SUMMARY
Staphylococcus aureus is a major human pathogen that causes a wide range of clinical infections. It is a leading cause of bacteremia and infective endocarditis as well as osteoarticular, skin and soft tissue, pleuropulmonary, and device-related infections. This review comprehensively covers the epidemiology, pathophysiology, clinical manifestations, and management of each of these clinical entities. The past 2 decades have witnessed two clear shifts in the epidemiology of S. aureus infections: first, a growing number of health care-associated infections, particularly seen in infective endocarditis and prosthetic device infections, and second, an epidemic of community-associated skin and soft tissue infections driven by strains with certain virulence factors and resistance to β-lactam antibiotics. In reviewing the literature to support management strategies for these clinical manifestations, we also highlight the paucity of high-quality evidence for many key clinical questions.
INTRODUCTION
Staphylococcus aureus is both a commensal bacterium and a human pathogen. Approximately 30% of the human population is colonized with S. aureus (1). Simultaneously, it is a leading cause of bacteremia and infective endocarditis (IE) as well as osteoarticular, skin and soft tissue, pleuropulmonary, and device-related infections. Our aim in this review is to summarize recent developments in the epidemiology, pathophysiology, clinical manifestations, and management of these key S. aureus clinical infection syndromes. We do not address in any significant depth issues regarding colonization or mechanisms of drug resistance and refer readers to recent reviews (1–6).
STAPHYLOCOCCUS AUREUS BACTEREMIA
Bacteremia is perhaps the best-described manifestation of S. aureus infection. Multiple studies have now documented the prevalence, prognosis, and outcome of S. aureus bacteremia (SAB) in industrialized regions of the world. However, many basic questions about the epidemiology of SAB, particularly in the world's nonindustrialized regions, remain unanswered. Furthermore, there continues to be a paucity of high-quality evidence to guide the management of SAB.
Epidemiology
Longitudinal trends.
In the industrialized world, the population incidence of SAB ranges from 10 to 30 per 100,000 person-years (7). Longitudinal data from Denmark provide considerable insight into the impact of changes in access to health care interventions on SAB incidence. Between 1957 and 1990, the incidence of SAB increased from 3 per 100,000 person-years to 20 per 100,000 person-years (8). Rates of both hospital admissions and invasive medical interventions increased exponentially in Denmark during the same period. As a result, nosocomial acquisition was a key contributor to these overall increases in the incidence of SAB. Since 1990, however, the overall SAB incidence in Denmark has been relatively stable at ∼21.8 per 100,000 person-years (9).
While overall rates of SAB may have stabilized over the past 20 years, the contribution of methicillin-resistant S. aureus (MRSA) has fluctuated. For example, in Quebec, Canada, the incidence of MRSA bacteremia increased from 0 per 100,000 person-years to 7.4 per 100,000 person-years from 1991 to2005, despite stable rates of methicillin-susceptible S. aureus (MSSA) bacteremia during the same period (10). Similar trends of increasing MRSA bacteremia incidence over this time period were seen in Minnesota from 1998 to 2005 (11); Calgary, Canada, from 2000 to 2006 (12); and Oxfordshire, United Kingdom, from 1997 to 2003 (13). In North America, epidemic community-associated clones of MRSA (e.g., USA300) have been largely responsible for the increase in the incidence of MRSA bacteremia (12, 14), while in the United Kingdom, epidemic health care-associated clones of MRSA (United Kingdom EMRSA-15 and EMRSA-16) have been responsible (15). Since 2005, most of these same regions have experienced significant reductions in rates of MRSA bacteremia, almost certainly linked to improvements in infection control procedures. These reductions were especially evident in the United Kingdom, where rates of MRSA bacteremia were halved between 2004 and 2011 (16, 17), but have also been documented in the United States (18), Australia (19), and France (20).
Nonindustrialized settings.
Far less is known about the incidence and burden of SAB in the nonindustrialized and newly industrialized regions of the world. Although the overall incidence of community-acquired SAB during 2004 to 2010 in northeast Thailand was 2.5 per 100,000 person-years (21), this study reported incidence rates for community-acquired SAB only. Incomplete case ascertainment may also have contributed to this low reported incidence. In contrast, the incidences of SAB were 27 per 100,000 person-years among children <5 years of age in Kilifi, Kenya (22); 48 per 100,000 person-years among children <15 years of age in Manhica District, Mozambique (23); and 26 per 100,000 person-years among children <13 years of age in Soweto, South Africa (24). Collectively, these reports underscore the clear need for population-based studies to determine the burden of S. aureus in nonindustrialized regions of the world.
Risk groups.
Age is a powerful determinant of SAB incidence, with the highest rates of infection occurring at either extreme of life (7, 10–12, 14, 25–28). Studies consistently demonstrate high rates in the first year of life, a low incidence through young adulthood, and a gradual rise in incidence with advancing age. For example, the incidence of SAB is >100 per 100,000 person-years among subjects >70 years of age (7) but is only 4.7 per 100,000 person-years in younger, healthier U.S. military personnel (29). Male gender is consistently associated with increased SAB incidence (10, 14, 25, 26, 29), with male-to-female ratios of ∼1.5. The basis for this increased risk is not understood.
The incidence of SAB is also associated with ethnicity. In the United States, the incidence of invasive MRSA in the black population (66.5 per 100,000 person-years) is over twice that in the white population (27.7 per 100,000 person-years) (14, 18). In Australia, the incidence of SAB in the indigenous population is 5.8 to 20 times that of nonindigenous Australians (30–32). Similarly, Maori and Pacific Island people have significantly higher rates of incidence of SAB than do those of European ethnicity in New Zealand (33, 34). Differences in markers of the socioeconomic status of indigenous compared to nonindigenous populations do not fully explain the disparity between these groups (31). The contribution of host genetic susceptibility to these ethnic differences has not yet been investigated.
The HIV-infected population has a significantly increased incidence of SAB. Two studies reported incidences of SAB in HIV-infected patients of 494 per 100,000 person-years (35) and 1,960 per 100,000 person-years (36), or 24 times that of the non-HIV-infected population (35). Although much of this increase results from high rates of injection drug use in the HIV-infected population, even the non-injection drug-using HIV-infected population exhibits higher rates of SAB than those in the non-HIV-infected population (35). Among HIV-infected individuals, a low CD4 count was independently associated with SAB. Also, compared to injection drug users (IDUs), men who have sex with men (MSM) were likely to have a low CD4 count and to have nosocomial SAB (35). Thus, HIV-infected IDUs tend to acquire community-onset SAB as a consequence of injection drug use, whereas MSM have higher rates of nosocomial SAB.
The high risk of SAB in the overall IDU population can be inferred from a Dutch study that monitored 758 IDUs for 1,640 person-years and determined that there were 10 confirmed episodes of S. aureus IE (37). Based on these figures, the incidence of SAB was at least 610 per 100,000 person-years. In the setting of injection of material into the bloodstream, additional factors contributing to the high incidence of SAB include an increased prevalence of S. aureus colonization compared to that in the general population (38), frequent skin and soft tissue infections (SSTIs) (39), and a drug-using environment that facilitates the person-to-person transmission of S. aureus (40).
Hemodialysis patients are also at a greatly increased risk of SAB. The incidences of SAB in hemodialysis-dependent patients were 3,064 per 100,000 person-years in Taiwan (41), 17,900 per 100,000 person-years in Ireland (42), and 4,045 to 5,015 per 100,000 person-years in the United States (18). The predominant risk factor for these patients is the presence of an intravascular access device and in particular the use of a cuffed, tunneled catheter (e.g., permacath) for dialysis (42). However, other host factors that result in an impairment of the host immune defense, including neutrophil dysfunction (43), iron overload (44), diabetes (45), and increased rates of colonization (45), may also increase the likelihood of invasive S. aureus infections. The infrequent vancomycin dosing strategy often used among hemodialysis-dependent patients may not maintain an adequate trough level in high-flux, large-pore-size artificial kidneys (46–48), increasing the risk for relapsing SAB.
Table 1 summarizes the incidences of SAB from the above-mentioned studies and other studies (49–55).
TABLE 1.
Incidence of S. aureus bacteremia per 100,000 person-years in different subpopulations and geographical regions
| Population | Region(s) | Time period (yr) | Incidence per 100,000 person-years for all S. aureus isolates (incidence for MRSA isolates)a | Reference |
|---|---|---|---|---|
| All | Denmark | 1957–1990 | 3–20 (NA) | 8 |
| Adults ≥21 yr of age | Denmark | 1981–2000 | 18.2–30.5 (NA) | 49 |
| All | Denmark | 1995–2008 | 22.7 (0.18) | 9 |
| Adults ≥18 yr of age | Iceland | 1995–2008 | 24.5 (0.15) | 25 |
| All | Finland | 1995–2001 | 14 (<0.14) | 27 |
| All | 7 countries | 2000–2008 | 26.1 (1.9) | 7 |
| All | Sweden | 2003–2005 | 33.9 (0) | 55 |
| All | Finland | 2004–2007 | 20 (NA) | 50 |
| All | Netherlands | 2009 | 19.3 (0.18) | 51 |
| All | North Rhine-Westphalia, Germany | 2009 | NA (5.76) | 51 |
| Adults ≥18 yr of age | Quebec | 1991–2005 | 24.1–32.4 (0–7.4) | 10 |
| Adults ≥18 yr of age | Olmsted County, MN, USA | 1998–2005 | 38.2 (12.4) | 11 |
| All | New Zealand | 1998–2005 | 21.5 (0.08) | 26 |
| All | Calgary, Canada | 2000–2006 | 19.7 (2.2) | 12 |
| All | USA | 2004–2005 | NA (31.8) | 14 |
| All, military | USA | 2005–2010 | 4.7 (2) | 29 |
| All | NT, Australia | 2006–2007 | 65 (16) | 30 |
| All | Australia | 2007–2010 | 11.2 (16) | 31 |
| All, CA | Northeast Thailand | 2004–2010 | 2.6 (0.1) | 21 |
| Children ≤20 yr of age | Denmark | 1971–2000 | 4.5–8.4 (NA) | 52 |
| Children ≤18 yr of age | Calgary | 2000–2006 | 6.5 (0.05) | 53 |
| Children <5 yr of age | Kenya | 1998–2002 | 27 (NA) | 22 |
| Children <15 yr of age | Mozambique | 2001–2006 | 48 (4.3) | 23 |
| Children <5 yr of age | Ghana | 2007–2009 | 630 (105) | 54 |
| Children <13 yr of age | South Africa | 2005–2006 | 26 (10) | 24 |
| HIV, ≥16 yr of age | Denmark | 1995–2007 | 494 (4.9) | 35 |
| HIV, adult | USA | 2000–2004 | 1,960 (850) | 36 |
| Hemodialysis | Ireland | 1998–2009 | 17,000 (5,600) | 42 |
| All dialysis | Taiwan | 2003–2008 | 1,809 (1,131) | 41 |
NA, not available.
Clinical Manifestations
Although there are many different primary clinical foci or manifestations of SAB, there are consistent patterns across cohorts. In several recent studies involving consecutive patients with either SAB (MSSA and MRSA) (12, 31, 32, 56–60) or only MRSA bacteremia (61–66), common primary clinical foci or sources of infection are vascular catheter-related infections, SSTIs, pleuropulmonary infections, osteoarticular infections, and IE (Table 2). These common primary clinical foci represent a subset of the common general clinical manifestations of S. aureus infections. However, a focus of infection is not found in ∼25% of cases.
TABLE 2.
Primary foci of infection in cohorts with S. aureus bacteremiaa
| Region (reference) | % of MRSA cases in cohort | % of HCA cases in cohort | No. (%) of cases with focus of infection |
Total no. of cases | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infective endocarditis | Osteoarticular | SSTI | Pleuropulmonary | Line related | No focus/unknown | Other | ||||
| Central Australia (32) | 21.6 | 25.6 | 9 (7.2) | 20 (16) | 42 (34) | 11 (8.8) | 9 (7.2) | 30 (24) | 4 (3.2) | 125 |
| Australia (59) | 24.8 | 79.1 | 433 (6) | 956 (13) | 1,415 (20) | 519 (7.2) | 1,387 (19) | 1,100 (15) | 1,421 (20) | 7,231 |
| Sydney, Australia (65) | 100 | 92 | 15 (3.8) | 37 (9.3) | 80 (20) | 52 (13) | 140 (35) | 40 (10) | 35 (8.8) | 399 |
| Calgary, Canada (12)b | 11.3 | 75.3 | 79 (5.5) | 227 (16) | 224 (16) | 220 (15) | 586 (41) | 104 (7.2) | 1,440 | |
| Missouri, USA (64) | 100 | 92.6 | 0 (0) | 0 (0) | 39 (24) | 0 (0) | 37 (23) | 70 (43) | 17 (10) | 163 |
| New York, USA (61) | 100 | 97.9 | 91 (14) | 72 (11) | 112 (17) | 55 (8.4) | 302 (46) | 0 (0) | 20 (3.1) | 652 |
| Birmingham, UK (66) | 100 | 99.5 | 6 (3.1) | 3 (1.5) | 37 (19) | 0 (0) | 73 (37) | 68 (35) | 8 (4.1) | 195 |
| Italy (57) | 53.9 | 85.5 | 0 (0) | 0 (0) | 14 (9.3) | 7 (4.6) | 23 (15) | 104 (69) | 3 (2) | 151 |
| Israel (56) | 42.8 | 100 | 55 (4.4) | 71 (5.6) | 294 (23) | 144 (11) | 172 (14) | 298 (24) | 227 (18) | 1,261 |
| Thailand (58) | 27.6 | 55.1 | 8 (11) | 9 (12) | 20 (27) | 16 (22) | 10 (14) | 0 (0) | 10 (14) | 73 |
| South Korea (63) | 100 | 95.1 | 9 (3.4) | 16 (6) | 35 (13) | 24 (9) | 132 (49) | 36 (13) | 16 (6) | 268 |
| Japan (62) | 100 | NA | 0 (0) | 0 (0) | 17 (15) | 10 (8.7) | 27 (23) | 23 (20) | 38 (33) | 115 |
| Multisite (60) | 11.7 | NA | 282 (8.3) | 456 (13) | 502 (15) | 178 (5.2) | 942 (28) | 641 (19) | 394 (12) | 3,395 |
| Total | 15,468 | |||||||||
The mean percentages of patients for each primary focus of infection from all the studies were as follows: 5% for infective endocarditis, 8% for osteoarticular, 19% for SSTI, 9% for pleuropulmonary, 26% for line related, 24% for no focus/unknown, and 11% for other foci. MRSA, methicillin-resistant S. aureus; HCA, health care associated; SSTI, skin and soft tissue infection.
Line-related bacteremia was not reported in this study.
As the clinical epidemiology of S. aureus infections changes, it is likely that the proportion of cases of SAB with these individual primary clinical foci will change. For example, reductions in catheter-related infections following improved infection control practices and implementation of central line bundles have resulted in catheter-related SAB contributing to a smaller fraction of all cases of SAB (67). Similarly, rates of SSTI-associated SAB are highest in communities with large numbers of cutaneous infections. Examples include an increase in the incidence of USA300 community-associated MRSA (CA-MRSA) bacteremia with the widespread emergence of USA300 MRSA SSTIs (68) as well as high incidences of both SSTI and SAB in indigenous populations (30).
SAB can be classified as “complicated” or “uncomplicated.” These designations have significant implications for the extent and type of diagnostic evaluation, duration of antibiotic treatment, and overall prognosis. A single-center study of 724 episodes of SAB defined complicated infection as one that resulted in attributable mortality, central nervous system (CNS) involvement, an embolic phenomenon, metastatic sites of infection, or recurrent infection within 12 weeks (69). Predictors of complicated SAB were community acquisition, positive follow-up blood cultures at 48 to 96 h, persistent fever at 72 h, and skin findings suggesting an acute systemic infection (petechiae, vasculitis, infarcts, ecchymoses, or pustules) (69). The association between positive follow-up blood cultures and persistent fever with complicated SAB and subsequently poorer outcomes has been independently validated, as recently reviewed (70). The primary source of infection also predicts 30-day mortality, with higher mortality rates for bacteremia without a focus (22 to 48%), IE (25 to 60%), and pulmonary infections (39 to 67%), compared to lower rates for catheter-related bacteremia (7 to 21%), SSTIs (15 to 17%), and urinary tract infections (UTIs) (10%) (70). Similar findings have recently been described in a pooled analysis of five prospective observational studies (60).
Outcomes and Management
In the preantibiotic era, the case fatality rate (CFR) for SAB was ∼80% (71). Although the introduction of penicillin to treat SAB immediately reduced this high mortality rate (72), CFRs for SAB have plateaued at 15 to 50% over the past several decades (70). This lack of improvement in patient outcomes reflects both a relative plateau in antibiotic efficacy and larger numbers of older, “sicker” patients that now acquire SAB. Indeed, predictors of mortality from SAB include increasing age; the presence of comorbid conditions; the source, extent, and persistence of infection; and failure to remove eradicable foci (70). Guidelines for the management of SAB are available (73–76), and evidence to support various recommendations has been comprehensively reviewed (77). A striking impression from these documents is the poor quality of evidence that informs clinical management of SAB. For example, in a recent systematic review of evidence for the role of transesophageal echocardiography (TEE) and optimal antibiotic therapy in SAB, only one study met GRADE (grading of recommendation, assessment, development, and evaluation) criteria for high-quality evidence (78). Robust clinical trials are needed to address many outstanding questions regarding the management and treatment of this common and potentially lethal infection.
Despite the need for further high-quality evidence, broadly accepted key tenets in the management of SAB include (i) defining patients as having either uncomplicated or complicated infection; (ii) identifying and removing infected foci; and (iii) applying appropriate antimicrobial therapy with regard to the agent, dose, and duration. The Infectious Diseases Society of America (IDSA) has published guidelines with the following criteria to define uncomplicated SAB: (i) exclusion of IE by echocardiography, (ii) no implanted prostheses, (iii) negative results of follow-up blood cultures drawn 2 to 4 days after the initial set, (iv) defervescence within 72 h after the initiation of effective antibiotic therapy, and (v) no evidence of metastatic infection (79). Any other patient should be considered to have complicated SAB. Establishing the status of individual patients with regard to each of these criteria allows appropriate decisions to be made about subsequent treatment duration.
Infectious diseases consultation.
An infectious diseases (ID) consultation can play a key role in facilitating the process of appropriate investigation and management of patients with SAB. ID consultation for patients with SAB is associated with higher rates of various quality-of-care metrics, including (i) obtaining follow-up blood cultures to assess the clearance of SAB (80–86), (ii) obtaining an echocardiograph (69, 81, 83, 85, 87, 88), (iii) removing infected foci (80, 86, 89), (iv) providing a longer duration of treatment for complicated SAB (80–84, 86–89), and (v) administering β-lactam antibiotics for MSSA infections (80, 81, 83, 86, 88, 89). Eleven studies also reported that ID consultation for SAB is associated with reduced patient mortality rates (61, 62, 80–85, 87, 88, 90). Collectively, these results suggest that ID consultation should be regarded as the standard of care in institutions where this subspecialty service is available.
Role of transesophageal echocardiography.
Imaging of the cardiac valves is required to determine if there is underlying IE present in a patient with SAB. However, whether transesophageal echocardiography (TEE) is required in all such patients is unresolved. Among four studies that evaluated IE with both TEE and transthoracic echocardiography (TTE), rates of detection of IE were higher with TEE (14 to 25%) than with TTE (2 to 14%) (91–94). However, the increased sensitivity of TEE for the detection of IE compared to that of TTE needs to be balanced by the associated costs, risks, and availability of TEE. Esophageal perforation occurs in ∼1 in 5,000 TEEs performed (95). To risk stratify situations where TEE may not be required, a number of studies have proposed criteria to identify a low-risk subset of patients with SAB: (i) negative TTE results (92, 96), (ii) nosocomial acquisition of bacteremia (96, 97), (iii) negative follow-up blood cultures (93, 98), (iv) absence of an intracardiac device (92, 93, 96–98), (v) absence of hemodialysis dependence (98), and (vi) no clinical signs of endocarditis or metastatic foci (92, 93, 97, 98). Currently, it may be reasonable to avoid TEE in patients meeting all of these criteria. However, such recommendations would clearly be strengthened by a prospective trial with robust clinical outcomes comparing universal TEE to only targeted TEE for those patients with low-risk features.
Antibiotics.
The recommended duration of intravenous (i.v.) antibiotics for uncomplicated SAB is at least 2 weeks. In a recent prospective cohort study of uncomplicated SAB (as defined by IDSA criteria), receipt of antibiotic therapy for <2 weeks was associated with a relapse rate of 8% (compared to 0% for those treated for at least 2 weeks) (99). This relapse rate is consistent with the 6% rate of late complications (inclusive of relapse and metastatic complications) for intravascular catheter-associated SAB treated for <2 weeks identified in a 1993 meta-analysis of 11 studies (100). Although a few observational studies have suggested that as little as 7 days of i.v. antibiotics may be adequate (reviewed by Thwaites et al. [77]), such abbreviated courses must be regarded as investigational pending robust, generalizable evidence. Until such evidence exists, all patients with uncomplicated SAB should receive at least 2 weeks of i.v. antibiotics (73, 78, 79). Two-week courses of therapy, both with (101–104) and without (102) adjunctive aminoglycosides, have also been used successfully for uncomplicated, IDU-associated, right-sided S. aureus IE. Cure rates in these studies ranged from 77 to 94% (101–103, 105) and were similar for those who did and those who did not receive adjunctive aminoglycosides. However, cure rates were lower for patients who received glycopeptides (e.g., vancomycin and teicoplanin) than for those receiving antistaphylococcal penicillins (101, 105). Thus, patients being treated with vancomycin for right-sided IE should receive >2 weeks of therapy. For complicated SAB, 4 to 6 weeks of i.v. therapy has been the standard practice for over half a century and continues to be recommended (73, 75, 79, 106).
There is evidence that β-lactam therapy is better than glycopeptides for MSSA bacteremia from both randomized controlled trials (RCTs) (104, 105, 107) and observational studies (108–118). Vancomycin and daptomycin are currently the only antibiotics that are approved by the U.S. Food and Drug Administration (FDA) for MRSA bacteremia and right-sided IE. The sole high-quality RCT involving patients with MRSA bacteremia demonstrated that for the MRSA subgroup, daptomycin at 6 mg/kg of body weight i.v. once daily was noninferior to vancomycin (119). Treatment success at 42 days after completion of therapy was found for 20/45 (44%) daptomycin recipients, versus 14/44 (32%) patients receiving vancomycin plus low-dose, short-course gentamicin (absolute difference, 12.6%; 95% confidence interval [CI], −7.4% to 32.6%; P = 0.28). Vancomycin has also been compared to teicoplanin (120), trimethoprim-sulfamethoxazole (TMP-SMX) (121), linezolid (122, 123), and dalbavancin (124) in open-label RCTs. None of these antibiotics were shown to be significantly superior to vancomycin. Thus, at this stage, vancomycin and daptomycin are the first-line therapies for MRSA bacteremia.
INFECTIVE ENDOCARDITIS
S. aureus is now the most common cause of IE in the industrialized world (125). Due to its propensity to cause severe disease and its frequent antibiotic resistance, S. aureus is a dreaded cause of IE. Although our ability to rigorously study IE was previously limited by its relative infrequency at any single institution, large multinational collaborations such as the International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) (126) and robust population-level studies (127–129) have provided critical insights into the epidemiology and prognosis of IE in general and S. aureus IE in particular.
Epidemiology
Traditionally, the overall incidence of IE was estimated to be 1.5 to 6 per 100,000 person-years. These figures were derived from a systematic review of studies from Europe and the United States with population-level data for the period from 1970 to 2000 (130). The proportion of IE cases due to S. aureus ranged from 16 to 34%, with no temporal trend to suggest microbiologic shifts. More recent studies, however, have identified important changes in the epidemiology of S. aureus IE. Based on data from a nationwide inpatient sample (NIS) in the United States, the incidence of IE was calculated to increase from 11.4 per 100,000 person-years in 1999 to 16.6 per 100,000 person-years in 2006 (131), with most of the increase in incidence being driven by an increase in the incidence of S. aureus IE. S. aureus IE was also associated with increased mortality compared to other causative pathogens, a finding in keeping with most contemporary studies (125, 132–135). In a separate analysis of this NIS data set, the incidence of IE increased from 9.3 per 100,000 person-years in 1998 to 12.7 per 100,000 person-years in 2009. The proportion of IE cases coded as being due to S. aureus increased from 24% to 32% between 1998 and 2009 (136).
Although the incidence of IE elsewhere in the industrialized world has been reported to be severalfold lower than that in the United States, S. aureus remains the most common causative agent in those regions. In France, the overall incidence of IE remained stable at ∼3.5 per 100,000 person-years from 1991 to 2008, but the proportion of S. aureus IE increased from 16% to 26% during the same period (127). In Veluto, Italy, the incidence of IE increased from 4.1 per 100,000 person-years to 4.9 per 100,000 person-years from 2000 to 2008. S. aureus predominated, causing ∼40% of cases (128). Similarly, the incidence of IE in New South Wales, Australia, from 2001 to 2005 was 4.7 per 100,000 person-years. Again, S. aureus was the most common cause (32%) (129). Collectively, these studies confirm the predominance of S. aureus as a cause of IE across different industrialized countries. In contrast, the epidemiology of IE in nonindustrialized or newly industrialized settings involves primarily viridans group streptococci as the major pathogen infecting rheumatic heart valves (137–141).
It is apparent from population-based studies in industrialized regions (127–129, 142) and the prospective cohort studies from the ICE-PCS cohort (125, 132, 133, 143, 144) that the prevalence of health care-associated IE, particularly due to S. aureus, has increased. For example, Benito and colleagues reported that over one-third (34%) of a large cohort of 1,622 non-IDU patients with native valve IE had health care-associated infections (133). Cases of health care-associated IE were more likely to be caused by S. aureus (125, 133, 142). Thus, in contrast to previous IE series where S. aureus comprised <10% of cases (71, 145), S. aureus is now consistently the cause of IE in >25% of cases (125–129, 146). In conclusion, S. aureus has emerged over the last decade to become the most common cause of IE in the industrialized world, with a primary risk factor for this infection being health care contact.
Prosthetic valve endocarditis.
For patients with an underlying prosthetic valve, the yearly incidence of prosthetic valve IE ranges from 0.8 to 3.6% (147–149). S. aureus is now the most common cause of prosthetic valve IE (150, 151), responsible for 23 to 33% of cases (150, 152). This development is due in part to the frequency of S. aureus as a cause of health care-associated bacteremia and the high risk of hematogenous seeding of prosthetic valves by S. aureus once it gains access to the bloodstream. For example, in one prospective cohort study of patients with a prosthetic cardiac valve who developed SAB, the risk of IE was ∼51% (153). Fang et al. (154) reported a similar risk for developing prosthetic valve IE (15 of 34 cases; 44%) in a subgroup of their patients with SAB. These results emphasize the high risk of prosthetic valve IE associated with SAB (153, 154) and indicate that all patients with a prosthetic valve who develop SAB should be evaluated for IE, preferably by TEE.
The probability of developing S. aureus prosthetic valve IE is highest within the first 12 months after valve replacement surgery (149, 150) and is likely associated with the incomplete endothelialization of the prosthetic valve after placement (150) and also ongoing health care contact (150). Two large studies found that patients with mechanical valves are at a significantly higher risk for early prosthetic valve IE than are patients with porcine prosthetic valves, although there was no difference in the cumulative 5-year risk (148, 149). In contrast, neither the location (e.g., aortic or mitral) nor the composition (e.g., mechanical versus bioprosthetic) of the valve appears to significantly increase the risk of having S. aureus prosthetic valve IE in bacteremic patients (149, 153).
Grover et al. found that the most significant predictor of prosthetic valve IE due to any pathogen was active IE at the time of implantation of the prosthetic valve (7.4% versus 0.9%) (147). Other risk factors for prosthetic valve IE are previous episodes of endocarditis, persistent bacteremia, health care-associated infections, and injection drug use (150). The presence of multivalvular disease as well as male sex are risk factors for early prosthetic valve IE (147, 149), while superficial wound infection (relative risk [RR], 3.5; P = 0.004) is a risk factor for late prosthetic valve IE (147). Although Wang et al. (150) found that the mean age of patients developing prosthetic valve IE is significantly older than that of patients with native valve IE (65 versus 56 years; P < 0.001), Guerrero et al. (146) reported no significant difference in age distribution regarding patients who have S. aureus native valve or prosthetic valve IE (60 versus 58 years; P > 0.05).
Pathophysiology
The formation of a nidus for bacterial colonization and infection begins with damage to the cardiac endothelium, either by direct trauma (e.g., intravascular catheters and electrodes, injected particulate matter from injection drug use, or turbulent blood flow resulting from valvular abnormalities) or inflammation (e.g., secondary to rheumatic heart disease or degenerative valvular disease). The exposure of subendothelial cells elicits the production of extracellular matrix proteins and tissue factor and the deposition of fibrin and platelets to form sterile vegetations. If these thrombotic vegetations become colonized by bacteria, IE can result (155).
S. aureus has a number of cell wall-associated factors that allow it to attach to extracellular matrix proteins, fibrin, and platelets (156). In particular, clumping factors A and B (ClfA and ClfB, respectively; also known as fibrinogen-binding proteins) are key for attachment to and colonization of the valvular tissue. Fibronectin-binding protein A (FnBPA) and FnBPB facilitate binding to both fibrinogen and fibronectin and also play a role in subsequent endothelial cell invasion and inflammation (157, 158). In addition, Clf, FnBP, and the serine-aspartate repeat protein SdrE induce platelet aggregation and activation (159, 160). These findings have been demonstrated in studies involving the knockout of genes encoding these proteins in S. aureus as well as experiments where the expression of these proteins in the normally nonpathogenic bacterium Lactococcus lactis results in the ability to cause IE (161, 162). More recent studies have determined the importance of host-derived ultralarge von Willebrand factor fibers in mediating adhesion (probably via cell wall teichoic acids) of S. aureus to intact endothelial cells (163) and the role of the prothrombin-activating proteins staphylocoagulase and von Willebrand factor-binding protein in binding prothrombin and converting fibrinogen into fibrin (164). Staphylococcal superantigens have also been shown to be critical to the formation of vegetations, probably through a combined effect of systemic hypotension and direct toxicity to endothelial cells (165).
Although in vitro and animal model studies have provided key experimental data in delineating the role of various virulence factors, studies involving large cohorts of patients are essential to link these clues with clinical disease. Several studies have described (166) and confirmed (167) that isolates with distinct bacterial genotypes are associated with specific disease phenotypes, including IE (166–168). For example, clinical S. aureus isolates within clonal complex 30 (CC30) have been shown to be significantly more likely to be associated with IE (166, 167) and are more likely to have adhesion- and superantigen-encoding genes such as clfB, cna, and eap (167). The relevance of this epidemiologic association was further strengthened by the recent observation that CC30 isolates were more likely to cause IE in a rabbit endocarditis model than other common, clinically relevant strains (169).
Clinical Manifestations and Outcomes
The clinical manifestations of S. aureus IE are now well understood through the ICE-PCS cohort (125) as well as national cohorts (170) and long-term single-center studies (146, 171, 172). Patient characteristics associated with S. aureus IE include injection drug use, health care-associated infections, a shorter duration of symptoms prior to diagnosis, persistent bacteremia, the presence of a presumed intravascular device source, stroke, and diabetes mellitus (125, 171).
Table 3 outlines the major demographic and clinical features of S. aureus IE. Left-sided valvular disease is more common than right-sided disease, and the mitral valve is more commonly involved than the aortic valve, in a ratio of ∼1.5:1. Right-sided disease is usually secondary to either injection drug use or the presence of a central catheter. However, S. aureus IE in IDUs is not restricted to the tricuspid valve. Approximately 30% of cases of IE in IDUs are left sided (125, 173). Complications for S. aureus IE are common, particularly for left-sided IE, in which embolism of the systemic circulation and heart failure frequently occur.
TABLE 3.
Clinical and demographic characteristics of large cohorts of patients with S. aureus infective endocarditisi
| Characteristic | Value reported in reference: |
|||||
|---|---|---|---|---|---|---|
| 125 | 170 | 146 | 173 | 171 | 172 | |
| No. of patients | 558 | 260 | 133 | 74 | 61 | 27 |
| Region | Multinational | Denmark | Spain | Finland | France | Australia |
| Study type | Multicenter | Multicenter | Single center | Multicenter | Single center | Single center |
| Period (yr) | 2000–2003 | 1982–1991 | 1985–2006 | 1999–2002 | 1990–2000 | 1991–2006 |
| Age (yr) | 57a | 68a | NA | 55b | 57b | 64a |
| No. (%) of patients | ||||||
| Males | 341 (61) | 145 (56) | 89 (77) | 47 (64) | 42 (69) | 18 (67) |
| With acquisition type | ||||||
| HCA | 218 (39) | 88c (33) | 29c (22) | 34c (46) | NA | 26 (96) |
| Community | 326 (58) | 172 (67) | 104 (78) | 40 (54) | NA | 1 (4) |
| IDU | 117 (21) | 0d (0) | 62 (47) | 20 (27) | NA | 1 (4) |
| On dialysis | 79 (14) | NA | NA | 6 (8) | 7 (11) | 1 (4) |
| With diabetes | 110 (20) | 35 (13) | 2 (6) | 19 (26) | 12 (20) | 8 (30) |
| With intravascular device | 159 (28) | 23 (9) | NA | 10 (14) | 11 (17) | 14 (52) |
| With native valve | 401 (72) | 215 (83) | 113 (85) | 57 (77) | 55 (90) | 17 (63) |
| With prosthetic valve | 86 (15) | 24 (9) | 20 (15) | 17 (23) | 6 (10) | 10 (37) |
| With location of vegetations | ||||||
| Aortic | 143 (29) | 84 (32) | 21 (16) | 26 (35) | 22 (36) | 6 (22) |
| Mitral | 224 (46) | 100 (38) | 42 (32) | 22 (30) | 28 (46) | 15 (56) |
| Tricuspid/pulmonary | 132 (27) | 13 (5) | 58 (47) | 16 (22) | 11 (18) | 2 (7) |
| With MRSA | 283/424 (67) | 0 (0) | 8 (6) | 0e (0) | NA | 27f (100) |
| With complication | ||||||
| Stroke | 119 (21) | 91 (35) | 30 (23) | 13 (18) | 21 (34) | 9 (35) |
| Cardiac failure | 161 (29) | 139 (53) | 36 (27) | NA | 19 (31) | 4 (15) |
| Intracardiac abscess | 71 (13) | NA | 12 (9) | 3 (4) | 14 (23) | 3 (12) |
| With in-hospital mortality | 125 (22) | 164g (63) | 37 (28) | 17h (23) | 21 (34) | 15 (66) |
| With surgery | 211 (38) | 27 (10) | 39 (29) | 7 (9) | 20 (33) | 16 (59) |
Median age.
Mean age.
For several studies, only nosocomial versus community-onset data were collected.
IDUs were excluded from the study.
MRSA was excluded from the study.
Only MRSA was reported in this study.
This study included 83 patients not clinically known to have S. aureus IE but who were subsequently diagnosed at autopsy.
This study referred to 28-day mortality.
HCA, health care associated; IDU, intravenous drug user; MRSA, methicillin-resistant S. aureus; NA, not available.
For those with SAB and a prosthetic valve, clinical manifestations suggesting S. aureus prosthetic valve IE are persistent fever (odds ratio [OR], 4.4; 95% CI, 1.0 to 19.1) and persistent SAB (OR, 11.7; 95% CI, 2.9 to 47.7) (153). Other clinical findings in S. aureus prosthetic valve IE are peripheral emboli, splenomegaly, or new regurgitant murmurs (174–177). El-Ahdab et al. (153) found that of patients with SAB and a prosthetic valve who underwent TEE, 23% showed valvular vegetation and 11% showed evidence of a valvular abscess. Patients with S. aureus prosthetic valve IE generally develop a new murmur less frequently than do patients with S. aureus native valve IE (146) and typically have a shorter duration of symptoms before a diagnosis is made (146).
Diagnosis of S. aureus IE is generally established by the application of modified Duke criteria (178), which incorporate a combination of factors, including history and physical exam, blood culture results, and echocardiography results. In a minority of cases, however, standard blood or tissue culture results will not detect S. aureus. Real-time PCR (RT-PCR) targeting 16S rRNA genes may be a useful adjunct for the microbiological diagnosis of endocarditis in this setting (179). In an analysis of 48 patients in France with culture-negative IE, S. aureus was detected by PCR in 10/48 (20.4%) patients (180). In a similar analysis of 69 patients in the United Kingdom and Ireland with culture-negative IE, 2 patients had S. aureus infection identified by PCR of explanted valve tissue (181).
The overall mortality rate for S. aureus IE ranges from 22 to 66% and is consistently higher than those for other causes of IE. Across the broad categories of S. aureus IE, left-sided IE has a poorer prognosis than right-sided IE, health care-associated IE has a poorer prognosis than community-associated IE, prosthetic valve IE has a poorer prognosis than native valve IE, and non-IDU-associated IE has a poorer prognosis than IDU-associated IE (125, 173). In addition, consistent predictors of mortality are increasing age, stroke, and heart failure (125, 170). Stroke is a grave but frequent complication arising from S. aureus prosthetic valve IE, afflicting 23 to 33% of patients (177, 182, 183), and is a significant prognostic indicator of mortality (146, 177, 182, 183). Sohail et al. found that of patients with S. aureus prosthetic valve IE, an American Society of Anesthesiologists class IV status and the presence of bioprosthetic (compared to mechanical) valves were also independent predictors of mortality (177).
Management
Antimicrobial therapy.
All patients with S. aureus IE require prolonged i.v. antibiotics. Detailed guidelines have been reported by professional societies in the United States and Europe (79, 106, 184, 185). An addition found in the most recent guidelines is the recognition of daptomycin as an option for treatment of S. aureus IE. In the key registrational trial, daptomycin was noninferior to standard therapy for SAB (119). On the basis of these results, daptomycin gained an indication for treatment of SAB and right-sided S. aureus IE, including infections due to MRSA. The relatively small number of patients in the trial with left-sided S. aureus IE (n = 18) prevented meaningful conclusions regarding daptomycin's utility in this setting. Nonsusceptibility to daptomycin developed in 5 of 45 patients with MRSA bacteremia (and 2 of 74 patients with MSSA) treated with daptomycin. Nonetheless, daptomycin treatment is now recommended in United Kingdom guidelines for native valve MRSA IE where the isolate has a vancomycin MIC of >2 mg/liter (106) and in IDSA guidelines for all cases of native valve MRSA IE (79). The recommended dose is 6 mg/kg, but higher doses (8 to 10 mg/kg) are increasingly being used and appear to be safe (186, 187). Registries for the use of daptomycin have included 86 patients with MRSA IE; outcomes appear favorable (186, 187). However, these were not comparative studies, and a large proportion of patients received concomitant therapy with other antibacterial agents. Carugati et al. (188) examined the ICE-Daptomycin substudy database and compared 29 patients (12 with S. aureus and 7 with MRSA) who received high-dose daptomycin (median, 9.2 mg/kg) with 149 patients (74 with S. aureus and 18 with MRSA) who received the standard of care for Gram-positive IE. Clearance of MRSA bacteremia was significantly faster in the daptomycin cohort than in the standard-of-care cohort (1.0 days versus 5.0 days).
The clinical syndrome of treatment-emergent nonsusceptibility to daptomycin in MRSA has been noted in a number of studies at rates of 11% (5 of 45 patients) (119), 11% (6/54) (187), 60% (6/10) (189), and 39% (7/18) (190). The risk of this phenomenon appears greatest in those patients without adequate source control (119), suboptimal daptomycin dosing (189), and persistent MRSA bacteremia (189, 190). To reduce the risk of treatment-emergent resistance and to provide the possibility for synergy, a number of investigators have evaluated the addition of a second antibiotic to daptomycin in vitro and in animal studies. These second agents have included gentamicin (191–198); rifampin (191–196, 198); β-lactam antibiotics (195, 199–204), including ceftaroline (202, 203); TMP-SMX (201); and linezolid (201). Clinical successes with combination therapy have also been reported with rifampin (205), TMP-SMX (206, 207), fosfomycin (208, 209), and β-lactams (210, 211). Unfortunately, an RCT comparing daptomycin to daptomycin combined with gentamicin was terminated after recruiting only 24 patients (ClinicalTrials.gov registration number NCT00638157). Thus, the role of combination therapy with daptomycin remains to be defined, and the development of treatment-emergent resistance to daptomycin must be closely monitored, particularly among patients with residual sites of infection or persistent bacteremia (212).
Various guidelines (79, 106, 184, 185) recommend that prosthetic valve MRSA IE be treated with a combination of vancomycin, gentamicin, and rifampin. These recommendations are based largely on expert opinion and on small retrospective studies of methicillin-resistant coagulase-negative staphylococci (CoNS) (213, 214). Given that neither rifampin nor gentamicin appears to improve outcomes for native valve S. aureus IE and that these antibiotics are in fact associated with adverse side effects (215, 216), there is a clear need for further research to determine the optimal antimicrobial therapy for prosthetic valve S. aureus IE.
Surgery.
Recent studies have underscored the importance of early surgery in the treatment of IE in general and S. aureus IE in particular. Following a period of controversy over the results of various cohort studies and the lack of adjustment for bias in these studies (217–223), the benefit of surgery for native valve IE was demonstrated in an analysis of the ICE-PCS cohort (224). This study used propensity-based matching to adjust for treatment selection bias, survivor bias, and hidden bias. The subgroups with S. aureus IE, as well as patients with paravalvular complications and those with systemic embolization, were found to benefit from early surgery (224). Early surgery reduced the risk of subsequent embolic events in an RCT for patients with native valve IE and large vegetations or severe valvular disease (225). However, there were only eight patients with S. aureus IE in this study, thus precluding conclusions specifically regarding the S. aureus subgroup.
The timing of surgery following stroke is controversial. For patients with intracerebral hemorrhage, there is consensus that surgery should be delayed by at least 1 month. For those patients with ischemic stroke, a number of studies (reviewed by Rossi et al. [226]) have suggested that surgery does not need to be delayed if there are indications for surgery. Although an analysis of the ICE cohort specifically addressing this question concluded that early surgery is not associated with increased mortality, concerns have been raised regarding the adjusted OR for in-hospital mortality being 2.3 (95% CI, 0.94 to 5.7) for those receiving surgery within 7 days of stroke compared to delayed surgery (227, 228). Further studies with more detailed stratification, including a subset of patients with S. aureus IE, and inclusion of data on long-term neurological outcomes will be required to determine which patients will truly benefit from early compared to delayed surgery following ischemic stroke.
Several studies have concluded that all patients with S. aureus prosthetic valve IE, regardless of whether they have complications, benefit from surgery, citing the lower mortality rates found with the combination of medical and surgical treatments (146, 152, 153, 183, 229–232). For example, Fernandez Guerrero et al. found that of the 65% of patients who underwent valve replacement surgery, only 15% died, whereas all of the 35% of patients who did not receive surgery died (146). An analysis of all patients with prosthetic valve IE in the ICE cohort found no overall benefit with early surgery compared to medical therapy after adjustment for treatment selection and survivor bias (151). In a post hoc analysis that did not adjust for survivor bias, improved survival was found for those with the highest probability of receiving surgery. Those with the highest probability for surgery typically had factors that current recommendations suggest should receive surgery, including heart failure and uncontrolled infection (including paravalvular abscesses) (106, 184, 185). The role of early valve surgery in S. aureus prosthetic valve IE was specifically addressed by Chirouze et al. (233) with the ICE-PCS cohort. As expected, the 1-year mortality rate was significantly higher among patients with S. aureus prosthetic valve IE than among patients with non-S. aureus prosthetic valve IE (48.2% versus 32.9%; P = 0.003), and patients with S. aureus prosthetic valve IE who underwent early valve surgery had a significantly lower 1-year mortality rate (33.8% versus 59.1%; P = 0.001) than did those who did not. However, in multivariate, propensity-adjusted models, receipt of early valve surgery for S. aureus prosthetic valve IE was not associated with reduced 1-year mortality rates. Based on these findings, the decision to pursue early valve surgery in cases of S. aureus prosthetic valve IE should be individualized for each patient based upon infection-specific characteristics rather than solely upon the identification of S. aureus as the causative pathogen.
In summary, one recent RCT and several well-designed cohort studies have now provided strong supportive evidence for early surgery in IE patients with heart failure, uncontrolled infection, and a high risk of emboli. It is likely that these findings apply to patients with S. aureus IE in particular. Given the poorer outcomes associated with S. aureus native valve IE, the absolute benefit of early surgery (and hence the number needed to treat to demonstrate a clinically meaningful difference) may be even more favorable.
SKIN AND SOFT TISSUE INFECTIONS
S. aureus causes a variety of SSTIs, ranging from the benign (e.g., impetigo and uncomplicated cellulitis) to the immediately life-threatening. It is the most common pathogen isolated from surgical site infections (SSIs), cutaneous abscesses, and purulent cellulitis. Here we review the epidemiology, pathophysiology, clinical features, and treatment of S. aureus SSTIs, with an emphasis on the recent epidemic of community-associated MRSA (CA-MRSA).
Epidemiology
While S. aureus has traditionally been the leading cause of SSTIs, its importance has ballooned in the past 15 years with the emergence of a worldwide epidemic of CA-MRSA SSTIs (234, 235). Because the rise of CA-MRSA was previously explored in detail (236), it is reviewed here briefly.
MRSA was described shortly after the introduction of methicillin but was uncommon outside the health care environment until the 1990s. Around that time, reports emerged of patients presenting with MRSA who did not have traditional health care risk factors. These reports included both children and adults in various geographic locations presenting predominately with SSTI (237–251), with community clusters among athletes, men who have sex with men, correctional facilities (252–254), homeless persons and IDUs (255), military personnel (256–258), and indigenous populations (30, 239, 250, 259).
Over time, it became apparent that the CA-MRSA epidemic was not simply replacing endemic SSTI strains but was significantly increasing the incidence of SSTIs. For example, Pallin et al. (260) estimated that the number of emergency department (ED) visits for SSTIs in the United States increased from 1.2 million in 1993 to 3.4 million in 2005. These data were corroborated by others. Hersh et al. (261) queried U.S. national survey data and found an increase in the number of coded SSTI encounters from 32.1 to 48.1 per 1,000 population from 1997 to 2005, largely in younger and black patients. Inpatient admissions for SSTIs exhibited the same trend. Edelsberg et al. (262) estimated that there were 675,000 admissions for SSTI in the United States in 2000, compared to 869,800 in 2004, with the most notable increases being seen for younger and urban patients. Frei et al. (263) found that among pediatric patients, the numbers of hospitalizations for both MSSA and CA-MRSA increased from 1996 to 2006. More recent U.S. data suggest that the MRSA SSTI incidence may have peaked around 2007 to 2008. For example, from 2005 to 2010, the proportion of all community-onset SSTIs due to MRSA in Department of Defense beneficiaries declined from 62% to 52%, although overall S. aureus SSTI rates did not change (29).
When CA-MRSA was first recognized in the United States in the late 1990s, molecular typing demonstrated that the predominant clone was USA400 (236, 264). Since 2000, USA400 has largely been supplanted by a single epidemic clone, USA300, which has been responsible for the rapid shift in epidemiology in the United States. King et al. (265) found that the USA300 clone was the cause of most community-onset S. aureus SSTIs. Among 389 patients in a Georgia health system, 72% of all S. aureus SSTIs were caused by MRSA, and ∼85% of these were caused by USA300. Similar findings were seen concurrently in cohorts of patients presenting to emergency departments elsewhere in the United States (266–270).
Increasing rates of SSTIs have also been noted in Australia and the United Kingdom. In the United Kingdom, from 1991 to 2006, there was a 3-fold increase in admission rates for abscesses and cellulitis and increases in the numbers of prescriptions for antistaphylococcal antibiotics from primary care settings (271, 272). In Australia, there was a 48% increase in the number of hospitalizations for cutaneous abscesses between 1999 and 2008 (273), with a concurrent increasing proportion of outpatient S. aureus strains attributed to CA-MRSA (251). Notably, the increasing incidence of SSTIs in these regions cannot be attributed to USA300, which is an infrequent cause of staphylococcal infections in Europe (274) and Australia (251).
Pathophysiology
The pathogenesis of S. aureus SSTI has been comprehensively reviewed elsewhere (275, 276) and is summarized briefly here. The primary defense against S. aureus infection is the neutrophil response. When S. aureus enters the skin, neutrophils and macrophages migrate to the site of infection. S. aureus evades this response in a multitude of ways, including blocking chemotaxis of leukocytes, sequestering host antibodies, hiding from detection via polysaccharide capsule or biofilm formation, and resisting destruction after ingestion by phagocytes.
With the rise in the number of SSTIs caused by CA-MRSA, there has been intense interest in understanding the enhanced pathogenicity of these strains. Multiple virulence factors appear to contribute, including Panton-Valentine leukocidin (PVL), alpha-hemolysin (also called alpha-toxin), phenol-soluble modulins (PSMs), the arginine catabolic mobile element (ACME), and a regulatory locus referred to as agr.
PVL causes lysis of human white blood cells (WBCs). In the early 1990s, it was linked to S. aureus cutaneous infections (277, 278) and has been epidemiologically associated with CA-MRSA infections, raising the question of whether it was responsible for increased virulence. Vandenesch et al. assessed 117 CA-MRSA isolates from a widespread geographic area and performed pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST) to look for common genetic markers (279). All isolates shared a type IV staphylococcal cassette chromosome mec (SCCmec) element and the pvl locus. Similar work in the United States (280) and France (281) established a correlation between the presence of pvl and CA-MRSA infections. In a meta-analysis of several studies (282–285), the presence of pvl was clearly associated with abscesses and furuncles, with an odds ratio of 10.5 (95% CI, 7.4 to 14.9) (286). However, the quantity of PVL produced in CA-MRSA infections has not been found to correlate with the severity of infection; for example, some high-PVL-producing strains were found in patients with uncomplicated abscesses, whereas patients with necrotizing fasciitis carried S. aureus strains with lower levels of PVL production (287). A study of isolates from 142 human infections in England and Wales was similarly unable to document an association between levels of PVL production in vitro and clinical severity of infection (288). Furthermore, laboratory models have not found PVL to be the predominant virulence determinant in skin infections. In mice, pvl-negative USA300 and USA400 strains caused skin disease comparable to that caused by pvl-positive strains (289). However, mice are not an optimal model for human SSTI, in that mouse neutrophils are resistant to the toxic effects of PVL compared to human and rabbit neutrophils (290, 291). In a rabbit model, compared to isogenic pvl knockout mutants, pvl-positive MRSA strains were also found to exert toxic effects on keratinocytes; after being taken up by host cells, the pvl-positive strains were able to escape and induce keratinocyte apoptosis, facilitating local spread and inflammation (292). In another rabbit experimental model (293), pvl-positive and pvl-negative strains produced similar disease. Thus, despite the strong epidemiological association between pvl and abscesses and furuncles (286), evidence from animal models does not conclusively link pvl and the pathogenesis of skin lesions.
Substantial attention has also been directed to alpha-hemolysin, a toxin that forms pores in various human cells, not limited to red blood cells, leading to cell lysis. Its role in S. aureus virulence has been appreciated for nearly a century (294). In the rabbit skin infection study mentioned above, virulence correlated with transcript levels of alpha-hemolysin and PSMα (293). More recently, it was discovered that alpha-hemolysin interacts with the ADAM10 receptor, and ADAM10-deficient mice are protected from severe skin infection (295). In the virulent Australian sequence type 93 (ST93) MRSA clone (296, 297), high levels of alpha-hemolysin have been associated with more severe cutaneous lesions (298). Alpha-hemolysin also appears to contribute to the penetration of keratinocytes in skin infection (299). Vaccination against alpha-hemolysin in mice led to less severe skin disease with subsequent challenge (300).
Phenol-soluble modulins are a family of small, amphipathic proteins found in S. aureus that lyse human cells, including neutrophils and erythrocytes. A growing body of evidence from both in vitro and in vivo studies suggests that PSMs may also be important in the development of SSTI. PSMs and their proteolytic products facilitate S. aureus colonization (301) and dispersion (302) on skin. PSM deletion in a mouse abscess model led to significantly decreased skin lesions, supporting a role in virulence (303). In a subsequent rabbit skin infection model, alpha-hemolysin, PSMα, and agr appeared to contribute to pathogenesis (304). The level of production of phenol-soluble modulins is also significantly higher in USA300 isolates than in other variants of MRSA (303). A recent investigation demonstrated that in vitro levels of PSM production were significantly higher among clinical MRSA isolates originating from an SSTI source than in geographically matched MRSA isolates from cases of hospital-acquired pneumonia (HAP) or IE (305).
The success of the USA300 strains has been linked to the presence of ACME (306, 307). Although USA500, the progenitor strain of USA300, has virulence similar to that of USA300 in animal models (308), it has proven less successful in terms of spread in the human population. USA500 notably lacks the ACME locus. Recently, Planet et al. (309) demonstrated that the speG gene in the ACME locus confers increased resistance to skin-produced polyamines that are toxic to other S. aureus strains, resulting in a likely selective advantage during skin colonization and infection.
Differential gene expression for proteins such as PVL, alpha-toxin, and PSMs appears to also contribute to the enhanced virulence of CA-MRSA. These elements are under the control of agr, a regulatory locus that controls the expression of S. aureus toxins. In a mouse model, less severe infection ensued after inoculation with agr-deleted S. aureus strains (310). Similarly, clinical ST93 strains with agr mutations produce less alpha-hemolysin and are less virulent than wild-type strains (298).
Other candidate virulence determinants continue to be discovered. The sasX gene was found in the S. aureus clone most common in Asia. In addition to a putative role in nasal colonization and pleural infection, the presence of this gene was correlated with larger cutaneous abscesses than those in mice infected with sasX mutant strains (311).
Clinical Features and Outcomes
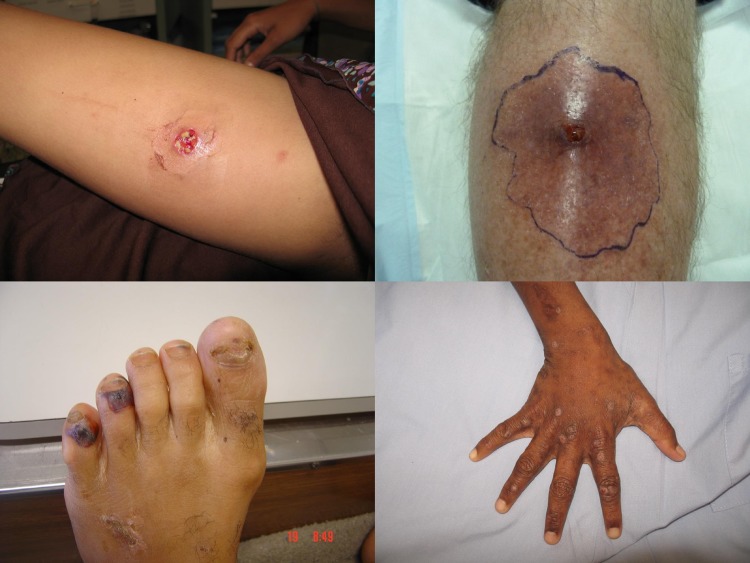
Even prior to the rise of CA-MRSA, S. aureus was a key contributor to SSTIs (see Fig. 1 for photos of classical S. aureus SSTIs).
FIG 1.
Staphylococcus aureus skin and soft tissue infections. Shown are abscess (top left), cellulitis surrounding a pustule (top right), embolic infarcts complicating infective endocarditis (bottom left), and impetigo complicating scabies infection (bottom right).
Impetigo is the most common bacterial skin infection of children (312). In general, impetigo presents as bullous or papular lesions that progress to crusted lesions, without accompanying systemic symptoms, on exposed areas of the body (usually the face or extremities). Recent studies of impetiginous lesions found recovery rates of 29 to 90% and 57 to 81% for Streptococcus pyogenes and S. aureus, respectively (313–316).
While the hallmark infection of S. aureus SSTI is generally regarded as the cutaneous abscess (30, 247, 248, 317–319), other manifestations of skin infection are also encountered clinically. Nonpurulent cellulitis may be caused by S. aureus in a minority of cases, although the lack of a diagnostic gold standard and variability introduced by different microbiological methods obscure the true microbiology of this condition (320). While S. aureus cellulitis most commonly involves the lower extremities, it may also involve other regions, including the upper extremities, abdominal wall, and face. It vies for primacy with streptococci as a cause of preseptal and orbital cellulitis (321–323).
Necrotizing fasciitis is another cutaneous syndrome caused by S. aureus. In a review of 843 patients with wound cultures positive for MRSA, 14 isolates were identified as being associated with necrotizing fasciitis or myositis. Coexisting conditions among those patients included injection drug use in 6/14 (43%) patients, previous MRSA infections in 3/14 (21%), diabetes mellitus in 3/14 (21%), and hepatitis C in 3/14 (21%) (324). In Taiwan, a review of 53 patients with necrotizing fasciitis revealed that 38% of infections were caused by S. aureus, 60% of which were caused by MRSA (325).
Pyomyositis can occur with both MSSA and MRSA. It has a tropical predilection, accounting for up to 1 to 4% of hospital admissions in some tropical countries, with S. aureus being responsible for an estimated 90% of these presentations (326). It is less common in temperate climates, where it occurs primarily in children and young adults (327, 328) and has been reported in association with HIV (329).
SSIs occur after 2 to 5% of all surgeries (330), although there is considerable heterogeneity depending on the type of procedure, population studied, comorbid illnesses, experience of the surgeon, setting, and antimicrobial prophylaxis utilized. According to 2009-2010 U.S. National Healthcare Safety Network data, S. aureus was the most common cause of SSIs overall, accounting for 30% of infections. Of these, 44% of isolates were methicillin resistant (331). In registrational trials of complicated SSTIs, even higher proportions of SSIs due to S. aureus have been found; for example, 49% of SSIs in ATLAS studies (telavancin versus vancomycin) were due to S. aureus (332).
A particularly devastating SSI is mediastinitis complicating median sternotomy for cardiac surgery. S. aureus is the most common cause of postoperative mediastinitis (333–338). Fowler et al. demonstrated that the presence of SAB in the postoperative period was highly predictive of a diagnosis of mediastinitis, with a likelihood ratio (LR) of 25, compared to blood cultures positive for other pathogens or negative blood cultures (338). These findings were subsequently independently validated (334, 336). Thus, the presence of SAB following sternotomy mandates aggressive investigation to exclude the possibility of postoperative mediastinitis.
Treatment
Numerous RCTs have been conducted for different subsets of SSTIs. These studies (339–361) are summarized in Table 4, and additional comments are presented below.
TABLE 4.
Randomized controlled trials involving skin and soft tissue infectionsa
| Type of SSTI and authors of study, yr (reference) | Population | Study design | No. of patients | Treatment(s) | Outcome | Description |
|---|---|---|---|---|---|---|
| Impetigo | ||||||
| Oranje et al., 2007 (339) | Children and adults with impetigo | Observer-blind RCT | 519 | 1% retapamulin ointment twice daily for 5 days vs 2% sodium fusidate ointment 3 times daily for 7 days | 99.1% and 94.0% clinical efficacy in per-protocol populations | 60.5% were culture positive for S. aureus, of which 3.5% were positive for MRSA |
| Koning et al., 2008 (340) | Children and adults with impetigo | Double-blind RCT | 210 | 1% retapamulin ointment twice daily vs placebo, each for 5 days | Retapamulin was superior to placebo in clinical success at 7 days (86% vs 52%) and 14 days (76% vs 39%) | 69.5% were culture positive for S. aureus |
| Bowen et al., 2014 (316) | Children with impetigo | Investigator-blind RCT | 508 | Benzathine penicillin i.m. in a single dose vs TMP-SMX twice daily for 3 days or daily for 5 days | TMP-SMX was noninferior to penicillin in mITT (84.7% vs 85.3%) or evaluable populations when assessed for improvement or cure at day 7 | 81% were culture positive for S. aureus, and 90% were culture positive for S. pyogenes; 13.3% of S. aureus isolates were MRSA |
| Uncomplicated SSTI | ||||||
| Tack et al., 1997 (341) | Children with uncomplicated SSTI, mostly impetigo (57%), infected dermatitis (9%), wound infection (8%), and cellulitis (7%) | Investigator-blind RCT | 394 | 7 mg/kg cefdinir twice daily vs 10 mg/kg cephalexin 4 times daily, each for 10 days | No difference; high cure rate in both arms (98.3% vs 93.8%) in microbiologically evaluable population | 72.1% were culture positive for S. aureus |
| Bucko et al., 2002 (342) | Adults and children at least 12 yr of age with uncomplicated SSTI | Double-blind RCT | 1,685 | 200 mg or 400 mg cefditoren vs either 250 mg cefuroxime or 500 mg cefadroxil, each given twice daily for 10 days | Similar clinical cure rates at TOC visit (85%, 83%, 88%, and 85%, respectively) | 31.1% were culture positive for S. aureus, of which 8% were positive for MRSA |
| Giordano et al., 2006 (343) | Adults and children at least 13 yr of age with uncomplicated SSTI | Investigator-blind RCT | 391 | 300 mg cefdinir twice daily vs 250 mg cephalexin 4 times daily, each for 10 days | No difference in clinical cure rate at TOC visit in ITT (83% vs 82%) or CE (89% vs 89%) populations; no difference between MRSA and MSSA subgroups was found | 38.6% were culture positive for S. aureus, of which 52.3% were positive for MRSA |
| Rajendran et al., 2007 (344) | Adults with uncomplicated skin abscess who underwent drainage procedure | Double-blind RCT | 166 | 500 mg cephalexin 4 times daily vs placebo, each for 7 days | No difference; high cure rates in both arms (84.1% vs 90.5%) | 70.4% were culture positive for S. aureus, of which 87.8% were positive for MRSA |
| Duong et al., 2010 (345) | Children with uncomplicated skin abscess who underwent drainage procedure | Double-blind RCT | 161 | TMP-SMX (10–12 mg trimethoprim/kg/day divided into 2 doses, with a maximum dose of 160 mg trimethoprim/dose) vs placebo, each for 7–10 days | No difference; high success rates in both arms (94.7% vs 95.9%); more new lesions at 10 days in placebo-treated group (26% vs 13%) but not at 3 mo | 88.2% were culture positive for S. aureus, of which 90.8% were positive for MRSA |
| Schmitz et al., 2010 (346) | Adults with uncomplicated skin abscess who underwent drainage procedure | Double-blind RCT | 212 | 2 tablets of 160/800 mg TMP-SMX twice daily vs placebo, each for 7 days | Nonsignificant difference in treatment failure (17% vs 26%), higher incidence of subsequent new lesions within 30 days in placebo group (28% vs 9%) | 62.3% were culture positive for S. aureus, of which 73.5% were positive for MRSA |
| Pallin et al., 2013 (347) | Adults and children with cellulitis without abscess | Double-blind RCT | 146 | TMP-SMX vs placebo, each in addition to cephalexin, for 7–14 days | No difference in 30-day cure rates (62% vs 60%) | |
| Miller et al., 2015 (369) | Adults and children with uncomplicated cellulitis or skin abscess | Double-blind RCT | 524 | Clindamycin vs TMP-SMX, each for 10 days | No difference in cure rates at 14 days in ITT population (80.3% vs 77.7%) or evaluable population | Among suppurative lesions, S. aureus was found in 218 (42%), 178 (82%) of which were MRSA isolates; there was 14% clindamycin resistance and 0% TMP-SMX resistance among S. aureus isolates |
| Complicated SSTI and ABSSSIc | ||||||
| Stevens et al., 2000 (348) | Adults with cSSTI suspected to be due to a Gram-positive organism | Double-blind RCT | 819 | 600 mg linezolid i.v. every 12 h vs 2 g oxacillin i.v. every 6 h, each for 10–21 days (mean, 13.4 days), with transition to oral linezolid or dicloxacillin, respectively, when clinically improving | Similar cure rates in ITT population (69.8% vs 64.9%), CE population (88.6% vs 85.8%), and ME population (88.1% vs 86.1%) | 23.9% were culture positive for S. aureus |
| Arbeit et al., 2004 (349) | Adults with cSSTI due to Gram-positive organism and requiring hospitalization and i.v. therapy for ≥4 days | Pooled analysis of 2 evaluator-blind RCTs | 1,082 | 4 mg/kg/day daptomycin vs vancomycin or a penicillinase-resistant penicillin (cloxacillin, nafcillin, oxacillin, or flucloxacillin), each for 7–14 days | No difference in clinical success in ITT population (71.5% vs 71.1%) | S. aureus was cultured in samples from 58.0% of patients, of which 13.9% were positive for MRSA |
| Weigelt et al., 2005 (350) | Adults with cSSTI requiring hospitalization. | Open-label RCT | 1,180 | Linezolid vs vancomycin, each for a goal of 7–14 days (minimum, 4 days; maximum, 21 days) | No difference in clinical response in ITT population (92.2% vs 88.5%); linezolid was superior (71% vs 55%) in the subgroup with MRSA | 71% culture positive for S. aureus, of which 59% were positive for MRSA |
| Ellis-Grosse et al., 2005 (351)b | Adults with cSSTI | Pooled analysis of 2 double-blind RCTs | 1,116 | Tigecycline vs vancomycin-aztreonam, each for up to 14 days | No significant difference in cure rates in clinically evaluable populations (86.5% vs 88.6%) at TOC visit | 28.6% were culture positive for S. aureus, of which 20.4% were positive for MRSA |
| Breedt et al., 2005 (352)b | Adults with cSSTI | Double-blind RCT | 546 | Tigecycline vs vancomycin-aztreonam, each for up to 14 days | Tigecycline was noninferior in clinical response in the clinically evaluable mITT population | 24.2% were culture positive for S. aureus, of which 9.1% were positive for MRSA |
| Sacchidanand et al., 2005 (353)b | Adults with known or suspected cSSSI who required ≥5 days of i.v. antibiotics | Double-blind RCT | 573 | Tigecycline vs vancomycin-aztreonam, each for up to 14 days | Tigecycline was noninferior in clinical response in the CE population (82.9% vs 82.3%) at TOC visit | 20.0% were culture positive for S. aureus, of which 36.5% were positive for MRSA |
| Jauregui et al., 2005 (354) | Adults with cSSSI suspected or confirmed to harbor a Gram-positive pathogen | Double-blind RCT | 854 | 2:1 distribution of dalbavancin at 1,000 mg on day 1 and 500 mg on day 8 vs 600 mg linezolid every 12 h, each for 14 days (with each dalbavancin dose defined as 7 days of therapy) | Dalbavancin was noninferior in clinical success at TOC visit (88.9% vs 91.2%) | 57.6% were culture positive for S. aureus, of which 56.5% were positive for MRSA |
| Noel et al., 2008 (361) | Adults with cSSSI | Double-blind RCT | 828 | Ceftobiprole vs vancomycin-ceftazidime, each for 7–14 days | Ceftobiprole was noninferior in cure rate in clinically evaluable (90.5% vs 90.2%) or ITT populations at 7- to 14-day TOC visit | 45.4% were culture positive for S. aureus, of which 33% were positive for MRSA |
| Noel et al., 2008 (355) | Adults with cSSSI | Double-blind RCT | 784 | Ceftobiprole vs vancomycin, each for 7–14 days | Ceftobiprole was noninferior in cure rate in clinically evaluable (93.3% vs 93.5%) or ITT populations at 7- to 14-day TOC visit | 60.9% were culture positive for S. aureus, of which 37.1% were positive for MRSA |
| Stryjewski et al., 2008 (356) | Adults with cSSSI | Pooled analysis of 2 double-blind RCTs (ATLAS 1 and 2) | 1,867 | Telavancin vs vancomycin, each for 7–14 days | Telavancin was noninferior in cure among the clinically evaluable population (88.3% vs 87.1%) at 7- to 14-day TOC visit | 61.2% were culture positive for S. aureus, of which 62.7% were positive for MRSA |
| Krievins et al., 2009 (357) | Adults with cSSTI | Double-blind RCT | 92 | 0.8 or 1.6 mg/kg iclaprim vs 1 g vancomycin, each given twice daily for 10 days | No difference in clinical cure rates at TOC visit (92.9% for lower-dose iclaprim, 90.3% for higher-dose iclaprim, and 92.9% for vancomycin) | 57% were culture positive for S. aureus, of which 10% were positive for MRSA |
| ASSIST 1 and 2 (828) | Adults with cSSSI | Pooled results of 2 investigator-blind RCTs (ASSIST 1 and 2) | 991 | 0.8 mg/kg iclaprim i.v. twice daily vs 600 mg linezolid i.v. twice daily, each for 10–14 days | Iclaprim did not meet prespecified noninferiority criteria for clinical cure rate at TOC visit in ITT and PP populations | 59.6% were culture positive for S. aureus, of which 39.4% were positive for MRSA |
| Craft et al., 2011 (358) | Adults with ABSSSI suspected or proven to be caused by a Gram-positive organism | Double-blind RCT | 198 | 600 mg fusidic acid p.o. twice daily (n = 43), fusidic acid at 1,500 mg twice daily for 2 loading doses and then 600 mg twice daily (n = 78), or 600 mg linezolid p.o. twice daily (n = 77); study outcomes were reported only for the “loading-dose” and linezolid groups | Similar clinical success rates between fusidic acid loading-dose and linezolid groups, among ITT (86% vs 95%), mITT (88% vs 93%), CE (92% vs 99%), and ME (96% vs 98%) populations | 71.6% were culture positive for S. aureus, of which 70.3% were positive for MRSA |
| Friedland et al., 2012 (359) | Adults with cSSSI | Pooled analysis of 2 double-blind RCTs (CANVAS 1 and 2) | 1,378 | Ceftaroline vs vancomycin-aztreonam, each for 5–14 days | Ceftaroline was noninferior in cure rates in clinically evaluable (91.6% vs 92.7%) and mITT (85.9% vs 85.5%) populations | 53.3% were culture positive for S. aureus, of which 36.9% were positive for MRSA |
| Prokocimer et al., 2013 (360) | Adults with ABSSSI | Double-blind RCT | 667 | 200 mg tedizolid daily for 6 days vs 600 mg linezolid twice daily for 10 days | Tedizolid was noninferior in early clinical response (79.5% vs 79.4%); results at the end of treatment and 1–2 wk posttherapy were also similar | 51.9% were culture positive for S. aureus, of which 51.4% were positive for MRSA |
| Moran et al., 2014 (373) | Patients aged ≥12 yr with ABSSSI | Double-blind RCT | 666 | 200 mg tedizolid i.v. daily for 6 days vs 600 mg linezolid i.v. twice daily for 10 days, with optional oral step-down therapy | Tedizolid was noninferior in early clinical response (85% vs 83%) | 48.8% were culture positive for S. aureus, of which 33.5% were positive for MRSA |
| Corey et al., 2014 (374) | Adults with suspected or proven ABSSSI requiring at least 7 days of i.v. therapy | Double-blind RCT | 954 | 1,200 mg oritavancin i.v. in a single dose vs vancomycin twice daily for 7–10 days | Oritavancin was noninferior in the mITT population (82.3% vs 78.9%) in a composite outcome of (i) cessation of spreading or reduction in lesion size, (ii) absence of fever, and (iii) no rescue antibiotic | 60.8% were culture positive, of which 73.8% were positive for S. aureus (47.7% MRSA, 52.3% MSSA) |
| Boucher et al., 2014 (372) | Adults with ABSSSI requiring at least 3 days of i.v. therapy | Pooled analysis of 2 double-blind RCTs (DISCOVER I and II) | 1,312 | Dalbavancin i.v. on days 1 and 8 vs vancomycin for at least 3 days +/− linezolid to complete 10–14 days of therapy | Dalbavancin was noninferior in early clinical response (79.7% vs 79.8%) | 50.8% were culture positive, of which 77.0% were positive for S. aureus (30.6% MRSA, 69.4% MSSA) |
SSTI, skin and soft tissue infection; cSSTI, complicated skin and soft tissue infection; ABSSSI, acute bacterial skin and skin structure infection; cSSSI, complicated skin and skin structure infection; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; TMP-SMX, trimethoprim-sulfamethoxazole; i.m., intramuscular; RCT, randomized controlled trial; ITT, intention to treat; mITT, modified intention to treat; TOC, test of cure; CE, clinically evaluable; ME, microbiologically evaluable; p.o., orally; PP, per protocol.
Note that tigecycline has subsequently received an FDA black box warning for an increased risk of death compared to other antibacterial drugs.
The FDA has released periodic guidance on clinical trial design for drug development for skin and skin structure infections. The studies enrolling patients with “cSSTI” were performed in accordance with the initial 1998 version of this guidance. This FDA guidance was updated in 2010 and included the newer designation ABSSSI. The oritavancin and dalbavancin trials were performed in accordance with this version of the guidelines. For a comparison of these guidelines, see reference 376. The guideline was updated again in October 2013 (375).
Impetigo.
A 2012 meta-analysis of 68 treatment trials for impetigo (362) concluded that topical antibiotics, including mupirocin, fusidic acid, and retapamulin, are more effective than placebo and as effective as or more effective than oral antibiotics. There was no difference when mupirocin and fusidic acid were compared head-to-head (362). Penicillin was inferior to erythromycin and cloxacillin. However, the majority of these studies were conducted in industrialized countries, and the findings may not be applicable to resource-limited settings, where the greater burden of impetigo lies (363) and where the severity of lesions and likelihood of development of resistance to topical agents are greater. Of the 68 trials included in the meta-analysis, only 5 were from resource-limited settings, and only 1 involved the extensive impetigo typically seen in these settings. Recently, an RCT of systemic therapy with short-course oral TMP-SMX versus intramuscular benzathine penicillin G in 508 indigenous Australian children with extensive impetigo provided high-quality evidence for the equivalent efficacies of these agents (316). The TMP-SMX regimens are particularly attractive due to the short courses of 3 or 5 days and significantly fewer side effects than with intramuscular benzathine penicillin G injections.
Uncomplicated SSTI.
It is not clear whether antibiotic therapy is required for other uncomplicated S. aureus SSTIs, especially for cases of abscess when incision and drainage are pursued. In an analysis of retrospective data, Lee et al. (364) noted that in children with culture-proven CA-MRSA skin abscesses (96% of whom underwent a drainage procedure), no significant differences in outcome were seen between those who received an effective antibiotic and those who did not; similar outcomes have been reported by others (365). In a larger cohort of 1,647 patients with SSTIs, 81% received antibiotics, but receipt of inactive initial therapy was not associated with a worse outcome, irrespective of whether a drainage procedure was done (247). This was in contrast to the findings of a subsequent analysis of 492 adults with community-onset uncomplicated SSTIs due to MRSA; in this retrospective review, receipt of active antimicrobial therapy was associated with lower rates of treatment failure (5% versus 13%) (366). However, 84% of those failures were because the patient required an additional incision-and-drainage procedure, leaving in question whether it was antibiotic failure per se, as opposed to inadequate surgical therapy, that led to treatment failures. Additionally, in a trial comparing two cephalosporins (cefdinir and cephalexin) for treatment of uncomplicated SSTIs, no difference in clinical cure rates between MSSA and MRSA subgroups was noted (343).
These findings laid the groundwork for several RCTs comparing antibiotics to placebo for the treatment of uncomplicated SSTIs. Rajendran et al. (344) randomized 166 patients with uncomplicated SSTIs to receive 7 days of cephalexin versus placebo, after incision and drainage. Seventy percent of cultures were positive for S. aureus, 88% of which were positive for MRSA and 93% of which were PVL positive. Clinical cure rates at 7 days were similar and were >90% in the placebo arm. Duong et al. (345) randomized 161 pediatric patients to receive 10 days of TMP-SMX versus placebo after incision and drainage, with in-person follow-up at 10 to 14 days and phone follow-up at 90 days. Failure rates were similar and were <6% for both groups. Eighty percent of patients had CA-MRSA isolated (100% TMP-SMX susceptible), whereas 9% had MSSA isolated. Patients on antibiotics developed fewer new lesions in the short term but not at the 90-day mark. Schmitz et al. (346) randomized 212 patients to receive TMP-SMX or placebo after incision and drainage. Treatment failure was seen in 17% of those who received TMP-SMX, versus 26% who received placebo, a difference that was not statistically significant. Others (367, 368) have noted that the point estimate of a 9% difference in the failure rate could be clinically significant if confirmed with an adequate sample size and extrapolated over the large number of S. aureus SSTIs each year. For uncomplicated cellulitis, the addition of TMP-SMX to cephalexin treatment did not provide benefit (347). Currently, there are larger ongoing clinical trials (ClinicalTrials.gov registration numbers NCT00730028 and NCT00729937) to answer this question more definitively. Miller et al. reported results of a study involving 524 patients with uncomplicated SSTIs, randomized to receive TMP-SMX versus clindamycin, each for 10 days. MRSA was the most common organism isolated. There was no significant difference between treatments (369).
There is some heterogeneity in the definition of “uncomplicated” in these studies; for example, Schmitz et al. (346) excluded immunocompromised patients and those with facial abscesses, whereas Rajendran et al. (344) included those patients. Duong et al. (345) excluded children with diabetes or other chronic health problems. There is consistency, however, in the exclusion of hemodynamically unstable patients or those with an extension of the abscess into deeper structures. A conservative definition of uncomplicated abscess would thus exclude those who are systemically unwell; have comorbidities, including immunosuppression or diabetes; or have abscesses in locations for which complete drainage is difficult, such as the face, hand, or genitalia. Taken together, studies to date suggest that for these uncomplicated cutaneous abscesses for which drainage is pursued, additional antimicrobial therapy may not be required. This is the position taken in IDSA guidelines for the treatment of MRSA infection, although those authors specify that for purulent cellulitis in the absence of a drainable focus of infection, empirical therapy for CA-MRSA is recommended (76). The 2014 IDSA guidelines for SSTI differentiate between mild infections (no systemic signs of infection), for which adjunctive antibiotics are not required, and moderate (systemic signs of infection) or severe (failed initial antibiotic therapy, impaired host defenses, or systemic signs of infection with hypotension) infections, for which antibiotics are indicated (370). For cases of uncomplicated cellulitis, generally defined as those in which the patient is systemically well, current IDSA SSTI guidelines recommend therapy aimed at streptococci, not S. aureus (76, 370), with therapy for CA-MRSA being reserved for those patients who do not respond to β-lactam treatment.
The long-held assumption that TMP-SMX is not effective for SSTIs involving S. pyogenes is being strongly challenged. It appears that TMP-SMX has in vitro efficacy against S. pyogenes (371). Two recent clinical trials suggest that TMP-SMX has clinical efficacy for nonsuppurative cellulitis (369) and impetigo due to S. pyogenes (316). Thus, TMP-SMX may be an appropriate treatment option for both S. pyogenes- and S. aureus-related SSTIs.
Complicated SSTI.
For complicated SSTI, a number of registrational trials have compared different antimicrobial agents (Table 4). Current IDSA MRSA treatment guidelines recommend vancomycin, linezolid, daptomycin, telavancin, or ceftaroline for patients hospitalized with a severe purulent SSTI (370). In the case of a nonpurulent SSTI, a β-lactam antibiotic is recommended for mild or moderate infection, whereas vancomycin is recommended as part of empirical therapy for severe nonpurulent SSTI. As shown in Table 4, a number of other agents have been studied; dalbavancin (372), tedizolid (373), and oritavancin (374) have obtained FDA approval in 2014 alone. The FDA has released periodic guidance on clinical trial design for drug development for SSTIs. Following the initial 1998 version of this guidance, updates were provided in 2010 (which included the newer designation of acute bacterial skin and skin structure infections [ABSSSIs]) and most recently in 2013 (375; for a discussion of these guidelines, see reference 376).
For necrotizing fasciitis, there are limited data to guide a treatment approach, both because the disease is uncommon and because patients with necrotizing fasciitis have been excluded from most trials. Empirical therapy should cover MRSA and anaerobes, and recommended combinations include vancomycin plus piperacillin-tazobactam or a carbapenem (370). Directed therapy for S. aureus should be an antistaphylococcal penicillin for MSSA or vancomycin for MRSA. Similar considerations extend to pyomyositis, for which S. aureus is the primary pathogen. Clindamycin is recommended for necrotizing fasciitis caused by S. pyogenes, based on its suppression of streptococcal toxins and two observational studies showing greater efficacy than with β-lactams (377, 378). In an additional observational cohort, the addition of clindamycin was associated with reduced mortality due to invasive S. pyogenes infections (379). There are no such data available for S. aureus necrotizing fasciitis, and IDSA guidelines do not specifically recommend the addition of clindamycin in this setting (370). Nonetheless, other groups recommend adding clindamycin for its antitoxin effect (380). The successful use of linezolid to switch off toxin production in a surgical wound with S. aureus-associated toxic shock syndrome (TSS) has also been reported (381). Our practice is to add clindamycin for S. aureus necrotizing fasciitis. There is currently no evidence to recommend a role for i.v. immunoglobulin for S. aureus necrotizing fasciitis. Aggressive surgical debridement and antistaphylococcal antibiotics are considered cornerstones of therapy (370).
OSTEOARTICULAR INFECTIONS
S. aureus is the most common pathogen in all three major classes of osteoarticular infection, namely, osteomyelitis (OM) (382–392), native joint septic arthritis (393–401), and prosthetic joint infection (PJI) (402–406). As staphylococcal osteoarticular infections in children are common and have distinctive clinical and management issues compared to those in adults, we include an in-depth discussion of this important subpopulation.
Osteomyelitis
Osteomyelitis is an infection of bone resulting in its inflammatory destruction, bone necrosis, and new bone formation. The Waldvogel classification system (407) describes three types of OM: hematogenous OM, contiguous-focus OM (from adjacent structures such as joint spaces or soft tissues or from trauma or surgery with direct implantation of organisms), and OM with vascular insufficiency (most commonly in patients with diabetes or peripheral vascular disease and generally involving the foot). S. aureus is the predominant cause of OM in all of these categories and is identified in 30 to 60% of cases (Table 5). Hematogenous OM generally involves the ends of long bones in children and adolescents and the axial skeleton in older adults (408), partly due to the blood supply to vertebrae in adults being more extensive than that to the long bones. This section principally focuses on hematogenous OM that most commonly manifests as vertebral OM in adults and long bone OM in children, where S. aureus is typically the key pathogen.
TABLE 5.
Osteoarticular infections and the percentage caused by Staphylococcus aureusa
| Type of osteoarticular infection and reference | Study type | No. of cases (no. of cases with microbiologic diagnosis) | Region | Time period (yr) | Population | Predominant causative organism | % of infections caused by S. aureus (% caused by MRSA) |
|---|---|---|---|---|---|---|---|
| Nonvertebral osteomyelitis | |||||||
| 382 | Prospective observational, single center | 166 | Oxford, UK | Chronic OM | MSSA | 32 | |
| 383 | Retrospective, single center | 454 (454) | USA | 1982–1998 | Adults treated at HITH; 52% diabetic foot infections, 6% vertebral infections, 19% long bone infections | MSSA | 54 |
| Vertebral osteomyelitis | |||||||
| 384 | Retrospective | 70 (44) | St. Louis, MO, USA | Adults with hematogenous VOM | MSSA | 55 (22) | |
| 385 | Retrospective | 137 | Denmark | 1978–1982 | Adults with hematogenous VOM | ||
| 386 | Retrospective, multicenter | 253 | Cleveland, OH, USA | 1950–1994 | All ages with VOM | MSSA | 49 |
| 387 | Retrospective, single center | 129 (74) | Cambridge, UK | 1998–2008 | All ages with VOM | MSSA | 51 |
| 388 | Retrospective, single center | 58 | Sweden | 1990–2005 | All ages with VOM | MSSA | 34 |
| Native joint septic arthritis | |||||||
| 393 | Retrospective | 233 | Switzerland | 1999–2008 | Adults with hematogenous NJSA | MSSA | 49.3 (4.7) |
| 394 | Retrospective | 81 (59) | Cambodia | 2007–2011 | Children with NJSA and OM | MSSA | 49 (2) |
| 395 | Retrospective, single center | 44 (24) | Victoria, Australia | Children with acute NJSA | MSSA | 76 (8) | |
| 396 | Retrospective, single center | 53 | Japan | 1955–2005 | Adults with hematogenous NJSA | MSSA | |
| 397 | Retrospective, single center | 65 (28) | Saudi Arabia | 1997–2006 | Children with NJSA | MSSA | 39 |
| 398 | Retrospective, single center | 110 | Israel | 1987–2003 | Adults with NJSA | MSSA | 40 |
| Prosthetic joint infection | |||||||
| 402 | Retrospective, 10 hospitals | 147 | Victoria, Australia | 2006–2008 | Early-onset infections | MSSA | 53 |
| 403 | Prospective, 9 hospitals | 50 | Spain | 2004–2006 | Adults with hematogenous PJI | MSSA | 38 |
| 404 | Prospective, single center | 38 (27) | Norway | 1998–2005 | Adults with early hip PJI | MSSA | 37 |
| 405 | Prospective, single center | 152 (90) | Oxford, UK | Adults with PJI having 2-stage replacements | CoNS | 18 | |
| 403 | Prospective, 9 hospitals | 139 (132) | Spain | 2004–2006 | Adults with early PJI | MSSA | 40 (12) |
| 406 | Retrospective, single center | 112 | Oxford, UK | 1998–2003 | Adults with PJI treated with DAIR | MSSA | 73 (8) |
OM, osteomyelitis; VOM, vertebral osteomyelitis; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; CoNS, coagulase-negative Staphylococcus; HITH, hospital in the home; PJI, prosthetic joint infection; DAIR, debridement and implant retention; NJSA, native joint septic arthritis.
Epidemiology.
The incidence of vertebral OM in adults is increasing. Scandinavian studies reported an incidence of 0.05 per 100,000 person-years in Denmark in 1978 to 1982 (385), compared with 2.2 per 100,000 person-years in Sweden in 1990 to 1995 (388). More recently, the incidence of vertebral OM in Funen County, Denmark, increased during a 14-year period (1995 to 2008), from 2.2 to 5.8 per 100,000 person-years (409). S. aureus caused 55% of cases, and the incidence of S. aureus vertebral OM increased from 1.6 to 2.5 per 100,000 person-years (409). Elsewhere, incidence rates are similar. In New Zealand, from 2000 to 2005, the incidence of vertebral OM was 9.8 per 100,000 person-years in the >65-year age group (410). In a nationwide Japanese study, the incidence increased from 5.3 per 100,000 person-years in 2007 to 7.4 per 100,000 person-years in 2010 (411). The major risk factors for vertebral OM are advancing age (389, 409, 411), diabetes mellitus (384, 386, 411, 412), injection drug use (412, 413), and immunosuppression (389, 412), and the growing incidence of these risk factors, together with increased access to advanced radiological modalities, may explain the increasing incidence of vertebral OM. The majority of patients with vertebral OM and concomitant SAB are >60 years of age (414).
Pathophysiology.
Animal models have demonstrated that healthy bone is generally highly resistant to infection and that either direct trauma or a large bacterial inoculum is needed to establish infection (415). S. aureus, however, has evolved to overcome the natural resistance of bone to infection. For example, it expresses numerous surface proteins that mediate adherence to components of bone matrix and collagen (416). These bacterial cell surface receptors are known as adhesins or MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) (417, 418). Strains of S. aureus lacking genes that encode certain MSCRAMMs are less likely to cause osteoarticular infections in animal models (417, 419, 420). S. aureus is also able to form biofilms on foreign materials that act as sanctuary sites, where it is relatively protected from antimicrobial agents and the host immune response (421). Finally, S. aureus can invade osteoblasts (422) and form small-colony variants (SCVs) (423) in the intracellular compartment, where they are able to survive in a metabolically inactive state while preserving the integrity of the host cell. Kalinka et al. (424) recently found that S. aureus clinical isolates cultured from samples from patients with chronic OM were better able to invade osteoblasts than those from samples from patients with sepsis and nasal colonization. Isolates from acute and chronic OM also formed more biofilm, and compared to isolates from acute OM, those from chronic OM were likely to develop a small-colony-variant phenotype (424).
SCVs of S. aureus are important in the pathogenesis of a number of chronic forms of staphylococcal infections, including osteomyelitis (424–426), relapsing prosthetic joint infection (427, 428), skin and soft tissue infection (429), endocarditis (430), and infections involving ventriculoperitoneal shunts (431) and nasal sinuses (432). SCVs are also important in patients with cystic fibrosis (CF) (see Pleuropulmonary Infections, below). SCVs are naturally occurring subpopulations possessing important metabolic and phenotypic differences from ordinary S. aureus isolates (comprehensively reviewed in reference 423). SCVs are slower growing, less pigmented, and less hemolytic and produce less coagulase than ordinary S. aureus isolates, making them difficult to identify in the laboratory. They are able to survive intracellularly within nonprofessional phagocytes such as fibroblasts and endothelial cells (433, 434) and are relatively resistant to cell wall-active antibiotics and aminoglycosides (435, 436). The basis of these phenotypic changes appears to be a defect in the electron transport chain and auxotrophy (dependence) on certain growth factors, including hemin, menadione, and thymidine (437). Given their slow growth and intracellular survival, SCVs are difficult to eradicate with standard antibiotic therapy. The ideal therapy for these variants is unclear, and there are no randomized trials on which to base recommendations. Most experts recommend the use of antibiotics such as rifampin and quinolones that penetrate host cells but do not act on bacterial cell wall synthesis (423, 438) and also emphasize the role of extensive surgical debridement in the treatment of SCV infections (439).
Clinical manifestations and outcomes.
Vertebral OM generally involves the endplates of two adjacent vertebrae and the intervening disc space. The most common route of spread is hematogenous seeding to the vertebral endplates, and from here, the infection spreads directly into the disc space (386). Hence, the term “discitis” is a misnomer, since disc space infection is secondary. The exception is where infection has been directly introduced to the disc space (e.g., following a surgical microdiscectomy). The hallmark of vertebral OM is back pain, being present in 85 to 100% of patients. Localized vertebral percussion tenderness is present in >80% of cases, but fever is present in anywhere from 18 to 83% of cases in various case series (Table 6). A significant minority of patients have signs of nerve compression (such as limb weakness) at presentation, and one-quarter of patients with vertebral OM will ultimately develop paralysis or significant neurologic dysfunction (386). Among 133 consecutive patients with SAB and concomitant vertebral OM, the most frequent primary foci or portals of entry of infection were the skin (21%) and urinary tract (10%). However, 71/133 patients (53%) had no identified primary focus (414). Comparisons between vertebral OM caused by MRSA and that caused by MSSA have found that patients with infections due to MRSA have more comorbidities and are more likely to have had recent nonspinal surgery (390). Lumbar vertebrae are most frequently affected, followed by thoracic and then cervical regions (384, 386, 412). Notably, the diagnosis of vertebral OM is frequently delayed, with an interval of >1 month from symptom onset to diagnosis for the majority of patients (386, 389, 391, 392, 414). Delayed diagnosis has been associated with poorer longer-term outcomes, including death, chronic pain, and residual disability (386).
TABLE 6.
Clinical manifestations of vertebral osteomyelitis and septic arthritis caused by S. aureusa
| Infection type and reference | No. of patients | Age of patients (yr) | Main symptom(s) (% of patients) | Main sign(s) (% of patients) | Laboratory finding(s) (% of patients) | Description |
|---|---|---|---|---|---|---|
| Vertebral osteomyelitis | ||||||
| 386 | 253 | 60b | Limb weakness (25) | Fever (78) | NA | |
| 389 | 111 | 60c | Back pain (91), limb weakness (33) | Fever (16) | Raised ESR (95) | |
| 390 | 49 | 65c | Back pain (96), limb weakness (53) | Fever (57), limb weakness (53) | NA | |
| 391 | 40 | 58c | Back pain (100), limb weakness (48) | Vertebral tenderness (83), fever (65) | Raised CRP level (98), raised ESR (95) | |
| 392 | 20 | 72c | Back pain (85), limb weakness (55) | Fever (30) | Raised ESR (95), raised CRP level (100) | |
| Native joint septic arthritis | ||||||
| 399 | 47 | 66.5b | Joint pain (83) | Joint swelling (79), fever (34) | Raised CRP level (98), raised ESR (100) | |
| 400 | 56 | 49b | Joint pain (100) | Restricted joint movement (100) | 95% single joint, 67% knee joint | |
| 401 | 75 | 2.5b | Joint pain (85) | Joint swelling (77), fever (44) | Raised CRP level (98), raised ESR (100) | 85% single joint, 56% knee joint |
NA, not available; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Median age.
Mean age.
The peripheral WBC count is raised in a variable proportion of patients with OM, but the erythrocyte sedimentation rate (ESR) and serum C-reactive protein (CRP) level are raised in 95 to 100% of patients with acute osteomyelitis (Table 6). A systematic review of 14 studies of vertebral OM found that the reported yield from blood cultures for a microbiological diagnosis was 58% (range, 30 to 78%) (412). Therefore, in the appropriate clinical and radiological settings, positive blood cultures can eliminate the need for diagnostic biopsy or aspiration of infected bone for culture. However, bone biopsy for culture and histology should be pursued if blood cultures are negative, as it provides a higher diagnostic yield (77%; range, 47 to 100%) (412). Where possible, the biopsy specimen is best obtained prior to antibiotic treatment. Several investigators have found that the microbiological yield from biopsy specimens of patients on antibiotics is ∼50% lower than that from biopsy specimens obtained prior to antibiotic treatment (440–442). Where an initial percutaneous biopsy specimen is negative, there may be value in obtaining a second percutaneous biopsy specimen. Gras et al. (443) examined a cohort of 136 patients with vertebral OM who were all blood culture negative and who had not received antibiotics in the previous 2 weeks. Performance of a second biopsy after an initial negative result led to a microbiological diagnosis in 80% (74/93) of cases, versus 44% (60/136) with only one biopsy specimen. While significantly more invasive, open surgical biopsy is also more likely to yield a diagnosis than needle biopsy. For example, in a series of 70 patients from Missouri, an open biopsy had a 93% diagnostic yield, compared with 48% for radiologically guided needle biopsy (384). In summary, a microbiological diagnosis should be sought to guide subsequent therapy. One suggested approach to the diagnosis of vertebral OM begins with blood cultures, followed by an initial percutaneous biopsy, a second percutaneous biopsy if the initial biopsy specimen is sterile, and an open biopsy if clinically indicated (441).
The short-term mortality rates for OM are substantial, at 2.8 to 7.7% for nonvertebral OM (383, 444) and 6 to 16% for vertebral OM (386, 389, 391, 411). Furthermore, health care costs (445, 446) and rates of long-term functional impairment (386, 395, 447) are also high. Akiyama et al. (411) found that for vertebral OM, higher mortality rates were significantly associated with older age (ORs of 2.78, 3.99, and 7.13 for patients aged 60 to 69, 70 to 79, and ≥80 years, respectively, compared with those aged ≤59 years; P < 0.001), hemodialysis use (OR, 10.56; P < 0.001), diabetes (OR, 2.37; P < 0.001), liver cirrhosis (OR, 2.63; P = 0.001), malignancy (OR, 2.68; P < 0.001), and IE (OR, 3.19; P < 0.001).
Management.
The management of vertebral OM caused by S. aureus requires prolonged courses of antibiotics. Jensen et al. (414) found that among 114 patients with bacteremic S. aureus OM, recurrence was more likely in patients receiving <8 weeks of antibiotic therapy than in those receiving ≥8 weeks of therapy. Park et al. (448) determined that for 62 patients with vertebral OM due to MRSA, patients receiving <8 weeks of antibiotic treatment were 4.8 times more likely to relapse than those receiving ≥8 weeks of therapy. There was no such association for 77 patients with MSSA infections (448). In contrast, Roblot et al. (449) determined that for 120 patients, outcomes were similar for 36 patients treated at one center with a median of 6 weeks of total therapy and for 84 patients treated at another center with a median of 10 weeks total therapy. These data and other observational data (386, 450) have led to recommendations that range from 6 weeks (449, 451) to 12 weeks (441) of antibiotic treatment. Most recently, Bernard et al. (452) compared antibiotic treatments for 6 weeks and 12 weeks for patients with pyogenic vertebral OM. In an open-label, noninferiority clinical trial design powered to achieve an absolute margin of 10%, 359 patients from 71 French medical care centers were randomly assigned to either of the treatment durations. Of these infections, 145 (41%) were due to S. aureus, 95% (n = 137) of which were due to MSSA. The primary endpoint was defined as the proportion of patients who were classified as being cured at 1 year by a masked independent validation committee. In an intention-to-treat analysis, 6 weeks of antibiotic treatment was noninferior to 12 weeks of antibiotic treatment, suggesting that the standard duration of antibiotic treatment for patients with this infection could be reduced to 6 weeks (452). Where clinical improvement is slow, undrained or unremoved foci exist, the CRP level is slow to normalize, or infection is due to MRSA, we consider it prudent to extend antibiotic therapy to at least 8 weeks. For patients responding well to initial i.v. therapy, observational data suggest that a switch to oral therapy after 2 weeks may be safe (451, 453). Results reported by Bernard et al. (452) are also supportive in that the overall rate of treatment success in their RCT was 91% with a median duration of 14 days of i.v. therapy.
Septic Arthritis
Epidemiology.
Native joint septic arthritis is uncommon, with a population-based incidence of 4 to 10 per 100,000 person-years in Western Europe (454–456) but a higher incidence in nonindustrialized countries and disadvantaged populations (e.g., 29.1 per 100,000 person-years in aboriginal Australians [457] and 13.8 per 100,000 person-years in children in Cambodia [394]). In adults, underlying joint diseases such as rheumatoid arthritis and crystal arthropathies are predisposing conditions for septic arthritis (458). In addition to rheumatoid arthritis, other risk factors identified in a prior systematic review included an age of >80 years, diabetes mellitus, recent joint surgery, and skin infection (459). In healthy children, septic arthritis is typically related to spreading from contiguous OM affecting the vascular growth plate of long bones. S. aureus is the most common causative organism in both children and adults, accounting for 39 to 76% of identified pathogens in various case series (Table 5). There appears to have been no significant change in the distribution of causative organisms in native joint septic arthritis over the past 20 years (460). MRSA remains uncommon in most recent case series, accounting for 2 to 8% of infections (Table 5), but its prevalence may be increasing in some areas, particularly the United States (461).
Pathophysiology.
S. aureus most commonly gains access to a joint space via bacteremic seeding of the vascular synovial lining of the joint, accounting for up to 70% of cases. Direct implantation, either through trauma or an iatrogenic event (e.g., following an intra-articular steroid injection), accounts for most of the remainder. Rarely, septic arthritis can occur following arthroscopy, the reported incidence of which is 0.01 to 0.23% (462).
As is the case in the pathogenesis of osteomyelitis, S. aureus MSCRAMMs mediate adherence to host proteins in the joint extracellular matrix, a process that is more likely to occur in damaged or healing joints (463). Upon seeding the synovial membrane, bacteria pass into the joint space. Synovial fluid inhibits S. aureus growth in vitro (464), an observation that is at odds with the substantial damage caused by S. aureus septic arthritis. Several avenues of investigation have clarified this apparent contradiction. In murine models, there is a rapid recruitment of neutrophils, mediated by formylated peptides (465), after S. aureus gains entry to the joint space. Activated macrophages and T cells are recruited as well, and a host of cytokines are induced, including tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-1 (IL-1), IL-2, IL-6, and IL-17 (466). Neutrophils, however, play a dual role. They are needed for bacterial clearance, and their absence in experimental models leads to higher mortality rates and worse arthritis (467); however, they also contribute to tissue damage via enzyme release and free radical formation. A study of human synovial fluid also demonstrated that S. aureus forms large aggregates with host-derived fibrin, a finding that suggests a possible explanation for in vivo resistance to killing by neutrophils, as these aggregates are too large for neutrophil phagocytosis (468).
If the host is able to contain the initial S. aureus invasion, the infection may resolve. However, in the absence of an effective early response, the inflammatory process leads to joint destruction. In addition to the damage inflicted by proteases and inflammatory cytokines released by cells of the synovial lining, ischemia due to increased intra-articular pressure may also result (417). Thus, the major deleterious consequence of septic arthritis is the destruction of articular cartilage, leading to degenerative osteoarthritis.
Clinical manifestations and outcomes.
Septic arthritis is most commonly monoarticular, but ∼10% of cases are polyarticular (469, 470), and this occurs mainly in the context of SAB. The knee is the most commonly involved joint in acute septic arthritis, comprising 50% of cases, followed by the hip and then the shoulder (471, 472). Septic arthritis involving the pubic symphysis or the sacroiliac joint accounts for ∼5% of cases and can be difficult to diagnose (473, 474). Sternoclavicular septic arthritis is strongly associated with IDUs but can occur in other settings (475). It accounts for 1% of septic arthritis cases in the general population but up to 17% in IDUs in one series (476).
Patients with septic arthritis generally present acutely with a single swollen, hot, red, and tender joint. Classically, an affected joint is said to be so inflamed, tense, and tender as to make any movement impossible. However, contrary to traditional teaching, a mobile joint does not rule out septic arthritis. Both joint pain and swelling are present in >80% of cases at presentation, but fever is present in only 30 to 50% of cases (Table 6).
Arthrocentesis is the definitive diagnostic test for septic arthritis. Synovial fluid leukocyte counts are generally in the range of 50,000 to 150,000 cells/mm3, with likelihood ratios (LRs) for bacterial septic arthritis of 7.7 and 28.0 for synovial leukocyte counts of >50,000 cells/mm3 and >100,000 cells/mm3, respectively (459). More than 90% of synovial fluid white blood cells are neutrophils in most cases of culture-confirmed septic arthritis. In a meta-analysis including 6,242 patients with septic arthritis (of all causes but most commonly S. aureus), a leukocyte differential consisting of >90% neutrophils was strongly associated with septic arthritis (LR, 3.4; 95% CI, 2.8 to 4.2), while a differential of <90% neutrophils suggested that septic arthritis was absent (LR, 0.34; 95% CI, 0.25 to 0.47) (459). In bacterial septic arthritis in general (including S. aureus and other pathogens), Gram stain is positive in 29 to 50% of cases (477), and synovial fluid culture is positive for the majority of patients who have not received prior systemic antibiotics. This is in contrast to gonococcal septic arthritis, where synovial fluid cultures are positive in only ∼50% of cases (478, 479). The major clinical differential diagnoses for septic arthritis are acute crystal arthropathies (gout and pseudogout) and acute hemarthrosis. Gout can coexist with septic arthritis, so the presence of crystals does not rule out the diagnosis of concomitant septic arthritis (480, 481).
Between 10 and 30% of patients with septic arthritis suffer long-term decreased joint function or mobility (395, 447, 454, 482). This proportion is higher with S. aureus than with other organisms and with delays in diagnosis or surgical intervention (483, 484). S. aureus septic arthritis is considered a medical emergency, as it can lead to rapid and irreversible joint damage if it is not treated promptly.
Management.
There are no adequately powered randomized trials to guide management of S. aureus septic arthritis in adults. Therefore, guidelines have relied primarily upon expert opinion, usually extrapolated from treatment of other invasive staphylococcal infections and from animal and observational human studies (76, 470, 485). Most experts agree that one or more episodes of drainage of the joint space are urgently required in all cases, followed by a minimum of 3 to 4 weeks of antistaphylococcal antibiotic treatment, the initial 2 weeks of which should be intravenously administered. A recent retrospective study from Switzerland suggests that shorter courses of antibiotics may be sufficient for adults with uncomplicated native joint septic arthritis (486). This study included 157 adult patients and found that a total antibiotic duration of 14 days (the initial 7 days being i.v.) was noninferior to longer courses of 4 to 6 weeks, including patients with S. aureus infections. In the daptomycin registrational trial for S. aureus bacteremia (119), there were 32 patients with osteoarticular infections, 16 of whom had native joint septic arthritis. Comparable success rates were observed among patients treated with daptomycin (7/11; 64%) or with standard therapy (3/5; 60%) (487). The best surgical approach for drainage of the joint is also unclear and depends on the situation. For deep joints such as the hip, arthrotomy and lavage are generally the preferred methods of drainage. For more accessible joints such as the knee, either arthroscopic drainage and lavage or repeated closed-needle drainage is recommended, with no clear evidence of superiority of either of these two approaches (488, 489). The role, if any, of adjuvant corticosteroids is also unknown; in a murine model, systemic corticosteroid administration was associated with favorable outcomes (490). Two randomized trials and one nonrandomized trial have suggested a benefit of adjunctive dexamethasone in children with native joint septic arthritis (482, 491, 492), but there are no data for adults.
Osteoarticular Infections in Children
Epidemiology.
The incidence of osteoarticular infections in children ranges from 7 to 22 per 100,000 person-years based on studies from Europe (493–495). These infections are more common in males than in females (with incidences in French children of 24 per 100,000 person-years for boys and 19 per 100,000 person-years for girls) and in toddlers than in other age groups (494, 495). Some ethnic groups may be at higher risk, with Maori and Pacific Islander populations being overrepresented in a study involving 813 cases of acute OM in New Zealand (496). In the United States, CA-MRSA has become considerably more prominent as a cause of acute osteoarticular infections since 2000. In a study of 158 cases in Tennessee, the proportion of osteoarticular infections due to CA-MRSA rose from 4% to 40% from 2000 to 2004 (497). Similarly, the proportion of cases of acute OM due to CA-MRSA was 6% in 1999 to 2001 compared to 31% in 2001 to 2003 in Dallas, TX (498). In Houston, TX, between 2001 and 2010, 195 of 376 (52%) cases of S. aureus OM were due to MRSA (499).
Clinical manifestations and outcomes.
Acute hematogenous OM in children presents with fever and malaise, local pain, and point tenderness and most commonly involves the metaphysis of the tibia or the femur, resulting in limping or an inability to walk (500). The pain is often poorly localized but becomes more focal over time. The hallmark of the pain is its constant nature. Overlying redness and swelling are often present, which may create diagnostic confusion. For diagnostic purposes, CRP analysis is highly sensitive and thus has value in excluding the diagnosis of acute osteoarticular infection. In a prospective study of 265 osteoarticular infections in children, using a cutoff of 20 mg/liter, CRP analysis had a sensitivity of 95% for the diagnosis of acute OM; and the combination of CRP and ESR (with a cutoff 20 mm/h) analyses provided a sensitivity of 98% (501). Additionally, CRP analysis can be used to monitor the response to antibiotic treatment (501). Peltola and Paakkonen provide an excellent diagnostic algorithm for acute OM in children (500).
With regard to acute OM caused by S. aureus, Ju et al. (502) found four clinical parameters that could predict the probability of acute OM in children due to MRSA compared to MSSA: temperature of >38°C, hematocrit level of <34%, white blood cell count of >12,000 cells/μl, and CRP level of >13 mg/liter. However, this study suffers from having only 11 patients with MRSA and a lack of genotyping data and from its single-center design (502). An attempt to validate this clinical prediction algorithm among 58 patients (MRSA, n = 16; MSSA, n = 42) in Phoenix, AZ, found the algorithm to have a poor predictive value (503). Without genotyping data, it seems likely that the study by Ju et al. and previous studies that also compared cases of acute OM due to MRSA versus MSSA (498, 502, 504, 505) found discriminators between USA300 and other S. aureus clones rather than between MRSA and MSSA per se.
With the emergence of CA-MRSA, deep venous thrombosis (DVT) adjacent to the site of OM has been described by several groups (506–510). DVT with acute OM has been associated with MRSA (506, 508), PVL-positive strains of S. aureus (510), and USA300 in particular (507). Compared to patients with acute OM but no DVT, those with DVT were consistently unwell, likely to be bacteremic, and likely to have pulmonary involvement (presumably due to septic pulmonary emboli), and MRSA was overrepresented (511, 512). Thus, a high index of suspicion for DVT is required for children with acute OM who are critically unwell or who have pulmonary involvement, and Doppler ultrasound screening near the site of infection should be considered (511).
Nonetheless, most large series suggest that for acute OM in children, outcomes are generally favorable. Only 1 child out of 1,000 with OM died in a case series from France (494), and of 131 prospectively monitored cases in Finland, only 2 children developed mild sequelae (varus deformity of tibia and ankle pain during exercise) (513). The mean length of hospital stay was 8.6 days in the French series (494).
Management.
Empirical treatment for acute OM in children is dictated by the local antibiogram of S. aureus. Where the prevalence of MRSA is <10% among community S. aureus strains, an antistaphylococcal penicillin or cephalosporin is recommended; where the prevalence of CA-MRSA is >10% and the rate of clindamycin resistance is <10%, clindamycin is recommended; and where both the prevalence of CA-MRSA and the rate of clindamycin resistance are >10%, vancomycin should be used (500). If the child is severely ill and has suspected acute OM or septic arthritis, it is prudent to treat the child with both vancomycin and an antistaphylococcal β-lactam until bacterial susceptibilities are known (499).
For pediatric acute OM caused by MSSA, an early switch to oral therapy appears safe. A prospective study of 70 children with either septic arthritis or OM demonstrated that an algorithmic approach resulted in 59% of children converting to oral therapy after 3 days of i.v. therapy and 86% converting to oral therapy after 5 days. All 70 children had good outcomes at 1 year of follow-up (514). Similarly, Peltola et al. switched 131 patients to oral therapy after a median of 3 to 4 days of i.v. therapy, with excellent outcomes (513). An early switch to oral therapy is particularly important for children, as the risk for central line-related complications is high. Ruebner et al. found that of 75 patients who received >2 weeks of treatment for acute OM through a central venous catheter (CVC), 41% developed at least one CVC-related complication (515).
A prospective, quasirandomized, controlled, open-label trial involving 252 children with osteoarticular infections (82/252 with OM and 189/252 with S. aureus [all MSSA]; the proportion of cases of OM with MSSA was not reported) in Finland determined that oral clindamycin or a first-generation cephalosporin was equally efficacious as follow-up therapy (516). The same investigators also determined in an RCT involving 131 children with acute OM (117 with S. aureus, all MSSA) that 20 days of total therapy resulted in outcomes equivalent to those with 30 days of total therapy (513). In children with septic arthritis, a separate Finnish study (517) found that a duration of 10 days of total antibiotic therapy was equivalent to 30 days of therapy. Of 130 cases, only 1 developed a late-onset infection following completion of therapy, and this case was in the 30-day arm of the study. A systematic review (518) noted that the above-mentioned RCTs are only of moderate quality, principally due to a lack of blinding, and concluded that the recommendations of treatment for acute OM of 3 to 4 days of i.v. therapy followed by oral antibiotics for a total treatment duration of 3 weeks should be regarded as being supported by only weak evidence (grade 2B).
Given the severity of osteoarticular infections caused by CA-MRSA and their conspicuous absence in the Finnish studies, it is unknown whether abbreviated i.v. and subsequent oral therapies can effectively treat acute OM due to MRSA. This point is acknowledged by Peltola and Paakkonen (500) and also in IDSA guidelines for MRSA infections (76). Both reports suggest that acute OM due to MRSA should be treated with a minimum of 4 to 6 weeks of total therapy. Additionally, it is recommended that infants <3 months of age receive a longer course of i.v. therapy due to concerns over the absorption and efficacy of oral antibiotics (518).
Only 12% of 130 patients with septic arthritis in a Finnish study (517) required a surgical procedure, and in a separate trial, 62/131 (47%) cases of acute OM received resectional surgery to the bone cortex (513). However, higher rates of surgery have been noted in the United States. For example, Tuason et al. found that of 57 cases of acute OM, 41 (72%) children required surgery, 12 of whom underwent ≥2 surgeries (519). Additional concerns are that hip septic arthritis in children can result in ischemic necrosis of the femoral head and that sequelae of septic arthritis may be more common than for OM. An Australian study including 44 children, in whom S. aureus was the causative organism in 76% of cases, found that 10% of children at 12 months had residual joint dysfunction (395). Thus, careful clinical assessment and monitoring are mandatory. For patients with extensive disease or where levels of inflammatory markers are not being reduced as expected, ongoing reassessment of the need for surgical intervention is advised.
Adjunctive dexamethasone appears to be a promising intervention to accelerate recovery and decrease residual morbidity in children with native joint septic arthritis. Odio et al. (482) randomized 123 children with septic arthritis (67% of whom had infections due to S. aureus) to receive 4 days of adjunctive i.v. dexamethasone in addition to antibiotics. They found that a significantly lower proportion of patients in the dexamethasone group had residual joint dysfunction after 12 months of follow-up (2% versus 26%). Harel et al. (491) randomized 49 children to receive 4 days of dexamethasone or placebo for septic arthritis and found more rapid resolution of fever and pain and a shorter duration of i.v. antibiotics in the dexamethasone group. However, it is unclear if these results apply to S. aureus infections, as 65% of patients had no pathogen isolated from joint fluid, and for the remainder of the patients, the most common pathogen was Kingella kingae. More rapid early recovery with dexamethasone was also recently found in a double-blind, nonrandomized, prospective clinical trial enrolling 60 children in Pakistan (492).
Prosthetic Joint Infection
Epidemiology.
Prosthetic joint replacement is common, with >600,000 procedures being performed in the United States (520) and >77,000 being performed in Australia annually (Australian Orthopaedic Association National Joint Replacement Registry [https://aoanjrr.dmac.adelaide.edu.au/]). Older observational studies reported rates of infection of 0.5 to 1% for hip arthroplasties and 1 to 2% for knees (521, 522); more recent data from a large U.S. Medicare data set (including ∼70,000 knee replacement patients and 40,000 hip replacement patients) estimate the risk of infection to be ∼2% for both hip and knee arthroplasties (523, 524). Hence, PJI is a very common problem and poses a large economic burden in industrialized countries (445, 525). The major risk factors for PJI are prior surgery on the index joint, obesity, rheumatoid arthritis, duration of implantation surgery, and immunosuppression (526–528).
PJIs are usually classified as early postoperative (within 30 days of implantation), late chronic (indolent presentation), and late acute hematogenous (explosive onset in a previously well-functioning joint). High-virulence organisms, primarily S. aureus but also beta-hemolytic streptococci, aerobic Gram-negative organisms, and mixed infections, are generally the cause of most early and hematogenous infections. Chronic infections are more likely to be caused by indolent organisms, including coagulase-negative staphylococci, Enterococcus spp., and Propionibacterium spp. In nearly all case series, for all forms of PJI, S. aureus is the most common causative organism, accounting for 18 to 73% of cases (Table 5). In patients with a prosthetic joint in situ who develop SAB, a PJI eventuates in 29 to 39% of cases (446, 529, 530).
Pathophysiology.
Within a biofilm, bacteria are contained in a polymeric matrix that adheres to prosthetic material. The biofilm acts as a sanctuary site where S. aureus is relatively protected from antimicrobial agents and the host immune response. In addition, organisms within a biofilm generally enter a stationary or stringent phase of growth and are thus much more resistant to antimicrobial killing than those in the active or vegetative phase (531). The presence of an implanted foreign body has been shown to reduce the inoculum of S. aureus required to establish an infection by a factor of 100,000 (532). An implanted joint prosthesis is avascular, and the bone-prosthesis interface is relatively poorly vascularized. These facts explain why PJIs are so difficult to treat, can occur despite all efforts at prevention, and generally require removal of the prosthesis for definitive cure.
Clinical manifestations.
Early PJIs (presenting within 30 days of implantation) generally present as a deep wound infection. The patient is usually acutely ill, with fever and joint inflammation and effusion. Clinical evidence of wound infection in the postoperative period is the strongest risk factor for early PJI, with an odds ratio of 52 (95% CI, 21 to 130) (521).
Chronic infections are more subtle. Often, there is a history of the joint “never being quite right,” with low-grade chronic pain and poor function but no obvious signs of infection. In one case series including 110 patients with PJI, fever was present in only 4.5% of cases (533). The exception is the presence of a discharging sinus. A PJI can be diagnosed with certainty if the sinus can be shown to communicate with the joint space. It can be very difficult to differentiate the chronic pain of the aseptic loosening of a prosthetic joint from that of a low-grade chronic infection. Hemarthrosis, gout, and metallic debris-induced synovitis can also be mistaken for PJI.
A definitive diagnosis of PJI due to S. aureus requires the isolation of the organism from operative specimens of joint fluid and/or periprosthetic tissue. When all causative organisms are considered as a group, positive cultures from three operative specimens represent a 95% probability of infection, whereas two positive specimens represent a 20% probability, and one specimen represents a 13% probability of infection (534). However, S. aureus is virtually never a nonpathogenic isolate or contaminant (e.g., in comparison to coagulase-negative staphylococci and propionibacteria). Thus, a single isolate of S. aureus in the appropriate clinical setting can be considered diagnostic.
For investigation of suspected PJI, culture of a preoperative closed-needle aspirate of the hip or knee joint for synovial fluid analysis is highly specific for infection (e.g., 95% in a study of 145 revision knee arthroplasties, 40 of which were found to be infected [535]) but lacks sensitivity (73% in the above-mentioned study and 75% in another series of 68 hip and knee replacements [536]). Gram stains of synovial fluid without accompanying growth are difficult to interpret, as fibrin and other artifacts may cause false-positive Gram stains, and the sensitivity is poor; Stirling et al. found a false-negative rate of 78% for 143 positive synovial fluid cultures (537). Hence, it is important if possible to avoid the use of any antibiotics (including preoperative prophylaxis) in the 1 to 2 weeks leading up to a diagnostic sampling of joint fluid or tissue. If PJI is suspected but not proven, the next step is operative exploration, with collection of at least 5 periprosthetic tissue specimens for culture and histology (538).
If the prosthesis itself or its components are removed, they should be cultured. Simple swab or broth cultures of explanted prostheses lack sensitivity because of biofilm-associated organisms, perioperative antibiotic use, or adjacent antibiotic-impregnated cement. The sensitivity can be improved by placing the prosthesis in a sonication bath and culturing the sonicate fluid. This sonication technique has been found to have a sensitivity of 75 to 77% for culturing of microorganisms, compared to 34 to 45% with culturing of multiple periprosthetic tissue specimens, and is particularly valuable if there has been recent antibiotic use (539, 540). However, it may cause problems with false-positive cultures of environmental organisms and other contaminants if appropriate cutoffs are not used (541). The diagnostic role of 16S PCR is uncertain, as in most cases, it appears to add little to culture and may lead to both false-positive and false-negative results (542).
One might expect this discussion to not be relevant to S. aureus infections, since S. aureus is a nonfastidious and easily cultured organism in most circumstances. However, in the setting of PJI, S. aureus is often associated with biofilm and may form SCVs (428), both of which may render it difficult to culture with usual microbiological methods. For further information on the diagnosis of PJI, readers are referred to recent IDSA guidelines (543) and other review articles (544–547).
Management.
Staphylococcal early postoperative PJI has been traditionally treated with 2-stage joint replacement, with resultant cure rates of >90% (405, 548). Over the past 2 decades, the debridement and implant retention (DAIR) procedure has been increasingly practiced, and there are accumulating data that this strategy leads to acceptable cure rates of 70 to 80% in appropriately selected patients (549, 550). However, some studies report lower success rates. Cobo et al. (551) retrospectively reported 117 cases of early PJI (most commonly caused by S. aureus) and found a 57% cure rate. A systematic review including data from 14 original articles and 710 patients treated with DAIR for “early” (within 6 weeks of implantation) PJI found pooled success rates of 46% for those undergoing a single debridement and 52% if multiple debridements were used, after a mean follow-up of 53 months (552). DAIR is seen as an attractive strategy as it is cheaper and more convenient, avoiding the multiple operations and prolonged immobility associated with 2-stage joint replacements. Zimmerli and colleagues (553) proposed an algorithm for the selection of a surgical treatment strategy for patients with a PJI, which suggests that DAIR should be considered only if the patient meets all of the following criteria: <3 weeks since the onset of symptoms, a stable implant, good soft tissue envelope, and an organism that is susceptible to rifampin and/or quinolones. Other patients should undergo one- or two-stage replacement or, for those who are unfit for any surgery, attempted long-term antibiotic suppression.
There are two main approaches to antibiotic treatment in patients with staphylococcal PJI treated with DAIR, with insufficient evidence to definitively recommend one over the other. The first approach uses 6 weeks of i.v. vancomycin (for MRSA or coagulase-negative staphylococci) or an antistaphylococcal penicillin (for MSSA), following adequate debridement. This approach has had high reported success rates in appropriately selected patients. For example, success rates of 70% at 2 years in early postoperative prosthetic hip joint infections (554) and 71% in 38 early postoperative prosthetic hip joint infections (404) have been reported. Neither of these studies used rifampin.
The second, and increasingly popular, approach uses shorter courses of i.v. vancomycin or an antistaphylococcal penicillin (2 to 6 weeks) along with 3 to 6 months of oral rifampin-based combination therapy (rifampin combined with a second oral agent, most commonly ciprofloxacin or fusidic acid). Zimmerli et al. (555) reported the only prospective controlled trial assessing the role of rifampin for treatment of PJI in 33 adults with staphylococcal infection of various orthopedic implants. Patients with <3 weeks of symptoms prior to initial debridement were randomized to receive either 2 weeks of i.v. flucloxacillin or vancomycin with rifampin or placebo, followed by either ciprofloxacin-rifampin or ciprofloxacin-placebo therapy for 3 to 6 months (555). Those authors reported a successful outcome at 24 months for 12 of 12 patients in the rifampin group, compared with 7 of 12 in the placebo group (P = 0.02). This study is problematic for a number of reasons: (i) it contained only 15 patients with PJI, 8 of whom received rifampin-based therapy; (ii) it was not analyzed by intention to treat, as when one includes the 9 patients who were excluded from the analysis due to having received <85% of the study drug, the cure rates are not significantly different between the two groups (P = 0.10); and (iii) the control arm received a treatment known to have a significant chance of failure, because a course of 3 to 6 months of ciprofloxacin monotherapy for S. aureus often leads to resistance (556). Observational studies of this approach are very heterogeneous; however, they report success rates ranging from 57% (551) to ≥85% (549, 550). Despite the flaws of the study by Zimmerli et al. (555), 2013 IDSA guidelines (543) on PJI recommend rifampin-based combination therapy for 3 to 6 months following DAIR, grading this recommendation as level A1 (good evidence from ≥1 properly randomized, controlled trial). This recommendation has been criticized (557), as there are actually no properly designed RCTs to support it.
It is important to note the adequacy of surgical debridement as a key source of heterogeneity in all studies of DAIR. This is probably more important than the choice of antibiotic regimen in determining cure rates and ranges from a single operation involving only arthroscopic lavage to multiple operations involving the removal of all infected periprosthetic tissue and loose cement, the exchange of the prosthesis liners, and high-volume lavage with antiseptic-containing solutions. Lora-Tamayo et al. (558) found that polyethylene liner exchange independently predicted treatment success (adjusted OR, 0.65; 95% CI, 0.44 to 0.95).
The role of DAIR for acute hematogenous infection (an explosive onset of symptoms in a previously well-functioning joint, often years after the original implantation surgery) is less certain. Cure rates in this setting appear to be lower than in early postoperative infection, ranging from 50 to 70% (554, 558, 559). As for early postoperative infections, in those for whom DAIR is not considered appropriate, the main curative option is one- or two-stage joint replacement.
Two-stage replacement has higher cure rates than DAIR and is the primary mode of treatment recommended for those with chronic PJI. This involves an initial operation with removal of the prosthesis and all infected bone and cement, followed by a period of i.v. antibiotics (2 to 8 weeks) and then a second operation where a new prosthesis is implanted. There are few data to guide the duration of therapy between the two stages or the choice of antibiotics. Most guidelines recommend vancomycin for MRSA infections and antistaphylococcal penicillins for MSSA, with or without adjunctive rifampin (543). Because vancomycin has poor bone penetration and low clinical cure rates, there is increasing interest in the use of alternative agents for MRSA osteoarticular infections, including linezolid (560, 561), daptomycin (562), and rifampin in combination with either quinolones or fusidic acid (549, 563). The only reported RCT addressing antibiotic choice for staphylococcal PJI in patients undergoing 2-stage replacement compared daptomycin with the “standard of care” (vancomycin, teicoplanin, or nafcillin) for 6 weeks in between the 2 stages in 75 adults with staphylococcal PJI (562). The primary safety outcome of this study was an elevation of creatinine kinase (CK) levels. A raised CK level was found more frequently in the daptomycin group (CK level of >500 in 19% of patients, compared with 8% in the control group). Based on a stringent definition of success, the rate of successful treatment was higher in the daptomycin group (60% versus 38%). This study is difficult to extrapolate, as the test of cure was at a very early time point (1 to 2 weeks following the second stage). While 2-stage replacement is the most common mode of exchange arthroplasty, one-stage joint replacement (where the infected prosthesis is removed and replaced with a new one at a single operation) appears to have similar success rates in experienced centers. In a meta-analysis including 62 observational studies and 2,500 patients comparing one- and two-stage joint replacements for PJI, successful outcomes at 24 months were reported for 91% of patients following one-stage replacement and for 90% of patients following two-stage replacement (548). For a more detailed discussion on the management of S. aureus PJI, the reader is referred to recent IDSA guidelines (543) and recent reviews (545, 564, 565).
OTHER PROSTHETIC DEVICE INFECTIONS
S. aureus is particularly adept at infecting foreign bodies within the human host. Infections of prosthetic cardiac valves and prosthetic joints are described above, but this section provides further background on the formation of biofilms, the pathognomonic feature of device infections. Device infections of implantable cardiac devices, intravascular catheters, and other prostheses are discussed in greater depth.
Formation of Biofilm
S. aureus forms a biofilm on the surface of a foreign device, making eradication of the infection without surgical removal of the device all but impossible. A biofilm is a community of sessile bacteria encased in an extracellular matrix of water, microbial cells, nutrients, polysaccharides, DNA, and proteins (566–568). The biofilm provides a protective matrix around the encased bacteria and is highly resistant to host immune defenses and antimicrobials (567, 569, 570). Within the endovascular system, the host deposits fibrin (571–573), fibronectin (573), fibrinogen (574), and collagen (575) in a sheath along the surface of an inserted device (571, 572, 576).
The formation of a biofilm occurs in the following 4 steps: (i) initiation, (ii) colonization, (iii) replication, and (iv) dispersal (576). The first step of biofilm formation is the reversible adherence of S. aureus to the device (576). The bacteria initially adhere to a protein-coated device via hydrogen bonds, van der Waals forces, and electrostatic interactions (567, 576). Integral to the ability of S. aureus to seed prosthetic devices is the MSCRAMM family of bacterial proteins. One of these MSCRAMMs, fibronectin-binding protein A (FnBPA), enables S. aureus to bind to fibronectin, a host extracellular matrix molecule that coats the surface of endovascular prostheses such as permanent pacemakers (PPMs) and implantable cardioverter defibrillators (ICDs) (156, 520). This binding of S. aureus FnBPA to human fibronectin is thought to be a critical initial step in the pathogenesis of prosthetic device infections (577). Lower et al. showed that specific single nucleotide polymorphisms in fnbA were associated with (i) greater in vitro binding to fibronectin, as assessed by atomic force microscopy; (ii) a higher number of hydrogen bonds between fibronectin and FnBPA in a simulated model system; and (iii) a higher risk of cardiac device infection (CDI) in patients with SAB (578).
In the second step, colonization, S. aureus upregulates the expression of genes necessary to synthesize the extracellular polymeric substance that forms the matrix, an effective barrier to antibiotics and host defenses (576, 579).
In the third step, the bacteria divide to form microcolonies, spreading nonuniformly along the surface of the device (576). In many S. aureus strains, the bacteria adhere to each other through polysaccharide intercellular adhesin, also referred to as poly-N-acetylglucosamine (PNAG), synthesized by icaADBG-encoded enzymes (579–585). Many MRSA isolates also have the capacity to form biofilms through an icaABDG-independent mechanism, such as FnBPA and FnBPB as well as major autolysin (Atl) (582, 586, 587). This biofilm phenotype is less virulent than the phenotype of PNAG-mediated biofilm (582).
In the fourth step, some bacteria will switch back to the planktonic state and disperse due to hemodynamic stress, a decrease in nutrient availability, or other unknown physiological causes, leading to bacteremia (576). Overall, S. aureus regulates biofilm formation via “quorum sensing,” a cell-cell communication process that enables the bacteria to communicate information about their environment, such as bacterial density, salt stress, and nutrient availability (567, 588, 589).
Cardiac Device Infections
Epidemiology.
PPMs and ICDs are critical in managing arrhythmias and hemodynamic instability in low-cardiac-output states and have improved the quality of life of patients worldwide. However, infection poses the threat of serious and significant complications (590). The reported incidence of CDI ranges from 0.7 to 2.2% (591–599). While the incidence of cardiac device implantation has continued to increase, with 42% more cardiac devices implanted in 1999 than in 1990 in U.S. Medicare beneficiaries, the rate of device infection has increased at a substantially higher rate, with a 124% increase in the infection rate over the same period (600). Similarly, Greenspon et al. found that the incidence of cardiac device implantation increased by 96% while the annual incidence of CDI increased by 210% from 1993 to 2008 in U.S. Medicare beneficiaries (597). This increase in the prevalence of CDIs was tracked with increasing rates of procedures, increasing numbers of medical centers implanting cardiac devices, and significant increases in patient comorbidity indicators in cardiac device recipients.
Permanent pacemakers and ICDs can become infected directly during initial implantation or indirectly via hematogenous seeding from a distant source. A temporal cutoff of 1 year after implantation or surgical manipulation is often used to indicate whether an episode of CDI is most likely due to direct inoculation (early infection, <1 year) or hematogenous seeding (late infection, >1 year) (601, 602). Although the short-term risk for CDI for an individual patient is greatest in the immediate postprocedure period, the majority of CDI cases in patients with SAB are actually late infections (601, 602). This finding is likely to reflect the larger number of patients with long-standing cardiac devices rather than higher rates of seeding. In the case of hematogenous CDI, seeding of the cardiac device usually originates from one of four sources: (i) the generator pocket, (ii) an intravascular catheter, (iii) non-device-related soft tissue infection, or (iv) pneumonia/lung infection (602). S. aureus is responsible for 23 to 46% of CDIs (594, 603–606), with up to 51% of these S. aureus infections being due to MRSA (602, 604, 607–609). Of patients with SAB and a preexisting cardiac device, there is a high risk of CDI. In a prospective study of 33 patients at Duke University, Chamis et al. found that the incidence of confirmed or possible CDIs in such patients was 45% (601). This high rate of CDIs was subsequently externally validated by Uslan et al. (estimated rate, 55%) (594) and Obeid et al. (estimated rate, 37%) (610). Collectively, these reports underscore that providers must be aware of the likelihood of CDI in patients with SAB who have a preexisting ICD or PPM (601, 602, 611). For patients with SAB and a cardiac device, the rate of CDI is significantly higher among patients with ICDs than among those with PPMs (602, 610). For example, Uslan et al. described the rate of ICD-related infection in patients with SAB to be 60%, versus 24% for patients with PPMs (602). Reasons for the disparity in CDI rates between ICD and PPM patients are unknown, although it has been speculated to be due in part to the higher comorbid burden of patients with ICDs (602, 610). Studies that externally validate this clinical phenomenon and establish its biological basis are needed.
The impact of CDI caused by S. aureus is high. The identification of SAB in patients with cardiac devices is associated with a 25% all-cause 12-week mortality rate and per-patient costs of up to $83,635 in 2004 (612). Le et al. found that S. aureus CDIs were associated with a longer duration of bacteremia, longer hospitalization, and greater mortality than similar infections caused by coagulase-negative staphylococci (CoNS) (609). Viola et al. conducted a multicenter, retrospective review of 160 patients with S. aureus CDI and nonstaphylococcal CDI and found that the mortality rate was not statistically different between the two groups (613). Cardiac device infections due to MRSA are generally, but not invariably, associated with higher mortality rates than CDIs caused by MSSA. For example, Chu et al. found the distinction to be statistically significant (612), while Chamis et al. and Roig et al. did not (44% versus 27% [P = 0.47] [601] and 24% versus 25% [607], respectively).
Risk factors for S. aureus CDI.
Cardiac device infections due to any bacterial species, including S. aureus, tend to occur in white males over the age of 60 years (596, 597, 604, 606, 613). However, in a Danish PPM registry study that included a large number of children, Johansen et al. also found high CDI rates in children and adolescents (595). Overall, the majority of patients with S. aureus CDI tend to have multiple medical comorbidities, such as coronary artery disease, diabetes mellitus, congestive heart failure, and prosthetic heart valves (602). A large retrospective study at the Mayo Clinic found that late S. aureus CDI (>1 year after implantation), compared to late CoNS CDI, was commonly associated with hemodialysis (17% versus 4%) and corticosteroid therapy (19% versus 5%) as well as the presence of a prosthetic valve (21% versus 8%), a central venous catheter (15% versus 4%), and a remote source of infection (40% versus 10%) (609). Viola et al. reported that patients with S. aureus CDI tended to have more comorbid conditions and more health care-associated infections (81% versus 49%; P < 0.001) than patients with nonstaphylococcal CDI. Additionally, S. aureus CDI was more frequently associated with a history of bacteremia within the preceding year than nonstaphylococcal CDI (21% versus 4%) (613).
A number of studies have confirmed that the rate of PPM infection is much higher for replacement PPMs (5.3 infections/1,000 device-years after PPM replacement) than for first-time PPM placements (1.82 infections/1,000 device-years after initial PPM placement) (591, 595, 596). The risk for S. aureus CDI is increased by recent instrumentation of the device (602). Underscoring this point, it is estimated that as many as 24% of S. aureus CDIs occur within 3 months of cardiac device implantation or revision (602).
Clinical manifestations.
The most common clinical manifestations of early S. aureus CDI are localized pain and erythema of the generator pocket (602, 603). In contrast, in late S. aureus CDI, the leads are most commonly involved, and the generator pocket typically appears normal (602). Patients with S. aureus CDI more commonly present with fever, leukocytosis, tachycardia, chills, hypotension, malaise, and anorexia than do patients with CDI due to CoNS (609) or other nonstaphylococcal bacterial causes of CDI (613). Similarly, patients with S. aureus CDI are more likely to present with leukocytosis, a higher mean WBC count, an elevated ESR, and bacteremia than are those with nonstaphylococcal CDI (613). It is important to emphasize that the absence of signs or symptoms of systemic infection does not exclude the possibility of S. aureus CDI, especially if the infection has been present for an extended period or if it is limited to the generator pocket. In such settings, clinical presentation may be limited to localized inflammatory signs at the site of the pocket, without any systemic symptoms (604, 614).
Management.
Sohail et al. proposed guidelines for the diagnosis and management of CDI in 2007 (604). They outlined that the first step in cases of suspected CDI is to obtain at least two blood cultures. In the case of positive blood cultures or prior antibiotic therapy, TEE is warranted. Should TEE demonstrate CDI, surgical removal of the device is warranted, regardless of clinical presentation. The explanted device and leads should be cultured and Gram stained (615), and postoperative blood cultures should be drawn (604). Generator pocket site tissue should be obtained, as culture of tissue has a higher yield than that of swabs of the pocket site (616). It should also be borne in mind that leads are usually extracted through an open generator pocket and thus prone to lead contamination. Antibiotic treatment without device and lead extraction for S. aureus CDI has a high failure rate (604, 611, 617). Therefore, complete device removal is integral to the treatment of CDI (615).
Current American Heart Association guidelines recommend that patients begin i.v. vancomycin until culture susceptibility test results return. Should the S. aureus isolate demonstrate susceptibility to methicillin, the patient may switch to a single antistaphylococcal β-lactam such as nafcillin or cefazolin (615). In the case of β-lactam allergy or MRSA infection, vancomycin should be continued (615). While no clinical trials have been conducted to determine the appropriate duration of antibiotic therapy for CDI, current guidelines for CDI recommend 7 to 10 days of antibiotics after device removal if the infection is limited to the pocket site and the extracted device shows only device erosion without inflammatory changes (615). If the CDI does not meet these criteria, the patient should undergo 10 to 14 days of antibiotics (615). A course of at least 2 weeks of antibiotic therapy is recommended after device extraction if the patient had bacteremia, and a course of at least 4 weeks is recommended for patients with >24 h of ongoing positive blood cultures despite device extraction (615). After device removal, the patient should be reevaluated as to whether reimplantation of the device is indicated (615). If replacement is warranted, the new site should be contralateral to the extraction site (615). Additionally, blood cultures should be negative for at least 72 h prior to replacement (615).
Intravascular Catheter Infections
Epidemiology.
Intravascular catheters may become directly infected at the hub site during insertion or manipulation (618–620), with the subsequent complication of central line-associated bloodstream infection (CLABSI). The reported incidence of CLABSI ranges from 1.3 to 6.8 events per 1,000 device-days (621–624). Notably, the incidence of CLABSI was found to be >3-fold higher for intensive care units (ICUs) in countries in Latin America, Asia, Africa, and Europe than for ICUs in the United States (622). S. aureus follows CoNS as the major bacterium causing CLABSI, responsible for 14 to 41% of CLABSIs (624–627). S. aureus CLABSI is associated with a 7 to 21% mortality rate (612, 628–632) and has been estimated to cost $9,830 to $14,136 per episode (633).
According to the Centers for Disease Control and Prevention (CDC), there has been a 57% reduction in the number of CLABSIs due to all pathogens over the past 9 years in the United States, with 25,000 fewer cases in 2009 than in 2001 (634). In particular, the number of S. aureus CLABSIs has declined by 73% over this time period (634), with reductions in MSSA and MRSA CLABSIs of 74% and 50%, respectively, between 1997 and 2007 (67). This decrease in the incidence of S. aureus CLABSI is attributed in part to the dissemination and uptake of MRSA transmission prevention guidelines (67, 635, 636) as well as improved central line insertion practices.
Central venous catheter (CVC) colonization and the subsequent development of CLABSI vary by the site of insertion, with the highest risk for femoral vein insertions and the lowest risk for subclavian vein insertions (625). CVC bacterial colonization increases with an increasing duration of insertion (625), and the presence of a central line extending beyond 30 days significantly increases the risk for contracting MRSA CLABSI (637). Sadoyma et al. found two independent risk factors for S. aureus CLABSI: (i) the presence of S. aureus at the insertion site (OR, 6.98; 95% CI, 2.42 to 21.90) and (ii) the presence of S. aureus in the tip of the catheter (OR, 7.95; 95% CI, 1.95 to 19.60) (624).
Catheter-related SAB is particularly prominent in hemodialysis patients. Marr et al. monitored 102 patients for 16,081 catheter-days and reported that 40% of patients developed bacteremia, with an incidence of 3.9 episodes per 1,000 catheter-days (638). S. aureus was the most commonly isolated organism, occurring in 44% of the cases. Risk factors for catheter-related SAB were a previous episode of bacteremia and an immunocompromised state (638). Rates of SAB in hemodialysis patients differ significantly by the type of vascular access, with the highest rates occurring in patients with a tunneled, cuffed catheter (59.5%), followed by patients with arteriovenous grafts (36%) and finally by patients with arteriovenous fistulas (4.5%) (639).
Clinical manifestations.
Clinical features of CLABSI are usually nonspecific. Fever, erythema, tenderness, induration, and/or purulence at the catheter insertion site may suggest catheter infection (628, 630, 632, 640). Purulence at the catheter insertion site is much more common in CLABSIs due to S. aureus than in those due to Gram-negative rods (50% versus 2.4%; P < 0.01) (641). Blood cultures positive for S. aureus in a patient with a catheter but lacking another identifiable source of infection likely indicate catheter infection (642).
Anywhere from 6 to 30% of patients with CLABSI have been reported to develop complications (630, 643, 644) such as native valve IE (9 to 18%) (640, 643, 645), prosthetic valve IE (146), septic arthritis (3.4%), and vertebral OM (2.2%) (643). Fowler et al. found the following five risk factors for hematogenous complications in S. aureus CLABSI: (i) the presence of a foreign device, such as a long-term catheter or prosthesis (RR, 4.0; 95% CI, 1.7 to 9.3); (ii) community-onset infection (RR, 2.3; 95% CI, 1.2 to 4.1); (iii) hemodialysis dependence (RR, 3.8; 95% CI, 2.1 to 7.1); (iv) increased symptom duration (OR for each day, 1.1; 95% CI, 1.1 to 1.2); and (v) a higher mean APACHE II score (P = 0.02) (643). Other studies have found that immunosuppression and preexisting valvular disease are also host factors that increase the risk for complications associated with S. aureus CLABSI (629). Methicillin resistance is also a risk factor for complications stemming from an S. aureus CLABSI (643).
Although any hematogenous complication can arise from an episode of S. aureus CLABSI, endovascular infections are particularly common (643). In particular, the possibility of septic thrombophlebitis should be considered in every patient with S. aureus CLABSI who exhibits persistent bacteremia. In a prospective cohort study, 48 consecutive patients with S. aureus CLABSI involving a catheter in the neck or upper torso underwent targeted physical examination (e.g., for upper arm circumference, asymmetric venous markings in the upper chest, and the presence of hand vein collapse when hands are raised above the heart level) and Doppler ultrasonography to evaluate the prevalence of septic thrombophlebitis. Importantly, an ultrasonogram was provided to all enrolled study subjects and was not dependent upon clinical suspicion of underlying septic thrombophlebitis by the treating physicians. Two key findings arose from this study. First, septic thrombophlebitis was common; 71% of the 48 patients had definite or possible hemodynamically significant thrombosis visualized by Doppler ultrasonography. Second, physical examination was inadequate to exclude the presence of underlying septic thrombophlebitis. The sensitivity of physical examination compared to Doppler ultrasonography was only 24%. Based upon these findings, ultrasonography should be performed on every patient with S. aureus CLABSI and persistent bacteremia or persistent fever, even in the setting of a normal physical examination (646, 647).
Prevention and treatment.
Rates of S. aureus CLABSI have declined significantly in the past decade (67). This reduction has been attributed to improved procedures for catheter insertion and care (67, 648–650). There are extensive and updated guidelines provided by the CDC for the prevention of line-related infections (651). The major areas of emphasis are “1) educating and training health care personnel who insert and maintain catheters; 2) using maximal sterile barrier precautions during central venous catheter insertion; 3) using a > 0.5% chlorhexidine skin preparation with alcohol for antisepsis; 4) avoiding routine replacement of central venous catheters as a strategy to prevent infection; and 5) using antiseptic/antibiotic impregnated short-term central venous catheters and chlorhexidine impregnated sponge dressings if the rate of infection is not decreasing despite adherence to other strategies” (651). The guidelines emphasize the implementation of bundled strategies and documenting and reported rates of compliance with all components of the bundle.
The key tenets for the management of S. aureus CLABSI (647) are the removal of the infected catheter, the use of appropriate antibiotics guided by antibiotic susceptibility testing, and a delineation of the SAB into complicated or uncomplicated infection. Uncomplicated SAB should be treated with at least 14 days of parenteral therapy (79, 647). Treatment of S. aureus CLABSI for <10 days has been associated with relapse of infection (629, 640) and is ill advised. Complicated SAB associated with a vascular catheter should be managed with 4 to 6 weeks of therapy. In all cases of S. aureus CLABSI, removal of the infected catheter is associated with a higher cure rate and a lower mortality rate (629, 632, 647, 652) and should be regarded as the standard of care (647). Rarely, catheter removal is sufficiently problematic in an individual patient with limited i.v. access that the risk imposed by attempting to retain the infected vascular access point is exceeded by the risk of removing it. In such desperate situations, adjunctive antibiotic lock therapy, in which antibiotics are instilled into the catheter lumen for extended periods, should be considered in addition to parenteral therapy (628, 647, 653).
Infections Involving Other Prosthetic Devices
Infection of breast implants.
(i) Epidemiology.
Approximately 1 to 2.5% of breast prostheses develop infection (654–657). Estimates for the proportion of these infections due to S. aureus range from 0 to 75% (655, 658, 659). For example, in a multicenter retrospective cohort of 106 patients with confirmed or suspected breast prosthesis infection, Feldman et al. found that 68% of the 31 culture-positive breast implants grew S. aureus, 37% of which grew MRSA (658). In contrast, none of the 65 culture-confirmed silicone breast infections reported by Ahn et al. grew S. aureus (659). The true incidence of S. aureus infection in breast prostheses is unknown, as there is currently no surveillance network that records the total number of mammoplasties and infections (660).
Breast implantation for reconstructive purposes is a significant risk factor for infection (658, 660, 661), as these patients typically have a higher degree of tissue scarring, ischemia, and delayed wound healing (660). Other risk factors for infection are (i) hematoma formation secondary to inadequate hemostasis, (ii) seroma formation, (iii) adjuvant chemotherapy or radiation, and (iv) skin irritation (658, 660).
(ii) Pathogenesis.
There are four possible routes of S. aureus breast implant infection: (i) contaminated implant or saline, (ii) contamination during the surgery, (iii) seeding from a hematogenous source, or (iv) contamination via the patient's skin and mammary ducts (660). The breast tissue is colonized with flora that is similar to that of the skin. The bacteria gain access to the deep breast tissue via the nipple ducts, which provide a passage for bacteria to enter the deep breast tissue from the skin surface (655, 660). This skin flora may be the source of infection during periareola or transareola breast surgeries (660).
(iii) Clinical manifestations.
Fever, localized pain, fluctuance, erythema, and accumulation of pus in the breast are all symptoms indicative of an infected breast implant (658, 660). Additionally, capsular contraction and change in breast shape may be seen (659).
(iv) Management.
A single dose of antistaphylococcal antibiotic prophylaxis prior to surgery is recommended to prevent implant infection (660). Additionally, there may be a role for antimicrobial-impregnated implants, as Darouiche et al. demonstrated the benefit of minocycline-rifampin-impregnated saline-filled silicone breast implants in reducing the incidence of S. aureus infection in a rabbit model (662).
The recommended management for an infected breast implant is surgical removal of the implant with postsurgical drainage, accompanied by 10 to 14 days of appropriate antibiotics (520, 660). Removal of the capsule surrounding the implant may be indicated in some cases, and immediate reimplantation is not recommended (660).
Infection of ventricular shunts.
(i) Epidemiology and clinical manifestations.
The reported frequency of CNS shunt infections ranges from 2 to 39% (663–665). S. aureus is the causative organism in 13 to 25% of infected shunts (663, 665, 666). In the most robust analysis of the epidemiology of CNS shunt infections to date, Schoenbaum et al. found that S. aureus was the second most common pathogen, accounting for 27% of ventriculoatrial shunts and 21% of ventriculoperitoneal shunts (663).
Some of the important risk factors for shunt infection are (i) the presence of a postoperative cerebrospinal fluid (CSF) leak (664), (ii) age of <6 months, (iii) poor condition of the skin, (iv) the presence of infection at the time of surgery, and (v) shunt reimplantation following removal secondary to infection (667).
Fever is a frequent but nonspecific sign of shunt infection (663). Other common symptoms are malaise, nausea, vomiting, and signs of increased intracranial pressure and meningeal irritation (663, 665).
(ii) Management.
Management of an infected ventricular shunt generally requires surgical removal and appropriate parenteral antibiotics (665, 668). Some experts advocate that the surgery should occur in two stages. First, the infected shunt is explanted while an external ventricular catheter is placed to drain CSF and monitor intracranial pressure (520, 666). This external ventricular catheter should be replaced every 5 to 10 days (520). Once CSF culture has confirmed that the infection has been cleared, a new shunt may be implanted on the contralateral side (520).
S. aureus infection of penile implants.
(i) Epidemiology, clinical manifestations, and risk factors.
The risk of penile prosthesis infection is ∼3 to 5%, with S. aureus causing ∼8% of those infections (520, 669). Symptoms are typically pain, swelling, and drainage (669). Fever, leukocytosis, and positive blood cultures are less common and typically occur later in the course of infection (669). Spinal injury and steroid use are two significant risk factors for infection of penile prosthesis (669).
(ii) Management.
Improved surgical approaches, including the use of antibiotic-coated hydrophilic implants and a “no-touch” surgical technique (with exchange of surgical instruments and gloves immediately prior to insertion of the prosthesis), have reduced the rates of penile implant infection (670). Management of an infected penile prosthesis without complications or bacteremia consists of 2 to 4 weeks of systemic antibiotics, and most experts now recommend a single-stage removal-and-replacement procedure with vigorous irrigation (671).
PLEUROPULMONARY INFECTIONS
S. aureus is an important cause of pneumonia. It was initially implicated as a devastating respiratory complication of influenza during the 1918 pandemic (672). It thereafter remained an infrequent but well-documented cause of community-acquired pneumonia (CAP), even in the absence of preceding influenza infection (673, 674). S. aureus has had a more predominant role in hospitalized patients with respiratory infections and has been implicated in each of the three other major subsets of pneumonia: hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), and health care-associated pneumonia (HCAP) (675). It is also a common pathogen in patients with cystic fibrosis.
Epidemiology
S. aureus is an important cause of pneumonia in both community-onset presentations and hospital-acquired infections. Hospitalized patients quickly develop oropharyngeal colonization with nosocomial flora and can subsequently manifest lower respiratory tract infections related to these organisms (676–678). Overall, S. aureus has consistently been shown to be one of the most common pathogens in these health care-associated infections. For example, S. aureus accounted for >40% of culture-positive HCAP cases in a large U.S. retrospective cohort, with a roughly equal distribution of MRSA and MSSA (679). MRSA was found to cause 28% of VAP cases in 31 U.S. community hospitals (680). In the SENTRY Antimicrobial Surveillance Program from 1997 to 2001, S. aureus was the most common pathogen isolated in the United States and in Europe and the second most common organism in Latin America (681). Pooled data for Asia (682) are similar albeit with an increased incidence of other Gram-negative pathogens competing for primacy with S. aureus and Pseudomonas aeruginosa. Patients with MRSA pneumonia incur an increased cost of care compared to patients with MSSA pneumonia (683). At present, then, S. aureus remains a well-known pathogen in both CAP and HCAP presentations, and modern isolates are a mix of methicillin-susceptible and methicillin-resistant strains (684–687).
Recently, a new clinical entity of severe necrotizing pneumonia caused by distinct strains of S. aureus has emerged. The first report was of fulminant respiratory illness in four children in Minnesota and North Dakota in the late 1990s. These cases were due to infection with a novel community-associated MRSA strain type, USA400, which carried genes for PVL (264). The association between S. aureus isolates carrying pvl and necrotizing pneumonia was also noted in France (688), and subsequent reports included otherwise healthy adults presenting with severe CAP (689–695). Despite the attention given to this new entity in both the scientific literature and the lay press, the incidence of S. aureus CAP is actually fairly low, estimated at 2 to 3% of all adult CAP cases in the United States, of which approximately half are caused by MRSA (696). For example, among 627 prospectively enrolled patients with CAP from 12 U.S. urban emergency departments in 2006 to 2007, 2.4% had culture-confirmed MRSA (a subset of these isolates were genotyped and were all USA300), and 1.5% had MSSA (697). Patients with MRSA isolated had a more severe clinical presentation. Prospective data from the United Kingdom are similar but with a lower proportion of cases that are due to MRSA. In 1,348 patients hospitalized with pneumonia from 2005 to 2009, a microbiologic diagnosis was made for ∼30% of CAP patients, of which 9.3% and 0.6% were due to MSSA and MRSA, respectively. These rates were similar to those in the HCAP population in the same study for MSSA (10.1%) and MRSA (2.2%) (698) but were higher than those reported for a cohort of 885 episodes of CAP from Australia. In the Australian study, a microbiologic diagnosis was made in 46% of episodes, of which 2.5% and 0.2% were due to MSSA and MRSA, respectively (699).
Historically, S. aureus has accounted for approximately one-third of cases of empyema (700). Furthermore, S. aureus pneumonia or empyema may occur as a result of hematogenous spread, as with septic emboli from an infected cardiac valve, or via local extension from another infected source (701).
Risk factors.
Risk factors for MSSA pneumonia are similar to those for pneumonia in general and include low body mass index, smoking, chronic lung disease, history of pneumonia, diabetes, and chronic liver disease. Risk factors for invasive MRSA disease (including, but not limited to, pneumonia) include history of hospitalization, history of surgery, and long-term-care residence (14). MRSA carriage also confers a risk of subsequent invasive disease. In a year-long surveillance study conducted by Huang et al., pneumonia was the most common form of invasive disease that subsequently developed in patients found to be carrying MRSA (702), corroborating similar data reported previously (703). In a study of patients hospitalized with S. aureus pneumonia, 37% of which were community-onset cases, risk factors for MRSA (versus MSSA) included tobacco use, illicit drug use, recent antibiotic use, chronic obstructive pulmonary disease (COPD), liver disease, and HIV infection (704).
While knowledge of the general factors that predispose patients to incident pneumonia is useful, the increasing prevalence of pneumonia due to resistant organisms, including MRSA, has spurred interest in identifying those patients at increased risk for these multidrug-resistant (MDR) pathogens (679). The most important risk factors are related to health care contact, which led to formal American Thoracic Society (ATS)/IDSA guidelines delineating the entity of HCAP (675), with corresponding recommendations for empirical broad-spectrum antibiotic therapy for patients meeting HCAP definitions. Patient characteristics that meet definitions for HCAP include residence in a nursing home or extended-care facility, recent hospitalization, recent antimicrobial exposure, the need for long-term hemodialysis, home infusion therapy or home wound care, and underlying immunosuppression. While these risk factors have been corroborated in other geographic settings (687, 705–708), the prevalence of potentially resistant pathogens causing HCAP in these regions varies considerably (709).
Nonetheless, the overall incidence of MDR pathogens, including MRSA, is relatively low in patients with HCAP (710). To help identify patients at risk of HCAP with MDR pathogens, Shorr et al. developed and validated a clinical prediction model: 4 points were assigned for recent hospitalization, 3 points were assigned if the patient presented from a long-term-care facility, 2 points were assigned if the patient was undergoing chronic hemodialysis, and 1 point was assigned if the patient was admitted to the ICU within 24 h of evaluation in the emergency department, for a possible maximum score of 10. Those patients with a score of zero had a prevalence of resistant organisms of ∼15%, whereas nearly 75% of those with a score of 6 to 10 had a resistant pathogen (711). This same group developed a separate score (derived from a different set of patients over the period of 2005 to 2009 and in a population with an overall prevalence of MRSA pneumonia of 14%) that aims to predict MRSA specifically (Table 7) (712). As might be expected, it is difficult to predict the presence of a single pathogen based on clinical features alone. While the latter scoring system could divide patients into low-risk (score of 0 or 1), medium-risk (score of 2 to 5), and high-risk (score of 6 to 10) strata, in the validation cohort, the low-risk group still had an MRSA prevalence of ∼10%, while prevalence in the high-risk group was just over 30%.
TABLE 7.
Risk score for methicillin-resistant S. aureus pneumoniaa
| Variable | No. of points assigned |
|---|---|
| Age of <30 or >79 yr | 1 |
| Recent hospitalization (≥2 days within the last 90 days) | 2 |
| Nursing home/long-term acute-care exposure within the last 90 days | 1 |
| Prior i.v. antibiotic therapy within the last 30 days | 1 |
| Intensive care unit admission | 2 |
| Cerebrovascular disease | 1 |
| Dementia | 1 |
| Female with diabetes mellitus | 1 |
Adapted from reference 712.
Rates of HCAP caused by MDR bacteria are lower in most parts of Europe than in the United States (713). For example, although more than half of a recent cohort of 935 Italian pneumonia patients had ≥1 HCAP risk factor, only 6.1% had an MDR pathogen isolated, the most common of which was MRSA. Notably, MSSA was more common than MRSA among those with no risk factors (12.4% versus 2.7% of all culture-positive patients). Among those with HCAP risk factors, MSSA and MRSA were equally prevalent (12.4% and 14.4%, respectively). Recent hospitalization, nursing home residency, and chronic renal failure were predictive of isolation of MDR pathogens. Overall, S. aureus was the second most common bacterium isolated. Thus, it was possible to develop another prediction score for this setting (714), an undertaking that has since been repeated in other geographic settings (715).
Pathophysiology
The presence of S. aureus in the respiratory tract can lead to a wide range of outcomes, from asymptomatic colonization to fulminant invasive disease, and the host immune response is a significant determinant of this outcome. Of the numerous virulence factors important in staphylococcal infections, several appear to be specifically implicated in the development of pleuropulmonary infections, especially in the most severe manifestation of S. aureus pneumonia, the hemorrhagic necrotizing phenotype. Massive polymorphonuclear leukocyte influx into the lung parenchyma and the formation of microabscesses are typical findings of S. aureus pneumonia (716) and have been associated with PVL positivity (688). In a murine model, Labandeira-Rey et al. demonstrated that PVL is sufficient to cause pneumonia and does so by inducing changes in the transcription of genes that encode secreted and cell wall-anchored proteins, including spa (717). Similar findings were noted in a rabbit model (718). However, a recent meta-analysis of studies examining the role of PVL in clinical staphylococcal disease found that PVL positivity, while common in SSTIs, was comparatively rare in pneumonia (286). Importantly, Bubeck Wardenburg et al. found that another pore-forming toxin, called alpha-hemolysin, but not PVL, was essential for the pathogenesis of clinical pneumonia (719). At the cellular level, proposed mechanisms for the action of alpha-hemolysin include activation of the NLRP3 inflammasome, leading to necrotic lung injury (720), and alpha-hemolysin promotion of platelet-neutrophil aggregates, which then dysregulate inflammatory responses and contribute to tissue destruction (721). Notably, immunization against alpha-hemolysin is protective against lethal pneumonia in mice (722, 723).
Clinical Features and Outcomes
There are distinct clinical phenotypes associated with health care-associated MRSA (HCA-MRSA) and CA-MRSA pneumonia. HCA-MRSA tends to occur in elderly patients with multiple comorbid illnesses and follows a clinical course similar to that of pneumonia caused by Gram-negative organisms. Bacteremia, when it occurs, is a poor prognostic indicator. In a retrospective review of 60 patients with nosocomial bacteremic S. aureus pneumonia (42 with MRSA and 18 with MSSA), the mortality rate was >50% in both MSSA and MRSA cases (724). Vidaur et al. noted higher mortality rates and higher medical resource utilization, including longer time on a ventilator, for patients with MRSA VAP than for patients infected other pathogens, including MSSA (725). This excess mortality with MRSA, however, has not been demonstrated by other investigators (726).
Staphylococcal PVL-positive necrotizing pneumonia was initially described in young, previously healthy patients with a preceding flu-like illness, with rapid onset of severe symptoms, high fever, leukopenia, cavitary pneumonia, and a fulminant course with mortality rates of 56% (727, 728). During the 2009 influenza A virus H1N1 pandemic in France, among 103 patients admitted to the ICU with confirmed influenza A virus H1N1 2009 infections, 48 had bacterial coinfection (54% due to Streptococcus pneumoniae and 31% due to S. aureus) (729). The median age of those with bacterial coinfection was 43 years, and the mortality rate was 21%. In this setting, procalcitonin levels of >0.8 μg/liter had a sensitivity of 91% for detecting bacterial coinfection (729). However, previously well patients with influenza virus infections are not the only patient group affected by PVL-positive necrotizing pneumonia: in a retrospective review of 15 patients with CA-MRSA CAP, only 1 had evidence of preceding influenza, there was no seasonal pattern, and half of the patients were immunocompromised. Pleural effusions were present in 9/15 patients, and the mortality rate was 13% (730). An examination in France of 133 PVL-producing S. aureus pneumonia cases found a mortality rate of 39%, and methicillin resistance was not an independent predictor of mortality (731), in keeping with similar findings from a prior meta-analysis of 107 cases (732).
In patients with CF, poor clearance of viscous airway secretions leads to colonization and subsequent clinical infections with pathogenic bacteria. S. aureus is the most common of these pathogens, an increasing proportion of which are MRSA (733). S. aureus may form biofilms in the airways of patients with CF (734), contributing to the persistence of colonization and antibiotic failure. SCVs are also found frequently in patients with CF; 17% of 252 CF patients in a German study (735) and 24% of 100 children with CF in a U.S. study (736) (15) had airways colonized with SCVs, which is associated with worse lung disease (735, 736). Colonization with MRSA appears to be an independent predictor of mortality in CF patients (737).
Management
With the advent of guidelines recommending empirical antibiotics targeting MRSA in high-risk patients with pneumonia, the question has naturally arisen as to whether such guideline-concordant therapy improves outcomes. In a retrospective review of 757 patients with HCAP (27% of whom had MRSA), inappropriate initial antimicrobial therapy and the absence of empirical anti-MRSA antibiotics were associated with a higher risk of mortality (738). In a similar investigation of patients admitted with pneumonia in Canada, only 10 of 1,220 patients with culture-positive infection had MRSA isolated, and guideline-concordant therapy did not influence HCAP mortality (739). Similar findings have been documented in other settings of low MRSA prevalence (740). Some authors have found higher mortality rates with the use of guideline-concordant empirical therapy, with a trend toward higher mortality rates even in the subset with MRSA (741, 742). A more nuanced view emerged from Madaras-Kelly et al., in which receipt of “guideline-similar therapy” increased the 30-day mortality rate overall but appeared to improve mortality in the subset of patients with an a priori increased risk of resistance to ceftriaxone or moxifloxacin (743).
Two major clinical trials have compared the uses of vancomycin and linezolid in known cases of MRSA pneumonia. Rubinstein et al. randomized patients with nosocomial pneumonia to receive vancomycin or linezolid, each in combination with aztreonam (744). Given the few MRSA patients in either group, the trial was then extended to include an additional 345 patients (745). Clinical cure rates did not differ between vancomycin and linezolid treatments, while the microbiologic success rate was slightly higher in the linezolid group. In a post hoc MRSA subgroup analysis, linezolid was associated with cure in 36/61 (59%) patients, compared to 22/62 (36%) for vancomycin (P < 0.01), and also improved survival of 60/75 (80%) patients in the linezolid group, versus 54/85 (64%) patients in the vancomycin group (P = 0.03) (746). Interpretation of these results was complicated by the use of a vancomycin dosing regimen that was less aggressive than would be recommended under current dosing guidelines (747). This uncertainty paved the way for ZEPHyR, a subsequent randomized, double-blind trial of 1,184 patients from 154 sites over 6 years that aimed to address these issues (748). In ZEPHyR, cure was defined as resolution of clinical signs and symptoms of pneumonia compared with the baseline, improvement or lack of progression determined by chest imaging, and no requirement for additional antibacterial treatment, and it was assessed at the end of the study (defined as 7 to 30 days after the end of therapy) by the investigators, with “occasional override by the sponsor.” Patients treated with linezolid had a higher clinical cure rate (58% versus 47% in the per-protocol population; P = 0.042), but the 60-day mortality rate was not significantly different between groups (15.7% in the linezolid group versus 17% in the vancomycin group) (748). Strengths of this study included the large sample size, the attention to vancomycin MIC values and trough levels, and extension of therapy in bacteremic patients (749). Limitations of ZEPHyR included an imbalance between per-protocol groups, as more patients in the vancomycin group had bacteremia and required mechanical ventilation. Additionally, vancomycin trough levels were suboptimal in >50% of patients (750–752). Therefore, there is uncertainty regarding the optimal antimicrobial agent for MRSA pneumonia.
In the setting of known MRSA pneumonia, ATS/IDSA guidelines recommend vancomycin, linezolid, or clindamycin, noting a stronger evidence base for vancomycin and linezolid than for clindamycin. The suggested duration of therapy is 7 to 21 days, depending on the clinical response. British Thoracic Society (BTS) guidelines advocate for vancomycin, linezolid, or teicoplanin (a glycopeptide antibiotic that is not available in the United States). However, a special case is made for PVL-producing S. aureus, for which the use of linezolid or clindamycin (depending on susceptibility results) in addition to rifampin is recommended, with the further addition of i.v. immunoglobulin for those with clinical deterioration or features of severe disease (753). The preference for linezolid and clindamycin over vancomycin in this setting is based upon the hypothesis that linezolid and clindamycin, which suppress toxin production, may improve survival with this specific infection, for which toxin production is a key virulence factor. This improved survival has been demonstrated in a rabbit model of necrotizing MRSA pneumonia (754) and in a pig pneumonia model (755). Two retrospective studies suggest that there may be a clinical benefit for suppression of toxins in such cases (756, 757).
Daptomycin should not be used for MRSA pneumonia, as it is inactivated by surfactant (758). However, in the case of hematogenous septic pulmonary emboli, as from right-sided endocarditis, daptomycin may be an option (119).
Ceftaroline fosamil is a broad-spectrum cephalosporin with bactericidal activity against Gram-positive pathogens, including MRSA, as well as some Gram-negative pathogens. It was studied in two identical registrational trials (FOCUS 1 and FOCUS 2) that compared ceftaroline with ceftriaxone in the treatment of adults hospitalized with CAP but not requiring ICU care and with a Pneumonia Outcomes Research Team risk class III or IV status (759). Known MRSA was an exclusion criterion (since ceftriaxone does not have significant activity against MRSA), and ultimately, only 2 patients, both in the ceftriaxone group, had cultures positive for MRSA. Ceftaroline was noninferior to ceftriaxone overall, and in the MSSA group, clinical cure was reported for 18/25 (72%) patients with ceftaroline, versus 18/30 (60%) patients with ceftriaxone. Thus, ceftaroline appears to have a potential role in the treatment of CAP, although it remains unstudied in MRSA pneumonia and in more severe CAP presentations necessitating ICU care.
For patients with CF, two ongoing studies are examining the role of decolonization (760, 761); currently, there is no clear role for attempted decolonization. It is also unknown whether a different treatment strategy should be used for acute flares due to MRSA in CF patients (762); vancomycin and linezolid are recommended first-line therapies, as for MRSA pulmonary infections in other patient populations.
OTHER STAPHYLOCOCCAL CLINICAL SYNDROMES
Epidural Abscess
Epidural abscesses can be intracranial or spinal. Intracranial epidural abscesses are much less common than spinal epidural abscesses and usually follow surgery or trauma. Discussion in this section is limited to spinal epidural abscesses.
Epidemiology.
Although a rare infection (∼1 in 20,000 hospitalized patients [763]), spinal epidural abscess is the second most common infectious cause of medical malpractice in the United States (764). The incidence of epidural abscess appears to have increased over the past 30 years (765). This is likely to be in part due to the increasing availability of magnetic resonance imaging (MRI) (which is much more sensitive than previous modalities) and partly due to the increasing use of epidural catheters and electrodes for pain management. S. aureus is the most common causative agent of spinal epidural abscess, accounting for 60 to 73% of all cases (766–768).
Pathophysiology and clinical manifestations.
An epidural abscess may arise by hematogenous seeding from an episode of SAB, by contiguous spread from an adjacent focus (such as psoas abscess or vertebral OM), or due to direct inoculation from trauma, spinal surgery, or the placement of epidural catheters (766). Although a spinal epidural abscess may occur anywhere from the cervical to the sacral spine, it is generally more likely to occur where the epidural space is larger. Thus, posterior epidural space involvement is more common than anterior epidural space involvement, and lumbar and lower thoracic epidural abscesses are more common than cervical epidural abscesses (769). Because the epidural space is a continuous vertical region, epidural abscesses generally spread over several vertebral levels and rarely may involve the entire spine. The most important potential consequence of spinal epidural abscesses is damage to the spinal cord and nerve roots, which can occur due to direct compression of the cord by an expanding collection of pus (770) or indirectly through arterial or venous ischemia. The major risk factors for S. aureus spinal epidural abscess include diabetes mellitus (766), injection drug use (766, 768), recent spinal surgery or trauma, and recent placement of epidural injections, catheters, or stimulating wires (771, 772). Approximately 2.5% of patients with SAB have epidural abscesses (69). Thus, any patient with SAB who complains of new or changing back pain should undergo spinal imaging, preferably with MRI, to evaluate the possibility of an epidural abscess.
The classic clinical triad for epidural abscess is back pain, fever, and neurological signs; however, the complete triad is present in only a minority of patients at presentation (773). For this reason, the diagnosis of spinal epidural abscess is often not initially considered. For example, only 40% of admitting diagnoses included spinal epidural abscess as the suspected diagnosis for one series of 43 patients ultimately found to have epidural abscess (763). Darouiche et al. (763, 765) described the following clinical staging system, which is useful for determining the timing and nature of management of spinal epidural abscesses: stage 1, back pain at the affected vertebral level; stage 2, nerve root pain radiating from the involved area; stage 3, objective motor and sensory loss and/or bladder and bowel dysfunction; and stage 4, paralysis. This staging system is important because once patients enter stages 2 and 3, their spinal cord is under threat, and urgent surgical decompression is required.
Management.
In general, surgical decompression (laminectomy, debridement of infected or necrotic tissue, and drainage of pus) is required to achieve a successful outcome in cases of S. aureus spinal epidural abscess. This is in conjunction with a long course of high-dose i.v. antibiotic therapy (766). Because of the possibility of permanent paralysis, spinal epidural abscess is a medical and surgical emergency. Once paralysis is established for >24 to 48 h, the damage is likely to be permanent. Thus, the key step is the early recognition of the possibility of spinal epidural abscess and rapid investigation to confirm the diagnosis. Most authors suggest that surgical decompression be performed urgently (within 24 h of diagnosis) in patients with S. aureus spinal epidural abscess (763, 774–776). However, it is increasingly being recognized that select patients may not require surgical intervention. Such patients include those in whom paralysis has been present for >48 h and those with early (clinical stage 1) infection with small abscesses, where a pathogen has been identified by blood culture or computed tomography (CT)-guided aspiration of the abscess (777, 778). MSSA spinal epidural abscesses should be treated with ∼6 weeks of a high-dose i.v. antistaphylococcal β-lactam (e.g., 2 g nafcillin every 4 h [q4h] i.v.). For MRSA, treatment with vancomycin for a similar duration is advised, aiming for plasma levels of 15 to 20 mg/liter.
Meningitis
Epidemiology and pathophysiology.
S. aureus is an uncommon cause of bacterial meningitis, accounting for 4.9 to 6.4% of cases (779–784). S. aureus meningitis may either arise by hematogenous spread from a non-CNS focus of infection or be secondary to neurosurgical intervention (785, 786). Hematogenous S. aureus meningitis is usually community acquired (780, 785) and, compared with postsurgical S. aureus meningitis, typically affects older individuals (mean age of 59 years versus 40 years; P = 0.04) (787) with severe medical comorbidities such as diabetes or chronic kidney disease (780, 787). The initial source of hematogenous S. aureus meningitis is generally IE, pneumonia, or SSTI (788). The mortality rate for hematogenous S. aureus meningitis is higher (43 to 50%) than that occurring postsurgically (14 to 25%) (780, 787). Among S. aureus meningitis cases, methicillin resistance has been increasing in recent years (786, 789–791). Specifically, Pintado et al. conducted a retrospective, multicenter study examining MRSA meningitis in adults over a 25-year period (1981 to 2005). This group found that nearly half of MRSA meningitis cases arose in the last 5 years of the study. The 30-day mortality rate for these patients was 31%. Most MRSA meningitis cases are nosocomial and postsurgical (786, 789).
Clinical manifestations and risk factors.
Because meningitis is a rare complication for patients with SAB, occurring in 1.7% of 724 prospectively identified patients with SAB, clinicians need to be alert to its possibility (69). S. aureus meningitis typically presents with one or more of the following signs or symptoms: persistent fever, headache, stiff neck, and vomiting (786, 787, 792). Fever and change in consciousness are the two most common clinical symptoms (782, 789). Patients with hematogenous S. aureus meningitis typically have a greater degree of CSF leukocytosis than do postsurgical patients (787).
The major predisposing factors for postsurgical meningitis are (i) the presence of an intrathecal device or ventriculoperitoneal shunt (780, 787, 792), (ii) recent neurosurgery, and (iii) a CSF leak (780). Of note, S. aureus is the second leading cause of bacterial meningitis among patients with a ventriculoperitoneal shunt (793) (see the section on S. aureus CNS shunt infection, above). Intravenous drug use is an important risk factor for hematogenous S. aureus meningitis, being present in 52% of patients in a recent series of 21 cases of hematogenous S. aureus meningitis from a single center in the United States (780, 794) but in only 12.5% of 96 cases from a nationwide Danish study (794).
Risk factors for mortality among patients with S. aureus meningitis include a hematogenous compared with a postsurgical source (e.g., 56% mortality for a hematogenous source versus 18% for a postsurgical source within a national Danish study [795]), increasing age and number of comorbidities (794), the presence of septic shock (787), and concurrent IE (782, 784).
Management.
The IDSA recommends high-dose i.v. nafcillin or oxacillin to treat MSSA meningitis and vancomycin for MRSA meningitis (668). Vancomycin has poor penetration into the CSF of ∼1% through uninflamed and 5% through inflamed meninges (782, 789), and in practice, it can be difficult to achieve therapeutic levels within the CSF. This is even more of an issue when the vancomycin MIC of an MRSA isolate approaches or exceeds 2 μg/ml. In such dire settings, consideration can be given to unproven adjunctive therapies such as the intrathecal administration of vancomycin (796, 797) or the addition of antibiotics such as linezolid (CSF penetration, 66% [79]), TMP-SMX (CSF penetration, ∼50% [798]), or daptomycin (CSF penetration, 6% [799]).
Toxic Shock Syndrome
Epidemiology.
S. aureus toxic shock syndrome (TSS) was first described in 1978 by Todd et al., who reported the illness in a group of 7 children (800). Shortly thereafter, S. aureus TSS became linked with superabsorbent tampons in menstruating women in the 1980s (801, 802), reaching an annual infection rate of 13.7 per 100,000 menstruating women (803). After the removal of highly absorbent tampons from the market, the annual incidences of S. aureus TSS declined to 1 per 100,000 menstruating women and 0.3 per nonmenstruating persons (804–806). The incidence of S. aureus TSS has remained stable since that time, with the current annual incidences reported to be 0.69 per 100,000 menstruating women and 0.32 per 100,000 total population (804). Currently, the numbers of menstrual and nonmenstrual cases of staphylococcal TSS are similar, and the most common foci of infection in nonmenstrual cases are SSTIs (804, 807). Nonmenstrual cases have been associated with a higher mortality rate than menstrual cases (807).
Pathophysiology, clinical manifestations, and diagnosis.
S. aureus TSS is a superantigen-mediated process. Some strains of S. aureus secrete an exotoxin called toxic shock syndrome toxin 1 (TSST-1) (808). TSST-1 cross-links the T-cell receptor with major histocompatibility complex class II (MHC-II) on antigen-presenting cells, triggering large-scale T-cell activation and massive cytokine release (802, 809, 810). This leads to an overwhelming systemic inflammatory response syndrome and is manifested by septic shock with organ failure. The CDC reported diagnostic criteria for staphylococcal TSS (811). A confirmed case must meet all of the following criteria: (i) a fever of >38.9°C, (ii) shock (systolic blood pressure of <90 mm Hg despite adequate fluid resuscitation), (iii) a diffuse macular erythematous rash (typically followed 1 to 2 weeks later by desquamation), and (iv) specific abnormalities involving at least three organ systems. The organ system involvements that can be included are as follows: (i) gastrointestinal, with vomiting or diarrhea; (ii) musculoskeletal, with severe myalgia or creatinine kinase levels >2 times the upper limit of normal (ULN); (iii) renal, with serum creatinine levels >2 times the ULN; (iv) hepatic, with bilirubin or transaminase levels >2 times the ULN; (v) hematologic, with platelet counts of <100,000 platelets/μl; and (vi) CNS, with delirium without focal signs.
Management.
The key aspects of treatment of S. aureus TSS include identifying and removing the source of S. aureus toxin production (e.g., tampon or surgical wound) and supportive care (812, 813). In the setting of postoperative S. aureus TSS, the involved surgical wound often appears normal (807, 814, 815). However, this benign appearance in no way reduces the need for surgical debridement in patients with wound-associated S. aureus TSS. Antibiotics, in contrast, play a secondary role in the management of TSS. Because it can block the production of exotoxins by the bacterial ribosome, clindamycin or linezolid is often added to standard antibiotic therapy (381). Intravenous immunoglobulin may also be effective, although clinical evidence of benefit is not well established, and there is less evidence supporting the use of i.v. immunoglobulin to treat staphylococcal TSS than for streptococcal TSS. Nonetheless, it is recommended that i.v. immunoglobulin be considered for patients who have had no clinical response to aggressive supportive therapy within 6 h (807, 813).
Urinary Tract Infection
Epidemiology, clinical manifestations, and risk factors.
S. aureus is a rare cause of urinary tract infection (UTI) in the community, accounting for only 0.5 to 1% of positive urine cultures (816, 817). S. aureus UTI is more frequent in patients with an indwelling urinary catheter (818). Muder et al. conducted a longitudinal study of 102 patients at a long-term veteran care facility with documented S. aureus bacteriuria and found that 33% of the patients with S. aureus isolated from their urine had UTI symptoms, and 13% were bacteremic (818). Importantly, when S. aureus is isolated from urine in patients without an obvious urinary focus, it can reflect SAB, and thus, it is imperative to perform blood cultures for any patient with S. aureus bacteriuria (819). The most common symptom of S. aureus UTI is fever (818). Other symptoms are hematuria, altered mental status, dysuria, suprapubic pain, and, less commonly, flank pain (818). MRSA UTI is associated with longer stays in health care facilities, recent antibiotic use, and urinary catheterization (818, 820).
Management.
Because catheters are a frequent cause of UTI, it is important to reduce the use of urinary catheterization to only individuals who have a clear indication for it and to remove the device as soon as clinically indicated (821). It is recommended that patients with catheter-associated UTI (once SAB is excluded) receive 10 to 14 days of appropriate antibiotics, as determined by culture susceptibility results, as well as removal and replacement of the catheter (821).
Septic Thrombophlebitis
S. aureus septic thrombophlebitis has been reported to occur in up to 8% of all patients with SAB (69). In selected populations, however, such as patients with CVC-associated SAB, the prevalence of associated thrombosis has been reported to be as high as 71% (646) (see the section on intravascular catheter infections, above, for further details on CLABSI and septic thrombophlebitis).
S. aureus thrombophlebitis less commonly manifests as Lemierre syndrome, involving the internal jugular veins (822–824), or septic pelvic thrombophlebitis (825). S. aureus Lemierre syndrome may manifest as severe neck pain, nausea, vomiting, and weakness (822). Septic pelvic thrombophlebitis manifests as high fever despite antibiotics and acute abdominal pain (825). In adults, it is almost entirely associated with pelvic procedures. In children, there have been a number of reports of deep venous thrombosis associated with CA-MRSA infections (507, 510).
Treatment with anticoagulation therapy and appropriate antibiotics is recommended for cases of pelvic septic thrombophlebitis (825). For patients with central venous catheter-associated septic thrombophlebitis, Crowley et al. found a trend toward lower mortality rates in those with who received anticoagulation therapy (646). A systematic review of the use of i.v. heparin for the treatment of all forms of septic thrombophlebitis concluded that heparin was a useful addition to the antimicrobial treatment regimen and that the risk of complications from anticoagulation therapy is low (826). Thus, guidelines for the management of CLABSI suggest that anticoagulation therapy with heparin should be considered for the treatment of individuals with septic thrombophlebitis (647).
CONCLUSIONS
Clinical infections with S. aureus will likely remain both common and serious. Not only have there been waves of increasing antimicrobial resistance (827), but the spectrum of clinical disease also continues to change. In the past 2 decades, we have witnessed two clear shifts in the epidemiology of S. aureus infections: first, a growing number of health care-associated infections, particularly seen in IE and prosthetic device infections, and second, an epidemic of community-associated SSTIs driven by strains with particular virulence factors. There is no doubt that there will continue to be a shifting landscape in the interactions between host and pathogen in the decades to come.
ACKNOWLEDGMENTS
S.Y.C.T. and J.S.D. are Australian National Health and Medical Council Career Development fellows (1065736 for S.Y.C.T. and 1083105 for J.S.D.).
V.G.F. is supported by grants R01-AI068804 and K24-AI093969 from the National Institutes of Health.
T.L.H. has been a paid consultant for The Medicines Company. V.G.F. served as Chair of the V710 Scientific Advisory Committee (Merck); has received grant support or has grants pending from Cerexa, Pfizer, Advanced Liquid Logic, MedImmune, and Cubist; has been a paid consultant for Merck, Astellas, Affinium, Theravance, Cubist, Cerexa, Durata, Pfizer, NovaDigm, Novartis, The Medicines Company, Biosynexus, MedImmune, Inimex, and Bayer; and has received honoraria from Merck, Astellas, Cubist, Pfizer, Theravance, and Novartis. S.Y.C.T., J.S.D., and E.E. have no conflicts of interest to declare.
Biographies

Steven Y. C. Tong is an infectious diseases physician based at the Royal Darwin Hospital in Darwin, Northern Territory, Australia. His clinical training mainly occurred in Melbourne, Australia, and was followed by a Ph.D. (2007 to 2010) at the Menzies School of Health Research in Darwin. During his postdoctoral training, he spent time at Duke University Medical Center, Durham, NC (2011), and at the Wellcome Trust Sanger Institute, Cambridge, United Kingdom (2012). As a principal research fellow at the Menzies School of Health Research (2013 to present), his research interests broadly cover infections that affect indigenous populations in northern Australia, including staphylococcal and streptococcal infections, hepatitis B, influenza, and rheumatic heart disease. He has particular interests in applying genomic techniques to understanding the epidemiology of staphylococcal disease and in conducting clinical trials to improve treatments for Staphylococcus aureus bacteremia.

Joshua S. Davis is an infectious diseases specialist at John Hunter Hospital in Newcastle, Australia, and a clinical research fellow at the Menzies School of Health Research in Darwin, Australia. He did his training in internal medicine and infectious diseases in Sydney, Newcastle, and Darwin. His Ph.D. was awarded in 2010 for work on the pathophysiology and epidemiology of severe sepsis. Since 2010, Dr. Davis's main research interests have been in investigator-initiated clinical trials in severe bacterial infections, with a focus on osteoarticular infections and S. aureus bacteremia.

Emily Eichenberger is a first-year Internal Medicine resident at New York Presbyterian/Weill Cornell Medical Center. She completed her undergraduate training at Dartmouth College in 2009, where she majored in Human Biology. She then attended Medical School at Duke University, where she spent a year researching the role of genetic polymorphisms in Staphylococcus aureus in prosthetic joint infections. She continues to have an ongoing interest in Infectious Diseases.

Thomas L. Holland is an infectious diseases physician at Duke University in Durham, NC. He received his medical degree from Wake Forest University and completed internal medicine residency followed by an infectious diseases fellowship at Duke University. His research interests include treatment of staphylococcal infections; he is currently engaged in clinical trials to evaluate treatment durations for staphylococcal bacteremia and to define the optimal vancomycin dosing strategy for MRSA bacteremia.

Vance G. Fowler, Jr., M.D., M.H.S., is Professor in the Division of Infectious Diseases, Department of Medicine, and Department of Molecular Genetics and Microbiology at Duke University Medical Center. His research interests focus on clinical and translational research in antibiotic-resistant bacteria, especially MRSA.
REFERENCES
- 1.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 2.Coates R, Moran J, Horsburgh MJ. 2014. Staphylococci: colonizers and pathogens of human skin. Future Microbiol 9:75–91. doi: 10.2217/fmb.13.145. [DOI] [PubMed] [Google Scholar]
- 3.Verhoeven PO, Gagnaire J, Botelho-Nevers E, Grattard F, Carricajo A, Lucht F, Pozzetto B, Berthelot P. 2014. Detection and clinical relevance of Staphylococcus aureus nasal carriage: an update. Expert Rev Anti Infect Ther 12:75–89. doi: 10.1586/14787210.2014.859985. [DOI] [PubMed] [Google Scholar]
- 4.Stryjewski ME, Corey GR. 2014. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis 58(Suppl 1):S10–S19. doi: 10.1093/cid/cit613. [DOI] [PubMed] [Google Scholar]
- 5.Malachowa N, DeLeo FR. 2010. Mobile genetic elements of Staphylococcus aureus. Cell Mol Life Sci 67:3057–3071. doi: 10.1007/s00018-010-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laupland KB, Lyytikainen O, Sogaard M, Kennedy KJ, Knudsen JD, Ostergaard C, Galbraith JC, Valiquette L, Jacobsson G, Collignon P, Schonheyder HC, International Bacteremia Surveillance Collaborative . 2013. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 19:465–471. doi: 10.1111/j.1469-0691.2012.03903.x. [DOI] [PubMed] [Google Scholar]
- 8.Frimodt-Moller N, Espersen F, Skinhoj P, Rosdahl VT. 1997. Epidemiology of Staphylococcus aureus bacteremia in Denmark from 1957 to 1990. Clin Microbiol Infect 3:297–305. doi: 10.1111/j.1469-0691.1997.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 9.Mejer N, Westh H, Schonheyder HC, Jensen AG, Larsen AR, Skov R, Benfield T, Danish Staphylococcal Bacteraemia Study Group . 2012. Stable incidence and continued improvement in short term mortality of Staphylococcus aureus bacteraemia between 1995 and 2008. BMC Infect Dis 12:260. doi: 10.1186/1471-2334-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allard C, Carignan A, Bergevin M, Boulais I, Tremblay V, Robichaud P, Duperval R, Pepin J. 2008. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991-2005. Clin Microbiol Infect 14:421–428. doi: 10.1111/j.1469-0691.2008.01965.x. [DOI] [PubMed] [Google Scholar]
- 11.El Atrouni WI, Knoll BM, Lahr BD, Eckel-Passow JE, Sia IG, Baddour LM. 2009. Temporal trends in the incidence of Staphylococcus aureus bacteremia in Olmsted County, Minnesota, 1998 to 2005: a population-based study. Clin Infect Dis 49:e130–e138. doi: 10.1086/648442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laupland KB, Ross T, Gregson DB. 2008. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. J Infect Dis 198:336–343. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 13.Wyllie DH, Peto TE, Crook D. 2005. MRSA bacteraemia in patients on arrival in hospital: a cohort study in Oxfordshire 1997-2003. BMJ 331:992. doi: 10.1136/bmj.38558.453310.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core Surveillance MRSA Investigators . 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AP, Pearson A, Duckworth G. 2005. Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother 56:455–462. doi: 10.1093/jac/dki266. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AP, Davies J, Guy R, Abernethy J, Sheridan E, Pearson A, Duckworth G. 2012. Mandatory surveillance of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in England: the first 10 years. J Antimicrob Chemother 67:802–809. doi: 10.1093/jac/dkr561. [DOI] [PubMed] [Google Scholar]
- 17.Stone SP, Fuller C, Savage J, Cookson B, Hayward A, Cooper B, Duckworth G, Michie S, Murray M, Jeanes A, Roberts J, Teare L, Charlett A. 2012. Evaluation of the national Cleanyourhands campaign to reduce Staphylococcus aureus bacteraemia and Clostridium difficile infection in hospitals in England and Wales by improved hand hygiene: four year, prospective, ecological, interrupted time series study. BMJ 344:e3005. doi: 10.1136/bmj.e3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, Ray SM, Harrison LH, Lynfield R, Dumyati G, Townes JM, Schaffner W, Patel PR, Fridkin SK. 2010. Health care-associated invasive MRSA infections, 2005-2008. JAMA 304:641–648. doi: 10.1001/jama.2010.1115. [DOI] [PubMed] [Google Scholar]
- 19.Grayson ML, Jarvie LJ, Martin R, Johnson PD, Jodoin ME, McMullan C, Gregory RH, Bellis K, Cunnington K, Wilson FL, Quin D, Kelly AM. 2008. Significant reductions in methicillin-resistant Staphylococcus aureus bacteraemia and clinical isolates associated with a multisite, hand hygiene culture-change program and subsequent successful statewide roll-out. Med J Aust 188:633-640. [DOI] [PubMed] [Google Scholar]
- 20.Jarlier V, Trystram D, Brun-Buisson C, Fournier S, Carbonne A, Marty L, Andremont A, Arlet G, Buu-Hoi A, Carlet J, Decre D, Gottot S, Gutmann L, Joly-Guillou ML, Legrand P, Nicolas-Chanoine MH, Soussy CJ, Wolf M, Lucet JC, Aggoune M, Brucker G, Regnier B. 2010. Curbing methicillin-resistant Staphylococcus aureus in 38 French hospitals through a 15-year institutional control program. Arch Intern Med 170:552–559. doi: 10.1001/archinternmed.2010.32. [DOI] [PubMed] [Google Scholar]
- 21.Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D. 2013. Epidemiology, microbiology and mortality associated with community-acquired bacteremia in northeast Thailand: a multicenter surveillance study. PLoS One 8:e54714. doi: 10.1371/journal.pone.0054714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JA. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 23.Sigauque B, Roca A, Mandomando I, Morais L, Quinto L, Sacarlal J, Macete E, Nhamposa T, Machevo S, Aide P, Bassat Q, Bardaji A, Nhalungo D, Soriano-Gabarro M, Flannery B, Menendez C, Levine MM, Alonso PL. 2009. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 24.Groome MJ, Albrich WC, Wadula J, Khoosal M, Madhi SA. 2012. Community-onset Staphylococcus aureus bacteraemia in hospitalised African children: high incidence in HIV-infected children and high prevalence of multidrug resistance. Paediatr Int Child Health 32:140–146. doi: 10.1179/1465328111Y.0000000044. [DOI] [PubMed] [Google Scholar]
- 25.Asgeirsson H, Gudlaugsson O, Kristinsson KG, Heiddal S, Kristjansson M. 2011. Staphylococcus aureus bacteraemia in Iceland, 1995-2008: changing incidence and mortality. Clin Microbiol Infect 17:513–518. doi: 10.1111/j.1469-0691.2010.03265.x. [DOI] [PubMed] [Google Scholar]
- 26.Huggan PJ, Wells JE, Browne M, Richardson A, Murdoch DR, Chambers ST. 2010. Population-based epidemiology of Staphylococcus aureus bloodstream infection in Canterbury, New Zealand. Intern Med J 40:117–125. doi: 10.1111/j.1445-5994.2009.01910.x. [DOI] [PubMed] [Google Scholar]
- 27.Lyytikainen O, Ruotsalainen E, Jarvinen A, Valtonen V, Ruutu P. 2005. Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995-2001. Eur J Clin Microbiol Infect Dis 24:399–404. doi: 10.1007/s10096-005-1345-3. [DOI] [PubMed] [Google Scholar]
- 28.Meyer A, Ladefoged K, Poulsen P, Koch A. 2008. Population-based survey of invasive bacterial diseases, Greenland, 1995-2004. Emerg Infect Dis 14:76–79. doi: 10.3201/eid1401.071240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landrum ML, Neumann C, Cook C, Chukwuma U, Ellis MW, Hospenthal DR, Murray CK. 2012. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 308:50–59. doi: 10.1001/jama.2012.7139. [DOI] [PubMed] [Google Scholar]
- 30.Tong SY, Bishop EJ, Lilliebridge RA, Cheng AC, Spasova-Penkova Z, Holt DC, Giffard PM, McDonald MI, Currie BJ, Boutlis CS. 2009. Community-associated strains of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus in indigenous northern Australia: epidemiology and outcomes. J Infect Dis 199:1461–1470. doi: 10.1086/598218. [DOI] [PubMed] [Google Scholar]
- 31.Tong SY, van Hal SJ, Einsiedel L, Currie BJ, Turnidge JD. 2012. Impact of ethnicity and socio-economic status on Staphylococcus aureus bacteremia incidence and mortality: a heavy burden in indigenous Australians. BMC Infect Dis 12:249. doi: 10.1186/1471-2334-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewagama S, Einsiedel L, Spelman T. 2012. Staphylococcus aureus bacteraemia at Alice Springs Hospital, Central Australia, 2003-2006. Intern Med J 42:505–512. doi: 10.1111/j.1445-5994.2011.02449.x. [DOI] [PubMed] [Google Scholar]
- 33.Hill PC, Birch M, Chambers S, Drinkovic D, Ellis-Pegler RB, Everts R, Murdoch D, Pottumarthy S, Roberts SA, Swager C, Taylor SL, Thomas MG, Wong CG, Morris AJ. 2001. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J 31:97–103. doi: 10.1111/j.1444-0903.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- 34.Hill PC, Wong CG, Voss LM, Taylor SL, Pottumarthy S, Drinkovic D, Morris AJ. 2001. Prospective study of 125 cases of Staphylococcus aureus bacteremia in children in New Zealand. Pediatr Infect Dis J 20:868–873. doi: 10.1097/00006454-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Larsen MV, Harboe ZB, Ladelund S, Skov R, Gerstoft J, Pedersen C, Larsen CS, Obel N, Kronborg G, Benfield T. 2012. Major but differential decline in the incidence of Staphylococcus aureus bacteraemia in HIV-infected individuals from 1995 to 2007: a nationwide cohort study. HIV Med 13:45–53. doi: 10.1111/j.1468-1293.2011.00937.x. [DOI] [PubMed] [Google Scholar]
- 36.Burkey MD, Wilson LE, Moore RD, Lucas GM, Francis J, Gebo KA. 2008. The incidence of and risk factors for MRSA bacteraemia in an HIV-infected cohort in the HAART era. HIV Med 9:858–862. doi: 10.1111/j.1468-1293.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spijkerman IJ, van Ameijden EJ, Mientjes GH, Coutinho RA, van den Hoek A. 1996. Human immunodeficiency virus infection and other risk factors for skin abscesses and endocarditis among injection drug users. J Clin Epidemiol 49:1149–1154. doi: 10.1016/0895-4356(96)00180-1. [DOI] [PubMed] [Google Scholar]
- 38.Tuazon CU, Sheagren JN. 1974. Increased rate of carriage of Staphylococcus aureus among narcotic addicts. J Infect Dis 129:725–727. doi: 10.1093/infdis/129.6.725. [DOI] [PubMed] [Google Scholar]
- 39.Palepu A, Tyndall MW, Leon H, Muller J, O'Shaughnessy MV, Schechter MT, Anis AH. 2001. Hospital utilization and costs in a cohort of injection drug users. CMAJ 165:415–420. [PMC free article] [PubMed] [Google Scholar]
- 40.Craven DE, Rixinger AI, Goularte TA, McCabe WR. 1986. Methicillin-resistant Staphylococcus aureus bacteremia linked to intravenous drug abusers using a “shooting gallery.” Am J Med 80:770–776. [DOI] [PubMed] [Google Scholar]
- 41.Wang IK, Chang YC, Liang CC, Chuang FR, Chang CT, Lin HH, Lin CC, Yen TH, Lin PC, Chou CY, Huang CC, Tsai WC, Chen JH. 2012. Bacteremia in hemodialysis and peritoneal dialysis patients. Intern Med 51:1015–1021. doi: 10.2169/internalmedicine.51.7111. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald SF, O'Gorman J, Morris-Downes MM, Crowley RK, Donlon S, Bajwa R, Smyth EG, Fitzpatrick F, Conlon PJ, Humphreys H. 2011. A 12-year review of Staphylococcus aureus bloodstream infections in haemodialysis patients: more work to be done. J Hosp Infect 79:218–221. doi: 10.1016/j.jhin.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Vanholder R, Ringoir S, Dhondt A, Hakim R. 1991. Phagocytosis in uremic and hemodialysis patients: a prospective and cross sectional study. Kidney Int 39:320–327. doi: 10.1038/ki.1991.40. [DOI] [PubMed] [Google Scholar]
- 44.Boelaert JR, Daneels RF, Schurgers ML, Matthys EG, Gordts BZ, Van Landuyt HW. 1990. Iron overload in haemodialysis patients increases the risk of bacteraemia: a prospective study. Nephrol Dial Transplant 5:130–134. doi: 10.1093/ndt/5.2.130. [DOI] [PubMed] [Google Scholar]
- 45.Zimakoff J, Bangsgaard Pedersen F, Bergen L, Baago-Nielsen J, Daldorph B, Espersen F, Gahrn Hansen B, Hoiby N, Jepsen OB, Joffe P, Kolmos HJ, Klausen M, Kristoffersen K, Ladefoged J, Olesen-Larsen S, Rosdahl VT, Scheibel J, Storm B, Tofte-Jensen P. 1996. Staphylococcus aureus carriage and infections among patients in four haemo- and peritoneal-dialysis centres in Denmark. The Danish Study Group of Peritonitis in Dialysis (DASPID). J Hosp Infect 33:289–300. [DOI] [PubMed] [Google Scholar]
- 46.Jeremiah CJ, Wills C, Bayly A, Perry GJ, Davis JS, Tong SY, Currie BJ. 2014. Vancomycin dosing nomogram for haemodialysis patients. Nephrology 19:513–514. doi: 10.1111/nep.12270. [DOI] [PubMed] [Google Scholar]
- 47.Vandecasteele SJ, De Vriese AS. 2011. Vancomycin dosing in patients on intermittent hemodialysis. Semin Dial 24:50–55. doi: 10.1111/j.1525-139X.2010.00803.x. [DOI] [PubMed] [Google Scholar]
- 48.Barth RH, DeVincenzo N. 1996. Use of vancomycin in high-flux hemodialysis: experience with 130 courses of therapy. Kidney Int 50:929–936. doi: 10.1038/ki.1996.393. [DOI] [PubMed] [Google Scholar]
- 49.Benfield T, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, Skov R, Westh H, Skinhoj P. 2007. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect 13:257–263. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 50.Skogberg K, Lyytikainen O, Ollgren J, Nuorti JP, Ruutu P. 2012. Population-based burden of bloodstream infections in Finland. Clin Microbiol Infect 18:E170–E176. doi: 10.1111/j.1469-0691.2012.03845.x. [DOI] [PubMed] [Google Scholar]
- 51.van Cleef BA, Kluytmans JA, van Benthem BH, Haenen A, Monen J, Daniels-Haardt I, Jurke A, Friedrich AW. 2012. Cross border comparison of MRSA bacteraemia between The Netherlands and North Rhine-Westphalia (Germany): a cross-sectional study. PLoS One 7:e42787. doi: 10.1371/journal.pone.0042787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frederiksen MS, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, Skov R, Westh H, Skinhoj P, Benfield T. 2007. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. Pediatr Infect Dis J 26:398–405. doi: 10.1097/01.inf.0000261112.53035.4c. [DOI] [PubMed] [Google Scholar]
- 53.Vanderkooi OG, Gregson DB, Kellner JD, Laupland KB. 2011. Staphylococcus aureus bloodstream infections in children: a population-based assessment. Paediatr Child Health 16:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nielsen MV, Sarpong N, Krumkamp R, Dekker D, Loag W, Amemasor S, Agyekum A, Marks F, Huenger F, Krefis AC, Hagen RM, Adu-Sarkodie Y, May J, Schwarz NG. 2012. Incidence and characteristics of bacteremia among children in rural Ghana. PLoS One 7:e44063. doi: 10.1371/journal.pone.0044063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobsson G, Dashti S, Wahlberg T, Andersson R. 2007. The epidemiology of and risk factors for invasive Staphylococcus aureus infections in western Sweden. Scand J Infect Dis 39:6–13. doi: 10.1080/00365540600810026. [DOI] [PubMed] [Google Scholar]
- 56.Bishara J, Goldberg E, Leibovici L, Samra Z, Shaked H, Mansur N, Paul M. 2012. Healthcare-associated vs. hospital-acquired Staphylococcus aureus bacteremia. Int J Infect Dis 16:e457–e463. doi: 10.1016/j.ijid.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Bassetti M, Trecarichi EM, Mesini A, Spanu T, Giacobbe DR, Rossi M, Shenone E, Pascale GD, Molinari MP, Cauda R, Viscoli C, Tumbarello M. 2012. Risk factors and mortality of healthcare-associated and community-acquired Staphylococcus aureus bacteraemia. Clin Microbiol Infect 18:862–869. doi: 10.1111/j.1469-0691.2011.03679.x. [DOI] [PubMed] [Google Scholar]
- 58.Nickerson EK, Hongsuwan M, Limmathurotsakul D, Wuthiekanun V, Shah KR, Srisomang P, Mahavanakul W, Wacharaprechasgul T, Fowler VG, West TE, Teerawatanasuk N, Becher H, White NJ, Chierakul W, Day NP, Peacock SJ. 2009. Staphylococcus aureus bacteraemia in a tropical setting: patient outcome and impact of antibiotic resistance. PLoS One 4:e4308. doi: 10.1371/journal.pone.0004308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turnidge JD, Kotsanas D, Munckhof W, Roberts S, Bennett CM, Nimmo GR, Coombs GW, Murray RJ, Howden B, Johnson PD, Dowling K. 2009. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust 191:368–373. [DOI] [PubMed] [Google Scholar]
- 60.Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG Jr, Hellmich M, Hopkins S, Kern WV, Llewelyn MJ, Rieg S, Rodriguez-Bano J, Scarborough M, Seifert H, Soriano A, Tilley R, Torok ME, Weiss V, Wilson AP, Thwaites GE, ISAC, INSTINCT, SABG, UKCIRG, and Colleagues . 2014. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 68:242–251. doi: 10.1016/j.jinf.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pastagia M, Kleinman LC, Lacerda de la Cruz EG, Jenkins SG. 2012. Predicting risk for death from MRSA bacteremia. Emerg Infect Dis 18:1072–1080. doi: 10.3201/eid1807.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Isobe M, Uejima E, Seki M, Yamagishi Y, Miyawaki K, Yabuno K, Masaoka M, Hamaguchi S, Yoshioka N, Tomono K. 2012. Methicillin-resistant Staphylococcus aureus bacteremia at a university hospital in Japan. J Infect Chemother 18:841–847. doi: 10.1007/s10156-012-0423-6. [DOI] [PubMed] [Google Scholar]
- 63.Park KH, Kim ES, Kim HS, Park SJ, Bang KM, Park HJ, Park SY, Moon SM, Chong YP, Kim SH, Lee SO, Choi SH, Jeong JY, Kim MN, Woo JH, Kim YS. 2012. Comparison of the clinical features, bacterial genotypes and outcomes of patients with bacteraemia due to heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-susceptible S. aureus. J Antimicrob Chemother 67:1843–1849. doi: 10.1093/jac/dks131. [DOI] [PubMed] [Google Scholar]
- 64.Honda H, Doern CD, Michael-Dunne W Jr, Warren DK. 2011. The impact of vancomycin susceptibility on treatment outcomes among patients with methicillin resistant Staphylococcus aureus bacteremia. BMC Infect Dis 11:335. doi: 10.1186/1471-2334-11-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Hal SJ, Jones M, Gosbell IB, Paterson DL. 2011. Vancomycin heteroresistance is associated with reduced mortality in ST239 methicillin-resistant Staphylococcus aureus blood stream infections. PLoS One 6:e21217. doi: 10.1371/journal.pone.0021217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis T, Chaudhry R, Nightingale P, Lambert P, Das I. 2011. Methicillin-resistant Staphylococcus aureus bacteremia: epidemiology, outcome, and laboratory characteristics in a tertiary referral center in the UK. Int J Infect Dis 15:e131–e135. doi: 10.1016/j.ijid.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. 2009. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997-2007. JAMA 301:727–736. doi: 10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- 68.Tattevin P, Schwartz BS, Graber CJ, Volinski J, Bhukhen A, Bhukhen A, Mai TT, Vo NH, Dang DN, Phan TH, Basuino L, Perdreau-Remington F, Chambers HF, Diep BA. 2012. Concurrent epidemics of skin and soft tissue infection and bloodstream infection due to community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis 55:781–788. doi: 10.1093/cid/cis527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fowler VG Jr, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, Cheng AC, Dudley T, Oddone EZ. 2003. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 163:2066–2072. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]
- 70.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. 2012. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 25:362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skinner D, Keefer CS. 1941. Significance of bacteremia caused by Staphylococcus aureus. Arch Intern Med 68:851–875. doi: 10.1001/archinte.1941.00200110003001. [DOI] [Google Scholar]
- 72.Spink WW, Hall WH. 1945. Penicillin therapy at the University of Minnesota hospitals: 1942-1944. Ann Intern Med 22:510–525. doi: 10.7326/0003-4819-22-4-510. [DOI] [Google Scholar]
- 73.Gould FK, Brindle R, Chadwick PR, Fraise AP, Hill S, Nathwani D, Ridgway GL, Spry MJ, Warren RE, MRSA Working Party of the British Society for Antimicrobial Chemotherapy . 2009. Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother 63:849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 74.Gemmell CG, Edwards DI, Fraise AP, Gould FK, Ridgway GL, Warren RE, Joint Working Party of the British Society for Antimicrobial Chemotherapy, Hospital Infection Society, Infection Control Nurses Association . 2006. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J Antimicrob Chemother 57:589–608. doi: 10.1093/jac/dkl017. [DOI] [PubMed] [Google Scholar]
- 75.Mitchell DH, Howden BP. 2005. Diagnosis and management of Staphylococcus aureus bacteraemia. Intern Med J 35(Suppl 2):S17–S24. doi: 10.1111/j.1444-0903.2005.00977.x. [DOI] [PubMed] [Google Scholar]
- 76.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Ryback MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 77.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Torok ME, Walker S, Wertheim HF, Wilson P, Llewelyn MJ, UK Clinical Infection Research Group . 2011. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis 11:208–222. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 78.Holland TL, Arnold C, Fowler VG Jr. 2014. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 312:1330–1341. doi: 10.1001/jama.2014.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Ryback MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 80.Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. 2009. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore) 88:263–267. doi: 10.1097/MD.0b013e3181b8fccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagao M, Iinuma Y, Saito T, Matsumura Y, Shirano M, Matsushima A, Takakura S, Ito Y, Ichiyama S. 2010. Close cooperation between infectious disease physicians and attending physicians can result in better management and outcome for patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect 16:1783–1788. doi: 10.1111/j.1469-0691.2010.03156.x. [DOI] [PubMed] [Google Scholar]
- 82.Choi SH, Cho SY, Park JH, Chung JW. 2011. Impact of infectious-disease specialist consultations on outcomes of Staphylococcus aureus bacteremia in a hospital with a low volume of patients with S. aureus bacteremia. J Infect 62:181–185. doi: 10.1016/j.jinf.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Robinson JO, Pozzi-Langhi S, Phillips M, Pearson JC, Christiansen KJ, Coombs GW, Murray RJ. 2012. Formal infectious diseases consultation is associated with decreased mortality in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 31:2421–2428. doi: 10.1007/s10096-012-1585-y. [DOI] [PubMed] [Google Scholar]
- 84.Fries BL, Licitra C, Crespo A, Akhter K, Busowski MT, Salazar D, Wallace MR. 2014. Infectious diseases consultation and the management of Staphylococcus aureus bacteremia. Clin Infect Dis 58:598–599. doi: 10.1093/cid/cit730. [DOI] [PubMed] [Google Scholar]
- 85.Tissot F, Calandra T, Prod'hom G, Taffe P, Zanetti G, Greub G, Senn L. 2014. Mandatory infectious diseases consultation for MRSA bacteremia is associated with reduced mortality. J Infect 69:226–234. doi: 10.1016/j.jinf.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Jenkins TC, Price CS, Sabel AL, Mehler PS, Burman WJ. 2008. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis 46:1000–1008. doi: 10.1086/529190. [DOI] [PubMed] [Google Scholar]
- 87.Rieg S, Peyerl-Hoffmann G, de With K, Theilacker C, Wagner D, Hubner J, Dettenkofer M, Kaasch A, Seifert H, Schneider C, Kern WV. 2009. Mortality of S. aureus bacteremia and infectious diseases specialist consultation—a study of 521 patients in Germany. J Infect 59:232–239. doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 88.Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. 2010. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 123:631–637. doi: 10.1016/j.amjmed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pragman AA, Kuskowski MA, Abraham JM, Filice GA. 2012. Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract (Baltim Md) 20:261–267. doi: 10.1097/IPC.0b013e318255d67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forsblom E, Ruotsalainen E, Ollgren J, Jarvinen A. 2013. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 56:527–535. doi: 10.1093/cid/cis889. [DOI] [PubMed] [Google Scholar]
- 91.Fowler VG Jr, Li J, Corey GR, Boley J, Marr KA, Gopal AK, Kong LK, Gottlieb G, Donovan CL, Sexton DJ, Ryan T. 1997. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol 30:1072–1078. doi: 10.1016/S0735-1097(97)00250-7. [DOI] [PubMed] [Google Scholar]
- 92.Van Hal SJ, Mathur G, Kelly J, Aronis C, Cranney GB, Jones PD. 2005. The role of transthoracic echocardiography in excluding left sided infective endocarditis in Staphylococcus aureus bacteraemia. J Infect 51:218–221. doi: 10.1016/j.jinf.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 93.Khatib R, Sharma M. 2013. Echocardiography is dispensable in uncomplicated Staphylococcus aureus bacteremia. Medicine 92:182–188. doi: 10.1097/MD.0b013e318294a710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sullenberger AL, Avedissian LS, Kent SM. 2005. Importance of transesophageal echocardiography in the evaluation of Staphylococcus aureus bacteremia. J Heart Valve Dis 14:23–28. [PubMed] [Google Scholar]
- 95.Khandheria BK, Seward JB, Tajik AJ. 1994. Transesophageal echocardiography. Mayo Clin Proc 69:856–863. doi: 10.1016/S0025-6196(12)61788-1. [DOI] [PubMed] [Google Scholar]
- 96.Joseph JP, Meddows TR, Webster DP, Newton JD, Myerson SG, Prendergast B, Scarborough M, Herring N. 2013. Prioritizing echocardiography in Staphylococcus aureus bacteraemia. J Antimicrob Chemother 68:444–449. doi: 10.1093/jac/dks408. [DOI] [PubMed] [Google Scholar]
- 97.Rasmussen RV, Host U, Arpi M, Hassager C, Johansen HK, Korup E, Schonheyder HC, Berning J, Gill S, Rosenvinge FS, Fowler VG Jr, Moller JE, Skov RL, Larsen CT, Hansen TF, Mard S, Smit J, Andersen PS, Bruun NE. 2011. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 12:414–420. doi: 10.1093/ejechocard/jer023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaasch AJ, Fowler VG Jr, Rieg S, Peyerl-Hoffmann G, Birkholz H, Hellmich M, Kern WV, Seifert H. 2011. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 53:1–9. doi: 10.1093/cid/cir320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chong YP, Moon SM, Bang KM, Park HJ, Park SY, Kim MN, Park KH, Kim SH, Lee SO, Choi SH, Jeong JY, Woo JH, Kim YS. 2013. Treatment duration for uncomplicated Staphylococcus aureus bacteremia to prevent relapse: analysis of a prospective observational cohort study. Antimicrob Agents Chemother 57:1150–1156. doi: 10.1128/AAC.01021-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jernigan JA, Farr BM. 1993. Short-course therapy of catheter-related Staphylococcus aureus bacteremia: a meta-analysis. Ann Intern Med 119:304–311. doi: 10.7326/0003-4819-119-4-199308150-00010. [DOI] [PubMed] [Google Scholar]
- 101.Chambers HF, Miller RT, Newman MD. 1988. Right-sided Staphylococcus aureus endocarditis in intravenous drug abusers: two-week combination therapy. Ann Intern Med 109:619–624. doi: 10.7326/0003-4819-109-8-619. [DOI] [PubMed] [Google Scholar]
- 102.Ribera E, Gomez-Jimenez J, Cortes E, del Valle O, Planes A, Gonzalez-Alujas T, Almirante B, Ocana I, Pahissa A. 1996. Effectiveness of cloxacillin with and without gentamicin in short-term therapy for right-sided Staphylococcus aureus endocarditis. A randomized, controlled trial. Ann Intern Med 125:969–974. [DOI] [PubMed] [Google Scholar]
- 103.Torres-Tortosa M, de Cueto M, Vergara A, Sanchez-Porto A, Perez-Guzman E, Gonzalez-Serrano M, Canueto J. 1994. Prospective evaluation of a two-week course of intravenous antibiotics in intravenous drug addicts with infective endocarditis. Eur J Clin Microbiol Infect Dis 13:559–564. doi: 10.1007/BF01971306. [DOI] [PubMed] [Google Scholar]
- 104.Fortun J, Perez-Molina JA, Anon MT, Martinez-Beltran J, Loza E, Guerrero A. 1995. Right-sided endocarditis caused by Staphylococcus aureus in drug abusers. Antimicrob Agents Chemother 39:525–528. doi: 10.1128/AAC.39.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fortun J, Navas E, Martinez-Beltran J, Perez-Molina J, Martin-Davila P, Guerrero A, Moreno S. 2001. Short-course therapy for right-side endocarditis due to Staphylococcus aureus in drug abusers: cloxacillin versus glycopeptides in combination with gentamicin. Clin Infect Dis 33:120–125. doi: 10.1086/320869. [DOI] [PubMed] [Google Scholar]
- 106.Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW, Working Party of the British Society for Antimicrobial Chemotherapy . 2012. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 67:269–289. doi: 10.1093/jac/dkr450. [DOI] [PubMed] [Google Scholar]
- 107.Calain P, Krause KH, Vaudaux P, Auckenthaler R, Lew D, Waldvogel F, Hirschel B. 1987. Early termination of a prospective, randomized trial comparing teicoplanin and flucloxacillin for treating severe staphylococcal infections. J Infect Dis 155:187–191. doi: 10.1093/infdis/155.2.187. [DOI] [PubMed] [Google Scholar]
- 108.Degener JE, Vogel M, Michel MF, Mutsaers MM, Hop WC. 1989. The efficacy of the combination of teicoplanin or flucloxacillin with netilmicin in the treatment of Staphylococcus aureus bacteraemia. J Antimicrob Chemother 23:899–904. doi: 10.1093/jac/23.6.899. [DOI] [PubMed] [Google Scholar]
- 109.Khatib R, Johnson LB, Sharma M, Fakih MG, Ganga R, Riederer K. 2009. Persistent Staphylococcus aureus bacteremia: incidence and outcome trends over time. Scand J Infect Dis 41:4–9. doi: 10.1080/00365540802441711. [DOI] [PubMed] [Google Scholar]
- 110.Walker TM, Bowler IC, Bejon P. 2009. Risk factors for recurrence after Staphylococcus aureus bacteraemia. A retrospective matched case-control study. J Infect 58:411–416. doi: 10.1016/j.jinf.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 111.Lodise TP Jr, McKinnon PS, Levine DP, Rybak MJ. 2007. Impact of empirical-therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 51:3731–3733. doi: 10.1128/AAC.00101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khatib R, Johnson LB, Fakih MG, Riederer K, Khosrovaneh A, Shamse Tabriz M, Sharma M, Saeed S. 2006. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis 38:7–14. doi: 10.1080/00365540500372846. [DOI] [PubMed] [Google Scholar]
- 113.Siegman-Igra Y, Reich P, Orni-Wasserlauf R, Schwartz D, Giladi M. 2005. The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia. Scand J Infect Dis 37:572–578. doi: 10.1080/00365540510038488. [DOI] [PubMed] [Google Scholar]
- 114.Chang FY, Peacock JE Jr, Musher DM, Triplett P, MacDonald BB, Mylotte JM, O'Donnell A, Wagener MM, Yu VL. 2003. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 82:333–339. doi: 10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 115.Stryjewski ME, Szczech LA, Benjamin DK Jr, Inrig JK, Kanafani ZA, Engemann JJ, Chu VH, Joyce MJ, Reller LB, Corey GR, Fowler VG Jr. 2007. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 44:190–196. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 116.Kim SH, Kim KH, Kim HB, Kim NJ, Kim EC, Oh MD, Choe KW. 2008. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 52:192–197. doi: 10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Small PM, Chambers HF. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 34:1227–1231. doi: 10.1128/AAC.34.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC, Thom KA, Cosgrove SE, Sakoulas G, Perencevich EN. 2011. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis 11:279. doi: 10.1186/1471-2334-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fowler VG Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fatkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 355:653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 120.Menichetti F, Martino P, Bucaneve G, Gentile G, D'Antonio D, Liso V, Ricci P, Nosari AM, Buelli M, Carotenuto M, Fasola G, Jacopino P, Montillo M, Barbabietola G, Girmenia C, Del Favero A, Gimema Infection Program . 1994. Effects of teicoplanin and those of vancomycin in initial empirical antibiotic regimen for febrile, neutropenic patients with hematologic malignancies. Antimicrob Agents Chemother 38:2041–2046. doi: 10.1128/AAC.38.9.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Markowitz N, Quinn EL, Saravolatz LD. 1992. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med 117:390–398. doi: 10.7326/0003-4819-117-5-390. [DOI] [PubMed] [Google Scholar]
- 122.Wilcox MH, Tack KJ, Bouza E, Herr DL, Ruf BR, Ijzerman MM, Croos-Dabrera RV, Kunkel MJ, Knirsch C. 2009. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 48:203–212. doi: 10.1086/595686. [DOI] [PubMed] [Google Scholar]
- 123.Shorr AF, Kunkel MJ, Kollef M. 2005. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother 56:923–929. doi: 10.1093/jac/dki355. [DOI] [PubMed] [Google Scholar]
- 124.Raad I, Darouiche R, Vazquez J, Lentnek A, Hachem R, Hanna H, Goldstein B, Henkel T, Seltzer E. 2005. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 40:374–380. doi: 10.1086/427283. [DOI] [PubMed] [Google Scholar]
- 125.Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 126.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duval X, Delahaye F, Alla F, Tattevin P, Obadia JF, Le Moing V, Doco-Lecompte T, Celard M, Poyart C, Strady C, Chirouze C, Bes M, Cambau E, Iung B, Selton-Suty C, Hoen B, AEPEI Study Group . 2012. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol 59:1968–1976. doi: 10.1016/j.jacc.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 128.Fedeli U, Schievano E, Buonfrate D, Pellizzer G, Spolaore P. 2011. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis 11:48. doi: 10.1186/1471-2334-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sy RW, Kritharides L. 2010. Health care exposure and age in infective endocarditis: results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J 31:1890–1897. doi: 10.1093/eurheartj/ehq110. [DOI] [PubMed] [Google Scholar]
- 130.Tleyjeh IM, Abdel-Latif A, Rahbi H, Scott CG, Bailey KR, Steckelberg JM, Wilson WR, Baddour LM. 2007. A systematic review of population-based studies of infective endocarditis. Chest 132:1025–1035. doi: 10.1378/chest.06-2048. [DOI] [PubMed] [Google Scholar]
- 131.Federspiel JJ, Stearns SC, Peppercorn AF, Chu VH, Fowler VG Jr. 2012. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med 172:363–365. doi: 10.1001/archinternmed.2011.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, Raoult D, Bayer A, Fowler VG Jr. 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis merged database. Clin Infect Dis 41:507–514. doi: 10.1086/431979. [DOI] [PubMed] [Google Scholar]
- 133.Benito N, Miro JM, de Lazzari E, Cabell CH, del Rio A, Altclas J, Commerford P, Delahaye F, Dragulescu S, Giamarellou H, Habib G, Kamarulzaman A, Kumar AS, Nacinovich FM, Suter F, Tribouilloy C, Venugopal K, Moreno A, Fowler VG Jr, ICE-PCS Investigators . 2009. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Intern Med 150:586–594. doi: 10.7326/0003-4819-150-9-200905050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chu VH, Cabell CH, Benjamin DK Jr, Kuniholm EF, Fowler VG Jr, Engemann J, Sexton DJ, Corey GR, Wang A. 2004. Early predictors of in-hospital death in infective endocarditis. Circulation 109:1745–1749. doi: 10.1161/01.CIR.0000124719.61827.7F. [DOI] [PubMed] [Google Scholar]
- 135.Thuny F, Di Salvo G, Belliard O, Avierinos JF, Pergola V, Rosenberg V, Casalta JP, Gouvernet J, Derumeaux G, Iarussi D, Ambrosi P, Calabro R, Riberi A, Collart F, Metras D, Lepidi H, Raoult D, Harle JR, Weiller PJ, Cohen A, Habib G. 2005. Risk of embolism and death in infective endocarditis. Prognostic value of echocardiography: a prospective multicenter study. Circulation 112:69–75. doi: 10.1161/CIRCULATIONAHA.104.493155. [DOI] [PubMed] [Google Scholar]
- 136.Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. 2013. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One 8:e60033. doi: 10.1371/journal.pone.0060033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kanafani ZA, Mahfouz TH, Kanj SS. 2002. Infective endocarditis at a tertiary care centre in Lebanon: predominance of streptococcal infection. J Infect 45:152–159. doi: 10.1053/jinf.2002.1041. [DOI] [PubMed] [Google Scholar]
- 138.Ikama MS, Nkalla-Lambi M, Kimbally-Kaky G, Loumouamou ML, Nkoua JL. 2013. Profile of infective endocarditis at Brazzaville University Hospital. Med Sante Trop 23:89–92. doi: 10.1684/mst.2013.0151. [DOI] [PubMed] [Google Scholar]
- 139.Yameogo NV, Kologo KJ, Yameogo AA, Yonaba C, Millogo GR, Kissou SA, Toguyeni BJ, Samadoulougou AK, Pignatelli S, Simpore J, Zabsonre P. 2014. Infective endocarditis in sub-Saharan African children, cross-sectional study about 19 cases in Ouagadougou at Burkina Faso. Ann Cardiol Angeiol (Paris) 63:7–10. doi: 10.1016/j.ancard.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 140.Trabelsi I, Rekik S, Znazen A, Maaloul I, Abid D, Maalej A, Kharrat I, Ben Jemaa M, Hammemi A, Kammoun S. 2008. Native valve infective endocarditis in a tertiary care center in a developing country (Tunisia). Am J Cardiol 102:1247–1251. doi: 10.1016/j.amjcard.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 141.Letaief A, Boughzala E, Kaabia N, Ernez S, Abid F, Ben Chaabane T, Ben Jemaa M, Boujnah R, Chakroun M, Daoud M, Gaha R, Kafsi N, Khalfallah A, Slimane L, Zaouali M. 2007. Epidemiology of infective endocarditis in Tunisia: a 10-year multicenter retrospective study. Int J Infect Dis 11:430–433. doi: 10.1016/j.ijid.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 142.Selton-Suty C, Celard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, Delahaye F, Alla F, Duval X, Hoen B, AEPEI Study Group . 2012. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 54:1230–1239. doi: 10.1093/cid/cis199. [DOI] [PubMed] [Google Scholar]
- 143.Fowler VG Jr, Sanders LL, Kong LK, McClelland RS, Gottlieb GS, Li J, Ryan T, Sexton DJ, Roussakis G, Harrell LJ, Corey GR. 1999. Infective endocarditis due to Staphylococcus aureus: 59 prospectively identified cases with follow-up. Clin Infect Dis 28:106–114. doi: 10.1086/515076. [DOI] [PubMed] [Google Scholar]
- 144.Cabell CH, Abrutyn E. 2002. Progress toward a global understanding of infective endocarditis. Early lessons from the International Collaboration on Endocarditis investigation. Infect Dis Clin North Am 16:255–272. doi: 10.1016/S0891-5520(01)00007-1. [DOI] [PubMed] [Google Scholar]
- 145.Thayer WS. 1931. Bacterial or infective endocarditis: Gibson lectures for 1930. Edinb Med J 38:237–265. [PMC free article] [PubMed] [Google Scholar]
- 146.Fernandez Guerrero ML, Gonzalez Lopez JJ, Goyenechea A, Fraile J, de Gorgolas M. 2009. Endocarditis caused by Staphylococcus aureus: a reappraisal of the epidemiologic, clinical, and pathologic manifestations with analysis of factors determining outcome. Medicine (Baltimore) 88:1–22. doi: 10.1097/MD.0b013e318194da65. [DOI] [PubMed] [Google Scholar]
- 147.Grover FL, Cohen DJ, Oprian C, Henderson WG, Sethi G, Hammermeister KE. 1994. Determinants of the occurrence of and survival from prosthetic valve endocarditis. Experience of the Veterans Affairs Cooperative Study on Valvular Heart Disease. J Thorac Cardiovasc Surg 108:207–214. [PubMed] [Google Scholar]
- 148.Ivert TS, Dismukes WE, Cobbs CG, Blackstone EH, Kirklin JW, Bergdahl LA. 1984. Prosthetic valve endocarditis. Circulation 69:223–232. doi: 10.1161/01.CIR.69.2.223. [DOI] [PubMed] [Google Scholar]
- 149.Calderwood SB, Swinski LA, Waternaux CM, Karchmer AW, Buckley MJ. 1985. Risk factors for the development of prosthetic valve endocarditis. Circulation 72:31–37. doi: 10.1161/01.CIR.72.1.31. [DOI] [PubMed] [Google Scholar]
- 150.Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Pare C, Almirante B, Munoz P, Rizzi M, Naber C, Logar M, Tattevin P, Iarussi DL, Selton-Suty C, Jones SB, Casabe J, Morris A, Corey GR, Cabell CH, International Collaboration on Endocarditis-Prospective Cohort Study Investigators . 2007. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 297:1354–1361. doi: 10.1001/jama.297.12.1354. [DOI] [PubMed] [Google Scholar]
- 151.Lalani T, Chu VH, Park LP, Cecchi E, Corey GR, Durante-Mangoni E, Fowler VG Jr, Gordon D, Grossi P, Hannan M, Hoen B, Munoz P, Rizk H, Kanj SS, Selton-Suty C, Sexton DJ, Spelman D, Ravasio V, Tripodi MF, Wang A, International Collaboration on Endocarditis-Prospective Cohort Study Investigators . 2013. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 173:1495–1504. doi: 10.1001/jamainternmed.2013.8203. [DOI] [PubMed] [Google Scholar]
- 152.Wolff M, Witchitz S, Chastang C, Regnier B, Vachon F. 1995. Prosthetic valve endocarditis in the ICU. Prognostic factors of overall survival in a series of 122 cases and consequences for treatment decision. Chest 108:688–694. [DOI] [PubMed] [Google Scholar]
- 153.El-Ahdab F, Benjamin DK Jr, Wang A, Cabell CH, Chu VH, Stryjewski ME, Corey GR, Sexton DJ, Reller LB, Fowler VG Jr. 2005. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med 118:225–229. doi: 10.1016/j.amjmed.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 154.Fang G, Keys TF, Gentry LO, Harris AA, Rivera N, Getz K, Fuchs PC, Gustafson M, Wong ES, Goetz A, Wagener MM, Yu VL. 1993. Prosthetic valve endocarditis resulting from nosocomial bacteremia. A prospective, multicenter study. Ann Intern Med 119:560–567. [DOI] [PubMed] [Google Scholar]
- 155.Que YA, Moreillon P. 2011. Infective endocarditis. Nat Rev Cardiol 8:322–336. doi: 10.1038/nrcardio.2011.43. [DOI] [PubMed] [Google Scholar]
- 156.Patti JM, Allen BL, McGavin MJ, Hook M. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol 48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 157.Que YA, Haefliger JA, Piroth L, Francois P, Widmer E, Entenza JM, Sinha B, Herrmann M, Francioli P, Vaudaux P, Moreillon P. 2005. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med 201:1627–1635. doi: 10.1084/jem.20050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Piroth L, Que YA, Widmer E, Panchaud A, Piu S, Entenza JM, Moreillon P. 2008. The fibrinogen- and fibronectin-binding domains of Staphylococcus aureus fibronectin-binding protein A synergistically promote endothelial invasion and experimental endocarditis. Infect Immun 76:3824–3831. doi: 10.1128/IAI.00405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fitzgerald JR, Loughman A, Keane F, Brennan M, Knobel M, Higgins J, Visai L, Speziale P, Cox D, Foster TJ. 2006. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol Microbiol 59:212–230. doi: 10.1111/j.1365-2958.2005.04922.x. [DOI] [PubMed] [Google Scholar]
- 160.O'Brien L, Kerrigan SW, Kaw G, Hogan M, Penades J, Litt D, Fitzgerald DJ, Foster TJ, Cox D. 2002. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol Microbiol 44:1033–1044. doi: 10.1046/j.1365-2958.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 161.Veloso TR, Chaouch A, Roger T, Giddey M, Vouillamoz J, Majcherczyk P, Que YA, Rousson V, Moreillon P, Entenza JM. 2013. Use of a human-like low-grade bacteremia model of experimental endocarditis to study the role of Staphylococcus aureus adhesins and platelet aggregation in early endocarditis. Infect Immun 81:697–703. doi: 10.1128/IAI.01030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Que YA, Francois P, Haefliger JA, Entenza JM, Vaudaux P, Moreillon P. 2001. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect Immun 69:6296–6302. doi: 10.1128/IAI.69.10.6296-6302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pappelbaum KI, Gorzelanny C, Grassle S, Suckau J, Laschke MW, Bischoff M, Bauer C, Schorpp-Kistner M, Weidenmaier C, Schneppenheim R, Obser T, Sinha B, Schneider SW. 2013. Ultralarge von Willebrand factor fibers mediate luminal Staphylococcus aureus adhesion to an intact endothelial cell layer under shear stress. Circulation 128:50–59. doi: 10.1161/CIRCULATIONAHA.113.002008. [DOI] [PubMed] [Google Scholar]
- 164.Vanassche T, Kauskot A, Verhaegen J, Peetermans WE, van Ryn J, Schneewind O, Hoylaerts MF, Verhamme P. 2012. Fibrin formation by staphylothrombin facilitates Staphylococcus aureus-induced platelet aggregation. Thromb Haemost 107:1107–1121. doi: 10.1160/TH11-12-0891. [DOI] [PubMed] [Google Scholar]
- 165.Salgado-Pabon W, Breshears L, Spaulding AR, Merriman JA, Stach CS, Horswill AR, Peterson ML, Schlievert PM. 2013. Superantigens are critical for Staphylococcus aureus infective endocarditis, sepsis, and acute kidney injury. mBio 4(4):e00494-13. doi: 10.1128/mBio.00494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fowler VG Jr, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, Federspiel J, Naidich S, Remortel B, Rude T, Brown P, Reller LB, Corey GR, Gill SR. 2007. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis 196:738–747. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- 167.Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, Barriere S, Woods CW, Chu VH, Marin M, Bukovski S, Garcia P, Corey GR, Korman T, Doco-Lecompte T, Murdoch DR, Reller LB, Fowler VG Jr. 2011. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis 204:704–713. doi: 10.1093/infdis/jir389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miller CE, Batra R, Cooper BS, Patel AK, Klein J, Otter JA, Kypraios T, French GL, Tosas O, Edgeworth JD. 2012. An association between bacterial genotype combined with a high-vancomycin minimum inhibitory concentration and risk of endocarditis in methicillin-resistant Staphylococcus aureus bloodstream infection. Clin Infect Dis 54:591–600. doi: 10.1093/cid/cir858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Spaulding AR, Satterwhite EA, Lin YC, Chuang-Smith ON, Frank KL, Merriman JA, Schaefers MM, Yarwood JM, Peterson ML, Schlievert PM. 2012. Comparison of Staphylococcus aureus strains for ability to cause infective endocarditis and lethal sepsis in rabbits. Front Cell Infect Microbiol 2:18. doi: 10.3389/fcimb.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Roder BL, Wandall DA, Frimodt-Moller N, Espersen F, Skinhoj P, Rosdahl VT. 1999. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch Intern Med 159:462–469. doi: 10.1001/archinte.159.5.462. [DOI] [PubMed] [Google Scholar]
- 171.Nadji G, Remadi JP, Coviaux F, Mirode AA, Brahim A, Enriquez-Sarano M, Tribouilloy C. 2005. Comparison of clinical and morphological characteristics of Staphylococcus aureus endocarditis with endocarditis caused by other pathogens. Heart 91:932–937. doi: 10.1136/hrt.2004.042648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rogers BA, Drake AK, Spelman D. 2009. Methicillin resistant Staphylococcus aureus endocarditis in an Australian tertiary hospital: 1991-2006. Heart Lung Circ 18:208–213. doi: 10.1016/j.hlc.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 173.Ruotsalainen E, Sammalkorpi K, Laine J, Huotari K, Sarna S, Valtonen V, Jarvinen A. 2006. Clinical manifestations and outcome in Staphylococcus aureus endocarditis among injection drug users and nonaddicts: a prospective study of 74 patients. BMC Infect Dis 6:137. doi: 10.1186/1471-2334-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Slaughter L, Morris JE, Starr A. 1973. Prosthetic valvular endocarditis. A 12-year review. Circulation 47:1319–1326. [DOI] [PubMed] [Google Scholar]
- 175.Ben Ismail M, Hannachi N, Abid F, Kaabar Z, Rouge JF. 1987. Prosthetic valve endocarditis. A survey. Br Heart J 58:72–77. doi: 10.1136/hrt.58.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tornos P, Almirante B, Olona M, Permanyer G, Gonzalez T, Carballo J, Pahissa A, Soler-Soler J. 1997. Clinical outcome and long-term prognosis of late prosthetic valve endocarditis: a 20-year experience. Clin Infect Dis 24:381–386. doi: 10.1093/clinids/24.3.381. [DOI] [PubMed] [Google Scholar]
- 177.Sohail MR, Martin KR, Wilson WR, Baddour LM, Harmsen WS, Steckelberg JM. 2006. Medical versus surgical management of Staphylococcus aureus prosthetic valve endocarditis. Am J Med 119:147–154. doi: 10.1016/j.amjmed.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 178.Fournier PE, Casalta JP, Habib G, Messana T, Raoult D. 1996. Modification of the diagnostic criteria proposed by the Duke Endocarditis Service to permit improved diagnosis of Q fever endocarditis. Am J Med 100:629–633. doi: 10.1016/S0002-9343(96)00040-X. [DOI] [PubMed] [Google Scholar]
- 179.Goldenberger D, Kunzli A, Vogt P, Zbinden R, Altwegg M. 1997. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol 35:2733–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Morel AS, Dubourg G, Prudent E, Edouard S, Gouriet F, Casalta JP, Fenollar F, Fournier PE, Drancourt M, Raoult D. 2015. Complementarity between targeted real-time specific PCR and conventional broad-range 16S rDNA PCR in the syndrome-driven diagnosis of infectious diseases. Eur J Clin Microbiol Infect Dis 34:561–570. doi: 10.1007/s10096-014-2263-z. [DOI] [PubMed] [Google Scholar]
- 181.Harris KA, Yam T, Jalili S, Williams OM, Alshafi K, Gouliouris T, Munthali P, NiRiain U, Hartley JC. 2014. Service evaluation to establish the sensitivity, specificity and additional value of broad-range 16S rDNA PCR for the diagnosis of infective endocarditis from resected endocardial material in patients from eight UK and Ireland hospitals. Eur J Clin Microbiol Infect Dis 33:2061–2066. doi: 10.1007/s10096-014-2145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chirouze C, Cabell CH, Fowler VG Jr, Khayat N, Olaison L, Miro JM, Habib G, Abrutyn E, Eykyn S, Corey GR, Selton-Suty C, Hoen B, International Collaboration on Endocarditis Study Group . 2004. Prognostic factors in 61 cases of Staphylococcus aureus prosthetic valve infective endocarditis from the International Collaboration on Endocarditis merged database. Clin Infect Dis 38:1323–1327. doi: 10.1086/383035. [DOI] [PubMed] [Google Scholar]
- 183.John MD, Hibberd PL, Karchmer AW, Sleeper LA, Calderwood SB. 1998. Staphylococcus aureus prosthetic valve endocarditis: optimal management and risk factors for death. Clin Infect Dis 26:1302–1309. doi: 10.1086/516378. [DOI] [PubMed] [Google Scholar]
- 184.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J, Lengyel M, Muller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL, ESC Committee for Practice Guidelines . 2009. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 30:2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 185.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA, Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association, Infectious Diseases Society of America . 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 186.Dohmen PM, Guleri A, Capone A, Utili R, Seaton RA, Gonzalez-Ramallo VJ, Pathan R, Heep M, Chaves RL. 2013. Daptomycin for the treatment of infective endocarditis: results from a European registry. J Antimicrob Chemother 68:936–942. doi: 10.1093/jac/dks467. [DOI] [PubMed] [Google Scholar]
- 187.Kullar R, Casapao AM, Davis SL, Levine DP, Zhao JJ, Crank CW, Segreti J, Sakoulas G, Cosgrove SE, Rybak MJ. 2013. A multicentre evaluation of the effectiveness and safety of high-dose daptomycin for the treatment of infective endocarditis. J Antimicrob Chemother 68:2921–2926. doi: 10.1093/jac/dkt294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carugati M, Bayer AS, Miro JM, Park LP, Guimaraes AC, Skoutelis A, Fortes CQ, Durante-Mangoni E, Hannan MM, Nacinovich F, Fernandez-Hidalgo N, Grossi P, Tan RS, Holland T, Fowler VG Jr, Corey RG, Chu VH, International Collaboration on Endocarditis . 2013. High-dose daptomycin therapy for left-sided infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Antimicrob Agents Chemother 57:6213–6222. doi: 10.1128/AAC.01563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sharma M, Riederer K, Chase P, Khatib R. 2008. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 27:433–437. doi: 10.1007/s10096-007-0455-5. [DOI] [PubMed] [Google Scholar]
- 190.Gasch O, Camoez M, Dominguez MA, Padilla B, Pintado V, Almirante B, Martin C, Lopez-Medrano F, de Gopegui ER, Blanco JR, Garcia-Pardo G, Calbo E, Montero M, Granados A, Jover A, Duenas C, Pujol M, REIPI/GEIH Study Groups . 2014. Emergence of resistance to daptomycin in a cohort of patients with methicillin-resistant Staphylococcus aureus persistent bacteraemia treated with daptomycin. J Antimicrob Chemother 69:568–571. doi: 10.1093/jac/dkt396. [DOI] [PubMed] [Google Scholar]
- 191.Rose WE, Leonard SN, Rybak MJ. 2008. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 52:3061–3067. doi: 10.1128/AAC.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Baltch AL, Ritz WJ, Bopp LH, Michelsen P, Smith RP. 2008. Activities of daptomycin and comparative antimicrobials, singly and in combination, against extracellular and intracellular Staphylococcus aureus and its stable small-colony variant in human monocyte-derived macrophages and in broth. Antimicrob Agents Chemother 52:1829–1833. doi: 10.1128/AAC.01480-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Baltch AL, Ritz WJ, Bopp LH, Michelsen PB, Smith RP. 2007. Antimicrobial activities of daptomycin, vancomycin, and oxacillin in human monocytes and of daptomycin in combination with gentamicin and/or rifampin in human monocytes and in broth against Staphylococcus aureus. Antimicrob Agents Chemother 51:1559–1562. doi: 10.1128/AAC.00973-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Credito K, Lin G, Appelbaum PC. 2007. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob Agents Chemother 51:1504–1507. doi: 10.1128/AAC.01455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Entenza JM, Giddey M, Vouillamoz J, Moreillon P. 2010. In vitro prevention of the emergence of daptomycin resistance in Staphylococcus aureus and enterococci following combination with amoxicillin/clavulanic acid or ampicillin. Int J Antimicrob Agents 35:451–456. doi: 10.1016/j.ijantimicag.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 196.LaPlante KL, Woodmansee S. 2009. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob Agents Chemother 53:3880–3886. doi: 10.1128/AAC.00134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tsuji BT, Rybak MJ. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 49:2735–2745. doi: 10.1128/AAC.49.7.2735-2745.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miro JM, Garcia-de-la-Maria C, Armero Y, Soy D, Moreno A, del Rio A, Almela M, Sarasa M, Mestres CA, Gatell JM, Jimenez de Anta MT, Marco F, Hospital Clinic Experimental Endocarditis Study Group . 2009. Addition of gentamicin or rifampin does not enhance the effectiveness of daptomycin in treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 53:4172–4177. doi: 10.1128/AAC.00051-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Leonard SN, Rolek KM. 2013. Evaluation of the combination of daptomycin and nafcillin against vancomycin-intermediate Staphylococcus aureus. J Antimicrob Chemother 68:644–647. doi: 10.1093/jac/dks453. [DOI] [PubMed] [Google Scholar]
- 200.Mehta S, Singh C, Plata KB, Chanda PK, Paul A, Riosa S, Rosato RR, Rosato AE. 2012. Beta-lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 56:6192–6200. doi: 10.1128/AAC.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Steed ME, Vidaillac C, Rybak MJ. 2010. Novel daptomycin combinations against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro model of simulated endocardial vegetations. Antimicrob Agents Chemother 54:5187–5192. doi: 10.1128/AAC.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rose WE, Schulz LT, Andes D, Striker R, Berti AD, Hutson PR, Shukla SK. 2012. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother 56:5296–5302. doi: 10.1128/AAC.00797-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Werth BJ, Sakoulas G, Rose WE, Pogliano J, Tewhey R, Rybak MJ. 2013. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 57:66–73. doi: 10.1128/AAC.01586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob Agents Chemother 54:3161–3169. doi: 10.1128/AAC.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rose WE, Berti AD, Hatch JB, Maki DG. 2013. Relationship of in vitro synergy and treatment outcome with daptomycin plus rifampin in patients with invasive methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother 57:3450–3452. doi: 10.1128/AAC.00325-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Di Carlo P, D'Alessandro N, Guadagnino G, Bonura C, Mammina C, Lunetta M, Novo S, Giarratano A. 2013. High dose of trimethoprim-sulfamethoxazole and daptomycin as a therapeutic option for MRSA endocarditis with large vegetation complicated by embolic stroke: a case report and literature review. Infez Med 21:45–49. [PubMed] [Google Scholar]
- 207.Avery LM, Steed ME, Woodruff AE, Hasan M, Rybak MJ. 2012. Daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus vertebral osteomyelitis cases complicated by bacteremia treated with high-dose daptomycin and trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother 56:5990–5993. doi: 10.1128/AAC.01046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Miro JM, Entenza JM, Del Rio A, Velasco M, Castaneda X, Garcia de la Maria C, Giddey M, Armero Y, Pericas JM, Cervera C, Mestres CA, Almela M, Falces C, Marco F, Moreillon P, Moreno A, Hospital Clinic Experimental Endocarditis Study Group . 2012. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 56:4511–4515. doi: 10.1128/AAC.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen LY, Huang CH, Kuo SC, Hsiao CY, Lin ML, Wang FD, Fung CP. 2011. High-dose daptomycin and fosfomycin treatment of a patient with endocarditis caused by daptomycin-nonsusceptible Staphylococcus aureus: case report. BMC Infect Dis 11:152. doi: 10.1186/1471-2334-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis 53:158–163. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moise PA, Amodio-Groton M, Rashid M, Lamp KC, Hoffman-Roberts HL, Sakoulas G, Yoon MJ, Schweitzer S, Rastogi A. 2013. Multicenter evaluation of the clinical outcomes of daptomycin with and without concomitant beta-lactams in patients with Staphylococcus aureus bacteremia and mild to moderate renal impairment. Antimicrob Agents Chemother 57:1192–1200. doi: 10.1128/AAC.02192-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Moise PA, North D, Steenbergen JN, Sakoulas G. 2009. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect Dis 9:617–624. doi: 10.1016/S1473-3099(09)70200-2. [DOI] [PubMed] [Google Scholar]
- 213.Karchmer AW, Archer GL, Dismukes WE. 1983. Rifampin treatment of prosthetic valve endocarditis due to Staphylococcus epidermidis. Rev Infect Dis 5(Suppl 3):S543–S548. [DOI] [PubMed] [Google Scholar]
- 214.Karchmer AW, Archer GL, Dismukes WE. 1983. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med 98:447–455. doi: 10.7326/0003-4819-98-4-447. [DOI] [PubMed] [Google Scholar]
- 215.Riedel DJ, Weekes E, Forrest GN. 2008. Addition of rifampin to standard therapy for treatment of native valve infective endocarditis caused by Staphylococcus aureus. Antimicrob Agents Chemother 52:2463–2467. doi: 10.1128/AAC.00300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cosgrove SE, Vigliani GA, Fowler VG Jr, Abrutyn E, Corey GR, Levine DP, Rupp ME, Chambers HF, Karchmer AW, Boucher HW. 2009. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 48:713–721. doi: 10.1086/597031. [DOI] [PubMed] [Google Scholar]
- 217.Aksoy O, Sexton DJ, Wang A, Pappas PA, Kourany W, Chu V, Fowler VG Jr, Woods CW, Engemann JJ, Corey GR, Harding T, Cabell CH. 2007. Early surgery in patients with infective endocarditis: a propensity score analysis. Clin Infect Dis 44:364–372. doi: 10.1086/510583. [DOI] [PubMed] [Google Scholar]
- 218.Vikram HR, Buenconsejo J, Hasbun R, Quagliarello VJ. 2003. Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis: a propensity analysis. JAMA 290:3207–3214. doi: 10.1001/jama.290.24.3207. [DOI] [PubMed] [Google Scholar]
- 219.Tleyjeh IM, Ghomrawi HM, Steckelberg JM, Hoskin TL, Mirzoyev Z, Anavekar NS, Enders F, Moustafa S, Mookadam F, Huskins WC, Wilson WR, Baddour LM. 2007. The impact of valve surgery on 6-month mortality in left-sided infective endocarditis. Circulation 115:1721–1728. doi: 10.1161/CIRCULATIONAHA.106.658831. [DOI] [PubMed] [Google Scholar]
- 220.Cabell CH, Abrutyn E, Fowler VG Jr, Hoen B, Miro JM, Corey GR, Olaison L, Pappas P, Anstrom KJ, Stafford JA, Eykyn S, Habib G, Mestres CA, Wang A. 2005. Use of surgery in patients with native valve infective endocarditis: results from the International Collaboration on Endocarditis merged database. Am Heart J 150:1092–1098. doi: 10.1016/j.ahj.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 221.Sy RW, Bannon PG, Bayfield MS, Brown C, Kritharides L. 2009. Survivor treatment selection bias and outcomes research: a case study of surgery in infective endocarditis. Circ Cardiovasc Qual Outcomes 2:469–474. doi: 10.1161/CIRCOUTCOMES.109.857938. [DOI] [PubMed] [Google Scholar]
- 222.Tleyjeh IM, Baddour LM. 2007. The potential impact of survivor treatment selection bias on the perceived efficacy of valve surgery in the treatment of infective endocarditis. Clin Infect Dis 44:1392–1393. doi: 10.1086/516609. [DOI] [PubMed] [Google Scholar]
- 223.Tleyjeh IM, Kashour T, Zimmerman V, Steckelberg JM, Wilson WR, Baddour LM. 2008. The role of valve surgery in infective endocarditis management: a systematic review of observational studies that included propensity score analysis. Am Heart J 156:901–909. doi: 10.1016/j.ahj.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 224.Lalani T, Cabell CH, Benjamin DK, Lasca O, Naber C, Fowler VG Jr, Corey GR, Chu VH, Fenely M, Pachirat O, Tan RS, Watkin R, Ionac A, Moreno A, Mestres CA, Casabe J, Chipigina N, Eisen DP, Spelman D, Delahaye F, Peterson G, Olaison L, Wang A. 2010. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 121:1005–1013. doi: 10.1161/CIRCULATIONAHA.109.864488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kang DH, Kim YJ, Kim SH, Sun BJ, Kim DH, Yun SC, Song JM, Choo SJ, Chung CH, Song JK, Lee JW, Sohn DW. 2012. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 366:2466–2473. doi: 10.1056/NEJMoa1112843. [DOI] [PubMed] [Google Scholar]
- 226.Rossi M, Gallo A, De Silva RJ, Sayeed R. 2012. What is the optimal timing for surgery in infective endocarditis with cerebrovascular complications? Interact Cardiovasc Thorac Surg 14:72–80. doi: 10.1093/icvts/ivr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tleyjeh IM, Baddour LM. 2013. Early cardiac surgery after ischemic stroke in patients with infective endocarditis may not be safe. Clin Infect Dis 56:1844–1845. doi: 10.1093/cid/cit097. [DOI] [PubMed] [Google Scholar]
- 228.Barsic B, Dickerman S, Krajinovic V, Pappas P, Altclas J, Carosi G, Casabe JH, Chu VH, Delahaye F, Edathodu J, Fortes CQ, Olaison L, Pangercic A, Patel M, Rudez I, Tamin SS, Vincelj J, Bayer AS, Wang A, International Collaboration on Endocarditis-Prospective Cohort Study Investigators . 2013. Influence of the timing of cardiac surgery on the outcome of patients with infective endocarditis and stroke. Clin Infect Dis 56:209–217. doi: 10.1093/cid/cis878. [DOI] [PubMed] [Google Scholar]
- 229.Bishara J, Leibovici L, Gartman-Israel D, Sagie A, Kazakov A, Miroshnik E, Ashkenazi S, Pitlik S. 2001. Long-term outcome of infective endocarditis: the impact of early surgical intervention. Clin Infect Dis 33:1636–1643. doi: 10.1086/323785. [DOI] [PubMed] [Google Scholar]
- 230.Wang A, Pappas P, Anstrom KJ, Abrutyn E, Fowler VG Jr, Hoen B, Miro JM, Corey GR, Olaison L, Stafford JA, Mestres CA, Cabell CH. 2005. The use and effect of surgical therapy for prosthetic valve infective endocarditis: a propensity analysis of a multicenter, international cohort. Am Heart J 150:1086–1091. doi: 10.1016/j.ahj.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 231.Yu VL, Fang GD, Keys TF, Harris AA, Gentry LO, Fuchs PC, Wagener MM, Wong ES. 1994. Prosthetic valve endocarditis: superiority of surgical valve replacement versus medical therapy only. Ann Thorac Surg 58:1073–1077. doi: 10.1016/0003-4975(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 232.Truninger K, Attenhofer Jost CH, Seifert B, Vogt PR, Follath F, Schaffner A, Jenni R. 1999. Long term follow up of prosthetic valve endocarditis: what characteristics identify patients who were treated successfully with antibiotics alone? Heart 82:714–720. doi: 10.1136/hrt.82.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chirouze C, Alla F, Fowler VG Jr, Sexton DJ, Corey GR, Chu VH, Wang A, Erpelding ML, Durante-Mangoni E, Fernandez-Hidalgo N, Giannitsioti E, Hannan MM, Lejko-Zupanc T, Miro JM, Munoz P, Murdoch DR, Tattevin P, Tribouilloy C, Hoen B, ICE Prospective Investigators . 2015. Impact of early valve surgery on outcome of Staphylococcus aureus prosthetic valve infective endocarditis: analysis in the International Collaboration of Endocarditis-Prospective Cohort Study. Clin Infect Dis 60:741–749. doi: 10.1093/cid/ciu871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Otto M. 2013. Community-associated MRSA: what makes them special? Int J Med Microbiol 303:324–330. doi: 10.1016/j.ijmm.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.David MZ, Daum RS. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 238.Frank AL, Marcinak JF, Mangat PD, Schreckenberger PC. 1999. Community-acquired and clindamycin-susceptible methicillin-resistant Staphylococcus aureus in children. Pediatr Infect Dis J 18:993–1000. doi: 10.1097/00006454-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 239.Groom AV, Wolsey DH, Naimi TS, Smith K, Johnson S, Boxrud D, Moore KA, Cheek JE. 2001. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 286:1201–1205. doi: 10.1001/jama.286.10.1201. [DOI] [PubMed] [Google Scholar]
- 240.Fergie JE, Purcell K. 2001. Community-acquired methicillin-resistant Staphylococcus aureus infections in south Texas children. Pediatr Infect Dis J 20:860–863. doi: 10.1097/00006454-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 241.Dufour P, Gillet Y, Bes M, Lina G, Vandenesch F, Floret D, Etienne J, Richet H. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis 35:819–824. doi: 10.1086/342576. [DOI] [PubMed] [Google Scholar]
- 242.Baggett HC, Hennessy TW, Leman R, Hamlin C, Bruden D, Reasonover A, Martinez P, Butler JC. 2003. An outbreak of community-onset methicillin-resistant Staphylococcus aureus skin infections in Southwestern Alaska. Infect Control Hosp Epidemiol 24:397–402. doi: 10.1086/502221. [DOI] [PubMed] [Google Scholar]
- 243.Buckingham SC, McDougal LK, Cathey LD, Comeaux K, Craig AS, Fridkin SK, Tenover FC. 2004. Emergence of community-associated methicillin-resistant Staphylococcus aureus at a Memphis, Tennessee children's hospital. Pediatr Infect Dis J 23:619–624. doi: 10.1097/01.inf.0000131981.67342.c4. [DOI] [PubMed] [Google Scholar]
- 244.Wang CC, Lo WT, Chu ML, Siu LK. 2004. Epidemiological typing of community-acquired methicillin-resistant Staphylococcus aureus isolates from children in Taiwan. Clin Infect Dis 39:481–487. doi: 10.1086/422642. [DOI] [PubMed] [Google Scholar]
- 245.Dietrich DW, Auld DB, Mermel LA. 2004. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 113:e347–e352. doi: 10.1542/peds.113.4.e347. [DOI] [PubMed] [Google Scholar]
- 246.Hisata K, Kuwahara-Arai K, Yamanoto M, Ito T, Nakatomi Y, Cui L, Baba T, Terasawa M, Sotozono C, Kinoshita S, Yamashiro Y, Hiramatsu K. 2005. Dissemination of methicillin-resistant staphylococci among healthy Japanese children. J Clin Microbiol 43:3364–3372. doi: 10.1128/JCM.43.7.3364-3372.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 248.Kaplan SL, Hulten KG, Gonzalez BE, Hammerman WA, Lamberth L, Versalovic J, Mason EO Jr. 2005. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis 40:1785–1791. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 249.Chen AE, Goldstein M, Carroll K, Song X, Perl TM, Siberry GK. 2006. Evolving epidemiology of pediatric Staphylococcus aureus cutaneous infections in a Baltimore hospital. Pediatr Emerg Care 22:717–723. doi: 10.1097/01.pec.0000236832.23947.a0. [DOI] [PubMed] [Google Scholar]
- 250.Udo EE, Pearman JW, Grubb WB. 1993. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J Hosp Infect 25:97–108. doi: 10.1016/0195-6701(93)90100-E. [DOI] [PubMed] [Google Scholar]
- 251.Nimmo GR, Coombs GW, Pearson JC, O'Brien FG, Christiansen KJ, Turnidge JD, Gosbell IB, Collignon P, McLaws ML. 2006. Methicillin-resistant Staphylococcus aureus in the Australian community: an evolving epidemic. Med J Aust 184:384–388. [DOI] [PubMed] [Google Scholar]
- 252.Centers for Disease Control and Prevention. 2003. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. MMWR Morb Mortal Wkly Rep 52:88. [PubMed] [Google Scholar]
- 253.Kazakova SV, Hageman JC, Matava M, Srinivasan A, Phelan L, Garfinkel B, Boo T, McAllister S, Anderson J, Jensen B, Dodson D, Lonsway D, McDougal LK, Arduino M, Fraser VJ, Killgore G, Tenover FC, Cody S, Jernigan DB. 2005. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 352:468–475. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 254.Begier EM, Frenette K, Barrett NL, Mshar P, Petit S, Boxrud DJ, Watkins-Colwell K, Wheeler S, Cebelinski EA, Glennen A, Nguyen D, Hadler JL. 2004. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis 39:1446–1453. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- 255.Young DM, Harris HW, Charlebois ED, Chambers H, Campbell A, Perdreau-Remington F, Lee C, Mankani M, Mackersie R, Schecter WP. 2004. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 139:947–951. doi: 10.1001/archsurg.139.9.947. [DOI] [PubMed] [Google Scholar]
- 256.Kallen AJ, Driscoll TJ, Thornton S, Olson PE, Wallace MR. 2000. Increase in community-acquired methicillin-resistant Staphylococcus aureus at a naval medical center. Infect Control Hosp Epidemiol 21:223–226. doi: 10.1086/501750. [DOI] [PubMed] [Google Scholar]
- 257.Zinderman CE, Conner B, Malakooti MA, LaMar JE, Armstrong A, Bohnker BK. 2004. Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg Infect Dis 10:941–944. doi: 10.3201/eid1005.030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Crum NF, Lee RU, Thornton SA, Stine OC, Wallace MR, Barrozo C, Keefer-Norris A, Judd S, Russell KL. 2006. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med 119:943–951. doi: 10.1016/j.amjmed.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 259.Tong SY, McDonald MI, Holt DC, Currie BJ. 2008. Global implications of the emergence of community-associated methicillin-resistant Staphylococcus aureus in indigenous populations. Clin Infect Dis 46:1871–1878. doi: 10.1086/588301. [DOI] [PubMed] [Google Scholar]
- 260.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA Jr. 2008. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 51:291–298. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 261.Hersh AL, Chambers HF, Maselli JH, Gonzales R. 2008. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 168:1585–1591. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 262.Edelsberg J, Taneja C, Zervos M, Haque N, Moore C, Reyes K, Spalding J, Jiang J, Oster G. 2009. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 15:1516–1518. doi: 10.3201/eid1509.081228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Frei CR, Makos BR, Daniels KR, Oramasionwu CU. 2010. Emergence of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections as a common cause of hospitalization in United States children. J Pediatr Surg 45:1967–1974. doi: 10.1016/j.jpedsurg.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 264.Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. JAMA 282:1123–1125. doi: 10.1001/jama.282.12.1123-JWR0922-2-1. [DOI] [PubMed] [Google Scholar]
- 265.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med 144:309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 266.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 267.Frazee BW, Lynn J, Charlebois ED, Lambert L, Lowery D, Perdreau-Remington F. 2005. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med 45:311–320. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 268.Hasty MB, Klasner A, Kness S, Denmark TK, Ellis D, Herman MI, Brown L. 2007. Cutaneous community-associated methicillin-resistant Staphylococcus aureus among all skin and soft-tissue infections in two geographically distant pediatric emergency departments. Acad Emerg Med 14:35–40. doi: 10.1197/j.aem.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 269.Jacobus CH, Lindsell CJ, Leach SD, Fermann GJ, Kressel AB, Rue LE. 2007. Prevalence and demographics of methicillin resistant Staphylococcus aureus in culturable skin and soft tissue infections in an urban emergency department. BMC Emerg Med 7:19. doi: 10.1186/1471-227X-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, Moran GJ, EMERGEncy ID Net Study Group . 2011. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis 53:144–149. doi: 10.1093/cid/cir308. [DOI] [PubMed] [Google Scholar]
- 271.Hayward A, Knott F, Petersen I, Livermore DM, Duckworth G, Islam A, Johnson AM. 2008. Increasing hospitalizations and general practice prescriptions for community-onset staphylococcal disease, England. Emerg Infect Dis 14:720–726. doi: 10.3201/eid1405.070153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Saxena S, Thompson P, Birger R, Bottle A, Spyridis N, Wong I, Johnson AP, Gilbert R, Sharland M, Improving Children's Antibiotic Prescribing Group . 2010. Increasing skin infections and Staphylococcus aureus complications in children, England, 1997-2006. Emerg Infect Dis 16:530–533. doi: 10.3201/eid1603.090809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Vaska VL, Nimmo GR, Jones M, Grimwood K, Paterson DL. 2012. Increases in Australian cutaneous abscess hospitalisations: 1999-2008. Eur J Clin Microbiol Infect Dis 31:93–96. doi: 10.1007/s10096-011-1281-3. [DOI] [PubMed] [Google Scholar]
- 274.Otter JA, French GL. 2010. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect Dis 10:227–239. doi: 10.1016/S1473-3099(10)70053-0. [DOI] [PubMed] [Google Scholar]
- 275.DeLeo FR, Diep BA, Otto M. 2009. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am 23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Foster TJ. 2005. Immune evasion by staphylococci. Nat Rev Microbiol 3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 277.Cribier B, Prevost G, Couppie P, Finck-Barbancon V, Grosshans E, Piemont Y. 1992. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology 185:175–180. [DOI] [PubMed] [Google Scholar]
- 278.Prevost G, Couppie P, Prevost P, Gayet S, Petiau P, Cribier B, Monteil H, Piemont Y. 1995. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J Med Microbiol 42:237–245. doi: 10.1099/00222615-42-4-237. [DOI] [PubMed] [Google Scholar]
- 279.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Diep BA, Sensabaugh GF, Somboonna N, Carleton HA, Perdreau-Remington F. 2004. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J Clin Microbiol 42:2080–2084. doi: 10.1128/JCM.42.5.2080-2084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 282.Hsu LY, Koh TH, Kurup A, Low J, Chlebicki MP, Tan BH. 2005. High incidence of Panton-Valentine leukocidin-producing Staphylococcus aureus in a tertiary care public hospital in Singapore. Clin Infect Dis 40:486–489. doi: 10.1086/427033. [DOI] [PubMed] [Google Scholar]
- 283.Nickerson EK, Wuthiekanun V, Wongsuvan G, Limmathurosakul D, Srisamang P, Mahavanakul W, Thaipadungpanit J, Shah KR, Arayawichanont A, Amornchai P, Thanwisai A, Day NP, Peacock SJ. 2009. Factors predicting and reducing mortality in patients with invasive Staphylococcus aureus disease in a developing country. PLoS One 4:e6512. doi: 10.1371/journal.pone.0006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tong SY, Lilliebridge RA, Bishop EJ, Cheng AC, Holt DC, McDonald MI, Giffard PM, Currie BJ, Boutlis CS. 2010. Clinical correlates of Panton-Valentine leukocidin (PVL), PVL isoforms, and clonal complex in the Staphylococcus aureus population of Northern Australia. J Infect Dis 202:760–769. doi: 10.1086/655396. [DOI] [PubMed] [Google Scholar]
- 285.Mesrati I, Saidani M, Ennigrou S, Zouari B, Ben Redjeb S. 2010. Clinical isolates of Pantone-Valentine leucocidin- and gamma-haemolysin-producing Staphylococcus aureus: prevalence and association with clinical infections. J Hosp Infect 75:265–268. doi: 10.1016/j.jhin.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 286.Shallcross LJ, Fragaszy E, Johnson AM, Hayward AC. 2013. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect Dis 13:43–54. doi: 10.1016/S1473-3099(12)70238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hamilton SM, Bryant AE, Carroll KC, Lockary V, Ma Y, McIndoo E, Miller LG, Perdreau-Remington F, Pullman J, Risi GF, Salmi DB, Stevens DL. 2007. In vitro production of Panton-Valentine leukocidin among strains of methicillin-resistant Staphylococcus aureus causing diverse infections. Clin Infect Dis 45:1550–1558. doi: 10.1086/523581. [DOI] [PubMed] [Google Scholar]
- 288.Boakes E, Kearns AM, Badiou C, Lina G, Hill RL, Ellington MJ. 2012. Do differences in Panton-Valentine leukocidin production among international methicillin-resistant Staphylococcus aureus clones affect disease presentation and severity? J Clin Microbiol 50:1773–1776. doi: 10.1128/JCM.06421-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, Kreiswirth BN, DeLeo FR. 2006. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis 194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 290.Hongo I, Baba T, Oishi K, Morimoto Y, Ito T, Hiramatsu K. 2009. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J Infect Dis 200:715–723. doi: 10.1086/605332. [DOI] [PubMed] [Google Scholar]
- 291.Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Chi CY, Lin CC, Liao IC, Yao YC, Shen FC, Liu CC, Lin CF. 2014. Panton-Valentine leukocidin facilitates the escape of Staphylococcus aureus from human keratinocyte endosomes and induces apoptosis. J Infect Dis 209:224–235. doi: 10.1093/infdis/jit445. [DOI] [PubMed] [Google Scholar]
- 293.Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. 2010. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis 202:1866–1876. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins 5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Inoshima N, Wang Y, Bubeck Wardenburg J. 2012. Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J Investig Dermatol 132:1513–1516. doi: 10.1038/jid.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tong SY, Sharma-Kuinkel BK, Thaden JT, Whitney AR, Yang SJ, Mishra NN, Rude T, Lilliebridge RA, Selim MA, Ahn SH, Holt DC, Giffard PM, Bayer AS, Deleo FR, Fowler VG Jr. 2013. Virulence of endemic nonpigmented northern Australian Staphylococcus aureus clone (clonal complex 75, S. argenteus) is not augmented by staphyloxanthin. J Infect Dis 208:520–527. doi: 10.1093/infdis/jit173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chua KY, Seemann T, Harrison PF, Monagle S, Korman TM, Johnson PD, Coombs GW, Howden BO, Davies JK, Howden BP, Stinear TP. 2011. The dominant Australian community-acquired methicillin-resistant Staphylococcus aureus clone ST93-IV [2B] is highly virulent and genetically distinct. PLoS One 6:e25887. doi: 10.1371/journal.pone.0025887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chua KY, Monk IR, Lin YH, Seemann T, Tuck KL, Porter JL, Stepnell J, Coombs GW, Davies JK, Stinear TP, Howden BP. 2014. Hyperexpression of alpha-hemolysin explains enhanced virulence of sequence type 93 community-associated methicillin-resistant Staphylococcus aureus. BMC Microbiol 14:31. doi: 10.1186/1471-2180-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Soong G, Chun J, Parker D, Prince A. 2012. Staphylococcus aureus activation of caspase 1/calpain signaling mediates invasion through human keratinocytes. J Infect Dis 205:1571–1579. doi: 10.1093/infdis/jis244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Mocca CP, Brady RA, Burns DL. 2014. Role of antibodies in protection elicited by active vaccination with genetically inactivated alpha hemolysin in a mouse model of Staphylococcus aureus skin and soft tissue infections. Clin Vaccine Immunol 21:622–627. doi: 10.1128/CVI.00051-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Joo HS, Cheung GY, Otto M. 2011. Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J Biol Chem 286:8933–8940. doi: 10.1074/jbc.M111.221382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tsompanidou E, Denham EL, Becher D, de Jong A, Buist G, van Oosten M, Manson WL, Back JW, van Dijl JM, Dreisbach A. 2013. Distinct roles of phenol-soluble modulins in spreading of Staphylococcus aureus on wet surfaces. Appl Environ Microbiol 79:886–895. doi: 10.1128/AEM.03157-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, Deleo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 304.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, Deleo FR. 2011. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis 204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Berlon NR, Sharma Kuinkel BK, Joo HS, Park LP, Otto M, Fowler VG Jr. 2014. Increased in vitro production of phenol soluble modulins (PSMs) are associated with a soft tissue infection source in clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA), abstr B-1996 Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 306.Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, des Etages SA, Jones A, Palazzolo-Ballance AM, Perdreau-Remington F, Sensabaugh GF, DeLeo FR, Chambers HF. 2008. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J Infect Dis 197:1523–1530. doi: 10.1086/587907. [DOI] [PubMed] [Google Scholar]
- 307.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 308.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. 2009. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 106:5883–5888. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Planet PJ, LaRussa SJ, Dana A, Smith H, Xu A, Ryan C, Uhlemann AC, Boundy S, Goldberg J, Narechania A, Kulkarni R, Ratner AJ, Geoghegan JA, Kolokotronis SO, Prince A. 2013. Emergence of the epidemic methicillin-resistant Staphylococcus aureus strain USA300 coincides with horizontal transfer of the arginine catabolic mobile element and speG-mediated adaptations for survival on skin. mBio 4(6):e00889-13. doi: 10.1128/mBio.00889-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. 2011. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun 79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Li M, Du X, Villaruz AE, Diep BA, Wang D, Song Y, Tian Y, Hu J, Yu F, Lu Y, Otto M. 2012. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med 18:816–819. doi: 10.1038/nm.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Bangert S, Levy M, Hebert AA. 2012. Bacterial resistance and impetigo treatment trends: a review. Pediatr Dermatol 29:243–248. doi: 10.1111/j.1525-1470.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- 313.McDonald M, Dougall A, Holt D, Huygens F, Oppedisano F, Giffard PM, Inman-Bamber J, Stephens AJ, Towers R, Carapetis JR, Currie BJ. 2006. Use of a single-nucleotide polymorphism genotyping system to demonstrate the unique epidemiology of methicillin-resistant Staphylococcus aureus in remote aboriginal communities. J Clin Microbiol 44:3720–3727. doi: 10.1128/JCM.00836-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Steer AC, Jenney AW, Kado J, Batzloff MR, La Vincente S, Waqatakirewa L, Mulholland EK, Carapetis JR. 2009. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis 3:e467. doi: 10.1371/journal.pntd.0000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Nagaraju U, Bhat G, Kuruvila M, Pai GS, Jayalakshmi, Babu RP. 2004. Methicillin-resistant Staphylococcus aureus in community-acquired pyoderma. Int J Dermatol 43:412–414. doi: 10.1111/j.1365-4632.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- 316.Bowen AC, Tong SY, Andrews RM, O'Meara IM, McDonald MI, Chatfield MD, Currie BJ, Carapetis JR. 2014. Short-course oral co-trimoxazole versus intramuscular benzathine benzylpenicillin for impetigo in a highly endemic region: an open-label, randomised, controlled, non-inferiority trial. Lancet 384:2132–2140. doi: 10.1016/S0140-6736(14)60841-2. [DOI] [PubMed] [Google Scholar]
- 317.Liu C, Graber CJ, Karr M, Diep BA, Basuino L, Schwartz BS, Enright MC, O'Hanlon SJ, Thomas JC, Perdreau-Remington F, Gordon S, Gunthorpe H, Jacobs R, Jensen P, Leoung G, Rumack JS, Chambers HF. 2008. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004-2005. Clin Infect Dis 46:1637–1646. doi: 10.1086/587893. [DOI] [PubMed] [Google Scholar]
- 318.Purcell K, Fergie J. 2005. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: a 14-year study at Driscoll Children's Hospital. Arch Pediatr Adolesc Med 159:980–985. doi: 10.1001/archpedi.159.10.980. [DOI] [PubMed] [Google Scholar]
- 319.Davis SL, Perri MB, Donabedian SM, Manierski C, Singh A, Vager D, Haque NZ, Speirs K, Muder RR, Robinson-Dunn B, Hayden MK, Zervos MJ. 2007. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol 45:1705–1711. doi: 10.1128/JCM.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chambers HF. 2013. Cellulitis, by any other name. Clin Infect Dis 56:1763–1764. doi: 10.1093/cid/cit126. [DOI] [PubMed] [Google Scholar]
- 321.Chaudhry IA, Shamsi FA, Elzaridi E, Al-Rashed W, Al-Amri A, Arat YO. 2008. Inpatient preseptal cellulitis: experience from a tertiary eye care centre. Br J Ophthalmol 92:1337–1341. doi: 10.1136/bjo.2007.128975. [DOI] [PubMed] [Google Scholar]
- 322.McKinley SH, Yen MT, Miller AM, Yen KG. 2007. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol 144:497–501. doi: 10.1016/j.ajo.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 323.Seltz LB, Smith J, Durairaj VD, Enzenauer R, Todd J. 2011. Microbiology and antibiotic management of orbital cellulitis. Pediatrics 127:e566–e572. doi: 10.1542/peds.2010-2117. [DOI] [PubMed] [Google Scholar]
- 324.Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Tang AW, Phung TO, Spellberg B. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 325.Lee YT, Lin JC, Wang NC, Peng MY, Chang FY. 2007. Necrotizing fasciitis in a medical center in northern Taiwan: emergence of methicillin-resistant Staphylococcus aureus in the community. J Microbiol Immunol Infect 40:335–341. [PubMed] [Google Scholar]
- 326.Chauhan S, Jain S, Varma S, Chauhan SS. 2004. Tropical pyomyositis (myositis tropicans): current perspective. Postgrad Med J 80:267–270. doi: 10.1136/pgmj.2003.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Martinez-Aguilar G, Avalos-Mishaan A, Hulten K, Hammerman W, Mason EO Jr, Kaplan SL. 2004. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr Infect Dis J 23:701–706. doi: 10.1097/01.inf.0000133044.79130.2a. [DOI] [PubMed] [Google Scholar]
- 328.Pannaraj PS, Hulten KG, Gonzalez BE, Mason EO Jr, Kaplan SL. 2006. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 43:953–960. doi: 10.1086/507637. [DOI] [PubMed] [Google Scholar]
- 329.Al-Tawfiq JA, Sarosi GA, Cushing HE. 2000. Pyomyositis in the acquired immunodeficiency syndrome. South Med J 93:330–334. [PubMed] [Google Scholar]
- 330.Lewis SS, Moehring RW, Chen LF, Sexton DJ, Anderson DJ. 2013. Assessing the relative burden of hospital-acquired infections in a network of community hospitals. Infect Control Hosp Epidemiol 34:1229–1230. doi: 10.1086/673443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network Team, Participating NHSN Facilities . 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 332.Wilson SE, O'Riordan W, Hopkins A, Friedland HD, Barriere SL, Kitt MM, ATLAS Investigators. 2009. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections associated with surgical procedures. Am J Surg 197:791–796. doi: 10.1016/j.amjsurg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 333.Steingrimsson S, Gottfredsson M, Kristinsson KG, Gudbjartsson T. 2008. Deep sternal wound infections following open heart surgery in Iceland: a population-based study. Scand Cardiovasc J 42:208–213. doi: 10.1080/14017430801919557. [DOI] [PubMed] [Google Scholar]
- 334.Nakamura T, Daimon T, Mouri N, Masuda H, Sawa Y. 2014. Staphylococcus aureus and repeat bacteremia in febrile patients as early signs of sternal wound infection after cardiac surgery. J Cardiothorac Surg 9:80. doi: 10.1186/1749-8090-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Russo PL, Bull A, Bennett N, Boardman C, Burrell S, Motley J, Friedman ND, Richards M. 2005. Infections after coronary artery bypass graft surgery in Victorian hospitals—VICNISS Hospital Acquired Infection Surveillance. Aust N Z J Public Health 29:244–248. doi: 10.1111/j.1467-842X.2005.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 336.San Juan R, Aguado JM, Lopez MJ, Lumbreras C, Enriquez F, Sanz F, Chaves F, Lopez-Medrano F, Lizasoain M, Rufilanchas JJ. 2005. Accuracy of blood culture for early diagnosis of mediastinitis in febrile patients after cardiac surgery. Eur J Clin Microbiol Infect Dis 24:182–189. doi: 10.1007/s10096-005-1302-1. [DOI] [PubMed] [Google Scholar]
- 337.Sharma M, Berriel-Cass D, Baran J Jr. 2004. Sternal surgical-site infection following coronary artery bypass graft: prevalence, microbiology, and complications during a 42-month period. Infect Control Hosp Epidemiol 25:468–471. doi: 10.1086/502423. [DOI] [PubMed] [Google Scholar]
- 338.Fowler VG Jr, Kaye KS, Simel DL, Cabell CH, McClachlan D, Smith PK, Levin S, Sexton DJ, Reller LB, Corey GR, Oddone EZ. 2003. Staphylococcus aureus bacteremia after median sternotomy: clinical utility of blood culture results in the identification of postoperative mediastinitis. Circulation 108:73–78. doi: 10.1161/01.CIR.0000079105.65762.DB. [DOI] [PubMed] [Google Scholar]
- 339.Oranje AP, Chosidow O, Sacchidanand S, Todd G, Singh K, Scangarella N, Shawar R, Twynholm M, TOC100224 Study Team . 2007. Topical retapamulin ointment, 1%, versus sodium fusidate ointment, 2%, for impetigo: a randomized, observer-blinded, noninferiority study. Dermatology 215:331–340. doi: 10.1159/000107776. [DOI] [PubMed] [Google Scholar]
- 340.Koning S, van der Wouden JC, Chosidow O, Twynholm M, Singh KP, Scangarella N, Oranje AP. 2008. Efficacy and safety of retapamulin ointment as treatment of impetigo: randomized double-blind multicentre placebo-controlled trial. Br J Dermatol 158:1077–1082. doi: 10.1111/j.1365-2133.2008.08485.x. [DOI] [PubMed] [Google Scholar]
- 341.Tack KJ, Keyserling CH, McCarty J, Hedrick JA. 1997. Study of use of cefdinir versus cephalexin for treatment of skin infections in pediatric patients. The Cefdinir Pediatric Skin Infection Study Group. Antimicrob Agents Chemother 41:739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Bucko AD, Hunt BJ, Kidd SL, Hom R. 2002. Randomized, double-blind, multicenter comparison of oral cefditoren 200 or 400 mg BID with either cefuroxime 250 mg BID or cefadroxil 500 mg BID for the treatment of uncomplicated skin and skin-structure infections. Clin Ther 24:1134–1147. doi: 10.1016/S0149-2918(02)80024-8. [DOI] [PubMed] [Google Scholar]
- 343.Giordano PA, Elston D, Akinlade BK, Weber K, Notario GF, Busman TA, Cifaldi M, Nilius AM. 2006. Cefdinir vs. cephalexin for mild to moderate uncomplicated skin and skin structure infections in adolescents and adults. Curr Med Res Opin 22:2419–2428. doi: 10.1185/030079906X148355. [DOI] [PubMed] [Google Scholar]
- 344.Rajendran PM, Young D, Maurer T, Chambers H, Perdreau-Remington F, Ro P, Harris H. 2007. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother 51:4044–4048. doi: 10.1128/AAC.00377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Duong M, Markwell S, Peter J, Barenkamp S. 2010. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med 55:401–407. doi: 10.1016/j.annemergmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 346.Schmitz GR, Bruner D, Pitotti R, Olderog C, Livengood T, Williams J, Huebner K, Lightfoot J, Ritz B, Bates C, Schmitz M, Mete M, Deye G. 2010. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med 56:283–287. doi: 10.1016/j.annemergmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 347.Pallin DJ, Binder WD, Allen MB, Lederman M, Parmar S, Filbin MR, Hooper DC, Camargo CA Jr. 2013. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim-sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis 56:1754–1762. doi: 10.1093/cid/cit122. [DOI] [PubMed] [Google Scholar]
- 348.Stevens DL, Smith LG, Bruss JB, McConnell-Martin MA, Duvall SE, Todd WM, Hafkin B. 2000. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother 44:3408–3413. doi: 10.1128/AAC.44.12.3408-3413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 38:1673–1681. doi: 10.1086/420818. [DOI] [PubMed] [Google Scholar]
- 350.Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch C. 2005. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother 49:2260–2266. doi: 10.1128/AAC.49.6.2260-2266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ellis-Grosse EJ, Babinchak T, Dartois N, Rose G, Loh E, Tigecycline 300 cSSSI Study Group, Tigecycline 305 cSSSI Study Group . 2005. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin Infect Dis 41(Suppl 5):S341–S353. doi: 10.1086/431675. [DOI] [PubMed] [Google Scholar]
- 352.Breedt J, Teras J, Gardovskis J, Maritz FJ, Vaasna T, Ross DP, Gioud-Paquet M, Dartois N, Ellis-Grosse EJ, Loh E, Tigecycline 305 cSSSI Study Group . 2005. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother 49:4658–4666. doi: 10.1128/AAC.49.11.4658-4666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sacchidanand S, Penn RL, Embil JM, Campos ME, Curcio D, Ellis-Grosse E, Loh E, Rose G. 2005. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: results from a phase 3, randomized, double-blind trial. Int J Infect Dis 9:251–261. doi: 10.1016/j.ijid.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 354.Jauregui LE, Babazadeh S, Seltzer E, Goldberg L, Krievins D, Frederick M, Krause D, Satilovs I, Endzinas Z, Breaux J, O'Riordan W. 2005. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis 41:1407–1415. doi: 10.1086/497271. [DOI] [PubMed] [Google Scholar]
- 355.Noel GJ, Strauss RS, Amsler K, Heep M, Pypstra R, Solomkin JS. 2008. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by Gram-positive bacteria. Antimicrob Agents Chemother 52:37–44. doi: 10.1128/AAC.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Stryjewski ME, Graham DR, Wilson SE, O'Riordan W, Young D, Lentnek A, Ross DP, Fowler VG, Hopkins A, Friedland HD, Barriere SL, Kitt MM, Corey GR. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis 46:1683–1693. doi: 10.1086/587896. [DOI] [PubMed] [Google Scholar]
- 357.Krievins D, Brandt R, Hawser S, Hadvary P, Islam K. 2009. Multicenter, randomized study of the efficacy and safety of intravenous iclaprim in complicated skin and skin structure infections. Antimicrob Agents Chemother 53:2834–2840. doi: 10.1128/AAC.01383-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Craft JC, Moriarty SR, Clark K, Scott D, Degenhardt TP, Still JG, Corey GR, Das A, Fernandes P. 2011. A randomized, double-blind phase 2 study comparing the efficacy and safety of an oral fusidic acid loading-dose regimen to oral linezolid for the treatment of acute bacterial skin and skin structure infections. Clin Infect Dis 52(Suppl 7):S520–S526. doi: 10.1093/cid/cir167. [DOI] [PubMed] [Google Scholar]
- 359.Friedland HD, O'Neal T, Biek D, Eckburg PB, Rank DR, Llorens L, Smith A, Witherell GW, Laudano JB, Thye D. 2012. CANVAS 1 and 2: analysis of clinical response at day 3 in two phase 3 trials of ceftaroline fosamil versus vancomycin plus aztreonam in treatment of acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 56:2231–2236. doi: 10.1128/AAC.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Prokocimer P, De Anda C, Fang E, Mehra P, Das A. 2013. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 309:559–569. doi: 10.1001/jama.2013.241. [DOI] [PubMed] [Google Scholar]
- 361.Noel GJ, Bush K, Bagchi P, Ianus J, Strauss RS. 2008. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin Infect Dis 46:647–655. doi: 10.1086/526527. [DOI] [PubMed] [Google Scholar]
- 362.Koning S, van der Sande R, Verhagen AP, van Suijlekom-Smit LW, Morris AD, Butler CC, Berger M, van der Wouden JC. 2012. Interventions for impetigo. Cochrane Database Syst Rev 1:CD003261. doi: 10.1002/14651858.CD003261.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect Dis 5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 364.Lee MC, Rios AM, Aten MF, Mejias A, Cavuoti D, McCracken GH Jr, Hardy RD. 2004. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 23:123–127. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 365.Paydar KZ, Hansen SL, Charlebois ED, Harris HW, Young DM. 2006. Inappropriate antibiotic use in soft tissue infections. Arch Surg 141:850–854; discussion 855–856. doi: 10.1001/archsurg.141.9.850. [DOI] [PubMed] [Google Scholar]
- 366.Ruhe JJ, Smith N, Bradsher RW, Menon A. 2007. Community-onset methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis 44:777–784. doi: 10.1086/511872. [DOI] [PubMed] [Google Scholar]
- 367.Spellberg B, Boucher H, Bradley J, Das A, Talbot G. 2011. To treat or not to treat: adjunctive antibiotics for uncomplicated abscesses. Ann Emerg Med 57:183–185. doi: 10.1016/j.annemergmed.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Broder JS. 2011. Randomized controlled trials, antibiotics, and cutaneous abscesses: has lack of statistical power prevented recognition of an effective therapy? Ann Emerg Med 57:185. doi: 10.1016/j.annemergmed.2010.08.036 (Reply, 57: 185–186, doi:. [DOI] [PubMed] [Google Scholar]
- 369.Miller LG, Daum RS, Creech CB, Young D, Downing MD, Eells SJ, Pettibone S, Hoagland RJ, Chambers HF, DMID 07-0051 Team. 2015. Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl J Med 372:1093–1103. doi: 10.1056/NEJMoa1403789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC. 2014. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59:e10–e52. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 371.Bowen AC, Lilliebridge RA, Tong SY, Baird RW, Ward P, McDonald MI, Currie BJ, Carapetis JR. 2012. Is Streptococcus pyogenes resistant or susceptible to trimethoprim-sulfamethoxazole? J Clin Microbiol 50:4067–4072. doi: 10.1128/JCM.02195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. 2014. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 370:2169–2179. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]
- 373.Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. 2014. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 14:696–705. doi: 10.1016/S1473-3099(14)70737-6. [DOI] [PubMed] [Google Scholar]
- 374.Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, Porwal A, Giordano P, Lucasti C, Perez A, Good S, Jiang H, Moeck G, O'Riordan W, SOLO I Investigators. 2014. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med 370:2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 375.Center for Drug Evaluation and Research, FDA, US Department of Health and Human Services. 2013. Guidance for industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. Center for Drug Evaluation and Research, FDA, US Department of Health and Human Services, Washington, DC: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm071185.pdf. [Google Scholar]
- 376.Corey GR, Stryjewski ME. 2011. New rules for clinical trials of patients with acute bacterial skin and skin-structure infections: do not let the perfect be the enemy of the good. Clin Infect Dis 52(Suppl 7):S469–S476. doi: 10.1093/cid/cir162. [DOI] [PubMed] [Google Scholar]
- 377.Zimbelman J, Palmer A, Todd J. 1999. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J 18:1096–1100. doi: 10.1097/00006454-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 378.Mulla ZD, Leaverton PE, Wiersma ST. 2003. Invasive group A streptococcal infections in Florida. South Med J 96:968–973. doi: 10.1097/01.SMJ.0000051060.95210.9A. [DOI] [PubMed] [Google Scholar]
- 379.Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. 2014. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group A streptococcal infections. Clin Infect Dis 59:358–365. doi: 10.1093/cid/ciu304. [DOI] [PubMed] [Google Scholar]
- 380.Gillet Y, Dumitrescu O, Tristan A, Dauwalder O, Javouhey E, Floret D, Vandenesch F, Etienne J, Lina G. 2011. Pragmatic management of Panton-Valentine leukocidin-associated staphylococcal diseases. Int J Antimicrob Agents 38:457–464. doi: 10.1016/j.ijantimicag.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 381.Stevens DL, Wallace RJ, Hamilton SM, Bryant AE. 2006. Successful treatment of staphylococcal toxic shock syndrome with linezolid: a case report and in vitro evaluation of the production of toxic shock syndrome toxin type 1 in the presence of antibiotics. Clin Infect Dis 42:729–730. doi: 10.1086/500265. [DOI] [PubMed] [Google Scholar]
- 382.Sheehy SH, Atkins BA, Bejon P, Byren I, Wyllie D, Athanasou NA, Berendt AR, McNally MA. 2010. The microbiology of chronic osteomyelitis: prevalence of resistance to common empirical anti-microbial regimens. J Infect 60:338–343. doi: 10.1016/j.jinf.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 383.Tice AD, Hoagland P, Shoultz DA. 2003. Outcomes of osteomyelitis among patients treated with outpatient parenteral antimicrobial therapy. Am J Med 114:723–728. doi: 10.1016/S0002-9343(03)00231-6. [DOI] [PubMed] [Google Scholar]
- 384.Bhavan KP, Marschall J, Olsen MA, Fraser VJ, Wright NM, Warren DK. 2010. The epidemiology of hematogenous vertebral osteomyelitis: a cohort study in a tertiary care hospital. BMC Infect Dis 10:158. doi: 10.1186/1471-2334-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Krogsgaard MR, Wagn P, Bengtsson J. 1998. Epidemiology of acute vertebral osteomyelitis in Denmark: 137 cases in Denmark 1978-1982, compared to cases reported to the National Patient Register 1991-1993. Acta Orthop Scand 69:513–517. doi: 10.3109/17453679808997789. [DOI] [PubMed] [Google Scholar]
- 386.McHenry M, Easly K, Locker G. 2002. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis 34:1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 387.Corrah TW, Enoch DA, Aliyu SH, Lever AM. 2011. Bacteraemia and subsequent vertebral osteomyelitis: a retrospective review of 125 patients. QJM 104:201–207. doi: 10.1093/qjmed/hcq178. [DOI] [PubMed] [Google Scholar]
- 388.Beronius M, Bergman B, Andersson R. 2001. Vertebral osteomyelitis in Goteborg, Sweden: a retrospective study of patients during 1990-95. Scand J Infect Dis 33:527–532. doi: 10.1080/00365540110026566. [DOI] [PubMed] [Google Scholar]
- 389.Carragee EJ. 1997. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 79:874–880. doi: 10.1302/0301-620X.79B5.8078. [DOI] [PubMed] [Google Scholar]
- 390.Inoue S, Moriyama T, Horinouchi Y, Tachibana T, Okada F, Maruo K, Yoshiya S. 2013. Comparison of clinical features and outcomes of Staphylococcus aureus vertebral osteomyelitis caused by methicillin-resistant and methicillin-sensitive strains. SpringerPlus 2:283. doi: 10.1186/2193-1801-2-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Chelsom J, Solberg CO. 1998. Vertebral osteomyelitis at a Norwegian university hospital 1987-97: clinical features, laboratory findings and outcome. Scand J Infect Dis 30:147–151. doi: 10.1080/003655498750003537. [DOI] [PubMed] [Google Scholar]
- 392.Torda AJ, Gottlieb T, Bradbury R. 1995. Pyogenic vertebral osteomyelitis: analysis of 20 cases and review. Clin Infect Dis 20:320–328. doi: 10.1093/clinids/20.2.320. [DOI] [PubMed] [Google Scholar]
- 393.Clerc O, Prod'hom G, Greub G, Zanetti G, Senn L. 2011. Adult native septic arthritis: a review of 10 years of experience and lessons for empirical antibiotic therapy. J Antimicrob Chemother 66:1168–1173. doi: 10.1093/jac/dkr047. [DOI] [PubMed] [Google Scholar]
- 394.Stoesser N, Pocock J, Moore CE, Soeng S, Hor P, Sar P, Limmathurotsakul D, Day N, Kumar V, Khan S, Sar V, Parry CM. 2013. The epidemiology of pediatric bone and joint infections in Cambodia, 2007-11. J Trop Pediatr 59:36–42. doi: 10.1093/tropej/fms044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Howard-Jones AR, Isaacs D, Gibbons PJ. 2013. Twelve-month outcome following septic arthritis in children. J Pediatr Orthop B 22:486–490. doi: 10.1097/BPB.0b013e32836027ca. [DOI] [PubMed] [Google Scholar]
- 396.Okano T, Enokida M, Otsuki R, Hagino H, Teshima R. 2011. Recent trends in adult-onset septic arthritis of the knee and hip: retrospective analysis of patients treated during the past 50 years. J Infect Chemother 17:666–670. doi: 10.1007/s10156-011-0244-z. [DOI] [PubMed] [Google Scholar]
- 397.Al Saadi MM, Al Zamil FA, Bokhary NA, Al Shamsan LA, Al Alola SA, Al Eissa YS. 2009. Acute septic arthritis in children. Pediatr Int 51:377–380. doi: 10.1111/j.1442-200X.2008.02791.x. [DOI] [PubMed] [Google Scholar]
- 398.Eder L, Zisman D, Rozenbaum M, Rosner I. 2005. Clinical features and aetiology of septic arthritis in northern Israel. Rheumatology (Oxford) 44:1559–1563. doi: 10.1093/rheumatology/kei092. [DOI] [PubMed] [Google Scholar]
- 399.Gupta M, Sturrock R, Field M. 2003. Prospective comparative study of patients with culture proven and high suspicion of adult onset septic arthritis. Ann Rheum Dis 62:327–331. doi: 10.1136/ard.62.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Khan FY, Abu-Khattab M, Baagar K, Mohamed SF, Elgendy I, Anand D, Malallah H, Sanjay D. 2013. Characteristics of patients with definite septic arthritis at Hamad General Hospital, Qatar: a hospital-based study from 2006 to 2011. Clin Rheumatol 32:969–973. doi: 10.1007/s10067-013-2211-9. [DOI] [PubMed] [Google Scholar]
- 401.Gupta MN, Sturrock RD, Field M. 2001. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford) 40:24–30. doi: 10.1093/rheumatology/40.1.24. [DOI] [PubMed] [Google Scholar]
- 402.Peel TN, Cheng AC, Choong PF, Buising KL. 2012. Early onset prosthetic hip and knee joint infection: treatment and outcomes in Victoria, Australia. J Hosp Infect 82:248–253. doi: 10.1016/j.jhin.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 403.Rodriguez D, Pigrau C, Euba G, Cobo J, Garcia-Lechuz J, Palomino J, Riera M, Del Toro MD, Granados A, Ariza X. 2010. Acute haematogenous prosthetic joint infection: prospective evaluation of medical and surgical management. Clin Microbiol Infect 16:1789–1795. doi: 10.1111/j.1469-0691.2010.03157.x. [DOI] [PubMed] [Google Scholar]
- 404.Westberg M, Grogaard B, Snorrason F. 2012. Early prosthetic joint infections treated with debridement and implant retention: 38 primary hip arthroplasties prospectively recorded and followed for median 4 years. Acta Orthop 83:227–232. doi: 10.3109/17453674.2012.678801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Bejon P, Berendt A, Atkins BL, Green N, Parry H, Masters S, McLardy-Smith P, Gundle R, Byren I. 2010. Two-stage revision for prosthetic joint infection: predictors of outcome and the role of reimplantation microbiology. J Antimicrob Chemother 65:569–575. doi: 10.1093/jac/dkp469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, Gundle R, Berendt A. 2009. One hundred and twelve infected arthroplasties treated with ‘DAIR' (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother 63:1264–1271. doi: 10.1093/jac/dkp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Waldvogel FA, Medoff G, Swartz MN. 1970. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med 282:198–206. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- 408.Espersen F, Frimodt-Moller N, Skinhoj P, Bentzon MW. 1991. Changing pattern of bone and joint infections due to Staphylococcus aureus: study of cases of bacteremia in Denmark, 1959-1988. Rev Infect Dis 13:945–948. [DOI] [PubMed] [Google Scholar]
- 409.Kehrer M, Pedersen C, Jensen TG, Lassen AT. 2014. Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. J Infect 68:313–320. doi: 10.1016/j.jinf.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 410.Hutchinson C, Hanger C, Wilkinson T, Sainsbury R, Pithie A. 2009. Spontaneous spinal infections in older people. Intern Med J 39:845–848. doi: 10.1111/j.1445-5994.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- 411.Akiyama T, Chikuda H, Yasunaga H, Horiguchi H, Fushimi K, Saita K. 2013. Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database. BMJ Open 3:e002412. doi: 10.1136/bmjopen-2012-002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. 2009. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum 39:10–17. doi: 10.1016/j.semarthrit.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 413.Ziu M, Dengler B, Cordell D, Bartanusz V. 2014. Diagnosis and management of primary pyogenic spinal infections in intravenous recreational drug users. Neurosurg Focus 37:E3. doi: 10.3171/2014.6.FOCUS14148. [DOI] [PubMed] [Google Scholar]
- 414.Jensen AG, Espersen F, Skinhoj P, Frimodt-Moller N. 1998. Bacteremic Staphylococcus aureus spondylitis. Arch Intern Med 158:509–517. doi: 10.1001/archinte.158.5.509. [DOI] [PubMed] [Google Scholar]
- 415.Lew DP, Waldvogel FA. 1997. Osteomyelitis. N Engl J Med 336:999–1007. [DOI] [PubMed] [Google Scholar]
- 416.Cunningham R, Cockayne A, Humphreys H. 1996. Clinical and molecular aspects of the pathogenesis of Staphylococcus aureus bone and joint infections. J Med Microbiol 44:157–164. doi: 10.1099/00222615-44-3-157. [DOI] [PubMed] [Google Scholar]
- 417.Shirtliff M, Mader J. 2002. Acute septic arthritis. Clin Microbiol Rev 15:527–544. doi: 10.1128/CMR.15.4.527-544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Darouiche RO, Landon GC, Patti JM, Nguyen LL, Fernau RC, McDevitt D, Greene C, Foster T, Klima M. 1997. Role of Staphylococcus aureus surface adhesins in orthopaedic device infections: are results model-dependent? J Med Microbiol 46:75–79. doi: 10.1099/00222615-46-1-75. [DOI] [PubMed] [Google Scholar]
- 419.Switalski L, Patti J, Butcher W, Gristina A, Speziale P, Hook M. 1993. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol Microbiol 7:99–107. doi: 10.1111/j.1365-2958.1993.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 420.Fischer B, Vaudaux M, Magnin Y, Mestikawy R, Lew D. 1996. Novel animal model for studying the molecular mechanisms of bacterial adhesion to bone-implanted metallic devices: role of fibronectin in Staphylococcus aureus adhesion. J Orthop Res 14:914–920. doi: 10.1002/jor.1100140611. [DOI] [PubMed] [Google Scholar]
- 421.Clauss M, Tafin UF, Bizzini A, Trampuz A, Ilchmann T. 2013. Biofilm formation by staphylococci on fresh, fresh-frozen and processed human and bovine bone grafts. Eur Cell Mater 25:159–166. [DOI] [PubMed] [Google Scholar]
- 422.Shi S, Zhang X. 2012. Interaction of Staphylococcus aureus with osteoblasts. Exp Ther Med 3:367–370. doi: 10.3892/etm.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 424.Kalinka J, Hachmeister M, Geraci J, Sordelli D, Hansen U, Niemann S, Oetermann S, Peters G, Loffler B, Tuchscherr L. 2014. Staphylococcus aureus isolates from chronic osteomyelitis are characterized by high host cell invasion and intracellular adaptation, but still induce inflammation. Int J Med Microbiol 304:1038–1049. doi: 10.1016/j.ijmm.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 425.Rolauffs B, Bernhardt TM, von Eiff C, Hart ML, Bettin D. 2002. Osteopetrosis, femoral fracture, and chronic osteomyelitis caused by Staphylococcus aureus small colony variants (SCV) treated by girdlestone resection—6-year follow-up. Arch Orthop Trauma Surg 122:547–550. [DOI] [PubMed] [Google Scholar]
- 426.von Eiff C, Bettin D, Proctor RA, Rolauffs B, Lindner N, Winkelmann W, Peters G. 1997. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin Infect Dis 25:1250–1251. doi: 10.1086/516962. [DOI] [PubMed] [Google Scholar]
- 427.Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. 2006. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis 43:961–967. doi: 10.1086/507633. [DOI] [PubMed] [Google Scholar]
- 428.Tande AJ, Osmon DR, Greenwood-Quaintance KE, Mabry TM, Hanssen AD, Patel R. 2014. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. mBio 5(5):e01910-14. doi: 10.1128/mBio.01910-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Abele-Horn M, Schupfner B, Emmerling P, Waldner H, Goring H. 2000. Persistent wound infection after herniotomy associated with small-colony variants of Staphylococcus aureus. Infection 28:53–54. doi: 10.1007/s150100050014. [DOI] [PubMed] [Google Scholar]
- 430.Bhattacharyya S, Roy S, Mukhopadhyay P, Rit K, Dey J, Ganguly U, Ray R. 2012. Small colony variants of Staphylococcus aureus isolated from a patient with infective endocarditis: a case report and review of the literature. Iran J Microbiol 4:98–99. [PMC free article] [PubMed] [Google Scholar]
- 431.Spanu T, Romano L, D'Inzeo T, Masucci L, Albanese A, Papacci F, Marchese E, Sanguinetti M, Fadda G. 2005. Recurrent ventriculoperitoneal shunt infection caused by small-colony variants of Staphylococcus aureus. Clin Infect Dis 41:e48–e52. doi: 10.1086/432577. [DOI] [PubMed] [Google Scholar]
- 432.Gitomer SA, Ramakrishnan VR, Malcolm KC, Kofonow JM, Ir D, Frank DN. 2015. Initial investigation of small colony variants of Staphylococcus aureus in chronic rhinosinusitis. Am J Rhinol Allergy 29:29–34. doi: 10.2500/ajra.2015.29.4133. [DOI] [PubMed] [Google Scholar]
- 433.Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, Peters G, Becker K, Loffler B. 2010. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J Infect Dis 202:1031–1040. doi: 10.1086/656047. [DOI] [PubMed] [Google Scholar]
- 434.von Eiff C, Becker K, Metze D, Lubritz G, Hockmann J, Schwarz T, Peters G. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin Infect Dis 32:1643–1647. doi: 10.1086/320519. [DOI] [PubMed] [Google Scholar]
- 435.Baumert N, von Eiff C, Schaaff F, Peters G, Proctor RA, Sahl HG. 2002. Physiology and antibiotic susceptibility of Staphylococcus aureus small colony variants. Microb Drug Resist 8:253–260. doi: 10.1089/10766290260469507. [DOI] [PubMed] [Google Scholar]
- 436.Garcia LG, Lemaire S, Kahl BC, Becker K, Proctor RA, Denis O, Tulkens PM, Van Bambeke F. 2013. Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J Antimicrob Chemother 68:1455–1464. doi: 10.1093/jac/dkt072. [DOI] [PubMed] [Google Scholar]
- 437.Proctor RA, Peters G. 1998. Small colony variants in staphylococcal infections: diagnostic and therapeutic implications. Clin Infect Dis 27:419–422. doi: 10.1086/514706. [DOI] [PubMed] [Google Scholar]
- 438.von Eiff C. 2008. Staphylococcus aureus small colony variants: a challenge to microbiologists and clinicians. Int J Antimicrob Agents 31:507–510. doi: 10.1016/j.ijantimicag.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 439.Neut D, van der Mei HC, Bulstra SK, Busscher HJ. 2007. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthop 78:299–308. doi: 10.1080/17453670710013843. [DOI] [PubMed] [Google Scholar]
- 440.Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA. 2004. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J 80:607–609. doi: 10.1136/pgmj.2003.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Grados F, Lescure FX, Senneville E, Flipo RM, Schmit JL, Fardellone P. 2007. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine 74:133–139. doi: 10.1016/j.jbspin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 442.de Lucas EM, Gonzalez Mandly A, Gutierrez A, Pellon R, Martin-Cuesta L, Izquierdo J, Sanchez E, Ruiz E, Quintana F. 2009. CT-guided fine-needle aspiration in vertebral osteomyelitis: true usefulness of a common practice. Clin Rheumatol 28:315–320. doi: 10.1007/s10067-008-1051-5. [DOI] [PubMed] [Google Scholar]
- 443.Gras G, Buzele R, Parienti JJ, Debiais F, Dinh A, Dupon M, Roblot F, Mulleman D, Marcelli C, Michon J, Bernard L. 2014. Microbiological diagnosis of vertebral osteomyelitis: relevance of second percutaneous biopsy following initial negative biopsy and limited yield of post-biopsy blood cultures. Eur J Clin Microbiol Infect Dis 33:371–375. doi: 10.1007/s10096-013-1965-y. [DOI] [PubMed] [Google Scholar]
- 444.Waldvogel FA, Medoff G, Swartz MN. 1970. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Engl J Med 282:316–322. [DOI] [PubMed] [Google Scholar]
- 445.Peel TN, Dowsey MM, Buising KL, Liew D, Choong PF. 2013. Cost analysis of debridement and retention for management of prosthetic joint infection. Clin Microbiol Infect 19:181–186. doi: 10.1111/j.1469-0691.2011.03758.x. [DOI] [PubMed] [Google Scholar]
- 446.Lalani T, Chu VH, Grussemeyer CA, Reed SD, Bolognesi MP, Friedman JY, Griffiths RI, Crosslin DR, Kanafani ZA, Kaye KS, Ralph Corey G, Fowler VG Jr. 2008. Clinical outcomes and costs among patients with Staphylococcus aureus bacteremia and orthopedic device infections. Scand J Infect Dis 40:973–977. doi: 10.1080/00365540802245146. [DOI] [PubMed] [Google Scholar]
- 447.Yuan HC, Wu KG, Chen CJ, Tang RB, Hwang BT. 2006. Characteristics and outcome of septic arthritis in children. J Microbiol Immunol Infect 39:342–347. [PubMed] [Google Scholar]
- 448.Park KH, Chong YP, Kim SH, Lee SO, Choi SH, Lee MS, Jeong JY, Woo JH, Kim YS. 2013. Clinical characteristics and therapeutic outcomes of hematogenous vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus. J Infect 67:556–564. doi: 10.1016/j.jinf.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 449.Roblot F, Besnier JM, Juhel L, Vidal C, Ragot S, Bastides F, Le Moal G, Godet C, Mulleman D, Azais I, Becq-Giraudon B, Choutet P. 2007. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin Arthritis Rheum 36:269–277. doi: 10.1016/j.semarthrit.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 450.Friedman JA, Maher CO, Quast LM, McClelland RL, Ebersold MJ. 2002. Spontaneous disc space infections in adults. Surg Neurol 57:81–86. doi: 10.1016/S0090-3019(01)00681-4. [DOI] [PubMed] [Google Scholar]
- 451.Zimmerli W. 2010. Clinical practice. Vertebral osteomyelitis. N Engl J Med 362:1022–1029. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
- 452.Bernard L, Dinh A, Ghout I, Simo D, Zeller V, Issartel B, Le Moing V, Belmatoug N, Lesprit P, Bru JP, Therby A, Bouhour D, Denes E, Debard A, Chirouze C, Fevre K, Dupon M, Aegerter P, Mulleman D, Duration of Treatment for Spondylodiscitis Study Group . 2015. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 385:875–882. doi: 10.1016/S0140-6736(14)61233-2. [DOI] [PubMed] [Google Scholar]
- 453.Babouee Flury B, Elzi L, Kolbe M, Frei R, Weisser M, Scharen S, Widmer AF, Battegay M. 2014. Is switching to an oral antibiotic regimen safe after 2 weeks of intravenous treatment for primary bacterial vertebral osteomyelitis? BMC Infect Dis 14:226. doi: 10.1186/1471-2334-14-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. 1999. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis 58:214–219. doi: 10.1136/ard.58.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. 1997. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis 56:470–475. doi: 10.1136/ard.56.8.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Kaandorp C, Van Schaardenburg D, Krijnen P, Habbema J, Van de Laar M. 1995. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheum 38:1819–1825. doi: 10.1002/art.1780381215. [DOI] [PubMed] [Google Scholar]
- 457.Morgan D, Fisher D, Merianos A, Currie BJ. 1996. Septic arthritis: an 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect 117:423–428. doi: 10.1017/S0950268800059070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Garcia-Arias M, Balsa A, Mola EM. 2011. Septic arthritis. Best Pract Res Clin Rheumatol 25:407–421. doi: 10.1016/j.berh.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 459.Margaretten ME, Kohlwes J, Moore D, Bent S. 2007. Does this adult patient have septic arthritis? JAMA 297:1478–1488. doi: 10.1001/jama.297.13.1478. [DOI] [PubMed] [Google Scholar]
- 460.Dubost J, Soubrier M, De Champs C, Ristori J, Bussiere J, Sauvezie B. 2002. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis 61:267–269. doi: 10.1136/ard.61.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Frazee BW, Fee C, Lambert L. 2009. How common is MRSA in adult septic arthritis? Ann Emerg Med 54:695–700. doi: 10.1016/j.annemergmed.2009.06.511. [DOI] [PubMed] [Google Scholar]
- 462.Babcock H, Matava M, Fraser V. 2002. Postarthroscopy surgical site infections: review of the literature. Clin Infect Dis 34:65–71. doi: 10.1086/324627. [DOI] [PubMed] [Google Scholar]
- 463.Shirtliff ME, Mader JT. 2002. Acute septic arthritis. Clin Microbiol Rev 15:527–544. doi: 10.1128/CMR.15.4.527-544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Gruber BF, Miller BS, Onnen J, Welling RD, Wojtys EM. 2008. Antibacterial properties of synovial fluid in the knee. J Knee Surg 21:180–185. doi: 10.1055/s-0030-1247816. [DOI] [PubMed] [Google Scholar]
- 465.Gjertsson I, Jonsson IM, Peschel A, Tarkowski A, Lindholm C. 2012. Formylated peptides are important virulence factors in Staphylococcus aureus arthritis in mice. J Infect Dis 205:305–311. doi: 10.1093/infdis/jir713. [DOI] [PubMed] [Google Scholar]
- 466.Colavite-Machado PM, Ishikawa LL, Franca TG, Zorzella-Pezavento SF, da Rosa LC, Chiuso-Minicucci F, da Cunha ML, Garlet GP, Sartori A. 2013. Differential arthritogenicity of Staphylococcus aureus strains isolated from biological samples. BMC Infect Dis 13:400. doi: 10.1186/1471-2334-13-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Verdrengh M, Tarkowski A. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun 65:2517–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. 2015. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis 211:641–650. doi: 10.1093/infdis/jiu514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Goldenberg D, Sexton D. 2014. Disseminated gonococcal infection. In Hynes NA. (ed), UpToDate. UpToDate, Waltham, MA: Accessed 24 September 2014. [Google Scholar]
- 470.Mathews CJ, Weston VC, Jones A, Field M, Coakley G. 2010. Bacterial septic arthritis in adults. Lancet 375:846–855. doi: 10.1016/S0140-6736(09)61595-6. [DOI] [PubMed] [Google Scholar]
- 471.Goldenberg D. 1998. Septic arthritis. Lancet 351:197–202. doi: 10.1016/S0140-6736(97)09522-6. [DOI] [PubMed] [Google Scholar]
- 472.Norden C, Gillespie W, Nade S. 1994. Infectious arthritis. In Norden C. (ed), Infections in bones and joints. Blackwell Scientific Publications, Boston, MA. [Google Scholar]
- 473.Andole SN, Gupta S, Pelly M. 2011. Septic arthritis affecting pubic symphysis. BMJ Case Rep 2011:bcr0420114089. doi: 10.1136/bcr.1104.2011.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Ross J, Hu L. 2003. Septic arthritis of the pubic symphysis—review of 100 cases. Medicine 82:340–345. doi: 10.1097/01.md.0000091180.93122.1c. [DOI] [PubMed] [Google Scholar]
- 475.Kak V, Chandresekar PH, Narula AP. 2002. Bone and joint infections in injection drug users. Infect Dis Clin North Am 16:681–695. doi: 10.1016/S0891-5520(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 476.Ross J, Shamsuddin H. 2004. Sternoclavicular septic arthritis—review of 180 cases. Medicine 83:139–148. doi: 10.1097/01.md.0000126761.83417.29. [DOI] [PubMed] [Google Scholar]
- 477.Mathews CJ, Coakley G. 2008. Septic arthritis: current diagnostic and therapeutic algorithm. Curr Opin Rheumatol 20:457–462. doi: 10.1097/BOR.0b013e3283036975. [DOI] [PubMed] [Google Scholar]
- 478.Wise CM, Morris CR, Wasilauskas BL, Salzer WL. 1994. Gonococcal arthritis in an era of increasing penicillin resistance. Presentations and outcomes in 41 recent cases (1985-1991). Arch Intern Med 154:2690–2695. [DOI] [PubMed] [Google Scholar]
- 479.O'Brien JP, Goldenberg DL, Rice PA. 1983. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine (Baltimore) 62:395–406. doi: 10.1097/00005792-198311000-00005. [DOI] [PubMed] [Google Scholar]
- 480.O'Connell PG, Milburn BM, Nashel DJ. 1985. Coexistent gout and septic arthritis: a report of two cases and literature review. Clin Exp Rheumatol 3:265–267. [PubMed] [Google Scholar]
- 481.Jarrett MP, Grayzel AI. 1980. Simultaneous gout, pseudogout, and septic arthritis. Arthritis Rheum 23:128–129. doi: 10.1002/art.1780230127. [DOI] [PubMed] [Google Scholar]
- 482.Odio CM, Ramirez T, Arias G, Abdelnour A, Hidalgo I, Herrera ML, Bolanos W, Alpizar J, Alvarez P. 2003. Double blind, randomized, placebo-controlled study of dexamethasone therapy for hematogenous septic arthritis in children. Pediatr Infect Dis J 22:883–888. doi: 10.1097/01.inf.0000091293.32187.7b. [DOI] [PubMed] [Google Scholar]
- 483.Lane J, Falahee M, Wojtys E, Hankin F, Kaufer H. 1990. Pyoarthrosis of the knee: treatment considerations. Clin Othop 252:198–204. [PubMed] [Google Scholar]
- 484.Matthews PC, Dean BJ, Medagoda K, Gundle R, Atkins BL, Berendt AR, Byren I. 2008. Native hip joint septic arthritis in 20 adults: delayed presentation beyond three weeks predicts need for excision arthroplasty. J Infect 57:185–190. doi: 10.1016/j.jinf.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 485.Coakley G, Mathews C, Field M, Jones A, Kingsley G, Walker D, Phillips M, Bradish C, McLachlan A, Mohammed R, Weston V, British Society for Rheumatology Standards, Guidelines and Audit Working Group . 2006. BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults. Rheumatology (Oxford) 45:1039–1041. doi: 10.1093/rheumatology/kel163a. [DOI] [PubMed] [Google Scholar]
- 486.Uckay I, Tovmirzaeva L, Garbino J, Rohner P, Tahintzi P, Suva D, Assal M, Hoffmeyer P, Bernard L, Lew D. 2013. Short parenteral antibiotic treatment for adult septic arthritis after successful drainage. Int J Infect Dis 17:e199–e205. doi: 10.1016/j.ijid.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 487.Lalani T, Boucher HW, Cosgrove SE, Fowler VG, Kanafani ZA, Vigliani GA, Campion M, Abrutyn E, Levine DP, Price CS, Rehm SJ, Corey GR, Karchmer AW, S. aureus Endocarditis and Bacteraemia Study Group . 2008. Outcomes with daptomycin versus standard therapy for osteoarticular infections associated with Staphylococcus aureus bacteraemia. J Antimicrob Chemother 61:177–182. doi: 10.1093/jac/dkm437. [DOI] [PubMed] [Google Scholar]
- 488.Goldenberg DL, Brandt KD, Cohen AS, Cathcart ES. 1975. Treatment of septic arthritis: comparison of needle aspiration and surgery as initial modes of joint drainage. Arthritis Rheum 18:83–90. doi: 10.1002/art.1780180116. [DOI] [PubMed] [Google Scholar]
- 489.Ravindran V, Logan I, Bourke BE. 2009. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology (Oxford) 48:1320–1322. doi: 10.1093/rheumatology/kep220. [DOI] [PubMed] [Google Scholar]
- 490.Sakiniene E, Bremell T, Tarkowski A. 1996. Addition of corticosteroids to antibiotic treatment ameliorates the course of experimental Staphylococcus aureus arthritis. Arthritis Rheum 39:1596–1605. doi: 10.1002/art.1780390921. [DOI] [PubMed] [Google Scholar]
- 491.Harel L, Prais D, Bar-On E, Livni G, Hoffer V, Uziel Y, Amir J. 2011. Dexamethasone therapy for septic arthritis in children: results of a randomized double-blind placebo-controlled study. J Pediatr Orthop 31:211–215. doi: 10.1097/BPO.0b013e3182092869. [DOI] [PubMed] [Google Scholar]
- 492.Arti H, Mousapour A, Alavi SM. 2014. The effect of intravenous dexamethasone in the treatment of septic arthritis. Pak J Med Sci 30:955–957. doi: 10.12669/pjms.305.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Mitha A, Boutry N, Nectoux E, Petyt C, Lagree M, Happiette L, Martinot A, Hospital Network for Evaluating the Management of Infectious Diseases in Children, Dubos F. 2015. Community-acquired bone and joint infections in children: a 1-year prospective epidemiological study. Arch Dis Child 100:126–129. doi: 10.1136/archdischild-2013-305860. [DOI] [PubMed] [Google Scholar]
- 494.Grammatico-Guillon L, Maakaroun Vermesse Z, Baron S, Gettner S, Rusch E, Bernard L. 2013. Paediatric bone and joint infections are more common in boys and toddlers: a national epidemiology study. Acta Paediatr 102:e120–e125. doi: 10.1111/apa.12115. [DOI] [PubMed] [Google Scholar]
- 495.Riise OR, Kirkhus E, Handeland KS, Flato B, Reiseter T, Cvancarova M, Nakstad B, Wathne KO. 2008. Childhood osteomyelitis—incidence and differentiation from other acute onset musculoskeletal features in a population-based study. BMC Pediatr 8:45. doi: 10.1186/1471-2431-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Street M, Puna R, Huang M, Crawford H. 20 October 2014. Pediatric acute hematogenous osteomyelitis. J Pediatr Orthop doi: 10.1097/BPO.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 497.Arnold SR, Elias D, Buckingham SC, Thomas ED, Novais E, Arkader A, Howard C. 2006. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop 26:703–708. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 498.Saavedra-Lozano J, Mejias A, Ahmad N, Peromingo E, Ardura MI, Guillen S, Syed A, Cavuoti D, Ramilo O. 2008. Changing trends in acute osteomyelitis in children: impact of methicillin-resistant Staphylococcus aureus infections. J Pediatr Orthop 28:569–575. doi: 10.1097/BPO.0b013e31817bb816. [DOI] [PubMed] [Google Scholar]
- 499.Kaplan SL. 2014. Recent lessons for the management of bone and joint infections. J Infect 68(Suppl 1):S51–S56. doi: 10.1016/j.jinf.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 500.Peltola H, Paakkonen M. 2014. Acute osteomyelitis in children. N Engl J Med 370:352–360. doi: 10.1056/NEJMra1213956. [DOI] [PubMed] [Google Scholar]
- 501.Paakkonen M, Kallio MJ, Kallio PE, Peltola H. 2010. Sensitivity of erythrocyte sedimentation rate and C-reactive protein in childhood bone and joint infections. Clin Orthop Relat Res 468:861–866. doi: 10.1007/s11999-009-0936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Ju KL, Zurakowski D, Kocher MS. 2011. Differentiating between methicillin-resistant and methicillin-sensitive Staphylococcus aureus osteomyelitis in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am 93:1693–1701. doi: 10.2106/JBJS.J.01154. [DOI] [PubMed] [Google Scholar]
- 503.Wade Shrader M, Nowlin M, Segal LS. 2013. Independent analysis of a clinical predictive algorithm to identify methicillin-resistant Staphylococcus aureus osteomyelitis in children. J Pediatr Orthop 33:759–762. doi: 10.1097/BPO.0b013e3182a11cf7. [DOI] [PubMed] [Google Scholar]
- 504.Hawkshead JJ III, Patel NB, Steele RW, Heinrich SD. 2009. Comparative severity of pediatric osteomyelitis attributable to methicillin-resistant versus methicillin-sensitive Staphylococcus aureus. J Pediatr Orthop 29:85–90. doi: 10.1097/BPO.0b013e3181901c3a. [DOI] [PubMed] [Google Scholar]
- 505.Erdem G, Salazar R, Kimata C, Simasathien T, Len KA, Bergert L, Melish M. 2010. Staphylococcus aureus osteomyelitis in Hawaii. Clin Pediatr (Phila) 49:477–484. doi: 10.1177/0009922809352805. [DOI] [PubMed] [Google Scholar]
- 506.Hollmig ST, Copley LA, Browne RH, Grande LM, Wilson PL. 2007. Deep venous thrombosis associated with osteomyelitis in children. J Bone Joint Surg Am 89:1517–1523. doi: 10.2106/JBJS.F.01102. [DOI] [PubMed] [Google Scholar]
- 507.Gonzalez BE, Teruya J, Mahoney DH Jr, Hulten KG, Edwards R, Lamberth LB, Hammerman WA, Mason EO Jr, Kaplan SL. 2006. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics 117:1673–1679. doi: 10.1542/peds.2005-2009. [DOI] [PubMed] [Google Scholar]
- 508.Bouchoucha S, Benghachame F, Trifa M, Saied W, Douira W, Nessib MN, Ghachem MB. 2010. Deep venous thrombosis associated with acute hematogenous osteomyelitis in children. Orthop Traumatol Surg Res 96:890–893. doi: 10.1016/j.otsr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 509.Crary SE, Buchanan GR, Drake CE, Journeycake JM. 2006. Venous thrombosis and thromboembolism in children with osteomyelitis. J Pediatr 149:537–541. doi: 10.1016/j.jpeds.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 510.Nourse C, Starr M, Munckhof W. 2007. Community-acquired methicillin-resistant Staphylococcus aureus causes severe disseminated infection and deep venous thrombosis in children: literature review and recommendations for management. J Paediatr Child Health 43:656–661. doi: 10.1111/j.1440-1754.2007.01153.x. [DOI] [PubMed] [Google Scholar]
- 511.Altobelli MG, Quinonez RA. 2012. When should DVT be suspected in children with osteomyelitis? Hosp Pediatr 2:167–172. doi: 10.1542/hpeds.2012-0011. [DOI] [PubMed] [Google Scholar]
- 512.Mantadakis E, Plessa E, Vouloumanou EK, Michailidis L, Chatzimichael A, Falagas ME. 2012. Deep venous thrombosis in children with musculoskeletal infections: the clinical evidence. Int J Infect Dis 16:e236–e243. doi: 10.1016/j.ijid.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 513.Peltola H, Paakkonen M, Kallio P, Kallio MJ, Osteomyelitis-Septic Arthritis Study Group . 2010. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J 29:1123–1128. doi: 10.1097/INF.0b013e3181f55a89. [DOI] [PubMed] [Google Scholar]
- 514.Jagodzinski NA, Kanwar R, Graham K, Bache CE. 2009. Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. J Pediatr Orthop 29:518–525. doi: 10.1097/BPO.0b013e3181ab472d. [DOI] [PubMed] [Google Scholar]
- 515.Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. 2006. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics 117:1210–1215. doi: 10.1542/peds.2005-1465. [DOI] [PubMed] [Google Scholar]
- 516.Peltola H, Paakkonen M, Kallio P, Kallio MJ, OM-SA Study Group . 2012. Clindamycin vs. first-generation cephalosporins for acute osteoarticular infections of childhood—a prospective quasi-randomized controlled trial. Clin Microbiol Infect 18:582–589. doi: 10.1111/j.1469-0691.2011.03643.x. [DOI] [PubMed] [Google Scholar]
- 517.Peltola H, Paakkonen M, Kallio P, Kallio MJ, Osteomyelitis-Septic Arthritis Study Group . 2009. Prospective, randomized trial of 10 days versus 30 days of antimicrobial treatment, including a short-term course of parenteral therapy, for childhood septic arthritis. Clin Infect Dis 48:1201–1210. doi: 10.1086/597582. [DOI] [PubMed] [Google Scholar]
- 518.Howard-Jones AR, Isaacs D. 2013. Systematic review of duration and choice of systemic antibiotic therapy for acute haematogenous bacterial osteomyelitis in children. J Paediatr Child Health 49:760–768. doi: 10.1111/jpc.12251. [DOI] [PubMed] [Google Scholar]
- 519.Tuason DA, Gheen T, Sun D, Huang R, Copley L. 2014. Clinical and laboratory parameters associated with multiple surgeries in children with acute hematogenous osteomyelitis. J Pediatr Orthop 34:565–570. doi: 10.1097/BPO.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 520.Darouiche RO. 2004. Treatment of infections associated with surgical implants. N Engl J Med 350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 521.Wymenga A, Van Horn J, Theeuwes A, Muytjens H, Sloof T. 1992. Perioperative factors associated with septic arthritis after arthroplasty. Acta Orthop Scand 63:665–671. doi: 10.1080/17453679209169732. [DOI] [PubMed] [Google Scholar]
- 522.Widmer AF. 2001. New developments in diagnosis and treatment of infection in orthopedic implants. Clin Infect Dis 33(Suppl 2):S94–S106. doi: 10.1086/321863. [DOI] [PubMed] [Google Scholar]
- 523.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. 2010. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 468:52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. 2009. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty 24:105–109. doi: 10.1016/j.arth.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 525.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. 2012. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27:61–65.e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 526.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. 1998. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 27:1247–1254. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 527.Peel TN, Dowsey MM, Daffy JR, Stanley PA, Choong PF, Buising KL. 2011. Risk factors for prosthetic hip and knee infections according to arthroplasty site. J Hosp Infect 79:129–133. doi: 10.1016/j.jhin.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 528.Berbari EF, Osmon DR, Lahr B, Eckel-Passow JE, Tsaras G, Hanssen AD, Mabry T, Steckelberg J, Thompson R. 2012. The Mayo prosthetic joint infection risk score: implication for surgical site infection reporting and risk stratification. Infect Control Hosp Epidemiol 33:774–781. doi: 10.1086/666641. [DOI] [PubMed] [Google Scholar]
- 529.Murdoch DR, Roberts SA, Fowler VG Jr, Shah MA, Taylor SL, Morris AJ, Corey GR. 2001. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis 32:647–649. doi: 10.1086/318704. [DOI] [PubMed] [Google Scholar]
- 530.Sendi P, Banderet F, Graber P, Zimmerli W. 2011. Periprosthetic joint infection following Staphylococcus aureus bacteremia. J Infect 63:17–22. doi: 10.1016/j.jinf.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 531.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37:1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. 1982. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis 146:487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- 533.Gomez J, Rodriguez M, Banos V, Martinex L, Claver MA, Ruiz J, Simarro E, Medina M, Clavel M. 2002. Infections in joint prostheses: epidemiology and clinical presentation. A prospective study 1992-1999. Enferm Infecc Microbiol Clin 20:74–77. (In Spanish.) doi: 10.1016/S0213-005X(02)72745-6. [DOI] [PubMed] [Google Scholar]
- 534.Atkins B, Athanasou N, Deeks J. 1998. Prospective evaluation of criteria for microbiologic diagnosis of prosthetic-joint infection at revision arthroplasty. J Clin Microbiol 36:2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Fink B, Makowiak C, Fuerst M, Berger I, Schafer P, Frommelt L. 2008. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J Bone Joint Surg Br 90:874–878. doi: 10.1302/0301-620X.90B7.20417. [DOI] [PubMed] [Google Scholar]
- 536.Virolainen P, Lahteenmaki H, Hiltunen A, Sipola E, Meurman O, Nelimarkka O. 2002. The reliability of diagnosis of infection during revision arthroplasties. Scand J Surg 91:178–181. [DOI] [PubMed] [Google Scholar]
- 537.Stirling P, Faroug R, Amanat S, Ahmed A, Armstrong M, Sharma P, Qamruddin A. 2014. False-negative rate of gram-stain microscopy for diagnosis of septic arthritis: suggestions for improvement. Int J Microbiol 2014:830857. doi: 10.1155/2014/830857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. 1998. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol 36:2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 540.Scorzolini L, Lichtner M, Iannetta M, Mengoni F, Russo G, Panni AS, Vasso M, Bove M, Villani C, Mastroianni CM, Vullo V. 2014. Sonication technique improves microbiological diagnosis in patients treated with antibiotics before surgery for prosthetic joint infections. New Microbiol 37:321–328. [PubMed] [Google Scholar]
- 541.Zhai Z, Li H, Qin A, Liu G, Liu X, Wu C, Li H, Zhu Z, Qu X, Dai K. 2014. Meta-analysis of sonication fluid samples from prosthetic components for diagnosis of infection after total joint arthroplasty. J Clin Microbiol 52:1730–1736. doi: 10.1128/JCM.03138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Panousis K, Grigoris P, Butcher I, Rana B, Reilly JH, Hamblen DL. 2005. Poor predictive value of broad-range PCR for the detection of arthroplasty infection in 92 cases. Acta Orthop 76:341–346. [PubMed] [Google Scholar]
- 543.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America . 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 544.Peel TN, Buising KL, Choong PF. 2012. Diagnosis and management of prosthetic joint infection. Curr Opin Infect Dis 25:670–676. doi: 10.1097/QCO.0b013e32835915db. [DOI] [PubMed] [Google Scholar]
- 545.Cobo J, Del Pozo JL. 2011. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther 9:787–802. doi: 10.1586/eri.11.95. [DOI] [PubMed] [Google Scholar]
- 546.Cataldo MA, Petrosillo N, Cipriani M, Cauda R, Tacconelli E. 2010. Prosthetic joint infection: recent developments in diagnosis and management. J Infect 61:443–448. doi: 10.1016/j.jinf.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 547.Matthews PC, Berendt AR, McNally MA, Byren I. 2009. Diagnosis and management of prosthetic joint infection. BMJ 338:b1773. doi: 10.1136/bmj.b1773. [DOI] [PubMed] [Google Scholar]
- 548.Beswick AD, Elvers KT, Smith AJ, Gooberman-Hill R, Lovering A, Blom AW. 2012. What is the evidence base to guide surgical treatment of infected hip prostheses? Systematic review of longitudinal studies in unselected patients. BMC Med 10:18. doi: 10.1186/1741-7015-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Peel TN, Buising KL, Dowsey MM, Aboltins CA, Daffy JR, Stanley PA, Choong PF. 2013. Outcome of debridement and retention in prosthetic joint infections by methicillin-resistant staphylococci, with special reference to rifampin and fusidic acid combination therapy. Antimicrob Agents Chemother 57:350–355. doi: 10.1128/AAC.02061-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Senneville E, Joulie D, Legout L, Valette M, Dezeque H, Beltrand E, Rosele B, d'Escrivan T, Loiez C, Caillaux M, Yazdanpanah Y, Maynou C, Migaud H. 2011. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis 53:334–340. doi: 10.1093/cid/cir402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Cobo J, Miguel LG, Euba G, Rodriguez D, Garcia-Lechuz JM, Riera M, Falgueras L, Palomino J, Benito N, del Toro MD, Pigrau C, Ariza J. 2011. Early prosthetic joint infection: outcomes with debridement and implant retention followed by antibiotic therapy. Clin Microbiol Infect 17:1632–1637. doi: 10.1111/j.1469-0691.2010.03333.x. [DOI] [PubMed] [Google Scholar]
- 552.Romano CL, Manzi G, Logoluso N, Romano D. 2012. Value of debridement and irrigation for the treatment of peri-prosthetic infections. A systematic review. Hip Int 22(Suppl 8):S19–S24. doi: 10.5301/HIP.2012.9566. [DOI] [PubMed] [Google Scholar]
- 553.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 554.Tsukayama DT, Estrada R, Gustilo RB. 1996. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am 78:512–523. [DOI] [PubMed] [Google Scholar]
- 555.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 279:1537–1541. [DOI] [PubMed] [Google Scholar]
- 556.Gilbert DN, Kohlhepp SJ, Slama KA, Grunkemeier G, Lewis G, Dworkin RJ, Slaughter SE, Leggett JE. 2001. Phenotypic resistance of Staphylococcus aureus, selected Enterobacteriaceae, and Pseudomonas aeruginosa after single and multiple in vitro exposures to ciprofloxacin, levofloxacin, and trovafloxacin. Antimicrob Agents Chemother 45:883–892. doi: 10.1128/AAC.45.3.883-892.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Eisen DP, Denholm JS. 2013. Recommendations for rifampicin therapy of staphylococcal infection in Infectious Diseases Society of America prosthetic joint infection guidelines are not supported by available literature. Clin Infect Dis 57:159–160. doi: 10.1093/cid/cit183. [DOI] [PubMed] [Google Scholar]
- 558.Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sanchez-Somolinos M, Baraia-Etxaburu JM, Rico A, Palomino J, Rodriguez-Pardo D, Horcajada JP, Benito N, Bahamonde A, Granados A, del Toro MD, Cobo J, Riera M, Ramos A, Jover-Saenz A, Ariza J, REIPI Group for the Study of Prosthetic Infection . 2013. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 56:182–194. doi: 10.1093/cid/cis746. [DOI] [PubMed] [Google Scholar]
- 559.Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. 1999. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am 81:1434–1445. [DOI] [PubMed] [Google Scholar]
- 560.Rao N, Ziran B, Hall R, Santa E. 2004. Successful treatment of chronic bone and joint infections with oral linezolid. Clin Orthop Relat Res 427:67–71. [DOI] [PubMed] [Google Scholar]
- 561.Lipsky B, Itani K, Norden C. 2004. Treating foot infections in diabetic patients: a randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin Infect Dis 38:17–24. doi: 10.1086/380449. [DOI] [PubMed] [Google Scholar]
- 562.Byren I, Rege S, Campanaro E, Yankelev S, Anastasiou D, Kuropatkin G, Evans R. 2012. Randomized controlled trial of the safety and efficacy of daptomycin versus standard-of-care therapy for management of patients with osteomyelitis associated with prosthetic devices undergoing two-stage revision arthroplasty. Antimicrob Agents Chemother 56:5626–5632. doi: 10.1128/AAC.00038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.El Helou OC, Berbari EF, Lahr BD, Eckel-Passow JE, Razonable RR, Sia IG, Virk A, Walker RC, Steckelberg JM, Wilson WR, Hanssen AD, Osmon DR. 2010. Efficacy and safety of rifampin containing regimen for staphylococcal prosthetic joint infections treated with debridement and retention. Eur J Clin Microbiol Infect Dis 29:961–967. doi: 10.1007/s10096-010-0952-9. [DOI] [PubMed] [Google Scholar]
- 564.Shuman EK, Urquhart A, Malani PN. 2012. Management and prevention of prosthetic joint infection. Infect Dis Clin North Am 26:29–39. doi: 10.1016/j.idc.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 565.Zimmerli W. 2000. Prosthetic joint infection: diagnosis and treatment. Curr Infect Dis Rep 2:377–379. doi: 10.1007/s11908-000-0059-z. [DOI] [PubMed] [Google Scholar]
- 566.Sutherland IW. 2001. The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol 9:222–227. doi: 10.1016/S0966-842X(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 567.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. 2012. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 33:5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 568.Houston P, Rowe SE, Pozzi C, Waters EM, O'Gara JP. 2011. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect Immun 79:1153–1165. doi: 10.1128/IAI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 570.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 571.Mehall JR, Saltzman DA, Jackson RJ, Smith SD. 2002. Fibrin sheath enhances central venous catheter infection. Crit Care Med 30:908–912. doi: 10.1097/00003246-200204000-00033. [DOI] [PubMed] [Google Scholar]
- 572.Hoshal VL Jr, Ause RG, Hoskins PA. 1971. Fibrin sleeve formation on indwelling subclavian central venous catheters. Arch Surg 102:353–358. doi: 10.1001/archsurg.1971.01350040115023. [DOI] [PubMed] [Google Scholar]
- 573.Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger UE, Lew DP, Waldvogel FA. 1989. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin. J Infect Dis 160:865–875. doi: 10.1093/infdis/160.5.865. [DOI] [PubMed] [Google Scholar]
- 574.Herrmann M, Vaudaux PE, Pittet D, Auckenthaler R, Lew PD, Schumacher-Perdreau F, Peters G, Waldvogel FA. 1988. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis 158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 575.Xiang DZ, Verbeken EK, Van Lommel AT, Stas M, De Wever I. 1998. Composition and formation of the sleeve enveloping a central venous catheter. J Vasc Surg 28:260–271. doi: 10.1016/S0741-5214(98)70162-4. [DOI] [PubMed] [Google Scholar]
- 576.Padera RF. 2006. Infection in ventricular assist devices: the role of biofilm. Cardiovasc Pathol 15:264–270. doi: 10.1016/j.carpath.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 577.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 578.Lower SK, Lamlertthon S, Casillas-Ituarte NN, Lins RD, Yongsunthon R, Taylor ES, DiBartola AC, Edmonson C, McIntyre LM, Reller LB, Que YA, Ros R, Lower BH, Fowler VG Jr. 2011. Polymorphisms in fibronectin binding protein A of Staphylococcus aureus are associated with infection of cardiovascular devices. Proc Natl Acad Sci U S A 108:18372–18377. doi: 10.1073/pnas.1109071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Fitzpatrick F, Humphreys H, O'Gara JP. 2005. The genetics of staphylococcal biofilm formation—will a greater understanding of pathogenesis lead to better management of device-related infection? Clin Microbiol Infect 11:967–973. doi: 10.1111/j.1469-0691.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 580.Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67:5427–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Arciola CR, Baldassarri L, Montanaro L. 2001. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol 39:2151–2156. doi: 10.1128/JCM.39.6.2151-2156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O'Gara JP. 2012. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog 8:e1002626. doi: 10.1371/journal.ppat.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS. 2004. Global gene expression in Staphylococcus aureus biofilms. J Bacteriol 186:4665–4684. doi: 10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Fitzpatrick F, Humphreys H, O'Gara JP. 2005. Evidence for icaADBC-independent biofilm development mechanism in methicillin-resistant Staphylococcus aureus clinical isolates. J Clin Microbiol 43:1973–1976. doi: 10.1128/JCM.43.4.1973-1976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Vergara-Irigaray M, Valle J, Merino N, Latasa C, Garcia B, Ruiz de Los Mozos I, Solano C, Toledo-Arana A, Penades JR, Lasa I. 2009. Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect Immun 77:3978–3991. doi: 10.1128/IAI.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.O'Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, Loughman A, Foster TJ, O'Gara JP. 2008. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol 190:3835–3850. doi: 10.1128/JB.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.O'Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O'Gara JP. 2007. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol 45:1379–1388. doi: 10.1128/JCM.02280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Vuong C, Saenz HL, Gotz F, Otto M. 2000. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis 182:1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- 589.Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick RP, Muir TW. 1999. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci U S A 96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Alter P, Waldhans S, Plachta E, Moosdorf R, Grimm W. 2005. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol 28:926–932. doi: 10.1111/j.1540-8159.2005.00195.x. [DOI] [PubMed] [Google Scholar]
- 591.Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N, Rey JL, Lande G, Lazarus A, Victor J, Barnay C, Grandbastien B, Kacet S, PEOPLE Study Group . 2007. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 592.Lekkerkerker JC, van Nieuwkoop C, Trines SA, van der Bom JG, Bernards A, van de Velde ET, Bootsma M, Zeppenfeld K, Jukema JW, Borleffs JW, Schalij MJ, van Erven L. 2009. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart 95:715–720. doi: 10.1136/hrt.2008.151985. [DOI] [PubMed] [Google Scholar]
- 593.Pfeiffer D, Jung W, Fehske W, Korte T, Manz M, Moosdorf R, Luderitz B. 1994. Complications of pacemaker-defibrillator devices: diagnosis and management. Am Heart J 127:1073–1080. doi: 10.1016/0002-8703(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 594.Uslan DZ, Sohail MR, St Sauver JL, Friedman PA, Hayes DL, Stoner SM, Wilson WR, Steckelberg JM, Baddour LM. 2007. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 167:669–675. doi: 10.1001/archinte.167.7.669. [DOI] [PubMed] [Google Scholar]
- 595.Johansen JB, Jorgensen OD, Moller M, Arnsbo P, Mortensen PT, Nielsen JC. 2011. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J 32:991–998. doi: 10.1093/eurheartj/ehq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Nery PB, Fernandes R, Nair GM, Sumner GL, Ribas CS, Menon SM, Wang X, Krahn AD, Morillo CA, Connolly SJ, Healey JS. 2010. Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J Cardiovasc Electrophysiol 21:786–790. doi: 10.1111/j.1540-8167.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 597.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 2011. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 58:1001–1006. doi: 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 598.Catanchin A, Murdock CJ, Athan E. 2007. Pacemaker infections: a 10-year experience. Heart Lung Circ 16:434–439. doi: 10.1016/j.hlc.2007.02.097. [DOI] [PubMed] [Google Scholar]
- 599.Rodriguez DJ, Afzal A, Evonich R, Haines DE. 2012. The prevalence of methicillin resistant organisms among pacemaker and defibrillator implant recipients. Am J Cardiovasc Dis 2:116–122. [PMC free article] [PubMed] [Google Scholar]
- 600.Cabell CH, Heidenreich PA, Chu VH, Moore CM, Stryjewski ME, Corey GR, Fowler VG Jr. 2004. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990-1999. Am Heart J 147:582–586. doi: 10.1016/j.ahj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 601.Chamis AL, Peterson GE, Cabell CH, Corey GR, Sorrentino RA, Greenfield RA, Ryan T, Reller LB, Fowler VG Jr. 2001. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 104:1029–1033. doi: 10.1161/hc3401.095097. [DOI] [PubMed] [Google Scholar]
- 602.Uslan DZ, Dowsley TF, Sohail MR, Hayes DL, Friedman PA, Wilson WR, Steckelberg JM, Baddour LM. 2010. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol 33:407–413. doi: 10.1111/j.1540-8159.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 603.Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. 2000. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med 133:604–608. doi: 10.7326/0003-4819-133-8-200010170-00011. [DOI] [PubMed] [Google Scholar]
- 604.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner S, Baddour LM. 2007. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 49:1851–1859. doi: 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 605.Trappe HJ, Pfitzner P, Klein H, Wenzlaff P. 1995. Infections after cardioverter-defibrillator implantation: observations in 335 patients over 10 years. Br Heart J 73:20–24. doi: 10.1136/hrt.73.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Smit J, Korup E, Schonheyder HC. 2010. Infections associated with permanent pacemakers and implanted cardioverter-defibrillator devices. A 10-year regional study in Denmark. Scand J Infect Dis 42:658–664. doi: 10.3109/00365548.2010.482943. [DOI] [PubMed] [Google Scholar]
- 607.Roig IL, Darouiche RO, Musher DM, Trautner BW. 2012. Device-related infective endocarditis, with special consideration of implanted intravascular and cardiac devices in a predominantly male population. Scand J Infect Dis 44:753–760. doi: 10.3109/00365548.2012.678882. [DOI] [PubMed] [Google Scholar]
- 608.Tarakji KG, Chan EJ, Cantillon DJ, Doonan AL, Hu T, Schmitt S, Fraser TG, Kim A, Gordon SM, Wilkoff BL. 2010. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 7:1043–1047. doi: 10.1016/j.hrthm.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 609.Le KY, Sohail MR, Friedman PA, Uslan DZ, Cha SS, Hayes DL, Wilson WR, Steckelberg JM, Baddour LM, Mayo Cardiovascular Infections Study Group . 2012. Clinical features and outcomes of cardiovascular implantable electronic device infections due to staphylococcal species. Am J Cardiol 110:1143–1149. doi: 10.1016/j.amjcard.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 610.Obeid KM, Szpunar S, Khatib R. 2012. Long-term outcomes of cardiovascular implantable electronic devices in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol 35:961–965. doi: 10.1111/j.1540-8159.2012.03438.x. [DOI] [PubMed] [Google Scholar]
- 611.Camus C, Leport C, Raffi F, Michelet C, Cartier F, Vilde JL. 1993. Sustained bacteremia in 26 patients with a permanent endocardial pacemaker: assessment of wire removal. Clin Infect Dis 17:46–55. doi: 10.1093/clinids/17.1.46. [DOI] [PubMed] [Google Scholar]
- 612.Chu VH, Crosslin DR, Friedman JY, Reed SD, Cabell CH, Griffiths RI, Masselink LE, Kaye KS, Corey GR, Reller LB, Stryjewski ME, Schulman KA, Fowler VG Jr. 2005. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am J Med 118:1416.e19–1416.e24. doi: 10.1016/j.amjmed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 613.Viola GM, Awan LL, Ostrosky-Zeichner L, Chan W, Darouiche RO. 2012. Infections of cardiac implantable electronic devices: a retrospective multicenter observational study. Medicine 91:123–130. doi: 10.1097/MD.0b013e31825592a7. [DOI] [PubMed] [Google Scholar]
- 614.Klug D, Wallet F, Lacroix D, Marquie C, Kouakam C, Kacet S, Courcol R. 2004. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart 90:882–886. doi: 10.1136/hrt.2003.010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, Masoudi FA, Okum EJ, Wilson WR, Beerman LB, Bolger AF, Estes NA III, Gewitz M, Newburger JW, Schron EB, Taubert KA, American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in Young, Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular Nursing, Council on Clinical Cardiology, Interdisciplinary Council on Quality of Care, American Heart Association . 2010. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 616.Dy Chua J, Abdul-Karim A, Mawhorter S, Procop GW, Tchou P, Niebauer M, Saliba W, Schweikert R, Wilkoff BL. 2005. The role of swab and tissue culture in the diagnosis of implantable cardiac device infection. Pacing Clin Electrophysiol 28:1276–1281. doi: 10.1111/j.1540-8159.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 617.Klug D, Lacroix D, Savoye C, Goullard L, Grandmougin D, Hennequin JL, Kacet S, Lekieffre J. 1997. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation 95:2098–2107. doi: 10.1161/01.CIR.95.8.2098. [DOI] [PubMed] [Google Scholar]
- 618.de Cicco M, Panarello G, Chiaradia V, Fracasso A, Veronesi A, Testa V, Santini G, Tesio F. 1989. Source and route of microbial colonisation of parenteral nutrition catheters. Lancet ii:1258–1261. [DOI] [PubMed] [Google Scholar]
- 619.Salzman MB, Isenberg HD, Shapiro JF, Lipsitz PJ, Rubin LG. 1993. A prospective study of the catheter hub as the portal of entry for microorganisms causing catheter-related sepsis in neonates. J Infect Dis 167:487–490. doi: 10.1093/infdis/167.2.487. [DOI] [PubMed] [Google Scholar]
- 620.Linares J, Sitges-Serra A, Garau J, Perez JL, Martin R. 1985. Pathogenesis of catheter sepsis: a prospective study with quantitative and semiquantitative cultures of catheter hub and segments. J Clin Microbiol 21:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Chitnis AS, Edwards JR, Ricks PM, Sievert DM, Fridkin SK, Gould CV. 2012. Device-associated infection rates, device utilization, and antimicrobial resistance in long-term acute care hospitals reporting to the National Healthcare Safety Network, 2010. Infect Control Hosp Epidemiol 33:993–1000. doi: 10.1086/667745. [DOI] [PubMed] [Google Scholar]
- 622.Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, Leblebicioglu H, Fisher D, Alvarez-Moreno C, Khader IA, Del Rocio Gonzalez Martinez M, Cuellar LE, Navoa-Ng JA, Abouqal R, Guanche Garcell H, Mitrev Z, Pirez Garcia MC, Hamdi A, Duenas L, Cancel E, Gurskis V, Rasslan O, Ahmed A, Kanj SS, Ugalde OC, Mapp T, Raka L, Yuet Meng C, Thu LTA, Ghazal S, Gikas A, Narvaez LP, Mejia N, Hadjieva N, Gamar Elanbya MO, Guzman Siritt ME, Jayatilleke K, INICC Members. 2012. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control 40:396–407. doi: 10.1016/j.ajic.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 623.Tao L, Hu B, Rosenthal VD, Gao X, He L. 2011. Device-associated infection rates in 398 intensive care units in Shanghai, China: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis 15:e774–e780. doi: 10.1016/j.ijid.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 624.Sadoyma G, Diogo Filho A, Gontijo Filho PP. 2006. Central venous catheter-related bloodstream infection caused by Staphylococcus aureus: microbiology and risk factors. Braz J Infect Dis 10:100–106. doi: 10.1590/S1413-86702006000200006. [DOI] [PubMed] [Google Scholar]
- 625.Collignon P, Soni N, Pearson I, Sorrell T, Woods P. 1988. Sepsis associated with central vein catheters in critically ill patients. Intensive Care Med 14:227–231. doi: 10.1007/BF00717995. [DOI] [PubMed] [Google Scholar]
- 626.Collignon PJ, Munro R, Sorrell TC. 1984. Systemic sepsis and intravenous devices. A prospective survey. Med J Aust 141:345–348. [DOI] [PubMed] [Google Scholar]
- 627.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 628.Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, Craven DE, Infectious Diseases Society of America, American College of Critical Care Medicine, Society for Healthcare Epidemiology of America . 2001. Guidelines for the management of intravascular catheter-related infections. Infect Control Hosp Epidemiol 22:222–242. doi: 10.1086/501893. [DOI] [PubMed] [Google Scholar]
- 629.Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. 1995. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med 155:1161–1166. [DOI] [PubMed] [Google Scholar]
- 630.Thomas MG, Morris AJ. 2005. Cannula-associated Staphylococcus aureus bacteraemia: outcome in relation to treatment. Intern Med J 35:319–330. doi: 10.1111/j.1445-5994.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 631.Mylotte JM, McDermott C. 1987. Staphylococcus aureus bacteremia caused by infected intravenous catheters. Am J Infect Control 15:1–6. doi: 10.1016/0196-6553(87)90069-1. [DOI] [PubMed] [Google Scholar]
- 632.Dugdale DC, Ramsey PG. 1990. Staphylococcus aureus bacteremia in patients with Hickman catheters. Am J Med 89:137–141. doi: 10.1016/0002-9343(90)90290-T. [DOI] [PubMed] [Google Scholar]
- 633.Rosen AB, Fowler VG Jr, Corey GR, Downs SM, Biddle AK, Li J, Jollis JG. 1999. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med 130:810–820. doi: 10.7326/0003-4819-130-10-199905180-00004. [DOI] [PubMed] [Google Scholar]
- 634.Centers for Disease Control and Prevention. 2011. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep 60:243–248. [PubMed] [Google Scholar]
- 635.Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force . 2002. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control 30:S1–S46. doi: 10.1067/mic.2002.130391. [DOI] [PubMed] [Google Scholar]
- 636.Larson EL, Quiros D, Lin SX. 2007. Dissemination of the CDC's Hand Hygiene Guideline and impact on infection rates. Am J Infect Control 35:666–675. doi: 10.1016/j.ajic.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Yoshida J, Ishimaru T, Kikuchi T, Matsubara N, Ueno T, Hirata N, Koyanagi N. 2010. Central line-associated bloodstream infection: is the hospital epidemiology of methicillin-resistant Staphylococcus aureus relevant? J Infect Chemother 16:33–37. doi: 10.1007/s10156-009-0018-Z. [DOI] [PubMed] [Google Scholar]
- 638.Marr KA, Sexton DJ, Conlon PJ, Corey GR, Schwab SJ, Kirkland KB. 1997. Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med 127:275–280. doi: 10.7326/0003-4819-127-4-199708150-00003. [DOI] [PubMed] [Google Scholar]
- 639.Inrig JK, Reed SD, Szczech LA, Engemann JJ, Friedman JY, Corey GR, Schulman KA, Reller LB, Fowler VG Jr. 2006. Relationship between clinical outcomes and vascular access type among hemodialysis patients with Staphylococcus aureus bacteremia. Clin J Am Soc Nephrol 1:518–524. doi: 10.2215/CJN.01301005. [DOI] [PubMed] [Google Scholar]
- 640.Ehni WF, Reller LB. 1989. Short-course therapy for catheter-associated Staphylococcus aureus bacteremia. Arch Intern Med 149:533–536. [PubMed] [Google Scholar]
- 641.Nielsen J, Ladefoged SD, Kolmos HJ. 1998. Dialysis catheter-related septicaemia—focus on Staphylococcus aureus septicaemia. Nephrol Dial Transplant 13:2847–2852. doi: 10.1093/ndt/13.11.2847. [DOI] [PubMed] [Google Scholar]
- 642.Pearson ML. 1996. Guideline for prevention of intravascular device-related infections. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 17:438–473. doi: 10.1086/647338. [DOI] [PubMed] [Google Scholar]
- 643.Fowler VG Jr, Justice A, Moore C, Benjamin DK Jr, Woods CW, Campbell S, Reller LB, Corey GR, Day NP, Peacock SJ. 2005. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 40:695–703. doi: 10.1086/427806. [DOI] [PubMed] [Google Scholar]
- 644.Libman H, Arbeit RD. 1984. Complications associated with Staphylococcus aureus bacteremia. Arch Intern Med 144:541–545. doi: 10.1001/archinte.1984.00350150137033. [DOI] [PubMed] [Google Scholar]
- 645.Shah M, Watanakunakorn C. 1979. Changing patterns of Staphylococcus aureus bacteremia. Am J Med Sci 278:115–121. doi: 10.1097/00000441-197909000-00002. [DOI] [PubMed] [Google Scholar]
- 646.Crowley AL, Peterson GE, Benjamin DK Jr, Rimmer SH, Todd C, Cabell CH, Reller LB, Ryan T, Corey GR, Fowler VG Jr. 2008. Venous thrombosis in patients with short- and long-term central venous catheter-associated Staphylococcus aureus bacteremia. Crit Care Med 36:385–390. doi: 10.1097/01.CCM.0B013E3181611F914. [DOI] [PubMed] [Google Scholar]
- 647.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Centers for Disease Control and Prevention. 2005. Reduction in central line-associated bloodstream infections among patients in intensive care units—Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep 54:1013–1016. [PubMed] [Google Scholar]
- 649.Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. 2000. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet 355:1864–1868. doi: 10.1016/S0140-6736(00)02291-1. [DOI] [PubMed] [Google Scholar]
- 650.Maki DG, Ringer M, Alvarado CJ. 1991. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet 338:339–343. doi: 10.1016/0140-6736(91)90479-9. [DOI] [PubMed] [Google Scholar]
- 651.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph A, Rupp ME, Saint S, Healthcare Infection Control Practices Advisory Committee . 2011. Guidelines for the prevention of intravascular cather-related infections, 2011. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. [Google Scholar]
- 652.Fowler VG Jr, Sanders LL, Sexton DJ, Kong L, Marr KA, Gopal AK, Gottlieb G, McClelland RS, Corey GR. 1998. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 27:478–486. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 653.Poole CV, Carlton D, Bimbo L, Allon M. 2004. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant 19:1237–1244. doi: 10.1093/ndt/gfh041. [DOI] [PubMed] [Google Scholar]
- 654.McGrath MH, Burkhardt BR. 1984. The safety and efficacy of breast implants for augmentation mammaplasty. Plast Reconstr Surg 74:550–560. doi: 10.1097/00006534-198410000-00019. [DOI] [PubMed] [Google Scholar]
- 655.Brand KG. 1993. Infection of mammary prostheses: a survey and the question of prevention. Ann Plast Surg 30:289–295. doi: 10.1097/00000637-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 656.Kjoller K, Holmich LR, Jacobsen PH, Friis S, Fryzek J, McLaughlin JK, Lipworth L, Henriksen TF, Jorgensen S, Bittmann S, Olsen JH. 2002. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 48:229–237. doi: 10.1097/00000637-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 657.De Cholnoky T. 1970. Augmentation mammaplasty. Survey of complications in 10,941 patients by 265 surgeons. Plast Reconstr Surg 45:573–577. [PubMed] [Google Scholar]
- 658.Feldman EM, Kontoyiannis DP, Sharabi SE, Lee E, Kaufman Y, Heller L. 2010. Breast implant infections: is cefazolin enough? Plast Reconstr Surg 126:779–785. doi: 10.1097/PRS.0b013e3181e5f7ff. [DOI] [PubMed] [Google Scholar]
- 659.Ahn CY, Ko CY, Wagar EA, Wong RS, Shaw WW. 1996. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstr Surg 98:1225–1229. doi: 10.1097/00006534-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 660.Pittet B, Montandon D, Pittet D. 2005. Infection in breast implants. Lancet Infect Dis 5:94–106. doi: 10.1016/S1473-3099(05)01281-8. [DOI] [PubMed] [Google Scholar]
- 661.Siggelkow W, Klosterhalfen B, Klinge U, Rath W, Faridi A. 2004. Analysis of local complications following explantation of silicone breast implants. Breast 13:122–128. doi: 10.1016/j.breast.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 662.Darouiche RO, Meade R, Mansouri MD, Netscher DT. 2002. In vivo efficacy of antimicrobe-impregnated saline-filled silicone implants. Plast Reconstr Surg 109:1352–1357. doi: 10.1097/00006534-200204010-00022. [DOI] [PubMed] [Google Scholar]
- 663.Schoenbaum SC, Gardner P, Shillito J. 1975. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis 131:543–552. doi: 10.1093/infdis/131.5.543. [DOI] [PubMed] [Google Scholar]
- 664.Kulkarni AV, Drake JM, Lamberti-Pasculli M. 2001. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg 94:195–201. doi: 10.3171/jns.2001.94.2.0195. [DOI] [PubMed] [Google Scholar]
- 665.Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP. 1984. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg 60:1014–1021. [DOI] [PubMed] [Google Scholar]
- 666.Kestle JR, Garton HJ, Whitehead WE, Drake JM, Kulkarni AV, Cochrane DD, Muszynski C, Walker ML. 2006. Management of shunt infections: a multicenter pilot study. J Neurosurg 105:177–181. [DOI] [PubMed] [Google Scholar]
- 667.Renier D, Lacombe J, Pierre-Kahn A, Sainte-Rose C, Hirsch JF. 1984. Factors causing acute shunt infection. Computer analysis of 1174 operations. J Neurosurg 61:1072–1078. [DOI] [PubMed] [Google Scholar]
- 668.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. 2004. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 39:1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 669.Wilson SK, Delk JR II. 1995. Inflatable penile implant infection: predisposing factors and treatment suggestions. J Urol 153:659–661. doi: 10.1016/S0022-5347(01)67678-X. [DOI] [PubMed] [Google Scholar]
- 670.Eid JF, Wilson SK, Cleves M, Salem EA. 2012. Coated implants and “no touch” surgical technique decreases risk of infection in inflatable penile prosthesis implantation to 0.46%. Urology 79:1310–1315. doi: 10.1016/j.urology.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 671.Darouiche RO, Bella AJ, Boone TB, Brock G, Broderick GA, Burnett AL, Carrion R, Carson C III, Christine B, Dhabuwala CB, Hakim LS, Henry G, Jones LA, Khera M, Montague DK, Nehra A. 2013. North American consensus document on infection of penile prostheses. Urology 82:937–942. doi: 10.1016/j.urology.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 672.Chickering HT, Park JH. 1919. Staphylococcus aureus pneumonia. JAMA 72:617–626. doi: 10.1001/jama.1919.02610090001001. [DOI] [Google Scholar]
- 673.Wallace HJ. 1937. Specimen from a case of staphylococcal pneumonia. Proc R Soc Med 30:885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Johnson A. 1944. Report on a case of staphylococcal pneumonia with staphylococcal septicaemia: treated with penicillin. Ulster Med J 13:122-3–122-4, 144–149. [PMC free article] [PubMed] [Google Scholar]
- 675.American Thoracic Society, Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 676.Safdar N, Crnich CJ, Maki DG. 2005. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care 50:725–739. [PubMed] [Google Scholar]
- 677.Nseir S, Di Pompeo C, Pronnier P, Beague S, Onimus T, Saulnier F, Grandbastien B, Mathieu D, Delvallez-Roussel M, Durocher A. 2002. Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur Respir J 20:1483–1489. doi: 10.1183/09031936.02.00012902. [DOI] [PubMed] [Google Scholar]
- 678.Rello J, Quintana E, Ausina V, Castella J, Luquin M, Net A, Prats G. 1991. Incidence, etiology, and outcome of nosocomial pneumonia in mechanically ventilated patients. Chest 100:439–444. doi: 10.1378/chest.100.2.439. [DOI] [PubMed] [Google Scholar]
- 679.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. 2005. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 680.Lee MS, Walker V, Chen LF, Sexton DJ, Anderson DJ. 2013. The epidemiology of ventilator-associated pneumonia in a network of community hospitals: a prospective multicenter study. Infect Control Hosp Epidemiol 34:657–662. doi: 10.1086/670991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Jones RN. 2003. Global epidemiology of antimicrobial resistance among community-acquired and nosocomial pathogens: a five-year summary from the SENTRY Antimicrobial Surveillance Program (1997-2001). Semin Respir Crit Care Med 24:121–134. doi: 10.1055/s-2003-37923. [DOI] [PubMed] [Google Scholar]
- 682.Chawla R. 2008. Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries. Am J Infect Control 36:S93–S100. doi: 10.1016/j.ajic.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 683.Shorr AF, Tabak YP, Gupta V, Johannes RS, Liu LZ, Kollef MH. 2006. Morbidity and cost burden of methicillin-resistant Staphylococcus aureus in early onset ventilator-associated pneumonia. Crit Care 10:R97. doi: 10.1186/cc4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Echols RM, Tillotson GS, Song JX, Tosiello RL. 2008. Clinical trial design for mild-to-moderate community-acquired pneumonia—an industry perspective. Clin Infect Dis 47(Suppl 3):S166–S175. doi: 10.1086/591399. [DOI] [PubMed] [Google Scholar]
- 685.Jones RN. 2010. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 51(Suppl 1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 686.Osiyemi O, Dickinson G. 2000. Gram-positive pneumonia. Curr Infect Dis Rep 2:207–214. doi: 10.1007/s11908-000-0037-5. [DOI] [PubMed] [Google Scholar]
- 687.Carratala J, Mykietiuk A, Fernandez-Sabe N, Suarez C, Dorca J, Verdaguer R, Manresa F, Gudiol F. 2007. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med 167:1393–1399. doi: 10.1001/archinte.167.13.1393. [DOI] [PubMed] [Google Scholar]
- 688.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piemont Y, Brousse N, Floret D, Etienne J. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 689.Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 690.Kallen AJ, Reed C, Patton M, Arnold KE, Finelli L, Hageman J. 2010. Staphylococcus aureus community-onset pneumonia in patients admitted to children's hospitals during autumn and winter of 2006-2007. Epidemiol Infect 138:666–672. doi: 10.1017/S095026880999135X. [DOI] [PubMed] [Google Scholar]
- 691.Hageman JC, Uyeki TM, Francis JS, Jernigan DB, Wheeler JG, Bridges CB, Barenkamp SJ, Sievert DM, Srinivasan A, Doherty MC, McDougal LK, Killgore GE, Lopatin UA, Coffman R, MacDonald JK, McAllister SK, Fosheim GE, Patel JB, McDonald LC. 2006. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis 12:894–899. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Centers for Disease Control and Prevention. 2007. Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006-January 2007. MMWR Morb Mortal Wkly Rep 56:325–329. [PubMed] [Google Scholar]
- 693.Gonzalez BE, Hulten KG, Dishop MK, Lamberth LB, Hammerman WA, Mason EO Jr, Kaplan SL. 2005. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis 41:583–590. doi: 10.1086/432475. [DOI] [PubMed] [Google Scholar]
- 694.Tong SY, Anstey NM, Lum GD, Lilliebridge RA, Stephens DP, Currie BJ. 2008. Fatal community-associated methicillin-resistant Staphylococcus aureus pneumonia after influenza. Med J Aust 188:61. [DOI] [PubMed] [Google Scholar]
- 695.Peleg AY, Munckhof WJ. 2004. Fatal necrotising pneumonia due to community-acquired methicillin-resistant Staphylococcus aureus (MRSA). Med J Aust 181:228–229. [DOI] [PubMed] [Google Scholar]
- 696.Wunderink RG. 2013. How important is methicillin-resistant Staphylococcus aureus as a cause of community-acquired pneumonia and what is best antimicrobial therapy? Infect Dis Clin North Am 27:177–188. doi: 10.1016/j.idc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 697.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, Albrecht V, Limbago B, Talan DA, EMERGEncy ID NET Study Group . 2012. Prevalence of methicillin-resistant Staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis 54:1126–1133. doi: 10.1093/cid/cis022. [DOI] [PubMed] [Google Scholar]
- 698.Chalmers JD, Taylor JK, Singanayagam A, Fleming GB, Akram AR, Mandal P, Choudhury G, Hill AT. 2011. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis 53:107–113. doi: 10.1093/cid/cir274. [DOI] [PubMed] [Google Scholar]
- 699.Charles PG, Whitby M, Fuller AJ, Stirling R, Wright AA, Korman TM, Holmes PW, Christiansen KJ, Waterer GW, Pierce RJ, Mayall BC, Armstrong JG, Catton MG, Nimmo GR, Johnson B, Hooy M, Grayson ML, Australian CAP Study Collaboration . 2008. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis 46:1513–1521. doi: 10.1086/586749. [DOI] [PubMed] [Google Scholar]
- 700.Bryant RE, Salmon CJ. 1996. Pleural empyema. Clin Infect Dis 22:747–762; quiz 763–744. doi: 10.1093/clinids/22.5.747. [DOI] [PubMed] [Google Scholar]
- 701.Oki T, Funai K, Sekihara K, Shimizu K, Shiiya N. 2013. Refractory methicillin-resistant Staphylococcus aureus empyema invasion from a cervical abscess: report of a case. Kyobu Geka 66:852–854. [PubMed] [Google Scholar]
- 702.Huang SS, Hinrichsen VL, Datta R, Spurchise L, Miroshnik I, Nelson K, Platt R. 2011. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PLoS One 6:e24340. doi: 10.1371/journal.pone.0024340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Datta R, Huang SS. 2008. Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis 47:176–181. doi: 10.1086/589241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Wooten DA, Winston LG. 2013. Risk factors for methicillin-resistant Staphylococcus aureus in patients with community-onset and hospital-onset pneumonia. Respir Med 107:1266–1270. doi: 10.1016/j.rmed.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 705.Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, Goto Y, Fukui Y, Iwaki M, Okumura J, Yamaguchi I, Yagi T, Tanikawa Y, Sugino Y, Shindoh J, Ogasawara T, Nomura F, Saka H, Yamamoto M, Taniguchi H, Suzuki R, Saito H, Kawamura T, Hasegawa Y. 2013. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med 188:985–995. doi: 10.1164/rccm.201301-0079OC. [DOI] [PubMed] [Google Scholar]
- 706.Venditti M, Falcone M, Corrao S, Licata G, Serra P, Study Group of the Italian Society of Internal Medicine . 2009. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med 150:19–26. doi: 10.7326/0003-4819-150-1-200901060-00005. [DOI] [PubMed] [Google Scholar]
- 707.Ma HM, Ip M, Woo J, Hui DS, Lui GC, Lee NL, Chan PK, Rainer TH. 2013. Risk factors for drug-resistant bacterial pneumonia in older patients hospitalized with pneumonia in a Chinese population. QJM 106:823–829. doi: 10.1093/qjmed/hct152. [DOI] [PubMed] [Google Scholar]
- 708.Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, So TM, Yasin RM, Hsueh PR, Carlos CC, Hsu LY, Buntaran L, Lalitha MK, Kim MJ, Choi JY, Kim SI, Ko KS, Kang CI, Peck KR, Asian Network for Surveillance of Resistant Pathogens Study Group . 2011. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med 184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 709.Yap V, Datta D, Metersky ML. 2013. Is the present definition of health care-associated pneumonia the best way to define risk of infection with antibiotic-resistant pathogens? Infect Dis Clin North Am 27:1–18. doi: 10.1016/j.idc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 710.Garcia-Vidal C, Viasus D, Roset A, Adamuz J, Verdaguer R, Dorca J, Gudiol F, Carratala J. 2011. Low incidence of multidrug-resistant organisms in patients with healthcare-associated pneumonia requiring hospitalization. Clin Microbiol Infect 17:1659–1665. doi: 10.1111/j.1469-0691.2011.03484.x. [DOI] [PubMed] [Google Scholar]
- 711.Shorr AF, Zilberberg MD, Reichley R, Kan J, Hoban A, Hoffman J, Micek ST, Kollef MH. 2012. Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clin Infect Dis 54:193–198. doi: 10.1093/cid/cir813. [DOI] [PubMed] [Google Scholar]
- 712.Shorr AF, Myers DE, Huang DB, Nathanson BH, Emons MF, Kollef MH. 2013. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect Dis 13:268. doi: 10.1186/1471-2334-13-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Aliberti S, Di Pasquale M, Zanaboni AM, Cosentini R, Brambilla AM, Seghezzi S, Tarsia P, Mantero M, Blasi F. 2012. Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis 54:470–478. doi: 10.1093/cid/cir840. [DOI] [PubMed] [Google Scholar]
- 714.Aliberti S, Cilloniz C, Chalmers JD, Zanaboni AM, Cosentini R, Tarsia P, Pesci A, Blasi F, Torres A. 2013. Multidrug-resistant pathogens in hospitalised patients coming from the community with pneumonia: a European perspective. Thorax 68:997–999. doi: 10.1136/thoraxjnl-2013-203384. [DOI] [PubMed] [Google Scholar]
- 715.Park SC, Kang YA, Park BH, Kim EY, Park MS, Kim YS, Kim SK, Chang J, Jung JY. 2012. Poor prediction of potentially drug-resistant pathogens using current criteria of health care-associated pneumonia. Respir Med 106:1311–1319. doi: 10.1016/j.rmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 716.Adem PV, Montgomery CP, Husain AN, Koogler TK, Arangelovich V, Humilier M, Boyle-Vavra S, Daum RS. 2005. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med 353:1245–1251. doi: 10.1056/NEJMoa044194. [DOI] [PubMed] [Google Scholar]
- 717.Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, Vandenesch F, Bowden MG. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 718.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 720.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. 2012. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Parimon T, Li Z, Bolz DD, McIndoo ER, Bayer CR, Stevens DL, Bryant AE. 2013. Staphylococcus aureus alpha-hemolysin promotes platelet-neutrophil aggregate formation. J Infect Dis 208:761–770. doi: 10.1093/infdis/jit235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.DeLeo FR, Otto M. 2008. An antidote for Staphylococcus aureus pneumonia? J Exp Med 205:271–274. doi: 10.1084/jem.20080167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, Keller A, O'Day T, Du Q, Cheng L, Damschroder M, Robbie G, Suzich J, Stover CK, Sellman BR. 2014. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58:1108–1117. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.DeRyke CA, Lodise TP Jr, Rybak MJ, McKinnon PS. 2005. Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest 128:1414–1422. doi: 10.1378/chest.128.3.1414. [DOI] [PubMed] [Google Scholar]
- 725.Vidaur L, Planas K, Sierra R, Dimopoulos G, Ramirez A, Lisboa T, Rello J. 2008. Ventilator-associated pneumonia: impact of organisms on clinical resolution and medical resources utilization. Chest 133:625–632. doi: 10.1378/chest.07-2020. [DOI] [PubMed] [Google Scholar]
- 726.Zahar JR, Clec'h C, Tafflet M, Garrouste-Orgeas M, Jamali S, Mourvillier B, De Lassence A, Descorps-Declere A, Adrie C, Costa de Beauregard MA, Azoulay E, Schwebel C, Timsit JF, Outcomerea Study Group . 2005. Is methicillin resistance associated with a worse prognosis in Staphylococcus aureus ventilator-associated pneumonia? Clin Infect Dis 41:1224–1231. doi: 10.1086/496923. [DOI] [PubMed] [Google Scholar]
- 727.Gillet Y, Vanhems P, Lina G, Bes M, Vandenesch F, Floret D, Etienne J. 2007. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin Infect Dis 45:315–321. doi: 10.1086/519263. [DOI] [PubMed] [Google Scholar]
- 728.Rubinstein E, Kollef MH, Nathwani D. 2008. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46(Suppl 5):S378–S385. doi: 10.1086/533594. [DOI] [PubMed] [Google Scholar]
- 729.Cuquemelle E, Soulis F, Villers D, Roche-Campo F, Ara Somohano C, Fartoukh M, Kouatchet A, Mourvillier B, Dellamonica J, Picard W, Schmidt M, Boulain T, Brun-Buisson C, A/H1N1 REVA-SRLF Study Group . 2011. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med 37:796–800. doi: 10.1007/s00134-011-2189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Lobo LJ, Reed KD, Wunderink RG. 2010. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest 138:130–136. doi: 10.1378/chest.09-1562. [DOI] [PubMed] [Google Scholar]
- 731.Sicot N, Khanafer N, Meyssonnier V, Dumitrescu O, Tristan A, Bes M, Lina G, Vandenesch F, Vanhems P, Etienne J, Gillet Y. 2013. Methicillin resistance is not a predictor of severity in community-acquired Staphylococcus aureus necrotizing pneumonia—results of a prospective observational study. Clin Microbiol Infect 19:E142–E148. doi: 10.1111/1469-0691.12022. [DOI] [PubMed] [Google Scholar]
- 732.Vardakas KZ, Matthaiou DK, Falagas ME. 2009. Comparison of community-acquired pneumonia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus producing the Panton-Valentine leukocidin. Int J Tuberc Lung Dis 13:1476–1485. [PubMed] [Google Scholar]
- 733.Cystic Fibrosis Foundation. 2013. Patient registry: annual data report 2013. Cystic Fibrosis Foundation, Bethesda, MD: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2013_CFF_Patient_Registry_Annual_Data_Report.pdf. [Google Scholar]
- 734.Cakir Aktas N, Erturan Z, Karatuna O, Karahasan Yagci A. 2013. Panton-Valentine leukocidin and biofilm production of Staphylococcus aureus isolated from respiratory tract. J Infect Dev Ctries 7:888–891. doi: 10.3855/jidc.4135. [DOI] [PubMed] [Google Scholar]
- 735.Besier S, Smaczny C, von Mallinckrodt C, Krahl A, Ackermann H, Brade V, Wichelhaus TA. 2007. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J Clin Microbiol 45:168–172. doi: 10.1128/JCM.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Wolter DJ, Emerson JC, McNamara S, Buccat AM, Qin X, Cochrane E, Houston LS, Rogers GB, Marsh P, Prehar K, Pope CE, Blackledge M, Deziel E, Bruce KD, Ramsey BW, Gibson RL, Burns JL, Hoffman LR. 2013. Staphylococcus aureus small-colony variants are independently associated with worse lung disease in children with cystic fibrosis. Clin Infect Dis 57:384–391. doi: 10.1093/cid/cit270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. 2010. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 738.Micek ST, Reichley RM, Kollef MH. 2011. Health care-associated pneumonia (HCAP): empiric antibiotics targeting methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa predict optimal outcome. Medicine (Baltimore) 90:390–395. doi: 10.1097/MD.0b013e318239cf0a. [DOI] [PubMed] [Google Scholar]
- 739.Grenier C, Pepin J, Nault V, Howson J, Fournier X, Poirier MS, Cabana J, Craig C, Beaudoin M, Valiquette L. 2011. Impact of guideline-consistent therapy on outcome of patients with healthcare-associated and community-acquired pneumonia. J Antimicrob Chemother 66:1617-1624. doi: 10.1093/jac/dkr176. [DOI] [PubMed] [Google Scholar]
- 740.Griffin AT, Peyrani P, Wiemken TL, Ramirez JA, Arnold FW. 2013. Empiric therapy directed against MRSA in patients admitted to the intensive care unit does not improve outcomes in community-acquired pneumonia. Infection 41:517–523. doi: 10.1007/s15010-012-0363-1. [DOI] [PubMed] [Google Scholar]
- 741.Torres A, Ferrer M, Badia JR. 2010. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis 51(Suppl 1):S48–S53. doi: 10.1086/653049. [DOI] [PubMed] [Google Scholar]
- 742.Attridge RT, Frei CR, Restrepo MI, Lawson KA, Ryan L, Pugh MJ, Anzueto A, Mortensen EM. 2011. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J 38:878–887. doi: 10.1183/09031936.00141110. [DOI] [PubMed] [Google Scholar]
- 743.Madaras-Kelly KJ, Remington RE, Sloan KL, Fan VS. 2012. Guideline-based antibiotics and mortality in healthcare-associated pneumonia. J Gen Intern Med 27:845–852. doi: 10.1007/s11606-012-2011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Rubinstein E, Cammarata S, Oliphant T, Wunderink R, Linezolid Nosocomial Pneumonia Study Group . 2001. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis 32:402–412. doi: 10.1086/318486. [DOI] [PubMed] [Google Scholar]
- 745.Wunderink RG, Cammarata SK, Oliphant TH, Kollef MH, Linezolid Nosocomial Pneumonia Study Group . 2003. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clin Ther 25:980–992. doi: 10.1016/S0149-2918(03)80118-2. [DOI] [PubMed] [Google Scholar]
- 746.Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. 2003. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 124:1789–1797. doi: 10.1378/chest.124.5.1789. [DOI] [PubMed] [Google Scholar]
- 747.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 49:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 748.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 749.Torres A. 2012. Antibiotic treatment against methicillin-resistant Staphylococcus aureus hospital- and ventilator-acquired pneumonia: a step forward but the battle continues. Clin Infect Dis 54:630–632. doi: 10.1093/cid/cir907. [DOI] [PubMed] [Google Scholar]
- 750.Lahey T. 2012. Questionable superiority of linezolid for methicillin-resistant Staphylococcus aureus nosocomial pneumonia: watch where you step. Clin Infect Dis 55:159–160. doi: 10.1093/cid/cis329. [DOI] [PubMed] [Google Scholar]
- 751.Wolff M, Mourvillier B. 2012. Linezolid for the treatment of nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus. Clin Infect Dis 55:160–161. doi: 10.1093/cid/cis330. [DOI] [PubMed] [Google Scholar]
- 752.Taccone FS, Vincent JL, Denis O, Jacobs F. 2012. Should we abandon vancomycin for treatment of methicillin-resistant Staphylococcus aureus pneumonia? Still questions to answer. Clin Infect Dis 55:161–163. doi: 10.1093/cid/cis332 .)Reply 55: 163–165, doi:.) [DOI] [PubMed] [Google Scholar]
- 753.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, Macfarlane JT, Read RC, Roberts HJ, Levy ML, Wani M, Woodhead MA, Pneumonia Guidelines Committee of the BTS Standards of Care Committee . 2009. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 64(Suppl 3):iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 754.Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. 2013. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J Infect Dis 208:75–82. doi: 10.1093/infdis/jit129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Martinez-Olondris P, Rigol M, Soy D, Guerrero L, Agusti C, Quera MA, Li Bassi G, Esperatti M, Luque N, Liapikou M, Filella X, Marco F, de la Bellacasa JP, Torres A. 2012. Efficacy of linezolid compared to vancomycin in an experimental model of pneumonia induced by methicillin-resistant Staphylococcus aureus in ventilated pigs. Crit Care Med 40:162–168. doi: 10.1097/CCM.0b013e31822d74a2. [DOI] [PubMed] [Google Scholar]
- 756.Li HT, Zhang TT, Huang J, Zhou YQ, Zhu JX, Wu BQ. 2011. Factors associated with the outcome of life-threatening necrotizing pneumonia due to community-acquired Staphylococcus aureus in adult and adolescent patients. Respiration 81:448–460. doi: 10.1159/000319557. [DOI] [PubMed] [Google Scholar]
- 757.Subedi S, Baird R, Tong SY. 2014. Does the addition of lincosamides have mortality benefit in severe staphylococcal infection?, abstr C-765 Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 758.Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 191:2149–2152. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 759.File TM Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley I, Thye D. 2010. Integrated analysis of FOCUS 1 and FOCUS 2: randomized, doubled-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin Infect Dis 51:1395–1405. doi: 10.1086/657313. [DOI] [PubMed] [Google Scholar]
- 760.Lo DKH, Hurley MN, Muhlebach MS, Smyth AR. 2013. Interventions for the eradication of methicillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst Rev 2:CD009650. doi: 10.1002/14651858.CD009650.pub3. [DOI] [PubMed] [Google Scholar]
- 761.Jennings MT, Boyle MP, Weaver D, Callahan KA, Dasenbrook EC. 2014. Eradication strategy for persistent methicillin-resistant Staphylococcus aureus infection in individuals with cystic fibrosis—the PMEP trial: study protocol for a randomized controlled trial. Trials 15:223. doi: 10.1186/1745-6215-15-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Fusco NM, Toussaint KA, Prescott WA Jr. 2015. Antibiotic management of methicillin-resistant Staphylococcus aureus-associated acute pulmonary exacerbations in cystic fibrosis. Ann Pharmacother 49:458–468. doi: 10.1177/1060028014567526. [DOI] [PubMed] [Google Scholar]
- 763.Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. 1992. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore) 71:369–385. [PubMed] [Google Scholar]
- 764.Bryan CS. 2002. Infectious diseases in primary care, p 111–152. WB Saunders, Philadelphia, PA. [Google Scholar]
- 765.Darouiche RO. 2006. Spinal epidural abscess. N Engl J Med 355:2012–2020. doi: 10.1056/NEJMra055111. [DOI] [PubMed] [Google Scholar]
- 766.Reihsaus E, Waldbaur H, Seeling W. 2000. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev 23:175–204; discussion 205. doi: 10.1007/PL00011954. [DOI] [PubMed] [Google Scholar]
- 767.Lohr M, Reithmeier T, Ernestus RI, Ebel H, Klug N. 2005. Spinal epidural abscess: prognostic factors and comparison of different surgical treatment strategies. Acta Neurochir (Wien) 147:159–166; discussion 166. doi: 10.1007/s00701-004-0414-1. [DOI] [PubMed] [Google Scholar]
- 768.Huang PY, Chen SF, Chang WN, Lu CH, Chuang YC, Tsai NW, Chang CC, Wang HC, Chien CC, Chen SH, Huang CR. 2012. Spinal epidural abscess in adults caused by Staphylococcus aureus: clinical characteristics and prognostic factors. Clin Neurol Neurosurg 114:572–576. doi: 10.1016/j.clineuro.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 769.Chen WC, Wang JL, Wang JT, Chen YC, Chang SC. 2008. Spinal epidural abscess due to Staphylococcus aureus: clinical manifestations and outcomes. J Microbiol Immunol Infect 41:215–221. [PubMed] [Google Scholar]
- 770.Feldenzer JA, McKeever PE, Schaberg DR, Campbell JA, Hoff JT. 1988. The pathogenesis of spinal epidural abscess: microangiographic studies in an experimental model. J Neurosurg 69:110–114. doi: 10.3171/jns.1988.69.1.0110. [DOI] [PubMed] [Google Scholar]
- 771.Sillevis Smitt P, Tsafka A, van den Bent M, de Bruin H, Hendriks W, Vecht C, Teng-van de Zande F. 1999. Spinal epidural abscess complicating chronic epidural analgesia in 11 cancer patients: clinical findings and magnetic resonance imaging. J Neurol 246:815–820. doi: 10.1007/s004150050460. [DOI] [PubMed] [Google Scholar]
- 772.Grewal S, Hocking G, Wildsmith JA. 2006. Epidural abscesses. Br J Anaesth 96:292–302. doi: 10.1093/bja/ael006. [DOI] [PubMed] [Google Scholar]
- 773.Davis DP, Wold RM, Patel RJ, Tran AJ, Tokhi RN, Chan TC, Vilke GM. 2004. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med 26:285–291. doi: 10.1016/j.jemermed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 774.Curry WT Jr, Hoh BL, Amin-Hanjani S, Eskandar EN. 2005. Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol 63:364–371. doi: 10.1016/j.surneu.2004.08.081. [DOI] [PubMed] [Google Scholar]
- 775.Tang HJ, Lin HJ, Liu YC, Li CM. 2002. Spinal epidural abscess—experience with 46 patients and evaluation of prognostic factors. J Infect 45:76–81. doi: 10.1053/jinf.2002.1013. [DOI] [PubMed] [Google Scholar]
- 776.Lu CH, Chang WN, Lui CC, Lee PY, Chang HW. 2002. Adult spinal epidural abscess: clinical features and prognostic factors. Clin Neurol Neurosurg 104:306–310. doi: 10.1016/S0303-8467(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 777.Wheeler D, Keiser P, Rigamonti D, Keay S. 1992. Medical management of spinal epidural abscesses: case report and review. Clin Infect Dis 15:22–27. doi: 10.1093/clinids/15.1.22. [DOI] [PubMed] [Google Scholar]
- 778.Siddiq F, Chowfin A, Tight R, Sahmoun AE, Smego RA Jr. 2004. Medical vs surgical management of spinal epidural abscess. Arch Intern Med 164:2409–2412. doi: 10.1001/archinte.164.22.2409. [DOI] [PubMed] [Google Scholar]
- 779.Pizon AF, Bonner MR, Wang HE, Kaplan RM. 2006. Ten years of clinical experience with adult meningitis at an urban academic medical center. J Emerg Med 30:367–370. doi: 10.1016/j.jemermed.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 780.Aguilar J, Urday-Cornejo V, Donabedian S, Perri M, Tibbetts R, Zervos M. 2010. Staphylococcus aureus meningitis: case series and literature review. Medicine (Baltimore) 89:117–125. doi: 10.1097/MD.0b013e3181d5453d. [DOI] [PubMed] [Google Scholar]
- 781.Dzupova O, Rozsypal H, Prochazka B, Benes J. 2009. Acute bacterial meningitis in adults: predictors of outcome. Scand J Infect Dis 41:348–354. doi: 10.1080/00365540902849391. [DOI] [PubMed] [Google Scholar]
- 782.Huang WC, Lee CH, Liu JW. 2010. Clinical characteristics and risk factors for mortality in patients with meningitis caused by Staphylococcus aureus and vancomycin minimal inhibitory concentrations against these isolates. J Microbiol Immunol Infect 43:470–477. doi: 10.1016/S1684-1182(10)60073-4. [DOI] [PubMed] [Google Scholar]
- 783.Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, Swartz MN. 1993. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med 328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 784.Schlesinger LS, Ross SC, Schaberg DR. 1987. Staphylococcus aureus meningitis: a broad-based epidemiologic study. Medicine (Baltimore) 66:148–156. [DOI] [PubMed] [Google Scholar]
- 785.Teh BW, Slavin MA. 2012. Staphylococcus aureus meningitis: barriers to treatment. Leuk Lymphoma 53:1443–1444. doi: 10.3109/10428194.2012.668685. [DOI] [PubMed] [Google Scholar]
- 786.Pintado V, Pazos R, Jimenez-Mejias ME, Rodriguez-Guardado A, Gil A, Garcia-Lechuz JM, Cabellos C, Chaves F, Domingo P, Ramos A, Perez-Cecilia E, Domingo D. 2012. Methicillin-resistant Staphylococcus aureus meningitis in adults: a multicenter study of 86 cases. Medicine (Baltimore) 91:10–17. doi: 10.1097/MD.0b013e318243442b. [DOI] [PubMed] [Google Scholar]
- 787.Pintado V, Meseguer MA, Fortun J, Cobo J, Navas E, Quereda C, Corral I, Moreno S. 2002. Clinical study of 44 cases of Staphylococcus aureus meningitis. Eur J Clin Microbiol Infect Dis 21:864–868. doi: 10.1007/s10096-002-0814-1. [DOI] [PubMed] [Google Scholar]
- 788.Lerche A, Rasmussen N, Wandall JH, Bohr VA. 1995. Staphylococcus aureus meningitis: a review of 28 consecutive community-acquired cases. Scand J Infect Dis 27:569–573. doi: 10.3109/00365549509047069. [DOI] [PubMed] [Google Scholar]
- 789.Lu CH, Chang WN. 2000. Adults with meningitis caused by oxacillin-resistant Staphylococcus aureus. Clin Infect Dis 31:723–727. doi: 10.1086/314034. [DOI] [PubMed] [Google Scholar]
- 790.Chang WN, Lu CH, Wu JJ, Chang HW, Tsai YC, Chen FT, Chien CC. 2001. Staphylococcus aureus meningitis in adults: a clinical comparison of infections caused by methicillin-resistant and methicillin-sensitive strains. Infection 29:245–250. doi: 10.1007/s15010-001-1092-z. [DOI] [PubMed] [Google Scholar]
- 791.Rodriguez Guardado A, Maradona Hidalgo JA, Perez Gonzalez F, Carton Sanchez JA, Blanco A, Rial JC, Asensi Alvarez V. 2005. Postsurgery meningitis by Staphylococcus aureus: comparison between methicillin-sensitive and resistant strains. Med Clin (Barc) 124:102–103. doi: 10.1157/13070850. [DOI] [PubMed] [Google Scholar]
- 792.Logigan C, Mihalache D, Dorneanu O, Turcu T. 2009. Study of nosocomial bacillary meningitis admitted in the Clinic of Infectious Diseases Iasi on a 20 years period. Rev Med Chir Soc Med Nat Iasi 113:721–726. [PubMed] [Google Scholar]
- 793.Givner LB, Kaplan SL. 1993. Meningitis due to Staphylococcus aureus in children. Clin Infect Dis 16:766–771. doi: 10.1093/clind/16.6.766. [DOI] [PubMed] [Google Scholar]
- 794.Pedersen M, Benfield TL, Skinhoej P, Jensen AG. 2006. Haematogenous Staphylococcus aureus meningitis. A 10-year nationwide study of 96 consecutive cases. BMC Infect Dis 6:49. doi: 10.1186/1471-2334-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Jensen AG, Espersen F, Skinhoj P, Rosdahl VT, Frimodt-Moller N. 1993. Staphylococcus aureus meningitis. A review of 104 nationwide, consecutive cases. Arch Intern Med 153:1902–1908. [DOI] [PubMed] [Google Scholar]
- 796.Matsubara H, Makimoto A, Higa T, Kawamoto H, Kanda Y, Kami M, Tanosaki R, Mineishi S, Ohira M, Takaue Y. 2003. Successful treatment of meningoencephalitis caused by methicillin-resistant Staphylococcus aureus with intrathecal vancomycin in an allogeneic peripheral blood stem cell transplant recipient. Bone Marrow Transplant 31:65–67. doi: 10.1038/sj.bmt.1703799. [DOI] [PubMed] [Google Scholar]
- 797.Goto K, Ohi T, Namba A, Uemura N, Kitaguchi H. 2011. Successful treatment of methicillin-resistant Staphylococcus aureus meningitis by intrathecal injection of vancomycin. Brain Nerve 63:417–421. [PubMed] [Google Scholar]
- 798.Dudley MN, Levitz RE, Quintiliani R, Hickingbotham JM, Nightingale CH. 1984. Pharmacokinetics of trimethoprim and sulfamethoxazole in serum and cerebrospinal fluid of adult patients with normal meninges. Antimicrob Agents Chemother 26:811–814. doi: 10.1128/AAC.26.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Gerber P, Stucki A, Acosta F, Cottagnoud M, Cottagnoud P. 2006. Daptomycin is more efficacious than vancomycin against a methicillin-susceptible Staphylococcus aureus in experimental meningitis. J Antimicrob Chemother 57:720–723. doi: 10.1093/jac/dkl007. [DOI] [PubMed] [Google Scholar]
- 800.Todd J, Fishaut M, Kapral F, Welch T. 1978. Toxic-shock syndrome associated with phage-group-I staphylococci. Lancet ii:1116–1118. [DOI] [PubMed] [Google Scholar]
- 801.Herzer CM. 2001. Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract 14:131–136. [PubMed] [Google Scholar]
- 802.Issa NC, Thompson RL. 2001. Staphylococcal toxic shock syndrome. Suspicion and prevention are keys to control. Postgrad Med 110:55–56, 59–62. [DOI] [PubMed] [Google Scholar]
- 803.Osterholm MT, Forfang JC. 1982. Toxic-shock syndrome in Minnesota: results of an active-passive surveillance system. J Infect Dis 145:458–464. doi: 10.1093/infdis/145.4.458. [DOI] [PubMed] [Google Scholar]
- 804.DeVries AS, Lesher L, Schlievert PM, Rogers T, Villaume LG, Danila R, Lynfield R. 2011. Staphylococcal toxic shock syndrome 2000-2006: epidemiology, clinical features, and molecular characteristics. PLoS One 6:e22997. doi: 10.1371/journal.pone.0022997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Gaventa S, Reingold AL, Hightower AW, Broome CV, Schwartz B, Hoppe C, Harwell J, Lefkowitz LK, Makintubee S, Cundiff DR, Sitze S, Toxic Shock Syndrome Study Group . 1989. Active surveillance for toxic shock syndrome in the United States, 1986. Rev Infect Dis 11(Suppl 1):S28–S34. [DOI] [PubMed] [Google Scholar]
- 806.Hajjeh RA, Reingold A, Weil A, Shutt K, Schuchat A, Perkins BA. 1999. Toxic shock syndrome in the United States: surveillance update, 1979 1996. Emerg Infect Dis 5:807–810. doi: 10.3201/eid0506.990611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Descloux E, Perpoint T, Ferry T, Lina G, Bes M, Vandenesch F, Mohammedi I, Etienne J. 2008. One in five mortality in non-menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbiol Infect Dis 27:37–43. doi: 10.1007/s10096-007-0405-2. [DOI] [PubMed] [Google Scholar]
- 808.Musser JM, Schlievert PM, Chow AW, Ewan P, Kreiswirth BN, Rosdahl VT, Naidu AS, Witte W, Selander RK. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc Natl Acad Sci U S A 87:225–229. doi: 10.1073/pnas.87.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Fraser J, Arcus V, Kong P, Baker E, Proft T. 2000. Superantigens—powerful modifiers of the immune system. Mol Med Today 6:125–132. doi: 10.1016/S1357-4310(99)01657-3. [DOI] [PubMed] [Google Scholar]
- 810.Kalyan S, Chow AW. 2008. Staphylococcal toxic shock syndrome toxin-1 induces the translocation and secretion of high mobility group-1 protein from both activated T cells and monocytes. Mediators Inflamm 2008:512196. doi: 10.1155/2008/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Centers for Disease Control and Prevention. 1997. Case definitions for infectious conditions under public health surveillance. MMWR Recommend Rep 46(RR-10):1–55. [PubMed] [Google Scholar]
- 812.Low DE. 2013. Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin 29:651–675. doi: 10.1016/j.ccc.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 813.Lappin E, Ferguson AJ. 2009. Gram-positive toxic shock syndromes. Lancet Infect Dis 9:281–290. doi: 10.1016/S1473-3099(09)70066-0. [DOI] [PubMed] [Google Scholar]
- 814.Bartlett P, Reingold AL, Graham DR, Dan BB, Selinger DS, Tank GW, Wichterman KA. 1982. Toxic shock syndrome associated with surgical wound infections. JAMA 247:1448–1450. doi: 10.1001/jama.1982.03320350052030. [DOI] [PubMed] [Google Scholar]
- 815.Reingold AL, Hargrett NT, Dan BB, Shands KN, Strickland BY, Broome CV. 1982. Nonmenstrual toxic shock syndrome: a review of 130 cases. Ann Intern Med 96:871–874. doi: 10.7326/0003-4819-96-6-871. [DOI] [PubMed] [Google Scholar]
- 816.Barrett SP, Savage MA, Rebec MP, Guyot A, Andrews N, Shrimpton SB. 1999. Antibiotic sensitivity of bacteria associated with community-acquired urinary tract infection in Britain. J Antimicrob Chemother 44:359–365. doi: 10.1093/jac/44.3.359. [DOI] [PubMed] [Google Scholar]
- 817.Demuth PJ, Gerding DN, Crossley K. 1979. Staphylococcus aureus bacteriuria. Arch Intern Med 139:78–80. doi: 10.1001/archinte.1979.03630380056019. [DOI] [PubMed] [Google Scholar]
- 818.Muder RR, Brennen C, Rihs JD, Wagener MM, Obman A, Stout JE, Yu VL. 2006. Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clin Infect Dis 42:46–50. doi: 10.1086/498518. [DOI] [PubMed] [Google Scholar]
- 819.Lee BK, Crossley K, Gerding DN. 1978. The association between Staphylococcus aureus bacteremia and bacteriuria. Am J Med 65:303–306. doi: 10.1016/0002-9343(78)90824-0. [DOI] [PubMed] [Google Scholar]
- 820.Coll PP, Crabtree BF, O'Connor PJ, Klenzak S. 1994. Clinical risk factors for methicillin-resistant Staphylococcus aureus bacteriuria in a skilled-care nursing home. Arch Fam Med 3:357–360. doi: 10.1001/archfami.3.4.357. [DOI] [PubMed] [Google Scholar]
- 821.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE, Infectious Diseases Society of America . 2010. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 822.Stauffer C, Josiah AF, Fortes M, Menaker J, Cole JW. 2013. Lemierre syndrome secondary to community-acquired methicillin-resistant Staphylococcus aureus infection associated with cavernous sinus thromboses. J Emerg Med 44:e177–e182. doi: 10.1016/j.jemermed.2012.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823.Bilal M, Cleveland KO, Gelfand MS. 2009. Community-acquired methicillin-resistant Staphylococcus aureus and Lemierre syndrome. Am J Med Sci 338:326–327. doi: 10.1097/MAJ.0b013e3181a9302b. [DOI] [PubMed] [Google Scholar]
- 824.Puymirat E, Biais M, Camou F, Lefevre J, Guisset O, Gabinski C. 2008. A Lemierre syndrome variant caused by Staphylococcus aureus. Am J Emerg Med 26:380.e5–380.e7. doi: 10.1016/j.ajem.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 825.Mostafavifar AM, Guilfoose J, Sarwari AR. 2009. Septic pelvic thrombophlebitis due to Staphylococcus aureus. W V Med J 105:20–22. [PubMed] [Google Scholar]
- 826.Falagas ME, Vardakas KZ, Athanasiou S. 2007. Intravenous heparin in combination with antibiotics for the treatment of deep vein septic thrombophlebitis: a systematic review. Eur J Pharmacol 557:93–98. doi: 10.1016/j.ejphar.2006.11.068. [DOI] [PubMed] [Google Scholar]
- 827.Chambers HF, Deleo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.FDA. 2008. Iclaprim for the treatment of complicated skin and skin structure infections. FDA briefing document for Anti-Infective Drugs Advisory Committee Meeting November 20, 2008. FDA, Washington, DC: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4394b3-01-FDA.pdf. [Google Scholar]