Abstract
The lack of a well-characterized biomarker for the diagnosis of chronic obstructive pulmonary disease (COPD) has increased interest toward finding one, because this would provide potential insight into disease pathogenesis and progression. Since persistent neutrophilia is an important hallmark in COPD Pro-Gly-Pro (PGP), an extracellular matrix-derived neutrophil chemoattractant, has been suggested to be a potential biomarker in COPD. The purpose of this review is to critically examine both biological and clinical data related to the role of PGP in COPD, with particular focus on its role as a clinical biomarker and potential therapeutic target in disease. The data provided in this review will offer insight into the potential use of PGP as end point for future clinical studies in COPD lung disease. Following PGP levels during disease might serve as a guide for the progression of lung disorders.
Keywords: biomarker, proline-glycine-proline, COPD, prolyl endopeptidase, lung disease
chronic obstructive pulmonary disease (COPD) is a progressive disorder that causes major problems worldwide (3). The prevalence of COPD is high and it is associated with increased morbidity and mortality (13). It is now globally ranked as the third leading cause of mortality with more than 300 million people suffering from the disease and nearly 3 million deaths each year (21, 39). COPD affects people of all social classes all over the world, with a prevalent increase in developing countries. The lifetime risk of developing COPD has been estimated to be as high as ∼25% (13).
COPD is characterized by the progressive development of airflow limitation. It encompasses chronic bronchitis (chronic inflammation and obstruction of small airways) and emphysema (parenchymal destruction), which lead to progressive narrowing of the airways and shortness of breath (5). The inflammation is resistant to corticosteroids and there are currently neither safe nor effective treatments available (3). COPD is directly related to the prevalence of tobacco smoking, but air pollution and other inhalational exposures are also risk factors for COPD development (28). Additionally, genetic risk factors may contribute to COPD development or progression. α1-Antitrypsin deficiency is the best-documented hereditary condition associated with COPD although there is not complete genetic penetrance for emphysema development in this population. Additionally, the COPDGene study has identified susceptibility loci in genomewide association studies that may contribute to disease progression or phenotypes (27).
Small airways disease and parenchymal destruction are two striking pathological features of COPD. In patients, the small airways are characterized by mucus accumulation in the lumen, peribronchiolar bronchitis, development of lymphoid follicles, and infiltration of both innate and adaptive immune cells (39). COPD is characterized by chronic airway inflammation. Inhalation of noxious particles may cause pulmonary inflammation and, in individuals who develop COPD, the normal response to inflammation in the lungs appears to be augmented. This chronic inflammatory response modulates ongoing tissue destruction in the lungs leading to emphysema. Additionally, chronic inflammation could lead to disruption of the normal repair and defense mechanisms, resulting in small airway fibrosis that leads to air trapping and progressive airflow limitation (35).
COPD is diagnosed when the postbronchodilator forced expiratory volume in 1 s (FEV1)-to-forced vital capacity (FVC) ratio (FEV1/FVC) is less than 0.70, which confirms the presence of persistent airflow limitation (35). The use of spirometry is required for diagnosis and to assess the severity of the disease; however, FEV1 is also commonly used as a biomarker in clinical trials. It is also the only marker widely accepted by regulatory agencies for new drug approval. Although FEV1 represents a robust measure of lung function, it does not account for the pathogenic processes behind the disease progression and the poor reversibility observed in COPD. Measuring the degree of reversibility of airflow limitation (e.g., postbronchodilator FEV1/FVC) is therefore no longer recommended (35, 39). Additionally, in COPD, the lack of modifiable biomarkers represents a major barrier to drug discovery. Finding a biomarker that would permit therapeutic and perhaps preventative measures to be introduced earlier in the disease progression is therefore of utmost importance (22). In this review, we discuss the matrikine Pro-Gly-Pro (PGP) as a potential biomarker in COPD.
Inflammation in COPD
Tobacco smokers with a normal lung function display increased pulmonary inflammation. It is likely that COPD represents an enhanced response of the respiratory mucosa to inhaled irritants (3). In mice, exposure to cigarette smoke causes irreversible lung damage. The inflammatory alterations in the airways are only partially reversed after smoking cessation, a phenomenon observed also in humans (8). Since the inflammation in COPD patients persist after smoking cessation, it is also likely that the inflammation is maintained by autonomous mechanisms (3).
The severity of the inflammation in COPD increases with disease progression. Most inflammation occurs in the peripheral airways and lung parenchyma, with increased numbers of neutrophils, macrophages, and lymphocytes present in the lungs. The molecular mechanism behind COPD is complex and not yet fully understood. In the case of environmental factors, chronic inhalation of irritants initially activates pattern recognition receptors such as Toll-like receptors (TLRs), leading to the activation of innate immune reactions. The innate immune response is characterized by the influx of neutrophils and macrophages to the lungs as well as the activation of airway epithelial cells and mucus secretion. As the disease progresses, the adaptive immune system becomes activated with upregulation and activation of B and T lymphocytes (3). In COPD, neutrophils are the most abundant leukocytes present in the bronchial walls and lumen. Neutrophils have an important role in the host's immunological defense toward pathogens. They are the hallmark of acute inflammation and form the first line of host defense (14). Upon microbial invasion, neutrophils are rapidly recruited to the site of invasion where the invaders are being destroyed through phagocytosis (16). However, neutrophils also release toxic products, and cessation of recruitment and clearance of neutrophils is therefore equally important to maintain homeostasis. Without active clearance, neutrophils are able to cause considerable bystander or collateral damage to the host tissue because of their toxicity. Chronic neutrophilic inflammation has been implicated in lung diseases such as COPD and cystic fibrosis (CF) (33). The increased accumulation of neutrophils in the COPD lung has suggested to be caused by a combination of enhanced neutrophilic recruitment and failure of clearance (16). Recruitment of neutrophils to the site of inflammation is controlled and directed by the release of chemoattractant signals that can be both endogenous and pathogen driven (32). The major chemoattractants for neutrophils are the Glu-Leu-Arg motif-containing ELR+ CXC chemokines. Classical endogenous chemoattractants include CXCL8 and GRO-α, GRO-β, and GRO-γ (CXCL1, CXCL2, and CXCL3, respectively) in humans, and KC (CXCL1) and MIP-2 (CXCL2) in mice. Neutrophil migration is facilitated once the ELR+ CXC chemokines bind to specific G protein-coupled receptors on the surface of neutrophils (primarily CXCR1/2) (19, 25, 36). The mediators may be derived from macrophages and epithelial cells but also neutrophils are major sources of CXCL8 (2). Upon activation, neutrophils are recruited from the circulation to the pulmonary circulation, from which they adhere to the endothelial cells in the alveolar wall before they enter the alveolar space. At the site, neutrophils secrete proteases such as neutrophil elastase, cathepsin G, proteinase-3 and matrix metalloproteinases (MMPs). These contribute to the alveolar destruction, the tissue breakdown in the lung parenchyma causing emphysema, and the mucus hypersecretion from mucosal glands (4). Over the years, much attention has been given to neutrophil elastase (NE) and proteinase 3 in the breakdown of lung parenchyma. However, increasing evidence has evolved, supporting MMPs derived from neutrophils to have a critical role in COPD due to their ability to generate chemotactic peptides promoting the recruitment of neutrophils to the lungs (5).
MMPs, PE, PGP, and LTA4H in Chemotaxis of Neutrophils
MMP-8 and MMP-9 are two MMPs implicated in the pathogenesis of tissue destruction in COPD. Although intrinsic lung cells are able to produce MMPs on their own, neutrophils are thought to be the primary source in chronic neutrophilic lung diseases. They are zinc-dependent proteases responsible for the breakdown of extracellular matrix (ECM) (38). It has been recognized for decades that fragments of matrix protein exert chemotactic activities on neutrophils and monocytes, and that they possibly also exert additional proinflammatory actions (22). In 1995, Pfister et al. (26) first discovered PGP in a rabbit model investigating alkali-induced damage to the eye. In the study, it was demonstrated that ulceration of the cornea was characterized by neutrophilic inflammation and led to the generation of two tripeptides: PGP and its acetylated form N-α-PGP. The collagen-derived matrikines were established to be neutrophil chemoattractants. N-α-PGP was determined to have the highest chemotactic potency (26, 38). MMP-8 and MMP-9 are both able to digest collagen, an important component of ECM. A final reaction of the digestion is driven by prolyl endopeptidase (PE), leading to the generation of PGP (11, 36).
PE is a serine protease that cleaves to the carboxyl side of proline residues in oligopeptides. It is a proline-specific endopeptidase found in mammals. PE differs significantly from classical serine proteases (e.g., trypsin) in both structure and selectivity. It is selective for small peptide substrates only and is not able to cleave substrates larger than 30–100 amino acids (22, 30). Classical serine proteases are rarely involved in the cleaving of peptide bonds containing proline residues since they do not fit into the catalytic site of the enzymes. Because many biologically active peptides contain proline, enzymes that cleave peptides at the proline residue may have an important biological effect (9). PE has been associated with the pathogenesis of neurological and cardiovascular conditions. It has also been implicated in respiratory disorders with chronic inflammation (22). Weathington and colleagues (36) identified a novel pathway involved in the neutrophil influx signaling to the lung in which PE plays a critical role. The most prominent chemokine in the COPD pathophysiology is CXCL8 (4). However, antagonizing CXCL8 by use of an α-CXCL8 antibody does not completely inhibit the neutrophil chemotaxis in COPD patients. The involvement of other chemoattractants has therefore been suggested (7). In recent years, the collagen-derived tripeptide PGP has been proposed as a plausible chemoattractant in COPD lungs.
Weathington et al. (36) elegantly demonstrated the molecular mechanism behind PGP's chemotactic effects. In their study, it was demonstrated that PGP shares structural homology with ELR+ chemokines such as CXCL8 and that it activates CXCR1/2 upon binding, causing neutrophilic chemotaxis, which consequently augments the inflammatory cascade in COPD.
Generation of PGP from tissue breakdown was investigated both in vitro and in vivo, and its chemotactic ability for neutrophils was displayed by using a mouse model. Instillation of N-α-PGP in murine models led to the recruitment of neutrophils to the airways and chronic administration of the tripeptide led to the development of COPD-like pathology in the lung (36, 39). Van Houwelingen et al. (34) confirmed that PGP induces neutrophil migration in a dose-dependent manner in vivo. They also demonstrated that PGP induces emphysema-like changes in form of alveolar enlargement and right ventricular hypertrophy. The complementary peptide l-arginine-threonine-arginine (RTR) that binds PGP was found to impede both migration and activation of neutrophils induced by PGP in vivo. RTR also completely inhibited PGP-induced emphysema in the lungs of the mice. In vitro, RTR impeded both PGP- and CXCL8-induced chemotaxis.
Furthermore, recently we have shown that the PE inhibitor valproic acid can diminish cigarette smoke-mediated inflammation in an acute murine smoking model (1).
In 2010, however, de Kruijf et al. (10) published data suggesting that N-α-PGP does not directly bind to human CXCR1/2. In their work they showed that N-α-PGP was not able to displace the CXCR1/2 radioligand [I125]CXCL8 from its receptors on either human neutrophils or the cell line HEK293T when incubated simultaneously. Also, with use of a G protein-dependent phospholipase C activation assay and a G protein-independent β-arrestin2 recruitment assay, N-α-PGP did not directly activate CXCR2 (10). Because these findings were in conflict with previous publications, other groups started investigating the receptor of N-α-PGP. Almost a year later, Kim et al. (18) using a fluorescein isothiocyanate (FITC)-labeled PGP showed that N-α-PGP does bind to CXCR2 after all. The FITC-labeled PGP bound to murine CXCR2-positive RBL-2H3-transfected cells but not to empty vector controls. To better understand the findings of de Kruijf et al., in 2012 Jackson et al. (17) published new data describing four chiral isomers of N-α-PGP. They showed that the isomer N-α-l-Pro-Gly-l-Pro (LL-N-α-PGP) is chemotactic for neutrophils, whereas the isomer N-α-d-Pro-Gly-d-Pro (DD-NAc-PGP) acts like an antagonist (17). Because de Kruijf et al. did not report what isomer they have used, it could be speculated that this might be in part the reason for the conflicting findings. Jackson et al. added that N-α-PGP should have been preincubated with neutrophils before addition of CXCL8 to measure receptor binding because CXCL8 has a much higher binding affinity to CXCR2 compared with N-α-PGP (10, 17).
The generation of PGP occurs after initial insult to the epithelial layer leading to exposure of collagen and subsequent collagen cleavage initiated by MMPs and completed by PE. Increased levels of PGP and PE have been observed in COPD and CF. The generation of the increased amounts of PGP in the sputum of these patients was PE dependent (11). Since MMP-8, MMP-9, and PE are present in neutrophils, and PE activity is increased in COPD serum and sputum, it is likely that the presence of these enzymes is responsible for the generation of PGP and the self-perpetuating cycle of neutrophilic inflammation in COPD (22). In neutrophils obtained from COPD patients, the intracellular basal PE activity was measured to be 25-fold higher compared with healthy donors. Additionally, PE proteins were expressed not only in neutrophils and macrophages but also in epithelial cells (24).
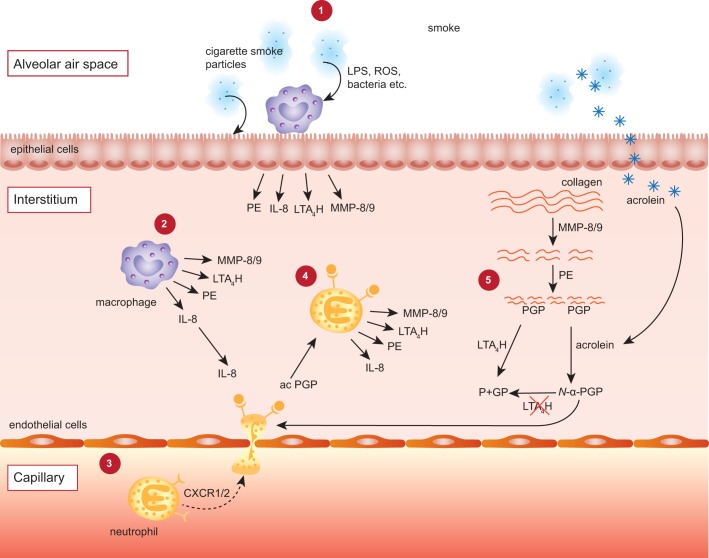
In physiological conditions, accumulation of PGP in the lungs is prevented by enzymatic degradation via leukotriene A4 hydrolase (LTA4H). LTA4H is a proinflammatory enzyme that generates the inflammatory mediator leukotriene B4 through hydrolase activity in the cytosol. However, LTA4H also possesses aminopeptidase activity; and PGP is its physiological substrate. Although LTA4H is able to degrade PGP, its more potent form N-α-PGP appears to be resistant to the enzymatic degradation. The exact molecular mechanism behind the acetylation of the NH2-terminal of PGP is still unknown but cigarette smoke condensate has the potential to do so. Also, cigarette smoke shifted the activity of LTA4H toward a proinflammatory phenotype since it appears to inhibit the peptidase activity but not the hydrolase activity (33, 39). In a translational study by Wells et al. (37), alterations to the LTA4H-PGP pathways in a murine model of chronic cigarette smoke exposure were successfully translated into clinical disease. The research group demonstrated a strong association between N-α-PGP and current cigarette smoking across all levels of COPD disease severity. Cigarette smoke selectively inhibits LTA4H aminopeptidase activity that consequently initiates PGP accumulation and chronic neutrophilic inflammation. Interestingly, inactivation of LTA4H aminopeptidase persists after smoking cessation. Endogenous generation of the reactive aldehyde acrolein at least partly causes the persistent inactivation of the aminopeptidase activity of the enzyme (37). In Fig. 1, the pathophysiology involving PGP is illustrated.
Fig. 1.
Illustration of the Pro-Gly-Pro (PGP) pathology in neutrophilic inflammation. 1: Cigarette smoke inhalation causes tissue resident cells such as macrophages and epithelial cells to release several mediators, including prolyl endopeptidase (PE), IL-8, leukotriene A4 hydrolase (LTA4H), and matrix metalloproteinase (MMP)8/9. 2 and 3: The neutrophilic chemokine IL-8 attracts neutrophils from the capillary via binding with CXCR1/2. 4 and 5: Neutrophils subsequently release MMPs and PE, which cleave collagen from the extracellular matrix to release the tripeptide and neutrophil chemoattractant PGP. LTA4H can cleave and inactivate PGP. However, components of cigarette smoke, such as acrolein, inhibit LTA4H and can acetylate the PGP to form the more potent acetylated form of PGP (N-α-PGP). Moreover, N-α-PGP cannot be cleaved by LTA4H. PGP-generating enzymes can now be released by neutrophils after recruitment and activation by PGP: a self-sustaining neutrophilic inflammation. ROS, reactive oxygen species; P+GP, proline + glycine-proline.
Clinical Investigation of PGP
The role of PGP in the vicious cycle leading to self-sustaining neutrophilic inflammation observed in chronic lung diseases such as COPD and CF has received more attention during the past years (39). The study of Weathington et al. (36) measured PGP peptides in patients with COPD, although the sample size was limited, consisting of 5 COPD patients and 18 control subjects. Nevertheless, detectable levels of PGP were found in 3 of 5 individuals in the patient group with an average of 363 pg/ml, and in 2 of 18 control samples with an average of 22 pg/ml. The difference between the positive samples was pronounced and significant (P < 0.01). Interestingly, two COPD patients negative for PGP were also tested negative for emphysema (36).
To further assess PGP as a potential biomarker, Gaggar et al. (11) examined N-α-PGP and PGP levels in sputum from CF patients with moderately severe lung disease (n = 10) compared with healthy controls (n = 10). Eight of 10 CF patients displayed N-α-PGP levels above detection threshold compared with 1 of 10 in the control group with average concentrations of 3.78 and 0.13 ng/ml, respectively (P < 0.01). For PGP, mean values were established to 204.8 ng/ml in CF samples and 16.2 ng/ml in sputum from healthy volunteers (P < 0.05). Additionally, a correlation between N-α-PGP and PGP levels was recognized, demonstrating a strong relationship between the presences of the collagen peptides in clinical samples. The initial results led to inquiries regarding the involvement of specific proteases involved in PGP generation in CF patients. PE was found to have a fivefold increase in activity in sputum of CF patients compared with control and increased PE activity was confirmed with PGP generation (11).
As described previously, MMPs have been assigned an emerging role in the destruction of ECM in the pathogenesis of chronic neutrophilic lung diseases. Together with human neutrophil elastase (HNE), MMP isoforms have an increased proteolytic activity in sputum of CF patients. MMP-9 was the predominantly active MMP isoform (12). Its presence correlated with the decline in lung function in CF patients (29). Since both HNE and MMP, together with PE, have an increased activity in the sputum of CF patients, Gaggar et al. (12) hypothesized that CF sputum contained the required components to generate PGP from collagen. By incubating CF and control sputum samples with either type I and type II collagen, it was demonstrated that CF sputum indeed did generate significantly more PGP from both collagen I and II compared with control. These findings led to consideration of whether this tripeptide could serve as a biomarker in chronic neutrophilic disease. Sputum samples from a CF inpatient cohort were therefore collected in the beginning of exacerbation (within 48 h of admission) and subsequently after ∼14 days of standard inpatient therapy at the end of the hospitalization. Over the course of treatment, PGP levels were significantly reduced. Also, a trend toward significance was observed in the correlation between PGP decline and improvements in FEV1 and FVC. Upon discharge of the patients, PGP levels were still fivefold higher than those of healthy controls. The elevated PGP levels after recovery were proposed to indicate ongoing inflammation and matrix degradation in the lungs of CF patients (11).
O'Reilly et al. (22) further evaluated N-α-PGP and PGP as a biomarker for COPD. PGP concentrations were determined in sputum samples of COPD patients (n = 16), severe asthmatics (n = 10) and controls (nonsmokers with no history of lung disease; n = 10). Sputum N-α-PGP levels above the detection limit were identified in 13 of 16 COPD subjects but not in the control or the asthma cohort. Positive PGP levels were detected in all COPD samples and in a minority of the controls (3 of 10). The PGP levels in sputum from COPD patients was reported to be significantly higher than those of asthmatic and healthy controls, further supporting the appreciation of N-α-PGP and PGP as potential biomarkers distinguishing COPD from other health states. In ex vivo experimentation, COPD sputum was incubated with collagen type I that was predialyzed to remove any necessary enzymes to thrive PGP generation de novo. Since much smaller amounts of N-α-PGP were generated, it was proposed that acetylation could be the rate-limiting reaction for N-α-PGP formation. In further ex vivo experimentation, the same group established that inhibition of MMP-1, MMP-9, and PE but not HNE reduces PGP generation, supporting the role of MMPs and PE in PGP generation and consequently COPD pathogenesis. The macrolide antibiotic azithromycin was also able to reduce the PGP levels. Additionally, PGP levels were found to be twice as high in serum samples of COPD patients compared with control, with acceptable correlation (r = 0.71; P = 0.11) between sputum and serum levels considering the sample size (n = 6), suggesting its potential usefulness as a serum biomarker as well a lung-based biomarker (22).
In a multicenter trial of azithromycin in addition to normal medication in stable COPD outpatients in 2013, an ancillary study was conducted to investigate whether sputum levels of PGP were altered by the treatment or associated with the frequency of exacerbations (23). Macrolide antibiotics accumulate in their host cells (e.g., macrophages and neutrophils) and exert anti-inflammatory effects via inhibition of inflammatory cytokines (e.g., CXCL8), reduction of neutrophil activation and induction of phagocytosis of apoptotic neutrophils (31). In a blinded fashion, O'Reilly et al. (23) performed several ex vivo experiments to examine PGP, myeloperoxidase (MPO), and MMPs and their ability to generate PGP, as well as the correlation with azithromycin use once the parent trial was unblinded. It should be noted that all patients were nonsmokers or ceased smoking at least 6 mo before the study since smoking is a known hypersensitivity to macrolides (23). Treatment with azithromycin successfully lowered the levels of PGP in the sputum of COPD patients compared with placebo, particularly when taken during a longer period and, as a result, the neutrophilic burden was reduced, measured as decreased levels of MPO. Also, the clinical response was improved with a reduced exacerbation frequency. However, most striking was the indication of elevated PGP levels exceeding acute COPD exacerbation for as long as 35 days before the onset of symptoms observed in a few specimens. Although no conclusion could be drawn on a functional relationship, the data suggest the idea of PGP as a possible important player in COPD pathogenesis, particularly in exacerbations (23). In the parent study, add-on treatment with azithromycin demonstrated a trend for reduced number of exacerbations as well as reduced number of neutrophilic airway inflammation markers (31).
In the study by Wells et al. (37), never smokers (n = 18), control smokers (no airflow obstruction; n = 18), current-smoking COPD patients (n = 13), and former-smoking COPD patients (n = 10) were investigated for alterations in N-α-PGP, LTA4H, and aminopeptidase levels. LTA4H levels were increased in sputum of control smokers compared with never smokers; however, a decrease of ∼65% of the aminopeptidase activity was observed for the control-smoking group. Since the LTB4 concentrations were higher for smoking subjects, it could be concluded that cigarette smoke selectively inhibits the aminopeptidase activity of the LTA4H enzyme without affecting the epoxide hydrolase function. Also, the neutrophil marker MPO was significantly elevated in control smokers compared with never smokers and PGP levels were threefold as high in the smoking population. Furthermore, in a pilot study, sputum LTA4H was significantly increased in COPD patients compared with smokers and never smokers. The LTA4H levels did not differ between current- and former-smoking subjects. Although enzyme concentrations were elevated for the COPD subjects, aminopeptidase activity was further inhibited compared with smokers and never smokers (P = 0.02 and P = 0.015, respectively). There was no difference between current and former smokers in the COPD groups. Sputum PGP levels were significantly higher among COPD subjects compared with never smokers; however, no statistical significant difference was found between COPD subjects and control smokers. Nevertheless, significantly elevated N-α-PGP levels were established in the sputum of all COPD patients compared with control smokers. The N-α-PGP concentrations remained elevated also in former-smoking COPD subjects compared with smoking controls. Additionally, sputum LTB4 and MPO levels were elevated in COPD subjects compared with never smokers and similar to those observed in smoking controls. A correlation was found between N-α-PGP and MPO levels. No correlation was observed between PGP/N-α-PGP and LTB4 amounts. To further investigate the PGP/LTA4H pathway, sputum samples from current- and former-smoking COPD subjects enrolled in another study (ECLIPSE) were investigated for N-α-PGP, LTA4H, and aminopeptidase activity. In the ECLIPSE cohort, N-α-PGP levels were elevated in current smokers compared with former smokers. No difference was observed between current and former COPD smokers in LTA4H levels or aminopeptidase activity. The results were similar to those obtained in the pilot study. In further investigations, log N-α-PGP levels were found to strongly correlate with cigarette smoking. The N-α-PGP levels increased with disease stage (GOLD levels) for current smokers compared with former smokers. LTA4H remained the same between the groups despite GOLD stage. This suggests that the increase in N-α-PGP is a result of increased N-α-PGP production and not due to alterations in the PGP/N-α-PGP breakdown process. Moreover, N-α-PGP levels were similar between current- and former-smoking COPD subject with emphysema alone and those with both emphysema and chronic bronchitis but trended toward elevated levels in smoking COPD subjects with chronic bronchitis alone. It can be concluded that smoke-mediated loss of LTA4H aminopeptidase activity and elevations in PGP/N-α-PGP amounts appear to have a role in chronic neutrophilic inflammation and in COPD pathophysiology (37).
It should be noted that all clinical studies reported in which PGP is measured have a small sample size. To better understand the role of PGP in human disease and the potential role as biomarker for COPD, more clinical studies are needed in which a larger population is investigated. These studies should point out whether PGP could be used as a more specific biomarker for certain patients and perhaps as a predictor for exacerbations as was suggested by O'Reilly et al. (23).
Also, several other diseases have been reported to show elevated PGP levels, such as CF, bronchiolitis obliterans syndrome, and inflammatory bowel disease (11, 15, 20). We speculate that PGP might be significantly elevated in any disease with a neutrophilic inflammation and a high cellular matrix turnover (11).
Although O'Reilly et al. (22, 23) have directly compared the PGP levels in COPD patients vs. patients with severe asthma, it could be useful to include other lung diseases as well. For example, the role of PGP in lung fibrosis as seen in in some types of interstitial lung disease (ILD) has not been investigated before. It would be interesting to see whether PGP plays any role in ILDs to further examine the specificity of PGP.
Currently, further studies are needed to investigate whether PGP could be used as a (prognostic) biomarker reflecting the progression of the disease rather than as a biomarker to diagnose a disease. This will require larger well-phenotyped COPD patient cohorts and examining PGP peptide levels and clinical parameters to accurately assess the sensitivity of this biomarkers with changes in disease status. Fortunately, such large cohorts are now available and present a unique opportunity to prospectively study PGP in various COPD phenotypes (i.e., chronic bronchitis vs. emphysema, frequent vs. nonfrequent exacerbators, etc.).
Conclusion
COPD remains a prevalent pulmonary disorder with high attributable morbidity and mortality. The determination of a robust biomarker that is easily measured, is highly reproducible, trends with clinical progression, and may serve as a surrogate marker for appropriate therapeutic intervention is critically needed in COPD lung disease (6). To date, PGP peptides have fulfilled all features of this paradigm of an biomarker in a COPD population and warrant ongoing evaluation in this deadly disorder. However, further clinical investigation with larger COPD patient populations and studies in additional lung disorders are needed to further examine the utility of PGP as potential biomarker.
GRANTS
This research was supported by a Mosaic Grant from the Netherlands Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek, The Hague, The Netherlands) Grant 017.008.029 (M. Abdul Roda) and by NHLBI grants HL110950, HL114439, and HL07783 (J. E. Blalock) and HL102371 (A. Gaggar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.A.R., A.M.F., and G.F. conception and design of research; M.A.R. prepared figures; M.A.R., A.M.F., F.A.M.R., J.E.B., A.G., and G.F. drafted manuscript; M.A.R., A.M.F., F.A.M.R., J.E.B., A.G., and G.F. edited and revised manuscript; M.A.R., A.M.F., F.A.M.R., J.E.B., A.G., and G.F. approved final version of manuscript.
REFERENCES
- 1.Abdul Roda M, Sadik M, Gaggar A, Hardison MT, Jablonsky MJ, Braber S, Blalock JE, Redegeld FA, Folkerts G, Jackson PL. Targeting prolyl endopeptidase with valproic acid as a potential modulator of neutrophilic inflammation. PLoS One 9: e97594, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altstaedt J, Kirchner H, Rink L. Cytokine production of neutrophils is limited to interleukin-8. Immunology 89: 563–568, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov 12: 543–559, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev 56: 515–548, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 343: 269–280, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ, Chowdhury B, Kharitonov SA, Magnussen H, Page CP, Postma D, Saetta M. Pulmonary biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 6–14, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Beeh KM, Kornmann O, Buhl R, Culpitt SV, Giembycz MA, Barnes PJ. Neutrophil chemotactic activity of sputum from patients with COPD: role of interleukin 8 and leukotriene B4. Chest 123: 1240–1247, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Braber S, Henricks PA, Nijkamp FP, Kraneveld AD, Folkerts G. Inflammatory changes in the airways of mice caused by cigarette smoke exposure are only partially reversed after smoking cessation. Respir Res 11: 99, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham DF, O'Connor B. Proline specific peptidases. Biochim Biophys Acta 1343: 160–186, 1997. [DOI] [PubMed] [Google Scholar]
- 10.de Kruijf P, Lim HD, Overbeek SA, Zaman GJ, Kraneveld AD, Folkerts G, Leurs R, Smit MJ. The collagen-breakdown product N-acetyl-Proline-Glycine-Proline (N-alpha-PGP) does not interact directly with human CXCR1 and CXCR2. Eur J Pharmacol 643: 29–33, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol 180: 5662–5669, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaggar A, Li Y, Weathington N, Winkler M, Kong M, Jackson P, Blalock JE, Clancy JP. Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. Am J Physiol Lung Cell Mol Physiol 293: L96–L104, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet 378: 991–996, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 56: 559–564, 1994. [PubMed] [Google Scholar]
- 15.Hardison MT, Galin FS, Calderon CE, Djekic UV, Parker SB, Wille KM, Jackson PL, Oster RA, Young KR, Blalock JE, Gaggar A. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J Immunol 182: 4423–4431, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoenderdos K, Condliffe A. The neutrophil in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 48: 531–539, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Jackson PL, Noerager BD, Jablonsky MJ, Hardison MT, Cox BD, Patterson JC, Dhanapal B, Blalock JE, Muccio DD. A CXCL8 receptor antagonist based on the structure of N-acetyl-proline-glycine-proline. Eur J Pharmacol 668: 435–442, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SD, Lee HY, Shim JW, Kim HJ, Yoo YH, Park JS, Baek SH, Zabel BA, Bae YS. Activation of CXCR2 by extracellular matrix degradation product acetylated Pro-Gly-Pro has therapeutic effects against sepsis. Am J Respir Crit Care Med 184: 243–251, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Abdul Roda M, Verspaget HW, Wolfkamp SC, te Velde AA, Jones CW, Jackson PL, Blalock JE, Sparidans RW, Kruijtzer JA, Garssen J, Folkerts G, Kraneveld AD. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut 63: 578–587, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Abdul Roda M, Verspaget HW, Wolfkamp SC, te Velde AA, Jones CW, Jackson PL, Blalock JE, Sparidans RW, Kruijtzer JA, Garssen J, Folkerts G, Kraneveld AD. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut 63: 578–587, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res 10: 38, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Reilly PJ, Jackson PL, Wells JM, Dransfield MT, Scanlon PD, Blalock JE. Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations. BMJ Open 3: e004140, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overbeek SA, Braber S, Koelink PJ, Henricks PA, Mortaz E, LoTam Loi AT, Jackson PL, Garssen J, Wagenaar GT, Timens W, Koenderman L, Blalock JE, Kraneveld AD, Folkerts G. Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation? PLoS One 8: e55612, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overbeek SA, Henricks PA, Srienc AI, Koelink PJ, de Kruijf P, Lim HD, Smit MJ, Zaman GJ, Garssen J, Nijkamp FP, Kraneveld AD, Folkerts G. N-acetylated Proline-Glycine-Proline induced G-protein dependent chemotaxis of neutrophils is independent of CXCL8 release. Eur J Pharmacol 668: 428–434, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfister RR, Haddox JL, Sommers CI, Lam KW. Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-proline-glycine-proline and N-methyl-proline-glycine-proline. Invest Ophthalmol Vis Sci 36: 1306–1316, 1995. [PubMed] [Google Scholar]
- 27.Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, Feng S, Hersh CP, Bakke P, Gulsvik A, Ruppert A, Lodrup Carlsen KC, Roses A, Anderson W, Rennard SI, Lomas DA, Silverman EK, Goldstein DB; ICGN Investigators. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 5: e1000421, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176: 532–555, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Sagel SD, Kapsner RK, Osberg I. Induced sputum matrix metalloproteinase-9 correlates with lung function and airway inflammation in children with cystic fibrosis. Pediatr Pulmonol 39: 224–232, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Shan L, Mathews II, Khosla C. Structural and mechanistic analysis of two prolyl endopeptidases: role of interdomain dynamics in catalysis and specificity. Proc Natl Acad Sci USA 102: 3599–3604, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson JL, Powell H, Baines KJ, Milne D, Coxson HO, Hansbro PM, Gibson PG. The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trial. PLoS One 9: e105609, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snelgrove RJ. Targeting of a common receptor shared by CXCL8 and N-Ac-PGP as a therapeutic strategy to alleviate chronic neutrophilic lung diseases. Eur J Pharmacol 667: 1–5, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, Blalock JE. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science 330: 90–94, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Houwelingen AH, Weathington NM, Verweij V, Blalock JE, Nijkamp FP, Folkerts G. Induction of lung emphysema is prevented by l-arginine-threonine-arginine. FASEB J 22: 3403–3408, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187: 347–365, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 12: 317–323, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Wells JM, O'Reilly PJ, Szul T, Sullivan DI, Handley G, Garrett C, McNicholas-Bevensee CM, Abdul Roda M, Miller BE, Tal-Singer R, Gaggar A, Rennard SI, Jackson PL, Blalock JE. An aberrant leukotriene A4 hydrolase-PGP pathway in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 190: 51–61, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X, Jackson PL, Tanner S, Hardison MT, Abdul Roda M, Blalock JE, Gaggar A. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS One 6: e15781, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon HI, Sin DD. Biomarkers of therapeutic response in patients with chronic obstructive pulmonary disease: a critical review of the literature. Drugs 71: 1821–1837, 2011. [DOI] [PubMed] [Google Scholar]