean level change. In contrast to the influences on stability of episodic memory, the different trajectories suggested by that change variability were almost entirely accounted for by non-overlapping sets of unique environmental influences. Efforts to discern the specific environmental events that drive change, if successful, may identify modifiable risk factors that reduce memory loss. For example, environmental factors such as activity level, engagement in intellectually stimulating activities, or diet, might have differential effects on different components of the brain regions described in the preceding paragraph. Examination of environmental factors may also need to be conducted in the context of cognitive process analyses in order to determine what accounts for the differential change patterns.
Micro- Versus Macro-Level Analysis
Researchers frequently employ a general/composite memory index or a single test-level index (one measure from a test or a composite of selected measures from a test). Our results indicate that most of the influences on memory stability or change are unlikely to be captured with general/composite measures, and that test-level indices still conflate influences on general memory ability and test-specific abilities. Analyses at a more macro level might include memory indices adjusted for some index of GCA, but those indices are not adjusted for general memory ability per se. Had we used a single general composite measure or composite measures from only one of the tests (as is most common), we would have drawn three entirely different conclusions about mean level memory change over this time period: it declines; it improves (suggesting practice effects); or it does not change. The different genetic influences on test-specific episodic memory factors, and the different directions of mean change for the test factors also highlight the value of extensive neurocognitive assessment.
Several studies have shown that both general and specific influences account for individual differences in cognitive change across multiple cognitive domains. Highlighting the value of our more micro-level focus, we found the same phenomenon at both the phenotypic and the genetic level within only one aspect of one specific cognitive domain. In the only similar multivariate longitudinal genetic analysis of which we are aware, multiple cognitive domains were examined (Tucker-Drob, Reynolds, et al., 2014). There was little structure for change factors in adults 50–65 years of age, in contrast to adults 65–96 years. As such, the authors performed the longitudinal genetic analysis only in the 65–96 year old part of the sample. The lack of structure in the 50–65 year old subgroup lends further support for the idea that our more micro-level approach may be particularly relevant for middle-aged adults. That said, we note that even more micro-level analysis is, of course, possible.
Strengths and Limitations
The large sample size, extensive memory testing, and narrow age cohort are strengths of the study for examining memory change in a particular period of adult life. All participants were men and were primarily Caucasian, so we cannot be certain about the generalizability of the results to women or to different racial/ethnic groups. As in any longitudinal study, not all participants returned for the follow-up. However, wave 1 data for all participants were included in our analyses so as to reduce bias in the estimates that can be caused by analyzing only individuals with data from both timepoints. We cannot yet know if the observed patterns will remain the same in continued follow-ups into later life, but the results do shed light on the understudied period of middle to late middle adulthood just prior to the important transition period to early old age. We did not examine specific neurobiological substrates, specific genes, or specific environmental factors that may underlie the observed patterns. However, by addressing the genetic complexity of memory, the present results may inform genome-wide association studies (GWAS) or more fine-grained neuroscientific studies (Bearden et al., 2012; Kremen et al., 2014; Papassotiropoulos & de Quervain, 2011). For example, combining samples is becoming indispensable for GWAS, and knowing the extent of common and unique genetic influences among different memory phenotypes should be valuable information prior to combining samples for large-scale analyses.
Summary
Our results indicate that memory stability over time is not simply a function of a general memory factor. For three of the most widely-used memory tests, there are—in addition to any common genetic influences—non-overlapping sets of genetic influences on their cross-time stability. There is also reliable change variance that is primarily due to test-specific unique environmental influences. Taken together, these longitudinal results highlight the complexity of episodic memory and memory change at the phenotypic and genetic level, the value of more micro-level analysis within specific cognitive domains, and the importance of using multiple measures for more than simply generating a composite summary index. Our narrow age cohort design also enabled us to illuminate the understudied midlife to early old age transition period. In sum, these findings are important for understanding genetic and environmental influences on cognition and age-related cognitive change, and for refining the search for underlying mechanisms.
Supplementary Material
Acknowledgments
This work was supported by National Institute on Aging Grants R01 AG018386, AG022381, and AG022982 (to William S. Kremen), R01 AG018384 (to Michael J. Lyons), and K08 AG047903 (to Matthew S. Panizzon). This material was, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health Healthcare System. The content is solely the responsibility of the authors and does not necessarily represent official views of the NIA, NIH, or VA. The Cooperative Studies Program of the U.S. Department of Veterans Affairs provided financial support for development and maintenance of the Vietnam Era Twin Registry. Numerous organizations provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible. We also appreciate the time and energy of many staff and students on the VETSA projects.
Contributor Information
Matthew S. Panizzon, Department of Psychiatry, Center for Behavioral Genomics, University of California, San Diego, and VA San Diego Healthcare System
Michael C. Neale, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University
Anna R. Docherty, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University
Carol E. Franz, Department of Psychiatry and Center for Behavioral Genomics, University of California, San Diego
Kristen C. Jacobson, Department of Psychiatry and Behavioral Neuroscience, University of Chicago
Rosemary Toomey, Department of Psychologcial and Brain Sciences, Boston University.
Hong Xian, Department of Biostatistics, St. Louis University, and Research Service, VA St. Louis Healthcare System.
Terrie Vasilopoulos, Department of Anesthesiology, University of Florida.
Brinda K. Rana, Department of Psychiatry and Center for Behavioral Genomics, University of California, San Diego
Ruth M. McKenzie, Department of Psychological and Brain Sciences, Boston University
Michael J. Lyons, Department of Psychological and Brain Sciences, Boston University
William S. Kremen, Department of Psychiatry, Center for Behavioral Genomics, University of California, San Diego, and VA San Diego Healthcare System
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Bayroff AG, Anderson AA. Development of Armed Forces Qualification Tests 7 and 8 (Technical Research Report 1122) Alexandria, VA: U.S. Army Research Institute; 1963. [Google Scholar]
- Bearden CE, Karlsgodt KH, Bachman P, van Erp TG, Winkler AM, Glahn DC. Genetic architecture of declarative memory: Implications for complex illnesses. Neuroscientist. 2012;18:516–532. doi: 10.1177/1073858411415113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S, Neale MC, Maes H, Wilde M, Spiegel M, Brick TJF. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fiecas M, Gutierrez ED, Panizzon MS, Eyler LT, Vuoksimaa E, Kremen WS. Genetic topography of brain morphology. Proc Natl Acad Sci U S A. 2013;110:17089–17094. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Gutierrez ED, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, Dale AM. Hierarchical genetic organization of human cortical surface area. Science. 2012;335:1634–1636. doi: 10.1126/science.1215330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nature Reviews Neuroscience. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test (CVLT-2) 2nd ed. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Eaves LJ, Last KA, Young PA, Martin NG. Model-fitting approaches to the analysis of human behavior. Heredity. 1978;41:249–320. doi: 10.1038/hdy.1978.101. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eisen, Neuman R, Goldberg J, Rice T. Determining zygosity in the Vietnam Era Twin Registry: An approach using questionnaires. Clinical Genetics. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF, MacDonald SW. Latent change models of adult cognition: Are changes in processing speed and working memory associated with changes in episodic memory? Psychology and Aging. 2003;18:755–769. doi: 10.1037/0882-7974.18.4.755. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Nesselroade JR. Assessing psychological change in adulthood: An overview of methodological issues. Psychology and Aging. 2003;18:639–657. doi: 10.1037/0882-7974.18.4.639. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Schaie KW. Stability and change in adult intelligence: 1. Analysis of longitudinal covariance structures. Psychology and Aging. 1986;1:159–171. doi: 10.1037//0882-7974.1.2.159. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Sliwinski MJ. Understanding ageing. An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology. 2001;47:341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- Keller MC, Medland SE, Duncan LE. Are extended twin family designs worth the trouble? A comparison of the bias, precision, and accuracy of parameters estimated in four twin family models. Behavior Genetics. 2010;40:377–393. doi: 10.1007/s10519-009-9320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Franz CE, Lyons MJ. VETSA: The Vietnam Era Twin Study of Aging. Twin Research and Human Genetics. 2013;16:399–402. doi: 10.1017/thg.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Panizzon MS, Franz CE, Spoon KM, Vuoksimaa E, Jacobson KC, Lyons MJ. Genetic complexity of episodic memory: A twin approach to studies of aging. Psychology and Aging. 2014;29:404–417. doi: 10.1037/a0035962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Panizzon MS, Xian H, Barch DM, Franz CE, Grant MD, Lyons MJ. Genetic architecture of context processing in late middle age: More than one underlying mechanism. Psychology and Aging. 2011;26:852–863. doi: 10.1037/a0025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, Lyons MJ. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Lange K, Westlake J, Spence MA. Extensions to pedigree analysis: III. Variance components by the scoring method. Annals of Human Genetics. 1976;39:485–491. doi: 10.1111/j.1469-1809.1976.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd ed. New York: Wiley; 2002. [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, Kremen WS. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychological Science. 2009;20:1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markon KE, Krueger RF. An empirical comparison of information-theoretic selection criteria for multivariate behavior genetic models. Behavior Genetics. 2004;3:593–610. doi: 10.1007/s10519-004-5587-0. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Nesselroade JR. Structuring data to study development and change. In: Cohen SH, Reese HW, editors. Life-Span Developmental Psychology: Methodological Innovations. Mahwah, NJ: Erlbaum; 1994. pp. 223–268. [Google Scholar]
- McArdle JJ, Plassman BL. A biometric latent curve analysis of memory decline in older men of the NAS-NRC twin registry. Behavior Genetics. 2009;39:472–495. doi: 10.1007/s10519-009-9272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer; 1992. [Google Scholar]
- Panizzon MS, Vuoksimaa E, Spoon KM, Jacobson KC, Lyons MJ, Franz CE, Kremen WS. Genetic and environmental influences on general congitive ability: Is g a valid latent construct? Intelligence. 2014;43:65–76. doi: 10.1016/j.intell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papassotiropoulos A, de Quervain DJ. Genetics of human episodic memory: Dealing with complexity. Trends in Cognitive Science. 2011;15:381–387. doi: 10.1016/j.tics.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, de Quervain DJ. Common Kibra alleles are associated with human memory performance. Science. 2006;314:475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- Reynolds CA, Finkel D, McArdle JJ, Gatz M, Berg S, Pedersen NL. Quantitative genetic analysis of latent growth curve models of cognitive abilities in adulthood. Developmental Psychology. 2005;41:3–16. doi: 10.1037/0012-1649.41.1.3. [DOI] [PubMed] [Google Scholar]
- Schedlbauer AM, Copara MS, Watrous AJ, Ekstrom AD. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci Rep. 2014;4:6431. doi: 10.1038/srep06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeneborn CA, Heyman KM. National Health Statistics Reports. Vol. 16. Hyattsville, MD: National Center for Health Statistics; 2009. Health characteristics of adults aged 55 years and over: United States, 2004–2007. National Health Statistics Reports; no. 16. [PubMed] [Google Scholar]
- Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annual Review of Psychology. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- Steiger JH, Shapiro A, Browne MW. On the multivariate asymptotic distribution of sequential chi-square statistics. Psychometrika. 1985;50:253–264. [Google Scholar]
- Tucker-Drob EM, Briley DA, Starr JM, Deary IJ. Structure and correlates of cognitive aging in a narrow age cohort. Psychology and Aging. 2014;29:236–249. doi: 10.1037/a0036187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Reynolds CA, Finkel D, Pedersen NL. Shared and unique genetic and environmental influences on aging-related changes in multiple cognitive abilities. Developmental Psychology. 2014;50:152–166. doi: 10.1037/a0032468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 381–403. [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275–1278. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale (WMS-III) San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Widaman KF, Ferrer E, Conger RD. Factorial invariance within longitudinal structural equation models: Measuring the same construct across time. Child Dev Perspect. 2010;4:10–18. doi: 10.1111/j.1750-8606.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LJ, Holahan PJ. Parsimony-based fit indices for multiple-indicator models: Do they work? Structural Equation Modeling. 1994;1:161–189. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.