Abstract
Multiple pulmonary conditions are characterized by an abnormal misbalance between various tissue components, for example, an increase in the fibrous connective tissue and loss/increase in extracellular matrix proteins (ECM). Such tissue remodeling may adversely impact physiological function of airway smooth muscle cells (ASMCs) responsible for contraction of airways and release of a variety of bioactive molecules. However, few efforts have been made to understand the potentially significant impact of tissue remodeling on ASMCs. Therefore, this study reports how ASMCs respond to a change in mechanical stiffness of a matrix, to which ASMCs adhere because mechanical stiffness of the remodeled airways is often different from the physiological stiffness. Accordingly, using atomic force microscopy (AFM) measurements, we found that the elastic modulus of the mouse bronchus has an arithmetic mean of 23.1 ± 14 kPa (SD) (median 18.6 kPa). By culturing ASMCs on collagen-conjugated polyacrylamide hydrogels with controlled elastic moduli, we found that gels designed to be softer than average airway tissue significantly increased cellular secretion of vascular endothelial growth factor (VEGF). Conversely, gels stiffer than average airways stimulated cell proliferation, while reducing VEGF secretion and agonist-induced calcium responses of ASMCs. These dependencies of cellular activities on elastic modulus of the gel were correlated with changes in the expression of integrin-β1 and integrin-linked kinase (ILK). Overall, the results of this study demonstrate that changes in matrix mechanics alter cell proliferation, calcium signaling, and proangiogenic functions in ASMCs.
Keywords: airways smooth muscle cells, asthma, matrix stiffness, hydrogel, integrin
in diseases such as asthma and chronic obstructive pulmonary disease (COPD), structural and functional changes in the airway are key characteristics. In this regard, airway smooth muscle cells (ASMCs) are important not only for normal airway function by virtue of their role in regulating airway tone and caliber but also to pathophysiology via their responses to inflammation and other insults that drive diseases such as asthma and COPD. For example, ASMCs can contribute to the thicker and stiffer airway of asthma by undergoing increased cell proliferation, as well as greater deposition of extracellular matrix (ECM) components (3). Furthermore, ASMCs serve as a source of growth factors including vascular endothelial growth factor (VEGF) that can have autocrine/paracrine function on the airways and on the pulmonary vasculature (13–14, 39).
While resident cell types such as ASMCs respond to inflammatory stimuli and even growth factors, smooth muscle cells are also responsive to changes in physical properties of tissues that occur in various chronic diseases. For instance, in a rabbit model, atherosclerosis increases the elastic modulus of the systemic vascular wall from ∼30 to 80 kPa (2, 22). Such stiffening of the vascular wall leads to an increased proliferation of vascular smooth muscle cells due to upregulation of platelet-derived growth factor receptor (PDGFR) signaling (2). In a similar context, several prior studies reported that lung diseases result in altered stiffness of pulmonary tissue (17, 23). However, few efforts have been made to systematically examine whether the change of matrix stiffness affects activities of ASMC characteristics.
In this study, we hypothesized that mechanical stiffness of a cell adhesion matrix would modulate factors implicated in the development of lung disease, and we focused on cell growth, calcium signaling, and proangiogenic growth factor secretion in ASMCs. To examine this hypothesis, we performed mechanical mapping of the ASMC layer in mouse pulmonary airways to gauge the stiffness of airways. We grew human AMSCs on collagen-conjugated polyacrylamide (CCP) hydrogels with different mechanical stiffness by tuning the elastic modulus of the hydrogels to span the range seen in normal airway tissue and exceeded this range to model pathological changes in matrix mechanical properties (7) ASMCs were cultured on these gels to analyze effects of matrix stiffness on cell adhesion, proliferation, and secretion of VEGF. Exposure of ASMCs to tumor necrosis factor (TNF-α) or transforming growth factor (TGF-β) was used to recapitulate inflammatory, extracellular microenvironment. In parallel, we examined expressions of integrin-β1 and integrin-linked kinase (ILK) and calcium signaling of cells to elucidate the underlying mechanism by which matrix rigidity modulates cellular activities.
MATERIALS AND METHODS
Atomic force microsopy-based mechanical mapping of airway tissue.
The mechanical mapping of the lung airways was performed in accordance with a protocol described previously (19). In short, fresh tissue samples were prepared by inflating mouse (C57BL/6) lungs with 2% agarose at 37°C (50 ml/kg body wt) followed by cooling to 4°C on ice. Tissue strips with a 400-μm thickness were cut with a razor blade and then washed in phosphate-buffered saline (PBS) at 37°C to remove the agarose. Intact airways running parallel to the cut surface of tissue strips were identified by staining fresh tissue with an antibody against α-smooth muscle actin (Sigma). Alternatively, strips of 400-μm thickness were cut by a VF-300 microtome (Precision Instruments, Greenville, NC), and airways cut transverse to their major axis were identified by characteristic morphology and presence of cuboidal epithelium. The tissue strip samples were adhered to poly-l-lysine-coated coverslips by gently pressing them with untreated coverslips. Mechanical properties of tissue samples were characterized as previously described (17, 19) using the MFP-3D atomic force microscopy (AFM; Asylum Research). A sphere-tipped probe (Novascan, Ames, IA) with a diameter of 5 μm and a nominal spring constant of ∼60 pN/nm was used. The cantilever spring constant was further confirmed by thermal fluctuation method, and the AFM system was calibrated following the manufacturer's instruction before each indentation measurement. Force-indentation profiles were acquired at an indentation depth of ∼240 nm and rate of 20 μm/s. Twelve airways in the mouse lung were examined in this analysis. Surface maps were assembled by sampling a 16 × 16 grid covering 80 × 80 μm area of airway wall surface. Transverse maps of airway cross sections were assembled by sampling along airway radii. The overall distribution of airway elastic properties was assessed by averaging elastic moduli measured at 3 locations around the circumference of 12 individual airways. The elastic modulus at each point was calculated from fitting force-indentation data using a Hertz sphere model (19) assuming a Poisson's ratio of 0.4.
Preparation of collagen-conjugated polyacrylamide hydrogels.
Bovine type I collagen solution (3 mg/ml; PureCol, Advanced Biomatrix) was first incubated with 50 mg/ml acryloyl poly(ethylene glycol) N-hydroxysuccinimide ester (acryloyl PEG-NHS; Jenkem Technology) at 4 °C for 2 h. Then, the collagen bound with acryloyl-PEG-NHS was mixed with the 40% (wt/wt) acrylamide and 2% (wt/wt) N,N′-methylenebis(acrylamide) stock solutions and with 1:100 (vol/vol) and 10% (wt/wt) ammonium persulfate (Sigma) and 10% (wt/wt) tetramethylethylenediamine (TEMED; Fluka). Egel was controlled by varying the molar ratio between N,N′-methylenebis(acrylamide) and acrylamide (Mc). Specifically, increasing Mc from 0.01 to 0.03 and 0.06 led to an increase of Egel from 15 to 27 and 93 kPa (Table 1). The molar ratio between 2% N,N′-methylenebis(acrylamide) and acrylamide was varied from 0.01 to 0.06. The mixture was poured in a space between two glass plates separated by a 2-mm spacer. After 10 min, the resulting gel disks with a 1-cm diameter were punched out using a puncher. The gel disks were further incubated in PBS at room temperature overnight before being plated ASMCs on the gel. PBS was replaced three times a day to wash out excess reagents.
Table 1.
Composition and properties of hydrogels
| Hydrogel | M | E, kPa | Q |
|---|---|---|---|
| 1 | 0.01 | 15 ± 1.1 | 43.2 ± 2.0 |
| 2 | 0.03 | 26.9 ± 0.4 | 24.1 ± 0.8 |
| 3 | 0.06 | 92.6 ± 5.9 | 16.0 ± 0.7 |
M, molar ratio of N,N′-methylenebis(acrylamide) to acrylamide; E, elastic modulus; Q, degree of swelling.
Analysis of stiffness and degree of swelling of the hydrogel.
Mechanical stiffness of the collagen-conjugated polyacrylamide gel was evaluated by measuring compressive elastic modulus. After incubation in PBS over 24 h, gels were compressed at a rate of 0.1 mm/min using a mechanical testing system (MTS Insight). Compressive elastic modulus was calculated from the slope of the curve between stress and the first 10% of strain. Three samples were measured per condition. To determine the degree of swelling, gels were incubated in PBS at 37°C for 24 h and weighed. Then, the gels were lyophilized to measure solid mass. The degree of swelling was calculated by dividing the weight of a fully rehydrated gel by the weight of the dry gel.
Cell culture.
In all experiments, we used primary human ASMCs derived from lungs of patients undergoing thoracic surgery at the Mayo Clinic for focal, noninfectious pathologies (37). Cells were used within passages 2–8 and were cultured in DMEM/F12 (Mediatech, CellGro) supplemented by 10% fetal bovine serum (Invitrogen), 1:100 GlutaMAX (Invitrogen), penicillin, and streptomycin (Invitrogen). The cells were seeded on hydrogels at a density of 10,000 cells/cm2, and then cell-adhered gel disks were incubated at 37°C and at 5% CO2. For the analysis of cell proliferation and VEGF secretion, 10,000 cells were seeded per gel. For all other experiments, we plated 1,000 cells per gel. We limited the passage number of ASMCs to eight, because of the significantly decreased cell proliferation rates after passaging cells more than eight times.
Analysis of intracellular actin filament organization.
Cells were washed twice with prewarmed neutral PBS. Then, cells were fixed with warm 4% formaldehyde in PBS for 10 min and washed twice with fresh PBS at room temperature. The fixed cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min. After blocking of cells in 3% bovine serum albumin (BSA) solution, Alexa Fluor 488 phalloidin (Invitrogen) was added as per manufacturer's instruction. Slides were mounted using Prolong Gold Antifade Reagent (Invitrogen). Images were acquired with a laser scanning confocal microscope (LSM 700; Zeiss).
Morphometric analysis.
Phase contrast images of proliferating cells were captured using an inverted microscope (DMI 4000B; Leica) equipped with a digital camera ORCA-ER (Hamamatsu Photonics). National Institutes of Health (NIH) ImageJ software was utilized for all morphometric analyses of ASMCs. Cell perimeter and cell area were measured by delineating the cell contour using NIH ImageJ software. The aspect ratio (the ratio of the cell's long axis to its short axis) was calculating after determining the length of each cell's long and short axis.
Immunocytochemical analysis of integrin-β1.
Cells were fixed with warm 4% buffered formaldehyde in PBS for 30 min and then incubated in 0.1% Triton-x (Sigma) solution for membrane permeabilization. Fixed samples were blocked with 3% BSA solution prepared with PBS and incubated overnight at 4°C in PBS dissolved with primary antibodies: mouse anti-integrin-β1 antibody (Abcam) and rabbit polyclonal anti-phospho-ILK (Ser246) antibody (EMD Millipore). The slides were washed three times with PBS and incubated with secondary antibodies: Alexa Fluor 568 goat anti-mouse antibody (Invitrogen) and Alexa Fluor 488 donkey anti-rabbit antibody (Invitrogen). All antibodies were applied in dilution 1:1,000. Images were prepared with a laser scanning confocal microscope (LSM 700; Zeiss) using the same laser intensity between different conditions. Specifically, integrin-β1 was imaged by exciting samples at 555 nm and collecting emission ranging from 560 to 600 nm. ILK was imaged by exciting samples at 488 nm and collecting emission ranging from 488 to 550 nm. For measurement of staining intensity, each cell was delineated along the perimeter using NIH ImageJ software, and mean gray value was determined for each cell. Then, averages were calculated, and the mean value obtained on the stiffest matrix (Egel = 93 kPa) was assumed to be a 100%. After the background was deducted, staining intensity values for the gels with Egel of 15 and 27 kPa were divided by the mean value for the stiffest matrix to calculate percent values. Gray values from at least 50 cells were processed for each experimental condition.
Analysis of cell proliferation.
Cell proliferation rate was evaluated with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (10). ASMCs were seeded on hydrogel disks at a density of 10,000 cells/cm2. MTT assay was conducted at 3 h and 72 h after cell plating. The results of the assay were based on the light absorbance at 570 nm measured using a microplate reader (Biotek Synergy HT). Measurements were repeated three times in triplicate. The absorbance values at 3 h postseeding were presumed to be 100% for each condition. To convert absorbance values into percentages, the absorbance values at 72 h postseeding were divided by the absorbance values at 3 h and multiplied by 100%.
Intracellular Ca2+ concentration imaging.
Human ASMCs cultured on hydrogels were incubated in 5 μM fura-2 acetoxymethyl (AM) ester (Invitrogen) for 45 min at room temperature, and the resulting fluorescence was visualized using a Nikon Eclipse TE2000-U inverted microscope with an extra-long working distance 20X Plan Flour lens (WD 6.9–8.2 mm) allowing us to image through the gels. Cells were perfused with Hank's balanced salt solution (HBSS) containing 2 mM Ca2+ while being alternately excited at 340 and 380 nm with a Lambda 10-2 filter changer (Sutter Instruments, Novato, CA). Baseline fluorescence was briefly established and intracellular Ca2+ concentration ([Ca2+]i) responses to histamine were measured with 5–10 cells in software-defined regions of interest. Fluorescence emissions were collected separately every 2 s for each wavelength with a 510-nm barrier filter. Images were acquired with a Photometric Cascade digital camera system (Roper Scientific, Tucson, AZ), and the ratio of emission yields at wavelengths of 340 and 380 nm was calculated. These experiments were repeated at least three times (i.e., 3 different gels) with multiple (>15) cells per field.
Analysis of cellular VEGF secretion.
VEGF secretion by the ASMCs was measured with a R&D Duo Set Human ELISA Kit according to the manufacturer's instructions. In brief, ASMCs were plated at 10,000 cells/cm2 on hydrogel disks, and hydrogels were placed into 12-well plates with 0.7 ml culture medium. A 100 μl-medium was collected at 72 h for ELISA after initiation of the experiment. For stimulation of VEGF secretion, 10 ng/ml TGF-β1, or 100 ng/ml TNF-α were added into the medium. The light absorbance of samples at 450 nm was measured using a microplate reader (Biotek Synergy HT). Then, the amount of secreted VEGF was back calculated from the light absorbance using a standard curve that was generated with standard samples. To account for a change in cell numbers over the 72-h culture period, the VEGF concentration values were divided by individual absorbance values of the MTT assay performed after collecting medium samples for VEGF ELISA.
Statistical analysis.
At least three replicates were tested for each experimental group, and 50 cells or more were analyzed for each condition unless indicated otherwise. Statistical significance was determined via ANOVA, where P < 0.05. Following ANOVA, we used Tukey's post hoc analysis to compare between individual groups. The data are presented with means ± SD unless indicated otherwise. Correlation was analyzed using the sample Pearson correlation coefficient. All statistical analysis was done using the SYSTAT software.
RESULTS
Airway tissue stiffness mapping with AFM.
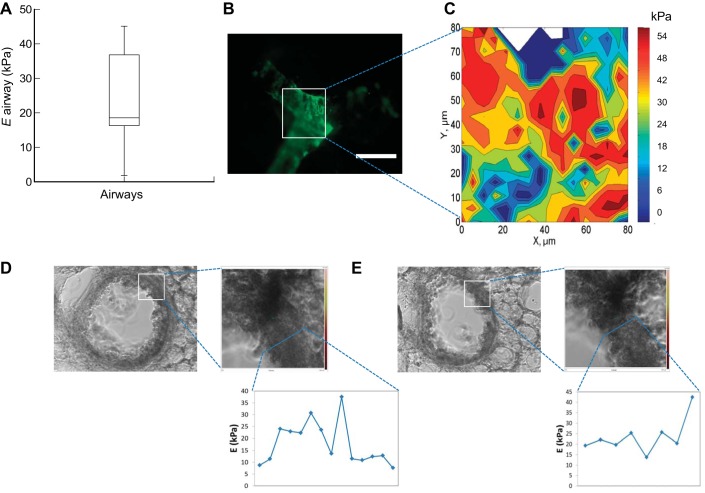
Using AFM, we evaluated stiffness of intrapulmonary airways in the mouse lung (Fig. 1). We measured an average elastic modulus of 23.1 ± 14 kPa (median 18.6 kPa), with a broad distribution (range 2–45 kPa), indicating substantial heterogeneity across airways. To further assess regional variations in elastic moduli, we focused first on an airway branch point exposed on the surface of a thin slice of fresh, unfixed lung tissue (Fig. 1, A–C). The airway was marked with an antibody against the α-smooth muscle actin, and the region of interest with dimension of 80 × 80 μm was mapped using AFM. The elastic modulus of the ASMC layer of mouse intrapulmonary airways was determined to be mostly in a range between 18 and 38 kPa in this longitudinal section, consistent with the overall trends observed across airways, and indicative of the substantial spatial variation. Such variation in mechanical properties could reflect variations in the airway wall mechanical properties, or sampling of different regions within the airway wall as it traverses the plane in which the tissue was cut. To address this issue, we also examined airways cut transverse to their long axis (Fig. 1, D and E). In these airways, variations in elastic modulus were observed throughout the thickness of the airway wall, with a range of modulus values spanning ∼2–45 kPa. Taken together these results demonstrate that the airways are substantially stiffer than previous reports for lung alveoli (elastic modulus ∼1–2 kPa) measured using identical methods (17).
Fig. 1.
Atomic force microscopy (AFM)-based mechanical analysis of airways. A: box-plot chart of the airway wall stiffness. The chart reflects the results of measurements done on 12 airways using indentation depth of 240 nm. B: fluorescent microscopy image of the lung airway stained with an antibody to α-smooth muscle actin. It highlights the smooth muscle cells that wrap around the airway. Scale bar = 100 μm. C: elastograph of the tissue area circumscribed in B. Color bar indicates a level of compressive elastic modulus in kPa. Axis labels indicate spatial scale of airways in μm. D and E: phase contrast photographs of 2 airways (bronchi) in the mouse lung, with corresponding graphs indicating the elastic modulus (E) of a bronchus throughout the entire thickness of the bronchial wall.
Preparation of collagen-conjugated polyacrylamide gels with controlled elastic moduli.
Cell-adherent polyacrylamide gels with controlled elastic moduli (Egel) were prepared by chemically conjugating the same concentration of bovine type I collagen to the hydrogel with varied cross-linking density. The resulting elastic moduli of 15, 27, and 93 kPa span the average range observed in normal airway tissue as reported above and exceed this range to also study values that may be approached in stiff, fibrotic remodeled tissue (9, 24, 40). Introduction of α-acryloyl poly(ethylene glycol) succinimidyl ester and collagen molecules into the gel-forming molecular mixture created the gel chemically conjugated with collagen. As expected, cells exclusively adhered to the gel chemically coupled with collagen molecules. Only a minimal number of cells attached to the collagen-free hydrogel.
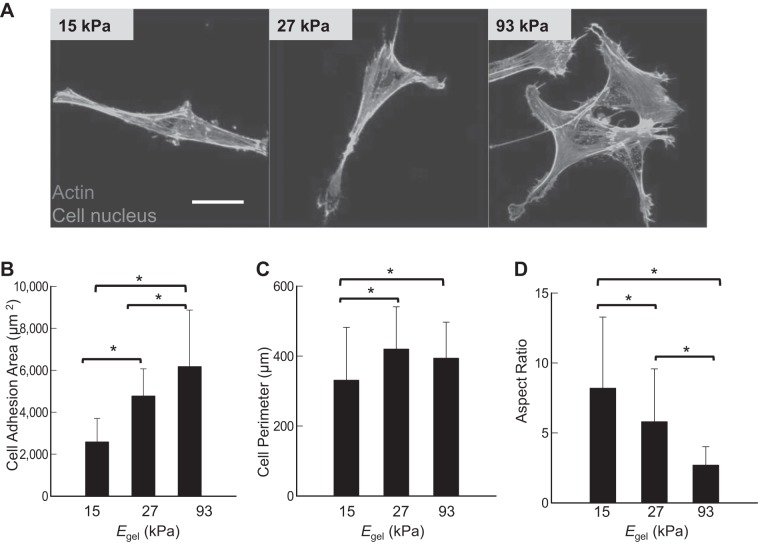
Effects of Egel on adhesion and actin polymerization of ASMCs.
We examined adhesion morphology of ASMCs adhered to gels with varying Egel (Fig. 2). Within 3 h after seeding, >90% of cells were attached to the gel, independent of Egel (not shown). However, the cell adhesion morphology was dependent on Egel (Fig. 2A). Intracellular cytoskeletal organization was also dependent of Egel. The cells adhering to the gels with Egel of 93 kPa exhibited fully stretched actin fibers, while those adhered to the gel with Egel of 15 kPa displayed limited number of short actin fibers. The cells on the gel with Egel of 27 kPa displayed similar actin organization to that on the gel of 15 kPa.
Fig. 2.
Effects of elastic moduli of the gels (Egel) on adhesion and cytoskeletal organization of airway smooth muscle cells (ASMCs). A: fluorescence images of human ASMCs seeded on collagen-conjugated polyacrylamide gels with Egel of 15, 27, or 93 kPa at a density 1,000 cells/cm2 and stained for actin microfilaments and nuclei with Alexa Fluor 488 phalloidin (green) and DAPI (blue), respectively. B: dependency of cell adhesion area on the Egel. C: dependency of cell perimeter on the Egel. D: inverse dependency of cell aspect ratio on the Egel. Approximately 100 cells were evaluated for each experimental condition. Bars and error bars represent the means ± SD. **P < 0.05, statistically significant difference of values between conditions in B–D. Scale bar in A = 20 μm.
The extent of cell spreading, quantified with cell adhesion area, was proportional to Egel, so that cells adhered to the gel with Egel of 93 kPa occupied 3.4 times larger surface than cells adhered to the gel with Egel of 15 kPa (Fig. 2B). Cell perimeter was 1.2 times lower on the gel with Egel of 15 kPa, compared with the cells on the gel with Egel of 27 kPa, whereas no significant difference was found between the gels with E of 27 and 93 kPa (Fig. 2C).
ASMCs adhered to the gel with Egel of 15 kPa were elongated to a spindle form, while those on the gel Egel of 93 kPa were spread isotropically. Therefore, an aspect ratio of cells was increased with decreasing Egel (Fig. 2D). The aspect ratio of the cells adhered to the gel with Egel of 15 kPa was almost 4.2 times larger than when adhered to the gel with Egel of 93 kPa.
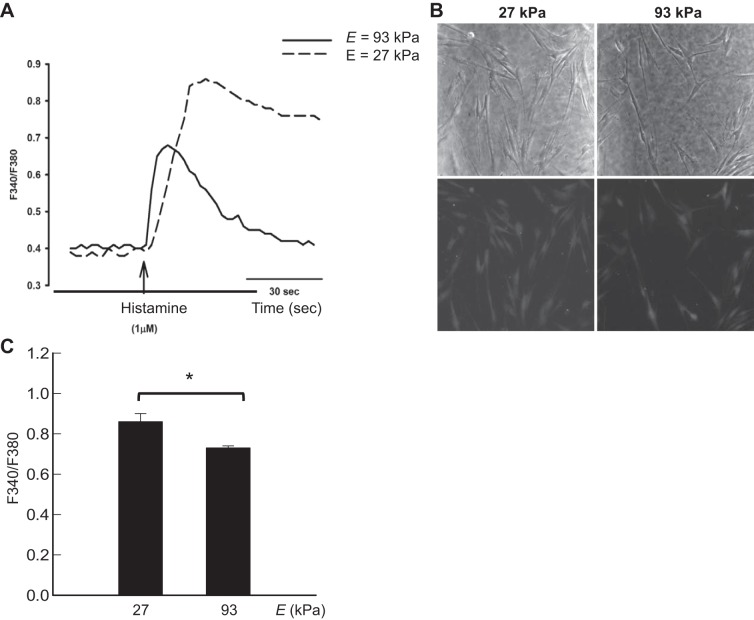
Effects of Egel on intracellular calcium responses.
Intracellular calcium responses to histamine were examined, given the relevance of calcium regulation to airway contractility (37) and the role of altered ASMC calcium homeostasis in airway hyperreactivity of asthma (8, 15, 21, 28, 31) As assessed by the F340/F380 ratio, ASMCs cultured on the gel with Egel of 27 kPa produced greater calcium responses than those adhered to the gel with Egel of 93 kPa (Fig. 3). Cells adhered to the gel with Egel of 15 kPa detached following exposure to histamine. There was substantial consistency in the [Ca2+]i responses to histamine across cells within a field and across cells on gels of the same stiffness.
Fig. 3.
Effects of elastic moduli of the gels (Egel) on intracellular Ca2+ concentration ([Ca2+]i) responses. A: [Ca2+]i response was evaluated on the gels with Egel of 27 and 93 kPa; 1 μM histamine was used as an agonist to induce increases in [Ca2+]i. Tracing of [Ca2+]i response is representative of >15 cells each from 3 separate gels, as shown in summary graph. Images in B demonstrate phase contrast (top row) and fluorescent images (bottom row) indicating similar basal levels of [Ca2+]i on the gels with Egel of 27 and 93 kPa, respectively. The summary graph in C depicts the [Ca2+]i peak response of >15 cells each from 3 separate gels. *P < 0.05, statistically significant difference of values between conditions in C.
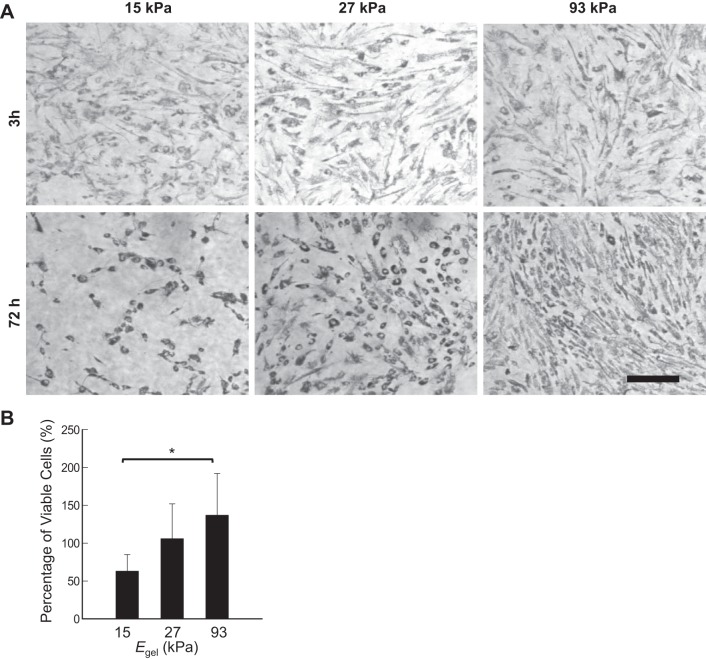
Effects of Egel on proliferation of AMSCs.
The effects of Egel on proliferation of AMSCs were evaluated by examining a change in the number of metabolically active cells using a MTT reagent at 3 and 72 h postseeding (Fig. 4, A and B). Specifically, after 72 h, only a minimal increase in the number of cells positively stained with the MTT reagent was observed on the gel with Egel of 27 kPa, whereas a 37% increase in the number of metabolically active cells was found on the gel with Egel of 93 kPa. Thus the cells adhered to the stiffest gel almost reached confluency during the cell culture over 72 h (Fig. 4A). The cells adhered to the gel with Egel of 15 kPa rather underwent a significant decrease in the numbers of live cells.
Fig. 4.
Effects of elastic moduli of the gels (Egel) on proliferation of ASMCs. A: phase contrast images of human ASMCs positively stained with the MTT reagent at 3 and 72 h postseeding. B: quantified analysis of the percentages of metabolically active cells at 72 h. The percentage of metabolically active cells at 3 h was taken as 100%. Bars and error bars represent the means ± SD. *P < 0.05, statistically significant difference of values between conditions in B. Scale bar = 20 μm.
Matrix stiffness-dependent VEGF secretion of ASMCs.
Effects of Egel on proangiogenic activities of ASMCs were first examined by measuring cellular secretion of VEGF in the absence and presence of inflammatory cytokines, such as TNF-α and TGF-β1 (Fig. 5). In the absence of inflammatory cytokines, ASMCs on a gel with Egel of 15 kPa secreted 2.7 and 5.8 times more VEGF than ASMCs adhered to the gel with Egel of 27 and 93 kPa, respectively. Overall, the VEGF secretion level of ASMCs when normalized to the cell number was inversely dependent on Egel.
Fig. 5.
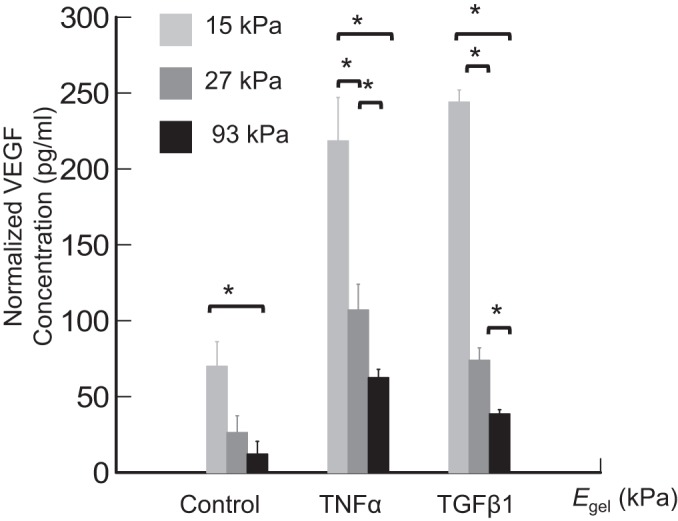
Effects of elastic moduli of the gels (Egel) on VEGF secretion of ASMCs. Human ASMCs were seeded on the collagen-conjugated polyacrylamide gels at a density 10,000 cells/cm2. VEGF secretion was evaluated with a human VEGF ELISA kit 72 h postseeding. TGF-β1 or TNF-α were added to stimulate VEGF secretion as indicated. Bars and error bars represent the means ± SD. *P < 0.05, statistically significant difference of values between indicated group pairs.
Further exposure of ASMCs to inflammatory cytokines including TNF-α and TGF-β1 for 72 h significantly elevated the amount of VEGF secreted by the cells. In the presence of TNF-α, the cells cultured on a gel with Egel of 15 kPa secreted 2 and 3.4 times more VEGF than the cells on the gels with Egel of 27 and 93 kPa, respectively (Fig. 5). In a similar manner, in a medium supplemented by TGF-β1, the softest gels stimulated cells to secrete 3.3 and 6.2 times more VEGF than the intermediate stiff and most rigid gel, respectively (Fig. 5).
Effects of Egel on integrin-β1 and phosphorylated ILK expressions of ASMCs.
We analyzed effects of Egel on cellular integrin-β1 and phosphorylated ILK expression to examine whether the inverse dependencies of calcium and VEGF secretions of ASMCs on Egel are related to the change in the number of receptor-ligand bonds. Integrin-β1 is a major integrin that facilitates attachment to collagen I substrate in fibroblasts and smooth muscles cells (12). It is known that the number of cell adhesion bonds is proportional to the cellular integrin expression, specifically phosphorylated integrins (6).
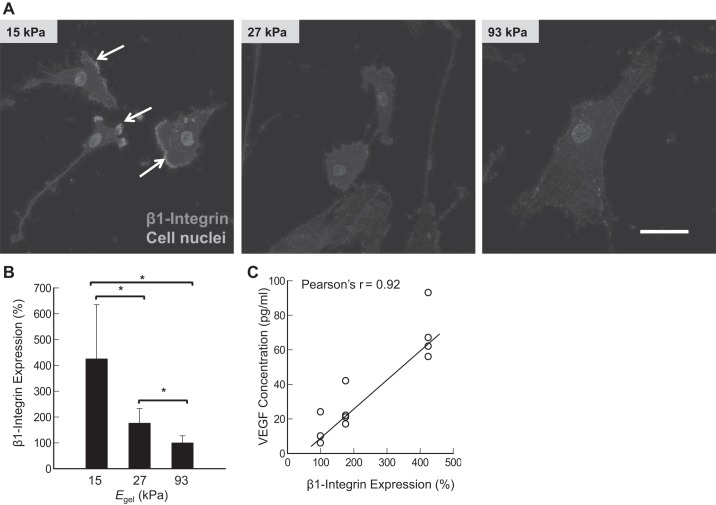
According to images of cells immunostained after 24 h of cell culture, cells adhered to the gel with Egel of 15 kPa displayed the localization of integrin-β1 in the moving front edge of the cellular membrane (Fig. 6A). In contrast, the cells adhered to the stiffer gels expressed reduced integrin-β1. Therefore, integrin-β1 expression level, quantified by antibody staining intensity, was inversely proportional to Egel. Specifically, ASMCs adhered to the gel with Egel of 15 kPa expressed 2.4 and 1.7 times more integrin-β1 than those adhered to the gel with Egel of 27 and 93 kPa, respectively (Fig. 6B). Therefore, the VEGF secretion level of ASMCs was linearly correlated to the integrin-β1 expression level of cells (Fig. 6C).
Fig. 6.
Effects of elastic moduli of gels (Egel) on integrin-β1 expression. A: confocal images of cells stained for the integrin-β1 (red color). White arrows indicate the leading edge of ASMCs. B: inverse dependency of integrin-β1 on Egel. C: correlation between the integrin-β1 expression level and the VEGF secretion level. Bars and error bars represent the means ± SD. Scale bar = 50 μm. In these experiments, human ASMCs were seeded on the collagen-conjugated polyacrylamide gels at a density 1,000 cells/cm2. Integrin-β1 expression was detected by staining with anti-β1-integrin antibodies at 24 h postseeding. *P < 0.05, statistically significant difference of values between conditions in B.
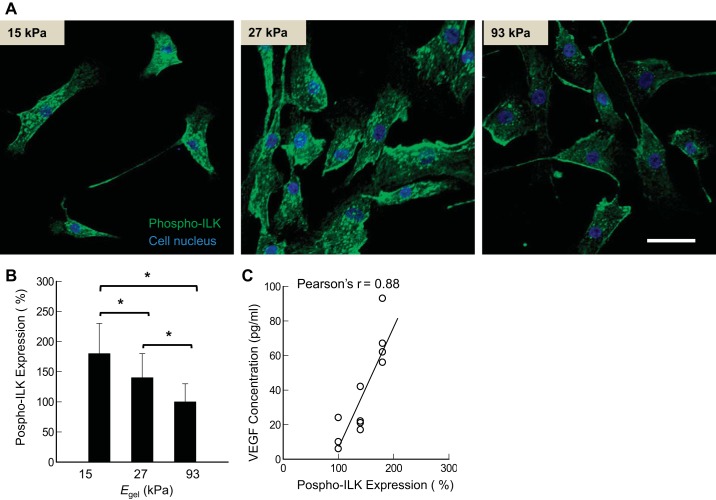
The phosphorylated ILK, which is involved in inside-out and outside-in signaling via integrin-β1, was also influenced by Egel, according to the immunostained images. The expression level of phosphorylated ILK (Ser246), analyzed by the intensity of staining with anti-ILK antibodies, was inversely related to Egel (Fig. 7, A and B). Specifically, the expression level was decreased by a factor of 1.8 when Egel was increased from 15 to 93 kPa. Therefore, the VEGF secretion level of ASMCs was linearly proportional to the cellular ILK expression level (Fig. 7C).
Fig. 7.
Effects of elastic moduli of gels (Egel) on phosphorylated integrin-linked kinase (ILK) expression. A: confocal images of ASMCs stained for phosphorylated ILK (green color). B: inverse dependency of the phosphorylated ILK expression level on Egel. C: correlation between the phosphorylated ILK expression level and VEGF secretion level. *P < 0.05, statistically significant difference between all groups in B. Bars and error bars represent the means ± SD. Scale bar = 50 μm. In these experiments, human ASMCs were seeded on the collagen-conjugated polyacrylamide gels at a density 1,000 cells/cm2. Phosphorylated ILK was detected with fluorescent antibodies, and its expression was quantified by the intensity of the fluorescent signal.
DISCUSSION
In summary, this study demonstrates that mechanical stiffness of a gel, to which ASMCs adhere, plays a significant role in modulating adhesion, proliferation, histamine-induced calcium responses, and inflammation-induced VEGF secretion. The extent of cell spreading was proportional to an elastic modulus of the gel (Egel). ASMCs cultured on the gel stiffer than normal airways displayed a faster cell proliferation, reduced reactivity to histamine, and a lower VEGF secretion than the cells cultured on a gel with stiffness of a normal airway. In contrast, cells cultured on the soft gel displayed a decrease in proliferation over time and also produced larger amounts of VEGF than the ASMCs cultured on the gel with stiffness of a normal airway (40). Additionally, VEGF secretion levels were in direct relationship to the cellular integrin and phosphorylated ILK expression levels, and calcium responses were significantly downregulated on the stiffest gel.
In the lung, ASMCs occupy a narrow compartment between respiratory epithelium and scarce fibrous connective tissue of adventitia. Changes in mechanical properties of ASMC niche caused by pathological processes happen in most chronic lung diseases, such as asthma, pulmonary fibrosis, and chronic bronchitis (7, 9, 17, 23). These diseases are accompanied by the softening or stiffening of the airways (7, 17, 23, 42). Furthermore, in asthma and chronic bronchitis, there is often an increase in the vascularity and the amount of fibrous connective tissue around the airways (3, 16). Because of paucity of the data describing the mechanical properties of ASMC niche, we reproduced mechanical environments characteristic for the ASMC niche using collagen-conjugated hydrogels with controlled stiffness.
Based on previous biomechanical studies examining the ASMC niche and our own AFM-based mechanical mapping of normal airways, our hydrogels were formulated to present Egel of 15, 27, and 93 kPa (1, 35). However, several limitations of our AFM methodology should be noted. First, examination of lung tissue by AFM requires removal of the lungs from the thorax and cutting of the tissue, thereby eliminating physiological prestress and air-liquid interface and precluding application of physiological variations in stretch. Secondly, while the fine spatial resolution of AFM provides insight into local mechanical properties and their variation, the sampling region is small (μm) and limited to the cut surface; therefore, it is difficult to extrapolate our results to more macroscale traditional measures of lung tissue mechanics. Finally, it should be noted that our examination of airways in transverse and longitudinal orientations yielded slightly different ranges of elastic modulus (Fig. 1). Such differences may reflect normal biological variability, may indicate tissue anisotropy, or could be a function of the airways being intact in longitudinal orientation vs. cut in the transverse orientation, thereby releasing prestress in the airway wall and reducing measured elastic properties. Therefore, additional careful study is warranted to investigate these issues, as well as to expand this methodology to directly study the mechanical properties of remodeled airways. Despite these limitations, the AFM methodology is the best approach currently available for detailing the local mechanical environment that cells experience in situ, and we believe the range of elastic moduli identified in our AFM work, and the hydrogel elastic moduli chosen for our in vitro studies, are both highly relevant for the study of airway smooth muscle biology.
Therefore, we propose that decreased proliferation rates of ASMCs on the gel with Egel of 15 kPa implicate the natural process of smooth muscle loss in small and terminal airways during emphysema as a result of the matrix softening (27). In contrast, fast ASMC proliferation on the gel with Egel of 93 kPa is in line with the pathological cellular hyperplasia that is often encountered in asthma patients. Thus stiffening of airways due to the ECM deposition and subepithelial fibrosis may contribute to the general ASMC hyperplasia in asthma (2).
We also suggest that integrin signaling, particularly integrin-β1, is crucial for ASMC adhesion and proliferation onto the collagen-conjugated polyacrylamide gel. Type I collagen is a major component of ECM in airways (25, 30, 41); and integrins-α2β1 and -α1β1 are responsible for adhesion and migration of smooth muscle cells on the collagen gel in vitro (12, 25, 30, 34). Interestingly, the integrin-β1 expression level was inversely proportional to Egel of the hydrogel in this study. It is also noteworthy that integrin-β1 was asymmetrically expressed in the cell membrane and localized to the presumptive leading edge of ASMCs when adhered to the gel with Egel of 15 kPa, similar to fibroblasts (12). In contrast, cells adhered to stiffer substrates displayed uniform distribution of integrins within cell bodies. Such local increase of the intracellular concentration of integrin-β1 can be explained by a high integrin expression, high turnover, and recycling of integrin-β1 molecules that occur at a faster rate on very soft gels (5). However, this inverse dependency of the integrin expression level on Egel may change; depending on the integrin involved, cell adhesion ligands linked to the gel as well as their spatial organization (4).
Additionally, mechanical responsiveness of ASMCs to histamine, characterized by calcium responses in this study, was altered by stiffness of the hydrogel to which cells adhered. Particularly, cells on a gel with similar stiffness to the normal airway exhibited a higher level of calcium response to histamine than cells on a stiffer gel. A lower responsiveness of AMSCs on the gel stiffer than normal airways may indicate an additional effect of the subepithelial fibrosis in asthma. The stiffened airways walls are more resistant to the deformation and narrowing compensating for excessive ASMC contraction.
Taken together, this study demonstrated that the inverse dependencies of calcium response and VEGF secretions of ASMCs on Egel is related to the cellular mechanosensing because it is known that cellular integrin-β1 and phosphorylated ILK expressions play important roles in transmitting an external mechanical signal. We found that the stiffer gel reduces the inflammatory cytokine-induced VEGF secretory function of ASMCs, so that the VEGF secretion level is linearly proportional to the integrin-β1 expression level. It was previously reported that the secretion of several cytokines by ASMCs is regulated by integrin-β1-ligand binding, particularly to collagen I (30). The relevance of integrin signaling to VEGF secretion level was further related to the phosphorylated ILK, a linkage between the integrin and VEGF signaling cascade. ILK is an upstream regulator of PKB/Akt pathway, which binds intracytoplasmic tails of β-integrins, and then, the subsequently phosphorylated ILK activates HIF-1α and VEGF expressions in at least one cell type, prostate cancer cells (36). The increase in ILK activity was reported to be positively dependent on integrin-β1 binding with type I collagen used in this study (26). We therefore suggest that the softer collagen-conjugated gel stimulates integrin expression by cells and subsequently increases the phosphorylated ILK level. Therefore, the matrix stiffness-modulated VEGF secretion of cells must be related to the ILK expression level.
In addition, such ILK-mediated cellular activities are attributed to the intracellular actin polymerization and organization. Cellular cytoskeleton composed of a variety of structural proteins serves to maintain the shape of a cell, participate in cell movement and intracellular transport, and contribute to the resistance to deformation. Actin being one of the main cellular structural proteins significantly contributes to the structural integrity of the cell. Actin fibers also orchestrate cellular stretching together with microtubules, while being connected to integrins (38). It is therefore suggested that the dependency between cellular activities and matrix stiffness is related to changes of cell-exerting force marked with intracellular organization of actin fibers. In a similar context, the stiff hydrogel matrix with E of 93 kPa likely increased the cellular traction force as confirmed with the fully stretched actin fibers, thus leading to increased cellular proliferation and decreased calcium response and VEGF secretion (11, 29). Conversely, the compliant matrix reduced force generated by cells, thus activating cellular calcium response to histamine and VEGF secretion while limiting cellular growth.
Here, we demonstrate that calcium response in ASMCs in vitro is altered by matrix stiffness. It was beyond the scope of the present study to specifically compare these in vitro results to ASMCs in vivo, where the adjacent presence of other cell types and resultant stiffness may affect the Ca2+ responses. Furthermore, the intrinsic Ca2+ responses of cultured ASMCs to agonists such as histamine and metacholine may differ from those in vivo (32–33). Nonetheless, the relevance of our results lies in establishing a link between matrix stiffness and the behavior of ASMCs. Additionally, there are certain limitations to the interpretation of data developed using ASMCs grown on static hydrogels. In natural conditions, ASMCs face rhythmic stretch and relaxation cycles that may change the stiffness of airways in response. Therefore, it is clear that the diapason of the optimal stiffness for the ASMC niche is not a fixed value. It is rather a certain range in the elastic modulus. Because of technological issues, the elastic modulus of maximally stretched and completely collapsed alveolar and bronchiolar tissue is still unknown, and most values describing airway stiffness were produced on the static lung tissue of cadavers (19, 23, 42). Hopefully, with the advancement of technology it will soon become possible to analyze and reproduce physical properties of lung tissue in live animals at different phases of respiratory cycle.
The analysis of longitudinal airway sections by AFM is inherently complicated by the distance between the cutting plane and the airway. Thus we combined both longitudinal and cross-sectional analysis of airways by AFM to gain insight into the spatial variations in airway wall stiffness and provide the first measurement of mechanical properties associated with the ASMC niche.
Conclusion.
Overall, ASMCs are very responsive to mechanical stiffness of the collagen-conjugated hydrogel, a model cell adhesion matrix. The cells adhering to the gel stiffer than the normal airway displayed an increased degree of spreading, an elevated rate of proliferation, and reduced calcium responses to histamine. Such result implicates fibrosis-induced hyperplasia of airways in asthmatic patients. In contrast, the cells adhering to the gel softer than normal airways exhibited reduced rates of proliferation and a temporary increased VEGF secretion following the exposure to inflammatory cytokines, which reflects pathological changes in emphysema. Specifically, the inverse dependence of calcium and VEGF secretion levels in ASMCs to the gel stiffness were related to the expression levels of integrin-β1 and phosphorylated ILK. Taken together, the results of this study will greatly contribute to a better understanding of ASMC physiology and further developing advanced strategies of various pulmonary diseases.
GRANTS
This work was supported by the National Science Foundation (STC-EBICS Grant CBET-0939511 and CBET-1403491), National Heart, Lung, and Blood Institute Grants R01-HL-092961, R01-HL-088029, and R01-HL-056470, and the Korean Institute of Industrial Technology (JE140004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S., K.M.C., D.S., D.H.K., D.J.T., Y.S.P., and H.K. conception and design of research; A.S., M.A.T., K.M.C., D.S., K.B., and D.J.T. performed experiments; A.S., M.A.T., K.M.C., D.S., K.B., D.J.T., and H.K. analyzed data; A.S., M.A.T., K.M.C., D.S., K.B., D.J.T., and H.K. interpreted results of experiments; A.S., M.A.T., and D.J.T. prepared figures; A.S. drafted manuscript; A.S., D.H.K., D.J.T., Y.S.P., and H.K. edited and revised manuscript; K.M.C., D.S., D.H.K., D.J.T., Y.S.P., and H.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Fei Liu who performed the AFM characterization of mouse airway.
REFERENCES
- 1.Ansell TK, McFawn PK, Noble PB, West AR, Fernandes L, Mitchell HW. Potent bronchodilation and reduced stiffness by relaxant stimuli under dynamic conditions. Eur Respir J 33: 844–851, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Brown XQ, Bartolak-Suki E, Williams C, Walker ML, Weaver VM, Wong JY. Effect of substrate stiffness and PDGF on the behavior of vascular smooth muscle cells: implications for atherosclerosis. J Cell Physiol 225: 115–122, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung KF. The role of airway smooth muscle in the pathogenesis of airway wall remodeling in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 347–354; discussion 371–342, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Du J, Chen X, Liang X, Zhang G, Xu J, He L, Zhan Q, Feng XQ, Chien S, Yang C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci USA 108: 9466–9471, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia AJ, Huber F, Boettiger D. Force required to break alpha5beta1 integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. J Biol Chem 273: 10988–10993, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Hinz B. Mechanical aspects of lung fibrosis: a spotlight on the myofibroblast. Proc Am Thorac Soc 9: 137–147, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J 30: 114–133, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Hocking DC. Fibronectin matrix deposition and cell contractility: implications for airway remodeling in asthma. Chest 122: 275S–278S, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Huang KT, Chen YH, Walker AM. Inaccuracies in MTS assays: major distorting effects of medium, serum albumin, and fatty acids. Biotechniques 37: 406, 408, 410–412, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci 124: 9–18, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones C, Ehrlich HP. Fibroblast expression of alpha-smooth muscle actin, alpha2beta1 integrin and alphavbeta3 integrin: influence of surface rigidity. Exp Mol Pathol 91: 394–399, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knox AJ, Corbett L, Stocks J, Holland E, Zhu YM, Pang L. Human airway smooth muscle cells secrete vascular endothelial growth factor: up-regulation by bradykinin via a protein kinase C and prostanoid-dependent mechanism. FASEB J 15: 2480–2488, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Knox AJ, Stocks J, Sutcliffe A. Angiogenesis and vascular endothelial growth factor in COPD. Thorax 60: 88–89, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koopmans T, Anaparti V, Castro-Piedras I, Yarova P, Irechukwu N, Nelson C, Perez-Zoghbi J, Tan X, Ward JP, Wright DB. Ca2+ handling and sensitivity in airway smooth muscle: emerging concepts for mechanistic understanding and therapeutic targeting. Pulm Pharmacol Ther 29: 108–120, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med 156: 229–233, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J Vis Exp 28: pii: 2911. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahn K, Ojo OO, Chadwick G, Aaronson PI, Ward JP, Lee TH. Ca(2+) homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax 65: 547–552, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto T, Abe H, Ohashi T, Kato Y, Sato M. Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol Meas 23: 635–648, 2002. [DOI] [PubMed] [Google Scholar]
- 23.McGee KP, Mariappan YK, Hubmayr RD, Carter RE, Bao Z, Levin DL, Manduca A, Ehman RL. Magnetic resonance assessment of parenchymal elasticity in normal and edematous, ventilator-injured lung. J Appl Physiol 113: 666–676, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan SE, Takle EJ, Holmes AJ. Vitamin A deficiency alters the pulmonary parenchymal elastic modulus and elastic fiber concentration in rats. Respir Res 6: 77, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen TT, Ward JP, Hirst SJ. beta1-Integrins mediate enhancement of airway smooth muscle proliferation by collagen and fibronectin. Am J Respir Crit Care Med 171: 217–223, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Nho RS, Xia H, Kahm J, Kleidon J, Diebold D, Henke CA. Role of integrin-linked kinase in regulating phosphorylation of Akt and fibroblast survival in type I collagen matrices through a beta1 integrin viability signaling pathway. J Biol Chem 280: 26630–26639, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Niu R, Liu H, Fu J. Effects of shenmai and aminophylline on apoptosis of small airway smooth muscle cells and the expression of relevant genes in rats with emphysema. J Huazhong Univ Sci Technolog Med Sci 22: 310–312, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Ozier A, Allard B, Bara I, Girodet PO, Trian T, Marthan R, Berger P. The pivotal role of airway smooth muscle in asthma pathophysiology. J Allergy (Cairo) 2011: 742710, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parameswaran H, Lutchen KR, Suki B. A computational model of the response of adherent cells to stretch and changes in substrate stiffness. J Appl Physiol (1985) 116: 825–834, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Q, Lai D, Nguyen TT, Chan V, Matsuda T, Hirst SJ. Multiple beta 1 integrins mediate enhancement of human airway smooth muscle cytokine secretion by fibronectin and type I collagen. J Immunol 174: 2258–2264, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol 305: L912–L933, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts JA, Raeburn D, Rodger IW, Thomson NC. Comparison of in vivo airway responsiveness and in vitro smooth muscle sensitivity to methacholine in man. Thorax 39: 837–843, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts JA, Rodger IW, Thomson NC. Airway responsiveness to histamine in man: effect of atropine on in vivo and in vitro comparison. Thorax 40: 261–267, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slepian MJ, Massia SP, Dehdashti B, Fritz A, Whitesell L. Beta3-integrins rather than beta1-integrins dominate integrin-matrix interactions involved in postinjury smooth muscle cell migration. Circulation 97: 1818–1827, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Soucy PA, Werbin J, Heinz W, Hoh JH, Romer LH. Microelastic properties of lung cell-derived extracellular matrix. Acta Biomater 7: 96–105, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan C, Cruet-Hennequart S, Troussard A, Fazli L, Costello P, Sutton K, Wheeler J, Gleave M, Sanghera J, Dedhar S. Regulation of tumor angiogenesis by integrin-linked kinase (ILK). Cancer Cell 5: 79–90, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol 303: L923–L928, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration–the actin connection. J Cell Sci 122: 199–206, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 290: L209–L221, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Wang JY, Mesquida P, Lee T. Young's modulus measurement on pig trachea and bronchial airways. Conf Proc IEEE Eng Med Biol Soc 2011: 2089–2092, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Wang R, Stromer MH, Huiatt TW. Integrin expression in developing smooth muscle cells. J Histochem Cytochem 46: 119–126, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Yuan H, Kononov S, Cavalcante FS, Lutchen KR, Ingenito EP, Suki B. Effects of collagenase and elastase on the mechanical properties of lung tissue strips. J Appl Physiol 89: 3–14, 2000. [DOI] [PubMed] [Google Scholar]