Abstract
Importance
The 2008 Physical Activity Guidelines for Americans recommended a minimum of 75 vigorous-intensity or 150 moderate-intensity minutes per week (7.5 metabolic equivalent hours per week (MET h/wk)) of aerobic activity for “substantial” health benefit, and suggested “additional” benefits by doing more than double this amount. However, the upper limit of longevity benefit or possible harm with more physical activity is unclear.
Objective
To quantify the dose-response association between leisure-time physical activity and mortality, and to define the upper limit of benefit or harm associated with more physical activity.
Design
We pooled data from six studies in the NCI Cohort Consortium (baseline 1992–2003). We used Cox proportional hazards regression with cohort stratification to generate multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CI). Median follow-up time was 14.2 years.
Setting
Population-based prospective cohorts in the U.S. and Europe with self-reported physical activity.
Participants
661,137 men and women (116,686 deaths); median age 62 (range 21–98) years.
Exposure
Leisure-time moderate- to vigorous-intensity physical activity
Main Outcome
Mortality
Results
Compared to those reporting no leisure-time physical activity, we observed a 20% lower mortality risk among those performing less than the recommended 7.5 MET h/wk minimum (HR=0.80, 95% CI 0.78–0.82), a 31% lower risk at 1–2 times the recommended minimum (0.69, 0.67–0.70), and a 37% lower risk at 2–3 times the minimum (0.63, 0.62–0.65). An upper threshold for mortality benefit occurred at 3–5 times the physical activity recommendation (0.61, 0.59–0.62), but compared to the recommended minimum, the additional benefit was modest (31% vs. 39%). There was no evidence of harm at 10+ times the recommended minimum (0.68, 0.59–0.78). A similar dose-response was observed for mortality due to cardiovascular disease and to cancer.
Conclusions and Relevance
Meeting the Guideline minimum by either moderate- or vigorous-intensity activities was associated with nearly the maximum longevity benefit. We observed a benefit threshold around 3–5 times the recommended leisure-time physical activity minimum, and no excess risk at 10+ times the minimum. In regard to mortality, clinicians should encourage inactive adults to perform leisure-time physical activity and do not need to discourage adults who already participate in high activity levels.
Introduction
Regular physical activity has consistently been associated with a reduced risk of mortality 1–3. However, the 2008 Physical Activity Guidelines for Americans were the first-ever recommendations published by the federal government to describe types and amounts of physical activity that offer health benefits. The 2008 Guidelines recommended 150–300 minutes of moderate-intensity or 75–150 minutes of vigorous-intensity aerobic activity weekly for substantial health benefits 4. However, the Guidelines noted that the upper threshold of benefit for aerobic activity and potential harms associated with very high levels of activity were undefined.
Few prospective cohorts have been able to examine the association between activity levels above these recommendations and mortality due to few deaths among participants reporting higher activity levels. However, in recent years endurance training has increased as indicated by a record 541,000 individuals in the U.S. completing a marathon in 2013 5 and 510,859 USA Triathlon members in 2012 6. Recent studies have suggested higher risk of arrhythmias with prolonged endurance training 7 or sudden death due to electrical and myocardial remodeling 8, raising concerns among individuals performing such activities and making health effects of very high levels of exercise a potential clinical concern. The 2008 Guidelines reviewed this evidence and concluded that while cardiac risk increases when an individual becomes more active than usual (e.g. someone inactive undertakes vigorous activities), these cardiac events are rare and individuals who are regularly physically active have the lowest risk of cardiac events even while active 9.
While a previous publication using these six pooled cohort studies showed lower mortality among those performing three times the recommended minimum10, in the present study, with additional follow-up, we tackle the not previous addressed question of upper limit of benefit from physical activity. In this pooled analysis we have a sufficient number of deaths to examine the shape of the mortality dose-response curve for adults performing more than the recommended physical activity minimum (i.e., 150 minutes/week of moderate- or 75 minutes/week of vigorous-intensity activity, or some combination expending equivalent energy) and up to 10+ times the recommended minimum.
To fill the gap in scientific knowledge of the dose-response relation between leisure-time physical activity and mortality, we aimed to quantify 1) the upper threshold for longevity benefit from leisure-time physical activity, and 2) mortality risks associated with very high levels of exercise. In secondary analyses, we also evaluated the mortality dose-response for moderate- and vigorous-intensity leisure-time physical activity separately.
Methods
Study population
The six cohorts in our pooled analysis previously participated in the National Cancer Institute (NCI) Cohort Consortium analyses of body mass index (BMI) or physical activity and mortality 10,11. We used the same inclusion criteria as those previous studies: a prospective design, at least 5 years of follow-up, at least 1,000 deaths among non-Hispanic white participants, baseline data collected in 1970 or later, and assessment of height, weight and smoking status, as well as leisure-time physical activity. Of the 19 studies in the BMI and mortality analysis, eight had information on time spent in moderate or vigorous-intensity leisure-time physical activity and five agreed to participate. The Cancer Prevention Study II (CPII) later joined the consortium, met the criteria and agreed to participate. We excluded individuals missing BMI or reporting a BMI <15 or >60 kg/m2. Each participating study was approved by the institutional review board of the host institute.
The included cohorts have been previously described. In short, the National Institutes of Health (NIH)–AARP Diet and Health Study collected information on diet and health risk factors among members of AARP 12. The CPS II assessed environmental and lifestyle cancer risk factors among U.S. and Puerto Rican individuals 13. CLUE II investigated cardiovascular and cancer risk factors among Washington County, Maryland, residents 14. In the U.S. Radiologic Technologists (USRT) Study, radiologic technologists residing in the U.S. and certified by the American Registry of Radiologic Technologists were recruited for the study of cancer risk factors 15. The Women's Health Study (WHS) is a completed randomized clinical trial testing low-dose aspirin and vitamin E for preventing cardiovascular disease and cancer in female health professionals from 1992–2004, after which participants were followed observationally 16–18. The Women's Lifestyle and Health Study (WLHS) is a population-based cohort study on disease risk among Swedish women sampled from the Uppsala Health Care Region 19.
Exposure assessment
Physical activity construct validity and intensity levels are presented in eTable 1. In short, CPS II, CLUE II, and WHS had 7–8 line items querying the average weekly time spent performing the following activities over the prior year: walking, jogging/running, swimming, tennis/racquetball, bicycling, aerobics and dance. The physical activity questionnaires were adapted from the Nurses’ Health Study questionnaire, which has shown correlation coefficients ranging from 0.79–0.83 compared to recalls and from 0.59–0.62 compared to diaries20.
NIH-AARP and WLHS used physical activity questionnaires that have not formally been validated, but have shown expected associations between physical activity and mortality in previous studies 12,21, and USRT has shown expected inverse associations with breast cancer 15. The NIH-AARP Study included a single line item for all moderate- or vigorous-intensity leisure time physical activities with categorical responses measured in hours per week (h/wk). The WLHS questionnaire included separate line items about hours per day in leisure-time physical activity such as walking, horseback riding, or in strenuous activities, and the U.S. Radiologic Technologists study had separate line items for h/wk spent walking for exercise and exercising strenuously. For all six studies, we calculated energy expended per activity by multiplying the estimated MET value 22 (multiple of resting metabolic rate) by the number of h/wk and summed across activities to estimate overall leisure-time physical activity energy expenditure in MET h/wk.
We used standardized categories to harmonize data between cohorts as follows: race/ethnicity (black, white, other), education (did not finish high school, finished high school, post-high school training, some college, finished college, missing), smoking status (never, former, current, missing), history of cancer (yes, no/missing), history of heart disease (yes, no/missing), alcohol consumption (0, >0-<15, 15-<30, 30+ grams/day), marital status (married, divorced, widowed, unmarried, missing) and BMI (<18.5, 18.5–25, 25-<30, 30-<35, 35+ kg/m2). We imputed the value for alcohol using the median value because non-drinkers and true missing values were grouped differently between studies. In subsequent analysis we tested associations using a missing category for alcohol instead of the imputed value and found no change in our physical activity results (all hazard ratios were within 0.02 of previous estimates). Questionnaires did not distinguish between “missing” and “no” for history of heart disease and cancer history; thus individuals were dichotomized into groups of yes or missing/no. Missing data was <5% for all covariates. We performed analyses calculating follow-up time in two ways: first, using age at study entry to age at death or end of follow up and second, calculating time from baseline questionnaire to date of death or end of follow-up. Because results did not differ from analyses using age as the time metric or using follow-up time and adjusting for age, in further analyses we used the latter method and adjusted for continuous age. The National Death Index, death certificates, or medical records were used to ascertain date of death (eTable 1).
Statistical analysis
We performed Cox proportional hazards regression stratified by cohort to generate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for leisure-time physical activity and mortality. Final models were adjusted for age, gender, race, education, smoking status, cancer history, heart disease, alcohol consumption, marital status, and BMI. We created the following MET h/wk categories: 0, 0.1-<7.5, 7.5-<15, 15-<22.5, 22.5-<40, 40-<75, and 75+. These categories were created to reflect multiples of the federal physical activity recommendations, ranging from 1–2 times the recommended minimum (7.5-<15 MET h/wk) to up to 10+ times the recommended levels (75+ MET h/wk). We further examined intensity in the five cohorts with line items for individual activities (CLUE II, CPS II, USRT, WHS and WLH) by separating moderate (3-<6 METs) and vigorous (6+ <METs) intensity activities and creating mutually adjusted models. In intensity analyses, MET h/wk categories were 0, 0.1-<7.5, 7.5-<15, 15-<30, and 30+ due to a lower range of MET h/wk for each intensity and fewer deaths in the highest categories. We also examined heart disease and cancer-specific mortality. The proportional hazards assumption was tested by creating an interaction term between continuous leisure-time physical activity and follow-up time and using the Wald test for significance of the interaction term.
To test for statistical significance of interaction we created interaction terms between continuous leisure-time physical activity and the exposures of interest and used the Wald test for dichotomous variables and the likelihood ratio test for multi-level variables. We also created separate models by cohort and used random effects meta-analyses to generate summary risk estimates and the I2 statistic for heterogeneity. To explore individual random effects we created Cox proportional hazards frailty models. To further test influence by specific cohorts, we performed analyses excluding one cohort at a time. We used a restricted cubic spline to explore whether mortality risk increased at the highest leisure-time physical activity levels 23. We excluded individuals reporting >100 MET h/wk to test the influence of outliers and also restricted the dataset to those performing >15 MET h/wk to test the p-trend for leisure-time physical activity and mortality at levels only among individuals reporting more than double the recommended minimum. We also stratified by potential effect modifiers age, sex, education, race, BMI, smoking status, heart disease and previous cancer.
All analyses were performed in SAS 9.3 other than the random effects meta-analysis (STATA 11). The figure was generated using GraphPad Prism 6.
Results
Our pooled dataset included 661,137 participants (291,485 men and 369,652 women). With a median 14.2 follow-up years (range 0–15.2), we observed 116,686 deaths. Descriptive characteristics of the six cohorts are included eTable 2. Median age at study entry was 62 years and the median physical activity level was 8 MET h/wk (interquartile range 4–22). Those performing the most leisure-time physical activity tended to be younger, never smokers, have a lower BMI, be married and have fewer comorbidities (Table 1).
Table 1.
Descriptive characteristics of study participants according to leisure time physical activity (LTPA) categories (n=661,137)
| Characteristic | Category (%) | Participants | LTPA level, MET h/wk (percentages in each category reported below)a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.1-<7.5 | 7.5-<15 | 15-<22.5 | 22.5-<40 | 40-<75 | 75+ | |||
| Participants, n | 661,137 | 52,848 | 172,203 | 170,563 | 118,169 | 124,446 | 18,831 | 4,077 | |
| Deaths, n | 116,686 | 11,523 | 33,511 | 28,957 | 19,979 | 21,114 | 1,390 | 212 | |
| Age, years | <60 | 276,418 | 50 | 45 | 43 | 39 | 36 | 65 | 76 |
| 60-<70 | 307,556 | 39 | 45 | 48 | 53 | 54 | 27 | 18 | |
| 70+ | 61,356 | 12 | 10 | 10 | 8 | 10 | 8 | 6 | |
| Gender | Men | 291,485 | 38 | 42 | 44 | 49 | 49 | 30 | 28 |
| Women | 369,652 | 62 | 58 | 56 | 51 | 51 | 70 | 72 | |
| Smoking Status | Never | 275,388 | 40 | 43 | 43 | 41 | 41 | 48 | 51 |
| Former | 298,256 | 41 | 44 | 46 | 49 | 49 | 42 | 38 | |
| Current | 74,977 | 19 | 13 | 11 | 10 | 10 | 10 | 11 | |
| Alcohol intake | Non-drinker | 179,676 | 38 | 30 | 26 | 22 | 25 | 23 | 24 |
| One drink/d | 376,861 | 51 | 56 | 59 | 59 | 56 | 65 | 66 | |
| Two drinks/d | 54,063 | 7 | 7 | 8 | 10 | 10 | 7 | 6 | |
| Education | College Graduate | 250,564 | 29 | 37 | 41 | 44 | 40 | 44 | 43 |
| Marital Status | Married | 474,338 | 77 | 77 | 77 | 74 | 76 | 81 | 76 |
| BMI, kg/m2 | <25 | 277,193 | 36 | 38 | 42 | 44 | 48 | 60 | 60 |
| 25-<30 | 256,713 | 37 | 39 | 40 | 40 | 40 | 32 | 30 | |
| 30+ | 119,988 | 27 | 23 | 18 | 15 | 12 | 9 | 10 | |
| Race | White | 627,393 | 96 | 96 | 97 | 96 | 97 | 96 | 93 |
| Co-morbidities | Heart disease | 61,158 | 8 | 9 | 9 | 11 | 10 | 4 | 3 |
| Cancer | 46,358 | 8 | 8 | 7 | 6 | 7 | 6 | 5 | |
Frequencies are column percentage.
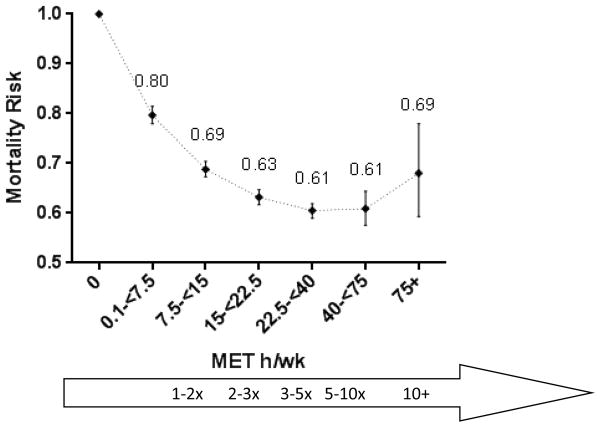
Compared to no baseline leisure-time physical activity, any level of activity was associated with a significantly lower risk of mortality (Figure 1, eTable 3). Specifically, among those performing less than the recommended leisure-time physical activity minimum (0.1-<7.5 MET h/wk), we observed 20% lower risk of mortality (HR=0.80, 95% CI 0.78–0.82). This inverse association grew stronger among those performing 1–2 times the recommended minimum (7.5-<15 MET h/wk HR=0.69, 95% CI 0.67–0.70) or 2–3 times the minimum (15-<22.5 MET h/wk HR=0.63, 95% CI 0.62–0.65), but appeared to reach a threshold of a 39% lower mortality risk among those performing 3–10 times the recommended minimum (22.5-<40 MET h/wk HR=0.61, 95% CI 0.59–0.62; 40-<75 MET h/wk HR=0.61, 95% CI 0.58–0.64). We observed a still reduced, but not as strong 32% lower mortality risk for those performing 10+ times the recommended minimum leisure-time physical activity levels (75+ MET h/wk HR=0.68, 95% CI 0.59–0.78) after adjustment for other known mortality risk factors (comparing 40–75 to 75+ MET h/wk p=0.127).
Figure 1. Hazard ratios (HRs) and 95% confidence intervals (CIs) for leisure time moderate- to vigorous-intensity physical activity and mortalitya-c.
The dose-response curve and category-specific hazard ratio estimates for leisure time moderate- to vigorous-intensity physical activity and mortality. Crude and adjusted risk estimates are presented in eTable3.
Exercise levels compared to the federally recommended minimum of 7.5 MET h/wk
aModels were stratified by cohort and use age as the underlying time scale. The model was adjusted for gender, smoking (never, former, current, missing), alcohol (none, <15 grams/day, 15–30 grams/day, 30+ grams/day), education (dropout, high school, post high school education, some college, college graduate, post-college, missing), marital status (married, divorced, widowed, single, missing), history of cancer, history of heart disease, and body mass index (<18.5, 18.5–25, 25-<30, 30-<35, 35+ kg/m2).
bThe dotted line between categories illustrates an assumed dose-response rather than individual data points.
cCrude and adjusted hazard ratios and 95% confidence intervals are presented in Supplemental Table 3.
To test for heterogeneity we modeled the association between leisure-time physical activity and mortality by random-effects meta-analysis. We found similar patterns of association, suggesting a relative threshold for benefit among those meeting or exceeding the leisure-time physical activity recommended minimum (eTable 4). Heterogeneity between cohorts was statistically significant for all leisure-time physical activity categories (p<0.05). We also created separate models for each cohort to examine cohort-specific risk estimates. The direction of association was inverse for all cohorts, and did not show evidence of additional mortality benefit at the highest leisure-time physical activity levels (eTable 4). Excluding cohorts from analysis one at a time to further test for influence showed results consistent with the main findings (eTable 5).
We also created separate moderate vs vigorous intensity categories ranging from 0–30+ MET h/wk and ran models mutually adjusted for both leisure-time physical activity intensities. In this subset analysis of 348,725 individuals, while we observed some mortality benefit with low levels of moderate-intensity activity (0.1–7.5 MET h/wk HR=0.80, 95% CI 0.78–0.83), the maximum observed mortality benefit accrued with meeting the recommended minimum of 7.5 MET h/wk (7.5-<15 MET h/wk HR=0.73, 95% CI 0.71–0.75), and higher moderate-intensity activity levels did not yield additional benefit (30+ MET h/wk HR=0.72, 95% CI 0.68–0.76) (Table 2). For vigorous-intensity leisure-time physical activity, any level of activity even below the recommended minimum was associated with an approximate 20% lower mortality risk. Associations were similar between men and women.
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for leisure time physical activity and mortality, by activity intensity (n=108,902 men and n=239,823 women)a
| MET h/wk | 0 | 0.1-<7.5 | 7.5-<15 | 15-<30 | 30+ |
|---|---|---|---|---|---|
| Moderate-intensity activity | |||||
| Participants | 53,376 | 122,522 | 100,687 | 59,304 | 12,836 |
| Deaths | 8,359 | 16,203 | 11,667 | 8,054 | 1,696 |
| Age-adjusted HR (95% CI)b | 1.00 | 0.70 (0.68–0.72) | 0.62(0.60–0.64) | 0.63 (0.61–0.65) | 0.63 (0.60–0.67) |
| Fully adjusted HR (95% CI)c | 1.00 | 0.80 (0.78–0.83) | 0.73 (0.71–0.75) | 0.71 (0.68–0.73) | 0.72 (0.68–0.76) |
| Men | 1.00 | 0.84 (0.81–0.87) | 0.77 (0.74–0.80) | 0.73 (0.71–0.76) | 0.74 (0.69–0.79) |
| Women | 1.00 | 0.76 (0.73–0.80) | 0.68 (0.65–0.71) | 0.67 (0.63–0.70) | 0.69 (0.64–0.75) |
| Vigorous-intensity activity | |||||
| Participants | 243,598 | 55,160 | 23,792 | 10,816 | 15,359 |
| Deaths | 40,229 | 3,525 | 638 | 892 | 695 |
| Age-adjusted HR (95% CI)b | 1.00 | 0.75 (0.73–0.78) | 0.72 (0.67–0.78) | 0.71 (0.67–0.76) | 0.72 (0.67–0.78) |
| Fully adjusted HR (95% CI)c | 1.00 | 0.80 (0.78–0.83) | 0.77 (0.71–0.84) | 0.78 (0.73–0.83) | 0.79 (0.73–0.85) |
| Men | 1.00 | 0.78 (0.75–0.82) | 0.69 (0.61–0.78) | 0.72 (0.66–0.79) | 0.77 (0.70–0.85) |
| Women | 1.00 | 0.83 (0.79–0.88) | 0.85 (0.77–0.94) | 0.86 (0.78–0.94) | 0.81 (0.72–0.91) |
AARP was not included in this analysis because the questionnaire did not distinguish between moderate and vigorous-intensity activities.
Models are stratified by cohort, are adjusted for age and are mutually adjusted for both moderate and vigorous-intensity activities.
Models are additionally adjusted for gender (in non-stratified models), smoking (never, former, current, missing), alcohol (none, <15 grams/day, 15–30 grams/day, 30+ grams/day), education (dropout, high school, post high school education, some college, college graduate, post-college, missing), marital status (married, divorced, widowed, single, missing), history of cancer, history of heart disease, and where indicated, body mass index (<18.5, 18.5–25, 25-<30, 30-<35, 35+ kg/m2).
In analyses of leisure-time physical activity and cause-specific mortality we found a monotonic inverse trend for cancer deaths, with a 31% lower cancer mortality risk (HR=0.69, 95% CI 0.55–0.87) among those performing 10+ times the recommended minimum (75+ MET h/wk) compared to those reporting no activity (Table 3). For CVD deaths, however, the upper threshold was observed among those reporting 3–5 times the recommended minimum (22.5-<40 MET h/wk HR=0.58, 95% CI 0.56–0.61). There was no additional benefit for those reporting 5–10 or 10+ times the recommended minimum (HR, 95% CIs = 0.61, 0.55–0.67 and 0.71, 0.56–0.91, respectively); There was no statistical difference between these two highest physical activity categories (p=0.226).
Table 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for moderate to vigorous-intensity physical activity and cause-specific mortality (n=661,137)
| MET h/wk | 0 | 0.1-<7.5 | 7.5-<15 | 15-<22.5 | 22.5-<40 | 40-<75 | 75+ |
|---|---|---|---|---|---|---|---|
| Participants, n | 52,848 | 172,203 | 170,563 | 118,169 | 124,446 | 18,831 | 4,077 |
| Cancer deaths, n | 3,143 | 8,584 | 7,375 | 4,373 | 5,187 | 557 | 75 |
| HR (95% CI)a | 1.00 | 0.87 (0.83–0.90) | 0.79 (0.75–0.82) | 0.75 (0.72–0.79) | 0.74 (0.71–0.77) | 0.72 (0.66–0.79) | 0.69 (0.55–0.87) |
| CVD deaths, n | 3238 | 7,952 | 6,316 | 3,293 | 4,044 | 457 | 69 |
| HR (95% CI)a | 1.00 | 0.80 (0.77–0.84) | 0.67 (0.65–0.70) | 0.59 (0.57–0.63) | 0.58 (0.56–0.61) | 0.61 (0.55–0.67) | 0.71 (0.56–0.91) |
Multivariable-adjusted models with stratification by cohort were adjusted for age, gender, smoking (never, former, current, missing), alcohol (none, <15 grams/day, 15–30 grams/day, 30+ grams/day), education (dropout, high school, post high school education, some college, college graduate, post-college, missing), marital status (married, divorced, widowed, single, missing), history of cancer, history of heart disease, and body mass index (<18.5, 18.5–25, 25-<30, 30-<35, 35+ kg/m2).
In stratified analyses we found that the upper limit of mortality benefit appeared to be consistent across covariate strata, although we observed some variation in point estimates where number of deaths was low (Table 4). While we found statistically significant differences by age, sex, education, BMI, smoking status and heart disease possibly attributable to the large sample sizes (p<0.001), examination of point estimates did not show results contradictory to our main findings. When we tested differences between the two highest physical activity categories that appeared to diverge (e.g. men reporting 40-<75 v. 75+ MET h/wk) the two categories were not statistically different (p=0.07).
Table 4.
Stratified hazard ratios and 95% confidence intervals for leisure time physical activity and mortality
| MET h/wk | 0 | 0.1-<7.5 | 7.5-<15 | 15-<22.5 | 22.5-<40 | 40-<75 | 75+ | p-interact |
|---|---|---|---|---|---|---|---|---|
| Age (years) | <0.001 | |||||||
| <50 | 1.00 | 0.73 (0.61–0.87) | 0.75 (0.62–0.90) | 0.81 (0.65–1.01) | 0.70 (0.56–0.89) | 0.80 (0.61–1.03) | 0.72 (0.47–1.11) | |
| 50-<60 | 1.00 | 0.78 (0.74–0.83) | 0.67 (0.64–0.71) | 0.66 (0.62–0.70) | 0.65 (0.61–0.69) | 0.63 (0.54–0.72) | 0.60 (0.43–0.84) | |
| 60-<70 | 1.00 | 0.81 (0.78–0.83) | 0.70 (0.68–0.72) | 0.63 (0.61–0.65) | 0.59 (0.57–0.61) | 0.61 (0.56–0.66) | 0.70 (0.57–0.86) | |
| 70+ | 1.00 | 0.77 (0.74–0.81) | 0.65 (0.62–0.68) | 0.62 (0.59–0.65) | 0.60 (0.57–0.62) | 0.54 (0.49––0.60) | 0.67 (0.52–0.85) | |
| Sex | 0.002 | |||||||
| Male | 1.00 | 0.82 (0.80–0.85) | 0.71 (0.69–0.73) | 0.63 (0.61–0.65) | 0.61 (0.59–0.63) | 0.62 (0.58–0.67) | 0.74 (0.62–0.89) | |
| Female | 1.00 | 0.77 (0.74–0.79) | 0.67 (0.64–0.69) | 0.64 (0.61–0.66) | 0.60 (0.57–0.62) | 0.59 (0.54–0.65) | 0.61 (0.49–0.75) | |
| Education | 0.087 | |||||||
| High school or less | 1.00 | 0.78 (0.76–0.81) | 0.65 (0.63–0.68) | 0.61 (0.59–0.64) | 0.58 (0.56–0.61) | 0.58 (0.52–0.66) | 0.58 (0.40–0.85) | |
| Some college or post-high school | 1.00 | 0.74 (0.71–0.77) | 0.63 (0.61–0.65) | 0.56 (0.54–0.59) | 0.53 (0.51–0.55) | 0.53 (0.48–0.59) | 0.56 (0.43–0.71) | |
| College graduate | 1.00 | 0.75 (0.72–0.79) | 0.63 (0.61–0.66) | 0.57 (0.54–0.59) | 0.56 (0.53–0.58) | 0.55 (0.50–0.60) | 0.68 (0.55–0.83) | |
| Race | 0.237 | |||||||
| Non-Hispanic white | 1.00 | 0.79 (0.78–0.81) | 0.68 (0.67–0.70) | 0.62 (0.61–0.64) | 0.60 (0.59–0.61) | 0.61 (0.57–0.64) | 0.68 (0.59–0.78) | |
| African American | 1.00 | 0.80 (0.71–0.91) | 0.66 (0.58–0.76) | 0.70 (0.61–0.81) | 0.60 (0.51–0.69) | 0.57 (0.38–0.85) | 0.80 (0.42–1.52) | |
| BMI (kg/m2) | <0.001 | |||||||
| 18.5-<25 | 1.00 | 0.78 (0.75–0.81) | 0.66 (0.63–0.68) | 0.59 (0.57–0.62) | 0.56 (0.54–0.58) | 0.54 (0.50–0.59) | 0.63 (0.52–0.76) | |
| 25-<30 | 1.00 | 0.79 (0.76–0.82) | 0.70 (0.67–0.72) | 0.64 (0.61–0.66) | 0.62 (0.59–0.64) | 0.67 (0.62–0.74) | 0.76 (0.60–0.95) | |
| 30+ | 1.00 | 0.83 (0.79–0.86) | 0.72 (0.68–0.75) | 0.67 (0.64–0.71) | 0.66 (0.63–0.69) | 0.72 (0.61–0.85) | 0.62 (0.39–1.01) | |
| Smoking status | <0.001 | |||||||
| Never | 1.00 | 0.80 (0.76–0.83) | 0.69 (0.66–0.72) | 0.66 (0.63–0.69) | 0.64 (0.61–0.67) | 0.67 (0.61–0.74) | 0.67 (0.53–0.86) | |
| Former | 1.00 | 0.77 (0.75–0.79) | 0.65 (0.63–0.67) | 0.59 (0.57–0.61) | 0.56 (0.54–0.58) | 0.55 (0.51–0.59) | 0.61 (0.51–0.74) | |
| Current | 1.00 | 0.85 (0.81–0.89) | 0.77 (0.73–0.81) | 0.72 (0.68–0.76) | 0.69 (0.65–0.72) | 0.72 (0.61–0.84) | 0.83 (0.59–1.18) | |
| Heart disease | <0.001 | |||||||
| No | 1.00 | 0.80 (0.78–0.82) | 0.70 (0.68–0.72) | 0.65 (0.63–0.66) | 0.62 (0.60–0.64) | 0.62 (0.58–0.66) | 0.69 (0.60–0.80) | |
| Yes | 1.00 | 0.79 (0.75–0.83) | 0.65 (0.62–0.68) | 0.58 (0.55–0.61) | 0.55 (0.52–0.58) | 0.56 (0.48–0.65) | 0.66 (0.45–0.98) | |
| Previous cancer | 0.217 | |||||||
| No | 1.00 | 0.80 (0.78–0.82) | 0.69 (0.68–0.71) | 0.64 (0.62–0.65) | 0.61 (0.59–0.63) | 0.63 (0.59–0.67) | 0.70 (0.61–0.82) | |
| Yes | 1.00 | 0.79(0.75–0.83) | 0.66 (0.63–0.70) | 0.61 (0.57–0.65) | 0.58 (0.54–0.61) | 0.54 (0.47–0.62) | 0.59 (0.41–0.84) |
Multivariable models are stratified by cohort, and adjusted for age, gender, smoking (never, former, current, missing), alcohol (none, <15 grams/day, 15–30 grams/day, 30+ grams/day), education (dropout, high school, post high school education, some college, college graduate, post-college, missing), marital status (married, divorced, widowed, single, missing), history of cancer, history of heart disease, and body mass index (<18.5, 18.5–25, 25-<30, 30-<35, 35+ kg/m2). The variable used for stratification was not included in the given model.
Interaction was tested using a continuous leisure-time physical activity term and the exposure of interest, using a Wald test for dichotomous variables and a likelihood ratio test for multi-level variables.
Excluding those reporting >100 MET h/wk from analyses to assess the influence of outliers did not change our conclusions. We also performed an analysis limited to those reporting 15+ MET h/wk and using a continuous term to see whether the risk trend existed beyond the 15 MET h/wk threshold, we found that the p-trend for continuous physical activity was highly significant (p< 0.001). Using a cubic spline showed that the association between physical activity and mortality was not linear, but the patterns of risk observed in the splines paralleled risk estimates in the categorical analyses (eFigure 1).
Discussion
Our findings on the shape of the physical activity-mortality dose-response curve offer three unique and important contributions to inform healthcare professionals and future Guidelines – 1) the currently recommended amounts of leisure-time physical activity provide the majority of longevity benefits, 2) the longevity benefit threshold appears around 3–5 times the recommended physical activity minimum, and 3), there does not appear to be an elevated mortality risk with leisure-time physical activity levels as high as 10+ times the recommended minimum. In our study population both moderate and vigorous-intensity activities were associated with longevity benefit.
Two recent studies quantified the minimal amounts of leisure-time physical activity for longevity benefit but did not estimate mortality risk beyond ~3 times the recommended minimum, and therefore were unable to quantify the upper threshold of benefit. A Taiwanese study found that compared to those who were inactive, those who were meeting U.S. federal physical activity recommendations had a 26% lower mortality risk (HR=0.74, 95% CI 0.70–0.77) 24. Our previous publication in these pooled cohorts, with a shorter follow-up, showed major longevity benefits with leisure-time physical activity 3+ times the recommended minimum compared to none (HR=0.59, 95% CI 0.57–0.61), but did not detail mortality benefits for physical activity above 22.5+ MET h/wk or estimate separate risk estimates by activity intensity 10. The present study confirms and extends previous research by quantifying the upper threshold of benefit, which in turn demonstrates that adults who perform leisure-time physical activity at recommended levels achieve the majority of the mortality benefits.
Previous studies on associations between intensity-specific leisure-time physical activity (i.e. moderate vs. vigorous) and mortality have shown equivocal results. A study of leisure-time physical activity and mortality in middle-aged British men supported an association between vigorous-intensity but not moderate-intensity physical activity and all-cause and CVD mortality 25. Other studies have shown reductions in mortality rate from moderate activities, but greater reductions associated with vigorous-intensity activities26. Few studies examining MET expenditures and types of high-intensity activities have been reported among older adults, and given the low prevalence of older individuals performing very high levels of activity or high intensity on a population level, previous epidemiologic studies are not available for comparison. On the other hand, comprehensive reviews of the literature on physical activity and mortality report that overall volume of physical activity is associated with lower mortality risk, but report mixed findings on relative contributions of moderate vs vigorous-intensity activities 27,28. Other studies also support associations between moderate activities including walking and lower risk of coronary heart disease 29–31 or mortality 24,26. While more research is needed on physical activity intensity vs. dose, our finding that moderate-intensity activities was associated with mortality benefit is consistent with the 2008 Guidelines.
An analysis in the 2011 Behavioral Risk Factor Surveillance Survey (BRFSS) reported that walking contributed 47.4% of overall aerobic exercise, while running/jogging accounted for 13.4% and conditioning exercises (including stationary biking, Stairmaster/stair climbing or active gaming) accounted for 8.5% and sports grouped together accounted for 9.2% of activity32. Walking for exercise was also the largest contributor to overall MET h/wk in our analysis, which is consistent with the BRFSS findings. Thus, the major activities listed on these questionnaires were prevalent both at time of data collection and at present.
Possible harms previously associated with high levels of leisure-time physical activity may be explained by cause of death. The 2008 Guidelines evaluation of harms stated that risk of cardiac events was transiently increased during vigorous-intensity physical activity, particularly among inactive individuals, but that on average physically active individuals have a lower risk of adverse cardiac events 4. Recent studies have suggested cardiac remodeling and higher CVD and mortality risk with extremely high physical activity levels 7,33–38, but these studies were largely performed in athletic populations with a younger median age. Our cohort was older, as average age for those <60 years old was still 52 years old. While our study is not poised to examine the risk during or immediately after exercise or co-morbid cardiac conditions such as atrial fibrillation, our findings do not support the hypothesis for increased mortality risk at leisure-time physical activity levels of 10+ times the federal guidelines. The present findings align with other studies that have shown lower risks of mortality among long-term, long distance runners 39 as well as Tour de France cyclists 40. Thus, current trends in increasing marathon or triathalon participation should not cause alarm, at least with regard to mortality.
Strengths of our study include the prospective nature of the cohorts, extended follow-up time and detailed covariate information. We were uniquely positioned to estimate the threshold for longevity benefit from high leisure-time physical activity levels and potential harms with respect to mortality because by pooling we had enough individuals reporting this high level of exercise. Our stratified results further strengthen our finding, indicating the upper threshold of benefit was consistent in men and women, different age groups, various lifestyle factors and in those with and without cardiovascular disease and cancer. However, the smaller sample size in our highest physical activity category led to wider confidence intervals in this category.
Limitations of our study include the reliance on self-reported physical activity, reported at only a single time point. We also did not examine non-leisure time activities or sedentary time, both of which have shown associations with mortality. Also, although we attempted to adjust for confounding by history of disease or other known mortality risk factors, unaccounted risk factors may have influenced our observed results. Validation studies were not specifically designed for older adults performing very high levels of physical activity. Still, self-reported activity has shown construct validity in our cohorts by showing expected associations with mortality and disease-specific outcomes (eTable 1). The prospective design minimizes recall bias, but measurement error in self-reported leisure-time physical activity is likely to result in attenuation of the associations observed. Differences in the questionnaires between cohorts, variation in baseline age, relative physical fitness, and length of follow-up may explain some of the heterogeneity we observed between individual study results. However, additional analyses excluding each individual cohort showed that estimates were not unduly influenced by a single cohort. Our median reported leisure-time physical activity levels were above average U.S. values, which may be due to the higher education level of our study participants. Also, MET h/wk intensities were assigned using absolute, compendium-derived values that may not account for inter-individual variation. Lastly, we cannot completely exclude the possibility of inflation in upper physical activity categories.
In summary, meeting the recommended guidelines by either moderate or vigorous-intensity activities was associated with nearly the maximum longevity benefit. We observed this benefit threshold around 3–5 times the recommended leisure-time physical activity minimum, and no excess risk at 10+ times the recommended minimum. These findings are informative for individuals at both ends of the physical activity spectrum: they provide important evidence to inactive individuals by showing that modest amounts of activity provide substantial benefit for postponing mortality, while at the same time reassuring very active individuals of no exercise-associated increase in mortality risk. These data will be useful to inform future updates on physical activity guidelines regarding appropriate amount of physical activity to recommend for longevity.
Supplementary Material
Acknowledgments
Funding: This study was supported [in part] by the Intramural Research Program in the Division of Cancer Epidemiology and Genetics and the Division of Cancer Control and Population Sciences, both of the US National Institutes of Health (NIH,) National Cancer Institute (NCI). The NIH-AARP Diet and Health study was supported by the Intramural Research Program of the NCI. CLUE was supported by the National Institute of Aging, grant number: U01 AG18033 and NCI, grant number: CA105069. CPS II was supported by the intramural research program at the American Cancer Society (Atlanta, Georgia). The US Radiologic Technologists study was supported by the Intramural Research Program, Division of Cancer Epidemiology and Genetics, NCI. The Women's Health Study was supported by grant numbers CA047988, HL043851, HL080467, and HL099355. The Swedish Women's Lifestyle and Health study is supported by the Swedish Research Council (grant 521–2011–2955).The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; nor in the decision to submit the manuscript for publication.
H. Arem had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We are indebted to all study participants for their outstanding cooperation. We also would also like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention National Program of Cancer Registries, and cancer registries supported by the National Cancer Institute Surveillance Epidemiology and End Results program. Certain CLUE II data were provided by the VSA, MD DHMH, Baltimore, MD. The DHMH disclaims responsibility for any analyses, interpretation or conclusions. We also thank Dr. Jerry Reid of the American Registry of Radiologic Technologists for continued study support, Diane Kampa and Allison Iwan of the University of Minnesota for study management and data collection, and Jeremy Miller of Information Management Services for biomedical computing.
References
- 1.Paffenbarger RS, Hyde RT, Wing AL, Hsieh C-C. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986 doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 2.Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality. JAMA. 1998;279(6):440–444. doi: 10.1001/jama.279.6.440. [DOI] [PubMed] [Google Scholar]
- 3.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee I-M, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328(8):538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 4.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, D.C: U.S. Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed June 4, 2014];Running USA Annual Marathon Report. http://www.runningusa.org/statistics.
- 6. [Accessed June 4, 2014];Triathlon Growth Trends. http://www.usatriathlon.org/about-multisport/demographics.aspx.
- 7.Andersen K, Farahmand B, Ahlbom A, et al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht188. [DOI] [PubMed] [Google Scholar]
- 8.Link MS, Mark Estes N. Sudden cardiac death in athletes. Prog Cardiovasc Dis. 2008;51(1):44–57. doi: 10.1016/j.pcad.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Physical Activity Guidelines for Americans. 2008 http://www.health.gov/paguidelines/
- 10.Moore SC, Patel AV, Matthews CE, et al. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med. 2012;9(11):e1001335. doi: 10.1371/journal.pmed.1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koster A, Harris TB, Moore SC, et al. Joint associations of adiposity and physical activity with mortality: The National Institutes of Health-AARP Diet and Health Study. Am J Epidemiol. 2009 Jun 1;169(11):1344–1351. doi: 10.1093/aje/kwp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002 May 1;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 14.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004 Dec 15;160(12):1223–1233. doi: 10.1093/aje/kwh339. [DOI] [PubMed] [Google Scholar]
- 15.Howard RA, Leitzmann MF, Linet MS, Freedman DM. Physical activity and breast cancer risk among pre- and postmenopausal women in the U.S. Radiologic Technologists cohort. Cancer Causes Control. 2009 Apr;20(3):323–333. doi: 10.1007/s10552-008-9246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005 Jul 6;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005 Jul 6;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005 Mar 31;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 19.Margolis KL, Mucci L, Braaten T, et al. Physical activity in different periods of life and the risk of breast cancer: the Norwegian-Swedish Women's Lifestyle and Health cohort study. Cancer Epidemiol Biomarkers Prev. 2005 Jan;14(1):27–32. [PubMed] [Google Scholar]
- 20.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 21.Trolle-Lagerros Y, Mucci LA, Kumle M, et al. Physical activity as a determinant of mortality in women. Epidemiology. 2005 Nov;16(6):780–785. doi: 10.1097/01.ede.0000181312.35964.22. [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 SUPP/1):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 23.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure–response relationships. Stat Med. 2007;26(20):3735–3752. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 24.Wen CP, Wai JPM, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. The Lancet. 2011;378(9798):1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 25.Yu S, Yarnell JWG, Sweetnam PM, Murray L. What level of physical activity protects against premature cardiovascular death? The Caerphilly study. Heart. 2003 May 1;89(5):502–506. doi: 10.1136/heart.89.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee IM, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol. 2000 Feb 1;151(3):293–299. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 27.Oguma Y, Sesso HD, Paffenbarger RS, Lee I-M. Physical activity and all cause mortality in women: a review of the evidence. British Journal of Sports Medicine. 2002 Jun 1;36(3):162–172. doi: 10.1136/bjsm.36.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee I, Paffenbarger R, Hennekens C. Physical activity, physical fitness and longevity. Aging Clinical and Experimental Research. 1997;9(1–2):2–11. doi: 10.1007/BF03340123. [DOI] [PubMed] [Google Scholar]
- 29.Lee I-M, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical Activity and Coronary Heart Disease in Women: Is No Pain, No Gain Passé? JAMA. 2001;285(11):1447–1454. doi: 10.1001/jama.285.11.1447. [DOI] [PubMed] [Google Scholar]
- 30.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. New England Journal of Medicine. 1999;341(9):650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 31.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. New England Journal of Medicine. 2002;347(10):716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 32.Watson KB, Frederick GM, Harris CD, Carlson SA, Fulton JE. U.S. Adults’ Participation in Specific Activities, Behavioral Risk Factor Surveillance System—2011. Journal of Physical Activity & Health. 2014 doi: 10.1123/jpah.2013-0521. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breuckmann F, Mohlenkamp S, Nassenstein K, et al. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009 Apr;251(1):50–57. doi: 10.1148/radiol.2511081118. [DOI] [PubMed] [Google Scholar]
- 34.Oxborough D, Birch K, Shave R, George K. "Exercise-induced cardiac fatigue"--a review of the echocardiographic literature. Echocardiography. 2010 Oct;27(9):1130–1140. doi: 10.1111/j.1540-8175.2010.01251.x. [DOI] [PubMed] [Google Scholar]
- 35.O'Keefe JH, Lavie CJ. Run for your life … at a comfortable speed and not too far. Heart. 2013 Apr 15;99(8):516–519. doi: 10.1136/heartjnl-2012-302886. [DOI] [PubMed] [Google Scholar]
- 36.O'Keefe JH, Patil HR, Lavie CJ, Magalski A, Vogel RA, McCullough PA. Potential Adverse Cardiovascular Effects From Excessive Endurance Exercise. Mayo Clinic Proceedings. 2012;87(6):587–595. doi: 10.1016/j.mayocp.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drca N, Wolk A, Jensen-Urstad M, Larsson SC. Atrial fibrillation is associated with different levels of physical activity levels at different ages in men. Heart. 2014 May 14; doi: 10.1136/heartjnl-2013-305304. [DOI] [PubMed] [Google Scholar]
- 38.Mons U, Hahmann H, Brenner H. A reverse J-shaped association of leisure time physical activity with prognosis in patients with stable coronary heart disease: evidence from a large cohort with repeated measurements. Heart. 2014 May 14; doi: 10.1136/heartjnl-2013-305242. [DOI] [PubMed] [Google Scholar]
- 39.Chakravarty EF, Hubert HB, Lingala VB, Fries JF. Reduced disability and mortality among aging runners: A 21-year longitudinal study. Arch Intern Med. 2008;168(15):1638–1646. doi: 10.1001/archinte.168.15.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marijon E, Tafflet M, Antero-Jacquemin J, et al. Mortality of French participants in the Tour de France (1947–2012) European Heart Journal. 2013 Sep 3; doi: 10.1093/eurheartj/eht347. [DOI] [PubMed] [Google Scholar]
- 41.Peters TM, Schatzkin A, Gierach GL, et al. Physical activity and postmenopausal breast cancer risk in the NIH-AARP diet and health study. Cancer Epidemiology Biomarkers & Prevention. 2009;18(1):289–296. doi: 10.1158/1055-9965.EPI-08-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gierach GL, Chang SC, Brinton LA, et al. Physical activity, sedentary behavior, and endometrial cancer risk in the NIH-AARP Diet and Health Study. International Journal of Cancer. 2009;124(9):2139–2147. doi: 10.1002/ijc.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howard RA, Freedman DM, Park Y, Hollenbeck A, Schatzkin A, Leitzmann MF. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2008;19(9):939–953. doi: 10.1007/s10552-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hauser TH, Ho KKL. Accuracy of on-line databases in determining vital status. Journal of Clinical Epidemiology. 2001 Dec;54(12):1267–1270. doi: 10.1016/s0895-4356(01)00421-8. [DOI] [PubMed] [Google Scholar]
- 45.Gallicchio L, Chang H, Christo DK, et al. Single nucleotide polymorphisms in inflammation-related genes and mortality in a community-based cohort in Washington County, Maryland. American journal of epidemiology. 2008;167(7):807–813. doi: 10.1093/aje/kwm378. [DOI] [PubMed] [Google Scholar]
- 46.Patel AV, Callel EE, Bernstein L, Wu AH, Thun MJ. Recreational physical activity and risk of postmenopausal breast cancer in a large cohort of US women. Cancer Causes Control. 2003 Aug;14(6):519–529. doi: 10.1023/a:1024895613663. [DOI] [PubMed] [Google Scholar]
- 47.Chao A, Connell CJ, Jacobs EJ, et al. Amount, Type, and Timing of Recreational Physical Activity in Relation to Colon and Rectal Cancer in Older Adults: the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiology Biomarkers & Prevention. 2004 Dec 1;13(12):2187–2195. [PubMed] [Google Scholar]
- 48.Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among Cancer Prevention Study II participants. American Journal of Epidemiology. 1993;137(2):235–241. doi: 10.1093/oxfordjournals.aje.a116664. [DOI] [PubMed] [Google Scholar]
- 49.Mohan AK, Hauptmann M, Freedman DM, et al. Cancer and other causes of mortality among radiologic technologists in the United States. International journal of cancer. 2003;103(2):259–267. doi: 10.1002/ijc.10811. [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Lof M, Veierød MB, Sandin S, Adami H-O, Weiderpass E. Ultraviolet Exposure and Mortality among Women in Sweden. Cancer Epidemiology Biomarkers & Prevention. 2011 Apr 1;20(4):683–690. doi: 10.1158/1055-9965.EPI-10-0982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.