Abstract
The microbiota that populates the mammalian intestine consists of hundreds of trillions of bacteria that are separated from underlying immune cells by a single layer of epithelial cells. The intestinal immune system effectively tolerates components of the microbiota that provide benefit to the host while remaining poised to eliminate those that are harmful. Antigen presenting cells, especially macrophages and dendritic cells, play important roles in maintaining intestinal homeostasis via their ability to orchestrate appropriate responses to the microbiota. Paramount to elucidating intestinal macrophage- and dendritic cell-mediated functions is the ability to effectively isolate and identify these cells from a complex cellular environment. In this review, we summarize methodology for the isolation and phenotypic characterization of macrophages and DCs from the mouse intestine and discuss how this may be useful for gaining insight into the mechanisms by which mucosal immune tolerance is maintained.
Keywords: intestine, antigen presenting cell, macrophage, dendritic cell
1. Introduction
From birth, the mammalian intestine is exposed to a variety of foreign antigens including the commensal microbiota, dietary factors and pathogens1. Intestinal antigen presenting cells (APCs), comprised primarily of dendritic cells (DCs) and macrophages, are integral components of the mucosal immune system that aid in maintaining coexistence with these foreign antigens2–9. The lamina propria (LP) contains a large population of macrophages and DCs positioned directly beneath the intestinal epithelial barrier10–12. This proximity to the intestinal lumen suggests these cells have an important role in modulating innate and adaptive immune responses towards the enormous commensal microbiota10–19. The mechanisms by which intestinal macrophages and DCs interact with foreign antigens and promote the differentiation of T cells are active areas of research20–25. A critical component of identifying the functions of intestinal APCs is the ability to efficiently isolate these cells from a complex cellular environment26. In this review we summarize our methodology for the isolation and purification of intestinal macrophages and DCs and how this may be applied to investigate the functions of these cells. Additionally, we review genetically modified mouse strains that are proving insight into the complex in vivo function of intestinal APCs.
2. Ex vivo isolation and purification of mouse intestinal APCs
In this section, we outline a detailed methodology to isolate and purify mouse intestinal DCs and macrophages for subsequent phenotypic characterization using multi-color flow cytometry and for enrichment and cell sorting to conduct functional studies.
2.1. Dissection and dissociation of intestinal epithelial cells
Following euthanasia, the small intestine (SI) and/or the large intestine (LI) are quickly harvested from mice. At this point, it is important to tease the mesentery and the fatty tissues around the intestine. The intestine is cut longitudinally and washed of fecal contents and mucus from the intestinal lumen in Ca2+/Mg2+-Free Phosphate Buffered Saline (CMF-PBS) at room temperature. For the SI, the Peyer’s patches along the anti-mesenteric surface, which can be observed with the naked eye, are removed by fine dissection. The SI/LI is separately cut into approximately 1.5 cm pieces and placed into separate 50 ml conical tubes containing 30 ml of pre-warmed (37°C) Ca2+/Mg2+-Free Hank’s Balanced Salt Solution with 5% of Fetal Bovine Serum (CMF-HBSS/FBS) and 2 mM EDTA. Each 50 ml conical tube is horizontally placed into an orbital shaker and shaken at 250 rpm for 20 min at 37°C. A single mesh wire strainer is placed over a waste bucket and the contents of each 50 ml conical tube are poured through to recover the 1.5 cm pieces of intestine denuded of epithelial cells. The recovered pieces are then placed in separate 50 ml conical tubes containing 30 ml of pre-warmed CMF-HBSS/FBS with 2 mM EDTA and the same shaking step is repeated once more to ensure thorough removal of intestinal epithelial cells.
2.2. Tissue digestion and isolation of intestinal APCs
After shaking twice to dissociate the epithelial cells, the contents of each 50 ml conical tube is poured through the strainer and the remaining 1.5 cm pieces of intestine are transferred onto small plastic weigh boats. The 1.5 cm pieces of intestine are then minced, using scissors, in the plastic weigh boat and transferred to 50 ml conical tubes containing 20 ml of RPMI media with 10% of FBS, 1 mg/ml collagenase type IV or type VIII and 40 ug/ml DNAse. (Note: In our experience, both collagenase type IV and type VIII obtained from Sigma-Aldrich have provided similarly excellent digestion of the mouse small intestine, while collagenase type IV provides superior digestion of the large intestine). The RPMI medium should be pre-warmed to 37°C in a water bath. Each 50 ml conical tube is placed into an orbital shaker and digested at 200 rpm for 10–20 min at 37°C. Following digestion, each tube can be vortexed for 10–20 seconds to ensure thorough dissociation of any remaining intestinal tissue. The contents are then filtered through a 100 μm cell strainer directly into a 50 ml conical tube. Each 50 ml conical tube is topped off with CMF-HBSS/FBS and centrifuged at 4°C. After repeating this step once more, the supernatant is poured off and the cell pellet is resuspended in ice-cold CMF-HBSS/FBS or another appropriate buffer. These cells are ready to be stained with specific antibodies and analyzed by flow cytometry. (Note: In our experience, use of a ~45/70% Percoll gradient to further enrich for macrophages and dendritic cells leads to reduced cell yield as a result of a fraction of these cells residing on top of the upper 45% layer). For further functional analysis, the targeted cells can be enriched using anti-CD11b and anti-CD11c magnetic-beads and subsequently used for cell sorting. It should be noted that cell yield, viability and surface antigen expression are affected by several factors including the source, type, concentration and enzymatic activity of collagenase, the duration of tissue digestion, the degree of mincing, the temperature of media and the status of inflammation in the intestine26. Based on our experience, the concentration of collagenase is usually in the range of 1–1.5 mg/ml, and optimization of the different factors mentioned above for tissue digestion is important to ensure the highest quality and reproducibility of data. Upon optimizing these parameters, a sufficient cell yield should be achieved, allowing intestinal APCs to be characterized and purified for further functional studies.
3. Phenotype of intestinal APCs
In addition to intestinal APCs, the single cell suspension of intestinal tissue produced after digestion yields epithelial cells, other innate and adaptive immune cells (T, B, NK), sub-mucosal cells, as well as cells that can adhere together (doublets). Therefore, it is important to first exclude these cells in order to properly analyze intestinal APCs by multi-color flow cytometry. After excluding dead cells by using a viability dye (cell viability is typically >85%; data not shown), cells were gated on forward and side scatter, followed by elimination of doublets and exclusion of cells expressing CD3 (T cells), CD19 (B cells) and NK1.1 (NK cells). To exclude non-hematopoietic cell types and further narrow analyses to APCs, a gate was set for CD45+MHCII+ cells (Fig. 1a). Subsequently, this CD45+MHCII+ population can be subdivided into cells expressing high levels of CD11c (CD11c+) or low-intermediate levels (CD11c−). These populations can further be separated using CD103, F4/80, and CD11b into four subsets: CD11b−CD103+ DCs (blue), CD11b+CD103+ DCs (red), CD11c+F4/80+ macrophages (orange), CD11c−F4/80+ macrophages (green; Fig. 1b)27. CD103 is recognized as an excellent marker for intestinal DCs, whereas F4/80 is expressed by mature macrophages. CD11b is considered to be a myeloid marker but is also expressed by NK cells and activated lymphocytes, however these cells are excluded using a “dump” gate as shown in Fig. 1a. Giemsa staining for each of the four FACS sorted cell subsets is shown in Fig. 1c. CD11b−CD103+ DCs (blue) and CD11b+CD103+ DCs (red) displayed classical DC morphology with numerous dendrites extending from the cell surface. On the other hand, the CD11c+F4/80+ (orange) and the CD11c−F4/80+ macrophages (green) displayed typical macrophage morphology characterized by abundant phagocytic vacuoles. Recently several additional markers including CD14, CD26 and CD64 have been used to discriminate intestinal DCs and macrophages28,29. As shown in Fig. 1d, each of the four populations were differentially stained with these markers. CD14 is expressed by macrophages in the mouse intestine and participates in the cellular response to bacterial LPS and Toll-Like Receptor 4 (TLR4) signaling. CD26 has recently been defined as a useful DC marker but it is also found on various types of immune and epithelial cells. CD64 is highly expressed by resident intestinal macrophages and can be used to distinguish them from intestinal DCs. Utilizing this additional marker, Mowat et al. recently described that CD64−CD103−CD11b+CD11c+ cells can be characterized as a population of DCs in the intestine29. Other markers such as CD68, CX3CR1 and CD272 may also be used to identify DCs and macrophages3,4,30. Interestingly, CD11c−F4/80+ macrophages are abundant in the muscularis propria of the intestine8 where they can interact with enteric neurons to regulate gastrointestinal motility31.
Figure 1.
Representative analysis for dendritic cells (DCs) and macrophages in the intestine by multi-color flow cytometry. a) Small intestinal viable cells were gated used forward scatter area (FSC-A) and side scatter area (SSC-A), followed by exclusion of doublet cells using side scatter height versus width (SSC-H vs. SSC-W) and forward scatter height versus width (FSC-H vs. FSC-W). Next, cells expressing CD3, CD19 and/or NK1.1 were excluded before gating on CD45+MHCII+ cells. (b) CD45+MHCII+ cells were subdivided into cells expressing high levels of CD11c (CD11c+) or low-intermediate levels (CD11c−). These populations were further separated using CD103, F4/80, followed by CD11b into four subsets: CD11b-CD103+ DCs (blue gate), CD11b+CD103+ DCs (red gate), CD11c+F4/80+ macrophages (orange gate), CD11c-F4/80+ macrophages (green gate; Fig. 1b). c) Indicated cell populations from b) were FACS-sorted and analyzed using Giemsa stain to detail cell morphology. Panel c) is reproduced with permission from The Journal of Immunology, vol. 187, pp. 733–747, 2011. Copyright 2011. The American Association of Immunologists, Inc. Expression of CD14, CD26 and CD64 by CD11b−CD103+ DCs (blue), CD11b+CD103+ DCs (red), CD11c+F4/80+ macrophages (orange), CD11c−F4/80+ macrophages (green) is shown in d).
In summary, after the proper isolation and purification of intestinal cells, the phenotype of intestinal DCs and macrophages can be analyzed by multi-color flow cytometry. Although intestinal cell suspensions obtained after the purification is a mixture of various cell types, specific populations of different cell types, including DCs and macrophages, can be characterized by eliminating non-hematopoietic cells and by using defined markers listed below.
(Antibodies used: CD45-PerCP-Cy5.5, CD103-PE, CD11c-eFluor 610, MHCII (I-Ab)-FITC, CD11b-eFluor 450, F4/80-PE-Cy7, CD14-APC, CD26-APC, CD64-APC)
4. Modulation of CD4+ T cell responses by intestinal APCs
Intestinal APCs play a key role in regulating host-defense and immune tolerance to the multitude of enteric antigens that occupy and translocate from the lumen. In response to invading pathogens, intestinal APCs orchestrate the innate and adaptive arms of immunity to prevent and clear infections while immunosuppressive and anergic mechanisms are elicited to promote tolerance to antigens derived from food, self, and the commensal microbiota. Macrophages and DCs represent important APCs in the intestine that contribute to maintaining immune homeostasis in part through the regulation of CD4+ T cells. In particular, recent studies from our group and others have elucidated the ability of intestinal macrophages and DCs to induce Foxp3+ regulatory T (Treg) cells and Th17 cells that shape the inflammatory environment of the intestine (Fig. 2)14–16,23,32,33.
Figure 2.
Modulation of CD4+ T cell responses by each subset of intestinal DCs and macrophages. a) FACS-sorted CD11b− lamina propria (LP) dendritic cells (DCs), CD11b+ LP DCs, CD11c+ LP macrophages, and CD11c− LP macrophages were cocultured for 4 days with OT-II CD4+ T cells and OVA and then restimulated for 6 hours with PMA and ionomycin and intracellular production of IL-17A by CD4+ T cells was analyzed by FACS. Numbers next to FACS plots indicate the percentage of IL-17+ cells in each plot. Data from three independent experiments are summarized as means +/− SEM and plotted in the right panel bar graph. b) Th17 cell differentiation by CD11b+CD103+ LP DCs is IL-6 dependent. Assays were performed as in a) in the presence of rat IgG (isotype control) or anti-IL-6 Ab. FACS data are representative of three independent experiments with the means from all of the experiments 6 SEM plotted in the lower panel bar graph. *p < 0.05. c) Foxp3+ Treg cell differentiation by LP DCs and macrophages is ratio dependent. Intracellular staining and flow cytometry to assess Foxp3 expression by naive OT-II CD4+ T cells cocultured with FACS-sorted CD11b− LP DCs, CD11b+ LP DCs, CD11c+ LP macrophages, CD11c− LP macrophages, and OVA in the presence of TGF-β for 4 days. Numbers along the top row indicate the T cell/APC ratio. Numbers next to the boxes indicate the percentage of CD4+Foxp3+ cells in each plot. Data are representative of three independent experiments. This figure is reproduced with permission from The Journal of Immunology, vol. 187, pp. 733–747, 2011. Copyright 2011. The American Association of Immunologists, Inc.
The ability of macrophages and DCs to promote an appropriate balance between these T cell responses occurs through a variety of mechanisms. For example, the presence of the microbiota stimulates macrophages to secrete IL-1β that supports steady-state Th17 cells34 and also promotes the production of Csf2 from group 3 ILCs, which contributes to Foxp3+ Treg cell homeostasis in the intestine35. Moreover, intestinal macrophages secrete IL-10 that facilitates the induction16, maintenance36, and expansion32 of Foxp3+ Treg cells, which function to suppress inflammation and are important in the induction of oral tolerance—a state in which systemic immune responses are dampened to previously orally inoculated antigens. Not only are IL-10 and IL-10 receptor important for the induction of Foxp3+ Treg cells, but recent studies have demonstrated this signaling axis to be important for the differentiation of CX3CR1+ macrophages in the intestine. For example, CX3CR1+ macrophages deficient in IL-10R signaling from Cx3cr1creIl10rafl/fl mice yielded a pro-inflammatory gene signature with elevations in Nos2 and Il23a37. Coinciding with this pro-inflammatory change in macrophages are the development of colitogenic CD4+ T cells and inhibition of Foxp3+ Treg cells. Cx3cr1creIl10rafl/fl mice develop spontaneous colitis, and the transfer of unfractionated CD4+ T cells into Rag2−/−Il10rb−/− mice induced severe colitis that was associated with increased Th1 cell number and impaired Foxp3+ Treg cell generation and suppression in vivo37,38. These observations demonstrate that in the absence of IL-10R signaling, intestinal macrophages fail to develop into CX3CR1+ anti-inflammatory cells, but instead acquire effector functions that promote inflammation and may augment colitogenic CD4+ T cell responses. Similar observations are made in CX3CR1-deficient mice that have a paucity of intestinal macrophages coinciding with decreased Foxp3+ Treg cell proliferation in vivo, a defect in oral tolerance, and exacerbated Th17 cell-driven colitis. Together, both in vivo and in vitro studies suggest CX3CR1+ macrophages to be important in maintaining intestinal immune homeostasis by supporting the development and function of Foxp3+ Treg cells, and helping to establish tolerance to enteric antigens.
Similar to macrophages, the heterogeneous subsets of DCs in the intestine contribute to oral tolerance by regulating the generation of Foxp3+ Treg cells. As the lymphatics from the LP and PP drain into the mLNs, several reports have indicated these secondary lymphoid organs (SLOs) to be a site of intestinal antigen presentation and oral tolerance. The ability of intestinal DCs to migrate to the mLNs appears to be important for the induction of oral tolerance as mice deficient in CCR7 are unable to establish oral tolerance and have a significant reduction of CD103+ DCs in the mLNs, but not in the SI LP39. Furthermore, CD103+ DCs from the SI LP or mLNs are efficient in promoting the differentiation of naïve CD4+ T cells into Foxp3+ Treg cells when cultured in the presence of TGF-β1 and retinoic acid (RA) in vitro14,15. These observations suggest that intestinal DCs may promote immunologic tolerance by carrying enteric antigens to the mLN whereupon they direct the induction of Foxp3+ Treg cells and help to establish oral tolerance.
Intestinal DCs are also necessary for inducing Th17 cells in the LP. In particular, the CD103+CD11b+ DC subset seems to be important in Th17 differentiation16,27 since a specific reduction or loss of this subset is associated with significant reductions of Th17 cells in the SI, LI, and mLNs23. One mechanism by which CD103+CD11b+ DCs may support intestinal Th17 cell development is through their enhanced ability to produce IL-6 in response to microbial signals (Fig. 2a,b). Indeed, a commensal microbe that is unique in its ability to preferentially induce Th17 cells is segmented filamentous bacteria (SFB; Fig. 3)25,40. This SFB-driven Th17 development in the intestine is dependent on MHC class II expression on CD11c+ cells but independent of SLOs—suggesting that intestinal DCs may provide antigenic stimulation of naïve CD4+ T cells directly in situ41–43. However, the contribution of the different DC subsets in regulating SFB-driven Th17 cell differentiation through antigenic stimulation or the secretion of specific factors remains to be elucidated.
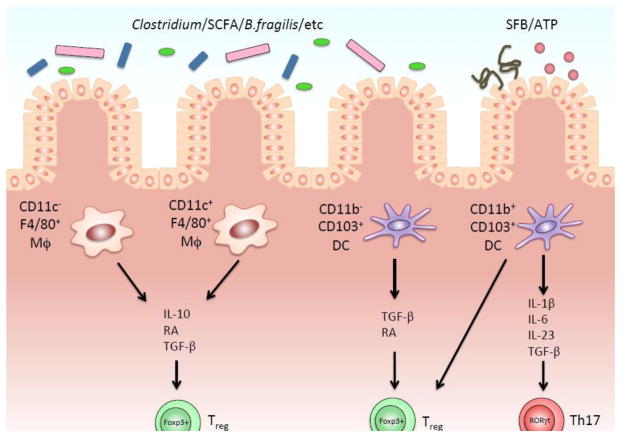
Figure 3.
Interactions between the gut microbiota, the dietary factors and intestinal immune system in each subset of antigen presenting cells of the lamina propria (LP). In response to the signals from microbiota such as Clostridium, Bacterioides fragilis, Segmented Filamentous Bacteria (SFB) or other luminal factors, intestinal dendritic cells (DCs) and macrophages are secreting a variety of cytokines that condition intestinal milieu. CD11b+ CD103+ DCs specifically contributes to induce Th17 differentiation, whereas all of 4 subsets have a potential to maintain Foxp3+ Tregs in LP.
5. Genetic mouse strains used to investigate intestinal APC function
In addition to ex vivo analysis, the use of genetically modified mouse strains has become an invaluable resource for determining the functions of intestinal DCs and macrophages in vivo. Mouse strains with global deletion of specific genes and their product have enabled researchers to investigate the function of different genes and proteins in intestinal APCs and their interaction with other immune cells. More selective transgenic mouse models, including Cre-LoxP systems and diphtheria toxin (DT) receptor (DTR) knock-in mice, have also allowed for gene deletion targeted to specific cell types. In addition to these models, insertion of fluorescent proteins, such as green fluorescent protein (GFP), into genes of interest has permitted the tracking of certain cell types in vivo. Below we describe some of the genetically modified mouse strains that have aided in the elucidation of DC and macrophage function, especially as it pertains to their role in regulating T cell responses in vivo.
Zbtb46
Zbtb46 is a transcription factor that is expressed by classical DCs (cDCs) but not macrophages, monocytes or plasmacytoid DCs (pDCs)44,45, and thus has aided in the development of useful animals models for discriminating the roles of cDCs from macrophages in immune responses in vivo. For example, mice with green fluorescent protein (GFP) under the control of the Zbtb46 promoter (Zbtb46-GFP), have allowed for selective visualization of cDCs. The examination of the intestine in these mice has confirmed that Zbtb46 is expressed only by cDCs and not macrophages in the lamina propria and mesenteric lymph nodes (mLN)45,46. Further studies have utilized mice with DTR inserted under control of the Zbtb46 promoter (Zbtb46-DTR), which allowed for selective depletion of cDCs after administration of diphtheria toxin, and indeed, these mice have reduced numbers of cDCs in the intestinal lamina propria while macrophage numbers are unaffected46. Zbtb46DTR mice have been utilized to demonstrate the requirement of Zbtb46-expressing cDCs in the protection against Citrobacter rodentium infection46. However, to date, the use of these aforementioned mice has not explored the role of Zbtb46-expressing cells in regulating intestinal T cell responses.
Batf3
Basic leucine zipper transcription factor ATF-like 3 (Batf3) is a transcription factor that is required for the development of CD11c+CD8α+ conventional DCs in lymphoid tissues47. This observation was originally reported in mice deficient for Batf3, and it has since been shown that Batf3−/− mice also lack CD103+CD11b− conventional DCs in peripheral organs including the intestine and mesenteric lymph nodes. Importantly, Batf3 deletion has no effect on the CD103+CD11b+ subset of DCs in the intestine and mesenteric lymph nodes. Thus, the use of this mouse strain has been important in determining which CD103+ DCs of the lamina propria and mLNs are important for the induction of Treg responses. Interestingly, the numbers of CD4+Foxp3+ Treg cells in both the lamina propria and mLNs were not affected by Batf3 depletion in mice. There was also no difference in the sensitivity of Batf3−/− and wild-type mice to the dextran sodium sulfate (DSS)-induced colitis, suggesting that CD103+CD11b− DCs are dispensable in tolerogenic Treg cell responses48. Other reports investigating Batf3−/− mice suggest that CD103+CD11b− DCs may be important for cross-presenting antigens to CD8 T-cells and promoting immune responses to different types of pathogens47,49.
Notch2
Notch is an evolutionarily conserved signaling pathway that dictates cell fate based on cues from the surrounding microenvironment. Global Notch depletion leads to embryonic lethality in mice50, however the use of mice with loxP sites floxed around Notch1 or 2 has allowed for deletion of this gene in specific cells types. For example, using mice with cre recombinase expressed under the promoter Vav1 (Vav1-Cre) leads to depletion of Notch1 or 2 in cells of hematopoietic origin including DCs and macrophages. CD11c-Cre mice have also been used for direct depletion of Notch1 or 2 in cells of myeloid origin. Both models have been employed to demonstrate the role of Notch2 signaling in the terminal stage of differentiation for cDCs46,33. Furthermore, in the intestine the loss of Notch2, but not Notch1 in CD11c+ cells resulted in the depletion of CD11b+CD103+ DCs in the lamina propria46,33. The absence of these DCs coincided with a decrease in the number of IL-17-producing CD4(+) T cells in the intestine highlighting the importance of intestinal CD11b+ DC subsets in Th17 cell homeostasis. CD11c-specific Notch2 depletion has also been utilized to demonstrate that CD11b+ cDCs, but not macrophages or CD103+CD11b− cDCs, are important for the defense against enteric pathogens in vivo46.
6. Concluding remarks
In this review, a precise methodology for investigating intestinal APCs as well as the latest findings concerning the interaction between APCs and CD4+ T cells are described. Numerous studies have reported that intestinal APCs are playing a crucial role in both health and disease as an integral part of the mucosal immune system. However, continued advancements in the identification and characterization of steady-state or inflammatory DCs and macrophages in mouse and humans are desired. This efficient method for isolation, purification and analysis of mouse APCs presented in this context should further facilitate ex vivo investigations on these fundamentally important cell populations involved in maintaining intestinal homeostasis.
Highlights.
Proper isolation and purification of mouse intestinal antigen presenting cells, including DCs and macrophages, is key to analyzing the role of these cells in mucosal immunology.
Advanced phenotypic analysis of intestinal antigen presenting cells can be performed by multi-color flow cytometry after isolation and purification of intestinal cells.
Intestinal DCs and macrophages play an important role in modulating CD4+ T-cell responses.
Several genetically engineered mouse strains are currently available to further investigate the function of intestinal DCs and macrophages.
Acknowledgments
We thank Aaron Rae (Emory University Department of Pediatrics and Children’s Healthcare of Atlanta Flow Core) for cell-sorting. This work was supported by grants from National Institutes of Health Grants 1R01DK097256 (to T.L.D.) and 1F30DK097904-03 (to D.G.).
Abbreviations
- APC
antigen presenting cells
- SI
small intestine
- LI
large intestine
- DC
dendritic cell
- Mϕ
macrophage
- LP
lamina propria
- SLOs
secondary lymphoid organs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Bain CC, Mowat AM. Intestinal macrophages - specialised adaptation to a unique environment. Eur J Immunol. 2011;41:2494–8. doi: 10.1002/eji.201141714. [DOI] [PubMed] [Google Scholar]
- 3.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102–17. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun. 2011;3:550–64. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt AM, Mowat AM. Mucosal macrophages and the regulation of immune responses in the intestine. Immunol Lett. 2008;119:22–31. doi: 10.1016/j.imlet.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 8.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–25. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–12. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Viney JL, Mowat AM, O’Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–25. [PubMed] [Google Scholar]
- 11.Hume DA, Robinson AP, MacPherson GG, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983;158:1522–36. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu LM, MacPherson GG. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993;177:1299–307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 14.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 17.Macpherson AJ, Lamarre A. BLySsful interactions between DCs and B cells. Nat Immunol. 2002;3:798–800. doi: 10.1038/ni0902-798. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–5. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 19.Macpherson AJ, Geuking MB, McCoy KD. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology. 2005;115:153–62. doi: 10.1111/j.1365-2567.2005.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pabst O, Bernhardt G. The puzzle of intestinal lamina propria dendritic cells and macrophages. Eur J Immunol. 2010;40:2107–11. doi: 10.1002/eji.201040557. [DOI] [PubMed] [Google Scholar]
- 22.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–69. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geem D, Medina-Contreras O, Kim W, Huang CS, Denning TL. Isolation and characterization of dendritic cells and macrophages from the mouse intestine. J Vis Exp. 2012:e4040. doi: 10.3791/4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R, Karp CL, Pulendran B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J Immunol. 2011;187:733–47. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150–66. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 29.Scott CL, Bain CC, Wright PB, Sichien D, Kotarsky K, Persson EK, Luda K, Guilliams M, Lambrecht BN, Agace WW, Milling SW, Mowat AM. CCR2CD103 intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerovic V, Bain CC, Mowat AM, Milling SW. Intestinal macrophages and dendritic cells: what’s the difference? Trends Immunol. 2014;35:270–7. doi: 10.1016/j.it.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–13. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–46. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, Ivanov II, Liu K, Merad M, Reizis B. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–91. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209:251–8. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–84. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, Brenner O, Krauthgamer R, Varol C, Muller W, Jung S. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–33. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, Mascanfroni ID, Al Adham Z, Lavoie S, Ibourk M, Nguyen DD, Samsom JN, Escher JC, Somech R, Weiss B, Beier R, Conklin LS, Ebens CL, Santos FG, Ferreira AR, Sherlock M, Bhan AK, Muller W, Mora JR, Quintana FJ, Klein C, Muise AM, Horwitz BH, Snapper SB. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–19. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geem D, Medina-Contreras O, McBride M, Newberry RD, Koni PA, Denning TL. Specific microbiota-induced intestinal Th17 differentiation requires MHC class II but not GALT and mesenteric lymph nodes. J Immunol. 2014;193:431–8. doi: 10.4049/jimmunol.1303167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lecuyer E, Rakotobe S, Lengline-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, Reynaud CA, Cerf-Bensussan N, Gaboriau-Routhiau V. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity. 2014;40:608–20. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–65. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–52. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satpathy AT, Briseno CG, Lee JS, Ng D, Manieri NA, Kc W, Wu X, Thomas SR, Lee WL, Turkoz M, McDonald KG, Meredith MM, Song C, Guidos CJ, Newberry RD, Ouyang W, Murphy TL, Stappenbeck TS, Gommerman JL, Nussenzweig MC, Colonna M, Kopan R, Murphy KM. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937–48. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–36. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, Murphy KM. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–59. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709–15. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]