Abstract
BACKGROUND:
Alloimmunization remains a significant complication of transfusion and has been associated with multiple factors, including inflammation, an important pathophysiologic mechanism in sickle cell disease (SCD). We explored whether alloimmunization is associated with disease severity in SCD.
STUDY DESIGN AND METHODS
Adult SCD patients were enrolled in a study of outcome modifying genes in SCD. Historical records of patients with SCD at two participating institutions were reviewed for data on antigen phenotype and alloimmunization. Differences in demographic, clinical and laboratory findings, end organ damage, and overall disease severity were then compared between alloimmunized and non-alloimmunized patients.
RESULTS
Of 319 patients, 87 (27%) were alloimmunized. Alloantibody specificities differed from those previously described, especially due to the significantly higher frequency of anti-S. Although alloimmunization was not associated with frequency of vaso-occlusive episodes, a higher percentage of alloimmunized patients had chronic pain, as defined by daily use of short acting narcotics (p=0.006), long acting narcotics (p=0.013), or both (p=0.03). Additionally, alloimmunized patients had poorer survival (HR=1.92, p=0.01) and were more likely to have avascular necrosis (p=0.024), end-organ damage (p=0.049) and red cell autoantibodies (p<0.001), even after controlling for the effects of age, gender, and hemoglobin diagnosis. Alloimmunization was not associated with other SCD related complications, such as acute chest syndrome or stroke.
CONCLUSIONS
Alloimmunization in SCD may be associated with chronic pain, risk of end-organ damage, and shorter survival. These novel findings suggest new directions for the investigation of immune response-mediated pathways common to alloimmunization and chronic pain.
Keywords: Immunohematology, Hematology - Red Cells, Transfusion Practices
INTRODUCTION
Patients with sickle cell disease (SCD) continue to be transfused frequently, compared with the general population. In the 2006 Nationwide Inpatient Sample (NIS) data of the Healthcare Cost and Utilization Project, 25% of nearly 100,000 admissions of patients with SCD involved transfusion. Twenty-seven percent of patients admitted for vaso-occlusive crisis were transfused, and 37.6% of patients treated for acute chest syndrome were transfused. (S. Kamble, S.D. Reed, C. Grussemeyer, and M.J. Telen, unpublished data)
Alloimmunization is a common complication of transfusion therapy among patients with SCD. Among different study populations with SCD, reported rates of alloimmunization have ranged from 18-47% of patients.1 This represents a much higher rate than in other chronically transfused populations.2,3 Alloimmune reactions can be associated with significant morbidity in SCD, including delay in urgent treatment of stroke and acute chest syndrome due to difficulty finding compatible blood, delayed transfusion reactions, hyperhemolysis,4,5 and autoantibody formation6-8.
It is unknown why some patients become alloimmunized while the majority of patients do not. Frequency of transfusion, along with differences between prevalent antigen phenotypes among SCD patients as compared to US blood donors,1,3,9 are thought to contribute to the relatively high rate of alloimmunization among patients with SCD. This is further supported by data showing lower rates of alloimmunization in SCD populations with more similar distribution of blood group antigens between donors and recipients.10
Antigenicity of the disparate antigens is also an important but potentially complex determinant.11 Other factors described as affecting the development of alloimmunization in patients with SCD include gender, number of transfusions,3,9 and age at which transfusion therapy began.12,13 More recent data have suggested that the increased incidence of alloimmunization in patients with SCD may also be related to altered immune responses, themselves associated with on-going inflammation14 or the frequency of certain HLA antigens.15,16 In animal studies, the magnitude and frequency of inflammation appears to affect alloimmunization.17 Further, a genetic polymorphism in Ro52 (SSA1;TRIM21) has been associated with age-dependent maturation of the immune system in response to red blood cell (RBC) antigen presentation.18 This could potentially be a marker to predict the ability of SCD patient to develop tolerance or produce antibodies beyond the neonatal period.
As our knowledge of the basic pathophysiology of SCD has expanded, so has our understanding that disease severity is dependent on a variety of complex interactions and multiple determinants, including genetic and environmental factors. We hypothesized that the development of alloantibodies in patients with SCD may therefore be associated with other SCD complications as well as overall disease severity.
MATERIALS and METHODS
This study was conducted as part of an Institutional Review Board-approved larger study of clinical outcome-modifying genes in adults with SCD. All participants gave written informed consent, and patient clinical and medical histories were obtained using standardized data collection forms that included questions about exposure to transfusion and measures of disease severity. In order to assess exposure to transfusion, patients were asked to estimate the number of units of blood received over their lifetime; documentation of lifetime transfusion episodes was generally unavailable. This history of transfusion was stratified into 5 categories: none, between 1-10 units, between 11-20 units, between 21-50 units, or >50 units. Patients were also asked if they were currently receiving chronic transfusion therapy or iron chelation treatment. Measures of disease severity included vaso-occlusion-related endpoints, such as the number of acute painful episodes requiring hospitalization in the past 12 months, and significant chronic pain, as assessed by use of daily narcotics, as well as the presence of documented end-organ damage. The overall disease severity of each patient was then determined using a modified version of a previously validated chronic disease severity score measuring cumulative end-organ damage.19-22 A score for each patient was calculated based on the presence or absence of each of the following: 1) pulmonary dysfunction as defined by the presence of tricuspid regurgitant jet velocity ≥ 2.5 m/sec or O2 saturation <92%; 2) radiologically demonstrated avascular necrosis (AVN) of the hip or shoulder; 3) central nervous system (CNS) abnormality, as defined by a history of stroke, seizure, or transient ischemic attack (TIA); 4) kidney dysfunction, as defined by proteinuria ≥ 1+ on urinalysis or creatinine >1.0 mg/ml; and 5) history of leg ulcers. For each item, one point was assigned if present. The points were added to obtain a final score, which ranged from 0 points (mildest disease) to a possible maximum of 5 points (most severe disease).
Steady-state laboratory values (mean of up to three values) were also obtained for all patients. For patients on hydroxyurea treatment when enrolled, baseline hematologic data, defined as the mean of up to three steady-state samples collected prior to hydroxyurea treatment, were used for the analysis.
Within this larger study, historical blood bank records of 338 adult patients with SCD enrolled at two participating institutions (Duke University Medical Center and the University of North Carolina at Chapel Hill) between March 2002 and January 2005 were reviewed to ascertain patient RBC phenotype and allo- and autoantibody data obtained through prior routine serologic testing. Identification of antigen phenotypes, alloantibodies and warm IgG autoantibodies had been performed using standard blood bank techniques. A patient was considered to be alloimmunized if their serum contained at least one antibody to an antigen absent from their own RBCs. Patients whose serum contained only cold-reacting autoantibodies, anti-I, anti-IH, anti-HLA, or so-called high titer low avidity (HTLA) antibodies, were not considered to be alloimmunized. Nineteen patients with incomplete blood bank records were excluded, leaving 319 subjects for analysis.
We then tested for differences between all immunized and non-alloimmunized patients with respect to demographic characteristics, history of transfusion exposures, clinical and laboratory findings, frequency of vaso-occlusive episodes, use of out-patient narcotics, the presence of end-organ damage, and overall disease severity, all of which had been ascertained using uniform case report forms as part of the larger study. Statistical analysis was performed using a standard statistical software package (SAS version 9.2, SAS Institute, Cary, NC). Chi-square tests were used for categorical variables, and the Fisher’s exact test was applied to data sets with cells containing less than five (5) observations. Logistic regression was then performed on nominally significant chi-square tests to obtain odds ratios. Analysis of Variance was used to compare the difference in means between all immunized and non-alloimmunized patients for continuous outcomes. Variables with significant departures from normality were log-transformed for analysis; these included HbF, creatinine, LDH, and ferritin. A p-value of <0.05 was considered significant. All tests were two-tailed.
RESULTS
Subject demographics and prevalence of alloimmunization
Of 319 adult patients with SCD and adequate information, 315 were self-identified as African American (99%) and 179 were female (56%). Patients ranged in age from 18 to 84 years old (mean 35.1 years ± 12.2 SD [standard deviation]). The majority of the sample consisted of patients with HbSS (246/319, 77%), followed by HbSC (41/319, 13%), HbSβ+ thalassemia (17/319, 5%), and HbSβ0 thalassemia (15/319, 5%).
Eighty-seven patients had alloantibodies, with an overall alloimmunization prevalence of 27%. Two of the three patients self-identified as Caucasian were alloimmunized; the one patient self-identified as Asian or Pacific Islander was not. Interestingly, within our adult cohort, alloimmunization was not related to age. The mean age of alloimmunized patients was 35.2 yrs, while that of non-alloimmunized patients was 35.1 yrs. Age at first alloimmunization was not available. Moreover, gender also did not have a statistically significant effect. Among women, 30.7% (55 of 179) were alloimmunized, while 22.9% of men (32 of 140) were alloimmunized (p>0.05). A comparison of the general demographic characteristics between alloimmunized and non-alloimmunized patients is shown in Table 1.
Table 1.
Demographics of Alloimmunized vs. Non-alloimmunized Patients With Sickle Cell Disease (n=319)
| Alloimmunized N=87 (%) |
Non-alloimmunized N=232 (%) |
P value |
|
|---|---|---|---|
| Mean Age (years) | 35.2 ± 10.1 | 35.1 ± 12.9 | 0.932 |
| Gender Male Female |
32 (23) 55 (31) |
108 (77) 124 (69) |
0.117 |
| History of Transfusion (n=285)*
None 1-10 11-20 21-50 >50 |
1 (1) 32 (41) 21 (27) 9 (11) 16 (20) |
19 (9) 98 (48) 32 (16) 29 (14) 28 (14) |
0.017 |
| Warm Autoantibody | 22 (25) | 2 (<1) | <0.001 |
Thirty-four (34) patients did not respond to this question.
Hemoglobin diagnosis and history of transfusion
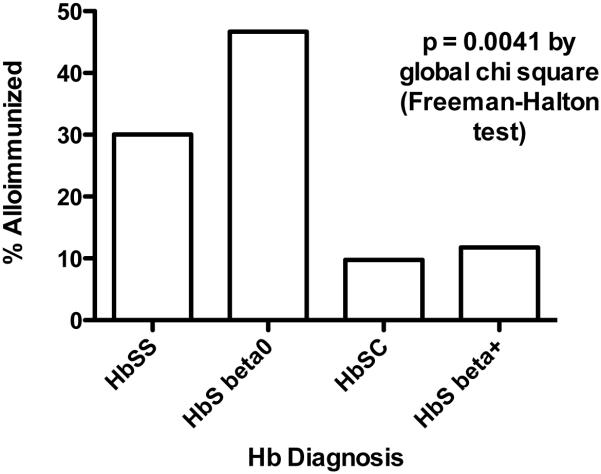
As shown in Figure 1, hemoglobin (Hb) diagnosis was strongly associated with the presence of alloimmunization (p=0.004), with the highest percentage of alloimmunized patients being those with HbSβ0 thalassemia (46.7%) and the lowest percentage occurring in patients with HbSC (9.8%). Furthermore, Hb diagnosis was significantly associated with a history of transfusion (p=0.002). As expected, a history of any prior transfusion was also strongly associated with alloimmunization (p=0.017). Of the 285 patients who provided a transfusion history, 78/79 (98.73%) alloimmunized patients and 187/206 (90.78%) non-alloimmunized patients reported that they had been transfused, and only one alloimmunized subject (1/79, 1.27%) had not been transfused. The one alloimmunized nontransfused subject had had two previous live births. However, after adjusting for the effects of Hb diagnosis, age, and gender, the association of alloimmunization with transfusion history no longer reached statistical significance. (OR=8.0; 95%CI 1.0 - 64; p=0.05), suggesting that Hb diagnosis was the driving factor for transfusion, and therefore for risk of alloimmunization. Perhaps surprisingly, alloimmunization did not appear to be directly related to the number of reported transfusions received, as the highest percentage of alloimmunized patients occurred among those who reported having received between 11 to 20 transfusions. In addition, the shape of distributions for transfusion history did not differ between alloimmunized and non-alloimmunized patients (Cochran-Armitage trend test p=0.364). We also examined the potential association of alloimmunization with ferritin levels, possibly a more objective measure of transfusions received; while mean ferritin levels were higher among alloimmunized patients (p=0.05), this association was also driven by Hb diagnosis (p=0.001). Likewise, comparison of mean ferritin levels between the two groups was only minimally significant, with p=0.042 (Wilcoxon-Mann-Whitney test). Current treatment with chronic transfusion therapy or iron chelation therapy was also not associated with higher risk of alloimmunization. Five (6%) of 80 alloimmunized patients were currently receiving chronic transfusion therapy, compared to 7 (3%) of 212 non-alloimmunized patients (p=0.258). Similarly, 5 (6%) of 79 alloimmunized patients compared to 7 (3%) of 211 non-alloimmunized patients were currently receiving iron chelation therapy (p=0.252).
Figure 1. Percent alloimmunized patients with specific hemoglobin diagnoses.
Patients with HbSS and HbS-β0 thalassemia were alloimmunized significantly more often than were patients with HbSC or HbS β+ thalassemia.
Antibody specificities and frequency of autoantibodies
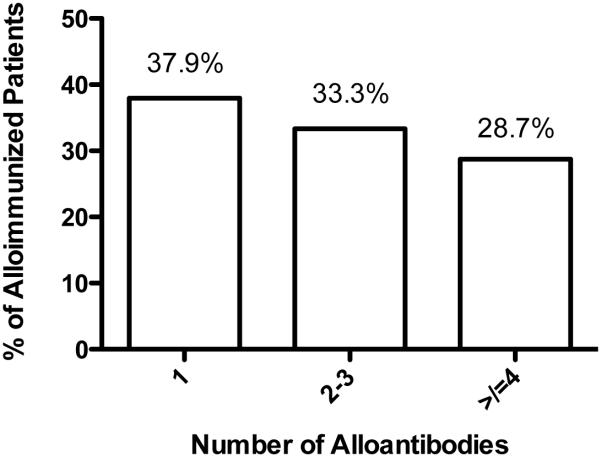
The most commonly occurring alloantibodies, in descending order of frequency, were anti-E, -C, -S, -Fya or -Fy3, -Jkb, -K, and -M. Compared to the frequency of antigen specificities observed for alloantibodies in the Cooperative Study of SCD (see Table 2), our patients experienced significantly more frequent alloimmunization to the S, M, Jkb and V antigens (p<0.0001, p=0.013, p=0.025, and p=0.046, respectively) and less frequently made alloantibodies to the K antigen, although the difference in frequency of alloimmunization to K was not significant (p=0.106). No other differences approached statistical significance. Although our population was older than that examined by the CSSCD, we did not find a markedly greater proportion of alloimmunized patients who were multiply alloimmunized (Figure 2). Alloimmunized patients who made only one alloantibody comprised 44.97% of the alloimmunized CSSCD cohort and 37.93% of our study’s alloimmunized cohort (p=0.238). However, in the study by Rosse et al., 17.75% of 338 alloimmunized patients made four or more alloantibodies, while in our cohort, 28.73% of 87 alloimmunized patients made four or more alloantibodies (p=0.022).
Table 2.
Specificity of Alloantibodies Among Alloimmunized Patients, Compared to the Cooperative Study of SCD (CSSCD)9
| Specificity | % of 338 alloimm CSSCD Pts |
% of 87 alloimm Duke- UNC Pts |
P value | Specificity | % of 338 alloimm CSSCD Pts |
% of 87 alloimm Duke- UNC Pts |
P value |
|---|---|---|---|---|---|---|---|
| D | 13.0 | 11.49 | 0.704 | M | 7.7 | 17.24 | 0.013* |
| C | 30.2 | 35.63 | 0.364 | N | 1.5 | 1.15 | 1.000 |
| c | 3.0 | 2.30 | 1.000 | S | 9.2 | 29.89 | <0.0001* |
| E | 42.3 | 45.98 | 0.538 | s | 1.5 | 2.30 | 0.636 |
| e | 4.4 | 2.30 | 0.543 | Jka | 1.8 | 3.45 | 0.398 |
| V | 2.4 | 6.90 | 0.046* | Jkb | 10.7 | 19.54 | 0.025* |
| K | 28.1 | 19.54 | 0.106 | Fya or Fy3 | 18.6 | 24.14 | 0.251 |
Statistically significant difference between the two studies
Figure 2. Prevalence of having multiple alloantibodies among alloimmunized patients.
Among patients who were alloimmunized, slightly less than 38% had only one alloantibody identified, while close to one-third had two or three alloantibodies, and nearly a third had four or more alloantibodies.
Alloimmunization was, nevertheless, strongly associated with the presence of a warm autoantibody, as 25% of alloimmunized patients had a warm autoantibody compared to <1% of non-alloimmunized patients (p<0.0001). Moreover, there was a very high prevalence of autoantibodies among patients alloimmunized against the E, V, S and Jkb antigens, and this reached statistical significance when compared to association with alloantibodies of other specificities. Among patients who made anti-E or anti-S, the prevalence of autoantibodies was 72.72% and 50%, respectively.
Relationship of alloimmunization to laboratory parameters
Mean steady-state laboratory values did not differ between alloimmunized and non-alloimmunized patients, except in a very few instances (Table 3). Alloimmunized patients as a whole had slightly lower baseline hemoglobin levels (8.3 ± 1.6) compared to non-alloimmunized patients (8.8 ± 2.0; p=0.04); while this difference was also observed within each hemoglobin diagnosis group, the differences within each diagnosis group were not statistically significant (data not shown). Ferritin levels, while higher in alloimmunized patients, demonstrated great variability of values within each group.
Table 3.
Mean Laboratory Values in Alloimmunized vs. Non-alloimmunized Patients
| N* | Alloimmunized |
Non-
alloimmunized |
P
value |
|
|---|---|---|---|---|
| Values ± S.D. | ||||
| Hematologic | ||||
| Hemoglobin (g/dL) | 230 | 8.3 ± 1.6 | 8.8 ± 2.0 | 0.040 |
| Hemoglobin F (%)† | 172 | 6.8 ± 5.2 | 6.3 ± 5.2 | 0.206 |
| Platelet count (× 109/L)‡ | 218 | 431.2 ± 172.1 | 426.3 ± 156.2 | 0.840 |
| WBC count (× 109/L) | 220 | 11.5 ± 4.1 | 11.6 ± 3.6 | 0.886 |
| Abs. Reticulocyte Count (× 109/L) |
201 | 301.0 ± 163.1 | 280.8 ± 135.8 | 0.375 |
| Renal | ||||
| Creatinine (mg/dL)ǁ | 280 | 0.8 ± 0.4 | 0.9 ± 0.5 | 0.620 |
| Glomerular Filtration Rate (mL/min/1.73 m2) |
270 | 100.4 ± 19.9 | 102.7 ± 20.4 | 0.402 |
| Other | ||||
| Lactate Dehydrogenase (U/L) ǁ |
277 | 1106.9 ± 691.6 | 1052.4 ± 687.7 | 0.333 |
| Ferritin (ng/mL) § | 143 | 1526.4 ± 2153.3 | 914.3 ± 1612.2 | 0.042 |
Number of subjects for whom data were available.
Statistical analysis based on log-transformed value. Excludes one patient with hemoglobin F percentage greater than 30%.
Excludes one patient with platelet counts greater than 1,000,000 X 109/L.
Statistical analysis based on log-transformed value.
statistical analysis based on log-transformed value. Excludes six patients (three alloimmunized and three non-alloimmunized) with creatinine values greater than 4.7 mg/dL.
Relationship of alloimmunization to pain and measures of disease severity
Alloimmunization was not associated with a more frequent history of acute vaso-occlusive episodes, as defined by number of painful episodes requiring medical attention and/or hospitalization during the year prior to enrolment (Table 4). However, chronic pain, as defined by daily home opioid use, was associated with alloimmunization. This association was seen in patients who reported daily use of long-acting opioids (p=0.013), short-acting opioids (p=0.006), and both long-acting and short-acting opioids (p=0.03) but was strongest for short-acting opioids.
Table 4.
Comparison of Pain-Related Outcomes in Alloimmunized vs. Non-alloimmunized SCD Patients
| N* | % of Alloimmunized Patients |
% of Non- alloimmunized Patients |
P value |
|
|---|---|---|---|---|
|
Vaso-occlusive Endpoints
Painful episodes requiring medical attention in past 12 months Painful episodes requiring hospitalization in past 12 months |
294 291 |
77 61 |
71 57 |
0.339 0.539 |
| Hydroxyurea use | 282 | 50 | 48 | 0.708 |
|
Daily home opioid use
Long-acting opioids Short-acting opioids Both long and short-acting opioids |
295 276 275 |
39 47 28 |
24 29 16 |
0.013 0.006 0.030 |
Number of subjects for whom data were available.
When the prevalence of SCD-related complications was measured (Table 5), we found that alloimmunization was significantly more common in patients with avascular necrosis (AVN) of the hip or shoulder, with 48% of alloimmunized patients having AVN compared with 33% of non-alloimmunized patients (p=0.024, Table 5). However, the prevalence of other disease complications did not vary between the two groups. In addition, the presence of AVN conferred an odds ratio of >2 for the daily use of opioids (p=0.005, p=0.002, and p=0.009 for association of AVN with long-acting, short-acting, and both).
Table 5.
Prevalence of SCD-Related Outcomes in Alloimmunized vs. Non-alloimmunized Patients
| N* | % of Alloimmunized Patients |
% of Non- alloimmunized Patients |
P value |
|
|---|---|---|---|---|
| Central Nervous System Endpoints | ||||
| Stroke Any cerebral vascular event† |
289 286 |
20 32 |
13 25 |
0.162 0.267 |
| Pulmonary Endpoints | ||||
| Acute chest syndrome Tricuspid regurgitant jet velocity ≥ 2.5 m/s Oxygen saturation < 92% |
295 187 272 |
83 40 9 |
76 35 5 |
0.221 0.560 0.260 |
| Other SCD-related Endpoints | ||||
| AVN of hip or shoulder Proteinuria ≥ 1 + Leg ulcers |
289 283 292 |
48 23 26 |
33 25 23 |
0.024
0.697 0.687 |
Number of subjects for whom data were available.
Includes stroke, transient ischemic attacks, and seizures.
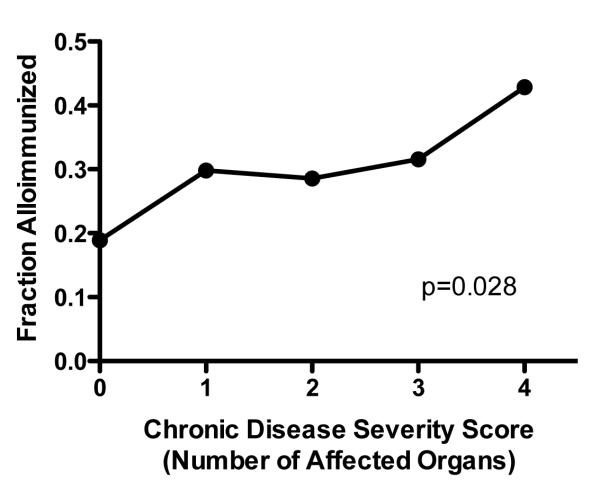
We also compared overall disease severity between alloimmunized and non-alloimmunized patients using a multi-organ specific chronic disease severity score.22 The highest score for any individual patient, alloimmunized or non-alloimmunized, was four out of a possible five points. Alloimmunized patients were 1.5 times more likely to have organ damage compared to non-alloimmunized patients. (31% vs. 20%), and this difference was statistically significant (p=0.049). Furthermore, the prevalence of alloimmunization increased with increasing degrees of end-organ damage (p=0.028, Figure 3).
Figure 3. Relationship of overall disease severity and alloimmunization, based on a multi-organ chronic disease severity score.
Scores were calculated based on the presence or absence of 1) pulmonary dysfunction 2) kidney dysfunction 3) CNS abnormality, 4) AVN of hips or shoulders, and 5) leg ulcers, for a total possible score of 5. The highest observed score for an individual patient, alloimmunized or non-alloimmunized, was 4 out of the possible 5. Alloimmunization was increasingly prevalent with increased disease severity (p=0.028). In addition, only 20% of alloimmunized patients had no organ damage, compared to 31% of non-alloimmunized patients and this difference was statistically significant (p=0.049, data not shown)).
The results of multiple regression modelling of alloimmunization and significant bivariate associations, taking into account age, gender, and Hb diagnosis as covariates, are shown in Table 6. Alloimmunization remained a strong predictor of daily opioid use, even after adjusting for these covariates, with alloimmunized patients having 2 times the odds of daily long-acting opioid use (p=0.016), daily short-acting opioid use (p=0.012) or both (p=0.048). However, female patients were only half as likely to be on long-acting opioids (OR=0.55, p=0.012) as males, regardless of alloimmunization status. Age was also a significant predictor of daily short-acting opioid use (OR 1.03, 95% CI=1.01-1.06, p=0.002), but its effect was modest compared to alloimmunization. A similar association was seen with alloimmunization and the presence of AVN. Alloimmunized patients had 2 times the odds of having AVN of the hip or shoulder, even after adjusting for age, gender, and Hb diagnosis (p=0.012). Age was a significant predictor of AVN (OR 1.05, 95% CI=1.03-1.07, p=<0.001), but its effect was modest compared to alloimmunization. While AVN independently affected the odds of daily narcotic use, its effect was small compared to the effect of alloimmunization, although its effect on the odds of using long-acting narcotics daily was somewhat greater.
Table 6.
Effect of Alloimmunization on Risk of Clinical Outcomes*
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Daily Narcotic Use Long Acting Short Acting Both Long & Short Acting |
2.01 2.05 1.91 |
1.14, 3.55 1.17, 3.61 1.01, 3.62 |
0.016 0.012 0.048 |
| AVN | 2.08 | 1.18, 3.66 | 0.012 |
| Chronic Organ Damage (any vs. none) |
1.53 | 0.809, 2.91 | 0.190 |
Results for alloimmunization (yes vs. no) adjusted for hemoglobin diagnosis (all types), gender (female vs. male) and age as a continuous variable.
Survival was strongly correlated with presence of alloimmunization, as shown in Figure 4. Despite similar frequency of painful episodes and hospitalizations, patients without alloimmunization had life expectancies about a decade longer than did alloimmunized patients.
Figure 4. Survival of alloimmunized and nonalloimmunized patients.
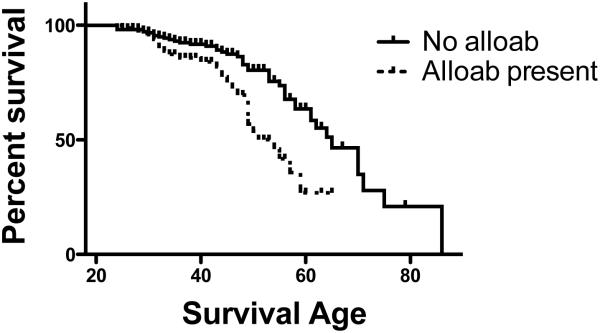
Kaplan-Meier estimation of survival in patients with and without alloimmunization showed a median life expectancy of 65 years in nonalloimmunized patients but only 54 in alloimmunized patients.
We also performed subanalyses in which we compared non-alloimmunized subjects to only those who made alloantibodies to antigens other than C, E, and K (n = 66). In these analyses, lower hemoglobin was no longer statistically significantly associated with alloimmunization (p=0.192), but we again found statistically significant associations of alloimmunization with long-acting opioids (p=0.009), short-acting opioids (p=0.023) and both long and short-acting opioids (p=0.015), as well as AVN (p=0.047 when adjusted for hemoglobin diagnosis, gender and age, as in Table 6). Furthermore, this subset of alloimmunized patients still had poorer survival (HR=1.7889, p=0.0388) than nonalloimmunized patients.
DISCUSSION
Our study of a contemporary adult population with SCD shows both significant differences as well as similarities in the prevalence and pattern of alloimmunization compared to the landmark CSSCD study published over 20 years ago.9 First, while the CSSCD study demonstrated a prevalence of alloimmunization of 18.6% in a U.S. SCD population, we documented a prevalence of 27.3%. This difference is likely a result of studying only an adult cohort rather than one that included newborns and young children with limited exposure to transfusions. Similarly, in the more recent PROACTIVE study, which enrolled subjects with a mean age of 19.3 years (range, 2.0-68.0), 75.8% of whom had received at least one transfusion, the prevalence of alloimmunization was 14.4%.23 Interestingly, in that study, 50% of the adult patients found to be alloimmunized had received <10 units of blood, and there was no difference in alloimmunization rate between centers that prospectively matched for C, E and K and those that did not. Thus, both our study and the smaller one of Miller et al.23 suggest that alloimmunization is not clearly related to the number of prior transfusions. While our study showed a higher prevalence of alloimmunization, this was likely related to the higher mean age of our study subjects (>35 years), and the nearly 100% frequency of a history of transfusion.
We also found interesting differences in the frequency of certain antibody specificities, including anti-S, anti-M, anti-Jkb, and anti-V, compared to the CSSCD report, while the frequency of antibodies to the C and E antigens were similar in the two studies. In both studies, however, anti-C, -E, -K, and –Fya were among the most frequent alloantibodies found. The increased frequency of anti-S in our study has also been found in some other studies, including one in Africa.24 Interestingly, the percentage of alloimmunized patients who had ≥4 alloantibodies was also significantly higher in our study compared to CSSCD (p=0.022). We cannot eliminate the possibility that the location of our study (two nearby institutions in North Carolina) or local transfusion practices contributed to both the frequency of alloimmunization as well as to the alloantibody specificities we found. In our state, the two university clinical centers that conducted this study have prospectively matched blood for C, E and K when transfusing SCD patients for > 30 years, while the local community hospitals in this quite rural state have not done so. Thus, our patients have often acquired anti-C and anti-E after transfusion at local community hospitals. Consequently, the prevalence of anti-C and anti-E are nearly identical to those reported in the CSSCD study, a study performed before most centers had adopted routine matching for C, E and K.
Recent studies have also shown that polymorphisms within the RH locus, but not routinely detected by D, C/c, E/e phenotyping, may be linked to alloimmunization to Rh antigens. Chou et al.25 reported 15 years’ experience transfusing 182 patients with SCD with donor units antigen-matched for RhD, C and E, and K, and selected primarily from African-American donors. Despite these strategies, 80 patients (44%) became alloimmunized, and 94 of 146 antibodies had Rh specificity. Thirty-five Rh alloantibodies occurred in antigen-negative patients receiving corresponding D-, C- or E- RBCs who should not have made the antibody in the absence of exposure to antigen-positive RBCs. Fifty-six Rh antibodies occurred in patients whose RBCs were positive for the antigen in serological tests and would therefore have not been expected to recognize the antigen as foreign. The authors concluded that variant RH alleles in both patients and donors could result in unexpected Rh alloantibodies. In a smaller study, O’Suoji and colleagues found that efforts to antigen match blood for C, E, and K nevertheless resulted in a significant alloimmunization rate to these antigens; they implicated both episodic transfusion in institutions that did not provide phenotypically matched blood as well as sensitization in the presence of rare RH variants.26
In addition, the association of alloimmunization with autoantibody formation was quite striking in this study. In the previous study by Castellino et al., only two of 14 autoantibodies were not associated with alloimmunization.6 As reviewed by Garratty,7 the mechanisms whereby alloimmunization and autoantibody formation may be associated are potentially of great interest and may include “epitope spreading” as well as more generalized activation of the immune system during alloantibody formation. Indeed, this association of alloimmunization and autoantibody formation is not limited to the SCD or even hemoglobinopathy population.8
Unlike the CSSCD study, our study has both sought to identify clinical characteristics associated with alloimmunization as well as to explore whether alloimmunization itself is a risk factor for other complications in SCD. We found that alloimmunization was strongly associated with the presence of chronic pain, as assessed by daily opioid use, a relatively common finding in adult patients. In addition, alloimmunization was associated with AVN among adults with SCD. These findings have not been previously reported. However, such associations do not provide evidence of causation or even directionality of possible relationships.
Pain is a hallmark of sickle cell disease. Pain occurs frequently in the context of acute vaso-occlusive episodes, and acute painful episodes are the predominant symptom associated with SCD. However, in adults pain is also often more chronic in nature and may have a neuropathic and/or inflammatory component.27 Thus, chronic pain syndromes in SCD are considered to be different in nature from acute vaso-occlusive episodes and have been associated with several disease complications, such as leg ulcers, bone and joint disease, or nerve disturbances.27 Among chronic pain syndromes, AVN is associated with deep somatic pain, often complicated by incident pain, that is, pain related to movement.28 Treatment of persistent moderate to severe pain generally relies on opioids.27
The mechanism of chronic pain in sickle cell disease is poorly understood, and current avenues of investigation include the PISCES study29,30 of the nature of pain in SCD as well as investigation of genetic polymorphisms that may explain predisposition to pain.31,32 Studies suggest that most patients treat pain at home and that only when severity and acuity of pain increases sufficiently do they seek medical attention. Based on PISCES data, more patients than previously thought have daily pain that they treat at home.29,30 Thus, at this time, we can only speculate as to why chronic pain and alloimmunization to red cell blood group antigens may be associated events. It is possible that immune system activation might contribute both to the risk of alloimmunization as well as to development of chronic pain.
As in other studies, factors associated with alloimmunization included a history of transfusion, although this relationship was not directly related to the number of transfusions reported by patients. This lack of a relationship, however, may have resulted from the retrospective nature of the transfusion data (patient report). We did find a weak relationship between alloimmunization and ferritin level, which is assumed to result from transfusional iron burden, and the differences in mean ferritin between the two groups was quite large (1526.4 ± 2153.3 vs 914.3 ± 1612.2 ng/mL). This result suggests that subjects’ recall of the number of previous transfusions was not accurate and that ferritin, which was markedly higher in alloimmunized patients compared to non-alloimmunized patients, may be a better reflection of actual number of transfusions received. However, ferritin is also an acute phase reactant and thus might also reflect a more pro-inflammatory status among patients who have been alloimmunized. Age was not associated with alloimmunization in this study. However, all of the patients in this study were adults, and age of first transfusion was not known. Therefore, the potential effect of tolerance induction could not be assessed. Gender, also identified as a factor related to risk of alloimmunization, was not seen as significant in this study. Again, this could be secondary to the age distribution of the subjects in our study as compared to the CSSCD, since older age (and thus higher prevalence of a positive transfusion history) would be anticipated to make less significant the potentially alloimmunizing effect of pregnancy.
Finally, the association of alloimmunization with shortened survival is particularly interesting, and this possible association has not been previously studied. Given that the prevalence of end-organ damage was only slightly increased in the alloimmunized cohort, and that the frequency of vaso-occlusive episodes requiring medical attention was not significantly increased, the mechanism of this difference in survival is particularly intriguing. We hypothesize that the pathways predisposing to chronic pain requiring daily opioids also lead to decreased survival, although what these pathways might be remains an open question at this time. Several lines of evidence implicate inflammatory pathways in chronic pain of SCD.25,26 Whether these mechanisms involve major genetic effects or result from stochastic disease-related events, the causes of the association of alloimmunization with decreased survival certainly merits further investigation.
ACKNOWLEDGMENTS
The authors thank Jude Jonassaint, RN, M. Dell Strayhorn, FNP, MPH, and Susan K. Jones, RN, for outstanding collection of clinical data on the study subjects.
Footnotes
CONFLICT OF INTEREST
All authors declare that they have no competing financial interests.
REFERENCES
- 1.Telen MJ. Principles and problems of transfusion in sickle cell disease. Semin Hematol. 2001;38:315–23. doi: 10.1016/s0037-1963(01)90025-3. [DOI] [PubMed] [Google Scholar]
- 2.Heddle NM, Soutar RL, O'Hoski PL, Singer J, McBride JA, Ali MA, Kelton JG. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91:1000–5. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 3.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–21. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 4.King KE, Shirey RS, Lankiewicz MW, Young-Ramsaran J, Ness PM. Delayed hemolytic transfusion reactions in sickle cell disease: simultaneous destruction of recipients' red cells. Transfusion. 1997;37:376–81. doi: 10.1046/j.1537-2995.1997.37497265337.x. [DOI] [PubMed] [Google Scholar]
- 5.Win N, New H, Lee E, de la Fuente J. Hyperhemolysis syndrome in sickle cell disease: case report (recurrent episode) and literature review. Transfusion. 2008;48:1231–8. doi: 10.1111/j.1537-2995.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 6.Castellino SM, Combs MR, Zimmerman SA, Issitt PD, Ware RE. Erythrocyte autoantibodies in paediatric patients with sickle cell disease receiving transfusion therapy: frequency, characteristics and significance. Br J Haematol. 1999;104:189–94. doi: 10.1046/j.1365-2141.1999.01127.x. [DOI] [PubMed] [Google Scholar]
- 7.Garratty G. Autoantibodies induced by blood transfusion. Transfusion. 2004;44:5–9. doi: 10.1111/j.0041-1132.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Young PP, Uzieblo A, Trulock E, Lublin DM, Goodnough LT. Autoantibody formation after alloimmunization: are blood transfusions a risk factor for autoimmune hemolytic anemia? Transfusion. 2004;44:67–72. doi: 10.1046/j.0041-1132.2003.00589.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosse WF, Gallagher D, Kinney TR, Castro O, Dosik H, Moohr J, Wang W, Levy PS. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–7. [PubMed] [Google Scholar]
- 10.Moreira Junior G, Bordin JO, Kuroda A, Kerbauy J. Red blood cell alloimmunization in sickle cell disease: the influence of racial and antigenic pattern differences between donors and recipients in Brazil. Am J Hematol. 1996;52:197–200. doi: 10.1002/(SICI)1096-8652(199607)52:3<197::AID-AJH11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Sela M. Antigenicity: some molecular aspects. Science. 1969;166:1365–74. doi: 10.1126/science.166.3911.1365. [DOI] [PubMed] [Google Scholar]
- 12.Gill FM, Sleeper LA, Weiner SJ, Brown AK, Bellevue R, Grover R, Pegelow CH, Vichinsky E. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86:776–83. [PubMed] [Google Scholar]
- 13.Murao M, Viana MB. Risk factors for alloimmunization by patients with sickle cell disease. Braz J Med Biol Res. 2005;38:675–82. doi: 10.1590/s0100-879x2005000500004. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickson JE, Hod EA, Perry JR, Ghosh S, Chappa P, Adisa O, Kean LS, Ofori-Acquah SF, Archer DR, Spitalnik SL, Zimring JC. Alloimmunization to transfused HOD red blood cells is not increased in mice with sickle cell disease. Transfusion. 2012;52:214–6. doi: 10.1111/j.1537-2995.2011.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alarif L, Castro O, Ofosu M, Dunston G, Scott RB. HLA-B35 is associated with red cell alloimmunization in sickle cell disease . Clin Immunol Immunopathol. 1986;38:178–83. doi: 10.1016/0090-1229(86)90136-4. [DOI] [PubMed] [Google Scholar]
- 16.Hoppe C, Klitz W, Vichinsky E, Styles L. HLA type and risk of alloimmunization in sickle cell disease. Am J Hematol. 2009;84:462–4. doi: 10.1002/ajh.21442. [DOI] [PubMed] [Google Scholar]
- 17.Zimring JC, Hendrickson JE. The role of inflammation in alloimmunization to antigens on transfused red blood cells. Curr Opin Hematol. 2008;15:631–5. doi: 10.1097/MOH.0b013e328313695e. [DOI] [PubMed] [Google Scholar]
- 18.Tatari-Calderone Z, Minniti CP, Kratovil T, Stojakovic M, Vollmer A, Barjaktarevic I, Zhang E, Hoang AL, Vukmanovic S. rs660 polymorphism in Ro52 (SSA1; TRIM21) is a marker for age-dependent tolerance induction and efficiency of alloimmunization in sickle cell disease. Mol Immunol. 2009;47:64–70. doi: 10.1016/j.molimm.2008.12.027. N.L.C. [DOI] [PubMed] [Google Scholar]
- 19.Gil KM, Abrams MR, Phillips G, Keefe FJ. Sickle cell disease pain: relation of coping strategies to adjustment. J Consult Clin Psychol. 1989;57:725–31. doi: 10.1037//0022-006x.57.6.725. [DOI] [PubMed] [Google Scholar]
- 20.Gil KM, Abrams MR, Phillips G, Williams DA. Sickle cell disease pain: 2. Predicting health care use and activity level at 9-month follow-up. J Consult Clin Psychol. 1992;60:267–73. doi: 10.1037//0022-006x.60.2.267. [DOI] [PubMed] [Google Scholar]
- 21.Wison Schaeffer JJ, Gil KM, Burchinal M, Kramer KD, Nash KB, Orringer E, Strayhorn D. Depression, disease severity, and sickle cell disease. J Behav Med. 1999;22:115–26. doi: 10.1023/a:1018755831101. [DOI] [PubMed] [Google Scholar]
- 22.Afenyi-Annan A, Kail M, Combs MR, Orringer EP, Ashley-Koch A, Telen MJ. Lack of Duffy antigen expression is associated with organ damage in patients with sickle cell disease. Transfusion. 2008;48:917–24. doi: 10.1111/j.1537-2995.2007.01622.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller ST, Kim HY, Weiner DL, Wager CG, Gallagher D, Styles LA, Dampier CD, Roseff SD. Red blood cell alloimmunization in sickle cell disease: prevalence in 2010. Transfusion. 2013;53:704–9. doi: 10.1111/j.1537-2995.2012.03796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natukunda B, Schonewille H, Ndugwa C, Brand A. Red blood cell alloimmunization in sickle cell disease patients in Uganda. Transfusion. 2010;50:20–5. doi: 10.1111/j.1537-2995.2009.02435.x. [DOI] [PubMed] [Google Scholar]
- 25.Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–71. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 26.O'Suoji C, Liem RI, Mack AK, Kingsberry P, Ramsey G, Thompson AA. Alloimmunization in sickle cell anemia in the era of extended red cell typing. Pediatr Blood Cancer. 2013;60:1487–91. doi: 10.1002/pbc.24530. [DOI] [PubMed] [Google Scholar]
- 27.Niscola P, Sorrentino F, Scaramucci L, De Fabritiis P, Cianciulli P. Pain Syndromes in Sickle Cell Disease: An Update. Pain Medicine. 2009;10:470–80. doi: 10.1111/j.1526-4637.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- 28.Tendas A, Scaramucci L, Giovannini M, Niscola P, De Sanctis V. Pain in blood cancers. Indian J Palliat Care. 2011;17:175–83. doi: 10.4103/0973-1075.92333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sogutlu A, Levenson JL, McClish DK, Rosef SD, Smith WR. Somatic symptom burden in adults with sickle cell disease predicts pain, depression, anxiety, health care utilization, and quality of life: The PiSCES Project. Psychosomatics. 2011;52:272–9. doi: 10.1016/j.psym.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 30.McClish DK, Smith WR, Dahman BA, Levenson JL, Roberts JD, Penberthy LT, Aisiku IP, Roseff SD, Bovbjerg VE. Pain site frequency and location in sickle cell disease: The PiSCES project. Pain. 2009;145:246–51. doi: 10.1016/j.pain.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira MCVC, Mendonça TF, Vasconcelos LRS, Moura P, Bezerra MAC, Santos MNN, Araújo AS, Cavalcanti MSM. Association of the MBL2 gene exon1 polymorphism and vasoocclusive crisis in patients with sickle cell anemia. Acta Haematol. 2009;121:212–5. doi: 10.1159/000220335. [DOI] [PubMed] [Google Scholar]
- 32.Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, Wu T, Allchorne A, Diatchenko L, Binshtok AM, Goldman D, Adolph J, Sama S, Atlas SJ, Carlezon WA, Parsegian A, Lötsch J, Fillingim RB, Maixner W, Geisslinger G, Max M, Woolf C. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–77. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]