Abstract
Misfolded membrane proteins are retained in the endoplasmic reticulum (ER) and are subject to ER-associated degradation, which clears the secretory pathway of potentially toxic species. While the transcriptional response to environmental stressors has been extensively studied, limited data exist describing the cellular response to misfolded membrane proteins. To this end, we expressed and then compared the transcriptional profiles elicited by the synthesis of three ER retained, misfolded ion channels: The α-subunit of the epithelial sodium channel, ENaC, the cystic fibrosis transmembrane conductance regulator, CFTR, and an inwardly rectifying potassium channel, Kir2.1, which vary in their mass, membrane topologies, and quaternary structures. To examine transcriptional profiles in a null background, the proteins were expressed in yeast, which was previously used to examine the degradation requirements for each substrate. Surprisingly, the proteins failed to induce a canonical unfolded protein response or heat shock response, although messages encoding several cytosolic and ER lumenal protein folding factors rose when αENaC or CFTR was expressed. In contrast, the levels of these genes were unaltered by Kir2.1 expression; instead, the yeast iron regulon was activated. Nevertheless, a significant number of genes that respond to various environmental stressors were upregulated by all three substrates, and compared with previous microarray data we deduced the existence of a group of genes that reflect a novel misfolded membrane protein response. These data indicate that aberrant proteins in the ER elicit profound yet unique cellular responses.
Keywords: ER-associated degradation (ERAD), heat shock response (HSR), unfolded protein response (UPR), molecular chaperone, iron regulon
the function of the cellular protein quality control (PQC) machinery is critical for ensuring organismal health, and not surprisingly numerous diseases, including cystic fibrosis, Parkinson's, Huntington's, and Alzheimer's diseases, diabetes, heart disease, alpha-1 antitrypsin deficiency, and nephrogenic diabetes insipidus, are associated with PQC (49). Several of these diseases are also linked to defects in the biogenesis of secreted and integral membrane proteins. When secreted and integral membrane proteins fail to properly fold or assemble in the endoplasmic reticulum (ER), they are targeted for ER-associated degradation (ERAD). During ERAD, substrates are selected by cytosolic and ER lumenal molecular chaperones, retrotranslocated (or dislocated) from the ER to the cytosol by the Cdc48/p97 complex, ubiquitinated, and then degraded by the 26S proteasome. Diseases such as cystic fibrosis result when a mutated yet partially functional protein is prematurely targeted for ERAD, thereby failing to mature and reach its final cellular destination (26, 68, 162). Other diseases, such as liver disease associated with α1-antitrypsin deficiency, result when a misfolded protein is poorly targeted for ERAD, which results in its aggregation in the ER and compromised cellular homeostasis (161). Misfolded soluble proteins in the cytoplasm, and even membrane proteins with misfolded cytoplasmic domains, are similarly selected by molecular chaperones, ubiquitinated, and degraded by the proteasome (14, 164). In select other cases these species are targeted for degradation by autophagy (28). Overall, there are numerous routes employed to ensure that protein homeostasis, or proteostasis, is maintained to ensure cellular and organismal health (9).
Even though most aberrant proteins are efficiently degraded, the transient accumulation of these polypeptides can trigger a cellular stress response. Two classical cellular stress response pathways are the unfolded protein response (UPR) and the heat shock response (HSR). In yeast, the UPR is induced in response to the accumulation of misfolded proteins in the ER lumen, which are sensed by the ER transmembrane kinase/endoribonuclease, Ire1 (30, 108, 140, 163). The activation of Ire1 leads to the splicing of an intron in the HAC1 pre-mRNA and subsequent translation of the Hac1 transcription factor (31, 109, 115, 117). Hac1 binds to UPR elements within the promoters of specific genes and activates the transcription of ∼380 genes, which represents ∼6% of the genome. Among these genes are molecular chaperones, which facilitate protein refolding, components of the ERAD machinery, and factors required for ER-to-Golgi transport, which clears the ER of damaged proteins, and for lipid synthesis, which enlarges the ER (154). Activation of the UPR also leads to a general repression of translational to lower the overall protein-folding burden of the cell (43, 52). In mammals, the UPR is more complex in that unfolded proteins are sensed by Ire1 as well as by ATF6 and PERK, which regulate some of the same processes as Ire1 and can also activate an apoptotic response (52, 55, 56, 142, 143).
In contrast to the UPR, the HSR is triggered by the accumulation of misfolded proteins in the cytosol (157, 164). In yeast the transcription factors Hsf1 along with Msn2/Msn4 account for nearly all HSR-induced gene transcription (107). Hsf1 resides primarily in the nucleus as a trimeric, DNA-bound protein that is activated by hyperphosphorylation in response to cytosolic stress (51, 100). Most studies support a model in which Hsf1 is repressed through interactions with molecular chaperones, such as Hsp70 and Hsp90 (37, 53, 113). When these chaperones are titrated away, either by misfolded nuclear proteins or upon the depletion of the nuclear chaperone pool, Hsf1 is activated (107). Hsf1 activity is also regulated by posttranslational modifications, such as sumoylation and ubiquitination (59, 62). In an analogous manner, Msn2/Msn4 are phosphorylated, which blocks translocation to the nucleus (32, 46), but are dephosphorylated in response to stress. Dephosphorylation results in nuclear translocation, which promotes the transcription of genes containing stress response elements (32, 83, 97, 99). In mammalian cells, if induction of the HSR fails to mitigate stress, then an apoptotic response is also initiated, as occurs when the UPR is insufficient to alleviate ER stress (10, 69, 150). An analogous programmed cell death pathway has also been observed in yeast (39, 54, 92, 93).
The retention of misfolded membrane proteins in the ER presents a unique challenge for the cell because the protein resides in three chemically unique compartments: the ER lumen, the cytosol, and the lipid bilayer. In principle, then, these proteins might induce the UPR, the HSR, and/or another ill-defined response. In one study, the transcriptional responses to three model misfolded proteins, an ER lumenal protein (CPY*), a cytosolic protein (VHL-L158P), and an integral membrane protein, Ste6* (103), were compared in yeast. The authors found that the misfolded cytosolic and ER lumenal proteins respectively induced cytosolic stress (HSR) and ER lumenal (UPR) reporter genes, as might be anticipated. Curiously, the misfolded membrane protein, Ste6*, induced a stress response that more closely resembled that of the misfolded cytosolic protein, but some Hac1 splicing was also evident, even though only ∼7% of the protein resides in the ER lumen prior to its degradation via the ERAD pathway. It is unknown, however, whether other misfolded membrane proteins, especially those with more substantial lumenal domains, elicit a similarly complex response. It is also unknown whether underlying principles associated with defective membrane proteins linked to specific diseases might be obtained by profiling transcriptional responses.
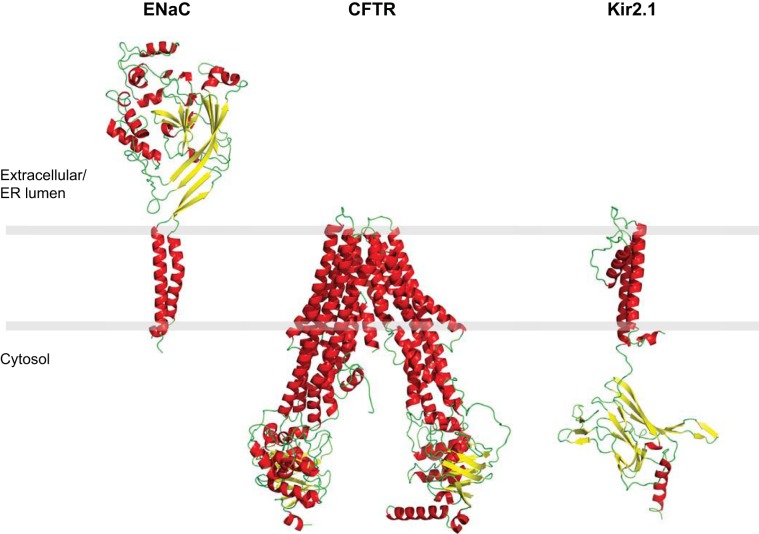
To these ends, we investigated the transcriptional responses to the expression of three topologically distinct, ERAD targeted, integral membrane proteins in yeast. The substrates we chose for this analysis are associated with human diseases and even the wild-type forms of these proteins fold inefficiently in yeast and human cells (15, 16, 68, 74, 79, 94, 101, 147, 158, 162). The substrates include the α subunit of the epithelial sodium channel, ENaC, an inwardly rectifying potassium channel, Kir2.1, and the cystic fibrosis transmembrane conductance regulator, CFTR (see Fig. 1). By expressing the proteins in yeast, factors required for the degradation, folding, and/or trafficking of these proteins were identified by employing genetic attacks. The requirements for many of the factors required to orchestrate the biogenesis of these substrates were then recapitulated in human cells (2, 15, 16, 74, 79, 88, 166).
Fig. 1.
Structural models of αENaC, Kir2.1, and CFTR. To generate the structural models, the amino acid sequence of each protein was modeled onto the structures of homologs using the Phyre2 server (76). Protein domains absent from the template structure are not modeled. Specifically, 1,185 amino acids of CFTR (80% of sequence) were modeled onto the multidrug resistance protein, pgp-1 (PDB:4F4C) with 100% confidence. Absent regions include amino acids 1–26, 687–845, 896–911, and 1173–1193. The model for αENaC contains 409 amino acids (60% coverage) modeled onto the ASIC1 crystal structure (PDB: 2QTS) with 100% confidence. Absent regions encompass amino acids 1–106, 601–699, and 199–272 and includes both cytosolic tails. The Kir2.1 model contains 318 amino acids (74% of the sequence) modeled onto the inward rectifying potassium channel Kir2.2 (PDB:3JYC) with 100% confidence. Regions absent correspond to amino acids 1–44 and 372–427.
The hypothesis underlying this work is that the expression of αENaC, CFTR, and Kir2.1 would induce the transcription of a gene network that reflects their diverse structures, assembly pathways, and/or topology. We also surmised that some of the induced genes would be required for the folding and/or degradation of each substrate. From our analysis we conclude that a unique transcriptional response results from the expression of each membrane protein. Also, while numerous UPR target genes were induced by the expression of αENaC and CFTR, a canonical UPR was absent. Instead, each of our datasets substantially overlapped with genes upregulated by a variety of environmental stressors (45). Kir2.1 was unique in its induction of the iron regulon. As outlined in previously published studies (2, 50, 79), we propose that the mining of the acquired datasets will provide a route to identify ill-characterized genes that impact ion channel biogenesis in human cells.
MATERIALS AND METHODS
Yeast growth conditions, RNA isolation, and transcriptional profiling methods.
Yeast strains were propagated at 26°C by standard methods for media preparation and transformation (1). The wild-type yeast strain used throughout these studies is a W303-like wild type strain [HLJ1/YDJ1 (120, 167)] unless otherwise noted. Wild-type yeast were transformed with pRS426GPD-αENaC-HA (15), pSM1152 (pRS426PPGK-CFTR-3HA) (171), pRS416TEF-myc-Kir2.1-HA (79) or a vector control, pRS426GPD (110). A total of 10 ml cultures were grown in selective medium at 26°C overnight. In the morning the cultures were diluted into 200 ml of selective medium and grown to midlog phase (OD600 = 0.5). Cells were harvested and stored at −80°C. To analyze protein expression, 1 ml of OD600 = 0.5 cells was harvested, protein lysates were prepared as previously described by alkaline lysis and TCA precipitation (15, 170), and Western blots were performed to confirm the expression of each heterologously expressed protein (αENaC, CFTR, Kir2.1) using the following antisera: anti-hemagglutinin-horseradish peroxidase (HA-HRP) (clone 3F10, Roche) or anti-glucose-6-phosphate dehydrogenase (G6P, Sigma) as a loading control. The G6P primary antibody was detected with a donkey horseradish peroxidase conjugated anti-rabbit IgG secondary (GE Healthcare). HRP-conjugated antibodies bound to the filter were imaged with enhanced chemiluminescence (Pierce) and visualized using a Kodak 440CF Image Station.
Total RNA from each strain was isolated using the hot phenol extraction method, essentially as described (29). In brief, yeast pellets (∼200 ml of OD600 = 0.5 cells) were resuspended in 12 ml TES (10 mM Tris·HCl, pH 7.5, 10 mM EDTA, 0.5% SDS) and agitated on a Vortex mixer for 10 s. A volume of 12 ml of acid phenol (Sigma) was added, and samples were incubated at 65°C for a total of 40 min and agitated on a Vortex mixer briefly every 10 min. Samples were placed on ice for 5 min and then centrifuged at 12,000 g for 5 min at 4°C. The aqueous layer was transferred to a new tube and re-extracted with 12 ml of acid phenol, placed on ice for 5 min, and centrifuged as above. The aqueous phase was again transferred to a clean tube and was then extracted with 12 ml of chloroform. After centrifugation at 12,000 g for 5 min the aqueous phase was transferred to a clean tube and RNA was precipitated with 1.2 ml of 3 M NaOAc, pH 5.3, and 32.5 ml of 100% EtOH. The RNA was pelleted by centrifugation at 12,000 g for 5 min, and the pellet was washed twice with 25 ml of 70% EtOH and centrifuged for 5 min at 12,000 g after each wash. The final RNA pellet was air dried at room temperature and resuspended in 3 ml of TE (10 mM Tris·HC, pH 7.0, 1 mM EDTA). A total of 1 mg of the recovered RNA was further purified using a Qiagen RNeasy midi kit and treated with on-column DNase (Qiagen) as described in the product manual. The RNA concentration and OD260/OD280 ratio were determined spectrophotometrically and 1 μg of RNA was run on a 1% agarose gel to confirm that the RNA species were not degraded.
The University of Pittsburgh Genomics & Proteomics Core Laboratory synthesized the labeled cRNA, performed array hybridization, and scanned the arrays as described below. Labeled cRNA was prepared using the 3′IVT Express kit (Affymetrix) according to the manufacturer's instructions. Total RNA (100 to 500 ng) and T7-(dT)24 primers were used to make ds-cDNA. The entire reaction was then used as input for in vitro transcription using biotinylated dNTPs. Next, the resulting biotinylated cRNA was purified using the supplied reagents. The cRNA was quantified by reading the OD260 of a 1:100 dilution. In addition, 1 ml of the cRNA was run on an Agilent Bioanalyzer to verify that most products represented full-length transcripts. After confirmation, an aliquot of 20 μg of cRNA was incubated at 94°C in fragmentation buffer (40 mM Tris-acetate, pH 8.1, 100 mM KOAc, 30 mM MgOAc) for 35 min to fragment the cRNA into segments of 35 to 200 bases. A 1 ml aliquot of this sample was then run on an Agilent Bioanalyzer to verify that fragmentation resulted in RNA species of the desired size distribution.
The fragmented RNA (15 μg) was added to a final volume of 300 ml in a hybridization cocktail: hybridization buffer, which contains 100 mM MES, 1 M NaCl, 20 mM EDTA, 0.01% Tween 20, and 100 mg/ml herring sperm DNA, 50 mg/ml acetylated BSA, 50 pM Affymetrix Control Oligo B2, and an Affymetrix Eukaryotic Hybridization Control. An appropriate volume of this sample was applied to the Yeast Genome 2.0 GeneChip Array, and the chips were incubated overnight at 45°C with rotation. Following hybridization the sample was removed and the GeneChip cassette was filled with nonstringent wash buffer. The chip was loaded onto an Affymetrix 450 Fluidics station for washing and staining. The wash and stain protocols were the double-stain protocols developed by Affymetrix for use with the Affymetrix 450 Fluidics Station. To remove unbound sample, arrays were first washed with nonstringent wash buffer (6× SSPE, 0.01% Tween 20) followed by a stringent wash in 100 mM MES, 0.1 M NaCl, 0.01% Tween 20. The GeneChips were then stained for 10 min in streptavidin-phycoerythrin (SAPE) solution (1× MES stain buffer, which contains 100 mM MES, 1 M NaCl, 0.05% Tween 20, 2 mg/ml acetylated BSA, 10 mg/ml SAPE). Nonstringent buffer was then used to wash off the first stain solution. Signal amplification was achieved by incubating 10 min with biotinylated anti-streptavidin (1× MES stain buffer, 2 mg/ml acetylated BSA, 0.1 mg/ml goat IgG, 3 mg/ml biotinylated anti-streptavidin) followed by a second 10 min incubation with SAPE. Next, the chip was washed and filled with array holding buffer (100 mM MES, 1 M NaCl, 0.01% Tween 20) before being removed from the fluidics station and scanned using the GeneArray 3000 scanner.
Transcriptome data analysis.
Analysis of the microarray data was conducted using efficiency analysis to optimize the methods of normalization and transformation and for the selection of the most reproducible test for differentially expressed genes (DEGs), and its threshold (71). Efficiency analysis identified the J5 test (123) as the most internally consistent test (compared to fold-change, the D1 test, the t-test, and random feature selection) after z-transform for the first two comparisons and after mean normalization for the third comparison. The J5 test is especially useful for datasets with a small number of replicates and may reduce the number of false positives generated through other analytical methods (19, 123), and this test has previously been used to analyze microarray data from diverse sources (18, 25, 42, 106, 118, 128, 131, 144). Missing values were imputed using k-nearest neighbors. False discovery risk estimate was calculated per comparison using the method described in Dobrowolski et al. (33a), simply FDRisk = (R/m)*[1 − (R/m)] where R is the number of DEGs per comparison and m is the number of genes on the array. This risk applies to the set, requires a fixed threshold (to define R), an assumption that the number of differentially expressed genes is equal in size to R (but need not be the actual set of genes found), and is the joint probability that a gene is not differentially expressed but is found to be anyway.
caGEDA was used to determine fold-ratio values [(M1 − M2)/M2]. αENaC used a z-transformation, CFTR used a z-transformation, and Kir2.1 was mean normalized, as described above. No threshold value was utilized in order to obtain values for all genes. Once the genes were obtained, a matching algorithm (Perl-script, entitled “MatchingKeepAll.pl”) was used to match DEGs with their corresponding fold-ratio values. All other data manipulations were performed manually using Microsoft Excel.
The data discussed in this publication have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO) (38) and are accessible through the GEO Series accession number GSE61246 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE61246).
Statistical comparisons between datasets.
For this analysis we used the subset of genes that were identified using the statistical analysis performed in each of the previous studies. The subsets of differentially expressed genes for each condition were compared with those genes that were induced more than twofold from datasets generated in response to heat shock (shift from 21 to 37°C for 20 min), 1.5 mM reducing agent (DTT) for 60 or 180 min, or an oxidizing agent (1.5 mM diamide for 60 min) (45). Comparisons to the environmental stress response (ESR) were made using the gene lists identified in Gasch et al. (45). Those genes that were differentially induced in response to Ste6* expression or alkaline stress were identified as described (103, 139). The UPR targets were identified using a multifactorial approach (154), and via the dataset provided with this publication we identified 422 genes that meet our criteria. The overlap between datasets was determined using BioVenn [http://www.cmbi.ru.nl/cdd/biovenn; (63)], and statistical significance of the overlap and the representation factor (RF) was determined using http://nemates.org/MA/progs/overlap_stats.html. An RF of >1 indicates more overlap than expected by chance, whereas an RF of <1 indicates less overlap than expected based on chance. RF = (# of overlapping genes between data sets)/(expected # of overlapping genes). Expected # of overlapping genes between datasets = [(# of genes in set 1) × (# of genes in set 2)]/(total # of genes on the microarray) (5,841). Probability was calculated using normal approximation (129). Calculations are described in more detail in Chapter 6.2 of Numerical Recipes in C: The Art of Scientific Computing (129).
Reverse transcription and qPCR.
Wild-type yeast cultures transformed with the appropriate plasmid were grown to log phase in selective medium, and RNA was isolated as described above. For treatment of cultures with bathophenanthroline disulfonate (BPS, Sigma), the cultures were split and either left untreated or treated with 100 μM BPS for 2 h prior to RNA isolation. Reverse transcription to generate cDNA was performed using 1 mg of RNA and the Ambion RETROscript kit (AM1710) as described in the product manual. cDNA levels were determined using SYBR-green (Thermo KO221) and an Applied Biosystems 7300 Real-Time PCR machine. Primers for each gene were validated using genomic DNA to confirm that primer efficiency was >90%. Differences in transcript level were determined using the Pfaffl methodology (124) with the gene encoding G6P (ZWF1) serving as our control.
β-Galactosidase reporter assays for the iron regulon.
Cultures of wild-type yeast were transformed with a FET3pr-LacZ reporter plasmid [pJLU62 (156)] and either a vector control (pRSGPD426) or the expression plasmid for αENaC, CFTR, or Kir2.1 (see above) and were grown at 24°C overnight in selective medium. The cultures were diluted to an OD600 of 0.4, 400 μM of BPS was added to the treatment group, and the cultures were incubated on a roller drum for 2 h at 24°C. A 1 ml aliquot of each culture was harvested, centrifuged at 12,000 g for 1 min, washed once with sterile water, and resuspended in 1 ml Z-buffer (0.06 M Na2HPO4/0.04 M Na2H2PO4, pH 7.0, 0.01 M KCl, 1 mM MgSO4, and 0.05 M β-mercaptoethanol). The OD600 of this suspension was then taken, 100 μl of chloroform and 50 μl 0.1% SDS were added, and the mixture was incubated at room temperature for 20 min with occasional agitation on a Vortex mixer. Next, a 200 μl aliquot of each sample was pipetted into a 96-well plate and 35 μl of 4 mg/ml ortho-nitrophenyl-β-galactoside was added. The OD420 and OD550 were then measured in a 96-well plate reader (Thermo Scientific, Multiskan GO) over time to determine optimal incubation time. Relative activity in Miller units was calculated as previously described (104) at the 30 min time point.
Protein stability assays.
A wild-type yeast strain harboring a green fluorescent protein (GFP)-tagged copy of Fet3 [DY150, FET3-GFP (40)], which was generously provided by the Kaplan lab (University of Utah), was transformed with either a vector control (pRS426GPD), pRSTEF416-myc-Kir2.1-HA, or pRS426GPD-αENaC-HA. Overnight cultures were grown in selective medium to an OD600 of 0.5–1.0. The cultures were then diluted to an OD600 of 0.5. Cycloheximide was added to a final concentration of 50 mg/ml and cultures were incubated at 30°C. A 1 ml aliquot of each culture was harvested at 0, 30, 60 and 90 min, and cell lysates were generated and samples processed and analyzed by SDS-PAGE and Western blotting as previously described (15). Anti-GFP antibody (Roche) was used to detect FET3-GFP, anti-HA-HRP (clone 3F10, Roche Applied Science) was used to detect αENaC and Kir2.1, and anti-G6P (Sigma) was used as a loading control.
Fluorescence microscopy.
The FET3-GFP yeast strain was transformed with the pRS426GPD, pRS426GPD-αENaC-HA, or pRS416TEF-myc-Kir2.1-HA expression vectors, as described above, or the pRS416TEF-Kir2.1AAA-HA expression vector [which encodes an inactive potassium channel, (152)], and overnight cultures were grown in selective medium. For BPS treatment cultures were incubated with 200 mM BPS for 2 h prior to imaging. Fluorescence microscopy was performed on an Olympus FV1000 microscope and Olympus FV10-ASW version 3.01 software. Identical imaging parameters were used within each experiment. The intensity of plasma membrane fluorescence was measured and images were formatted with ImageJ (v1.44o) software. Data represent the mean values of >80 individual cells for each condition that were normalized to the average fluorescence of control cells ± SE. Significance was determined by a one-way ANOVA test with Tukey's post hoc comparison (70).
Generation of structural models.
Structural models of CFTR, Kir2.1, and αENaC were generated by uploading each amino acid sequence to the Phyre2 server (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index), which generated a homology based model for each heterologously expressed protein (76). Pymol was used to format and resize the models (136). Regions that were not included in the models are noted in the figure legends.
RESULTS AND DISCUSSION
Identification of substrates used for transcriptional profiling.
To define the transcriptional response to the expression of misfolded membrane proteins, we chose three substrates, αENaC, CFTR, and Kir2.1, that can be expressed in yeast, undergo ERAD, and exhibit unique topologies (Fig. 1, and see below). Several requirements for the degradation of the proteins were first established in the yeast model and were then confirmed in higher cell types (2, 3, 15, 16, 47, 74, 79, 167, 168, 170). In addition, even the wild-type versions of each of these proteins, as used in this study, fold inefficiently in the ER in both yeast and mammals.
ENaC is a heterotrimeric sodium channel composed of three homologous subunits, α, β, and γ, and is responsible for sodium absorption in the kidney and other epithelia, thus playing a critical role in salt and electrolyte balance. Each ENaC subunit (∼75–80 kDa) is composed of two transmembrane helices, a large extracellular domain (∼65% of the protein), and a short NH2- and COOH-terminal tail (∼25–30% of the protein's mass) (17, 133, 145). The α-, β-, and γ-ENaC subunits assemble in the ER and subsequently traffic to the plasma membrane. ENaC expression can be regulated by transcription, and in at least some epithelial cells the β- and γ-subunits are expressed at high levels, but in the absence of the α-subunit they are subject to ERAD (146, 147, 158) until the α-subunit is expressed in response to aldosterone (6, 98). Similarly, when the α-subunit is expressed in the absence of the β- and γ-subunits, this results in ERAD (158). These data can be explained by the fact that there are extensive contacts between the extracellular domains of the α-, β-, and γ-subunits (67, 75, 149). Loss-of-function mutations in αENaC result in renal salt-wasting, hypotension, and hyperkalemia (48). We showed that the cytosolic small heat shock proteins (sHsps), ER lumenal Hsp40s (Scj1 and Jem1), and an ER lumenal Hsp70-like protein, Lhs1, facilitate αENaC degradation (15, 16, 74). Importantly, the roles of each of these factors are conserved in higher cells (15, 16, 74).
Unlike αENaC, CFTR is a chloride transporter and contains 12 transmembrane helices, two nucleotide-binding domains, and a regulatory domain. It has a molecular mass of 165–180 kDa, depending on the glycosylation status. The majority of CFTR is located in the cytosol (∼77% of the mass), whereas only 4% of the protein is extracellular/ER lumenal and 19% lies within the lipid bilayer (22, 23, 134). CFTR folds slowly, and up to 80% of wild-type CFTR can be targeted for ERAD (68, 101, 162). The most common disease-causing mutation is the deletion of a phenylalanine at position 508 (ΔF508). ΔF508-CFTR is ER retained and quantitatively destroyed by ERAD (34, 90, 141, 171). The targeting of CFTR for ERAD in yeast requires cytosolic chaperones including Hsp70 (Ssa1p), Hsp40s (Ydj1 and Hlj1), as well as the sHsps, each of which is also vital for maximal degradation in mammalian cells (2, 3, 47, 167, 168, 170). Other experiments using our yeast model system provided evidence that an FK-506 binding protein and the Hsp90 chaperone network act in opposition to the ERAD machinery and promote CFTR folding and stabilization (64, 167).
The third substrate we chose is Kir2.1, an inwardly rectifying potassium channel that forms a homotetramer. Each Kir2.1 subunit is ∼49 kDa and contains two transmembrane domains, a short NH2-terminal tail, and a much longer COOH-terminal cytosolic tail. An extracellular/ER lumenal loop dips back into the membrane to form the potassium-conducting pore. Like CFTR, the majority (∼75%) of the Kir2.1 mass lies within the cytoplasm (116, 137). Loss-of-function mutations in Kir2.1 that block Golgi export are linked to Andersen-Tawil syndrome (91) and can result in periodic paralysis, ventricular arrhythmias, and skeletomuscular dysplasia (127). Through the development of a yeast expression system for Kir2.1, we found that a significant population of the channel is targeted for degradation in the vacuole/lysosome, as observed in mammalian cells (66, 79, 112). However, another population of Kir2.1 is targeted for ERAD through the action of the cytosolic Hsp70, Ssa1 (79).
αENaC, CFTR, and Kir2.1 induce unique cellular stress responses in yeast.
To transcriptionally profile the cellular responses to these three proteins, we grew yeast cells expressing αENaC, CFTR, or Kir2.1 along with yeast harboring a vector control alone under identical conditions to log phase and isolated RNA as described in materials and methods. At least three isolates of each strain were examined, a Western blot was performed to confirm protein expression, and we found that αENaC and CFTR were expressed at similar levels although Kir2.1 expression was slightly lower (data not shown). Nevertheless, each protein is rapidly targeted for ERAD in yeast (2, 15, 16, 64, 74, 79, 167, 170). The doubling times for each strain were determined over a 16 h time-course (n = 4) and are as follows: vector containing = 2.3 h ± 0.26, αENaC expressing = 3.3 h ± 0.10, CFTR expressing = 2.6 h ± 0.25, and Kir2.1 expressing = 2.2 h ± 0.011.
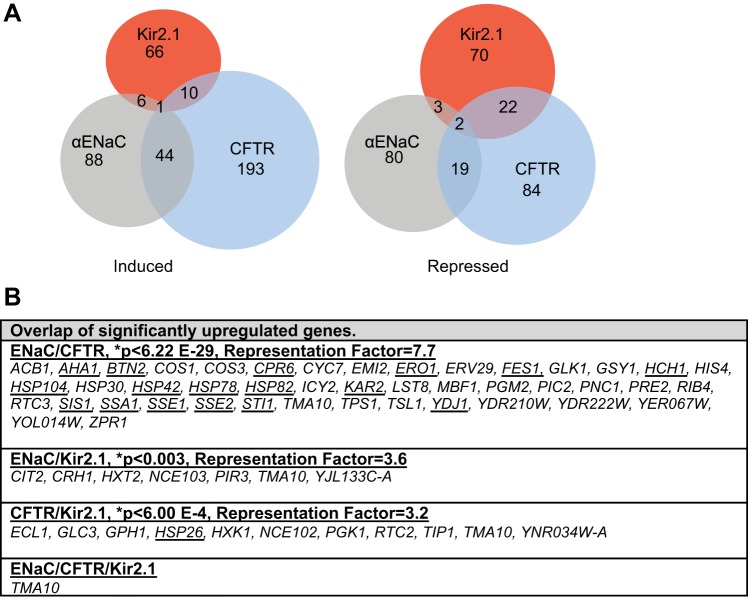
An overview of the microarray analysis of yeast expressing αENaC, Kir2.1, and CFTR is presented as both a heat map (Fig. 2) and a Venn diagram in Fig. 3A. The gene order, category as well as the intensity value, based on J5 score, is included as Supplemental File S1.1 As demonstrated in the heat map the overall transcriptional responses of each of these proteins compared with a vector control are dramatically different. However, there is clearly more overlap between the genes induced when αENaC and CFTR were expressed than between either αENaC or CFTR and the Kir2.1 dataset. This overlap is surprising because αENaC and Kir2.1 are perhaps more topologically similar than αENaC and CFTR. We determined that 243 genes (139 induced, 104 repressed; FDRisk = 0.0221) were differentially expressed in the αENaC dataset, 375 genes were differentially expressed in the CFTR dataset (248 induced and 127 repressed; FDRisk = 0.0337), and 180 genes were differentially expressed in the Kir2.1 dataset (83 induced and 97 repressed; FDRisk = 0.0165). A complete list of the differentially expressed genes can be found in Supplemental File S2 (J5 score and fold change for comparison).
Fig. 2.
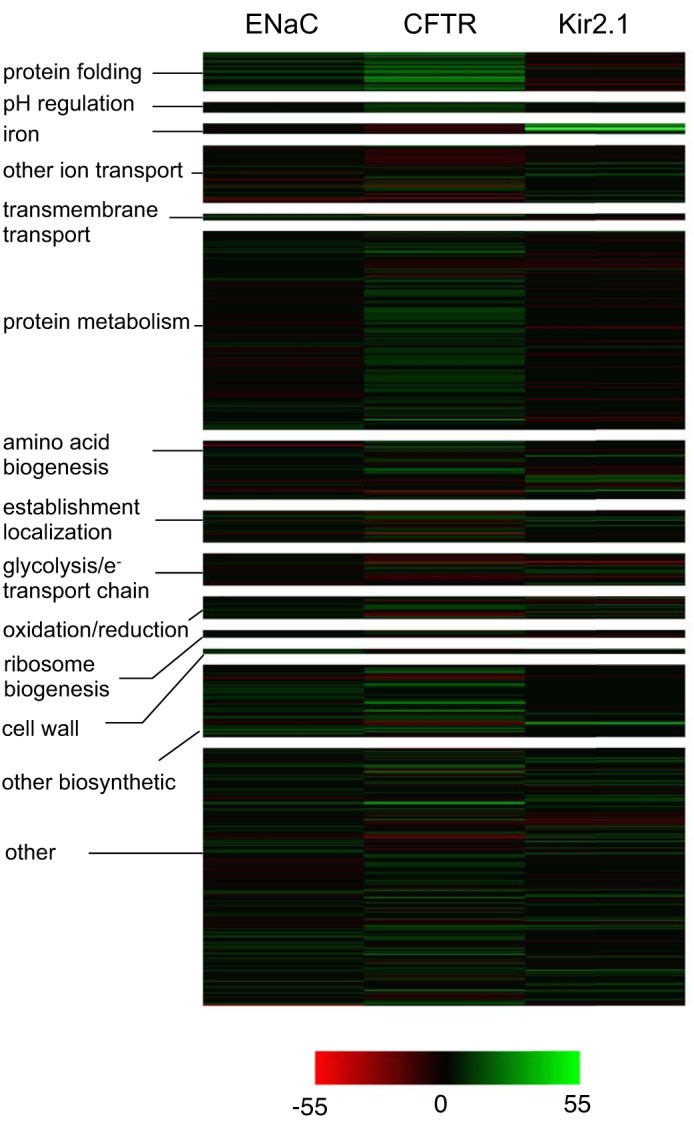
Heat map illustrating changes in genomic expression upon the expression of αENaC, Kir2.1, or CFTR. Differentially expressed genes for yeast expressing αENaC, CFTR, or Kir2.1 were sorted based on Gene Ontology (GO) terms into broad categories that are labeled on the left. GO term priority was assigned based on the category ranking on the left (i.e., genes associated with GO term “protein folding” were included in the top category and excluded from other categories with lower priority). Intensity values were assigned based on J5 score and centered at an intensity of 0. GO terms contained in each of the categories are: protein folding (de novo protein folding, protein folding, protein refolding), pH regulation (regulation of pH, pH reduction, intracellular pH reduction, vacuolar acidification, regulation of cellular pH, regulation of intracellular pH), iron (iron assimilation, iron coordination entity transport, iron assimilation by chelation, siderophore transport), other ion transport (cation transport, ion transmembrane transport, proton transport, hydrogen ion transmembrane transport, ion homeostasis, cation homeostasis, cellular ion homeostasis, monovalent inorganic cation transport, cellular cation homeostasis), transmembrane transport (transmembrane transport), protein metabolism (protein metabolism), amino acid biogenesis (cellular amino acid metabolic process), establishment of localization (establishment of localization), glycolysis/e− transport (glycolytic process, electron transport chain, respiratory electron transport, oxidative phosphorylation), oxidation/reduction (oxidation-reduction process), ribosome biogenesis (ribosome biogenesis, ribosome assembly, ribosomal small subunit biogenesis, ribosomal small subunit assembly, maturation of SSU-rRNA from rRNA transcript), cell wall (cell wall macromolecule cell process), other biosynthetic (cellular biosynthetic process, ribonucleotide biosynthetic process).
Fig. 3.
Expression of αENaC, Kir2.1 or CFTR in yeast results in unique transcriptional responses. A: Venn diagram illustrating the number of genes that are induced or repressed upon αENaC, CFTR, or Kir2.1 expression as determined from the microarray analysis. The number of genes that overlap between each dataset is noted. B: the significantly induced genes that are found in at least 2 of our datasets (αENaC, CFTR, and Kir2.1) are listed. P values and the representation factor (RF) are based on those found at http://Nemates.org as described in materials and methods. Genes associated with protein folding are underlined.
In Fig. 3B, we show the specific genes that were upregulated in at least two datasets. In this report, we have placed the greatest emphasis on genes that were induced rather than those that were repressed for two reasons. First, the expression of genes that are induced by ER stress (i.e., the UPR) or cytoplasmic stress (the HSR) provides a diagnostic for compensatory responses that augment protein turnover. For example, ERAD is enhanced by the UPR (20, 114, 154), and the activity of the cytoplasmic protein folding machinery is enhanced by the HSR (reviewed in Ref. 157). Second, genes that are downregulated may reflect repression of the transcription machinery or enhanced mRNA turnover. In mammalian cells, enhanced mRNA turnover results from the regulated Ire1-dependent decay pathway that has been described in both Drosophila and mammalian cell systems (60, 61). And third, our prior work indicates that enhanced genes can act as ERAD facilitators (2, 79), and an increasing number of physiologically relevant substrates appear to utilize such facilitators to regulate cellular homeostasis (12).
On the basis of the data presented in Fig. 3B, and the analysis of a file containing the complete dataset (Supplemental File S2), we have determined that the majority of genes that are present in both the αENaC and CFTR-induced gene datasets are involved in protein folding (underlined in Fig. 3B and illustrated in Fig. 2). Both ER lumenal (KAR2, ERO1) and cytosolic (AHA1, FES1, HSP104, HSP42, HSP82, SIS1, SSA1, SSE2, STI1, YDJ1) chaperones and cochaperones are induced when αENaC or CFTR are expressed. This result was somewhat surprising as the majority of αENaC resides in the ER lumen, whereas the majority of CFTR resides in the cytosol (Fig. 1). We had hypothesized that αENaC would induce ER lumenal chaperones, whereas CFTR would induce the cytosolic chaperone network. One explanation for this result is that CFTR is titrating out a factor that is required for ERAD, which leads to inhibition of the ERAD pathway and to a build-up of misfolded lumenal proteins. This phenomenon was observed when the cytoplasmic, aggregation-prone huntingtin protein was expressed in both yeast and neuronal cells (36). In addition, it is worth noting that genes encoding factors known to directly affect the early biogenesis or degradation of CFTR were evident in the dataset of upregulated genes, including SSA1, YDJ1, HSP82, HSP26, and HSP42 (2, 167, 170). Finally, data indicate that high-level expression of ΔF508-CFTR can induce the UPR in mammalian cells (11).
Interestingly, only one gene, TMA10, was found in all three induced gene datasets. TMA10 encodes a protein of unknown function that is associated with the translation machinery (41) and is upregulated by the DNA damage response (153), potentially reflecting the presence of the heterologous expression vectors used in this study. Nevertheless, there was a statistically significant overlap between each of the pairwise datasets for the induced genes: The RF is the number of genes that overlap between the datasets divided by the number of genes predicted to overlap by chance based on the number of genes in the yeast genome (Fig. 3B). From this analysis, a comparison of the induced genes in the αENaC and CFTR datasets has an RF = 7.7, so that there are 7.7 times more genes that overlap between these sets than predicted by chance. The overlap between CFTR and Kir2.1, while still significant (P < 0.0006), is only 3.2 times more than expected by chance (RF = 3.2), and a similarly significant overlap between the αENaC and Kir2.1 dataset was observed (RF = 3.6, P < 0.003). As discussed below, the lower level of significance for the CFTR/Kir2.1 and αENaC/Kir2.1 datasets compared with the CFTR/αENaC results reflects the unique profile exhibited upon Kir2.1 expression.
A second analysis of the differentially expressed, induced genes was completed using the Gene Ontology (GO) Term Finder tool in the Saccharomyces Genome Database (http://www.yeastgenome.org/) (27). A select list of the enriched terms and representative genes is summarized in Table 1. Genes associated with metabolism and transcription as well as those that are fungal-specific have been omitted to narrow our discussion; however, all genes are included in the heat map in Fig. 2. In addition, those GO terms in which the set of enriched genes exhibited significant overlap (e.g., “iron ion homeostasis” and “cellular iron ion homeostasis”) were merged. As suggested by the data in Fig. 3B and Table 1, the αENaC and CFTR datasets are enriched for similar genes, most notably those involved in protein folding and refolding. In contrast, few of these genes were also induced in the Kir2.1 dataset, and the only “protein folding” gene that was induced by Kir2.1 is a sHsp, Hsp26. In fact, the sHsps Hsp26 and Hsp42 are the only molecular chaperones represented in all three data sets. Hsp26 expression was also induced in the CFTR dataset, and the Hsp42 message rose in both the αENaC and CFTR datasets. Previous work determined that the sHsps facilitate the ERAD of αENaC and CFTR (2, 74). Therefore, we investigated whether the sHsps play a role in targeting Kir2.1 for ERAD by monitoring the Kir2.1 degradation rate in a yeast strain lacking both sHsps (Δhsp26Δhsp42). However, unlike αENaC and CFTR, deletion of the sHsps had no effect on Kir2.1 stability (data not shown). Either another protein “holdase,” such as Hsp104, acts in conjunction with the sHsps, as suggested by other studies (21, 105) or the increased level of HSP26 reflects its control by Hsf1 (4, 155). If so, it is surprising that other Hsf1 targets were not induced by Kir2.1 expression. Future studies will identify genes that may act in conjunction with the sHsps during Kir2.1 biogenesis.
Table 1.
Selected GO terms enriched by biological process
| Biological Process | P Value | Gene # | Genes | |
|---|---|---|---|---|
| ENaC | protein refolding | 1.42 E-05 | 8 | SSA1, SSE2, HSP78, HSP104, CPR6, YDJ1, SSE1, HSP82 |
| protein folding | 0.00013 | 15 | SSA1, SSE2, AHA1, HSP78, BTN2, CHS7, HSP104, CPR6, ERO1, SIS1, YDJ1, HCH1, STI1, SSE1, HSP82 | |
| CFTR | protein folding | 3.10 E-11 | 24 | SSA1, HSP26, SSE2, PDI1, SSB1, AHA1, HSP78, SSA4, MDJ1, BTN2, EGD2, SBA1, SSA2, HSP104, CPR6, HSP60, ERO1, HSC82, SIS1, YDJ1, HCH1, STI1, SSE1, HSP82 |
| protein refolding | 2.40 E-09 | 11 | SSA1, SSE2, HSP78, MDJ1, HSP104, CPR6, HSP60, HSC82, YDJ1, SSE1, HSP82 | |
| de novo protein folding | 3.01 E-05 | 7 | SSB1, MDJ1, EGD2, HSP60, HSC82, YDJ1, HSP82 | |
| regulation of pH | 0.00014 | 10 | VMA2, VMA9, VMA1, VMA3, VMA8, VMA7, VMA10, VMA5, VMA4, BTN2 | |
| pH reduction | P < 0.00034 | 9 | VMA2, VMA9, VMA1, VMA3, VMA8, VMA7, VMA10, VMA5, VMA4 | |
| monovalent inorganic cation homeostasis | 0.0004 | 11 | VMA2, VMA9, VMA1, VMA3, VMA8, VMA7, VMA10, VMA5, VMA4, BTN2, COS3 | |
| cellular monovalent inorganic cation homeostasis | 0.00083 | 10 | VMA2, VMA9, VMA1, VMA3, VMA8, VMA7, VMA10, VMA5, COS3, VMA4 | |
| Kir2.1 | iron assimilation | 7.41 E-10 | 7 | SIT1, FTR1, ARN1, ARN2, FET3, FIT2, FIT3 |
| iron ion homeostasis | 1.47 E-07 | 9 | SIT1, FTR1, ARN1, ARN2, TIS11, FET3, FIT2, FIT3, ISU1 | |
| iron coordination entity transport | 6.32 E-05 | 5 | SIT1, ARN1, ARN2, FIT2, FIT3 | |
| iron ion transmembrane transport | 0.00166 | 3 | FTR1, FET3, FET4 | |
| siderophore transport | 2.88 E-06 | 5 | SIT1, ARN1, ARN2, FIT2, FIT3 | |
| cell wall organization or biogenesis | 8.25 E-06 | 14 | TIP1, SED1, ACT1, CRH1, BGL2, HSP150, CWP1, CWP2, PIR3, CCW12, EXG1, CCW14, HPF1, SRL1 | |
| metal ion/transition ion/cellular cation homeostasis | P < 0.00018 | 10 | IZH1, SIT1, FTR1, ARN1, ARN2, TIS11, FET3, FIT2, FIT3, ISU1 | |
| chemical and ion homeostasis | 2.60 E-05 | 12 | PMP3, IZH1 SIT1, FTR1, ARN1, ARN2, CWP2, TIS11, FET3, FIT2, FIT3, ISU1 | |
| cation homeostasis | 2.74 E-05 | 11 | IZH1, SIT1, FTR1, ARN1, ARN2, CWP2, TIS11, FET3, FIT2, FIT3, ISU1 |
Enriched gene ontology (GO) terms obtained after transcriptionally profiling αENaC, CFTR, and Kir2.1 expressing yeast. The subset of induced genes under each condition was analyzed for significant enrichment of GO terms by biological process (P < 0.05) as described in materials and methods. The list of GO terms was edited to eliminate those arising from potential secondary effects, as described in the text. A complete list of GO terms can be found in Supplemental File S3.
Quantitative PCRs validate the upregulation of select genes.
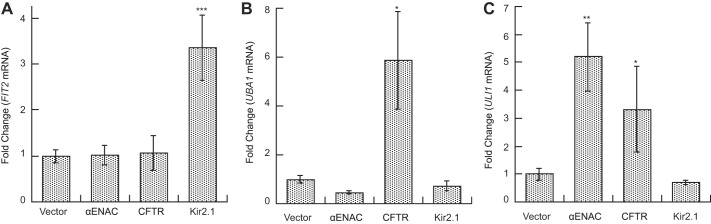
To confirm that select genes were differentially expressed, we performed quantitative PCR (qPCR). For this analysis, we assessed a single gene that was specifically induced by each substrate. Consistent with our microarray analysis (Supplemental File S2), and as shown in Fig. 4A, FIT2 [facilitator of iron transport; (130)] expression was significantly higher when Kir2.1 was expressed than in cells containing a vector control or when αENaC or CFTR was expressed. The qPCR analysis also verified that the levels of the UBA1 transcript, which encodes the yeast ubiquitin activating enzyme, rose dramatically upon CFTR expression but was lower or unaffected in the other cells (Fig. 4B). Further confirmation for our dataset was obtained by our previous demonstration that the HSP26, HSP82, and FES1 transcripts were upregulated in yeast expressing CFTR (2), as observed in our microarray dataset (Supplemental File S2). Finally, we examined the levels of the ULI1 message in yeast containing a vector control or the αENaC, CFTR, or Kir2.1 expression vectors. The function of the Uli1 protein is unknown, but its message has been used as a marker for ER stress (103, 154). As anticipated from our microarray dataset, ULI1 message levels significantly increased when αENaC was expressed, but the levels were unchanged in response to Kir2.1 expression (Fig. 4C). However, we also found that ULI1 mRNA levels were also somewhat higher in CFTR expressing yeast, albeit not to the extent resulting from αENaC expression. Although these data suggest that the microarray analyses might have missed transcripts that were only marginally above our cut-off, we were able to confirm the increased expression of select candidates.
Fig. 4.
Confirmation of selected induced genes by quantitative PCR (qPCR). Wild-type yeast containing either a vector control (pRS426GPD) or vectors engineered for the expression of αENaC (pRS426GPD-αENaC-HA), CFTR (pRS426PPGK-CFTR-3HA), or Kir2.1 (pRS416TEF-myc-Kir2.1-HA) were grown to log phase and harvested, and RNA was prepared and reverse transcription performed as described in materials and methods. cDNA was then subjected to qPCR analysis. A: FIT2, B: UBA1, and C: ULI1. Data represent the means of 4–7 experiments ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Ion channel expression triggers the ESR.
Gasch et al. (45) defined a set of ∼900 genes that respond similarly to a range of environmental stresses, including high and low temperature, oxidative stress (i.e., hydrogen peroxide, menadione, and diamide), reductant (DTT), hyper- and hypo-osmotic stress, nitrogen depletion, and progression into stationary phase. Among this set, 283 genes were induced and ∼600 genes were repressed. The authors termed this set the ESR (45). Based on the fact that misfolded proteins en route to the proteasome may lead to a stress response, we hypothesized that our dataset and genes induced by the ESR may overlap. As hypothesized, there was a reasonable degree of overlap (Table 2, RF values of 2.4–4.3). A number of the overlapping genes are heat shock proteins or are induced by heat shock (HSP104, HSP12, HSP42, HSP26, HSP78, SSA4, SSE2). However, it is worth noting that the CFTR and αENaC datasets match up with only 10% of the ESR genes and Kir2.1 dataset contains only 3.5% of the ESR genes (Supplemental File S4).
Table 2.
Significance of overlap between datasets
| αENaC | CFTR | Kir2.1 | |
|---|---|---|---|
| ESR-induced (Gasch et al. 2000) | P < 1.0 E-11 RF = 4.3 % overlap (29/283) | P < 6.7 E-6 RF = 2.4% overlap (29/283) | P < 0.006 RF = 2.5% overlap (10/283) |
| ESR-repressed (Gasch et al. 2000) | P < 7.1 E-9 RF = 3% overlap (31/585) | P < 0.003 RF = 0.3% overlap (4/585) | P < 8.0 E-11 RF = 3.4% overlap (33/585) |
| Heat shock (37°C)-induced (Gasch et al. 2000) | P < 9.2 E-30 RF = 7.3% overlap (49/295) | P < 2.84 E-17 RF = 4% overlap (48/295) | P < 1.6 E-6 RF = 4% overlap (17/295) |
| DTT (180 min)-induced (Gasch et al. 2000) | P < 3.23 E-22 RF = 11.5% overlap (27/101) | P < 5.82 E-07 RF = 4.1% overlap (17/101) | P < 0.002 RF = 4.3% overlap (6/101) |
| DTT (60 min)-induced (Gasch et al. 2000) | P < 8.5 E-11 RF = 12.4% overlap (12/43) | P < 0.007 RF = 3.5% overlap (7/43) | P < 0.44 RF = 1.8% overlap (1/43) |
| Oxidative stress (diamide)-induced (Gasch et al. 2000) | P < 2.55 E-21 RF = 11.5% overlap (26/98) | P < 6.68 E-08 RF = 4.5% overlap (18/98) | P < 6.25 E-06 RF = 6.7% overlap (9/98) |
| UPR-induced (Travers et al. 2000) | P < 0.09 RF = 1.5% overlap (14/422) | P < 0.42 RF = 1% overlap (18/422) | P < 0.02 RF = 0.2% overlap (1/422) |
| Ste6* expression-induced (Metzger and Michaelis, 2009) | P < 4.84 E-4 RF = 3.5% overlap (10/120) | P < 0.004 RF = 2.9% overlap (12/120) | P <0.028 RF = 2.4% overlap (5/120) |
| Alkaline pH-induced (Serrano et al. 2002) | P < 8.3 E-5 RF = 3.7% overlap (12/144) | P < 0.002 RF = 2.5% overlap (14/144) | P < 3.9 E-10 RF = 7.9% overlap (15/144) |
Comparisons between the datasets generated from αENaC (139 induced and 106 repressed), CFTR (248 induced and 129 repressed genes), and Kir2.1 (83 induced and 97 repressed genes) expressing yeast vs. those listed on the left are shown. P values and representation factors (RF) were calculated as described in Fig. 1. RF < 2, roman type; 2 ≤ RF ≤ 4, underlined; 4 < RF ≤ 7 boldfaced; RF < 7 boldfaced + underlined. The number of genes that overlap between the datasets was divided by the total number of genes within the comparison dataset to calculate the percent overlap. ESR, environmental stress response.
We next compared our datasets to those generated under individual ESR stress conditions: oxidative stress (diamide), DTT treatment (at 60 and 120 min), and heat shock. We found that there was more significant similarity between all but one of these datasets and each of our datasets, with calculated RF values between 3.5 and 12.4 (Table 2). The exception was the absence of overlap between the Kir2.1 expression and 60 min DTT treatment datasets. In every case the αENaC dataset overlapped more strongly than that for either CFTR or Kir2.1, with the strongest overlap (RF = 12.4) for αENaC and DTT treatment. DTT triggers the dissolution of disulfide bonds in the ER and is a potent inducer of the UPR (154). Thus, ER stress triggered by Kir2.1 may be more subtle relative to that induced by αENaC and CFTR. We emphasize again that an ER stress response upon CFTR expression was surprising, given that only a minor fraction of the protein resides in the ER, but as described above this effect may be due to the titration of an ERAD or other PQC-requiring factor.
To further examine whether the expression of the three ion channels induced a UPR-like response, we compared our datasets to those identified in a prior study (154) in which mRNA levels were measured after treatment with either DTT or tunicamycin (Table 2), which compromises the addition of fully assemble glycans on secreted proteins (80, 138, 151). Perhaps because this dataset was more rigorously culled compared with the DTT/ESR-induced genes, the overlap in this case was not statistically significant. However, two of the substrates examined in our work augmented the expression of a number of established UPR targets: αENaC (COS4, ULI1, ACB1, ERV29, ERO1, COS3, ADD37, YIL108W, YGL117W, KAR2, HIS4, SEC26, ERG7, INO1), and CFTR (COS8, COS4, HEM13, ADE5, HXK2, ACB1, ERV29, PM140, PDI1, ADH5, AAT2, HIS4, FRD1, HEM13, HMG2, ERO1, COS3, DPM1, KAR2). Consistent with these data, we showed that yeast expressing αENaC exhibit a UPR, as assessed with a UPR reporter (15). And, consistent with the ESR analysis, above, only one UPR target (THI20) was induced by Kir2.1. In fact, there was a significant underrepresentation of UPR targets within the genes found in the Kir2.1 dataset (P = 0.02, RF = 0.2). As described below, the downregulation of UPR target genes may reflect the fact that Kir2.1 induces another stress response in yeast, one that might negatively regulate the UPR.
We also noted that the datasets obtained with αENaC, CFTR, and Kir2.1 overlapped significantly with the subset of genes induced by oxidative stress (45), with RF values between 4.5 and 11.5 (Table 2 and Supplemental File S4) as well as with the heat shock-inducible genes (RF = 4–7.3). In fact, when we compared αENaC and the heat shock and oxidative stress responses, the RF values (7.3 and 11.5, respectively) were higher than those for CFTR (4 and 4.5, respectively) or Kir2.1 (4 and 6.7, respectively). Notably, 26 genes from the heat shock-responsive genes are found in both the αENaC and CFTR datasets. Therefore, contrary to our predictions, induction of the HSR did not correlate with the relative amount of the protein found in a distinct compartment. This could, again, reflect the titration of a general PQC factor, or it could reflect the fact that the αENaC termini, which are deposited in the cytoplasm, are sufficiently unfolded in the absence of the β- and γ-subunits that the HSR is activated. As discussed above, structural predictions of the ENaC channel posit the existence of multiple contacts between the extracellular domains (67, 75, 149), which would be absent when only one subunit is expressed. Collectively, our analysis reveals that misfolded membrane proteins induce a stress response that is most similar to the ESR, and the expression of αENaC results in a stronger ESR (and specifically an HSR) than either CFTR or Kir2.1.
The translation machinery is differentially regulated by αENaC, CFTR and Kir2.1.
Differential effects were observed among genes associated with the GO terms “translation/ribosome biogenesis” upon expression of αENaC, CFTR, and Kir2.1 (Supplemental File S3). Even though we have not focused on repressed genes, it is notable that αENaC repressed 104 genes, and many of these genes were associated with translation (58) and/or ribosome biogenesis (29) (see Supplemental File S3). Kir2.1 synthesis similarly repressed a significant number of genes associated with translation (35), whereas CFTR did not. Repression of ribosome biogenesis/translation-associated genes could be associated with the slower-growing phenotype of αENaC-expressing yeast (doubling time = 3.3 h vs. 2.3 h for vector expressing); however, these genes were also repressed in Kir2.1-expressing yeast, which actually grow faster than control yeast (doubling time = 2.2 h). In contrast, expression of CFTR led to the induction of 22 genes that are repressed when αENaC is expressed, 18 of which are associated with translation and ribosome biogenesis (RPS18A, RPS25A, RPL25, RPS13, RPL32, RPS12, RPS15, RPS27B, RPP2B, RPP1B, RPS14A, RPS16B, RPL35A, RPL2B, RPL1A, RPL15A). In the case of αENaC and Kir2.1, we envision that reducing translation may lower the overall protein-folding burden on the cell, as observed in mammalian cells in response to the UPR (43, 52, 126). Although it is mysterious why this process is induced upon CFTR expression the data might fit with a model where CFTR titrates essential PQC factors (see above), which in turn leads to a rise in translation efficiency as a compensatory response.
Kir2.1 expression activates the iron regulon.
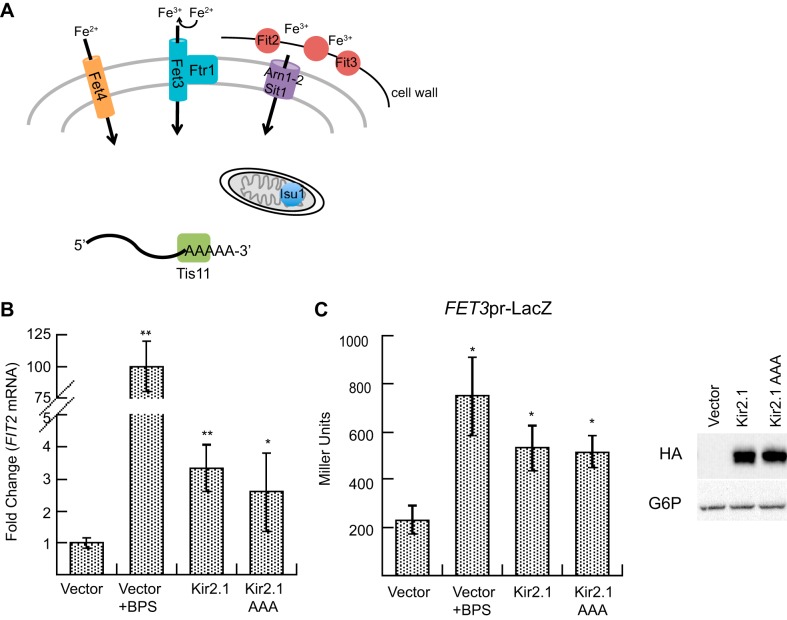
As highlighted in Table 1, GO terms associated with Kir2.1 expression differ significantly from those associated with αENaC and CFTR expression. Specifically, the expression of numerous genes involved in iron transport and storage (SIT1, FTR1, ARN1, ARN2, TIS11, FIT2, FIT3, ISU1, FET3, FET4) are induced. The yeast iron regulon is transcribed when iron is depleted, as well as by treatment with zinc, hydroxyurea, and cisplatin and during the alkaline response (35, 73, 78, 82, 119). Iron regulon transcription is primarily controlled by the AFT1 transcription factor (125), and activated genes include the high-affinity iron transport complex (FET3 and FTR1), a low-affinity iron transporter (FET4), three siderophore iron-chelate transporters (SIT1, ARN1, ARN2), an mRNA binding protein (TIS11), two cell wall-associated proteins that retain siderophore iron (FIT2, FIT3), and a scaffold protein that helps assemble iron-sulfur clusters in the mitochondria (ISU1) (Fig. 5A) (7, 33, 44, 57, 58, 130, 132, 148, 169). Unlike αENaC and CFTR, a population of Kir2.1 traffics to the plasma membrane in yeast, where it forms a functional potassium channel (79). Therefore, we hypothesized that Kir2.1 expression interferes with iron transport, perhaps by increasing the concentration of potassium in cells.
Fig. 5.
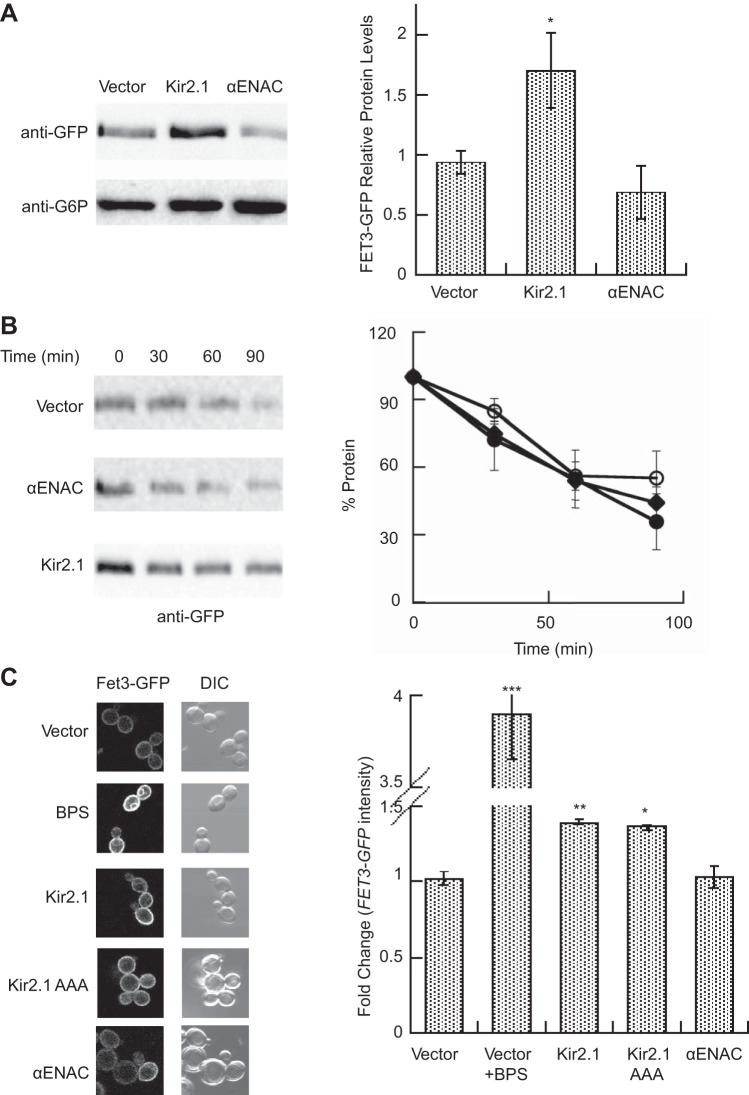
The iron regulon is induced when a potassium transport-deficient form of Kir2.1 is expressed. A: a model illustrating the function of the iron regulon genes induced by Kir2.1. Fet4 is the low-affinity iron transporter; Fet3 and Ftr1 form the high-affinity iron transporter. Fit2 and Fit3 bind siderophore iron in the cell wall. Arn2-3 and Sit1 are iron chelate transporters. Isu1 assists in the assembly of iron sulfur clusters in the mitochondria, and Tis11 is an mRNA binding protein that represses a subset of genes when iron is limiting (7, 33, 44, 57, 58, 130, 132, 148, 169). B: qPCR was performed with RNA extracted from wild-type yeast expressing either an hemagglutinin (HA) epitope-tagged version of Kir2.1 or Kir2.1 AAA or a vector control or vector-containing yeast treated with 200 μM bathophenanthroline disulfonate (BPS). Data represent the means of 3–6 experiments ± SE. *P < 0.03, **P < 0.001 C: a β-galactosidase reporter assay using a FET3 promoter driven LacZ construct was performed using cells containing a vector control, or in the presence of 400 μM BPS, or expressing Kir2.1 or Kir2.1 AAA. Data reported were gathered 30 min after substrate addition and represent the means from a representative experiment (n = 4) ± SE, *P < 0.05. To confirm protein expression, cells were harvested prior to the experiment, lysates were prepared, and the samples were then subject to SDS-PAGE and Western blotting. Blots were probed with anti-HA to detect Kir2.1 and Kir2.1 AAA or anti-glucose-6-phosphate-dehyrogenase (G6P) as a loading control.
To test this hypothesis, we first asked if a mutant form of Kir2.1, Kir2.1 AAA, in which the potassium selectivity filter has been mutated from GYG to AAA to block potassium transport (79, 152), would still lead to iron regulon induction. We first performed a qPCR analysis to measure FIT2 mRNA levels (Fig. 5B). As a positive control, FIT2 expression was analyzed in the presence of the iron chelator, BPS. As expected, BPS treatment lead to a dramatic increase in the level of FIT2 mRNA (Fig. 5B). Expression of either Kir2.1 or the Kir2.1 AAA similarly increased the FIT2 message, to a lesser extent than BPS. Nevertheless, it appears that potassium conduction is dispensable for induction of the iron regulon genes by Kir2.1.
To confirm these data, we transformed yeast with a plasmid that contains the LacZ gene driven by the FET3 promoter (156), which is a known target of the iron regulon and was significantly upregulated upon Kir2.1 expression (Table 1 and Supplemental File S2). BPS treatment again served as a positive control and significantly induced the activity of the FET3 promoter; Western blot confirmed that Kir2.1 and Kir2.1 AAA were expressed to similar levels (Fig. 5C). As above, the expression of either Kir2.1 or Kir2.1 AAA led to a similar increase in FET3 promoter activity.
Kir2.1 expression increases plasma membrane levels of Fet3.
Given that the potassium-conducting properties of Kir2.1 appeared to be dispensable for iron regulon induction, we next asked whether Kir2.1 expression interfered with the assembly and/or stability of one or more of the proteins that are required for cellular iron homeostasis. We first asked whether Kir2.1 expression affected the stability of the high-affinity iron transporter complex, which is composed of Fet3 and Ftr1. To begin to address this question we assessed the levels and stability of Fet3-GFP in either the presence or absence of Kir2.1. As a negative control Fet3 stability was monitored in the presence of αENaC expression or when cells contained a vector control. As shown in Fig. 6A, Kir2.1 expression increased the steady-state pool of Fet3-GFP compared with the vector control or αENaC-expressing yeast. However, analysis of cycloheximide chase data revealed that the expression of neither Kir2.1 nor αENaC significantly affected the rate of Fet3 degradation (Fig. 6B). Because the failure of Fet3 to reach the plasma membrane will induce the iron regulon (84), we next tested whether Kir2.1 expression prevented the delivery of Fet3 to the plasma membrane. Fet3 localization was monitored using a yeast strain harboring a GFP-tagged version of Fet3 (40) and was transformed with either a vector lacking an insert or with one encoding Kir2.1, Kir2.1 AAA, or αENaC. As a positive control, yeast were treated with BPS, which resulted in a robust increase in the intensity of Fet3 at the plasma membrane (Fig. 6C). A number of BPS-treated cells also exhibited ER staining, which was observed by other groups (40) and is likely due to the fact that overexpression of plasma membrane proteins can overwhelm the ER exit machinery (13). The observation that both Kir2.1 and Kir2.1 AAA expression significantly increased the intensity of Fet3-GFP signal at the plasma membrane was unexpected, an effect that was absent with cells contained a vector control or expressed αENaC (Fig. 6C). These data indicate that Kir2.1 expression actually augments trafficking of Fet3 to the plasma membrane. Future experiments to determine whether the newly trafficked Fet3 forms a functional iron transporter will be informative, because the possibility remains that Kir2.1 is interfering with the proper assembly of Fet3 and its partner Ftr1. Increased levels of nonfunctional Fet3 at the plasma membrane could lead to the induction of iron responsive genes.
Fig. 6.
Kir2.1 expression increases Fet3 levels and plasma membrane localization. A: lysates of wild-type FET3-GFP yeast transformed with either a vector control or a Kir2.1 or αENaC expression plasmid were immunoblotted with anti-GFP or anti-G6P as a loading control. Data represent the means of 4 experiments ± SE; *P < 0.05 B: cycloheximide chase assays were performed at 30°C as described in materials and methods using the yeast described in A: vector control (closed circles), Kir2.1 expressing (open circles), αENaC expressing (diamonds). Data represent the means of 4–7 experiments ± SE. C: the intensity of plasma membrane fluorescence in the yeast isolates described in A was measured. Data represent the mean values from at least 80 cells, normalized to the average fluorescence of control cells ± SE. Significance was determined by a 1-way ANOVA test with Tukey's post hoc comparison (70); *P < 0.05, **P < 0.01, ***P < 0.0001.
Previous work indicated that the iron regulon can still be induced in the presence of physiological iron concentrations if the formation of iron-sulfur clusters is instead disabled. Moreover, the iron regulon is permanently activated when iron-sulfur cluster assembly is compromised (24, 135). Thus, we were intrigued by the fact that the transcription of eight genes, among them Ssc1, are repressed only in response to Kir2.1 expression (Supplemental Files S2 and S3). Ssc1, a mitochondrial Hsp70, facilitates the transport of Yfh1 into the mitochondria (160), and Yfh1 is required for the assembly of iron-sulfur clusters (8). Together, these data suggest that the expression of Kir2.1 may compromise the formation of iron-sulfur clusters, which in turn leads to the observed response. Future experiments to determine whether overexpression of Ssc1 and/or Yfh1 block Kir2.1 induction of the iron regulon should be considered.
Another possible explanation for the induction of the iron regulon is that yeast vacuole function is compromised by Kir2.1 expression. The vacuole is the major site of iron storage in yeast (85). When we compared the list of genes induced by Kir2.1 to those induced by alkaline stress (Table 2), which neutralizes the pH in the vacuole, we found significant overlap between the two datasets (RF = 7.9). It will be important to define at the molecular level whether specific aspects of vacuole function (e.g., nutrient accumulation, autophagy, hydrolase activity, protein trafficking) are impaired by Kir2.1 expression.
Misfolded membrane proteins induce a novel stress response.
In a previous study, PQC substrates residing in the cytosol (VHL-L158P), the ER lumen (CPY*), and the ER membrane (Ste6*) were expressed in yeast, and the induction of specific reporter genes was monitored (103). The ULI1 and KAR2 transcripts served as reporters of ER lumenal stress (UPR) and were induced in response to CPY* expression, even though this ERAD substrate is rapidly degraded. As anticipated, the SSA4 and STI1 messages reported on cytosolic stress and were induced in response to VHL-L158P expression. Ste6* had no effect on UPR targeted genes but increased the levels of the cytosolic stress transcripts, although to lower levels than VHL-L158P. Similarly, we found that αENaC expression induced the UPR marker genes, ULI1 and KAR2, as well as the cytosolic stress marker gene, STI1 (Fig. 1 and Supplemental File S2). This result is consistent with the data discussed above. Also in accordance with the data outlined above, Kir2.1 expression failed to induce the UPR or cytosolic stress reporters. In turn, Ste6* is a truncated version of an ABC transporter, like CFTR, and is a well-characterized ERAD substrate (65, 86, 159). Therefore, we reasoned that CFTR would induce a related subset of genes as Ste6*. As hypothesized, CFTR expression induced cytosolic stress reporter transcripts, SSA1 and STI1, as well as the ER stress marker gene, KAR2 (Table 1 and File S2).
Metzger and Michaelis (103) also performed a microarray analysis of yeast expressing Ste6*. Surprisingly, there was overlap (RF = 2.4–3.5) between each of our datasets and the messages induced by Ste6* (see Table 2 and File S4), even though Kir2.1 expression failed to induce the reporter genes itemized above. Among the Ste6*-induced genes were 23 that are regulated by the RPN4 transcription factor, which activates the expression of genes encoding proteasomal subunits (87, 96, 103, 165). Of these 23 genes, αENaC induced two (UBI4, RPN1) and CFTR induced one (SGT2). Interestingly, among the overlapping genes that we identified (19 in total), eight were found in at least two of our datasets (underlined in Table 3). We propose that this select group represents a set of misfolded membrane protein stress-responders and includes: 1) PRE2, which encodes the chymotrypsin-like subunit of the proteasome; 2) HSP30, which encodes a membrane-associated heat shock protein that regulates the yeast plasma membrane H+-ATPase (102, 121); 3) BTN2, which encodes a v-SNARE required for endosome-to-Golgi trafficking (72) and interacts with the Sis1 (Hsp40) and Hsp42 (sHsp) molecular chaperones (95); and 4) HCH1, which encodes an Hsp90 partner, activates the chaperone's ATPase activity, and regulates cellular sensitivity to Hsp90 inhibitor (5, 89, 122). Like Hsp90, Hch1 is also heat shock responsive, and Hsp90 stabilizes both CFTR and Ste6* in yeast and human cells (88, 111, 167). Two genes, YJL133C-A and YNRO34W-A, which encode uncharacterized proteins, were also induced. The YNR034W-A gene is regulated by Msn2/4 (81) and the gene product interacts with ubiquitin, which is also upregulated in both the Ste6* and αENaC datasets (Table 3). Future studies will examine the functions of these proteins in PQC.
Table 3.
Misfolded membrane proteins induce a discrete set of genes
| ENaC/Ste6* P< 4.86 E-4; RF = 3.5 |
| PRE2, CMK2, CIT2, RPN1, BTN2, UBI4, HCH1, YIP3, HSP30, YJL133C-A |
| CFTR/Ste6* P< 0.004; RF = 2.9 |
| DLD3, PRE2, YRO2, ADE17, BTN2, UBA1, HCH1, SGT2, ECL1, HSP30, ADE13, YNRO34W-A |
| Kir2.1/Ste6*P < 0.028; RF = 2.4 |
| CIT2, ECL1, FET4, YNR034W-A, YJL133C-A |
Genes that are induced in response to Ste6* and αENaC, and CFTR and Kir2.1 are presented. Within this set of genes we underline those that are found in at least 3 of the datasets (αENaC, CFTR, Kir2.1, and/or Ste6*). P values and RFs are based on those found at Nemates.org and are described in materials and methods. Genes associated with protein folding are underlined.
Conclusions
We have characterized the transcriptional responses to three exogenously expressed ion channels in the yeast S. cerevisiae, a model organism that lacks the channels and in which the PQC and stress-responsive pathways are largely conserved. Because all three channels are known targets of the quality control machinery, we predicted that their expression would induce genes associated with PQC and folding. While αENaC and CFTR induced the expression of a significant number of genes linked to these processes, Kir2.1 did not. Instead, Kir2.1 triggered the transcription of genes associated with the iron regulon. We also hypothesized that the heterologous expression of αENaC, CFTR, and Kir2.1 would induce a well-characterized stress response, such as the UPR or HSR. However, a transcriptional response was evident that most closely resembled the ESR, which is triggered in response to a wide range of environmental stressors including heat shock, DTT, and oxidative stress. These results indicate that a misfolded membrane protein-induced stress response is quite complex and affects a number of transcriptional regulators in the cell. These data are especially intriguing given the fact that αENaC, CFTR, and Kir2.1 do not accumulate in yeast but are rapidly degraded. Therefore, the transient accumulation of these aberrant substrates is sufficient to reconfigure cellular homeostasis. It will be exciting in future work to characterize the functions of the many genes that are induced in response to the presence of these misfolded membrane proteins and to define how this stress response and the ESR are controlled at the molecular level.
GRANTS
This work was funded by National Institute of Health (NIH) Grants DK-090195 to T. M. Buck, DK-65161 to T. R. Kleyman, GM-75061 to J. L. Brodsky, and DK-79307 (University of Pittsburgh George O'Brien Kidney Research Center). The Bioinformatics Analysis Core was a pilot project partly funded by NIH/US Department of Health and Human Services Grant 5 UL1 RR-024153 to Dr. Stephen Reis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.M.B., T.R.K., and J.L.B. conception and design of research; T.M.B., R.J., J.L.-W., and P.G.N. performed experiments; T.M.B., R.J., J.L.-W., and J.L.A. analyzed data; T.M.B., R.J., J.L.-W., and J.L.B. interpreted results of experiments; T.M.B., R.J., J.L.-W., and J.L.A. prepared figures; T.M.B. drafted manuscript; T.M.B., P.G.N., T.R.K., and J.L.B. edited and revised manuscript; T.M.B., R.J., J.L.-W., J.L.A., P.G.N., T.R.K., and J.L.B. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Allyson O'Donnell, Chris Guerriero, and all the members of the Brodsky lab, as well as the Arndt lab, Jerry Kaplan, Deborah Hollingshead, Tamanna Sultana, and the Genomics and Proteomics Core Laboratory at the University of Pittsburgh, Patrick Gibney, Meredith Metzger, and Tom Harper for helpful discussions, reagents, and/or technical assistance.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Adams A, Gottschling DE, Kaiser CA, Stearns T. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual (1997 Ed.) 1997. [Google Scholar]
- 2.Ahner A, Nakatsukasa K, Zhang H, Frizzell RA, Brodsky JL. Small heat-shock proteins select ΔF508-CFTR for endoplasmic reticulum-associated degradation. Mol Biol Cell 18: 806–814, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahner A, Gong X, Schmidt BZ, Peters KW, Rabeh WM, Thibodeau PH, Lukacs GL, Frizzell RA. Small heat shock proteins target mutant cystic fibrosis transmembrane conductance regulator for degradation via a small ubiquitin-like modifier-dependent pathway. Mol Biol Cell 24: 74–84, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amoros M, Estruch F. Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Mol Microbiol 39: 1523–1532, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong H, Wolmarans A, Mercier R, Mai B, LaPointe P. The co-chaperone Hch1 regulates Hsp90 function differently than its homologue Aha1 and confers sensitivity to yeast to the Hsp90 inhibitor NVP-AUY922. PLoS One 7: e49322, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+ channel subunits. Am J Physiol Cell Physiol 271: C605–C611, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Askwith C, Eide D, Van Ho A, Bernard PS, Li L, Davis-Kaplan S, Sipe DM, Kaplan J. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76: 403–410, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Babcock M, de Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, Pandolfo M, Kaplan J. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 276: 1709–1712, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 319: 916–919, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol 40: 2353–2362, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Bartoszewski R, Rab A, Jurkuvenaite A, Mazur M, Wakefield J, Collawn JF, Bebok Z. Activation of the unfolded protein response by deltaF508 CFTR. Am J Respir Cell Mol Biol 39: 448–457, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodsky JL, Wojcikiewicz RJ. Substrate-specific mediators of ER associated degradation (ERAD). Curr Opin Cell Biol 21: 516–521, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodsky JL, Skach WR. Protein folding and quality control in the endoplasmic reticulum: recent lessons from yeast and mammalian cell systems. Curr Opin Cell Biol 23: 464–475, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell 40: 238–252, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Buck TM, Kolb AR, Boyd C, Kleyman TR, Brodsky JL. The ER associated degradation of the epithelial sodium channel requires a unique complement of molecular chaperones. Mol Biol Cell 21: 1047–1058, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buck TM, Plavchak L, Roy A, Donnelly BF, Kashlan OB, Kleyman TR, Subramanya AR, Brodsky JL. The Lhs1/GRP170 chaperones facilitate the endoplasmic reticulum-associated degradation of the epithelial sodium channel. J Biol Chem 288: 18366–18380, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol Cell Physiol 267: C1682–C1690, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Carlson PE Jr, Carr KA, Janes BK, Anderson EC, Hanna PC. Transcriptional profiling of Bacillus anthracis Sterne (34F2) during iron starvation. PLoS One 4: e6988, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson PE Jr, Horzempa J, O'Dee DM, Robinson CM, Neophytou P, Labrinidis A, Nau GJ. Global transcriptional response to spermine, a component of the intramacrophage environment, reveals regulation of Francisella gene expression through insertion sequence elements. J Bacteriol 191: 6855–6864, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casagrande R, Stern P, Diehn M, Shamu C, Osario M, Zuniga M, Brown PO, Ploegh H. Degradation of proteins from the ER of S. cerevisiae requires an intact unfolded protein response pathway. Mol Cell 5: 729–735, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Cashikar AG, Duennwald M, Lindquist SL. A chaperone pathway in protein disaggregation. Hsp26 alters the nature of protein aggregates to facilitate reactivation by Hsp104. J Biol Chem 280: 23869–23875, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang XB, Hou YX, Jensen TJ, Riordan JR. Mapping of cystic fibrosis transmembrane conductance regulator membrane topology by glycosylation site insertion. J Biol Chem 269: 18572–18575, 1994. [PubMed] [Google Scholar]
- 23.Chen M, Zhang JT. Membrane insertion, processing, and topology of cystic fibrosis transmembrane conductance regulator (CFTR) in microsomal membranes. Mol Membr Biol 13: 33–40, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Chen OS, Crisp RJ, Valachovic M, Bard M, Winge DR, Kaplan J. Transcription of the yeast iron regulon does not respond directly to iron but rather to iron-sulfur cluster biosynthesis. J Biol Chem 279: 29513–29518, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J, Kane LP. Global identification of genes and pathways regulated by Akt during activation of T helper cells. F1000Res 2: 109, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63: 827–834, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, Christie KR, Costanzo MC, Dwight SS, Engel SR, Fisk DG, Hirschman JE, Hitz BC, Karra K, Krieger CJ, Miyasato SR, Nash RS, Park J, Skrzypek MS, Simison M, Weng S, Wong ED. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Res 40: D700–D705, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 368: 651–662, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Collart MA, Oliviero S. Preparation of yeast RNA. Curr Protoc Mol Biol 13: Unit13.12, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73: 1197–1206, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87: 391–404, 1996. [DOI] [PubMed] [Google Scholar]
- 32.De Wever V, Reiter W, Ballarini A, Ammerer G, Brocard C. A dual role for PP1 in shaping the Msn2-dependent transcriptional response to glucose starvation. EMBO J 24: 4115–4123, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dix DR, Bridgham JT, Broderius MA, Byersdorfer CA, Eide DJ. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J Biol Chem 269: 26092–26099, 1994. [PubMed] [Google Scholar]
- 33a.Dobrowolski SF, Lyons-Weiler J, Biery A, Spridik K, Vockley G, Kranik E, Skvorak K, Sultana T. Methylome repatterning in a mouse model of Maternal PKU Syndrome. Mol Genet Metab 113: 194–199, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Du K, Sharma M, Lukacs GL. The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol 12: 17–25, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Dubacq C, Chevalier A, Courbeyrette R, Petat C, Gidrol X, Mann C. Role of the iron mobilization and oxidative stress regulons in the genomic response of yeast to hydroxyurea. Mol Genet Genom 275: 114–124, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Duennwald ML, Lindquist S. Impaired ERAD and ER stress are early and specific events in polyglutamine toxicity. Genes Dev 22: 3308–3319, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duina AA, Kalton HM, Gaber RF. Requirement for Hsp90 and a CyP-40-type cyclophilin in negative regulation of the heat shock response. J Biol Chem 273: 18974–18978, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farrugia G, Balzan R. Oxidative stress and programmed cell death in yeast. Front Oncol 2: 64, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felice MR, De Domenico I, Li L, Ward DM, Bartok B, Musci G, Kaplan J. Post-transcriptional regulation of the yeast high affinity iron transport system. J Biol Chem 280: 22181–22190, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Fleischer TC, Weaver CM, McAfee KJ, Jennings JL, Link AJ. Systematic identification and functional screens of uncharacterized proteins associated with eukaryotic ribosomal complexes. Genes Dev 20: 1294–1307, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta 30: 15–24, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol 5: a013169, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garland SA, Hoff K, Vickery LE, Culotta VC. Saccharomyces cerevisiae ISU1 and ISU2: members of a well-conserved gene family for iron-sulfur cluster assembly. J Mol Biol 294: 897–907, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 11: 4241–4257, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, Ruis H, Schuller C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev 12: 586–597, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grove DE, Fan CY, Ren HY, Cyr DM. The endoplasmic reticulum-associated Hsp40 DNAJB12 and Hsc70 cooperate to facilitate RMA1 E3-dependent degradation of nascent CFTRDeltaF508. Mol Biol Cell 22: 301–314, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grunder S, Firsov D, Chang SS, Jaeger NF, Gautschi I, Schild L, Lifton RP, Rossier BC. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J 16: 899–907, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev 92: 537–576, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haass FA, Jonikas M, Walter P, Weissman JS, Jan YN, Jan LY, Schuldiner M. Identification of yeast proteins necessary for cell-surface function of a potassium channel. Proc Natl Acad Sci USA 104: 18079–18084, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol 24: 5249–5256, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397: 271–274, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Harris N, MacLean M, Hatzianthis K, Panaretou B, Piper PW. Increasing Saccharomyces cerevisiae stress resistance, through the overactivation of the heat shock response resulting from defects in the Hsp90 chaperone, does not extend replicative life span but can be associated with slower chronological ageing of nondividing cells. Mol Genet Genomics 265: 258–263, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Hauptmann P, Riel C, Kunz-Schughart LA, Frohlich KU, Madeo F, Lehle L. Defects in N-glycosylation induce apoptosis in yeast. Mol Microbiol 59: 765–778, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10: 3787–3799, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haze K, Okada T, Yoshida H, Yanagi H, Yura T, Negishi M, Mori K. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J 355: 19–28, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heymann P, Ernst JF, Winkelmann G. Identification of a fungal triacetylfusarinine C siderophore transport gene (TAF1) in Saccharomyces cerevisiae as a member of the major facilitator superfamily. Biometals 12: 301–306, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Heymann P, Ernst JF, Winkelmann G. Identification and substrate specificity of a ferrichrome-type siderophore transporter (Arn1p) in Saccharomyces cerevisiae. FEMS Microbiol Lett 186: 221–227, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci USA 103: 45–50, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313: 104–107, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186: 323–331, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu Y, Mivechi NF. Promotion of heat shock factor Hsf1 degradation via adaptor protein filamin A-interacting protein 1-like (FILIP-1L). J Biol Chem 286: 31397–31408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hulsen T, de Vlieg J, Alkema W. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genom 9: 488, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutt DM, Roth DM, Chalfant MA, Youker RT, Matteson J, Brodsky JL, Balch WE. FK506 binding protein 8 peptidylprolyl isomerase activity manages a late stage of cystic fibrosis transmembrane conductance regulator (CFTR) folding and stability. J Biol Chem 287: 21914–21925, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huyer G, Piluek WF, Fansler Z, Kreft SG, Hochstrasser M, Brodsky JL, Michaelis S. Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multispanning membrane protein and a soluble luminal protein. J Biol Chem 279: 38369–38378, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Jansen JA, de Boer TP, Wolswinkel R, van Veen TA, Vos MA, van Rijen HV, van der Heyden MA. Lysosome mediated Kir2.1 breakdown directly influences inward rectifier current density. Biochem Biophys Res Commun 367: 687–692, 2008. [DOI] [PubMed] [Google Scholar]
- 67.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Jensen TJ, Loo MA, Pind S, Williams DB, Goldberg AL, Riordan JR. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83: 129–135, 1995. [DOI] [PubMed] [Google Scholar]
- 69.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 92: 1564–1572, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Jones LV, Tukey JW. A sensible formulation of the significance test. Psychol Meth 5: 411–414, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Jordan R, Patel S, Hu H, Lyons-Weiler J. Efficiency analysis of competing tests for finding differentially expressed genes in lung adenocarcinoma. Cancer Inform 6: 389–421, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kama R, Robinson M, Gerst JE. Btn2, a Hook1 ortholog and potential Batten disease-related protein, mediates late endosome-Golgi protein sorting in yeast. Mol Cell Biol 27: 605–621, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaplan J, McVey Ward D, Crisp RJ, Philpott CC. Iron-dependent metabolic remodeling in S. cerevisiae. Biochim Biophys Acta 1763: 646–651, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Kashlan OB, Mueller GM, Qamar MZ, Poland PA, Ahner A, Rubenstein RC, Hughey RP, Brodsky JL, Kleyman TR. Small heat shock protein alphaA-crystallin regulates epithelial sodium channel expression. J Biol Chem 282: 28149–28156, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kashlan OB, Kleyman TR. Epithelial Na(+) channel regulation by cytoplasmic and extracellular factors. Exp Cell Res 318: 1011–1019, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4: 363–371, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Kellis M, Birren BW, Lander ES. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature 428: 617–624, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Kimura A, Ohashi K, Naganuma A. Cisplatin upregulates Saccharomyces cerevisiae genes involved in iron homeostasis through activation of the iron insufficiency-responsive transcription factor Aft1. J Cell Physiol 210: 378–384, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Kolb AR, Needham PG, Rothenberg C, Guerriero CJ, Welling PA, Brodsky JL. ESCRT regulates surface expression of the Kir2.1 potassium channel. Mol Biol Cell 25: 276–289, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuo SC, Lampen JO. Inhibition by 2-deoxy-D-glucose of synthesis of glycoprotein enzymes by protoplasts of Saccharomyces: relation to inhibition of sugar uptake and metabolism. J Bacteriol 111: 419–429, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lai LC, Kosorukoff AL, Burke PV, Kwast KE. Dynamical remodeling of the transcriptome during short-term anaerobiosis in Saccharomyces cerevisiae: differential response and role of Msn2 and/or Msn4 and other factors in galactose and glucose media. Mol Cell Biol 25: 4075–4091, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamb TM, Xu W, Diamond A, Mitchell AP. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J Biol Chem 276: 1850–1856, 2001. [DOI] [PubMed] [Google Scholar]
- 83.Lenssen E, James N, Pedruzzi I, Dubouloz F, Cameroni E, Bisig R, Maillet L, Werner M, Roosen J, Petrovic K, Winderickx J, Collart MA, De Virgilio C. The Ccr4-Not complex independently controls both Msn2-dependent transcriptional activation–via a newly identified Glc7/Bud14 type I protein phosphatase module–and TFIID promoter distribution. Mol Cell Biol 25: 488–498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Kaplan J. Defects in the yeast high affinity iron transport system result in increased metal sensitivity because of the increased expression of transporters with a broad transition metal specificity. J Biol Chem 273: 22181–22187, 1998. [DOI] [PubMed] [Google Scholar]
- 85.Li L, Chen OS, McVey Ward D, Kaplan J. CCC1 is a transporter that mediates vacuolar iron storage in yeast. J Biol Chem 276: 29515–29519, 2001. [DOI] [PubMed] [Google Scholar]
- 86.Loayza D, Tam A, Schmidt WK, Michaelis S. Ste6p mutants defective in exit from the endoplasmic reticulum (ER) reveal aspects of an ER quality control pathway in Saccharomyces cerevisiae. Mol Biol Cell 9: 2767–2784, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.London MK, Keck BI, Ramos PC, Dohmen RJ. Regulatory mechanisms controlling biogenesis of ubiquitin and the proteasome. FEBS Lett 567: 259–264, 2004. [DOI] [PubMed] [Google Scholar]
- 88.Loo MA, Jensen TJ, Cui L, Hou Y, Chang XB, Riordan JR. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J 17: 6879–6887, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lotz GP, Lin H, Harst A, Obermann WM. Aha1 binds to the middle domain of Hsp90, contributes to client protein activation, and stimulates the ATPase activity of the molecular chaperone. J Biol Chem 278: 17228–17235, 2003. [DOI] [PubMed] [Google Scholar]
- 90.Lukacs GL, Mohamed A, Kartner N, Chang XB, Riordan JR, Grinstein S. Conformational maturation of CFTR but not its mutant counterpart (delta F508) occurs in the endoplasmic reticulum and requires ATP. EMBO J 13: 6076–6086, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma D, Taneja TK, Hagen BM, Kim BY, Ortega B, Lederer WJ, Welling PA. Golgi export of the Kir2.1 channel is driven by a trafficking signal located within its tertiary structure. Cell 145: 1102–1115, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madeo F, Frohlich E, Ligr M, Grey M, Sigrist SJ, Wolf DH, Frohlich KU. Oxygen stress: a regulator of apoptosis in yeast. J Cell Biol 145: 757–767, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Madeo F, Herker E, Maldener C, Wissing S, Lachelt S, Herlan M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S, Frohlich KU. A caspase-related protease regulates apoptosis in yeast. Mol Cell 9: 911–917, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Malik B, Schlanger L, Al-Khalili O, Bao HF, Yue G, Price SR, Mitch WE, Eaton DC. Enac degradation in A6 cells by the ubiquitin-proteosome proteolytic pathway. J Biol Chem 276: 12903–12910, 2001. [DOI] [PubMed] [Google Scholar]
- 95.Malinovska L, Kroschwald S, Munder MC, Richter D, Alberti S. Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol Biol Cell 23: 3041–3056, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mannhaupt G, Schnall R, Karpov V, Vetter I, Feldmann H. Rpn4p acts as a transcription factor by binding to PACE, a nonamer box found upstream of 26S proteasomal and other genes in yeast. FEBS Lett 450: 27–34, 1999. [DOI] [PubMed] [Google Scholar]
- 97.Martinez-Pastor MT, Marchler G, Schuller C, Marchler-Bauer A, Ruis H, Estruch F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J 15: 2227–2235, 1996. [PMC free article] [PubMed] [Google Scholar]
- 98.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayordomo I, Estruch F, Sanz P. Convergence of the target of rapamycin and the Snf1 protein kinase pathways in the regulation of the subcellular localization of Msn2, a transcriptional activator of STRE (Stress Response Element)-regulated genes. J Biol Chem 277: 35650–35656, 2002. [DOI] [PubMed] [Google Scholar]
- 100.McDaniel D, Caplan AJ, Lee MS, Adams CC, Fishel BR, Gross DS, Garrard WT. Basal-level expression of the yeast HSP82 gene requires a heat shock regulatory element. Mol Cell Biol 9: 4789–4798, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol 3: 100–105, 2001. [DOI] [PubMed] [Google Scholar]
- 102.Meena RC, Thakur S, Chakrabarti A. Regulation of Saccharomyces cerevisiae plasma membrane H(+)-ATPase (Pma1) by dextrose and Hsp30 during exposure to thermal stress. Ind J Microbiol 51: 153–158, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Metzger MB, Michaelis S. Analysis of quality control substrates in distinct cellular compartments reveals a unique role for Rpn4p in tolerating misfolded membrane proteins. Mol Biol Cell 20: 1006–1019, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1972. [Google Scholar]
- 105.Mogk A, Deuerling E, Vorderwulbecke S, Vierling E, Bukau B. Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol Microbiol 50: 585–595, 2003. [DOI] [PubMed] [Google Scholar]
- 106.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119: 756–766, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morano KA, Grant CM, Moye-Rowley WS. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 190: 1157–1195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74: 743–756, 1993. [DOI] [PubMed] [Google Scholar]
- 109.Mori K, Kawahara T, Yoshida H, Yanagi H, Yura T. Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells 1: 803–817, 1996. [DOI] [PubMed] [Google Scholar]
- 110.Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156: 119–122, 1995. [DOI] [PubMed] [Google Scholar]
- 111.Nakatsukasa K, Huyer G, Michaelis S, Brodsky JL. Dissecting the ER-associated degradation of a misfolded polytopic membrane protein. Cell 132: 101–112, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nalos L, de Boer TP, Houtman MJ, Rook MB, Vos MA, van der Heyden MA. Inhibition of lysosomal degradation rescues pentamidine-mediated decreases of K(IR)2.1 ion channel expression but not that of K(v)111. Eur J Pharmacol 652: 96–103, 2011. [DOI] [PubMed] [Google Scholar]
- 113.Nelson RJ, Ziegelhoffer T, Nicolet C, Werner-Washburne M, Craig EA. The translation machinery and 70 kd heat shock protein cooperate in protein synthesis. Cell 71: 97–105, 1992. [DOI] [PubMed] [Google Scholar]
- 114.Ng DT, Spear ED, Walter P. The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control. J Cell Biol 150: 77–88, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nikawa J, Akiyoshi M, Hirata S, Fukuda T. Saccharomyces cerevisiae IRE2/HAC1 is involved in IRE1-mediated KAR2 expression. Nucleic Acids Res 24: 4222–4226, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nishida M, MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell 111: 957–965, 2002. [DOI] [PubMed] [Google Scholar]
- 117.Nojima H, Leem SH, Araki H, Sakai A, Nakashima N, Kanaoka Y, Ono Y. Hac1: a novel yeast bZIP protein binding to the CRE motif is a multicopy suppressor for cdc10 mutant of Schizosaccharomyces pombe. Nucleic Acids Res 22: 5279–5288, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.O'Donoghue LE, Ptitsyn AA, Kamstock DA, Siebert J, Thomas RS, Duval DL. Expression profiling in canine osteosarcoma: identification of biomarkers and pathways associated with outcome. BMC Cancer 10: 506, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pagani MA, Casamayor A, Serrano R, Atrian S, Arino J. Disruption of iron homeostasis in Saccharomyces cerevisiae by high zinc levels: a genome-wide study. Mol Microbiol 65: 521–537, 2007. [DOI] [PubMed] [Google Scholar]
- 120.Pagant S, Kung L, Dorrington M, Lee MC, Miller EA. Inhibiting endoplasmic reticulum (ER)-associated degradation of misfolded Yor1p does not permit ER export despite the presence of a diacidic sorting signal. Mol Biol Cell 18: 3398–3413, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Panaretou B, Piper PW. The plasma membrane of yeast acquires a novel heat-shock protein (hsp30) and displays a decline in proton-pumping ATPase levels in response to both heat shock and the entry to stationary phase. Eur J Biochem 206: 635–640, 1992. [DOI] [PubMed] [Google Scholar]
- 122.Panaretou B, Siligardi G, Meyer P, Maloney A, Sullivan JK, Singh S, Millson SH, Clarke PA, Naaby-Hansen S, Stein R, Cramer R, Mollapour M, Workman P, Piper PW, Pearl LH, Prodromou C. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol Cell 10: 1307–1318, 2002. [DOI] [PubMed] [Google Scholar]
- 123.Patel S, Lyons-Weiler J. caGEDA: a web application for the integrated analysis of global gene expression patterns in cancer. Appl Bioinformat 3: 49–62, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Philpott CC, Protchenko O. Response to iron deprivation in Saccharomyces cerevisiae. Eukaryot Cell 7: 20–27, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pincus D, Aranda-Diaz A, Zuleta IA, Walter P, El-Samad H. Delayed Ras/PKA signaling augments the unfolded protein response. Proc Natl Acad Sci USA 111: 14800–14805, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir21 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell 105: 511–519, 2001. [DOI] [PubMed] [Google Scholar]
- 128.Pomerantz RG, Mirvish ED, Erdos G, Falo LD Jr, Geskin LJ. Novel approach to gene expression profiling in Sezary syndrome. Br J Dermatol 163: 1090–1094, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C: The Art of Scientific Computing. Cambridge, UK: Cambridge University Press; 1993, p. 994. [Google Scholar]
- 130.Protchenko O, Ferea T, Rashford J, Tiedeman J, Brown PO, Botstein D, Philpott CC. Three cell wall mannoproteins facilitate the uptake of iron in Saccharomyces cerevisiae. J Biol Chem 276: 49244–49250, 2001. [DOI] [PubMed] [Google Scholar]
- 131.Ptitsyn AA, Weil MM, Thamm DH. Systems biology approach to identification of biomarkers for metastatic progression in cancer. BMC Bioinformat 9, Suppl 9: S8, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Puig S, Askeland E, Thiele DJ. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120: 99–110, 2005. [DOI] [PubMed] [Google Scholar]
- 133.Renard S, Lingueglia E, Voilley N, Lazdunski M, Barbry P. Biochemical analysis of the membrane topology of the amiloride-sensitive Na+ channel. J Biol Chem 269: 12981–12986, 1994. [PubMed] [Google Scholar]
- 134.Rosenberg MF, O'Ryan LP, Hughes G, Zhao Z, Aleksandrov LA, Riordan JR, Ford RC. The cystic fibrosis transmembrane conductance regulator (CFTR): three-dimensional structure and localization of a channel gate. J Biol Chem 286: 42647–42654, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rutherford JC, Ojeda L, Balk J, Muhlenhoff U, Lill R, Winge DR. Activation of the iron regulon by the yeast Aft1/Aft2 transcription factors depends on mitochondrial but not cytosolic iron-sulfur protein biogenesis. J Biol Chem 280: 10135–10140, 2005. [DOI] [PubMed] [Google Scholar]
- 136.Schrodinger L. The PyMOL Molecular Graphics System, version 1.5.0.4 2010. [Google Scholar]
- 137.Schwalbe RA, Rudin A, Xia SL, Wingo CS. Site-directed glycosylation tagging of functional Kir2.1 reveals that the putative pore-forming segment is extracellular. J Biol Chem 277: 24382–24389, 2002. [DOI] [PubMed] [Google Scholar]
- 138.Schwarz RT, Rohrschneider JM, Schmidt MF. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol 19: 782–791, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Serrano R, Ruiz A, Bernal D, Chambers JR, Arino J. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol Microbiol 46: 1319–1333, 2002. [DOI] [PubMed] [Google Scholar]
- 140.Shamu CE, Walter P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J 15: 3028–3039, 1996. [PMC free article] [PubMed] [Google Scholar]
- 141.Sharma M, Benharouga M, Hu W, Lukacs GL. Conformational and temperature-sensitive stability defects of the delta F508 cystic fibrosis transmembrane conductance regulator in post-endoplasmic reticulum compartments. J Biol Chem 276: 8942–8950, 2001. [DOI] [PubMed] [Google Scholar]
- 142.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol 18: 7499–7509, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shi Y, An J, Liang J, Hayes SE, Sandusky GE, Stramm LE, Yang NN. Characterization of a mutant pancreatic eIF-2alpha kinase, PEK, and co-localization with somatostatin in islet delta cells. J Biol Chem 274: 5723–5730, 1999. [DOI] [PubMed] [Google Scholar]
- 144.Shin SS, Bales JW, Yan HQ, Kline AE, Wagner AK, Lyons-Weiler J, Dixon CE. The effect of environmental enrichment on substantia nigra gene expression after traumatic brain injury in rats. J Neurotrauma 30: 259–270, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem 269: 24379–24383, 1994. [PubMed] [Google Scholar]
- 146.Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology 146: 5079–5085, 2005. [DOI] [PubMed] [Google Scholar]
- 147.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J 16: 6325–6336, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stearman R, Yuan DS, Yamaguchi-Iwai Y, Klausner RD, Dancis A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 271: 1552–1557, 1996. [DOI] [PubMed] [Google Scholar]
- 149.Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life 60: 620–628, 2008. [DOI] [PubMed] [Google Scholar]
- 150.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184–190, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Takatsuki A, Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 24: 785–794, 1971. [DOI] [PubMed] [Google Scholar]
- 152.Tinker A, Jan YN, Jan LY. Regions responsible for the assembly of inwardly rectifying potassium channels. Cell 87: 857–868, 1996. [DOI] [PubMed] [Google Scholar]
- 153.Tkach JM, Yimit A, Lee AY, Riffle M, Costanzo M, Jaschob D, Hendry JA, Ou J, Moffat J, Boone C, Davis TN, Nislow C, Brown GW. Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat Cell Biol 14: 966–976, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101: 249–258, 2000. [DOI] [PubMed] [Google Scholar]
- 155.Treger JM, Schmitt AP, Simon JR, McEntee K. Transcriptional factor mutations reveal regulatory complexities of heat shock and newly identified stress genes in Saccharomyces cerevisiae. J Biol Chem 273: 26875–26879, 1998. [DOI] [PubMed] [Google Scholar]
- 156.Urbanowski JL, Piper RC. The iron transporter Fth1p forms a complex with the Fet5 iron oxidase and resides on the vacuolar membrane. J Biol Chem 274: 38061–38070, 1999. [DOI] [PubMed] [Google Scholar]
- 157.Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol 2: a004390, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Valentijn JA, Fyfe GK, Canessa CM. Biosynthesis and processing of epithelial sodium channels in Xenopus oocytes. J Biol Chem 273: 30344–30351, 1998. [DOI] [PubMed] [Google Scholar]
- 159.Vashist S, Ng DT. Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J Cell Biol 165: 41–52, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Voisine C, Schilke B, Ohlson M, Beinert H, Marszalek J, Craig EA. Role of the mitochondrial Hsp70s, Ssc1 and Ssq1, in the maturation of Yfh1. Mol Cell Biol 20: 3677–3684, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Y, Perlmutter DH. Targeting intracellular degradation pathways for treatment of liver disease caused by alpha1-antitrypsin deficiency. Pediatr Res 75: 133–139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83: 121–127, 1995. [DOI] [PubMed] [Google Scholar]
- 163.Welihinda AA, Kaufman RJ. The unfolded protein response pathway in Saccharomyces cerevisiae. Oligomerization and trans-phosphorylation of Ire1p (Ern1p) are required for kinase activation. J Biol Chem 271: 18181–18187, 1996. [DOI] [PubMed] [Google Scholar]
- 164.Wolff S, Weissman JS, Dillin A. Differential scales of protein quality control. Cell 157: 52–64, 2014. [DOI] [PubMed] [Google Scholar]
- 165.Xie Y, Varshavsky A. RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: a negative feedback circuit. Proc Natl Acad Sci USA 98: 3056–3061, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang Y, Janich S, Cohn JA, Wilson JM. The common variant of cystic fibrosis transmembrane conductance regulator is recognized by hsp70 and degraded in a pre-Golgi nonlysosomal compartment. Proc Natl Acad Sci USA 90: 9480–9484, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Youker RT, Walsh P, Beilharz T, Lithgow T, Brodsky JL. Distinct roles for the Hsp40 and Hsp90 molecular chaperones during cystic fibrosis transmembrane conductance regulator degradation in yeast. Mol Biol Cell 15: 4787–4797, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Younger JM, Ren HY, Chen L, Fan CY, Fields A, Patterson C, Cyr DM. A foldable CFTRDeltaF508 biogenic intermediate accumulates upon inhibition of the Hsc70-CHIP E3 ubiquitin ligase. J Cell Biol 167: 1075–1085, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yun CW, Ferea T, Rashford J, Ardon O, Brown PO, Botstein D, Kaplan J, Philpott CC. Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake. J Biol Chem 275: 10709–10715, 2000. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Y, Nijbroek G, Sullivan ML, McCracken AA, Watkins SC, Michaelis S, Brodsky JL. Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol Biol Cell 12: 1303–1314, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang Y, Michaelis S, Brodsky JL. CFTR expression and ER-associated degradation in yeast. Methods Mol Med 70: 257–265, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.