Abstract
Patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) not fit for intensive treatment need novel therapy options. Vascular endothelial growth factor (VEGF) receptor inhibition is one potential mechanism by which AML and MDS could be treated. The receptor tyrosine kinase inhibitor AZD2171 (cediranib) has activity against VEGF receptors KDR and FLT-1. This multicenter phase II study was designed to test cediranib's activity in patients with AML or high-risk MDS. The primary endpoint was confirmed disease response defined as a composite of complete remission, partial remission or hematologic improvement. The study enrolled 23 subjects in the AML cohort and 16 subjects in the MDS cohort. There were no confirmed responses in either group. Since the study met the stopping rule after the first stage of enrollment, the trial was closed to further accrual. Common adverse events in both cohorts included thrombocytopenia, neutropenia, anemia, fatigue, dyspnea, diarrhea, nausea and dehydration.
Keywords: Myeloid leukemias and dysplasias, pharmacotherapeutics, signal transduction
Introduction
Acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) are clonal disorders of hematopoietic stem cells characterized by a hypercellular bone marrow, increased blast count and the presence of circulating blasts along with peripheral blood cytopenias [1,2]. There are a variety of treatment and management strategies for these diseases. Otherwise healthy patients with AML can be treated with intensive induction chemotherapy using an anthracycline and cytarabine. If remission is achieved, patients will then move on to consolidation chemotherapy, often with cytarabine, or an allogeneic stem cell transplant. Less fit patients with AML can be treated with off-label approaches such as the hypomethylating drugs azacitidine or decitabine, or with the chemotherapy agent clofarabine. A supportive care and transfusion-only plan may be suitable for many patients who are older or who have a poor performance status [3]. Treatment of MDS generally relies on the Food and Drug Administration (FDA)-approved drug therapies azacitidine, decitabine and lenalidomide as well as growth factor support and transfusions. Hematopoietic cell transplant is potentially curative for patients with MDS, but the procedure requires a human leukocyte antigen (HLA)-matched donor and is unsuitable for a majority of patients because of advanced age and comorbidities. Therefore, there is a recognized need to develop new treatments for AML and MDS that are both effective and well tolerated, particularly for older patients.
It has been demonstrated that growth of AML is dependent upon close interactions with leukemic blasts and stromal cells within the bone marrow microenvironment. An increased number of endothelial cells are present in AML bone marrow biopsy samples when compared with patients who do not have leukemia [4]. AML blasts have been shown to secrete vascular endothelial growth factor (VEGF), and also express VEGF receptors, creating a potential autocrine loop. AML blasts express receptors for VEGF receptor-2 (VEGFR2) in 10 – 20% of cases [5]. These receptors may be involved in leukemic cell proliferation and resistance to apoptosis by responding to growth stimuli provided by endothelial cells from the bone marrow microenvironment [6,7]. Two high-affi nity receptors for VEGF with associated tyrosine kinase activity have been identified, KDR (kinase insert domain-containing receptor = VEGFR2) and Flt-1 (FMS-like tyrosine kinase 1 = VEGFR1).
AZD2171 (cediranib) has been developed as an orally available, small molecule receptor tyrosine kinase (RTK) inhibitor. The compound has activity against the VEGF receptors KDR and Flt-1, with a 50% inhibitory concentration (IC 50) of < 2 nM and 5 nM in vitro, respectively [8]. It has been active in a number of solid tumor preclinical models and human clinical trials, and is being evaluated in several ongoing trials in combination with cytotoxic chemotherapy. Commonly reported side effects include nausea, fatigue, diarrhea and hypertension [9].
Given the VEGF pathway's involvement with proliferation and resistance to apoptosis in myeloid diseases, we designed and conducted this single-agent phase II study to evaluate the activity of cediranib in patients with relapsed/refractory AML, in selected patients with untreated AML, and in patients with high-risk MDS.
Materials and methods
Patient eligibility
Adults older than 18 years with histologically confirmed AML or high grade MDS were eligible. Patients with AML with good risk inv(16), t(8;21) or t(15;17) cytogenetics were eligible if in second or greater relapse. Patients with t(15;17) must have failed all-trans retinoic acid (ATRA) and arsenic therapy, with progression within 12 months of treatment. Any patient with AML who did not achieve complete remission (CR) after two induction regimens was eligible. Previously untreated patients with AML older than 60 years were eligible for enrollment if the treating physician deemed them unfit for intensive induction chemotherapy.
Patients with MDS were eligible if they had intermediate-2 or high risk disease based on an International Prognostic Scoring System (IPSS) score of 1.5 or higher [10].
All subjects were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2. Subjects also had to have a normal bilirubin, aspartate aminotransferase (AST; or serum glutamic oxaloacetic transaminase [SGOT]) and alanine aminotransferase (ALT; or serum glutamic pyruvic transaminase [SGPT]) ≤ 2.5 × the institutional normal limit, and a normal creatinine or glomerular filtration rate (GFR) > 60 mL/min/1.73 m 2 . Women of childbearing potential were required to have a negative serum pregnancy test within 7 days of registration. Any prior chemotherapy or growth factor support had to be completed more than 4 weeks prior to registration. Potential subjects with disseminated intravascular coagulation, allergy to compounds similar to cediranib, a mean QTc > 500 ms or history of familial long QT syndrome, human immunodeficiency virus (HIV) positive status or ejection fraction < 45% were not eligible for enrollment. Because proteinuria was seen in animal studies of AZD2171 and is a known class effect of other antiangiogenic agents, greater than 1 + proteinuria on two consecutive urinalyses taken more than 1 week apart at baseline was an exclusion criterion. In addition, potential subjects with active central nervous system (CNS) leukemia, those with symptomatic leukostasis requiring leukapheresis and those with any other uncontrolled intercurrent illness were not eligible for enrollment. Baseline patient characteristics are listed in Table I.
Table I.
Baseline patient characteristics.
| AML (n = 23) | MDS (n = 16) | Total (n = 39) | |
|---|---|---|---|
| Age (years) | |||
| n | 23 | 16 | 39 |
| Median | 70 | 73 | 71 |
| Range | (61.0-86.0) | (61.0-87.0) | (61.0-87.0) |
| Gender | |||
| Female | 12 (52.2%) | 6 (37.5%) | 18 (46.2%) |
| Male | 11 (47.8%) | 10 (62.5%) | 21 (53.8%) |
| ECOG performance score | |||
| 0 | 4 (17.4%) | 4 (25.0%) | 8 (20.5%) |
| 1 | 12 (52.2%) | 11 (68.8%) | 23 (59.0%) |
| 2 | 7 (30.4%) | 1 (6.3%) | 8 (20.5%) |
| Race | |||
| White | 23 (100.0%) | 16 (100.0%) | 39 (100.0%) |
| Months from bone marrow diagnosis to on-study | |||
| n | 23 | 16 | 39 |
| Median | 1.1 | 17.6 | 8.7 |
| Range | (–0.4-42.4) | (0.4-87.9) | (–0.4-87.9) |
| Associated diseases | |||
| Yes | 7 (30.4%) | 1 (6.3%) | 8 (20.5%) |
| No | 16 (69.6%) | 15 (93.8%) | 31 (79.5%) |
| Complication hemorrhage within last 3 months | |||
| Yes | 1 (4.3%) | 0 (0.0%) | 1 (2.6%) |
| No | 22 (95.7%) | 16 (100.0%) | 38 (97.4%) |
| Infection within last 3 months | |||
| Yes | 11 (47.8%) | 1 (6.3%) | 12 (30.8%) |
| No | 12 (52.2%) | 15 (93.8%) | 27 (69.2%) |
| Hematopoietic diagnosis WHO | |||
| Not WHO AML classified at diagnosis, relapsed | 1 (4.3%) | 0 (0.0%) | 1 (2.6%) |
| AML with t(8;21)(q22;q22), (AML1/ETO) | 1 (4.3%) | 0 (0.0%) | 1 (2.6%) |
| AML with abnormal bone marrow eosinophils | 1 (4.3%) | 0 (0.0%) | 1 (2.6%) |
| Following MDS or MDS/MPD | 3 (13.0%) | 0 (0.0%) | 3 (7.7%) |
| Alkylating agent/radiation-related type | 1 (4.3%) | 0 (0.0%) | 1 (2.6%) |
| AML without maturation | 4 (17.4%) | 0 (0.0%) | 4 (10.3%) |
| AML with maturation | 7 (30.4%) | 0 (0.0%) | 7 (17.9%) |
| Acute myelomonocytic leukemia | 4 (17.4%) | 0 (0.0%) | 4 (10.3%) |
| Acute monoblastic/acute monocytic leukemia | 1 (4.3%) | 0 (0.0%) | 1 (2.6%) |
| Refractory cytopenia with multilineage dysplasia | 0 (0.0%) | 2 (12.5%) | 2 (5.1%) |
| Refractory anemia with excess blasts-1 (RAEB-1) | 0 (0.0%) | 5 (31.3%) | 5 (12.8%) |
| Refractory anemia with excess blasts-2 (RAEB-2) | 0 (0.0%) | 3 (18.8%) | 3 (7.7%) |
| Myelodysplastic syndrome, unclassified (MDS-U) | 0 (0.0%) | 6 (37.5%) | 6 (15.4%) |
| Prior stem transplant | |||
| No | 23 (100.0%) | 16 (100.0%) | 39 (100.0%) |
| Cytogenetic risk group | |||
| 4 (17.4%) | 3 (18.8%) | 7 (17.9%) | |
| Intermediate | 8 (34.8%) | 6 (37.5%) | 14 (35.9%) |
| High | 9 (39.1%) | 7 (43.8%) | 16 (41.0%) |
| Other | 2 (8.7%) | 0 (0.0%) | 2 (5.1%) |
| Stage of treatment | |||
| De novo AML | 11 (47.8%) | ||
| Relapsed/refractory disease | 12 (52.2%) | ||
| IPSS risk group | |||
| Intermediate-2 | 10 (62.5%) | ||
| High risk | 6 (37.5%) |
ECOG, Eastern Cooperative Oncology Group; WHO, World Health Organization; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; IPSS, International Prognostic Scoring System.
The study was a multicenter phase II trial conducted through the Mayo Clinic-led Phase 2 Consortium (P2C). The protocol was approved by the Institutional Review Board (IRB) at each of the participating institutions.
Study treatment
Subjects with AML were initially treated with cediranib 45 mg by mouth once daily. Due to toxicities seen in the first seven subjects, the starting dose was decreased to 30 mg daily for the remaining 16 subjects with AML. All 16 subjects with MDS began treatment with the 30 mg daily dose. Treatment cycle length was 28 days with continuous dosing. Subjects could remain on treatment for up to 26 cycles or until one of the following events: disease progression; intercurrent illness that prevented further therapy; unacceptable adverse events; the subject's decision to withdraw; or the treating investigator's decision to withdraw the subject from study. Dose delays and modifications were pre-specified based on the observation of hematologic toxicities or grade 3 or higher non-hematologic adverse events (AEs) related to study drug. Given the known association between VEGF pathway inhibitors and hypertension, the treatment protocol specified the use of antihypertensive therapy for grade 2 hypertension and dose delays/modifications in the event of grade 3 hypertension. Subjects experiencing grade 4 hypertension were withdrawn from the trial. Proteinuria was assessed during treatment, and dose adjustments were made or subjects withdrawn in the event of persistent proteinuria. All toxicities were graded based on the Common Terminology Criteria for Adverse Events v3.0 (CTCAE v3.0).
Statistical analysis
Subjects with AML and MDS were analyzed separately as pre-specified in the protocol. The primary endpoint was confirmed disease response noted on two consecutive evaluations performed at least 8 weeks apart. Response could be complete remission (CR), partial remission (PR) or hemato-logic improvement (HI) as defined by International Working Group (IWG) criteria [11,12]. Secondary endpoints included toxicity, response duration, time to treatment failure (TTF), overall survival (OS) and hematologic response. TTF and OS were evaluated using the Kaplan – Meier method.
The study used a two-stage design for each of the two patient groups (AML and MDS). A minimum of 16 and a maximum of 30 evaluable subjects could be enrolled in each group, with a planned interim analysis 6 months after the 16th subject enrolled. Subjects with AML treated at the initial 45 mg daily dose were not included in the final stopping rule decision, but their results are reported here in full. The largest response rate that would cause the regimen to be considered ineffective was 10%, and the smallest response rate that would be considered worthy of subsequent study was 30%. The decision rule to continue enrollment after interim analysis was based on a modified Fleming design [13]. If two or more of the first 16 subjects were classified as confirmed responders, then accrual to a total of 30 patients in that group would continue. If one or no subjects had a confirmed response, then the treatment would be deemed ineffective in that population, and accrual would be stopped.
Results
Patient characteristics and treatment AML cohort
A total of 23 subjects with AML were enrolled, and all were evaluable. The baseline characteristics are described in Table I. Median baseline marrow blasts were 30% (range 6 – 89%). Prior therapy before study enrollment included azacitidine (n = 3), induction chemotherapy (n = 12) and no prior treatment (n = 11). The first seven patients with AML were treated with 45 mg daily, although the study was amended to use a 30 mg initial daily dose due to grade 3 toxicity in four of seven patients (one mucositis, two fatigue, one hypertension). The subsequent 16 subjects were initially treated with the 30 mg daily dose. Subjects received a median of 1 treatment cycle (range 1 – 5 cycles). A total of 39 cycles were administered. One cycle was delayed due to proteinuria, and one cycle was delayed due to mucositis and resolved renal insuffi ciency due to urosepsis. Two subjects had dose reductions, one for grade 4 thrombocytopenia and one for proteinuria. Subjects discontinued therapy for alternative treatment (n = 6), disease progression (n = 6), adverse events (n = 4), other medical problems (n = 1), and the treating physician's discretion (n = 6).
MDS cohort
A total of 16 subjects with MDS were enrolled, and all were evaluable. All 16 subjects with MDS were treated with 30 mg daily. Baseline cytogenetic risk and IPSS risk groups are listed in Table I. Six subjects had no previous therapy for MDS. Ten subjects had received prior treatment including azacitidine (n = 7), decitabine (n = 2), cytarabine (n = 2), erythropoietin-stimulating agents (n = 2) and lenalidomide (n = 1). Median baseline marrow blasts were 12% (range 2 – 18%). Subjects received a median of 2 treatment cycles (range 1 – 11 cycles). A total of 45 cycles were administered. Five subjects had a total of seven treatment delays due to the following reasons: grade 4 neutropenia (n = 2), proteinuria (n = 2), nausea (n = 1), diarrhea (n = 1) and patient choice (n = 1). Six subjects had a total of seven dose reductions for the following reasons: grade 4 neutropenia (n = 1), dyspnea (n = 2), diarrhea (n = 1), proteinuria (n = 2) and muscle weakness (n = 1). Subjects discontinued therapy for disease progression (n = 4), adverse event (n = 2), patient refusal of further therapy (n = 6) and the treating physician's discretion (n = 4).
Responses
AML cohort
Twelve of the 23 (52%) subjects with AML completed 4 weeks of therapy and underwent a bone marrow aspirate and biopsy after cycle 1. Three of these subjects completed three cycles of therapy and underwent another bone marrow aspirate and biopsy at 3 months. None of these subjects showed a response. Of the 11 subjects who did not complete the first treatment cycle, four came off study and were treated with further non-protocol therapy; three experienced disease progression and received no additional treatment; and four experienced AEs including infection, fatigue, hematuria and the development of granulocytic sarcoma. Three subjects (13%) were alive and 20 subjects (87%) died. Figure 1 shows overall survival in all patients. Figure 2 shows overall survival by AML and MDS cohort. Subjects still alive at last follow-up had survival times of 35.6 months, 36.5 months and 41.7 months since study enrollment. All subjects (100%) who died had progressive disease. Median OS was 3.7 months (95% confidence interval [CI]: 2.2 – 5.7). Median time to treatment failure was 0.9 months (95% CI: 0.5 – 1.2). Figure 3 shows time to treatment failure in all patients. Figure 4 shows time to treatment failure by disease cohort. Since the study met the stopping rule after the first stage of enrollment, no further subjects were accrued after 23 subjects in the AML cohort.
Figure 1.
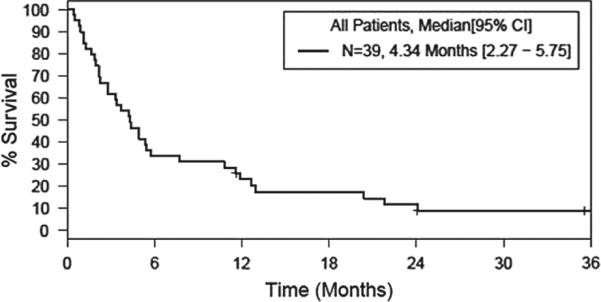
Overall survival, all patients.
Figure 2.

Overall survival by disease group.
Figure 3.
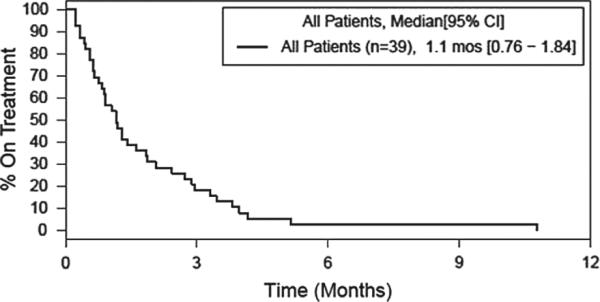
Time to treatment failure, all patients.
Figure 4.
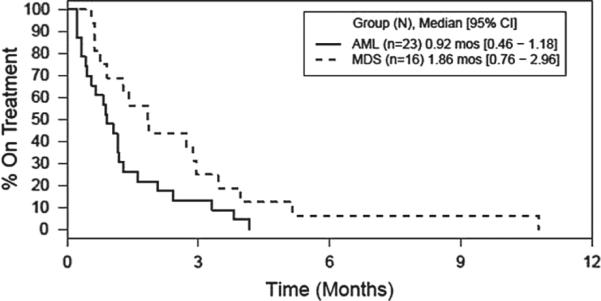
Time to treatment failure by disease group.
MDS cohort
Eleven of the 16 (69%) subjects with MDS completed 4 weeks of therapy and underwent a bone marrow aspirate and biopsy after cycle 1. Two of these subjects completed three cycles of therapy and underwent an additional bone marrow aspirate and biopsy at 3 months. None of the subjects showed a response. One subject (6.3%) was alive and 15 subjects (93.7%) died. The subject still alive at last follow-up had survived for 11.7 months after study enrollment. Thirteen of 15 subjects (86.7%) died of progressive MDS. One subject died of myocardial infarction/cardiac arrest and one subject died after a fall and subsequent hemorrhagic stroke. Median OS was 4.7 months (95% CI: 2.0 – 10.8). Median time to treatment failure was 1.9 months (95% CI: 0.8 – 3.0). Since the study met the stopping rule after the first stage of enrollment, no further subjects were accrued after 16 subjects in the MDS cohort. Best blast responses for both the AML and MDS cohorts are listed in Table II.
Table II.
Best blast response in subjects with AML and MDS.
| Group | Baseline BM biopsy (%) | Minimum bone marrow blasts (%) | Greatest % change from baseline |
|---|---|---|---|
| AML | 42 | 16 | –61.9 |
| AML | 54 | 27 | –50 |
| AML | 23 | 16 | –30.43 |
| AML | 82 | 65 | –20.73 |
| AML | 20 | 16 | –20 |
| AML | 85 | 76 | –10.59 |
| AML | 25 | 24 | –4 |
| AML | 29 | 42 | 44.83 |
| AML | 30 | 50 | 66.67 |
| AML | 6 | 13 | 116.67 |
| AML | 25 | 57 | 128 |
| AML | 25 | 77 | 208 |
| MDS | 10 | 4 | –60 |
| MDS | 12 | 8 | –33.33 |
| MDS | 15 | 10 | –33.33 |
| MDS | 15 | 11 | –26.67 |
| MDS | 9 | 8 | –11.11 |
| MDS | 2 | 2 | 0 |
| MDS | 6 | 6 | 0 |
| MDS | 12 | 15 | 25 |
| MDS | 15 | 19 | 26.67 |
| MDS | 2 | 3 | 50 |
| MDS | 18 | 55 | 205.56 |
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; BM, bone marrow.
Adverse events and toxicity
AML cohort
In 23 subjects with AML treated with either the 45 mg or 30 mg daily dose, grade 3 – 4 toxicities that were at least possibly related to cediranib included neutropenia (n = 2), thrombocytopenia (n = 8), anemia (n = 1), fatigue (n = 3), asthenia (n = 1), mucositis (n = 1), diarrhea (n = 2), proteinuria (n = 1), dyspnea (n = 1), leukopenia (n = 1) and hypertension (n = 1). Of the first seven subjects treated with the 45 mg daily dose, four experienced grade 3 toxicities (one mucositis, two fatigue, one hypertension). Therefore, the study was amended to use a 30 mg daily starting dose for the subsequent 16 subjects enrolled.
MDS cohort
In 16 subjects with MDS treated with the 30 mg daily dose, grade 3 – 4 toxicities that were at least possibly related to cediranib included anemia (n = 2), neutropenia (n = 4), thrombocytopenia (n = 6), leukopenia (n = 1), asthenia (n = 1), hypertension (n = 1), dyspnea (n = 3), fatigue (n = 4), dehydration (n = 2), diarrhea (n = 2) and nausea (n = 2). Adverse events for both the AML and MDS cohorts are listed in Table III.
Table III.
Adverse events in subjects with AML and MDS*, deemed to be at least possibly related.
| Patients with at least one | Arm | n | % |
|---|---|---|---|
| Grade 3 + adverse event | AML | 14 | 60.9 |
| MDS | 11 | 68.8 | |
| Grade 4 + adverse event | AML | 7 | 30.4 |
| MDS | 5 | 31.3 | |
| Grade 3 + hem adverse event | AML | 10 | 43.5 |
| MDS | 7 | 43.8 | |
| Grade 4 + hem adverse event | AML | 7 | 30.4 |
| MDS | 5 | 31.3 | |
| Grade 3 + non-hem adverse event | AML | 7 | 30.4 |
| MDS | 37.5 |
| Grade |
|||||
|---|---|---|---|---|---|
| Adverse event Type | 3 |
4 |
|||
| Arm | n | % | n | % | |
| Platelet count decreased | AML | 2 | 8.7 | 6 | 26.1 |
| MDS | 2 | 12.5 | 4 | 25 | |
| Fatigue | AML | 3 | 13 | ||
| MDS | 4 | 25 | |||
| Neutrophil count decreased | AML | 1 | 4.3 | 1 | 4.3 |
| MDS | 2 | 12.5 | 2 | 12.5 | |
| Diarrhea | AML | 2 | 8.7 | ||
| MDS | 2 | 12.5 | |||
| Dyspnea | AML | 1 | 4.3 | ||
| MDS | 3 | 18.8 | |||
| Hemoglobin decreased | AML | 1 | 4.3 | ||
| MDS | 2 | 12.5 | |||
| Dehydration | MDS | 2 | 12.5 | ||
| Hypertension | AML | 1 | 4.3 | ||
| MDS | 1 | 6.3 | |||
| Leukocyte count decreased | AML | 1 | 4.3 | ||
| MDS | 1 | 6.3 | |||
| Muscle weakness | AML | 1 | 4.3 | ||
| MDS | 1 | 6.3 | |||
| Nausea | MDS | 2 | 12.5 | ||
| Mucositis oral | AML | 1 | 4.3 | ||
| Protein urine positive | AML | 1 | 4.3 | ||
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; hem, hematologic.
Evaluable subjects: 23 in AML arm, 16 in MDS arm.
Discussion
Ever since Dr. Judah Folkman's seminal article describing “ tumor angiogenesis factor (TAF), ” inhibition of new blood vessel formation has been studied and used to treat a variety of cancers [14,15]. Canonical angiogenesis inhibitors work by binding to and inhibiting VEGF, the VEGFR or the RTKs involved with VEGF signaling. Examples of approved angiogenesis inhibitors and the cancers they treat include the VEGF inhibitor bevacizumab (colorectal cancer, glioblastoma, non-small cell lung cancer), the VEGFR inhibitor aflibercept (colorectal cancer) and the RTK inhibitors sunitinib (renal cell cancer) and sorafenib (renal cell cancer, hepatocellular cancer) [15]. A significant challenge to the clinical development of angiogenesis inhibitors is the current lack of established in vivo effi cacy measures. However, there are several ongoing avenues of biomarker research, including measuring circulating endothelial cells, hyper-tensive response and assessment of molecular and imaging responses [16].
There have been previously published MDS/AML studies that incorporate small molecule inhibitors of VEGFRs. In a phase II study using the RTK inhibitor SU5416 as a single agent, 33 patients with relapsed/refractory AML and 22 patients with MDS were treated with twice-weekly infusions. Three patients (5%) achieved a PR and one patient (2%) had HI. Median OS was 12 weeks for the AML cohort and was not reached for the MDS group [17]. In a separate study, SU5416 was administered to 43 patients with refractory AML or in untreated patients deemed unfit for intensive induction chemotherapy. Seven patients (16%) achieved a PR. Those patients whose blasts expressed higher levels of VEGF mRNA were observed to have a higher response rate and reduced bone marrow microvessel density [18].
The monoclonal antibody bevacizumab has been used in conjunction with chemotherapy to treat AML. In a single-arm phase II study, 48 adults with relapsed/refractory AML were given bevacizumab on day 8, after receiving cytarabine and mitoxantrone in a timed sequential therapy (TST) approach. The overall response rate was 48%, with 33% achieving CR. Median OS was 16 months for those who went into remission, while median disease-free survival (DFS) was 7 months. At 1 year, OS was 64% and DFS was 35%, comparing favorably to historical controls [7]. In a separate, randomized phase II trial conducted by the Dutch – Belgian and Swiss cooperative groups, 171 patients older than 60 years with untreated AML or high-grade MDS were randomized to treatment with daunorubicin and cytarabine ± bevacizumab on day 1 and day 15. Induction treatment was then followed by twice-daily cytarabine for 6 days ± bevacizumab. The investigators showed no difference in CR rate (65% each group), EFS at 12 months (33% standard arm vs. 30% bevacizumab arm) or EFS at 24 months (22% standard arm vs. 16% bevacizumab arm). Overall, their conclusions were that the addition of bevacizumab to standard chemotherapy did not improve outcomes in this patient population [19].
Bevacizumab has also been studied as a single-agent treatment in patients with MDS. In a phase II study, Legros and colleagues enrolled 21 patients with MDS and treated them with single-agent bevacizumab using a dose of 5 mg/ kg intravenously every 2 weeks for 12 weeks [20]. The study showed that therapy was well tolerated. Furthermore, correlative studies showed expected bevacizumab-induced effects including decreased plasma VEGF levels and reduced bone marrow vessel density. However, the clinical response rate was disappointing, with only one patient (5%) showing transfusion independence [20].
The Alliance conducted a single-agent phase II trial of the VEGFR tyrosine kinase inhibitor vatalanib in patients with MDS. Altogether 155 patients were treated in two dose-defined cohorts (a 1250 mg daily and 750 – 1250 mg daily dose-escalation cohort) for a 28-day treatment cycle. Hematologic improvement occurred in seven patients (5%), and all responders were on study drug for at least 3 months. Toxici-ties were significant, and included fatigue, nausea, vomiting, dizziness and anorexia [21].
Taken as a whole, the experience with single-agent therapies to inhibit angiogenesis has been disappointing for the treatment of AML and MDS. While angiogenesis is important for the pathogenesis of these diseases, as described in the “ Introduction, ” the complexity of MDS and AML biology (including key self-renewal pathways and uncontrolled cellular proliferation) makes it unlikely that targeting the VEGF pathway alone will become a clinically useful strategy. Combining angiogenesis inhibition with cytotoxic chemotherapy or other targeted agents (such as FLT3 inhibitors or agents that affect leukemia stem cell maintenance) could be considered in future trials.
In the present study, there were no objective responses to single-agent cediranib treatment in either the AML or MDS treatment arms. The toxicities seen in these patient populations using the 45 mg and 30 mg daily dosing regimens were significant, particularly with regard to thrombocytopenia (grade 3 – 4 in 35% of subjects with AML and 38% of subjects with MDS). The lack of response seen in this trial does not justify further development of cediranib as a single agent in patients with AML or MDS.
Acknowledgements
This trial was supported by the National Cancer Institute, N01-CM-2011-00099 and CA15083. The authors would like to thank Adam Pettinger for providing biostatistical support and Emery Bresnick for critical review and comments.
Footnotes
Potential conflict of interest:
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–1885. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 3.O'Donnell MR, Abboud CN, Altman J, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984–1021. doi: 10.6004/jnccn.2012.0103. [DOI] [PubMed] [Google Scholar]
- 4.Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95:309–313. [PubMed] [Google Scholar]
- 5.Fiedler W, Graeven U, Ergun S, et al. Vascular endothelial growth factor, a possible paracrine growth factor in human acute myeloid leukemia. Blood. 1997;89:1870–1875. [PubMed] [Google Scholar]
- 6.Dias S, Shmelkov SV, Lam G, et al. VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood. 2002;99:2532–2540. doi: 10.1182/blood.v99.7.2532. [DOI] [PubMed] [Google Scholar]
- 7.Karp JE, Gojo I, Pili R, et al. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res. 2004;10:3577–3585. doi: 10.1158/1078-0432.CCR-03-0627. [DOI] [PubMed] [Google Scholar]
- 8.Hennequin LF, Ple P, Stokes ES, et al. Structure-activity relationship, physicochemical and pharmacokinetic properties of AZD2171: a highly potent inhibitor of VEGF receptor tyrosine kinases. Proc Am Assoc Cancer Res. 2004 Abstract 1048. [Google Scholar]
- 9.Sahade M, Caparelli F, Hoff PM. Cediranib: a VEGF receptor tyrosine kinase inhibitor. Future Oncol. 2012;8:775–781. doi: 10.2217/fon.12.73. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 11.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 14.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 15.Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson PM, LaBonte MJ, Lenz HJ. Assessing the in vivo effi cacy of biologic antiangiogenic therapies. Cancer Chemother Pharmacol. 2013;71:1–12. doi: 10.1007/s00280-012-1978-8. [DOI] [PubMed] [Google Scholar]
- 17.Giles FJ, Stopeck AT, Silverman LR, et al. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood. 2003;102:795–801. doi: 10.1182/blood-2002-10-3023. [DOI] [PubMed] [Google Scholar]
- 18.Fiedler W, Mesters R, Tinnefeld H, et al. A phase 2 clinical study of SU5416 in patients with refractory acute myeloid leukemia. Blood. 2003;102:2763–2767. doi: 10.1182/blood-2002-10-2998. [DOI] [PubMed] [Google Scholar]
- 19.Ossenkoppele GJ, Stussi G, Maertens J, et al. Addition of bevacizumab to chemotherapy in acute myeloid leukemia at older age: a randomized phase 2 trial of the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON) and the Swiss Group for Clinical Cancer Research (SAKK). Blood. 2012;120:4706–4711. doi: 10.1182/blood-2012-04-420596. [DOI] [PubMed] [Google Scholar]
- 20.Legros L, Slama B, Karsenti JM, et al. Treatment of myelodysplastic syndromes with excess of blasts by bevacizumab is well tolerated and is associated with a decrease of VEGF plasma level. Ann Hematol. 2012;91:39–46. doi: 10.1007/s00277-011-1242-z. [DOI] [PubMed] [Google Scholar]
- 21.Gupta P, Mulkey F, Hasserjian RP, et al. A phase II study of the oral VEGF receptor tyrosine kinase inhibitor vatalanib (PTK787/ZK222584) in myelodysplastic syndrome: Cancer and Leukemia Group B study 10105 (Alliance). Invest New Drugs. 2013;31:1311–1320. doi: 10.1007/s10637-013-9978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


