Abstract
While supraspinal mechanisms underlying respiratory pattern formation are well characterized, the contribution of spinal circuitry to the same remains poorly understood. In this study, we tested the hypothesis that intraspinal GABAergic circuits are involved in shaping phrenic motor output. To this end, we performed bilateral phrenic nerve recordings in anesthetized adult rats and observed neurogram changes in response to knocking down expression of both isoforms (65 and 67 kDa) of glutamate decarboxylase (GAD65/67) using microinjections of anti-GAD65/67 short-interference RNA (siRNA) in the phrenic nucleus. The number of GAD65/67-positive cells was drastically reduced on the side of siRNA microinjections, especially in the lateral aspects of Rexed's laminae VII and IX in the ventral horn of cervical segment C4, but not contralateral to microinjections. We hypothesize that intraspinal GABAergic control of phrenic output is primarily phasic, but also plays an important role in tonic regulation of phrenic discharge. Also, we identified respiration-modulated GABAergic interneurons (both inspiratory and expiratory) located slightly dorsal to the phrenic nucleus. Our data provide the first direct evidence for the existence of intraspinal GABAergic circuits contributing to the formation of phrenic output. The physiological role of local intraspinal inhibition, independent of descending direct bulbospinal control, is discussed.
Keywords: GABA, siRNA, interneurons, motoneurons, phrenic nerve
breathing provides the gas exchange essential to life and is, therefore, under automatic control, responding to afferent feedback streams from many sources, such as central and peripheral chemoreceptors, as well as mechanical stretch receptors in the airways and lungs. Because of the importance of basic mechanisms generating respiratory rhythms (23, 51, 78, 80), many efforts have been made to advance our knowledge about the organization of respiratory motor outputs at different levels of the neuraxis (5, 11, 34, 63, 42). According to the conventional view, phrenic motoneurons (PMNs) receive monosynaptic excitatory (inspiratory) and inhibitory (expiratory) inputs from supraspinal respiratory neurons located at the pontomedullary level (10, 12, 16, 20, 22–24, 58, 66–69, 73, 78, 79, 81). The role of spinal interneurons (SpINs) in shaping motor output has been largely overlooked. Moreover, the distribution, synaptic relationships, electrophysiological characteristics, and neurotransmitter phenotype of respiratory related SpINs in the phrenic nucleus (PNucl) are likewise inadequately defined. Published studies have either characterized respiration-related SpINs anatomically (44, 45, 47, 87) or electrophysiologically (3, 4, 8, 14, 15, 28, 59, 60) in a variety of species.
A methodological limitation inherent to pharmacological antagonism is its inability to selectively differentiate the roles mediated by different sources of synaptic input. Thus, although local microinjection of antagonists improves the ability to spatially localize antagonist effects, interpretation of results using this approach is not definitive because all receptors in the region will be affected, and the source of synaptic drive (local vs. descending) is impossible to identify. Fortunately, in recent years, 20–25 base-pair short interference RNA (siRNA), assembled into endoribonuclease-containing complexes, has been used to selectively cleave mRNA, thereby knocking down protein synthesis. The present study specifies the role of local spinal GABAergic neurons suggested (but not proven) by our previous pharmacological study (53), by making use of microinjections of anti-glutamate decarboxylase siRNA (anti-GAD siRNA) directly into the PNucl to silence spinal GABAergic interneurons. This approach has the advantage over other preparations because the inhibitory GABA-ergic inputs of descending respiratory drive are unaffected by the microinjection of anti-GAD siRNA into the PNucl, thereby increasing specificity of the source of the inhibitory drive. In addition, electrophysiological recording and subsequent phenotypic classification of GABAergic interneurons allowed us to identify definitive locations and roles for these cells. Their role in controlling phrenic nerve discharge is evaluated and discussed.
METHODS
Animal Preparation
General surgical preparation.
All procedures were approved by the Drexel University Institutional Animal Care and Use Committee, which oversees Drexel University's AAALAC-accredited animal program. Sixteen (11 with anti-GAD siRNA and 5 with negative control siRNA) spontaneously breathing adult Sprague-Dawley male rats (340–380 g) were initially anesthetized with isoflurane (4–5% vaporized in O2, Matrix) via a snout mask. Anesthetic depth was maintained at the level that prevented limb withdrawal reflexes and changes in heart rate and blood pressure in response to noxious stimulation of the distal hind limbs. Following tracheotomy and intubation, animals were artificially ventilated with the same gas mixture (2–2.5% of isoflurane in O2, 60 cycles/min, 2.3–3.0 ml of tidal volume; Columbus Apparatus) throughout the experiment. EKG was recorded via subcutaneous electrodes using conventional amplification and filtering (Neurolog; Digitimer, Hertfordshire, UK) and monitored using an audio amplifier (model AM10; Grass Instruments) and oscilloscope. One femoral artery and vein were cannulated for measurement of arterial pressure and infusion of drugs and saline, respectively. During all surgical procedures, core temperature was maintained at 37.0 ± 0.1°C via a servocontrolled heating blanket coupled to a rectal thermometer (Harvard Apparatus). Using a ventral approach, the phrenic nerves (PNs) were dissected free from the surrounding tissue, transected, desheathed, and covered with mineral oil-soaked cotton. Rats were then placed supine in a stereotaxic frame. Arterial and tracheal cannulas were connected to pressure transducers (CDXII; Argon Medical) to monitor arterial blood pressure and lung inflation pressure, respectively, using conventional amplifiers (Gould Statham). In all animals, end-tidal CO2 was maintained between 4.5 and 5% (Capstar CWE) and locked to ±0.1% during injection and recording epochs (see below) by adjusting minute volume.
Spinal surgery.
Infrathyroid portions of the trachea and esophagus were removed. The C3–C5 vertebrae were exposed using a ventral approach by removal of the rectus capiti, superior oblique, and ventral portions of the longus colli muscles. The ventral surface of the C3–C5 spinal cord was exposed by grinding of the vertebral bodies using a variable-speed drill, and sealed with bone wax. The dura was then opened using iridectomy scissors.
Recording.
Prior to neuronal recording, bilateral pneumothorax was performed to eliminate lung inflation-related motion artifact and chest wall mechanoreceptor feedback to the respiratory central pattern generator. Animals were paralyzed by an intravenous bolus injection of 2 mg/kg, followed by continuous infusion (3–4 mg·kg−1·h−1) of vecuronium bromide (Abbott Laboratories) dissolved in Ringer-Locke solution. A positive end-expiratory pressure of 1.0 cm H2O was applied to prevent atelectasis. The central ends of the PN were placed on bipolar silver electrodes and immersed in a mineral oil pool formed by skin flaps. Electrical activity from left and right PNs was amplified and filtered (10–5, 000 Hz; Neurolog, Digitimer). The extracellular activity of spinal respiration-related neurons was recorded (200–3,000 Hz; Neurolog) from the phrenic nucleus (Fig. 1, B and C) using glass microelectrodes with a tip outer diameter of 2–2.5 μm (7–10 MΩ) pulled from borosilicate glass capillaries (World Precision Instruments; cat. no. 1B120-F4) and filled with 0.5 M NaCl and 2.5% Neurobiotin (Invitrogen). The microelectrode was held in a stepper motor assembly (T-NA08A50; Zaber Technologies), attached to the rail of the stereotaxic frame via a micromanipulator, and advanced in steps of 1.5–2.0 μm to a depth 1.0–1.25 mm from ventral surface and 1.0–1.2 mm lateral to the midsagittal plane. Recorded SpINs were labeled juxtacellularly by iontophoresis of neurobiotin with positive current (5–10 nA, 20 Hz, 20 ms; Axoclamp 2A) for 20–25 min (Fig. 1, A1). The electrical activity of the two PNs, extracellular discharges of respiratory neurons in PNucl, expiratory CO2 level, arterial blood pressure, and lung inflation pressure were recorded onto the hard disk of a personal computer at a sampling rate of 10,000 kHz using a 16-bit A/D converter with visualization software (AD Instruments).
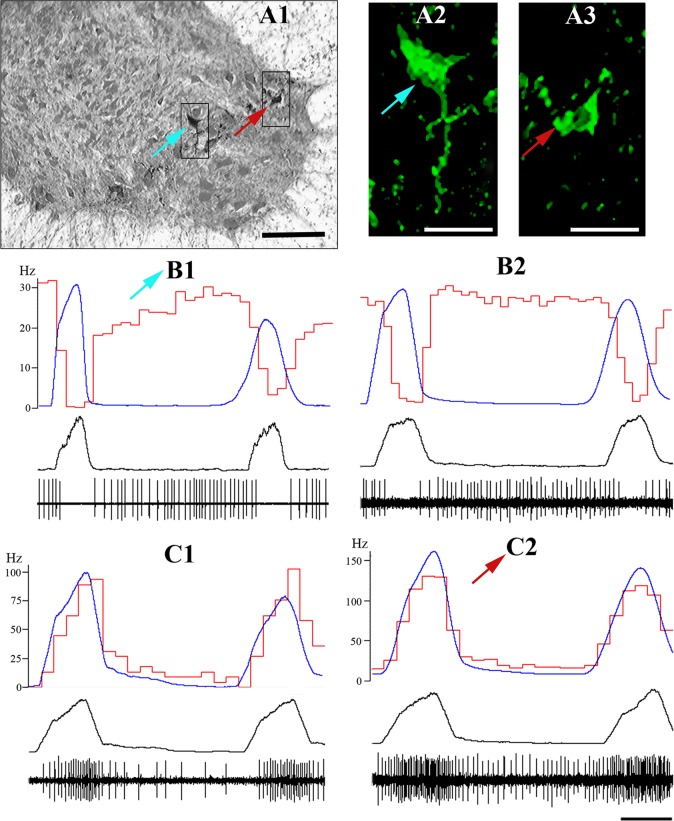
Fig. 1.
Immunohistochemical identification and firing patterns of respiratory interneurons recorded from C3–C5 ventral horn. A1–A3: one expiratory interneuron (light blue arrow; see B1 for firing pattern) and one inspiratory interneuron with tonic extended expiratory activity (red arrow; see C2 for firing pattern) double-labeled with neurobiotin (subpanel A1) and FITC-immunofluorescence secondary anti-GAD65/67 antibodies (subpanels A2 and A3). Scale bars: A1, 100 μm; A2 and A3, 60 μm. B1, B2, C1, C2: examples of recorded expiratory (top) and inspiratory interneurons (bottom) with extended expiratory activity. Averaged phrenic triggered integrated phrenic nerve activity (blue traces, arbitrary units, 100–120 sweeps, integration τ = 50 ms), cycle-triggered histograms (CTHs, red traces, 50–75 ms bin size) of analyzed interneurons (left scale, Hz), integrated phrenic nerve (PN) and spiking activity of recorded interneurons (low two black traces) are shown. Time bar = 250 ms, applied to B1–C2.
Application of siRNA.
siRNA targeting glutamate decarboxylase (both the 65- and 67-kDa isoforms; anti-GAD65/67 siRNA), designed for in vivo experiments with new “stealth” technology (Invitrogen, cat. no. 1330003; 3 oligos—RSS302157, RSS302158, RSS302159 against the 65-kDa isoform, and 3 oligos—RSS302154, RSS302155, RSS302156 against the 67-kDa isoform), was dissolved in saline (pH = 7.4) and injected unilaterally into one PNucl at the C4 level (n = 11). The majority of PMNs (∼64%) at this level have been described previously (26). Prior to treatment with siRNA, the PNucl was identified via microinjection of 10 nl of 10 mmol l-glutamate using triple-barrel pipettes (18–20-μm outer diameter for each barrel) when an immediate response in the phrenic neurogram was observed (53). A second barrel was used for anti-GAD 65/67 siRNA microinjections (20 μmol, 5 nl every 2 min for 2.5 h, 20 psi, 8–10-s pulse duration; Picospritzer III from Parker Instruments). A third barrel was filled with 2% Pontamine sky blue and was used for histological identification (10 nl) of the microinjection site (Fig. 2C). Additionally, in five rats negative control random sequence siRNA (Stealth RNAi siRNA negative control Hi GC, Invitrogen cat. no. 12935-400), and in another five rats, saline (pH = 7.4) (second control) was injected in the same manner as active siRNA.
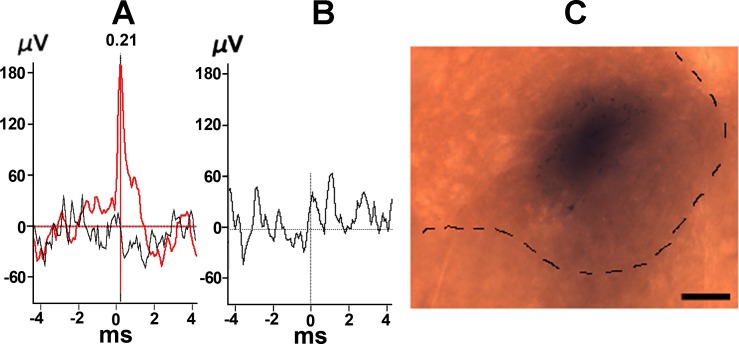
Fig. 2.
Examples of interneuron identification and marking of microinjection site. A and B: Phrenic motoneurons (PMN; A, red) and interneuron (B) identification using spike-triggering averaging (STA) techniques (see Marchenko and Rogers, 2007), demonstrating the presence or absence of a STA peak in the PN, respectively. Black trace in A: STA after shifting PN record by one respiratory cycle. C: histological identification of the siRNA microinjection site using 2% Pontamine sky blue (10 nl). Scale bar is 250 μm.
Immunohistochemistry.
At the end of each experiment, animals were transcardially perfused with 500 ml of normal saline (20°C, pH 7.4) with heparin (600 U/l) followed by 500 ml 4% paraformaldehyde in 0.1 M PBS. The spinal cord was removed and postfixed in the same fixative for 24 h at 4°C. Prior to sectioning, spinal cord was cryoprotected by incubation in 20% sucrose in PBS for 24 h. 60-μm sections were cut the following day to determine the number of GAD-positive cells and the position of juxtacellularly labeled units. In the case of neurobiotin-labeled neurons, sections were then processed with Vectastain ABC kit (Vector Labs; PK-6100). The recording electrode position for each neuron was defined in micrometers lateral from the midline and by depth from the ventral surface of spinal cord. Location of nonlabeled units was identified by their distance to labeled cells. The positions of all neurons were corrected according to tissue shrinkage after fixation. Labeled and unlabeled cells were mapped onto a standardized stereotaxic atlas (62).
GAD65/67 determination.
Six frozen sections (60 μm) from each C4 spinal segment were cut and collected in serial order in PBS at the center of injections marked with Pontamine sky blue (see above). This number of sections was chosen on the basis of calculation of the projected volume of spread (356 μm in diameter) of 5 nl of drug in brain tissues (56) and to cover the area (360 μm in diameter) where pharmacological effect is expected to be stable and repeatable. According to the Eq. 13 in Ref. 56, this volume will immediately occupy the area that is larger (356 μm in diameter) than the PNucl [∼150–200 μm; see Fig. 1 from Furicchia and Goshgarian (25)] but less than the average (760 μm) dendritic expanse of phrenic motoneurons (25). Free-floating sections were washed in PBS (3 × 10 min), preincubated in 10% of normal goat serum (NGS; Vector Labs, cat. no. S-100) for 2 h, and placed in primary rabbit polyclonal anti-GAD65/67 antibodies (1:250 with 5% of NGS in 0.1 M PBS; Millipore, cat. no. AB1511) for 48 h in a shaker at 4°C. Following this, sections were washed in PBS (3 × 10 min) and incubated in goat anti-rabbit biotinylated secondary antibodies (1:500; Vector Labs, cat. no. BA-1000) for 2 h. GAD65/67-positive sections were then processed with Vectastain ABC kit (Vector Labs, PK-6100), washed in PBS again (3 × 10 min), and visually explored using a microscope (Olympus-Bx51). In the case of neurobiotin-labeled neurons, double-labeling techniques were applied: neurobiotin-positive cells were identified (Fig. 1A1) with standard Vectastain ABC kit (Vector Labs, PK-6100), and GAD65/67-positive cells were detected using FITC-conjugated secondary antibodies (1:250, Vectors Labs, cat. no. FI-5000). Double-labeled cells were detected by superimposition of GAD65/67- and neurobiotin-positive cells from digital photomicrographs (Fig. 1, A2 and A3). Sections were mounted on gelatin-coated slides and covered with water-based mounting medium (Ted Pella, cat. no. 27212), to prevent fading of fluorescence and stored at 4°C in the dark.
Data Analysis
Electrophysiology.
Single-unit activity and general respiratory output variables, including respiratory rate (RR), inspiratory (Ti), and expiratory (Te) durations, inspiratory burst amplitude, and tonic activity duration and amplitude were determined using ≥50 respiratory cycles. Spike2 (version 5, Cambridge Electronic Design), MatLab (version R2011a, MathWorks), IBM SPSS STATISTICS 20, and custom-written scripts for measurement of parameters from PN and single-unit activity were used for data analysis. Event markers for single-neuron action potentials and for onset and offset of integrated (τ = 50 ms) phrenic nerve activity were derived from the raw recordings. Spike patterns of single units were analyzed by creating PN onset-triggered histograms (i.e., cycle-triggered histograms). PMNs were positively identified by the presence of a waveform peak in the unit's spike-triggered average (STA; >1,000 respiratory cycle) of the ipsilateral phrenic nerve and the absence of this waveform in a one respiratory cycle-shifted STA of the same (Fig. 2A; see also Ref. 54). Presumed interneurons (INs) were identified by the absence of uncorrelated waveform in the STA (Fig. 2B). The changes in amplitude for inspiratory and expiratory phases were calculated and reported as a percentage of control of maximal peak of averaged integrated activity during inspiration and background level during expiration before and 30, 45, 60, 75, 90, 105, and 120 min (“timestamps”) after the onset of siRNA microinjections (Fig. 3). To investigate dynamic changes in integrated PN activity over inspiratory and expiratory phase across all animals, respiratory phases were divided in 15 bins for each animal. The absolute value of inspiratory integrated activity and its relative increase (by subtracting of control values at each timestamp) was calculated with and without background influences by subtraction of averaged expiratory background level from inspiratory phase at each timestamp with corresponding P values calculated for each bin (Fig. 4, A–C). The normality of the data distribution was confirmed by applying Lilliefors test in MatLab. Depending on how well the data conformed to a normal distribution, either parametric (t-test) or nonparametric (Mann-Whitney U) tests were applied to compare two groups of data (e.g., pre-siRNA vs. post-siRNA injection).
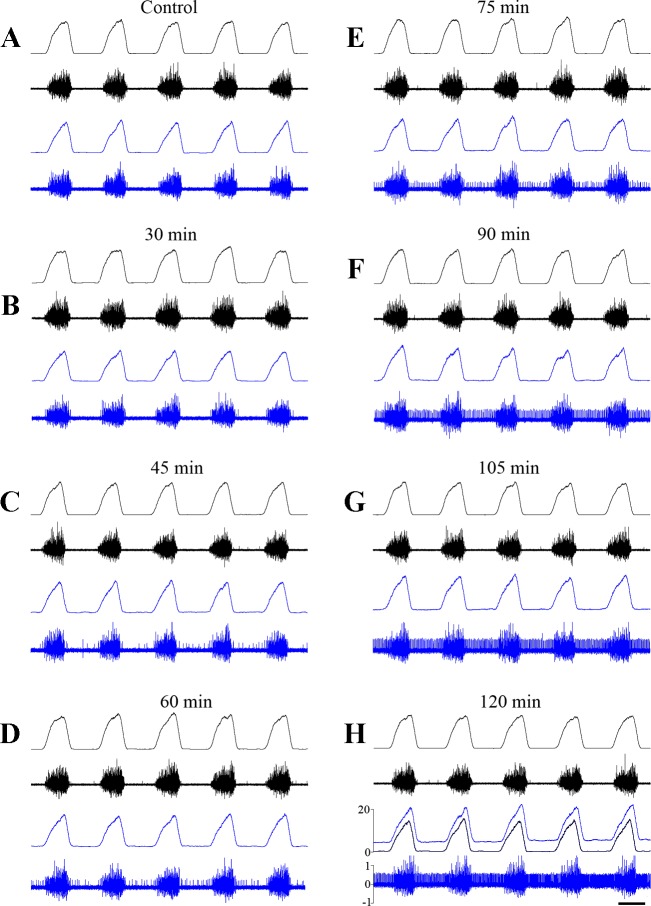
Fig. 3.
Dynamics of phrenic nerve activity during anti-GAD65/67 siRNA microinjection into the phrenic nucleus. Raw and integrated ipsilateral (blue) and contralateral (black) PN activity at different time points relative to the beginning of anti-GAD65/67 siRNA microinjections. A: before (Control) microinjections, and after: 30 min (B); 45 min (C); 60 min (D); 75 min (E); 90 min (F); 105 min (G); and 120 min (H). H, traces: overlapping of integrated ipsilateral PN traces before (black) and after 2 h (blue) of siRNA microinjections. Integrated PN activity in arbitrary units; raw activity in mV (×105). Time bar = 250 ms.
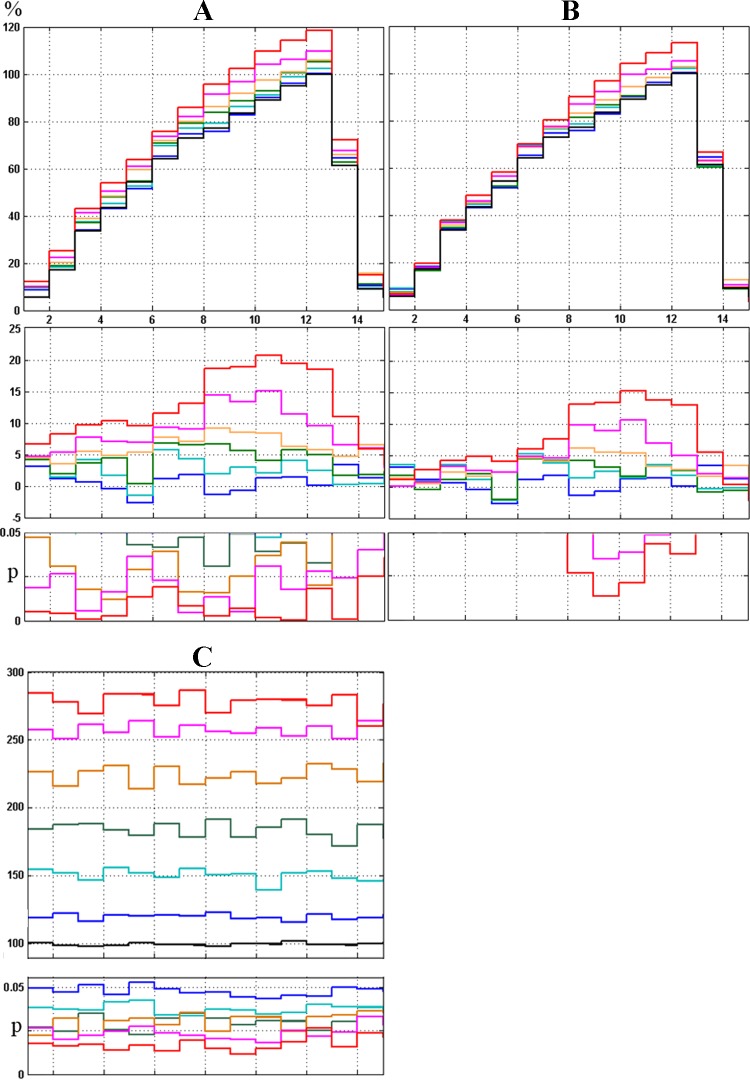
Fig. 4.
Dynamic changes in phrenic nerve phasic (inspiratory) and tonic background (expiratory) activity during anti-GAD65/67 siRNA microinjection. Across-animal normalized (in % to control preinjection state) inspiratory (A, top) and expiratory (C, top) integrated PN activity ipsilateral to siRNA injection at 0 (black), 45 (blue), 60 (cyan), 75 (green), 90 (orange), 105 (pink) and 120 (red) min following siRNA injection. A, middle: same as top, but with control values subtracted. A and C, bottom: bin P values with significance level of P < 0.05, compared with control. B, same as A, except that expiratory background activity has been subtracted. For correct averaging, respiratory phases were divided in 15 bins to avoid their differences in length in individual animals and across animal set (see Electrophysiology).
Histology.
GAD65/67-positive cells were counted from six sections on both sides in anti-GAD65/67 siRNA injection experiments and in control animals. Only neurons with identifiable nucleoli were counted. Photomicrographs of the selected neurons were made with a digital camera (Infinity-2; Lumenera) attached to an Olympus BX51 microscope and saved in TIFF format (300 dpi in space and 16 bits in color resolution, Photoshop 12.04, Adobe). GAD-positive cell outlines in all six sections were counted on these digital photomicrographs. Only cells with homogenous staining in the cytoplasmic compartment were identified as GAD-positive. The total average number of GAD-positive cells was counted from six sections (the average cumulative sum). These data were then normalized (in %) and analyzed in SigmaPlot for each Rexed's layer VII-IX in the ventral horn of spinal segments C3–C5, according to Paxinos and Watson's atlas of the rat brain (62). As we were not able to test for normality of cell distribution from six sections (due to insufficient number of samples), we assumed a priori a normal distribution of data. As negative control, GAD65/67-positive cells were also counted and analyzed from sections of five animals with injections of negative control siRNA and injections of saline.
For comparisons between multiple results, we used parametric (one-way and repeated-measures ANOVA) and nonparametric (Kruskal-Wallis and Friedman) tests. All values are indicated as means ± SD, or as means ± SE, as appropriate. SE measures are given when groups of standard deviations are compared. Differences were considered significant at the 95% confidence limit (P < 0.05).
RESULTS
Changes in Phrenic Nerve Activity During Anti-GAD-65/67 siRNA Microinjection into the C4 Ventral Horn
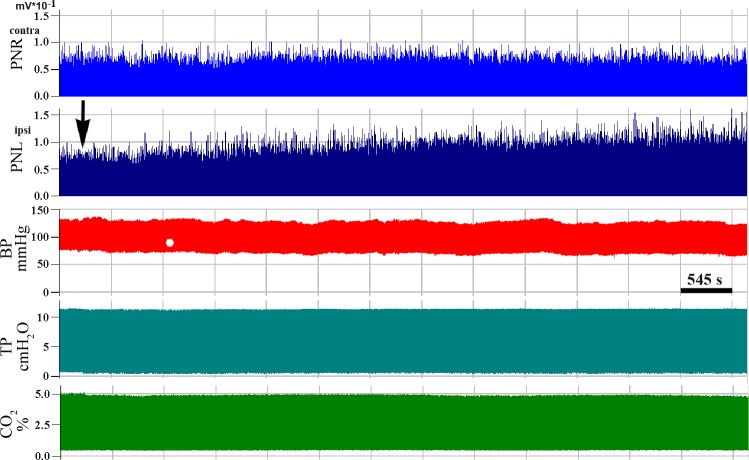
No changes in PN activity were observed during the first 30–45 min during injection, save for some irregular expiratory activity in the ipsilateral PN (Fig. 3, A–C). After 60–90 min (Figs. 3, D–F, and 7C), this low-amplitude expiratory discharge transformed into a regular tonic pattern [145 ± 12.7% of background (BG) preinjection level; % BG] and saturated after 2 h when a significant expiratory discharge became evident (281 ± 38.7% BG). An increase in PN burst amplitude during inspiration was also noted after 60–90 min (Figs. 3, D–F, and 4A) and plateaued after 120 min of injections. However, after subtraction of BG, the significant increase in PN inspiratory amplitude was found only during the second half of inspiration, 90–120 min after injections (Fig. 4B). No changes in phrenic nerve activity were detected on the contralateral side or in control experiments (data not shown). Also, we did not detect any changes in blood pressure during siRNA microinjections (Fig. 5).
Fig. 5.
Compressed record of phrenic nerve activity before and during anti-GAD65/67 siRNA monolateral microinjection into the phrenic nucleus. Traces, from top to bottom: integrated contralateral (contra, untreated right side, PNR) and ipsilateral (ipsi, treated left side, PNL) PN discharges. BP, blood pressure, TP, tracheal pressure, CO2, end-tidal CO2 level. Arrow indicates beginning of siRNA microinjection.
siRNA Reduction/Elimination of GAD-65/67 Expression in Ventral Horn
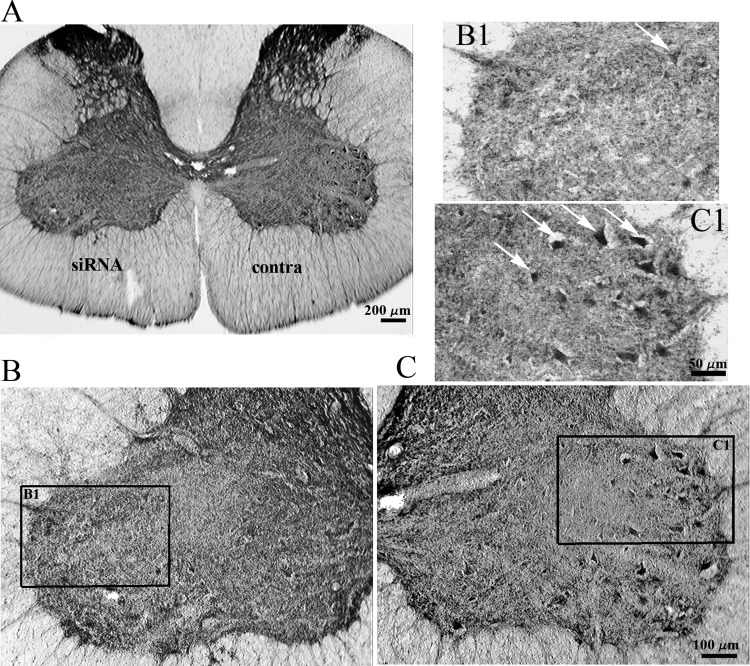
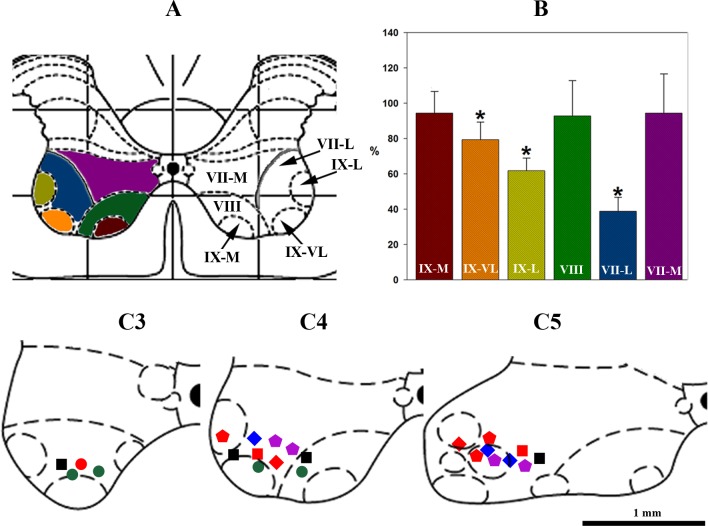
The average cumulative sum of GAD-positive cells in control (saline injections) experiments was 56.44 ± 11.13 in VII-L, 84.34 ± 14.9 in VII-M, 44.28 ± 10.65 in VIII, 35.1 ± 9.72 in IX-VL, and 24.78 ± 7.48 in IX-M. The number of GAD65/67-positive cells in the ventral horn of the C4 spinal segment was drastically reduced on the side of anti-GAD65/67 siRNA microinjections (Figs. 6B1 and 7B). The most prominent reduction in GAD65/67-positive cells was found in the lateral part of VII (38.87 ± 7.95% of control, VII-L) and IX (62.4 ± 9.7% of control, IX-L), and in ventrolateral part of IX (78.5 ± 6.9% of control, IX-VL). No significant changes in GAD65/67 immunofluorescence were detected in Rexed's laminae VIII, medial part of IX (IX-M), or the medial aspect of VII (VII-M). The distribution of GAD65/67-positive cells did not show significant differences on the contralateral side and in control experiments with negative control siRNA and saline microinjections.
Fig. 6.
Distribution of GAD65/67-containing spinal interneurons after unilateral anti-GAD65/67 siRNA microinjection into the C4 ventral horn. A: C4 transverse section following a 2-h unilateral anti-GAD65/67 siRNA block (siRNA) vs. contralateral side (contra). B and C: magnified view of ventral horn on ipsi (B) and contralateral side (C) showing clearly labeled GAD-positive neurons (white arrows indicate some in focal plane on high power magnification panels B1 and C1).
Firing Patterns of GABAergic Respiratory Interneurons of C3–C5 Ventral Horn
Eleven inspiratory interneurons with extended expiratory activity were recorded from the ventral horn at spinal levels C3–C5 (e.g., Fig. 1, C1 and C2 and Fig. 7, C3–C5). Six of them showed uniform (Fig. 7, C3–C5, I+E, squares) and five exhibited decrementing (Fig. 7, C3–C5, I-Dec+E, circles) firing frequency patterns during inspiration. Of these 11 cells, three I+E and two I-Dec+E interneurons were labeled with neurobiotin. Of the neurobiotin-labeled cells, two I+E and one I-Dec+E cells were GAD65/67-positive (Fig. 7, C3–C5, red squares and circles, respectively).
Fig. 7.
Changes in GAD65/67-immunopositive cells after anti-GAD65/67 siRNA injection into the C4 ventral horn, and location of respiratory interneurons. A: C4 transverse spinal section taken from Paxinos and Watson stereotaxic rat brain atlas (55), where from VII to IX are Rexed's layers in C4 ventral horn and M, L, and VL are medial, lateral, and ventrolateral layer subdivisions, respectively. Grid spacing is 1 mm. B: normalized (% of saline-microinjected control set of animals) number of GAD-65/67-positive cells after anti-GAD65/67 siRNA injection into the C4 ventral horn (colors of bars corresponded to layers in A); asterisks (*) show significant (P < 0.05) difference in GAD-65/67 expression compared with control. C3–C5: distribution of respiratory interneurons recorded from C3–C5 ventral horn: squares, inspiratory with extended expiratory activity (I+E; n = 6); circles, decrementing inspiratory with extended expiratory activity (I-Dec+E; n = 5); pentagons, augmenting expiratory (E-Aug; n = 7); diamonds, slow decrementing expiratory (E-Dec; n = 5). GAD65/67-positive (n = 8) cells are indicated in red and GAD65/67-negative cells are indicated by other solid colors (I+E, black squares; I-Dec+E, green circles; E-Aug, violet pentagons; E-Dec, blue diamonds), respectively. Scale bar is 1 mm.
Twelve interneurons that fired during expiration (Figs. 1, B1 and B2, and 7, C3–C5) were recorded. Seven of these exhibited augmenting activity during expiration (Fig. 7, C3–C5, E-Aug, pentagons), and five demonstrated slowly decrementing firing patterns (Fig. 7, C3–C5, E-Dec, diamonds). Of these 12 neurons, 7 were labeled with neurobiotin, 4 of which exhibited an augmenting pattern and 3 showed decrementing expiratory discharge. Of the neurobiotin-labeled cells, three of the E-Aug and two of the E-Dec neurons were GAD65/67-positive (Fig. 1, A1–A3 and Fig. 7, C3–C5, red pentagons and diamonds, respectively). On the basis of these results, five of the seven (∼71%) neurobiotin-labeled expiratory and three of the five (∼60%) inspiratory interneurons were GAD-positive.
DISCUSSION
General Findings
In the present study, we provide the first direct evidence for the existence of intraspinal GABAergic circuits contributing to the formation of phrenic output. There are three principal findings in this study. First, we found that GABAergic control of phrenic output is primarily phasic and contributes to regulation of phrenic discharge in late inspiration. Second, we demonstrated the existence of respiratory-modulated GABAergic SpINs (∼2/3 of those labeled with neurobiotin), both inspiratory and expiratory cells, in C3–C5 ventral horns located slightly dorsal to the phrenic nucleus. Lastly, changes in GAD65/67 expression were localized only on the side ipsilateral to siRNA microinjections. Finally, we detected tonic inhibitory influences on phrenic nerve discharge, which may arise from a single or multiple sources. These findings demonstrate that SpINs exert local, largely unilateral GABAergic control over phrenic output.
Although GABAergic inhibitory mechanisms have been implicated in respiratory pattern formation (13, 61, 77), the role of local spinal inhibitory neurons in controlling phrenic output is emerging. It has previously been shown that local GABAA receptor blockade in the PNucl causes increased phrenic nerve amplitude in all phases of respiration in decerebrate rats (53). In contrast, phrenic microinjections of the glycine receptor antagonist strychnine increased phrenic activity only during inspiration and postinspiration (53). In urethane-anesthetized rats, single injections of GABAA and GABAB antagonists into the PNucl caused transient increases in phrenic nerve amplitude (7). However, because these studies blocked receptors, it was not possible to determine the source (i.e., distal vs. local) of the inhibitory drive. The present study suggests that the source is within and around the PNucl.
Methodological Considerations
Methodological considerations played a crucial role in allowing us to characterize local GABAergic interneurons physiologically, vis-à-vis the effects of their functional downregulation. In contrast to pharmacological antagonists, which block target receptors and nonselectively interfere with descending and local GABAergic neurotransmission, we assume that siRNA caused the local downregulation of GABA synthesis in interneurons. Furthermore, the ability to reduce translation of a protein using RNA interference sufficiently to observe physiological effects requires the targeted protein to have a relatively short half-life of ∼2–3 h (75). In this study, we used local microinjections of siRNA against both (65 and 67 kDa) isoforms of glutamate decarboxylase, the enzyme responsible for synthesizing GABA, which allowed for selective neurotransmitter silencing of local intraspinal GABAergic interneuronal activity without affecting either respiratory rhythm generation, descending inhibitory pathways, or contralateral PN activity (see results). Thus, siRNA is a viable tool for silencing selective neuronal phenotypes if they express a specific neurotransmitter-synthesizing enzyme.
GAD-65/67 Expression
The number of GAD65/67-positive cells was drastically reduced on the side of siRNA microinjections (Figs. 6 and 7B), especially in the lateral aspects of layer VII. No significant changes in GAD65/67 immunoreactivity were observed in Rexed layers VIII, medial aspects of VII, or IX, which are located further from the injection site. Most likely, this was because an efficacious concentration of siRNA was not achieved in these regions.
In the mammalian CNS, the two forms of GAD—GAD65 and GAD67—are encoded by separate genes with 70% sequence homology (17, 18, 39). GAD65 and GAD67 colocalize in most GABAergic cells, but their expression levels vary in different subcellular compartments; GAD65 preferentially accumulates in axon terminals, whereas GAD67 is localized in both terminals and cell bodies (19). However, within the ventral horn of the spinal cord, there are a greater number of GAD67-immunoreactive profiles, mostly located around motoneurons (21).
The cervical level of spinal cord in adult rats contains few GAD67 neurons of medium or large size (>25 μm)—they were visible in layers VII, VIII, and IX. Fewer number of GAD65-positive neurons were also detected in ventral horn (21). The same distribution of GAD65 and GAD67 subunits among layers of spinal cord was demonstrated by Mackie et al. (52). As we used immunodetection of both 65- and 67-kDa GAD isoforms, these data are generally in agreement with our result showing a similar distribution of GAD-positive cells in the ventral horn.
Role of Ventrolateral Spinal GABAergic Circuits in Controlling Phrenic Output
The specific role of local GABAergic regulation of phrenic output was also investigated, with the purpose of ascribing this class of cells with tonic and/or phasic influence. In the PN ipsilateral to the siRNA-treated phrenic nucleus, a low-amplitude expiratory discharge became evident after 30–45 min of microinjections, increasing and becoming stable at 60–90 min, and finally plateaued at 2 h (Fig. 3). This demonstrates that local GABAergic neurons in the siRNA-affected regions (Fig. 7B) are involved in the control of phrenic motoneuron discharge during post-I and E2 phases of respiration. Additionally, we observed an increase in integrated PN inspiratory amplitude ipsilateral to the siRNA-treated phrenic nucleus. This increase became apparent at 60–90 min of microinjections, consistent with a role for these interneurons in phasic gain control, as it was shown for PMNs (61), bulbospinal (11), and propriobulbar respiratory neurons (77). Parkis et al. (61) suggested bulbospinal pathways as the main source of GABA-ergic inspiratory inhibition of PMNs but did not exclude involvement of spinal interneurons.
After subtraction of averaged expiratory background activity (which was increased by this treatment; see Fig. 3), the increase in inspiratory activity retained significance only during the second half of inspiration (Fig. 4). Our present results demonstrate a role of local GABAergic interneurons in controlling both tonic and phasic PN activity, which is consistent with (albeit more specific than) our previous results, where microinjections of the GABAA antagonist gabazine into the PNucl increased phrenic activity in all phases of respiration (53).
In the most simplified scheme, there are two possible connectivity patterns for these local GABAergic interneurons, which may exist in parallel (Fig. 8). GABAergic interneurons may be interposed in a three-neuron (or greater) descending relay, where bulbospinal units synapse on interneurons, which, in turn, synapse on phrenic motoneurons. Consistent with this potential model, bulbospinal projections to intercostal and abdominal respiratory motoneurons have been demonstrated extensively in the cat and rat (10, 12, 16, 20, 22, 40, 58, 72, 73) and, together with interneurons (4, 37, 42, 43, 74, 76, 84), may play an important role in integrating supraspinal and spinal inputs (63, 82). The interesting results considering medullary respiratory GABA-ergic neurons as exclusively propriobulbar (but not bulbospinal or cranial motoneurons) were demonstrated by Yamazaki et al. (86) and Okazaki et al. (57) in cats and rats. Taking into account these observations, we may suggest an important role of intraspinal inhibitory circuits in the control of respiratory motoneurons under normal (eupneic) and pathological conditions. Alternatively, inhibitory inspiratory interneurons may represent Renshaw cells, first described in the cat (75) and located in Rexed layer VII, which receive motoneuronal excitation and recurrently inhibit the same. The existence of Renshaw recurrent inhibition has been demonstrated for phrenic (30, 31, 49) and intercostal respiratory motoneurons (30, 41). These parallel models are further complicated by potential supraspinal drive of Renshaw cells (31). Additionally, it has been also shown that some spinal interneurons corelease both glycine and GABA to activate functionally distinct receptors in their postsynaptic target cells (38), and these “mixture” synapses represent about 20–22% of total GABAergic and glycinergic inputs to motoneurons (50). GABAA receptor subunit composition in lamina IX exhibits extraordinary complexity, suggesting the presence of multiple receptor subtypes within single motoneurons and a rather significant heterogeneity in the expression of these subtypes among the different neurons (70).
Fig. 8.
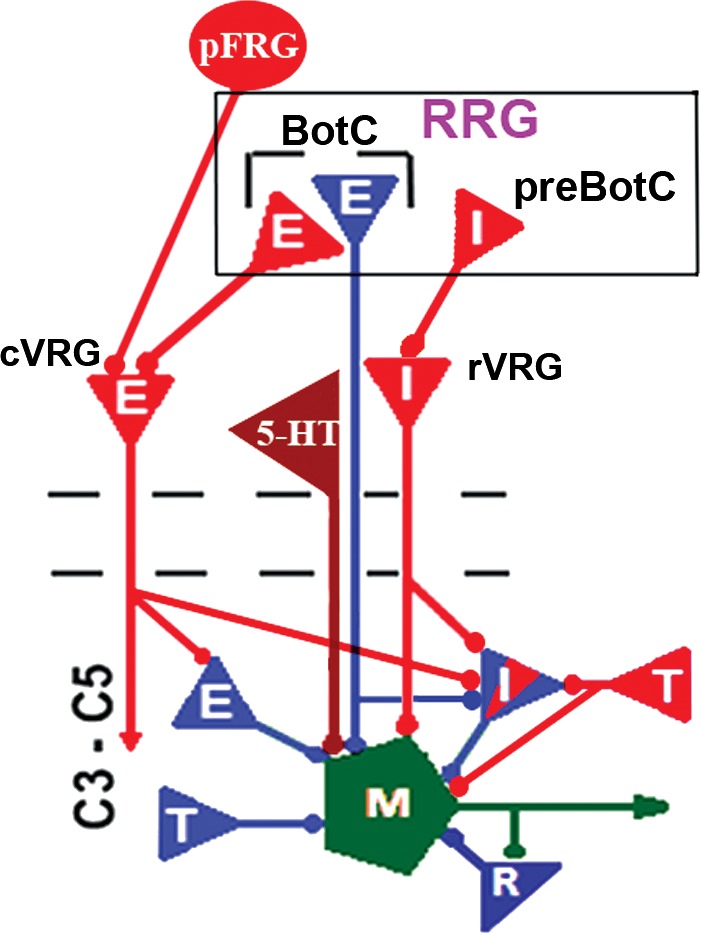
Hypothetical scheme of interactions between spinal respiration-related neurons and descending bulbospinal commands. Excitatory and inhibitory elements (somata and axons) are shown in red and blue, respectively. I, E, T, M (green) and R denote inspiratory, expiratory, tonic, phrenic motoneuron and Renshaw neuronal somata and axons, respectively. pFRG, parafacial respiratory group (possible generator for active expiration; see Refs. 29 and 35). BötC, Bötzinger complex; preBötC, preBötzinger complex; rVRG, rostral ventral respiratory group. cVRG, caudal ventral respiratory group; 5-HT, raphe-spinal (tonic excitatory) pathways. Phrenic motoneurons receive excitatory inspiratory drive from bulbospinal neurons located in rVRG (10, 12, 16, 20, 22, 71). rVRG neurons, in turn, are excited by preinspiratory preBötC neurons, part of the kernel of respiratory rhythm generation (RRG). Phrenic motoneurons receive direct inhibitory drive from BötC (84) during expiration (blue E). Inspiratory bulbospinal drive from the rVRG may activate excitatory and inhibitory (for e.g., GABAergic- and glycine-ergic) local inspiratory interneurons (blue and red I). During the expiratory phase, pFRG and BötC also excites (red E) expiratory bulbospinal neurons located in cVRG (1, 6). Descending cVRG axons may excite (red E) spinal intercostal and abdominal expiratory motoneurons (not pictured), and their collaterals may excite GABAergic expiratory (blue E) interneurons located in the ventral horn of C3–C5. Raphe-spinal 5-HT-containing pathways (dark red 5-HT) may play an important role in the maintenance of tonic excitatory support of phrenic motoneurons. Renshaw cells, and tonic inhibitory (e.g., GABAergic- and glycine-ergic, blue T) and excitatory (red T) interneurons are shown as additional inhibitory intraspinal circuit elements.
Proposed Function of Spinal Interneurons in Phrenic Pattern Formation
All recorded interneurons were located within and dorsal to the PNucl, which is in agreement with similar results obtained from cats (3, 14) and rabbits (60). The most consistent finding was that almost all respiratory interneurons possessed some degree of tonic activity throughout all phases of respiration and exhibited preferential phasic firing during specific respiratory phases (inspiratory, expiratory).
A potential functional role of respiratory spinal interneurons is also suggested by the finding that rhythmic bursting sometimes has been reported in phrenic nerve discharge following high cervical (C1–C2 and C2–C3) spinalization (2, 9, 64, 85). This indicates some rhythmogenic capability inherent in the local high cervical (C1–C2) and/or phrenic circuits. Another potential role, perhaps in limiting the range of excitability in the local circuit, is suggested by the induction of spinal phrenic reflexes following PN stimulation (81). It has been proposed that spinal interneurons, including Renshaw cells, play an important role in the integration of segmental and suprasegmental inputs (3, 28, 33, 82, 83) and may be involved in plasticity mechanisms involved in recovery of respiration following spinal cord injury (44–48).
Conclusion
In conclusion, we demonstrate the functional role of local GABAergic interneurons in the C4 ventral horn regulating tonic and phasic activity of phrenic output. This is only the first step in more thoroughly differentiating local vs. bulbospinal circuit interactions involved in respiratory pattern formation. The existence of excitatory SpINs as parallel regulators of motoneuron excitability, integrating supraspinal and spinal inputs, may also play a role in the same. It is evident that highly complex supraspinal and intraspinal networks appear to interact to affect a precise and flexible control of phrenic output. Moreover, it is clear that the phrenic motoneurons and interneurons are not simple relays of respiratory drive, as they actively and dynamically process descending and local inputs. On the basis of our results, a hypothetical schematic of possible interactions between spinal respiration-related neurons and descending bulbospinal inputs is proposed (Fig. 8). Further studies are needed to more thoroughly characterize and delineate the roles of descending and local fast synaptic inhibition in phrenic pattern formation.
Perspectives and Significance
Our results show that selective blockade of GAD65/67 synthesis with siRNA opens a new approach to the investigation of the role of spinal GABA-ergic circuits involved in pattern formation of respiratory motor output and may be applied to studying other CNS microcircuits. Silencing via siRNA is a powerful experimental approach, and simpler than e.g., optogenetic techniques, in the manipulation of neurotransmitter-specific cell populations in selected brain regions. The future direction of the current project includes more thorough study of SpINs located in different layers of ventral spinal cord in combination with transsynaptic and monosynaptic retrograde tracing (pseudorabies virus and cholera toxin B) and identification of neurotransmitter (GABA-, glycine-ergic or glutamatergic) and spiking patterns of labeled cells. Detailed information about SpINs connected to respiratory motoneurons may change our basic view on the spinal mechanisms involved in breathing pattern formation during eupnoea and in challenges, such as hypercapnia, hypoxia, and resistive loading of upper airways. The role of SpINs in compensatory (plastic) adaptation of breathing during different pathological conditions (e.g., chronic obstructive pulmonary diseases, amyotrophic lateral sclerosis, and spinal cord injury) may also be addressed, as has already been discussed (27, 32, 44–47), and may lead to developing new treatment strategies.
GRANTS
This work was supported by the grant from Craig H. Neilsen Foundation (CHNF Grant 224089), National Institutes of Health (1R01NS069220-01A1) and Drexel University College of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.M. conception and design of research; V.M., M.G.Z.G., and R.F.R. performed experiments; V.M., M.G.Z.G., and R.F.R. analyzed data; V.M., M.G.Z.G., and R.F.R. interpreted results of experiments; V.M. prepared figures; V.M., M.G.Z.G., and R.F.R. drafted manuscript; V.M., M.G.Z.G., and R.F.R. edited and revised manuscript; V.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address: Robert F. Rogers, CHDI Management, 156 Village Blvd., Ste. 200, Princeton, NJ 08540.
REFERENCES
- 1.Anders K, Ballantyne D, Bischoff AM, Lalley PM, and Richter DW. Inhibition of caudal medullary expiratory neurones by retrofacial inspiratory neurones in the cat. J Physiol 437: 1–25, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki M, Watanabe H, Ebata N, Mori S. Spontaneous respiratory activity in spinal cats. Brain Res 157: 376–380, 1978. [DOI] [PubMed] [Google Scholar]
- 3.Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res 533: 141–146, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol 82: 1224–1232, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol 42: 76–90, 1979. [DOI] [PubMed] [Google Scholar]
- 6.Bongianni F, Corda M, Fontana G, Pantaleo T. Expiration-related neurons in the caudal ventral respiratory group of the cat: influences of the activation of Bötzinger complex neurons cat: influences of the activation of Bötzinger complex neurons. Brain Res 526: 299–302, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Chitravanshi VC, Sapru HN. GABA receptors in the phrenic nucleus of the rat. Am J Physiol Regul Integr Comp Physiol 276: R420–R428, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Cleland CI, Getting PA. Respiratory-modulated and phrenic afferent-driven neurons in the cervical spinal cord (C4–C6) of the fluorocarbon-perfused guinea pig. Exp Brain Res 93: 307–311, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Coglianese CJ, Peiss CN, Wurster RD. Rhythmic phrenic nerve activity in spinal dogs. Respir Physiol 29: 247–257, 1977. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MI, Piercey MF, Gootman PM, Wolotsky P. Synaptic connections between medullary inspiratory neurons and phrenic motoneurons as revealed by cross-correlation. Brain Res 81: 319–324, 1974. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MI, Feldman JL. Discharge properties of dorsal medullary inspiratory neurons: relation to pulmonary afferent and phrenic efferent discharge. J Neurophysiol 51: 753–776, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Davies JG, Kirkwood PA, Sears TA. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol 368: 63–87, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogas Z, Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Differential effects of GABAA receptor antagonists in the control of respiratory neuronal discharge patterns. J Neurophysiol 80: 2368–2377, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Douse MA, Duffin J. Axonal projections and synaptic connections of C5 segment expiratory interneurones in the cat. J Physiol 470: 431–444, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffin J, Iscoe S. The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp Brain Res 112: 35–40, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Ellenberger HH, Feldman JL, Goshgarjan HG. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J Comp Neurol 302: 707–714, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res 16: 215–226, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron 7: 91–100, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci 14: 1834–1855, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedorko L, Merrill EG, Lipski J. Two descending medullary inspiratory pathways to phrenic motoneurones. Neurosci Lett 43: 285–291, 1983. [DOI] [PubMed] [Google Scholar]
- 21.Feldblum S, Dumoulin A, Anoal M, Sandillon F, Privat A. Comparative distribution of GAD65 and GAD67 mRNAs and proteins in the rat spinal cord supports a differential regulation of these two glutamate decarboxylases in vivo. J Neurosci Res 42: 742–757, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci 5: 1993–2000, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–242, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75: 423–452, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furicchia JV, Goshgarian HG. Dendritic organization of phrenic motoneurons in the adult rat. Exp Neurol 96: 62l–634, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and golgi study. J Comp Neurol 201: 441–456, 1981. [DOI] [PubMed] [Google Scholar]
- 27.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 4: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Grelot L, Milano S, Portillo F, Miller AD. Respiratory interneurons of the lower cervical (C4–C5) cord: membrane potential changes during fictive coughing, vomiting, and swallowing in the decerebrate cat. Pflügers Arch 425: 313–320, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Guyenet PG, Mulkey DC. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol 173: 244–255, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilaire G, Khatib M, Monteau R. Spontaneous respiratory activity of phrenic and intercostal Renshaw cells. Neurosci Lett 43: 97–101, 1983. [DOI] [PubMed] [Google Scholar]
- 31.Hilaire G, Khatib M, Monteau R. Central drive on Renshaw cells coupled with phrenic motoneurons. Brain Res 376: 133–139, 1986. [DOI] [PubMed] [Google Scholar]
- 32.Hossaini M, Cano SC, Vera van Dis Haasdijk ED, Hoogenraad CC, Holstege JC, Jaarsma D. Spinal inhibitory interneuron pathology follows motor neuron degeneration independent of glial mutant superoxide dismutase 1 expression in SOD1-ALS mice. J Neuropathol Exp Neurol 8: 662–677, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Iscoe S, Duffin J. Effects of stimulation of phrenic afferents on cervical respiratory interneurones and phrenic motoneurones in cats. J Physiol 497: 803–812, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir Physiol 26: 113–128, 1976. [DOI] [PubMed] [Google Scholar]
- 35.Janczewski WA, Feldman JL. Novel data supporting the two respiratory rhythm oscillator hypothesis. Focus on “Respiration-Related Rhythmic Activity in the Rostral Medulla of Newborn Rats”. J Neurophysiol 96: 1–2, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol 58: 105–124, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Johnson IP, Sears TA. Ultrastructure of interneurons within motor nuclei of the thoracic region of the spinal cord of the adult cat. J Anat 161: 171–85, 1988. [PMC free article] [PubMed] [Google Scholar]
- 38.Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science 281: 419–424, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman DL, Houser CR, Tobin AJ. Two forms of the γ-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem 56: 720–723, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkwood PA, Sears TA. Proceedings: Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol 234: 87P–89P, 1973. [PubMed] [Google Scholar]
- 41.Kirkwood PA, Sears TA, Westgaard RH. Recurrent inhibition of intercostal motoneurones in the cat. J Physiol 319: 111–130, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirkwood PA, Munson JB, Sears TA, Westgaard RH. Respiratory interneurons in the thoracic spinal cord of the cat. J Physiol 395: 161–192, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkwood PA, Schmid K, Sears TA. Functional identities of thoracic respiratory interneurones in the cat. J Physiol 461: 667–687, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci 31: 538–547, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol 169: 123–132, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol 179: 3–13, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Lee KZ, Dougherty BJ, Sandhu MS, Lane MA, Reier PJ, Fuller DD. Phrenic motoneuron discharge patterns following chronic cervical spinal cord injury. Exp Neurol 249: 20–32, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipski J, Fyffe RE, Jodkowski J. Recurrent inhibition of cat phrenic motoneurons. J Neurosci 5: 1545–1555, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu TT, Bannatyne BA, Maxwell DJ. Organization and neurochemical properties of intersegmental interneurons in the lumbar enlargement of the adult rat. Neuroscience 171: 461–484, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Lumsden T. Observations on the respiratory centres in the cat. J Physiol 57: 153–160, 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackie M, Hughes DI, Maxwell DJ, Tillakaratne NJ, Todd AJ. Distribution and colocalisation of glutamate decarboxylase isoforms in the rat spinal cord. Neuroscience 119: 461–472, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Marchenko V, Rogers RF. GABAAergic and glycinergic inhibition in the phrenic nucleus organizes and couples fast oscillations in motor output. J Neurophysiol 101: 2134–2145, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Marchenko V, Ghali MG, Rogers RF. Motoneuron firing patterns underlying fast oscillations in phrenic nerve discharge in the rat. J Neurophysiol 108: 2134–2143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merrill EG. Finding a respiratory function for the medullary neurons. In: Essays on the Nervous System, edited by Bellaris R and Gray EG. Oxford, UK: Clarendon Press: 1974, p. 451–486. [Google Scholar]
- 56.Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res 333: 325–329, 1985. [DOI] [PubMed] [Google Scholar]
- 57.Okazaki M, Takeda R, Haji A, Yamazaki H. Glutamic acid decarboxylase-immunoreactivity of bulbar respiratory neurons identified by intracellular recording and labeling in rats. Brain Res 914: 34–47, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Onai T, Saji M, Miura M. Projections of supraspinal structures to the phrenic motor nucleus in rats studied by a horseradish peroxidase microinjection method. J Auton Nerv Syst 21: 233–239, 1987. [DOI] [PubMed] [Google Scholar]
- 59.Palises R, Viala D. Existence of respiratory interneurons in the cervical spinal cord of the rabbit. CR Acad Sci III 305: 321–324, 1987. [PubMed] [Google Scholar]
- 60.Palisses R, Perségol L, Viala D. Evidence for respiratory interneurones in the C3–C5 cervical spinal cord in the decorticate rabbit. Exp Brain Res 78: 624–632, 1989. [DOI] [PubMed] [Google Scholar]
- 61.Parkis MA, Dong XW, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. J Neurosci 19: 2368–2380, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam, The Netherlands: Elsevier, 2007. [Google Scholar]
- 63.Qin C, Chandler MJ, Foreman RD, Farber JP. Upper thoracic respiratory interneurons integrate noxious somatic and visceral information in rats. J Neurophysiol 88: 2215–2223, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Reinoso MA, Sieck GC, Hubmayr RD. Respiratory muscle coordination in acute spinal dogs. Respir Physiol 104: 29–37, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Renshaw B. Central effects of centripetal impulses in axons of spinal ventral roots. J Neurophysiol 9: 191–204, 1946. [DOI] [PubMed] [Google Scholar]
- 66.Richter DW, Ballantyne D. A three phase theory about the basic respiratory pattern generator. In: Central Neurone Environment, edited by Schlafke M, Koepchen H, and See W. Berlin, Germany: Springer, 1983, p. 164–174. [Google Scholar]
- 67.Rybak IA, Paton JFR, Rogers RF, St-John WM. Generation of the respiratory rhythm: State- dependency and switching. Neurocomputing 44–46: 605–614, 2002. [Google Scholar]
- 68.Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and statedependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res 165: 201–220, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rybak IA, Smith JC. Computational modeling of the respiratory network. In: Encyclopedia of Neuroscience, edited by Binder MD, Hirokawa N, Windhorst U, Hirsch MC et al., Berlin, Germany: Springer-Verlag, 2009, p. 824–832. [Google Scholar]
- 70.Ruano D, Létang V, Biton B, Avenet P, Benavides J, Scatton B, Vitorica J. Subunit composition of rat ventral spinal cord GABA(A) receptors, assessed by single cell RT-multiplex PCR. Neuroreport 11: 3169–3173, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Saether K, Hilaire G, Monteau R. Dorsal and ventral respiratory groups of neurons in the medulla of the rat. Brain Res 419: 87–96, 1987. [DOI] [PubMed] [Google Scholar]
- 72.Sasaki SI, Uchino H, Uchino Y. Axon branching of medullary expiratory neurons in the lumbar and the sacral spinal cord of the cat. Brain Res 648: 229–238, 1994. [DOI] [PubMed] [Google Scholar]
- 73.Saywell SA, Anissimova NP, Ford TW, Meehan CF, Kirkwood PA. The respiratory drive to thoracic motoneurones in the cat and its relation to the connections from expiratory bulbospinal neurones. J Physiol 579: 765–782, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saywell SA, Ford TW, Meehan CF, Todd AJ, Kirkwood PA. Electrophysiological and morphological characterization of propriospinal interneurons in the thoracic spinal cord. Neurophysiology 105: 806–826, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schlosser M, Walschus U, Klöting I, Walther R. Determination of glutamic acid decarboxylase (GAD65) in pancreatic islets and its in vitro and in vivo degradation kinetics in serum using a highly sensitive enzyme immunoassay. Dis Markers 24: 191–198, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmid K, Kirkwood PA, Munson JB, Shen E, Sears TA. Contralateral projections of thoracic respiratory interneurones in the cat. J Physiol 461: 647–665, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmid K, Foutz AS, Denavit-Saubie M. Inhibitions mediated by glycine and GABAa receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res 710: 150–160, 1996. [DOI] [PubMed] [Google Scholar]
- 78.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci 364: 2577–2587, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith JC, Abdala APL, Borgmann A, Rybak IA, Paton JFR. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci 36: 152–162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith JC, Richter DW. Respiratory rhythm generation in vivo. Physiology 29: 58–71, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Speck DF, Revelette WR. Attenuation of phrenic motor discharge by phrenic nerve afferents. J Appl Physiol 62: 941–945, 1987. [DOI] [PubMed] [Google Scholar]
- 82.Sumi T, Kotani S. Excitatory and inhibibitory reflexogenic skin areas for the intercostal respiratory neurons in the dog. Acta Med Okayama 13: 301–313, 1959. [Google Scholar]
- 83.Sumi T. Organization of spinal respiratory neurons. Ann NY Acad Sci 109: 561–570, 1963. [DOI] [PubMed] [Google Scholar]
- 84.Tian GF, Peever JH, Duffin J. Bötzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Exp Brain Res 122: 149–156, 1998. [DOI] [PubMed] [Google Scholar]
- 85.Viala D, Freton E. Evidence for respiratory and locomotor pattern generators in the rabbit cervico-thoracic cord and for their interactions. Exp Brain Res 49: 247–256, 1983. [DOI] [PubMed] [Google Scholar]
- 86.Yamazaki H, Haji A, Okazaki M, Takeda R. Immunoreactivity for glutamic acid decarboxylase and N-methyl-d-aspartate receptors of intracellularly labeled respiratory neurons in the cat. Neurosci Lett 293: 61–64, 2000. [DOI] [PubMed] [Google Scholar]
- 87.Yates BJ, Smail JA, Stocker SD, Card JP. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience 90: 1501–1513, 1999. [DOI] [PubMed] [Google Scholar]