Abstract
Objective
We evaluated the potential of methylphenidate to improve antidepressant response to citalopram in elderly depressed patients with respect to clinical and cognitive outcomes.
Methods
We conducted a 16-week randomized double-blind placebo-controlled trial for geriatric depression in 143 older outpatients diagnosed with major depression comparing treatment response in three groups: 1) methylphenidate and placebo (N=48); 2) citalopram and placebo (N=48); 3) methylphenidate and citalopram (N=47). Primary outcome was defined as the change in depression severity. Remission was defined as Hamilton Depression Rating Scale (HDRS-24) score of 6 or below. Secondary outcomes included measures of anxiety, apathy, quality of life, and cognition.
Results
Citalopram daily doses ranged between 20–60 mg (mean 32 mg); methylphenidate daily doses ranged between 5–40 mg (mean 16 mg). All groups showed significant improvement in the severity of depression. However, the improvement in depression severity and the clinical global impression was more prominent in the methylphenidate and citalopram group compared to methylphenidate and placebo and citalopram and placebo (P<0.05). Additionally, the rate of improvement in the methylphenidate and citalopram group was significantly faster than that in the citalopram and placebo in the first 4 weeks of the trial. The groups did not differ on cognitive improvement or the number of side-effects.
Conclusions
Combined treatment with citalopram and methylphenidate demonstrated an enhanced clinical response profile in the mood and wellbeing, and the rate of response compared to either drug. All treatments led to an improvement in cognitive functioning, without additional benefit from the use of methylphenidate.
Keywords: geriatric depression, enhanced antidepressant response, accelerated rate of response, stimulants, methylphenidate, citalopram, SSRI, augmentation, combination, cognition
Despite progress in antidepressant therapies, a considerable number of depressed elderly patients develop either a chronic course, or relapse frequently after periods of improvement.(1,2) Elderly patients appear to have a less robust response compared to younger adults with a higher rate of recurrence, and lower effect sizes and remission rates in response to first-line antidepressant treatment of about 30%. (1–6)
Cognitive impairment in late-life depression is common and is associated with frontostriatal systems dysfunction, inadequate treatment response, (7, 8) functional impairment in instrumental activities of daily living, (9) and increased risk of conversion to dementia. (10) Cognitive deficits often persist despite successful treatment of depression. (11–15)
Methylphenidate is a dopamine reuptake inhibitor, and as a single agent, has been shown to be effective and safe in a few open and controlled studies in the elderly.(16–21) The use of dopaminergic agents like methylphenidate in geriatric depression could be important due to reduction in the dopamine neurotransmission with aging.(16–18) Methylphenidate has demonstrated efficacy in the executive dysfunction targets including deficits in attention, apathy and withdrawal. (16–18)
Our trial is the first randomized trial to assess the use of methylphenidate to improve antidepressant response in geriatric depression. We compared clinical efficacy and safety of three treatment groups representing three different mechanisms of action with respect to dopaminergic function: 1) methylphenidate and placebo)- mostly dopaminergic; 2) citalopram and placebo -mostly serotonergic; and 3) methylphenidate and citalopram – mixed. We have hypothesized based on our preliminary observations that the combined use of citalopram and methylphenidate would lead to faster and improved antidepressant response with improvements in mood, function, and cognition.
METHODS
Study Procedures
From August 2008 to September 2012, 510 were screened by phone, and 213 individuals were recruited for a diagnostic interview. After describing the details of the study to interested and eligible subjects, written informed consent was obtained in accordance with the procedures set by the UCLA Institutional Review Board (IRB).
Inclusion criteria were: 1) current episode of unipolar major depressive disorder according to DSM-IVTR criteria; 2) Hamilton Depression Rating Scale (HDRS-24) score ≥ 16 (22); and 3) Mini-Mental State Exam (MMSE) (23) score ≥ 26. Exclusion criteria were: 1) history of any other psychiatric disorders (other than unipolar major depressive disorder with or without comorbid anxiety symptoms); 2) severe or acute unstable medical illness, including the presence of either atrial or ventricular arrhythmia, or acute ischemic changes on the baseline electrocardiogram (ECG); 3) acute suicidal or violent behavior or history of suicide attempt within the last year; or 4) any other central nervous system diseases. Patients were free of psychotropic medications for at least two weeks before starting the trial.
Primary Outcomes
Improvement in residual depressive symptoms in the three treatment groups over time using the continuous HDRS scores was the primary outcome measure for this study. We also analyzed the differences in the rate of response by week 4 of treatment to characterize the rate of response based on the methylphenidate titration schedule that ended at week 4.
Co-morbid symptoms and secondary outcomes
We measured co-morbid symptoms of anxiety, apathy, medical and vascular risk factors, health-related quality of life, and cognitive performance. Remission rates were analyzed as secondary outcomes and defined as the HDRS score of 6 or below.
Randomization procedures
Randomization was performed using a computer-generated schedule. Because there were three groups we used block randomization to maintain balance over the course of the study with a random mix of block lengths of 3 and 6 to help further preserve the blind. Allocation concealment was implemented using sealed, sequentially numbered boxes that were identical in appearance for the three treatment groups. In order, to monitor the internal validity of the randomization and blinding in the trial, we created a guessing scale for the study staff in the first year of the trial (as we did in our pilots) and the accuracy of our guessing for group assignment in two independent trials were 35%.
Intervention procedures
Participants were seen in-person weekly for the first 4 weeks, while methylphenidate dose was titrated for evaluation of safety and detection of accelerated response, and every 2 weeks thereafter for the remainder of 16 weeks. Treatment with both drugs was initiated simultaneously after the baseline assessment in order to track accelerated response. Participants were given a weekly supply of the given study medications prepared and dispensed by the UCLA Pharmacy in matching capsules: 20 mg/day citalopram and methylphenidate 2.5 mg (or 1 cap) twice a day (recommended at 9 am and 3 pm), or the matching number of capsules of placebo as a starting dose. We used a 5–40 mg flexible dose of methylphenidate of that was increased based on the response and tolerability assessment during each weekly visit in the first 4 weeks of treatment. The dose range was established in two of our pilot studies that were dedicated to the dose-finding and safety evaluation of the optimal methylphenidate dose in older adults. (18, 19) The dose of the methylphenidate was increased at each visit if subjects had the Clinical Global Impression (CGI)-improvement (24) scores of 3 and greater and they showed no serious adverse effects. The increment increase of methylphenidate occurred in the first 4 weeks of the trial by 2.5 mg twice a day every 4 days between days 4 and 28 of treatment, or until subjects were able to achieve CGI score of 1 or 2. After day 28 of methylphenidate titration, subjects remained on the same dose through the end of the trial. If subjects showed minimal improvement with CGI improvement score of 3 or greater by Day 28 of treatment, citalopram dose was increased to 40 mg and continued to the end of the trial in the majority of subjects, with the exception of 13 subjects, who received another increase in citalopram dose to 60 mg at weeks 7–8 of the trial due to insufficient response.
The allowed dose adjustment for methylphenidate was decreasing by 2 pills, to a minimum of 5 mg a day, and decreasing citalopram dose to 20 mg. If subjects could not tolerate the minimum allowed dose, they were discontinued from the trial. The use of concomitant rescue medications during the treatment trial was restricted to the use of lorazepam up to 1 mg day.
Assessment Instruments
Subjects were evaluated using validated assessment instruments that included the Hamilton Depression Rating Scale (HDRS-24), Montgomery-Asberg Depression Rating Scale (MADRS),(25) and Clinical Global Impression (CGI) (24) to measure depression severity and change over time. Measures of co-morbid psychiatric symptoms that could be affected by the use of methylphenidate included the Hamilton Anxiety Rating Scale (22) and Apathy Evaluation Scale (26). Cerebrovascular Risk Factor Prediction Chart, (27) and Cumulative Illness Rating Scale-Geriatrics (28) assessed medical co-morbidity. Other instruments, and the Medical Outcomes Study Short Form 36-Item Health Survey (SF-36). The primary outcome measures were administered at all visits. The rest of the clinical measures were administered at baseline and the end of the study by two raters (HL and NSC).
Cognitive Assessment
At baseline and endpoint, participants also completed a comprehensive neuropsychological test battery (29) to assess five cognitive domains: memory (California Verbal Learning Test-II [long delayed free recall], Rey–Osterrieth Complex Figure Test [30-minute delayed recall]), language (Boston Naming Test, Verbal Fluency Task [F-A-S], Animal Naming Test]), attention/processing speed (WAIS-III Digit Span, Trail Making Test A, Stroop Color Trial [Golden Version]), executive functioning (Trail Making Test B, Stroop interference [Golden version]), and visuospatial functioning (WAIS-III Block Design, Rey–Osterrieth Complex Figure Test [copy]).
We transformed raw scores to z-scores for each test score of interest for each participant, and then averaged the z-scores. Z-scores were calculated from published normative data (29–31). For variables in which good performance was represented by lower values (e.g., Trail Making Test), z-scores were reversed so that high z-scores represented good performance for all measures. These Z-scores were averaged within each neuropsychological domain to produce composite scores and then averaged over all tests to calculate a global performance score. We computed the following Cronbach’s alpha coefficients for each domain: language (0.86), visuospatial (0.89), memory (0.87), executive (0.76), and attention/speed of information processing (0.82).
Safety and Adherence Assessments
Vital signs and weight were measured at baseline and at each visit in addition to a 12-lead ECG performed at baseline, and at weeks 3 and 16, if any cardiac complaints were present. A physical examination was administered at baseline and week 16, or upon early termination. Side-effects were assessed at all visits by the Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale. (32) Treatment compliance was assessed by employing indirect measures of adherence including questioning of the patients, returned pill count, and drug level measures at weeks 3, 8, and 16. Plasma levels of citalopram and metabolite, as well as ritalin and ritalinic acid levels, were obtained. We explored the relationship between measures of adherence and outcomes.
Statistical analysis
All data were entered into the database at the time of their collection and analyzed after completion of the trial. Safety analyses were performed using descriptive statistics and frequency distribution of dropouts. Patients in the three treatment groups were compared using ANOVAs (for continuous variables) or chi-squared tests (for categorical variables) on all demographic and clinical measures at baseline to assess the success of the randomization procedures. All outcome results used Intent-to-Treat (ITT) analyses. Mean longitudinal trajectories of HDRS scores for patients who dropped out were not found to be different from patients who did not dropout. Hence, continuous HDRS scores were analyzed using a mixed effects general linear model, as implemented in SAS PROC MIXED, under the missing at random assumption. Two mixed modeling approaches were used in modeling longitudinal HDRS scores in the three groups. The first approach made less stringent assumptions on the shape of the HDRS trajectories in time and included treatment group as the between-subject factor, time as the within-subject factor, and the interaction term between time and treatment group. Based on the shape of the HDRS trajectories from the first analysis, the second mixed model included time as a continuous variable to target rates of change in HDRS scores directly, and was compromised of a broken line model, with different slopes in the three groups, from baseline to 4 weeks and from 4 weeks to end of study. Analysis included testing whether groups were significantly different in the slopes of these two linear segments (from 0–4 weeks and 4–16 weeks). In addition to the above two analyses for HDRS scores, the proportion of subjects who achieved remission was also analyzed using a chi-squared test.
The secondary outcome measures were also analyzed using mixed effects models, with group, time and group*time as predictors. Post hoc analyses determined the significance of specific pair-wise group differences and within-group changes. The level of significance for primary outcomes was set at the alpha level of p ≤ 0.05, two-tailed.
RESULTS
Figure 1 presents the CONSORT flow.
Table 1 presents the baseline demographic, clinical and cognitive characteristics of the intent-to-treat sample of the three treatment groups. Of the 143 randomized participants, the average age of the study sample was 69.7 (SD=7.3) years, the mean baseline depression severity was 18.9 (SD=2.9) on the 24-item HDRS, and the average MMSE score of the sample at baseline was 28.7 (SD=1.3). Fifty nine (41. 3%) met the criteria for treatment resistance after having had two adequate trials with antidepressants of two different classes with no group difference in proportion. At baseline, the groups differed by the proportion of women in the three groups (p=0.05). They also significantly differed in their baseline HDRS and the Cumulative Illness Rating Scale scores (p≤0.05). We therefore controlled for these variables in the subsequent analyses. The use of lorazepam as a rescue drug was minimal, 0.5–1 mg was used only in 12 subjects: 4 in citalopram+placebo, 6 in methylphenidate+ placebo and 2 in methylphenidate+ citalopram subjects with no group differences detected (chi-sq(2) = 2.3, p = .3), and controlling for it did not change our results.
Table 1.
Comparison of baseline clinical and demographic characteristics between the three treatment groups
| Baseline Variable | Citalopram + Placebo (n=48) |
Methylphenidate + Placebo (n=47) |
Methylphenidate + Citalopram (n=48) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | % | Mean | SD | N | % | Mean | SD | N | % | P value | |
| Demographics | |||||||||||||
| Age | 70.1 | 7.1 | 70.0 | 7.1 | 68.9 | 7.6 | .7 | ||||||
| Women (%) | 31 | 65.0 | 19 | 40.0 | 28 | 58.0 | .05 | ||||||
| Education | 15.8 | 2.7 | 16.0 | 2.4 | 15.2 | 3.0 | .3 | ||||||
| Race (%) | .9 | ||||||||||||
| Caucasian | 36 | 75.0 | 35 | 74.4 | 37 | 77.0 | |||||||
| African American | 5 | 10.5 | 5 | 10.6 | 5 | 10.4 | |||||||
| Asian | 1 | 2.0 | 2 | 4.4 | 3 | 6.3 | |||||||
| Hispanic | 6 | 12.5 | 5 | 10.6 | 3 | 6.3 | |||||||
| Age at depression onset | 42.7 | 23.5 | 40.4 | 25.1 | 45.6 | 22.3 | .6 | ||||||
| Number of episodes | 3.8 | 3.8 | 4.0 | 5.4 | 3.3 | 2.9 | .7 | ||||||
| Chronic course >24 month (%) | 41 | 85.0 | 37 | 78.1 | 37 | 78.1 | .6 | ||||||
| Duration of episode (month) | 51.6 | 45.6 | 50.2 | 47.2 | 38.6 | 31.8 | .3 | ||||||
| Psychiatric Symptoms | |||||||||||||
| HDRS-24* | 18.3 | 2.3 | 19.8 | 3.6 | 18.7 | 2.9 | .04 | ||||||
| Montgomery Asberg | 18. | 3.1 | 18.2 | 4.1 | 17.5 | 3.4 | .3 | ||||||
| Depression Rating Scale | 6 | ||||||||||||
| Apathy Evaluation Scale | 31.3 | 8.6 | 31.0 | 10.6 | 30.0 | 10.1 | .8 | ||||||
| Hamilton Anxiety Scale | 8.9 | 2.4 | 9.9 | 3.2 | 8.7 | 2.9 | .07 | ||||||
| Comorbid Medical Symptoms | |||||||||||||
| Cumulative Illness | 5.8 | 4.6 | 5.5 | 3.5 | 4.0 | 3.2 | .04 | ||||||
| Rating Scale-Geriatrics | |||||||||||||
| Cerebrovascular Risk Factor | 12.2 | 5.8 | 10.9 | 5.0 | 9.7 | 4.5 | .06 | ||||||
| Health-related quality of life | |||||||||||||
| Short-Form Health Survey | |||||||||||||
| Energy | 30.9 | 18.8 | 33.3 | 20.0 | 31.3 | 20.3 | .8 | ||||||
| Wellbeing | 44.2 | 17.6 | 47.3 | 15.6 | 45.6 | 17.2 | .7 | ||||||
| Role-Emotional | 34.0 | 27.9 | 37.6 | 28.3 | 31.2 | 26.1 | .5 | ||||||
Note: *HDRS-24- Hamilton Depression Rating Scale-24 Item
SD-standard deviation
The treatment groups did not differ in mean dosage of citalopram or methylphenidate (citalopram+placebo group: mean citalopram dose = 35.0 (SD=14.6) mg/day; methylphenidate+placebo group: mean methylphenidate dose =16.4 mg/day (SD=7.2); methylphenidate+citalopram group: mean citalopram dose = 32.3 (SD=13.5) mg/day, and mean methylphenidate dose = 16.2 (SD=8.1) mg/day). There was a statistically significant association in each group for a greater proportion of remission with greater citalopram daily dose: remission rates with no citalopram (in the methylphenidate group)=29.79%‚ in those on 20 mg of citalopram remission rate was 41.86%‚ in those on 40 mg −56.41%‚ in those on 60 mg-69.23% (Chi square=9.70; df= 3; P=0. 02). However, the trend for remission rate did not follow dose increase for the methylphenidate: methylphenidate dose of 0= 41.67%‚ methylphenidate dose of 5–10 mg=24.24% ‚ methylphenidate dose of 15–20 mg per day=58.33%; and dose > 20 mg per day= 53.85% (Chi square= 9.82; df=3; p= 0.02).
Analyses of change in HDRS scores over time
The mixed model, using time as a categorical effect and adjusting for gender and baseline medical burden, indicated that there was a statistically significant difference between the three treatment groups in change in HDRS score from baseline to study end (F(20,137)=2.5, p=.0008). Post-hoc analyses revealed that the change in HDRS score was significantly greater in the methylphenidate+citalopram group compared to both the citalopram+placebo group (t(137) = 2.4, p = .02) and the methylphenidate+placebo group (t(137) = 2.8, p = .005). Adjustment for the measures of drug levels and adherence measures, or medical burden did not change the results. Change in HDRS scores over time for the three treatment groups is graphically depicted in Figure 2.
Figure 2.
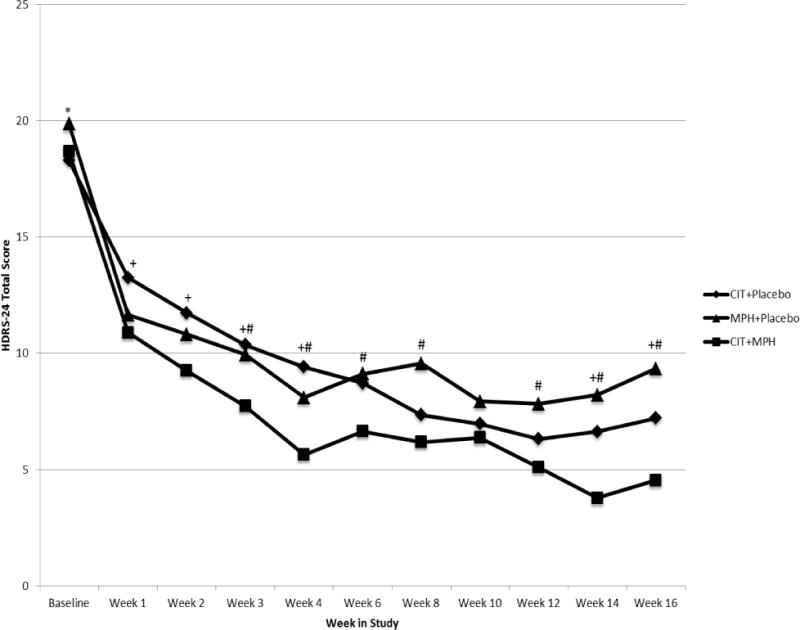
Change in HDRS-24 scores by treatment condition over the 16-week period
Citalopram=CIT; Methylphenidate=MPH; Placebo=PBO
*Statistically significant difference between CIT+PBO and MPH+PBO, p<.05
+ Statistically significant difference between CIT+PBO and CIT+MPH, p<.05
# Statistically significant difference between MPH+PBO and CIT+MPH, p<.05
Rate of change in response
Figure 2 reveals that there are two separate rates of change from baseline to 4 weeks and from 4 weeks to 16 weeks in the HDRS trajectories in time. Hence in order to target the rate of change in the response directly, we used a broken-line mixed effects model, with separate slopes for the three groups from baseline to 4 weeks and then from 4 weeks to 16 weeks. From baseline to 4 weeks, the methylphenidate+citalopram group exhibits a significantly faster decrease in HDRS scores compared to citalopram+placebo (slope difference = 0.54 (SE = 0.24), t(125) = 2.21, p = .03), but not compared to methylphenidate+placebo (slope difference = 0.07 (SE = 0.24), t(125) = 0.3, p = .7); and the difference between citalopram+placebo and methylphenidate+placebo groups does not reach statistical significance (slope difference = 0.46 (SE = 0.24), t(125) = 1.9, p = .06). After 4 weeks, the methylphenidate+citalopram group shows a significantly faster decrease in HDRS scores compared to methylphenidate+placebo: slope difference = 0.19 (SE = 0.09), t(105) = 2.11, p = .04; but not compared to citalopram+placebo: slope difference = −0.01 (SE = 0.09), t(105) = −0.14, p = .8. The HDRS scores decrease significantly faster in the citalopram+placebo group compared to the methylphenidate+placebo group also after week 4: slope difference = 0.20 (SE = 0.09), t(105) = 2.23, p = .03. The predicted values of HDRS scores obtained using this model are plotted in Supplemental Figure 1.
Secondary Remitter analyses
Twenty of 48 (41.7%) in the citalopram+ placebo group, 14 of 47 (29.8%) in the methylphenidate+placebo group, and 29 of 48 (60.4%) in the methylphenidate +citalopram group met remission criteria (HDRS ≤ 6) at study end. These differences were statistically significant (χ2 (2)=9.2, p=.01), and were driven mostly by the differences in the remission rates between methylphenidate +citalopram and methylphenidate +placebo (χ2(1)=9.0, p=.003), while the difference between methylphenidate +citalopram and citalopram and placebo groups showed a trend for significance (χ2(1)=3.4, p=.07). Note that group differences in remission rates were not found significant at 14 or 12 weeks. Due to high dropout rates observed significant differences at 16 weeks need to be interpreted with caution. The analyses of group differences by the status of remitters, partial responders and non-responders also demonstrated significant differences favoring methylphenidate+citalopram (χ2 (4)=9.9, p=.04). In the citalopram and placebo group, 20 subjects were remitters, 15 were partial responders and 13 were non-responders; in the methylphenidate +placebo group, 14 were remitters; 18 were partial responders, and 15 were non-responders; and in the methylphenidate +citalopram group, 29 remitted; 8 were partial responders and 11 were non-responders. Accelerated remission after 4 weeks of treatment was achieved by relatively few subjects with no observed group differences (8 or 17% in citalopram and placebo; 7 or 15% in methylphenidate and placebo; and 10 or 21% in the methylphenidate+citalopram groups; χ2 (2)=0.8, p=.7).
Secondary outcomes
Clinical global improvement
We combined those with CGI-I score of 1 and 2 (very much and much improved, compared to those with minimal improvement or no change, or minimally worse). In the combination group, 2/32 or 84.4% improved much or very much compared to either of the control groups (methylphenidate+ placebo [13/33 or 39.4%] and citalopram and placebo [17/30 or 56.7%]) (Chi square=13.9 (2); p= 0.001); while no significant difference was found between citalopram and placebo and methylphenidate+ placebo (chi square=1.9; P=0.17)
Analyses of change in secondary outcomes over time
Changes in SF-36 Wellbeing scores also showed significant between-group differences (F (2,136)=4.8; p=0.01) favoring the combination group (Table 2). The change in the severity of anxiety, apathy, and psychological resilience measures did not differ between the treatment groups.
Table 2.
Comparison of change scores between the three treatment groups
| Variable | CIT+PBO (n=48) |
MPH+PBO (n=48) |
MPH+CIT (n=47) |
P value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Psychiatric Symptoms | |||||||
| Hamilton Anxiety Rating Scale | −4.4 | .6 | −5.4 | .5 | −6.2 | .6 | .1 |
| Apathy Evaluation Scale | 7.1 | 1.5 | 7.1 | 1.4 | 9.1 | 1.4 | .5 |
| CGI- improvement* | −1.4 | .2 | −0.8 | 0.2 | −1.4 | .2 | .001 |
| Health-related quality of life | |||||||
| Short-Form Health Survey | |||||||
| Energy | 17.1 | 4.1 | 18.2 | 3.9 | 24.1 | 3.9 | .4 |
| Wellbeing | 25.0 | 3.6 | 13.9 | 3.5 | 28.5 | 3.5 | .01 |
| Role-Emotional | 28.8 | 6.7 | 26.3 | 6.4 | 42.6 | 6.4 | .2 |
Note: HDRS-24- Hamilton Depression Rating Scale-24 Item, CGI-Improvement – Clinical Global Impression-Improvement Scale; +A negative change score corresponds to an improvement from baseline to endpoint, except for the Apathy Evaluation Scale and Short-Form Health Survey scores.
Citalopram=CIT; Methylphenidate=MPH; Placebo=PBO; SD- standard deviation
Supplemental Table 2 presents estimated effect sizes on the selected measures over time.
Cognitive Outcomes
There were no significant differences between treatment groups on baseline neuropsychological performance. Table 2 presents baseline to endpoint change scores (using z-scores) for each composite domain, and for the global performance score.
Between-treatment analyses of cognitive change
Between treatment analyses revealed no significant differences in cognitive change (across all neuropsychological domains and the global performance score) from baseline to endpoint.
Within-treatment analyses of cognitive change
As can be seen in Table 3, we observed variable improvement in cognitive functioning within treatment groups. First, both the methylphenidate+placebo and citalopram+methylphenidate groups demonstrated significant improvement on the global performance score (methylphenidate+placebo: t(136)=−2.91, p=.004; citalopram+methylphenidate: t(136)=−2.04, p=.04). All treatment groups significantly improved in language (citalopram+placebo: t(136)=−2.81, p=.01; methylphenidate+placebo: t(136)=−2.61, p=.01); citalopram+methylphenidate: t(136)=−3.25, p=.002). The methylphenidate+placebo group additionally improved in executive functioning (t(136)=−2.45, p=.02). Finally, the citalopram+placebo group demonstrated significant improvement in attention (t(136)=−2.43, p=.02). No within treatment changes were noted in memory or visuospatial functioning. Across comparisons, change in HDRS-24 score from baseline to endpoint did not moderate improvement in cognitive functioning.
Table 3.
Between-treatment comparison of cognitive z-score change by composite domain, and raw-score change by neuropsychological test
| Variable | Citalopram+ Placebo (n=48) |
Methylphenidate+ Placebo (n=48) |
Citalopram+ Methylphenidate (n=47) |
P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw-score change by test | Z-score change | SE | Raw-score change by test | Z-score change | SE | Raw-score change by test | Z-score change | SE | ||
| Attention/Processing Speed | .24 | .10 | −.006 | .09 | .05 | .09 | .2 | |||
| WAIS-III Digit Span | 2.07 | .25 | .37 | |||||||
| Trail Making Test A (sec)+ | −6.25 | −1.78 | −.36 | |||||||
| Stroop Color Trial | 3.82 | .52 | −.4 | |||||||
| Language | .26 | .09 | .23 | .09 | .27 | .08 | .9 | |||
| FAS | 3.5 | 4.09 | 3.48 | |||||||
| Animal Naming Test | 1.29 | −.47 | .73 | |||||||
| Boston Naming Test | 3.2 | 1.84 | 1.36 | |||||||
| Executive Functioning | .13 | .12 | .28 | .12 | −.02 | .11 | .2 | |||
| Trail Making Test B (sec)+ | −21.71 | −8.98 | 8.0 | |||||||
| Stroop Color-Word | 2.55 | .82 | −.19 | |||||||
| Visuospatial Functioning | −.10 | .11 | .01 | .11 | .16 | .10 | .2 | |||
| WAIS-III Block Design | 1.56 | 2.55 | −.1 | |||||||
| RCFT Copy | .05 | −.41 | .71 | |||||||
| Memory | −.03 | .10 | .12 | .09 | -.05 | .09 | .4 | |||
| CVLT-2 Long Delay Free | .31 | .11 | −.39 | |||||||
| RCFT 30-Minute Delay | 3.09 | 2.34 | 1.28 | |||||||
| Global Performance Score | .10 | .05 | .15 | .05 | .10 | .05 | .7 | |||
Note: The Stroop Interference score was used to develop the Executive Functioning composite score. That variable was not included in the raw-score change, as no raw score is generated. We instead include the raw-score change for the Color-Word trial as a reference. However, unlike the Stroop Interference score, this does not correct for time completed on control trials (color and word naming trials) of the Stroop Color and Word Test.
A negative change score corresponds to an improvement from baseline to endpoint
Citalopram=CIT; Methylphenidate=MPH; Placebo=PBO; SE= standard error
Dropout and tolerability analysis
The three groups did not differ significantly in their dropout times (citalopram+palcebo: mean(SD) 33.2 (21.2) days; range 8–86); methylphenidate+palcebo: 27.8 (15.1); 7–48); and citalopram+methylphenidate: 41.6 (31.3); 7–98; F(2,42) = 1.24, p = .3). The groups did not differ by the number of side-effects, dropout rates (χ2 (2)=0.6, p=0.7), or dropout reasons (χ2(8)=9.1, p=0.3). Post-randomization, 16 dropped out due to side effects (7 each in the citalopram+placebo and methylphenidate+placebo groups and 2 in the citalopram+methylphenidate group), 3 due to lack of efficacy, and 26 due to other causes (Supplemental Table 1).
Discussion
Our study is the first randomized placebo-controlled trial aimed to test the clinical efficacy and tolerability of methylphenidate in combination with citalopram used to improve antidepressant response in geriatric depression compared to citalopram and placebo and methylphenidate and placebo. We detected improved response in the citalopram+methylphenidate group as evidenced by greater improvement in the two continuous measures of depression and the global clinical improvement. We also observed a faster rate of response in depression symptoms in the methylphenidate and citalopram group in the first four weeks of treatment compared to citalopram and placebo, and compared to methylphenidate and placebo for the remainder of the trial, while the rate of response for the methylphenidate+placebo and citalopram+ placebo differed statistically in the last 12 weeks of the trial. We found greater remission rates in the methylphenidate+ citalopram compared to the methylphenidate+ placebo group, with a trend in comparison to the citalopram+placebo group. This potentially gives guidance to the clinicians on the use of these drugs to achieve faster remission in depressed older adults, but may not translate into greater remission rates over a longer period of time compared to citalopram and placebo.
Antidepressant treatment appeared beneficial for cognition, though augmentation with methylphenidate did not offer additional benefits. However, the participants taking methylphenidate demonstrated improvement in the global cognitive performance score. We also report improvements in the SF-36 wellbeing scale of the combination of methylphenidate and citalopram versus comparison groups. We did not find group differences in other clinical measures or side-effects. Overall, the outcomes are encouraging for mental health providers given the limited number of successful treatment strategies to enhance antidepressant response with additional benefits in function in geriatric depression.
To-date, a very limited number of studies have suggested stimulants might be especially useful in older adults (18–21, 33–36). Two large series of medically ill mixed-age patients (34, 36) suggested the value of adjunctive dextroamphetamine (range: 2.5 mg to 30 mg) and methylphenidate (range: 5–30 mg/d) in relieving depression with the rapid onset of response within 48 hours. Our findings on cognitive outcomes are generally consistent with the literature, as they suggest that cognition improves following acute antidepressant treatment (37), although some studies do not support it (12, 15).
There are several limitations of our study. We used a convenience sample of outpatients with moderate major depression. Therefore, the results may not be applicable to the acutely medically ill patients, and those with more severe depression. Due to the use of methylphenidate, we excluded subjects with prior history of substance abuse and severe anxiety disorders that may limit generalizability of the results. Although we were interested in acceleration of response with the use of methylphenidate, the titration was relatively slow because of the concerns for safety in elderly subjects, but the rate of remission within the first four weeks or methylphenidate use was still more rapid than the expected after 16 weeks of the use of citalopram. Finally, although we found a relationship between increased dose of citalopram and the best response for the doses of methylphenidate between 15–20 mg per day, our results with regard to the citalopram dosing should be interpreted cautiously with consideration of the 2011 FDA warning (38) about citalopram maximum dosing recommendations to be limited to 40 mg in younger adults, and to 20 mg in the elderly due to potential cardiac side-effects, and recently published data confirming increase in the QTc interval prolongation with citalopram use in older patients with dementia.(39) In addition, ideally, the comparison of different drug dosing on the remission rates would require a fixed dose comparison trial. Finally, high dropout rate is a limitation of the study. Our analysis did not suggest apparent indication of missing not at random, and hence mixed effects modeling of our primary outcome HDRS scores has been carried out under the missing at random assumption. However, group differences at 16 weeks in remission rates should be interpreted with caution due to high dropout and further studies are needed to ascertain the reasons for group differences in the remission rates.
Despite these limitations, our study is the first comprehensive and well-controlled study to address the methylphenidate potential to enhance clinical and cognitive outcomes. In this study the combination of citalopram and methylphenidate resulted in higher and faster achieved remission rates than citalopram plus placebo (60 vs. 42%), while adverse events were no more common with combination treatment. The combination may offer a method of improving the efficacy and rate of response to treatment in late life depression and can inform research and clinical practice.
Supplementary Material
Supplemental Figure 1. Predicted HDRS scores over time.
Figure 1.
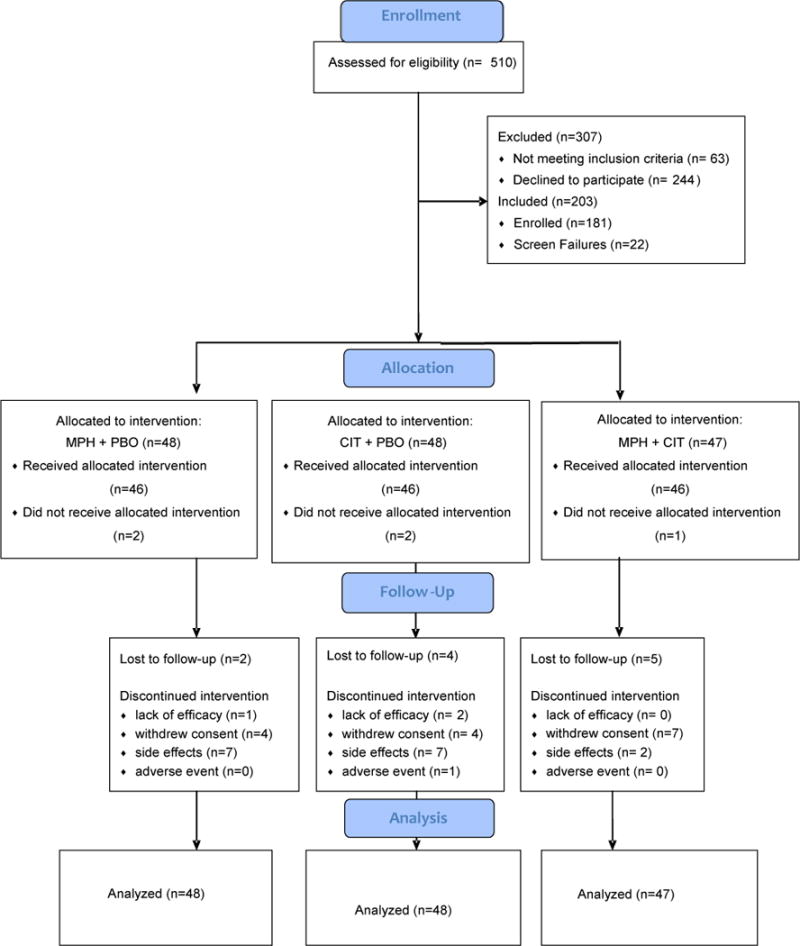
Consort Diagram (Citalopram=CIT; Methylphenidate=MPH; Placebo=PBO).
Acknowledgments
Drs. Helen Lavretsky, Michelle Reinlieb, Linda Ercoli, Damla Senturk, and Prabha Siddarth had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of support: This work was supported by NIH grants MH077650 and MH086481 to Dr. Lavretsky.
Dr. Lavretsky reports having received research grants from Forest Research Institute and the Alzheimer’s Research and Prevention Foundation.
Footnotes
Potential conflicts of interest: Drs. Reinlieb, Siddarth, Senturk, Ercoli and Ms. St. Cyr have no financial conflicts of interest.
Trial Registration: NCT00602290. Effectiveness of Methylphenidate in Improving Cognition and Function in Older Adults With Depression.
References
- 1.Alexopoulos GS, Young RC, Abrams RC, Meyers B, Shamoian CA. Chronicity and relapse in geriatric depression. Biol Psychiatry. 1989;26(6):551–64. doi: 10.1016/0006-3223(89)90080-2. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds CF, 3rd, Dew MA, Pollock BG, Mulsant BH, Frank E, Miller MD, et al. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354(11):1130–8. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JC, Delucchi K, Schneider LS. Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am J Geriatr Psychiatry. 2008;16(7):558–67. doi: 10.1097/JGP.0b013e3181693288. Epub 2008/07/02. [DOI] [PubMed] [Google Scholar]
- 4.Nelson JC, Delucchi KL, Schneider LS. Moderators of outcome in late-life depression: a patient-level meta-analysis. Am J Psychiatry. 2013;170(6):651–9. doi: 10.1176/appi.ajp.2012.12070927. Epub 2013/04/20. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. Am J Psychiatry. 2013;170(7):723–33. doi: 10.1176/appi.ajp.2012.12040474. Epub 2013/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sackeim HA, Roose SP, Burt T. Optimal length of antidepressant trials in late-life depression. J Clin Psychopharmacol. 2005;25(4 Suppl 1):S34–7. doi: 10.1097/01.jcp.0000170683.25802.12. Epub 2005/07/20. [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–10. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry. 2010;18(2):128–35. doi: 10.1097/JGP.0b013e3181c796d2. Epub 2010/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry. 2005;13(3):244–9. doi: 10.1176/appi.ajgp.13.3.244. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of neurology. 1999;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 11.Bhalla RK, Butters MA, Becker JT, Houck PR, Snitz BE, Lopez OL, et al. Patterns of mild cognitive impairment after treatment of depression in the elderly. Am J Geriatr Psychiatry. 2009;17(4):308–16. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157(12):1949–54. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 13.Culang ME, Sneed JR, Keilp JG, Rutherford BR, Pelton GH, Devanand DP, et al. Change in cognitive functioning following acute antidepressant treatment in late-life depression. Am J Geriatr Psychiatry. 2009;17(10):881–8. doi: 10.1097/jgp.0b013e3181b4bf4a. Epub 2009/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr. 2007;19(1):125–35. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- 15.Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37(2):99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 16.Lavretsky H, Park S, Siddarth P, Kumar A, Reynolds CF., 3rd Methylphenidate-enhanced antidepressant response to citalopram in the elderly: a double-blind, placebo-controlled pilot trial. Am J Geriatr Psychiatry. 2006;14(2):181–5. doi: 10.1097/01.JGP.0000192503.10692.9f. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155(3):344–9. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 18.Lavretsky H, Kim MD, Kumar A, Reynolds CF., 3rd Combined treatment with methylphenidate and citalopram for accelerated response in the elderly: an open trial. J Clin Psychiatry. 2003;64(12):1410–4. doi: 10.4088/jcp.v64n1202. [DOI] [PubMed] [Google Scholar]
- 19.Lavretsky H, Kumar A. Methylphenidate augmentation of citalopram in elderly depressed patients. Am J Geriatr Psychiatry. 2001;9(3):298–303. [PubMed] [Google Scholar]
- 20.Satel SL, Nelson JC. Stimulants in the treatment of depression: a critical overview. J Clin Psychiatry. 1989;50(7):241–9. [PubMed] [Google Scholar]
- 21.Murray GB, Cassem E. Use of stimulants in depressed patients with medical illness. In: Nelson JG, editor. Geriatric Psychopharmacology. New York-Basel-Hong Kong: Marcel Decker, Inc; 1998. pp. 245–58. [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Guy W. Clinical Global Impressions (CGI) In: Services UDoHaH, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville: Alcohol Drug Abuse and Mental Health Administration, NIMPH Psychopharmacology Research Branch; 1976. pp. 218–22. [Google Scholar]
- 25.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. The assessment of anxiety states by rating. The British journal of medical psychology. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 27.Truelsen T, Lindenstrom E, Boysen G. Comparison of probability of stroke between the Copenhagen City Heart Study and the Framingham Study. Stroke. 1994;25(4):802–7. doi: 10.1161/01.str.25.4.802. [DOI] [PubMed] [Google Scholar]
- 28.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 29.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York: Oxford; 2004. [Google Scholar]
- 30.Selnes OA, Jacobson L, Machado AM, Becker JT, Wesch J, Miller EN, et al. Normative data for a brief neuropsychological screening battery. Multicenter AIDS Cohort Study. Percept Mot Skills. 1991;73(2):539–50. doi: 10.2466/pms.1991.73.2.539. Epub 1991/10/01. [DOI] [PubMed] [Google Scholar]
- 31.Van Gorp WG, Satz P, Mitrushina M. Neuropsychological processes associated with normal aging. Developmental Neuropsychology. 1990;6:279–90. [Google Scholar]
- 32.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 33.Darvill FT, Jr, Woolley S. Double blind evaluation of methylphenidate (ritalin) hydrochloride; its use in the management of institutionalized geriatric patients. J Am Med Assoc. 1959;169(15):1739–41. doi: 10.1001/jama.1959.03000320041011. Epub 1959/04/11. [DOI] [PubMed] [Google Scholar]
- 34.Masand P, Pickett P, Murray GB. Psychostimulants for secondary depression in medical illness. Psychosomatics. 1991;32(2):203–8. doi: 10.1016/S0033-3182(91)72093-8. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 35.Nelson JC. Augmentation strategies for treatment of unipolar major depression. Modern problems of pharmacopsychiatry. 1997;25:34–55. doi: 10.1159/000061657. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 36.Woods SW, Tesar GE, Murray GB, Cassem NH. Psychostimulant treatment of depressive disorders secondary to medical illness. J Clin Psychiatry. 1986;47(1):12–5. Epub 1986/01/01. [PubMed] [Google Scholar]
- 37.Doraiswamy PM, Krishnan KR, Oxman T, Jenkyn LR, Coffey DJ, Burt T, et al. Does antidepressant therapy improve cognition in elderly depressed patients? J Gerontol A Biol Sci Med Sci. 2003;58(12):M1137–44. doi: 10.1093/gerona/58.12.m1137. Epub 2003/12/20. [DOI] [PubMed] [Google Scholar]
- 38.FDA. FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. 2011 [March 5, 2014] Available from: http://www.fda.gov/drugs/drugsafety/ucm297391.htm.
- 39.Drye LT, Spragg D, Devanand DP, Frangakis C, Marano C, Meinert CL, et al. Changes in QTc interval in the citalopram for agitation in Alzheimer’s disease (CitAD) randomized trial. PloS one. 2014;9(6):e98426. doi: 10.1371/journal.pone.0098426. Epub 2014/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Predicted HDRS scores over time.


