Abstract
The high affinity Sigma Receptor 1 (σR1) ligand (+)-pentazocine ((+)-PTZ) affords profound retinal neuroprotection in vitro and in vivo by a yet-unknown mechanism. A common feature of retinal disease is Müller cell reactive gliosis, which includes cytokine release. Here we investigated whether LPS stimulates cytokine release by primary mouse Müller cells and whether (+)-PTZ alters release. Using a highly sensitive inflammatory antibody array we observed significant release of macrophage inflammatory proteins (MIP1γ, MIP2, MIP3α) and interleukin-12 (IL12 (p40/p70)) in LPS-treated cells compared to controls, and a significant decrease in secretion upon (+)-PTZ treatment. Müller cells from σR1 knockout mice demonstrated increased MIP1γ, MIP2, MIP3α and IL12 (p40/p70) secretion when exposed to LPS compared to LPS-stimulated WT cells. We investigated whether cytokine secretion was accompanied by cytosolic-to-nuclear NFκB translocation and whether endothelial cell adhesion/migration was altered by released cytokines. Cells exposed to LPS demonstrated increased NFκB nuclear location, which was reduced significantly in (+)-PTZ-treated cells. Media conditioned by LPS-stimulated-Müller cells induced leukocyte-endothelial cell adhesion and endothelial cell migration, which was attenuated by (+)-PTZ treatment. The findings suggest that release of certain inflammatory cytokines by Müller cells can be attenuated by σR1 ligands providing insights into the retinal neuroprotective role of this receptor.
Keywords: (+)-pentazocine, cytokine array, mouse, radial glial cell, retinal disease, retinal degeneration
Introduction
Sigma receptor 1 (σR1) is a 25.3 kDa transmembrane protein that shares no homology with any other known mammalian protein. Within cells, σR1 is present at the plasma membrane, nuclear, endoplasmic reticulum (ER), and mitochondrial-associated membranes (Aydar et al., 2002; Jiang et al., 2006; Hayashi and Su, 2007). σR1 was cloned initially by Hanner from guinea pig (Hanner et al., 1996) and subsequently by members of our lab from rat, mouse and human (Kekuda et al., 1996; Seth et al., 1997; Seth et al., 1998). It is expressed in numerous tissues including CNS, liver, kidney; however, neither its endogenous ligand nor its function is known. The location of σR1 at the ER-mitochondrial interface prompted the hypothesis that σR1 may function as a molecular chaperone (Hayashi and Su, 2007). In spite of the gaps in our understanding of the physiological role of σR1, there has been considerable interest in this protein because ligands for σR1 have anti-nociceptive (Wuensch, 2012), anti-depressant (Sabino et al., 2009), memory-enhancing (Maurice and Lockhart, 1997) and neuroprotective properties (Maurice and Su, 2009).
The neuroprotective properties of σR1 have been reported not only in brain, but also in ocular tissues. Among ocular tissues, retina is particularly vulnerable to numerous sight-threatening degenerative diseases including diabetic retinopathy, macular degeneration, ischemic retinopathy, retinitis pigmentosa. σR1 is expressed abundantly in retina (Ola et al, 2001, Liu et al., 2010). Studies from our lab have investigated the neuroprotective properties of the high affinity σR1 ligand (+)-pentazocine ((+)-PTZ) in vitro and in vivo. Apoptotic retinal neuronal cell death involving ganglion cells (RGCs) was reduced significantly and profound preservation of retinal architecture was observed in vivo in a mouse model of diabetic retinopathy (Ins2Akita/+) treated with (+)-PTZ for many weeks (Smith et al., 2008). Most studies investigating σR1 and its retinal neuroprotective function have focused on its role in neurons, particularly RGCs using cell lines (Dun et al., 2007; Tchedre et al., 2008), primary RGCs (Ha et al., 2011a; Mueller et al., 2013) and an optic nerve crush model of RGC death (Mavlyutov et al., 2011). Recently, we explored the role of σR1 as a mediator of ER stress in the retina of σR1−/− mice and found no changes in ER gene expression when the whole retina was analyzed, but marked upregulation of several key ER stress genes when Müller glial cells from σR1−/− mice were analyzed (Ha et al., 2014). The data suggest that σR1 may mediate its neuroprotective function through actions on glial cells. Very recently, the role of σR1 in modulating the inflammatory response of retina-derived microglia, the resident retinal immune cell, was investigated and the data showed that (+)-PTZ suppressed inflammatory responses in this cell type (Zhao et al., 2014). This finding is important given that microglial cells may play a key role in glaucoma. In studies of brain, specifically striatum, Robson et al (2013) reported that the σR1 ligand SN79 mitigated methamphetamine-induced microglial activation and associated increases in cytokine expression in a rodent model of methamphetamine-induced neurotoxicity. Behensky and co-workers reported that stimulation of σR1 receptors prevented activation of microglia in a model of Alzheimer’s disease (Behensky et al, 2013).
The current study focused on retinal radial Müller glial cells and the role of σR1 in regulating cytokine release under inflammatory stress. Müller cells are the major glial population of the retina (reviewed by Reichenbach and Bringmann, 2013). They offer stability to the complex retinal architecture and support the function and metabolism of retinal neurons and blood vessels. Müller cells play a key role in normal retinal function and become activated in response to pathological stimuli. They hypertrophy and proliferate under pathologic conditions leading to formation of glial scars, which fill the spaces left by dying neurons and dysfunctional synapses. Indeed, in diseases such as retinal detachment and proliferative retinopathies they may release factors that can be protective or deleterious.
In the present study, we investigated the role of σR1 in modulating inflammatory mediators secreted by retinal Müller glia. We used lipopolysaccharide (LPS), a major structural component of the outer wall of gram-negative bacteria and a potent activator of the immune system to induce inflammation. Taking advantage of cytokine array technology we detected cytokines that were secreted upon LPS treatment, some of which decreased when cells were treated with (+)-PTZ. We investigated several of these cytokines in more detail and also examined cytokine release in Müller cells in σR1−/− mice. Our data support a role for σR1 in mediating cytokine modulation by retinal Müller glial cells.
Methods and Materials
Isolation of mouse retinal Müller glial cells
C57BL/6J (wildtype (σR1+/+)) mouse breeding pairs (Jackson Laboratories, Bar Harbor, ME) and σR1−/− (homozygous knockout) mice were used. σR1−/− mice were generated as described (Sabino et al., 2009) and the colony was established at Georgia Regents University. Mice were genotyped as described (Ha et al., 2011b). They were maintained according to our protocol approved by the Institutional Animal Care and Use Committee and in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Müller cells were isolated from 5–7 day mice per our method (Ha et al., 2014). Briefly, eyeballs were removed, incubated in DMEM containing penicillin/streptomycin (pen/strep) overnight at room temperature in the dark. They were rinsed in PBS, incubated in buffer containing trypsin, EDTA, and collagenase. Retinas were removed taking care to avoid contamination by the retinal pigment epithelium (RPE), transferred to DMEM media containing 10% fetal bovine serum (FBS) plus pen/strep and pipetted into small aggregates of 10–16 retinas per 100 mm dish. Isolated cells were detected within 1 to 3 days and within 3 to 5 days substantial cell growth ensued. Cultures were washed with medium until only a strong adherent flat cell population remained. Cells were passaged 1 to 3 days after washing and were seeded into culture flasks (50,000 cells/cm2); culture media was changed three times per week.
Immunocytochemical confirmation of σR1 in retinal Müller cells
Purity of Müller cell cultures has been verified (Umapathy et al., 2005). The cells are positive for vimentin and CRALBP, known Müller cell markers; they are negative for neuronal, endothelial and microglial markers and for RPE-65, a protein expressed by RPE cells. Immunocytochemical detection of σR1 and vimentin was performed in the current study. Primary Müller cells from wildtype were seeded on coverslips, grown for 24 h, fixed with icecold 4% paraformaldehyde, washed with PBS-Triton X-100, incubated with Power Block and incubated overnight at 4°C with rabbit polyclonal σR1 (1:1000, Ola et al 2002) or with goat polyclonal anti-vimentin (1:200) antibody. To verify culture purity, especially to investigate contamination by microglia, the cells were incubated with rabbit polyclonal Iba1 (1:50, Wako Chemicals, Richmond, VA) and mouse monoclonal CD11b (1:50, AbCam, Cambridge, MA) , two established markers for microglial cells (Ito et al, 1998; Perego et al, 2011). Cells were washed with PBS-Triton X-100 followed by incubation with appropriate secondary antibodies (goat anti-rabbit IgG coupled to Alexa Fluor 488, donkey anti-goat IgG coupled to Alexa Fluor 488, goat anti-mouse IgG coupled to Alexa Fluor 555 from InVitrogen, Eugene, OR) (1:1000) for 1 h at 37 °C. Cells were washed, coverslipped with Fluoroshield with DAPI (Sigma-Aldrich, St. Louis, MO) to label nuclei. Negative controls were treated identically except that PBS replaced the primary antibodies. Immunofluorescent signals were visualized using an Axioplan-2 fluorescent microscope (Carl Zeiss, Göttingen, Germany) equipped with an HRM camera. Images were captured and processed using Zeiss Axiovision digital image processing software (version 4.7).
Cell viability assay following incubation with LPS
Müller cells were seeded (10,000 cells/well) in 96 well plates, and adhered overnight at 37°C in a humidified chamber (95% O2/5% CO2). They were treated in serum-free media containing LPS over a concentration range: [0.1µg/ml – 20 µg/ml] for 24 h. The supernatant was removed and replaced with fresh media containing MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) provided as part of the Vybrant MTT Cell Proliferation Assay Kit (V-13154, Molecular Probes, Grand Island, NY). Optical density of the supernatant was assessed at 570 nm and cell viability was expressed as percentage of control.
Detection of reactive oxygen species (ROS)
Müller glial cells harvested from σR1+/+ and σR1−/− mice were seeded (50,000 cells/well) in 8-well chamber slides, adhered overnight at 37°C in a humidified chamber (95% O2/5% CO2). They were pre-treated 1 h with (+)-PTZ [3 µM], exposed to LPS [0.1µg/ml] in the presence or absence of (+)-PTZ for 24 h. (Pre-treatment was used to maintain consistency with previous studies showing robust neuroprotection when cells were pre-treated with the (+)-PTZ (Dun et al, 2007)). ROS were detected using CellROX® Green Reagent (Life Technologies, Grand Island, NY, USA). CellROX® green reagent [final 5µM] was added to cells, incubated 30 min at 37°C, and washed three times with PBS. Cells were fixed with 4% paraformaldehyde 10 min, washed and ROS detected by immunofluorescence using the Axioplan-2 fluorescent microscope. Images were captured and processed using Zeiss Axiovision digital image processing software as described above.
Analysis of inflammatory cytokines
Müller glial cells harvested from σR1+/+ mice were seeded at a density of 350,000 cells per well and adhered overnight at 37°C. There were four treatment groups: cells incubated in serum-free media (control); cells incubated in serum-free media containing (+)-PTZ [3 µM]; cells incubated in serum-free media containing LPS [0.1µg/ml]; or cells incubated in serum-free media for a 1 h pre-treatment with (+)PTZ followed by incubation with LPS [0.1µg/ml] in the presence of (+)-PTZ [3 µM]. The cells were incubated for 18 h at 37°C. The cell culture supernatant was collected and assayed for secreted inflammatory cytokines using the Mouse Cytokine Array C3 platform (RayBiotech, Norcross, GA, USA). These arrays are highly sensitive and allow assessment of 62 cytokines. The membranes were blocked with 1X assay diluent followed by incubation with the media harvested from the cells (referred to as “conditioned media”) overnight at 4°C. The membranes were incubated with biotin-labeled anti-cytokines followed by incubation with avidin-HRP (1:1000). The membranes were developed using ECL (Thermo Scientific, Pittsburgh, PA, USA) and detected on HyBlot CL™ autoradiography film (Denville Scientific, NJ, USA). The spots on membranes were quantified using ImageJ (NIH, Version 1.45) and the density (representing a different cytokine) was normalized against the internal standards, provided as part of the array. The density determined using ImageJ was an arbitrary unit value (integrated density value) and fold-differences in cytokine secretion were noted between treatment groups.
Real time quantitative RT-PCR (RT-qPCR) analysis
Expression levels of mRNA transcripts specific for genes encoding MIP1γ, MIP2, MIP3α, IL12 (p40/p70), σR1 and GAPDH were examined in Müller cells. There were three treatment groups of cells incubated in 1% serum-containing media: cells incubated in media (control); cells incubated in media containing LPS [0.1µg/ml]; or cells incubated in media for a 1 h pre-treatment with (+)-PTZ [3µM] followed by incubation with LPS [0.1µg/ml] in the presence of (+)-PTZ [3 µM]. The cells were incubated at 37°C for 1, 6 or 24 h. RT-qPCR was performed per our method (Ha et al, 2014). Total RNA was isolated using TRIzol™ Reagent (Invitrogen, Carlsbad, CA) and quantified. 2 µg of RNA was reverse transcribed using the iScript™ cDNA Synthesis Kit (#170–8891, BioRad Laboratories, Hercules, CA). cDNAs were amplified for 40 cycles using Absolute SYBR Green Fluorescein (BioRad) and gene specific primers (Table 1) using the BioRad CFX96 Real-time System. Expression levels were calculated by comparison of Ct values (delta-delta Ct) (Schmittgen and Livak, 2008).
Table 1.
Primers used for qRT-PCR analysis of genes encoding cytokines.
| Gene | NCBI Accession No. |
Primer Sequence | Predicted band size (bp) |
|---|---|---|---|
| MIP 1γ | NM_011338 | Forward: 5’-CAACAGAGACAAAAGAAGTCCAGAG −3’ Reverse: 5’- CTTGCTGATAAAGATGATGCCC-3’ |
193 |
| MIP2 | NM_009140.2 | Forward: 5’-CGCCCAGACAGAAGTCATAG-3’ Reverse: 5’-TCCTCCTTTCCAGGTCAGTTA-3’ |
132 |
| MIP 3α | NM_016960 | Forward: 5’- GCAGCCAGGCAGAAGCAGC −3’ Reverse: 5’- TCACAGCCCTTTTCACCCAGTTC-3’ |
198 |
| IL12 p40 | NM_008353 | Forward: 5’- TGGTTTGCCATCGTTTTGCTG −3’ Reverse: 5’- ACAGGTGAGGTTCACTGTTTCT −3’ |
123 |
| σR1 | NM_030996 | Forward: 5’-CATTCGGGACGATACTGGGC-3’ Reverse: 5’-CCTGGGTAGAAGACCTCACTTTT −3’ |
101 |
| GAPDH | NM_008084.2 | Forward: 5’-CATGGCCTCCAAGGAGTAAGA-3’ Reverse: 5’-GAGGGAGATGCTCAGTGTTGG-3’ |
104 |
Assessment nuclear translocation of NF-κB)
To determine NF-κB cellular localization, Müller cells harvested from σR1+/+ and σR1−/− mice were seeded in 8-well Lab-Tek chamber slides (Fisher Scientific, Pittsburgh, PA), and allowed to adhere overnight at 37°C. Cells were either not treated (control), were stimulated with 0.1µg/ml LPS for 15 min or were pretreated with (+)-PTZ [3µM] 30 min followed by treatment with LPS [0.1µg/ml] in the presence of (+)-PTZ [3 µM] for 15 min. Cells were fixed in 4% paraformaldehyde, washed three times with 0.1% Triton X-100, blocked (PowerBlock, BioGenex, San Ramon, CA) for 1 h, and incubated with anti-rabbit NF-κB (1:300, Cell Signaling, Danvers, MA, USA) overnight at 4°C followed by 1 h incubation with donkey anti-rabbit Alexafluor 555 (1:1000, In Vitrogen, Eugene, OR). Each treatment (control, LPS, LPS-(+)-PTZ) was performed in triplicate; ten images were captured per well using the Zeiss Axioplan-2 fluorescent microscope (yielding thirty (30) images for analysis per treatment). For each image, the number of cells in which NF-κB localized solely to the nucleus (rather than nucleus and cytoplasm) was determined and the data were expressed as a ratio to the total number of cells in the field.
ELISA detection of MIP1γ, MIP2, MIP3α and IL12 (p40/p70) in Müller cells
Müller cells harvested from σR1+/+ and σR1−/− mice were incubated in serum-free media in presence/absence of 1.0 µg/ml LPS for 24 h. Conditioned media was used in ELISA assays to detect secretion of MIP1γ, MIP2, MIP3α and IL12 (p40/p70). ELISA assays were performed using RayBiotech Mouse ELISA kits: ELM-MIP1γ, ELM-MIP2, ELM-MIP3α and ELM-IL12 (p40/p70) following the manufacturer’s protocol. The 96-well plate (provided with the kit) is coated with immobilized antibody. After the sample was applied to the coated plate, the specific biotinylated antibody (provided with the kit) was added. The HRP-conjugated streptavidin was added followed by tetramethylbenzidine to yield a color reaction, which was detected at an absorbance of 450 nm using the VERSAmax Tunable Microplate Reader (Molecular Devices, Sunnyvale, CA).
Leukocyte adhesion assay
We determined whether conditioned media (harvested as described below) from LPS-treated Müller cells altered the adherence of leukocytes to endothelial cells using human leukocytes purchased from Sanguine Bioscience (Valencia, CA). The viability of leukocytes was assessed using the trypan blue exclusion assay and were labeled with Leukotracker (Cell Biolabs, San Diego, CA), a leukocyte-specific immunofluorescent dye. Human umbilical vein endothelial cells (HUVEC) (ATCC, Manassas, VA) were grown to confluence and were treated with conditioned media collected from Müller cells treated 24 h with 0.1 µg/ml LPS in the presence/absence of 3µM (+)-PTZ. Following treatment, Leukotracker-labeled leukocytes were added to the confluent HUVEC monolayer and incubated for 90 min at 37°C. The HUVEC monolayer was washed three times with RPMI-1640 medium (Life Technology) to remove non-adherent leukocytes. Cells were lysed and fluorescence of the lysate was determined using a Synergy H1 Microplate Reader (BioTek, Winooski, VT) at 480 nm/520 nm; values were plotted as relative fluorescence units (RFU).
HUVEC cell migration assay
To determine whether conditioned media from LPS-treated Müller cells altered endothelial cell migration, HUVEC were seeded in 500µl of serum-free, phenol red-free vascular cell basal medium (ATCC) in the upper chamber of BD Biocoat control inserts (BD Biosciences, Bedford, MA) with 8-µm pore membrane filters following the manufacturer’s guidelines for migration assays. To the lower chamber was added 500µl of conditioned media from Müller cells that had been treated 24 h with 0.1µg/ml LPS in the presence/absence of (+)-PTZ. FBS was added to the conditioned media at the time of the migration experiment to a final concentration of 5%. HUVEC cells were incubated in the conditioned media for 18 h after which non-adherent cells were removed from the upper chamber using a cotton swab. Cells that had migrated through the permeable filter were fixed with 100% methanol, stained using 1% toluidine blue/borax stain (Electron Microscopy Sciences, Hatfield, PA), and washed three times in distilled water. After drying, inserts were visualized using a Zeiss Axiovert S100 inverted microscope and images captured using the Axiovision software. Data represent the number of migrating cells per field examined using the 10x objective. For each well, four fields were quantified. Experiments were performed in triplicate.
Generation of conditioned media
Primary Müller cells harvested from σR1+/+ and σR1−/− mice (350,000 cells per well in a six-well plate) were seeded and allowed to adhere overnight at 37°C. There were three treatment groups: control denotes cells that were maintained in serum-free media 18 h; LPS-treated denotes cells exposed to LPS [0.1 µg/ml] 18 h in serum-free media; LPS + (+)-PTZ denotes (+)-PTZ pre-treatment 1 h in serum-free media followed by incubation in serum-free media in the presence of LPS [0.1µg/ml] + (+)-PTZ [3 µM] for 18 h at 37°C. The cell culture media was removed by pipetting, transferred to a 15 ml tube and centrifuged 1000 rpm for 3 min to remove any cell debris. The supernatant, referred to as “conditioned media” has all the factors released by the Müller cells in response to the treatment. To assess whether the reagents (LPS or (+)-PTZ) directly altered leukocyte adhesion or endothelial cell migration, additional experiments were conducted with serum-free media only, serum-free media containing LPS [0.1 µg/ml] or serum-free media containing LPS [0.1µg/ml] + (+)-PTZ [3 µM].
Statistical analysis
The cytokine array, gene expression, the NF-κB nuclear location analysis, ELISA, leukocyte adhesion and migration data were analyzed by multifactor ANOVA using GraphPad Prism software (Version 6.0, La Jolla, CA). Tukey was the post-hoc test and a p value <0.05 was considered significant.
RESULTS
The σR1 ligand (+)- PTZ inhibits ROS production in Müller glial cells
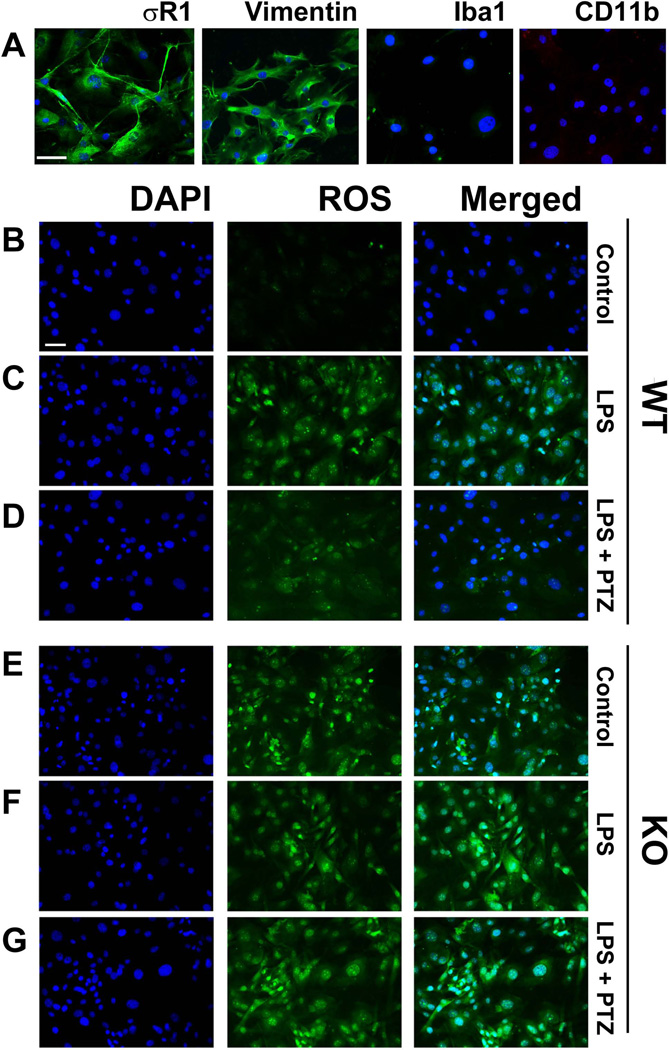
Immunocytochemical analysis confirmed the presence of σR1 in primary Müller cells harvested from wildtype mice. σR1 is detected at the plasma membrane and within the cells (Fig. 1A). The cells were positive for the glial marker vimentin (Fig. 1A, second panel), but were negative for microglial cell markers Iba1 and CD11b (Fig. 1A, third and fourth panels, respectively). Cell viability was not altered in cells incubated 24 h in media containing LPS at a concentration range of 0.1 – 20 µg/ml (data not shown). ROS production was evaluated in σR1+/+ Müller cells exposed 24 h to 0.1 µg/ml LPS and there was marked increase in ROS (Fig. 1C) compared to control cells (Fig. 1B). The concentration of LPS (0.1µg/ml) was sufficient to induce ROS production and unless otherwise stated was the LPS concentration used for the remaining experiments. We observed marked inhibition of ROS production in LPS-incubated cells that were treated with 3µM (+)-PTZ (Fig. 1D). We also examined ROS production in Müller cells harvested from σR1−/− mice. Interestingly, there was an increase in ROS levels detected in cells that received no treatment (Fig. 1E) suggesting an endogenous increase in oxidative stress in the σR1−/− Müller cells. We subjected σR1−/− Müller cells to 0.1 µg/ml LPS in the absence or presence of 3µM (+)-PTZ. ROS levels were elevated in σR1−/− Müller cells treated with LPS (Fig. 1F); there was no effect in ROS production when the σR1−/− Müller cells were treated with (+)-PTZ (Fig. 1G) confirming earlier reports that σR1 is required for (+)-PTZ to mediate effects (Ha et al, 2012; Zhao et al, 2014).
Fig 1. Examination of σR1, vimentin and microglial marker expression and ROS production in primary mouse Müller cells.
(A) Immunolabeling of primary Müller cells with antibodies against σR1 (green florescence); vimentin (green fluorescence); Iba1 (green fluorescence); and CD11b (red fluorescence). Nuclei were stained with DAPI (blue). Panels B – G: Fluorescent detection of ROS (green florescence), nuclei were stained with DAPI (blue florescence) in σR1+/+ (wildtype, WT) cells (B-D) and σR1−/− (knockout, KO) cells (E-G). Müller cells receiving no LPS exposure (control, B and E)); Müller cells exposed 24 h to LPS [0.1 µg/ml] (C and F); Müller cells exposed 24 h to LPS [0.1 µg/ml] pre-and co-treated with (+)-PTZ [3µM] (D and G). Calibration bar = 100 µM.
Analysis of cytokine release
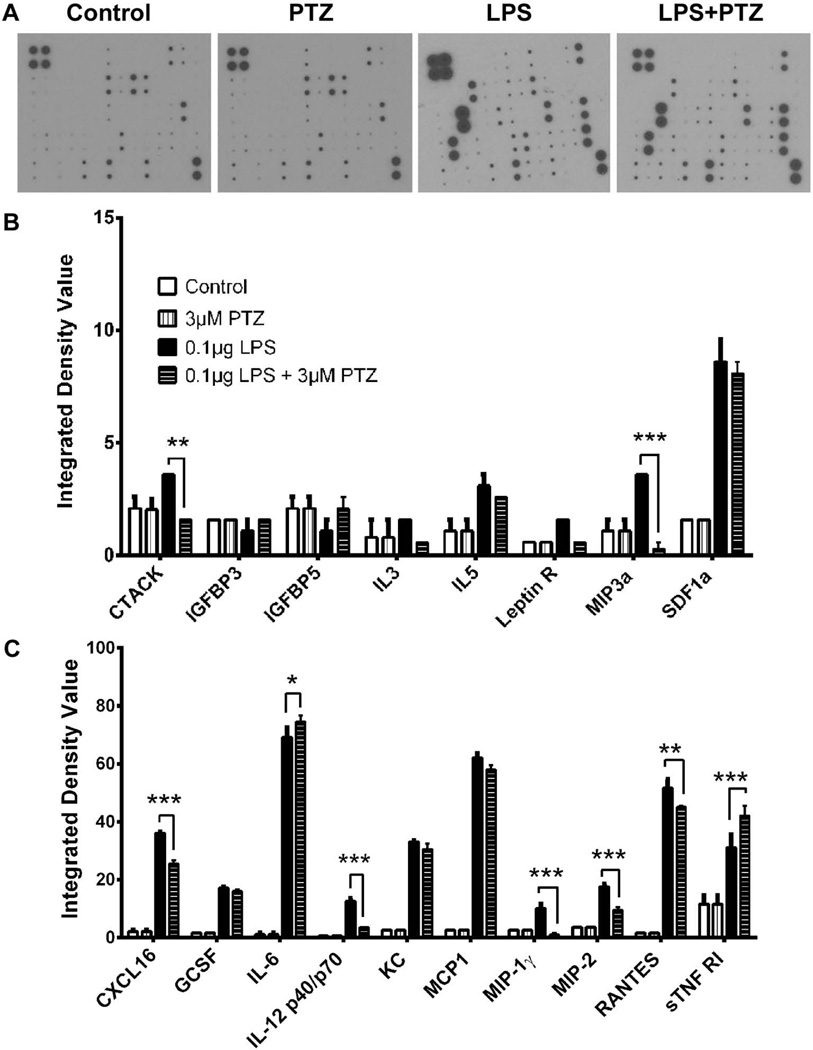
In retinal diseases, cytokines and chemokines are secreted by retinal Müller glial cells as part of an inflammatory response. We utilized antibody array membranes to determine which cytokines/chemokines were secreted in response to LPS stimulation and whether the secretion was altered by treatment with (+)-PTZ. Representative samples of the arrays of conditioned media from control, (+)-PTZ treated, LPS-exposed and LPS-(+)-PTZ treated cells are shown in Fig. 2A. The intensity of each cytokine on the membrane was calculated as an integrated density values (IDV), which is the density of each spot compared to the density of the internal positive controls present on each membrane. Exposure of Müller cells to LPS induced secretion of several inflammatory cytokines that are crucial chemoattractants and activators of macrophages and leukocytes including CXCL16, IL-6, MCP1, RANTES by more than 20 fold; GCSF, IL12 (p40/p70), KC by more than 10 fold; IL-5, MIP3α, SDF1α, MIP1γ, MIP2, sTNFR1 by more than 3 fold (Fig. 2B and 2C). Examination of cytokine release in LPS-(+)-PTZ-treated cells compared to LPS incubation alone revealed a significant decrease in release of CTACK, MIP3α, CXCL16, IL12 (p40/p70), MIP1γ, MIP2, and RANTES. While there was a substantial increase in LPS-induced release of SDF1α, MCP1 and IL-6, the (+)-PTZ treatment did not alter secretion of these cytokines. Thus, (+)-PTZ appears to play a role in modulating Müller cell release of specific cytokines. Incubation of Müller cells with (+)-PTZ alone (absence of any LPS treatment) yielded no difference in cytokine release compared to non-treated cells (Fig. 2B, 2C). Interestingly, we found two factors whose secretion increased by LPS-exposure and then increased further (rather than decreased) when LPS-exposed cells were treated with (+)-PTZ: IL-6 and sTNFR1. Based on the data that the most significant reversal of LPS-stimulated cytokine release following (+)-PTZ treatment involved MIP1γ, MIP2, MIP3α and IL12 (p40/p70), we examined these cytokines in more detail.
Fig 2. Detection of cytokines secreted by primary mouse Müller cells in response to LPS and (+)-PTZ.
(A) Representative arrays used to assess cytokines secreted by primary Müller cells that received no treatment (control), were treated with (+)-PTZ [3µM], were exposed 24 h to LPS [0.1 µg/ml] (LPS) or were exposed 24 h to LPS [0.1 µg/ml] and were pre-and co-treated with (+)-PTZ [3µM] (LPS+PTZ). Spots on each array represent individual cytokines. The upper four spots on the left corner of each array are internal standards provided with the array. Spot intensities were quantified densitometrically and were compared to the density of the internal standards yielding an integrated density value (IDV) for each cytokine. Of the 62 cytokines that can be detected using the array, (B) and (C) provide quantitative data for the cytokines whose secretion was detected in Müller cells (*p<0.05, ** p<0.01, *** p<0.001).
Analysis of gene expression of MIP1γ, MIP2, MIP3α and IL12 (p40/p70)
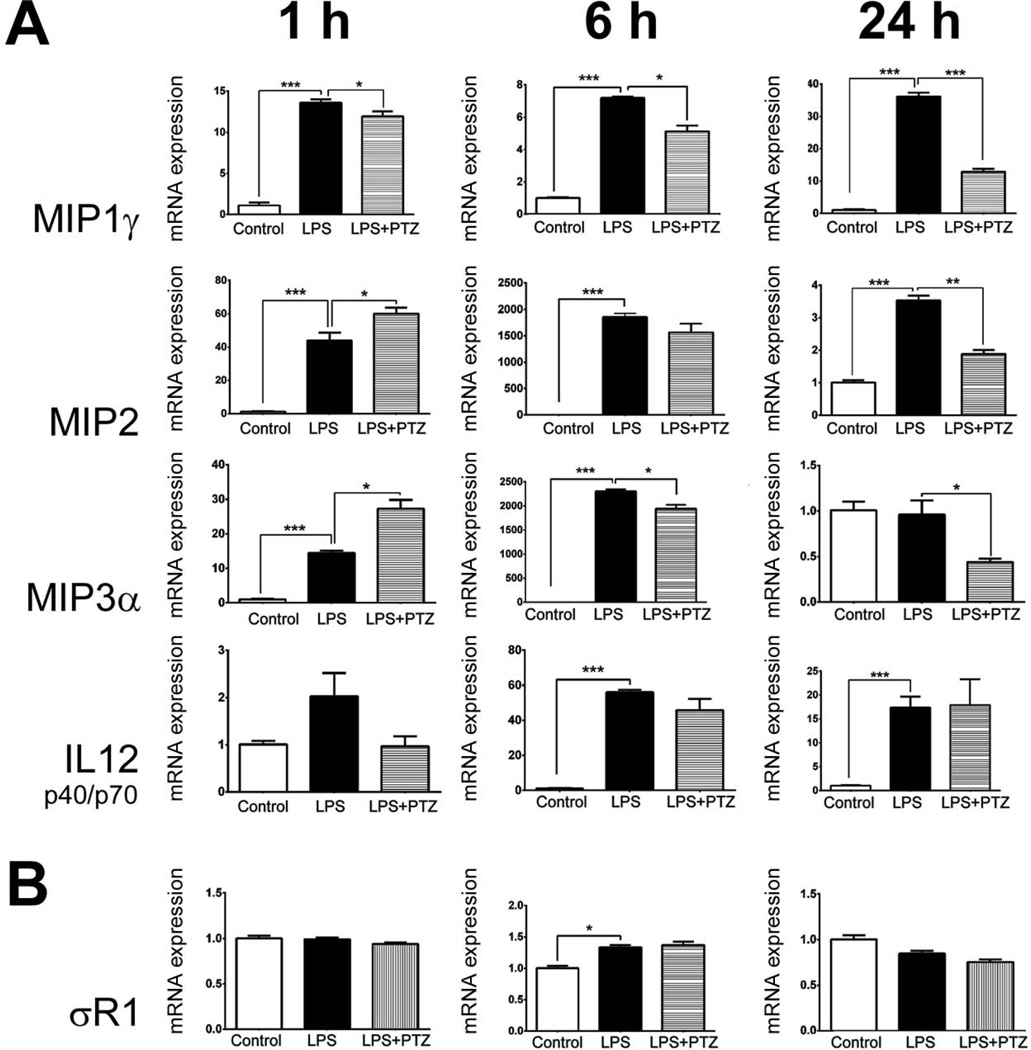
We examined the expression of genes encoding MIP1γ, MIP2, MIP3α and IL12 (p40/p70) in Müller cells exposed to 0.1 µg/ml LPS for 1, 6, or 24 h in the presence/absence of 3 µM (+)-PTZ. For all four genes the level of expression in untreated (control) cells was very low (Fig. 3A), but exposure to LPS led to a significant increase in expression for MIP1γ, MIP2, MIP3α within 1 h and all four of the cytokines within 6 h (and 24 h). Treatment of LPS-incubated cells with (+)-PTZ led to a decrease in expression of MIP1γγ and MIP2 within 6 h, by 24 h the level of genes encoding MIP1γ, MIP2, MIP3α had decreased significantly in LPS-exposed, (+)-PTZ treated cells. There was no significant effect of (+)-PTZ treatment on mRNA levels of IL12 (p40/p70) following LPS incubation. There was no alteration of σR1 mRNA levels in Müller cells exposed to LPS or to LPS-(+)-PTZ (Fig. 3B).
Fig 3. Analysis of genes encoding MIP1γ, MIP2, MIP3α, IL12 (p40/p70) and σR1 in Müller cells treated with LPS and (+)-PTZ.
mRNA was isolated from mouse Müller cells that received no treatment (control), were exposed 1, 6, or 24 h to LPS [0.1 µg/ml] (LPS) or were exposed 1, 6, or 24 h to LPS [0.1 µg/ml] plus pre-and co-treated with (+)-PTZ [3µM] (LPS+PTZ); qRT-PCR was performed to analyze the expression of (A) MIP1γ, MIP2, MIP3α, IL12 p40, (B) σR1 using primer pairs listed in Table 1 Gene levels were expressed relative to gene expression for control (non-LPS treated) condition (arbitrarily assigned a value of 1) and were normalized to GAPDH. Each experiment was performed in triplicate (*p<0.05, ** p<0.01, *** p<0.001).
Analysis of NFκB activation
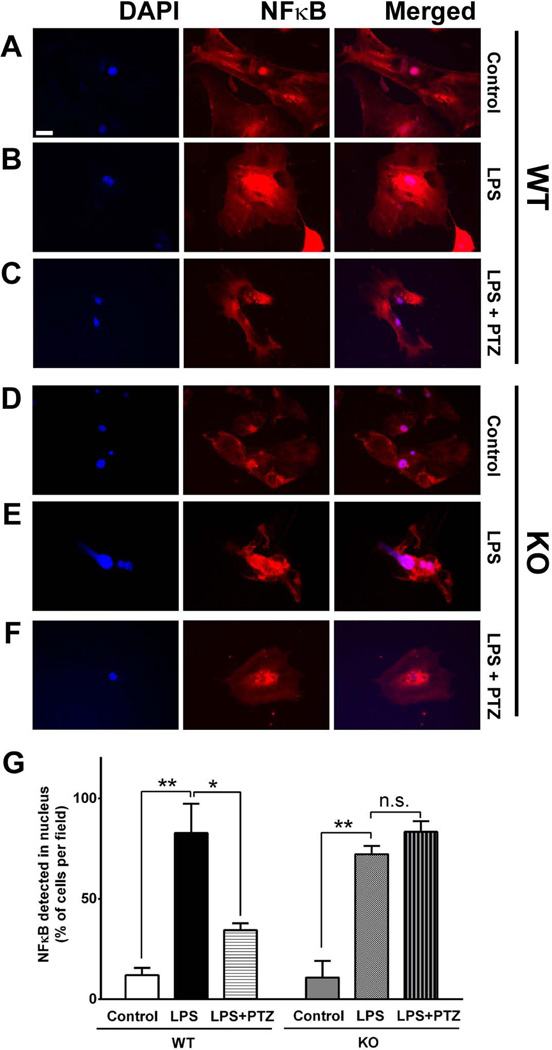
During cellular homeostasis NFκB resides primarily in the cytosol. Upon inflammation NFκB is translocated to the nucleus, where it binds to its promoter to initiate the transcription of inflammatory cytokines. To evaluate the cellular location of NF-κB in Müller cells treated with LPS and consequences on this translocation with (+)-PTZ, we performed an immunofluorescent detection study. Cells harvested from either σR1+/+ and σR1−/− mice were either untreated (control) or were pre-treated with (+)-PTZ followed by 15 min LPS incubation (in the presence or absence of (+)-PTZ). Regarding Müller cells harvested from σR1+/+ mice, there is a basal level of NFκB present in nucleus (co-localized with the nuclear marker DAPI) as well as cytosol of control cells (Fig. 4A). When cells were incubated with LPS there was a marked translocation of NFκB from cytosol to nucleus, such that the fluorescent intensity in the nuclear region was markedly increased (Fig. 4B). In cells incubated with LPS and treated with (+)-PTZ, nuclear NFκB levels decreased markedly (Fig. 4C). For Müller cells harvested from σR1−/− mice, under control conditions the NFκB distributed to the nucleus was similar (Fig. 4D) to that observed in in cells harvested from σR1+/+ mice (Fig. 4A). Following incubation with LPS, there was a marked translocation of NFκB to the nucleus (Fig. 4E), that was not reversed upon (+)-PTZ treatment (Fig. 4F). We quantified these observations by counting the cells in which NFκB had translocated to the nucleus and expressed the data per number of cells in the field (10 fields per treatment; each treatment performed in triplicate). For the Müller cells harvested from σR1+/+ mice, there were significantly more cells with NFκB localized to the nucleus of LPS-incubated cells (~75%) compared to control cells (~10%). However, in LPS-incubated cells treated with (+)-PTZ the number of cells with nuclear NFκB decreased to ~30% (Fig. 4G). The data suggest a role for (+)-PTZ in NFκB nuclear translocation. In experiments using Müller cells harvested from σR1−/− mice, there were significantly more cells with NFκB localized to the nucleus of LPS-incubated cells (~70%) compared to control cells (~10%), but approximately the same number of cells with NFκB localized to the nucleus in the LPS-incubated cells treated with (+)-PTZ (~70%) (Fig. 4G). The data are consistent with notion that σR1 is required for (+)-PTZ to mediate effects (Ha et al, 2012; Zhao et al, 2014).
Fig 4. Immunocytochemical analysis of LPS-induced NFκB translocation.
Immunofluorescent labeling of σR1+/+ (wildtype, WT) and σR1−/− (knockout, KO) mouse Müller cells that (A, C) received no treatment (control), (B, E) were exposed 15 min to LPS [0.1 µg/ml] (LPS), or (C, F) were exposed 15 min to LPS [0.1 µg/ml] plus pre-and co-treated with (+)-PTZ [3µM] (LPS+PTZ) with an antibody against NFκB (p65), detected with fluorescent dye (red); nuclei stained with DAPI (blue). (G) Quantitation of the data: ten fields were imaged per well and cells in which NFκB was located in the nucleus were counted, these data were expressed as a ratio to the total number of cells in the field. The experiments were performed in triplicate and values expressed as percentage of cells per field that localized NF-κB to the nucleus (*p<0.05, ** p<0.01, n.s. = not significant). Calibration bar = 100 µM.
ELISA analysis of cytokine release in WT and σR1−/− Müller cells
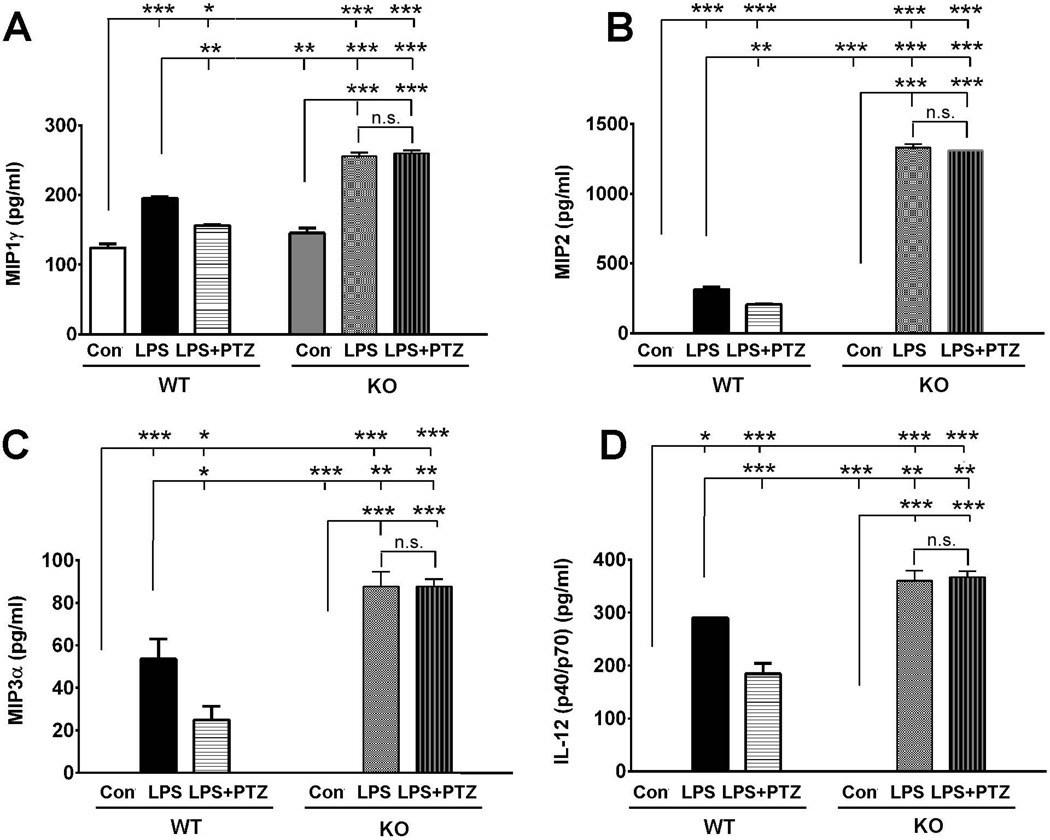
The above-mentioned data indicated that cytokine release by Müller cells, which was stimulated with LPS, could be attenuated by (+)-PTZ raising the question whether there is any alteration in cytokine expression when σR1 is not present. We isolated Müller cells from σR1+/+ and σR1−/− mice and incubated the cells with 0.1µg LPS for 24 h and then used ELISA to analyze secretion of MIP1γ, MIP2, MIP3α and IL12 (p40/p70). When we analyzed the secretion of these cytokines between the σR1+/+ and σR1−/− Müller cells, we did not observe significant differences in cytokine release. (For example, with MIP1γ, the baseline secretion for σR1+/+ cells was ~124.1 pg/ml and for σR1−/− cells was ~145.3 pg/ml (Fig. 5A)). A similar trend was observed with other cytokines examined (MIP2, MIP3α and IL12 (p40/p70) (Fig. 5B–D). However, when cells were incubated with LPS, we noted significant differences in cytokine secretion in the presence versus absence of σR1. For example there was a 24% (~1.3 fold) increase in MIP1γ secretion in σR1−/− cells (~255.7 pg/ml) compared to σR1+/+ cells (~195.1 pg/ml) (Fig. 5A). For MIP2, there was a 5 fold increase in release by the LPS-stimulated σR1−/− cells compared to σR1+/+ (Fig. 5B). For MIP3α the σR1−/− cells exposed to LPS showed ~1.6 fold increase in secretion compared to the σR1+/+ cells (Fig. 5C). For IL12 (p40/p70) there was ~1.25 fold increase in secretion for σR1−/− LPS-exposed cells compared with σR1+/+ LPS-exposed cells (Fig. 5D). Additionally, we examined the release of MIP2, MIP3α and IL12 (p40/p70) in cells exposed to LPS in the presence of (+)-PTZ. While we observed attenuation of cytokine release in LPS-incubated σR1+/+ cells treated with (+)-PTZ, we did not observe a change in the release of these cytokines in LPS-incubated σR1−/− cells when treated with (+)-PTZ (Fig. 5A–D).
Fig 5. ELISA detection of MIP1γ, MIP2, MIP3α, and IL12 (p40/p70) secretion by WT and σR1−/− Müller cells.
Müller cells harvested from either σR1+/+ (wildtype, WT) or σR1−/− (knockout, KO) mice were incubated with or without LPS [1.0 µg/ml] for 24 h in the presence/absence of (+)-PTZ [3µM] (LPS+PTZ) following which the conditioned media was collected and subjected to ELISA to quantify secretion of (A) MIP1γ, (B) MIP2, (C) MIP3α,and (D) IL12 (p40/p70). Data are presented as pg/ml. Each experiment was performed in triplicate (*p<0.05, ** p<0.01, *** p<0.001, n.s. = not significant).
Leukocyte adhesion and endothelial cell migration
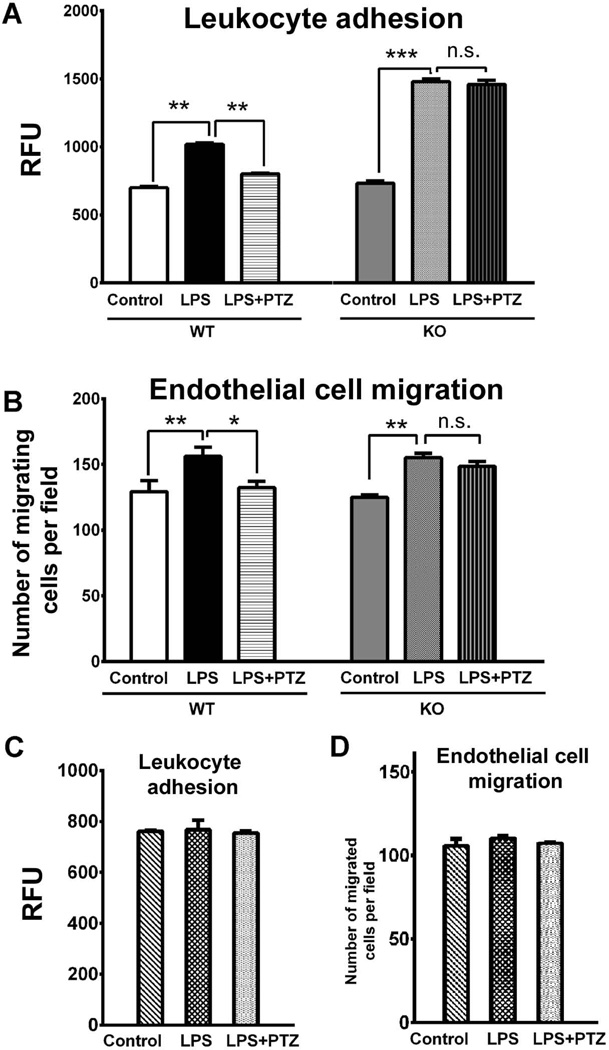
Adhesion of leukocytes to endothelial cells disrupts the blood-retinal barrier, damages endothelial cells and impairs capillary perfusion (Stone et al., 1996, Joussen et al., 2001; Miyamoto et al., 2000). To examine the role of proinflammatory cytokines secreted by Müller cells on leukocyte recruitment and infiltration during inflammation, we used human leukocytes and examined their adherence to HUVEC when exposed to media conditioned by Müller cells harvested from σR1+/+ and σR1−/− mice incubated with LPS in the presence/absence of (+)-PTZ. When HUVEC were incubated for 24 h with the conditioned medium of LPS-exposed σR1+/+ Müller cells, leukocyte adhesion increased by two fold (Fig. 6A). The LPS-induced leukocyte adhesion was inhibited by (+)-PTZ treatment (Fig. 6A). When HUVEC were incubated for 24 h with the conditioned medium of LPS-exposed σR1−/− Müller cells, leukocyte adhesion increased by two fold (Fig. 6A), however the adhesion was not inhibited by (+)-PTZ treatment (Fig. 6A).
Fig 6. Assessment of leukocyte adhesion and endothelial cell migration as a consequence of LPS stimulation of Müller cells.
Media conditioned by either σR1+/+ (wildtype, WT) or σR1−/− (knockout, KO) Müller cells that received no treatment (control), were exposed 24 h to LPS [0.1 µg/ml] (LPS) or were exposed 24 h to LPS [0.1 µg/ml] and were pre-and co-treated with (+)-PTZ [3µM] (LPS+PTZ) was used to evaluate: (A) Effects on the adhesion of leukocytes to endothelial cells. The graph depicts level of fluorescence of the lysate detected at 480 nm/520 nm; values were plotted as relative fluorescence units (RFU). (B) Extent of migration of HUVEC cells through a porous filter. The graph depicts the number of cells that had migrated through the porous filter detected by immunofluorescence. Data are expressed as mean ± S.E.M. Each experiment was performed in triplicate; *p<0.05, ** p<0.01, n.s. = not significant. The effects on leukocyte adhesion (C) or endothelial cell migration (D) when exposed to serum-free media (control), or media containing LPS, or media containing LPS and (+)-PTZ were investigated in the same manner as described for the conditioned media studies. There were no significant effects on leukocyte adhesion or endothelial cell migration when incubated under these conditions.
The proliferation and migration of endothelial cells is required to form capillary tubes and several anti-angiogenic studies have focused on delaying or inhibiting these processes (Kwon et al., 2005). We examined whether exposing HUVEC to media harvested from σR1+/+ and σR1−/− Müller cells that had been exposed to LPS in the presence/absence of (+)-PTZ would alter cell migration through a porous filter. Conditioned media from LPS-exposed σR1+/+ cells induced ~1.8 fold increase in endothelial migration compared with control (non-LPS treated) cells (Fig. 6B); media harvested from σR1+/+ cells treated with LPS in the presence of (+)-PTZ showed endothelial cell migration that was similar to control cells (Fig. 6B). Conditioned media from LPS-exposed σR1−/− cells induced ~1.8 fold increase in endothelial migration compared with non-LPS treated cells (Fig. 6B); however, media harvested from σR1−/− cells treated with LPS in the presence of (+)-PTZ did not alter the LPS-induced migration (Fig. 6B). Since our conditioned media from these incubations also contained LPS or LPS + (+)-PTZ, we investigated whether the reagents (LPS or (+)-PTZ) directly altered leukocyte adhesion or endothelial cell migration. Additional experiments were conducted with serum-free media only, serum-free media containing LPS [0.1 µg/ml] or serum-free medial containing LPS [0.1µg/ml] + (+)-PTZ [3 µM]. We observed no differences in leukocyte adhesion (Fig. 6C) or endothelial cell migration (Fig. 6D) when the experiments were conducted using serum containing LPS in the presence or absence of (+)-PTZ.
Discussion
This study represents the first analysis of σR1 modulation of retinal Müller glial cell cytokine secretion. Four major findings emerge from this work. First, activation of σR1 by its high-affinity ligand (+)-PTZ attenuated LPS-induced secretion of certain cytokines and their gene expression. Second, activation of σR1 by (+)-PTZ was accompanied by an inhibition of NFκB nuclear translocation. Third, there was a significant difference in LPS-stimulated cytokine secretion by Müller glial cells lacking σR1 compared to those expressing the receptor. Fourth, following LPS exposure, factors secreted into the media by Müller cells stimulated leukocyte adhesion and endothelial migration, which were attenuated by (+)-PTZ treatment.
Many sight-threatening retinal diseases feature inflammation as a contributor to pathology including diabetic retinopathy and macular degeneration (de Oliveira Dias et al., 2011; Zarbin and Rosenfeld, 2010). A component of the inflammatory process is cytokine release. Our study used LPS, an inducer of inflammation to determine cytokines secreted by Müller cells during inflammation and whether treatment with (+)-PTZ would impact cytokine release. We took advantage of highly sensitive array technology that permits simultaneous assessment of 62 secreted cytokines and found that LPS exposure triggered secretion of several cytokines. The secretion of some of these was reduced when the cells were treated with (+)-PTZ. We investigated comprehensively three members of the large macrophage inflammatory protein (MIP) family (MIP1γ, MIP2, MIP3α) and IL12 (p40/p70). MIP1γ (CCL9) is secreted by dendritic cells, macrophages and myeloid cell lines (Hara et al., 1995) that are crucial for monocytes and T-cell chemotaxis from the circulation to inflamed tissue. It plays an important role in regulation of transendothelial migration of monocytes, dendritic cells, and natural killer cells (Maurer and Von Stebut, 2004). MIP2 (CXCL2) is a major inducible chemokine secreted by macrophages (Wolpe et al., 1999), epithelial cells, vascular endothelial cells, astrocytes, mast cells and neutrophils (Zhao et al., 2000; Mancardi et al., 2000; Biedermann et al., 2000; Armstrong et al., 2004). In retina, MIP-2 expression was induced in a mouse model of ischemia induced by central retinal artery occlusion (Kramer et al., 2009). Additionally, LPS exposure upregulated MIP-2 in retinal microglia and Müller cells (Kochan et al., 2012; Lin et al., 2013). MIP3α (CCL20) is constitutively expressed by epithelial (Li et al., 2011) and immune cells (Maddur et al., 2012) in normal, homeostatic conditions and is upregulated in inflammatory conditions (Schutyser et al., 2003). Increased expression of MIP-3α stimulates T-cell migration and accelerates the rate of dry eye disease; blocking MIP-3α reduces the immune response and ocular surface inflammation (Dohlman et al., 2013). Expression of genes encoding MIP-1γ, MIP-2, MIP3α was increased dramatically within 1 h of LPS exposure and remained elevated over 24 h. Gene expression was attenuated within 6–24 h in those LPS-exposed cells treated with (+)-PTZ, suggesting that σR1 activation regulates the transcription of these cytokines.
We investigated IL12 (p40/p70), whose LPS-induced secretion in Müller glial cells was attenuated by treatment with (+)-PTZ. IL12 (p40/p70) is an interleukin secreted by antigen presenting cells including dendritic cells and macrophages in response to T cell engagement of the MHC class II and CD40 molecules (Cella et al., 1996; Koch et al., 1996). Increased levels of IL12 (p40/p70) have been implicated in branch retinal vein occlusion (BRVO); blockade of IL12 (p40/p70) significantly improved visual acuity and retinal thickness in BRVO (Kaneda et al., 2011). LPS-induced increase of IL12 (p40/p70) was reported in retinal Müller glia in a study evaluating the inflammatory-inhibiting effects of endocannabinoids (Krishnan and Chatterjee, 2012). Increased expression of IL12 (p40/p70) has also been observed in experimental autoimmune uveitis (Sun et al., 1999).
Several studies reported the neuroprotective role (e.g. survival of RGCs) upon exposure to IL-6 after ischemia-reperfusion injury in vivo and in vitro (Inomata et al., 2003,Sanchez et al., 2003; Mendonca Torres et al., 2001). Thus, our observation that (+)-PTZ induced an increase in IL-6 secretion in Müller cells upon LPS stimulation may suggest a role in protecting retina from inflammatory insults and warrant further study. We observed an increase also in secretion of soluble tumor necrosis factor 1 (sTNFR1), a natural homeostatic regulator of TNF-α (Aderka et al., 1992) in LPS-exposed Müller cells treated with (+)-PTZ. However, we did not observe LPS-induced secretion of TNFα in our model system, so the significance of the upregulation of sTNFR1 is not clear and awaits further study. It is noteworthy that there were cytokines, whose secretion was stimulated by LPS that were not affected by the (+)-PTZ treatment, for example MCP1. MCP1 has been implicated in retinal disease (Zhao et al., 2014), but it appears that activation σR1 does not modulate its secretion, at least in our experimental system. The modulation of cytokine secretion by (+)-PTZ appears to be cytokine-specific rather than a pan-cytokine effect.
NFκB is a ubiquitous redox sensitive transcription factor regulating the transcription of a wide range of genes. It is a central regulator of microglial response to activating stimuli including LPS and cytokines (Pahl, 1999). In the cytosol, NFκB exists as a p65/p50 heterodimer in complex with an inhibitor protein, IκB. Upon activation, the IκB dissociates from the complex and is degraded by the proteasome. The p65/p50 complex translocates to the nucleus, which can trigger a variety of cellular responses including the release of cytokines and chemokines. We investigated the location of NFκB under LPS-stimulated conditions in the presence/absence of σR1 activation using immunofluorescence methods to detect the p65 portion of the protein. Our data showed a marked increase in NFκB nuclear translocation that was attenuated by σR1 activation. The observation may have relevance to retinal disease since NFκB has been implicated in diabetic retinopathy, experimental immunouveitis and oxidative stress-induced retinal degeneration (Kern, 2007; Kubota et al., 2009; Fang et al., 2013, Krishnan and Chatterjee, 2012). We observed no reversal of LPS-induced nuclear NFκB translocation when σR1−/− cells were treated with (+)-PTZ, emphasizing the requirement for σR1 for (+)-PTZ to mediate effects on NFκB.
To investigate further the role of σR1 in secretion of cytokines by Müller glial, we harvested Müller cells from σR1−/− mice and maintained them in the same manner as Müller cells isolated from σR1+/+ mice. We analyzed the secretion of MIP-1γ, MIP-2, MIP3α and IL12 (p40/p70) by ELISA and found that cells that had not been exposed to LPS were comparable in the secretion of these cytokines regardless of whether they were isolated from σR1+/+ or σR1−/− mice. However, when σR1−/− cells were exposed to LPS, there was a marked increase in release of these cytokines compared to σR1+/+ cells. This supports the notion that σR1 may function as a mediator of cellular stress. This has been suggested by earlier studies. σR1−/− demonstrate a late onset degeneration of retinal ganglion cells (RGCs) (Ha et al., 2011a). Mavlyutov et al (2011) subjected σR1+/+ and σR1−/− mice to optic nerve crush and reported more RGC death in σR1−/− mice compared to σR1+/+ mice. Ha and colleagues induced diabetes in σR1+/+ and σR1−/− mice and detected normal retinal structure and function at young ages in both groups; however, when subjected to the chronic stress of diabetes, there was an acceleration of retinal functional deficits in σR1−/− mice such that ganglion cell dysfunction was observed at a much earlier age than non-diabetic σR1−/− mice (Ha et al, 2012). These reports, in concert with the present data showing increased stress-induced cytokine release in the absence of σR1, support the hypothesis that σR1 plays a role in modulating retinal stress.
Glial cells interact with vascular cells. Proinflammatory cytokines and adhesion molecules produced during inflammation can induce migration of endothelial cells, which is a pathogenic feature of age-related macular degeneration and proliferative diabetic retinopathy (de Oliveira Dias et al., 2011; Zarbin and Rosenfeld, 2010). Leukocyte adhesion can lead to blood-retinal barrier breakdown, endothelial damage and impaired capillary perfusion (Stone et al., 1996, Joussen et al., 2001). Leukocyte recruitment and infiltration is an essential and common feature of inflammation especially related to endothelial cells. To explore the functional relevance of our findings of σR1 modulation of cytokine release, we utilized conditioned media (from LPS-exposed σR1+/+ and σR1−/− Müller glial cells) in experiments with leukocytes and endothelial cells and observed increased leukocyte-endothelial cell adhesion and endothelial cell migration. These phenomena were attenuated when cells were subjected to media of LPS-exposed σR1+/+ Müller cells treated with (+)-PTZ (but not the media from LPS-incubated, (+)-PTZ treated σR1−/− cells). These experiments represent functional assessment of the consequences on vascular cells when Müller cells are subjected to inflammatory stimulation and suggest a new avenue for investigation, namely the role of σR1 in glial-vascular interactions. Our observation is noteworthy given previous reports that selective activation of σR1 reduced leukocyte invasion and the levels of inflammatory cytokines in vivo (Gardner et al., 2004).
In conclusion, our study demonstrates that activation of σR1 can attenuate LPS-induced release of inflammatory cytokines in primary mouse Müller glial cells and that it may involve NFκB. Media conditioned by Müller cells treated with LPS and (+)-PTZ can inhibit leukocyte adhesion and endothelial cell migration, which occurs in LPS-stimulated cells. Müller cells are the principal glial cells in the retina crucial in supporting neurons under normal and pathological conditions. While earlier studies of σR1-mediated retinal neuroprotection considered the neuron as the target for beneficial effects, the current data raise the important possibility that σR1 ligands may mediate neuroprotection via their actions on retinal Müller glial cells.
Acknowledgments
We thank Dr. M. Al-Shabrawey and members of his lab for use of the microplate reader and for discussions about leukocyte adhesion assay. This work was supported by the National Eye Institute (NIH) R01EY014560.
Glossary
List of abbreviations
- CRALBP
Cellular retinaldehyde binding protein
- CTACK
Cutaneous T-cell-attracting chemokine
- CXCL16
Chemokine (C-X-C motif) ligand 16
- ELISA
Enzyme-linked immunosorbent assay
- ER
Endoplasmic Reticulum
- FBS
fetal bovine serum
- GCSF
Granulocyte colony-stimulating factor
- HRP
Horseradish peroxidase
- IκB
Inhibitor of kappa B
- IDV
Integrated density values
- IGFBP3
Insulin-like growth factor-binding protein 3
- IGFBP5
Insulin-like growth factor-binding protein 5
- IL12(p40/p70)
Interleukin 12 (p40/p70)
- IL16
Interleukin 16
- IL3
Interleukin 3
- IL5
Interleukin 5
- KC
Keratinocyte chemoattractant
- LPS
Lipopolysaccharide
- MCP1
Monocyte chemotactic protein
- MIP1γ
Macrophage Inflammatory Protein 1 gamma
- MIP2
Macrophage inflammatory protein 2
- MIP3α
Macrophage Inflammatory Protein-3alpha
- NFκB
Nuclear factor kappa B
- RANTES
Regulated on activation, normal T cell expressed and secreted
- RFU
Relative fluorescence units
- RGC
Retinal ganglion cell
- ROS
Reactive oxygen species
- RPE
Retinal pigment epithelium
- SDF1α
Stromal cell-derived factor 1alpha
- sTNFR1
Soluble TNF receptor 1
- (+)-PTZ
(+)-Pentazocine
- σR1
sigma receptor 1
References
- Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DA, Major JA, Chudyk A, Hamilton TA. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J Leukoc Biol. 2004;75:641–648. doi: 10.1189/jlb.0803370. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, Kunkel SL, Hültner L, Röcken M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behensky AA, Yasny IE, Shuster AM, Seredenin SB, Petrov AV, Cuevas J. Stimulation of sigma receptors with afobazole blocks activation of microglia and reduces toxicity caused by amyloid-β25–35. J Pharmacol Exp Ther. 2013;347:458–467. doi: 10.1124/jpet.113.208348. [DOI] [PubMed] [Google Scholar]
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Dias JR, Rodrigues EB, Maia M, Magalhães O, Jr, Penha FM, Farah ME. Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol. 2011;95:1631–1637. doi: 10.1136/bjo.2010.186361. [DOI] [PubMed] [Google Scholar]
- Dohlman TH, Chauhan SK, Kodati S, Hua J, Chen Y, Omoto M, Sadrai Z, Dana R. The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci. 2013;54:4081–4091. doi: 10.1167/iovs.12-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun Y, Thangaraju M, Prasad P, Ganapathy V, Smith SB. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine, a sigma receptor-1 specific ligand. Invest Ophthalmol Vis Sci. 2007;48:4785–4794. doi: 10.1167/iovs.07-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang IM, Yang CH, Yang CM, Chen MS. Chitosan oligosaccharides attenuates oxidative-stress related retinal degeneration in rats. PLoS One. 2013;8:e77323. doi: 10.1371/journal.pone.0077323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner B, Zhu LX, Roth MD, Tashkin DP, Dubinett SM, Sharma S. Cocaine modulates cytokine and enhances tumor growth through sigma receptors. J Neuroimmunol. 2004;147:95–98. doi: 10.1016/j.jneuroim.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Ha Y, Dun Y, Thangaraju M, Duplantier J, Dong Z, Liu K, Ganapathy V, Smith SB. Sigma receptor 1 modulates endoplasmic reticulum stress in retinal neurons. Invest Ophthalmol Vis Sci. 2011a;52:527–540. doi: 10.1167/iovs.10-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Saul A, Tawfik A, Williams C, Bollinger K, Smith R, Tachikawa M, Zorrilla E, Ganapathy V, Smith SB. Late-onset inner retinal dysfunction in mice lacking sigma receptor 1 (σR1) Invest Ophthalmol Vis Sci. 2011b;52:7749–7760. doi: 10.1167/iovs.11-8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Saul A, Tawfik A, Zorrilla EP, Ganapathy V, Smith SB. Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1. Mol Vis. 2012;18:2860–2870. [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Shanmugam AK, Markand S, Zorrilla E, Ganapathy V, Smith SB. Sigma receptor 1 modulates ER stress and Bcl2 in murine retina. Cell Tissue Res. 2014;356:15–27. doi: 10.1007/s00441-013-1774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site Proc Natl Acad Sci USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER- mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Inomata Y, Hirata A, Yonemura N, Koga T, Kido N, Tanihara H. Neuroprotective effects of interleukin-6 on NMDA-induced rat retinal damage. Biochem Biophys Res Commun. 2003;302:226–232. doi: 10.1016/s0006-291x(03)00127-x. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, Ganapathy V, Smith SB. Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Invest Ophthalmol Vis Sci. 2006;47:5576–5582. doi: 10.1167/iovs.06-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda S, Miyazaki D, Sasaki S, Yakura K, Terasaka Y, Miyake K, Ikeda Y, Funakoshi T, Baba T, Yamasaki A, Inoue Y. Multivariate analyses of inflammatory cytokines in eyes with branch retinal vein occlusion: relationships to bevacizumab treatment. Invest Ophthalmol Vis Sci. 2011;52:2982–2988. doi: 10.1167/iovs.10-6299. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem Biophys Res Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:1–14. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan T, Singla A, Tosi J, Kumar A. Toll-like receptor 2 ligand pretreatment attenuates retinal microglial inflammatory response but enhances phagocytic activity toward Staphylococcus aureus. Infect Immun. 2012;80:2076–2088. doi: 10.1128/IAI.00149-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M, Dadon S, Hasanreisoglu M, Monselise Y, Avraham BR, Feldman A, Eldar I, Weinberger D, Goldenberg-Cohen N. Proinflammatory cytokines in a mouse model of central retinal artery occlusion. Mol Vis. 2009;15:885–894. [PMC free article] [PubMed] [Google Scholar]
- Krishnan G, Chatterjee N. Endocannabinoids alleviate proinflammatory conditions by modulating innate immune response in muller glia during inflammation. Glia. 2012;60:1629–1645. doi: 10.1002/glia.22380. [DOI] [PubMed] [Google Scholar]
- Kubota S, Kurihara T, Mochimaru H, Satofuka S, Noda K, Ozawa Y, Oike Y, Ishida S, Tsubota K. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50(7):3512–3519. doi: 10.1167/iovs.08-2666. [DOI] [PubMed] [Google Scholar]
- Kwon YS, Hong HS, Kim JC, Shin JS, Son Y. Inhibitory effect of rapamycin on corneal neovascularization in vitro and in vivo. Invest Ophthalmol Vis Sci. 2005;46:454–460. doi: 10.1167/iovs.04-0753. [DOI] [PubMed] [Google Scholar]
- Li Z, Burns AR, Miller SB, Smith CW. CCL20, cd T cells, and IL- 22 in corneal epithelial healing. FASEB J. 2011;25:2659–2668. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Fang D, Zhou H, Su SB. The expression of Toll-like receptors in murine Müller cells, the glial cells in retina. Neurol Sci. 2013;34:1339–1346. doi: 10.1007/s10072-012-1236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LL, Wang L, Zhong YM, Yang XL. Expression of sigma receptor 1 mRNA and protein in rat retina. Neuroscience. 2010;167:1151–1159. doi: 10.1016/j.neuroscience.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Mancardi S, Vecile E, Dusetti N, Calvo E, Stanta G, Burrone OR, Dobrina A. Evidence of CXC, CC and C chemokine production by lymphatic endothelial cells. Immunology. 2003;108:523–530. doi: 10.1046/j.1365-2567.2003.01613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligands. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Nickells RW, Guo LW. Accelerated retinal ganglion cell death in mice deficient in the Sigma-1 receptor. Mol Vis. 2011;17:1034–1043. [PMC free article] [PubMed] [Google Scholar]
- Mendonca Torres PM, de Araujo EG. Interleukin-6 increases the survival of retinal ganglion cells in vitro. J Neuroimmunol. 2001;117:43–50. doi: 10.1016/s0165-5728(01)00303-4. [DOI] [PubMed] [Google Scholar]
- Mueller BH, Park Y, Daudt DR, Ma HY, Akopova I, Stankowska DL, Clark AF, Yorio T. Sigma-1 receptor stimulation attenuates calcium influx through activated L-type Voltage Gated Calcium Channels in purified retinal ganglion cells. Exp Eye Res. 2013;107:21–31. doi: 10.1016/j.exer.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Ola MS, Moore P, El-Sherbeny A, Roon P, Agarwal N, Sarthy VP, Casellas P, Ganapathy V, Smith SB. Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Brain Res Mol Brain Res. 2001;95:86–95. doi: 10.1016/s0169-328x(01)00249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola MS, Moore PM, Maddox D, El-Sherbeny A, Huang W, Roon P, Agarwal N, Ganapathy V, Smith SB. Analysis of Sigma Receptor (σR1) expression in retinal ganglion cells cultured under hyperglycemic conditions and in diabetic mice. Brain Res Mol Brain Res. 2002;107:97–107. doi: 10.1016/s0169-328x(02)00444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Turner RC, Naser ZJ, McCurdy CR, Huber JD, Matsumoto RR. SN79, a sigma receptor ligand, blocks methamphetamine-induced microglial activation and cytokine upregulation. Exp Neurol. 2013;247:134–142. doi: 10.1016/j.expneurol.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype Behav. Brain Res. 2009;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RN, Chan CK, Garg S, Kwong JM, Wong MJ, Sadun AA, Lam TT. Interleukin-6 in retinal ischemia reperfusion injury in rats. Invest Ophthalmol Vis Sci. 2003;44:4006–4011. doi: 10.1167/iovs.03-0040. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative Ct method. Nat Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a σ receptor from rat brain. J Neurochem. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun. 1997;241:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- Smith SB, Duplantier J, Dun Y, Mysona B, Roon P, Martin PM, Ganapathy V. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci. 2008;49:4154–4161. doi: 10.1167/iovs.08-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Chan-Ling T, Pe’er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1996;37:290–299. [PubMed] [Google Scholar]
- Sun B, Sun SH, Chan CC, Wiggert B, Caspi RR. Autoimmunity to a pathogenic retinal antigen begins as a balanced cytokine response that polarizes towards type 1 in a disease-susceptible and towards type 2 in a disease-resistant genotype. Int Immunol. 1999;11:1307–1312. doi: 10.1093/intimm/11.8.1307. [DOI] [PubMed] [Google Scholar]
- Tchedre KT, Yorio T. Sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Invest Ophthalmol Vis Sci. 2008;49:2577–2288. doi: 10.1167/iovs.07-1101. [DOI] [PubMed] [Google Scholar]
- Umapathy NS, Li W, Mysona BA, Smith SB, Ganapathy V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Müller cells. Invest Ophthalmol Vis Sci. 2005;46:3980–3987. doi: 10.1167/iovs.05-0488. [DOI] [PubMed] [Google Scholar]
- Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuensch B. The σ1 receptor antagonist S1RA is a promising candidate for the treatment of neurogenic pain. J Med Chem. 2012;55:8209–8210. doi: 10.1021/jm3011993. [DOI] [PubMed] [Google Scholar]
- Zarbin MA, Rosenfeld PJ. Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina. 2010;30(9):1350–1367. doi: 10.1097/IAE.0b013e3181f57e30. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ha Y, Liou GI, Gonsalvez GB, Smith SB, Bollinger KE. Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Invest Ophthalmol Vis Sci. 2014;55:3375–3384. doi: 10.1167/iovs.13-12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MQ, Stoler MH, Liu AN, Wei B, Soguero C, Hahn YS, Enelow RI. Alveolar epithelial cell chemokine expression triggered by antigen-specific cytolytic CD8(+) T cell recognition. J Clin Invest. 2000;106:R49–R58. doi: 10.1172/JCI9786. [DOI] [PMC free article] [PubMed] [Google Scholar]