Abstract
ANG II increases fetal blood pressure and stimulates fetal heart growth; however, little is known regarding its direct effects on cardiomyocytes in vivo. We sought to determine whether ANG II stimulates heart growth and cardiomyocyte hypertrophy and/or hyperplasia in utero in the immature fetal heart independent of the effects on cardiac afterload. In twin gestation, fetal sheep at ∼100 days gestation (term 145 days), one fetus received a chronic (6 days) infusion of ANG II alone (50 μg·kg−1·min−1) or ANG II plus nitroprusside (NTP) to attenuate the increase in blood pressure; noninstrumented twins served as controls. ANG II alone, but not ANG II + NTP resulted in a significant increase in heart mass (left and right ventricle + septum, corrected for body weight) compared with controls. ANG II, but not ANG II+NTP, also significantly increased cardiomyocyte area compared with control and increased the percentage of binucleated myocytes. ANG II with or without concomitant infusion of NTP increased cardiac PCNA expression, a marker of proliferation. Steady-state protein expression of terminal mitogen-activated protein kinases, cyclin B1, cyclin E1, and p21 were similar among groups. We conclude that in vivo, ANG II increases fetal cardiac mass via cardiomyocyte hypertrophy, differentiation, and to a lesser extent hyperplasia. The effects of ANG II on hypertrophy appear dependent upon the increase in blood pressure (mechanical load), whereas effects on proliferation are load-independent.
Keywords: heart, cardiomyocyte, hypertrophy, hyperplasia
studies of the fetal sheep heart, long used as a model of human cardiac development, demonstrate marked changes of the myocardium during the last one-third of gestation. In addition to developmental changes in vascularity and myocyte myofibrillar and mitochondrial content, there is a marked increase in the number of cardiomyocytes within the heart, as well as a transition from mononucleated to binucleated (terminally differentiated) myocytes (19, 38). In the sheep heart, the transition from mononucleated to binucleated cells begins around 100 days of gestation (term ∼145 days), such that, at term, ∼70% of cardiomyocytes are binucleated, or terminally differentiated (19). Both humoral and hemodynamic forces influence cardiac growth and cardiomyocyte proliferation and maturation, although mechanisms governing the normal heart growth process are not well understood (6, 10, 16–18, 26, 37). Regulation of this developmental process is essential, given that cardiomyocyte endowment is essentially determined by birth and that postnatal proliferative capacity is limited. Disruption of this process, with resultant underendowment of the heart with myocytes, as may occur with fetal hypoxia or undernutrition, may increase susceptibility to cardiac failure later in life (4, 21, 22). Thus, understanding perinatal influences on heart growth and the biological mechanisms regulating cardiomyocyte proliferation and transition to a terminally differentiated state is important.
In the adult heart, ANG II has direct effects on cardiac function and structure, inducing several growth-promoting genes, protein synthesis, and cell growth (32, 35). In the mature heart, infusion of ANG II stimulates the development of cardiac hypertrophy independently of effects on blood pressure (8, 20), whereas blockade of the renin-angiotensin system (RAS) with a converting-enzyme inhibitor or an ANG II type 1 (AT1)-receptor antagonist attenuates the development of pressure overload-induced hypertrophy and inhibits many of the molecular and cellular adaptations to pressure-overload states (29, 30). The responsiveness of the immature myocardium to ANG II is less well studied. In vitro, ANG II stimulates fetal cardiomyocyte proliferation, but not hypertrophy (39). In vivo, infusion of ANG II results in late-gestation fetal cardiomyocyte proliferation and hypertrophy; however, whether this is a direct or indirect effect, resulting from increased blood pressure and mechanical load is not known (26). Therefore, we sought to determine whether ANG II stimulates heart growth and cardiomyocyte hyperplasia and/or hypertrophy in the immature fetal heart independent of the effects of cardiac load. Fetal sheep were directly infused with ANG II at 100 days of gestation to target the transition from mononuclear to binucleated (terminally differentiated) cardiomyocytes in the fetal heart (19). Coinfusion of the vasodilator sodium nitroprusside (NTP) was utilized to normal blood pressure during ANG II administration. We found that fetal ANG II infusion increased cardiomyocyte binucleation and also resulted in increased fetal heart mass via cardiomyocyte hypertrophy. These effects were attenuated by NTP administration, suggesting mechanical forces are responsible for the effects of ANG II on the fetal heart.
MATERIALS AND METHODS
Ethical approval.
All procedures were performed within the regulations of the Animal Welfare Act and the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals” and were approved by the University of Iowa Animal Care and Use Committee. Time-bred pregnant ewes of mixed Dorset-Suffolk breed with twin fetuses were obtained from a local supplier and acclimated to the laboratory over several days.
Animals and surgical preparation.
Pregnant ewes at 97 days gestation (term 145 days) with twin fetal pregnancies were used for the study (n = 18 ewes). Anesthesia was induced with 12 mg/kg of thiopental sodium (Pentothal Sodium; Abbott Laboratories, Abbott Park, IL) and maintained with a mixture of isoflurane (1–3%), oxygen (30%), and nitrous oxide. Under sterile conditions, the uterus was opened, the fetal head was exteriorized, and the indwelling catheters were placed in the right carotid artery and jugular vein (BB3175-V/5, 0.86-mm internal diameter; Scientific Commodities, Lake Havasu City, AZ). Fetal blood gases were monitored throughout the surgery following catheter placement. A catheter for measurement of amniotic pressure was secured to the fetal skin, and the uterus was closed in two layers. Control fetuses were not instrumented to avoid a second uterine incision and inadvertent manipulation of the first fetus that would increase the risk for loss of the surgical preparation. Maternal flank incisions were closed in separate layers, and all catheters were exteriorized through a subcutaneous tunnel and placed in a cloth pouch on the ewe's flank. Ampicillin sodium (Wyeth Laboratories, Philadelphia, PA) was administered intra-amniotically at the completion of surgery (2 g) and to the ewe prior to surgery (2 g) and daily for 3 days. Pregnant ewes were returned to individual pens and allowed free access to food and water. Butorphanol (0.1 mg/kg iv; Torbugesic; Fort Dodge Animal Health, Fort Dodge, IA) was given for 24 h postoperatively for analgesia. Animals were allowed 24 h after surgical preparation before physiological measurements began.
Experimental protocol.
Physiological measurements were taken 24 h after surgical preparation. Ewes were confined to stanchions each day for recording of fetal physiological measurements and were afforded free access to food and water. Fetal pressures were recorded with Transpac pressure transducers (Abbott) on a calibrated computerized system (MacLab, ADInstruments, Colorado Springs, CO; Apple, Cupertino, CA). Fetal arterial pressures were referred to amniotic fluid pressure and reported as arithmetic mean from computer tracings. Fetal heart rate was monitored with a cardiotachometer triggered from the arterial pressure wave. Arterial blood samples were obtained for determination of blood gases and pH (Gem Premier 3000, Instrumentation Laboratory, Bedford MA). After the initial fetal monitoring period (day 0), catheterized fetuses received either a continuous infusion of ANG II (50 μg·kg−1·min−1; Sigma-Aldrich, St. Louis, MO) for 6 days or a coinfusion of ANG II (50 μg·kg−1·min−1) and NTP (Sigma-Aldrich, starting dose of 5 μg·kg−1·min−1) using a battery-operated Pegasus VARIO micropiston pump (Instech Lab, Plymouth Meeting, PA). Physiological measurements were obtained daily for 6 days. The dose of NTP was adjusted daily to maintain MABP at day 0 values. Nitroprusside doses ranged from 5 to 15 μg·kg−1·min−1 over the duration of the study.
Tissue collection.
On the 7th postoperative day, ewes were again anesthetized with general anesthesia, the fetuses were exteriorized, and administered heparin (5,000 U iv) and saturated potassium chloride (10 ml iv) to arrest their hearts in diastole. Fetuses were weighed, and their hearts were removed. In a subset of fetuses (n = 5 for each group), the hearts were dissected into anatomical components, and each component was weighed and immediately frozen in liquid nitrogen. For the remaining fetuses (n = 4 for each group), fetal hearts were enzymatically dissociated on a Langendorff apparatus, and the cardiomyocytes were fixed for morphometric analysis, as previously described (19). Ewes were euthanized by an intravenous administration of pentobarbital sodium and phenytoin sodium (120 mg/kg barbiturate, euthasol solution; Virbac, Fort Worth, TX).
Cardiac dissociation and cardiomyocyte analysis.
For determination of ploidy, cells were stained with hematoxylin and eosin, and no fewer than 300 myocytes from each ventricle of each fetus were analyzed at ×40 magnification on a light microscope (Zeiss Axiophot, Bartels and Stout, Bellevue, WA) to determine the number of nuclei per cardiomyocyte. For cell morphology, long-axis (length) and maximal cross-sectional diameter (width) dimensions and area of cardiomyocytes were measured, as previously described (26). Fixed myocytes were prepared in a wet mount with methylene blue and were selected for measurement, according to a random, nonrepeating, and unbiased method employing a counting frame. Myocytes were photographed at ×40, and photomicrographs were immediately analyzed using calibrated Optimas software (Optimas, Seattle, WA) and ImageJ software (http://rsbweb.nih.gov/ij/). At least 100 mononucleated cells were measured per ventricle per fetus. Because of the paucity of binucleated cells, cardiomyocyte morphometry was unable to be reliably performed on this cell population.
Quantitative immunoblot.
Immunoblots were performed as described previously to quantify protein expression in left ventricle tissues (27). Cardiac samples were homogenized and then sonicated in a buffer containing soybean trypsin inhibitor, leupeptin, and PMSF in 50 mM Tris, 10 mM EDTA, 150 mM NaCl, and 0.1% mercaptoethanol. Cellular debris was removed by centrifugation, and samples were quantified spectrophotometrically. Protein was separated by SDS-PAGE and transferred to nitrocellulose membranes. These were blocked for 1 h in 5% nonfat milk, and then incubated with primary antibodies overnight at 5°C. Bound antibody was detected by incubation with infrared-labeled secondary antibodies (IRDye 800 or IRDye 700 700DX, Li-Cor Biotechnology, Lincoln, NE). Blots were read and quantified on a Li-Cor Odyssey Imaging System (Li-Cor Biotechnology). All immunoblots were performed in duplicate with the results for each sample being averaged. Primary antibodies included antibodies from Santa Cruz Biotechnology (Santa Cruz, CA) specific to total ERK1/2 (sc-93), phosphorylated ERK1/2 (sc-7383), total JNK1/2 (sc-1648), phosphorylated JNK1/2 (sc-6254), from Cell Signaling Technology (Beverly, MA) specific to AKT (9272), phosphorylated AKT (9275), PCNA (2586), cyclin B1 (4135), cyclin E (4129), and p21 (2978).
Data analysis.
All values are presented as means ± SE. Statistical comparisons were performed by Student's unpaired, two-tailed t-test or ANOVA with a post hoc Tukey test when the F statistic was found to be significant. A value of P < 0.05 was considered significant.
RESULTS
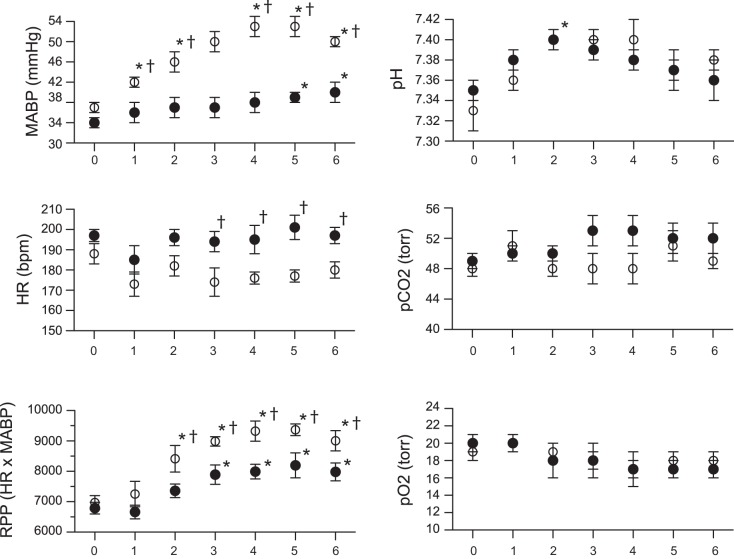
A total of 36 fetuses from 18 pregnant ewes were studied, with nine fetuses receiving a continuous infusion of ANG II, nine fetuses receiving a coinfusion of ANG II and NTP, and 18 fetuses serving as twin-matched controls. Infusion of ANG II produced a significant increase in fetal mean arterial pressure from 37 ± 1 on day 0 to 50 ± 1 mmHg on day 6 (Fig. 1). No significant change in heart rate was identified over this period of time. Because we did not catheterize control fetuses, hemodynamic data are not available for comparison. However, other investigators have reported fetal baseline blood pressure of 37–38 mmHg and heart rates of 182–191 beats per minute (bpm) at a similar gestational age (2, 41). Coadministration of NTP with ANG II was also accompanied by an increase in blood pressure, although significantly less than with ANG II alone (Fig. 1). No change in heart rate was detected over time during administration of NTP and ANG II, although heart rate was significantly higher in this group compared with ANG II-alone fetuses. Rate pressure product (HR × mean arterial blood pressure), a measure of workload on the myocardium, increased above day 0 values in ANG II and ANG II + NTP animals, although the increase was significantly greater in ANG II-infused fetuses (Fig. 1). Arterial pH and blood gas values remained relatively stable in both groups during the duration of the study.
Fig. 1.
Hemodynamic and arterial blood gas values from fetal sheep infused with ANG II (50 μg/kg) or ANG II plus sodium NTP, with the dose adjusted daily to attenuate changes in blood pressure. Day 0 values obtained prior to starting infusions. MABP, mean arterial blood pressure; HR, heart rate: RPP, rate pressure product (HR × MABP). *P < 0.05 compared with day 0 value in same group. †P < 0.05 ANG II group compared with ANG II + NTP group. Identical values between groups result in appearance of missing data for selected time points. ○, ANG II; ●, ANG II + NTP.
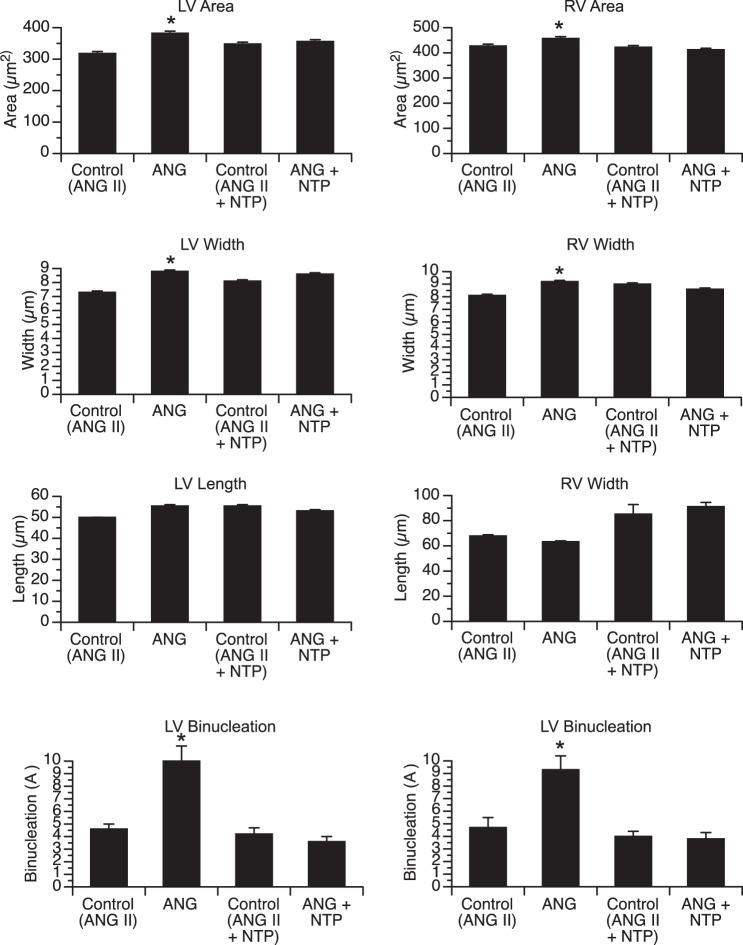
Fetal weight was similar among groups, while absolute ventricular weight [left ventricle (LV) free wall + right ventricle (RV) free wall + septum] and ventricle-to-body weight ratio were significantly increased in ANG II-infused animals compared with twin controls (Table 1). ANG II resulted in increased LV and RV mass, as evidenced by the increase in LV/body weight and RV/body weight ratios. In contrast, fetuses receiving a coinfusion of ANG II + NTP demonstrated no increase in LV, RV, or total ventricle weight compared with control twins. Left and right ventricular cardiomyocyte area and width were significantly increased in ANG II-infused fetuses compared with twin controls, alhough no difference in cardiomyocyte length was identified (Fig. 2). There was no statistically significant difference in left or right ventricular cardiomyocyte dimensions between ANG II + NTP-infused fetuses and twin controls. The percentage of binucleated cardiomyocytes (a marker of terminal differentiation) was significantly increased in the LV and RV of ANG II-infused fetuses compared with controls, but not significantly affected in ANG II + NTP-infused animals (Fig. 2).
Table 1.
Effects of ANG II and ANG II + NTP on cardiac mass
| ANG II | Control (ANG II) | ANG II + NTP | Control (ANG II + NTP) | |
|---|---|---|---|---|
| Gestational age, days | 105 ± 1 | 105 ± 1 | 104 ± 1 | 104 ± 1 |
| Fetal weight (FW), g | 1289 ± 61 | 1409 ± 60 | 1455 ± 73 | 1450 ± 58 |
| Ventricle mass, g | 9.34 ± 0.81 | 7.23 ± 0.62 | 8.71 ± 0.71 | 7.96 ± 0.64 |
| Ventricle mass/FW, g/kg | 7.26 ± 0.21* | 5.27 ± 0.20 | 6.24 ± 0.15 | 5.67 ± 0.18 |
| LV mass/FW, g/kg | 2.84 ± 0.18* | 2.03 ± 0.10 | 2.58 ± 0.13 | 2.36 ± 0.10 |
| RV mass/FW, g/kg | 3.32 ± 0.06* | 2.48 ± 0.16 | 2.78 ± 0.07 | 2.49 ± 0.10 |
Ventricle mass = left ventricle (LV) + right ventricle (RV) + septum weight.
P < 0.05, compared with paired controls. Paired controls are unoperated twins.
Fig. 2.
Cardiomyocyte dimensions and nucleation in fetal sheep infused with ANG II (50 μg/kg) or ANG II + NTP, with the dose adjusted daily to attenuate changes in blood pressure and twin-paired controls [Control (ANG II), Control (ANG II +NTP)]. Comparisons made between treatment group and respective paired controls. *P < 0.05 compared with paired control group.
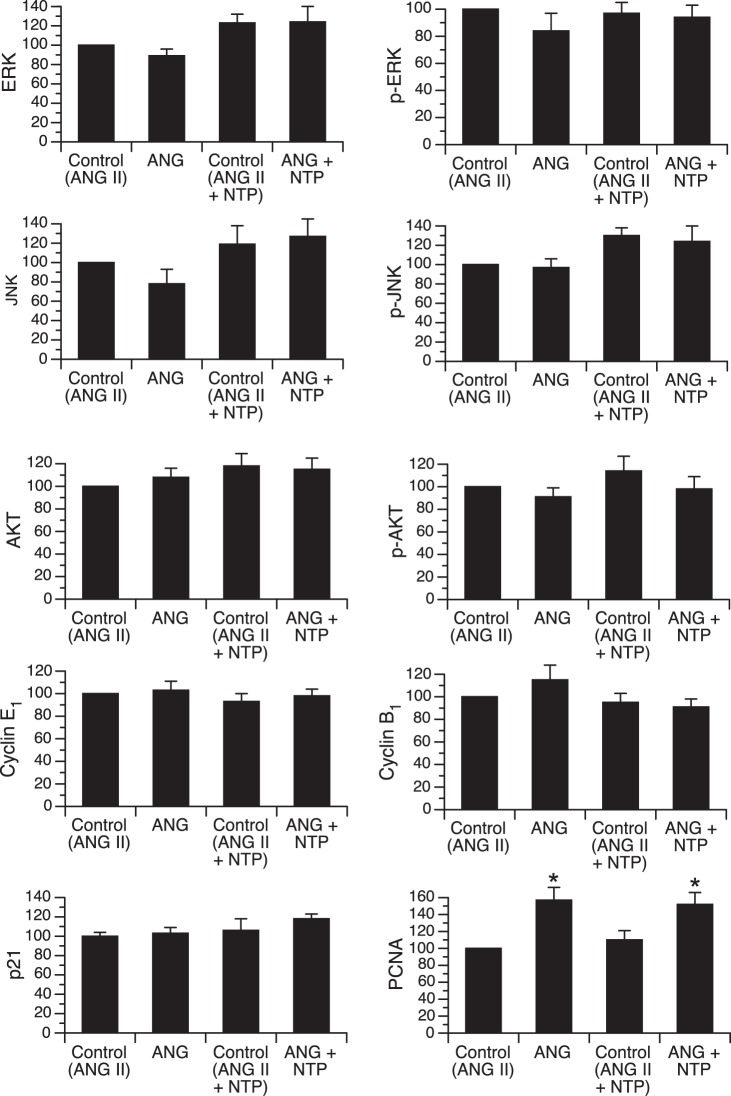
To determine whether MAPK or PI3K/AKT pathways are activated in the early-gestation fetal heart in response to increased systolic load, resulting from prolonged ANG II infusion, we measured the steady-state protein levels of total and phosphorylated (activated) ERK, JNK, and AKT in left ventricular myocardium (Fig. 3). No significant differences in the level of any of these proteins were identified among groups. Levels of cell cycle regulator proteins cyclin E1 and B1 and the cyclin-dependent kinase inhibitor p21 were also unchanged by infusion of either ANG II alone or ANG II + NTP. However, PCNA, a marker of cell proliferation, was significantly increased by ANG II infusion in the absence and presence of NTP coinfusion.
Fig. 3.
Myocardial steady-state protein levels of total and phosphorylated ERK, JNK, AKT, cyclin E1, cyclin B1, p21, and PCNA in fetal sheep infused with ANG II (50 μg/kg) or ANG II plus sodium NTP, with the dose adjusted daily to attenuate changes in blood pressure and twin-paired controls [Control (ANG II); Control (ANG II +NTP)]. Comparisons made between treatment group and respective paired controls. All values were normalized to controls for ANG II group (defined at 100). *P < 0.05 compared with paired control group.
DISCUSSION
In previous studies in late-gestation fetal sheep, we demonstrated that a 5–7-day continuous infusion of ANG II, which significantly increased fetal mean blood pressure by 15–20 mmHg, resulted in increased cardiac mass via cardiomyocyte hypertrophy and proliferation (26, 36). However, in these studies, we were unable to differentiate the mechanisms by which ANG II affects cardiomyocytes. More specifically, we could not separate the direct effects of activation of cardiac ANG receptors from indirect effects on the heart by changes in cardiac mechanical load. Furthermore, the studies were done at a developmental time point when the fetal heart is composed of both mononucleated cardiomyocytes, capable of proliferation, hypertrophy, and terminal differentiation and binucleated cardiomyocytes, which are already terminally differentiated and only capable of hypertrophy. In the present study, we similarly found that ANG II infused in fetal sheep at a developmental stage when the heart is almost entirely composed of mononucleated cardiomyocytes increased fetal cardiac mass, cardiomyocyte area, and proliferation. However, in fetuses receiving a coinfusion of NTP to attenuate the ANG II-mediated increase in blood pressure, cardiac mass and cardiomyocyte area were similar to control fetuses, although expression of PCNA, a marker of proliferation remained elevated. These findings suggest that increases in fetal cardiac mass and cardiomyocyte area resulting from exogenous ANG II are dependent upon increased cardiac load and not a direct effect of ANG II upon cardiomyocytes. On the other hand, cardiomyocyte proliferation may result from a direct effect of ANG II upon the heart.
Previous studies have found that fetal hypertension or increases in cardiac mechanical load results in cardiac growth (16–18, 26, 37). Banding of the late-gestation fetal sheep pulmonary artery, which increased mean pulmonary artery pressure by ∼24 mmHg for 10 days resulted in right-ventricle myocyte enlargement and increased percentage of binucleated cells, consistent with cardiomyocyte hypertrophy and maturation (1). Banding of the proximal aorta immediately distal to the ductus arteriosus in late-gestation fetal sheep similarly led to an increase in cardiac mass (28). Preductal banding of the aortic arch in midgestation fetal sheep, which resulted over time in obstruction of the isthmic aorta, produced an increased left ventricular mass 27–37 days after surgery (33). These authors concluded that a biphasic response was present, with initial cardiomyocyte hypertrophy (<30 days following banding) followed by hyperplasia (>30 days following banding). Their conclusions regarding hyperplasia stem from the increase in cardiac mass in the absence of evidence of increased cardiomyocyte size. Markers of proliferation were either similar among groups (PCNA expression) or not identified (Ki67 antigen). Induction of hypertension in late-gestation fetal sheep by daily plasma infusion also resulted in significantly increased heart weights and identified two phases of cardiomyocyte growth and maturation (16). After 4 days of hypertension, cell cycle activity and cardiomyocyte area increased, whereas after 8 days, a marked increase in cellular binucleation was also observed, along with increased cell cycle activity and hypertrophy. Interestingly, reduced systolic pressure load resulting from angiotensin-converting enzyme inhibition slowed fetal heart growth by decreasing cardiomyocyte hyperplasia. Taken together, these studies demonstrate the responsiveness of the fetal heart to prevailing blood pressure and cardiac mechanical load and suggest that the developmentally regulated changes in systemic vasoregulatory tone (40) may, in part, modulate physiological cardiac growth and maturation.
Study of the direct effects of ANG II on isolated cardiomyocytes from fetal, neonatal, and adult hearts reveals clear developmental differences (34). In embryonic chick cardiomyocytes, ANG II acts through classical signal transduction pathways of G protein-coupled receptor to stimulate hypertrophy (11). In contrast, in neonatal rodent cardiomyocytes, ANG II-mediated hypertrophic effects are dependent upon stimulation of NADPH oxidase and activation of p38 MAPK, in addition to activation of ERK via a PKC-dependent pathways (3, 12, 25). In isolated fetal sheep cardiomyocytes, 48-h exposure to ANG II stimulates hyperplastic, but not hypertrophic, growth via an ERK-dependent mechanism (39). We similarly found that, in vivo, ANG II stimulates fetal cardiomyocyte proliferation, as determined by increased PCNA expression, independent of the effects of systemic hemodynamics although ERK was not activated (see below). On the other hand, ANG II-induced increased cardiac mass and cardiomyocyte hypertrophy and terminal differentiation appeared to result from an indirect effect via cardiac mechanical load.
Despite a growing understanding of the pathological changes that occur in the fetal heart with alterations in load, the molecular mechanisms regulating these changes remain poorly defined. We recently utilized gene expression arrays to explore changes in the late-gestation fetal cardiac transcriptome associated with ANG II-induced increased cardiac mass (26). Chief among the biological pathways identified as highly impacted by fetal RAS modulation are those involved in cytoskeletal remodeling, transcriptional regulation, and cell cycle activity. However, the ability to discern biological pathways regulating cardiomyocyte hyperplasia from those of hypertrophy was limited by the mixed population (mononucleated and binucleated) of cardiomyocytes present in the fetal heart during late gestation. The present study allowed us to examine the response to a hypertrophic/hyperplastic stimuli in a more homogeneous population of cardiomyoctyes. Although a transcriptome approach was not taken, we did explore MAPK and AKT signaling pathways, which have been implicated in regulating cardiac modeling in a variety of models and studies. No differences in the expression of terminal MAPK proteins or activated AKT among groups were identified. However, we recognize our study measured this outcome at a single time point and did not allow for determination of earlier and perhaps transient changes in expression of signaling proteins.
We chose NTP infusion as an approach to attenuate the increase in blood pressure caused by ANG II administration because of the need to carefully titrate fetal hemodynamics and because of limited vasodilator alternatives. Specifically, we opted against adrenergic receptor antagonists because of known direct effects on the myocardium (14). Nitroprusside functions as a vasodilator by releasing nitric oxide (NO) and has a short half-life, allowing dose adjustment to modulate blood pressure. The majority of the effects of NO are mediated through stimulation of soluble guanylate cyclase, with resultant increases in cGMP. cGMP then exerts its physiological actions via a number of cGMP-regulated pathways, many of which are present in cardiomyocytes, in addition to vascular smooth muscle (13). Endogenous nitric oxide appears to exert antihypertrophic effects, as overexpresson of myocardial endothelial NOS (eNOS) attenuated the development of pressure overload hypertrophy in adult rats, whereas inhibition of eNOS-derived NO formation induced cell growth and p38 MAPK activation (42). eNOS-deficient mice with chronic pressure overload resulting from aortic constriction exhibit enhanced LV hypertrophy compared with wild-type, while restoration of eNOS protein and activity in cardiomyocytes of eNOS-deficient mice attenuated LV hypertrophy (5, 31). Finally, supplementation of l-arginine, the substrate for NOS, reduced LV hypertrophy in spontaneously hypertensive rats, and rats treated with the β-agonist isoprenaline (23). However, whether exogenous NO (such as that derived from sodium NTP infusion) has a direct effect on cardiomyocytes, is unclear. Endogenous NO production in cardiomyocytes is highly compartmentalized (24) as a result of NOS localization within the same subcellular component as soluble guanylate cyclase. Because of the high myoglobin content of cardiomyocytes, which acts as a scavenger of NO, NO diffusion distances within myocytes are likely limited (9, 13). Thus, the direct impact of sodium NTP on cardiomyocytes in vivo has not been fully elucidated. Additionally, desensitization to NO donors, as determined by guanylate cyclase activity, occurs in rat cardiomyocytes following prolonged (24 h) exposure to low-dose NTP (7). Studies are required to determine whether the prolonged administration of NTP has a direct effect of myocardial remodeling.
Workload on the myocardium, as measured by rate pressure product (RPP), increased in both groups of ANG II-infused fetuses, although to a far lesser extent in animals coinfused with NTP. It is possible that more distinct morphological and molecular differences between the ANG II and ANG II+NTP group would exist if the increase in RPP in the ANG II + NTP fetuses could have been avoided. The increase in RPP in ANG II + NTP fetuses relates primarily to the significantly greater heart rate in this group. Reasons for this relative tachycardia are unclear, as MABP also increased slightly in these animals, although the latter may be related to effects of ANG II on baroreflex function (15).
Because of normal maturational changes in gene expression and signaling pathways and the different populations of cardiomyocytes in immature and mature fetuses, our findings regarding the effects on ANG II on the heart may not be applicable at other time points. We should also acknowledge additional limitations of the study include possible toxic effects of cyanide and thiocyanate, products of sodium nitroprusside metabolism. Cyanide may limit oxygen utilization by inhibiting cytochrome-c oxidase, although no metabolic acidosis was seen in fetuses administered nitroprusside. Thiocyanate toxicity typically results in constitutional symptoms, which we could not assess. It is likely that cyanide and thiocyanate are cleared, to a certain extent, across the placenta into the maternal circulation as transplacental passage of metabolites is evident when sodium nitroprusside is administered to the mother.
In summary, our studies indicate that ANG II indirectly induces cardiac growth, cardiomyocyte hypertrophy, and maturation in early-gestation fetal sheep primarily through load-dependent mechanisms. ANG II also has a direct effect on cardiomyocyte proliferation, although the degree of proliferation is not sufficient to significantly increase cardiac mass. Additional studies of the developing myocardium are necessary to delineate the signaling pathways regulating physiological and pathological cardiomyocyte hypertrophy and proliferation and to further understand cardiac health implications of altered myocyte endowment.
Perspectives and Significance
We have studied the physiological mechanisms by which ANG II increases fetal sheep heart mass. We observed that ANG II infusion stimulates cardiomyocytes hypertrophy and promotes binucleation (terminal differentiation) primarily through effects on increasing mechanical load, whereas effects on proliferation are independent of hemodynamics. Elucidation of pathological processes affecting the fetal heart is necessary to identify and ultimately ameliorate risks for cardiac disease later in life.
GRANTS
This work supported by National Institutes of Health Grant HL-080657 (to J. L. Segar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.S., T.D.S., and J.L.S. conception and design of research; J.S. and J.L.S. performed experiments; J.S., T.D.S., and J.L.S. analyzed data; J.S. and J.L.S. interpreted results of experiments; J.S. and J.L.S. drafted manuscript; J.S., T.D.S., and J.L.S. approved final version of manuscript; T.D.S. and J.L.S. edited and revised manuscript; J.L.S. prepared figures.
REFERENCES
- 1.Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ, Thornburg KL. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol 279: R1157–R1164, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Booth LC, Malpas SC, Barrett CJ, Guild SJ, Gunn AJ, Bennet L. Is baroreflex control of sympathetic activity and heart rate active in the preterm fetal sheep? Am J Physiol Regul Integr Comp Physiol 296: R603–R609, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Booz GW, Day JN, Baker KM. Angiotensin II effects on STAT3 phosphorylation in cardiomyocytes: evidence for Erk-dependent Tyr-705 dephosphorylation. Basic Res Cardiol 98: 33–38, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Botting KJ, McMillen IC, Forbes H, Nyengaard JR, Morrison JL. Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. J Am Heart Assoc 3: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buys ES, Raher MJ, Blake SL, Neilan TG, Graveline AR, Passeri JJ, Llano M, Perez-Sanz TM, Ichinose F, Janssens S, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Cardiomyocyte-restricted restoration of nitric oxide synthase 3 attenuates left ventricular remodeling after chronic pressure overload. Am J Physiol Heart Circ Physiol 293: H620–H627, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL, Thornburg KL. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J 26: 397–408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis JP, Vo XT, Sulakhe PV. Altered responsiveness of guanylyl cyclase to nitric oxide following treatment of cardiomyocytes with S-nitroso-d,l-acetylpenicillamine and sodium nitroprusside. Biochem Biophys Res Commun 238: 351–356, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Dostal DE, Baker KM. Angiotensin II stimulation of left ventricular hypertrophy in adult rat heart. Am J Hypertens 5: 276–280, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Flogel U, Merx MW, Godecke A, Decking UK, Schrader J. Myoglobin: A scavenger of bioactive NO. Proc Natl Acad Sci USA 98: 735–740, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology 147: 3643–3649, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Goutsouliak V, Rabkin SW. Angiotensin II-induced inositol phosphate generation is mediated through tyrosine kinase pathways in cardiomyocytes. Cell Signal 9: 505–512, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, Shah AM. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol 47: 817–826, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Hammond J, Balligand JL. Nitric oxide synthase and cyclic GMP signaling in cardiac myocytes: from contractility to remodeling. J Mol Cell Cardiol 52: 330–340, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Hata JA, Williams ML, Koch WJ. Genetic manipulation of myocardial β-adrenergic receptor activation and desensitization. J Mol Cell Cardiol 37: 11–21, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Ismay MJ, Lumbers ER, Stevens AD. The action of angiotensin II on the baroreflex response of the conscious ewe and the conscious fetus. J Physiol 288: 467–479, 1979. [PMC free article] [PubMed] [Google Scholar]
- 16.Jonker SS, Faber JJ, Anderson DF, Thornburg KL, Louey S, Giraud GD. Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. Am J Physiol Regul Integr Comp Physiol 292: R913–R919, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Jonker SS, Scholz TD, Segar JL. The effect of adrenalectomy on the cardiac response to subacute fetal anemia. Can J Physiol Pharmacol 89: 79–88, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonker SS, Scholz TD, Segar JL. Transfusion effects on cardiomyocyte growth and proliferation in fetal sheep after chronic anemia. Pediatr Res 69: 485–490, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol 102: 1130–1142, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Ohta K, Hamaguchi A, Yukimura T, Miura K, Iwao H. Angiotensin II induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension 25: 1252–1259, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol 286: H1712–H1719, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig 10: 265–274, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Lin Y, Wang LN, Xi YH, Li HZ, Xiao FG, Zhao YJ, Tian Y, Yang BF, Xu CQ. l-arginine inhibits isoproterenol-induced cardiac hypertrophy through nitric oxide and polyamine pathways. Basic Clin Pharmacol Toxicol 103: 124–130, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Loyer X, Heymes C, Samuel JL. Constitutive nitric oxide synthases in the heart from hypertrophy to failure. Clin Exp Pharmacol Physiol 35: 483–488, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Nishida M, Tanabe S, Maruyama Y, Mangmool S, Urayama K, Nagamatsu Y, Takagahara S, Turner JH, Kozasa T, Kobayashi H, Sato Y, Kawanishi T, Inoue R, Nagao T, Kurose H. G alpha 12/13- and reactive oxygen species-dependent activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase by angiotensin receptor stimulation in rat neonatal cardiomyocytes. J Biol Chem 280: 18, 434–18, 441, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Norris AW, Bahr TM, Scholz TD, Peterson ES, Volk KA, Segar JL. Angiotensin II-induced cardiovascular load regulates cardiac remodeling and related gene expression in late-gestation fetal sheep. Pediatr Res 75: 689–696, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson AK, Protheroe KN, Scholz TD, Segar JL. The mitogen-activated protein kinases and Akt are developmentally regulated in the chronically anemic fetal sheep heart. J Soc Gynecol Investig 13: 157–165, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Olson AK, Protheroe KN, Scholz TD, Segar JL. The mitogen-activated protein kinases and Akt are developmentally regulated in the chronically anemic fetal sheep heart. J Soc Gynecol Invest 13: 157–165, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Pan J, Fukuda K, Kodama H, Makino S, Takahashi T, Sano M, Hori S, Ogawa S. Role of angiotensin II in activation of the JAK/STAT pathway induced by acute pressure overload in the rat heart. Circ Res 81: 611–617, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Rockman HA, Wachhorst SP, Mao L, Ross JJ. ANG II receptor blockade prevents ventricular hypertrophy and ANF gene expression with pressure overload in mice. Am J Physiol Heart Circ Physiol 266: H2468–H2475, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Ruetten H, Dimmeler S, Gehring D, Ihling C, Zeiher AM. Concentric left ventricular remodeling in endothelial nitric oxide synthase knockout mice by chronic pressure overload. Cardiovasc Res 66: 444–453, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Sadoshima JI, Izumo S. Signal transduction pathways of angiotensin II-induced c-fos gene expression in cardiac myocytes in vitro. Circ Res 73: 424–438, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Samson F, Bonnet N, Heimburger M, Rucker-Martin C, Levitsky DO, Mazmanian GM, Mercadier JJ, Serraf A. Left ventricular alterations in a model of fetal left ventricular overload. Pediatr Res 48: 43–49, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Schluter KD, Wenzel S. Angiotensin II: a hormone involved in and contributing to pro-hypertrophic cardiac networks and target of anti-hypertrophic cross-talks. Pharmacol Ther 119: 311–325, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Schunkert H, Sadoshima J, Cornelius T, Kagaya Y, Weinberg EO, Izumo S, Riegger G, Lorell BH. Angiotensin II-induced growth responses in isolated adult rat hearts. Evidence for load-independent induction of cardiac protein synthesis by angiotensin II. Circ Res 76: 489–497, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Segar JL, Dalshaug GB, Bedell KA, Smith OM, Scholz TD. Angiotensin II in cardiac pressure-overload hypertrophy in fetal sheep. Am J Physiol Regul Integr Comp Physiol 281: R2037–R2047, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Segar JL, Volk KA, Lipman MH, Scholz TD. Thyroid hormone is required for growth adaptation to pressure load in the ovine fetal heart. Exp Physiol 98: 722–733, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smolich JJ, Walker AM, Campbell GR, Adamson TM. Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am J Physiol Heart Circ Physiol 257: H1–H9, 1989. [DOI] [PubMed] [Google Scholar]
- 39.Sundgren NC, Giraud GD, Stork PJ, Maylie JG, Thornburg KL. Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J Physiol 548: 881–891, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unno N, Wong CH, Jenkins SL, Wentworth RA, Ding XY, Li C, Robertson SS, Smotherman WP, Nathanielsz PW. Blood pressure and heart rate in the ovine fetus: ontogenic changes and effects of fetal adrenalectomy. Am J Physiol Heart Circ Physiol 276: H248–H256, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Wassink G, Bennet L, Booth LC, Jensen EC, Wibbens B, Dean JM, Gunn AJ. The ontogeny of hemodynamic responses to prolonged umbilical cord occlusion in fetal sheep. J Appl Physiol (1985) 103: 1311–1317, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel S, Rohde C, Wingerning S, Roth J, Kojda G, Schluter KD. Lack of endothelial nitric oxide synthase-derived nitric oxide formation favors hypertrophy in adult ventricular cardiomyocytes. Hypertension 49: 193–200, 2007. [DOI] [PubMed] [Google Scholar]