Abstract
Background
Rhegmatogenous retinal detachment (RRD) is a full-thickness break in the sensory retina, caused by vitreous traction on the retina. While pneumatic retinopexy, scleral buckle, and vitrectomy are the accepted surgical interventions for eyes with RRD, their relative effectiveness has remained controversial.
Objectives
The objectives of this review were to assess the effectiveness and safety of pneumatic retinopexy versus scleral buckle or pneumatic retinopexy versus a combination treatment of scleral buckle and vitrectomy for people with RRD. The secondary objectives were to summarize any data on economic measures and quality of life.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 12), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to January 2015), EMBASE (January 1980 to January 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to January 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 13 January 2015.
Selection criteria
We included all randomized or quasi-randomized controlled trials comparing the effectiveness of pneumatic retinopexy versus scleral buckle (with or without vitrectomy) for eyes with RRD.
Data collection and analysis
After screening for eligibility, two review authors independently extracted study characteristics, methods, and outcomes. We followed systematic review standards as set forth by The Cochrane Collaboration.
Main results
We included two randomized controlled trials (218 eyes of 216 participants) comparing the effectiveness of pneumatic retinopexy versus scleral buckle for eyes with RRD. We identified no studies investigating the comparison of pneumatic retinopexy versus a combination treatment of scleral buckle and vitrectomy. Of the two included studies, one was a small study with 20 participants enrolled in Ireland and followed for an average of 16 months. The second study was larger with 196 participants (198 eyes) enrolled in the United States and followed for at least 6 months. Cautious interpretation of the results is warranted, since we graded the evidence as low to moderate quality due to insufficient reporting of study methods and imprecision and inconsistency among study results.
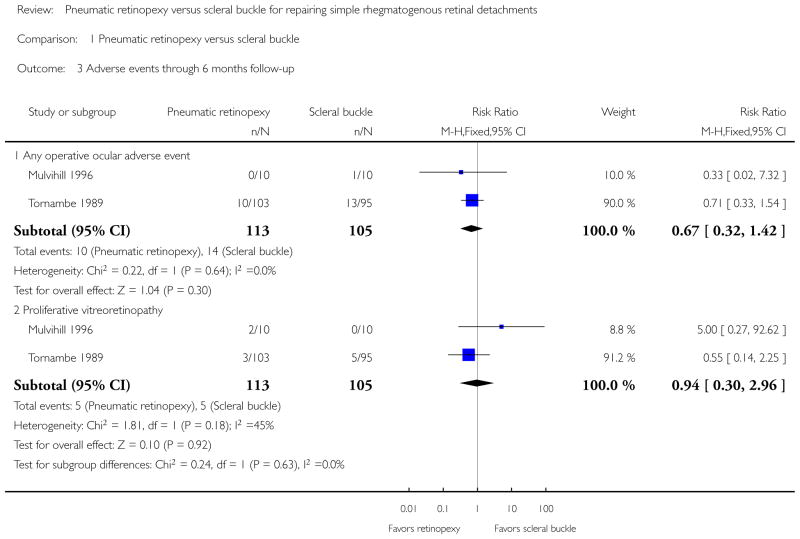
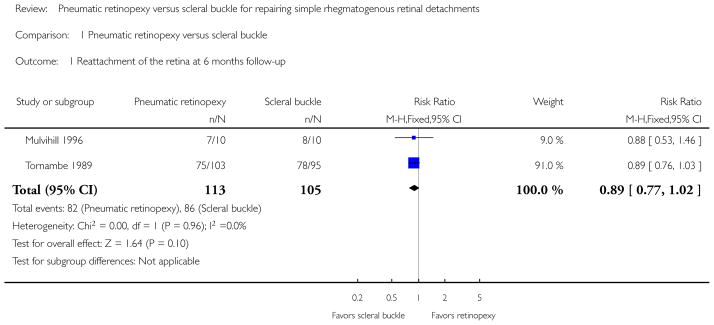
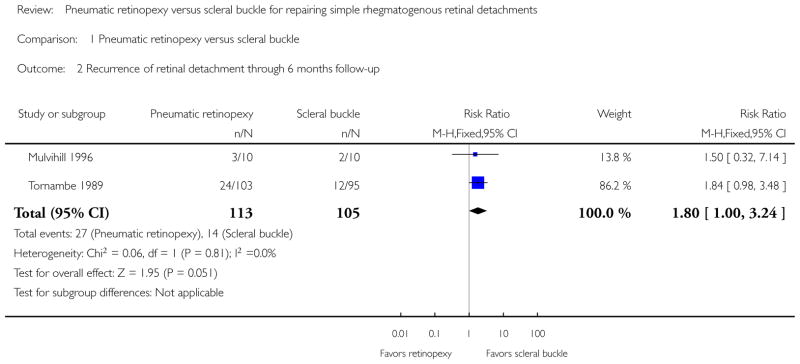
Both studies showed fewer eyes achieving retinal reattachment in the pneumatic retinopexy group compared with the scleral buckle group by six-months follow-up (risk ratio (RR) 0.89, 95% confidence interval (CI) 0.77 to 1.02, 218 eyes); however, we are uncertain as to whether the intervention has an important effect on reattachment because the results are imprecise. Eyes in the pneumatic retinopexy group also were more likely to have had a recurrence of retinal detachment by six-months follow-up (RR 1.80, 95% CI 1.00 to 3.24, 218 eyes); however, we are uncertain as to whether the intervention has an important effect on recurrence because the lower CI equals no difference. Neither study reported mean change in visual acuity, quality of life data, or economic measures. Differences between the pneumatic retinopexy group and scleral buckle group were uncertain due to small numbers of events with respect to operative ocular adverse events (RR 0.67, 95% CI 0.32 to 1.42, 218 eyes), development of cataract (RR 0.92, 95% CI 0.06 to 14.54, 198 eyes), glaucoma (RR 0.31, 95% CI 0.03 to 2.91, 198 eyes), macular pucker (RR 0.74, 95% CI 0.20 to 2.67, 198 eyes), and proliferative vitreoretinopathy (RR 0.94, 95% CI 0.30 to 2.96, 218 eyes). Fewer eyes in the pneumatic retinopexy group compared with the scleral buckle group experienced choroidal detachment (RR 0.17, 95% CI 0.05 to 0.57, 198 eyes) or myopic shift equal to or greater than 1 diopter spherical equivalent (RR 0.04, 95% CI 0.01 to 0.13, 198 eyes).
Authors’ conclusions
The evidence suggests that pneumatic retinopexy may result in lower rates of reattachment and higher rates of recurrence than scleral buckle for eyes with RRD, but does not rule out no difference between procedures. The relative safety of the procedures is uncertain and the relative effects of these procedures in terms of other patient-important outcomes, such as visual acuity and quality of life, is unknown. Due to the limited information available between pneumatic retinopexy and scleral buckle procedures, future research addressing these evidence gaps are warranted.
PLAIN LANGUAGE SUMMARY
Surgical interventions for rhegmatogenous retinal detachment
Research question
In this review we aimed to determine whether pneumatic retinopexy or scleral buckle is a better surgical treatment for rhegmatogenous retinal detachment (RRD).
Background
Retinal detachment is the separation of the retina, the light-sensitive tissue at the back of the eye, from its underlying layer attached to the inner back surface of the eye. RRD is when the separation results from retinal breaks or tears, usually due to pulling (traction) from the vitreous, the substance that fills the center of the eye.
Three surgical interventions are used to repair the retinal break(s) in RRD: pneumatic retinopexy, scleral buckle, and vitrectomy. In pneumatic retinopexy, a gas bubble is injected into the vitreous cavity in the center of the eye to provide a mechanical seal (tamponade) for the retinal breaks until the breaks can be sealed with heat (laser) or cold (cryotherapy). In scleral buckle, local pressure is applied to retinal breaks by suturing material onto the outer part of the eye (sclera) to indent (buckle) it inward. In vitrectomy, the vitreous is removed to relieve traction on the retina from the vitreous and a gas tamponade may be used to facilitate healing.
Study characteristics
We found two randomized trials that had enrolled a total of 216 participants (218 eyes) from Ireland and the United States. Both studies evaluated whether pneumatic retinopexy or scleral buckle was a better treatment for RRD. The study in the US had 198 participants with 6 months to 2 years of follow-up. The study in Ireland had 20 participants with 5 to 27 months of follow-up. The evidence is current to 13 January 2015.
Key results
Results from both studies suggested that scleral buckle may perform better or as well as pneumatic retinopexy in terms of reattachment rates and reducing the risk of recurrence of detachment. Few ocular adverse events occurred during either procedure and differences in some adverse events occurring after the surgeries could not be determined. More eyes in the scleral buckle group experienced choroidal detachment (separation of the choroid, the layer between the retina and sclera, from the sclera) and myopic shift (change to nearsightedness that may be a sign of developing cataract) than eyes in the pneumatic retinopexy group.
Quality of the evidence
The quality of the evidence was assessed as low to moderate due to poor reporting of how the studies were done. Further, there was lack of information regarding important outcomes that may be useful when choosing which procedure to use in terms of vision, quality of life, and cost.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON [Explanation]
| Pneumatic retinopexy versus scleral buckle for repairing simple rhegmatogenous retinal detachments | ||||||
|---|---|---|---|---|---|---|
| Population: participants with simple rhegmatogenous retinal detachments Settings: ophthalmology centers Intervention: pneumatic retinopexy Comparison: scleral buckle | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Eyes (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Scleral buckle | Pneumatic retinopexy | |||||
|
Reattachment of the retina at 6 months follow-up |
819 per 1000 | 729 per 1000 (631 to 835) | RR 0.89 (0.77 to 1.02) | 218 (2 studies) | ⊕⊕⊕○ moderate† |
RR <1 favors scleral buckle |
|
Recurrence of retinal detachment through 6 months follow-up |
133 per 1000 | 240 per 1000 (133 to 431) | RR 1.80 (1.00 to 3.24) | 218 (2 studies) | ⊕⊕⊕○ moderate† |
RR > 1 favors scleral buckle |
|
Any operative ocular adverse event through 6 months follow-up |
133 per 1000 | 89 per 1000 (43 to 189) | RR 0.67 (0.32 to 1.42) | 218 (2 studies) | ⊕⊕○○ low†‡ |
RR <1 favors pneumatic retinopexy |
CI: confidence interval; RR: risk ratio
The basis for the assumed risk is the scleral buckle group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
The confidence interval crosses or equals no difference and does not rule out an effect; many study details from both included studies were not reported resulting in unclear risk of bias assessments for most domains.
Imprecision and inconsistency among study results.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group grades of evidence:
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
BACKGROUND
Description of the condition
A retinal detachment is the separation of the sensory retina from the underlying retinal pigment epithelium (Sodhi 2008). Retinal detachments are classified according to the cause of this separation; the four categories are rhegmatogenous, tractional, combined tractional/rhegmatogenous, and exudative (serous) (Sodhi 2008). In rhegmatogenous retinal detachment (RRD), the primary pathology is a full-thickness break in the sensory retina caused by vitreous traction on the retina. This retinal break allows fluid from the vitreous cavity to enter the subretinal space; RRD is the result of these two factors, along with a liquefied vitreous (Ghazi 2002). RRD is usually treated with surgery, the urgency of which depends on the status of the macula (attached or detached). In tractional retinal detachment, a vitreoretinal membrane generates tractional strain without a full-thickness tear in the retina. In exudative retinal detachment, serous fluid accumulates underneath the sensory retina. Common causes of exudative retinal detachment include inflammatory conditions such as posterior uveitis; therapy involves treatment of the underlying ocular condition. In this review, we considered the surgical interventions for repairing RRD.
In the United States, roughly 36,000 cases of RRD occur annually, as estimated from regional studies. Worldwide, the reported incidence rate of RRD varies dramatically in different countries. It was reported as 14 per 100,000 people per year in Sweden (Algvere 1999); 12.6 per 100,000 people per year in Minnesota, United States (Rowe 1999); and 7.98 per 100,000 people per year in Beijing (Li 2003). RRD occurs most commonly in people aged 40 to 70 years with pre-existing or concurrent posterior vitreous detachments that lead to retinal tears. While some studies report a success rate of up to 90% to 95% for surgical reattachment of the retina, as many as 40% of these participants have final visual acuities of 20/50 or lower. The occurrence of RRD in the general population is low (12 per 100,000 people, 0.01% annual risk, 0.06% lifetime risk), but there are several factors that increase the risk of experiencing RRD, including lattice degeneration, extreme myopia, cataract surgery, and ocular trauma or infection (Sodhi 2008).
Vitreoretinal traction and underlying weakness in the peripheral retina combine to cause the retinal breaks responsible for RRD. However, not all retinal breaks will in turn cause a retinal detachment (Byer 1998). In fact, there are numerous adhesive forces that can counteract the deleterious effects of retinal breaks and maintain the stability of the vitreous-retina border. For instance, the movement of ions and fluid by retinal pigment epithelium cells, choroid-subretinal oncotic pressure differentials, intraocular pressure-associated hydrostatic forces, and subretinal adhesive-like mucopolysaccharides all can work to offset a retinal break, thus preventing progression to RRD (Ghazi 2002). When these adhesive forces are not sufficient to compensate for vitreoretinal traction, fluid can enter the subretinal space and RRD can occur (Sodhi 2008).
People with RRD often have a history of flashing lights, vitreous floaters, or both, caused by an acute posterior vitreous detachment. After a variable period of time, the person may notice a peripheral visual field defect, which may progress to involve central vision (Gariano 2004). However, some people do not experience these premonitory symptoms. In these people, the first sign of RRD can be a black shadow that may or may not affect visual acuity. Involvement of the macula in a RRD, which is a common cause of decreased vision in a retinal detachment, is an important prognostic marker; people without macular involvement will have better visual outcomes.
In the clinic, examination of RRD patients may reveal pigmented cells, also known as tobacco dust, in the vitreous and the anterior chamber. Other clinical findings include transparent subretinal fluid and an opaque, furrowed-appearing retina that may ripple with the patient’s eye movements (Ross 2000).
Description of the intervention
Three separate surgical interventions are used in current clinical practice to repair retinal break(s) in RRD: pneumatic retinopexy, scleral buckle, and vitrectomy.
Pneumatic retinopexy may be performed as an outpatient clinical procedure. In this procedure, cryotherapy or laser is applied to the area of the retinal tear and a gas bubble is injected into the vitreous cavity to provide tamponade for the detached retina. Eyes with RRD meeting the following criteria are ideal cases for surgery: single retinal tear less than or equal to one clock hour in size, tear located in the superior half of the retina, and no associated peripheral retinal degeneration. However, eyes not meeting these criteria (e.g., larger breaks, limited lattice degeneration) also may be candidates for pneumatic retinopexy.
The scleral buckle procedure involves localizing the position of all retinal breaks, treating all retinal breaks with the cryoprobe, and supporting them with the scleral buckle. The scleral buckle can be positioned radially, in a segmental fashion, or it can encircle the entire eye.
Vitrectomy involves operating inside the eye and removing the vitreous to relieve vitreoretinal traction. The retina is reattached by various techniques depending on the location and extent of the detachment. At the conclusion of the vitrectomy, a gas bubble is usually injected into the eye to provide tamponade for the retina to heal (reattach). Scleral buckle surgery can be combined with vitrectomy when the retinal detachment is complex.
How the intervention might work
Surgical intervention, while of dubious benefit to asymptomatic RRD patients, is the clear course of action for those who experience symptoms; if symptomatic RRD is not treated, the affected eyes will be at risk for involvement of the entire retina and further vision loss.
In pneumatic retinopexy, retinal breaks are tamponaded by the intravitreal gas bubble, closed, and sealed by the chorioretinal adhesion induced by cryotherapy. The scleral buckle indents the eye wall, brings the detached retina closer to the eye wall, and relieves vitreoretinal traction. In vitrectomy, vitreous is removed and all of the vitreoretinal traction on any of the breaks excised. The patient’s retina is flattened intraoperatively by using a gas bubble.
Why it is important to do this review
RRD can progress to significant loss of visual acuity. Despite the wide array of surgical interventions available for management of RRD, repair success rates remained stable from 1979 to 1999, and likely to the present (Minihan 2001). This plateau indicates that choosing the most effective surgical approach for people with RRD is crucial. While various studies have proposed different paradigms for management of retinal detachment, few sufficiently powered randomized controlled trials (RCTs) have established any one therapy as clearly superior (Sodhi 2008). In this review we systematically examined the evidence on the effectiveness of pneumatic retinopexy versus scleral buckle as two major surgical treatments for RRD. A separate Cochrane review is under way that compares vitrectomy versus scleral buckle for repairing RRD (Znaor 2012).
OBJECTIVES
The objectives of this review were to assess the effectiveness and safety of pneumatic retinopexy versus scleral buckle or pneumatic retinopexy versus a combination treatment of scleral buckle and vitrectomy for people with RRD. The secondary objectives were to summarize any data on economic measures and quality of life.
METHODS
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi-RCTs, as specified in our published protocol (Ramchand 2010).
Types of participants
We included studies in which participants with RRD were enrolled for surgical treatment. Because RRD most commonly occurs in people aged 40 to 70 years with pre-existing or concurrent posterior vitreous detachments leading to retinal tears, we planned to consider studies with participants in this age range. However, we did not exclude studies with participants outside this age range nor studies that did not provide information on ages of participants.
Types of interventions
We included studies that compared pneumatic retinopexy with scleral buckle. We found no studies that compared pneumatic retinopexy with a combination of scleral buckle and vitrectomy surgery. As future studies are reported, we plan to include studies that have compared pneumatic retinopexy with a combination of scleral buckle and vitrectomy surgery.
Types of outcome measures
Primary outcomes
The primary outcome for this review was the proportion of participants who had successful reattachment of the retina after their initial surgery.
Secondary outcomes
The secondary outcomes for this review were the following.
Mean change in best-corrected visual acuity from baseline.
Proportion of participants with recurrence of retinal detachment.
The time period for assessing primary and secondary outcomes was one year after surgery, as specified in the protocol for this review. We also stated that we would include RCTs with at least six months of follow-up to allow for reporting of early adverse events and report outcomes at other times as reported by included studies.
Adverse effects
We compared adverse effects of surgery such as postoperative increase in intraocular pressure, choroidal detachments, proliferative vitreoretinopathy, cystoid macular edema, strabismus following the scleral buckling, macular pucker, progression of cataract in phakic eyes, and others reported in the included studies.
Quality-of-life measures
We planned to include data on quality of life measures, however no included study reported these data.
Economic data
Included studies did not report economic data. When new studies are added, we plan to summarize any available data on economic measures.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 12), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to January 2015), EMBASE (January 1980 to January 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to January 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 13 January 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We handsearched the reference lists of the included trials to identify other possible trials. We sought to obtain information about any ongoing study by contacting the relevant trial investigators.
Data collection and analysis
Selection of studies
Two review authors independently selected the studies for inclusion using a two-stage process. In the first stage, two review authors independently screened the titles and abstracts of all the records identified by electronic searches and handsearching. Each review author classified each record as follows: (1) definitely relevant, (2) possibly relevant, or (3) definitely not relevant. In the second stage, we retrieved the full-text report for all records classified by at least one review author as (1) definitely relevant or (2) possibly relevant. Two review authors then independently assessed each full-text report and classified as: (a) include, (b) awaiting classification, or (c) exclude. For studies classified as (b), we requested additional information from study investigators. The same two review authors compared their individual classifications, and then resolved any differences or requested review by a third review author. We documented all studies classified as (c) exclude. We retrieved and reviewed all pertinent references from each included study to provide the most complete published information possible about study design, methods, and findings.
Data extraction and management
Two review authors independently extracted data from studies included in the review using data extraction forms developed by the Cochrane Eyes and Vision Group. We resolved any discrepancies through discussion and by consulting a third review author when necessary. One review author entered data into Review Manager software (RevMan 2014), and a second review author verified the entries. Categories of information to be extracted for each study included methods (for example study design, number of participants, and setting), intervention details, outcomes (definitions and endpoints), and results for each outcome (sample size, missing data, summary data for each intervention). We contacted study authors whenever we needed additional information or clarification.
Assessment of risk of bias in included studies
We assessed the risk of bias as recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Two review authors assessed the risk of bias independently. This assessment required a description and a judgment on questions about selection bias, performance bias, detection bias, attrition bias, and reporting bias. We assessed each study as being at “low”, “high,” or “unclear” risk of bias. We judged studies as being at unclear risk of bias whenever lack of information or our uncertainty over the potential for bias rendered another classification impossible. Specific questions for assessing risk of bias focused on adequate sequence generation, allocation concealment before randomization, masking (blinding), adequate handling of incomplete outcome data, absence of selective outcome reporting, and absence of other potential sources of bias. Whenever the information available in the published trial reports was inadequate to assess risk of bias, we contacted the study investigators for clarification. We classified the trial on the basis of the available information if they did not respond within two weeks. We resolved discrepancies through discussion and by consulting a third review author when necessary.
Measures of treatment effect
Data analysis followed guidelines set forth in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We presented dichotomous data as risk ratios (RR) with 95% confidence intervals (CI). We reported outcomes based on the follow-up times reported for each study. Planned dichotomous outcome measures included the proportion of participants who had successful reattachment of the retina after their initial surgery, the proportion of participants with recurrence of retinal detachment after surgical reattachment, the proportion of participants with an adverse event, and the proportion of participants with improvement (yes or no) in quality-of-life measures (for example relief of symptoms). We planned to calculate the mean difference and 95% CI for continuous outcome measures, however neither of the included studies reported continuous outcome data. Planned continuous outcome measures included mean change in best-corrected visual acuity, mean change in quality-of-life scores, and economic (cost) outcomes.
Unit of analysis issues
The unit of analysis was the individual (one eye of each participant included), with the exception of two participants for which both eyes were included.
Dealing with missing data
We conducted analysis including studies with missing data in accordance with the guidelines in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We conducted the primary analysis based on the data as reported, although there was a mismatch between times of reported outcomes and our time points of interest. Whenever information was missing from the published trial reports or unclear, we contacted the primary trial investigators. We used the data available in the trial reports and described limitations of this method when applicable if they did not respond within two weeks. Fewer than 1% of participants in the included trials had missing outcome data, therefore we did not conduct sensitivity analyses to investigate the influence of missing data. We did not impute data.
Assessment of heterogeneity
We assessed heterogeneity by examining study characteristics and forest plots of the results. We used the I2 value to assess the impact of statistical heterogeneity and considered an I2 value of 50% or more as indicating substantial statistical heterogeneity.
Assessment of reporting biases
We included only two studies, therefore we could not examine funnel plots. When future studies are added to yield at least 10 studies in meta-analysis, we plan to examine funnel plots for each outcome to assess for publication bias. We assessed for selective outcome reporting as part of the ’Risk of bias’ assessment for each included study.
Data synthesis
We planned to analyze data using a random-effects model unless there were fewer than three trials. As we included only two trials in the review, we used a fixed-effect model. When meta-analysis was not appropriate due to substantial clinical or methodological heterogeneity, we reported results for each study individually and did not pool data across trials.
Subgroup analysis and investigation of heterogeneity
We were not able to perform subgroup analysis due to there being insufficient data available. When future studies are added and subgroup data becomes available, we plan to perform subgroup analysis based on the size and location of retinal detachment, that is whether the detachment involves the macula.
Sensitivity analysis
As we included only two studies in meta-analysis, there was no need for a sensitivity analysis. When future studies are added, we plan to conduct a sensitivity analysis to determine the impact of exclusion of studies with lower methodological quality, unpublished studies, and industry-funded studies. We would classify studies as of lower methodological quality based on the research design, such as studies that did not document how randomization was performed.
Summary of findings
We prepared a summary of findings table which includes relative and absolute risks based on the risks across intervention groups in the included studies. Two authors graded independently the overall quality of the evidence for each outcome using the GRADE classification (www.gradeworkinggroup.org/).
RESULTS
Description of studies
Results of the search
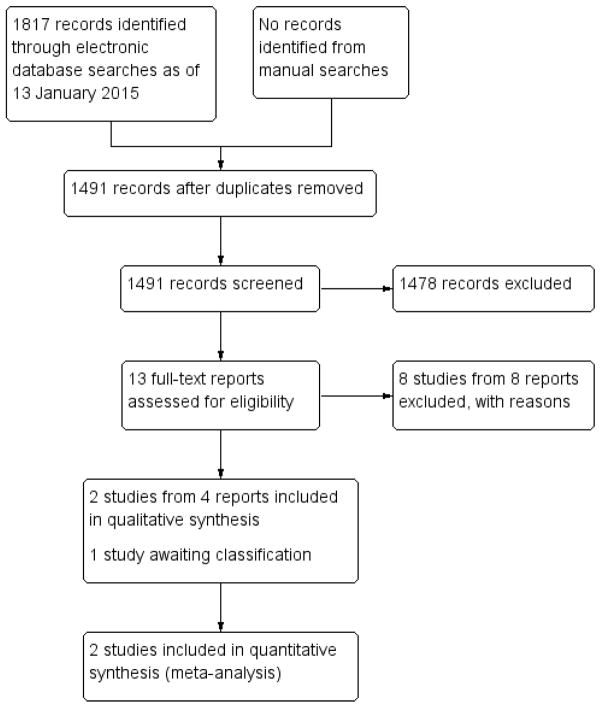
We identified 1806 titles and abstracts through bibliographic database searches and 11 ongoing trials from clinical trial registers (Figure 1). Removing duplicate records left 1484 titles and abstracts and 7 ongoing trials to be screened. We excluded 1478 records and assessed 13 full-text reports for eligibility. We excluded eight full-text reports from eight studies and recorded reasons for exclusion (see ’Excluded studies’ section). We included the remaining five reports: four reports referred to two studies that we included (Mulvihill 1996; Tornambe 1989), and one record (one study) is awaiting classification (Betran-Loustaunau 1997). When we contacted the authors of Betran-Loustaunau 1997, the only additional information they provided was that participants had been recruited from Mexico.
Figure 1.
Results of searching for studies for inclusion in the review
Included studies
We included two RCTs (218 eyes of 216 participants) in this review (see Characteristics of included studies).
Table 2.
Characteristics of included studies [ordered by study ID]
| Mulvihill 1996 | ||
| Methods |
Study design: parallel group randomized controlled trial Number randomized: 20 participants total 10 participants in the pneumatic retinopexy group 10 participants in the scleral buckle group Exclusions after randomization: none reported Unit of analysis: individual (1 eye of each participant included) Number analyzed: 20 participants total 10 participants in the pneumatic retinopexy group 10 participants in the scleral buckle group Losses to follow-up: none reported Power calculation: not reported |
|
| Participants |
Country: Ireland Age: not reported Gender: not reported Inclusion criteria: participants diagnosed with rhegmatogenous retinal detachment fulfilling certain criteria:
|
|
| Interventions |
Intervention 1: pneumatic retinopexy Intervention 2: scleral buckle “Pneumatic retinopexy was performed under local anaesthesia in the operating theatre in all cases. Following peribulbar anaesthesia, the eye was softened using Honan’s Balloon and the periorbital skin cleaned with 0.1% chlorhexidine and then draped. The retinal breaks were identified and gas then injected into the vitreous cavity 4 mm posterior to the limbus using a 30-gauge needle. The gas used was either 0.6 ml of sulphur hexafluoride (SF6) or 0.3 ml of perfluoropropane (C3F8). Following rejection of gas the patency of the central retinal artery was evaluated with the indirect ophthalmoscope. The retinal break was then sealed using either transconjunctival cryopexy or indirect (argon) laser. Cryopexy was used when a satisfactory reaction could not be obtained with laser. In some large bullous RDs the procedure was performed in two stages: the retina was allowed to flatten after gas injection and then the retinal break was sealed one to two days later. After gas injection the intraocular pressure was checked by applanation tonometry three and six hours later. Postoperatively the participants were instructed to posture for at least sixteen hours per day and for at least four days so the retinal breaks were uppermost, tamponaded by the intraocular gas bubble. Topical antibiotic/steroid (Betnesol-N) was administered for four weeks postoperatively. Briefly, scleral buckle was performed as follows. The conjunctiva and Tenon’s capsule were opened and the subretinal fluid drained via an opening in the sclera to allow the retina to move back into position after injection of sterile air into the vitreous cavity. The retinal break was then sealed with trans-scleral cryopexy. Finally, a hard silicone explant was sutured onto the sclera in the region of the retinal break to create a permanent scleral indentation. All of the scleral buckle procedures were carried-out under general anaesthesia.” Length of follow-up: Planned: not reported Actual: average follow-up 16.7 months (range 5 to 27 months) in the pneumatic retinopexy group and 16.0 months (range 8 to 23 months) in the scleral buckle group |
|
| Outcomes |
Primary outcome: reattachment of the retina Secondary outcomes: visual acuity, recurrence of retinal detachment, and adverse events Intervals at which outcomes assessed: not reported |
|
| Notes |
Type of report: published article Funding sources: not reported Disclosures of interest: not reported Study period: November 1991 to February 1994 Subgroup analyses: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | “Participants were randomised by a closed envelope technique into one of two groups.” |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel not reported, but surgeons could not have been masked |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All participants included and randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. |
| Other bias | Unclear risk | Funding source not reported. |
| Tornambe 1989 | ||
| Methods |
Study design: parallel group randomized controlled trial Number randomized: 200 eyes of 198 participants total; number not reported by treatment group Exclusions after randomization: none reported Unit of analysis: individual (1 eye of each participant included, except for 2 participants for which both eyes were included) Number analyzed: 198 eyes of 196 participants total; 103 eyes in the pneumatic retinopexy group (41 eyes with macular-attached; 62 eyes with macular-detached) 95 eyes in the scleral buckle group (35 eyes with macular-attached; 60 eyes with macular-detached) Losses to follow-up: 2 eyes of 2 participants; 1 participant died of a myocardial infarction 2 months after scleral buckle and 1 was lost to follow-up Power calculation: not reported |
|
| Participants |
Country: United States Age: not reported Gender: 64/103 (62%) men and 39/103 (38%) women in pneumatic retinopexy group; 65/95 (68%) men and 30/95 (32%) women in scleral buckle group Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention 1: pneumatic retinopexy Intervention 2: scleral buckle “When pneumatic retinopexy was used, the surgeon recorded the type and volume of gas injected (the type of gas selected was not randomized), the number of cryopexy or laser photocoagulation applications, the incidence and duration of central retinal artery closure, paracentesis, intraocular pressures at 5, 10, 20, 30 and 60 minutes after gas introduction, and all intraoperative complications. When scleral buckle was used, the surgeon recorded the type of buckle material, the number of cryopexy applications, drainage of subretinal fluid, the incidence and duration of central retinal artery closure, paracentesis, the type and volume of gas injected, and all intraoperative complications.” Length of follow-up: Planned: not reported Actual: 24 months |
|
| Outcomes | Primary and secondary outcomes not specified Outcome, as define d: surgical success (retinal reattachment at 6 months after 1 surgical intervention), visual acuity, recurrence of retinal detachment, morbidity, and adverse events Intervals at which outcomes assessed: surgical success (6, 12, and 24 months), visual acuity (1, 6, 12, and 24 months), and adverse events (1, 3, 7, and 14 days; 1, 2, 4, and 6 months) |
|
| Notes |
Type of report(s): published articles and conference abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Subgroup analyses: macular-attached and macular-detached (participants stratified before being randomized for treatment) Publication language: English |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported; however, “A separate randomization schedule was prepared for each center.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before randomization not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel not reported, but surgeons could not have been masked |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of personnel assessing retinal reattachment not reported; “Visual acuity was evaluated by an examiner, in a masked manner, who was unacquainted with the case.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 2 eyes (2 participants) of 198 eyes excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. |
| Other bias | Unclear risk | Funding source not reported. |
DD: disc diameter
ETDRS: Early Treatment Diabetic Retinopathy Study
RD: retinal detachment
Settings and participants
Characteristics of participants recruited in the two included studies were similar. Both studies included participants who were generally good candidates for pneumatic retinopexy (e.g., single retinal tear less than or equal to one clock hour in size, tear located in the superior half of the retina, absence of proliferative vitreoretinopathy, absence of uncontrolled glaucoma). There was no reason to expect differences among participants with simple RRD from different countries or settings. Tornambe 1989 enrolled participants from a teaching hospital in the United States; Mulvihill 1996 was conducted in Ireland, but did not report how participants were recruited.
Interventions
Both studies compared pneumatic retinopexy with scleral buckle surgery. In both studies, surgeons could inject either sulphur hexafluoride or perfluoropropane gas tamponades and use either cryopexy or laser to seal retinal breaks when performing pneumatic retinopexy. Both studies also employed similar procedures for scleral buckle surgery. Procedures generally included draining of the subretinal fluid, cryopexy to seal retinal breaks, gas injections, and suturing of the scleral buckle.
Outcomes
Both studies assessed reattachment of the retina, visual acuity, recurrence of retinal detachment, and adverse events as outcomes. Additionally, Tornambe 1989 assessed morbidity (time to resolution of symptoms, changes in refractive error, length of hospital stay). Neither study assessed quality-of-life or economic measures. Mulvihill 1996 had a minimum of 5 months follow-up for all participants (average follow-up of 16 months), while Tornambe 1989 reported outcomes at a primary endpoint of 6 months follow-up (198 of 200 eyes, 99%) and secondary endpoint of 24 months follow-up (169 of 200 eyes, 85%).
Excluded studies
We excluded eight potentially relevant studies after review of the full-text report (see Characteristics of excluded studies). Our reasons for exclusion included lack of randomization (four studies) and interventions not eligible (four studies).
Table 3.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avitabile 2004 | Not intervention of interest: RCT comparing cryopexy versus frequency-doubled Nd:YAG laser for retinopexy in eyes with RRD undergoing scleral buckle surgery |
| Barr 1995 | Not a RCT: review article of pneumatic retinopexy and scleral buckling surgery |
| Figueroa 2000 | Not intervention of interest: RCT comparing scleral buckle with retinopexy versus scleral buckle alone |
| Hsu 2014 | Not intervention of interest: RCT comparing dorzolamide plus timolol eye drops versus no eye drops in eyes undergoing retinal detachment surgery (pneumatic retinopexy or scleral buckle) |
| Maia 2007 | Not a RCT: prospective cohort study to determine postoperative macular function in eyes with RRD after surgery (pneumatic retinopexy or scleral buckle) |
| Massin 1971 | Not a RCT: historical cohort study comparing two techniques for scleral buckle surgery |
| Topbas 2013 | Not a RCT: prospective cohort study to determine visual outcomes and optical coherence tomography findings in eyes after successful retinal detachment surgery (pneumatic retinopexy, scleral buckle, or pars plana vitrectomy) |
| Veckeneer 2001 | Not intervention of interest: RCT comparing cryopexy versus laser photocoagulation for retinopexy in eyes with RRD undergoing scleral buckle surgery |
Nd:YAG: neodymium-doped yttrium aluminium garnet
RCT: randomized controlled trial
RRD: rhegmatogenous retinal detachment
Risk of bias in included studies
The quality of evidence was unclear as neither study reported key methodological details. Most of the domains had unclear risk of bias, but both studies had a low risk of attrition bias (Figure 2).
Figure 2.
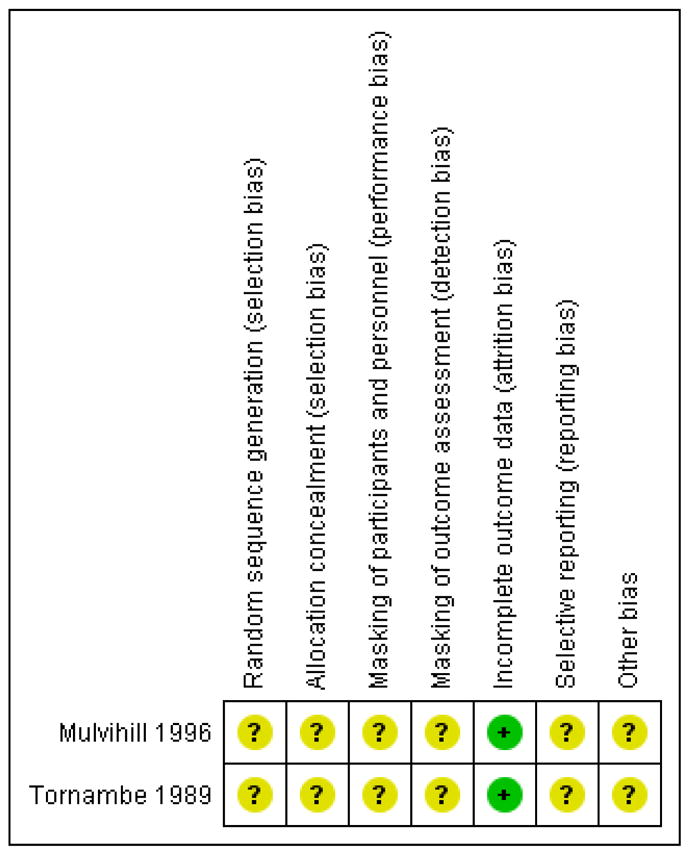
’Risk of bias’ summary: review authors’ judgments about each ’Risk of bias’ item for each included study.
Allocation
We assessed the risk of selection bias as unclear in both studies, as neither reported adequate methods of random sequence generation or allocation concealment. Although Mulvihill 1996 reported using closed envelopes to assign groups, it was unclear whether the envelopes were sequentially numbered or opaque to ensure treatment assignments were concealed.
Masking (performance bias and detection bias)
Although it was not possible to mask the surgeons performing the procedures, it is reasonable that participants and outcome assessors could have been masked. We judged both studies to have unclear risk of performance bias, as they did not report masking of participants. We also judged both studies to have unclear risk of detection bias, as they did not report masking of surgeons who assessed outcomes for retinal reattachment, recurrence of retinal detachment, and adverse events. Tornambe 1989 masked visual acuity examiners to treatment groups.
Incomplete outcome data
There was minimal attrition, as both studies had missing follow-up data for less than 1% of participants. We thus judged both studies to be at low risk of attrition bias.
Selective reporting
We planned to compare outcomes set forth in study protocols with outcomes reported in study reports to assess for selective outcome reporting. We judged both studies to have unclear risk of reporting bias, as we were unable to procure the protocol for either study.
Other potential sources of bias
We judged both studies to have unclear risk of bias as neither Mulvihill 1996 nor Tornambe 1989 reported sources of funding or conflicts of interest.
Effects of interventions
See: Summary of findings for the main comparison
We have been cautious when interpreting the evidence. We assessed the quality of the evidence as low to moderate and follow-up times differed between studies. Neither study reported outcomes at one-year follow-up. Mulvihill 1996 had a minimum follow-up of 5 months for all participants (average of 16 months), while Tornambe 1989 reported outcomes at 6- and 24-months follow-up. Tornambe 1989 (200 eyes of 198 participants) was a much larger study than Mulvihill 1996 (20 participants). While neither study showed statistical differences for effectiveness outcomes after pneumatic retinopexy and scleral buckle surgeries, neither study reported an a priori sample size or power calculation. Tornambe 1989 excluded from their analyses 2 eyes of 2 participants with missing data at 6-months follow-up and 31 eyes at 24-months follow-up.
Reattachment of the retina
We defined successful reattachment of the retina as reattachment after the initial surgery. As reattachment is a beneficial effect, RRs less than 1 favor scleral buckle and RRs greater than 1 favor pneumatic retinopexy for this outcome. We considered reattachment results from Mulvihill 1996 to approximate six-month outcomes, as most instances of reattachment would have been observed by this time. At six months, fewer participants in the pneumatic retinopexy group had retinal reattachment compared with participants in the scleral buckle group (RR 0.89, 95% CI 0.77 to 1.02; Analysis 1.1). Tornambe 1989 did not report retinal reattachment at 24 months.
Visual acuity
Neither study reported mean change in best-corrected visual acuity (BCVA) from baseline. Mulvihill 1996 reported that 8 of 10 participants (80%) in the pneumatic retinopexy group and 9 of 10 participants (90%) in the scleral buckle group had improvement in BCVA at final follow-up examination (improvement in BCVA not defined). At 6 months, Tornambe 1989 reported that 71 of 103 eyes (69%) in the pneumatic retinopexy group and 50 of 95 eyes (53%) in the scleral buckle group had BCVA of 20/40 or better, and 101 of 103 eyes (98%) in the pneumatic retinopexy group and 90 of 95 eyes (95%) in the scleral buckle group had BCVA of 20/200 or better. At 24 months, Tornambe 1989 reported that 81 of 92 eyes (88%) in the pneumatic retinopexy group and 57 of 77 eyes (74%) in the scleral buckle group had BCVA of 20/40 or better, and 90 of 92 eyes (98%) in the pneumatic retinopexy group and 74 of 77 eyes (96%) in the scleral buckle group had BCVA of 20/200 or better.
Recurrence of retinal detachment
Since recurrence of retinal detachment is a negative effect, RRs less than 1 favor pneumatic retinopexy and RRs greater than 1 favor scleral buckle for this outcome. We considered results from Mulvihill 1996 to approximate six-month outcomes, as most instances of redetachment would have occurred within the first six months. At six months, recurrence of retinal detachment (breaks) was more common in the pneumatic retinopexy group than in the scleral buckle group (RR 1.80, 95% CI 1.00 to 3.24; Analysis 1.2). Tornambe 1989 additionally reported that 1 eye in the pneumatic retinopexy group detached at 7 months after the initial surgery and 1 eye in the scleral buckle group detached at 11 months after initial surgery.
Adverse events
Mulvihill 1996 reported 2 of 10 participants (20%) in the pneumatic retinopexy group developed proliferative vitreoretinopathy (1 of these eyes became phthisical) and 1 of 10 participants (10%) in the scleral buckle group developed subretinal hemorrhage at the drainage site. No other adverse events were reported by Mulvihill 1996. Tornambe 1989 presented operative and postoperative adverse events up to six-months follow-up (Table 1).
Table 1.
Ocular adverse events through 6 months follow-up
| Ocular adverse event | Pneumatic retinopexy group | Scleral buckle group | Risk ratio (95% confidence interval); number of studies | ||
|---|---|---|---|---|---|
| Number with event | Total number | Number with event | Total number | ||
| Any operative adverse event* | 10 | 113 | 14 | 105 | 0.67 (0.32 to 1.42); 2 studies |
| Cataract | 1 | 103 | 1 | 95 | 0.92 (0.06 to 14.54); 1 study |
| Choroidal detachment | 3 | 103 | 16 | 95 | 0.17 (0.05 to 0.57); 1 study |
| Macular pucker | 4 | 103 | 5 | 95 | 0.74 (0.20 to 2.67); 1 study |
| Myopic shift ≥ 1 diopter | 3 | 103 | 65 | 95 | 0.04 (0.01 to 0.13); 1 study |
| Proliferative vitreoretinopathy | 5 | 113 | 5 | 105 | 0.94 (0.30 to 2.96); 2 studies |
includes retinal or vitreous incarceration, anterior hyaloidal or subretinal gas injection, anterior lens capsule touch, choroidal detachment, vitreous or subretinal hemorrhage, retinal perforation, or hyphema
In the pneumatic retinopexy group, 10 of 113 eyes (9%) were reported to have had an operative adverse event, including vitreous incarceration in paracentesis site, anterior hyaloidal gas injection, anterior lens capsule touch, choroidal detachment, vitreous hemorrhage, subretinal hemorrhage, or hyphema. In the scleral buckle group, 14 of 105 eyes (13%) were reported to have had an operative adverse event, including retinal incarceration, retinal perforation, choroidal detachment, vitreous hemorrhage, subretinal hemorrhage, or subretinal gas. The RR when comparing groups was 0.67 (95% CI 0.32 to 1.42) (Analysis 1.3). Fewer postoperative adverse events were observed in the pneumatic retinopexy group, specifically for choroidal detachment (RR 0.17, 95% CI 0.05 to 0.57) and myopic shift equal to or greater than 1 diopter spherical equivalent (RR 0.04, 95% CI 0.01 to 0.13). Five eyes in the pneumatic retinopexy group and five eyes in the scleral buckle group developed proliferative vitreoretinopathy (RR 0.94, 95% 0.30 to 2.96) (Analysis 1.3). Four eyes in the pneumatic retinopexy group had asymptomatic macular pucker; in the scleral buckle group, three eyes had asymptomatic macular pucker and two eyes had symptomatic macular pucker (RR 0.74, 95% CI 0.20 to 2.67). One eye in each group developed cataract (RR 0.92, 95% CI 0.06 to 14.54). Postoperative increase in intraocular pressure, cystoid macular edema, and strabismus following the scleral buckling were not reported.
Quality-of-life and economic outcomes
Neither of the included studies reported quality-of-life and economic data.
DISCUSSION
Summary of main results
We identified two studies comparing pneumatic retinopexy with scleral buckle for eyes with RRD. Fewer eyes achieved retinal reattachment by six-months follow-up with pneumatic retinopexy compared with scleral buckle. Eyes treated with pneumatic retinopexy also were more likely to have had a recurrence of retinal detachment by six-months follow-up compared with eyes treated with scleral buckle. However, the confidence intervals for both these outcomes do rule out no difference between procedures. For most adverse events there was uncertainty in the effect between groups, although eyes in the scleral buckle group were more susceptible to choroidal detachment and myopic shift than eyes in the pneumatic retinopexy group. Neither study reported mean change in visual acuity, quality of life data, or economic measures.
Overall completeness and applicability of evidence
Outcome data were available for more than 99% of participants in the included studies. The majority of participants were in the larger study from the United States; Tornambe 1989 enrolled 10 times as many participants and followed all participants for more than twice as long as Mulvihill 1996. We were not able to assess the effect of each surgery on the change of visual acuity, as this outcome was defined only as “improvement” in Mulvihill 1996 and reported categorically at a time point in Tornambe 1989. However, assuming the pre-operative BCVAs were similar at time of randomization in Tornambe 1989 and recognizing that the post-surgery BCVAs were similar, one could conclude that the changes in BCVA were similar between treatment groups.
Quality of the evidence
The quality of the evidence is largely unknown due to inadequate reporting. We judged each of the included studies to be at unclear risk of bias for most of the bias domains assessed. The potential for bias regarding rates of retinal reattachment and recurrence of retinal detachment was minimal, as the number of participants with these outcomes was similar at 6- and 24-month follow-up in Tornambe 1989 (all participants in Mulvihill 1996 had at least 5 months follow-up, average of 16 months).
Potential biases in the review process
We followed standard Cochrane systematic review methodology to minimize potential biases in the review process. We used no language or date restrictions in the electronic search for trials. We also searched clinical trial registries for ongoing trials.
Agreements and disagreements with other studies or reviews
Our conclusions agree with other studies and reviews. The American Academy of Ophthalmology preferred practice guidelines do not specify a preferred modern vitreoretinal surgical technique for retinal reattachment. Instead, they emphasize clinical judgment based on “careful preoperative examination and choice of an appropriate surgical procedure” (AAO 1996; Stuart 2014). Authors of a Cochrane systematic review of interventions for the related topic of giant retinal tear identified no studies comparing retinopexy with scleral buckle (Ang 2012). The authors of a systematic review of all surgical interventions for RRD that included the same studies as we included performed a subgroup analysis comparing pneumatic retinopexy with scleral buckle and concluded “unknown effectiveness” (Steel 2014; search date September 2013).
AUTHORS’ CONCLUSIONS
Implications for practice
There is an absence of sufficient high-quality evidence from randomized controlled trials comparing the risks and benefits of pneumatic retinopexy versus scleral buckle for RRD. Decisions must be based on clinical judgment, patient preferences, and the surgeon’s skill and experience with each procedure.
Implications for research
There is a need for well-designed, adequately powered randomized controlled trials to evaluate patient-important outcomes of pneumatic retinopexy versus scleral buckle as the two major surgical treatments for RRD. We recommend that randomization in such trials be stratified by history of previous surgical intervention in each eye, status of the fellow eye, and whether the RRD is a recurrence. A design in which participants are randomized to a surgical expert in pneumatic retinopexy or scleral buckle would address issues related to potential surgeon effect.
Because people who undergo surgical interventions for RRD already have reduced vision due to the underlying condition, relevant outcomes such as quality of life should be considered in addition to visual acuity and other clinical measures of vision. Analyses should include outcomes with specified lengths of follow-up. Both short-term and long-term outcomes should be examined. Short-term outcomes include early adverse events such as postoperative increase in intraocular pressure, choroidal detachment, or progression of cataract in the first six months following the surgery. Long-term outcomes include the proportion of participants who had successful reattachment of the retina after their initial surgery, change in BCVA from baseline to each scheduled follow-up examination, and the proportion of participants with recurrence of retinal detachment by one year after the surgery. Investigators of future randomized controlled trials should report on all issues that affect trial quality, for example those noted in the ’Risk of bias’ section of this review. They should also make their protocols available, for example on clinicaltrials.gov, to allow for comparison between protocol and reports from the randomized controlled trials.
Ethical concerns regarding randomization to different surgical approaches include potential complications, prognosis for recovery of visual acuity, and risk of recurrence of retinal detachment. These outcomes are important considerations in establishing equipoise. Although the evidence regarding these issues is weak, there is no evidence regarding outcomes important to patients, such as vision-related quality of life.
Table 4.
Characteristics of studies awaiting assessment [ordered by study ID]
| Betran-Loustaunau 1997 | |
| Methods |
Study design: parallel group randomized controlled trial Number randomized: 30 participants total; number not reported by treatment group Exclusions after randomization: not reported Unit of analysis: not reported Number analyzed: not reported Losses to follow-up: not reported Power calculation: not reported |
| Participants |
Country: Mexico Age: not reported Gender: not reported Inclusion criteria:
|
| Interventions |
Intervention 1: pneumatic retinopexy Intervention 2: scleral buckle Intervention 3: vitrectomy Length of follow-up: Planned: not reported Actual: mean length of follow-up was 4.3 months |
| Outcomes |
Outcome, as defined: surgical success (reattached retina with first surgery until final follow-up) Intervals at which outcomes assessed: not reported |
| Notes |
Type of report: conference abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Subgroup analyses: none reported Publication language: English Study author contacted. Author’s response was that the participants were recruited from Mexico; he was not able to provide more information |
Acknowledgments
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
National Eye Institute, National Institutes of Health, USA. Methodological support provided by the Cochrane Eyes and Vision Group, US Project funded by grant 1 U01 EY020522
National Institute for Health Research (NIHR), UK.
Richard Wormald, Co-ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
We acknowledge:
Dr. Kanchan Ramchand and Dr. Sumit Dhingra for contributions to the protocol of this review;
Iris Gordon, Trials Search Co-ordinator for CEVG, for devising and running the electronic search strategies;
Dr. Swaroop Vedula, Andrew Law, and Kristina Lindsley, CEVG@US; and Anupa Shah, Managing Editor for CEVG, for support and guidance in completing this review;
Dr. Stephen Schwartz and Dr. Jennifer Evans for providing comments;
Dr. Millena Bittencourt for the review of Portuguese articles;
Dr. Wataru Yano and Dr. Yuichiro Watanabe for the review of Japanese articles;
Dr. Xue Wang for review of abstracts, data abstraction, and guidance during the review process.
APPENDICES
Appendix 1. CENTRAL search strategy
-
#1
MeSH descriptor: [Retinal Detachment] explode all trees
-
#2
MeSH descriptor: [Retinal Perforations] explode all trees
-
#3
MeSH descriptor: [Vitreous Detachment] explode all trees
-
#4
retina* near/2 break*
-
#5
retina* near/2 tear*
-
#6
retina* near/2 detach*
-
#7
retina* near/2 perforat*
-
#8
#1 or #2 or #3 or #4 or #5 or #6 or #7
-
#9
MeSH descriptor: [Scleral Buckling] explode all trees
-
#10
scleral near/2 buckl*
-
#11
scleral near/2 encircl*
-
#12
encircling band
-
#13
MeSH descriptor: [Vitrectomy] explode all trees
-
#14
vitrectom* or PPV*
-
#15
MeSH descriptor: [Cryotherapy] explode all trees
-
#16
MeSH descriptor: [Cryosurgery] explode all trees
-
#17
cryotherap* or cryosurg*
-
#18
MeSH descriptor: [Light Coagulation] explode all trees
-
#19
MeSH descriptor: [Laser Coagulation] explode all trees
-
#20
laser near/2 photocoagulat*
-
#21
retinopex*
-
#22
#9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21
-
#23
#8 and #22
Appendix 2. MEDLINE (OvidSP) search strategy
randomized controlled trial.pt.
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1–7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
exp retinal detachment/
exp retinal perforation/
exp vitreous detachment/
(retina$ adj2 break$).tw.
(retina$ adj2 tear$).tw.
(retina$ adj2 detach$).tw.
(retina$ adj2 perforat$).tw.
or/13–19
exp cryotherapy/
exp cryosurgery/
(cryotherap$ or cryosurg$).tw.
exp light coagulation/
exp laser coagulation/
(laser adj2 photocoagulat$).tw.
retinopex$.tw.
or/21–27
20 and 28
12 and 29
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
exp randomized controlled trial/
exp randomization/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1–5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12–21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25–28
29 not 10
30 not (11 or 23)
11 or 24 or 31
retina tear/
retinal detachment/
vitreous body detachment/
(retina$ adj2 break$).tw.
(retina$ adj2 tear$).tw.
(retina$ adj2 detach$).tw.
(retina$ adj2 perforat$).tw.
or/33–39
cryotherapy/
cryosurgery/
(cryotherap$ or cryosurg$).tw.
retinopexy/
retinopex$.tw.
exp laser coagulation/
(laser adj2 photocoagulat$).tw.
or/41–47
40 and 48
32 and 49
Appendix 4. LILACS search strategy
retina$ and retinopexy
Appendix 5. ISRCTN search strategy
“(Condition: retina AND Interventions: retinopexy)”
Appendix 6. ClinicalTrials.gov search strategy
retina AND retinopexy
Analysis 1.1.
Comparison 1 Pneumatic retinopexy versus scleral buckle, Outcome 1 Reattachment of the retina at 6 months follow-up.
Analysis 1.2.
Comparison 1 Pneumatic retinopexy versus scleral buckle, Outcome 2 Recurrence of retinal detachment through 6 months follow-up.
Analysis 1.3.
Comparison 1 Pneumatic retinopexy versus scleral buckle, Outcome 3 Adverse events through 6 months follow-up.
Appendix 7. ICTRP search strategy
retinal AND retinopexy
DATA AND ANALYSES
Comparison 1. Pneumatic retinopexy versus scleral buckle
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reattachment of the retina at 6 months follow-up | 2 | 218 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 2 Recurrence of retinal detachment through 6 months follow-up | 2 | 218 | Risk Ratio (M-H, Fixed, 95% CI) | 1.80 [1.00, 3.24] |
| 3 Adverse events through 6 months follow-up | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Any operative ocular adverse event | 2 | 218 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.32, 1.42] |
| 3.2 Proliferative vitreoretinopathy | 2 | 218 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.30, 2.96] |
Footnotes
CONTRIBUTIONS OF AUTHORS
Conceiving and designing the review: EH, DS, DVD
Undertaking manual searches: EH, KAF, DS
Screening search results: DS, EH, KAF
Organizing retrieval of papers: EH, DS, KAF
Screening retrieved papers against inclusion criteria: DS, EH, KAF
Appraising risk of bias: EH, DS, KAF
Abstracting data from papers: EH, DS, KAF
Writing to authors of papers for additional information: EH
Providing additional data about papers: EH
Obtaining and screening data on unpublished studies: EH
Data management for the review: EH
Entering data into RevMan: EH, DS, KAF
Analysis of data: EH
Interpretation of data: EH, DVD
Writing the review: EH, DVD, DS, JC
Performing previous work that was the foundation of current study: EH, DVD
Guarantor for the review: EH
DECLARATIONS OF INTEREST
None known.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
We did not follow all methods as set forth in the protocol for the review due to there being insufficient quantitative data to conduct subgroup and sensitivity analyses (Ramchand 2010).
References to studies included in this review
* Indicates the major publication for the study
- Mulvihill 1996 {published data only}.Mulvihill A, Fulcher T, Datta V, Acheson R. Pneumatic retinopexy versus scleral buckling: a randomised controlled trial. Irish Journal of Medical Science. 1996;165(4):274–7. doi: 10.1007/BF02943089. [DOI] [PubMed] [Google Scholar]
- Tornambe 1989 {published data only}.Tornambe PE, Hilton GF. Pneumatic retinopexy. A multicenter randomized controlled clinical trial comparing pneumatic retinopexy with scleral buckling. The Retinal Detachment Study Group. Ophthalmology. 1989;96(6):772–83. doi: 10.1016/s0161-6420(89)32820-x. [DOI] [PubMed] [Google Scholar]; * Tornambe PE, Hilton GF, Brinton DA, Flood TP, Green S, Grizzard WS, et al. Pneumatic retinopexy. A two-year follow-up study of the multicenter clinical trial comparing pneumatic retinopexy with scleral buckling. Ophthalmology. 1991;98(7):1115–23. [PubMed] [Google Scholar]; Tornambe PE, Hilton GF, Grizzard WS, Hammer ME, Poliner LS, Yarian DL, et al. Pneumatic retinopexy: a two-year randomized controlled follow-up study comparing pneumatic retinopexy with scleral buckling. American Academy of Ophthalmology. 1990;123 [Google Scholar]
References to studies excluded from this review
- Avitabile 2004 {published data only}.Avitabile T, Bartolotta G, Torrisi B, Reibaldi A. A randomized prospective study of rhegmatogenous retinal detachment cases treated with cryopexy versus frequency-doubled Nd:YAG laser-retinopexy during episcleral surgery. Retina. 2004;24(6):878–82. doi: 10.1097/00006982-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Barr 1995 {published data only}.Barr CC. Pneumatic retinopexy and balloon buckles as alternatives to conventional scleral buckling surgery. Seminars in Ophthalmology. 1995;10(1):2–8. doi: 10.3109/08820539509059974. [DOI] [PubMed] [Google Scholar]
- Figueroa 2000 {published data only}.Figueroa M, Schirru A, Corte MD, Sbordone S, Romano A. Scleral buckling technique without retinopexy for treatment of rhegmatogenous retinal detachment. American Academy of Ophthalmology. 2000:123. doi: 10.1097/00006982-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Hsu 2014 {published data only}.Hsu J, Gerstenblith AT, London NJ, Garg SJ, Spirn MJ, Maguire JI, et al. Effect of topical aqueous suppression on intraocular gas duration after pure perfluoropropane injection in nonvitrectomized eyes with retinal detachment. Retina. 2014;34(12):2458–61. doi: 10.1097/IAE.0000000000000244. [DOI] [PubMed] [Google Scholar]
- Maia 2007 {published data only}.Maia OO, Jr, Takahashi WY, Chizzotti Bonanomi MT, Arantes TE. Rhegmatogenous retinal detachment: a postoperative study of the macula [Descolamento regmatogênico de retina: avaliação pós–operatória da mácula] Arquivos Brasileiros de Oftalmologia. 2007;70(6):996–1000. doi: 10.1590/s0004-27492007000600021. [DOI] [PubMed] [Google Scholar]
- Massin 1971 {published data only}.Massin M, Dubois-Poulsen A, Damois F. Comparison between 2 methods of treatment of retinal detachment (Advantages of H.A Lincoff ’s method) Archives d’Ophtalmologie et Revue Generale d’Ophtalmologie. 1971;31 (11):793–804. [PubMed] [Google Scholar]
- Topbas 2013 {published data only}.Topbas S, Gursoy H, Erol N, Usalp Z. Spectral-domain optical coherence tomographic analysis of macula in cases with successfully repaired macula-off retinal detachment. Retina-Vitreus. 2013;21(2):113–8. [Google Scholar]
- Veckeneer 2001 {published data only}.Veckeneer M, Van Overdam K, Bouwens D, Feron E, Mertens D, Peperkamp E, et al. Randomized clinical trial of cryotherapy versus laser photocoagulation for retinopexy in conventional retinal detachment surgery. American Journal of Ophthalmology. 2001;132(3):343–7. doi: 10.1016/s0002-9394(01)01026-1. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Betran-Loustaunau 1997 {published data only}.Betran-Loustaunau MA, Troconis D, Morales-Canton V, Ochoa-Contreras D, Dalma-Weiszhausz J, Jimenez-Sierra JM, et al. Comparative study of vitrectomy, pneumatic retinopexy, and scleral buckling for primary rhegmatogenous retinal-detachment. Investigative Ophthalmology and Visual Science. 1997 ARVO E-Abstract 3156. [Google Scholar]
Additional references
- AAO 1996.American Academy of Ophthalmology. The repair of rhegmatogenous retinal detachments. A report by the American Academy of Ophthalmology Committee on Ophthalmic Procedure Assessment Ophthalmology. 1996;103(8):1313–24. doi: 10.1016/s0161-6420(96)30505-8. [DOI] [PubMed] [Google Scholar]
- Algvere 1999.Algvere PV, Jahnberg P, Textorius O. The Swedish Retinal Detachment Register. I. A database for epidemiological and clinical studies. Graefe’s Archive for Clinical and Experimental Ophthalmology. 1999;237(2):137–44. doi: 10.1007/s004170050208. [DOI] [PubMed] [Google Scholar]
- Ang 2012.Ang GS, Townend J, Lois N. Interventions for prevention of giant retinal tear in the fellow eye. Cochrane Database of Systematic Reviews. 2012;(2) doi: 10.1002/14651858.CD006909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byer 1998.Byer NE. What happens to untreated asymptomatic retinal breaks, and are they affected by posterior vitreous detachment? Ophthalmology. 1998;105(6):1045–50. doi: 10.1016/S0161-6420(98)96006-7. [DOI] [PubMed] [Google Scholar]
- Deeks 2011.Deeks JJ, Higgins JPT, Altman DG, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Chapter 9: Analysing data and undertaking meta-analyses. Version 5.1.0 [updated March 2011] Available from www.cochrane-handbook.org. [Google Scholar]
- Gariano 2004.Gariano RF, Kim CH. Evaluation and management of suspected retinal detachment. American Family Physician. 2004;69(7):1691–8. [PubMed] [Google Scholar]
- Ghazi 2002.Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye. 2002;16(4):411–21. doi: 10.1038/sj.eye.6700197. [DOI] [PubMed] [Google Scholar]
- Glanville 2006.Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94 (2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Higgins 2011a.Higgins JPT, Altman DG, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Chapter 8: Assessing risk of bias in included studies. Version 5.1.0 [updated March 2011] Available from www.cochrane-handbook.org. [Google Scholar]
- Higgins 2011b.Higgins JPT, Deeks JJ, Altman DG, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Chapter 16: Special topics in statistics. Version 5.1.0 [updated March 2011] Available from www.cochrane-handbook.org. [Google Scholar]
- Li 2003.Li X Beijing Rhegmatogenous Retinal Detachment Study Group. Incidence and epidemiological characteristics of rhegmatogenous retinal detachment in Beijing, China. Ophthalmology. 2003;110(12):2413–7. doi: 10.1016/s0161-6420(03)00867-4. [DOI] [PubMed] [Google Scholar]
- Minihan 2001.Minihan M, Tanner V, Williamson TH. Primary rhegmatogenous retinal detachment: 20 years of change. British Journal of Ophthalmology. 2001;85(5):546–8. doi: 10.1136/bjo.85.5.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RevMan 2014.Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- Ross 2000.Ross WH, Stockl FA. Visual recovery after retinal detachment. Current Opinion in Ophthalmology. 2000;11 (3):191–4. doi: 10.1097/00055735-200006000-00007. [DOI] [PubMed] [Google Scholar]
- Rowe 1999.Rowe JA, Erie JC, Baratz KH, Hodge DO, Gray DT, Butterfield L, et al. Retinal detachment in Olmsted County, Minnesota, 1976 through 1995. Ophthalmology. 1999;106 (1):154–9. doi: 10.1016/S0161-6420(99)90018-0. [DOI] [PubMed] [Google Scholar]
- Sodhi 2008.Sodhi A, Leung LS, Do DV, Gower EW, Schein OD, Handa JT. Recent trends in the management of rhegmatogenous retinal detachment. Survey of Ophthalmology. 2008;53(1):50–67. doi: 10.1016/j.survophthal.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Steel 2014.Steel D. Retinal detachment. Clinical Evidence. 2014;3:710. [PMC free article] [PubMed] [Google Scholar]
- Stuart 2014.Stuart A. [accessed 12 November 2014];Pseudophakic retinal detachment repair: understand the options. www.aao.org/publications/eyenet/201105/retina3.cfm.
- Znaor 2012.Znaor L, Medic A, Marin J, Binder S, Lukic I, George J. Pars plana vitrectomy versus scleral buckle for repairing simple rhegmatogenous retinal detachments. Cochrane Database of Systematic Reviews. 2012;(1) doi: 10.1002/14651858.CD009562. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Ramchand 2010.Ramchand K, Hatef E, Sena DF, Fallano KA, Do DV. Pneumatic retinopexy versus scleral buckle for repairing simple rhegmatogenous retinal detachments. Cochrane Database of Systematic Reviews. 2010;(2) doi: 10.1002/14651858.CD008350. [DOI] [PMC free article] [PubMed] [Google Scholar]