Abstract
Oxidative stress and inflammation are risk factors for hypertension in pregnancy. Here, we examined the 24-h mean arterial pressure (MAP) via telemetry and the nitric oxide (NO) and redox systems in the kidney cortex, medulla, and aorta of virgin and pregnant rats treated with a high-fat/prooxidant Western diet (HFD), ANG II, and TNF-α. Female Sprague-Dawley rats were given a normal diet (ND) or a HFD for 8 wk before mating. Day 6 of pregnancy and age-matched virgins were implanted with minipumps infusing saline or ANG II (150 ng·kg−1·min−1) + TNF-α (75 ng/day) for 14 days. Groups consisted of Virgin + ND + Saline (V+ND) (n = 7), Virgin + HFD +ANG II and TNF-α (V+HFD) (n = 7), Pregnant + ND + Saline (P+ND) (n = 6), and Pregnant + HFD + ANG II and TNF-α (P+HFD) (n = 8). After day 6 of minipump implantation, V+HFD rats displayed an increase in MAP on days 7, 8, and 10–15 vs. V+ND rats. P+HFD rats, after day 6 of minipump implantation, showed an increase in MAP only on day 7 vs. P+ND rats. P+HFD rats had a normal fall in 24-h MAP, hematocrit, plasma protein concentration, and osmolality at late pregnancy. No change in kidney cortex, medulla, or aortic oxidative stress in P+HFD rats. P+HFD rats displayed a decrease in nNOSβ abundance, but no change in kidney cortex NOx content vs. P+ND rats. Pregnant rats subjected to a chronic HFD and prooxidant and proinflammatory insults have a blunted increase in 24-h MAP and renal oxidative stress. Our data suggest renal NO bioavailability is not altered in pregnant rats treated with a HFD, ANG II, and TNF-α.
Keywords: nitric oxide, Western diet, oxidative stress, ANG II, tumor necrosis factor-α
normal pregnancy is a state of chronic vasodilation with elevated nitric oxide (NO) production but also increased systemic oxidative stress (2, 4, 8, 12, 13, 16, 20, 37, 42, 44). In contrast, the renal cortex displays enhanced total antioxidant capacity at midterm (12) which, we suggest, enables increased renal NO to vasodilate the kidney, despite increased renal metabolism and reactive oxygen species generation due to renal sodium retention. This combination of increased renal sodium retention and increased glomerular filtration rate (GFR) is necessary for plasma volume expansion and an increased GFR (for removal of excess metabolic waste). In late pregnancy, the renal cortical total antioxidant capacity declines to virgin levels (12), which may account for the partial restoration of GFR to nonpregnant values (3, 10). Nevertheless, the renal cortical total antioxidant capacity is never reduced below nonpregnant values during normal pregnancy, thus protecting the kidney from the increased oxidative stress seen in the systemic circulation during normal pregnancy.
In preeclamptic pregnancy the delicate balance between oxidant and antioxidant pathways is switched further toward a prooxidant state, resulting in impaired vasodilation of the systemic and renal circulations, as well as impaired renal sodium retention, leading to the lack of plasma volume expansion (38, 47). Preeclamptic pregnancies are characterized by increased markers and mediators of oxidative stress and inflammation (20, 27, 31, 36, 37, 49, 41, 46, 50) and, in Western societies, are common in women of low socioeconomic status and who are overweight/obese (22, 29, 30, 49). In this study, we attempted to create a model of diet-induced systemic and renal oxidative stress by feeding a high-fat, high-refined carbohydrate diet depleted of antioxidants, which resembles the “fast food” or cafeteria-style Western diet. In addition, we administered TNF-α and ANG II during middle and late pregnancy since both are inflammatory and prooxidant agents (7, 9, 14, 49). TNF-α levels are elevated in preeclamptic pregnancies in women (7, 9), and administration of TNF-α to pregnant (but not nonpregnant) baboons causes hypertension and proteinuria (43). Furthermore, administration of TNF-α to late-pregnant rats results in loss of the normal gestational fall in blood pressure and impaired cGMP-dependent vasorelaxation (1, 19, 23, 25, 26, 32). Normal pregnant women become refractory to the vasoconstrictor actions of ANG II, but preeclamptic women develop increased sensitivity to ANG II (18). There have been reports that an autoantibody to the angiotensin receptor type 1 (AT1) is elevated in preeclamptic pregnancy (49) and that administration of the AT1 autoantibody to pregnant rats results in hypertension, increased ANG II sensitivity, and endothelial dysfunction (6, 24, 32, 33).
In this study, we measured blood pressure (by telemetry) in rats on a normal diet (ND) and a high-fat diet (HFD), over 8–9 wk and then during pregnancy together with administration of either vehicle (+ND) or ANG II + TNF-α (+HFD), from day 6 to 20. Virgins were followed over a similar time course. Indices of renal cortical and aortic NO and antioxidant/oxidant status were measured. We also made similar measurements in the renal medulla of all animals, since the medulla is particularly susceptible to oxidative stress.
METHODS
Animal Usage
All rat experiments were approved by the Institutional Animal Care and Use Committee at the University of Florida. Twenty-eight female Sprague-Dawley (SD) rats aged 15–21 wk were used in this study. Rats were placed into two groups of 14, where they were either fed a high-fat diet (HFD) or a normal diet (ND) throughout the 12-wk study. Telemetry probes were placed into the rats between weeks 2 and 4 on the diets (Fig. 1). After 5 or 6 wk of being on the ND or the HFD, baseline arterial blood pressure was obtained via telemetry for 4 days. At ∼8 wk, eight HFD and six ND rats were mated with fertile male rats to generate pregnancy. Day 1 of pregnancy was confirmed by the presence of sperm in vaginal smears. On day 6 of pregnancy, rats received a minipump infusion of either saline or ANG II and TNF-α. All HFD rats received ANG II and TNF-α, while all ND rats received saline via minipump. The final groups were Virgin rats + ND + Saline (V+ND) (n = 7), Virgin rats + HFD + ANG II/TNFα (V+HFD) (n = 7), Pregnant rats + ND + Saline (P+ND) (n = 6), and Pregnant rats + HFD + ANG II/TNF-α (P+HFD) (n = 8) (see Fig. 1).
Fig. 1.
Experimental setup. Four groups of rats completed the experimental timeline displayed above. The four groups consisted of Virgin + Normal Diet (ND) + saline (V+ND) (n = 7), Virgin + High-Fat/Western diet (HFD) + ANG II and TNF-α (V+HFD) (n = 7), Pregnant + ND + Saline (P+ND) (n = 5), and Pregnant + HFD + ANG II and TNF-α rats (P+HFD) (n = 8).
Dietary Administration
Female rats were given a normal chow (ND) (2018S) or a special prooxidant and HFD (TD.110489) from Harlan Laboratories. The HFD is composed of high amounts of refined carbohydrates and fats derived from sucrose, milk fat, and cholesterol. The HFD also lacks several antioxidants, such as vitamin C, vitamin E, selenium, and ethoxyquin. Diets and drinking water were given ad libitum.
Surgeries
Telemetry probe implantation.
Blood pressure in conscious and freely moving rats was measured by PA-C40 telemetry transmitter implants (Data Sciences International, St. Paul, MN). SD rats were anesthetized by isoflurane (IsoFlo; Abbott Laboratories, North Chicago, IL), and temperature and respiration rate were monitored. Two small skin incisions were made, one at the midline of the abdomen near the lowest rib and the other near the groin area of the left leg. The left femoral artery was carefully isolated, without damage to any nerves or the femoral vein. The PA-C40 transmitter catheter was inserted through the abdominal incision down to the groin incision, via trochar, and inserted into the left femoral artery. The transmitter battery was sutured to the outside of the abdominal wall. After the surgery, all rats were housed separately and given 7–10 days to recover before any telemetry data were acquired. For each rat, the systolic pressure, diastolic pressure, and mean arterial pressure (MAP) were recorded continuously for 5 min/h, after which an average of the 5-min recording was obtained. All data collection and collation were performed with the DSI equipment and software (St. Paul, MN). Only MAP is presented in this study.
Minipump implantation.
Rats were lightly anesthetized with a 3–5% isoflurane and a minipump (Alzet model 2ML2; Durect, Cupertino, CA), which delivered 5 μl/h for 14 days and was inserted into a subcutaneous pocket on the back. The minipump was filled with saline or ANG II and TNF-α (0.45 μg/μl and 0.625 ng/μl to deliver 150 ng·kg−1·min−1 and 75 ng/day, respectively). The ANG II dose of 150 ng·kg−1·min−1 was chosen, because it is a slow pressor dose that generates a delayed and modest rise in blood pressure after 2–3 wk of ANG II infusion (39). A 50% increase in TNF-α (75 ng/day) above the dose given in other studies (50 ng/day) (23, 25, 26) was administered on the basis of our preliminary experiments, in which a dose of 50 ng/day of TNF-α was administered and produced no responses. Full sterile technique was used for all recovery surgery.
Terminal tissue harvest.
All tissue harvesting was conducted under 5% isoflurane anesthesia. An incision was made along the midline of the abdomen. Next, a needle (20 G) was inserted into the abdominal aorta bifurcation, and blood was collected (using heparin as anticoagulant). After determination of hematocrit, the plasma was separated, and plasma osmolality and plasma protein concentrations were measured. The remaining plasma was aliquoted and stored at −80°C for future analyses. The kidney was removed and was dissected into the cortex and medulla. The aorta, consisting of both the aortic arch and thoracic aorta, was cleared of all adventitia. Tissues were snap frozen in liquid nitrogen and stored at −80°C for later analysis. During the terminal tissue harvest, the fetuses were removed from the uterus, counted, and weighed.
Western Blot Analysis
Kidney cortex, kidney medulla, and aorta abundance of proteins were detected using Western blot analysis, as previously described (11, 12) Tissue samples (200 μg of kidney cortex, 100 μg of kidney medulla, and 200 μg of aorta) were loaded on 6%, 7.5%, or 12% polyacrylamide gels and separated by electrophoresis. Proteins were transferred onto a nitrocellulose membrane and stained with Ponceau red (Sigma-Aldrich, St. Louis, MO) to verify protein transfer and equal loading among samples. Membranes were then blocked, washed, and incubated overnight at 4°C with the primary antibody of interest. Blots were then probed for eNOS, nNOSα, nNOSβ, ecSOD, MnSOD, Cu/ZnSOD, P22phox, and nitrotyrosine, as described in Table 1. Membranes were then incubated with the appropriate secondary antibody (Table 1) for 1 h at room temperature and then developed with enhanced chemiluminescent reagents (Thermo Scientific Pierce, Rockford, IL). For each protein probed, we ran multiple gels with 15 or 25 lanes that consisted of a molecular ladder, an internal positive control (Table 1), and tissue samples from each group. The bands were quantified by densitometry using the VersaDoc Imaging System and Software (Bio-Rad, Hercules, CA). Densitometry was normalized to Ponceau staining (total protein loaded) and an internal positive control, which allows for densitometric comparisons between samples among different membranes (11, 12). Mean values for the Virgin rats + ND + Saline (V+ND) group were set at 100% for comparisons between groups. In the results, we show only densitometry data, because of space limitations and since we have previously published representative Western blots for all proteins examined (11, 12).
Table 1.
Western blot details
| Primary Antibody |
Secondary Antibody |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proteins | %Gel | Blocking | Company | Species | Conc. | Company | Species | Conc. | MW, kDa | Pos. Control |
| Cu/Zn SOD | 12 | 5% NFM TBS-T 0.05% | Assay Designs/Stressgen (SOD-101) | Rabbit | 1:2,000 | Bio-Rad (170-6516) | Goat | 1:3,000 | ∼19 | Rat Brain (20 μg) |
| ecSOD | 12 | 5% NFM TBS | Abcam (ab21974) | Rabbit | 1:250 | Bio-Rad (170-6516) | Goat | 1:2,000 | ∼30 | Rat kidney cortex (200 μg) |
| eNOS | 12 | Sigma Tris 3% Milk-T 0.05% | BD Transduction | Mouse | 1:250 | Bio-Rad (170-6516) | Goat | 1:2,000 | ∼140 | Endothelial cell lysate (5 μg) |
| MnSOD | 12 | 5% NFM TBS-T 0.05% | Assay Designs/Stressgen (SOD-111) | Rabbit | 1:2,000 | Bio-Rad (170-6516) | Goat | 1:3,000 | ∼25 | Rat brain (20 μg) |
| Nitrotyrosine | 7.5 | 5% NFM TBS-T 0.05% | Upstate/Millipore (05-233) | Mouse | 1:500 | Bio-Rad (170-6516) | Goat | 1:2,000 | Multiple bands | Rat kidney medulla 5/6 Ablation infarction (100 μg) |
| nNOSα | 7.5 | Sigma Tris 2% Milk-T 0.5% | Santa Cruz (s5302) | Mouse | 1:50 | Bio-Rad (170-6516) | Goat | 1:3,000 | ∼160 | Rat cerebellum (2 μg) |
| nNOSβ | 6 | Sigma Tris 2% Milk-T 0.5% | ABR/Thermo Scientific (PA1-033) | Rabbit | 1:500 | Bio-Rad (170-6515) | Goat | 1:3,000 | ∼140 | Rat kidney medulla 5/6 Ablation infarction (100 μg) |
| P22phox | 12 | 5% NFM TBS-T 0.05% | Santa Cruz (sc-11712) | Goat | 1:50 | Santa Cruz (sc-2020) | Donkey | 1:2,000 | ∼25 | Rat lung (25 μg) |
Conc., concentration; MW, molecular weight; Pos., positive control; TBS, Tris-buffered saline; NFM, nonfat milk; T, Tween; ABR, Affinity BioReagents.
Total Antioxidant Capacity Assay
Total antioxidant capacity was measured using the antioxidant assay kit (709001; Cayman Chemical, Ann Harbor, MI), according to the manufacturer's instructions and as previously described by us (11, 12).
Plasma Creatinine
Plasma creatinine was measured by the mouse creatinine kit (Crystal Chem, Downers Grove, IL).
Plasma and Tissue NOx
Plasma and tissue NOx levels were measured by the Griess assay, as previously described by us (44). Final plasma NOx levels were calculated and normalized to plasma creatinine (μM) concentrations.
Statistical Analysis
All data are expressed as means ± SE. A two-way ANOVA and multiple-comparison test with Bonferroni adjustments were used to compare 24-h MAP, changes of body weight, daily food intake, and tissue protein abundance among the groups. The Student's t-test was used to compare changes in 24-h MAP between treatment (HFD vs. ND) in both virgin and pregnant rats. A three-way analysis of covariance (ANCOVA) with repeated measures was applied to determine whether there is an interaction effect among treatment (HFD vs. ND), pregnancy status (P vs. V), and experimental days (day 2 to day 19) on 24-h MAP. Body weight was adjusted in the model as a covariate. The two-way interaction effect between treatment and pregnancy status was analyzed at each time point after significant three-way interaction was identified. The adjusted means and the differences of the adjusted means in the three-way interaction were calculated and compared using independent t-test. P value ≤ 0.05 was considered significant. All statistical analyses were conducted using SAS 9.4 (Cary, NC) and GraphPad Prism 6 (La Jolla, CA).
RESULTS
Body Weight
There was no significant difference in body weights at day 0 between rats in each group before they were administered the HFD. The body weight at day 0, before the diet was administered, was 246 ± 7, 264 ± 6, 260 ± 3, and 268 ± 5 g for V+ND, V+HFD, P+ND, and P+HFD, respectively (not significant, ns). Body weights in all rats increased over time (Fig. 2A). Rats on HFD displayed an accelerated rate of body weight gain over the first 15 days on the HFD vs. rats on ND in virgins (1.2 ± 0.2 vs. 0.1 ± 0.2 g/day; P < 0.05 V+HFD vs. V+ND) and in rats destined to be pregnant (1.3 ± 0.2 vs. 0.3 ± 0.2 g/day; P < 0.05 P+HFD vs. P+ND). This was associated with an initial rapid increase in food intake in rats on the HFD. However, by day 15, HFD rats reduced their caloric intake to equal rats on a ND (Fig. 2B). As a result, after day 15, the growth curve continued to rise in parallel for rats fed the HFD and ND. After mating, pregnant rats on both diets exhibited an acceleration of body weight gain vs. virgin rats on either diet (Fig. 2A). Furthermore, pregnant rats on the HFD displayed a greater increase in body weight vs. pregnant rats receiving the ND, from days 10 through day 20 (6.8 ± 0.2 vs. 5.4 ± 0.2 g/day; P < 0.05). In virgin rats on both diets, the growth curves remained parallel and displayed little change in body weight.
Fig. 2.
A: Body weights (g) over time in virgin (V) and pregnant (P) rats given a normal diet and saline mini-pump infusion (V+ND and P+ND, respectively) or a high-fat/Western diet, ANG II, and TNF-α mini-pump infusion (V+HFD and P+HFD). □ denotes: V+ND (n = 7), ■ denotes V+HFD (n = 7), open triangles denote P+ND (n = 5), and ▲ denotes P+HFD (n = 8). *Significant difference in the rate of rise of body weight (BW) over the first 15 days on the diet, between normal diet (ND) and high-fat diet (HFD) groups in both virgins and pregnant rats. **Significant difference in the rate of rise of BW between virgin and pregnant groups with and without treatment, between experimental days 10 and 20. *P < 0.05 and **P < 0.05, two-way ANOVA with multiple-comparison test with Bonferroni adjustments. B: daily food intake (in kilocalories) over time in virgin (V) and pregnant (P) rats given a normal diet and saline minipump infusion (V+ND and P+ND, respectively) or a high-fat/Western diet, ANG II, and TNF-α minipump infusion (V+HFD and P+HFD, respectively).White bars denote V+ND rats (n = 7); gray bars denote V+HFD rats (n = 7), white-hatched bars denote P+ND (n = 5), and gray-hatched bars denote P+HFD rats (n = 8). *P < 0.05 vs. V+ND, †P < 0.05 vs. P+ND, ‡P < 0.05 vs. V+HFD, using two-way ANOVA with multiple-comparison test with Bonferroni adjustments.
Blood Pressure
Figure 3 summarizes 24-h mean arterial pressure (MAP) at baseline (i.e., the blood pressure that was obtained for 4 days after 5–6 wk on the diet and before pregnancy; see Fig. 1). As shown, there was no significant difference in MAP between rats on the ND and HFD, or in MAP at baseline between rats that remained virgins, and rats destined to become pregnant (Fig. 3).
Fig. 3.
Baseline 24 h. Mean arterial pressure (MAP), before mating, in virgin (V) and pregnant (P) rats given a normal diet [V+ND (n = 7) and P+ND (n = 5); white and white hatched histogram, respectively] or a high-fat/Western diet [V+HFD (n = 7) and P+HFD (n = 8): gray and gray-hatched histogram, respectively].
Figure 4 summarizes the 24-h adjusted least square means MAP profile in virgin and pregnant rats at baseline and over experimental days 2–19. The results from the three-way ANCOVA with repeated measures indicate that the three-way interaction among diet treatment, pregnancy status, and experimental day was statistically significant (P < 0.01) for 24-h MAP. We further examined the two-way interaction of treatment and pregnancy status at each time point. The MAP was similar and constant for the first six experimental days of the study and increased in all groups when the minipumps were implanted on day 6. This increase in MAP was transient in virgin and pregnant rats fed the ND with the saline minipump (Fig. 4, A and B), presumably due to surgical stress. After the minipump containing ANG II/TNF-α was implanted in rats on HFD, MAP remained elevated above virgin ND rats for experimental days 7, 8 and 10–15 (Fig. 4A). In contrast, MAP in pregnant rats on the HFD was only elevated on day 7 above pregnant rats on the ND (Fig. 4B). Figure 4C gives the difference of the adjusted means of 24-h MAP between HFD and ND in two groups, as well as the exact P values. The final MAP for each of the groups is 95 ± 3, 100 ± 3, 90 ± 4, 92 ± 4 mmHg for V+ND, V+HFD, P+ND, and P+HFD, respectively. Furthermore, the change in 24-h MAP from baseline 24-h MAP was elevated in virgin HFD vs. virgin ND rats from days 7–14 and 18–19 (Fig. 5A). In contrast, the changes in 24-h MAP in pregnant HFD rats vs. pregnant ND rats were only elevated on days 7, 11, and 12 (Fig. 5B). Furthermore, the blood pressure profiles in pregnant and virgin rats from days 15–19 were similar (Fig. 5B). Although only MAP is given, we also measured systolic and diastolic blood pressures, and the patterns were similar to the MAP.
Fig. 4.
MAP averaged over 24 h, shown as adjusted least squares means from three-way analysis of covariance, at baseline and experimental days 2–19 in virgin rats (A) and pregnant rats (B) given a normal diet and saline minipump infusion (ND) or a high-fat/Western diet, ANG II, and TNF-α minipump infusion (HFD) rats for 14 days. *Significant difference between ND (open symbol) and HFD (closed symbol), virgin (square) and pregnant (triangle) rats by independent t-test on each day. C: table gives the adjusted mean difference in MAP and the P value, for days 7–19. The groups are V+ND (n = 7), V+HFD (n = 7), P+ND (n = 5), and P+HFD (n = 8).
Fig. 5.
Change in MAP vs. baseline averaged in virgin rats (A) and pregnant rats (B) given a normal diet and saline minipump infusion (ND) or a high-fat/Western diet, ANG II, and TNF-α minipump infusion (HFD) rats for 14 days. *Significant difference between ND (open symbol) and HFD (closed symbol), virgin (square) and pregnant (triangle) rats by independent t-test on each day. The groups are V+ND (n = 7), V+HFD (n = 7), P+ND (n = 5), and P+HFD (n = 8).
Maternal/Fetal Outcome
As shown in Table 2, both groups of pregnant rats showed the normal hemodilution due to PVE, with falls in hematocrit and plasma protein concentration. Plasma osmolality also decreased significantly in both groups of pregnant rats compared with virgins, while plasma creatinine was not different between the groups (Table 2). The plasma (factored for Pcr) was elevated in the ND pregnant vs. virgin rats, but not in the HFD pregnant vs. virgin rats (Table 2). The pregnant rats on the HFD had fewer pups/litter compared with ND rats (10 ± 2 vs. 13 ± 2 pups, P < 0.05). However, the average pup weights were similar in both HFD and ND groups (3.87 g ± 0.21 vs. 4.52 g ± 0.23, ns), while the total fetal mass was higher in ND rats vs. HFD rats (57 ± 3 g vs. 39 ± 4 g, P < 0.05).
Table 2.
Maternal/fetal outcomes
| V + ND | V + HFD | P + ND | P + HFD | |
|---|---|---|---|---|
| Hematocrit | 37.5 ± 0.5 | 39.1 ± 1.1 | 28.8 ± 1.4* | 30.0 ± 2.2† |
| Plasma Protein, g/dl | 5.8 ± 0.1 | 5.9 ± 0.2 | 5.1 ± 0.2* | 5.1 ± 0.2† |
| Osmolality, mol/l | 306 ± 1 | 305 ± 3 | 293 ± 5* | 289 ± 2† |
| Plasma creatinine, mg/dl | 0.34 ± 0.04 | 0.42 ± 0.04 | 0.36 ± 0.04 | 0.38 ± 0.03 |
| Plasma NOx/Creatinine Ratio, μM | 1.3 ± 0.3 | 1.2 ± 0.2 | 2.3 ± 0.4* | 1.6 ± 0.3 |
| Tissue (kidney cortex) NOx μM/mg of protein | 9.6 ± 0.5 | 12.6 ± 0.4 | 19.0 ± 6.2 | 25.2 ± 8.2 |
Values are expressed as means ± SE. Percent blood hematocrit, plasma protein concentration, plasma osmolality, plasma creatinine, and plasma NOx/Cr ratio, and kidney cortex NOx in virgin (V) and pregnant (P) rats given a normal diet and saline minipump infusion (ND) or a high-fat/Western diet, ANG II, and TNF-α minipump infusion (HFD) for 14 days.
P < 0.05. vs. V + ND,
P < 0.05. vs V + HFD.
Nitric Oxide/Oxidant/Antioxidant Status
Normal virgins vs. HFD fed + ANG II and TNF-α virgins.
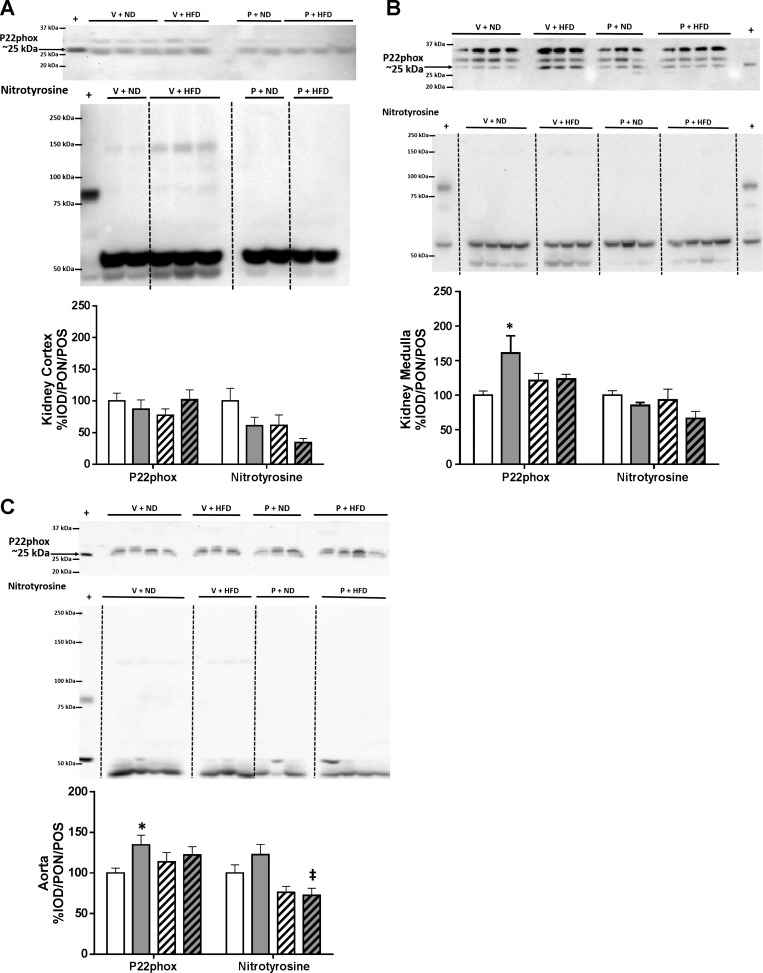
The HFD + ANG II/TNF-α treatment had no impact on the abundance of eNOS, nNOSα, or nNOSβ in the kidney cortex or medulla of virgin rats, while aortic eNOS was lower vs. ND virgins (Fig. 6, A–C). Diet had no effect on the abundance of ecSOD, MnSOD, and Cu/Zn SOD, or total antioxidant capacity in kidney cortex, medulla, or aorta of virgin rats (Fig. 7). There was no difference in the abundance of p22phox in kidney cortex; however, p22phox was increased in both the kidney medulla and aorta of V+HFD rats (Fig. 8). The nitrotyrosine levels were similar in kidney and aorta of virgin rats on both diets (Fig. 8).
Fig. 6.
Representative blots and densitometric analysis for eNOS, nNOSα, and nNOSβ in the kidney cortex (A), kidney medulla (B), and the aorta (C) of virgin and pregnant rats given a normal diet and saline minipump infusion (V+ND or P+ND) or a high-fat/Western diet, ANG II, and TNF-α minipump (V+HFD or P+HFD) infusion for 14 days. Densitometry analysis was quantified as integrated optical density (IOD) normalized to Ponceau (PON) staining and the positive control (POS) for each protein of interest. White bars represent V+ND rats (n = 7), gray bars represent V+HFD rats (n = 7), white hatched bars represents P+ND (n = 5), and gray hatched bars represent P+HFD rats (n = 8).*P < 0.05 vs. V+ND, †P < 0.05 vs. P+ND, ‡P < 0.05 vs. V+HFD; Two-way ANOVA with multiple comparison test with Bonferroni adjustments.
Fig. 7.
Representative blots and densitometric analysis for extracellular SOD (ecSOD), MnSOD, and Cu/Zn SOD in the kidney cortex (A), kidney medulla (B), and the aorta (C) of virgin (V) and pregnant (P) rats given a normal diet and saline minipump infusion (V+ND or P+ND) or a high-fat/Western diet, ANG II, and TNF-α minipump infusion (V+HFD or P+HFD) for 14 days. Densitometry analysis was quantified as integrated optical density (IOD) normalized to Ponceau (PON) staining and the positive control (POS) for each protein of interest in each tissue. The tissue total antioxidant capacity was quantified and expressed as micromole of Trolox per milligram of protein for each tissue. White bars represent V+ND rats (n = 7), gray bars represent V+HFD rats (n = 7), white hatched bars represent P+ND (n = 5), and gray hatched bars represent P+HFD rats (n = 8). †P < 0.05 vs. P+ND, ‡P < 0.05 vs.V+HFD. Two-way ANOVA with multiple-comparison test with Bonferroni adjustments.
Fig. 8.
Representative blots and densitometric analysis for P22phox and nitrotyrosine in the kidney cortex (A), kidney medulla (B), and the aorta (C) of virgin and pregnant rats given a normal diet and saline mini-pump infusion (V+ND or P+ND) or a high fat/western diet, ANGII, and TNFα mini-pump infusion (V+HFD or P+HFD) for 14 days. Densitometry analysis was quantified as integrated optical density (IOD) normalized to ponceau (PON) staining and the positive control (POS) for each protein of interest in each tissue. White histograms represents V+ND rats (n = 7), grey histograms represent V+HFD rats (n = 7), white hatched histograms represents P+ND (n = 5), and grey hatched histograms represent P+HFD rats (n = 8). *P < 0.05 vs. V+ND, ‡P < 0.05 vs.V+HFD; Two-way ANOVA with multiple-comparison test with Bonferroni adjustments.
Normal pregnancy vs. normal virgins.
In the present study, as reported by us earlier (12), the abundance of nNOSβ in the kidney cortex increased in pregnant rats on the ND compared with virgin rats (Fig. 6A). Aortic eNOS abundance decreased, and both nNOS isoforms tended to rise in the aorta in late pregnancy [as in our earlier publication (12), and in the present study], the increase in nNOSβ is now significant (Fig. 6C). There was no difference in aorta in normal pregnancy in abundance of any of the SOD isoforms, nor in total antioxidant capacity, as reported by us earlier (12) (Fig. 7). The data on the kidney medulla are novel, and we found no change in the abundance of any of the NOS or SOD isoforms, and total antioxidant capacity (Figs. 6 and 7). There is an increase in p22phox abundance, but no differences in nitrotyrosine levels in both the kidney medulla and the aorta (Fig. 7).
Normal pregnancy vs. HFD fed + ANG II and TNF-α pregnancy.
The major change in NOS isoform protein abundance was the suppression of the normal gestational rise in renal cortical nNOSβ, by the administration of the HFD, ANG II, and TNF-α in late-pregnant rats (Fig. 6A) compared with pregnant rats on ND. The nNOSβ abundance was also lower in the kidney medulla of pregnant rats fed HFD vs. virgins (Fig. 6B). Furthermore, aortic nNOSβ abundance was decreased in pregnant rats on HFD vs. pregnant rats on the ND (Fig. 6C). Although there was a small decrease in Cu/Zn SOD abundance in the kidney cortex of pregnant HFD rats vs. pregnant controls, the kidney cortex total antioxidant capacity was similar in both groups of late-pregnant rats (Fig. 7A). In the kidney medulla, there was a lower abundance of MnSOD in pregnant rats on HFD vs. pregnant controls, although there was no difference in total antioxidant capacity (Fig. 7B). In the aorta, there were no differences in any abundances of eNOS, nNOSα, or any of the SOD's enzymes along with no change in total antioxidant capacity between pregnant HFD and ND rats (Figs. 6C and 7C). There was no difference in p22phox or in nitrotyrosine abundances in kidney cortex, medulla, or aorta, between the two pregnant groups although aortic nitrotyrosine abundance was lower in pregnant vs. virgin rats on HFD (Fig. 8C).
DISCUSSION
The major new finding in this study was that despite feeding rats a high-fat, high-refined carbohydrate, and prooxidant diet, together with infusion of proinflammatory TNF-α and ANG II throughout most of pregnancy, late-pregnant rats did not develop maternal hypertension. In contrast, the virgin rats that were subjected to the same prooxidant, proinflammatory protocol did exhibit late increases in blood pressure compared with control (ND) virgins. This suggests that pregnancy in these SD rats was protective and opposed the hypertensive response to the prooxidant and proinflammatory protocol.
One unexpected finding was that the HFD did not result in obesity. The rate of rise in body weight was greater for the first 15 days on the HFD compared with ND-fed virgin rats and rats destined to be pregnant after mating in the protocol (Fig. 1). However, by day 15, HFD rats reduced their food intake to become isocaloric with ND rats. Thus, the body weight in HFD rats was only mildly elevated after ∼6 wk on the diet. Several studies have shown a variable response of SD rats to a HFD, with some being obesity-prone, while others being obesity-resistant (17, 28, 34). SD rats from different vendors have different susceptibilities to develop obesity on HFD (28), and female SD rats are more resistant to obesity vs. males given a HFD for 6–8 wk (44). Unfortunately, the rats used in our study (Harlan; Dublin facility) were clearly not obesity-prone, and this may explain why the baseline systolic (data not shown), diastolic (data not shown), and mean arterial pressure did not change after 5 to 6 wk on the HFD.
Although the blood pressures were similar in virgin rats on a ND or HFD before the minipump implant, the HFD rats receiving ANG II, and TNF-α, exhibited increases in 24-h MAP vs. ND rats receiving saline for experimental days 7, 8, and 10–15. After day 15, the blood pressure was still slightly elevated in virgin rats receiving the HFD, ANG II, and TNF-α above virgin rats receiving the ND. This indicates that the combination of ANG II + TNF-α administered for 14 days leads to a sustained, mild pressor effect in virgin rats. In contrast, in the pregnant rats, after a transient rise due to presumably surgical stress of minipump implantation, the 24-h MAP returned to baseline in ND and HFD-fed rats administered ANG II and TNF-α. Furthermore, after day 15, both groups of pregnant rats treated and untreated displayed the same fall in blood pressure. At day 19 of pregnancy, there was a significant drop in 24-h MAP compared with prepregnancy values in treated (105 ± 3 vs. 92 ± 4 mmHg, P < 0.05 P+HFD at day 0 vs. P+HFD at day 19) and untreated (104 ± 3 vs. 90 ± 4 mmHg, P < 0.05 P+ND at day 0 vs. P+ND at day 19) rats. Moreover, the change in 24-h MAP was elevated in virgin HFD rats vs. virgin ND rats after day 7. The increase in the change in 24-h MAP in HFD-pregnant rats was blunted, and in late pregnancy, they showed the same decline in MAP as ND rats. Thus, together, these data suggest that the pregnant rats are mildly “protected” against the increase in blood pressure seen in virgin HFD-fed rats with ANG II and TNF-α. In addition, these results imply that pregnant rats treated may have become refractory to the pressor actions of ANG II and TNF-α. Furthermore pregnant rats fed the HFD + ANG II and TNF-α, displayed the normal hemodilution of pregnancy (with falls in hematocrit and plasma protein concentration), suggesting that normal PVE occurred in treated pregnant rats. These data taken together suggest that maternal gestational adaptations are largely unaffected by the HFD + ANG II and TNF-α treatment and that only a small impact is seen on blood pressure.
There are previous studies in which superimposition of pregnancy on states of chronic hypertension and oxidative stress in the rat appears protective. For example, blood pressure falls during pregnancy in both the spontaneously hypertensive rat and the rat with 5/6th ablation/infarction of renal mass (15, 21). However, we had anticipated that the combination of chronic high-fat and prooxidant diet with ANG II + TNF-α administration during pregnancy would lead to an adverse “preeclamptic”-like response. The studies by LaMarca, Granger, and colleagues have shown that administration of TNF-α to late-pregnant rats results in a suppression of the late-gestational fall in blood pressure (1, 19, 23, 25, 26). These studies employed a lower dose than ours (50 vs. 75 ng/day) and the TNF-α was given during late pregnancy, from day 15 or 16 to day 19 to 20. Perhaps the circulating level of TNF-α had fallen by late pregnancy in our study? Administration of a much higher dose of TNF-α (2.5 μg·kg−1·min−1) by minipump to male rats resulted in a fall in TNF-α levels to baseline between days 10 and 15 of the infusion (5). Together, these findings suggest that TNF-α levels must be elevated late in pregnancy to compromise the normal peripheral vasodilation.
A primary goal of this study was to follow up on our earlier observations that during normal pregnancy in the rat, the kidney cortex is protected from oxidative stress (12). We chose to study late pregnancy here (when renal vascular resistance is returning toward nonpregnant values), since this is when “preeclamptic” like symptoms have been reported in several experimental rat models of compromised pregnancy (1, 6, 19, 24, 25, 26, 32, 33, 43). However, as indicated above, despite our best attempts, with rats fed a HFD along with ANG II and TNF-α, the treated rats did relatively well during pregnancy. Many of the responses of the renal and aortic NOS and prooxidant and antioxidant systems to pregnancy were similar in the ND and HFD rats in the present study. The only systematic difference was the loss of nNOSβ abundance in kidney cortex and aorta in the late-pregnant rats on the HFD with ANG II/TNF-α vs. normal late-pregnant rats on a ND + saline infusion. However, kidney cortex tissue NOx content was similar in both groups (ND and HFD) of late-pregnant rats (Table 1). The PNOx/PCr (indices of systemic NO production) was elevated in P+ND rats vs. V+ND but was absent in the P+HFD rats vs. V+HFD. Importantly, neither blood pressure nor kidney function (from plasma creatinine) was compromised by lack of nNOSβ abundance and PNOx/PCr levels during late pregnancy. Furthermore, the prooxidant and antioxidant systems generally responded similarly in the two groups of late-pregnant rats, with no evidence of increased oxidative stress in the HFD-fed rats administered ANG II/TNF-α. In fact, the only “negative” effect of the HFD was that litter number and total fetal mass were lower than the litter number and total fetal mass in ND rats.
Perspectives and Significance
In contrast to our hypothesis, pregnant rats exposed to a HFD with prooxidant and proinflammatory insults throughout the course of pregnancy did not develop preeclampsia or gestational hypertension. In fact, the pressor response to HFD + ANG II + TNF-α was less marked in pregnant vs. virgin rats, suggesting that pregnancy was mildly protective in this model. This agrees with earlier studies in rats with renal mass reduction and in the spontaneously hypertensive rats, where pregnancy lowered blood pressure (3). On the basis of our findings in this study, the maintained renal antioxidant capacity and renal NO bioavailability during pregnancy may have contributed to this protection.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant RO1-DK-56843 and the Robert and Mary Cade endowed Professorship in Physiology (to C. Baylis). Funding for M. Cunningham was provided by the National Institutes of Health NIDDK T32 training grant (T32DK-751825) awarded to the Division of Nephrology, University of Florida, as well as the Division of Nephrology Gatorade Research funds. Funding for C. West was provided by the Hypertension T32 training grant (T32 HL083810) at the University of Florida. A. Deng and X. Wen were supported by NIDDK Grant RO1-DK-56843 and the Division of Nephrology, Hypertension and Renal Transplantation Department of Medicine, respectively.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.W.C. and C.B. conception and design of research; M.W.C., C.A.W., and A.D. performed experiments; M.W.C. and X.W. analyzed data; M.W.C. and X.W. interpreted results of experiments; M.W.C. prepared figures; M.W.C. drafted manuscript; M.W.C., C.A.W., X.W., and C.B. edited and revised manuscript; M.W.C. and C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank B. Cunningham and R. Smith for expert technical assistance. We also thank the University of Florida Hypertension Center Telemetry Core for blood pressure recordings.
REFERENCES
- 1.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-α-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 15: 170–175, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Baylis C, Engels K. Adverse interactions between pregnancy and a new model of systemic hypertension produced by chronic blockade of EDRF in the rat. Clin Exp Hypertens B11: 117–129, 1992. [Google Scholar]
- 3.Baylis C. Glomerular filtration and volume regulation in gravid animal models. Baillieres Clin Obstet Gynaecol 8: 235–264, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Belo L, Caslake M, Santos-Silva A, Castro EMB, Pereira-Leite L, Quintanilha A, Rebelo I. LDL size, total antioxidant status and oxidized LDL in normal human pregnancy: a longitudinal study. Atherosclerosis 177: 361–399, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bozkurt B, Kribbs SB, Clubb FJ Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation 97: 1382–1391, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Brewer J, Liu R, Lu Y, Scott J, Wallace K, Wallukat G, Moseley J, Herse F, Dechend R, Martin JN Jr, LaMarca B. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension 62: 886–892, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 37: 240–249, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Chavez JE, Mosher MD. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J 7: 566–571, 1993. [PubMed] [Google Scholar]
- 9.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol 40: 102–111, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Conrad KP. Renal hemodynamics during pregnancy in chronically catheterized, conscious rats. Kidney Int 26: 24–29, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham MW Jr, Sasser JM, West CA, Milani CJ, Baylis C, Mitchell KD. Renal nitric oxide synthase and antioxidants preservation in CYP1a1-Ren-2 transgenic rats with inducible malignant hypertension. Am J Hypertens 26: 1242–1249, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham MW Jr, Sasser JM, West CA, Baylis C. Renal redox response to normal pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 304: R443–R449, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest 96: 482–490, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dechend R, Viedt C, Müller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 107: 1632–1639, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Deng A, Baylis C. Glomerular hemodynamic responses to pregnancy in rats with severe reduction of renal mass. Kidney Int 48: 39–44, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Deng A, Engels K, Baylis C. Impact of nitric oxide deficiency on blood pressure and glomerular hemodynamic adaptations to pregnancy in the rat. Kidney Int 50: 1132–1138, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11: 845–851, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682–2689, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giardina JB, Green GM, Cockrell Kl Granger JP, Khalil Ra. TNF-α enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol 283: R130–R143, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Hung TH, Lo LM, Chiu TH, Li MJ, Yeh YL, Chen SF, Hsieh TT. A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reprod Sci 17: 401–409, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Iacono A, Bianco G, Mattace Raso G, Esposito E, d'Emmanuele di Villa Bianca R, Sorrentino R, Cuzzocrea S, Calignano A, Autore G, Meli R. Maternal adaptation in pregnant hypertensive rats: improvement of vascular and inflammatory variables and oxidative damage in the kidney. Am J Hypertens 22: 777–783, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev Suppl 1: S18–S25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor-α in pregnant rats. Hypertension 52: 1168–1172, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type 1 receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 54: 905–909, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-α. Hypertension 46: 1022–1025, 2005. [DOI] [PubMed] [Google Scholar]
- 26.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46: 82–86, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Laresgoiti-Servitje E. A leading role for the immune system in the pathology of preeclampsia. J Leukoc Biol 94: 247–257, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Mbah AK, Kornosky JL, Kristensen S, August EM, Alio AP, Marty PJ, Belogolovkin V, Bruder K, Salihu HM. Super-obesity and risk for early and late pre-eclampsia. BJOG 117: 997–1004, 2010. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology 14: 368–374, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Orhan H, Onderoglu L, Yucel A, Sahin G. Circulating biomarkers of oxidative stress in complicated pregnancies. Arch Gynecol Obstet 267: 189–195, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Parrish MR, Ryan MJ, Glover P, Brewer J, Ray L, Dechend R, Martin JN Jr, Lamarca BB. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gend Med 8: 184–188, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parrish MR, Wallace K, Tam KB, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, Martin JN, Dechend R, LaMarca B. Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. Am J Hypertens 24: 835–840, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Pecoraro N, Ginsberg AB, Warne JP, Gomez F, la Fleur SE, Dallman MF. Diverse basal and stress-related phenotypes of Sprague Dawley rats from three vendors. Physiol Behav 89: 598–610, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Podjarny E, Losonczy G, Baylis C. Animal models of preeclampsia. Semin Nephrol 24: 596–606, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redman CWG, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response: a review. Placenta 17: S21–S27, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Sacks GP, Studens K, Sargent K, Redman CWG. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 179: 80–86, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 47: 203–208, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Sasser JM, Moningka NC, Cunningham MW Jr., Croker B, Baylis C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Regul Integr Comp Physiol 298: R740–R746, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serdar Z, Gur E, Colakoethullary M, Develioethlu O, Sarandol E. Lipid and protein oxidation and antioxidant function in women with mild and severe preeclampsia. Arch Gynecol Obstet 268: 19–25, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui IA, Jaleel A, Tamimi W, Kadri HM AL. Role of oxidative stress in the pathogenesis of preeclampsia. Arch Gynecol Obstet 282: 469–474, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Smith CA, Santymire B, Erdely A, Venkat V, Losonczy G, Baylis C. Renal nitric oxide production in rat pregnancy: role of constitutive nitric oxide synthases. Am J Physiol Renal Physiol 299: F830–F836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A. Tumor necrosis factor-α induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 56: 192–199, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Suto T, Losonczy G, Qiu C, Hill C, Samsell L, Ruby J, Charon N, Venuto R, Baylis C. Acute changes in urinary excretion of nitrite + nitrate do not necessarily predict renal vascular NO production. Kidney Int 48: 1272–1277, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Taraschenko OD, Maisonneuve IM, Glick SD. Sex differences in high fat-induced obesity in rats: effects of 18-methoxycoronaridine. Physiol Behav 103: 308–314, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf) 57: 609–613, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Verdonk K, Visser W, Van Den Meiracker AH, Danser AH. The renin-angiotensin-aldosterone system in pre-eclampsia: the delicate balance between good and bad. Clin Sci (Lond) 126: 537–544, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Vinikoor-Imler LC, Messer LC, Evenson KR, Laraia BA. Neighborhood conditions are associated with maternal health behaviors and pregnancy outcomes. Soc Sci Med 73: 1302–1311, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103: 945–952, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wisdom SJ, Wilson R, McKillop JH, Walker JJ. Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am J Obstet Gynecol 165: 1701–1704, 1991. [DOI] [PubMed] [Google Scholar]