Abstract
Interstitial fluid flow in and around the tumor tissue is a physiologically relevant mechanical signal that regulates intracellular signaling pathways throughout the tumor. Yet, the effects of interstitial flow and associated fluid shear stress on the tumor cell function have been largely overlooked. Using in vitro bioengineering models in conjunction with molecular cell biology tools, we found that fluid shear (2 dyn/cm2) markedly upregulates matrix metalloproteinase 12 (MMP-12) expression and its activity in human chondrosarcoma cells. MMP-12 expression is induced in human chondrocytes during malignant transformation. However, the signaling pathway regulating MMP-12 expression and its potential role in human chondrosarcoma cell invasion and metastasis have yet to be delineated. We discovered that fluid shear stress induces the synthesis of insulin growth factor-2 (IGF-2) and vascular endothelial growth factor (VEGF) B and D, which in turn transactivate MMP-12 via PI3-K, p38 and JNK signaling pathways. IGF-2-, VEGF-B- or VEGF-D-stimulated chondrosarcoma cells display markedly higher migratory and invasive potentials in vitro, which are blocked by inhibiting MMP-12, PI3-K, p38 or JNK activity. Moreover, recombinant human MMP-12 or MMP-12 overexpression can potentiate chondrosarcoma cell invasion in vitro and the lung colonization in vivo. By reconstructing and delineating the signaling pathway regulating MMP-12 activation, potential therapeutic strategies that interfere with chondrosarcoma cell invasion may be identified.
INTRODUCTION
Chondrosarcoma is a malignant bone tumor of chondrogenic origin. Interstitial fluid flow is a relevant mechanical signal in cartilage and bone (patho)physiology.1,2 Interstitial fluid flow in and around the normal or tumor tissue alters the local mechanical microenvironment and regulates intracellular signaling pathways. Jain and Chary3 have reported that interstitial fluid velocities in tumors vary between 0.1 and 2 µm/s, corresponding to fluid shear stresses of up to ~ 1 dyn/cm2.4 Others have measured fluid flow velocities in tumors of up to 4 µm/s.5 To date, the effects of interstitial flow and associated fluid shear on chondrosarcoma cell function have been largely overlooked.
Chondrosarcomas have the capacity to invade locally and generate metastases with a predilection for the lungs.6 Low or intermediate grade (I or II) chondrosarcoma have indolent growth and low metastatic potential, whereas grade III lesions exhibit high metastatic potential. Chondrosarcoma cell invasion into normal tissue is facilitated by the degradation of the extracellular matrix (ECM) within and around the tumor. The ECM of chondrosarcomas is comprised primarily of fibrillar collagens. Collagen types II and X correspond to a differentiated cell phenotype associated with good prognosis, whereas collagen type I is associated with a more proliferative, dedifferentiated phenotype and poorer clinical prognosis.7 Collagen degradation is mediated by the action of matrix metalloproteinases (MMPs), which comprise a multigene family with over 22 members. MMP-1, MMP-2, MMP-3, MMP-9 and MMP-13 are expressed in human chondrosarcoma cells.8 MMP-1 is the best-studied metalloproteinase in chondrosarcoma; it is upregulated in locally invasive tumors and its expression correlates with chondrosarcoma recurrence.9 It has also been reported that MMP-12 expression is induced in human chondrocytes during the malignant transformation.10 Yet, little is known regarding the potential contribution of MMP-12 to chondrosarcoma cell invasion.
MMP-12 is upregulated in human SW1353 chondrosarcoma cells upon treatment with growth factors or cytokines, such as transforming growth factor β or tumor necrosis factor α.10 Enhanced expression of MMP-12 is associated with glioma and endometrial adenocarcinoma cell invasion.11,12 More importantly, MMP-12 correlates with local recurrence and metastatic disease in non-small cell lung cancer patients.13 In contrast, MMP-12 expression by the carcinoma cells is associated with increased survival of colon cancer patients.14 In line with this outcome, Houghton et al.15 also reported that MMP-12 knock in suppresses lung metastasis of Lewis lung carcinoma and B16-F10 melanoma cells. Thus, MMP-12 exerts divergent effects on tumor metastasis that are tumor cell type specific.
In this study, we report that application of physiologically relevant fluid shear stress (2 dyn/cm2) markedly upregulates MMP-12 expression and its activity in human chondrosarcoma cells. We also delineated the signaling mechanism by which fluid shear activates MMP-12 and its effects on chondrosarcoma cell migration and invasion in vitro. Because chondrosarcoma display a predilection for metastasis to the lungs, we investigated the effects of recombinant human (rh)MMP-12 on the lung colonization in vivo using a tail vein injection model.
RESULTS
MMP-12 is markedly upregulated in human chondrosarcoma tissues and shear-activated chondrosarcoma cells
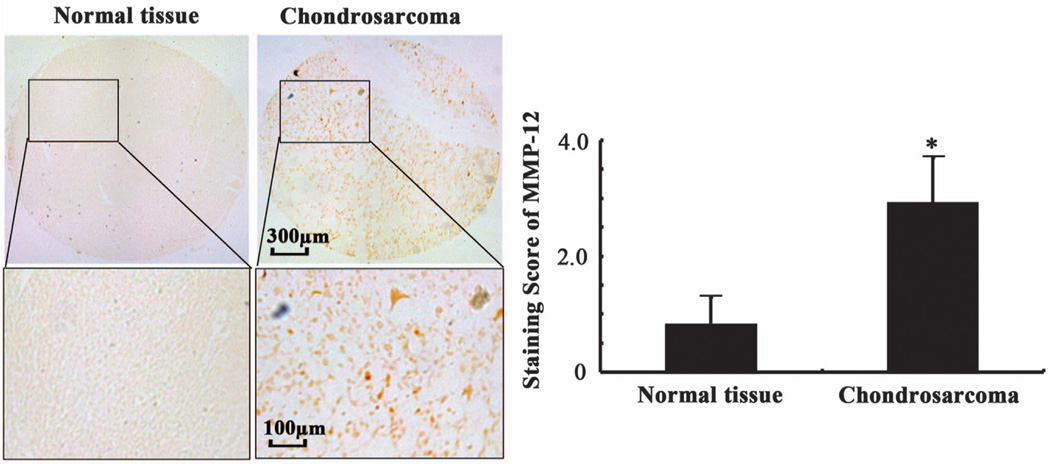
In light of prior data, suggesting that MMP-12 expression is induced in human chondrocytes during the fetal development and malignant transformation,10 we evaluated the expression levels of MMP-12 in human chondrosarcoma tissues relative to normal controls. As shown in Figure 1, MMP-12 immunostaining was detected in the cytoplasm and membrane of human chondrosarcomas. Importantly, MMP-12 expression was markedly (>4.5-fold) increased in human chondrosarcomas compared with normal tissues (Figure 1).
Figure 1.
MMP-12 expression is increased in human chondrosarcoma tissues relative to normal controls. MMP-12 immunoreactivity was determined by immunohistochemistry using an anti-MMP-12 antibody. These images are representative of six independent experiments, all revealing similar results. Lower panels show a close-up of the boxed areas. The intensity of MMP-12 immunostaining was determined using an immunohistochemical staining kit. Data represent the mean ± s.e.m. of six independent experiments. *P < 0.01 with respect to normal tissue specimens.
Fluid shear stress has been reported to influence the invasive potential of glioma cells through the modulation of MMP-1 and MMP-2,4 which degrade the ECM within and around the tumor. We thus investigated the effects of fluid shear on the regulation of MMPs in human chondrosarcoma cells. The human SW1353 chondrosarcoma cell line was chosen as a model system because it is the most commonly used cell line for bone tumor research.16 We also verified select data using Hs 819.T and CH2879 chondrosarcoma cells as models. Our data revealed that the messenger RNA (mRNA) expression levels of MMP-12 increased progressively with increasing shear stress, reaching a maximum at 2 dyn/cm2 after 48 h, and returned to baseline levels at 12–20 dyn/cm2 (Supplementary Table S1). Thus, subsequent experiments focused on analyzing cell responses after application of 2 dyn/cm2 for 48 h. Of all known MMPs, MMP-12 displayed the highest (35-fold) increase in the mRNA expression in shear-activated (2 dyn/cm2 for 48 h) human SW1353 chondrosarcoma cells (Supplementary Table S2). Smaller, yet significant, shear-mediated increases were detected in MMP-1 (eightfold) and MMP-7 (fivefold) mRNA levels (Supplementary Table S2). Interestingly, exposure of SW1353 cells to a higher shear stress level (20 dyn/cm2 for 48 h) failed to upregulate the mRNA levels of any MMP relative to static control specimens (Supplementary Table S2). Similar observations were made using Hs 819.T chondrosarcoma cells (Supplementary Table S3). In addition to the regulation of mRNA levels, exposure of human chondrosarcoma cells to low (2 dyn/cm2), but not high (20 dyn/cm2), shear stress markedly upregulated the protein expression and enzymatic activity of MMP-12 (Figure 2a). Collectively, these results suggest that fluid shear stress may contribute to chondrosarcoma cell invasion primarily via the induction of MMP-12.
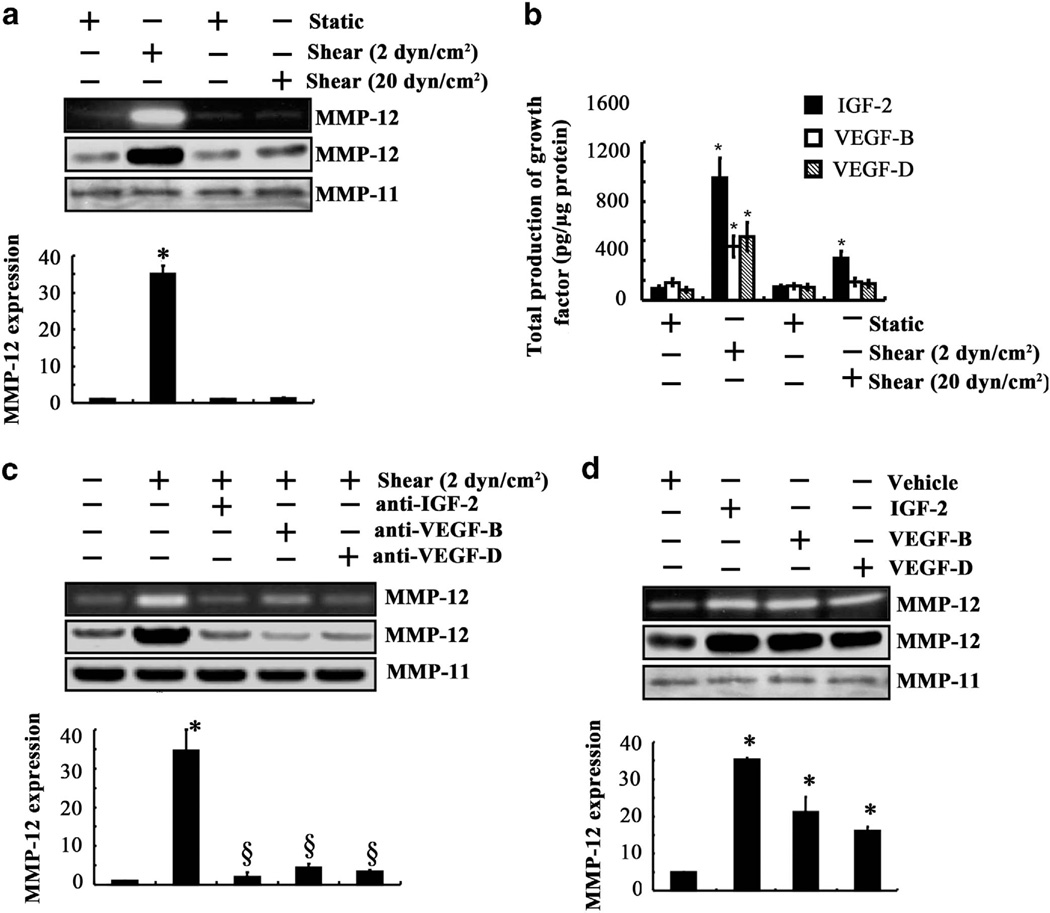
Figure 2.
IGF-2, VEGF-B and VEGF-D induced by fluid shear upregulate the mRNA, protein and enzymatic activity levels of MMP-12 in human chondrosarcoma cells. (a and b) SW1353 chondrosarcoma cells were exposed to either fluid shear stress (2 or 20 dyn/cm2) or static conditions (0 dyn/cm2) for 48 h. (c) In select experiments, cells were subjected to shear stress (2 dyn/cm2) or static conditions (0 dyn/cm2) for 48 h in the absence or presence of a blocking antibody (1 µg/ml) specific for IGF-2, VEGF-B or VEGF-D. (d) In other experiments, SW1353 cells were treated with exogenous IGF-2, VEGF-B or VEGF-D (100 ng/ml) for 48 h. (a, c and d) MMP-12 mRNA (bar graphs), protein and activity levels were determined by quantitative reverse transcription PCR (qRT-PCR), western blot and zymography, respectively. GAPDH and MMP-11 served as internal controls in qRT-PCR and western blot/zymography, respectively. These gels are representative of three experiments, all revealing similar results. (b) The production of shear-induced IGF-2, VEGF-B and VEGF-D was determined using enzyme immunoassay kits. Data represent the mean ± s.e.m. of three independent experiments. *P < 0.05 with respect to static or untreated control.
IGF-2, VEGF-B and VEGF-D mediate the shear-dependent activation of MMP-12 in human chondrosarcoma cells
Fluid shear stress (12 dyn/cm2) induces G2/M cell cycle arrest in human chondrosarcoma SW1353 cells.17 Along these lines, high (20 dyn/cm2), but not low (2 dyn/cm2), levels of fluid shear induce chondrocyte apoptosis.18,19 In view of these observations, we hypothesized that growth factors secreted by chondrosarcoma cells subjected to low levels of shear facilitate their survival and promote cell invasion via the upregulation of MMP-12. To test our hypothesis, we evaluated the effects of shear stress on the mRNA expression of different growth factors that have been implicated in chondrosarcoma cell invasion. Our analysis reveals that low (2 dyn/cm2), but not high (20 dyn/cm2), shear stress levels induced a pronounced increase in the mRNA expression levels of insulin growth factor-2 (IGF-2) (10-fold), vascular endothelial growth factor B (VEGF-B) and VEGF-D (3.5-fold) in human chondrosarcoma cells (Supplementary Table S4). Along these lines, low fluid shear stimulated IGF-2, VEGF-B and VEGF-D secretion (Figure 2b). As a next step, we assessed the influence of blocking antibodies specific for IGF-2, VEGF-B or VEGF-D on shear-induced MMP-12 expression and its activity. Our data reveal that the use of anti-IGF-2, anti-VEGF-B or anti-VEGF-D antibodies markedly inhibited the upregulation of MMP-12 mRNA, protein and activity levels in shear-activated SW1353 chondrosarcoma cells (Figure 2c). These blocking antibodies also suppressed the baseline levels of MMP-12 in static control chondrosarcoma cells (data not shown). To further establish the involvement of IGF-2, VEGF-B and VEGF-D in MMP-12 regulation, SW1353 cells were incubated with each of these growth factors for 48 h at a concentration of 100 ng/ml. As shown in Figure 2d, exogenously added IGF-2, VEGF-B or VEGF-D markedly induced MMP-12 mRNA, protein and activity levels. Taken altogether, our data suggest that MMP-12 induction in response to low shear stress proceeds through the upregulation of IGF-2, VEGF-B and VEGF-D in human chondrosarcoma cells.
Shear-induced and exogenously added IGF-2, VEGF-B and VEGF-D regulate MMP-12 expression and its activity via PI3-K, p38 and JNK signaling pathways in human chondrosarcoma cells
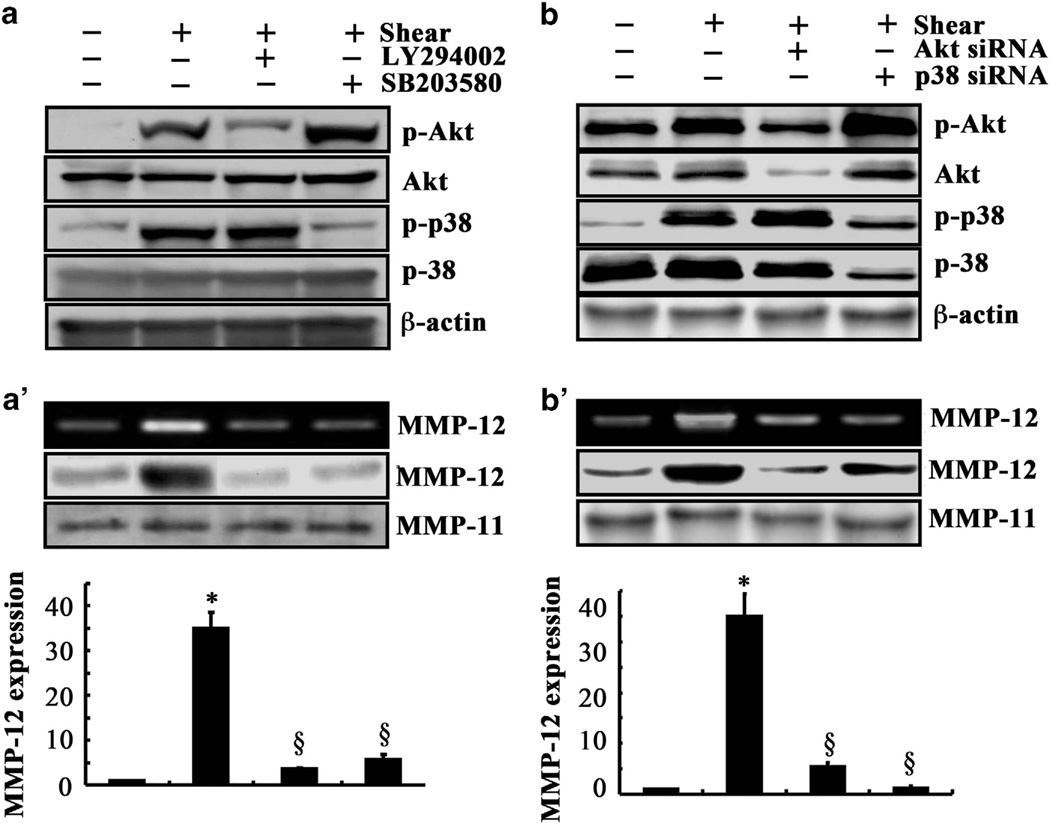
We next aimed to delineate the signaling mechanism of MMP-12 upregulation in sheared human chondrosarcoma cells. In view of our recent observations showing that shear-induced MMP-9 synthesis in human chondrocytes proceeds via PI3-K- and MAPK (ERK1/2, JNK)-dependent pathways,20 we examined the potential contribution of these signaling pathways to MMP-12 induction in sheared chondrosarcoma cells. Exposure of SW1353 cells to a shear stress level of 2 dyn/cm2 for 48 h augmented the phosphorylation levels of Akt at Ser 473 and p38 at Thr180/Tyr182 without affecting total Akt and p38 levels (Figure 3a). To establish the involvement of the PI3-K/Akt and p38 signaling pathways in shear-induced MMP-12 activation, SW1353 cells were treated with the selective PI3-K inhibitor LY294002 (10 µm) or the p38 inhibitor SB203580 (10 µm). LY294002 and SB203580 were effective in inhibiting the phosphorylation of Akt and p38, respectively, without altering their total expression levels (Figure 3a). They also markedly suppressed shear-induced MMP-12 mRNA, protein and activity levels (Figure 3a’). To verify the involvement of PI3-K/Akt and p38 pathways in MMP-12 induction in sheared chondrosarcoma cells, the experiments were carried out using SW1353 cells transfected with a small interfering RNA oligonucleotide sequence specific for Akt or p38, which effectively knocked down Akt or p38 expression, respectively, relatively to cells transfected with a scramble small interfering RNA control (Figure 3b). Depletion of Akt or p38 markedly attenuated shear-induced MMP-12 mRNA, protein and activity levels (Figure 3b’).
Figure 3.
Fluid shear regulates MMP-12 expression and its activity via PI3-K and p38 signaling pathways in human chondrosarcoma cells. (a and a’) SW1353 cells were exposed to either fluid shear (2 dyn/cm2) or static conditions (0 dyn/cm2) for 48 h in the absence or presence of the PI3-K inhibitor LY294002 (10 µm) or the p38 inhibitor SB203580 (10 µm). (b and b’) In select experiments, SW1353 cells were transfected with a small interfering RNA oligonucleotide sequence specific for Akt or p38 before being subjected to either fluid shear (2 dyn/cm2) or static conditions for 48 h. Phosphorylated Akt (Ser473) and p38 (Thr180/Tyr182) were detected by western blotting using specific antibodies. Equal loading in each lane is ensured by the similar intensities of total Akt, p38 and β-actin. MMP-12 mRNA (bar graphs), protein and activity levels were determined by quantitative reverse transcription PCR (qRT-PCR), western blot and zymography, respectively. GAPDH and MMP-11 served as internal controls in qRT-PCR and western blot/zymography, respectively. These gels are representative of three independent experiments, all revealing similar results. Data represent the mean ± s.e.m. of three experiments. *P < 0.05 relative to static control. §P < 0.05 compared with shear control.
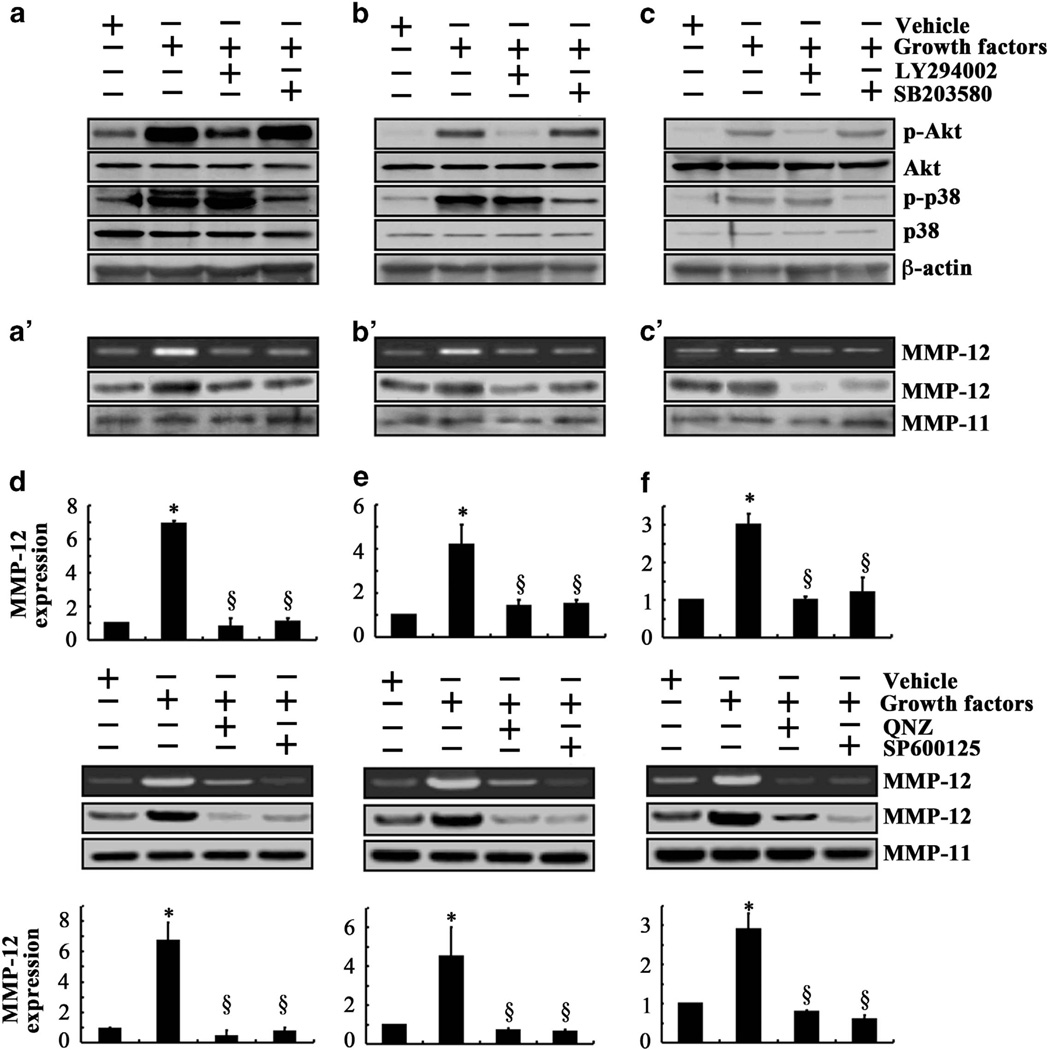
Given that the low fluid shear upregulates IGF-2, VEGF-B and VEGF-D expression, we sought to determine whether exogenously added IGF-2, VEGF-B and VEGF-D induce MMP-12 expression and its activity via PI3-K/Akt- and p38-dependent pathways. Chondrosarcoma cell treatment with each of the aforementioned growth factors augmented the phosphorylation levels of Akt at Ser 473 and p38 at Thr180/Tyr182 without affecting total Akt and p38 levels (Figures 4a–c). Treatment of SW1353 cells with the selective PI3-K inhibitor LY294002 or p38 inhibitor SB203580 blocked IGF-2, VEGF-B or VEGF-D induced phosphorylation of Akt and p38 (Figures 4a–c) and abrogated MMP-12 induction (Figures 4a’–c’).
Figure 4.
Exogenous IGF-2, VEGF-B and VEGF-D induce MMP-12 expression and its activity via PI3-K, p38 and JNK signaling pathways in human chondrosarcoma cells. SW1353 cells were treated with 100 ng/ml of IGF-2 (a and a’), VEGF-B (b and b’), VEGF-D (c and c’) or vehicle control in the absence or presence of the PI3-K inhibitor LY294002 (10 µm) or the p38 inhibitor SB203580 (10 µm). The levels of phospho-Akt (Ser473) and phospho-p38 (Thr180/Tyr182) in the cell lysates were determined by immunoblotting using specific antibodies. Equal loading in each lane is ensured by the similar intensities of total Akt, p38 and β-actin (a–c). In separate experiments, SW1353 cells were treated with 100 ng/ml of IGF-2 (d), VEGF-B (e), VEGF-D (f) or vehicle control in the absence or presence of the JNK inhibitor SP600125 (10 µm) or the NF-κB inhibitor quinazoline (1 µm). The protein expression of MMP-12 in the cell lysates was determined by western blotting. The enzymatic activity of MMP-12 in SW1353 cell cultured media was assessed by casein zymography. MMP-11 served as loading control (a’–c’ and d–f). Blots are representative of at least three independent experiments, all revealing similar results. MMP-12 mRNA levels (bar graphs) were determined by quantitative reverse transcription PCR using GAPDH as an internal control. Data represent the mean ± s.e.m. of at least three independent experiments. *P < 0.05 with respect to untreated control. §P < 0.05 compared with growth factor treatment in the absence of a pharmacological inhibitor.
In view of our recent observations showing the involvement of JNK and NF-κB signaling pathways in the induction of MMP-9 in chondrocytic cells,20 we examined their potential contributions to MMP-12 regulation in human chondrosarcoma cells. Treatment of SW1353 cells with the JNK inhibitor SP600125 (10 µm) or the NF-κB inhibitor 6-amino-4-(4-phenoxyphenylethylamino) quinazoline (1 µm) nearly abolished MMP-12 expression and its activity in response to IGF-2, VEGF-B or VEGF-D stimulation (Figures 4d–f). Collectively, our data suggest that low shear stress upregulates IGF-2, VEGF-B and VEGF-D expression, which in turn stimulate MMP-12 expression and activation via PI3-K-, p38-, JNK- and NF-κB-dependent pathways.
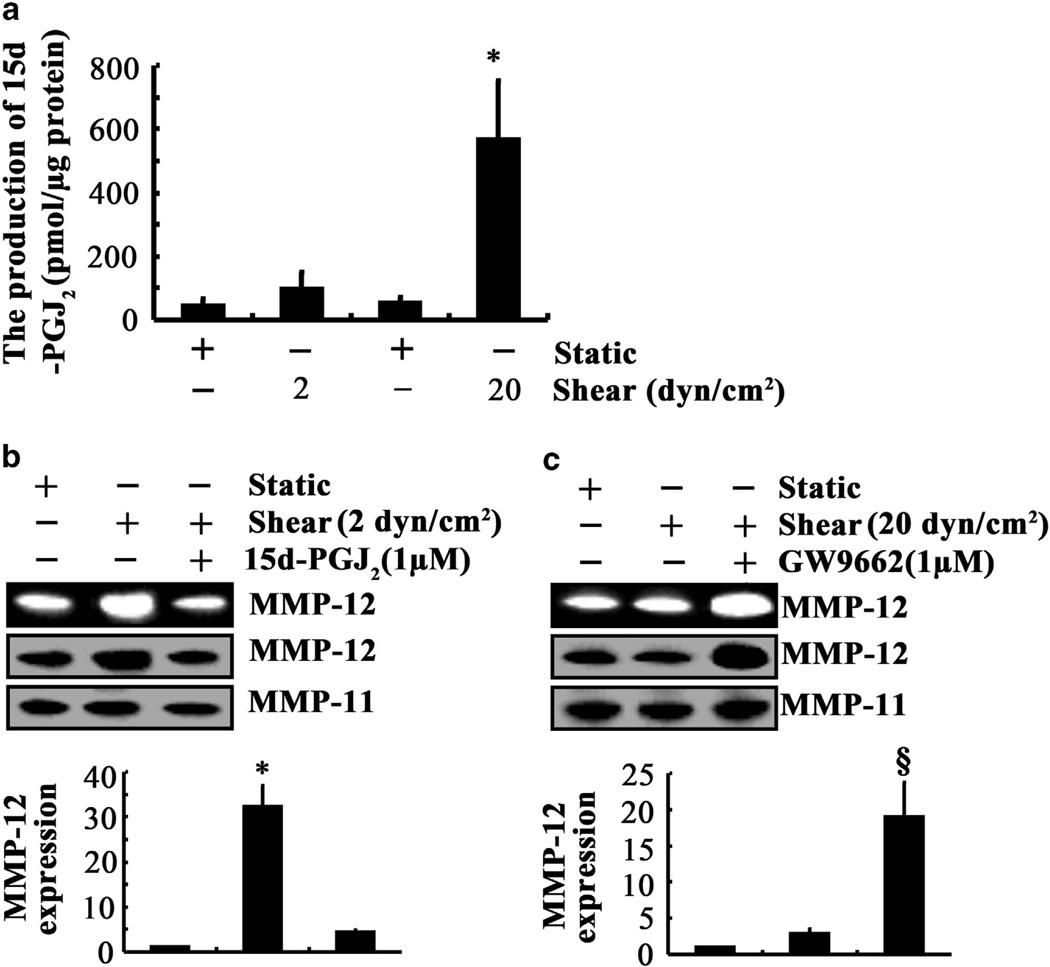
In contrast to low shear, high fluid shear stress fails to markedly upregulate MMP-12 expression in human chondrosarcoma cells (Figure 2a and Supplementary Tables S1). In light of prior findings showing that 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) exerts an antagonistic effect on cytokine-mediated PI3-K- and JNK-dependent NF-kB activation,20 and given the involvement of these signaling molecules in shear-induced MMP-12 expression, we first measured 15d-PGJ2 production in shear-activated SW1353 cells. Application of high, but not low, shear stress for 48 h markedly increased 15d-PGJ2 secretion (Figure 5a). Importantly, exogenous 15d-PGJ2 (1 µm) was sufficient to markedly inhibit MMP-12 mRNA, protein and activity levels induced by low (2 dyn/cm2) shear stress (Figure 5b). 15d-PGJ2 exerts its effects via the peroxisome proliferator-activated receptor γ.21 Interestingly, treatment of SW1353 cells with the peroxisome proliferator-activated receptor γ antagonist GW9662 (1 µm) before their exposure to high fluid shear for 48 h resulted in a 20-fold upregulation of MMP-12 mRNA, protein and activity levels (Figure 5c). Together, these data suggest the critical role of 15d-PGJ2 in the suppression of MMP-12 expression in chondrosarcoma cells subjected to high shear.
Figure 5.
The effects of 15d-PGJ2 on shear-induced MMP-12 expression and its activity. SW1353 chondrosarcoma cells were subjected to either fluid shear (2 or 20 dyn/cm2) or static conditions (0 dyn/cm2) for 48 h in the absence or presence of 15d-PGJ2 (1 µm) or the peroxisome proliferator-activated receptor γ antagonist GW9662 (1 µm). (a) 15d-PGJ2 production was quantified using an enzyme immunoassay kit. (b and c) MMP-12 mRNA (bar graphs), protein and activity levels were determined by quantitative reverse transcription PCR (qRT-PCR), western blot and zymography, respectively. GAPDH and MMP-11 served as internal controls in qRT-PCR and western blot/zymography, respectively. These gels are representative of three independent experiments, all revealing similar results. Data represent the mean ± s.e.m. of three experiments. *P < 0.05 with respect to untreated control. §P < 0.05 compared with sheared specimen.
IGF-2, VEGF-B and VEGF-D promote chondrosarcoma cell migration and invasion by activating MMP-12 via PI3-K, p38 and JNK signaling pathways
A key step of chondrosarcoma cell invasion into normal tissue is the MMP-dependent degradation of the ECM. Prior work suggests that MMP-12 facilitates the invasion of glioma11 and endometrial adenocarcinoma cells12 though the underlying mechanisms of this process remain obscure. We thus sought to determine whether MMP-12 has the ability to regulate chondrosarcoma migration and invasion, and to delineate the underlying signaling pathways. To this end, we used a microfluidic-based migration chamber, which consists of four-walled microchannels of prescribed dimensions through which cells are induced to migrate in response to chemoattractant.22–26 The cross-sectional area of the microchannels (W × H=6 × 10 µm2) was chosen to be comparable to the 8 µm pore size in typical transwell assays. Single cell motility through collagen I-coated microchannels was tracked in real time by phase contrast microscopy. In separate experiments, matrigel (3.3 mg/ml) was used instead of collagen I and was allowed to polymerize and fill up the microchannels. The invasive ability of chondrosarcoma cells to degrade the matrigel and migrate in these microchannels was monitored in real time.
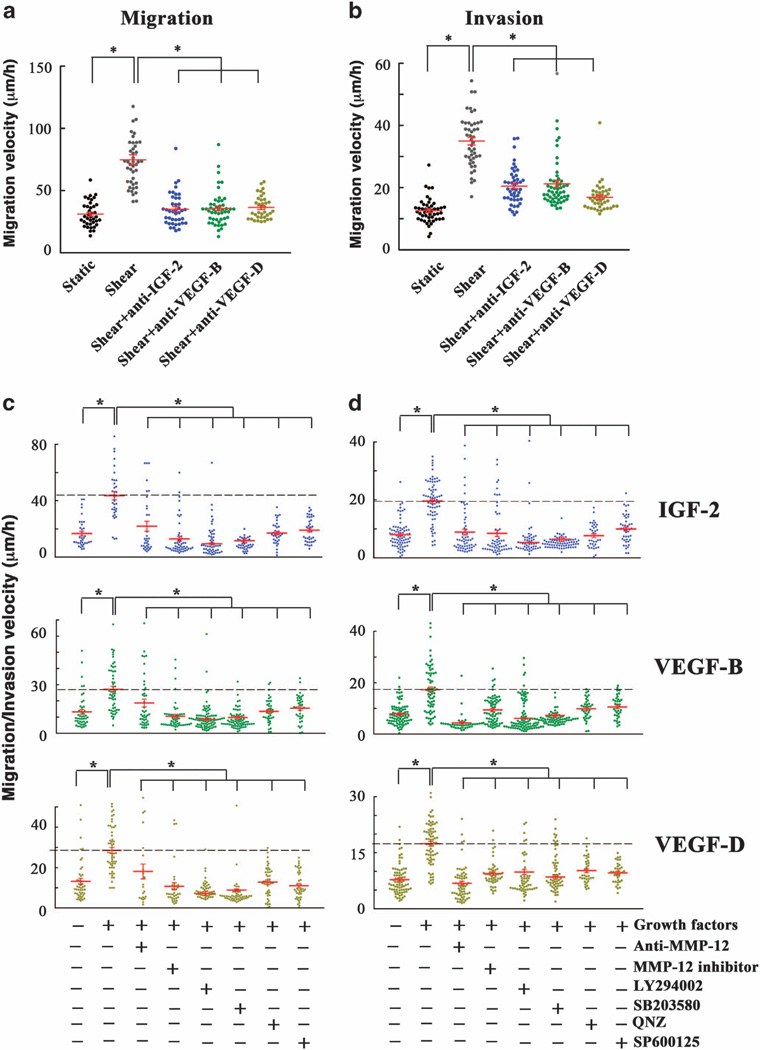
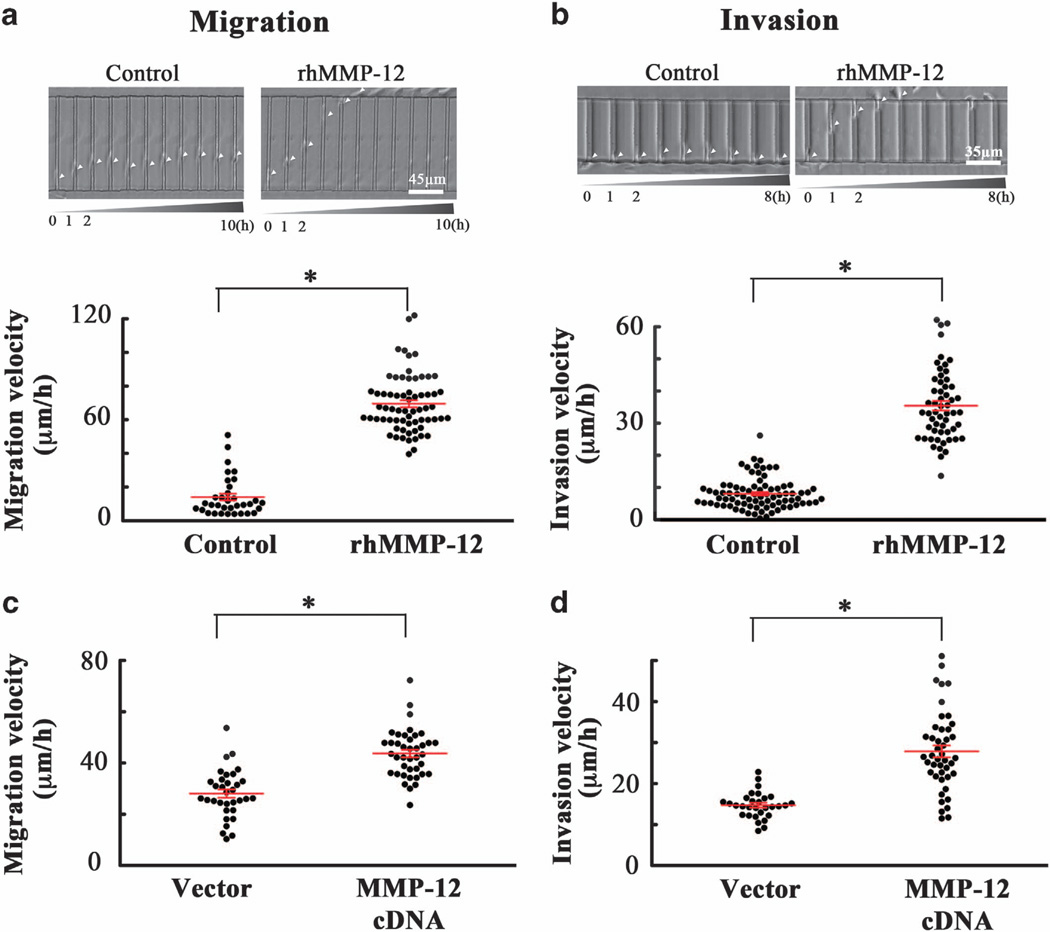
Incubation of human SW1353 chondrosarcoma static control cells with shear-conditioned medium resulted in a pronounced increase of their migratory (Figure 6a) and invasive (Figure 6b) velocities relative to cells incubated with static-conditioned medium. Pretreatment of cells with antibodies specific for IGF-2, VEGF-B or VEGF-D before their incubation with shear-conditioned medium significantly suppressed both migration and invasion (Figures 6a and b). These data illustrate the critical roles of endogenous shear-induced IGF-2, VEGF-B and VEGF-D in chondrosarcoma cell migration and invasion. Along these lines, treatment of human SW1353 chondrosarcoma cells with exogenous IGF-2, VEGF-B or VEGF-D markedly enhanced their migratory (Figure 6c) and invasive (Figure 6d) velocities. Interestingly, incubation of SW1353 cells with either an MMP-12 inhibitor (20 µm) or an anti-MMP-12 antibody (20 µg/ml) reversed the effects of IGF-2, VEGF-B and VEGF-D on cell migration and invasion (Figures 6c and d). To further demonstrate the critical role of MMP-12 in human chondrosarcoma cell migration and invasion, rhMMP-12 (50 ng/ml) was added to all inlets of the microchannel. Our data reveal that rhMMP-12 markedly enhanced SW1353 cell migration and invasion (Figures 7a and b). Similar results were obtained using CH2879 chondrosarcoma cells (Supplementary Figure S1). Along these lines, ectopic expression of MMP-12 increases both the migratory and invasive ability of human SW1353 chondrosarcoma cells in our microchannel device (Figures 7c and d). To further establish the role of MMP-12 in chondrosarcoma cell migration and invasion, and given that activated PI3-K, p38, JNK and NF-κB signaling pathways regulate MMP-12 expression and its activity, we evaluated the effects of selective pharmacological inhibitors on cell migration and invasion. Treatment of SW1353 cells with the PI3-K inhibitor LY294002 (10 µm) or the p38 inhibitor SB203580 (10 µm) blocked the enhancement of chondrosarcoma cell migration and invasion induced by exogenously added IGF-2, VEGF-B or VEGF-D (Figures 6c and d). Similar observations were made using the transwell migration and invasion assays (Supplementary Figure S2). Along these lines, the JNK inhibitor SP600125 and the NF-κB inhibitor quinazoline also inhibited chondrosarcoma cell migration and invasion stimulated by exogenous IGF-2, VEGF-B or VEGF-D (Figures 6c and d). Collectively, these data suggest that low levels of fluid shear (2 dyn/cm2) induce IGF-2, VEGF-B and VEGF-D upregulation, which in turn augments MMP-12 expression and its activity via PI3-K-, p38-, JNK- and NF-κB-dependent pathways, thereby promoting human chondrosarcoma cell migration and invasion.
Figure 6.
IGF-2, VEGF-B and VEGF-D enhance the human chondrosarcoma cell migration and invasion by activating MMP-12 via PI3-K-, p38-and JNK-dependent pathways. (a and b) SW1353 chondrosarcoma cells were incubated with either static- or shear-conditioned medium in the absence or presence of an antibody specific for IGF-2, VEGF-B or VEGF-D (20 µg/ml). (c and d) In select experiments, SW1353 chondrosarcoma cells were treated with 100 ng/ml IGF-2, VEGF-B, or VEGF-D in the absence or presence of an anti-MMP-12 antibody (20 µg/ml), MMP-12 inhibitor (20 µm), the PI3-K inhibitor LY294002 (10 µm), the p38 inhibitor SB203580 (10 µm), the NF-κB inhibitor quinazoline (1 µm) or the JNK inhibitor SP600125 (10 µm). Cells were induced to migrate using 10% fetal bovine serum as chemoattractant through collagen type I-coated microchannels (a and c) or matrigel-filled microchannels (b and d). The shear- or static-conditioned medium, the growth factors, pharmacological inhibitors and/or blocking antibodies were present in the media for the entire duration of the experiment. The average cell migration (a and c) and invasion (b and d) velocities were determined over a 10 (migration) or 8 h (invasion) period for > 30 cells from n > 3 independent experiments. Data represent the mean (µm/h) ± s.e.m. *P < 0.05.
Figure 7.
MMP-12 enhances the migration and invasion of human chondrosarcoma cells. (a and b) SW1353 cells were incubated with rhMMP-12 (50 ng/ml) or vehicle control before being seeded to the microchannel device. rhMMP-12 was present in the media for the entire duration of the experiment. (c and d) In select experiments, SW1353 cells transfected with either MMP-12 complementary DNA or the empty vector were seeded to the microchannel device. Cells were induced to migrate using 10% fetal bovine serum as chemoattractant through collagen type I-coated (a and c) or matrigel-filled (b and d) microchannels. The average cell migration (a and c) and invasion (b and d) velocities were determined over a 10 (migration) or 8 h (invasion) period for > 30 cells. Images are representative of six independent experiments, all revealing similar results. Data represent the mean ± s.e.m. *P < 0.05 relative to control cells.
MMP-12 promotes the lung colonization in vivo
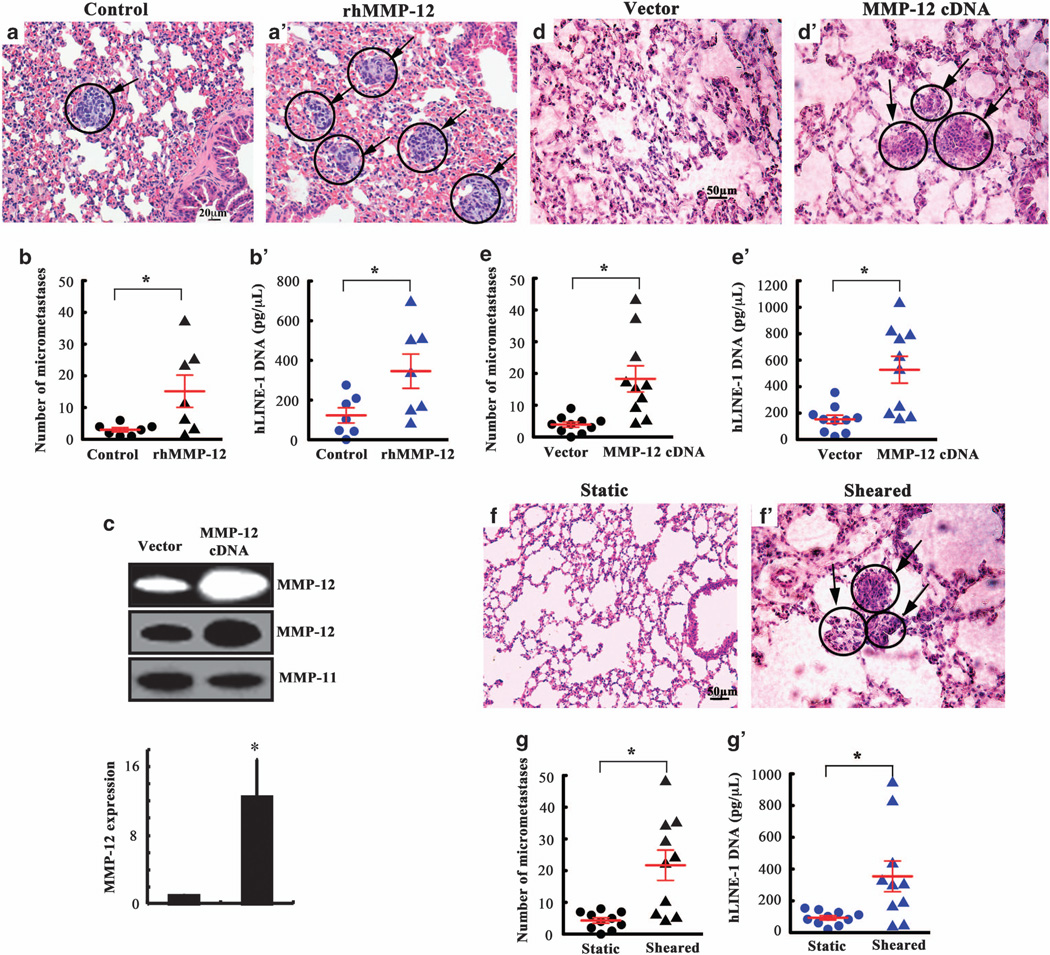
Because induction of MMP-12 promotes the migration and invasion of human chondrosarcoma cells in vitro (Figure 6 and Supplementary Figure S1), we examined the potential of rhMMP-12 on forming lung tumors in a tail vein injection model. To this end, 105 human CH2879 chondrosarcoma cells suspended in 100 µl serum-free medium (106 cells/ml) were injected into immunodeficient NOD/SCID/IL2 receptor gamma knockout (NSG) mice via the tail vein followed by tail vein injections with either rhMMP-12 (10 µg/ml) or vehicle control on the day of the cell injection (t = 0 weeks) and weekly thereafter for 5 weeks. All mice (7/7) injected with vehicle control developed only mild micrometastases containing ≤ 6 lung metastatic foci (Figure 8a). In contrast, only 3/7 mice injected with rhMMP-12 displayed mild micrometastases, whereas 4/7 mice exhibited moderate/severe lung micrometastases (11–37 foci per section) (Figures 8a’ and b). hLINE-1 (human long interspersed nuclear element-1) analysis27,28 quantitatively verified the higher levels of human DNA in the lungs of mice injected with rhMMP-12 (Figure 8b’). To further confirm these observations, mice were injected via the tail vein with CH2879 chondrosarcoma cells transfected with either MMP-12 complementary DNA or the empty vector. Ectopic expression of MMP-12 (Figure 8c) markedly increased the lung colonization in vivo (Figures 8d and d’), as evidenced by the higher number of micrometastases (Figure 8e) and higher human DNA content in the lungs of mice (Figure 8e’). Collectively, these data reveal that MMP-12 enhances the lung colonization of human chondrosarcoma cells in vivo. Because low fluid shear (2 dyn/cm2) induces the secretion of IGF-2, VEGF-B and VEGF-D, which are in turn is responsible for the upregulation of MMP-12 expression and its activity (Figure 2), we evaluated the ability of sheared chondrosarcoma cells and shear-conditioned medium on the lung colonization. To this end, CH2879 cells were exposed to either static or shear (2 dyn/cm2) conditions for 48 h. Next, the cells suspended in their corresponding conditioned medium were injected into mice via tail vein (t = 0 weeks); the conditioned medium was injected via the tail vein every 3 days for 5 weeks. As shown in Figures 8f–g’, shear-activated chondrosarcoma cells established significantly more lung micrometastases than the static controls.
Figure 8.
MMP-12 promotes the lung colonization in vivo. (a, a’, d, d’, f and f’) Representative histology of the lungs following tail vein injection. The lungs were isolated 5 weeks after tail vein injection of CH2879 chondrosarcoma cells into immunodeficient NSG mice. (a and a’) Mice were injected via the tail vein with either rhMMP-12 (10 µg/ml) or vehicle control at the day of the cell injection (t = 0 weeks) and weekly thereafter for 5 weeks. (d and d’) In select experiments, mice were injected with CH2879 cells transfected with either MMP-12 complementary DNA or the empty vector. In separate experiments, (g and g’) CH2879 cells were exposed to either static or shear (2 dyn/cm2) conditions for 48 h. Cells suspended in their corresponding conditioned medium were injected into mice via tail vein (t = 0 weeks); the conditioned medium was injected via the tail vein every 3 days for 5 weeks. The right lung lobes from each animal were fixed, stained with hematoxylin and eosin and examined for signs of lung micrometastases (indicated by arrowheads). (b, e and g) Quantification of the number of micrometastases present in the lungs of mice following tail vein injection in the absence or presence of rhMMP-12 (b) or MMP-12 overexpression (e) or static- or shear-conditioned medium as described above; n = 7–10 mice per group, *P < 0.05; t-test. (b’, e’ and f’) Presence of human DNA quantified in the lungs of mice injected with CH2879 chondrosarcoma cells via quantitative PCR of hLINE-1 DNA. n = 7–10 mice per group, *P < 0.05; t-test.
DISCUSSION
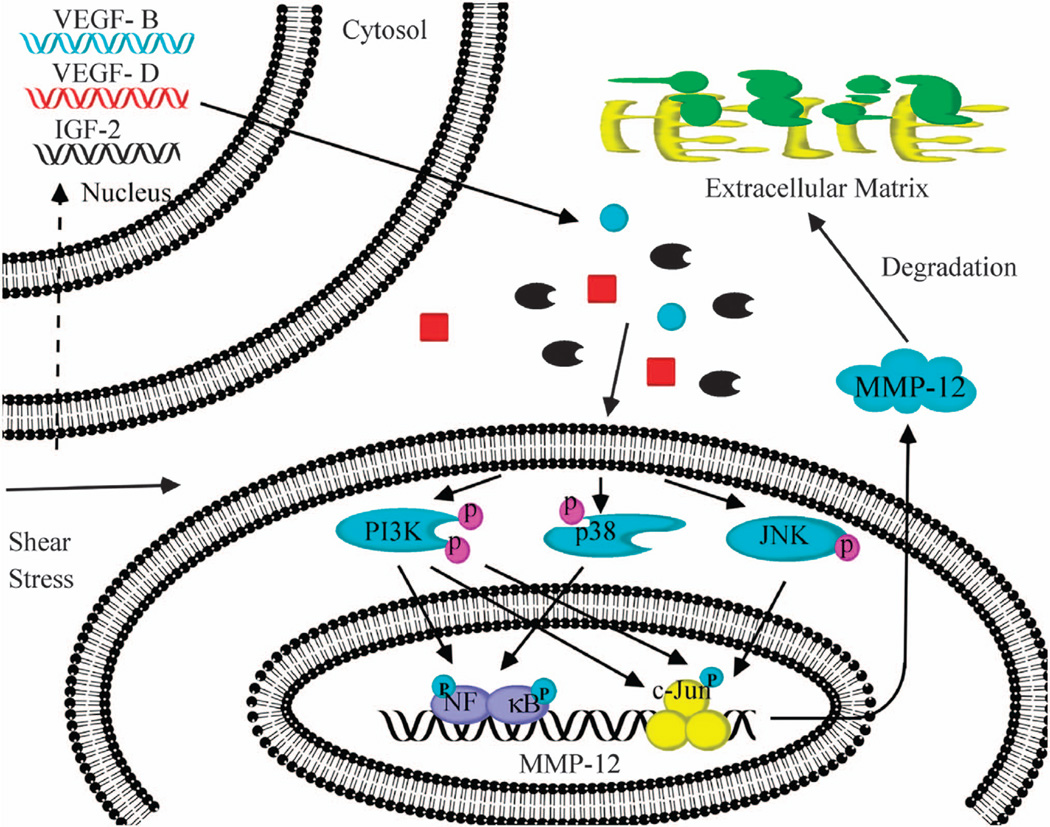
MMP-12 has divergent effects on tumor invasion and metastasis that are tumor cell type specific. For instance, MMP-12 overexpression correlates with local recurrence and metastatic disease in non-small cell lung cancer patients13 and with glioma and endometrial adenocarcinoma cell invasion.11,12 In contrast, MMP-12 expression is associated with better prognosis in colon and gastic cancer patients.14,29 To date, the potential role of MMP-12 in chondrosarcoma invasion and metastasis has yet to be delineated. Given that interstitial fluid flow and associated shear stress represent relevant mechanical signals in cartilage and bone (patho)physiology,1 we also investigated the effects of fluid shear on MMP-12 regulation in human chondrosarcoma cells. In line with a prior report, showing that MMP-12 is induced in human chondrocytes during the fetal development and malignant transformation,10 we found that MMP-12 expression is >4.5-fold higher in human chondrosarcomas than normal tissues. Low (2 dyn/cm2), but not high (20 dyn/cm2), fluid shear markedly (22–35-fold) upregulates MMP-12 expression and its activity in all chondrosarcoma cell lines examined in this study. We further demonstrate that fluid shear induces the synthesis of IGF-2, VEGF-B and VEGF-D, which in turn transactivate MMP-12 via PI3-K-, p38-, JNK- and NF-κB-dependent signaling pathways, leading to the increased chondrosarcoma cell invasion and lung colonization (Figure 9).
Figure 9.
Cascade of signaling events regulating MMP-12 expression and its activity in shear-activated human chondrosarcoma cells. Fluid shear (2 dyn/cm2) induces IGF-2, VEGF-B and VEGF-D expression and release in human chondrosarcoma cells. Elevated levels of IGF-2, VEGF-B and VEGF-D stimulate the activity of PI3-K/Akt, p38 and JNK signaling pathways, which in turn induce MMP-12 expression via transactivation of NF-κB and c-Jun. MMP-12 activation enhances chondrosarcoma cell migration and invasion in vitro and in vivo.
Although there is no prior direct evidence suggesting that fluid shear induces IGF-2 synthesis, others have reported that shear stress enhances VEGF expression in endothelial cells and skeletal muscles in vivo.30,31 VEGF has been identified to protect endothelial cells from apoptosis in response to shear stress.30 Because high (20 dyn/cm2), but not low (2 dyn/cm2), fluid shear induces apoptosis in human chondrocytes,18,19 and given that VEGF-B and VEGF-D are only induced by low, but not high, shear stress (Supplementary Table S4), we speculate that VEGF-B and VEGF-D along with IGF-2 may protect human chondrosarcoma cells from apoptosis and stimulate MMP-12 activity, thereby enhancing cell invasion and metastasis.
VEGF-B has been detected in breast carcinoma, melanoma and fibrosarcoma.32 Moreover, VEGF-B is associated with lymph node metastasis in breast cancer.33 Along these lines, VEGF-D promotes breast cancer metastasis via the lymphatics.34 In addition, increased IGF-2 activity has been associated with many types of cancer.35 In accord with these observations, we herein report that shear-induced (endogenous) or exogenous IGF-2, VEGF-B or VEGF-D potentiates the invasion of human chondrosarcoma cells by activating MMP-12, leading eventually to the increased lung colonization.
IGF-2, VEGF-B or VEGF-D augments MMP-12 expression and its activity in human chondrosarcoma cells via PI3-K-, p38-, JNK- and NF-kB-dependent signaling pathways (Figure 9). In line with these observations, IGF-2 has been reported to activate the PI3-K pathway during the chondrogenesis.36 VEGF-D can also stimulate PI3-K-dependent signaling in endothelial cells.37 VEGF has also been reported to regulate p38 activation in endothelial cells, albeit in a negative manner.38 This difference may be attributed to the divergent responses of distinct cell types. JNK and NF-κB signaling pathways have been implicated in the regulation of MMP-9 expression in shear-activated chondrocytic cells.20 Through the use of pharmacological inhibitors, we herein report the involvement of the JNK and NF-κB pathways in MMP-12 induction and invasion of human chondrosarcoma cells. We and others have shown that PI3-K, JNK and p38 can transactivate NF-κB.20,39 Because inhibition of NF-κB activity blocks IGF-2- and VEGF-induced MMP-12 synthesis, we propose that binding of phosphorylated NF-κB to its putative site(s) on the mmp-12 promoter is critical to the induction of MMP-12 expression in human chondrosarcomas. However, we cannot exclude the possibility that binding of phosphorylated c-Jun to its putative AP1 binding sites on the mmp-12 promoter might also contribute to MMP-12 expression.
The critical role of MMP-12 in growth factor-mediated chondrosarcoma cell invasion in vitro was demonstrated through the use of an anti-MMP-12 antibody or an MMP-12 inhibitor, which markedly suppressed cell invasion in a standard Transwell invasion assay and a novel three-dimensional microfluidic assay. Moreover, ectopic expression of MMP-12 was sufficient to increase the invasive ability of human chondrosarcoma cells. rhMMP-12, as well as MMP-12 overexpression enhanced the cell invasion in vitro and lung colonization in a tail vein injection model in vivo. Elevation of MMP-12 activity may potentiate chondrosarcoma cell invasion via intracellular signaling and release of ECM-bound growth factors following MMP-12-dependent ECM degradation.
Although interstitial fluid flow moves around the cell–matrix interface in all directions rather than only on the apical side, both interstitial flow and shear flow can drive cell responses via mechanical (that is, shear stress on the cell surface) and non-mechanical effects (that is, transport effects).40 Detailed studies comparing the effects of interstitial flow versus shear flow are lacking. However, laminar shear flow experiments have been widely performed to study the mechanobiology of bone- and cartilage-derived cells.1,17
We postulate that interstitial fluid flow, present in the bone and the cartilage, stimulates the production of IGF-2, VEGF-B and VEGF-D in human chondrosarcoma cells, which in turn upregulates MMP-12 expression and its activity, thereby enhancing their migratory and invasive potentials in vitro and in vivo. To establish distant metastatic foci, chondrosarcoma cells need to migrate through normal three-dimensional ECMs or longitudinal channels41 that inherently lack fluid flow or interstitial pressure. Of note, it is well established that interstitial fluid flow (IFF) is close to zero in most normal tissues.42 Thus, our migration/invasion assays in the absence of shear flow replicate aspects of the normal tissue microenvironment.
In summary, MMP-12 is significantly upregulated in human chondrosarcoma tissues relative to normal bone controls. Of all metalloproteinases, MMP-12 is the one that exhibits the most drastic (22–35-fold) upregulation in shear-activated chondrosarcoma cells. We also delineated the signaling pathway by which MMP-12 promotes chondrosarcoma cell invasion in vitro and demonstrated the role of MMP-12 in enhancing the lung colonization/metastasis in vivo. By delineating the signaling pathway regulating MMP-12 induction, potential therapeutic targets that repress chondrosarcoma cell invasion and metastasis may be identified.
MATERIALS AND METHODS
Cell culture and shear stress exposure
Human cell lines derived from chondrosarcoma grade II (SW1353 and HS 819.T obtained from American Type Culture Collection, Manassas, VA, USA) and chondrosarcoma grade III (CH2879 kindly provided by Dr Judith Bovée) were grown (37°C in 5% CO2) on glass slides in the appropriate medium supplemented with 10% fetal bovine serum. Before exposure to fluid shear stress, cells were incubated for 18 h in serum-free medium supplemented with 1% Nutridoma-SP (Roche Applied Science; Indianapolis, IN, USA).18,43,44 Cells were subjected to shear stress for up to 48 h in medium containing 1% Nutridoma-SP using a streamer gold flow device.19,20 In select experiments, pharmacological agents or blocking antibodies were added to the medium at the indicated concentrations just before the onset of shear or static exposure.
Transient transfection, quantitative real-time reverse transcription PCR, western blot and casein zymography assays were performed as previously described by Zhu et al.19 and Wang et al.20 IGF-2, VEGF-B and VEGF-D levels in static and sheared media were measured using enzyme immunoassay kits.
Microchannel migration and invasion assays
The fabrication of the microchannel device for cell migration and invasion was previously described.22–26 Microchannels of prescribed dimensions (W × H × L = 6 × 10 × 200 µm) were either pre-coated with collagen I (20 µg/ml) or filled with matrigel (3.3 mg/ml), which was allowed to polymerize at 37°C for at least 1 h, before seeding chondrosarcoma cells at the cell inlet. Cells were pretreated with 100 ng/ml of IGF-2, VEGF-B, VEGF-D or vehicle controls for 1 h in the absence or presence of an anti-MMP-12 antibody (R&D Systems, Minneapolis, MN, USA), MMP-12 inhibitor (Calbiochem, La Jolla, CA, USA), the PI3-K inhibitor LY294002, the p38 inhibitor SB203580, the JNK inhibitor SP600125 or the NF-κB inhibitor quinazoline. The aforementioned growth factors, pharmacological agents or antibodies were also added to all four inlets of the migration/invasion device22–26 and maintained for the entire duration of the migration or invasion assay. In select experiments, cells were pretreated with either shear- or static-conditioned media. In separate experiments, cells were treated with rhMMP-12 or vehicle control. In others, cells transfected with either MMP-12 complementary DNA or the empty vector were used in our assay. Cell migration was induced using 10% fetal bovine serum as chemotactic stimulus. To calculate the cell migration or invasion velocity, the cell center was identified as the midpoint between the poles of the cell body and was tracked by phase contrast microscopy for changes in the Y position at 20 min intervals over a 10 h period to generate an average value. Standard transwell migration and invasion assays were performed as previously described.23
Immunohistochemistry
Human bone tissue microarrays (US Biomax Inc., Rockville, MD, USA), encompassing 69 chondrosarcoma tissue cores and 24 normal bone tissue cores, were fixed with paraformaldehyde on tissue microarray slides. Slides were first deparaffinized with xylene, rehydrated in a graded series of ethanol and submerged in 3% hydrogen peroxide to eliminate endogenous peroxidase activity. MMP-12 level was determined using an immunohistochemical staining kit (Invitrogen, Carlsbad, CA, USA).
Tail vein injections
NOD/SCID/IL2 receptor gamma knockout mice (7–10 mice per experimental group; obtained from Jackson Lab, strain 005557, Bar Harbor, ME, USA) were injected via the tail vein with 105 CH2879 chondrosarcoma cells in a volume of 100 µl of serum-free medium followed by tail vein injection with either rhMMP-12 (100 µl of 10µg/ml) or vehicle control at the day of the cell injection (t = 0 weeks) and weekly thereafter for 5 weeks. In select experiments, CH2879 cells transfected with MMP-12 complementary DNA or the empty vector were injected to mice via the tail vein. In separate experiments, CH2879 cells were exposed to either static or shear (2 dyn/ cm2) conditions for 48 h. Next, cells suspended in their corresponding conditioned medium were injected into mice via the tail vein (t = 0 weeks); the conditioned medium was injected via the tail vein every 3 days for 5 weeks. Mice were euthanized 36 days post cell injection.
Histopathology of the lung tissue
The lung samples for pathology were fixed in 10% buffered formalin. The left lung lobe was used for hLINE analysis and the remaining lobes were for histopathological analysis of metastastatic foci.27,28 Tissues were collected, embedded in paraffin, sectioned at 5 µm, stained with hematoxylin and eosin and analyzed for histopathology analysis (PW and DLH, pathologists). Metastatic foci were counted in three lung histology sections from each mouse.
Quantification of hLINE-1 gene
DNA was extracted from the mouse lung tissue as previously described27,28 using the DNeasy blood and tissue kit (Qiagen, Valencia, CA, USA) in a sterile biological safety cabinet to minimize the risk of human DNA contamination. Elutions were analyzed via quantitative PCR as reported previously.27,28
Statistics
Data represent the mean ± s.e.m. of at least three independent experiments. Statistical significance of differences between means was determined by Student’s t-test or one-way analysis of variance, wherever appropriate. If means were shown to be significantly different, multiple comparisons by pairs were performed by the Tukey test.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part or in whole by the NCI (R01 CA186286, U54 CA143868), the Kleberg Foundation, the National Science Foundation (1159823), the Natural Science Foundation of China (31300777), the Fundamental Research Funds of China (N120520001, N120320001 and N130120002) and the Liaoning Provincial Talent Support Program (LJQ2013029).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
REFERENCES
- 1.Hillsley MV, Frangos JA. Bone tissue engineering: the role of interstitial fluid flow. Biotechnol Bioeng. 1994;43:573–581. doi: 10.1002/bit.260430706. [DOI] [PubMed] [Google Scholar]
- 2.Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech. 2009;41:347–374. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci USA. 1989;86:5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qazi H, Shi ZD, Tarbell JM. Fluid shear stress regulates the invasive potential of glioma cells via modulation of migratory activity and matrix metalloproteinase expression. PLoS ONE. 2011;6:e20348. doi: 10.1371/journal.pone.0020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dafni H, Israely T, Bhujwalla ZM, Benjamin LE, Neeman M. Overexpression of vascular endothelial growth factor 165 drives peritumor interstitial convection and induces lymphatic drain: magnetic resonance imaging, confocal microscopy, and histological tracking of triple-labeled albumin. Cancer Res. 2002;62:6731–6739. [PubMed] [Google Scholar]
- 6.Tang C-H. Molecular mechanisms of chondrosarcoma metastasis. Bio Medicine. 2012;2:92–98. [Google Scholar]
- 7.Aigner T, Muller S, Neureiter D, Illstrup DM, Kirchner T, Bjornsson J. Prognostic relevance of cell biologic and biochemical features in conventional chondrosarcomas. Cancer. 2002;94:2273–2281. doi: 10.1002/cncr.10461. [DOI] [PubMed] [Google Scholar]
- 8.Soderstrom M, Aro HT, Ahonen M, Johansson N, Aho A, Ekfors T, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human chondrosarcomas. APMIS. 2001;109:305–315. doi: 10.1034/j.1600-0463.2001.d01-125.x. [DOI] [PubMed] [Google Scholar]
- 9.Berend KR, Toth AP, Harrelson JM, Layfield LJ, Hey LA, Scully SP. Association between ratio of matrix metalloproteinase-1 to tissue inhibitor of metalloproteinase-1 and local recurrence, metastasis, and survival in human chondrosarcoma. J Bone Joint Surg Am. 1998;80:11–17. [PubMed] [Google Scholar]
- 10.Kerkela E, Bohling T, Herva R, Uria JA, Saarialho-Kere U. Human macrophage metalloelastase (MMP-12) expression is induced in chondrocytes during fetal development and malignant transformation. Bone. 2001;29:487–493. doi: 10.1016/s8756-3282(01)00595-6. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar S, Nuttall RK, Liu S, Edwards DR, Yong VW. Tenascin-C Stimulates Glioma Cell Invasion through Matrix Metalloproteinase-12. Cancer Res. 2006;66:11771–11780. doi: 10.1158/0008-5472.CAN-05-0470. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Dong Y, Zhao J, Sun H, Deng Y, Fan J, et al. Increased expression of human macrophage metalloelastase (MMP-12) is associated with the invasion of endometrial adenocarcinoma. Pathol Res Pract. 2007;203:499–505. doi: 10.1016/j.prp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann HS, Hansen G, Richter G, Taege C, Simm A, Silber RE, et al. Matrix metalloproteinase-12 expression correlates with local recurrence and metastatic disease in non-small cell lung cancer patients. Clin Cancer Res. 2005;11:1086–1092. [PubMed] [Google Scholar]
- 14.Yang W, Arii S, Gorrin-Rivas MJ, Mori A, Onodera H, Imamura M. Human macrophage metalloelastase gene expression in colorectal carcinoma and its clinicopathologic significance. Cancer. 2001;91:1277–1283. [PubMed] [Google Scholar]
- 15.Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, et al. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–6155. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 16.Ottaviano L, Schaefer KL, Gajewski M, Huckenbeck W, Baldus S, Rogel U, et al. Molecular characterization of commonly used cell lines for bone tumor research: a trans-European EuroBoNet effort. Genes Chromosomes Cancer. 2010;49:40–51. doi: 10.1002/gcc.20717. [DOI] [PubMed] [Google Scholar]
- 17.Chang SF, Chang CA, Lee DY, Lee PL, Yeh YM, Yeh CR, et al. Tumor cell cycle arrest induced by shear stress: roles of integrins and Smad. Proc Natl Acad Sci USA. 2008;105:3927–3932. doi: 10.1073/pnas.0712353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Healy ZR, Lee NH, Gao X, Goldring MB, Talalay P, Kensler TW, et al. Divergent responses of chondrocytes and endothelial cells to shear stress: cross-talk among COX-2, the phase 2 response, and apoptosis. Proc Natl Acad Sci USA. 2005;102:14010–14015. doi: 10.1073/pnas.0506620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu F, Wang P, Kontrogianni-Konstantopoulos A, Konstantopoulos K. Prostaglandin (PG)D2 and 15-deoxy-[Delta]12,14-PGJ2, but not PGE2, mediate shear-induced chondrocyte apoptosis via protein kinase A-dependent regulation of polo-like kinases. Cell Death Differ. 2010;17:1325–1334. doi: 10.1038/cdd.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Zhu F, Konstantopoulos K. The antagonistic actions of endogenous interleukin-1beta and 15-deoxy-Delta12,14-prostaglandin J2 regulate the temporal synthesis of matrix metalloproteinase-9 in sheared chondrocytes. J Biol Chem. 2012;287:31877–31893. doi: 10.1074/jbc.M112.362731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 22.Balzer EM, Tong Z, Paul CD, Hung W-C, Stroka KM, Boggs AE, et al. Physical confinement alters tumor cell adhesion and migration phenotypes. Faseb J. 2012;26:4045–4056. doi: 10.1096/fj.12-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep. 2013;3:1870. doi: 10.1038/srep01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung WC, Chen SH, Paul CD, Stroka KM, Lo YC, Yang JT, et al. Distinct signaling mechanisms regulate migration in unconfined versus confined spaces. J Cell Biol. 2013;202:807–824. doi: 10.1083/jcb.201302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong ZQ, Balzer EM, Dallas MR, Hung W-C, Stebe KJ, Konstantopoulos K. Chemotaxis of cell populations through confined spaces at single-cell resolution. PLoS ONE. 2012;7:e29211. doi: 10.1371/journal.pone.0029211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroka KM, Jiang H, Chen SH, Tong Z, Wirtz D, Sun SX, et al. Water permeation drives tumor cell migration in confined microenvironments. Cell. 2014;157:611–623. doi: 10.1016/j.cell.2014.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dallas MR, Liu G, Chen WC, Thomas SN, Wirtz D, Huso DL, et al. Divergent roles of CD44 and carcinoembryonic antigen in colon cancer metastasis. FASEB J. 2012;26:2648–2656. doi: 10.1096/fj.12-203786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rago C, Huso DL, Diehl F, Karim B, Liu G, Papadopoulos N, et al. Serial assessment of human tumor burdens in mice by the analysis of circulating DNA. Cancer Res. 2007;67:9364–9370. doi: 10.1158/0008-5472.CAN-07-0605. [DOI] [PubMed] [Google Scholar]
- 29.Cheng P, Jiang FH, Zhao LM, Dai Q, Yang WY, Zhu LM, et al. Human macrophage metalloelastase correlates with angiogenesis and prognosis of gastric carcinoma. Dig Dis Sci. 2010;55:3138–3146. doi: 10.1007/s10620-010-1127-3. [DOI] [PubMed] [Google Scholar]
- 30.dela Paz NG, Walshe TE, Leach LL, Saint-Geniez M, D'Amore PA. Role of shearstress- induced VEGF expression in endothelial cell survival. J Cell Sci. 2012;125:831–843. doi: 10.1242/jcs.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milkiewicz M, Brown MD, Egginton S, Hudlicka O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation. 2001;8:229–241. doi: 10.1038/sj/mn/7800074. [DOI] [PubMed] [Google Scholar]
- 32.Salven P, Lymboussaki A, Heikkila P, Jaaskela-Saari H, Enholm B, Aase K, et al. Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol. 1998;153:103–108. doi: 10.1016/S0002-9440(10)65550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunningham SP, Currie MJ, Han C, Robinson BA, Scott PA, Harris AL, et al. VEGF-B expression in human primary breast cancers is associated with lymph node metastasis but not angiogenesis. J Pathol. 2001;193:325–332. doi: 10.1002/path.814. [DOI] [PubMed] [Google Scholar]
- 34.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181–195. doi: 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Chao W, D'Amore PA. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev. 2008;19:111–120. doi: 10.1016/j.cytogfr.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamamura K, Zhang P, Yokota H. IGF2-driven PI3 kinase and TGFbeta signaling pathways in chondrogenesis. Cell Biology Int. 2008;32:1238–1246. doi: 10.1016/j.cellbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia H, Bagherzadeh A, Bicknell R, Duchen MR, Liu D, Zachary I. Vascular endothelial growth factor (VEGF)-D and VEGF-A differentially regulate KDR-mediated signaling and biological function in vascular endothelial cells. J Biol Chem. 2004;279:36148–36157. doi: 10.1074/jbc.M401538200. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz A, Kliche S, Mayr-Beyrle U, Fellbrich G, Waltenberger J. p38 MAPK inhibition is critically involved in VEGFR-2-mediated endothelial cell survival. Biochem Biophys Res Commun. 2003;306:730–736. doi: 10.1016/s0006-291x(03)01064-7. [DOI] [PubMed] [Google Scholar]
- 39.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by anti-angiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Healy ZR, Zhu F, Stull JD, Konstantopoulos K. Elucidation of the signaling network of COX-2 induction in sheared chondrocytes: COX-2 is induced via a Rac/MEKK1/MKK7/JNK2/c-Jun-C/EBPbeta-dependent pathway. Am J Physiol Cell Physiol. 2008;294:C1146–C1157. doi: 10.1152/ajpcell.00542.2007. [DOI] [PubMed] [Google Scholar]
- 44.Abulencia JP, Gaspard R, Healy ZR, Gaarde WA, Quackenbush J, Konstantopoulos K. Shear-induced cyclooxygenase-2 via a JNK2/c-Jun-dependent pathway regulates prostaglandin receptor expression in chondrocytic cells. J Biol Chem. 2003;278:28388–28394. doi: 10.1074/jbc.M301378200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.