Abstract
Neuronal activity regulates AMPA receptor trafficking, a process that mediates changes in synaptic strength, a key component of learning and memory. This form of plasticity may be induced by stimulation of the NMDA receptor which, among its activities, increases cyclic guanosine monophosphate through the nitric oxide synthase pathway. cGMP-dependent protein kinase type II (cGKII) is ultimately activated via this mechanism and AMPA receptor subunit GluA1 is phosphorylated at serine 845. This phosphorylation contributes to the delivery of GluA1 to the synapse, a step that increases synaptic strength. Previous studies have shown that cGKII-deficient mice display striking spatial learning deficits in the Morris Water Maze compared to wild-type littermates as well as lowered GluA1 phosphorylation in the postsynaptic density of the prefrontal cortex (Serulle, Zhang, Ninan, Puzzo, McCarthy, Khatri, Arancio, and Ziff, 2007; Wincott, Kim, Titcombe, Tukey, Girma, Pick, Devito, Hofmann, Hoeffer, and Ziff, 2013). In the current study, we show that cGKII knockout mice exhibit impaired working memory as determined using the prefrontal cortex-dependent Radial Arm Maze (RAM). Additionally, we report reduced repetitive behavior in the Marble Burying task (MB), and heightened anxiety-like traits in the Novelty Suppressed Feeding Test (NSFT). These data suggest that cGKII may play a role in the integration of information that conveys both anxiety-provoking stimuli as well as the spatial and environmental cues that facilitate functional memory processes and appropriate behavioral response.
1. Introduction
It is generally accepted that experience-dependent behavioral formation relies upon the modification of synaptic strength in the brain regions whose activities underlie the expression of behavior. Precise communication between the prefrontal cortex (PFC), striatum, amygdala, and hippocampus among other regions is critical in memory formation and retrieval as well as decision-making and appropriate action selection in processes such as executive function (Floresco, Seamans, and Phillips, 1997). The role of the PFC has been well studied in working memory, a component of executive function, and this controlled processing of information is necessary to achieve temporary goals (Baddeley, 1992; Ragozzino, Adams, and Kesner, 1998; Shimamura, 1995). The working memory system is thought to integrate perceptual information and long-term memory in order to generate motor commands and drive performance on complex tasks (Baddeley, 1992; 1996; 1998; Faw, 2003; Jones, 2002; Prabhakaran, Narayanan, Zhao, and Gabrieli, 2000). The PFC has a prominent role in the assimilation and communication of spatial information during tasks such as the Radial Arm Maze (RAM), a test designed to assess working memory (Floresco et al., 1997; Goldman-Rakic, 1990; Olton and Samuelson, 1976).
At the cellular level, memory is thought to result from long-lasting changes in synaptic function, a process also known as synaptic ‘plasticity’ (Eccles, 1964; Hebb, 1949; Malinow and Malenka, 2002). Neural activity regulates the molecular changes that underlie these synaptic alterations and involve the insertion and removal of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) (Kessels and Malinow, 2009). These ionotropic glutamate receptors are tetrameric and consist of GluA1-4 subunits (Hollmann and Heinemann, 1994; Malinow and Malenka, 2002). Receptors that lack the GluA2 subunit are Ca2+ -permeable and addition of these receptors postsynaptically may be triggered by N-Methyl-D-aspartic acid (NMDA) receptor activation. In one such pathway, a signaling cascade induced by the influx of Ca2+ promotes nitric oxide-dependent formation of cyclic guanosine monophosphate (cGMP) (Garthwaite and Boulton, 1995). cGMP-dependent protein kinase II (cGKII), a target of cGMP, phosphorylates serine 845 (S845) on the C-terminus of GluA1 which leads to increased levels of GluA1 in the plasma membrane (Incontro, Ciruela, Ziff, Hofmann, Sanchez-Prieto, and Torres, 2013; Serulle et al., 2007). Phosphorylation of S845, as well as other critical residues on the C-terminus of GluA1, lead to increases in excitatory neuronal transmission and are believed to play a role in memory processes (Boehm, Kang, Johnson, Esteban, Huganir, and Malinow, 2006; Lee, Takamiya, Han, Man, Kim, Rumbaugh, Yu, Ding, He, Petralia, Wenthold, Gallagher, and Huganir, 2003).
Appropriate behavioral responses are reliant upon functional memory processes as well as top-down control over emotional reactions to stimuli (Bishop, 2007). Previous behavioral studies of cGKII knockout (KO) animals suggest that this kinase is important for emotional responses (Werner, Raivich, Cowen, Strekalova, Sillaber, Buters, Spanagel, and Hofmann, 2004). Animals deficient in cGKII display heightened anxiety-like behaviors in the light-dark (L/D) box test as well as increased ethanol consumption in a two-bottle free choice test (Werner et al., 2004). cGKII may also be involved in learning and memory. Recent studies have shown striking learning and memory deficits in the Morris Water Maze in cGKII KO animals when compared to wild type (WT) controls (Wincott et al., 2013). Additionally, previous tests revealed a heightened acoustic startle response in cGKII KO animals as well as significant decreases in phosphorylation of GluA1 in the postsynaptic density (PSD) of PFC fractions (Wincott et al., 2013).
In the current study, we further characterize the role of cGKII in the PFC-dependent behavior. We have investigated cGKII KO mice in a PFC-dependent, working memory task: the RAM (D'Ardenne, Eshel, Luka, Lenartowicz, Nystrom, and Cohen, 2012; Fuster, 1973; Olton and Samuelson, 1976). This version of the task used in this study also assesses reference memory, a hippocampal-dependent process (Schmitt, Deacon, Reisel, Sprengel, Seeburg, Rawlins, and Bannerman, 2004). Using spatial cues, animals learn which of four of the eight arms are baited over the course of shaping and acquisition phases (Borroni, Fichtenholtz, Woodside, and Teyler, 2000). Re-entry into arms from which baits have been consumed is recorded as working memory errors (WMEs) and entry into arms where baits have never been discovered are recorded as reference memory errors (RMEs).
Based on our recent findings showing spatial learning deficits and lowered phosphorylation of GluA1 in the PSD of PFC fractions, we hypothesized that disrupted functioning of cGKII-mediated phosphorylation of S845 of GluA1 and the effects on AMPA receptor trafficking in the PFC of cGKII KO animals would result in increased working memory errors in the RAM in cGKII KO animals (Wincott et al., 2013). Additionally, we performed other behavioral tasks dependent in part on the PFC as well as other structures where cGKII is highly expressed, such as the amygdala (Werner et al., 2004). The Novelty Suppressed Feeding Test (NSFT) is a conflict test used to assess anxiety-like behaviors in animals (Shephard and Broadhurst, 1982) but has also been shown to be dependent on PFC function (Banasr and Duman, 2008; Sun, Liu, Yuan, Li, and Chen, 2012). We performed the NSFT to further characterize the reported anxiety-like phenotype in cGKII KO mice (Werner et al., 2004). Food-deprived animals are presented with a choice whereby they can either consume a pellet of food that has been positioned in the center of a brightly lit novel arena or they can remain around the perimeter of the arena and refrain from eating. Longer latency to consume food in this assay is considered to display an anxious phenotype (Fukumoto, Iijima, and Chaki, 2014; Iijima, Fukumoto, and Chaki, 2012). We also performed experiments using the Marble Burying (MB) assay. While this test has been used to investigate anxiety-like traits, more recently it has been shown to more accurately assess perseverative and repetitive behaviors, in which the prefrontal cortex plays a role (Burguiere, Monteiro, Feng, and Graybiel, 2013; Ichimaru, Egawa, and Sawa, 1995; Okada, Ota, Iida, Kishimoto, and Kishimoto, 2013; Thomas, Burant, Bui, Graham, Yuva-Paylor, and Paylor, 2009). MB is a frequently used assay in animal models of obsessive-compulsive disorder, a disorder thought to arise from inadequate function of the PFC (Okada et al., 2013; Rauch, Savage, Alpert, Dougherty, Kendrick, Curran, Brown, Manzo, Fischman, and Jenike, 1997; Shinomiya, Fujii, Sugimoto, Azuma, Tokunaga, Kitazumi, and Kamei, 2005).
The proceeding set of experiments was conducted to gain further insight into the consequences of cGKII gene deletion on PFC-dependent behaviors. Building on our previous work with cGKII KO mice, we find that cGKII KO animals exhibit increased anxiety-like traits, lowered perseverative behavior, and spatial learning and memory deficits, suggesting that cGKII plays a role in behaviors that involve the PFC.
2. Material and Methods
2.1. Statistics
Analysis of variance (ANOVA) and t tests were applied to behavior tests as necessary and each analysis is identified in the representative Results section. For the RAM, statistical analyses are two-tailed at a significance level of 0.05 and data on the graphs display mean ± SEM. Statistical analyses were performed using Graphpad Prism software, Version 6.0c (La Jolla, CA, USA).
2.2. Mice
cGKII KO generation has been previously described (Pfeifer, Aszodi, Seidler, Ruth, Hofmann, and Fassler, 1996): animals were backcrossed to C57BL/6 and bred at Taconic Farms in Germantown, NY. When the mice reached eight weeks of age, they were transferred to Memorial Sloan Kettering Cancer Center (MSKCC), Zuckerman Building, where they were housed under standard conditions of ~2-3 animals per cage (20-24 °C, 30-70% relative humidity, 12-h dark : 12-h light cycle, 0700 to 1900 hrs). All behavioral assays were conducted on adult male mice from the ages of 10-12 weeks at the beginning of testing during the latter part of the light cycle. Animal experiments were conducted in compliance with the Institutional Animal Care and Use Committee at the New York University School of Medicine. Assays took place in the Zuckerman Building Barrier Facility at MSKCC and were conducted in this order: MB, NSFT, RAM, Grip Strength, Wire-Hang and Nest-Building in two cohorts. For both cohorts, the time from the first test to the last was approximately five weeks.
2.3. Marble Burying
In MB (Broekkamp, Rijk, Joly-Gelouin, and Lloyd, 1986), each mouse was acclimated in a cage alone for half an hr in the testing room before being placed into a 22 × 16 inch rectangular Tupperware tub filled with sterile off-white mouse bedding tamped down to make a flat, even surface. Atop the bedding, twelve black marbles (Land of Marbles) had been carefully aligned in a uniform manner (3 × 4 in the center). The distance between marbles was approximately two inches. The distance of each outer marble was approximately two inches from the perimeter of the container wall. Each mouse was allowed to explore the environment freely for 30 mins. At the conclusion of the 30 mins, each mouse was removed from the container and the number of marbles buried to ½ of their depth was counted.
2.4. Novelty Suppressed Feeding Test
In the NSFT (Shephard and Broadhurst, 1982), each mouse was food deprived for ~24 hrs. At the end of this period, mice were acclimated in cages alone for half an hr in the testing room before being placed into a novel, brightly lit rectangular arena (12 × 18 inches). In the center of the arena, a food pellet was positioned atop a circular platform elevated one cm. Latency to eat was recorded, with ten mins as the maximum amount of time the animal was exposed to the new environment. Promptly after the animal began to eat or at the expiration of the ten mins, the mouse was placed back into its acclimation cage with a new food pellet that had been weighed. The amount of food the animal consumed in five mins following the test was measured.
2.5. Radial Arm Maze
The RAM task (Borroni et al., 2000; Olton and Samuelson, 1976) consisted of three stages: shaping, acquisition, and probe test, with a seven-day period between the end of the acquisition stage and the probe. Before the beginning of the shaping phase, animals were food restricted to 90% of their body weight. The apparatus used to test the animals consisted of an octagonal hub (maze center) with eight 36.8 cm arms protruding from the hub (Med Associates). At the end of each arm, a circular feeding receptacle was positioned where bait was placed at the beginning of each trial. Visible cues were positioned on walls surrounding the maze. Cues remained constant throughout testing.
Prior to testing, animals were removed from their housing room and brought to the behavioral testing area in their home cages. Each mouse was placed in an acclimation cage for 30 mins alone prior to testing. All animals were given two trials per day separated by one hr. For each mouse, four of the eight arms were selected randomly; those arms were baited and kept constant over the course of shaping, acquisition, and probe testing. This protocol was modified from Borroni and colleagues (Borroni et al., 2000) whereby our training protocol consisted of nine days during which mice were put in the maze for as long as it took them to find and consume baits from four arms that were randomly chosen to be baited (shaping stage). These same selected arms for each mouse were baited for each trial every day. The shaping stage was ended when the group of animals consumed, on average, greater than three baits over the course of two trials in a day. To follow, there were five days of an acquisition stage and the memory was probed seven days after this stage (probe test) during which the animals were housed normally. The type of bait used was a single chocolate sprinkle (Cake Mate) positioned in the center of the food receptacle at the end of each arm. Between trials, the maze was cleaned with a 70% ethanol solution and two trials were run per day.
Mice were allowed to explore freely until all four baits had been consumed or until ten mins had expired. The experimenter observed each mouse and recorded entries into baited arms as well as non-baited arms. Working memory errors (WMEs) were scored as re-entries into arms from which an animal had already consumed the bait and reference memory errors (RMEs) were recorded as entries into arms that had never been baited. “Entry” was defined as all four of the animals’ paws placed inside the arm. Also recorded was the amount of time taken to complete the task and the number of baits consumed, at maximum ten mins.
2.6. Grip Strength
The Grip Strength Test was conducted using an apparatus outfitted with a metal grid sensor and electronic device (Bioseb, Vitrolles Cedex, France) that measured maximum peak force of the animal's forepaws. Each animal was gently pulled across the grid three times in succession and the maximum force was measured.
2.7. Wire-Hang Test
For the Wire-Hang test, animals were placed atop a wire cage lid (Ray, Trammell, Verhulst, Ran, and Toth, 2011). The lid was shaken gently three times to allow mice to grip the wire with forepaws and hind paws before being inverted. Latency to fall was recorded, with the maximum hang time of 60 s.
2.8. Nest-Building
Nest-Building was conducted as described (Deacon, 2006). Mice were transferred to individual cages with wood-chip bedding approximately an hr before the dark cycle. Approximately 3 grams of nestlet (square white material shredded by animals during the dark cycle) were placed into the cages. Nests were assessed the following morning on a scale of 1-5, with the score of 1 assigned to a nestlet that had not been noticeably touched and was more than 90% intact and the score of 5 assigned to a crater-shaped nest with greater than 90% of the nestlet shredded. Scores in the middle of those numbers were assigned as described (Deacon, 2006).
3. Results
3.1. cGKII KO mice exhibit lowered perseverative behavior in MB
In Figure 1A, we show that the number of marbles buried in cGKII KO is significantly lower than WT. An unpaired Student's t test showed that the number of marbles buried by cGKII KO was significantly less than WT [t(12.08) = 3.39, p < 0.01]. Also presented is the average weight of cGKII KO and WT animals at the beginning of testing (Figure 1B). An unpaired Student's t test revealed a significant difference of weight in grams between cGKII KO and WT, with cGKII KO weighing less [t(15.09) = 4.15, p < 0.001]. At the conclusion of the battery of tests, weights were also recorded. Animals weighed on average the following: cGKII KO: 23.20 ± 0.46g and WT: 26.39 ± 0.65g. An unpaired Student's t test showed cGKII KO animals weighed significantly less than their WT littermates [t(13.24) = 4.01, p < 0.01]. cGKII KO animals’ weights ranged from 21.07g – 24.83g and WT animals’ weights ranged from 23.48g - 28.66g.
Figure 1.
MB and Weight. (A.) Number of marbles buried in the Marble Burying Test. Presented is the average (± SEM) number of marbles buried in an arena filled with bedding by wild type (WT), n=7, (black bar) and knockout cGKII (KO), n=11 (gray bar) animals during a 30 min period. (B.) Weight in grams. Presented is the average (± SEM) weight in grams of wild type (WT), n=8, (black bar) and knockout cGKII (KO), n=11 (gray bar) animals.
3.2. cGKII KO animals exhibit heightened anxiety-like behavior in the NSFT
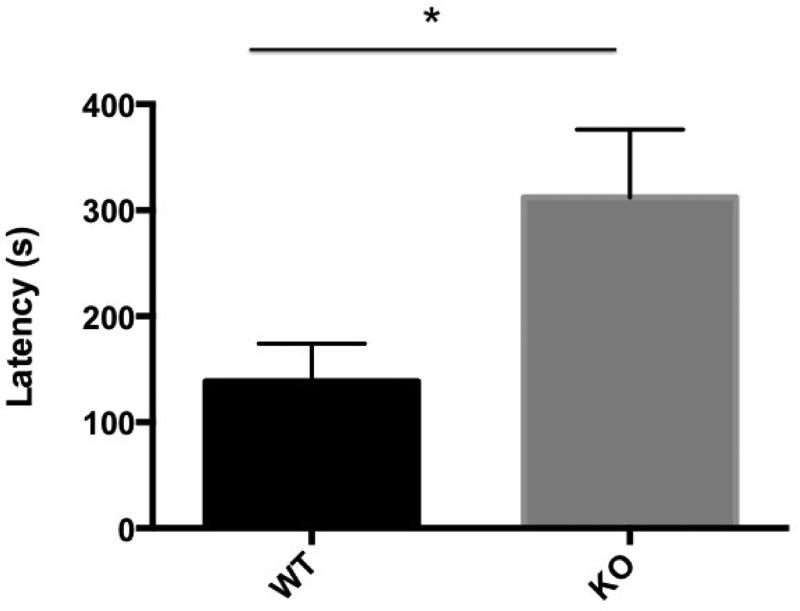
In Figure 2, we show latency for WT and cGKII KO to begin consumption of the food pellet positioned in the center of the arena. An unpaired Student's t test revealed that there were significant differences between cGKII KO and WT in latency to eat the exposed pellet. cGKII KO animals displayed significantly increased time to consume the pellet [t(15.15) = 4.01, p < 0.05]. This difference was not explained by overall hunger, because an unpaired Student's t test showed that the amount of food in grams consumed in the five mins after the test was not significantly different between cGKII KO and WT [t(8.39) = 0.60, p = 0.5635].
Figure 2.
Latency to eat in the Novelty Suppressed Feeding Test. Presented is the average (± SEM) latency in seconds (s) for food-deprived wild type (WT), n=8, (black bar) and knockout cGKII (KO), n=11 (gray bar) animals to eat a pellet positioned in the middle of a brightly lit novel arena.
3.3. cGKII KO mice display a greater number of working memory errors on the RAM
Our RAM testing revealed significant differences between cGKII KO and WT mice in working memory, with cGKII KO mice displaying deficits. A two-way ANOVA with repeated measures shown in Figure 3A revealed a significant main effect of genotype [F1,28 = 8.592, p = 0.007] as well as trial [F5,140 = 3.569, p = 0.005], with the greatest difference most evident on Day 1. There was not a significant Trial X Genotype interaction [F5,140 = 1.664, p = 0.147]. Also revealed were significant differences between cGKII KO and WT mice in reference memory, with cGKII KO animals showing impairments. A two-way ANOVA with repeated measures shown in Figure 3B revealed a significant main effect of genotype [F1,28 = 5.027, p = 0.033] as well as trial [F5,140 = 8.331, p < 0.0001]. There was a not a significant Trial X Genotype interaction [F5.140 = 1.709, p = 0.136]. The number of baits consumed during acquisition and the probe trial was not significantly different [F1,26 = 0.0086, p = 0.9266] between genotypes (3C). Also, latency to find baits was not significantly different between genotype as revealed by a two-way ANOVA with repeated measures [F1,28 = 1.365, p = 0.2525] but decreased significantly for both groups as the trials continued [F5,140 = 10.26, p < 0.0001] (Figure 3D).
Figure 3.
Learning and Memory in the Radial Arm Maze. (A). Across 5 days of acquisition (Acqu.) and 1 day memory probe (Pr.), presented is the average (± SEM) number of working memory errors (# WME) for wild type (WT), n=7, (dotted line) and knockout cGKII (KO), n=8 (solid line) animals. Days 1-5 of acquisition is presented after nine days of shaping. A memory probe of two trials was conducted seven days following the acquisition stage (Day 12). Working memory errors are re-entries into arms from which an animal has already collected bait. (B) Across 5 days of acquisition and 1 day memory probe, presented is the average (± SEM) number of reference memory errors (# RME) for WT (dotted line) and KO (solid line) animals. Days 1-5 of acquisition is presented after nine days of shaping. A memory probe of two trials was conducted seven days following the acquisition stage (Day 12). Reference memory errors are entries into arms from which an animal has never collected bait. (C). Across 5 days of acquisition and 1 day memory probe presented is the average (± SEM) number of baits found for WT (dotted line) and KO (solid line) animals. (D) Across 5 days of acquisition and 1 day memory probe, presented is the average (± SEM) number of seconds (s) it took for WT (dotted line) and KO (solid line) animals to collect all four baits. Four of the eight arms were selected at random to be baited for each animal and those same arms were baited each trial every day.
3.4. cGKII KO animals show lowered grip strength but no differences in the Wire-Hang test or Nest-Building
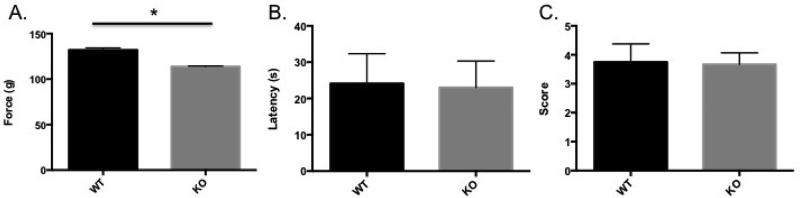
To assess the potential impact of reduced size and strength on the phenotypes we observed, we examined cGKII mice using the grip strength test. We found that cGKII KO mice had reduced grip strength compared to their wild-type littermates (Figure 4A). A two-way mixed ANOVA (genotype between subject/trial within subject) revealed a main effect of genotype [F1,16 = 6.727, p = 0.0196] on grip strength but no effect for trial [F2,32 = 0.1215, p = 0.8860] nor an interaction [F2,32 = 0.05422, p = 0.9473] between the two factors. Next we examined the cGKII KO mice using the Wire-Hang test, a test of coordination and strength that requires forepaws and hindpaws (Figure 4B). An unpaired Student's t test revealed no significant differences in latency to fall from the wire [t(15.10) = 0.10, p = 0.919]. Finally, we performed Nest-Building, which assesses motor coordination (Figure 4C). An unpaired Student's t test also showed no differences between cGKII KO and WT animals in this task [t(13.44) = 0.53, p = 0.605].
Figure 4.
Grip Strength, Wire-Hang test, and Nest-Building. A. Grip Strength. Presented is the average (± SEM) force in grams across three trials exerted by forepaws of wild type (WT), n=8, (black bar) and knockout cGKII (KO), n=10 (gray bar) animals. B. Wire-Hang test. Presented is the average (± SEM) latency in seconds (s) for WT (black bar) and KO (gray bar) animals to fall from an inverted cage lid. C. Nest-Building. Presented is the average (± SEM) score for WT (black bar) and KO (gray bar) animals in Nest-Building. Nests were assessed after 12 hrs of dark cycle on a scale of 1-5, with the score of 1 assigned to a nestlet that had not been noticeably touched, 2: with the nestlet partially torn, 3: nestlet mostly shredded, 4: with a flat but identifiable nest, and 5: with a crater-shaped nest with greater than 90% of the nestlet shredded.
4. Discussion
In this study, we report that cGKII KO mice display abnormal behaviors dependent in different degrees on PFC function. Specifically, we find that cGKII KO mice show working memory deficits in the RAM assay, a behavioral task heavily reliant on the PFC. We also find evidence, to a lesser degree, of reference memory deficits in cGKII KO animals in the RAM. Additionally, we provide evidence that cGKII activity is required for the PFC-dependent expression of anxiety-like behaviors. We corroborate and extend earlier findings of increased anxiety-like behavior in cGKII deficient mice using the conflict-based NSFT. Other studies have shown that the PFC plays a role in the display of anxiety-like traits as assessed by this assay (Banasr and Duman, 2008; Sun et al., 2012), bolstering our hypothesis that cGKII KO mice may have altered PFC function. Finally, we also report a lowered number of marbles buried in the MB test in cGKII KO animals as compared to WT suggesting lowered perseverative behavior. This study provides evidence that behaviors that involve the PFC may be altered in mice that are deficient in GKII.
One possible interpretation for these behavioral findings is proposed that there may be problems in the integration of information in cGKII KO. This may result from disrupted GluA1 phosphorylation and/or AMPAR trafficking in the PFC, an area where information from the hippocampus and amygdala converge (Ishikawa and Nakamura, 2003). This idea is supported by our previous biochemical findings which showed lowered phosphorylation of GluA1 in the PSD of PFC fractions (Wincott et al., 2013). These data combined with data from the current study suggest that cGKII may play a role in the PFC-mediated integration of perceptual information and environmental cues that facilitates adequate behavioral responses.
S845 phosphorylation on the C-terminus of GluA1 facilitates AMPAR trafficking, a process that plays a critical role in synapse potentiation and is necessary for learning and for memory retrieval (Serulle et al., 2007). Previous studies have shown that cGKII KO animals display pronounced spatial learning and memory deficits in the Morris Water Maze (Wincott et al., 2013). This result conflicted somewhat with earlier studies that reported that synaptic plasticity, as measured by long-term potentiation (LTP) in cGKII KO hippocampal slices appeared normal (Kleppisch, Pfeifer, Klatt, Ruth, Montkowski, Fassler, and Hofmann, 1999). However, in a later study, it was shown that LTP was greatly reduced in WT hippocampal slices by acute inhibition of cGKII using pharmacological agents (Serulle et al., 2007). It is possible that compensatory mechanisms exist in cGKII KO animals; another kinase, Protein Kinase A (PKA), phosphorylates the same critical residue on GluA1 (Roche, O'Brien, Mammen, Bernhardt, and Huganir, 1996) and it is possible that in some brain regions, other kinase activity may partially and/or imperfectly compensate for the loss of cGKII. To test the hypothesis that possible compensatory mechanisms are insufficient and that cGKII may indeed be required for the expression of hippocampal–dependent behavior, we tested cGKII KO mice in hippocampus-dependent learning and memory behavioral tasks. In support of this notion, we found learning and memory deficits in the Morris Water Maze (Wincott et al., 2013). We extended the results of this study to investigate the possibility that cGKII-signaling in other brain regions could also play a role in the Morris Water Maze learning and memory deficits (Kleppisch et al., 1999; Wincott et al., 2013). Biochemical studies revealed lowered phosphorylation of GluA1 S845 in the PSD of PFC fractions in cGKII KO animals compared to WT, indicating that this brain region may be involved (Wincott et al., 2013).
These biochemical data led us to perform additional assays examining behavioral tasks that were PFC-dependent (Banasr and Duman, 2008; Chamberlain, Menzies, Hampshire, Suckling, Fineberg, del Campo, Aitken, Craig, Owen, Bullmore, Robbins, and Sahakian, 2008; Fritts, Asbury, Horton, and Isaac, 1998; Ragozzino et al., 1998; Sun et al., 2012). We selected the RAM assay to assess working memory in cGKII KO animals, a process that has been well-studied as relying upon various subregions of the PFC (D'Ardenne et al., 2012; Funahashi and Kubota, 1994; George, Mandyam, Wee, and Koob, 2008; McCarthy, Puce, Constable, Krystal, Gore, and Goldman-Rakic, 1996; Nielsen-Bohlman and Knight, 1999; Perlstein, Dixit, Carter, Noll, and Cohen, 2003). Lesions to dorsal anterior cingulate cortex have produced deficits in acquisition of this task in rats and others have shown that the prelimbic region is also important for RAM working memory tasks (Joel, Tarrasch, Feldon, and Weiner, 1997; Taylor, Latimer, and Winn, 2003). These data are also consistent with other studies showing that inhibition of PKA in the PFC, another kinase that phosphorylates the same residue on GluA1, also produces working memory deficits (Aujla and Beninger, 2001; Serulle et al., 2007). Combined, these earlier findings are consistent with our biochemical studies and central hypothesis: that cGKII plays a role in PFC-dependent cognitive function.
The loss of cGKII activity may link lowered phosphorylation of GluA1 in the PSD of PFC and the working memory deficits we observe (Figure 3). Our study of working memory as assessed by the RAM bolsters the implications of our previous studies indicating that learning and memory are disrupted in cGKII KO animals despite the fact that LTP appears normal in cGKII KO hippocampal slices (Kleppisch et al., 1999; Wincott et al., 2013). Another possibility is that the differences we observed during the acquisition period of the task (Figure 3A) are related to differences in learning rates or problems acquiring strategy, rather than working memory deficits in cGKII KO animals as compared to WT (Joel et al., 1997). Nonetheless, our findings support the notion that cGKII activity is required for the normal expression of PFC-dependent behavior. To more fully understand the nature of the observed learning and memory phenotypes of these animals, it will be informative to follow up these studies with tasks such as the delayed-spatial win-shift task wherein animals keep information available and “on-line” when solving the task in the moment as well as across a delay (Taylor et al., 2003). cGKII may also play a role in anxiolytic responses that may rely on PFC activity (Banasr and Duman, 2008; Sun et al., 2012). cGKII KO animals have been reported to show increased anxiogenic responses in the L/D box test as well as the Elevated Zero maze (Werner et al., 2004). In contrast to these findings, we did not observe differences between cGKII KO and WT animals in the Open Field Assay (OFA) in center to total time ratio (Wincott et al., 2013), and thus to clarify this apparent discrepancy, we sought to characterize anxiety-like phenotypes further. cGKII is highly expressed in the amygdala, which sends afferents to the PFC, so therefore we conducted another assay used to measure anxiety-like behaviors, the NSFT, to clarify the reported phenotype (Werner et al., 2004). Consistent with the notion that cGKII is required for normal anxiolytic responses, cGKII KO mice displayed longer latencies to feed in a brightly lit arena (Figure 2). In contrast to our OFA data, our NSFT results agree with previous reports using the L/D box test (Werner et al., 2004). One possibility to explain the apparent discrepancy is that the L/D box test and NFST may assess behavior involving different circuits than OFA in the display of anxiety-like traits. Additionally, there is some controversy regarding which behavioral traits OFA accurately assesses (Careau, Bininda-Emonds, Ordonez, and Garland, 2012; Stanford, 2007). Combined, our data suggest that cGKII deficiency and the subsequent reduction of S845 phosphorylation of GluA1 promotes increased anxiety-like behaviors.
Traits associated with obsessive-compulsive disorder such as repetitive behavior are controlled in part by subregions of the PFC (Burguiere et al., 2013; Chamberlain et al., 2008; Milad and Rauch, 2012). To investigate repetitive behavior in cGKII KO mice, we employed the MB task. Because MB has also been used as measure of anxiety-related behavior, based on previous findings (Werner et al., 2004; Wincott et al., 2013) and our RAM data (Figure 3A), we hypothesized that we would observe enhanced marble burying in cGKII KO mice. Interestingly however, we observed a lower number of marbles buried in cGKII KO animals compared to WT. While these findings do support the idea that cGKII activity is linked to the normal display of PFC-mediated repetitive behaviors, these data conflict with what may have been expected if MB accurately reports innate anxiogenic responses. An elegant study by Thomas and colleagues (Thomas et al., 2009) revealed that MB did not correlate with two other anxiety tests, OFA and the L/D box test in a variety of mouse strains. In a series of comprehensive analyses, these researchers showed that MB is more accurately a test of perseverative and repetitive behavior; we therefore propose that our observed MB results in cGKII KO animals reflect a lowered perseveration phenotype, which is congruent with altered PFC function (Burguiere et al., 2013; Chamberlain et al., 2008; Milad and Rauch, 2012; Thomas et al., 2009). Pharmacological modulators of AMPARs have been shown to inhibit marble burying (Iijima, Kurosu, and Chaki, 2010). If cGKII removal modifies the number and/or function of synaptic AMPARs, knockout of the gene may mimic the effects of these drugs and reduce marble burying. Another potential explanation for these results is the previously described dwarfism present in the cGKII KO (Pfeifer et al., 1996); reduced size and strength may simply promote reduced marble burying activity in the cGKII KO mouse. The weights of cGKII KO animals in comparison to WT were significantly less (Figure 1B). To reduce the possibility that our behavioral results were confounded by dwarfism, we performed a pair of tests to assess neuromuscular function, the Grip Strength and the Wire-Hang test. As expected, the grip strength of forepaws in the cGKII KO was significantly lower than WT although there were no differences in the Wire-Hang test, in which the animal was assessed for latency to fall from an inverted cage lid while clinging by both its forepaws and hind paws. While the lowered grip strength of cGKII KO animals should be taken into consideration, it is unclear whether propensity to bury marbles is reflected by grip strength or whether the marbles simply fall through the bedding as an animal burrows throughout the arena (Thomas et al., 2009).
To further investigate the MB phenotype, motor coordination, and possible brain regions involved in the observed behaviors, the Nest-Building assay was employed. No differences were observed between cGKII KO and WT, indicating that the motor skills necessary to build nests in cGKII KO animals may be intact. Additionally, because cGKII expression is widespread, loss of function in regions other than the PFC may contribute the observed behavioral phenotype. In future studies, it would be highly beneficial to perform additional experiments conducted using mice where cGKII is conditionally eliminated post-developmentally in a region or cell-specific manner in the forebrain so as to assess whether the size and weight of animals could account for deficits in the behaviors we tested. This approach would allow us to conclusively eliminate the potential dwarfism confound from interpretation of these and earlier findings in which cGKII is removed from the brain.
Studies have shown a relationship between memory and anxiety and it has long been suggested that there is overlap between emotion-linked processes and cognitive function (Kalueff and Murphy, 2007; Venault and Chapouthier, 2007). Although our results and those from similar studies investigating GluA1 phosphorylation (Aujla and Beninger, 2001) suggest that cGKII regulation of GluA1 is important for working memory, we cannot rule out that the anxiety-like traits we observed in cGKII KO animals are not at least partially involved in the reported RAM learning and memory phenotypes. To address this possibility, we provided animals an extended shaping period to become acclimated with the new environment including to the experimenters during our modified version of the task. Nevertheless, it will be useful in future studies to conduct memory tests in which experimenters are not present, such as is possible with the use of touch screens/operant conditioning chambers. In any case, studies of human subjects have revealed a correlation between learning disabilities and anxiety (Nelson and Harwood, 2011). It may be of interest to investigate the anxiety and memory relationship further using cGKII KO mice as a mouse model for this observed interaction in human patients.
Differences in coping styles may also play a role in the expression of the observed behaviors (Coppens, de Boer, and Koolhaas, 2010; Koolhaas, Korte, De Boer, Van Der Vegt, Van Reenen, Hopster, De Jong, Ruis, and Blokhuis, 1999). The methods by which animals cope with novel and/or anxiety-provoking stimuli, as in the MB task, are thought to rely upon strategy implementation. If cGKII KO animals exhibit problems with implementing strategy, as is formally possible with our current RAM data (Figure 3A) and previous Morris Water Maze studies (Wincott et al., 2013), this may account for the apparent discrepancies observed in OFA and MB (Figure 1A). To further address this possibility, other tests such as the 5-choice-serial-reaction-time task (Muir, Everitt, and Robbins, 1996; Robbins, Everitt, Marston, Wilkinson, Jones, and Page, 1989) and the social interaction test (File and Hyde, 1978) can be used to assess traits such as behavioral flexibility and anxiety-like behavior. What is clear from the current study is that the removal of cGKII from the brain has a multi-faceted impact on the expression of numerous behaviors. cGKII activity is likely involved with coordinating the function within and between multiple brain regions to allow the normal expression of learning and memory.
5. Conclusions
Here we report that mice deficient in cGKII display working memory deficits, decreased perseverative behavior, and increased anxiety-like traits in the conflict-based NSFT. Our previous studies showed lowered phosphorylation of GluA1 in the PSD of PFC in cGKII KO animals as well as spatial learning and memory deficits, indicating an important role for this kinase in memory-based tasks as reflected by the PFC dysfunction of the cGKII KO reported here. We propose that converging signals in the PFC of cGKII KO are disrupted as the result of lowered GluA1 phosphorylation and that this disruption may account for the inadequate processing of environmental information necessary for appropriate behavioral response. The cGKII KO mouse may serve as a useful model for intellectual disabilities and other neurological disorders involving PFC function in humans. Understanding the importance of AMPAR trafficking and S845 phosphorylation in these disorders may help identify therapeutic targets for learning disabilities.
Highlights.
* cGKII KO are significantly deficient in working memory in the Radial Arm Maze
* cGKII KO show decreased repetitive behavior in Marble Burying
* cGKII KO exhibit increased anxiety-like traits as assessed by the Novelty-Suppressed Feeding Test
Acknowledgements
This work was supported by National Institutes of Health Grant R01MH067229 (to EBZ), Alzheimer's Association Grant MNIRGDP-12-258900 and a Simons Foundation Autism Investigator Award SFARI 274443 (to CAH). CW was supported by NIMH Training Grant T32MH019524. We would like to thank Natasha Tirko, Patrick Bader, and Richard Tsien for allowing us the use of their RAM and providing us with invaluable assistance. We would also like to thank Adam Mar for his helpful assistance in the interpretation of our data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aujla H, Beninger RJ. Hippocampal-prefrontocortical circuits: PKA inhibition in the prefrontal cortex impairs delayed nonmatching in the radial maze in rats. Behavioral neuroscience. 2001;115:1204–1211. [PubMed] [Google Scholar]
- Baddeley A. Working Memory: The Interface between Memory and Cognition. Journal of cognitive neuroscience. 1992;4:281–288. doi: 10.1162/jocn.1992.4.3.281. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory. C R Acad Sci III. 1998;321:167–173. doi: 10.1016/s0764-4469(97)89817-4. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial Loss in the Prefrontal Cortex Is Sufficient to Induce Depressive-like Behaviors. Biological psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in cognitive sciences. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Borroni AM, Fichtenholtz H, Woodside BL, Teyler TJ. Role of voltage-dependent calcium channel long-term potentiation (LTP) and NMDA LTP in spatial memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:9272–9276. doi: 10.1523/JNEUROSCI.20-24-09272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. European journal of pharmacology. 1986;126:223–229. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Burguiere E, Monteiro P, Feng GP, Graybiel AM. Optogenetic Stimulation of Lateral Orbitofronto-Striatal Pathway Suppresses Compulsive Behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careau V, Bininda-Emonds OR, Ordonez G, Garland T., Jr. Are voluntary wheel running and open-field behavior correlated in mice? Different answers from comparative and artificial selection approaches. Behavior genetics. 2012;42:830–844. doi: 10.1007/s10519-012-9543-0. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Aitken M, Craig K, Owen AM, Bullmore ET, Robbins TW, Sahakian BJ. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Coppens CM, de Boer SF, Koolhaas JM. Coping styles and behavioural flexibility: towards underlying mechanisms. Philosophical Transactions of the Royal Society B-Biological Sciences. 2010;365:4021–4028. doi: 10.1098/rstb.2010.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD. Feature Article: Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19900–19909. doi: 10.1073/pnas.1116727109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nature protocols. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Eccles JC. The physiology of synapses. Springer; Berlin: 1964. [Google Scholar]
- Faw B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: a tutorial review. Consciousness and cognition. 2003;12:83–139. doi: 10.1016/s1053-8100(02)00030-2. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? British journal of pharmacology. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. Journal of Neuroscience. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritts ME, Asbury ET, Horton JE, Isaac WL. Medial prefrontal lesion deficits involving or sparing the prelimbic area in the rat. Physiology & behavior. 1998;64:373–380. doi: 10.1016/s0031-9384(98)00096-1. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S. Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology. 2014 doi: 10.1007/s00213-013-3378-0. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Kubota K. Working memory and prefrontal cortex. Neuroscience research. 1994;21:1–11. doi: 10.1016/0168-0102(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Fuster J. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. Journal of neurophysiology. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annual review of physiology. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and Circuit Basis of Working Memory in Prefrontal Cortex of Nonhuman-Primates. Progress in brain research. 1990;85:325–336. doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior; a neuropsychological theory. Wiley; New York: 1949. [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annual review of neuroscience. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Ichimaru Y, Egawa T, Sawa A. 5-HT1A-receptor subtype mediates the effect of fluvoxamine, a selective serotonin reuptake inhibitor, on marble-burying behavior in mice. Japanese journal of pharmacology. 1995;68:65–70. doi: 10.1254/jjp.68.65. [DOI] [PubMed] [Google Scholar]
- Iijima M, Fukumoto K, Chaki S. Acute and sustained effects of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. Behavioural brain research. 2012;235:287–292. doi: 10.1016/j.bbr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Iijima M, Kurosu S, Chaki S. Effects of agents targeting glutamatergic systems on marble-burying behavior. Neuroscience letters. 2010;471:63–65. doi: 10.1016/j.neulet.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Incontro S, Ciruela F, Ziff E, Hofmann F, Sanchez-Prieto J, Torres M. The type II cGMP dependent protein kinase regulates GluA1 levels at the plasma membrane of developing cerebellar granule cells. Biochimica et biophysica acta. 2013;1833:1820–1831. doi: 10.1016/j.bbamcr.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. Journal of Neuroscience. 2003;23:9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Tarrasch R, Feldon J, Weiner I. Effects of electrolytic lesions of the medial prefrontal cortex or its subfields on 4-arm baited, 8-arm radial maze, two-way active avoidance and conditioned fear tasks in the rat. Brain research. 1997;765:37–50. doi: 10.1016/s0006-8993(97)00334-x. [DOI] [PubMed] [Google Scholar]
- Jones MW. A comparative review of rodent prefrontal cortex and working memory. Curr Mol Med. 2002;2:639–647. doi: 10.2174/1566524023361989. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Murphy DL. The importance of cognitive phenotypes in experimental modeling of animal anxiety and depression. Neural plasticity. 2007;2007:52087. doi: 10.1155/2007/52087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppisch T, Pfeifer A, Klatt P, Ruth P, Montkowski A, Fassler R, Hofmann F. Long-term potentiation in the hippocampal CA1 region of mice lacking cGMP-dependent kinases is normal and susceptible to inhibition of nitric oxide synthase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:48–55. doi: 10.1523/JNEUROSCI.19-01-00048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and biobehavioral reviews. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annual review of neuroscience. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cerebral cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends in cognitive sciences. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cerebral cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Nelson JM, Harwood H. Learning disabilities and anxiety: a meta-analysis. J Learn Disabil. 2011;44:3–17. doi: 10.1177/0022219409359939. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal cortical involvement in visual working memory. Brain research. Cognitive brain research. 1999;8:299–310. doi: 10.1016/s0926-6410(99)00035-x. [DOI] [PubMed] [Google Scholar]
- Okada K, Ota T, Iida J, Kishimoto N, Kishimoto T. Lower prefrontal activity in adults with obsessive-compulsive disorder as measured by near-infrared spectroscopy. Progress in neuro-psychopharmacology & biological psychiatry. 2013;43:7–13. doi: 10.1016/j.pnpbp.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of Places Passed - Spatial Memory in Rats. Journal of Experimental Psychology-Animal Behavior Processes. 1976;2:97–116. [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biological psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JDE. Integration of diverse information in working memory within the frontal lobe. Nature neuroscience. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behavioral neuroscience. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Dougherty D, Kendrick A, Curran T, Brown HD, Manzo P, Fischman AJ, Jenike MA. Probing striatal function in obsessive-compulsive disorder: a PET study of implicit sequence learning. The Journal of neuropsychiatry and clinical neurosciences. 1997;9:568–573. doi: 10.1176/jnp.9.4.568. [DOI] [PubMed] [Google Scholar]
- Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a mouse model for assessing fatigue during chemotherapy. Comp Med. 2011;61:119–130. [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, Marston HM, Wilkinson J, Jones GH, Page KJ. Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behavioural brain research. 1989;35:221–240. doi: 10.1016/s0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Schmitt WB, Deacon RM, Reisel D, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Spatial reference memory in GluR-A-deficient mice using a novel hippocampal-dependent paddling pool escape task. Hippocampus. 2004;14:216–223. doi: 10.1002/hipo.10168. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard RA, Broadhurst PL. Hyponeophagia and arousal in rats: effects of diazepam, 5-methoxy-N,N-dimethyltryptamine, d-amphetamine and food deprivation. Psychopharmacology. 1982;78:368–372. doi: 10.1007/BF00433744. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Memory and the prefrontal cortex. Annals of the New York Academy of Sciences. 1995;769:151–159. doi: 10.1111/j.1749-6632.1995.tb38136.x. [DOI] [PubMed] [Google Scholar]
- Shinomiya K, Fujii Y, Sugimoto Y, Azuma N, Tokunaga S, Kitazumi K, Kamei C. Effect of paroxetine on marble-burying behavior in mice. Methods and findings in experimental and clinical pharmacology. 2005;27:685–687. doi: 10.1358/mf.2005.27.10.948883. [DOI] [PubMed] [Google Scholar]
- Stanford SC. The Open Field Test: reinventing the wheel. Journal of psychopharmacology. 2007;21:134–135. doi: 10.1177/0269881107073199. [DOI] [PubMed] [Google Scholar]
- Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1305–1320. doi: 10.1038/npp.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CL, Latimer MP, Winn P. Impaired delayed spatial win-shift behaviour on the eight arm radial maze following excitotoxic lesions of the medial prefrontal cortex in the rat. Behavioural brain research. 2003;147:107–114. doi: 10.1016/s0166-4328(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venault P, Chapouthier G. Plasticity and anxiety. Neural plasticity. 2007;2007:75617. doi: 10.1155/2007/75617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C, Raivich G, Cowen M, Strekalova T, Sillaber I, Buters JT, Spanagel R, Hofmann F. Importance of NO/cGMP signalling via cGMP-dependent protein kinase II for controlling emotionality and neurobehavioural effects of alcohol. The European journal of neuroscience. 2004;20:3498–3506. doi: 10.1111/j.1460-9568.2004.03793.x. [DOI] [PubMed] [Google Scholar]
- Wincott CM, Kim S, Titcombe RF, Tukey DS, Girma HK, Pick JE, Devito LM, Hofmann F, Hoeffer C, Ziff EB. Spatial memory deficits and motor coordination facilitation in cGMP-dependent protein kinase type II-deficient mice. Neurobiol Learn Mem. 2013;99:32–37. doi: 10.1016/j.nlm.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]