Abstract
Relapse after clinical remission remains a leading cause of cancer-associated death. Although the mechanisms of tumor relapse are complex, the ability of cancer cells to survive physiologic stress is a prerequisite for recurrence. Ewing sarcoma (ES) and neuroblastoma (NB) are aggressive cancers that frequently relapse after initial remission. In addition, both tumors over-express the polycomb group (PcG) proteins BMI-1 and EZH2, which contribute to tumorigenicity. We have discovered that ES and NB resist hypoxic stress-induced death and that survival depends on PcG function. Epigenetic repression of developmental programs is the most well established cancer-associated function of PcG proteins. However, we noted that voltage-gated potassium (Kv) channel genes are also targets of PcG regulation in stem cells. Given the role of potassium in regulating apoptosis, we reasoned that repression of Kv channel genes might play a role in cancer cell survival. Here, we describe our novel finding that PcG-dependent repression of the Kv1.5 channel gene, KCNA5, contributes to cancer cell survival under conditions of stress. We show that survival of cancer cells in stress is dependent upon suppression of Kv1.5 channel function. The KCNA5 promoter is marked in cancer cells with PcG-dependent chromatin repressive modifications that increase in hypoxia. Genetic and pharmacologic inhibition of BMI-1 and EZH2, respectively, restore KCNA5 expression, which sensitizes cells to stress-induced death. In addition, ectopic expression of the Kv1.5 channel induces apoptotic cell death under conditions of hypoxia. These findings identify a novel role for PcG proteins in promoting cancer cell survival via repression of KCNA5.
Keywords: KCNA5, potassium channel, Kv1.5, polycomb, stress, cancer
Introduction
The ability to resist cell death is a hallmark of cancer (1). Cancer cells are able to survive despite exposure to cell intrinsic (e.g. metabolic and genotoxic) and extrinsic (e.g. hypoxia, nutrient deprivation) stress (1). This ability to survive conditions of stress allows cancer cells to escape physiologic death responses that are induced upon exposure to hostile microenvironments. In rapidly growing solid tumors the cancer outstrips its blood supply, thereby subjecting the cancer cells to a hostile microenvironment that is characterized by oxygen, growth factor and nutrient deprivation (1, 2). Furthermore, chemotherapy and radiation exacerbate the hostile local microenvironment by inducing tumor necrosis. Despite exposure to these tremendous stresses, however, solid tumors often recur after clinical remission and relapse remains a leading cause of cancer-associated death. The mechanisms of tumor relapse are both diverse and complex but elucidating how cancer cells resist stress-induced death has the potential to uncover novel opportunities for cancer therapy.
Stem cells harbor the ability to self-renew indefinitely and are epigenetically programmed to resist differentiation and to survive in hypoxic niche environments (3). Tumor cells often hijack normal stem cell processes to support their propagation and this is particularly evident in cancer cell populations that display tumor-initiating properties (1, 4). Although the mechanisms that support maintenance of stem cell traits are complex (3), chromatin repressive complexes are essential mediators of stemness and also crucial contributors to cancer pathogenesis (5). Among the best characterized of the chromatin repressive complexes are the polycomb group protein complexes PRC1 and PRC2 (reviewed in (5) and (6)). Polycomb proteins function to silence target gene loci via direct post-translational modification of histones. In particular, the PRC1 complex proteins BMI-1 and RING1B cooperate to induce ubiquitination of histone 2A at lysine residue 119 (H2AubK119), while the PRC2 member EZH2 mediates methylation of histone 3 at lysine residue 27 (H3K27me3) (5). Together these chromatin marks support maintenance of a repressed chromatin state that inhibits transcriptional activation (6). Both BMI-1 and EZH2 are highly over-expressed by many human cancers and play central roles in tumor initiation and tumor progression (6). In particular, over-expression of polycomb proteins is evident in tumor-initiating cell populations (7) and in the aggressive pediatric solid tumors Ewing sarcoma (ES) and neuroblastoma (NB) (8–12). The precise targets of polycomb-dependent regulation are cell type and context specific but, in general, polycomb repressive complexes support maintenance of stemness and oncogenesis by suppressing the expression of tumor suppressor genes and developmental regulators (6, 13)
Controlled regulation of intracellular levels of elemental ions is essential for normal cellular homeostasis. Transmembrane channels control ion flux across cellular membranes and there is abundant evidence that deregulation of calcium and sodium channel function can contribute to cancer pathogenesis in diverse fashions (14, 15). In addition, altered expression, regulation and function of potassium ion channels has been implicated in several cancer hallmarks including abnormal proliferation, resistance to cell death, and enhanced migration (16). In the current study we have identified the voltage-gated potassium channel Kv1.5-encoding gene, KCNA5, as a novel target of polycomb-dependent repression in aggressive cancer cells. Significantly, our studies show that epigenetic repression of KCNA5 contributes to selective survival of cancer cells under conditions of hypoxic stress and implicate activation of the Kv1.5 channel as a central mediator of hypoxia-induced apoptotic cell death.
Results
Polycomb proteins promote cancer cell survival under conditions of hypoxic stress
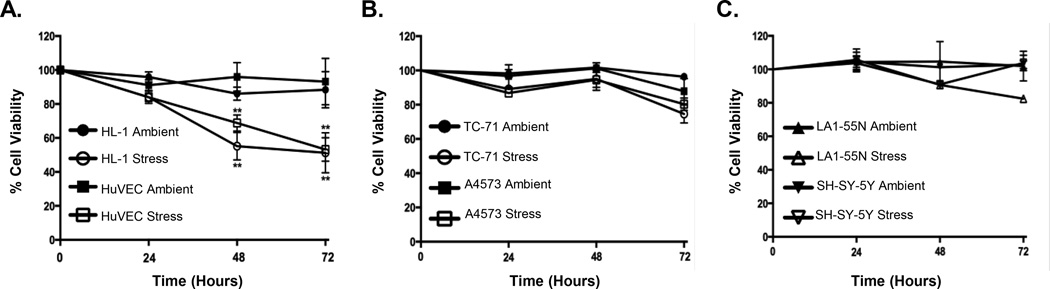
Most pediatric solid tumors, including NB and ES, respond to chemotherapy and tumors exhibit extensive necrosis at the time of surgery. However, a significant number of patients relapse following initial clinical remission demonstrating that at least some cells are capable of surviving the stress of a necrotic microenvironment. In order to explore the potential mechanisms that underlie resistance to stress-induced death we studied non-malignant and cancer cells in in vitro conditions that mimic the hostile microenvironment of a necrotic solid tumor. Specifically, cells were exposed to either ambient, unstressed conditions (21% oxygen, 10% FBS) or microenvironmental stress (1% oxygen, 0% FBS) and cell viability monitored over time. Exposure of non-malignant endothelial (HUVEC) and atrial (HL-1) cells to stress resulted in significant cell death that was evident within 24 hours and increased over time (Figure 1A). In contrast, ES (Figure 1B) and NB (Figure 1C) cells exhibited no significant loss of viability after up to 72 hours. Thus, these studies confirmed that ES and NB cells are relatively resistant to microenvironmental stress.
Figure 1. ES and NB cancer cells survive physiologic stress.
Under conditions of stress (serum starvation and hypoxia) non-malignant HuVEC and HL-1 cells experience a significant reduction in cell viability over a 72-hour time course (A). In contrast, ES cells, TC-71 and A4573, (B) and NB cells, LA1-55N and SH-SY-5Y, (C) survive. ** p<0.005 (mean ± SEM, n=3).
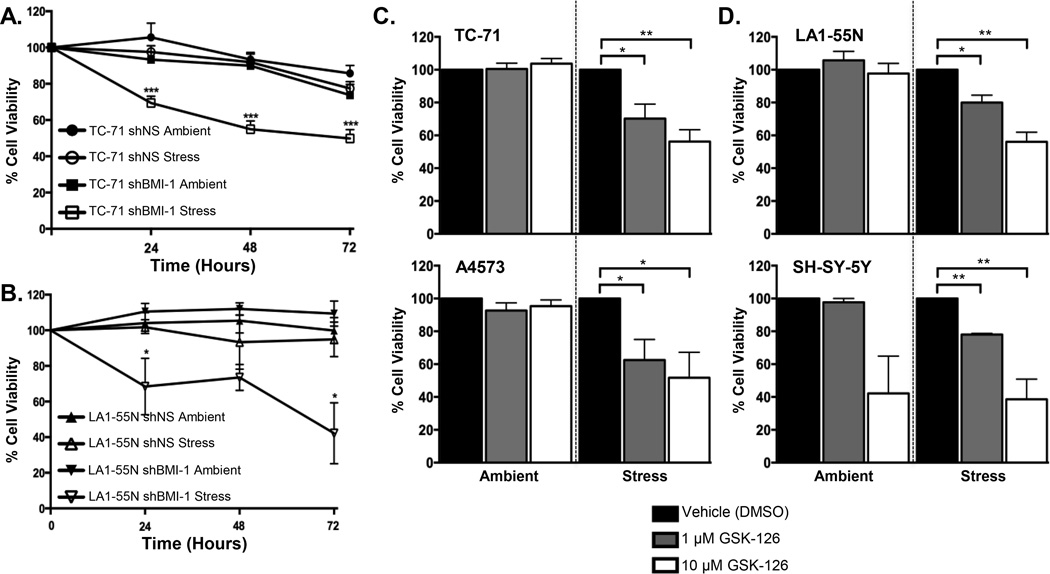
NB and ES are highly undifferentiated tumors that are thought to arise from stem and progenitor cells of neural crest (NB, ES) and/or mesenchymal (ES) origin. Stem cells thrive in conditions of hypoxia, leading us to hypothesize that the ability of NB and ES to survive stress may be linked to their primitive stem-like biology. Both ES and NB cells express high levels of the polycomb complex proteins BMI-1 and EZH2 and over-expression of these proteins contributes to stemness, tumorigenicity and tumor progression (8–12, 17). To test whether polycomb proteins contribute to survival of cancer cells under conditions of stress we evaluated viability in NB and ES cells that had been modified to down-regulate polycomb function. First, we assessed survival of cells that had been engineered to down-regulate BMI-1 as a result of RNA interference (Figure S1A). Significantly, NB and ES cells with reduced levels of BMI-1 showed no change in viability in ambient conditions but died upon transfer to stress conditions (Figure 2A–B). Next, we used the pharmacologic inhibitor GSK-126 to inhibit the methyltransferase activity of EZH2 (18). Once again, inhibition of polycomb function had no impact on cell survival under ambient conditions but resulted in loss of cell viability under conditions of stress (Figure 2C–D). Thus, survival of both ES and NB cells under conditions of hypoxia and nutrient deprivation is, at least in part, dependent on the continued activity of the polycomb proteins BMI-1 and EZH2.
Figure 2. Polycomb proteins promote survival of cancer cells under conditions of stress.
Analysis of cell survival in control (shNS) and BMI-1 knockdown (shBMI-1) ES (A) and NB (B) cells after transfer to stress conditions shows loss of viability in shBMI-1 cells over a 72-hour time course. Exposure of ES cells, TC-71 and A4573, (C) and NB cells, LA1-55N and SH-SY-5Y, (D) to the EZH2 inhibitor GSK-126 at 1 µM and 10 µM for 72 hours results in loss of viability when cells are exposed to stress for 24 hours. * p<0.05, ** p<0.005 and *** p<0.001 (mean ± SEM, n=3).
KCNA5 is repressed by polycomb in cancer cells
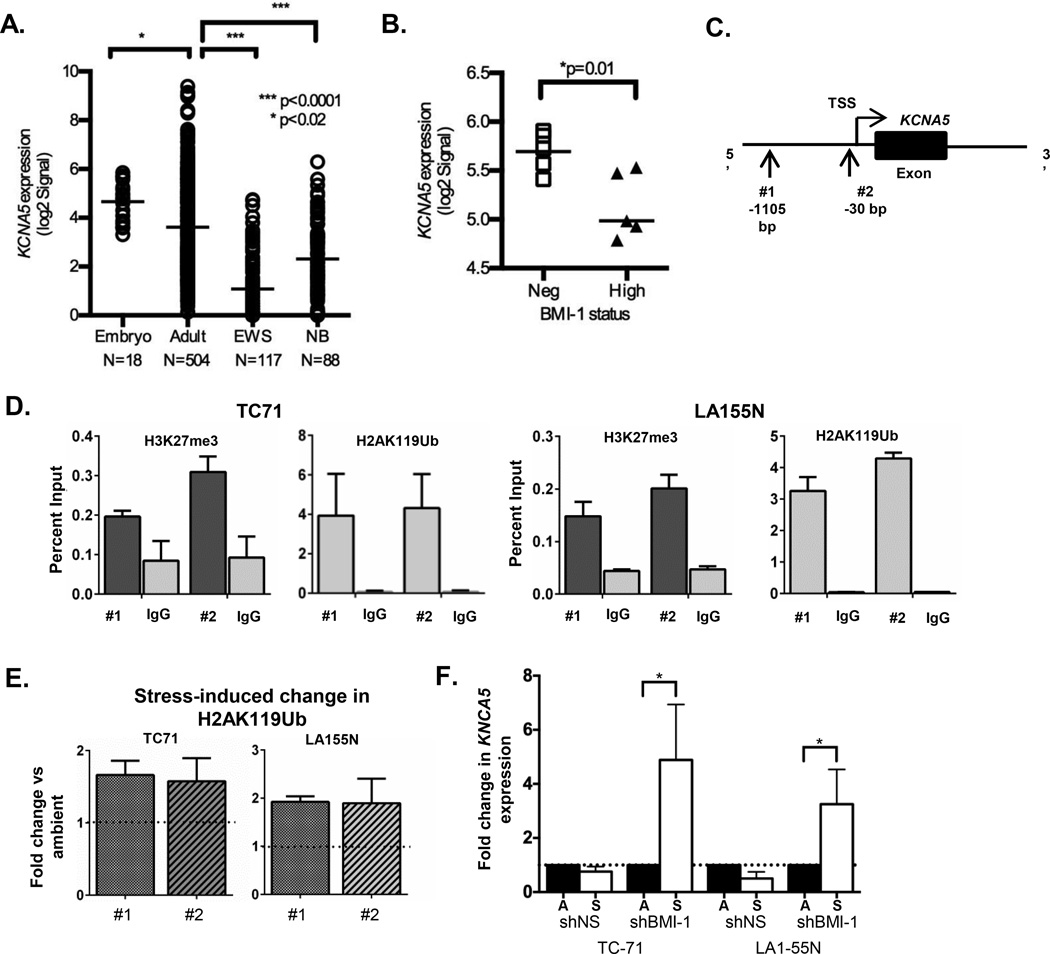
Having established that polycomb proteins promote cancer cell survival under conditions of stress we next sought to define the mechanism underlying the death-resistant phenotype. Polycomb proteins repress thousands of gene loci in a highly context specific manner. In human embryonic stem cells they specifically bind the promoters of nearly 2,000 protein-encoding genes involved in orchestration of normal embryonic development (13). In addition, in stem cells, fibroblasts and cancer cells the most prevalent targets of polycomb regulation are transcription factors that instruct cell fate and organogenesis and genes that are known to function as tumor suppressors, such as the cell cycle inhibitor CDKN2A (6, 13, 19). However, we noted on further analysis of the published data that promoters of potassium ion channel genes were also discovered to be highly enriched (p<3.8×10−11) among directly bound polycomb targets in stem cells (See Supplementary Table 10 in (13)) and also figured prominently among polycomb-regulated targets in human embryonic fibroblasts (See Supplementary Table 3 in (19)). Potassium flux out of a cell is a key requirement for apoptosis (20, 21) and, although altered expression of over 20 potassium ion channels has been described in human cancers, only two (Kv1.5 and Kv7.1) are down-regulated in tumors (16). Significantly, BMI-1 dependent silencing of the KCNA5 gene, which encodes Kv1.5, was recently shown to promote ischemic tolerance in neurons (22). Thus, we hypothesized that tolerance of hypoxic stress in cancer cells might be mediated by polycomb-dependent repression of the KCNA5 locus. To address this we first interrogated publicly available microarray databases to determine if expression of the KCNA5 transcript is altered in NB and ES. As shown in Figure 3A, basal levels of KCNA5 are reduced in both tumor types compared to non-malignant embryonic and adult tissues. In addition, levels of KCNA5 expression were found to be higher in a subset of ES tumors that did not express high levels of BMI-1 (23), thus providing indirect evidence that BMI-1 might repress KCNA5 in ES (Figure 3B). Further support for this relationship was evidenced by an inverse relationship between BMI-1 and EZH2 expression and KCNA5 expression in primary NB tumors (Figure S1B). To directly assess whether KCNA5 is targeted for polycomb-mediated silencing in ES and NB, we performed chromatin immunoprecipitation (ChIP) experiments using antibodies to the polycomb-dependent histone modifications H2AubK119 and H3K27me3. H2A119ub is mediated by the BMI-1 partner protein RING1B and H3K27me3 by EZH2. In each of the cancer types we detected enrichment of both polycomb-dependent modifications at the KCNA5 promoter, in particular H2A ubiquitination was highly enriched over background in both ES and NB cells (Figure 3C–D). Moreover, exposure of the cells to stress resulted in near two-fold increase in the H2A119Ub mark demonstrating that the cells acutely respond to microenvironmental stress by further repressing the KCNA5 promoter (Figure 3E). Consistent with polycomb-dependent repression of the locus in ES and NB cells, we detected little to no KCNA5 transcript in ambient conditions and no measurable increase was detected following exposure to stress (Figure 3F and Figure S1C). In contrast, KCNA5 transcription was increased in BMI-1-knockdown (Figure 3F) and EZH2 inhibitor-treated cells (Figure S1D) following transfer to stress conditions. Thus, the KCNA5 promoter is subject to epigenetic regulation by polycomb group proteins in ES and NB and persistent polycomb-dependent repression of the locus prevents up-regulation of the transcript when cells are exposed to microenvironmental stress.
Figure 3. Polycomb group proteins repress KCNA5 in ES and NB cells.
(A) Publicly available microarray data were analyzed using the R2: microarray analysis and visualization platform (http://r2.amc.nl). Expression of KCNA5 is significantly lower in ES (GSE34620) and NB (GSE16476) tumors compared to normal embryonic (GSE15744) and adult (GSE7307) tissues. (B) Expression of KCNA5 is increased in ES tumors that do not express high levels of BMI-1 (GSE16016). * p<0.02 and *** p<0.001 (C) Site of PCR primers for evaluation of histone modifications at the KCNA5 promoter. (D) Chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) of ES and NB cells in ambient conditions shows enrichment of both H3K27me3 and H2AK119Ub marks at the KCNA5 promoter (relative to IgG control ChIP). (E) ChIP of ES and NB cells in ambient and stress conditions shows a near 2-fold increase in enrichment of H2AK119Ub at the KCNA5 promoter after exposure to stress. Data are from 2 independent experiments and are expressed as mean +/− SEM. (F) qRT-PCR analysis demonstrates significant upregulation of KCNA5 expression in BMI-1 knockdown ES and NB cells after 8 hours in stress conditions. Expression normalized to the geometric mean of HPRT and GAPDH in each sample and expressed as fold change in stress relative to ambient conditions. * p<0.05 by Mann-Whitney Test (mean ± SEM, n=3).
The Kv1.5 channel regulates hypoxic stress-induced cell death
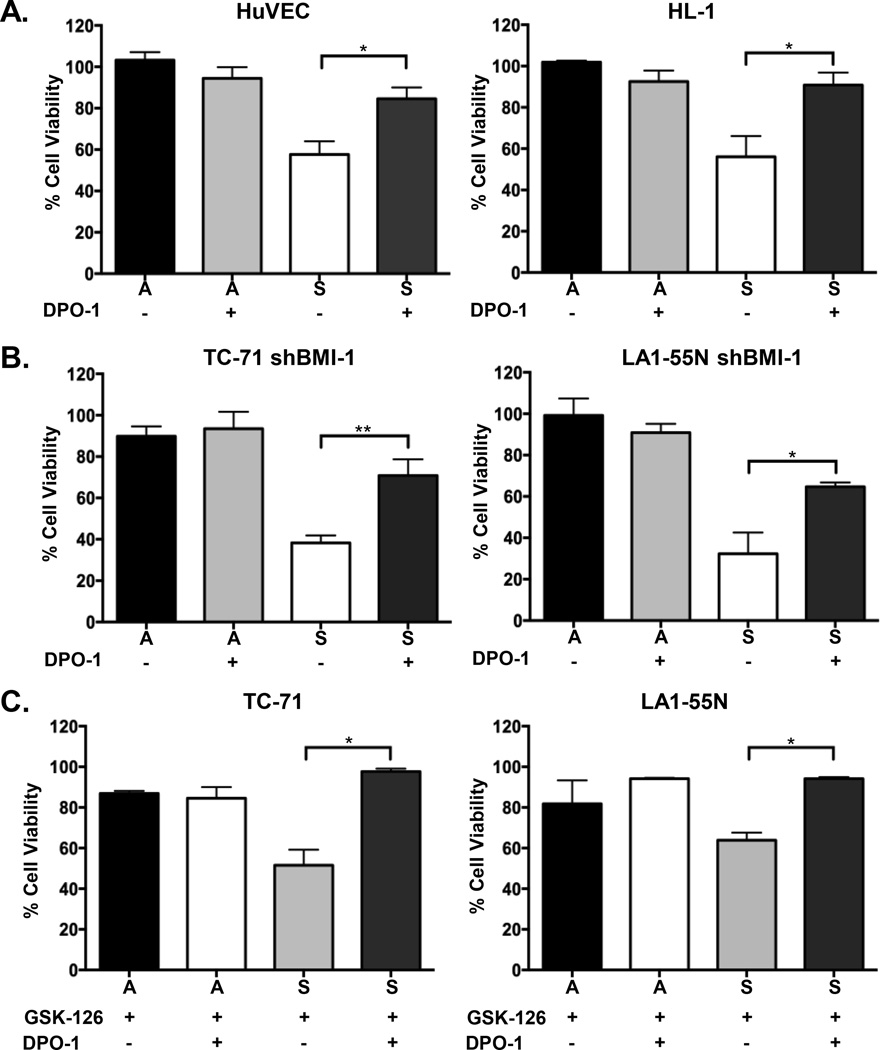
After confirming that polycomb proteins epigenetically repress KCNA5 in ES and NB cells we next sought to define the functional consequences of this repression. Specifically, we wished to determine whether loss of Kv1.5 channel function contributes to cell survival under conditions of hypoxic stress. To address this we took advantage of the pharmacologic compound diphenyl phosphine oxide-1 (DPO-1), a highly specific inhibitor of the Kv1.5 channel which effectively blocks potassium efflux through the channel with an IC50 = 310 nM (24) (Figure S2A). Non-malignant cells were exposed to DPO-1 and then transferred to stress conditions as above. Consistent with Kv1.5 being a key mediator of stress-induced death, channel blockade using DPO-1 rescued cell viability in both HUVEC and HL-1 cells (Figure 4A). Viability of cells under ambient conditions was unaffected by channel blockade (Figure 4A). To confirm these results we next exposed cells to 4’aminopyridine (4’AP), another potent inhibitor of the Kv1.5 channel (IC50 = 50 M) (25) (Figure S2A). Pharmacologic blockade of Kv1.5 using 4’AP also largely prevented death of stress-exposed HUVEC and HL-1 cells (Figure S2B). Thus, these findings indicate that hypoxic stress-induced death of non-malignant cells is, at least in part, dependent on a functional Kv1.5 channel.
Figure 4. Stress-induced death is prevented by pharmacologic blockade of Kv1.5 channel function.
Cell viability analysis demonstrates that treating cells with 310 nM DPO-1 largely prevents stress-induced death in non-malignant cells (A). Death is also prevented in cancer cells engineered to express reduced levels of BMI-1 (shBMI-1) (B) and in cancer cells that have been exposed to 10 µM GSK-126 prior to transfer to stress conditions (C). Viability in ambient conditions is unchanged by exposure to 310 nM DPO-1. * p<0.05 and ** p<0.005 (mean ± SEM, n=3).
Next, we tested the impact of Kv1.5 channel blockade on cancer cells that had been sensitized to stress-induced death as a consequence of polycomb modulation. Significantly, exposure of BMI-1 knockdown cancer cells to DPO-1 prevented stress-induced cell death (Figure 4B). Viability of ES cells was restored by DPO-1 while partial rescue was evident in the NB cells (Figure 4B). To test whether blockade of Kv1.5 would also restore the viability of EZH2-inhbited cells we exposed GSK-126 treated cells to DPO-1 prior to transfer to stress conditions. As shown (Figure 4C), DPO-1 restored cell viability in GSK-126 treated cells. Exposure of unmodified ES and NB cells to DPO-1 had no significant impact on cell death and cells remained viable in both ambient and stress conditions (Figure S2C). Together these data reveal the critical nature of polycomb proteins in promoting cancer cell viability under conditions of stress. In particular, they confirm that survival under conditions of hypoxic stress is, at least in part, dependent on polycomb-dependent suppression of the Kv1.5 channel. In addition, they further implicate both PRC1 and PRC2 in mediating epigenetic repression of the KCNA5 locus.
Ectopic expression of Kv1.5 restores sensitivity of cancer cells to hypoxic stress-induced apoptosis
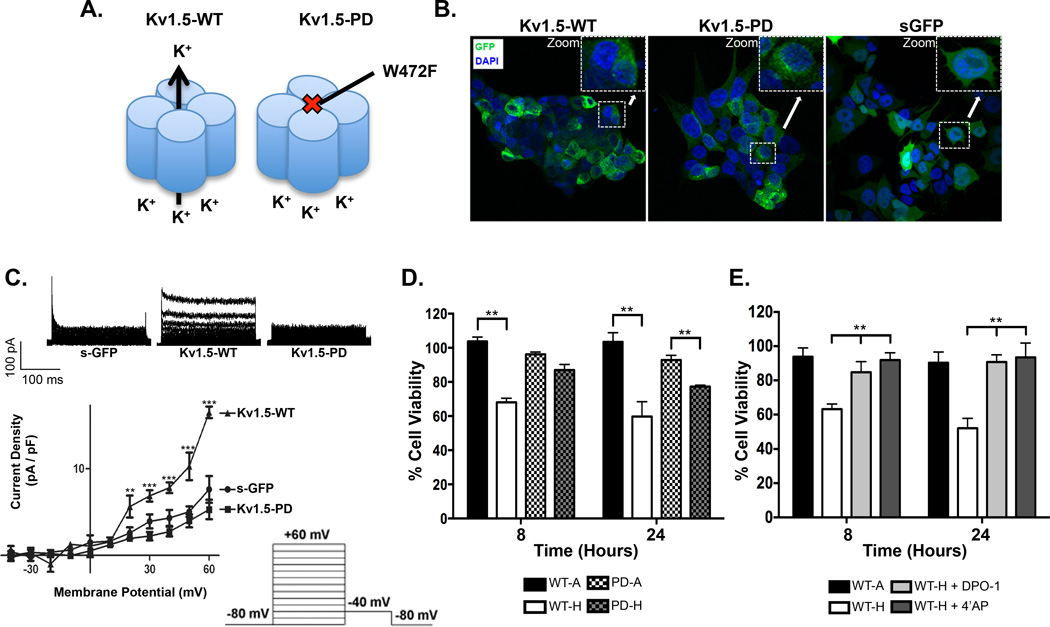
Potassium ion channels can affect cellular physiology through mechanisms that are both dependent or independent of their role as regulators of ion flux across the cell membrane (16) Having established that pharmacologic blockade of Kv1.5 prevents cell death under conditions of stress, we next sought to more directly test if this effect on cell survival was mediated by blockade of potassium efflux. To address this question we transduced TC-71 ES cells with wild-type (WT) and non-conducting, pore-dead (PD) Kv1.5 mutant constructs or a control soluble GFP (sGFP) using adenoviral infection. Both Kv1.5 constructs carry full-length human KCNA5 cDNA, tagged in the extracellular loop with GFP, and the trafficking and biophysical properties of both WT and PD ectopic proteins recapitulate those of the endogenous channel (26, 27). However, unlike Kv1.5-WT, which is fully functional, Kv1.5-PD generates a non-functional channel that cannot conduct potassium across the cell membrane as a result of an amino acid change (W472F) within the pore region (Figure 5A) (26). Confocal microscopy confirmed successful infection of ES cells with all constructs and cytoplasmic membrane localization of both Kv1.5-WT and Kv1.5-PD proteins (Figure 5B). Whole cell patch clamp experiments confirmed the presence of a robust and functional channel in Kv1.5-WT expressing cells and only background current in Kv1.5-PD that was equivalent to that of control sGFP infected cells (Figure 5C). To determine if the presence of ectopic channel altered cell viability we cultured infected cells in either room air (21% O2) or hypoxia (1% O2) for 24 hours. In these experiments, stress was limited to hypoxia alone without serum deprivation in order to more precisely assess the impact of channel re-expression on the hypoxic response. Expression of either Kv1.5-WT or Kv1.5-PD had no impact on cell viability in ambient conditions but transfer to hypoxic conditions resulted in death of the Kv1.5-WT cells (Figure 5D). In contrast, Kv1.5-PD cells experienced no loss of viability in hypoxia (Figure 5D). To confirm that the hypoxia-associated death of Kv1.5-WT cells was due to efflux of potassium through the ectopic channel we exposed cells to DPO-1 and 4’AP. Significantly, survival of Kv1.5-WT cells in hypoxia was largely restored if cells were treated with either of the two compounds (Figure 5E). Finally, to test the specificity of Kv1.5 as a mediator of hypoxia-induced death we tested whether ectopic expression of two other voltage-gated potassium channels, Kv1.4 and Kv1.3, would mimic the effect of Kv1.5. In contrast to Kv1.5, ectopic expression of Kv1.4 and Kv1.3 induced no appreciable death in cells that were exposed to hypoxia (Figure S3A). After 24 hours the Kv1.3 infected cells began to die but death was less than that of Kv1.5-transduced cells and equivalent to that of cells that were infected with control vectors sGFP and Kv1.5-PD (Figure S3A, B). Kv1.4 infected remained viable at 24 hours, suggesting that the lower titer of the Kv1.4 virus afforded protection against the non-specific adenoviral death that we observed with Kv1.5-PD, Kv1.3 and sGFP infection (Figure S3A, B). Thus, these data demonstrate that the effects of Kv1.5 in mediating hypoxia-induced death are not generalizable to other Kv channels.
Figure 5. Ectopic expression of the Kv1.5 channel restores stress-induced death in ES cells.
(A) Cartoon of the wild-type (Kv1.5-WT) and pore-dead (Kv1.5-PD) channels, depicting the amino acid change W472F which prevents efflux of potassium out of the Kv1.5 channel. (B) GFP-fluorescent immunocytochemistry and confocal microscopy reveals robust expression of ectopic Kv1.5-WT, Kv1.5-PD and soluble GFP (sGFP) in TC-71 cells 24 hours post adenoviral infection. (C) Electrophysiology confirms induction of functional Kv1.5 current in TC-71 cells transduced with Kv1.5-WT but not with Kv1.5-PD or sGFP. (D) TC-71 cells expressing Kv1.5-WT channel have a significant reduction in cell viability at 8 and 24 hours in hypoxia while the TC-71 cells expressing the Kv1.5-PD channel are unaffected, in particular at 8 hours. (E) Pharmacological block of the Kv1.5 channel with either 50 µM 4’AP or 310 nM DPO-1 largely prevents hypoxia-induced death in Kv1.5-WT expressing ES cells. ** p<0.005 and *** p<0.001 (mean± SEM, n=3).
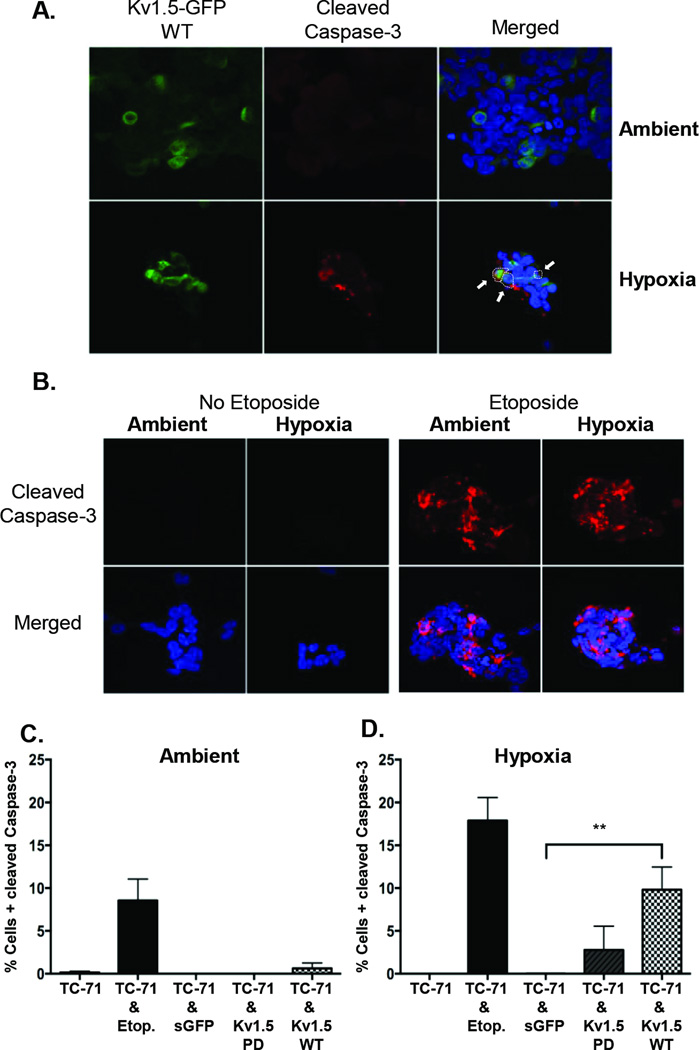
Finally, we investigated the mechanism of cell death in Kv1.5 expressing cells. It has been previously shown that potassium efflux promotes caspase activation and apoptosis (28, 29). To determine if TC-71 cells expressing Kv1.5-WT protein were dying by apoptosis we assessed cleavage of caspase-3 in ambient and hypoxic conditions. Whereas Kv1.5-WT cells showed little evidence of caspase-3 cleavage in ambient conditions, after 8 hours in hypoxia cells with cleaved caspase-3 were readily detected (Figure 6A). By comparison, unmodified TC-71 cells showed no evidence of caspase cleavage in either ambient or hypoxic conditions but apoptosis could be dramatically induced in both conditions by etoposide, a cytotoxic topoisomerase II inhibitor that is routinely used in ES therapeutic regimens (Figure 6B). By contrast, cells with cleaved caspase-3 were only rarely detected in Kv1.5-PD and sGFP transduced populations in both ambient and hypoxic conditions (Figure 6C & D). Thus, these studies together demonstrate the specificity of Kv1.5 as a key mediator of hypoxia-associated cell death and confirm that, under conditions of cell stress, efflux of potassium through the channel promotes apoptotic cell death.
Figure 6. The Kv1.5-WT channel mediates cell death through caspase-3 activation.
(A) Immunocytochemistry and confocal microscopy of Kv1.5-WT expressing TC-71 cells shows induction of caspase-3 cleavage in Kv1.5+ (GFP+) cells (Indicated by white arrows) following exposure to hypoxia. No significant cleavage is detected in Kv1.5+ cells in ambient conditions. (B) Cleaved caspase-3 staining is not detected in parent TC-71 cells in either ambient or hypoxic conditions. To serve as a positive control for cleaved caspase-3 staining, cells were exposed to 4 µg Etoposide. This exposure resulted in robust induction of caspase-3 cleavage in both ambient and hypoxic conditions. Quantification of cells with cleaved caspase-3 is shown for cells in A & B, under ambient (C) (21% O2) and hypoxic (1%O2) (D) conditions. ** p<0.005 (mean ± SEM, n=2–3. 150–400 cell nuclei were counted for each condition).
Discussion
Oxygen tension in normal tissues ranges from 2–9% but is often considerably less in stem cell niches and solid tumors (2). Normal sensing of oxygen tension is critical for cell proliferation and cell survival and cells that exist in hypoxic niches, such as cancer cells and stem cells, utilize sophisticated molecular tools to withstand the relatively ischemic microenvironment (3, 30–32). Cell proliferation and survival are also intimately linked to the concentration of intracellular ions including potassium, sodium and calcium (28, 33, 34). In particular, high levels of intracellular potassium inhibit caspase activation and promote cell survival, revealing a role for potassium efflux in execution of apoptotic cell death (29, 35, 36). Intracellular potassium concentrations are controlled by transmembrane ion channels, which actively regulate the flux of potassium ions through central pores (37, 38). There are 78 different potassium ion channels (16, 39) and each is expressed in a context specific manner and alters potassium flux in response to different cellular signals (37, 38). Interestingly, recent studies of ion channel expression in cancer have suggested that deregulation of potassium channels is a characteristic of human tumors (reviewed in (16) and (40)). Moreover, recent high-throughput drug screening studies showed that the potassium ionophore salinomycin is selectively cytotoxic to cancer stem cell populations (41, 42). These findings together support further investigation of ion-channel targeted approaches as novel opportunities for cancer therapy.
In the current study we have focused on defining the mechanism of Kv1.5 channel suppression in aggressive pediatric solid tumors and the contribution of this suppression to tumor pathogenesis. Our results implicate polycomb-dependent epigenetic repression of KCNA5 as a key mechanism of channel inhibition and reveal the critical role of this inhibition in promoting cancer cell survival under conditions of microenvironmental stress, in particular hypoxic stress. In addition, the inability of an ectopic pore-dead Kv1.5 channel to restore stress-induced death demonstrates that survival of Kv1.5 suppressed cancer cells is linked to its canonical function as a potassium conductor rather than to other potential non-canonical roles that might also impact on cell signaling (16)
The Kv1.5 channel is a voltage-gated potassium channel that conducts potassium in response to changes in cell membrane potential (39). In addition, Kv1.5 is a putative oxygen sensor and contributes to the regulation of cell proliferation and survival under conditions of hypoxia by altering intracellular potassium levels in response to shifts in redox states (25, 43–46). The role of Kv1.5 as an oxygen-sensing channel is well established in both cardiac and pulmonary physiology and aberrant expression and regulation of Kv1.5 channels contributes to pulmonary hypertension and cardiac arrhythmias (45–49). It was recently shown that exposure of neuronal cells to acute hypoxia and glucose deprivation leads to apoptosis and that this ischemia-induced cell death is associated with up-regulation of Kv1.5 expression (22). Significantly, tolerance to the ischemic insult could be elicited in this study if cells were first exposed to repetitive low-dose stress rather than an acute insult. At a molecular level this ischemic tolerance was found to be due to up-regulation of BMI-1 and recruitment of polycomb to the KCNA5 promoter (22). Thus, BMI-1 dependent repression of the KCNA5 gene can be an adaptive, physiologic response to chronic stress. Our current studies reveal that, in BMI-1 over-expressing cancer cells, this adaptive physiologic response has been coopted to promote the survival of the cancer cells under conditions of acute stress. Whether this survival mechanism is an inherent feature of the cancer cell of origin (e.g. a stem cell) or an acquired characteristic that arises during the process of malignant transformation remains to be determined and will likely vary between different cancer types.
There have been several reports that Kv1.5 expression is reduced in human cancers (50–54) and in gliomas loss of expression is directly correlated with tumor aggression, suggesting that loss of the channel might contribute to tumor progression (55). Moreover, recent studies demonstrated that restoration of normal mitochondrial function and redox tension by use of the pyruvate dehydrogenase kinase (PDK) inhibitor dichloroacetate (DCA), resulted in de-repression of KCNA5 transcription and induction of Kv1.5-dependent apoptosis (21). Unfortunately, although these initial findings suggested that DCA might be useful as a relatively non-toxic anti-cancer agent, later studies failed to show significant impact of DCA in other preclinical models (56). In particular, pediatric tumors including NB and ES were found to be completely insensitive to the cytotoxic effects of DCA (56, 57). Our discovery that the KCNA5 locus is targeted for polycomb-dependent repression in these tumors suggests that the disappointing results that have been encountered with DCA as an anti-cancer therapy may be due to the inaccessible chromatin state of the KCNA5 promoter in cancer cells that over-express BMI-1 and other polycomb proteins, such as pediatric solid tumor and cancer stem cells,
In summary, we have identified that epigenetic repression of the KCNA5 gene by polycomb proteins, in particular BMI-1, inhibits Kv1.5 expression and function in ES and NB. These findings provide new evidence that the Kv1.5 channel plays a seminal role in execution of hypoxia-induced apoptotic cell death in aggressive cancers. In addition, they suggest that cancer cells hijack physiologic regulation of the Kv1.5 channel to promote their survival in hostile microenvironments. Future studies are now needed to determine how this knowledge about altered epigenetic regulation of KCNA5 can be therapeutically exploited to maximize cell death in the context of aggressive solid tumors that are prone to relapse.
Materials and Methods
Cell Culture and Viability
ES cells were cultured in RPMI-1640 media (Gibco, Grand Island, NY, USA) and NB cells in MEM 1× media (Gibco, Grand Island, NY, USA). Media was supplemented with 10% FBS (Atlas Biologicals, Inc., Fort Collins, CO, USA) and 6 mM L-glutamine (Life Technologies, Grand Island, NY, USA). Identities were confirmed by short tandem repeat profiling. HuVEC cells were cultured in EBM-2 Basal Medium (Lonza, Pittsburgh, PA, USA) supplemented with EGM-2 SingleQuot Kit Suppl. & Growth Factors (Lonza, Pittsburgh, PA, USA). HL-1 cells were cultured in Claycomb media (Sigma-Aldrich, St. Louis, MO, USA-Aldrich) supplemented in 10% FBS (Sigma-Aldrich, St. Louis, MO, USA-Aldrich), 2 mM L-glutamine, 100 µg/mL Penicillin/Streptomycin and 0.1 mM Norepinephrine (Sigma-Aldrich, St. Louis, MO, USA-Aldrich). Cells were maintained in ambient conditions at 37°C in 5% CO2. For growth factor deprivation and hypoxia studies FBS was removed and cells were placed in 1% O2 in an xVivo system (Biospherix, Lacona, NY, USA). Viability was determined by cell counting and trypan blue. Data were normalized relative to 0 hours.
Pharmacologic inhibitor studies
4’Aminopyridine (4’AP) (50 µM, Sigma-Aldrich, St. Louis, MO, USA-Aldrich) was prepared in an aqueous solution and diphenyl phosphine oxide-1 (DPO-1) (310 nM, Tocris Bioscience, Bristol, UK) and GSK-126 (1 µM, 10 µM, Active Biochem, Maplewood, NJ, USA) were diluted in dimethyl sulfoxide (DMSO). Cells were pre-treated for 72 hours.
Generation of genetically modified cells
Cell lines were transduced with pLKO.1-puro vectors (Sigma-Aldrich, St. Louis, MO, USA-Aldrich) containing one of two short hairpin RNAs targeting BMI-1 (shBMI-1#156: 5’-CCTAATACTTTCCAGATTGAT-3’ or shBMI-1 #157: 5’-CGGAAAGTAAACAAAGACAAA-3’) or a non-silencing control sequence (shNS: 5’-CAACAAGATGAAGAGCACCAA-3’). Cells were selected in puromycin (2 µg/mL, Sigma-Aldrich, St. Louis, MO, USA-Aldrich) for 48 hours prior to experimentation.
Adenoviral constructs were generated using pAD/V5/-dest (Invitrogen, Grand Island, NY, USA). Virus was produced using ViraPower (Invitrogen, Grand Island, NY, USA) and purified using ViraPur Adenovirus mini purification (ViraPur, San Diego, CA, USA). Cells were infected with Kv1.5-GFP-WT, KV1.5-GFP-PD and soluble GFP 24 hour’s prior to use in an experiment. Response of the infected cells to hypoxia was tested by transferring cells to a 1% O2 chamber as above.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA extraction was performed using RNeasy® Plus Mini kit (Qiagen, Valencia, CA, USA) and cDNA generated using iScript (Bio-Rad, Hercules, CA, USA). qRT-PCR was performed using validated SYBR primers (sequences available on request). Analysis was performed in triplicate using the Lightcycler® 480 System (Roche Applied Science, Indianapolis, IN, USA). Data were analyzed by normalizing average Ct values of the gene of interest to the geometric mean of reference genes (HPRT and GAPDH) using ΔΔCt method.
Western Blot and Immunocytochemistry
Levels of BMI-1 (Millipore, Mouse mAb #05-637), EZH2 (Cell Signaling Technology, Rabbit mAb #4905), or GAPDH (Cell Signaling Technology, Rabbit mAb #2118) were determined using standard western blot assays as previously described (58).
For apoptosis studies TC-71 cells were treated with 4 µg Etoposide or infected with Kv1.5-WT, Kv1.5-PD or sGFP. After 24 hours, cells were placed in ambient (21% O2) or hypoxic (1% O2) conditions. After 8 hours, cells were fixed with 4% paraformaldehyde (10 min.), permeabilized with 0.1% Triton X-100 (10 min.) and blocked with 2% goat serum (20 min.). Cells were incubated with polyclonal anti-cleaved capase-3 (1:200 dilution, Cell Signaling Technology, Rabbit mAb #9661) in 2% goat serum (45 min.), followed by incubation with goat anti-rabbit Alexa Fluor-594 antibody (1:200 dilution, Life Technologies, Grand Island, NY, USA, Grand Island, NY, USA) in 2% goat serum (40 min.). Nuclei were labeled with DAPI (Life Technologies, Grand Island, NY, USA, Grand Island, NY, USA) and mounted with ProLong Gold (Invitrogen, Grand Island, NY, USA, Grand Island, NY, USA).
Cell images were acquired on a Nikon TI81 A1R confocal microscope with a 60× by 1.40× N.A. oil objective. For every experiment 5–10 images were acquired for each condition and 50–100 cells were analyzed. Z-stack images were compiled with ImageJ software (NIH, Bethesda, MD). The resolution obtained in these imaging experiments was 512 by 512 pixels with a z resolution of 0.5µm for each filter set.
Electrophysiology
The isolation and recording of the Ikur current, specific current of the Kv1.5 channel, was recorded by a whole-cell patch clamping protocol as previously described (59). Total potassium current was recorded using 10mV step depolarizations, from a holding potential of −80mV, to +60mV. The intracellular pipette solution contained (in mM): KCl 148, MgCl2 1, EGTA 5, HEPES 5, K2ATP 5; and was adjusted to pH 7.2 with KOH. The bath solution contained (in mM): NaCl 148, NaH2PO4 0.4, MgCl2 1, KCl 5.4, CaCl2 1, HEPES 15; and was adjusted to pH 7.4 with NaOH.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) was performed using established protocols, with modifications. Cells were fixed with 1% formaldehyde, lysed, and sonicated (Qsonica cup horn sonicator, 100% amplitude). DNA fragments were in the 200–700 bp range. The following antibodies were then added to the pre-cleared sample and incubated overnight at 4°C: Anti-Trimethyl Histone H3Lys27 (Millipore 07-449, 2g) and Anti-Ubiquityl-Histone H2ALys119 D27C4 XP (Cell Signaling Technology #8240, 10l). The complexes were purified using protein-G dynabeads (Invitrogen, Grand Island, NY, USA) followed by elution and crosslink reversal. DNA was recovered using QIAquick PCR Purification Kit (Qiagen, Valenica, CA, USA). Target sequences were amplified by PCR using iTaq SYBR green (Bio-Rad, Hercules, CA, USA). Primer sequences for the KCNA5 promoter were KCNA5 #1 (Forward: 5’-TCCAGCATCATCAGTTTCCA -3’ and Reverse: 5’-TGGCTCTCATTATGCACCAG-3’) and KCNA5 #2 (Forward: 5’-GCTGAAGGTTGCATCTGCT-3’ and Reverse: 5’- GGCCCTGACGTCAAGAAG-3’). Data were analyzed using the percent input method where Percent input = 100*2^(Average Ct Input – Average Ct IP).
Statistical Analysis
Statistics were performed using GraphPad Prism 6 (San Diego, CA, USA) and values of p<0.05 between groups were considered significant.
Supplementary Material
Acknowledgements
The authors wish to thank members of the Lawlor and Martens labs for helpful discussion. This research was supported by grants from the CureSearch Children’s Oncology Group and Nick Currey Fund, National Institutes of Health grants 1R01 CA134604 (E.R.L.), R01HL070973 (J.R.M.), AACR-SU2C IRG-1309 (ERL), Pharmacological Sciences Training Program T32 GM007767 (K.E.R.) and UM Cancer Biology Training Grant 5T32 CA009676-20 (L.K.S).
Footnotes
Conflict of Interest
The authors have no potential conflicts of interest to disclose.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nature reviews Cancer. 2008;8(12):967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nature reviews Molecular cell biology. 2014;15(4):243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15(9):1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 5.Laugesen A, Helin K. Chromatin Repressive Complexes in Stem Cells, Development, and Cancer. Cell stem cell. 2014;14(6):735–751. doi: 10.1016/j.stem.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nature reviews Cancer. 2009;9(11):773–784. doi: 10.1038/nrc2736. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas D, Abdueva D, van Doorninck J, Peng G, Shimada H, Tritche T. BMI-1 Promotes Ewing Sarcoma Tumorigenicity Independent of CDKN2A Repression. Cancer Research. 2008;68(16):6507–6515. doi: 10.1158/0008-5472.CAN-07-6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak K, Kerl K, Fehr D, Kramps C, Gessner C, Killmer K, et al. BMI1 is a target gene of E2F-1 and is strongly expressed in primary neuroblastomas. Nucleic Acids Research. 2006;34(6):1745–1754. doi: 10.1093/nar/gkl119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter GH, Plehm S, Fasan A, Rössler S, Unland R, Bennani-Baiti IM, et al. EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci U S A. 2009;106(13):5324–5329. doi: 10.1073/pnas.0810759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riggi N, Suva M-L, Suva D, Cironi L, Provero P, Tercier S, et al. EWS-FLI-1 Expression Triggers a Ewing's Sarcoma Initiation Program in Primary Human Mesenchymal Stem Cells. Cancer Research. 2008;68(7):2176–2185. doi: 10.1158/0008-5472.CAN-07-1761. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Liu Z, Woo C-W, Li Z, Wang L, Wei J, et al. EZH2 Mediates Epigenetic Silencing of Neuroblastoma Suppressor Genes CASZ1, CLU, RUNX3, and NGFR. Cancer Research. 2012;72(1):315–324. doi: 10.1158/0008-5472.CAN-11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125(2):301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser SP, Ozerlat-Gunduz I, Brackenbury WJ, Fitzgerald EM, Campbell TM, Coombes RC, et al. Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369(1638):20130105. doi: 10.1098/rstb.2013.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nature reviews Cancer. 2007;7(7):519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Jan LY. Targeting potassium channels in cancer. The Journal of cell biology. 2014;206(2):151–162. doi: 10.1083/jcb.201404136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Levetzow C, Jiang X, Gwye Y, von Levetzow G, Hung L, Cooper A, et al. Modeling initiation of Ewing sarcoma in human neural crest cells. PLoS One. 2011;6(4):e19305. doi: 10.1371/journal.pone.0019305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2013;492(7427):108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 19.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20(9):1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang F, Hoffmann EK. Role of ion transport in control of apoptotic cell death. Compr Physiol. 2012;2(3):2037–2061. doi: 10.1002/cphy.c110046. [DOI] [PubMed] [Google Scholar]
- 21.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, et al. A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell. 2007;11(1):37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Stapels M, Piper C, Yang T, Li M, Stowell C, Xiong Z-G, et al. Polycomb Group Proteins as Epigenetic Mediators of Neuroprotection in Ischemic Tolerance. Science Signaling. 2010;3(111):ra15-ra. doi: 10.1126/scisignal.2000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper A, van Doorninck J, Ji L, Russell D, Ladanyi M, Shimada H, et al. Ewing tumors that do not overexpress BMI-1 are a distinct molecular subclass with variant biology: a report from the Children's Oncology Group. Clin Cancer Res. 2011;17(1):56–66. doi: 10.1158/1078-0432.CCR-10-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stump G, Wallace A, Regan C, Lynch J. In Vivo Antiarrhythmic and Cardiac Electrophysiologic Effects of a Novel Diphenylphosphine Oxide IKur Blocker (2-Isopropyl-5-methylcyclohexyl) Diphenylphosphine Oxide. Journal of Pharmacology and Experimental Therapeutics. 2005;315(3):1362–1367. doi: 10.1124/jpet.105.092197. [DOI] [PubMed] [Google Scholar]
- 25.Fedida D, Bouchard R, Chen FSP. Slow gating charge immobilization in the human potassium channel Kv1.5 and its prevention by 4-aminopyridine. American Journal of Physiology - Cell Physiology. 1995;494(2):377–387. doi: 10.1113/jphysiol.1996.sp021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher SM, McEwen D, Zhang L, Arendt K, Van Genderen K, Martens JR. Antiarrhythmic Drug-Induced Internalization of the Atrial-Specific K+ Channel Kv1.5. Circulation Research. 2009;104(12):1390–1398. doi: 10.1161/CIRCRESAHA.108.192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwen D, Schumacher SM, Li Q, Benson M, Iniguez-Lluhi J, Van Genderen K, et al. Rab-GTPase-dependent Endocytic Recycling of KV1.5 in Atrial Myocytes. J Biol Chem. 2007;282(40):29612–29620. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- 28.Burg ED, Remillard CV, Yuan JXJ. K+ Channels in Apoptosis. J Membrane Biol. 2006;209(1):3–20. doi: 10.1007/s00232-005-0838-4. [DOI] [PubMed] [Google Scholar]
- 29.Hughes FM, Jr, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem. 1997;272(48):30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- 30.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell stem cell. 2010;7(2):150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell stem cell. 2011;9(4):298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene. 2013;32(35):4057–4063. doi: 10.1038/onc.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA. Voltage-sensitive ion channels and cancer. Cancer metastasis reviews. 2006;25(3):493–500. doi: 10.1007/s10555-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 34.Urrego D, Tomczak AP, Zahed F, Stuhmer W, Pardo LA. Potassium channels in cell cycle and cell proliferation. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369(1638):20130094. doi: 10.1098/rstb.2013.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cain K, Langlais C, Sun XM, Brown DG, Cohen GM. Physiological concentrations of K+ inhibit cytochrome c-dependent formation of the apoptosome. J Biol Chem. 2001;276(45):41985–41990. doi: 10.1074/jbc.M107419200. [DOI] [PubMed] [Google Scholar]
- 36.Karki P, Seong C, Kim JE, Hur K, Shin SY, Lee JS, et al. Intracellular K(+) inhibits apoptosis by suppressing the Apaf-1 apoptosome formation and subsequent downstream pathways but not cytochrome c release. Cell death and differentiation. 2007;14(12):2068–2075. doi: 10.1038/sj.cdd.4402221. [DOI] [PubMed] [Google Scholar]
- 37.Miller C. An overview of the potassium channel family. Genome biology. 2000;1(4) doi: 10.1186/gb-2000-1-4-reviews0004. REVIEWS0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacological reviews. 2000;52(4):557–594. [PubMed] [Google Scholar]
- 39.Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, et al. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacological reviews. 2003;55(4):583–586. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- 40.Pardo LA, Stuhmer W. The roles of K(+) channels in cancer. Nature reviews Cancer. 2014;14(1):39–48. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- 41.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sachlos E, Risueno RM, Laronde S, Shapovalova Z, Lee JH, Russell J, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149(6):1284–1297. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 43.Caouette D, Dongmo C, Berube J, Fournier D, Daleau P. Hydrogen peroxide modulates the Kv1.5 channel expressed in a mammalian cell line. Naunyn-Schmiedeberg's archives of pharmacology. 2003;368(6):479–486. doi: 10.1007/s00210-003-0834-0. [DOI] [PubMed] [Google Scholar]
- 44.Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. The Journal of membrane biology. 1996;154(2):91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- 45.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. American journal of physiology Heart and circulatory physiology. 2008;294(2):H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 46.Schumacher SM, Martens JR. Ion channel trafficking: a new therapeutic horizon for atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2010;7(9):1309–1315. doi: 10.1016/j.hrthm.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80(6):772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993;73(2):276–285. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- 49.Platoshyn O, Brevnova EE, Burg ED, Yu Y, Remillard CV, Yuan JX. Acute hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. American journal of physiology Cell physiology. 2006;290(3):C907–C916. doi: 10.1152/ajpcell.00028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stuhmer W. Role of voltage-gated potassium channels in cancer. The Journal of membrane biology. 2005;205(3):115–124. doi: 10.1007/s00232-005-0776-1. [DOI] [PubMed] [Google Scholar]
- 51.Bielanska J, Hernandez-Losa J, Moline T, Somoza R, Ramon y Cajal S, Condom E, et al. Differential expression of Kv1.3 and Kv1.5 voltage-dependent K+ channels in human skeletal muscle sarcomas. Cancer investigation. 2012;30(3):203–208. doi: 10.3109/07357907.2012.654872. [DOI] [PubMed] [Google Scholar]
- 52.Bielanska J, Hernandez-Losa J, Perez-Verdaguer M, Moline T, Somoza R, Ramon y Cajal S, et al. Voltage-Dependent Potassium Channels Kv1.3 and Kv1.5 in Human Cancer. Current Cancer Drug Targets. 2008;9:904–914. doi: 10.2174/156800909790192400. [DOI] [PubMed] [Google Scholar]
- 53.Felipe A, Bielanska J, Comes N, Vallejo A, Roig S, Ramon YCS, et al. Targeting the voltage-dependent K(+) channels Kv1.3 and Kv1.5 as tumor biomarkers for cancer detection and prevention. Current medicinal chemistry. 2012;19(5):661–674. doi: 10.2174/092986712798992048. [DOI] [PubMed] [Google Scholar]
- 54.Vallejo-Gracia A, Bielanska J, Hernandez-Losa J, Castellvi J, Ruiz-Marcellan MC, Ramon y Cajal S, et al. Emerging role for the voltage-dependent K+ channel Kv1.5 in B-lymphocyte physiology: expression associated with human lymphoma malignancy. Journal of leukocyte biology. 2013;94(4):779–789. doi: 10.1189/jlb.0213094. [DOI] [PubMed] [Google Scholar]
- 55.Arvind S, Arivazhagan A, Santosh V, Chandramouli BA. Differential expression of a novel voltage gated potassium channel--Kv 1.5 in astrocytomas and its impact on prognosis in glioblastoma. British journal of neurosurgery. 2012;26(1):16–20. doi: 10.3109/02688697.2011.583365. [DOI] [PubMed] [Google Scholar]
- 56.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. British journal of cancer. 2008;99(7):989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heshe D, Hoogestraat S, Brauckmann C, Karst U, Boos J, Lanvers-Kaminsky C. Dichloroacetate metabolically targeted therapy defeats cytotoxicity of standard anticancer drugs. Cancer chemotherapy and pharmacology. 2011;67(3):647–655. doi: 10.1007/s00280-010-1361-6. [DOI] [PubMed] [Google Scholar]
- 58.Lawlor ER, Scheel C, Irving J, Sorensen PH. Anchorage-independent multi-cellular spheroids as an in vitro model of growth signaling in Ewing tumors. Oncogene. 2002;21(2):307–318. doi: 10.1038/sj.onc.1205053. [DOI] [PubMed] [Google Scholar]
- 59.Martens JR, Navarro-Polanco R, Coppock EA, Nishiyama A, Parshley L, Grobaski TD, et al. Differential targeting of Shaker-like potassium channels to lipid rafts. J Biol Chem. 2000;275(11):7443–7446. doi: 10.1074/jbc.275.11.7443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.