Abstract
Background
Heavy alcohol use during adolescence may alter the trajectory of normal brain development. Whether developmental trajectories of regional cortical volume and white matter structures are differentially affected in heavy drinkers relative to non-drinking controls has not been studied over extended periods or with sample sizes adequate to address potential sex differences.
Methods
This longitudinal study examined gray and white matter volume trajectories in 134 adolescents (75 who transitioned into heavy drinking and 59 who remained light to non-drinkers over roughly 3.5 years). Each underwent magnetic resonance imaging (MRI) on a 3T system two to six times (390 total scans) between ages 12 to 24 and was followed up to 8 years. Volumes of neocortex, allocortex, and white matter structures were measured using atlas-based parcellation with longitudinal registration. Linear mixed-effects models described differences in trajectories of drinkers and controls over age; secondary analyses considered the contribution of other drug use to identified alcohol use effects.
Results
Heavy-drinking adolescents showed accelerated gray matter reduction in cortical lateral frontal and temporal volumes and attenuated white matter growth of the corpus callosum and pons relative to controls. These results were essentially the same when marijuana and other drug use were examined. Male and female drinkers showed similar patterns of development trajectory abnormalities.
Discussion
Longitudinal analysis enabled detection of accelerated typical volume decline in frontal and temporal cortical volumes and attenuated growth in principal white matter structures in adolescents who started to drink heavily. These results provide a call for caution regarding heavy alcohol use during adolescence, whether heavy alcohol drinking is the cause or one of many factors in a constellation of causes of these alterations in brain development.
Keywords: adolescent, alcohol, brain development, longitudinal, mixed linear effects, prospective
INTRODUCTION
Alcohol is among the most commonly used intoxicating substance during adolescence, with 43% of youth between ages 12 and 18 reporting past year alcohol use and 25% reporting past year drunkenness (1). By age 18, almost a quarter of youth report recent heavy episodic drinking, defined as consuming five or more drinks on one occasion during the past two weeks (1). These high rates of heavy alcohol use are concerning, as the adolescent brain undergoes extensive morphometric and functional maturation involving decreases in gray matter and increases in white matter volume (2, 3). Cross-sectional studies have shown that cortical gray matter volume reduction begins during preadolescence (approximately age 5–10) (3, 4) and is generally considered to be related to pruning of excess neurons, changes in the extracellular matrix, and white matter encroachment (5), beginning primarily in posterior brain regions and progressing to more anterior regions (6), with decreases in dorsal prefrontal cortical volume continuing into early adulthood (mid-20s) (7). In concert with cortical thinning, white matter volume increases over adolescence, due in part to myelination of white matter tracts and axonal extension for connectivity (3, 8). These co-occurring neural processes are integral components of functional development, creating localized and enhanced efficient information processing required for mature complex cognitive and motor abilities (9). Because of these extensive maturational changes, the developing adolescent brain may be especially vulnerable to the deleterious effects of exogenous agents, including alcohol (10).
Cross-sectional studies using structural magnetic resonance imaging (MRI) report smaller hippocampal, prefrontal cortical, and cerebellar volumes in heavy-drinking compared with non-drinking teens (10). Given the dynamic neural events of adolescence, controlled longitudinal study is essential for determining whether group differences can be explained by development change itself or as a result of interactions with other apparent causes. Using a longitudinal design, a recent study examined youth before (age ~17 years) and after (age ~19 years) initiating heavy alcohol use. Adolescents who began heavy drinking (n=30) over the follow-up period showed accelerated cortical thinning of right middle frontal gyrus and decreased white matter volume subjacent to precentral gyral and middle temporal gyral cortices compared with demographically matched non-drinking teens (n=25) (11). In a similar study of adolescents followed from age ~15 to 18, participants who initiated heavy drinking over the follow-up (n=20) showed significantly greater volume reduction in the left ventral diencephalon, left inferior and middle temporal gyrus, and left caudate and brain stem than youth who remained substance-naïve over the follow-up (n=20) (12). Yet to be addressed are whether heavy drinking during adolescence alters the trajectories of regional volume declines in cortical gray matter and growth of white matter and whether potential changes differ between the sexes.
The goal of this controlled, longitudinal study was to measure within-subject changes in regional brain morphometry and quantify cortical and white matter volume changes over longer intervals and in larger samples of youth than previously reported who remained non-drinkers compared with those who drank heavily during adolescence. Examination of youth over multiple MRI sessions (upwards of 6 sessions) enabled modeling of normal development of cortical and white matter volumes in nondrinkers and deviations from growth trajectories in youth who went on to be heavy drinkers. Accordingly, we tested the following hypotheses: 1) adolescents who refrained from drinking would have cortical gray matter volume decline and volume expansion of white matter brain structures; 2) adolescents who transitioned into heavy drinking would show deviations from the normal developmental volume trajectories, specifically, accelerated regional cortical volume loss and slowed white matter expansion relative to non-drinking youth. Secondary analyses considered the potential compounding effect of other drug use with heavy alcohol drinking.
METHODS
Participants
The sample was obtained from a larger ongoing neuroimaging study of 296 adolescents examining youth at-risk for substance use disorders (R01 AA013419; PI: Susan Tapert). Participants were recruited through flyers sent to households of students attending local middle schools, which included major eligibility criteria, financial compensation, and contact information. Informed consent and assent were obtained and included approval for youth and parents to be contacted for follow-up interviews and scans. Eligibility criteria, substance use history, family history of substance use, developmental, and mental health functioning data were obtained from the youth, their biological parent, and one other parent or close relative. The study protocol was executed in accordance with the standards approved by the University of California, San Diego Human Research Protections Program.
Exclusionary criteria included any neurological or DSM-IV (13) Axis I disorder, determined by the NIMH Diagnostic Interview Schedule for Children –version 4.0 (14); any history of head trauma or loss of consciousness (>2 minutes); history of chronic medical illness; learning disability or mental retardation; use of medications potentially affecting the brain; premature birth (prior to 35th gestational week); any suggestion of prenatal alcohol (>2 drinks within a week) or illicit drug exposure; contraindication to MRI (e.g., braces); inadequate comprehension of English; non-correctable sensory problems; and clinically abnormal brain anatomy as determined by neuroradiologist review. After screening 1,987 responders, approximately 15% remained eligible (see Table 1).
Table 1.
Demographic and substance use information at baseline and follow-up. Substance use information on heavy drinkers is divided by sex.
| Continuous Non-drinkers (n=59) | Heavy Drinkers (n=75) | |||
|---|---|---|---|---|
| M | SD or % | M | SD or % | |
|
| ||||
| Baseline | ||||
|
| ||||
| Age (range: 12–19) * | 13.74 range: 12–19 | 1.42 | 15.68 range: 12–19 | 1.96 |
|
| ||||
| Sex (% females) | 48% (n=28) | 40% (n=30) | ||
|
| ||||
| Race (% Caucasian) | 64% (n=38) | 79% (n=59) | ||
|
| ||||
| Family history of alcoholism density (range 0–2) | 0.21 | 0.33 | 0.35 | 0.54 |
|
| ||||
| Conduct disorder positive (%) * | 3% (n=2) | 13% (n=10) | ||
|
| ||||
| Hollingshead Index of Social Position score | 26.25 | 17.61 | 21.73 | 13.82 |
|
| ||||
| Parent salary ($ in thousands) a | 120.28 | 77.57 | 144.16 | 80.85 |
|
| ||||
| Years of education * | 7.12 | 1.44 | 9.05 | 2.09 |
|
| ||||
| Females’ Pubertal Development Scale Tanner stage equivalent* | 2.86 | 0.60 | 3.45 | 0.63 |
|
| ||||
| Males’ Pubertal Development Scale Tanner stage equivalent* | 2.31 | 0.67 | 2.92 | 0.68 |
|
| ||||
| Beck Depression Inventory total * | 1.46 | 2.97 | 2.73 | 4.11 |
|
| ||||
| CBCL/ASR Internalizing T-score b | 44.48 | 8.24 | 44.47 | 9.72 |
|
| ||||
| CBCL/ASR Externalizing T-score b * | 41.33 | 7.64 | 46.00 | 9.34 |
|
| ||||
| Grade point average | 3.45 | 0.68 | 3.45 | 0.64 |
|
| ||||
| Follow-up | ||||
|
| ||||
| Age (range: 13–24) * | 17.28 range: 13–22 | 2.01 | 19.64 range: 15–24 | 1.91 |
|
| ||||
| Number of scans completed (range: 2–6)* | 2.54 | 0.80 | 3.20 | 1.23 |
|
| ||||
| Years between first and last scans | 3.54 | 1.70 | 3.95 | 1.73 |
|
| ||||
| Females’ Pubertal Development Scale total | 3.74 | 0.45 | 3.89 | 0.27 |
|
| ||||
| Males’ Pubertal Development Scale total * | 3.41 | 0.54 | 3.67 | 0.46 |
|
| ||||
| Years of education* | 10.49 | 1.89 | 12.61 | 1.76 |
|
| ||||
| Beck Depression Inventory total * | 1.15 | 1.98 | 3.19 | 5.70 |
|
| ||||
| CBCL/ASR Internalizing T-score c | 39.84 | 6.96 | 42.01 | 10.17 |
|
| ||||
| CBCL/ASR Externalizing T-score c * | 40.38 | 7.25 | 48.23 | 9.54 |
|
| ||||
| Grade point average | 3.50 | 0.56 | 3.28 | 0.73 |
Continuous non-drinkers ≠ heavy drinkers, p<.05
Collected for 126 participants (8 refused answer).
Data acquired for 120 participants.
Data acquired for 116 participants.
Abbreviations: CBCL, Child Behavior Checklist; ASR, Adult Self Report.
Ethnicity was: 22% Latino; race was: 72% Caucasian, 15% multiracial, 2% Black/African-American, 2% Asian, 9% unknown (no significant between-group differences).
This longitudinal study was begun in July 2002 with imaging conducted on a 1.5T scanner that was replaced with a 3T system in June 2005. Attempts to merge data across 1.5T and 3T field strengths were unsatisfactory. Therefore, only participants who had multiple valid 3T scans that could be quantified with a longitudinal, atlas-based parcellation method (15, 16) were included in this study. Participants for this study (N=134) all had at least two 3T brain scans acquired over the course of the study, for a total of 390 scans. Participants completed substance use interviews every 3 months, and at each annual time point were defined as heavy drinkers or controls, based on previously reported classification schemes (17, 18) (see Figure 1). The final sample for this analysis consisted of 59 continuous controls (had minimal to no lifetime drinking throughout all follow-ups) and 75 heavy drinkers (had initiated heavy drinking over the follow-up). See Figure 1 and Table 1 for substance use information.
Figure 1.
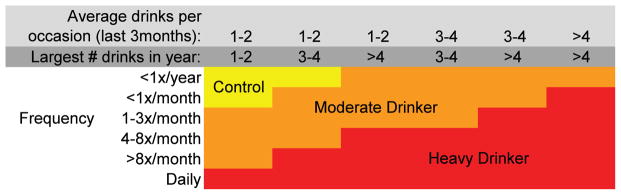
Substance use classification chart. Moderate drinkers were excluded from analyses.
Measures
Substance use measures
The Customary Drinking and Drug Use Record (19) obtained self-reported quantity and frequency of lifetime and past 3-month alcohol, tobacco, and other drug use (i.e., amphetamines, barbiturates, hallucinogens, cocaine, inhalants, opiates, spice, benzodiazepines, ecstasy, ketamine, gamma hydroxybutyrate, and other misused prescription medications), withdrawal/hangover symptoms, and endorsement of substance use disorder criteria. The Timeline Followback assessed substance use for the 30 days prior to the scan, and a parent or informant (e.g., roommate, sibling, friend) report of youth substance use was collected as collateral evidence. Each participant was categorized as a control or heavy drinker at each time point (17, 18) (see Figure 1). Moderate drinkers were not included in these analyses. Breathalyzer and urine toxicology screens confirmed self-report data.
Family background
The Family History Assessment Module (20) ascertained familial density of alcohol and other drug use disorders by adding 0.5 for each biological parent and 0.25 per biological grandparent endorsed by either youth or parent report as meeting criteria. Family history data were collected from one parent plus the other parent or a close relative. Socioeconomic background (i.e., educational attainment, occupation, and salary of each parent) was obtained from parents.
Development
The Pubertal Development Scale (21) provided a reliable and valid 5-item self-report measure of pubertal maturation, correlating well with physician ratings and Tanner Sexual Maturation Scale self-ratings (22). Scores ranged from 1 (prepubertal) to 5 (postpubertal).
Psychopathology and mood
The Child Behavior Checklist (23) was completed by parents for youth under age 18. The Adult Self Report (23) was completed by youth over age 18 to obtain levels of adolescent psychopathological syndromes (e.g., internalizing and externalizing behaviors). The Beck Depression Inventory-II assessed depressive symptoms (24).
Image acquisition
All imaging data included in the longitudinal statistical analysis of group differences reported in the Results were collected with the same 3 Tesla CXK4 short-bore Excite-2 MR system (General Electric, Milwaukee, WI), with an 8-channel phase-array head coil at the UCSD Keck FMRI Center. Eight high-bandwidth receivers for ultra-short TR times reduced signal distortions and signal dropout. Sessions involved a scout scan for head placement and slice selection, followed by a sagittal high-resolution 3d T1-weighted anatomical MRI (FOV 24 cm, 256 × 256 × 192 matrix, 0.94 × 0.94 × 1 mm voxels, 176 slices, TR=20 ms, TE=4.8 ms; flip angle 12°; 9 minutes).
Image processing
All images were first corrected for intensity bias using a second-order polynomial correction function computed by minimization of image entropy (25). Each bias-corrected image was then skull stripped using ROBEX (http://www.nitrc.org/projects/robex) (26). A brain mask was constructed from images acquired at the first MRI session. Each baseline brain mask was visually inspected and when necessary manually corrected.
Next, each follow-up image was aligned with the first image from the same subject in a two-step procedure: first, the skull-stripped follow-up image was linearly aligned with the skull-stripped initial image; second, the bias-corrected whole-head follow-up image was nonrigidly aligned with the bias-corrected whole-head initial image via nonrigid registration (27). By using skull-stripped images for initial, linear alignment, we ensured robust registration regardless of pose and shape changes in non-brain soft tissue. By using the whole-brain images for nonrigid registration, we ensured consistently good alignment quality throughout the brain, up to its surface, regardless of inconsistencies in the independently-computed brain masks.
Following alignment across time, the brain mask from each baseline image was resliced to each of the follow-up images from the same subject. This ensured temporally-consistent brain masks for all times points for the subsequent processing steps.
Skull-stripped images acquired at 1.5T were intensity normalized by histogram matching with respect to the first-acquired 3T image of the same subject. The 3T baseline or 1.5T image-intensity normalized baseline image was then aligned (first linearly, then non-linearly) with the T1-weighted (SPGR) channel of the SRI24 atlas (28). Using these alignment transformations, the tissue probability maps of the SRI24 atlas were then resliced into baseline image space of each subject. The probability maps were furthermore resliced into the space of each follow-up image for each subject via a concatenation of the atlas-to-baseline and the baseline-to-follow-up transformation for that subject and follow-up. Likewise, cortical and subcortical parcellation maps of the SRI24 atlas were resliced into the space of each baseline and follow-up image.
Using the resliced tissue probability maps as both initializers and priors during segmentation, tissue classification maps were then computed for each image using FSL’s FAST tool (29). Finally, the FAST tissue map for each image was combined with the SRI24 parcellation maps to obtain regional tissue volumes for all regions of interest in the parcellation maps.
Fitting of age trajectories after segmentation and parcellation resulted in discrete discontinuities across scanner strength within a subject. Therefore, subsequent data analyses used only 3T data from subjects with multiple 3T scans. Those whose initial scan was at 1.5T were included in the analysis only if they had two or more subsequent scans at 3T, and their first 3T scan was used as the baseline for trajectory analysis.
Regions of Interest (Figure 2)
Figure 2.
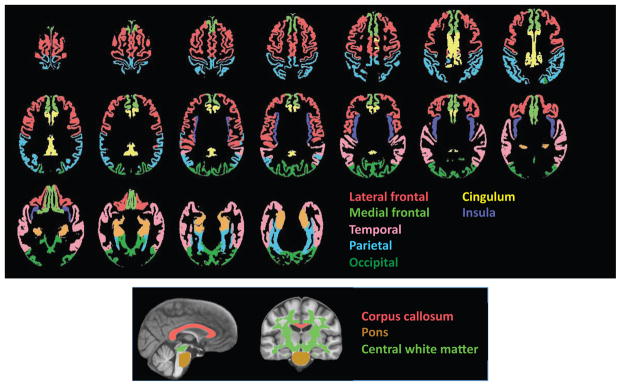
Top: Axial slices displaying the cortical gray matter regions of interest used to derive volumes for quantification. Bottom: Sagittal and coronal slices displaying the white matter regions of interest. All regions were determined with SRI24 atlas-based parcellation procedure.
All regions of interest were identified with the SRI24 atlas with tissue segmented into gray matter and white matter (16). Gray matter regions of interest included the total neocortex and lobar regions (frontal including the lateral and medial frontal cortex, temporal, parietal, and occipital cortices) and allocortex (cingulum and insula). The white matter regions of interest were the pons, corpus callosum, and central white matter, which was a large volume of subcortical white matter including much of the centrum semiovale and excluding white matter subjacent to neocortex. Subcortical structural measurement presented particular challenge in these data and will be pursued in the future.
Statistical analysis
Brain volumes of each region were first transformed into standardized Z-scores, which adjusted regional volumes for differences in supratentorial volume modeled from all subjects at all times, thereby adjusting for sex-related volume differences (3, 30, 31). As described previously (16), change trajectories of individual participants were calculated using the lmer function for linear mixed-effects modeling in the lme4 R statistical package (http://www.r-project.org/). The lmer function also allowed for testing of nested random effects; herein, the effects of age and drinking status were tested first to identify group differences in slopes of regional brain volumes adjusted for supratentorial volume. To examine longitudinal trajectories of volume change independent of age at data acquisition, we computed the trajectory slopes of each participant for each brain region by first removing the subject’s average age across the acquisitions to create a “centered-age” variable (i.e., the slope across the years of observation regardless of chronological age). We also entered the participant’s mean age across acquisitions (mean-age) into the model and sought group (alcohol_use)-by-centered-age interactions, (i.e., did the slope of volume change differ between alcohol users and non-users?):
| Model 1 |
RESULTS
The primary analysis model calculated trajectories of change over age, indicative of development, with the overarching hypothesis that heavy alcohol use during adolescence would affect the normal developmental trajectories of gray matter and white matter volume changes with aging. Outputs of interest were group differences in (1) mean slope of each volume over time, indicative of normal development in nondrinkers; and (2) alcohol use-by- centered-age trajectory (i.e., slope) interactions, indicative of deviation from normal development.
Developmental trajectories of neocortical volumes in heavy drinkers and controls
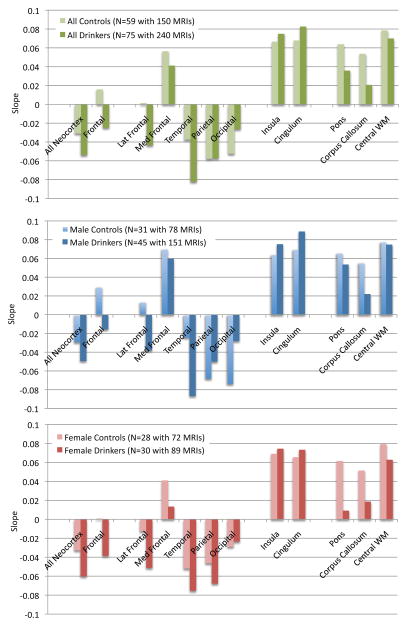
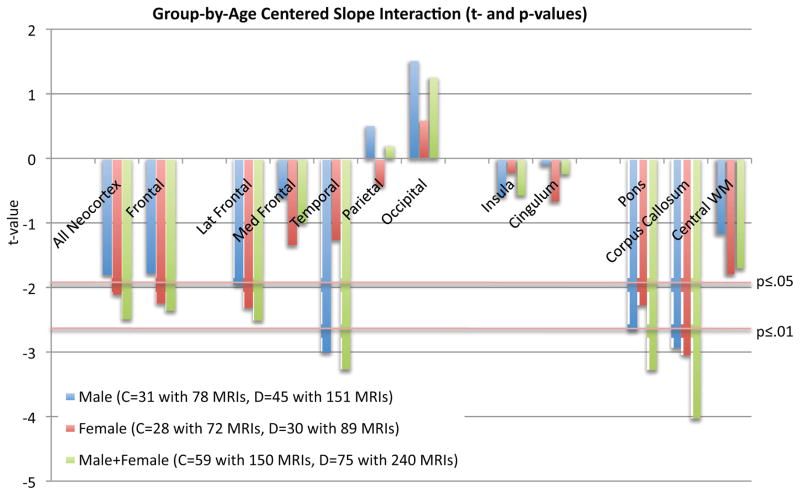
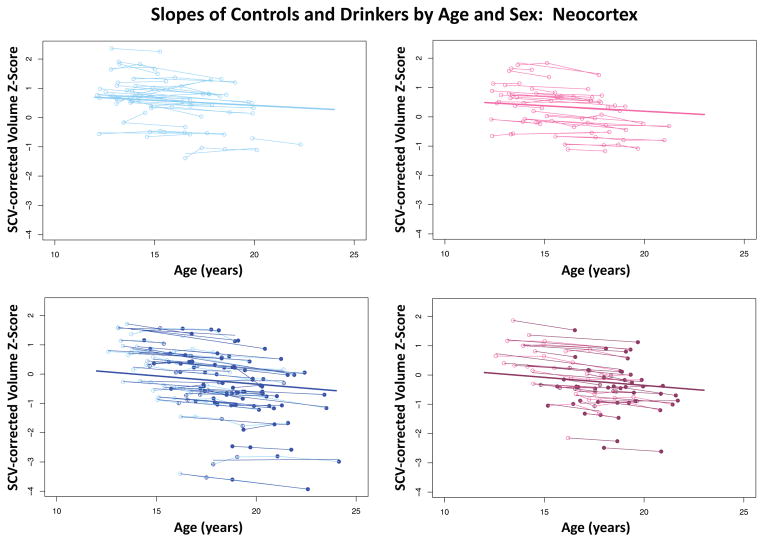
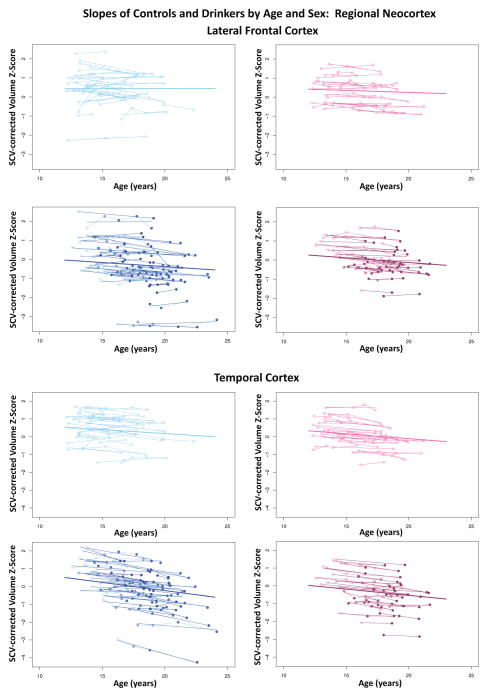
During the adolescent and young-adult years examined (age 12.13 to 24.14 with follow-up intervals of 0.85 to 8.4 years), neocortical volumes in most regions measured decreased over time in both non-drinking and heavy drinking youth (negative mean slopes in Figures 3–4). Relative to non-drinking controls, heavy drinkers exhibited greater volume reduction in the total neocortex (p=.0133) (Figure 5) and specifically in the frontal (p=.0191), lateral frontal (p=.0127), and temporal cortices (p=.0012) (Figure 6). Dividing the groups by sex yielded a similar pattern of results in the same regions observed in the total group, although differences were at trend levels between male controls and drinkers for the total neocortical (male p=.0729; female p=.0411) and lateral frontal (male p=.0519; female p=.0255) slopes (Figures 3–6). Sex differences were greater in the temporal lobe, which showed significantly decreasing volume in the male (p=.0031) but not female (p=.2106) drinkers (Figure 6). The medial prefrontal cortex was the single exception to neocortical gray matter volume shrinkage in these adolescents: although both drinkers and nondrinkers, whether male or female, showed volume expansion, the group-by- centered-age interactions were not significant (Figures 3–4).
Figure 3.
Mean slopes of each region of interest for all controls and all drinkers (top, green) and for each group divided by sex (middle: blue for male participants; bottom: pink for female participants). Negative slopes are in the direction of declining volume over age.
Figure 4.
Values from t-tests for group-by-age centered slope interactions: blue=male, red=female, green=male+female; c=control, d=drinker. Negative t-values indicate drinkers had greater volume reduction than controls.
Figure 5.
Plots of individual (N=134) supratentorial cranial volume (SCV)-corrected Z-scores by age for each control (empty dots) and drinker (colored dots) for gray matter in the overall neocortex. Male (blue) and female (pink) participant trajectories are shown separately. Each participant’s values are connected over time, and the age-centered slope of each participant is overlaid on his or her longitudinal data points. The long solid colored regression line is the expected volume-by-age regression based on the controls.
Figure 6.
Plots of individual (N=134) supratentorial cranial volume (SCV)-corrected Z-scores by age for each control (empty dots) and drinker (colored dots) for lateral frontal and temporal cortices. Male (blue) and female (pink) participant trajectories are shown separately. Each participant’s values are connected over time, and the age-centered slope of each participant is overlaid on his or her longitudinal data points. The long solid colored regression line is the expected volume-by-age regression based on the controls
Developmental trajectories of allocortical volumes in heavy drinkers and controls
Both non-drinking and heavy-drinking male and female participants exhibited significant volume enlargement of the insula and cingulum over the course of the study (Figure 3). Neither structure, however, showed growth attenuation in the heavy drinkers relative to non-drinkers, indicated by the absence of group-by- centered-age interactions (Figure 4).
Developmental trajectories of regional white matter volume in heavy drinkers and controls
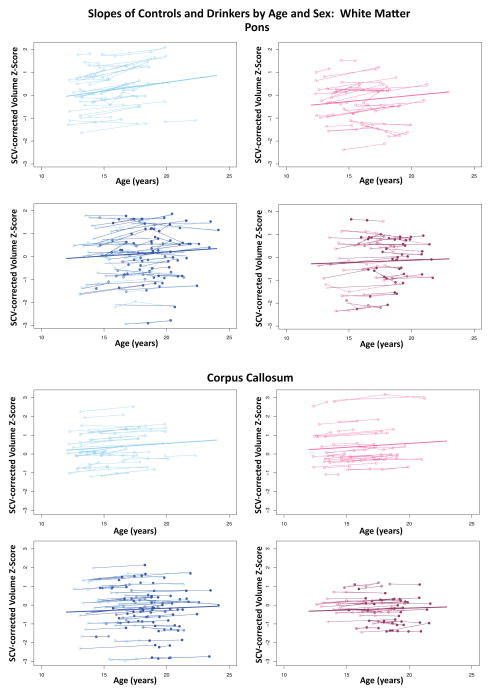
All three white matter regions showed volume growth in both heavy-drinking and non-drinking male and female participants, with the enlargement significant for the pons and corpus callosum (Figures 3–4). Growth trajectories were attenuated, however, in the heavy drinkers relative to nondrinkers in the pons (male p=.0085, female p=.0250, male+female p=.0012) and corpus callosum (male p=.0039, female p=.0029 male+female p=.0001) (Figures 4 and 7). The group trajectory differences in the central white matter volume of heavy drinkers did not reach statistical significance (Figure 4).
Figure 7.
Plots of individual (N=134) supratentorial cranial volume (SCV)-corrected Z-scores by age for each control (empty dots) and drinker (colored dots) for the pons and corpus callosum. Male (blue) and female (pink) participant trajectories are shown separately. Each participant’s values are connected over time, and the age-centered slope of each participant is overlaid on his or her longitudinal data points. The long solid colored regression line is the expected volume-by-age regression based on the controls
Contribution of other substance use on developmental trajectories
Illicit drug use was limited primarily to marijuana with the exception of two heavy drinking marijuana users: one also used amphetamines and the other used cocaine and opiates. Drug use was non-normally distributed, thereby precluding it as a continuous variable. Instead, we employed three different criteria (based on drug-use cut-points) in secondary analyses to examine the role of drugs other than alcohol on the results. 1) The number of days of marijuana use per year of observation interval (first to last scan used in the analyses) was discontinuous at ≥100 in 11 male and 6 female heavy drinkers. 2) A liberal criterion of 20 drug_use_days_per_year comprised 15 male and 7 female heavy drinkers. 3) A stricter criterion of 100 drug_use_days_per_year comprised 8 male and 4 female heavy drinkers. The mixed effects Model 1 was extended to Model 2 to include the interaction of centered-age and non-alcohol drug use (drug_use) as a dichotomous variable and tested with the three criteria.
| Model 2 |
Adding drug_use x centered-age to the model confirmed the regional original alcohol use effects with insubstantial changes in significance values (e.g., Figure 8). In only the corpus callosum, using the 100 drug_use_days_per_year criterion, was there a significant centered-age x alcohol_use and a significant centered-age x drug_use interaction (male+female, t=−4.514, p=.00001 for alcohol; t=2.142, p=.033 for drug+alcohol). Here, the alcohol users exhibited retarded corpus callosum growth, whereas the alcohol plus drug using participants had accelerated corpus callosum growth. This unpredicted result did not reach statistical significance with the other two drug use criteria.
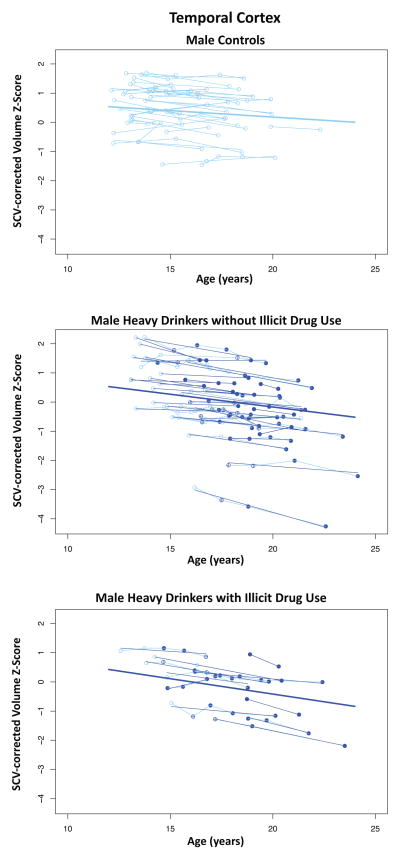
Figure 8.
Temporal cortical volumes and trajectories for the male controls (top), male heavy drinkers without illicit drug use (middle), and male heavy drinkers with illicit drug use (bottom).
In two regions where the alcohol x age-centered effect was not significant, the drug_use by centered-age effect was, suggesting attenuation of the normal trajectory. Using the 100 drug_days_per_year criterion and the combined group of both sexes, the alcohol+drug group was significantly different from controls (medial frontal t=−2.0302, p=0.043; insula: t=−2.1205, p=0.035) but the alcohol only group was not (medial frontal t=−0.3364, p=0.737; insula: t=0.0904, p=0.928).
DISCUSSION
Adolescents who had experienced episodes of heavy drinking had faster declining volumes in selective neocortical gray matter regions and smaller increases in regional white matter volumes relative to continuously non-drinking youth. This study is novel with respect to the number of assessments participants completed (two to six scans), the length of the study (multiple observations between ages 12 to 24), and the large sample size, allowing for comparison of sex-linked differences. The non-drinking adolescents studied over the same period served as a control group for estimating typical developmental trajectories over the same early- to late-adolescent age range as the heavy drinkers.
Dynamic brain growth and differential trajectories of gray and white matter volumes (3, 8) were notable in both the heavy drinkers and non-drinking adolescents. To the extent that the cortical volume reduction reflects normal, beneficial processes of neuronal pruning (3, 5, 8) and cortical restructuring, one might have expected a lesser change with age in the heavy drinkers; however, this was not the case because they exhibited accelerated declining volume trajectories. Possible interpretations of this pattern include accelerated but non-beneficial pruning or, alternatively, premature cortical gray matter decline similar to senescent volume declines seen in adult alcoholics (30) or even “normal” aging (3, 16). Over this same period, white matter volume increased in the corpus callosum and the pons, with a trend toward increases in a large sample of white matter of the centrum semiovale. The attenuated volume growth among heavy drinkers’ callosal and pontine white matter suggests a widespread effect, which has also been observed in chronically alcoholic adults (32). Longitudinal studies of adults with chronic alcohol dependence who sustained sobriety report normalization of white matter volumes (33) and microstructural integrity of selective fiber systems (34). To the extent that excessive alcohol consumption contributed to attenuated white matter volume growth in our heavy drinking adolescents, and given white matter fibers’ capacity for repair (21), one might speculate that constituent white matter processes could resume a normal growth trajectory and regain volume with abstinence from drinking.
This is the first study with a large sample size to measure macrostructural brain development over extended periods using an atlas-based parcellation and segmentation approach for longitudinal registration and quantification of major neocortical and allocortical regions and white matter structures in heavy drinking and non-drinking adolescents. The accelerated cortical volume regression together with slowed white matter volume growth in high-alcohol-consuming youth is largely consistent with previous findings in heavy-drinking youth that reported focal gray matter thinning in right middle frontal and left inferior and middle temporal cortical and other subcortical regions as well as slower expansion of white matter in right hemisphere precentral gyrus, lingual gyrus, middle temporal gyrus and anterior cingulate, relative to alcohol-naive adolescents (11, 12). Differences in specific loci showing significant growth deviations between groups might be attributable to differences related to measurement, sex, subject sampling, or to population differences with respect to behavioral attributes distinguishing drinkers from nondrinkers. Whether exposure to high doses of alcohol during critical periods of brain development in adolescence puts youth at risk for developing alcohol use disorders, or for exacerbated brain structural or functional abnormalities should youth progress to alcohol dependence, remains to be determined. Animal models of youthful drinking could help address this void. One series of studies in adolescent rodents exposed to high, intermittent doses of alcohol exhibited increased neuroimmune expression of the receptor for advanced glycation end products (RAGE) in prefrontal cortex only after extended periods without alcohol (35). This delayed response suggests that the effect of binge-like bouts of alcohol, even if not initially detectable, might reflect a form of neuroadaptation that has potential for later expression.
Previous cross-sectional findings have suggested that women may be more negatively affected by alcohol use than men (36). Our sample was adequately large to address possible sex differences in adolescent drinkers. While our male drinkers had endorsed drinking more drinks on each occasion, they were obtaining similar peak blood alcohol content levels to female drinkers. Despite their similar drinking levels, the only sex difference observed in our adolescents was for the temporal lobe group-by-age interaction, which was significant for the male but not the female youth, although the trajectory of the female drinkers showed a nonsignificant trend to deviate from normal in the same direction as male drinkers. These findings suggest that male and female heavy drinkers can sustain similar alterations in cortical brain volume growth during adolescence, taking into account potential differences in normal brain growth trajectories related to sex and pubertal development. However, smaller volume regions such as the amygdala, hippocampus and other sexually dimorphic regions known to be affected by alcohol were not examined in this analysis and should therefore be examined in future studies.
Disruption of the normal developmental trajectory of brain volume maturation during adolescence may have the potential to exert a negative effect on normal performance and development of cognitive and motor abilities (37). Deviations in structural brain development may explain in part previous longitudinal findings that adolescent heavy drinkers show worsening performance on behavioral tasks of visuospatial processing, attention, and working memory after initiating alcohol use (17, 38, 39). Further, abnormalities of neural growth patterns in heavy drinkers may contribute to short-term or long-term negative effects on cognitive, social, and academic functioning. Even longitudinal study, however, precludes discerning whether such functional difficulties are the result of excessive drinking alone or occur in interaction with the burden of a family history of alcohol dependence and a heightened likelihood of engaging in externalizing behaviors, including risk for consuming licit and illicit drugs along with alcohol, which occurs with greater incidence in heavy-drinking youth (40). Indeed, a large proportion of heavy drinkers in this study also consumed small to large amounts of marijuana and other drugs. Comparing only the drug-using heavy drinkers to controls produced results similar to the comparison of all heavy users. For instance, as seen in Figure 8, for the temporal cortex, the heavy alcohol drinkers with or without illicit drug use had similarly accelerated trajectories. There were, however, a few measures in which an effect for other substance use could be demonstrated over and above that of heavy alcohol use, and one (the corpus callosum) was counter intuitive. Despite substantial drug use among a few participants, the small sample size may have been inadequate to detect a compounded alcohol and drug effect.
Familial density of alcoholism did not differ between groups, and although externalizing symptoms were higher in heavy drinkers, the vast majority of participants in both groups were, not surprisingly, in the normal range. Rates of conduct disorder were higher in the heavy drinkers compared to controls (10 total heavy drinkers vs. 2 controls). Future studies with larger sample sizes and higher rates of youth with externalizing disorders will be able to disentangle the effects of heavy drinking from predisposing factors like comorbid psychological disorders and genetics.
Despite their strengths, controlled longitudinal studies suffer limitations, including interpretation of causality underlying observed brain volume changes. Nonetheless, our longitudinal analysis enabled detection of acceleration of normal volume decline in anterior and temporal cortical volumes and attenuation of growth in principal white matter structures in heavy-drinking adolescents. These results provide a call for caution regarding heavy alcohol use during adolescence, whether heavy alcohol drinking is the cause or one of many factors in a constellation of causes of these alterations in brain development.
Table 2.
| Continuous Non-drinkers (n=59) | Heavy Drinkers (n=75) | Heavy Drinking Females (n=30) | Heavy Drinking Males (n=45) | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
|
| ||||||||
| Baseline | ||||||||
|
| ||||||||
| Lifetime alcohol use occasions a | 0.07 | 0.37 | 15.75 | 30.66 | 15.53 | 24.67 | 15.89 | 34.35 |
|
| ||||||||
| Lifetime cannabis use occasions a | 0.00 | 0.00 | 10.95 | 36.57 | 10.43 | 27.28 | 11.29 | 41.93 |
|
| ||||||||
| Lifetime other drug use occasions | 0.00 | 0.00 | 0.56 | 2.41 | 0.87 | 3.49 | 0.36 | 1.26 |
|
| ||||||||
| Follow-up | ||||||||
|
| ||||||||
| Lifetime alcohol use occasions a | 0.85 | 1.95 | 210.08 | 236.47 | 165.10 | 219.42 | 240.07 | 244.98 |
|
| ||||||||
| Peak drinks on an occasion, past year a,b | 0.22 | 0.49 | 9.77 | 6.32 | 7.47 | 2.78 | 11.31 | 7.48 |
|
| ||||||||
| Peak drinks on an occasion, past 3 months a,b | 0.19 | 0.47 | 7.56 | 6.71 | 5.33 | 2.94 | 9.04 | 8.03 |
|
| ||||||||
| Estimated peak blood alcohol content, past 3 months a | 0.01 | 0.02 | 0.21 | 0.14 | 0.20 | 0.11 | 0.21 | 0.16 |
|
| ||||||||
| Drinking days per month, past 3 month average a | 0.15 | 0.36 | 9.69 | 9.48 | 9.97 | 9.54 | 9.51 | 9.54 |
|
| ||||||||
| Days since last alcohol use a | n/a | 30.60 | 88.96 | 22.80 | 36.45 | 35.80 | 111.20 | |
|
| ||||||||
| Tobacco cigarettes per day, past month a | 0.00 | 0.00 | 0.52 | 1.63 | 0.23 | 0.68 | 0.71 | 2.02 |
|
| ||||||||
| Lifetime cannabis use occasions a | 0.08 | 0.65 | 109.15 | 217.55 | 62.33 | 106.82 | 140.36 | 263.79 |
|
| ||||||||
| Cannabis use days per month, past 3 months a | 0.00 | 0.00 | 4.99 | 8.60 | 2.66 | 5.44 | 6.49 | 9.90 |
|
| ||||||||
| Lifetime other drug use occasions a | 0.00 | 0.00 | 46.44 | 223.59 | 21.00 | 77.28 | 63.40 | 281.80 |
Continuous non-drinkers ≠ heavy drinkers, p<.05
Female heavy drinkers ≠ male heavy drinkers, p<.05
Acknowledgments
Funding Support: R01 AA13419, U01 AA021692 (NCANDA-San Diego, PI: Tapert); F32 AA021610, K12 DA031794 (PI: Squeglia); F32 DA032188 (PI: Jacobus), U01 AA021697 (NCANDA-Data Analysis, PI: Pfefferbaum); and K05 AA017168 (PI: Sullivan).
References
- 1.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 1975–2013: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- 2.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021 doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 3.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 4.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 5.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Science. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TFr, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowell ER, Thompson PM, Tessner K, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2 doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 9.Squeglia LM, Jacobus J, Sorg SF, Jernigan TL, Tapert SF. Early adolescent cortical thinning is related to better neuropsychological performance. Journal of the International Neuropsychological Society. 2013;19:962–970. doi: 10.1017/S1355617713000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annual Review of Clinical Psychology. 2013;9 doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. The American Journal of Drug and Alcohol Abuse. 2013;39:345–355. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Developmental and Cognitive Neuroscience. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- 14.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan EV, Pfefferbaum A, Rohlfing T, Baker FC, Padilla ML, Colrain IM. Developmental change in regional brain structure over 7 months in early adolescence: Comparison of approaches for longitudinal atlas-based parcellation. NeuroImage. 2011;57:214–224. doi: 10.1016/j.neuroimage.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: Influence of initiating heavy drinking. Journal of Studies on Alcohol and Drugs. 2012;73:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 20.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JIJ, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 21.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17 doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 22.Miller CL, Tucker ML, Pasch L, Eccles JS. Measuring pubertal development: A comparison of different scales and different sources. Paper presented at the biennial meeting of the Society for Research in Child Development; Alexandria, VA. 1988. [Google Scholar]
- 23.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 24.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 25.Likar B, Viergever MA, Pernus F. Retrospective correction of MR intensity inhomogeneity by information minimization. IEEE Transactions on Medical Imaging. 2001;20:1398–1410. doi: 10.1109/42.974934. [DOI] [PubMed] [Google Scholar]
- 26.Iglesias JE, Liu CY, Thompson PM, Tu Z. Robust brain extraction across datasets and comparison with publicly available methods. IEEE Transactions on Medical Imaging. 2011;30:1617–1634. doi: 10.1109/TMI.2011.2138152. [DOI] [PubMed] [Google Scholar]
- 27.Rohlfing T, Maurer CRJ. Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Transactions on Information Technology in Biomedicine. 2003;7:16–25. doi: 10.1109/titb.2003.808506. [DOI] [PubMed] [Google Scholar]
- 28.Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multichannel atlas of normal adult human brain structure. Human Brain Mapping. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magnetic Resonance in Imaging. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- 30.Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical & Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 31.Luders E, Toga AW, Thompson PM. Why size matters: Differences in brain volume account for apparent sex differences in callosal anatomy: The sexual dimorphism of the corpus callosum. NeuroImage. 2014;84:820–824. doi: 10.1016/j.neuroimage.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, Harris GJ. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcoholism: Clinical & Experimental Research. 2013;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological Psychiatry. 2006;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, Gongvatana A, Grant I. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcoholism: Clinical & Experimental Research. 2012;36:1922–1923. doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of Disease. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgaleta M, Johnson W, Waber DP, Colom R, Karama S. Cognitive ability changes and dynamics of cortical thickness development in healthy children and adolescents. NeuroImage. 2014;84:810–819. doi: 10.1016/j.neuroimage.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of adolescent alcohol and drug use on neuropsychological functioning in young adulthood: 10-year outcomes. Journal of Child and Adolescent Substance Abuse. 2011;20:135–154. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- 40.Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for alcohol use disorders in men. Twin Research and Human Genetics. 2012;14:1–15. doi: 10.1375/twin.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]